- 1Department of Pharmacology, College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Clinical Pharmacy, Shaanxi Provincial Hospital of Tuberculosis Prevention and Treatment, Xi’an, China
- 3Department of Bioengineering, Zhuhai Campus of Zunyi Medical University, Zhuhai, China
- 4Key Laboratory of Veterinary Pharmaceutical Development of Ministry of Agriculture, Key Laboratory of New Animal Drug Project, Lanzhou Institute of Husbandry and Pharmaceutical Sciences, Chinese Academy of Agricultural Sciences, Lanzhou, China
Juglans mandshurica Maxim., also known as “Manchurian walnut” (Chinese) and “Onigurumi” (Japanese), is a medicinal plant widely distributed in Western and Central Asia, especially in China. It has been traditionally used to treat cancer, gastric ulcers, diarrhea, dysentery, dermatosis, uterine prolapse, and leukopenia. To date, more than 400 constituents including quinones (e.g. naphthoquinones, anthraquinones, naphthalenones, tetralones), phenolics, flavonoids, triterpenoids, coumarins, lignans, phenylpropanoids, diarylheptanoids, and steroids, were isolated and structurally identified from different plant parts of J. mandshurica. Among them, quinones, phenolics, triterpenoids, and diarylheptanoids, as the major bioactive substances, have been extensively studied and displayed significant bioactivity. Previous studies have demonstrated that J. mandshurica and a few of its active components exhibit a wide range of pharmacologically important properties, such as antitumor, immunomodulatory, anti-inflammatory, neuroprotective, anti-diabetic, antiviral, antimicrobial, and anti-melanogenesis activities. However, many investigations on biological activities were mainly based on crude extracts of this plant, and the major bioactive ingredients responsible for these bioactivities have not been well identified. Further in vitro and in vivo studies on the mechanisms of action of the pure bioactive compounds, and more elaborate toxicity studies as well as clinical studies are needed to ensure safety and effectiveness of the plant for human use. Taken together, the present review will provide some specific useful suggestions guide to further investigations and applications of this plant in the preparation of medicines and functional foods.
Introduction
Juglans mandshurica Maxim, known as Manchurian walnut and Onigurumi, is a perennial and fast-growing deciduous broad-leaf tree reaching up to 20 m in the family Juglandaceae. It is extensively cultivated and distributed on a large scale throughout China, India, Japan, Siberia, Russia, and Korean Peninsula, etc. (Son, 1995; Machida et al., 2005; Bai et al., 2010; Wang et al., 2015; Hu et al., 2016; Li et al., 2018; Zhao et al., 2018; Zhao et al., 2019). In China, as hardwood tree species together with Fraxinus mandshurica Rupr. and Phellodendron amurense Rupr., it is mainly distributed in temperate to warm-temperate zones, and thus itgrown throughout many regions of northeast China, such as Heilongjiang and Liaoning provinces (Editorial Committee of Flora of China, 1979; Wang et al., 2020a). Now, it is officially listed as a national level Ⅱ rare tree species and is also ranked as a rare and endangered tree species in China (Zhu et al., 2018). More importantly, every plant parts of J. mandshurica, including roots, stems, barks, branches, leaves, green husks, and immature fruits have important medical and health protection values, and have been used to prevent or treat multiple diseases for hundreds of years (see Figure 1; Zhao et al., 2019). As an example, “Bei-Qing–Long–Yi” (BQLY), the epicarp of immature fruits of J. mandshurica, has been used as traditional medicine for the treatment of cancer, gastric ulcers, diarrhea, dysentery, dermatosis, uterine prolapse, and leukopenia in northern China and Korea (Park et al., 2012; Liu et al., 2017; Park et al., 2017; Zhang et al., 2017; Huo et al., 2018; Zhou et al., 2019b). Currently, it is attracting increasing interest worldwide due to its various health-promoting effects. Nevertheless, overdosage or unreasonable use of BQLY can lead to some adverse reaction, such as nausea, vomiting, dizziness, dyspnea, palpitation, and even shock and death (Huo et al., 2017).
Phytochemical investigations on the different medicinal parts (roots, stems, barks, branches, leaves, and immature fruits) led to the isolation and identification of more than 400 compounds, including quinones, phenolics, flavonoids, lignans, coumarins, phenylpropanoids, triterpenoids, diarylheptanoids, and steroids. Among these compounds, quinones, phenolics, triterpenoids, and diarylheptanoids have been extensively studied and displayed the best bioactivity. As an example, naphthoquinone compounds obtained from green walnut husks of J. mandshurica were recognized as major active component that is mainly responsible for the anticancer activity, and the study on the bioactivity of these components has become a hotspot and attracted widespread attention from domestic and foreign researchers (Zhang et al., 2019). The kernels of the nuts of J. mandshurica also have high nutritional value, containing lipids (60–66%), proteins (15–20%), carbohydrates (1–15%), vitamins, and minerals (Wang et al., 2017b; Fang et al., 2018; Wang et al., 2020a). The lipids are also considered to be the main source for bioactivities owing to their abundant polyunsaturated fatty acids (Carey et al., 2020). Recent pharmacological studies have revealed that the active components and/or crude extracts of J. mandshurica display various biological activities, such as antitumor, immunoregulatory, anti-inflammatory, neuroprotective, anti-diabetic, antiviral, antimicrobial and anti-melanogenesis activities. More importantly, most of these claimed effects are consistent with those observed therapeutic actions of J. mandshurica in folk medicine.
Until recently, scientists have made a great contribution to report the chemical constituents and biological properties of J. mandshurica. However, no systematic review covering all-important aspects on this plant is available. In order to provide new insights for the in-depth exploration and comprehensive utilization of this plant, we systematically and critically summarize the current findings on botanical description, traditional usages, phytochemistry, pharmacology, and toxicology as well as the potential molecular mechanisms of J. mandshurica. Available information on this plant in this review enables people to explore their therapeutic potential, to highlight the gaps as well as provide the scientific basis for future study of this plant.
Botanical Description and Traditional Usages
Botanical Description
J. mandshurica is a tree with gray bark that can grow up to a height of approximately 20 m. The odd-pinnate compound leaves can grow up to 80 cm on the sprout, the petiole is 9–14 cm in length, the leaflets are 6–17 cm in length and 2–7 cm in width. The shape of the leaflets is elliptical, oblong, ovate-elliptic or oblong-lanceolate, serrated, first sparsely pubescent on top, the underside is flat pilose with stellate hairs, the lateral leaflets are sessile, the apex is acuminate, and the base is truncated or heart-shaped. The male catkin inflorescence is 9–20 cm long, the inflorescence rachis is pubescent and usually has 12 stamens, the drug septum is gray-black pilose, the female spike is 5–6 mm in length and usually has 4–10 flowers, and the rachis is pubescent. The infructescence is approximately 10–15 cm in length, and infructescence pendulous with up to 5–7 fruits. The fruit is globular, ovate or elliptical with a sharp tip, and it is densely covered with glandular pubescence. Generally, it is approximately 3.5–7.5 cm in length and 3–5 cm in diameter. The fruit nucleus is 2.5–5 cm long with 8 longitudinal ridges on the surface, two of which are more prominent. The flowering period is in May and the fruit period from August to September (http://ppbc.iplant.cn/sp/10792).
Traditional Usages
Local and traditional usages of J. mandshurica in China can be traced back to the Han dynasty over 2000 years ago. Available literature shows that J. mandshurica has been used as popular herbal medicine and food by ethnic groups in many regions of the world, especially in Asian countries, such as China, Japan, and Korea to treat the various diseases like leucorrhoea, diarrhea, gastritis, leukopenia, dermatosis, and uterine prolapse (Liu et al., 2004a; Li et al., 2005; Xu et al., 2010; Park et al., 2012; Park and Oh, 2014; Yao et al., 2015b; Li et al., 2017b; Park et al., 2017; Chaudhary et al., 2019).
In China, J. mandshurica, bitter and pungent in taste, was firstly listed and recorded as the “highest-grade” medicine in the famous Chinese ancient classical book “Compendium of Materia Medica” (Simplified Chinese: 本草纲目) compiled by pharmacologist Shizhen Li (1518–1593 CE) in the Ming Dynasty (Zhang et al., 2018). According to another TCM monograph of “Kaibao Bencao” (Simplified Chinese: 开宝本草) in the Song Dynasty, BQLY has the functions of nourishing lungs and relieving asthma. Moreover, the decoction of kernels, barks, roots, and immature pericarps of J. mandshurica has been used as folk remedy for treating cancer, which was consistent with their heat clearing and detoxification effects (Lee et al., 2002; Li et al., 2003; Park et al., 2012; Yao et al., 2012; Xu et al., 2013; Gao et al., 2016; Wang et al., 2017a; Zhang et al., 2019). Interestingly, J. mandshurica is traditionally decocted together with chicken eggs to effectively prevent and treat multiple tumors in Chinese folk medicine (Wang et al., 2017a; Wang et al., 2017c).
It is important that various parts of this plant, including the green walnut husks, green peels, roots, stems, barks, branches, leaves and immature fruits have a great medicinal value in indigenous medicine. The green peels were extensively used as folk remedy for removing heat and detoxication, relieving dysentery, and improving eyesight (Li et al., 2017a). The barks were commonly used to treat urinary stones, lichen planus circumscriptus, chronic bronchitis, blurred vision, shigellosis, and HIV (Xin et al., 2014; Yao et al., 2017). Its fresh rejuvenated fruit has been used traditionally as a medicine for treatment of cancer and dermatosis, and as an anodyne to relieve aches in China (Liu et al., 2004a). The nuts are extensively used as food because of its considerable nutritional value (Wang et al., 2017b; Mu et al., 2017). In Japan, several parts of this plant have been used in folk medicines and the fruits have been commonly used for the treatment of chilblains and athlete’s foot (Machida et al., 2005).
Phytochemical Constituents
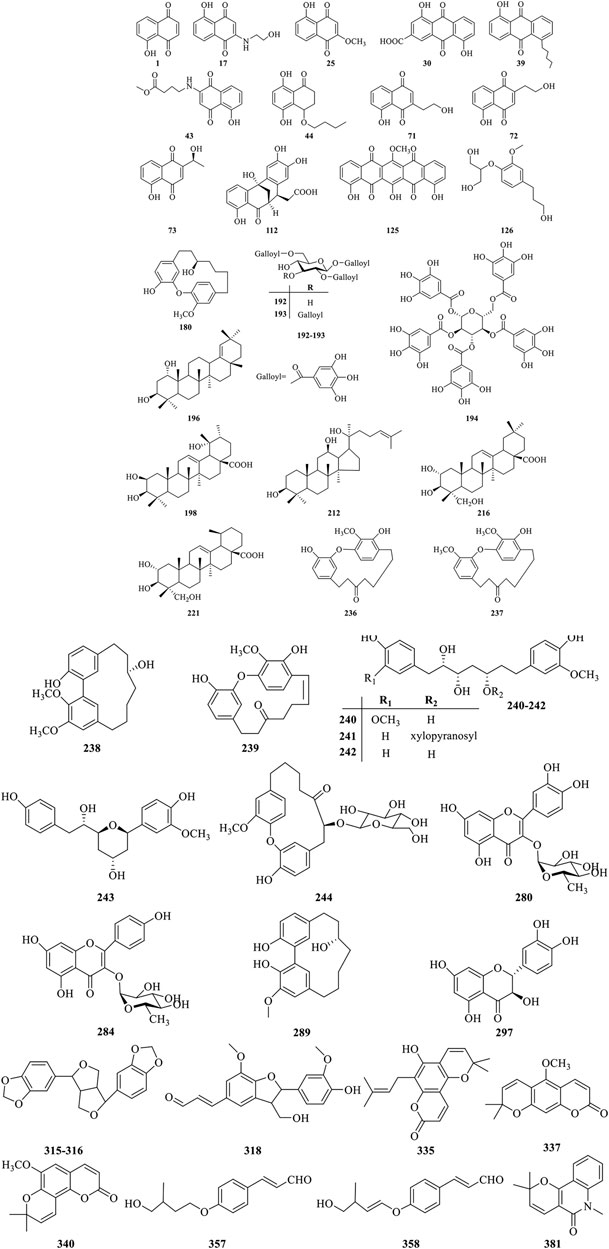
Currently, more than 400 comounds including quinones, phenolics, triterpenoids, diarylheptanoids, flavonoids, coumarins, lignans, phenylpropanoids, and steroids, etc. have been isolated and identified from different organs of J. mandshurica Among them, quinones, phenolics, triterpenoids, and diarylheptanoids are the most important and abundant bioactive constituents, which have been considered as the promising ingredients for future evaluation. Many ingredients with significant biological activities such as juglone, juglanthraquinone C, juglonol A, juglanin B, and juglansoside C might be used as markers for quantitative validatio and quality control of the plant in the future. The chemical compounds isolated and identified from J. mandshurica are summarized in Table 1, and structures of major bioactive compounds are presented in Figure 2.
