
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 19 November 2020
Sec. Renal Pharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.564281
 Ebenezeri Erasto Ngowi1,2,3
Ebenezeri Erasto Ngowi1,2,3 Muhammad Sarfraz1,2,4,5
Muhammad Sarfraz1,2,4,5 Attia Afzal1,2,5
Attia Afzal1,2,5 Nazeer Hussain Khan2,6
Nazeer Hussain Khan2,6 Saadullah Khattak1,2
Saadullah Khattak1,2 Xin Zhang6,7
Xin Zhang6,7 Tao Li1,2
Tao Li1,2 Shao-Feng Duan2,6,7*
Shao-Feng Duan2,6,7* Xin-Ying Ji1,2,8*
Xin-Ying Ji1,2,8* Dong-Dong Wu1,2,9*
Dong-Dong Wu1,2,9*Hydrogen sulfide (H2S) plays a key role in the regulation of physiological processes in mammals. The decline in H2S level has been reported in numerous renal disorders. In animal models of renal disorders, treatment with H2S donors could restore H2S levels and improve renal functions. H2S donors suppress renal dysfunction by regulating autophagy, apoptosis, oxidative stress, and inflammation through multiple signaling pathways, such as TRL4/NLRP3, AMP-activated protein kinase/mammalian target of rapamycin, transforming growth factor-β1/Smad3, extracellular signal-regulated protein kinases 1/2, mitogen-activated protein kinase, and nuclear factor kappa B. In this review, we summarize recent developments in the effects of H2S donors on the treatment of common renal diseases, including acute/chronic kidney disease, renal fibrosis, unilateral ureteral obstruction, glomerulosclerosis, diabetic nephropathy, hyperhomocysteinemia, drug-induced nephrotoxicity, metal-induced nephrotoxicity, and urolithiasis. Novel H2S donors can be designed and applied in the treatment of common renal diseases.
The involvement of hydrogen sulfide (H2S) in a variety of health disorders has gained increased attention from researchers and scientists in recent years. This rotten egg-smelling, colorless, and poisonous gas is the third gasotransmitter to be associated with body homeostasis and disease progression. Others include carbon monoxide (Kaide et al., 2001) and nitric oxide (NO) (Wever et al., 1999). H2S levels have been established as a promising disease indicator and treatment for a number of health conditions including cancers (Szabo et al., 2013), neurodegenerative diseases (Vandini et al., 2019), cardiovascular diseases (Peter et al., 2013), osteoporosis (Ma et al., 2019), kidney diseases (Aminzadeh and Vaziri, 2011), obesity (Katsouda et al., 2018), and aging (Kasinath, 2018). The gas shows protective roles by mediating cellular activities such as autophagy (Ling et al., 2017), inflammation, apoptosis (Tan et al., 2015), and oxidative stress (Cheung and Lau, 2018). Tempering with H2S level exerts direct effects on several cellular pathways such as nuclear factor kappa B (NF-κB) (Du et al., 2019), transforming growth factor-β (TGF-β) (Huang et al., 2018), extracellular signal-regulated protein kinases 1/2 (ERK1/2) (Shi et al., 2019), AMP-activated protein kinase (AMPK), and the mammalian target of rapamycin (mTOR) (Wu et al., 2017).
H2S is synthesized both enzymatically and nonenzymatically in mammalian cells (Nagahara et al., 1998; Shibuya et al., 2009). Three enzymes, namely, cystathionine γ-lyase (CSE) (Chiku et al., 2009), cystathionine β-synthase (CBS) (Braunstein et al., 1971), and 3-mercaptopyruvate sulfurtransferase (3-MPST) (Shibuya et al., 2009), are involved in the production of H2S. CBS and CSE are specifically located in the cytoplasm, while 3-MPST is found in both cytoplasm and mitochondria (Nagahara et al., 1998; Banerjee et al., 2015). 3-MPST catalyzes the production of H2S from 3-mercaptopyruvate (3-MP), which is synthesized by cysteine aminotransferase (CAT) from cysteine (Cys) and α-ketoglutarate (Kuo et al., 1983; Shibuya et al., 2009). Meanwhile, CSE and CBS produce H2S by catalyzing homocysteine (Hcy) and Cys in the transsulfuration pathway (Chen et al., 2004; Chiku et al., 2009). Low production of H2S and downregulation of CBS, CSE, and 3-MPST expressions have been reported in common renal disorders (Aminzadeh and Vaziri, 2011; Han et al., 2017; Chen Q. et al., 2018; Chen Y. et al., 2018; Zhou et al., 2020). Although the mechanism in the reduction and underproduction of H2S in common renal diseases is not yet clear, the supplementation with H2S donors can effectively restore H2S levels and ameliorate the disease state associated with low production of H2S (Song et al., 2014; Weber et al., 2017; Chen et al., 2018; Khukhlina et al., 2018; Akbari et al., 2019). Hence, understanding of the mechanism of H2S production in the body at the normal physiological condition and low production in the disease state can be beneficial in renal disease management. Generally, the protective effects of H2S can be attributed to its interactions with cell receptors and channels (Zhao et al., 2001) and its roles in posttranslation modification of proteins via sulfuration/persulfidation (Sen et al., 2012) and phosphorylation (Cao et al., 2018).
Renal system is one of the key and complex body systems involved in the regulation of body fluid and electrolytes. Apart from its basic functions, the system is also a target for numerous disease conditions of different etiology ranging from inflammations, acute injuries to chronic injuries, and cancer. In 2017, a systematic analysis of the global burden of chronic kidney diseases (CKD) reveals that over 1.2 million deaths occurred worldwide due to CKD, which is an increase of more than 40% from 1990 (Bikbov et al., 2020). Renal diseases can be classified into primary or secondary renal diseases. Primary renal diseases are caused by less severe diseases such as glomerulonephritis and tubulointerstitial fibrosis that interferes the normal kidney functions, whereas secondary kidney diseases involve the diseases that affect the kidney as a result of the complication of their long-duration and poor management of diabetes type 2 mellitus, hypertension, and primary kidney diseases (Schiffl et al., 2002). The pathology of the kidney is categorized into four main anatomical subsets which include the diseases of the glomerulus (diabetes and neoplasia), interstitium (obstructions and urinary tract infections), tubules (renal tubular acidosis and cystinuria), and blood vessels (renal vascular diseases) (Levey and Coresh, 2012). The risk factors for renal diseases include smoking, aging, environmental pollutants, and diseases (such as obesity, diabetes, anemia, and hypertension). Their initiation and development involve several mechanisms such as inflammation, oxidative stress, apoptosis, matrix production, autophagy, and cell injury. H2S is involved in the regulation of these mechanisms, suggesting that H2S may have potential in the treatment of kidney diseases.
In this review, we will analyze recent developments on the roles of H2S donors in common renal disorders. In addition, we summarize H2S-associated cellular pathways to show possible target sites for these compounds.
The main source of H2S in the human body is Cys metabolism. Cys is nonessential sulfur, containing amino acid that contributes to the synthesis of other amino acids including glutathione (GSH) which is involved in regulation of oxidative stress and detoxification (Beatty and Reed, 1980; Baker and Czarnecki-Maulden, 1987; Sekhar et al., 2011). Besides, it also participates in the synthesis of nonprotein essential compounds such as taurine and coenzyme A (Ondarza et al., 1974; Penttilä, 1990). H2S is synthesized as a byproduct of the Cys metabolism. The enzymatic production of H2S involves two systems, including pyridoxal 5′-phosphate-dependent (CBS and CSE) and pyridoxal 5′-phosphate-independent (3-MPST) systems (Stipanuk and Beck, 1982; Mikami et al., 2011). CBS mediates the conversion of Hcy and serine to produce cystathionine, which is then converted by CSE into Cys, α-ketobutyrate, and ammonia (Stipanuk and Ueki, 2011). CBS facilitates β replacement of thiol (-SH) group of the Cys with Hcy to produce H2S; meanwhile, CSE facilitates α, β, and γ elimination of cystathionine, Hcy, and Cys to form H2S (Chiku et al., 2009). On the other hand, 3-MPST and CAT catalyze the production of H2S from cysteine and α-ketoglutarate (Shibuya et al., 2009). In addition, H2S can be produced nonenzymatically from several biological reactions involving Cys, thiosulfate, elemental sulfur, GSH, glucose, and phosphogluconate (Koj et al., 1967; Searcy and Lee, 1998; Kolluru et al., 2013; Yang et al., 2019). The enzymatic and nonenzymatic mechanisms involved in the production of H2S are summarized in Figures 1, 2, respectively.
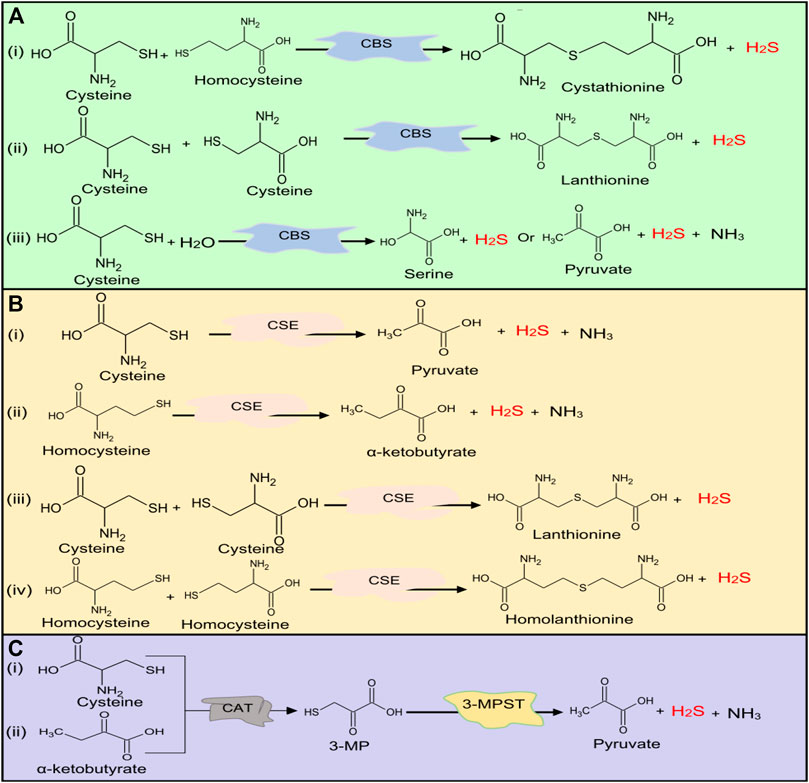
FIGURE 1. H2S production at normal physiological conditions in the body by (A) CBS, (B) CSE, and (C) 3-MPST. Both CBS and CSE produce H2S from cysteine and homocysteine, while 3-MPST produces H2S from 3-MP generated by CAT from Cys and α-ketobutyrate. H2S, hydrogen sulfide, CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; 3-MPST, 3-mercaptopyruvate sulfurtransferase; 3-MP, 3-mercaptopyruvate; CAT, cysteine aminotransferase; Cys, cysteine.
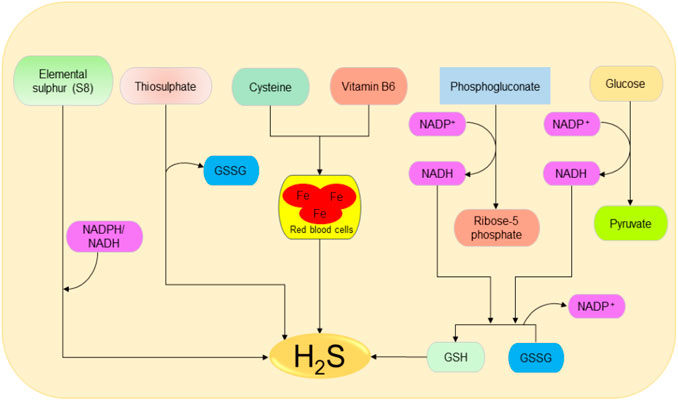
FIGURE 2. Possible spontaneous/nonenzymatic pathways involved in the production of H2S from elemental sulfur, thiosulfate, glutathione, cysteine, glucose, and phosphogluconate. Starting from the right-hand side, the two processes of glycolysis and NADPH oxidase facilitate the reduction of GSSG to GSH, which can be directly reduced to H2S. Next, in the presence of vitamin B6, cysteine can be catalyzed by iron contained in the red blood cells to produce H2S. Thiosulfate and elemental sulfur can also be reduced to H2S. H2S, hydrogen sulfide; NADPH, nicotinamide adenine dinucleotide phosphate oxidase; GSSG, oxidized glutathione; GSH, reduced glutathione.
CSE, CBS, and 3-MPST are localized in the kidney. In human renal tissues, CSE enzyme is reported to be localized in the glomerulus and tubulointerstitium (Bos et al., 2013). In addition, 75% of the renal cells and 87% of endothelial cells express the enzymes. On the other hand, CBS is highly expressed in renal proximal tubular cells (Yuan et al., 2017). Similarly, in mice kidneys, CBS and CSE proteins are well expressed with the former reported to be predominantly in the outer renal cortex (mainly in the proximal convoluted tubule) and the later in the inner cortex and medulla (specifically in the proximal straight tubule) (House et al., 1997). In addition, 3-MPST is localized in the proximal tubular epithelium (Nagahara et al., 1998). The deficiency of H2S-producing enzymes and H2S levels has been reported in human patients and rat models, which can be correlated with the severity of kidney diseases (Yuan et al., 2017). The above evidence indicates that the kidneys are enriched with all three enzymes and confirm their potential roles in kidney function.
Glomeruli are the basic units of the kidney. They are tiny filters that play crucial roles in the removal of waste material and urine formation (Pollak et al., 2014). Low glomerular filtration rate (GFR) has been reported in several renal diseases including acute kidney injury (AKI) and CKD (Marsik et al., 2008; Hsu and Hsu, 2011). Decreased GFR affects the removal of waste products by the kidneys. In a recent study, the reduced plasma levels of H2S in CKD patients could be significantly correlated with the reduction in GFR which confirms the importance of the gas in regulating the activities of the glomerulus (Kuang et al., 2018). It has been revealed that treatment of anesthetized rat models with CBS and CSE inhibitors, aminooxyacetic acid, and DL-propargylglycine (PAG) respectively, can impede the GFR by reducing vasodilation of the preglomerular arterioles; however, treatment with low doses of NaHS (a fast H2S-releasing donor) can protect the glomerulus functions by inducing opposite effects (Xia et al., 2009). Thus, treatment with H2S donors can improve kidney condition in patients by improving GFR (Figure 3).
