- 1Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China
- 2College of Pharmacy, Fujian Medical University, Fuzhou, China
- 3Department of Pharmacy, Fuzhou Second Hospital Affiliated to Xiamen University, Fuzhou, China
- 4Department of Information, Fujian Medical University Union Hospital, Fuzhou, China
Antiplatelet drugs may increase the risk of gastrointestinal bleeding. Currently, there is no specific score for predicting the risk of gastrointestinal bleeding caused by oral antiplatelet drugs. In this study, the gastrointestinal bleeding risk score was established and compared with the CRUSADE score in order to reduce the occurrence of clinical gastrointestinal bleeding events. Our study included 4052 patients who received oral antiplatelet drugs. Data were obtained from the patient medical records inpatient system. Cases of acute gastrointestinal bleeding and mortality were recorded. The bleeding score was established by logistic regression, area under the receiver operating characteristic curve, and the Hosmer–Lemeshow test. Finally, 171 patients had acute gastrointestinal bleeding. The mortality rates of patients in the bleeding and nonbleeding groups were 24.6 and 4.7%, respectively. A multivariate analysis revealed that an age of >65 years, anemia, recent major bleeding, a history of gastrointestinal bleeding, combined oral anticoagulants, and dual antiplatelet therapy are risk factors, and combined proton pump inhibitors are protective factors for acute gastrointestinal bleeding. We used these risk factors to establish a score for predicting acute gastrointestinal bleeding, named (ABC)2D score. The area under the curve for (ABC)2D score was 0.857 (p < 0.001), higher than the CRUSADE score of 0.693 (p < 0.001). The Hosmer–Lemeshow p value was 0.324. We developed the (ABC)2D score based on seven risk factors (i.e., age, anemia, recent major bleeding, a history of gastrointestinal bleeding, no-proton pump inhibitors use, combined oral anticoagulants, and dual antiplatelet therapy). (ABC)2D score was superior to the CRUSADE score. This new risk-scoring model may help to identify patients at a significant risk of gastrointestinal bleeding.
Introduction
Antiplatelet drugs are the cornerstone of cardiovascular disease prevention and treatment worldwide (Sostres et al., 2019). Although antiplatelet drugs successfully reduce the risk of recurrent ischemic events, they increase the risk of severe bleeding (Antithrombotic Trialists’ Collaboration et al., 2009; Li et al., 2017), of which gastrointestinal bleeding is common (Yamada et al., 2012). Studies have shown that after treatment with antiplatelet drugs, during a 2.5-year follow-up, the incidence of acute gastrointestinal bleeding is approximately 11.9% (Pannach et al., 2017), the prognosis of patients who have experienced bleeding events is poor, and these patients are at an increased risk of mortality from cardiovascular disease. Patients with moderate or severe bleeding increased cardiovascular mortality to 2.05 times (HR 2.05, 95% CI 1.38–3.04) (Berger et al., 2011). Therefore, in order to prevent and reduce the risk of gastrointestinal bleeding, it is important to use risk models for acute prediction before patients receive antiplatelet drug treatment.
Given the widespread use of antiplatelet therapy and the serious consequences of bleeding events, it is important to predict the risk factors for gastrointestinal bleeding associated with antiplatelet drugs and to construct a risk score. Currently, there are several bleeding risk scores, such as the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) score (Subherwal et al., 2009) and the S2TOP-BLEED score (Hilkens et al., 2017). The main outcome of these scores is major bleeding, including intraocular hemorrhage, gastrointestinal bleeding, and intracranial hemorrhage. The severity of bleeding events may be affected by organs themselves or by the changes that occur in organs (Laine and Jensen, 2012; Strate and Gralnek, 2016), so the above models used to independently predict gastrointestinal bleeding may not be accurate. There is currently no specific score for predicting the risk of gastrointestinal bleeding caused by oral antiplatelet drugs.
In our study, patients administered oral antiplatelet drugs were followed up for 6 months to study the risk factors for antiplatelet drug-induced gastrointestinal bleeding and to establish a score to assess the risk of gastrointestinal bleeding. In addition, comparing our score with the CRUSADE score, which is currently recommended by Chinese experts to assess bleeding risk after antiplatelet therapy (Chinese Society of Cardiology of Chinese Medical Association and Editorial Board of Chinese Journal of Cardiology 2013), allows clinicians to better predict the risk of gastrointestinal bleeding, thereby optimizing antiplatelet therapy and reducing the incidence of gastrointestinal bleeding events.