Quinones
Until now, approximately 125 quinones and their derivatives have been identified from the different plant organs of J. mandshurica. Quinones found in this plant can be structurally divided into naphthoquinones (1–29), anthraquinones (30–40), naphthalenones (41–54), tetralones (55–123), and benzoquinones (124–125) based on the structural characteristics. In recent years, the study on the bioactivity of naphthoquinone compounds obtained from J. mandshurica has become a hotspot, which was recognized as major active components for the anticancer activity (Zhang et al., 2019). However, few in vivo pharmacological activity evaluation and even clinical trials of these ingredients were still reported recently.
Phenolics
Nowadays, a total of 69 phenolics constituents (126–194) have been isolated and structurally characterized from the different parts of J. mandshurica. Nevertheless, only few bioactive phenolic compounds of this plant have been reported in recent years. To fully utilize the phenolics constituents of J. mandshurica in the development and application of cosmetic, functional foods and pharmaceutical products, more in-depth research on chemical ingredients and bioactivities are urgently needed.
Triterpenoids
To date, approximately forty-one triterpenoids (195–235) have been isolated and identified from the different parts of J. mandshurica. Among of them, dammarane-type triterpenoids isolated and identified from different medicinal parts of J. mandshurica, have captured more and more attention around the world due to their potent pharmacological activities, especially in antitumor properties (Salehi et al., 2019).
Diarylheptanoids
Diarylheptanoids own multiple pharmacological activities, raising ncreasingly attention over the last few decades (Sun et al., 2020). Currently, a total of 40 diarylheptanoids (236–275) were identified from the different parts of J. mandshurica. Among of them, compound 237–239, showed outstanding cytotoxicity against the A549 and HeLa cells (Wang et al., 2019a).
Flavonoids
Flavonoids are widespread in the plant kingdom in free form or as glycosides, and many of them are natural drugs with various medical functions (Luan et al., 2019). Up to date, a total of 39 flavonoids (276–314) have been obtained and purified from the green peel, epicarp, stem barks, roots, green walnut husks, and pericarps of J. mandshurica. Amongst the isolated compounds, taxifolin (297) exhibited the strongest anti-HIV-1 activity against MT-4 cells (Min et al., 2002). However, pharmacological investigations on other flavonoids from J. mandshurica are very limited in the existing literature, and need to urgently conduct in future study.
Lignans
Lignans with chiral carbon atoms are usually consisted of a pair of enantiomers or several pairs of stereoisomers with different amount in nature, and the biological activities of enantiomers are not identical due to the chiral nature of the biological receptors (Pereira et al., 2011). Until now, 20 lignans (315–334) have been structurally identified from the barks, roots, and fruits of J. mandshurica.
Coumarins
Coumarins refer to the general term of o-hydroxycinnamic acid lactones with the basic skeleton of benzoben-α-pyranone parent nucleus, which is one of the main components of TCM (Jiang et al., 2020). At present, 19 coumarins (335–353) have been isolated and characterized from the stem barks of J. mandshurica, and mainly include simple coumarins and pyranocoumarins.
Phenylpropanoids
Phenylpropanoids displayed various biological effects including defending against herbivores, microbial attack, or other sources of injury. Nowadays, a total of 16 phenylpropanoids (354–369) have been isolated and structurally identified from the barks, leaves, pericarps, and green walnut husks of J. mandshurica. However, studies on biological effects of phenylpropanoids from J. mandshurica are very limited.
Steroids
So far, phytochemical investigations from the green walnut husks, roots, and epicarp of J. mandshurica have shown the presence of 11 steroids (370–380) including daucosterol (370), daucosterin (371), 24(R)-5α-stigmasterol (372), β-sitosterol (373), stigmast-5-en-3β,7α-diol (374), stigmast-5-en-3β,7β-diol (375), stigmast-5-en-3β-ol (376), stigmast-4-en-3-one (377), 24(R)-5α-stigmastane-3,6- dione (378), ligstroside (379), and oleuropein (380). However, few bioactive steroids have been reported recently.
Alkaloids
Alkaloids is an important secondary metabolite and represent a relatively small class of compounds from this plant and possess remarkable antitumor activity. Until now, 7 alkaloids (381–387) have been isolated and structurally elucidated from the barks of J. mandshurica. However, there are not many studies on the biological activity of these alkaloids and therefore further research need to be explored.
Other Compounds
A few other classes of compounds (388–407) have been isolated from J. mandshurica. Among them, siaresinolic acid (391), dihydrophaseic acid (392), epi-dihydrophaseic acid (393), nodulisporone (394), 1-ethyl malate (395), 1-buthyl malate (396), succinic acid (397), ethyl-O-β-d-glucopyranoside, 3β,20-dihydroxy- 5β-pregnant (398) were first isolated from green walnut husks of this plant (Zhang et al., 2009; Qiu et al., 2017).
Pharmacological Properties
To date, J. mandshurica have been explored for multiple pharmacological activities, such as antitumor, immunoregulatory, anti-inflammatory, neuroprotective, antidiabetic, antiviral, antimicrobial, and anti-melanogenesis activities. Next, these biological activities were discussed one by one in the following paragraphs, and the recapitulative summary was also presented in Table 2. The mechanism of the typical and representative pharmacological activities like antitumor, immune immunoregulation, antioxidant and neuroprotective activities of J. mandshurica are summarized and presented in the following Figures 3–6, respectively.
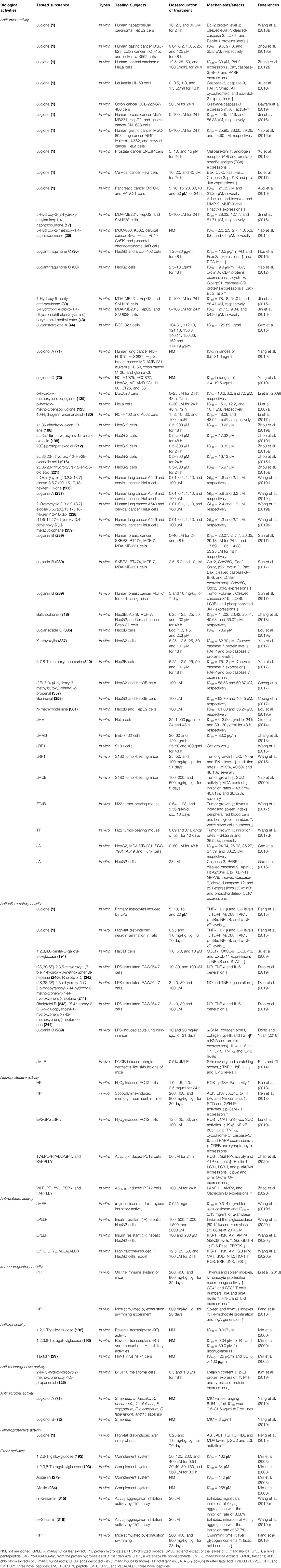
TABLE 2. The pharmacological activities of bioactive compounds and extracts of J. mandshurica ("↓", decrease; "↑", increase).
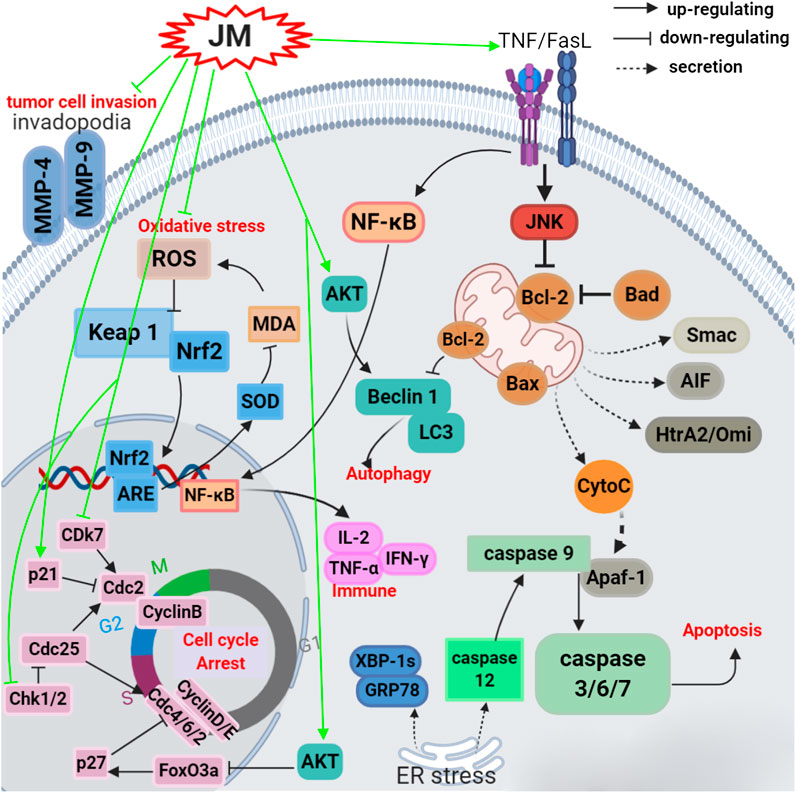
FIGURE 3. Schematic representation of the possible mechanism of antitumor activity of J. mandshurica.
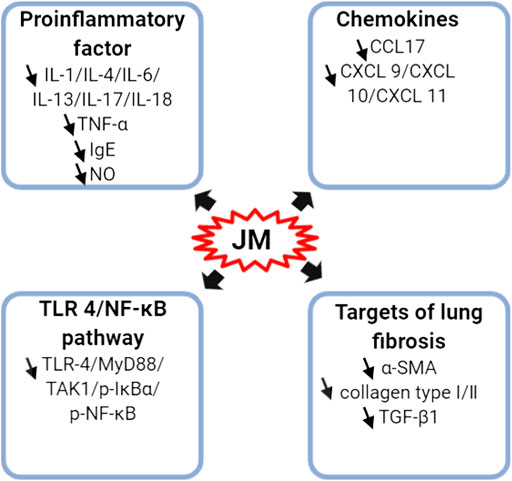
FIGURE 4. Schematic representation of the molecular mechanism of anti-inflammatory of J. mandshurica.
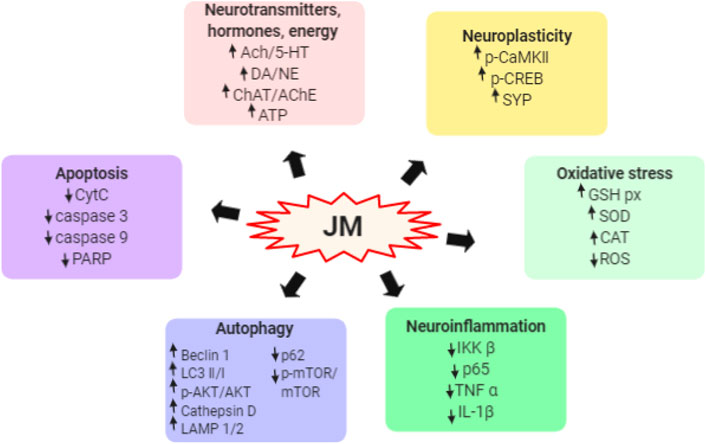
FIGURE 5. Schematic representation the underlying mechanism of neuroprotective activity of J. mandshurica.
