- 1Department of Pharmacology, College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2College of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, China
- 3Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
With the increase of the aging population, the high mortality and disability rates caused by ischemic stroke are some of the major problems facing the world, and they dramatically burden the society. Panax notoginseng (Burk) F. H. Chen, a traditional Chinese medicine, is commonly used for promoting blood circulation and removing blood stasis, and its main bioactive components are Panax notoginseng saponins (PNS). Therefore, we performed a meta-analysis on focal cerebral ischemia-reperfusion animal models established with middle cerebral artery occlusion (MCAO) surgery to evaluate the therapeutic effect of PNS. We systematically searched the reports of PNS in MCAO animal experiments in seven databases. We assessed the study quality using two literature quality evaluation criteria; evaluated the efficacy of PNS treatment based on the outcomes of the neurological deficit score (NDS), cerebral infarct volume (CIV), and biochemical indicators via a random/fixed-effects model; and performed a subgroup analysis utilizing ischemia duration, drug dosage, intervention time, and administration duration. We also compared the efficacy of PNS with positive control drugs or combination treatment. As a result, we selected 14 eligible studies from the 3,581 searched publications based on the predefined exclusion-inclusion criteria. PNS were significantly associated with reduced NDS, reduced CIV, and inhibited release of the inflammatory factors IL-1β and TNF-α in the focal MCAO rat models. The PNS combination therapy outperformed the PNS alone. In addition, ischemia time, drug dosage, intervention time, and administration duration in the rat models all had significant effects on the efficacy of PNS. Although more high-quality studies are needed to further determine the clinical efficacy and guiding parameters of PNS, our results also confirmed that PNS significantly relieves the focal cerebral ischemia-reperfusion in rat models. In the animal trials, it was suggested that an early intervention had significant efficacy with PNS alone or PNS combination treatment at a dosage lower than 25 mg/kg or 100–150 mg/kg for 4 days or longer. These findings further guide the therapeutic strategy for clinical cerebral ischemic stroke.
Introduction
As the population ages, stroke, especially ischemic stroke, has become a serious disease that threatens human life (Benjamin et al., 2018). The global prevalence of cerebrovascular disease was 80.1 million people in 2016. Of these patients, 67.6 million were affected by ischemic stroke (Benjamin et al., 2018; Benjamin, 2020). There was a 2.7% increase in the prevalence of ischemic stroke and a 1.7% decrease in the prevalence of hemorrhagic stroke from 2006 to 2016. Two point seven (2.7) million cases died of ischemic stroke and 2.8 million died of hemorrhagic ischemic stroke. Based on comparisons in the past few decades, the prevalence and mortality rates of stroke have generally declined in high-income countries, but no significant changes have been found in low- and middle-income countries (Nogles and Galuska, 2020). This situation is likely related to advancements in the awareness, treatment, and control rates of modifiable risk factors in higher-income countries. In the United States (US), nearly 795,000 people suffer from a new or recurrent stroke each year, with 87% having experienced ischemic strokes, 10% having experienced intracerebral hemorrhage (ICH) strokes, and 3% having experienced subarachnoid hemorrhage (SAH) strokes. In China, stroke is the leading cause of death among all other causes (Li et al., 2019). The incidence of hemorrhagic stroke has decreased by 1.7% annually, and the ischemic stroke has increased by 8.7% from 1984 to 2004 (Boehme et al., 2017).
The middle cerebral artery (MCA) is the most common artery involved in acute ischemic stroke (AIS) (Nogles and Galuska, 2020), which causes severe nerve damage and poor prognosis. Studies (Ng et al., 2007) have shown that the two most severe ischemic strokes are those in more than one vascular territory (MVT) and MCA. These strokes both cause severe neurocognitive impairments encompassing executive functions, memory, attention, and behavioral issues, such as depression, agitation, and abulia (van Lieshout et al., 2019; Busk et al., 2020; Chen et al., 2020; Draaisma et al., 2020; Syafrita et al., 2020). MCA strokes may even lead to further visuospatial cognitive impairments and impaired language. The small proportion of patients with MCA stroke who are discharged home is most probably because of reduced main functional measure scores and increased disability (Ng et al., 2007). Both treatment and rehabilitation for stroke pose enormous economic cost and cause severe nursing and financial burdens to patients and families (Johnson et al., 2019).
Early effective prevention and emergency treatment can maximize the prevention of stroke and minimize stroke cerebral nerve injury. The three broad levels of stroke prevention (Boehme et al., 2017) are primordial prevention to form a healthy lifestyle, primary prevention to protect nonstroke and transient ischemic attack (TIA) individuals from risk factors, and secondary prevention to prevent stroke recurrence. The suitable treatments for an acute ischemic stroke may be medical thrombolysis (intravenous alteplase) within 4.5 h, antiplatelet therapy within 12 h, endovascular thrombectomy (EVT) within 24 h, and early management of patients considered for hemicraniectomy within 48 h (Boulanger et al., 2018; Nogles and Galuska, 2020). There is no curative therapy if the treatment window is missed, and treatments will only improve symptoms and prevent relapse (Nogles and Galuska, 2020).
Although the conventional treatment has significant effects on acute ischemic stroke, ethnic medicine also plays an important role, especially in the field of traditional Chinese medicine (TCM). TCM plays an essential role in the diagnosis, prevention, and treatment of patients suffering from AIS in China for more than 2,000 years. Modern scholars of TCM also point out that blood stasis is the principal problem in the process of occurrence, development, and outcome of ischemic stroke (Wang et al., 2014; Zhong et al., 2015; Sun et al., 2016; Chai and Zhang, 2018). Therefore, in the prevention (Chang et al., 2016; Tsai et al., 2016; Song et al., 2019), treatment (Chen et al., 2019; Hsu et al., 2019; Luo et al., 2019), and rehabilitation (Huang et al., 2018; Zhao et al., 2018) of ischemic stroke, conventional treatment combined with drugs that promote blood circulation and remove blood stasis can be an effective intervention.
In the clinical practice of TCM, Chinese herbal medicine is generally considered nontoxic and potentially therapeutic for patients and is extensively used in stroke patients, although it is lacking in high-quality supportive literature (Yu et al., 2019; Wei et al., 2020). Panax notoginseng (Burkill) F. H. Chen (P. notoginseng), as well as its extract components or pharmaceutical preparations, is a commonly used medicine for promoting blood circulation and removing blood stasis (He et al., 2011; Gao et al., 2013; Gui et al., 2013; Liu et al., 2014a; Luo et al., 2018). P. notoginseng has platelet aggregation inhibition, anti-inflammation, and antioxidation effects and improves the absorption of intracranial hematoma and promotes the recovery of nerve function. Given the high morbidity and mortality of stroke, animal models have been developed over 4 decades to replicate as many aspects of human stroke as possible to further understand the underlying pathophysiological response and to explore the potential treatments (Kwolek-Klimkiewicz et al., 1996; Makkiyah and Sadewo, 2019; Wei et al., 2019).
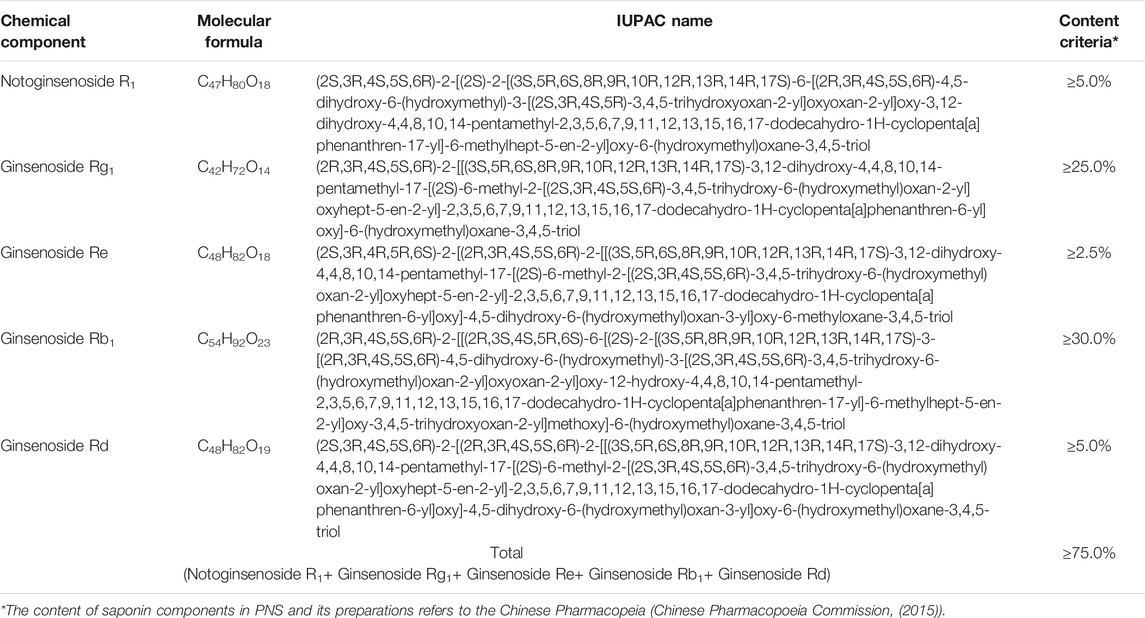
Panax notoginseng saponins (PNS) are recognized as the main bioactive ingredients of P. notoginseng, including different dammarane-type saponins (Yoshikawa et al., 2003; Xu et al., 2019). A series of commercial medicinal products of PNS-related preparations have been widely used in China, such as Xuesaitong injections, Xuesaitong capsules, Xuesaitong granules, and Xueshuantong capsules. According to the Chinese Pharmacopeia (Chinese Pharmacopoeia Commission, (2015)) for PNS-related preparations, the main chemical constituents of PNS contain five dammarane-type saponins, including notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd (Figure 1 and Table 1). The total content of the five ingredients is not less than 75% by high-performance liquid chromatography (HPLC) (Table 1).
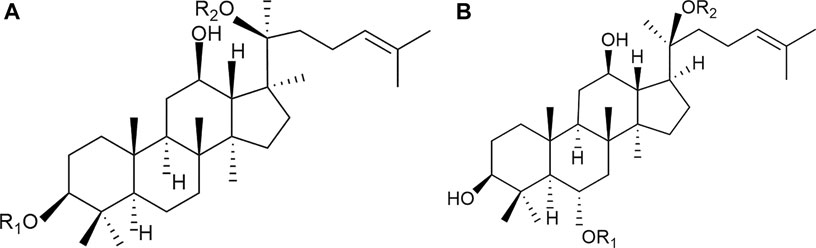
FIGURE 1. Chemical structures of the main saponins in PNS. The main five saponins were divided into two different dammarane-type saponins, namely, (A) 20(S)-protopanaxatriol saponins (PTS), including notoginsenoside R1, ginsenoside Rg1, and Re, and (B) 20(S)-protopanaxadiol saponins (PDS), including ginsenoside Rb1 and Rd.