Methods
Study Design and Population
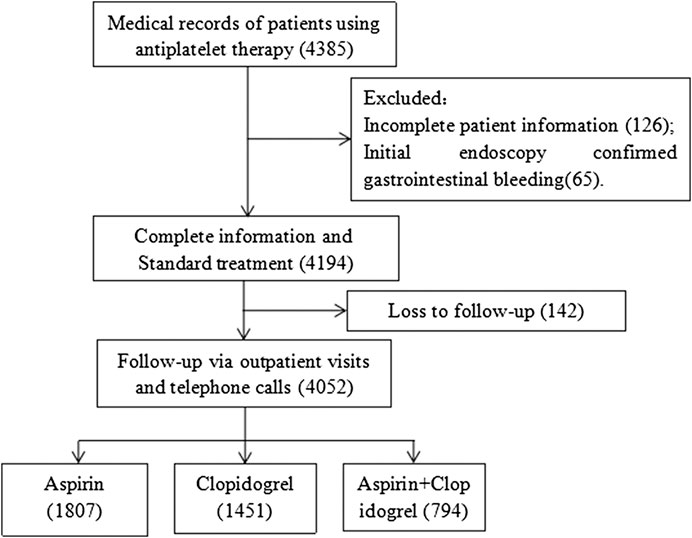
We retrospectively analyzed patients who used antiplatelet drugs for at least 6 months at two medical centers (Fujian Medical University Union Hospital (one of the largest emergency hospitals in China) and Fuzhou Second Hospital (affiliated to Xiamen University)) from May 2015 to December 2018. Data were obtained from the patient medical records inpatient system. We searched the electronic database using keywords such as “aspirin,” “clopidogrel,” and “antiplatelet drugs.” There were 4,385 patients taking antiplatelet drugs (aspirin 100 mg/day, clopidogrel 75 mg/day, or aspirin 100 mg/day plus clopidogrel 75 mg/day) continuously. The exclusion criteria were as follows: (1) age <18 years; (2) gastrointestinal bleeding before medication confirmed by endoscopy; (3) no follow-up or <6 months of follow-up; (4) insufficient data preventing subsequent analysis. Finally, 4,052 patients were included in the study (Figure 1). The study was approved by the Fujian Medical University Union Hospital Ethics Committee. Informed consent was obtained from all individual participants included in the study through telephone follow-up.
Data Collection
We collected data on each patient from the electronic database at the hospital. Data were clinically organized and recorded by professional doctors and nurses in a timely manner, including demographic information (e.g., age and sex), concomitant illnesses (e.g., hypertension, diabetes, chronic kidney or liver disease, history of gastrointestinal bleeding, history of peptic ulcers, recent major bleeding (defined as major bleeding <30 days before starting antiplatelet drugs), and anemia (defined as a hemoglobin concentration of <13 g/dl in males or <12 g/dl in females)), and other medications (e.g., nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and oral anticoagulants).
Endpoints and Follow-Up
Patients were followed up at the antithrombotic clinic and by telephone for approximately 6 months. Patients were generally required to undergo regular outpatient follow-up every month to facilitate monitoring of symptoms and clinical signs. For patients with a bleeding tendency, further examination was recommended. The main outcomes were acute gastrointestinal bleeding and death. Gastrointestinal bleeding events were defined as bleeding with clinical evidence (i.e., guaiac-positive stool, melena, hematochezia, hematemesis, or the presence of blood in the gastrointestinal tract on endoscopic evaluation) and a documented decrease in hemoglobin of ≥2 g/dl (Chan et al., 2010; Yin et al., 2018). The cause of death was determined by consulting clinical information records and death certificates in the electronic medical records system.
Statistical Analysis
SPSS 25.0 (IBM Corp., Armonk, NY, United States) statistical software was used to analyze data. Continuous variables are presented as mean ± standard deviation or median, and categorical variables are presented as percentages. Pearson’s χ2 test or Fisher’s exact test was used for univariate analysis of categorical variables as appropriate. The variables related to acute gastrointestinal hemorrhage with a univariate analysis (p < 0.20) were included in the logistic regression analysis to confirm whether they were independent predictors of acute gastrointestinal hemorrhage. If the p value was <0.05, they were retained in the final model to establish a risk score. The weight of each predictor, according to the coefficients in the model, was determined. The area under the curve (AUC) was used to evaluate the discriminatory power of the risk-scoring model (bleeding group vs. nonbleeding group 1:1). The Hosmer–Lemeshow test was used to assess the calibration ability of the model. The logrank test and Kaplan–Meier curves were used to estimate the cumulative mortality of patients with gastrointestinal bleeding and patients without bleeding. A p value of ≤0.05 was considered statistically significant.