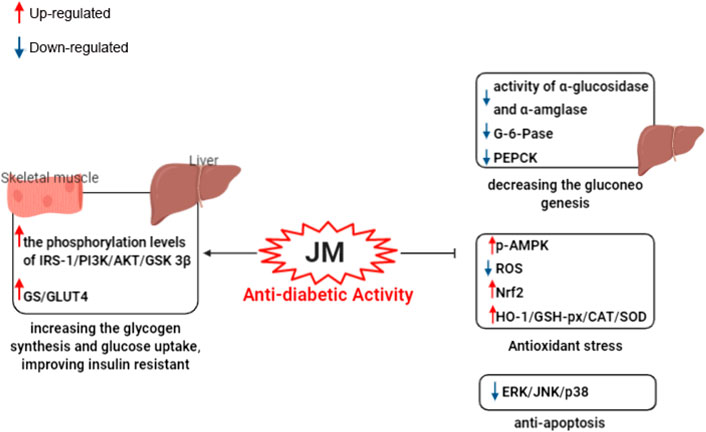
FIGURE 6. Schematic representation of underlying the mechanism of anti-dabite activity of J. mandshurica.
Antitumor Activity
A variety of the crude extracts, isolated compounds, and polysaccharides from J. mandshurica displayed significant antitumor activity both in vitro and in vivo. The underlying mechanisms of action of these components included induction of cell apoptosis and autophagy, cell cycle arrest, promotion of cell differentiation and inhibition of cell adhesion and invasion. Effects on telomerase activity and regulation of mRNA and protein expression levels of tumor-related factors were observed (see Table 2 and Figure 3). In general, the antitumor activity of J. mandshurica has been effectively demonstrated in various human cancer cell lines, such as hepatocellular carcinoma HepG2, Hep3B, Huh7, and BEL-7402 cells (Yao et al., 2012; Zhang et al., 2013; Zhou et al., 2015a; Gao et al., 2016; Hou et al., 2016; Jin et al., 2016; Cheng et al., 2017; Yao et al., 2017; Wang et al., 2018a; Zhang et al., 2018; Lou et al., 2019a; Zhou et al., 2019a; Lou et al., 2019b), lung cancer A549, NCI-H460, and NCI-H1975 cells (Yao et al., 2014; Yao et al., 2015b; Gao et al., 2016; Jin et al., 2016; Li et al., 2017a; Zhang et al., 2018; Yang et al., 2019), breast cancer SKBR3, BT474, MCF-7, Bcap-37, and MDA-MB-231 cells (Gao et al., 2016; Jin et al., 2016; Sun et al., 2017; Zhang et al., 2018), cervical cancer Hela, SiHa, and CaSKi cells (Li et al., 2007a; Zhang et al., 2012a; Xin et al., 2014; Yao et al., 2014; Yao et al., 2015b; Lu et al., 2017; Wang et al., 2019a), gastric cancer SNU638, BGC-803, SGC-7901, and BGC-823 cells (Li et al., 2009; Yao et al., 2014; Yao et al., 2015b; Guo et al., 2015; Gao et al., 2016; Jin et al., 2016; Zhou et al., 2019b), prostate cancer LNCaP cells (Xu et al., 2013), pancreatic cancer BxPC-3 and PANC-1 cells (Avcı et al., 2016), colon cancer HCT 15 and CCL-228-SW 480 cells (Bayram et al., 2019; Zhou et al., 2019b), leukemia K562 and HL-60 cells (Xu et al., 2010; Yao et al., 2014; Yao et al., 2015b; Li et al., 2017a; Zhou et al., 2019b), placental choriocarcinoma JAR cells (Yao et al., 2014), and glioma C6 cells (Yang et al., 2019). It is worth noting that the isolated compounds 1, 17, 25, 30, 39, 43, 44, 71, 72, 73, 125, 180, 196, 198, 212, 216, 221, 236, 237, 238, 239, 289, 318, 335, 337, 340, 357, 358, and 381 displayed significant antitumor activity against on HepG2, A549, MCF-7, Hela, SiHa, MDA-MB-231, BGC-803, SGC-7901, BGC-823, LNCaP, BxPC-3, and PANC-1 in vitro. Besides, the antitumor activity of the compounds with mother nucleus of 1, 4-naphthoquinone substituted by hydroxy is stronger than that of methoxy substitution at the same position, and the compounds with 5-and 8-hydroxy groups have the strongest antitumor activity. The anti-tumor activity of naphthoquinone type compounds is generally stronger than that of naphthone, naphthol and thier glycosides, and the naphthone glycosides showed the weakest antitumor activity (Zhang et al., 2019).
In vivo in mouse models, it has been demonstrated that J. mandshurica and its secondary products showed protective activity on MCF-7 tumor-bearing mice (Sun et al., 2017), S180 tumor-bearing mice (Yao et al., 2009; Wang et al., 2015), and H22 tumor-bearing mouse (Wang et al., 2017c; Wang et al., 2017d). A polysaccharide, namely JRP1, purified form the fruits, at doses of 25, 50 and 100 mg/kg, i.p., for 21 days, inhibited the tumor growth with inhibition rates of 35.3%, 40.6% and 48.1%, respectively, and decreased the index of spleen and thymus and increased the serum levels of immune regulatory markers such as IL-2, TNF-α and IFN-γ with a dose-dependent manner in S180 tumor-bearing mice (Wang et al., 2015). Orally administration with JMCE (at doses of 100, 200, and 500 mg/kg) to S180 tumor-bearing mice once a day for 8 days significantly elevated the indexes thymus and spleen, inhibited the growth of tumor with inhibition rates of 48.37%, 40.81%, and 36.52%, respectively. JMCE also increased the activity of SOD and decreased the content of MDA in the serum of tumor-bearing mice (Yao et al., 2009).
In traditional Chinese medicine as described by “Zhongguo Minjian Liaofa”, branches of J. mandshurica are decocted together with chicken eggs. The eggs should be initially administered and the decoction should be administered when there are no obvious side effects. Eggs decocted with J. mandshurica branches (EDJB), at doses of 0.64, 1.28, and 2.56 g/kg i.p. once a day for 10 days, suppressed the growth of tumor tissues and increased the body weights in H22 tumor-bearing mouse in a dose- and time-dependent manner. Moreover, EDJB dramatically elevated the thymus index and spleen index of tumor mice, improved the peripheral red blood cells and hemoglobin numbers as well as reduced the white blood cells numbers (Wang et al., 2017c), suggested EDJB has good anti-tumor effect against H22 cell. In addition, total tannins (TT) obtained from J. mandshurica, at doses of 0.09 and 0.18 g/kg once a day for 10 days, prominently inhibited the growth of tumor tissues in H22 tumor bearing mouse with an inhibition rate of 34.22% and 36.92%, respectively (Wang et al., 2017d).
Multidrug resistance (MDR) is a major obstacle that hinders the treatment of cancer. Wen et al. (2017) developed a self-assembled polyjuglanin nanoparticle, namely DOX/PJAD-PEG-siRNA, and evaluated its anticancer activity both in vitro and in vivo. In vitro results showed that it improved the cytotoxicity of doxorubicin (DOX) to A549/DOX and H69/CIS cell lines with MDR. Meanwhile, at concentrations of 2, 4, and 8 μg/ml, it significantly down-regulated the mRNA expressions of Kras, P-gp, and c-Myc in a dose-dependent manner (Wen et al., 2017). Moreover, DOX/PJAD-PEG-siRNA at 2 mg/kg for 21 days, significantly suppressed the growth of tumor, decreased the volume and weight of tumor, KI-67 positive levels and expressions of RAS and c-Myc, and increased the TUNEL positive levels and protein levels of p-JNK and p53 in drug-resistant xenografted nude mice when compared to the free DOX at same dose (Wen et al., 2017). These antitumor activities reported are consistent with the traditional usage such as the treatment of liver cancer, lung cancer, breast cancer, cervical cancer, and gastric cancer, etc.
Overall, J. mandshurica has prominent antitumor potential and has a good health benefit for human. Nevertheless, it is worth noting that most of the research conducted to study antitumor activity stay in the primary stage, and has employed in vitro-based methods and further more in-depth in vivo and mechanism of action investigations as well as clinical studies should therefore be encouraged and strengthened.
Immunoregulatory Activity
Li et al. (2018) first evaluated the immunoregulatory functions of the three protein hydrolyzates (PH), namely albumin, glutelin, and globin (molecular weights: 11–35 kDa) obtained from J. mandshurica in mice. The three compounds, glutelin, albumin, and globin at doses of 200, 400, and 800 mg/kg/d, for 35 days significantly increased the thymus and spleen indexes, lymphocyte proliferation, macrophage activity, CD4+ and CD8+ T cells numbers, IgA and sIgA levels, and dose-dependently up-regulated mRNA and protein expression levels of IFN-α and IL-6 relative to that of the control group (Li et al., 2018). Simultaneously, a hydrolysate peptide (HP) isolated from J. mandshurica (molecular weight <3 kDa), at dose of 800 mg/kg/d for 28 days, obviously elevated the spleen and thymus indexes and promoting the spleen T-lymphocyte proliferation and sIgA generation in the intestinal tract of mice stimulated by exhaustion swimming experiment (Li et al., 2018).
Anti-Inflammatory Activity
A variety of isolated compounds and crude extracts from J. mandshurica displayed anti-inflammatory activity in various inflammatory related models, and the possible mechanism of action of active compounds were showed in Figure 4. In HaCaT cells induced by IFN-γ, 1.0, 5.0, and 10 μM 1,2,3,4,6-penta-O-galloyl-β- d-glucose (PGG, 194) notably inhibited the protein and mRNA expression levels of CCL17, reduced the protein expression of CXCL-9, CXCL-10, and CXCL-11, and prominently repressed the NF-κB activation as well as STAT1 activation (Ju et al., 2009). Furthermore, PGG obviously reduced the protein expression of CXCL-9, CXCL-10, and CXCL-11 (Ju et al., 2009). Peng et al. (2015) revealed that juglone (1), at doses of 0.25 and 1.0 mg/kg, i.g., daily, for 70 days, significantly decreased the levels of TNF-α, IL-1β and IL-6 both in serum and hypothalamus tissues in rats with high-fat diet-induced neuroinflammation. Further investigations demonstrated that juglone suppressed the inflammatory responses via inhibition of TLR4/NF-κB signaling pathway by reducing the protein expressions of TLR4, MyD88, TAK1, p-IκBα, NF-κB, and p-NF-κB (Peng et al., 2015). In LPS-induced primary astrocytes, juglone at doses of 5, 10, 15, and 20 μM, could prominently down-regulate the expressions of these indicators involved in TLR4/NF-κB signaling pathway (Peng et al., 2015). Similarly, in LPS-stimulated acute lung injury mice model, juglanin B (289), at dosages of 10 and 20 mg/kg, i.g., daily, for 21 days, significantly alleviated the lung fibrosis and inflammation cell infiltration via decreasing the mRNA and protein expressions of α-SMA, collagen type I, collagen type III, and TGF-β1 (Dong and Yuan, 2018). Moreover, juglanin B (289) notably decreased the levels of IL-4, IL-6, IL-17, IL-18, TNF-α and IL-1β as well as down-regulated the expression of phosphorylated NF-κB via suppressing the IKKα/IκBα signaling pathway (Dong and Yuan, 2018). In addition, five diarylheptanoids and their glycosides, (2S,3S,5S)- 2,3,5-trihydroxy-1,7-bis-(4-hydroxy-3-methoxyphenyl)-heptane (240), (2S,3S,5S)- 2,3-dihydroxy-5-O-β-d-xylopyranosyl-7-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)-heptane (241), rhoiptelol C (242), rhoiptelol B (243), and 3′,4″-epoxy -2-O-β-d-glucopyanosyl-1-hydroxyphenyl)-7-(3-methoxy-phenyl)-heptan-3-one (244) significantly and dose-dependently repressed the NO, IL-6 and TNF-α generation in LPS-stimulated RAW264.7 cells (Diao et al., 2019).
Besides, J. mandshurica leaves ethanol extract (JMLE) is particularly effective against allergic dermatitis. After treatment with 0.5% JMLE, the clinical skin severity scores (1.50%) were significantly decreased relative to that of the control group (3.83%), and scratching scores (96.33%) also remarkedly reduced relative to that of the control group (325.01%) in DNCB-induced allergic dermatitis-like skin lesions of mice (Park and Oh, 2014). Further study showed that JMLE obviously decreased the serum levels of TNF-α, IgE, IL-1, and IL-13 (Park and Oh, 2014), suggesting that JMLE might provide the theoretical basis for the further study of active ingredients against allergic dermatitis.