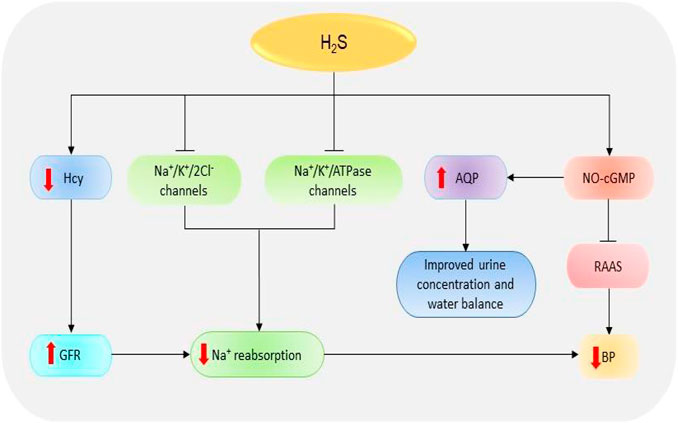
FIGURE 3. The dramatic presentation of the kidney functions regulated by H2S. H2S stimulates NO-cGMP pathway resulting in an inhibition of RAAS and subsequent reduction in BP. The activation of NO-cGMP can also increase the expressions of AQP-2, thereby improving urine concentration and water balance. Besides, H2S induces the inhibition of Na+/K+/2Cl- and Na+/K+/atpase channels, thence reducing Na+ reabsorption and consequently decreasing the BP. H2S also reduces Hcy levels resulting in an increase in GFR, suppression of Na+, and finally decline in BP. H2S, hydrogen sulfide; Hcy, homocysteine; Na+, sodium; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; GFR, glomerular filtrate rate; BP, blood pressure; AQP-2, aquaporin-2; RAAS, renin-angiotensin-aldosterone system.
The reabsorption of sodium (Na+) is one of the primary functions of the renal system. The failure of the kidney to remove excess Na+ has detrimental pathological implications due to its involvement in the regulation of blood volume, blood pressure (BP), and fluid balance. In addition, the accumulation of Na+ facilitates kidney damage by increasing fibrosis and oxidative stress (Thijssen et al., 2008). NaHS treatment can decrease Na+ reabsorption through several mechanisms including the elevation of GFR and the inhibition of Na+/K+/2Cl- and Na+/K+/ATPase channels, leading to the high urinary Na+ concentration (Xia et al., 2009). It has also been shown that treatment with H2S donor NaHS prevents the activation of epithelial Na+ channels by regulating the reactive oxygen species (ROS)/phosphatidylinositol 3-kinase/phosphatase and tensin homolog signaling pathway (Zhang et al., 2013; Wang et al., 2015). Together, these data imply that H2S is involved in Na+ balance and the regulation of kidney functions.
Another key function of the kidney is the regulation of BP. Kidney works together with the renin-angiotensin-aldosterone system (RAAS) to regulate BP and maintain electrolyte balance (Cortinovis et al., 2016). The decline in BP and Na+ level or the stimulation of β1-adrenoreceptors can result in the secretion of renin, which in turn converts angiotensinogen (produced in the liver) into angiotensin-I which is then converted to angiotensin II by angiotensin-I converting enzyme (ACE) (Soubrier et al., 1993; Deschepper, 1994). Angiotensin II acts on several receptors to mediate the downstream effects. For instance, angiotensin II facilitates the secretion of aldosterone hormone which is involved in the elevation of BP by promoting the reabsorption Na+ and excretion of potassium via Na+/K+/ATPase channels (Ikeda et al., 1991; Giacchetti et al., 1996). Treatment with a phosphorothioate-based synthetic H2S donor JK-1 suppresses the activation of RAAS, reduces BP, and improves renal functions (Li et al., 2018). It has also been shown that the treatment of anesthetized rat models with H2S donor Na2S can significantly decrease the BP and mesenteric resistance; meanwhile, the administration of CSE inhibitors (β-cyanoalanine) and PAG could increase BP and resistance in both mesenteric and renal circulations (Morales-Loredo et al., 2019). Another component involved in BP regulation is cyclic guanosine monophosphate (cGMP). The NO-cGMP pathway participates in the regulation of BP by attenuating the expression of kidney renin levels and the synthesis of aldosterone, ACE, and angiotensin II-type I receptor, resulting in the downstream reduction of BP (Shesely et al., 1996; Takemoto et al., 1997; Usui et al., 1998; Gambaryan et al., 2003). A recent report indicates that treatment with NaHS can also facilitate the release of NO and prevent cGMP breakdown by inactivating phosphodiesterase type 5, thereby maintaining the activation of NO-cGMP pathway (Coletta et al., 2012). In contrast, CSE inhibition inactivates the pathway and the downstream responses. Further evidence indicates that cGMP is downregulated in CSE knock-out mice and that the administration of NaHS results in its stimulation and the subsequent activation of protein kinase G (PKG), thus leading to vasodilation (Bucci et al., 2012). In addition, H2S can mediate nonclassical cGMP-independent activation of PKG via antioxidants to induce BP regulation (Stubbert et al., 2014). It has also been reported that PKG-inhibitor could not reduce GYY4137 (a slow H2S-releasing donor)-induced vasodilation of mouse aorta which suggests that the donor signals via PKG-independent pathway (Bucci et al., 2012). The data suggest that H2S regulates BP by stimulating both cGMP-dependent/independent and PKG-independent pathways.
Through its involvement in urine formation, the kidney actively participates in water regulation. Its role in water balance is supported by high expressions of water regulating proteins known as aquaporin (AQP) (Ishibashi et al., 2009). AQP-2 has been reported to be downregulated in animal models of kidney disorders including AKI and unilateral ureteral occlusion (UUO) (Suh et al., 2015; Wang et al., 2015). A recent animal study demonstrates that administration of H2S inhibitors, aminooxyacetic acid and PAG, can cause urinary concentration defects and reduce the expressions of AQP-2; however, treatment with GYY4137 and NaHS improves the urine concentration and upregulates AQP-2 proteins possibly via cAMP-dependent protein kinase signaling pathway (Luo et al., 2019). This implies that H2S plays a vital role in water balance and urine formation; however, further studies are needed to illuminate the mechanism involved.
Oxidative stress simply refers to the cellular redox imbalance in favor of oxidation which leads to the formation of radicals (Sies, 1997). Defects in antioxidant status result in higher levels of free oxygen radicals and superoxide dismutase, GSH peroxidase, and lipid peroxide have been identified as potential indicators for AKI (Dubey et al., 2000). These radicals cause DNA, protein, and lipid oxidations, thereby affecting their structure and functions. Evidence shows that the inhibition of CSE in mice models is critical for the production ROS and mitochondria dysfunction (Han et al., 2017). However, the elevation of H2S levels can restore the functions and reduce oxidative levels (Shan et al., 1990). One of the main mechanisms used to destroy unfolded and oxidized proteins is an adenosine triphosphate-independent 20S proteasome of the ubiquitin proteolytic pathway (Davies, 2001). It has been revealed that the inhibition of 20S proteasome promotes kidney malfunctioning, therefore exacerbating the progression of AKI and renal ischemia/reperfusion (I/R) (Huber et al., 2009; Zhang et al., 2020). However, NaHS could improve kidney condition by elevating the levels of proteasome subunit alpha type 6 and proteasome subunit beta type 7 (the key subunits of 20S proteasome), thereby reducing endoplasmic reticulum (ER) stress in rat models (Yi et al., 2018). In summary, H2S can regulate kidney activities via its interaction with ROS and 20S ubiquitin degradation pathway (Figure 4).
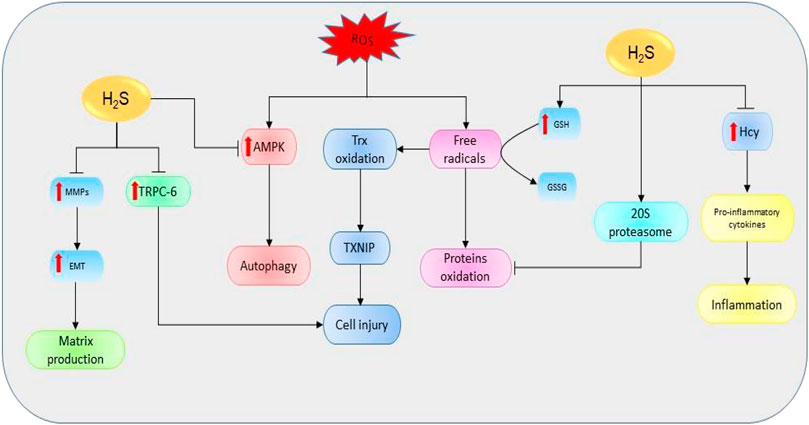
FIGURE 4. The diagrammatic illustration of the influence of H2S on the mechanisms involved in the initiation and development of renal diseases. H2S suppresses the elevation of Hcy resulting in the downregulation of proinflammatory cytokines (such as IL-1β, IL-6, and NF-қB) and the corresponding reduction of proinflammatory activities. Moreover, H2S increases the expressions of 20S proteasome which then reduces the oxidation of proteins by mediating proteolytic degradation of the oxidized proteins. Next, H2S can also stimulate the oxidation of GSH to GSSG resulting in the reduction of free oxygen radicals and subsequent suppression of protein oxidation. By reducing free oxygen radicals level, H2S also prevents the oxidations of Trx and its binding into TXNIP resulting in reduction of cell injury. Next, H2S prevents the ROS-mediated activation of AMPK pathway and its downstream effect on the induction of autophagy. Similarly, H2S reduces the expressions of MMPs and their resulting effects in EMT and matrix production. Also, H2S suppresses the levels of TRPC-6 leading to a reduction in cell injury. Alternatively, the decline in H2S levels could reverse the protective effects, resulting in the promotion of mechanisms favoring the development of kidney diseases (H2S, hydrogen sulfide; Hcy, homocysteine; GSH, glutathione; GSSG, oxidized glutathione; ROS, reactive oxygen species; TRPC-6, transient receptor potential cation channel, subfamily C, member 6; MMPs, matrix metalloproteinases; EMT, epithelial-mesenchymal transition; Trx, thioredoxin; TXNIP, Trx-interacting protein; AMPK, AMP-activated protein kinase).
Inflammation is one of the key players in kidney pathology. In patients with interstitial fibrosis and end-stage renal disease (ESRD), the plasma levels of inflammatory-mediated receptors and tumor necrosis factor receptor-1 and -2 are highly elevated, and the event is associated with the severity of the diseases (Niewczas et al., 2012; Sonoda et al., 2015). Moreover, the serum levels of several proinflammatory cytokines including interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) are also reported to be increased (Goldstein et al., 2006). With respect to H2S, the expression of CBS is reduced in renal tissues of patients with obstructive nephropathy compared to the normal ones (Yuan et al., 2017). Further analysis reveals that the knockdown of CBS in HK-2 human proximal tubular derived cells results in the elevation of proinflammatory markers while its overexpression shows opposite effects (Yuan et al., 2017). Moreover, both CSE and CBS negatively regulate Hcy levels via transsulfuration pathway; hence, their deficiency can cause the elevation of Hcy and the corresponding activation of proinflammatory responses leading to the progression of fibrosis (Chiku et al., 2009; Shastry and James, 2009; Yang et al., 2016). These results indicate that the reduction of the deficiency of H2S-producing enzymes in kidney diseases corresponds to the accumulation of Hcy and associated inflammatory features; thence, its supplementation could be useful in treating these diseases.
Autophagy is an important process associated with the destruction of unwanted cellular components and nutrients recycling. It plays a key role in protecting renal cells from apoptosis and associated loss of functions (Jiang et al., 2012). The deletion of autophagy-related gene 5 in mice results in the accumulation of oxidized and unfolded proteins, proteinuria, and subsequently podocyte loss (Hartleben et al., 2010). Besides, autophagy plays a crucial role in maintaining autosomal biogenesis and kidney functions and its loss could lead to the promotion of kidney damage (Maejima et al., 2013). However, further evidence suggests that excessive autophagy can stimulate apoptotic activities, leading to renal tubular atrophy (Chen et al., 2018). In UUO-induced mice, CBS and CSE levels have been shown to be reduced; meanwhile, the levels of autophagy and apoptotic markers are considerably elevated (Li et al., 2010). Treatment with exogenous H2S could protect the kidneys from ROS-induced autophagy and renal damage by inhibiting the ROS-AMPK pathway (Chen et al., 2018). In brief, the data above suggest that the decline in H2S levels in renal diseases can enhance autophagy activities through the elevation of ROS levels, resulting in disease progression; however, supplementation of H2S can rescue the condition.
Extracellular matrix (ECM) accumulation drives the loss of renal function and progression of renal damage. The activation of transforming growth factor-β1 (TGF-β1) is highly associated with the advancement of kidney fibrosis through its influence on the deposition of ECM (Yamamoto et al., 1994). Mechanistically, TGF-β1 increases the expressions of matrix metalloproteinases (MMPs) including MMP-2 and MMP-9, resulting in the promotion of epithelial-mesenchymal transition (EMT) and associated profibrotic activities (Turck et al., 1996; Strutz et al., 2002; Zhao et al., 2013). It has been suggested that the downregulation of CSE and CBS can induce matrix remodeling by negatively regulating the expressions of MMP-9, connexins, and N-methyl D-aspartate receptors in mice (Kundu et al., 2013). However, the effect could be ameliorated with NaHS treatment (Kundu et al., 2015). Furthermore, the treatment of diabetic mice model with tadalafil (a phosphodiesterase 5 inhibitor and H2S donor) reduces matrix production in podocytes by regulating NO-H2S-AMPK-mTORC1 signaling cascades (Lee et al., 2015). Treatment with GYY4137 has also been indicated to stabilize the levels of MMP-9, MMP-13, and MMP-14 and reduce ROS-induced kidney fibrosis by elevating the level of microRNA-194 (John et al., 2017). In summary, the decline in H2S levels facilitates the production of a matrix by regulating the associated pathways; however, the upregulation of H2S can prevent the event and improve kidney functions.