Results
Patient Characteristics
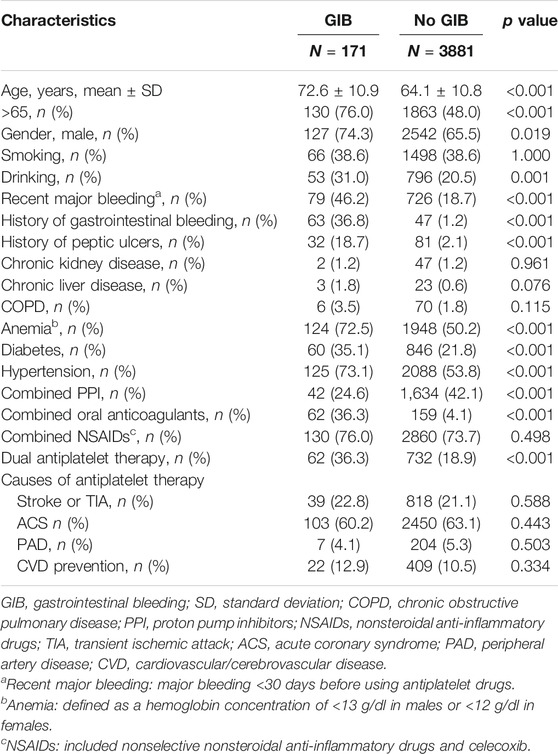
A total of 4,052 patients were included. Table 1 shows the baseline characteristics of patients taking antiplatelet drugs. The average age of patients in the bleeding group was greater than the average age of patients in the nonbleeding group (mean age, 72.6 vs. 64.1 years, respectively; p < 0.001). Certain factors, including an age of >65 years (76.0 vs. 48.0%, p < 0.001), male sex (74.3 vs. 65.5%, p = 0.019), drinking (31.0 vs. 20.5%, p = 0.001), recent major bleeding events (46.2 vs. 18.7%, p < 0.001), a history of gastrointestinal bleeding (36.8 vs. 1.2%, p < 0.001), a history of peptic ulcers (18.7 vs. 2.1%, p < 0.001), anemia (72.5 vs. 50.2%, p < 0.001), diabetes (35.1 vs. 21.8%, p < 0.001), hypertension (73.1 vs. 53.8%, p < 0.001), combined use of proton pump inhibitors (24.6 vs. 42.1%, p < 0.001), combined use of oral anticoagulants (36.3 vs. 4.1%, p < 0.001), and dual antiplatelet therapy (36.3 vs. 18.9%, p < 0.001) correlated with acute gastrointestinal bleeding (p < 0.05).
Occurrence of Gastrointestinal Bleeding and Death
During the 6-month follow-up period, the incidence of acute gastrointestinal bleeding was 4.2% (171/4,052). The mortality rate in the bleeding group was 24.6% (42/171), and the mortality rate in the nonbleeding group was 4.7% (182/3,881). The mortality rate in the bleeding group was approximately five times that of the nonbleeding group (Figure 2).
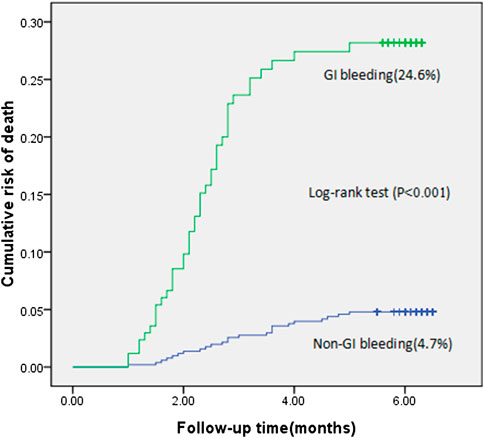
FIGURE 2. Cumulative risk of death in patients with gastrointestinal bleeding compared with patients without gastrointestinal bleeding (the logrank test, p < 0.001).