Based on a large-scale trove of experimental animal data, this study systematically evaluated and meta-analyzed the efficacy of PNS in middle cerebral artery occlusion (MCAO) animal models with focal cerebral ischemia-reperfusion (I/R).
Methods
Review Protocol
This article is a meta-analysis based on Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We registered the review protocol at the International Prospective Register of Systematic Reviews (PROSPERO) (registration no. CRD42020182383).
Search Strategy
We selected relevant studies from the publications of February 2020 in PubMed, Embase, Medline, Web of Science, Chinese National Knowledge Infrastructure (CNKI), and Wanfang Data information site. The language was restricted to Chinese and English. The subject terms for the Chinese literature search were Panax notoginseng (三七) and ischemia (缺血) OR cerebral ischemia (脑缺血). The MeSH terms and text words of Panax notoginseng, brain ischemia, stroke, and cerebrovascular disorder, were used to search English literature combinatorially. After the document retrieval strategy was formulated, two investigators (Tao Sun and Xing-Bao Tao) independently performed document retrieval. Disputes in the selection process were discussed and resolved together if any.
Inclusion and Exclusion Criteria
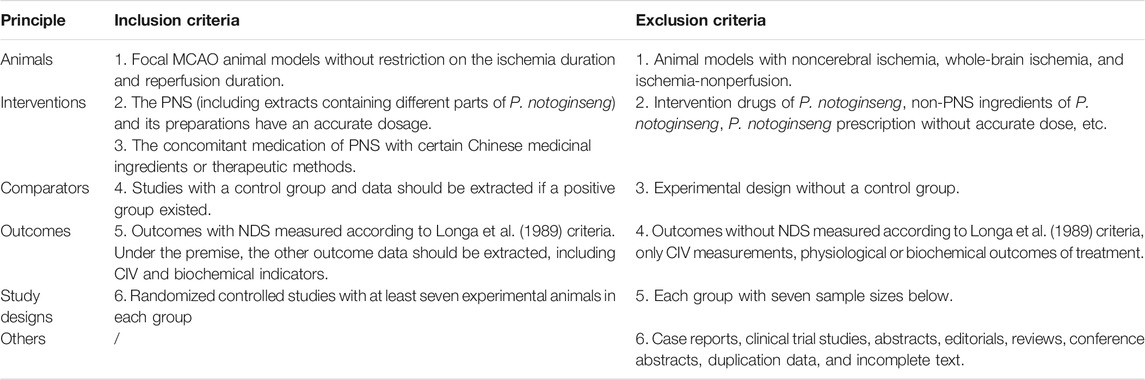
This systematic evaluation investigated the pharmacodynamics of PNS in animal models with focal cerebral I/R. According to the literature title, abstract, and full-text article, we rated the studies as eligible based on the predetermined inclusion and exclusion criteria (Table 2). The inclusion and exclusion criteria were based entirely on, but not limited to, animals, interventions, comparators, outcomes, and study designs. In this work, a professional document management software (Endnote X9) was used by two investigators independently, and disagreements were addressed by a third investigator (YX).
Quality Assessment
The SYRCLE’s Risk of Bias tool was adopted to evaluate the risk of bias for all included study (Hooijmans et al., 2014), including (1) random sequence generation; (2) baseline characteristics; (3) allocation concealment; (4) random housing; (5) blinded investigators; (6) blinded outcome assessment; (7) blinded outcome; (8) incomplete outcome data; (9) selective outcome reporting; and (10) ethical considerations. As the weights of the ten entries might vary depending on the outcome and review, it is challenging to justify the weights assigned. The total score inevitably assigned the “weights” to specific domains in the tool. Therefore, we just evaluated the items rather than summarizing them.
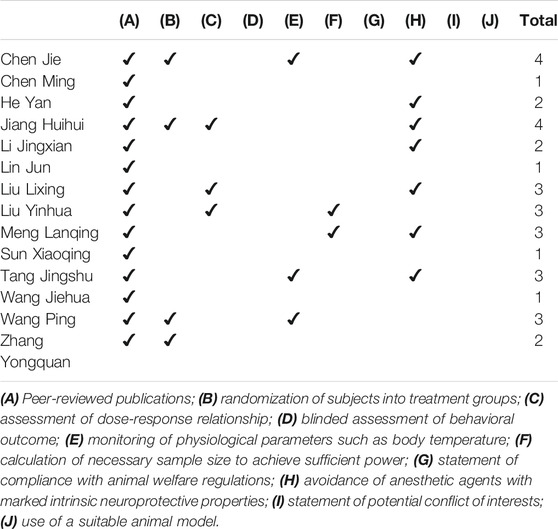
In the present study, we examined the animal models with cerebral ischemia-reperfusion, and we employed a revised tool of the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) (Macleod et al., 2004) checklist to assess the quality of the included studies. The leading evaluation indicators include (1) peer-reviewed publications; (2) presence of randomization of subjects into treatment groups; (3) assessment of dose-response relationship;(4) blinded assessment of behavioral outcome; (5) monitoring of physiological parameters such as body temperature; (6) calculation of necessary sample size to achieve sufficient power; (7) statement of compliance with animal welfare regulations; (8) avoidance of anesthetic agents with marked intrinsic neuroprotective properties; (9) statement of potential conflict of interests; and (10) use of a suitable animal model.
If the study was exclusively described as “completed as an experimental article” and “carried out taking into concern previous experiments,” the quality evaluation basis was further analyzed. If the reference belonged to the same team, the modeling data in the reference were directly extracted and considered of “low risk”; otherwise, it was considered “unclear risk.” The above two evaluation methods were consistent in determining the same indicators and were assessed by two investigators (PW and TD), and the differences were resolved by discussion.
Data Extraction
The following data were extracted from the included literature: (1) document elements: first author and year of publication; (2) experimental animal elements: animal species, sex, and weight; (3) MCAO model elements: anesthetic type and dose, number of experimental animals, ischemia duration, and reperfusion duration; (4) intervention elements: intervention group, administration route, dosage, intervention time point, and treatment duration; and (5) experimental outcomes: each outcome indicator.
In this meta-analysis study, neurological deficit score (NDS) and cerebral infarct volume (CIV) were used as the effect-quantity index, whereas the animal biochemical experiment indicators were used as possible drug mechanisms. If several independent intervention groups were included, all were included as study groups (e.g., different doses of PNS intervention groups in the same control group). In the control group, samples were divided into subgroups without affecting the sample size. If the data from the study literature were incomplete or only image data were displayed, the original data were discussed with the author. If necessary, data were estimated from graphics or recalculated from available data. The article was removed due to lack of exact data. The data were extracted from each article by two investigators independently.
Statistical Analysis
A meta-analysis was performed for NDS reported in 10 or more articles. For subgroup analysis, a minimum of two studies per subgroup was required. The NDS, CIV, and biochemical indicators were analyzed as continuous variables. We calculated the pooled estimates of the mean differences (SD)/standardized mean difference (SMD) between PNS and control groups, together with 95% confidence interval (CI). We used the Cochran Q test and I2 testing to assess heterogeneity between studies. To further explore the potential efficacy and pharmacological mechanism of PNS, we analyzed the drug effects of the PNS group, PNS combination group, and positive drug group on NDS and/or CIV indicators.
Heterogeneity was considered significant at p < 0.1. I2 testing values of 25, 50, and 75% were considered of low, moderate, and high inconsistency, respectively. When the heterogeneity was moderate or high, the sensitivity analyses of the literature study were investigated and conducted with subgroup analysis. The preset factors for the subgroup analysis included ischemia duration (1, 2 h, and unknown), drug dosage (D1 < 25 mg/kg, 25 mg/kg ≤ D2 < 50 mg/kg, 50 mg/kg ≤ D3 < 100 mg/kg, and 100 mg/kg ≤ D4 < 150 mg/kg), intervention time [(1) before occlusion, representing primordial prevention; (2) before and after occlusion, representing primary prevention; and (3) after occlusion, representing secondary prevention], and administration duration (1–3, 4–7, and >7 days). When the heterogeneity was strong and could not be eliminated, the random-effect model was employed to obtain an overall MD/SMD and 95% CI instead of the fixed-effect model. The funnel plot and Egger’s test were used to detect publication bias. In case of any deviation, the trim-and-fill computation was used to detect potential publication bias. All the analyses were performed with RevMan 5.3 and STATA 14.0.
Results
Study Selection
We identified a total of 3,581 search results. Six hundred and fifty-nine (659) duplications were ruled out by software (Endnote X9), and 501 duplications were manually removed. According to the inclusion and exclusion criteria, a total of 2,241 articles were deleted based on full-text reading, title and abstract. Among these articles, 1,930 failed to meet the requirements for experimental subjects, intervention measures, and experimental methods. Through full-text reading and reconfirmation in 180 articles, 166 articles were excluded because they were nonstandard. Among them, 18 were reviewed or not available, 61 subjects or interventions were inconsistent, 78 outcome indicators failed to fulfill the inclusion criteria, 2 had documented valid data that could not be provided, and 7 had small sample size and did not meet the requirements. A total of 14 studies were included in the meta-analysis (Figure 2).
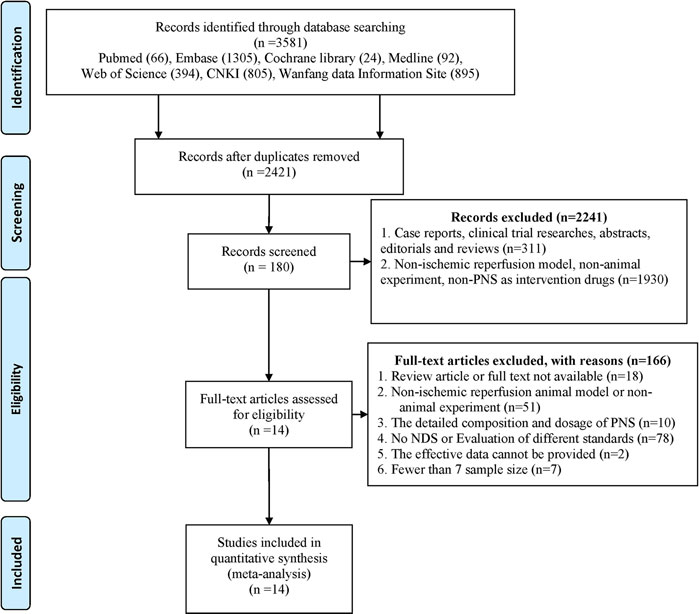
FIGURE 2. Flow diagram of the Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We screened all studies in the database based on the search terms and set the exclusion criteria in advance. The first round of screening was based on the title and abstract of the publication. We carefully studied the full text to make a judgment where insufficient data from the relevant abstracts were available for our decision.