Neuroprotective Activity
Neurodegenerative diseases are characterized by a severe and progressive loss of neurons in the central nervous system, leading to cognitive, behavioral, and motor dysfunctions (Liu et al., 2019). Natural-derived peptides are effective substances in alleviating the oxidative stress and preventing neurotoxicity (Zhao et al., 2020). The hydrolyzed peptide (HP) obtained from J. mandshurica displayed important neuroprotective activity both in vitro and in vivo, and the underlying mechanism was displayed in Figure 5.
Three different molecular-weight HP (<3 kDa; 3–10 kDa; >10 kDa) obtained from J. mandshurica, and their antioxidant capacity were evaluated in vitro after treated with different concentrations (1.0, 1.5, 2.0, and 2.5 mg/ml). Results found that the lower molecular-weight HP (<3 kDa) exhibit higher and significant antioxidant activities via repressing the production of ROS and increasing the activity of glutathione peroxidase (GSH-Px) in the H2O2-induced PC12 cells, which than those of higher molecular-weight HP, suggesting that the antioxidant capacity of HP might be relate to molecular-weight (Ren et al., 2018). Similarly, in vivo, orally administrated with HP at doses of 200, 400, and 800 mg/kg daily for 30 days in scopolamine-induced memory impairment in mice, the total path for searching the platform was significantly shortened, the escape latency was significantly decreased, and the dwelling distance and time in the coverage zone were notably increased in the Morris water maze test. HP also extended the latency and lessened errors in the passive avoidance response tests (Ren et al., 2018). Mechanically, HP increased the contents of ACh, ChAT, AChE, 5-HT, DA, and NE, elevated the activities of the SOD and GSH-Px as well as up-regulated the protein expression of p-CaMK II in brain tissues of mice (Ren et al., 2018). Subsequently, another antioxidant peptide obtained from J. mandshurica, namely EVSGPGLSPN, at concentrations of 12.5, 25, 50, and 100 μM, dose-dependently decreased the production of ROS, and enhanced the activities of CAT, GSH-px, and SOD in H2O2-induced PC12 cells (Liu et al., 2019). Simultaneously, EVSGPGLSPN inhibited the IKKβ and p65 expressions to repress the NF-κB pathway activation, alleviated the neurotoxic cascade by overexpression of IL-1β and TNF-α. Furthermore, EVSGPGLSPN significantly inhibited the apoptosis of PC12 cells by down-regulating the expression of cytochrome C, caspase-3/9, and PARP as well as up-regulating the expression of p-CREB and synaptophysin in oxidatively damaged PC12 cells (Liu et al., 2019). These results indicated that EVSGPGLSPN may protect against H2O2-induced neurotoxicity by increasing the activity of antioxidant enzymes and blocking the NF-κB/caspase pathways.
In a recent study, three peptides, namely YVLLPSPK, TWLPLPR, and KVPPLLY, obtained from J. mandshurica, at a concentration of 50 μM for 24 h, prominently inhibited the generation of ROS, increased the activity of GSH-Px and contents of ATP, and alleviated apoptosis in Aβ25–35-induced PC12 cells. It also promoted autophagy and affected the Akt/mTOR signaling pathway through up-regulating the protein expression levels of Beclin-1, LC3-I, LC3-II, LAMP1, LAMP2, Cathepsin and p-Akt/Akt as well as down-regulating the protein expression level of p62 and p-mTOR/mTOR at molecule levels (Zhao et al., 2020). Results from above studies indicated that J. mandshurica may serves as sustainable dietary supplement to further develop novel functional food to prevent or defer oxidation-incurred memory impairment damage and ageing/or age-related neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD).
Antidiabetic Activity
Recent findings have demonstrated that J. mandshurica possess significant hypoglycemic activity in vitro and the possible mechanism of this action was showed in Figure 6. The ethyl acetate fractions extracted from ethanol extract of J. mandshurica leaves (JMEE) showed significant α-glucosidase and α-amylase inhibitory activity in vitro with IC50 of 14 and 130 μg/ml, which were stronger than that of the positive drug acarbose with IC50 of 44 and 158 μg/ml, respectively (Wang et al., 2019c). In insulin resistant (IR) hepatic HepG2 cells, LPLLR (Leu-Pro-Leu-Leu-Arg), a novel pentapeptide from the protein hydrolysates of J. mandshurica, at concentrations of 100 and 200 μM, increased the phosphorylation levels of insulin receptor substrate 1 (IRS-1), phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt), AMPK and GSK3β, and up-regulated the expression levels of GS and glucose transporter type 4 (GLUT4), while down-regulated the expression levels of G-6-Pase and PEPCK in IR hepatic HepG2 cells (Wang et al., 2020a). These findings suggested that LPLLR exerts anti-diabetic effect through increasing the glycogen synthesis and glucose uptake, as well as decreasing the gluconeogenesis. In addition, the peptide LPLLR possesses good stability under in vitro simulated gastrointestinal digestion, and the low molecular weight (610.4 Da) of LPLLR may be beneficial for its intestinal absorption. Nevertheless, more in-depth in vivo investigation is needed to explore the stability and absorption of LPLLR. Subsequently, in high glucose-induced IR and oxidative stress in HepG2 cells, three novel peptides, namely Leu-Val-Arg-Leu (LVRL), Leu-Arg-Tyr-Leu (LRYL), and Val-LeuLeu-Ala-Leu-Val-Leu-Leu-Arg (VLLALVLLR) from J. mandshurica at 12.5–100 μM, significantly improve glucose consumption, glucose uptake, GLUT4 translocation, and elevated the phosphorylation of IRS-1, PI3K, and Akt. The activities of GSH-Px, CAT, and SOD, the nuclear transport of Nrf2, and the protein expression of HO-1 were also increased. Furthermore, these peptides reduced high glucose-induced ROS overproduction and the phosphorylation of ERK, JNK, and p38 (Wang et al., 2020b). These results suggested that peptides from J. mandshurica could protect HepG2 cells from high glucose-induced IR and oxidative stress by activating IRS-1/PI3K/Akt and Nrf2/HO-1 signaling pathways.
Antimicrobial Activity
Three new juglone derivatives, namely juglonol A (71), B (72), and C (73), isolated from the immature exocarps of J. mandshurica by Yang and his colleagues (2019) and their antimicrobial activity against Gram-positive (S. aureus and E. faeculis) and Gram-negative (E. coli and K. pneumonia) bacteria, yeast (C. albicans), and fungi (F. oxysporum, F. oxysporium, C. lagenarium, and P. asparagi) were evaluated. The results showed that juglonol A (71) obviously suppressed all tested strains except for E. coli. with the MIC values ranging 8 from 64 μg/ml However, juglonol B (72) only significantly inhibited the S. aureus with MIC value of 8 μg/ml (Yang et al., 2019). Juglonol A have also been demonstrated to exhibit modestly inhibitory activity against the non-small-cell lung carcinoma (NCI-H1975), lung adenocarcinoma (HCC827), hepatocellular carcinoma (HepG2), triple-negative breast cancer (MD-AMB-231), leukemia (HL-60), mouse colon cancer (CT26) and rat glioma (C6), and IC50 values were ranging from 9.5 to 31.6 μg/ml (Yang et al., 2019). These results suggested that the presence of juglone core or hydroxyethyl side chain is essential to the molecules’ biological activity and that the position of substitution has a marked impact on the potency. Hence, juglonol A, as pan-inhibitors, might be cytotoxic.
Antiviral Activity
Min et al. (2000) found that 1,2,6-trigalloylglucose (192) and 1,2,3,6- tetragalloylglucose (193) isolated from barks of J. mandshurica showed the most potent anti-reverse transcriptase (RT) activity of HIV-1 with the IC50 values of 67 and 40 nM, respectively. In addition, compound 192 notably suppressed the ribonuclease H (RNase H) activity with IC50 values of 39 μM when used illimaquinone as a positive control (Min et al., 2000). Simultaneously, Min and his colleagues further found that taxifolin (297) displayed the most potent anti-HIV-1 activity against MT-4 cells with the IC100 value of 25 μg/ml and CC100 value of above 100 μg/ml (Min et al., 2002). However, the certain mechanism of anti-HIV-1 activity should be performed at molecule level in the future.
Anti-Melanogenesis Activity
Recently, Kim et al. (2019) obtained three phenolic ingredients from fruit of J. mandshurica and evaluated their anti-melanogenesis activity in B16F10 melanoma cells and primary human epidermal melanocytes. It was found that compound 2-[4-(3-hydroxypropyl)-2-methoxyphenoxy]-1,3-propanediol (126) at concentrations of 0.5 and 1.0 μM, showed the highest inhibitory effect through reducing the melanin content, increasing the p-ERK protein expression and decreasing MITF and tyrosinase protein expressions. These effects also could immediately reverse by PD98059, which a potent ERK inhibitor, indicated compound 126 effectively curbed melanogenesis mainly through p-ERK-associated MITF degradation (Kim et al., 2019). Therefore, J. mandshurica has the potential to suppress melanogenesis and can become a useful resource for developing novel skin-whitening agents to cure hyperpigmentation disorders.
Pharmacokinetics
Neither systemic evidences regarding the pharmacokinetics extracts from this plant nor evaluations of its target-organ toxicity have been performed. Few investigations have studied the pharmacokinetics parameters of J. mandshurica and its bioactive compounds in animal experiments. Chen et al. (2018) first measured the gallic acid and syringic acid concentrations in rat plasma after the intragastric administration of the aqueous extracts of J. mandshurica at dose of 12 g/kg using high performance liquid chromatography (HPLC). The maximum plasma concentration (Cmax) was 0.64 μg/ml, while the time to reach peak concentration (Tmax) and elimination half-life (T1/2) were 61.80 and 184.21 min, respectively. The area under the plasma concentration-time curve (AUC0-t) and AUC0-∞ of gallic acid was 96.37 μg min/mL, and 121.59 μg min/mL. Additionally, the Cmax, Tmax, T1/2, AUC0-t, and AUC0-∞ of syringic acid was 0.43 μg/ml, 30.67 min, 99.63 min, 40.33 μg min/mL, 47.02 μg min/mL, respectively (Chen et al., 2018).
Additionally, Chen et al. (2018b) studied the chemical ingredients distribution of the ethanol extracts of exocarp from J. mandshurica after orally administrated at concentration of 1.35 g/ml to rats. The results showed that a total of 54 ingredients have been identified, including 41 archetypes and 13 metabolites. The archetypes included 17 naphthoquinones, 9 diarylheptanoids, 7 flavonoids, 5 triterpenoids, and 3 polyphenols. The metabolites comprised 4 naphthoquinones, 3 diarylheptanoids, and 1 flavonoid, etc, were detected in rats’ gastric tissues by UPLC-Q-TOF/MS technology for the first time (Cheng et al., 2018b). Similarly, 24 chemical components including 12 naphthoquinones, 5 flavonoids, 3 diarylheptanoids, and 4 triterpenoids were also detected in rats’ kidney tissues by UPLC-Q-TOF/MS technology after orally administration of the ethanol extract of J. mandshurica at a dose of 1.35 g/ml to rats (Wang et al., 2018b).
Overall, these results might be contributed to explain the body's metabolic process and relative mechanism of action of various components from J. mandshurica, and provide a methodological reference for the evaluation of the safety and effectiveness of compounds in the accumulation in gastric and kidney tissues and relational adverse reactions as well as composition and tissue distribution. It also provides more comprehensive information for clarifying the substance basis of anti-tumor effects in J. mandshurica. Further investigations are required to explore the pharmacokinetics, metabolic stability, and the drug delivery system of J. mandshurica and its active components.