Risk Factors for Gastrointestinal Bleeding and Establishment and Evaluation of the Risk-Scoring Model
With a univariate analysis, factors related to acute gastrointestinal bleeding were an age of >65 years (p < 0.001), male sex (p = 0.019), drinking (p = 0.001), recent major bleeding (p < 0.001), a history of gastrointestinal bleeding (p < 0.001), a history of peptic ulcers (p < 0.001), chronic liver disease (p = 0.076), chronic obstructive pulmonary disease (p < 0.115), anemia (p < 0.001), diabetes (p < 0.001), hypertension (p < 0.001), combined use of proton pump inhibitors (p < 0.001), combined use of oral anticoagulants (p < 0.001), and dual antiplatelet therapy (p < 0.001).
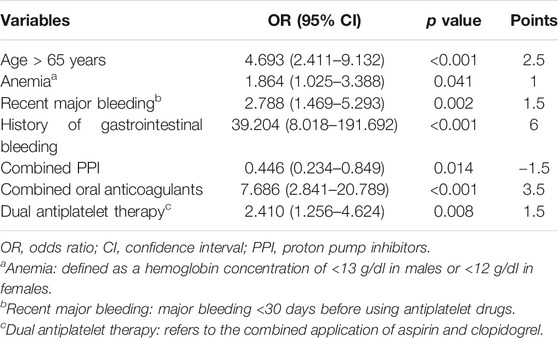
With a multivariate analysis, the risk-scoring model of gastrointestinal bleeding was established using seven risk factors, including an age of >65 years, anemia, recent major bleeding, a history of gastrointestinal bleeding, no-proton pump inhibitors use, combined use of oral anticoagulants, and dual antiplatelet therapy (Table 2). The score was named the (ABC)2D score. According to the regression coefficient of the final model, an age of >65 years, anemia, recent major bleeding, a history of gastrointestinal bleeding, combined use of proton pump inhibitors, combined use of oral anticoagulants, and dual antiplatelet therapy were assigned 2.5, 1, 1.5, 6. −1.5, 3.5, and 1.5 points, respectively.
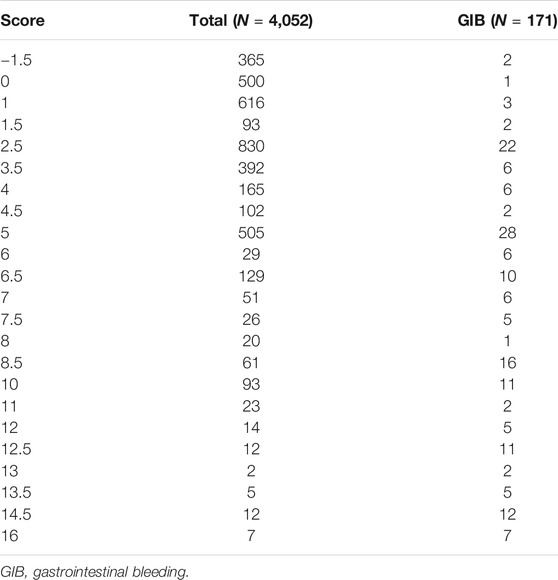
The AUC for the (ABC)2D score was 0.857 (95% confidence interval (CI) 0.818–0.896; p < 0.001), which is higher than the CRUSADE score (0.693, 95% CI 0.637–0.749; p < 0.001; Table 3). The p value for the Hosmer–Lemeshow test was 0.324. The Hosmer–Lemeshow test indicated that the risk-scoring model had a good calibration capability (p > 0.05). Detailed score distribution of (ABC)2D score is shown in Table 4.
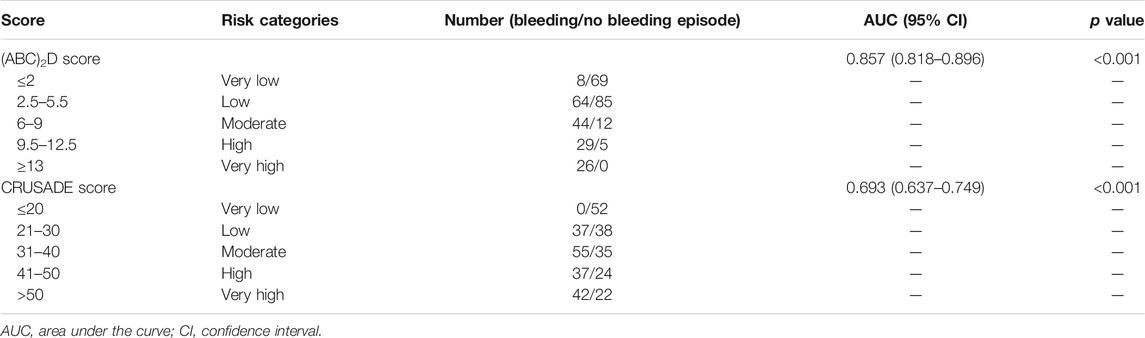
TABLE 3. Comparison of the predictive ability and performance of the (ABC)2D score and the CRUSADE score.