Cell injury affects the tissue repair process by inducing further infiltrations and loss of function. One of the main mediators of cell injury is hypoxia-induced mitochondria dysfunction (Zhang et al., 2007; Su et al., 2013; Zhu et al., 2014). Hypoxia-inducible factors (HIFs) play essential roles in kidney cell repair by reducing apoptosis, promoting cell proliferation, and facilitating the release of specific genes associated with tissue repair (Zhang et al., 2003). Although primary injuries can be well repaired, the defects in the repair mechanism can augment the condition, thus resulting in tissue and organ injury. For instance, HIF activation can prevent the degeneration of tissue by improving oxygen balance and promoting cell repair (Bernhardt et al., 2006); however, its prolonged stimulation can result in the progression of tissue injury by increasing ECM accumulation and inflammatory responses (Haase, 2012; Conde et al., 2017). The injuries are the result of high apoptosis, podocyte loss, accumulation of ECM, and inflammation. Besides, another study shows that immortalized proximal tubule cells release kidney injury molecule-1 which stimulates the secretion of monocyte chemoattractant protein-1 and its macrophage-dependent activities thereby promoting kidney fibrosis (Humphreys et al., 2013). A previous report indicates that the downregulation of CSE enhances Hcy-mediated podocyte injury by regulating Wnt pathway (Liu et al., 2015). Moreover, the inhibition of CSE promotes cell injury by facilitating the oxidation of thioredoxin (Trx) and its binding into Trx-interacting protein, resulting in the inhibition of apoptosis signal-regulating kinase 1 and its effect on P38 mitogen-activated protein kinase (MAPK) pathway; however, treatment with NaHS could reverse the effects (Mao et al., 2019). It has also been shown that the treatment of diabetic kidney disease mice model with taurine (a H2S-releasing sulfur-containing amino acids) significantly elevates the CSE level, thereby increasing H2S production and downregulating the protein levels of podocyte homeostasis regulator, transient receptor potential cation channel, subfamily C, member 6, and consequently the reduction of glucose-induced podocytes injuries (Zhang et al., 2020). Taken together, the above information implies that the downregulation of H2S-producing enzymes in kidney diseases could cause cell injury; however, the problem can be rescued with supplementation of H2S.
AKI is the condition caused by the drastic rise in urine levels of creatinine and urea as a result of decreased GFR (Hilton, 2006). The causes include infections, sepsis, liver failure, heart failure, cancer, kidney stones, I/R, glomerulonephritis, and medications. Oxidative stress and inflammation have been indicated as the main targets for H2S in kidney injury treatment induced by sepsis and renal I/R (Bos et al., 2009; Chiku et al., 2009; Wu et al., 2017; Qiu et al., 2018). Sepsis-associated AKI patients have high levels of creatinine, urea nitrogen, IL-1β, TNF-α, myeloperoxidase, and malondialdehyde concentrations, and these features are significantly associated with low H2S levels (Chen et al., 2018). In addition, treatment with NaHS effectively attenuates inflammation and oxidative activities by regulating Toll-like receptor 4/NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) pathway in lipopolysaccharide-induced AKI mice model (Chen et al., 2018) (Figure 5). Considering the above findings, regardless of the causes, the protective effect of H2S donors in AKI is conserved, which confirms the key role played by H2S in kidney injury and its therapeutic potential.
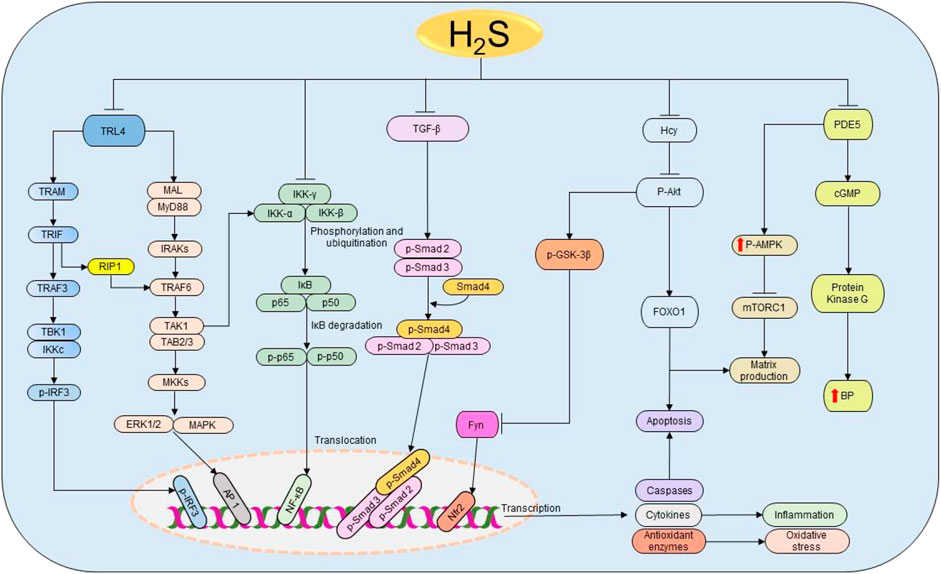
FIGURE 5. The cellular pathways regulated by H2S in renal diseases. Starting from the right-hand side, H2S suppresses the expressions of PDE5, thereby enhancing the production of NO and the activation of cGMP and PKG resulting in the subsequent reduction of BP. Simultaneously, PDE5 inhibition facilitates the phosphorylation of AMPK, resulting in the inactivation of mTORC1 and the corresponding reduction of matrix production. Next, H2S reduces Hcy levels, thereby inducing the dephosphorylation of Akt and the downstream activation of FOXO 1 signaling pathway, leading to the reduction of apoptosis and matrix production. The deactivation of Akt also triggers the activation of GSK3β and inhibition of Fyn leading to the elevation on Nfr2 and consequent rise in the antioxidant status. Similarly, H2S attenuates the activation of TGF-β and its downstream effectors Smad2/3 resulting in the promotion of anti-inflammatory activities. Moreover, H2S prevents the phosphorylation and ubiquitination of IKK complex, thereby facilitating the inhibition of NF-κB and subsequently reducing inflammation. H2S also inhibits TRL4-induced activation of ERK1/2, p38 MAPK, and NLRP3 pathways resulting in the suppression of apoptosis, oxidative stress, and inflammation. H2S, hydrogen sulfide; PDE5, phosphodiesterase type 5; cGMP, cyclic guanosine monophosphate; BP, blood pressure; p-AMPK, phosphorylated AMP-activated protein kinase; mTORC1, the mammalian target of rapamycin complex 1; FOXO1, forkhead box protein O1; Akt, protein kinase B; GSK3β, glycogen synthase kinase β; TGF-ꞵ1, transforming growth factor-beta 1; Smad 2/3/4, mothers against decapentaplegic homolog 2/3/4; IKK, IκB kinase; IκB, inhibitor of nuclear factor kappa B; p50 and p65, nuclear factor kappa B subunits; NF-κB, nuclear factor kappa B; TRL4, Toll-like receptor 4; MyD88, myeloid differentiation primary response 88; MAL, MyD88 adapter-like proteins; IRAK, interleukin-1 receptor-associated kinases; TRAF, tumor necrosis factor receptor-associated factor; TAK1, TGF-α activating kinase 1; TAB 2/3, TAK 1 binding protein 2/3; MKK, MAPK kinases; TRAM, translocating chain-associating membrane protein; TRIF, TIR-domain-containing adapter-inducing interferon β; RIP1, receptor-interacting serine/threonine-protein kinase 1; TBK1, TANK-binding kinase 1; IRF3, interferon regulatory factor 3; ERK1/2, extracellular signal-regulated kinase 1/2; MAPK, mitogen-activated protein kinase; AP-1, activator protein 1; NLPR3, NOD-, LRR-, and pyrin domain-containing protein 3.
CKD is one of the common health diseases with more than 675 million cases and 1.2 million deaths recorded in 2017 (Bikbov et al., 2020). The term CKD covers all five stages of the gradual loss of kidney functions. The higher stages (3–5) are usually referred to as chronic renal failure (CRF) (Padmanabhan et al., 2017). Based on the estimated glomerulus filtration rate (eGFR), CKD stages are classified as follows: eGFR, stage 5 (eGFR ≤14), stage 4 (15 ≤ eGFR ≤29), stage 3 (30 ≤ eGFR ≤59), stage 2 (60 ≤ eGFR ≤89), and stage 1 (eGFR ≥90) (Yassine et al., 2016). CKD mainly results from mild-to-severe range of prerenal, intrinsic renal, and/or postrenal processed diseases. Reduced level of H2S has been reported in both CKD patients and experimental models (Aminzadeh and Vaziri, 2011; Kuang et al., 2018). In 5/6 nephrectomy mice model, NaHS could effectively improve kidney functions by promoting antioxidant, antiapoptotic, and anti-inflammatory responses via the activation and deactivation of nuclear factor erythroid 2-related factor 2 (Nfr2) and mTOR pathways, respectively (Shirazi et al., 2019). Moreover, treatment with 30 µM NaHS for 8 weeks significantly decreases the expressions of cleaved caspase-3, p-NF-κB, and urine concentrations of neutrophil gelatinase-associated lipocalin in 5/6 nephrectomy mice (Askari et al., 2018). A recent study indicates that NaHS mitigates the protein levels of proinflammatory and proapoptotic markers in adenine-induced CRF in mice model by regulating MAPK and NF-κB pathways (Wu et al., 2017). Collectively, low level of H2S contributes to CKD progression by promoting apoptosis, autophagy, inflammation, and oxidative stress, and increasing the level of H2S could prevent these events, suggesting that H2S may serve as a new treatment option for CKD.
Renal fibrosis is regarded as the hallmark for all progressive CKD (Zhou and Liu, 2016). The causes for the disease include diabetes, obstructive urinary, glomerulonephritis, glomerulosclerosis, hypertrophy, and mutation (Nogueira et al., 2017). Several factors such as autophagy, inflammation, accumulation of collagen, oxidative stress, and apoptosis are known to play roles in the progression of the disease. H2S treatment reverses the streptozotocin (STZ)-induced accumulation of collagen II, tissue inhibitor of metalloproteinases 2, kidney hydroxyproline, and downregulation of connexins and MMP1/2 in mice (Zeng et al., 2016). Furthermore, NaHS reduces the elevated apoptotic and inflammatory activities in STZ diabetic rats by regulating TGF-β1 and ERK1/2 signaling pathways (Li et al., 2017). A recent study shows that NaHS administration in diabetic mice restores the levels of serum creatinine, blood urea nitrogen, and inflammatory cytokines as well as inhibiting the activation of TGF-β1/Smad 3 pathway (Jia et al., 2018). The data indicate a potential link between H2S and ECM remodeling activities, through which it suppresses renal fibrosis.
Obstructive uropathy is a renal disorder characterized by the disruption of the normal tubular fluid/urine flow from the kidney to the bladder as a result of structural/functional defects in the urinary tract (Klahr, 1991; Klahr, 2000). UUO is the common form of obstructive uropathy and an outstanding prototype for investigating tubulointerstitial fibrosis and obstructive nephropathy leading to CKD (Vaughan Jr et al., 2004). High inflammatory response and interstitial macrophage infiltration are associated with the induction of UUO (Silverstein et al., 2003). Growing evidence indicates that UUO diminishes the production of H2S and the expressions of CSE, CBS, and 3-MPST proteins (Han et al., 2017; Chen Q. et al., 2018; Chen Y. et al., 2018; Zhou et al., 2020). The deletion of CSE gene intensifies UUO-induced kidney fibrosis by increasing the interstitial collagen deposition, microphages infiltration, proinflammatory cytokine TNF-α and neutrophil marker Ly6G, mitochondrial damage, oxidative stress, and apoptosis (Han et al., 2017). However, the administration of H2S donors could restore UUO-induced alterations of the renal functions (Jiang et al., 2014). For instance, NaHS reverses the UUO-induced changes by suppressing the corresponding pathways, namely, NLRP3 (Chen et al., 2018), TGF-β1 (Jung et al., 2013; Song et al., 2014), and ROS-AMPK signaling cascades (Zhou et al., 2020). Likewise, GYY4137 can significantly reduce renal fibrosis, inflammation, and apoptosis in UUO-induced male Lewis rats by reducing the levels of EMT markers and inhibiting TGF-β1/Smad and MAPK pathways (Lin et al., 2018). In summary, the induction of UUO decreases the production of H2S and the inhibition of H2S-producing genes in UUO model could exacerbate the condition. However, the situation can be intervened and restored with the administration of H2S donors. Therefore, H2S donors may serve as a novel therapeutic option for the treatment of renal diseases associated with UUO.
Focal segmental glomerulosclerosis (FSGS) is the glomerular disease caused by the formation of scars in the glomeruli and podocyte loss (Wharram et al., 2005; Woroniecki and Kopp, 2007). In an attempt to compensate for the loss, the remaining podocytes undergo hypertrophy (Wiggins et al., 2005); however, the insufficiency of this process to fulfill the increased demand of the glomerulus volume leads to the progression of FSGS (Kriz, 2012). Glomerular extracts from FSGS patients’ biopsy show elevated levels of mTOR and parietal epithelial cells (PEC)-activation associated genes (Puelles et al., 2019). In animal models, prolonged activation of mTOR pathway is positively correlated with PEC activation and FSGS progression (Zschiedrich et al., 2017; Puelles et al., 2019). Furthermore, the complete inhibition of the pathway aggravates the condition; meanwhile, partial inhibition restores glomerulus integrity and maintains podocyte hypertrophy (Zschiedrich et al., 2017). Treatment of hyperhomocysteinemia (HHcy)-induced mice with NaHS prevents glomerulosclerosis by improving GFR and reducing macrophage infiltration and proinflammatory activities (Sen et al., 2009; Sen et al., 2010). Another study indicates that NaHS can alleviate glomerulosclerosis symptoms in aging mice mainly by reducing urinary albumin, serum cystatin c, proinflammatories, and renal cortical levels of laminin γ1 (a matrix protein involved in glomerulosclerosis) and by activating AMPK pathway (Lee et al., 2018). In summary, H2S supplementation decreases matrix deposition in glomerulosclerosis by regulating AMPK-mTOR signaling cascades; however, further studies are needed to elucidate the mechanism involved.