Study Characteristics
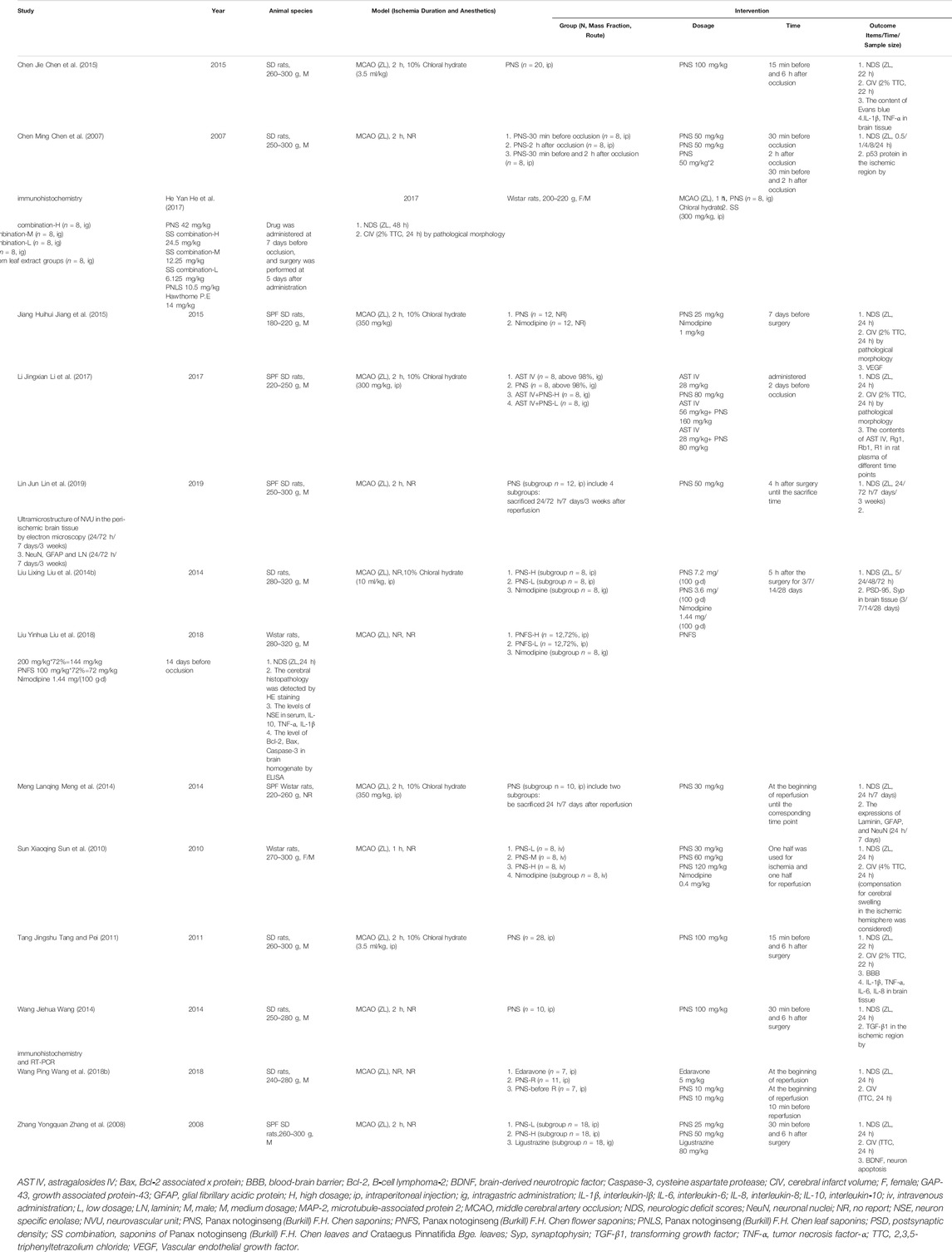
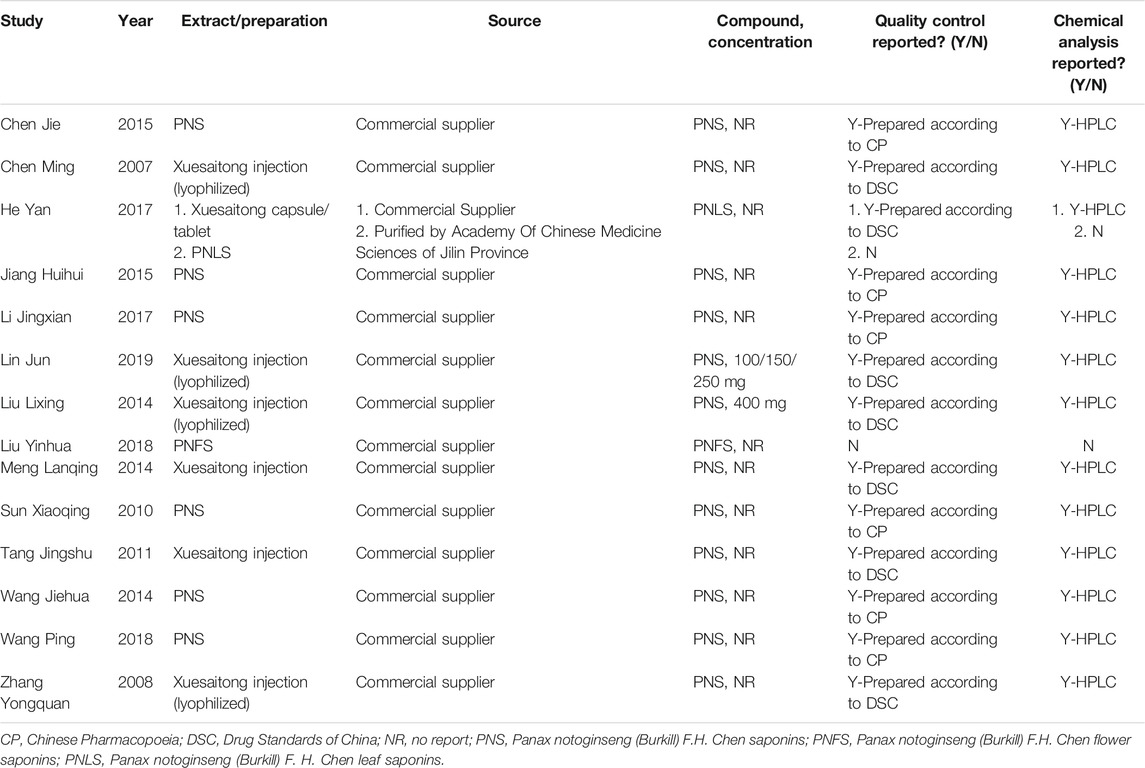
A total of 14 studies were included in this study, and all were published in Chinese (Table 3). Among the well-established studies, all the experimental animal models were rats, and 11/14 (78.57%) used only male rats. Seven of fourteen (7/14) included the experiments in which the rats were anesthetized with chloral hydrate without apparent neuroprotective effects, and the remaining seven articles did not illustrate the type of anesthetic drug used. In the included studies, nine articles (64.29%) had cerebral ischemia for 2 h and two articles (14.29%) for 1 h, and the remaining three articles had no specific ischemic duration. Three publications studied the effects of different reperfusion time on the effective index of the rat model after ischemia (Liu et al., 2014b; Meng et al., 2014; Lin et al., 2019). Of the 14 studies, six compared the medication of positive control drugs (nimodipine, edaravone, and ligustrazine), two compared the efficacy of concomitant medication (Crataegus pinnatifida Bge. leaf extract and astragalosides IV), and six compared different dosage groups of PNS (four articles with positive drug comparison and two articles with concomitant medication at dosage of 10–144 mg/kg). Regarding the route of administration, 10 studies included intraperitoneal injections (71.43%), two included intragastric administration, one included intravenous injection (Sun et al., 2010), and one did not clearly state the route of administration (Jiang et al., 2015). In terms of drug intervention time points, one article conducted a study on the time point of intervention (Chen et al., 2007), three articles had administration before occlusion, four articles had administration after occlusion, and six articles had administration before and after occlusion. Additionally, five articles used Xuesaitong injection (lyophilized), one study used P. notoginseng flower saponins (PNFS) (Liu et al., 2018), and one study used P. notoginseng leaves saponins (PNLS) (He et al., 2017). Eight of the articles used 2,3,5-triphenyltetrazolium chloride (TTC) to measure the CIV, and only five quantitatively analyzed the percentage. Table 4 shows a summary of the preparations used in the 14 selected articles.
The Methodological Quality of the Included Studies
With the SYRCLE’s Risk of Bias tool for research and analysis, 4/14 (28.57%) reports illustrated the randomization method, 1 (7.14%) of the reports did not express the randomization method, and the other reports only described “randomization” without specifying the randomization method, which was judged as “unclear risk of bias.” For the rat models with I/R, six articles (42.86%) clearly expressed that rats could be included in the experiment only after being evaluated and qualified in order to ensure uniform experimental baseline standards. Other articles did not clearly explain this requirement (for example, no statement was made for the randomness of the surgical process in the group undergoing surgery). In the field of random housing, only one document detailed the consistency and randomness of the environment in which experimental rats were housed. Seven articles (50.00%) tested all the test rats in all groups for the target index of this meta-analysis, while the other documents were not completely tested based on the initial grouping and did not explain the randomness of the test results. Nine articles (64.29%) described the absence of lost follow-up data. None of the studies expressed any other deviation, so they were all evaluated as “low risk.” Additionally, none of the literatures showed whether allocation was concealed and whether they were blinded for caregivers and investigators, blinded outcome assessment or selective reporting (Figure 3). The published literatures also have limitations in the design and implementation of animal experiment methodology and need to be ameliorated to ensure the feasibility of transforming basic research into clinical study.