Discussion
We studied the incidence and mortality of gastrointestinal bleeding in patients taking oral antiplatelet drugs. Patients who experienced an episode of acute gastrointestinal bleeding had a significantly higher risk of subsequent death when compared with the nonbleeding group (24.6 vs. 4.7%, respectively; p < 0.001). Through univariate and multivariate analyses, it was found that an age of >65 years, anemia, recent major bleeding, a history of gastrointestinal bleeding, combined use of oral anticoagulants, and dual antiplatelet therapy increased the risk of gastrointestinal bleeding, while combined use of proton pump inhibitors reduced the risk of gastrointestinal bleeding. Therefore, it can help remind clinicians that proton pump inhibitors should be initiated for those with a high score or obvious risk factors. Furthermore, if the patient’s score is high, then the need for dual antiplatelet therapy or combined anticoagulant should be reviewed.
During the 6-month follow-up of patients taking antiplatelet drugs, the death rate of patients with gastrointestinal bleeding was 24.6%. Such a high mortality rate reflects the importance of developing an accurate and simple method to predict which patients are at a high risk of gastrointestinal bleeding. Therefore, we used seven factors to build a new gastrointestinal bleeding risk-scoring model, which has a better predictive power than the CRUSADE score. According to the AUC, this new risk-scoring model can distinguish the risk of gastrointestinal bleeding. We aim to use the (ABC)2D scoring system in clinical practice as it may be a useful method to reduce the risk of bleeding in patients taking oral antiplatelet drugs and to provide a certain reference for clinical antiplatelet therapy.
In our study, age and a history of gastrointestinal bleeding influenced gastrointestinal bleeding. This is consistent with the findings of Lin et al. (2013) who stated that age and a history of gastrointestinal bleeding are independent risk factors for gastrointestinal bleeding in patients taking aspirin and clopidogrel.
We also found that recent major bleeding events (odds ratio (OR) = 2.788; p = 0.002) and anemia (OR = 1.864; p = 0.041) increased the risk of gastrointestinal bleeding. Ruíz-Giménez et al., 2008 found that major bleeding events (e.g., gastrointestinal bleeding and intracranial hemorrhage) were related to recent major bleeding (OR = 2.7; p < 0.001) and anemia (OR = 2.1; p < 0.001), which is similar to our findings.
We also found that combined use of oral anticoagulants greatly increased the risk of gastrointestinal bleeding. Related studies have also shown that combined use of antiplatelet drugs and anticoagulant drugs is a risk factor for gastrointestinal bleeding. Like traditional anticoagulant drugs, new oral anticoagulant drugs may increase the risk of gastrointestinal bleeding (Abrignani et al., 2020).
In addition, related studies have shown that dual antiplatelet therapy has a higher bleeding risk than single antiplatelet therapy (Diener et al., 2004; Bhatt et al., 2006). Compared with aspirin alone, clopidogrel combined with aspirin causes a significantly higher bleeding rate (Yusuf et al., 2001). In two randomized controlled trials, the relative risk of gastrointestinal bleeding with dual antiplatelet therapy was almost twice that with single antiplatelet therapy (Yusuf et al., 2001; ACTIVE Investigators et al., 2009). We also found that patients taking aspirin in combination with clopidogrel had a higher risk of gastrointestinal bleeding (OR = 2.410; p = 0.008) compared with patients taking either aspirin or clopidogrel alone.
Finally, our study showed that the use of combined proton pump inhibitors reduced the risk of gastrointestinal bleeding by approximately half (OR = 0.446; p = 0.014), which closely reflects the findings of Hu et al., 2018, who stated that patients taking aspirin and clopidogrel used combined proton pump inhibitors, and the incidence of gastrointestinal bleeding decreased (OR = 0.58; p = 0.022). Although the combined use of proton pump inhibitors is not included in the CRUSADE (Subherwal et al., 2009) and S2TOP-BLEED scores (Hilkens et al., 2017), combined use of proton pump inhibitors is an important means of preventing gastrointestinal bleeding in the long term.