HHcy refers to the elevated plasma level of Hcy usually above 15 µM (Seshadri et al., 2002). High level of HHcy is considered as a risk factor for various diseases including end-stage renal disease and neurodegenerative disorders (Seshadri et al., 2002; Nair et al., 2005). HHcy mice show reduced levels of CSE and CBS; conversely increasing H2S level decreases Hcy levels (Sen et al., 2010). HHcy promotes the dephosphorylation of Akt and FOXO1 signaling cascades, resulting in the subsequent activation of FOXO1 (Zhang et al., 2005), a vital pathway involved in apoptosis, oxidative stress, and inflammation (Ponugoti et al., 2013; Shi et al., 2018). Treatment with GYY4137 downregulates proapoptotic caspases, MMPs, collagens, and fibronectin one in HHcy-treated mouse mesangial cells through the regulation of Akt-FOXO1 pathways (Majumder et al., 2019). Similarly, GYY4137 reduces the expressions of caveolin 1 and connexins and increases the activities of endothelial NO synthase (eNOS) and tissue inhibitors of metalloproteinases (TIMPs) in HHcy mice (John and Sen, 2019). Furthermore, the treatment of HHcy mice with 30 µM NaHS protects renal tissues from HHcy-induced inflammation and ROS by restoring MMPs/TIMPs and oxidized GSH/GSH status (Sen et al., 2009). NaHS also reduces the homocysteinylation of eNOS and stabilizes systolic BP, GFR, and connexins levels in HHcy mice (Pushpakumar et al., 2019). Based on the above information, there is a strong link between H2S and Hcy levels. Therefore, H2S supplementation could be a potential therapeutic option for treating HHcy-associated diseases.
Diabetic nephropathy (DN) is a condition characterized by the presence of abnormal levels of protein in urine (proteinuria) (Østerby et al., 1993; Parving, 2001). DN is considered as an independent risk factor for ESRD (Ruggenenti et al., 1998). Reduced levels of H2S have been extensively documented in DN patients and mouse model (Yamamoto et al., 2013; Li et al., 2014). A recent study indicates that treatment with NaHS restores behavioral and biochemical changes in STZ-induced diabetic mice, while pretreatment with PAG (a CSE inhibitor) prevents the protective effects of NaHS (Kaur et al., 2015), which confirms the crucial role of H2S in the development and progression of DN. In addition, NaHS improves the GFR and blood flow by reducing angiotensin II level and regulating NF-κB, MAPK, and Nfr2 pathways (Zhou et al., 2014). Similarly, treatment with S-propargyl-cysteine improves inflammatory responses, oxidant/antioxidant status by inhibiting the activation of TGF β1/Smad3 and the phosphorylation of ERK, p38, and signal transducers and activators of transduction-3 signaling cascades in STZ-induced diabetic mice (Qian et al., 2016).
The overexpression of energy-sensing protein sirtuin 1 (SIRT1) impedes the progression of DN and reduces podocyte loss and oxidative stress in DN mice (Hong et al., 2018). SIRT1 regulates cellular activities by deacetylating several transcription factors including p53, p65, and FOXO (Solomon et al., 2006; Hariharan et al., 2010; Yang et al., 2012). Administration of 100 μmol/kg/day NaHS protects renal cells from diabetes mellitus-induced damage by upregulating SIRT1 (Ahmed et al., 2019). Overall, H2S donors can prevent the progression of DN by regulating the associated cellular pathways, indicating that these donors could be a potential option for the treatment of DN.
The pathological effects of drugs are the main cause of hospital-acquired AKI (Kaufman et al., 1991). Some of these drugs include acetaminophen (APAP), aspirin, cisplatin, and indinavir (Naughton, 2008). Cisplatin, an anticancer drug, induces nephrotoxicity notably through the reaction involving platinum and thiol protein groups (Levi et al., 1980). One of the most investigated reactions is that of cisplatin and GSH (Dedon and Borch, 1987; Jansen et al., 2002). H2S protects the kidney from cisplatin-induced nephrotoxicity by increasing GSH level and regulating key cellular activities such as oxidative stress, inflammation, and apoptosis (Dwivedi et al., 1996; Chiarandini et al., 2008; Ahangarpour et al., 2014; Liu et al., 2016). A previous study indicates that pretreatment with 21 mg/kg/day GYY4137 for 3 days followed by the administration of cisplatin aggravates the renovascular damage by promoting apoptotic, oxidative stress, and inflammatory activities (Liu et al., 2016). Despite the problem observed in the methodology of this study, pretreatment with GYY4137 could not prevent the cytotoxicity of cisplatin. Alternatively, it has been reported that treatment with GYY4137 and NaHS exert antitoxicity effects through the reduction of ROS and the inhibition of nicotinamide adenine dinucleotide phosphate and MAPK activities in cisplatin-administered porcine renal proximal tubule cell line LLC-PK1 (Cao et al., 2018). NaHS can also upregulate the expression of nephrin and reduce the level of desmin (Karimi and Absalan, 2017). In addition, treatment with diallyl disulfide (DADS) alleviates nephrotoxic effects of cisplatin by increasing antioxidant defense and suppressing apoptotic, oxidative, and inflammatory activities (Elkhoely and Kamel, 2018). Collectively, treatment with H2S donors induces anti-inflammatory, antiapoptotic, and antioxidant activities, thereby reducing the toxicity of cisplatin.
APAP/paracetamol-induced nephrotoxicity is amongst the least causatives of renal injury (Hiragi et al., 2018). The overdose of paracetamol is associated with elevated serum levels of creatinine and urea, oxidative stress (Canayakin et al., 2016), apoptosis, and DNA damage (Lorz et al., 2004). Cytochrome P450 2E1 (CYP2E1) plays a vital role in the bioactivation of APAP (Lee et al., 1996). Treatment with DADS inhibits the APAP-induced increase in oxidative stress, apoptosis, and inflammation in rats by downregulating the expressions of NF-қB, TNF-α, and cyclooxygenase-2 (Cox-2) in the kidney and the level of CYP2E1 in the liver and kidney (Ko et al., 2017). It has been shown that treatment with 50 μM/kg NaHS improves glomerular structures and functions by suppressing apoptosis and promoting anti-inflammatory and antioxidant activities in APAP-treated mice (Ozatik et al., 2019). These data suggest that H2S donors can prevent the bioactivation of APAP together with the associated apoptotic, inflammatory, and oxidative activities by regulating their respective pathways (Figure 6).
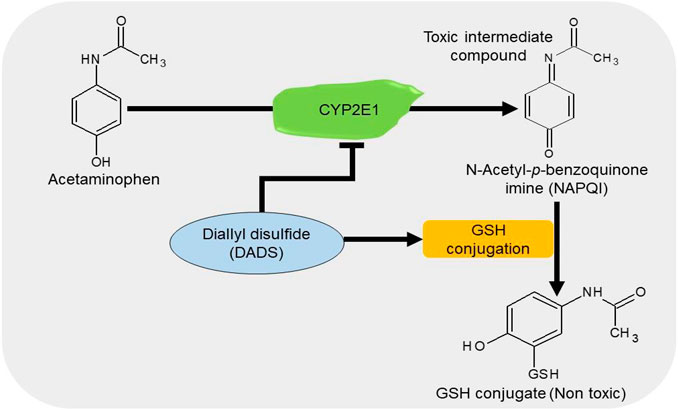
FIGURE 6. The diagrammatic presentation of the inhibitory effect of H2S donors on cytochrome p450 2E1 (CYP2E1). H2S donors increase the GSH levels and suppress the expression of CYP2E1, an enzyme responsible for the metabolism of APAP. By reducing the enzyme, H2S donors prevent the formation of a toxic intermediate metabolite NAPQI and by increasing GSH level, it facilitates the conjugation of NAPQI to form a nontoxic metabolite. H2S, hydrogen sulfide; CYP2E1, cytochrome p450 2E1; GSH, glutathione; APAP, acetaminophen; NAPQI, N-acetyl-p-benzoquinone imine.
Gentamicin is a common antibiotic for the treatment of Gram-negative bacteria infections (Becker and Cooper, 2013). However, high risk of nephrotoxicity has been associated with the drug (Morin et al., 1980; Weir and Mazumdar, 1994). H2S protects the kidney from gentamicin-induced renal failure by regulating oxidative stress and inflammatory cytokines (Pedraza-Chaverrı́ et al., 2003; Pedraza-Chaverrí et al., 2003; Kalayarasan et al., 2009). It has been shown that treatment with NaHS could significantly reduce serum urea and creatinine levels, as well as improving glomerulus morphology in gentamicin-treated mice (Otunctemur et al., 2014). In gentamicin-treated rats, administration of 50 mg/kg/day DADS for 4 days induces renoprotective effects by promoting antioxidant and anti-inflammatory activities (Pedraza-Chaverrı́ et al., 2003). Correspondingly, treatment with 150 mg/kg/day diallyl sulfide for 6 days improves renal functions by downregulating NF-κB, inducible NO synthase (iNOS), and TNF-α and activating Nfr2 pathway (Kalayarasan et al., 2009). In summary, the antioxidant and anti-inflammatory effects observed following the inhibition or elevation of H2S levels play a key role in reducing the toxicity induced by gentamicin (Figure 7). However, more studies are needed to investigate the underlying mechanisms.
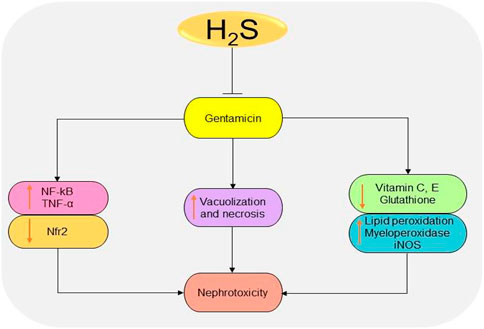
FIGURE 7. The diagrammatic illustration of the inhibitory effects of H2S donors on gentamicin-induced nephrotoxicity. H2S donors prevent gentamicin-induced oxidative stress, inflammation, and necrosis by reducing superoxide formation, lipid peroxidation, iNOS, NF-κB, TNF-α, and malondialdehyde levels. H2S, hydrogen sulfide; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor kappa B; TNF-α, tumor necrosis factor-alpha; ROS, reactive oxygen species; Nfr2, nuclear factor erythroid 2-related factor 2.
The accumulation of heavy metals such as lead, uranium (U), cadmium (Cd), mercury, zinc, and arsenic (As) has been suggested to induce toxicity in the kidney especially the nephron (Diamond and Zalups, 1998). H2S treatment attenuates U-induced ROS production, inflammation, ER stress, and caspase-dependent apoptosis (Yi et al., 2018). In the U-intoxicated rat model, NaHS alleviates the inflammatory and oxidative responses by inhibiting the activation of NF-қB and downstream levels of TNF-α, iNOS, and Cox-2 and promoting the activation of Nfr2 (Zheng et al., 2015). In addition, NaHS reduces the ER stress-mediated activation of apoptotic pathways by regulating Akt/glycogen synthase kinase-3β/Fyn/Nfr2 pathways in U-induced rats (Yi et al., 2018). Furthermore, diallyl trisulfide (DATS) can prevent As-induced elevation of apoptotic, inflammatory, and ROS activities in rats by activating Akt and Nfr2 antioxidant response element pathways (Miltonprabu et al., 2017). Similarly, diallyl tetrasulfide (DTS) significantly decreases the accumulation of Cd and the associated biochemical changes (Pari and Murugavel, 2005). The renoprotective effects of DTS are associated with metal chelating and antioxidant properties (Pari et al., 2007). Collectively, H2S donors can restore the reduced H2S levels and regulate key cellular activities altered by the toxicity of heavy metals.
Urolithiasis is the condition originated from the kidney, characterized by the accumulation of hard deposits of mineral crystals or salts usually made up of calcium salts, uric acids, and cysteine (Spivacow et al., 2016). Kidney stone results from the collection of calcium oxalate (CaOx), is termed calcium oxalate urolithiasis, and is one of the most common types of urolithiasis in human (Gisselman et al., 2009). Two forms of CaOx, namely, calcium oxalate monohydrate (COM) and calcium oxalate dihydrate (COD), are known to participate in body physiology and pathology, with COM being the nonpathogenic form and COD the pathogenic form (Manissorn et al., 2017). In addition, low pH favors COM formation, whereas the vice versa favors COD. A previous study shows that treatment with H2S donors could significantly reduce calcium oxalate stones agglomeration by adjusting the pH and facilitating calcium complex formations (Vaitheeswari et al., 2015). Treatment with 1.75 and 3.5 mM NaHS and Na2S2O3 could show an inhibition of 30–40% and 27–45%, respectively, following the analysis of calcium oxalate urolithiasis crystals from patients’ urine. In a CaOx rat model, administration of allicin, DADS, and DATS can prevent the accumulation of CaOx crystals and increase the expression of connexin 43 (Cx43) and the activities of gap junction, the effects which can be associated with CaOx stone progression (Lai et al., 2019). These data suggest that H2S donors possess potential in the treatment of kidney stones by regulating Cx43 level, pH, and the function of gap junction.
Several clinical trials have been conducted to check the potential of H2S donors in patients. It has been demonstrated that treatment with H2S donor SG-1002 significantly increases the blood levels of H2S and brain natriuretic peptide and NO bioavailability in heart failure patients (Polhemus et al., 2015). Moreover, treatment with sulfhydryl-containing ACE inhibitor, zofenopril, induces vasculoprotective effects in essential hypertensive patients by reducing the oxidation of low-density lipids, atherosclerosis, and stabilizing NO signaling pathway (Napoli et al., 2004). In mild-to-moderate hypertension patients, the treatment with 30 mg/day zofenopril induces much reduction in BP and less severe adverse events compared to the treatment with 20 mg/day enalapril (Mallion, 2007). The drug also shows similar effects on other antihypertensive drugs such as candesartan (Leonetti et al., 2006), irbesartan (Malacco et al., 2015), and hydrochlorothiazide (Lacourciere and Provencher, 1989). Besides, the administration of the drug in combination with hydrochlorothiazide exhibits stronger effects in BP regulation compared to individual drugs alone (Malacco and Omboni, 2007). It has also been shown that tadalafil can effectively improve kidney functions and quality of life in patients with ESRD who undergoes hemodialysis (Bolat et al., 2017). Administration of ATB-346 in healthy individuals shows less adverse events and no hematological, cardiovascular, or renal effects in phase 1 clinical trials for the drug (Wallace, 2015). The drug is also confirmed in phase 2 clinical trial to induce anti-inflammatory responses and inhibits cyclooxygenase enzyme more efficiently than a nonsteroidal anti-inflammatory drug, naproxen with less gastrointestinal toxicity (Wallace et al., 2020). Despite the availability of a few clinical studies, H2S-containing drugs have indicated promising potential in the treatment of kidney diseases and the reduction of inflammatory responses.