Toxicological Information
When evaluating the efficacy of drugs, toxicity and safety should be firstly taken into account. Although J. mandshurica as a popular Chinese herbal medicine is frequently used in TCM, information on the side effects and safety evaluations for this plant are seldom reported and insufficient to support their safety. Liu et al. (2004a) reported the acute toxicity of total extracts (TE), petroleum ether extracts (PEE), n-butanol extracts (nBE), aqueous extracts (AE), chloroform extracts (CE), and acetic ether extracts (AEE) from BQLY in mice by administering the increasing doses orally and intraperitoneal injection (TE, PEE, nBE, and AE at doses of 3.62, 4.25, 5.00, 5.88, and 6.29 g/kg, respectively; CE at doses of 400.2, 470.6, 553.6, 651.3, and 766.3 mg/kg; AEE at doses of 930.2, 1,094.4, 1,287.4, 1,514.7, and 1781.9 mg/kg) for 14 days. The results found that the treatment by gavage did not cause any deaths or side effects. However, the intraperitoneal injection with CE and AEE resulted in dose-dependent mortality with signs of toxicity, and the median lethal dose (LD50) of CE and AEE were 575.38 mg/kg and 1,303.59 mg/kg, respectively. Simultaneously, the LD50 of TE, PEE, nBE, and AE were more than 5 g/kg both in intragastrical and intraperitoneal administration (Liu et al., 2004b). These findings suggested that intraperitoneally injected with chloroform extracts and acetic ether extracts from BQLY were toxic to mice. Recently, Ju et al. (2019) investigated the acute toxicity of aqueous extracts from the stem-barks of J. mandshurica in mice by orally administering the at maximum dose of 227.27 g/kg daily for continuous 14 days. They found that the treatment by aqueous extracts did not cause any deaths or side effects (Ju et al., 2019). Therefore, these results further confirmed that the aqueous extracts of J. mandshurica did not present the apparent toxicity, and might be relatively safe for human.
Additionally, studies showed that BQLY contain many toxic compounds, such as juglone (Huo et al., 2017). In previous study, Westfall et al. (1961) reported that the LD50 of juglone in mice was 2.5 mg/kg by gavage, the LD50 of intraperitoneal injection was 25 mg/kg, and the LD50 of rats was 112 mg/kg by gavage (Westfall et al., 1961). Chen et al. (2005) speculated that the reason for the toxicity of juglone was that it combines with blood components after entering the blood, causing a high concentration of juglone in the blood. Moreover, juglone can react with the sulfhydryl compounds in the gastrointestinal contents, resulting in low absorption of juglone during intragastric administration, which accumulates in the cardia antrum, causing toxicity. In addition, juglone and its metabolites can covalently bind to cytosolic proteins in the kidney, causing renal toxicity (Chen et al., 2005).
The toxicity studies regarding the J. mandshurica and its active components are still in the exploratory stage and mainly focused on acute toxicity study. Therefore, apart from the classical toxicological evaluation, research on chronic toxicity, toxicity mechanism, and toxicokinetics should be further conducted in several animal models and provide scientific explanations for its toxicity and safety applications in the future.
Conclusion and Future Perspectives
The present review systematically summarizes the findings of the latest research on the traditional usages, phytochemical constituents, pharmacological properties, and toxicities of different extracts and ingredients of J. mandshurica. As a historical herbal medicine, it has been traditionally and popularly used in indigenous populations to treat cancer in China, Japan, Korea, and India more than 2000 years. Recent investigations have focused primarily on evaluating the anticancer activities of the extracts or isolated compounds of this plant. Until now, more than 400 chemical constituents have been isolated and identified from the different parts of J. mandshurica. Through a comprehensive analysis, we found that the quinones, phenolics, triterpenoids, and diarylheptanoids are major and important active compounds of J. mandshurica with numerous pharmacological activities shown in vivo and in vitro investigations.
However, there are also some points and aspects that need to be noted and researched further: (1) The quinones from J. mandshurica with prominent antitumor activity have captured researcher’s attention increasingly, and further study on these compounds should be a priority. Until recently, however, J. mandshurica was still considered as folk medicine for the treatment of cancer and the related preclinical experiments results are questioned and unpersuasive, future studies are necessary to address issues regarding composition of the extract, explicability of preclinical experiments, and lack of transformation of the preclinical results to clinical efficacy. Hence, the clinical trial evaluations of J. mandshurica, including animal models and should be conducted urgently. (2) Although a great number of chemical ingredients had been isolated and identified from this plant, pharmacological evaluations on these compounds are limited to few compounds such as juglone, juglanstetralone A, p-hydroxymethoxybenzobijuglone, juglanthraquinone C, and juglanin. Therefore, deep phytochemical studies of J. mandshurica and its pharmacological properties, especially the mechanism of action of its bioactive constituents to illustrate the correlation between ethnomedicinal uses and biological activities will undoubtedly be the focus of further research. (3) Toxicological investigations are crucial to understand the safety of herbal drugs, but data on toxicological aspects of J. mandshurica were still rarely. Although research confirmed that many medicinal parts of J. mandshurica have little or no toxicity, BQLY has some adverse reactions, which may cause harm to human health. Thence, toxicity and safety assessment studies on BQLY extract and other effective extracts are necessary to ensure the full use of medicinal resources, to meet the Western evidence-based medicine standards, and to provide accurate evidence for clinical applications. Besides, the crude drugs should be strictly in accordance with traditional processing theories and subjected to ancient processing techniques (Pao Zhi), including cleaning, cutting, drying, and digesting, which can reduce their toxicity and exert maximal therapeutic efficacy by transforming the secondary plant metabolites. (4) Pharmacokinetics is an indispensable part of new drug development and rational clinical drug use. However, data on the pharmacokinetics of active compounds and crude extracts of J. mandshurica remain unclear.
Overall, J. mandshurica is a source for nutritional and medical compounds and is worthy of further studty owing to its health-promoting properties and its potential for further development in food industry. However, the existing health-related evidence on J. mandshurica is insufficient, and its clinical value has not been adequately studied. Therefore, comprehensive investigations on biological properties, especially the underlying mechanism of bioactiveties of J. mandshurica and its isolated compounds, should be conducted in order to support its ethnomedicinal uses. Besides, the development of healthcare products of J. mandshurica will undoubtedly be the focus of further research. Lastly, this study will help scientists to created additional potential health-promoting pharmaceuticals and functional foods based on J. mandshurica.
Author Contributions
HL, KH, DL, and XS obtained and analyzed the literatures. FL, ZW, YJ, and YY wrote the manuscript. XH and NZ gave ideas and edited the manuscript. All authors read and approved the final version of the manuscript for publication.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82074094), the Open Research Fund of Chengdu University of Traditional Chinese Medicine Key Laboratory of Systematic Research of Distinctive Chinese Medicine Resources in Southwest China (Grant No. 2020XSGG002), the Xinglin Scholar Research Promotion Project of Chengdu University of Traditional Chinese Medicine (Grant No. CDTD2018014) and the Science and Technology Project of Zunyi (Grant No. ZSKH-HZ-(2020)-78).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
ABTS 2,2′-azino-bis-(3-ethylbenzenthiazoline-6-sulphonic) acids
Ach acetylcholine
AIF apoptosis-inducing factor
ATP adenosine 5′-triphosphate
A549/DOX DOX-resistant A549
AChE acetylcholinesterase
BQLY the epicarp of immature fruits
CXCL-9/10/11 chemokines
CCL-17 activation-regulated chemokine
ChAT choline acetyltransferase
CC100 maximum cytotoxic concentration
CDK-2 cyclin-dependent kinase 2
CAT catalase
DOX doxorubicin
DOX/PJAD-PEG-siRNA amphiphilic poly(juglanin (Jug) dithiodipropionic acid (DA))-b-poly(ethylene glycol) (PEG)-siRNA Kras with DOX
DNCB 2,4-dinitrochlorobenzene
DA dopamine
DPPH 1,1′-diphenyl-1-picrylhydrazyl
EDJB eggs decocted with J. mandshurica branches
ERK extracellular signal-regulated kinase
GSH glutathione
GSH-px glutathione peroxidase
5-HT 5-hydroxytryptamine
HIV human immunodeficiency virus
HO-1 heme oxygenase-1
H96/CIS Cisplatin-resistant H96
HP hydrolyzed peptide
IC50 50% inhibitory concentrations
IC100 complete inhibitory concentration
JNK c-Jun N-terminal kinase
JMEE J. mandshurica ethanol extracts
JMM6 a separated fraction of ethanol extract from J. mandshurica
JRP1 a water-soluble polysaccharide
JA ω-9 polyunsaturated fatty acid
JMCE chloroform extracts of J. mandshurica roots;
JMLE J. mandshurica leaves ethanol extract
KVPPLLY Lys-Val-Pro-Pro-Leu-Leu-Tyr
LPS lipopolysaccharide
IgA immunoglobulin A
IL-2 interleukin-2
IL-1β interleukin-1β
IL-4 interleukin-4
IL-6 interleukin-6
IL-13 interleukin-13
IL-17 interleukin-17
IL-18 interleukin-18
IFN-α interferon-α
IFN-γ interferon-γ
LAMP1/2 lysosome-associated membrane protein 1/2
mTOR mammalian target of serine/ threonine protein kinase rapamycin
MDR multidrug resistance
Nrf2 nuclear factor E2-related factor 2
NF-κB nuclear factor-κB
NE noradrenaline
p62 sequestosome 1
p-CaMK II phosphorylation of CaM-dependent protein kinase II
ROS reactive oxygen species
sIgA secretory IgA
SOD superoxide dismutase
α-SMA α-smooth muscle-actin
TCM Traditional Chinese Medicine
TWLPLPR Thr-Trp-Leu-Pro-Leu-Pro-Arg
TNF-α tumor necrosis factor-α
TGF-β1 transforming growth factor-β1
TLR-4 Toll like receptor-4
YVLLPSPK Tyr-Val-Leu-Leu-Pro-Ser-Pro-Lys
△Ψm mitochondrial membrane potential
References
Avcı, E., Arıkoğlu, H., and Kaya, D. E. (2016). Investigation of juglone effects on metastasis and angiogenesis in pancreatic cancer cells. Gene. 588, 74–78. doi:10.1016/j.gene.2016.05.001