Our study is advantageous because of its relatively large sample size (N = 4,052). Our study presents the first risk-scoring model for predicting antiplatelet-induced gastrointestinal bleeding. Second, the statistical methods used in this study were rigorous. The AUC was used to evaluate the predictive ability of the model, while the Hosmer–Lemeshow test was used to assess the calibration ability of the model.
Our research also has some limitations. First, the study was conducted at two centers. Second, although (ABC)2D score is a better predictor than the alternatives, this would have to be proven on a new independent cohort in the future. Our next study will continue to collect more data from more centers, establish a new cohort, and further verify the scoring system. Third, our scoring tool has not yet been applied to the clinic on a large scale, so its versatility also requires further investigation in large-scale prospective multicenter research studies.
Conclusion
We developed a new risk-scoring model based on seven factors (i.e., an age of >65 years, anemia, recent major bleeding, a history of gastrointestinal bleeding, no-proton pump inhibitors use, combined use of oral anticoagulants, and dual antiplatelet therapy) to evaluate the risk of acute gastrointestinal bleeding. The predictive ability of (ABC)2D is superior to that of the CRUSADE score. This new risk-scoring model may help to identify patients at a significant risk of gastrointestinal bleeding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Fujian Medical University Union Hospital Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JZ initiated the study. All the authors were involved in the literature search and data collection. ML and XZ performed the statistical analysis. ML drafted the first version of the manuscript. JZ and ML critically reviewed the manuscript and revised it.
Funding
The work was supported by the National Natural Science Foundation of Fujian Province of China (grant no. 2018Y0037).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank C. Fenglin’s team for their help in verifying the results of outpatient endoscopy of some patients with gastrointestinal bleeding.
References
Abrignani, M. G., Zullo, A., Gabrielli, D., Milazzo, G., Francesco, V. D, Luca, L. D., et al. (2020). ANMCO/AIGO Intersocietary consensus document: gastroprotection in patients receiving antiplatelet and/or anticoagulant drugs. G. Ital. Cardiol. 21 (3), 228–241. doi:10.1714/3306.32772
ACTIVE Investigators Connolly, S. J., Pogue, J., and Hart, R. G., Hohnloser Hohnlose, S. H., Pfeffer Pfeffer, M., et al. (2009). Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N. Engl. J. Med. 360, 2066–2078. doi:10.1056/NEJMoa0901301
Antithrombotic Trialists’ (ATT) Collaboration Baigent, C., and Blackwell, L., Collins Collins, R., Emberson Emberson, J., Godwin Godwin, J., et al. (2009). Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373, 1849–1860. doi:10.1016/S0140-6736(09)60503-1
Berger, J. S., Bhatt, D. L., Steg, P. G., Steinhubl, S. R., Montalescot, G., Shao, M., et al. (2011). Bleeding, mortality, and antiplatelet therapy: results from the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance (CHARISMA) trial. Am. Heart J. 162, 98–105. doi:10.1016/j.ahj.2011.04.015
Bhatt, D. L., Fox, K. A., Hacke, W., Berger, P. B., Black, H. R., Boden, W. E., et al. (2006). Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N. Engl. J. Med. 354, 1706–1717. doi:10.1056/NEJMoa060989
Chan, F. K., Cryer, B., Goldstein, J. L., Lanas, A., Peura, D. A., Scheiman, J. M., et al. (2010). A novel composite endpoint to evaluate the gastrointestinal (GI) effects of nonsteroidal antiinflammatory drugs through the entire GI tract. J. Rheumatol. 37, 167–174. doi:10.3899/jrheum.090168
Chinese Society of Cardiology of Chinese Medical AssociationEditorial Board of Chinese Journal of Cardiology (2013). Expert consensus of antiplatelet therapy in cardiovascular disease. Zhonghua Xin Xue Guan Bing Za Zhi. 41(3), 183–194. doi:10.3760/cma.j.issn.0253-3758.2013.03.004