H2S is a ubiquitous gasotransmitter that plays an important role in cell signaling and is associated with the development and progression of numerous diseases. Several studies have revealed low levels of CBS, CSE, and 3-MPST in various renal diseases and demonstrated that H2S donor treatments can effectively improve the disease states. H2S donors generally restore normal renal functions by regulating inflammatory, apoptotic, oxidative stress, and autophagy pathways. Herein, we summarize the effects of H2S-releasing compounds on common renal diseases in animal models (Table 1). Improved therapeutic outcomes have been demonstrated in CKD. However, there is a deficiency of drug toxicity data on whether the treatment with H2S donors can impair kidney functions especially when they are used for a long time in treating CKD. The mechanisms of action of H2S donors in common renal diseases need to be further investigated. In addition, the respective effects of underproduction or overproduction as a result of the treatment with H2S inhibitors/donors need to be determined. Consistent with the summarized available data, we propose that reduced level of H2S in blood can be considered as a diagnostic tool or disease severity indicator for renal diseases after setting limit for H2S in normal and disease state. Regardless, considering that H2S is a promising option in treating normal renal diseases, further studies are needed to test the hypothesis at the clinical level. Furthermore, novel H2S donors can be designed and applied in the treatment of common renal diseases.
Conceptualization: EN, MS, AA, DW, SD, XJ; data curation: EN, MS, AA, NK, SK, XZ, TL; funding acquisition: XJ, DW; writing-original draft: EN, NK, SK; visualization and supervision: MS, AA, SD, DW, XJ; writing-review and editing: EN, MS, AA, DW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81802718, 81670088, U1504817), the Foundation of Science & Technology Department of Henan Province, China (Nos. 202102310480, 182102310335, 192102310151), the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038), and the Science Foundation for Young Talents of Henan University College of Medicine, China (No. 2019013).
Ahangarpour, A., Fard, A. A., Gharibnaseri, M. K., Jalali, T., and Rashidi, I. (2014). Hydrogen sulfide ameliorates the kidney dysfunction and damage in cisplatin-induced nephrotoxicity in rat. Vet. Res. Forum. 5, 121–127.
Ahmed, H. H., Taha, F. M., Omar, H. S., Elwi, H. M., and Abdelnasser, M. (2019). Hydrogen sulfide modulates SIRT1 and suppresses oxidative stress in diabetic nephropathy. Mol. Cell. Biochem. 457, 1–9. doi: 10.1007/s11010-019-03506-x
Akbari, M., Sogutdelen, E., Juriasingani, S., and Sener, A. (2019). Hydrogen sulfide: emerging role in bladder, kidney, and prostate malignancies. Oxid. Med. Cell. Longev. 2019, 2360945. doi: 10.1155/2019/2360945
Aminzadeh, M. A., and Vaziri, N. D. (2011). Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol. Dial. Transplant. 27 (2), 498–504. doi: 10.1093/ndt/gfr560
Askari, H., Seifi, B., Kadkhodaee, M., Sanadgol, N., Elshiekh, M., Ranjbaran, M., and Ahghari, P. (2018). Protective effects of hydrogen sulfide on chronic kidney disease by reducing oxidative stress, inflammation and apoptosis. EXCLI J. 17, 14. doi: 10.17179/excli2017-711
Baker, D. H., and Czarnecki-Maulden, G. L. (1987). Pharmacologic role of cysteine in ameliorating or exacerbating mineral toxicities. J. Nutr. 117, 1003–1010. doi: 10.1093/jn/117.6.1003
Banerjee, R., Chiku, T., Kabil, O., Libiad, M., Motl, N., and Yadav, P. K. (2015). “Assay methods for H2S biogenesis and catabolism enzymes,” in Methods in enzymology.(Academic Press), Vol. 554, 189–200. doi: 10.1016/bs.mie.2014.11.016
Beatty, P. W., and Reed, D. J. (1980). Involvement of the cystathionine pathway in the biosynthesis of glutathione by isolated rat hepatocytes. Arch. Biochem. Biophys. 204, 80–87. doi: 10.1016/0003-9861(80)90009-0
Becker, B., and Cooper, M. A. (2013). Aminoglycoside antibiotics in the 21st century. ACS Chem. Biol. 8, 105–115. doi: 10.1021/cb3005116
Bernhardt, W. M., Câmpean, V., Kany, S., Jürgensen, J.-S., Weidemann, A., Warnecke, C., et al. (2006). Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J. Am. Soc. Nephrol. 17, 1970–1978. doi: 10.1681/asn.2005121302
Bikbov, B., Purcell, C. A., Levey, A. S., Smith, M., Abdoli, A., Abebe, M., et al. (2020). Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395, 709–733.
Bolat, M. S., Özer, İ., Cinar, O., Akdeniz, E., and Aşcı, R. (2017). The efficacy of low-dose tadalafil in patients undergoing hemodialysis with end-stage renal disease. Ren. Fail. 39, 582–587. doi: 10.1080/0886022x.2017.1349678
Bos, E. M., Leuvenink, H. G. D., Snijder, P. M., Kloosterhuis, N. J., Hillebrands, J.-L., Leemans, J. C., et al. (2009). Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 20, 1901–1905. doi: 10.1681/asn.2008121269
Bos, E. M., Wang, R., Snijder, P. M., Boersema, M., Damman, J., Fu, M., et al. (2013). Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 24, 759–770. doi: 10.1681/asn.2012030268
Braunstein, A. E., Goryachenkova, E. V., Tolosa, E. A., Willhardt, I. H., and Yefremova, L. L. (1971). Specificity and some other properties of liver serine sulphhydrase: evidence for its identity with cystathionine β-synthase. Biochim. Biophys. Acta Enzymol. 242, 247–260. doi: 10.1016/0005-2744(71)90105-7
Bucci, M., Papapetropoulos, A., Vellecco, V., Zhou, Z., Zaid, A., Giannogonas, P., et al. (2012). cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS One 7, e53319. doi: 10.1371/journal.pone.0053319
Canayakin, D., Bayir, Y., Kilic Baygutalp, N., Sezen Karaoglan, E., Atmaca, H. T., and Kocak Ozgeris, F. B., et al. (2016). Paracetamol-induced nephrotoxicity and oxidative stress in rats: the protective role of Nigella sativa. Pharmaceut. Biol. 54, 2082–2091. doi: 10.3109/13880209.2016.1145701
Cao, L., Cao, X., Zhou, Y., Nagpure, B. V., Wu, Z.-Y., et al. (2018). Hydrogen sulfide inhibits ATP-induced neuroinflammation and Aβ1-42 synthesis by suppressing the activation of STAT3 and cathepsin S Brain Behav. Immun. 73, 603–614. doi: 10.1016/j.bbi.2018.07.005
Cao, X., Xiong, S., Zhou, Y., Wu, Z., Ding, L., Zhu, Y., et al. (2018). Renal protective effect of hydrogen sulfide in cisplatin-induced nephrotoxicity. Antioxid. Redox Signal. 29, 455–470. doi: 10.1089/ars.2017.7157
Chen, Q., Yu, S., Zhang, K., Zhang, Z., Li, C., and Gao, B., et al. (2018). Exogenous H2S inhibits autophagy in unilateral ureteral obstruction mouse renal tubule cells by regulating the ROS-AMPK signaling pathway. Cell. Physiol. Biochem. 49, 2200–2213. doi: 10.1159/000493824
Chen, X., Jhee, K.-H., and Kruger, W. D. (2004). Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 279, 52082–52086. doi: 10.1074/jbc.c400481200
Chen, Y., Jin, S., Teng, X., Hu, Z., Zhang, Z., Qiu, X., et al. (2018). Hydrogen sulfide attenuates LPS-induced acute kidney injury by inhibiting inflammation and oxidative stress. Oxid. Med. Cell Longev. 2018, 1–10. doi: 10.1155/2018/6717212
Cheung, S. H., and Lau, J. Y. W. (2018). Hydrogen sulfide mediates athero-protection against oxidative stress via S-sulfhydration. PLoS One. 13, e0194176. doi: 10.1371/journal.pone.0194176
Chiarandini, J. P. F, de Ferreyra, E. C., and Castro, J. A. (2008). Diallyl disulfide prevention of cis-diamminedichloroplatinum-induced nephrotoxicity and leukopenia in rats: potential adjuvant effects. Nutr. Cancer. 60, 784–791. doi: 10.1080/01635580802100869
Chiku, T., Padovani, D., Zhu, W., Singh, S., Vitvitsky, V., and Banerjee, R. (2009). H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612. doi: 10.1074/jbc.m808026200
Coletta, C., Papapetropoulos, A., Erdelyi, K., Olah, G., Módis, K., Panopoulos, P., et al. (2012). Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. U.S.A. 109, 9161–9166. doi: 10.1073/pnas.1202916109
Conde, E., Giménez-Moyano, S., Martín-Gómez, L., Rodríguez, M., Ramos, M. E., Aguado-Fraile, E., et al. (2017). HIF-1α induction during reperfusion avoids maladaptive repair after renal ischemia/reperfusion involving miR127-3p. Sci. Rep. 7, 41099. doi: 10.1038/srep41099
Cortinovis, M., Ruggenenti, P., and Remuzzi, G. (2016). Progression, remission and regression of chronic renal diseases. Nephron. 134, 20–24. doi: 10.1159/000445844
Davies, K. J. A. (2001). Degradation of oxidized proteins by the 20S proteasome. Biochimie. 83, 301–310. doi: 10.1016/s0300-9084(01)01250-0
Dedon, P. C., and Borch, R. F. (1987). Characterization of the reactions of platinum antitumor agents with biologic and nonbiologic sulfur-containing nucleophiles. Biochem. Pharmacol. 36, 1955–1964. doi: 10.1016/0006-2952(87)90494-1
Deschepper, C. F. (1994). Angiotensinogen: hormonal regulation and relative importance in the generation of angiotensin II: hormonal regulation and relative importance in the generation of angiotensin II. Kidney Int. 46, 1561–1563. doi: 10.1038/ki.1994.446
Diamond, G. L., and Zalups, R. K. (1998). Understanding renal toxicity of heavy metals. Toxicol. Pathol. 26, 92–103. doi: 10.1177/019262339802600111
Du, Y., Liu, X. H., Zhu, H. C., Wang, L., Wang, Z. S., Ning, J. Z., et al. (2019). Hydrogen sulfide treatment protects against renal ischemia-reperfusion injury via induction of heat shock proteins in rats. Iran. J. Basic Med. Sci. 22, 99–105. doi: 10.22038/ijbms.2018.29706.7170
Dubey, N. K., Yadav, P., Dutta, A. K., Kumar, V., Ray, G. N., and Batra, S. (2000). Free oxygen radicals in acute renal failure. Indian Pediatr. 37, 153–158.
Dwivedi, C., Abu-Ghazaleh, A., and Guenther, J. (1996). Effects of diallyl sulfide and diallyl disulfide on cisplatin-induced changes in glutathione and glutathione-S-transferase activity. Anti Cancer Drugs. 7, 792–794. doi: 10.1097/00001813-199609000-00012
Elkhoely, A., and Kamel, R. (2018). Diallyl sulfide alleviates cisplatin-induced nephrotoxicity in rats via suppressing NF-κB downstream inflammatory proteins and p53/Puma signalling pathway. Clin. Exp. Pharmacol. Physiol. 45, 591–601. doi: 10.1111/1440-1681.12910
Gambaryan, S., Butt, E., Marcus, K., Glazova, M., Palmetshofer, A., Guillon, G., et al. (2003). cGMP-dependent protein kinase type II regulates basal level of aldosterone production by zona glomerulosa cells without increasing expression of the steroidogenic acute regulatory protein gene. J. Biol. Chem. 278, 29640–29648. doi: 10.1074/jbc.m302143200
Giacchetti, G., Opocher, G., Sarzani, R., Rappelli, A., and Mantero, F. (1996). Angiotensin II and the adrenal. Clin. Exp. Pharmacol. Physiol. 3, S119–S124.
Gisselman, K., Langston, C., Palma, D., and McCue, J. (2009). Calcium oxalate urolithiasis. Compendium. 31, 496–502.