Bai, W. N., Liao, W. J., and Zhang, D. Y. (2010). Nuclear and chloroplast DNA phylogeography reveal two refuge areas with asymmetrical gene flow in a temperate walnut tree from East Asia. New Phytol. 188, 892–901. doi:10.1111/j.1469-8137.2010.03407.x
Bayram, D., Özgöçmen1, M., Armagan, I., Sevimli, M., Türel, G. Y., and Şenol, N. (2019). Investigation of apoptotic effect of juglone on CCL-228-SW 480 colon cancer cell line. J. Cancer Res. Therapeut. 15, 68–74. doi:10.4103/jcrt.JCRT_880_17
Carey, A. N., Fisher, D. R., Bielinski, D. F., Cahoon, D. S., and Shukitt-Hale, B. (2020). Walnut-associated fatty acids inhibit LPS-induced activation of BV-2 microglia. Inflammation. 43, 241–250. doi:10.1007/s10753-019-01113-y
Chaudhary, N., Sasaki, R., Shuto, T., Watanabe, M., Kawahara, T., Suico, M. A., et al. (2019). Transthyretin amyloid fibril disrupting activities of extracts and fractions from Juglans mandshurica Maxim. var. cordiformis (Makino) Kitam. Molecules. 24, 500. doi:10.3390/molecules24030500
Chen, C., Wang, T. M., Di, X., and Kang, T. G. (2018). A pharmacokinetic study of gallic acid and syringic acid in the water extraction of Juglans mandshurica Maxim. in rat plasma. Chin. J. Ethnomed. Ethnopharmacy. 27, 28–33
Chen, G., Pi, X. M., and Yu, C. Y. (2015). A new naphthalenone isolated from the green walnut husks of Juglans mandshurica Maxim. Nat. Prod. Res. 29, 174–179. doi:10.1080/14786419.2014.971789
Chen, L. J., Lebetkin, E. H., and Burka, L. T. (2005). Metabolism and disposition of juglone in male F344 rats. Xenobiotica. 35, 1019–1034. doi:10.1080/00498250500356621
Cheng, T., Wang, G. L., Huo, J. H., and Wang, W. M. (2018b). Distribution of exocarp components of Juglans mandshurica in rats’ gastric tissues based on UPLC-Q-TOF/MS. Chin. Tradit. Herb. Drugs. 49, 2527–2539. doi:10.7501/j.issn.0253-2670.2018.11.007
Cheng, Z. Y., Du, Y. Q., Zhang, Q., Lin, B., Gao, P. Y., Huang, X. X., et al. (2018a). Two pairs of new alkaloid enantiomers with a spiro [benzofuranonebenzazepine] skeleton from the bark of Juglans mandshurica. Tetrahedron Lett. 59, 2050–2053. doi:10.1016/j.tetlet.2018.04.037
Cheng, Z. Y., Yao, G. D., Guo, R., Huang, X. X., and Song, S. J. (2017). Phenylpropanoids from Juglans mandshurica exhibit cytotoxicities on liver cancer cell lines through apoptosis induction. Bioorg. Med. Chem. Lett. 27, 597–601. doi:10.1016/j.bmcl.2016.12.005
Diao, S. B., Jin, M., Sun, J. F., Zhou, Y., Ye, C., Jin, Y., et al. (2019). A new diarylheptanoid and a new diarylheptanoid glycoside isolated from the roots of Juglans mandshurica and their anti-inflammatory activities. Nat. Prod. Res. 33, 701–707. doi:10.1080/14786419.2017.1408100
Dong, Z. W., and Yuan, Y. F. (2018). Juglanin suppresses fibrosis and inflammation response caused by LPS in acute lung injury. Int. J. Mol. Med. 41, 3353–3365. doi:10.3892/ijmm.2018.3554
Editorial Committee of Flora of China (1979). Chinese Academy of Sciences. Flora of China., Vol. 21. (Beijing: Science Press), 31–33
Fang, L., Ren, D. Y., Cui, L. Y., Liu, C. L., Wang, J., Liu, W., et al. (2018). Antifatigue, antioxidant and immunoregulatory effects of peptides hydrolyzed from Manchurian Walnut (Juglans mandshurica Maxim.) on mice. Grain Oil Sci. Technol. 1, 44–52. doi:10.3724/SP.J.1447.GOST.2018.18028
Fu, Q. F., Song, H. J., Zhu, L., Gao, H. R., Ma, D. N., Zhang, X. J., et al. (2020). Study on phenolic acid compounds of Juglans mandshurica. Inf. Trad. Chin. Med. 37, 44–47. doi:10.19656/j.cnki.1002-2406.200009
Gao, X. L., Lin, H., Zhao, W., Hou, Y. Q., Bao, Y. L., Song, Z. B., et al. (2016). JA, a new type of polyunsaturated fatty acid isolated from Juglans mandshurica Maxim, limits the survival and induces apoptosis of heptocarcinoma cells. Apoptosis. 21, 340–350. doi:10.1007/s10495-015-1202-5
Guo, L. N., Zhang, R., Guo, X. Y., Cui, T., Dong, W., Huo, J. H., et al. (2015). Identification of new naphthalenones from Juglans mandshurica and evaluation of their anticancer activities. Chin. J. Nat. Med. 13–0710. doi:10.1016/S1875-5364(15)30070-4
Hou, Y. Q., Yao, Y., Bao, Y. L., Song, Z. B., Yang, C., Gao, X. L., et al. (2016). Juglanthraquinone C induces intracellular ROS increase and apoptosis by activating the Akt/Foxo signal pathway in HCC cells. Oxid. Med. Cell. Longev., 494, 1623. doi:10.1155/2016/4941623
Hu, Z., Zhang, T., Gao, X. X., Wang, Y., Zhang, Q., Zhou, H. J., et al. (2016). De novo assembly and characterization of the leaf, bud, and fruit transcriptome from the vulnerable tree Juglans mandshurica for the development of 20 new microsatellite markers using Illumina sequencing. Mol. Genet. Genom. 291, 849–862. doi:10.1007/s00438-015-1147-y
Huo, J. H., Du, X. W., Sun, G. D., Dong, W. T., and Wang, W. M. (2018). Identification and characterization of major constituents in Juglans mandshurica using ultra performance liquid chromatography coupled with time-of-flight mass spectrometry (UPLC-ESI-Q-TOF/MS). Chin. J. Nat. Med. 16–0545. doi:10.1016/S1875-5364(18)30089-X
Huo, J. H., Du, X. W., Sun, G. D., Meng, Y. L., and Wang, W. M. (2017). Comparison of the chemical profiles of fresh-raw and dry-processed Juglans mandshurica. J. Separ. Sci. 40, 646–662. doi:10.1002/jssc.201600877
Jiang, H., Yang, L., Hou, A., Zhang, J., Wang, S., Man, W., et al. (2020). Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of Bupleuri Radix: a systematic review. Biomed. Pharmacother. 131, 110679. doi:10.1016/j.biopha.2020.110679
Jiang, Z., Diao, S. B., Li, R., Zhou, W., Sun, J. F., Zhou, Y., et al. (2018). One new 1, 4-napthoquinone derivative from the roots of Juglans mandshurica. Nat. Prod. Res. 32 (9), 1017–1021. doi:10.1080/14786419.2017.1375921
Jin, M., Diao, S. B., Zhang, C. H., Cao, S., Sun, J. F., Li, R., et al. (2015). Two new diarylheptanoids isolated from the roots of Juglans mandshurica. Nat. Prod. Res. 29, 1839–1844. doi:10.1080/14786419.2015.1009063
Jin, M., Sun, J. F., Li, R., Diao, S. B., Zhang, C. H., Cui, J. M., et al. (2016). Two new quinones from the roots of Juglans mandshurica. Arch. Pharm. Res. (Seoul). 39, 1237–1241. doi:10.1007/s12272-016-0781-1
Ju, S. M., Song, H. Y., Lee, S. J., Seo, W. Y., Sin, D. H., Goh, A. R., et al. (2009). Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta- O-galloyl-β-D-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 387, 115–120. doi:10.1016/j.bbrc.2009.06.137
Ju, X. C., Chen, C., Di, X., Xiao, H. H., Zhang, H., Zhai, Y. J., et al. (2019). Acute toxicity and in vitro anti-tumor activity of Juglans mandshurica. Central South Pharm. 17, 360–364. doi:10.7539/j.issn.1672-2981.2019.03.008
Kim, J. Y., Lee, E. J., Ahn, Y., Park, S., Kim, S. H., and Oh, S. H. (2019). A chemical compound from fruit extract of Juglans mandshurica inhibits melanogenesis through p-ERK-associated MITF degradation. Phytomedicine. 57, 57–64. doi:10.1016/j.phymed.2018.12.007
Kim, S. H., Lee, K. S., Son, J. K., Je, G. H., Lee, J. S., Lee, C. H., et al. (1998). Cytotoxic compounds from the roots of Juglans mandshurica. J. Nat. Prod. 61, 643–645. doi:10.1021/np970413m
Lee, K. S., Li, G., Kim, S. H., Lee, C. S., Woo, M. H., Lee, S. H., et al. (2002). Cytotoxic diarylheptanoids from the roots of Juglans mandshurica. J. Nat. Prod. 65, 1707–1708. doi:10.1021/np0201063
Lee, S. W., Lee, K. S., and Son, J. K. (2000). New naphthalenyl glycosides from the roots of Juglans mandshurica. Planta. Med. 66, 184–186. doi:10.1055/s-0029-1243129
Li, G., Cui, J. M., Kwon, Y., Seo, C. S., Lee, C. S., Woo, M. H., et al. (2005). Two new diarylheptanoids from Juglans mandshurica. Bull. Kor. Chem. Soc. 26, 1878–1880. doi:10.1002/chin.200616203
Li, G., Xu, M. L., Choi, H. G., Lee, S. H., Jahng, Y. D., Lee, C. S., et al. (2003). Four new diarylheptanoids from the roots of Juglans mandshurica. Chem. Pharm. Bull. 51, 262–264. doi:10.1248/cpb.51.262
Li, J., Wang, J., Liu, C. L., Fang, L., and Min, W. H. (2018). Protein hydrolyzates from Changbai mountain Walnut (Juglans mandshurica Maxim.) boost mouse immune system and exhibit immunoregulatory activities. Evid. Based Complement. Alternat. Med., 457, 6561. doi:10.1155/2018/4576561
Li, J., Xu, K. P., Zou, H., Long, H. P., Zou, Z. X., Kuang, J. W., et al. (2013). Chemical constituents in the green peel of Juglans mandshurica maxim. Central South Pharm. 11, 1–3. doi:10.7539/j.issn.1672-2981.2013.01.001
Li, J., Xu, K. P., Zou, Z. X., Zou, H., Long, H. P., Tan, L. H., et al. (2017a). Two new compounds from the green peel of Juglans mandshurica. J. Asian. Nat. Prod. Res. 19, 1087–1092. doi:10.1080/10286020.2017.1295228
Li, J., Xu, P. S., Zou, Z. X., Zou, H., Long, H. P., Tan, L. H., et al. (2017b). Three new compounds from the roots of Juglans mandshurica Maxim. Phytochem. Lett. 20, 40–44. doi:10.1016/j.phytol.2017.03.014
Li, Z. B., Bao, Y. M., Chen, H. B., Wang, J. Y., Hu, J. H., and An, L. J. (2007b). A cytotoxic compound from the leaves of Juglans mandshurica. Chin. Chem. Lett. 18, 846–848. doi:10.1016/j.cclet.2007.05.043
Li, Z. B., Wang, J. Y., Jiang, B., Zhang, X. L., An, L. J., and Bao, Y. M. (2007a). Benzobijuglone, a novel cytotoxic compound from Juglans mandshurica, induced apoptosis in HeLa cervical cancer cells. Phytomedicine. 14, 846–852. doi:10.1016/j.phymed.2007.09.004
Li, Z. B., Wang, J. Y., Yang, J., Zhang, X. L., An, L. J., and Bao, Y. M. (2009). Apoptosis of BGC823 cell line induced by p-hydroxymethoxybenzobijuglone, a novel compound from Juglans mandshurica. Phytother. Res. 23, 551–557. doi:10.1002/ptr.2685
Lin, H., Zhang, Y. W., Bao, Y. L., Wu, Y., Sun, L. G., Yu, C. L., et al. (2013). Secondary metabolites from the stem bark of Juglans mandshurica. Biochem. Systemat. Ecol. 51, 184–188. doi:10.1016/j.bse.2013.08.010
Lin, H., Zhang, Y. W., Hua, Y., Bao, Y. L., Wu, Y., Sun, L. G., et al. (2014). Three new compounds from the stem bark of Juglans mandshurica. J. Asian. Nat. Prod. Res. 16, 819–824. doi:10.1080/10286020.2014.923406
Liu, C. G., Wang, Y., Guo, S., Liu, Y. X., Sun, Y. P., Fu, L., et al. (2017). Study on chemical constituents from pericarps of Juglans mandshurica. Inf. Trad. Chin. Med. 34, 4–6. doi:10.19656/j.cnki.1002-2406.2017.04.002
Liu, C. L., Guo, Y., Zhao, F. R., Qin, H. X., Lu, H. Y., Fang, L., et al. (2019). Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 10, 3491–3501. doi:10.1039/c8fo02557f
Liu, G. R., Xu, K. P., Shen, J., Yang, F., Zou, H., and Tan, G. S. (2009). Antitumor chemical constituents from the roots of Juglans mandshurica Maxim. Central South Pharm. 7, 644–646
Liu, L. J., Li, W., Loike, K. Z., Zhang, S. J., and Nikaido, T. (2004a). New α-Tetralonyl glucosides from the fruit of Juglans mandshurica. Chem. Pharm. Bull. 52, 566–569. doi:10.1248/cpb.52.566
Liu, W., Lin, W. H., and Ji, Y. B. (2004b). Study on the acute toxicity experiment of mice and anti-tumor function in vitro of the qinglongyi. China J. Chin. Mater. Med. 29, 887–890.