Diener, H. C., Bogousslavsky, J., Brass, L. M., Cimminiello, C., Csiba, L., Kaste, M., et al. (2004). Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 364, 331–337. doi:10.1016/S0140-6736(04)16721-4
Ruíz-Giménez, N., Suárez, C., González, R., Neito, J. A., Todolí, J. A., Samperiz, A. L., et al. (2008). Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb. Haemost. 100 (1), 26–31. doi:10.1160/TH08-03-0193
Hikens, N. A., Algra, A., Diener, H. C., Reitsma, J. B., Bath, P. M., Csiba, L., et al. (2017). Predicting major bleeding in patients with noncardioembolic stroke on antiplatelets: S2TOP-BLEED. Neurology 89 (9), 936–943. doi:10.1212/WNL.0000000000004289
Hu, W., Tong, J., Kuang, X., Chen, W., and Liu, X. (2018). Influence of proton pump inhibitors on clinical outcomes in coronary heart disease patients receiving aspirin and clopidogrel: a meta-analysis. Medicine 97 (3), e9638. doi:10.1097/MD.0000000000009638
Laine, L., and Jensen, D. M. (2012). Management of patients with ulcer bleeding. Am. J. Gastroenterol. 107, 345–361. doi:10.1038/ajg.2011.480
Lin, C. C., Hu, H. Y., Luo, J. C., Hou, M. C., Lin, H. C., and Lee, F. Y. (2013). Risk factors of gastrointestinal bleeding in clopidogrel users: a nationwide population-based study. Aliment. Pharmacol. Ther. 38 (9), 1119–1128. doi:10.1111/apt.12483
Li, L., Geraghty, O. C., Mehta, Z., and Rothwell, P. M.Oxford Vascular Study (2017). Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 390, 490–499. doi:10.1016/S0140-6736(17)30770-5
Pannach, S., Goetze, J., Marten, S., Schreier, T., Tittl, L., and Westendorf, J. B. (2017). Management and outcome of gastrointestinal bleeding in patients taking oral anticoagulants or antiplatelet drugs. J. Gastroenterol. 52 (12), 1211–1220. doi:10.1007/s00535-017-1320-7
Sostres, C., Marcén, B., Laredo, V., Alfaro, E., Ruiz, L., Camo, P., et al. (2019). Risk of rebleeding, vascular events and death after gastrointestinal bleeding in anticoagulant and/or antiplatelet users. Aliment. Pharmacol. Ther. 50 (8), 919–929. doi:10.1111/apt.15441
Strate, L. L., and Gralnek, I. M. (2016). ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am. J. Gastroenterol. 111, 459–474. doi:10.1038/ajg.2016.41
Subherwal, S., Bach, R. G., and Chen, A. Y., Gage Gage, B. F., Rao Rao, S. V., Newby Newby, L. K., et al. (2009). Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with Early implementation of the ACC/AHA Guidelines) bleeding score. Circulation 119 (14), 1873–1882. doi:10.1161/CIRCULATIONAHA.108.828541
Yamada, Y., Eto, M., Yamamoto, H., Akishita, M., and Ouchi, Y. (2012). Gastrointestinal hemorrhage and antithrombotic drug use in geriatric patients. Geriatr. Gerontol. Int. 12 (4), 751–752. doi:10.1111/j.1447-0594.2012.00835.x
Yin, M. Y., Ruckel, S., Kfoury, A. G., Mckellar, S. H., Taleb, L., Gilbert, E. M., et al. (2018). Novel model to predict gastrointestinal bleeding during left ventricular assist device support. Circ. Heart Fail 11, e005267. doi:10.1161/CIRCHEARTFAILURE.118.005267
Keywords: acute gastrointestinal bleeding, risk factor, score, aspirin, clopidogrel
Citation: Lv M, Zheng X, Wu T, Chen W, Jiang S, Zhang H, Xu F and Zhang J (2021) A New Score for Predicting Acute Gastrointestinal Bleeding in Patients Administered Oral Antiplatelet Drugs. Front. Pharmacol. 11:571605. doi: 10.3389/fphar.2020.571605
Received: 08 September 2020; Accepted: 02 December 2020;
Published: 14 January 2021.
Edited by:
Nicolau Beckmann, Novartis Institutes for BioMedical Research, SwitzerlandReviewed by:
Jonathan Hoare, Imperial College London, United KingdomMatthew Hawks, Uniformed Services University of the Health Sciences, United States
Copyright © 2021 Lv, Zheng, Wu, Chen, Jiang, Zhang, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhua Zhang, pollyzhang2006@126.com
 Meina Lv1,2
Meina Lv1,2 Tingting Wu
Tingting Wu Jinhua Zhang
Jinhua Zhang