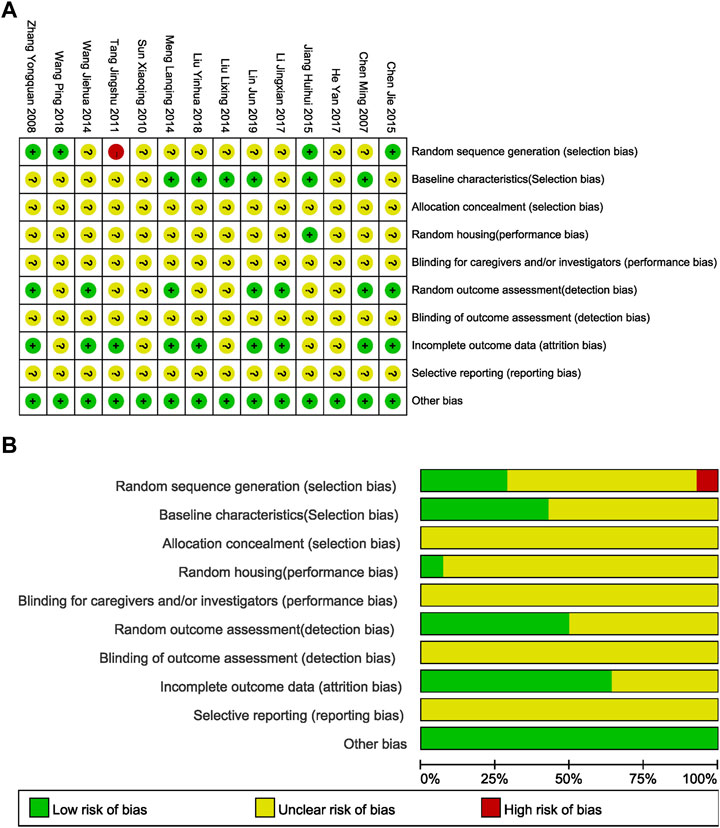
FIGURE 3. Evaluation of literature quality results obtained through SYRCLE’s Risk of Bias based on the Cochrane tool. (A) Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. (B) Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
With the modified CAMARADES for quality evaluation, the included studies scored an average of 2.36 (1–4 points) out of 10 points, indicating that the animal trials remain evolving to improve consistency and guidance. For cerebral I/R rat testing, only three articles considered the monitoring of physiological parameters during surgery, three articles examined the influence of doses of cerebral ischemia effect indicators, and two articles calculated the necessary sample size to achieve the specified quality of the test. No studies clearly stated the blinded assessment of behavioral outcome, potential conflict of interests, compliance with animal welfare regulations, and the use of a suitable animal model (Table 5).
Overall Efficacy of PNS on NDS
Forty-six (46) study groups were included in this analysis. There was moderate heterogeneity (I2 = 72%, p < 0.1). A sensitivity analysis of the included literature revealed that two documents (Meng et al., 2014; Jiang et al., 2015) had a greater impact on heterogeneity, so two outlier documents were removed by reevaluation. Finally, 42 study groups with 701 participants reported data on NDS, and the treatment with PNS reduced the score of NDS by 12.75% compared with the control group. The difference was statistically significant (MD −0.51, 95% CI: −0.62 to −0.40; p < 0.01) with heterogeneity (I2 = 36%, p = 0.01) (Figure 4A). The symmetrical funnel plot (Figure 4B) showed no published bias, and Egger’s regression test (Figure 4C) for symmetry was not significant (p = 0.098).
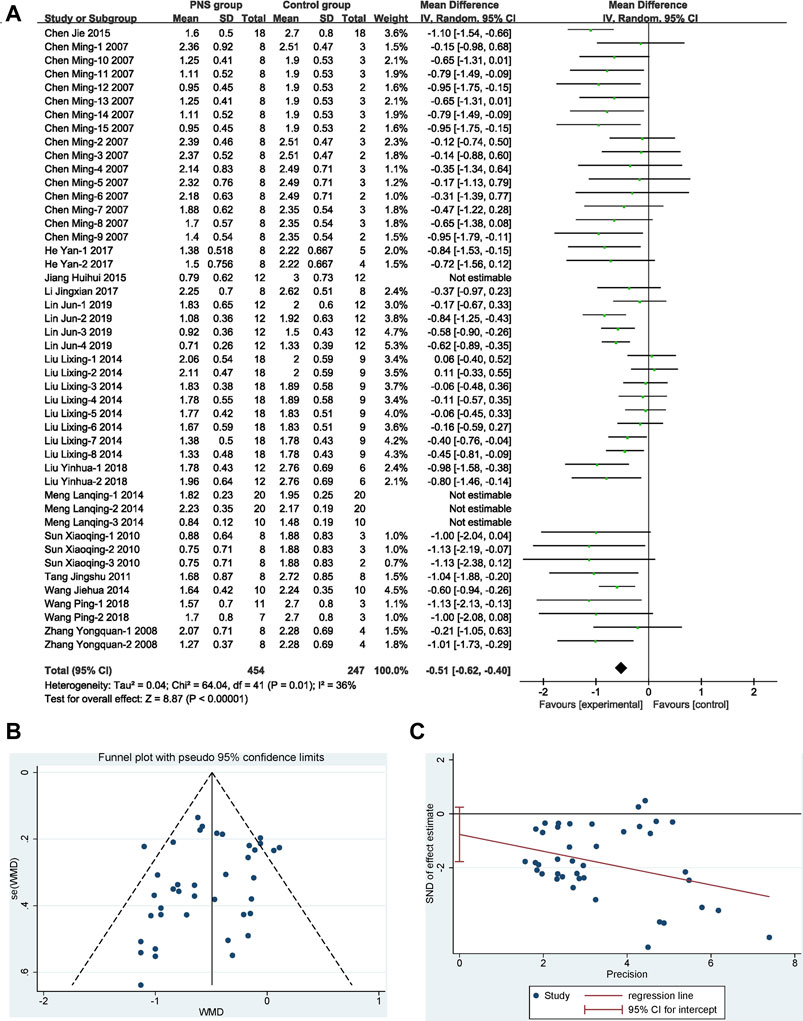
FIGURE 4. Overall efficacy of PNS on NDS. (A) Results of the meta-analysis visualized in a forest plot. Forest plot adopted mean difference and its corresponding 95% CI based on the random-effects model (Inverse Variance). It is showed that PNS significantly reduced the score of NDS with mild heterogeneity by removing the outlier studies. (B) Assessing of publication bias in a funnel plot. No publication bias could be detected from the symmetrical funnel plot. (C) Bias assessment plot by Egger’s test. Egger’s weighted regression suggested no publication bias for all analyses.
Prespecified Subgroup Analysis
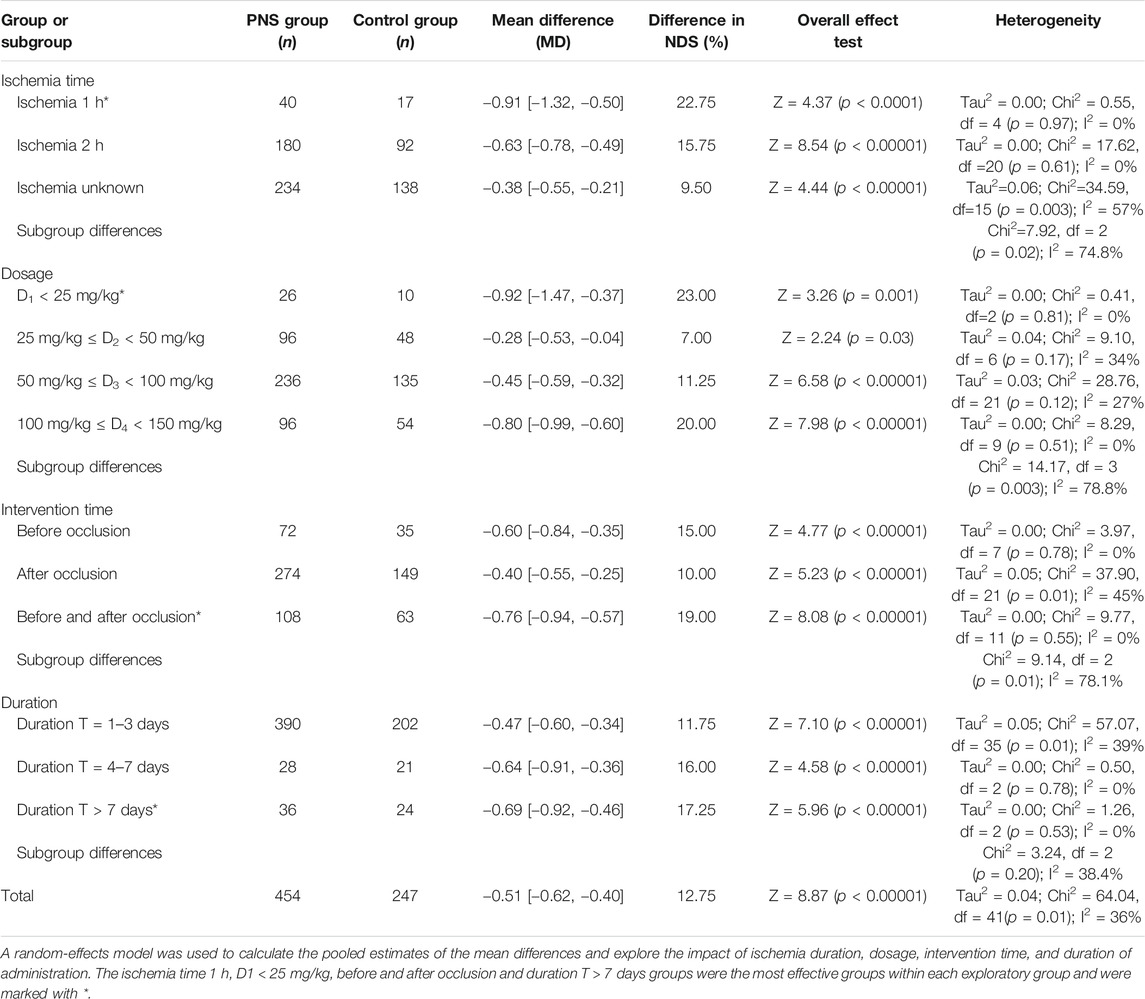
In the subgroup factors analysis, the NDS was used as a surrogate marker for efficacy. The factors of ischemic duration, drug dosage, and intervention time had significant impacts on NDS (subgroup heterogeneity values were I2 = 74.8, 78.8, and 78.1%, respectively) (Table 6), while the administration duration had nonsignificant impact (subgroup heterogeneity I2 = 38.4%).
Sorts of ischemic durations showed different treatment effects. In the rat experiments, restoration of blood perfusion and intervention within 1 h (22.75%) and 2 h (15.75%) improved the NDS compared with the average value (12.75%). This finding also suggested that the shorter the ischemic time is, the more prominent the treatment advantage would be in the later treatment sessions.
Drug dosage is one of the key factors that reflect the different efficacy of PNS. When the dose given to rats was D1 (23.0%) or D4 (20.0%), its therapeutic effect on NDS was better than that in the D2/D3 dosage groups (7 and 11.25%). That is to say, the therapeutic effect lower than 25 or 100–150 mg/kg was better than 25–100 mg/kg. It is shown that the association between the PNS dosage and NDS is materialized by a characteristic inverted U-shaped dose-response curve, revealing that the MCAO models with low- and high-dosage PNS both had better efficacy of NDS.