Liu, L. J., Li, W., Sasaki, T., Asada, Y., and Koike, K. (2010). Juglanone, a novel α-tetralonyl derivative with potent antioxidant activity from Juglans mandshurica. J. Nat. Med. 64, 496–499. doi:10.1007/s11418-010-0435-4
Lou, L. L., Guo, R., Cheng, Z. Y., Zhao, P., Yao, G. D., Wang, X. B., et al. (2018). Coumarins from Juglans Mandshurica Maxim and their apoptosis-inducing activities in hepatocarcinoma cells. Phytochem. Lett. 15–20. doi:10.1016/j.phytol.2018.01.005
Lou, L. L., Zhao, P., Cheng, Z. Y., Guo, R., Yao, G. D., Wang, X. B., et al. (2019a). A new coumarin from Juglans mandshurica Maxim induce apoptosis in hepatocarcinoma cells. Nat. Prod. Res. 33, 1791–1793. doi:10.1080/14786419.2018.1434646
Lou, L. L., Cheng, Z. Y., Guo, R., Yao, G. D., and Song, S. J. (2019b). Alkaloids from Juglans mandshurica maxim induce distinctive cell death in hepatocellular carcinoma cells. Nat. Prod. Res. 33, 911–914. doi:10.1080/14786419.2017.1413571
Lu, Z., Chen, H., Zheng, X. M., and Chen, M. L. (2017). Experimental study on the apoptosis of cervical cancer Hela cells induced by juglone through c-Jun N-terminal kinase/c-Jun pathway. Asian Pac. J. Trop. Med. 10, 572–575. doi:10.1016/j.apjtm.2017.06.005
Luan, F., Han, K. Q., Li, M. X., Zhang, T., Liu, D. H., Yu, L. H., et al. (2019). Ethnomedicinal uses, phytochemistry, pharmacology, and toxicology of species from the genus Ajuga L.: a systematic review. Am. J. Chin. Med. 47, 959–1003. doi:10.1142/S0192415X19500502
Machida, K., Matsuoka, E., Kasahara, T., and Kikuchi, M. (2005). Studies on the constituents of Juglans species. I. Structural determination of (4S)- and (4R)-4-hydroxy-alpha-tetralone derivatives from the fruit of Juglans mandshurica MAXIM. var. sieboldiana. MAKINO. Chem. Pharm. Bull. 53, 934–937. doi:10.1248/cpb.53.934
Machida, K., Yogiashi, Y., Matsuda, S., Suzuki, A., and Kikuchi, M. (2009). A new phenolic glycoside syringate from the bark of Juglans mandshurica MAXIM. var. sieboldiana MAKINO. J. Nat. Med. 63, 220–222. doi:10.1007/s11418-009-0312-1
Min, B. S., Lee, H. K., Lee, S. M., Kim, Y. H., Bae, K. H., Otake, T., et al. (2002). Anti-human immunodeficiency virus-type 1 activity of constituents from Juglans mandshurica. Arch. Pharm. Res. (Seoul). 25, 441–445. doi:10.1007/bf02976598
Min, B. S., Lee, S. Y., Kim, J. H., Lee, J. K., Kim, T. J., Kim, D. H., et al. (2003). Anti-complement activity of constituents from the stem-bark of Juglans mandshurica. Biol. Pharm. Bull. 26, 1042–1044. doi:10.1248/bpb.26.1042
Min, B. S., Nakamura, N., Miyashiro, H., Kim, Y. H., and Hattori, M. (2000). Inhibition of human immunodeficiency virus type 1 reverse transcriptase and ribonuclease H activities by constituents of Juglans mandshurica. Chem. Pharm. Bull. 48, 194–200. doi:10.1248/cpb.48.194
Mu, X. Y., Sun, M., Yang, P. F., and Lin, Q. W. (2017). Unveiling the identity of Wenwan walnuts and phylogenetic relationships of Asian Juglans species using restriction site-associated DNA-sequencing. Front. Plant Sci. 8, 1708. doi:10.3389/fpls.2017.01708
Ngoc, T. M., Hung, T. M., Thuong, P. T., Kim, J. C., Choi, J. S., Bae, K., et al. (2008). Antioxidative activities of galloyl glucopyranosides from the stem-bark of Juglans mandshurica. Biosci. Biotechnol. Biochem. 72, 2158–2163. doi:10.1271/bbb.80222
Park, G., Jang, D. S., and Oh, M. S. (2012). Juglans mandshurica leaf extract protects skin fibroblasts from damage by regulating the oxidative defense system. Biochem. Biophys. Res. Commun. 421, 343–348. doi:10.1016/j.bbrc.2012.04.013
Park, G., and Oh, M. S. (2014). Inhibitory effects of Juglans mandshurica leaf on allergic dermatitis-like skin lesions-induced by 2,4-dinitrochlorobenzene in mice. Exp. Toxicol. Pathol. 66, 97–101. doi:10.1016/j.etp.2013.10.001
Park, S., Kim, N., Yoo, G., Kim, S. N., Kwon, H. J., Jung, K., et al. (2017). Phenolics and neolignans isolated from the fruits of Juglans mandshurica Maxim. and their effects on lipolysis in adipocytes. Phytochemistry. 137, 87–93. doi:10.1016/j.phytochem.2017.01.019
Peng, X. H., Nie, Y., Wu, J. J., Huang, Q., and Cheng, Y. Q. (2015). Juglone prevents metabolic endotoxemia-induced hepatitis and neuroinflammation via suppressing TLR4/NF-κB signaling pathway in high-fat diet rats. Biochem. Biophys. Res. Commun. 462, 245–250. doi:10.1016/j.bbrc.2015.04.124
Pereira, A. C., Magalhães, L. G., Gonçalves, U. O., Luz, P. P., Moraes, A. C., Rodrigues, V., et al. (2011). Schistosomicidal and trypanocidal structure-activity relationships for (±)-licarin A and its (-)- and (+)-enantiomers. Phytochemistry. 72, 1424–1430. doi:10.1016/j.phytochem.2011.04.007
Qiu, J. Y., Wang, W. M., Li, J., Zhao, M., Wang, J. L., and Zhang, S. J. (2017). Chemical constituents in green walnut husks of Juglans regia. Chin. Trad. Herb. Drugs. 48, 2385–2389. doi:10.7501/j.issn.0253-2670.2017.12.005
Ren, D. Y., Zhao, F. R., Liu, C. L., Wang, J., Guo, Y., Liu, J. S., et al. (2018). Antioxidant hydrolyzed peptides from Manchurian walnut (Juglans mandshurica Maxim.) attenuate scopolamine-induced memory impairment in mice. J. Sci. Food. Agric. 98, 5142–5152. doi:10.1002/jsfa.9060
Salehi, B., Sener, B., Kilic, M., Sharif-Rad, J., Naz, R., Yousaf, Z., et al. (2019). Dioscorea plants: a genus rich in vital nutrapharmaceuticals-A review. Iran. J. Pharm. Res. 18, 68–89. doi:10.22037/ijpr.2019.112501.13795
Son, J. K. (1995). Isolation and structure determination of a new tetralone glucoside from the roots of Juglans mandshurica. Arch Pharm. Res. (Seoul). 18, 203–205.
Sun, D. J., Zhu, L. J., Zhao, Y. Q., Zhen, Y. Q., Zhang, L., Lin, C. C., et al. (2020). Diarylheptanoid: a privileged structure in drug discovery. Fitoterapia. 142, 104490. doi:10.1016/j.fitote.2020.104490
Sun, Z. L., Dong, J. L., and Wu, J. (2017). Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed. Pharmacother. 85, 303–312. doi:10.1016/j.biopha.2016.11.030
Wang, R. J., Wang, S., Xia, Y. J., Tu, M., Zhang, L. J., and Wang, Y. M. (2015). Antitumor effects and immune regulation activities of a purified polysaccharide extracted from Juglan regia. Int. J. Biol. Macromol. 72, 771–775. doi:10.1016/j.ijbiomac.2014.09.026
Wang, T. M., Fu, Y., Yu, W. J., Chen, C., Di, X., Zhang, H., et al. (2017a). Identification of polar constituents in the decoction of Juglans mandshurica and in the medicated egg prepared with the decoction by HPLC-Q-TOF MS2. Molecules. 22, 1452. doi:10.3390/molecules22091452
Wang, T. M., Liu, J., Yi, T., Zhai, Y. J., Zhang, H., Chen, H. B., et al. (2017b). Multiconstituent identification in root, branch, and leaf extracts of Juglans mandshurica using ultra high-performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Separ. Sci. 40, 3440–3452. doi:10.1002/jssc.201700521
Wang, T. M., Yu, W. J., Fu, Y., Di, X., Zhai, Y. J., Zhang, H., et al. (2017c). Inhibitory effect of the eggs decocted with branches of Juglans mandshurica on solid tumor of murine H22 hepatocarcinoma cell in mice. Drugs Clin. 32, 365–369. doi:10.7501/j.issn.1674-5515.2017.03.002
Wang, T. M., Yu, W. J., Fu, Y., Di, X., Zhai, Y. J., Kang, T. G., et al. (2017d). Vivo anti-tumor activity of total tannins from Juglans mandshurica, China Medical Herald. 14, 16–19.
Wang, P., Gao, C., Wang, W., Yao, L. P., Zhang, J., Zhang, S. D., et al. (2018a). Juglone induces apoptosis and autophagy via modulation of mitogen-activated protein kinase pathways in human hepatocellular carcinoma cells. Food Chem. Toxicol. 116, 40–50. doi:10.1016/j.fct.2018.04.004
Wang, G. L., Cheng, T., Huo, J. H., and Wang, W. M. (2018b). Analysis on chemical constituents from rat kidney tissues of Juglans mandshurica based on UPLC-Q-TOF/MS. Chin. Tradit. Herb. Drugs. 49, 3763–3769. doi:10.7501/j.issn.0253-2670.2018.16.006
Wang, A. D., Xie, C. J., Zhang, Y. Q., Li, M. C., Wang, X., Liu, J. Y., et al. (2019a). α-Tetralonyl glucosides from the green walnut husks of Juglans mandshurica and their antiproliferative effects. Planta. Med. 85, 335–339. doi:10.1055/a-0832-2328
Wang, J., Zhou, L., Cheng, Z. Y., Wang, Y. X., Yan, Z. Y., Huang, X. X., et al. (2019b). Chiral resolution and bioactivity of enantiomeric furofuran lignans from Juglans mandshurica Maxim. Nat. Prod. Res. 1-4. doi:10.1080/14786419.2019.1577839
Wang, H., Liu, H., Zhang, N. X., Zhang, H., Gao, W. Y., and Sun, J. M. (2019c). Screening and chemical analysis of hypoglycemic and antioxidant effective fractions from leaves of Juglans mandshurica. Mod. Chin. Med. 21, 312–315. doi:10.13313/j.issn.1673-4890.20180926005
Wang, J., Wu, T., Fang, L., Liu, C. L., Liu, X. T., Li, H. M., et al. (2020a). Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods. 69, 103944. doi:10.1016/j.jff.2020.103944
Wang, J., Wu, T., Fang, L., Liu, C. L., Liu, X. T., Li, H. M., et al. (2020b). Peptides from walnut (Juglans mandshurica Maxim.) protect hepatic HepG2 cells from high glucose-induced insulin resistance and oxidative stress. Food Funct. 11, 8112–8121. doi:10.1039/d0fo01753a
Wen, Z. M., Jie, J., Zhang, Y., Liu, H., and Peng, L. P. (2017). A self-assembled polyjuglanin nanoparticle loaded with doxorubicin and anti-Kras siRNA for attenuating multidrug resistance in human lung cancer. Biochem. Biophys. Res. Commun. 493, 1430–1437. doi:10.1016/j.bbrc.2017.09.132
Westfall, B. A., Russell, R. L., and Auyong, T. K. (1961). Depressant agent from walnut hulls. Science. 134, 1617. doi:10.1126/science.134.3490.1617
Xin, N., Hasan, M., Li, W., and Li, Y. (2014). Juglans mandshurica Maxim extracts exhibit antitumor activity on HeLa cells in vitro. Mol. Med. Rep. 9, 1313–1318. doi:10.3892/mmr.2014.1979
Xu, H. L., Yu, X. F., Qu, S. C., and Sui, D. Y. (2013). Juglone, isolated from Juglans mandshurica Maxim, induces apoptosis via down-regulation of AR expression in human prostate cancer LNCaP cells. Bioorg. Med. Chem. Lett. 23, 3631–3634. doi:10.1016/j.bmcl.2013.04.007