Goldstein, S. L., Leung, J. C., and Silverstein, D. M. (2006). Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clin. J. Am.Soc.Nephrol. 1, 979–986. doi: 10.2215/cjn.02291205
Haase, V. H. (2012). Hypoxia-inducible factor signaling in the development of kidney fibrosis. Fibrogene. Tissue Rep. 5, S16. doi: 10.1186/1755-1536-5-s1-s16
Han, S. J., Noh, M. R., Jung, J.-M., Ishii, I., Yoo, J., Kim, J. I., and Park, K. M. (2017). Hydrogen sulfide-producing cystathionine γ-lyase is critical in the progression of kidney fibrosis. Free Radic. Biol. Med. 112, 423–432. doi: 10.1016/j.freeradbiomed.2017.08.017
Hariharan, N., Maejima, Y., Nakae, J., Paik, J., DePinho, R. A., and Sadoshima, J. (2010). Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ. Res. 107, 1470–1482. doi: 10.1161/circresaha.110.227371
Hartleben, B., Gödel, M., Meyer-Schwesinger, C., Liu, S., Ulrich, T., Köbler, S., et al. (2010). Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 120, 1084–1096. doi: 10.1172/jci39492
Hiragi, S., Yamada, H., Tsukomoto, T., Yoshida, K., Kondo, N., Matsubara, T., et al. (2018). Acetaminophen administration and the risk of acute kidney injury: a self-controlled case series study. Clin. Epidemiol. 10, 265–276. doi: 10.2147/clep.s158110
Hong, Q., Zhang, L., Das, B., Li, Z., Liu, B., Cai, G., et al. 2018). Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 93, 1330–1343. doi: 10.1016/j.kint.2017.12.008
House, D. J., Brosnan, E. M., and Brosnan, T. J. (1997). Characterization of homocysteine metabolism in the rat kidney. Biochem. J. 328, 287–292. doi: 10.1042/bj3280287
Hsu, R. K., and Hsu, C.-y. (2011). Proteinuria and reduced glomerular filtration rate as risk factors for acute kidney injury. Curr. Opin. Nephrol. Hypertens. 20, 211–217. doi: 10.1097/mnh.0b013e3283454f8d
Huang, Y., Zhang, Z., Huang, Y., Mao, Z., Yang, X., Nakamura, Y., Sawada, N., et al. (2018). Induction of inactive TGF-β1 monomer formation by hydrogen sulfide contributes to its suppressive effects on Ang II- and TGF-β1-induced EMT in renal tubular epithelial cells. Biochem. Biophys. Res. Commun. 501, 534–540. doi: 10.1016/j.bbrc.2018.05.032
Huber, J. M., Tagwerker, A., Heininger, D., Mayer, G., Rosenkranz, A. R., and Eller, K. (2009). The proteasome inhibitor bortezomib aggravates renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 297, F451–F460. doi: 10.1152/ajprenal.90576.2008
Humphreys, B. D., Xu, F., Sabbisetti, V., Grgic, I., Naini, S. M., Wang, N., et al. (2013). Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Invest. 123, 4023–4035. doi: 10.1172/jci45361
Ikeda, U., Hyman, R., Smith, T. W., and Medford, R. M. (1991). Aldosterone-mediated regulation of Na+, K(+)-ATPase gene expression in adult and neonatal rat cardiocytes. J. Biol. Chem. 266, 12058–12066.
Ishibashi, K., Hara, S., and Kondo, S. (2009). Aquaporin water channels in mammals. Clin. Exp. Nephrol. 13, 107–117. doi: 10.1007/s10157-008-0118-6
Jansen, B. A. J., Brouwer, J., and Reedijk, J. (2002). Glutathione induces cellular resistance against cationic dinuclear platinum anticancer drugs. J. Inorg. Biochem. 89, 197–202. doi: 10.1016/s0162-0134(02)00381-1
Jia, Q., Wang, L., Wang, Q. Y., Liu, X. F., Ma, S. F., and Yang, R. (2018). Effects of hydrogen sulfide on renal fibrosis in diabetic rats and its mechanism. Chin. J. Appl. Physiol. 34, 572–576 [in Chinese]. doi: 10.12047/j.cjap.5734.2018.128
Jiang, D., Zhang, Y., Yang, M., Wang, S., Jiang, Z., and Li, Z. (2014). Exogenous hydrogen sulfide prevents kidney damage following unilateral ureteral obstruction. Neurourol. Urodyn. 33, 538–543. doi: 10.1002/nau.22450
Jiang, M., Wei, Q., Dong, G., Komatsu, M., Su, Y., and Dong, Z. (2012). Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 82, 1271–1283. doi: 10.1038/ki.2012.261
John, A., Kundu, S., Pushpakumar, S., Fordham, M., Weber, G., Mukhopadhyay, M., et al. (2017). GYY4137, a hydrogen sulfide donor modulates miR194-dependent collagen realignment in diabetic kidney. Sci. Rep. 7, 10924. doi: 10.1038/s41598-017-11256-3
John, A. S. P., and Sen, U. (2019). GYY4137 modulates renal remodeling in hyperhomocysteinemia. FASEB J. 33, 570–573. doi: 10.1096/fasebj.2019.33.1_supplement.570.3
Jung, K.-J., Jang, H.-S., Kim, J. I., Han, S. J., Park, J.-W., and Park, K. M. (2013). Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim. Biophys. Acta. Mol. Basis Dis. 1832, 1989–1997. doi: 10.1016/j.bbadis.2013.06.015
Kaide, J.-I., Zhang, F., Wei, Y., Jiang, H., Yu, C., Wang, W., et al. (2001). Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J. Clin. Invest. 107, 1163–1171. doi: 10.1172/jci11218
Kalayarasan, S., Prabhu, P. N., Sriram, N., Manikandan, R., Arumugam, M., and Sudhandiran, G. (2009). Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur. J. Pharmacol. 606, 162–171. doi: 10.1016/j.ejphar.2008.12.055
Karimi, A., Absalan, F., Khorsandi, l., Valizadeh, A., and Mansouri, E. (2017). Sodium hydrogen sulfide (NaHS) ameliorates alterations caused by cisplatin in filtration slit diaphragm and podocyte cytoskeletal in rat kidney. J. Nephropathol. 6, 150–156. doi: 10.15171/jnp.2017.26
Kasinath, B. (2018). Hydrogen sulfide ameliorates kidney changes in aging and diabetes. Innov. Aging. 2, 348. doi: 10.1093/geroni/igy023.1280
Katsouda, A., Szabo, C., and Papapetropoulos, A. (2018). Reduced adipose tissue H 2 S in obesity. Pharmacol. Res. 128, 190–199. doi: 10.1016/j.phrs.2017.09.023
Kaufman, J., Dhakal, M., Patel, B., and Hamburger, R. (1991). Community-acquired acute renal failure. Am. J. Kidney Dis. 17, 191–198. doi: 10.1016/s0272-6386(12)81128-0
Kaur, M., Sachdeva, S., Bedi, O., Kaur, T., and Kumar, P. (2015). Combined effect of hydrogen sulphide donor and losartan in experimental diabetic nephropathy in rats. J. Diabetes Metab. Disord. 14, 63. doi: 10.1186/s40200-015-0185-7
Khukhlina, O. S., Gerush, I. V., Antoniv, A. A., Mandryk, O. Y., Kopchuk, T. G., Melnychuk, S. P., et al. (2018). The role of hydrogen sulfide in the progression mechanisms of non-alcoholic steatohepatitis and chronic kidney disease. Wiad. Lek. 71, 474–478.
Klahr, S. (1991). New insights into the consequences and mechanisms of renal impairment in obstructive nephropathy. Am. J. Kidney Dis. 18, 689–699. doi: 10.1016/s0272-6386(12)80611-1
Klahr, S. (2000). Obstructive nephropathy. Intern. Med. 39, 355–361. doi: 10.2169/internalmedicine.39.355
Ko, J.-W., Shin, J.-Y., Kim, J.-W., Park, S.-H., Shin, N.-R., Lee, I.-C., et al. (2017). Protective effects of diallyl disulfide against acetaminophen-induced nephrotoxicity: a possible role of CYP2E1 and NF-κB. Food Chem. Toxicol. 102, 156–165. doi: 10.1016/j.fct.2017.02.021
Koj, A., Frendo, J., and Janik, Z. (1967). [35S]thiosulphate oxidation by rat liver mitochondria in the presence of glutathione. Biochem. J. 103, 791–795. doi: 10.1042/bj1030791
Kolluru, G. K., Shen, X., Bir, S. C., and Kevil, C. G. (2013). Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 35, 5–20. doi: 10.1016/j.niox.2013.07.002
Kriz, W. (2012). Podocyte hypertrophy mismatch and glomerular disease. Nat. Rev. Nephrol. 8 (11), 618–619. doi: 10.1038/nrneph.2012.198
Kuang, Q., Xue, N., Chen, J., Shen, Z., Cui, X., Fang, Y., et al. (2018). Low plasma hydrogen sulfide is associated with impaired renal function and cardiac dysfunction. Am. J. Nephrol. 47, 361–371. doi: 10.1159/000489606
Kundu, S., Pushpakumar, S. B., Tyagi, A., Coley, D., and Sen, U. (2013). Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am. J. Physiol. Endocrinol. Metabol. 304, E1365–E1378. doi: 10.1152/ajpendo.00604.2012
Kundu, S., Pushpakumar, S., and Sen, U. (2015) MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: hydrogen sulfide is a key modulator. Nitric. Oxid. 46, 172–185. doi: 10.1016/j.niox.2015.02.003
Kuo, S.-M., Lea, T. C., and Stipanuk, M. H. (1983). Developmental pattern, tissue distribution, and subcellular distribution of cysteine: α-ketoglutarate aminotransferase and 3-mercaptopyruvate sulfurtransferase activities in the rat. Neonatology. 43, 23–32. doi: 10.1159/000241634
Lacourciere, Y., and Provencher, P. (1989). Comparative effects of zofenopril and hydrochlorothiazide on office and ambulatory blood pressures in mild to moderate essential hypertension. Br. J. Clin. Pharmacol. 27, 371–376. doi: 10.1111/j.1365-2125.1989.tb05379.x
Lai, Y., Liang, X., Zhong, F., Wu, W., Zeng, T., Huang, J., et al. (2019). Allicin attenuates calcium oxalate crystal deposition in the rat kidney by regulating gap junction function. J. Cell. Physiol. 234, 9640–9651. doi: 10.1002/jcp.27651
Lee, H. J., Feliers, D., Barnes, J. L., Oh, S., Choudhury, G. G., et al. (2018). Hydrogen sulfide ameliorates aging-associated changes in the kidney. GeroScience. 40 163–176. doi: 10.1007/s11357-018-0018-y
Lee, H. J., Feliers, D., Mariappan, M. M., Sataranatarajan, K., Choudhury, G. G., Gorin, Y., et al. (2015). Tadalafil integrates nitric oxide-hydrogen sulfide signaling to inhibit high glucose-induced matrix protein synthesis in podocytes. J. Biol. Chem. 290, 12014–12026. doi: 10.1074/jbc.m114.615377
Lee, S. S. T., Buters, J. T. M., Pineau, T., Fernandez-Salguero, P., and Gonzalez, F. J. (1996). Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 271, 12063–12067. doi: 10.1074/jbc.271.20.12063
Leonetti, G., Rappelli, A., and Omboni, S., and on Behalf of the Study Group fnm (2006). A similar 24‐h blood pressure control is obtained by zofenopril and candesartan in primary hypertensive patients. Blood Pres. 15, 18–26. doi: 10.1080/08038020510046689
Levey, A. S., and Coresh, J. (2012). Chronic kidney disease. Lancet. 379, 165–180. doi: 10.1016/s0140-6736(11)60178-5
Levi, J., Jacobs, C., Kalman, S. M., McTigue, M., and Weiner, M. W. (1980). Mechanism of cis-platinum nephrotoxicity: I. Effects of sulfhydryl groups in rat kidneys. J. Pharmacol. Exp. Therapeut. 213, 545–550.
Li, H., Feng, S. J., Zhang, G. Z., and Wang, S. X. (2014). Correlation of lower concentrations of hydrogen sulfide with atherosclerosis in chronic hemodialysis patients with diabetic nephropathy. Blood Purificat. 38 (3–4), 188–194. doi: 10.1159/000368883
Li, L., Zepeda-Orozco, D., Black, R., and Lin, F. (2010). Autophagy is a component of epithelial cell fate in obstructive uropathy. Am. J. Pathol. 176 1767–1778. doi: 10.2353/ajpath.2010.090345
Li, Y., Li, L., Zeng, O., Liu, J. M., and Yang, J. (2017). H2S improves renal fibrosis in STZ-induced diabetic rats by ameliorating TGF-β1 expression. Ren. Fail. 39, 265–272. doi: 10.1080/0886022x.2016.1257433
Li, Z., Organ, C. L., Kang, J., Polhemus, D. J., Trivedi, R. K., and Sharp, T. E., et al. (2018). Hydrogen sulfide attenuates renin angiotensin and aldosterone pathological signaling to preserve kidney function and improve exercise tolerance in heart failure. J. Am. Coll. Cardiol. 3, 796–809. doi: 10.1016/j.jacbts.2018.08.011
Lin, S., Lian, D., Liu, W., Haig, A., Lobb, I., Hijazi, A., et al. (2018). Daily therapy with a slow-releasing H 2 S donor GYY4137 enables early functional recovery and ameliorates renal injury associated with urinary obstruction. Nitric Oxid. 76, 16–28. doi: 10.1016/j.niox.2018.03.002
Ling, Q., Yu, X., Wang, T., Wang, S.-G., Ye, Z.-Q., and Liu, J.-H. (2017). Roles of the exogenous H2S-mediated SR-A signaling pathway in renal ischemia/reperfusion injury in regulating endoplasmic reticulum stress-induced autophagy in a rat model. Cell. Physiol. Biochem. 41, 2461–2474. doi: 10.1159/000475915
Liu, M., Jia, Z., Sun, Y., Zhang, A., and Yang, T. (2016). A H2S donor GYY4137 exacerbates cisplatin-induced nephrotoxicity in mice. Mediat. Inflamm. 2016, 8145785. doi: 10.1155/2016/8145785
Liu, Y., Zhao, H., Qiang, Y., Qian, G., Lu, S., Chen, J., et al. (2015). Effects of hydrogen sulfide on high glucose-induced glomerular podocyte injury in mice. Int. J. Clin. Exp. Pathol. 8, 6814–6820.
Lorz, C., Justo, P., Sanz, A., Subirá, D., Egido, J., and Ortiz, A. (2004). Paracetamol-induced renal tubular injury: a role for ER stress. J. Am. Soc. Nephrol. 15, 380–389. doi: 10.1097/01.asn.0000111289.91206.b0
Luo, R., Hu, S., Liu, Q., Han, M., Wang, F., Qiu, M., et al. (2019). Hydrogen sulfide upregulates renal AQP‐2 protein expression and promotes urine concentration. FASEB J. 33, 469–483. doi: 10.1096/fj.201800436r
Ma, J., Shi, C., Liu, Z., Han, B., Guo, L., Zhu, L., and Ye, T. (2019). Hydrogen sulfide is a novel regulator implicated in glucocorticoids-inhibited bone formation. Aging. 11, 7537–7552. doi: 10.18632/aging.102269
Maejima, I., Takahashi, A., Omori, H., Kimura, T., Takabatake, Y., Saitoh, T., et al. (2013). Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347. doi: 10.1038/emboj.2013.171
Majumder, S., Ren, L., Pushpakumar, S., and Sen, U. (2019). Hydrogen sulphide mitigates homocysteine-induced apoptosis and matrix remodelling in mesangial cells through Akt/FOXO1 signalling cascade. Cell Signal. 61, 66–77. doi: 10.1016/j.cellsig.2019.05.003
Malacco, E., and Omboni, S. (2007). Antihypertensive efficacy of zofenopril plus hydrochlorothiazide fixed combination for treatment in metabolic syndrome. Adv. Ther. 24, 1006–1015. doi: 10.1007/BF02877705.