The time of PNS intervention is one of the factors affecting the late recovery of MCAO. The before occlusion intervention group (15.0%) and the before and after occlusion intervention group (19.0%) had higher NDS-improving efficacy than the after occlusion intervention group (10.0%), and the before and after occlusion intervention group was even more efficacious. The results suggested that preventive and comprehensive PNS administration had better therapeutic effects on the neurobehavioral effects caused by cerebral ischemic injury compared with the therapeutic administration. Additionally, there was moderate heterogeneity in the treatment administration group and the comprehensive treatment group. The reason may be that they were inconsistent in postoperative observation time, and the recovery status fluctuated greatly, while the preventive administration group observed time concentration within 24 h.
The administration duration is also related to the prognosis of MCAO and the level of NDS. The effect of NDS in the subgroups given drugs for more than 4 days (16.0 and 17.25%) was better than that of the subgroups given drugs for 1–3 days (11.75%), and the heterogeneity among subgroups was low. It is suggested that clinical medication could be taken for a long time according to the patient conditions.
Possible Protection Efficacy and Mechanism Analysis
Figure 5A compares the NDS between PNS group and positive control drug group. By comparing the effects of PNS and positive control drugs on the NDS of MCAO rats, nine studies with 357 rats (PNS group: n = 238, positive drug group: n = 119) were included. PNS had no statistical significance compared with the positive control drugs, and there was no heterogeneity in the study, suggesting that the PNS has a better effect on I/R than positive control drugs.
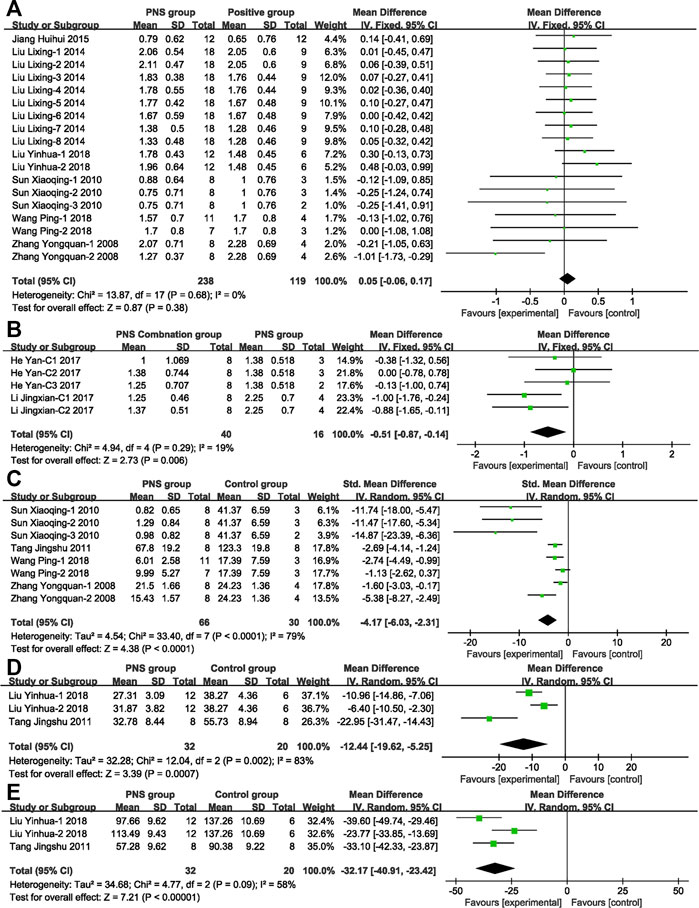
FIGURE 5. Forest plots of possible protection efficacy and mechanism analysis. (A) PNS vs. positive drug by using fixed-effect model (MD) analysis on NDS exhibits no statistical significance or heterogeneity. (B) PNS concomitant medication vs. PNS by using fixed-effect model analysis (MD) on NDS exhibits statistical significance and no heterogeneity. (C) PNS vs. control group by using random-effect model analysis (SMD) on CIV exhibits statistical significance and high heterogeneity. (D) PNS vs. control group by using random-effect model analysis (MD) on IL-1β exhibits statistical significance and high heterogeneity. (E) PNS vs. control group by using random-effect model analysis (MD) on TNF-α exhibits statistical significance and moderate heterogeneity.
Figure 5B compares the NDS score in PNS combination group and PNS group. By comparing the efficacy of PNS combination treatment (combined with Crataegus Pinnatifida Bge. leaf extract or astragalosides IV) and PNS alone treatment, five studies with 56 participants were included (PNS combination group: n = 40, PNS group n = 16). The PNS combination group was 12.75% better than the PNS alone group, indicating that the clinical application of PNS combinations, according to the patient conditions, can effectively improve adverse symptoms.
Figure 5C compares the CIV of the PNS group and positive control drug group. Nine articles with 132 participants showed that PNS could significantly reduce the area of cerebral ischemic infarction by 4.17 and alleviate the pathological symptoms of the ischemic model. Various studies have analyzed the mechanism of PNS to protect nerves.
The mechanisms of the drugs were analyzed. A meta-analysis of the inflammatory factors IL-1β (Figure 5D) and TNF-α (Figure 5E) in the included literature was performed. The study showed that PNS significantly inhibited the release of the inflammatory factors up to 12.44 pg ml−1 (IL-1β) and 32.17 pg ml−1 (TNF-α).
Discussion
We analyzed the focal MCAO rat model (Longa et al., 1989), which is relatively noninvasive with reversible regional cerebral ischemia. The model simulated the clinical pathogenesis and treatment of focal cerebral I/R patients and showed good evaluation effect. To reduce bias and ensure the validity of the data entered, we selected the unilateral MCAO model instead of the bilateral vertebral artery occlusion model, which also developed regional infarction accompanied by a series of pathological changes. The clinical evaluation of stroke includes a neurological examination, monitoring of vital signs, blood analysis, immediate neurovascular (vascular and brain) imaging, and cardiovascular investigations (Boulanger et al., 2018). All suspected patients with acute stroke should be assessed for neurological impairments and functional limitations (cognitive evaluation, screening for depression, and assistance with activities of daily living) and undergo brain imaging with NCCT or MRI (Boulanger et al., 2018). Therefore, we determine the efficacy of PNS with NDS and CIV, which was indicated to be significantly effective in the meta-analysis.
Statistical experiments (Perel et al., 2007; Blocker et al., 2019) showed that there was a significant difference between animal study results and clinical treatment effects in the treatment of stroke. Indeed, it objectively presents the probability of inconsistency between preclinical study and clinical disease treatment results. Although we cannot accurately project the value of the results of preclinical experiments, animal experiments are needed to further examine the biological mechanisms (Pound and Ritskes-Hoitinga, 2020). On the other hand, the lack of consistency between preclinical experiments and clinical trials may be due to random errors, bias, or the differentiation of animal models and human diseases. Consequently, in order to bridge the preclinical and clinical study, we need to further improve the quality of animal study methods, explore more systematic analysis of experimental data, and strengthen joint study between clinical and animal investigators (Bahadoran et al., 2020).
Based on the above analysis, the PNS drug dosage and NDS are characterized by an inverted U-shaped effect curve, which not only provides evidences for clinical applications, but also provides ideas for in-depth research of drug mechanisms. The study by Gold et al. (Gold and Van Buskirk, 1976) showed that the administration of adrenocorticotrophic hormone has an inverted-U dose-response curve characteristic on the memory enhancement of animals. Knauber et al. (Knauber and Muller, 2000) found that when prazosin was subchronically given to the elder rats to evaluate the learning capabilities and memory retention, the drug dose and the results of passive avoidance learning showed the characteristics of U-shaped dose-response curve by upregulating the α1-receptor density. Regarding the characteristics of drug dosage, it may be the potential way to further explore the mechanism of PNS and cerebral ischemia. Concomitantly, the dosage analysis of the animal experiments shows that human doses below 4.03 or 16.13–24.19 mg/kg can exert significant influence, but they are inconsistent with the current clinical utility and cause individual adverse effects at high doses. According to the 2008 clinical analysis of PNS in the Cochrane Database of Systematic Reviews, PNS can significantly reduce mortality and drug dependence in the clinic, reduce patient symptoms, and improve behavior scores. PNS are mainly used as an injection, and the drug dosage range is nearly 6.67–13.33 mg/kg over 14–28 days (Jiliang et al., 2005; Xi-hong and Min-jiu, 2009; Jin and Zheng, 2011; Gui et al., 2013; Li et al., 2016). This dose is not included in the optimal drug range from the experimental animal analysis. In Liu HL’s study (Jiliang et al., 2005), the drug was used at a dose of 400–800 mg/day, and three cases (a total of 120 cases in the group) had allergic dermatitis. In addition, the adverse reactions of PNS injections (Wan, 2013; Huang, 2018) from 1998 to 2017 showed that the damage of PNS injections involved multiple systems in the body, mainly damage to the skin and digestive, respiratory, and nervous systems and anaphylactic shock in severe cases. No effect of the dose was stated. Therefore, it is necessary to further explore the optimal dosage of PNS in the clinic, strengthen the monitoring of adverse reactions, and reduce the risk of medication for patients.
The study proves that PNS interventions at various stages of the disease can achieve significant results, covering primordial prevention (before occlusion), primary prevention (before and after occlusion), and secondary prevention (after occlusion). Cheng (Cheng et al., 2015) points out that intervention in the early diagnosis of lacunar infarction (within 24 h) increases the relative cerebral blood flow at discharge and improves neurological deficits in elderly patients. Zhang (Zhang et al., 2010) shows that the recurrence rate of ischemic stroke in the PNS group (5.83%) is significantly lower than that in the control group (17.82%).
The rat experiments together show that the effects are more prominent as the PNS treatment prolongs. Significant effects were achieved in rat models after treatment for more than four consecutive days, and the effect of more than 7 days improved even more. Clinically, the PNS medication cycle varies from 7 to 21 days, and all durations showed the protective effect on brain nerve cells (Wu et al., 2002; Feng et al., 2011; Gao et al., 2015). Dong XL (Dong et al., 2005) compared the drug usage of PNS for 7 and 21 days and measured blood specific viscosity (BSV), plasma fibrinogen, erythrocyte rigidity, and K value of erythrocyte sedimentation rate equation, extrapolating to the cumulative effects of treatments over time.