Xu, H. L., Yu, X. F., Qu, S. C., Zhang, R., Qu, X. R., Chen, Y. P., et al. (2010). Anti-proliferative effect of Juglone from Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing apoptosis through the mitochondria-dependent pathway. Eur. J. Pharmacol. 645, 14–22. doi:10.1016/j.ejphar.2010.06.072
Yang, B. Y., Jiang, Y. Q., Meng, Y., Liu, Y. X., Liu, Z. X., Xiao, H. B., et al. (2015). Studies on chemical constituents in n-butanol extracts from epicarp of green fruit of Juglans mandshurica. Chin. Tradit. Herb. Drugs. 46, 481–485. doi:10.7501/j.issn.0253-2670.2015.04.004
Yang, Q., Yao, Q. S., Kuang, Y., Zhang, Y. Z., Feng, L. L., Zhang, L., et al. (2019). Antimicrobial and cytotoxic juglones from the immature exocarps of Juglans mandshurica. Nat. Prod. Res. 33, 3203–3209. doi:10.1080/14786419.2018.1468326
Yao, D. L., Jiang, L. J., Zhou, W., Xu, T., Pen, L., and Li, G. (2009). Study on the inhibitory effect of chloroform extract of Juglans mandshurica root on mouse S180 sarcoma. J. Chin. Med. Mater. 32, 595–596. doi:10.13863/j.issn1001-4454.2009.04.042
Yao, D. L., Jin, M., Zhang, C. H., Luo, J., Li, R., Zheng, M. S., et al. (2014). A new phenolic glycoside from Juglans mandshurica. Nat. Prod. Res. 28, 998–1002. doi:10.1080/14786419.2014.902946
Yao, D. L., Zhang, C. H., Li, R., Luo, J., Jin, M., Piao, J. H., et al. (2015a). Two new conjugated ketonic fatty acids from the stem bark of Juglans mandshurica. Chin. J. Nat. Med. 13, 0299–0302. doi:10.1016/S1875-5364(15)30018-2
Yao, D. L., Zhang, C. H., Luo, J., Jin, M., Zheng, M. S., Cui, J. M., et al. (2015b). Chemical constituents from the leaves of Juglans mandshurica. Arch Pharm. Res. (Seoul). 38, 480–484. doi:10.1007/s12272-014-0398-1
Yao, G. D., Cheng, Z. Y., Shang, X. Y., Gao, P. Y., Huang, X. X., and Song, S. J. (2017). Coumarins from the bark of Juglans mandshurica exhibited anti-hepatoma activities via inducing apoptosis. J. Asian. Nat. Prod. Res. 19, 1134–1142. doi:10.1080/10286020.2017.1292256
Yao, Y., Zhang, Y. W., Sun, L. G., Liu, B., Bao, Y. L., Lin, H., et al. (2012). Juglanthraquinone C, a novel natural compound derived from Juglans mandshurica Maxim, induces S phase arrest and apoptosis in HepG2 cells. Apoptosis. 17, 832–841. doi:10.1007/s10495-012-0722-5
Zhang, J. B., Liu, J. X., Zha, F., and Di, L. D. (2009). Chemical constituents in green walnut husks of Juglans regia. Chin. Tradit. Herb. Drugs. 40, 847–849
Zhang, W., Liu, A. H., Li, Y., Zhao, X. Y., Lv, S. J., Zhu, W. H., et al. (2012a). Anticancer activity and mechanism of juglone on human cervical carcinoma HeLa cells. Can. J. Physiol. Pharmacol. 90, 1553–1558. doi:10.1139/y2012-134
Zhang, Y. W., Lin, H., Bao, Y. L., Wu, Y., Yu, C. L., Huang, Y. X., et al. (2012b). A new triterpenoid and other constituents from the stem bark of Juglans mandshurica. Biochem. Systemat. Ecol. 44, 136–140. doi:10.1016/j.bse.2012.04.015
Zhang, Y. L., Cui, Y. Q., Zhu, J. Y., Li, H. Z., Mao, J. W., Jin, X. B., et al. (2013). The anti-tumor effect and biological activities of the extract JMM6 from the stem-barks of the Chinese Juglans mandshurica Maxim on human hepatoma cell line BEL-7402. Afr. J. Tradit. Complement. Altern. Med. 10, 258–269. doi:10.4314/ajtcam.v10i2.10
Zhang, X. N., Bai, M., Cheng, Z. Y., Yu, Z. G., and Huang, X. X. (2018). Cytotoxic lignans from the barks of Juglans mandshurica. J. Asian. Nat. Prod. Res. 20, 494–499. doi:10.1080/10286020.2017.1374256
Zhang, Y. C., Ge, P. L., Chen, J. H., Zhou, Y. Y., and Liu, Y. X. (2019). Advances in studies on naphthoquinones from green walnut husks of Juglans mandshurica and their anticancer activities. Chin. Tradit. Herb. Drugs. 50, 2251–2256. doi:10.7501/j.issn.0253-2670.2019.09.035
Zhang, Y. W., Lin, H., Li, S. S., Chen, J. B., Sun, Y. S., and Li, Y. X. (2017). High-speed counter-current chromatography assisted preparative isolation of bioactive compounds from stem bark of Juglans mandshurica. J. Separ. Sci. 40, 767–778. doi:10.1002/jssc.201601043
Zhao, P., Zhou, H. J., Potter, D., Hu, Y. H., Feng, X. J., Dang, M., et al. (2018). Population genetics, phylogenomics and hybrid speciation of Juglans in China determined from whole chloroplast genomes, transcriptomes, and genotyping-bysequencing (GBS). Mol. Phylogenet. Evol. 126, 250–265. doi:10.1016/j.ympev.2018.04.014
Zhao, Y., Zhou, W., Diao, S. B., Jiang, Z., Jin, M., and Li, G. (2019). Phytochemical investigation on the roots of Juglans mandshurica and their chemotaxonomic significance. Biochem. Systemat. Ecol. 87, 103957. doi:10.1016/j.bse.2019.103957
Zhao, F. R., Wang, J., Lu, H. Y., Fang, L., Qin, H. X., Liu, C. L., et al. (2020). Neuroprotection by walnut-derived peptides through autophagy promotion via Akt/mTOR signaling pathway against oxidative stress in PC12 cells. J. Agric. Food Chem. 68, 3638–3648. doi:10.1021/acs.jafc.9b08252
Zhou, Y. Y., Meng, Y., Jiang, Y. Q., Liu, Z. X., and Yang, B. Y. (2014a). Study on anti-tumor chemical constituents from pericarps of Juglans mandshurica. J. Chin. Med. Mater. 37, 1998–2001. doi:10.13863/j.issn1001-4454.2014.11.022
Zhou, Y. Y., Liu, Z. X., Meng, Y., Jiang, Y. Q., and Yang, B. Y. (2014b). Chemical constituents from active fraction in pericarps of Juglans mandshurica. Chin. Tradit. Herb. Drugs. 45, 2303–2306. doi:10.7501/j.issn.0253-2670.2014.16.004
Zhou, Y. Y., Yang, B. Y., Liu, Z. X., Jiang, Y. Q., Liu, Y. X., Fu, L., et al. (2015a). Cytotoxicity of triterpenes from green walnut husks of Juglans mandshurica Maxim in HepG-2 cancer cells. Molecules. 20, 19252–19262. doi:10.3390/molecules201019252
Zhou, Y. Y., Yang, B. Y., Jiang, Y. Q., Liu, Z. X., Liu, Y. X., Wang, X. L., et al. (2015b). Studies on cytotoxic activity against HepG-2 cells of naphthoquinones from green walnut husks of Juglans mandshurica Maxim. Molecules. 20, 15572–15588. doi:10.3390/molecules200915572
Zhou, Y. Y., Liu, Y. X., Jiang, Y. Q., Liu, Z. X., Niu, F., Yang, B. Y., et al. (2015c). Chemical constituents from pericarp of Juglans mandshurica Maxim. Chin. Tradit. Pat. Med. 37, 2669–2673. doi:10.3969/j.issn.1001-1528.2015.12.021
Zhou, Y. Y., Jiang, Y. Q., Meng, Y., Liu, Z. X., and Yang, B. Y. (2015d). Active parts constituents from the pericarps of Jugland mandshurica Maxim. Chin. Tradit. Pat. Med. 37, 332–335. doi:10.3969/j.issn.1001-1528.2015.02.024
Zhou, Y. Y., Liu, Y. X., Meng, Y., Jiang, Y. Q., Liu, Z. X., Yang, B. Y., et al. (2015e). Quinones of Juglans mandshurica maxim. Acta Chinese Medicine and Pharmacology. 43, 8–10. doi:10.19664/j.cnki.1002-2392.2015.03.004
Zhou, Y. Y., Liu, Y. X., Jiang, Y. Q., Liu, Z. X., Yang, B. Y., and Xiao, H. B. (2016). Studies on anti-tumor chemical constituents in exocarps of Juglans mandshurica. Chin. Tradit. Herb. Drugs. 47, 2979–2983. doi:10.7501/j.issn.0253-2670.2016.17.004
Zhou, Y. Y., Liu, Q. Y., Yang, B. Y., Jiang, Y. Q., Liu, Y. X., Wang, Y., et al. (2017). Two new cytotoxic glycosides isolated from the green walnut husks of Juglans mandshurica Maxim. Nat. Prod. Res. 31, 1237–1244. doi:10.1080/14786419.2016.1233412
Zhou, Y. Y., Wang, Y., Song, H. J., Guo, S., Liu, Y., Liu, Y. X., et al. (2018a). Chemical constituents of n-butanol fraction from green walnut husks of Juglans mandshurica. Chin. Tradit. Herb. Drugs. 49, 4220–4225. doi:10.7501/j.issn.0253-2670.2018.18.004
Zhou, Y. Y., Song, H. J., Guo, S., Zhang, X. J., Fu, L., and Fu, Q. F. (2018b). Chemical constituents from EtOAc extract of Juglans mandshurica maxim. Information on Traditional Chinese Medicine. 35, 46–48. doi:10.19656/j.cnki.1002-2406.180146
Zhou, Y. Y., Song, H. J., Guo, S., Wang, Y., Gao, H. R., Zhang, X. J., et al. (2019a). A new triterpene from the green walnut husks of Juglans mandshurica Maxim. J. Nat. Med. 73, 800–804. doi:10.1007/s11418-019-01309-4
Zhou, Y. Y., Guo, S., Wang, Y., Song, H. J., Gao, H. R., Zhang, X. J., et al. (2019b). α-Tetralone glycosides from the green walnut husks of Juglans mandshurica Maxim. and their cytotoxic activities. Nat. Prod. Res. 1-9. doi:10.1080/14786419.2018.1561681
Zhou, Y. Y., Wang, Y., Guo, S., Song, H. J., Zhang, X. J., Liu, Y., et al. (2019c). Two new tetralone glycosides from the green walnut husks of Juglans mandshurica Maxim. Nat. Prod. Res. 33, 2932–2938. doi:10.1080/14786419.2018.1510397
Zhou, Y. Y., Song, H. J., Chen, H., Guo, S., Wang, Y., Gao, H. R., et al. (2019d). Study on flavonoids from green walnut husks of Juglans mandshurica. Chin. Tradit. Herb. Drugs. 50, 3588–3592. doi:10.7501/j.issn.0253-2670.2019.15.011
Zhou, Y. Y., Song, H. J., Li, J., Guo, S., Wang, Y., Gao, H. R., et al. (2020). Chemical constituents from the green walnut husks of Juglans mandshurica. Chin. Tradit. Pat. Med. 42, 375–381. doi:10.3969/j.issn.1001-1528.2020.02.020
Zhou, Y. Y., Wang, D., and Niu, F. (2010). Studies on constituents from pericarps of Juglans mandshurica with anti-tumor activity. Chin. Tradit. Herb. Drugs. 41, 11–14
Keywords: Juglans mandshurica, traditional uses, phytochemistry, pharmacology, antitumor activities
Citation: Luan F, Wang Z, Yang Y, Ji Y, Lv H, Han K, Liu D, Shang X, He X and Zeng N (2021) Juglans mandshurica Maxim.: A Review of Its Traditional Usages, Phytochemical Constituents, and Pharmacological Properties. Front. Pharmacol. 11:569800. doi: 10.3389/fphar.2020.569800
Received: 05 June 2020; Accepted: 04 December 2020;
Published: 21 January 2021.
Edited by:
Bey Hing Goh, Monash University Malaysia, MalaysiaReviewed by:
Javad Sharifi-Rad, Shahid Beheshti University of Medical Sciences, IranJayanta Kumar Patra, Dongguk University Seoul, South Korea
Copyright © 2021 Luan, Wang, Yang, Ji, Lv, Han, Liu, Shang, He and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xirui He, eGlydWloZTYxMDUxOTRAMTYzLmNvbQ==; Nan Zeng, MTk5MzIwMTVAY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Fei Luan1,2†
Fei Luan1,2†