Malacco, E., Omboni, S., and Parati, G. (2015). Blood pressure response to zofenopril or irbesartan each combined with hydrochlorothiazide in high-risk hypertensives uncontrolled by monotherapy: a randomized, double-blind, controlled, parallel group, noninferiority trial. Int. J. Hypertens. 2015, 139465. doi: 10.1155/2015/139465
Mallion, J. M. (2007). An evaluation of the initial and long‐term antihypertensive efficacy of zofenopril compared with enalapril in mild to moderate hypertension. Blood Pressure. 16, 13–18. doi: 10.1080/08038020701561703
Manissorn, J., Fong-ngern, K., Peerapen, P., and Thongboonkerd, V. (2017). Systematic evaluation for effects of urine pH on calcium oxalate crystallization, crystal-cell adhesion and internalization into renal tubular cells. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-01953-4
Mao, Z., Huang, Y., Zhang, Z., Yang, X., Zhang, X., Huang, Y., et al. (2019). Pharmacological levels of hydrogen sulfide inhibit oxidative cell injury through regulating the redox state of thioredoxin. Free Radic. Biol. Med. 134, 190–199. doi: 10.1016/j.freeradbiomed.2019.01.009
Marsik, C., Endler, G., Gulesserian, T., Wagner, O. F., and Sunder-Plassmann, G. (2008). Classification of chronic kidney disease by estimated glomerular filtration rate. Eur. J. Clin. Invest., 38 (4), 253–259. doi: 10.1111/j.1365-2362.2008.01934.x
Miltonprabu, S., Sumedha, N. C., and Senthilraja, P. (2017). RETRACTED: diallyl trisulfide, a garlic polysulfide protects against As-induced renal oxidative nephrotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int. Immunopharm. 50, 107–120. doi: 10.1016/j.intimp.2017.06.011
Mikami, Y., Shibuya, N., Kimura, Y., Nagahara, N., Ogasawara, Y., and Kimura, H. (2011). Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 439, 479–485. doi: 10.1042/bj20110841
Morales-Loredo, H., Barrera, A., Garcia, J. M., Pace, C. E., Naik, J. S., Gonzalez Bosc, L. V., et al. (2019). Hydrogen sulfide regulation of renal and mesenteric blood flow. Am. J. Physiol. Heart Circ. Physiol. 317, H1157–H1165. doi: 10.1152/ajpheart.00303.2019
Morin, J. P., Viotte, G., Vandewalle, A., Hoof, F. V., Tulkens, P., and Fillastre, J. P. (1980). Gentamicin-induced nephrotoxicity: a cell biology approach. Kidney International. 18, 583–590. doi: 10.1038/ki.1980.176
Zschiedrich, S., Bork, T., Liang, W., Wanner, N., Eulenbruch, K., Munder, S., et al. (2017). Targeting mTOR signaling can prevent the progression of FSGS. J. Am. Soc. Nephrol. 28, 2144–2157. doi: 10.1681/asn.2016050519
Nagahara, N., Ito, T., Kitamura, H., and Nishino, T. (1998). Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem. Cell Biol. 110, 243–250. doi: 10.1007/s004180050286
Nair, A. P., Nemirovsky, D., Kim, M., Geer, E. B., Farkouh, M. E., Winston, J., et al. (2005). Elevated homocysteine levels in patients with end-stage renal disease. Mt. Sinai J. Med. 72, 365–373.
Napoli, C., Sica, V., de Nigris, F., Pignalosa, O., Condorelli, M., Ignarro, L. J., et al. (2004). Sulfhydryl angiotensin-converting enzyme inhibition induces sustained reduction of systemic oxidative stress and improves the nitric oxide pathway in patients with essential hypertension. Am. Heart J. 148, e5. doi: 10.1016/j.ahj.2004.03.025
Niewczas, M. A., Gohda, T., Skupien, J., Smiles, A. M., Walker, W. H., Rosetti, F., et al. (2012). Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 23, 507–515. doi: 10.1681/asn.2011060627
Nogueira, A., Pires, M. J., and Oliveira, P. A. (2017). Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In vivo (Athens, Greece). 31, 1–22. doi: 10.21873/invivo.11019
Ondarza, R. N., Escamilla, E., Gutiérrez, J., and de la Chica, G. (1974). CoAS-Sglutathione and GSSG reductases from rat liver. Biochim. Biophys. Acta Enzymol. 341, 162–171. doi: 10.1016/0005-2744(74)90076-x
Østerby, R., Gall, M.-A., Schmitz, A., Nielsen, F. S., Nyberg, G., and Parving, H.-H. (1993). Glomerular structure and function in proteinuric type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 36, 1064–1070. doi: 10.1007/bf02374500
Otunctemur, A., Ozbek, E., Dursun, M., Sahin, S., Besiroglu, H., Ozsoy, O. D., et al. (2014). Protective effect of hydrogen sulfide on gentamicin-induced renal injury. Ren. Fail. 36, 925–931. doi: 10.3109/0886022x.2014.900599
Ozatik, F. Y., Teksen, Y., Kadioglu, E., Ozatik, O., and Bayat, Z. (2019). Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int. Urol. Nephrol. 51, 745–754. doi: 10.1007/s11255-018-2053-0
Padmanabhan, A., Gohil, S., Gadgil, N., and Sachdeva, P. (2017). Chronic renal failure: an autopsy study. Saudi J. Kidney Dis. Transpl. 28, 545–551. doi: 10.4103/1319-2442.206441
Pari, L., and Murugavel, P. (2005). Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ. Toxicol. Pharmacol. 20, 493–500. doi: 10.1016/j.etap.2005.05.009
Pari, L., Murugavel, P., Sitasawad, S. L., and Kumar, K. S. (2007). Cytoprotective and antioxidant role of diallyl tetrasulfide on cadmium induced renal injury: an in vivo and in vitro study. Life Sci. 80, 650–658. doi: 10.1016/j.lfs.2006.10.013
Parving, H.-H. (2001). Diabetic nephropathy: prevention and treatment. Kidney Int. 60, 2041–2055. doi: 10.1046/j.1523-1755.2001.00020.x
Pedraza-Chaverrí, J., González-Orozco, A. E., Maldonado, P. D., Barrera, D., Medina-Campos, O. N., and Hernández-Pando, R. (2003). Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur. J. Pharmacol. 473, 71–78. doi: 10.1016/s0014-2999(03)01948-4
Pedraza-Chaverrí, J., Maldonado, P. D., Barrera, D., Cerón, A., Medina-Campos, O. N., and Pando, R. H. (2003). Protective effect of diallyl sulfide on oxidative stress and nephrotoxicity induced by gentamicin in rats. Mol. Cell. Biochem. 254, 125–130. doi: 10.1023/A:1027372102135
Penttilä, K. E. (1990). Role of cysteine and taurine in regulating glutathione synthesis by periportal and perivenous hepatocytes. Biochem. J. 269, 659–664. doi: 10.1042/bj2690659
Peter, E. A., Shen, X., Shah, S. H., Pardue, S., Glawe, J. D., Zhang, W. W., et al. (2013). Plasma free H 2 S levels are elevated in patients with cardiovascular disease. J. Am. Heart Assoc. 2, e000387. doi: 10.1161/jaha.113.000387
Polhemus, D. J., Li, Z., Pattillo, C. B., Gojon, G., Gojon, G., Giordano, T., et al. (2015). A novel hydrogen sulfide prodrug, SG 1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc. Ther. 33, 216–226. doi: 10.1111/1755-5922.12128
Pollak, M. R., Quaggin, S. E., Hoenig, M. P., and Dworkin, L. D. (2014). The glomerulus: the sphere of influence. Clin. J. Am. Nephrol. 9, 1461–1469. doi: 10.2215/cjn.09400913
Ponugoti, B., Xu, F., Zhang, C., Tian, C., Pacios, S., and Graves, D. T. (2013). FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J. Cell Biol. 203, 327–343. doi: 10.1083/jcb.201305074
Puelles, V. G., van der Wolde, J. W., Wanner, N., Scheppach, M. W., Cullen-McEwen, L. A., Bork, T., et al. (2019). mTOR-mediated podocyte hypertrophy regulates glomerular integrity in mice and humans. JCI insight. 4, e99271. doi: 10.1172/jci.insight.99271
Pushpakumar, S., Kundu, S., and Sen, U. (2019). Hydrogen sulfide protects hyperhomocysteinemia-induced renal damage by modulation of caveolin and enos interaction. Sci. Rep. 9, 1–13. doi: 10.1038/s41598-018-38467-6
Qian, X., Li, X., Ma, F., Luo, S., Ge, R., and Zhu, Y. (2016). Novel hydrogen sulfide-releasing compound, S-propargyl-cysteine, prevents STZ-induced diabetic nephropathy. Biochem. Biophys. Res. Commun. 473, 931–938. doi: 10.1016/j.bbrc.2016.03.154
Qiu, H., Chen, X., Luo, Z., Zhao, L., Zhang, T., Yang, N., et al. (2018). Inhibition of endogenous hydrogen sulfide production exacerbates the inflammatory response during urine-derived sepsis-induced kidney injury. Exp. ther. med. 16, 2851–2858. doi: 10.3892/etm.2018.6520
Ruggenenti, P., Perna, A., Perna, A., Mosconi, L., Pisoni, R., and Remuzzi, G. (1998). Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. Kidney Int. 53, 1209–1216. doi: 10.1046/j.1523-1755.1998.00874.x
Schiffl, H., Lang, S. M., and Fischer, R. (2002). Stopping smoking slows accelerated progression of renal failure in primary renal disease. J. Nephrol. 15, 270.
Searcy, D. G., and Lee, S. H. (1998). Sulfur reduction by human erythrocytes. J. Exp. Zool. 282, 310–322. doi: 10.1002/(sici)1097-010x(19981015)282:3<310::aid-jez4>3.0.co;2-p
Sekhar, R. V., Patel, S. G., Guthikonda, A. P., Reid, M., Balasubramanyam, A., Taffet, G. E., et al. (2011). Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 94, 847–853. doi: 10.3945/ajcn.110.003483
Sen, N., Paul, B. D., Gadalla, M. M., Mustafa, A. K., Sen, T., Xu, R., Kim, S., and Snyder, S. H. (2012). Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell. 45, 13–24. doi: 10.1016/j.molcel.2011.10.021
Sen, U., Basu, P., Abe, O. A., Givvimani, S., Tyagi, N., Metreveli, N., et al. (2009). Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am. J. Physiol. Ren. Physiol. 297, F410–F419. doi: 10.1152/ajprenal.00145.2009
Sen, U., Munjal, C., Qipshidze, N., Abe, O., Gargoum, R., and Tyagi, S. C. (2010). Hydrogen sulfide regulates homocysteine-mediated glomerulosclerosis. Am. J. Nephrol. 31, 442–455. doi: 10.1159/000296717
Seshadri, S., Beiser, A., Selhub, J., Jacques, P. F., Rosenberg, I. H., D'Agostino, R. B., et al. (2002). Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 346, 476–483. doi: 10.1056/nejmoa011613
Shan, X., Aw, T. Y., and Jones, D. P. (1990). Glutathione-dependent projection against oxidative injury. Pharmacol. Therapeut. 47, 61–71. doi: 10.1016/0163-7258(90)90045-4
Shastry, S., and James, L. R. (2009). Homocysteine-induced macrophage inflammatory protein-2 production by glomerular mesangial cells is mediated by PI3 Kinase and p38 MAPK. J. Inflamm. 6, 27. doi: 10.1186/1476-9255-6-27
Shesely, E. G., Maeda, N., Kim, H. S., Desai, K. M., Krege, J. H., Laubach, V. E., et al. (1996). Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 93, 13176–13181. doi: 10.1073/pnas.93.23.13176
Shi, G., Liao, P.-Y., Cai, X.-L., Pi, X.-X., Zhang, M.-F., Li, S.-J., et al. (2018). FoxO1 enhances differentiation and apoptosis in human primary keratinocytes. Exp. Dermatol. 27, 1254–1260. doi: 10.1111/exd.13775
Shi, L., Liu, X. Y., Huang, Z. G., Ma, Z. Y., Xi, Y., Wang, L. Y., et al. (2019). Endogenous hydrogen sulfide and ERK1/2-STAT3 signaling pathway may participate in the association between homocysteine and hypertension. J. Geriatr. cardiol. 16, 822–834. doi: 10.11909/j.issn.1671-5411.2019.11.007
Shibuya, N., Mikami, Y., Kimura, Y., Nagahara, N., and Kimura, H. (2009a). Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 146, 623–626. doi: 10.1093/jb/mvp111
Shibuya, N., Tanaka, M., Yoshida, M., Ogasawara, Y., Togawa, T., et al. (2009b). 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants Redox Signal. 11, 703–714. doi: 10.1089/ars.2008.2253
Shirazi, M. K., Azarnezhad, A., Abazari, M. F., Poorebrahim, M., Ghoraeian, P., Sanadgol, N., et al. (2019). The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: involvement of oxidative stress, autophagy and apoptosis. J. Cell. Physiol. 234, 11411–11423. doi: 10.1002/jcp.27797
Sies, H. (1997). Oxidative stress: oxidants and antioxidants. Exp. Physiol. 82, 291–295. doi: 10.1113/expphysiol.1997.sp004024
Silverstein, D. M., Travis, B. R., Thornhill, B. A., Schurr, J. S., Kolls, J. K., Leung, J. C., et al. (2003). Altered expression of immune modulator and structural genes in neonatal unilateral ureteral obstruction. Kidney Int. 64, 25–35. doi: 10.1046/j.1523-1755.2003.00067.x
Solomon, J. M., Pasupuleti, R., Xu, L., McDonagh, T., Curtis, R., DiStefano, P. S., et al. (2006). Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol. Cell. Biol. 26, 28–38. doi: 10.1128/mcb.26.1.28-38.2006
Song, K., Wang, F., Li, Q., Shi, Y.-B., Zheng, H.-F., Peng, H., et al. (2014). Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney Int. 85, 1318–1329. doi: 10.1038/ki.2013.449
Sonoda, Y., Gohda, T., Suzuki, Y., Omote, K., Ishizaka, M., Matsuoka, J., et al. (2015). Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PloS One. 10, e0122212. doi: 10.1371/journal.pone.0122212
Soubrier, F., Hubert, C., Testut, P., Nadaud, S., Alhenc-Gelas, F. o., and Corvol, P. (1993). Molecular biology of the angiotensin I converting enzyme: I. Biochemistry and structure of the gene. J. Hypertens. 11, 471–476. doi: 10.1097/00004872-199305000-00001
Spivacow, F. R., Valle, Del., , E. E., Lores, E., and Rey, P. G. (2016). Kidney stones: composition, frequency and relation to metabolic diagnosis. Medicina. 76, 343–348.