This study indicates that PNS has excellent therapeutic effects that are analogous to those of positive control drugs, but its mechanism is worthy of extensive study and verification. Our study shows that PNS can alleviate MCAO symptoms by reducing the inflammatory response. However, AIS causes neuronal cell death through complex pathological changes, including the excessive influx of Ca2+, the formation of reactive oxygen species (ROS), and the dysfunction of mitochondrial. Edaravone captures and reduces excessive ROS to prevent brain damage (Matsumoto et al., 2018). Nimodipine is a calcium channel blocker that can significantly reduce the protein levels of caspase-3 and Bax in rat brain tissue and may inhibit the release of Ca2+ (Yang et al., 2006). The therapeutic mechanism of PNS (Wang et al., 2016; Zhao et al., 2016; Wang et al., 2018a) was based on the decrease in the levels of ROS and malondialdehyde (MDA); the increase in the activity of glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD); and the increase in cell viability and mitochondrial membrane potential. Additionally, PNS (Li et al., 2009) significantly attenuated the expression of caspase-3 and caspase-1 compared with the model group to achieve the neuroprotective effect on focal ischemia. There is increasing evidence that PNS treats MCAO by protecting nerve cells through multiple pathways and multiple targets.
In the present study, the favorable evidence of the concomitant medication also strengthens our confidence in treating MCAO with PNS combined with other traditional Chinese medicine ingredients (combined with Crataegus Pinnatifida Bge. leaf extract or astragalosides IV). By synthetically analyzing the animal and clinical trials, it is found that the concomitant medication of PNS can indeed synergistically enhance the efficacy and has favorable safety. Feng CL (Feng et al., 2011) has confirmed that AST IV combined with PNS improves cerebral vascular microcirculation, increases cerebral vascular blood flow, enhances the ability of brain cells to resist hypoxia, etc. AST IV combined with PNS has a protective effect on ischemic brain cells. Xue (Xue, 2018) has shown that edaravone combined with PNS significantly reduces the score of National Institutes of Health Stroke Scale (NIHSS). Hyperbaric oxygenation (HBO) (Wu et al., 2002; Dong et al., 2005) associated with PNS has rapid effects with few adverse reactions in the treatment of abnormal hemorheology in patients with ischemia cerebral vessel disease. The PNS administration plus intravenous thrombolysis with recombinant tissue-type plasminogen activator (rt-PA) (Li et al., 2016) can reduce I/R injury in patients with AIS by reducing the occurrence of hemorrhagic transformation and improving the prognosis with good safety. Zhang (Zhang et al., 2010) has showed that the combination of PNS and aspirin is better than aspirin alone in preventing recurrence of ischemic stroke.
In addition to being an effective adjuvant therapy in clinical ischemic stroke patients, PNS exhibits broad biological activities (Zhang, 2004; Zhang et al., 2019) and has obvious effects on cardiovascular system diseases, the endocrine system, and improved nerve function. Spontaneous intracerebral hemorrhage (ICH) patients (Luo et al., 2010a; Luo et al., 2010b; Luo et al., 2018) who were treated with Xueshuantong injection (175 mg/day) for two weeks exhibited increased hematoma absorption, improved recovery of neurological function, and reduced inflammatory response. Song et al. (2013) showed that photodynamic therapy (PDT) combined with PNS in the treatment of age-related macular degeneration (AMD) and choroidal neovascularization (CNV) has a reliable effect and can be used in clinical application. PNS (Deng, 2010) can significantly improve ischemia in diabetic foot patients by reducing the amount of angiotensin, reducing purulent secretions, and promoting healing of the ulcer area. Therefore, PNS can play a comprehensive protective role in patient management.
Limitations
In this study analysis, all the studies included the rat models treated with PNS for ischemic stroke. The rats have relatively low cost, good physiological responses, and available genetic techniques. However, there have been failure cases of translation from preclinical studies to clinical trials reported (Perel et al., 2007; Hossmann, 2009). In addition, no animal models of complications were selected in this systematic research literature, and this differed from the actual clinical situation. Therefore, other animal models need to be considered; investigators should choose the models that are more comparable to the development of human physiology and pathology, that are related to the pathogenesis of ischemic stroke, that have a more accurate experimental design of treatment window duration, and that have a sufficient basis for the translation of preclinical findings (Kaiser and West, 2020).
The included literature has problems with methodological irregularities and incomplete study reports, which may affect the validity of the results and conclusions. The common limitations of these studies are lack in describing participant randomization, allocation, results evaluation by the evaluators, limited available data on the main results, and unclear methods and dosage of traditional Chinese formulation. Therefore, the included reports are rated as high-risk or unclear-risk, which requires investigators to focus on improving their methodology and report quality in animal experiments to provide high-quality evidence.
Conclusion
A total of 14 articles were included in this meta-analysis. After heterogeneity judgment and sensitivity analysis, 43 study groups with 749 participants were finally included in our study. More reliable preclinical evidence was obtained based on the analysis of the NDS, CIV, and inflammatory factors TNF-α and IL-1β.
The comprehensive analysis of PNS intervention in MCAO rat models shows that early application of PNS intervention at all stages of the disease has significant effects on disease treatment, including primordial prevention, primary prevention, and secondary prevention. If the dosage of administration in rats is controlled within lower than 25 or 100–150 mg/kg and the drug is administered for more than 4 days, rat behavioral scores can be significantly changed, brain tissue can be protected from pathological changes, and the content of inflammatory factors in the body can be reduced. The effect of combined PNS and astragalosides IV or Crataegus pinnatifida Bge. leaf extract was more significant than that of PNS alone. It is worthwhile to transform PNS from laboratory to clinic.
Most of the studies included could not be fully assessed since there is an unclear risk of bias for the vast majority of the parameters. Consequently, the overarching outcome of this meta-analysis remains inconclusive and we urgently need much better designed studies.
Author Contributions
TS designed the study and wrote the main body. PW and TD collected the data, performed data curation, and conducted the analyses. XT and BL prepared the pictures and tables. YX revised and reviewed the manuscript. All authors contributed to manuscript revision and reading and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (nos. 81704024 and 81773906).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bahadoran, Z., Mirmiran, P., Kashfi, K., and Ghasemi, A. (2020). Importance of systematic reviews and meta-analyses of animal studies: challenges for animal-to-human translation. J. Am. Assoc. Lab. Anim. Sci. 59 (5), 469–477. doi:10.30802/AALAS-JAALAS-19-000139
Benjamin, E. J., Virani, S. S., Callaway, C. W., Chamberlain, A. M., Chang, A. R., Cheng, S., et al. (2018). Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation 137 (12), E67–E492. doi:10.1161/Cir.0000000000000558
Benjamin, (2020). Heart disease and stroke statistics-2019 update: a report from the American heart association (vol 139, pg e56, 2019). Circulation 141 (2), E33. doi:10.1161/Cir.0000000000000746
Blocker, S. J., Mowery, Y. M., Holbrook, M. D., Qi, Y., Kirsch, D. G., Johnson, G. A., et al. (2019). Bridging the translational gap: implementation of multimodal small animal imaging strategies for tumor burden assessment in a co-clinical trial. PLoS One 14 (4), e0207555. doi:10.1371/journal.pone.0207555
Boehme, A. K., Esenwa, C., and Elkind, M. S. (2017). Stroke risk factors, genetics, and prevention. Circ. Res. 120 (3), 472–495. doi:10.1161/Circresaha.116.308398
Boulanger, J., Lindsay, M., Gubitz, G., Smith, E., Stotts, G., Foley, N., et al. (2018). Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int. J. Stroke 13 (9), 949–984. doi:10.1177/1747493018786616
Busk, H., Stausholm, M. B., Lykke, L., and Wienecke, T. (2020). Electrical stimulation in lower limb during exercise to improve gait speed and functional motor ability 6 months poststroke. a review with meta-analysis. J. Stroke Cerebrovasc. Dis. 29 (3), 104565. doi:10.1016/j.jstrokecerebrovasdis.2019.104565
Chai, G. F., and Zhang, Q. P. (2018). Clinical research progress on treatment of ischemic stroke with Huoxue Huayu method. Clin. J. Tradit. Chin. Med. 30 (8), 1397–1400. doi:10.16448/j.cjtcm.2018.0426
Chang, C. C., Chen, T. L., Chiu, H. E., Hu, C. J., Yeh, C. C., Tsai, C. C., et al. (2016). Outcomes after stroke in patients receiving adjuvant therapy with traditional Chinese medicine: a nationwide matched interventional cohort study. J. Ethnopharmacol. 177, 46–52. doi:10.1016/j.jep.2015.11.028
Chen, B., Zhao, M., Chen, B., Yang, Z., Yu, X., Lin, X., et al. (2020). Effectiveness and safety of acupuncture in post-stroke depression (PSD). Medicine 99 (12), e18969. doi:10.1097/MD.0000000000018969
Chen, J., Yuan, J., Han, Z. C., and Cao, Y. L. (2015). Neuroprotective role and mechanism research of Panax notoginseng saponins on cerebral ischemia reperfusion injury in rats. China Pharm. 24 (24), 23–25, Available at: http://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjEwMTI1Eg16Z3l5MjAxNTI0MDEwGggxdW50amloeQ%3D%3D
Chen, M., Wang, K. H., Zeng, X. F., Lu, H., and Zhang, Y. Q. (2007). Effects of Panax notoginseng saponins on the expression of p53 protein after cerebral ischemia-reperfusion injury in rats. Capital Food Med. (22), 45–46. doi:10.3969/j.issn.1005-8257.2007.22.028
Chen, Z. Z., Gong, X., Guo, Q., Zhao, H., and Wang, L. (2019). Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 α, VEGF and promotion β-ENaC expression. J. Ethnopharmacol. 228, 70–81. doi:10.1016/j.jep.2018.09.017
Cheng, L. J., Wang, Z. D., and Pan, X. (2015). Effect of early intravenous administration of Panax notoginseng on elderly with acute lacunar infarction. J. Am. Geriatr. Soc. 63, S371. doi:10.1111/jgs.13704
Chinese Pharmacopoeia Commission (2015). Pharmacopoeia of the People's Republic of China. Beijing, China: China Medical Science Press.