Stipanuk, M. H., and Beck, P. W. (1982). Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 206, 267–277. doi: 10.1042/bj2060267
Stipanuk, M. H., and Ueki, I. (2011). Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 34, 17–32. doi: 10.1007/s10545-009-9006-9
Strutz, F., Zeisberg, M., Ziyadeh, F. N., Yang, C.-Q., Kalluri, R., Müller, G. A., et al. 2002). Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 61, 1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x
Stubbert, D., Prysyazhna, O., Rudyk, O., Scotcher, J., Burgoyne, J. R., and Eaton, P. (2014). Protein kinase G iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension. 64, 1344–1351. doi: 10.1161/hypertensionaha.114.04281
Su, M., Dhoopun, A.-R., Yuan, Y., Huang, S., Zhu, C., Ding, G., et al. (2013). Mitochondrial dysfunction is an early event in aldosterone-induced podocyte injury. Am. J. Physiol. Ren. Physiol. 305, F520–F531. doi: 10.1152/ajprenal.00570.2012
Suh, S. H., Lee, K. E., Kim, I. J., Kim, O., Kim, C. S., Choi, J. S., et al. (2015). Alpha-lipoic acid attenuates lipopolysaccharide-induced kidney injury. Clin. Exp. Nephrol. 19, 82–91. doi: 10.1007/s10157-014-0960-7
Szabo, C., Coletta, C., Chao, C., Módis, K., Szczesny, B., Papapetropoulos, A., et al. (2013). Tumor-derived hydrogen sulfide, produced by cystathionine- -synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 12474–12479. doi: 10.1073/pnas.1306241110
Takemoto, M., Egashira, K., Usui, M., Numaguchi, K., Tomita, H., Tsutsui, H., et al. (1997). Important role of tissue angiotensin-converting enzyme activity in the pathogenesis of coronary vascular and myocardial structural changes induced by long-term blockade of nitric oxide synthesis in rats. J. Clin. Invest. 99, 278–287. doi: 10.1172/jci119156
Tan, Z., Shi, Y., Yan, Y., Liu, W., Li, G., and Li, R. (2015). Impact of endogenous hydrogen sulfide on toll-like receptor pathway in renal ischemia/reperfusion injury in rats. Ren. Fail. 37 (4), 727–733. doi: 10.3109/0886022x.2015.1012983
Thijssen, S., Kitzler, T. M., and Levin, N. W. (2008). Salt: its role in chronic kidney disease. J. Ren. Nutr. 18, 18–26. doi: 10.1053/j.jrn.2007.10.006
Turck, J., Pollock, A. S., Lee, L. K., Marti, H.-P., and Lovett, D. H. (1996). Matrix metalloproteinase 2 (gelatinase A) regulates glomerular mesangial cell proliferation and differentiation. J. Biol. Chem. 271, 15074–15083. doi: 10.1074/jbc.271.25.15074
Usui, M., Ichiki, T., Katoh, M., Egashira, K., and Takeshita, A. (1998). Regulation of angiotensin II receptor expression by nitric oxide in rat adrenal gland. Hypertension. 32, 527–533. doi: 10.1161/01.hyp.32.3.527
Vaitheeswari, S., Sriram, R., Brindha, P., and Kurian, G. A. (2015). Studying inhibition of calcium oxalate stone formation: an in vitro approach for screening hydrogen sulfide and its metabolites. Int. Braz J. Urol. 41, 503–510. doi: 10.1590/s1677-5538.ibju.2014.0193
Vandini, E., Ottani, A., Zaffe, D., Calevro, A., Canalini, F., Cavallini, G. M., et al. (2019). Mechanisms of hydrogen sulfide against the progression of severe alzheimer's disease in transgenic mice at different ages. Pharmacology. 103, 50–60. doi: 10.1159/000494113
Vaughan, E. D., Marion, D., Poppas, D. P., and Felsen, D. (2004). Pathophysiology of unilateral ureteral obstruction: studies from Charlottesville to New York. J. Urol. 172, 2563–2569. doi: 10.1097/01.ju.0000144286.53562.95
Wallace, J. L. (2015). THU0474 A phase 1 clinical trial of ATB-346, A gastrointestinal-ssafe nonsteroidal anti-inflammatory drug. Ann. Rheum. Dis. 74, 371–372.
Wallace, J. L., Nagy, P., Feener, T. D., Allain, T., Ditrói, T., Vaughan, D. J., et al. (2020). A proof‐of‐concept, phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide‐releasing anti‐inflammatory drug. Br. J. Pharmacol. 177, 769–777. doi: 10.1111/bph.14641
Wang, Q., Song, B., Jiang, S., Liang, C., Chen, X., Shi, J., et al. (2015). Hydrogen sulfide prevents advanced glycation end-products induced activation of the epithelial sodium channel. Oxid. Med. Cell Longev. 2015, 976848. doi: 10.1155/2015/976848
Wang, W., Luo, R., Lin, Y., Wang, F., Zheng, P., Levi, M., et al. (2015). Aliskiren restores renal AQP2 expression during unilateral ureteral obstruction by inhibiting the inflammasome. Am. J. Physiol. Ren. Physiol. 308, F910–F922. doi: 10.1152/ajprenal.00649.2014
Weber, G. J., Pushpakumar, S. B., and Sen, U. (2017). Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am. J. Physiol. Heart Circ. Physiol. 312, H874–H885. doi: 10.1152/ajpheart.00637.2016
Weir, B. A., and Mazumdar, D. C. (1994). Aminoglycoside nephrotoxicity following single-dose cystoscopy prophylaxis. Ann. Pharmacother. 28, 199–201. doi: 10.1177/106002809402800209
Wever, R., Boer, P., Hijmering, M., Stroes, E., Verhaar, M., Kastelein, J., et al. (1999). Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler. Thromb. Vasc. Biol. 19, 1168–1172. doi: 10.1161/01.atv.19.5.1168
Wharram, B. L., Goyal, M., Wiggins, J. E., Sanden, S. K., Hussain, S., Filipiak, W. E., et al. (2005). Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 16, 2941–2952. doi: 10.1681/asn.2005010055
Wiggins, J. E., Goyal, M., Sanden, S. K., Wharram, B. L., Shedden, K. A., Misek, D. E., et al. (2005). Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J. Am. Soc. Nephrol. 16, 2953–2966. doi: 10.1681/asn.2005050488
Woroniecki, R. P., and Kopp, J. B. (2007). Genetics of focal segmental glomerulosclerosis. Pediatr. Nephrol. 22, 638–644. doi: 10.1007/s00467-007-0445-y
Wu, D., Luo, N., Wang, L., Zhao, Z., Bu, H., Xu, G., et al. (2017). Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Sci. Rep. 7, 455. doi: 10.1038/s41598-017-00557-2
Xia, M., Chen, L., Muh, R. W., Li, P.-L., and Li, N. (2009). Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J. Pharmacol. Exp. Therapeut. 329, 1056–1062. doi: 10.1124/jpet.108.149963
Yamamoto, J., Sato, W., Kosugi, T., Yamamoto, T., Kimura, T., Taniguchi, S, et al. (2013). Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clin. Exp. Nephrol. 17, 32–40. doi: 10.1007/s10157-012-0670-y
Yamamoto, T., Noble, N. A., Miller, D. E., and Border, W. A. (1994) Sustained expression of TGF-β1 underlies development of progressive kidney fibrosis. Kidney Int. 45, 916–927. doi: 10.1038/ki.1994.122
Yang, H., Zhang, W., Pan, H., Feldser, H. G., Lainez, E., Miller, C., et al. (2012). SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS One. 7, e46364. doi: 10.1371/journal.pone.0046364
Yang, J., Fang, P., Yu, D., Zhang, L., Zhang, D., Jiang, X., et al. (2016). Chronic kidney disease induces inflammatory CD40 + monocyte differentiation via homocysteine elevation and DNA hypomethylation. Circ. Res. 119, 1226–1241. doi: 10.1161/circresaha.116.308750
Yang, J., Minkler, P., Grove, D., Wang, R., Willard, B., Dweik, R., et al. (2019). Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2, 194. doi: 10.1038/s42003-019-0431-5
Yassine, H. N., Trenchevska, O., Dong, Z., Bashawri, Y., Koska, J., Reaven, P. D., et al. (2016). The association of plasma cystatin C proteoforms with diabetic chronic kidney disease. Proteome Sci. 14, 7. doi: 10.1186/s12953-016-0096-7
Yi, J., Yuan, Y., Zheng, J., and Hu, N. (2018). Hydrogen sulfide alleviates uranium-induced kidney cell apoptosis mediated by ER stress via 20S proteasome involving in Akt/GSK-3β/Fyn-Nrf2 signaling. Free Rad. Res. 52, 1020–1029. doi: 10.1080/10715762.2018.1514603
Yuan, X., Zhang, J., Xie, F., Tan, W., Wang, S., Huang, L., et al. (2017). Loss of the protein cystathionine β-synthase during kidney injury promotes renal tubulointerstitial fibrosis. Kidney Blood Press. Res. 42, 428–443. doi: 10.1159/000479295
Zeng, O., Li, F., Li, Y., Li, L., Xiao, T., Chu, C., et al. (2016). Effect of Novel Gasotransmitter hydrogen sulfide on renal fibrosis and connexins expression in diabetic rats. Bioengineered. 7, 314–320. doi: 10.1080/21655979.2016.1197743
Zhang, H.-S., Cao, E.-H., and Qin, J.-F. (2005). Homocysteine induces cell cycle G1 arrest in endothelial cells through the PI3K/Akt/FOXO signaling pathway. Pharmacology. 74, 57–64. doi: 10.1159/000083684
Zhang, H., Akman, H. O., Smith, E. L. P., Zhao, J., Murphy-Ullrich, J. E., and Batuman, O. A. (2003). Cellular response to hypoxia involves signaling via Smad proteins. Blood. 101, 2253–2260. doi: 10.1182/blood-2002-02-0629
Zhang, H., Gao, P., Fukuda, R., Kumar, G., Krishnamachary, B., Zeller, K. I., et al. (2007). HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Canc. Cell. 11, 407–420. doi: 10.1016/j.ccr.2007.04.001
Zhang, J., Chen, S., Liu, H., Zhang, B., Zhao, Y., Ma, K., et al. (2013). Correction: hydrogen sulfide prevents hydrogen peroxide-induced activation of epithelial sodium channel through a PTEN/PI(3,4,5)P3Dependent pathway. PLoS One. 8, e64304. doi: 10.1371/journal.pone.0064304
Zhang, R., Wang, X., Gao, Q., Jiang, H., Zhang, S., Lu, M., et al. (2020). Taurine supplementation reverses diabetes-induced podocytes injury via modulation of the CSE/TRPC6 Axis and improvement of mitochondrial function. Nephron. 144, 84–95. doi: 10.1159/000503832
Zhang, X.-z., Han, F., Ding, C.-g., Dou, M., Wang, Y.-x., Xue, W.-j., et al. (2020). Different roles of bortezomib and ONX 0914 in acute kidney injury. Int. Immunopharm. 82, 106259. doi: 10.1016/j.intimp.2020.106259
Zhao, H., Dong, Y., Tian, X., Tan, T. K., Liu, Z., Zhao, Y., et al. (2013). Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J. Nephrol. 2, 84–89. doi: 10.5527/wjn.v2.i3.84
Zhao, W., Zhang, J., Lu, Y., and Wang, R. (2001). The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 20, 6008–6016. doi: 10.1093/emboj/20.21.6008
Zheng, J., Zhao, T., Yuan, Y., Hu, N., and Tang, X. (2015). Hydrogen sulfide (H2S) attenuates uranium-induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-κB pathways. Chem. Biol. Interact. 242, 353–362. doi: 10.1016/j.cbi.2015.10.021
Zhou, D., and Liu, Y. (2016). Understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 12, 68–70. doi: 10.1038/nrneph.2015.215
Zhou, X., Feng, Y., Zhan, Z., and Chen, J. (2014). Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J. Biol. Chem. 289, 28827–28834. doi: 10.1074/jbc.m114.596593
Zhou, Y., Zhu, X., Wang, X., Peng, Y., Du, J., Yin, H., et al. (2020). H2S alleviates renal injury and fibrosis in response to unilateral ureteral obstruction by regulating macrophage infiltration via inhibition of NLRP3 signaling. Exp. Cell Res. 387, 111779. doi: 10.1016/j.yexcr.2019.111779
Keywords: H2S donors, physiological process, renal dysfunction, signaling pathways, common renal diseases
Citation: Ngowi EE, Sarfraz M, Afzal A, Khan NH, Khattak S, Zhang X, Li T, Duan S-F, Ji X-Y and Wu D-D (2020) Roles of Hydrogen Sulfide Donors in Common Kidney Diseases. Front. Pharmacol. 11:564281. doi: 10.3389/fphar.2020.564281
Received: 21 May 2020; Accepted: 30 September 2020;
Published: 19 November 2020.
Edited by:
Guangdong Yang, Laurentian University, CanadaReviewed by:
Albert Ong, The University of Sheffield, United KingdomCopyright © 2020 Ngowi, Sarfraz, Afzal, Hussain Khan, Khattak, Zhang, Li, Duan, Ji and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Dong Wu, ZGR3dWJpb21lZDIwMTBAMTYzLmNvbQ==, Shao-Feng Duan, ZHNmXzIwMDdAMTYzLmNvbQ==, Xin-Ying Ji, MTAxOTAwOTZAdmlwLmhlbnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.