Deng, X. Y. (2010). Effect of total notoginseng glycoside on 31 cases of diabetic foot. J. Hainan Med. Univ. 21 (1), 73–75. doi:10.3969/j.issn.1003-6350.2010.01.028
Dong, X. L., Niu, S. Y., Xu, H. B., and Han, M. Y. (2005). Effects of hyperbaric oxygenation associated with Panax notoginseng saponins therapy on hemorrhology indexes in patients with ischemic cerebrovascular disease. Chin. J. Clin. Rehabil. 9 (41), 6–7. doi:10.3321/j.issn:1673-8225.2005.41.004
Draaisma, L. R., Wessel, M. J., and Hummel, F. C. (2020). Non-invasive brain stimulation to enhance cognitive rehabilitation after stroke. Neurosci. Lett. 719, 133678. doi:10.1016/j.neulet.2018.06.047
Feng, C. L., Lin, S. Z., Fu, Z. L., Liu, X. Y., Lv, J. S., and Shen, W. B. (2011). Clinical study of astragalus injection combined with Xuesaitong injection in the treatment of ischemic stroke. J. Changchun Univ. Chin. Med. 27 (6), 924–926. doi:10.13463/j.cnki.cczyy.2011.06.018
Gao, L., Zhao, H., Liu, Q., Song, J., Xu, C., Liu, P., et al. (2013). Corrigendum to “improvement of hematoma absorption and neurological function in patients with acute intracerebral hemorrhage treated with Xueshuantong” [J Neurol Sci 323 (2012) 236–240]. J. Neurol. Sci. 325 (1–2), 190. doi:10.1016/j.jns.2012.12.007
Gao, Z. Q., Zhang, P., Dai, Y., Gao, J. F., Jia, P., Sun, G. H., et al. (2015). Short-term efficacy and safety of combined antithrombotic therapy for acute ischemic stroke. J. Nan Jing Med. Univ. 35 (8), 1152–1154. doi:10.7655/nydxbns20150821
Gold, P. E., and Van Buskirk, R. (1976). Enhancement and impairment of memory processes with post-trial injections of adrenocorticotrophic hormone. Behav. Biol. 16 (4), 387–400. doi:10.1016/s0091-6773(76)91539-x
Gui, Q., Yang, Y., Ying, S., and Zhang, M. (2013). Xueshuantong improves cerebral blood perfusion in elderly patients with lacunar infarction. Neural Regen. Res. 8 (9), 792–801. doi:10.3969/j.issn.1673-5374.2013.09.003
He, L., Chen, X., Zhou, M., Zhang, D., Yang, J., Yang, M., et al. (2011). Radix/rhizoma notoginseng extract (sanchitongtshu) for ischemic stroke: a randomized controlled study. Phytomedicine 18 (6), 437–442. doi:10.1016/j.phymed.2010.10.004
He, Y., Wen, F., Ji, F., Ding, T., Liu, B., Wang, X., et al. (2017). Protective effect on cerebral ischemia of Panax notoginseng leaves and Crataegus pinnatifida Bge. leaves. Lishizhen Med. Mater. Med. Res. 28 (03), 581–584. doi:10.3969/j.issn.1008-0805.2017.03.025
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43. doi:10.1186/1471-2288-14-43
Hossmann, K. A. (2009). Pathophysiological basis of translational stroke research. Folia Neuropathol. 47 (3), 213–227, Available at: https://www.termedia.pl/Pathophysiological-basis-of-translational-stroke-research,20,13176,1,1.html
Hsu, W.-H., Shen, Y.-C., Shiao, Y.-J., Kuo, C.-H., Lu, C.-K., Lin, T.-Y., et al. (2019). Combined proteomic and metabolomic analyses of cerebrospinal fluid from mice with ischemic stroke reveals the effects of a Buyang Huanwu decoction in neurodegenerative disease. PLoS One 14 (1), e0209184. doi:10.1371/journal.pone.0209184
Huang, A. Q. (2018). Adverse drug reactions caused by injection of Panax notoginseng saponins. Shenzhen J. Integr. Tradit. Chin. West. Med. 28 (6), 72–73. doi:10.16458/j.cnki.1007-0893.2018.06.035
Huang, W., Liao, X., Tian, J., Wu, J., Shan, Y., and Zhou, W. (2018). Traditional Chinese medicine for post-stroke depression: a systematic review and network meta-analysis (protocol). Medicine 97 (52), e13840. doi:10.1097/MD.0000000000013840
Jiang, H. H., Wang, Y. Y., Hu, D. H., and Jiang, R. Y. (2015). Effects of Panax notogiseng saponin on the expression of vascular endothelial growth factor after the brain ischemia-reperfusion injury in rats. J. Yangtze Univ. (Nat. Sci. Ed.) 12 (12), 1–4. doi:10.16772/j.cnki.1673-1409.2015.12.001
Jiliang, L. I. U., Fengzhi, Z., and Cunfeng, S. (2005). Efficacy and safety of saponins from Panax notoginseng in adults with progressive cerebral infarction. Chin. J. New Drugs 14 (11), 1352–1354. doi:10.3321/j.issn:1003-3734.2005.11.033
Jin, C. Y., and Zheng, S. F. (2011). Effects of ginkgo biloba leaves, Panax notoginseng saponins and buflomedil on platelet activation in patients with acute cerebral infarction. Med. Inform. 24 (5), 1861–1862. doi:10.3969/j.issn.1006-1959.2011.05.211
Johnson, C. O., Nguyen, M., Roth, G. A., Nichols, E., Alam, T., Abate, D., et al. (2019). Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 18 (5), 439–458. doi:10.1016/S1474-4422(19)30034-1
Kaiser, E. E., and West, F. D. (2020). Large animal ischemic stroke models: replicating human stroke pathophysiology. Neural Regen. Res. 15 (8), 1377–1387. doi:10.4103/1673-5374.274324
Knauber, J., and Müller, W. E. (2000). Subchronic treatment with prazosin improves passive avoidance learning in aged mice: possible relationships to alpha1-receptor up-regulation. J. Neural. Transm. 107 (12), 1413–1426. doi:10.1007/s007020070005
Kwolek-Klimkiewicz, J., Ciszek, B., Aleksandrowicz, R., Mazurowski, W., Zabek, M., and Górski, R. (1996). Applied anatomy of the extracerebral part of the arteria centralis longa. Folia Morphol. 55 (4), 371–372, Available at: https://pubmed.ncbi.nlm.nih.gov/9243913/
Li, C. S., Gao, Y. H., Chang, J. J., and Li, H. (2016). Effect of Panax notoginseng saponins on efficacy and hemorrhagic transformation of rt-PA intravenous thrombolysis in patients with acute ischemic stroke. Chin. J. Contemp. Neurol. Neurosurg. 16 (11), 784–792. doi:10.3969/j.issn.1672-6731.2016.11.012
Li, H., Deng, C. Q., Chen, B. Y., Zhang, S. P., Liang, Y., and Luo, X. G. (2009). Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J. Ethnopharmacol. 121 (3), 412–418. doi:10.1016/j.jep.2008.10.042
Li, J. X., Yang, X. Q., Tang, B., Liu, X. D., Tang, Y. H., Deng, C. Q., et al. (2017). [Effect of astragaloside Ⅳ combined with Panax notoginseng saponins on cerebral ischemia-reperfusion injury and study of pharmacokinetics in rats]. Zhongguo Zhongyao Zazhi 42 (19), 3786–3794 [in Chinese, with English summary]. doi:10.19540/j.cnki.cjcmm.20170901.013
Li, Z., Jiang, Y., Li, H., Xian, Y., and Wang, Y. (2019). China’s response to the rising stroke burden. BMJ 364, l879. doi:10.1136/bmj.l879
Lin, J., Liang, P., Huang, Q., Li, C., Nong, L. Y., Huang, J. M., et al. (2019). Effect of Panax notoginseng sapoIlins on neuro vascular unit in rats after cerebral ischemia-reperfusion. China Pharm. 28 (13), 10–14. doi:10.3969/j.issn.1006-4931.2019.13.003
Liu, L., Zhu, L., Zou, Y., Liu, W., Zhang, X., Wei, X., et al. (2014a). Panax notoginseng saponins promotes stroke recovery by influencing expression of Nogo-A, NgR and p75NGF, in vitro and in vivo. Biol. Pharm. Bull. 37 (4), 560–568. doi:10.1248/bpb.b13-00770
Liu, L. X., Zhu, L. Q., Liu, W., Wu, J. H., Zou, Y. H., Chen, J. X., et al. (2014b). Effect of Xuesaitong injection on PSD-95 and syp protein expression in cortex of focal cerebral infarction rats. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 12 (2), 217–219. doi:10.3969/j.issn.1672-1349.2014.02.046
Liu, Y. H., Yang, H. W., Zhang, Y. L., and Yang, J. (2018). Protective effect of panax notoginseng saponins on focal cerebral ischemia-reperfusion injury model in rats. J. Trop. Med. 18 (8), 1060–1064. doi:10.3969/j.issn.1672-3619.2018.08.018
Longa, E. Z., Weinstein, P. R., Carlson, S., and Cummins, R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20 (1), 84–91. doi:10.1161/01.str.20.1.84
Luo, L., Kang, J., He, Q., Qi, Y., Chen, X., Wang, S., et al. (2019). A NMR-based metabonomics approach to determine protective effect of a combination of multiple components derived from Naodesheng on ischemic stroke rats. Molecules 24 (9), 1831. doi:10.3390/molecules24091831
Luo, Y., Gao, L., and Song, J. (2010a). Efficacy of Panax notoginseng saponins on patients with intracerebral hemorrhage. Int. J. Stroke 5, 137–138. doi:10.1111/j.1747-4949.2010.00480.x
Luo, Y., Liu, Q., Song, J., Liu, P., and Gao, L. (2010b). Focused conference group: PW19-influence of degeneration and repair in the CNS and periphery efficacy of Panax notoginseng saponins on patients with intracerebral hemorrhage. Basic Clin. Pharmacol. Toxicol. 107, 427. doi:10.1111/j.1742-7843.2010.00600.x
Luo, Z.-J., Guo, T.-M., Tu, Q., Cheng, X.-L., Huang, Y., and Xiang, M.-Q. (2018). Therapeutic effect of integrating Chinese patent medicine Xuesaitong injection and Western medicine in treating patients with hypertensive intracerebral hemorrhage: a prospective randomized controlled trial. Eur. J. Integr. Med. 23, 26–31. doi:10.1016/j.eujim.2018.09.005
Macleod, M. R., O’Collins, T., Howells, D. W., and Donnan, G. A. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35 (5), 1203–1208. doi:10.1161/01.STR.0000125719.25853.20
Makkiyah, F. A., and Sadewo, W. (2019). Technical report: simple method of animal stroke model of luminal occlusion of middle cerebral artery in Indonesia. Surg. Neurol. Int. 10, 143. doi:10.25259/SNI_62_2019
Matsumoto, S., Murozono, M., Kanazawa, M., Nara, T., Ozawa, T., and Watanabe, Y. (2018). Edaravone and cyclosporine A as neuroprotective agents for acute ischemic stroke. Acute Med. Surg. 5 (3), 213–221. doi:10.1002/ams2.343
Meng, L. Q., Lu, W. X., Huang, J. M., Huang, X. H., Huang, Q., and Yuang, S. S. (2014). Effects of Panax notoginseng Saponins on neurovascular unit in rats with brain ischemia. J. Youjiang Med. Univ. Nationalities 36 (1), 7–9. doi:10.3969/j.issn.1001-5817.2014.01.003
Ng, Y. S., Stein, J., Ning, M., and Black-Schaffer, R. M. (2007). Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke 38 (8), 2309–2314. doi:10.1161/STROKEAHA.106.475483
Nogles, T. E., and Galuska, M. A. (2020). Middle cerebral artery stroke. Treasure Island, FL: StatPearls Publishing.
Perel, P., Roberts, I., Sena, E., Wheble, P., Briscoe, C., Sandercock, P., et al. (2007). Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ 334 (7586), 197–200. doi:10.1136/bmj.39048.407928.BE
Pound, P., and Ritskes-Hoitinga, M. (2020). Can prospective systematic reviews of animal studies improve clinical translation?. J. Transl. Med. 18 (1), 15. doi:10.1186/s12967-019-02205-x
Song, J., Xu, C., Zhang, J., and Gao, L. (2019). From clinical appearance to accurate management in acute ischemic stroke patients: with the guidance of innovative traditional Chinese medicine diagnosis. Brain Behav. 9 (10), e01411. doi:10.1002/brb3.1411
Song, M. X., Li, S., Wang, L., Sun, F., Jiang, Y. Q., and Li, H. (2013). Photodynamic therapy combined with the Sanqi Panax notoginseng for patients with age-related macular degeneration and choroidal neovascularization. Int. Eye Sci. 13 (8), 1628–1630. doi:10.3980/j.issn.1672-5123.2013.08.34
Sun, W. X., Xie, Y. M., Yang, W., Wang, Y. Y., Zhao, W., and Zhuang, Y. (2016). Distribution of TCM syndromes of ischemic stroke patients based on electronic medical data. Liaoning J. Tradit. Chin. Med. 43 (11), 2243–2246. doi:10.13192/j.issn.1000-1719.2016.11.002
Sun, X. Q., Zhao, M., and Liu, Z. P. (2010). Protective effect of Panax notoginseng saponins on cerebral ischemia-reperfusion injury in rats. Nei Mongol J. Tradit. Chin. Med. 29 (18), 40–41. doi:10.3969/j.issn.1006-0979.2010.18.048
Syafrita, Y., Amir, D., Susanti, R., and Fadhilah, I. (2020). Relationship of brain-derived neurotrophic factor, malondialdehyde, and 8-hydroxy 2-deoxyguanosine with post-ischemic stroke depression. Dement Neuropsychol. 14 (1), 41–46. doi:10.1590/1980-57642020dn14-010007
Tang, J. S., and Pei, Q. H. (2011). Neuroprotective role of Panax notoginseng saponins on cerebral ischemia-reperfusion injury in rats. Chin. J. Exp. Tradit. Med. Formulae 17 (15), 210–213. doi:10.13422/j.cnki.syfjx.2011.15.072
Tsai, T. Y., Li, C. Y., Livneh, H., Lin, I. H., Lu, M. C., and Yeh, C. C. (2016). Decreased risk of stroke in patients receiving traditional Chinese medicine for vertigo: a population-based cohort study. J. Ethnopharmacol. 184, 138–143. doi:10.1016/j.jep.2016.03.008
van Lieshout, E. C. C., van der Worp, H. B., Visser-Meily, J. M. A., and Dijkhuizen, R. M. (2019). Timing of repetitive transcranial magnetic stimulation onset for upper limb function after stroke: a systematic review and meta-analysis. Front. Neurol. 10, 1269. doi:10.3389/fneur.2019.01269
Wan, X. F. (2013). Adverse drug reactions induced by Xuesaitong injection: literature analysis of 68 cases. Evaluation and analysis of drug-use in hospitals of China. 13 (09), 846–848. doi:10.14009/j.issn.1672-2124.2013.09.008
Wang, F. J., Wang, S. X., Chai, L. J., Zhang, Y., Guo, H., and Hu, L. M. (2018a). Xueshuantong injection (lyophilized) combined with salvianolate lyophilized injection protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress. Acta Pharmacol. Sin. 39 (6), 998–1011. doi:10.1038/aps.2017.128
Wang, P., Yan, D. M., Huang, X., Zheng, X. Q., Ren, C., Yang, Z. X., et al. (2018b). The neuroprotection molecular basis and mechanism of Panax notoginseng saponins in the treatment of ischemic stroke. Chin. Pharmacol. Bull. 34 (12), 1750–1755. doi:10.3969/j.issn.1001-1978.2018.12.025
Wang, J. H. (2014). Effects of Panax notoginseng saponins on the expression of TGF-β1 in rats with focal cerebral ischemia-reperfusion. J. North Pharm. (12), 104–105, Available at: http://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjEwMTI1Eg1iZnl4MjAxNDEyMDg5GghobWNqeDh1bg%3D%3D
Wang, M. X., Zhao, J. Y., Sun, D. M., Meng, X. B., Sun, G. B., and Sun, X. B. (2016). [Protective effects and underlying mechanisms of Panax notoginseng saponins against SH-SY5Y cell apoptosis induced by 6-hydroxydopamine]. Yao Xue Xue Bao 51 (6), 898–906. doi:10.16438/j.0513-4870.2015-1082
Wang, X. L., Li, Q. H., Cao, K. G., Chen, X. Y., and Fan, J. P. (2014). Theoretical discussion of cause and pathogenesis of apoplexy. Shandong J. Tradit. Chin. Med. 33 (3), 165–167. doi:10.16295/j.cnki.0257-358x.2014.03.002
Wei, L., Shi, H., Lin, X., Zhang, X., Wang, Y., Liu, G., et al. (2019). Impact of cholesterol on ischemic stroke in different human-like hamster models: a new animal model for ischemic stroke study. Cells 8 (9), 1028. doi:10.3390/cells8091028
Wei, M., Wang, D., Kang, D., Lee, M. S., Choi, T. Y., Ang, L., et al. (2020). Overview of cochrane reviews on Chinese herbal medicine for stroke. Integr. Med. Res. 9 (1), 5–9. doi:10.1016/j.imr.2019.11.009
Wu, B. D., Zhang, J. L., Li, H. Y., and Dong, X. L. (2002). Effect of hyperbaric oxygen combined with Xuesaitong on hemorheology in ischemic cerebrovascular disease. Chin. J. Primary Med. Pharm. 9 (3), 248–249. doi:10.3760/cma.j.issn.1008-6706.2002.03.040
Xi-hong, Z., and Min-jiu, S. (2009). Effect of Panax notoginseng on high sensitivity C-reactive protein in patients with acute cerebral infarction. China J. Mod. Med. 19 (1), 120–121. doi:10.3969/j.issn.1005-8982.2009.01.032
Xu, C., Wang, W., Wang, B., Zhang, T., Cui, X., Pu, Y., et al. (2019). Analytical methods and biological activities of Panax notoginseng saponins: recent trends. J. Ethnopharmacol. 236, 443–465. doi:10.1016/j.jep.2019.02.035
Xue, J. Y. (2018). Efficacy of edaravone combined with thrombocytophan lyophilized powder in the treatment of senile cerebral infarction. Chin Med. J. Metall. Ind. 35 (5), 565–566. doi:10.13586/j.cnki.yjyx1984.2018.05.066
Yang, Y. T., Xu, A. Q., and Li, G. H. (2006). The protective effects of nimodipine on acute cerebral ischemic reperfusion injury in the rats. J. Qiqihar Med. Univ. (02), 139–140. doi:10.3969/j.issn.1002-1256.2006.02.006
Yoshikawa, M., Morikawa, T., Kashima, Y., Ninomiya, K., and Matsuda, H. (2003). Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J. Nat. Prod. 66 (7), 922–927. doi:10.1021/np030015l
Yu, J., Lv, Y., Yu, X., Li, Z. M., Liu, X. M., and Dong, H. W. (2019). Clinical study on treatment of cerebral hemorrhages with Chinese herbs for promoting blood circulation. Indian J. Pharmaceut. Sci. 81 (1), S68, Available at: https://www.ijpsonline.com/supplementary-files/supplement-to-issue-jan-feb-2019.pdf.
Zhang, D., Wei, F., Zhang, H. L., and Wang, Y. L. (2019). Advances in research on clinical application of Xueshuantong injection. J. Mod. Med. Health 35 (23), 3626–3628. doi:10.3969/j.issn.1009-5519.2019.23.018
Zhang, J. Y., Peng, L., Liu, D. B., and Liu, D. H. (2010). Panax notoginseng saponins combined with aspirin to prevent recurrence of cerebral infarction. J. Pract. Med. 26 (8), 1424–1425. doi:10.3969/j.issn.1006-5725.2010.08.064
Zhang, Q. (2004). Progress of clinical application of Xuexuantong injection. Hunan Guiding J. TCM (4), 63–64. doi:10.3969/j.issn.1672-951X.2004.04.032
Zhang, Y. Q., Mo, G. H., Chen, M., Zeng, X. F., and Lu, H. (2008). Effect of Panax notoginseng saponinsa on the expression of BDNF in rats with cerebral ischemia reperfusion. Chin. Tradit. Pat. Med. 30 (7), 958–961. doi:10.3969/j.issn.1001-1528.2008.07.007
Zhao, A.-m., Qiu, W.-r., Mao, L.-j., Ren, J.-g., Xu, L., Yao, M.-j., et al. (2018). The efficacy and safety of Jiedu Tongluo granules for treating post-stroke depression with qi deficiency and blood stasis syndrome: study protocol for a randomized controlled trial. Trials 19 (1), 275. doi:10.1186/s13063-018-2633-4
Zhao, J. Y., Wang, M. X., Zhao, Z. M., Meng, X. B., Sun, G. B., and Sun, X. B. (2016). Protective effect of Panax notoginseng saponins of Nrf2 signaling pathway on apoptosis of PC12 cells induced by Aβ25-35. Chin. Pharmacol. Bull. 32 (3), 343–349.
Keywords: Panax notoginseng saponins, ischemic stroke, middle cerebral artery occlusion, focal cerebral ischemia-reperfusion, animal model
Citation: Sun T, Wang P, Deng T, Tao X, Li B and Xu Y (2021) Effect of Panax notoginseng Saponins on Focal Cerebral Ischemia-Reperfusion in Rat Models: A Meta-Analysis. Front. Pharmacol. 11:572304. doi: 10.3389/fphar.2020.572304
Received: 13 June 2020; Accepted: 29 December 2020;
Published: 09 February 2021.
Edited by:
Takashi Sato, Tokyo University of Pharmacy and Life Sciences, JapanReviewed by:
You Yun, China Academy of Chinese Medical Sciences, ChinaDayun Feng, Fourth Military Medical University, China
Copyright © 2021 Sun, Wang, Deng, Tao, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xu, eHV5aW5nLmRvY3RvckBnbWFpbC5jb20=
 Tao Sun1
Tao Sun1 Ping Wang
Ping Wang Xingbao Tao
Xingbao Tao Ying Xu
Ying Xu