- 1Center for Evidence-Based and Translational Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Department of Rehabilitation, Taihe Hospital, Hubei University of Medicine, Shiyan, China
- 3Institutes of Evidence-Based Medicine and Knowledge Translation, Henan University, Kaifeng, China
- 4Administrative Office of Hospital Director, Zhongnan Hospital of Wuhan University, Wuhan, China
- 5Center for Primary Health Care Research, Lund University/Region Skåne, Malmö, Sweden
Objective: The quality and rationality of many recently registered clinical studies related to coronavirus disease 2019 (COVID-19) needs to be assessed. Hence, this study aims to evaluate the current status of COVID-19 related registered clinical trial.
Methods: We did an electronic search of COVID-19 related clinical studies registered between December 1, 2019 and February 21, 2020 (updated to May 28, 2020) from the ClinicalTrials.gov, and collected registration information, study details, recruitment status, characteristics of the subjects, and relevant information about the trial implementation process.
Results: A total of 1,706 studies were included 10.0% of which (n=171) were from France, 943 (55.3%) used an interventional design, and 600 (35.2%) used an observational design. Most of studies (73.6%) aimed to recruit fewer than 500 people. Interferon was the main prevention program, and antiviral drugs were the main treatment program. Hydroxychloroquine and chloroquine (230/943, 24.4%) were widely studied. Some registered clinical trials are incomplete in content, and 37.4% of the 1,706 studies may have had insufficient sample size.
Conclusion: The quality of COVID-19 related studies needs to be improved by strengthening the registration process and improving the quality of clinical study protocols so that these clinical studies can provide high-quality clinical evidence related to COVID-19.
Introduction
COVID-19, which broke out at the beginning of 2020, has spread rapidly (Zhou P. et al., 2020). Its clinical manifestations are very similar to Severe Acute Respiratory Syndrome (SARS). In severe cases, patients may go on to develop acute respiratory distress syndrome (ARDS). Patients with severe COVID-19 need intensive care to decrease mortality (Huang et al., 2020). As of July 13, 2020, there have been more than 12.8 million confirmed cases and 568,000 deaths globally (Johns Hopkins University, 2020).
COVID-19 is an emerging infectious disease for which, there is no specific treatment to date. Healthcare professionals have only been able to alleviate patients’ symptoms based on their experience (Jin et al., 2020) as up to now they have had insufficient knowledge of this disease. Hence, randomized clinical trials (RCTs) are necessary to verify the safety and effectiveness of the proposed drugs. Many scientists and clinicians have conducted clinical investigations, diagnostic accuracy tests, and treatment evaluations to understand the progress of COVID-19 and to improve clinical diagnosis and treatment. It is thus essential to evaluate the rationality and the potential value of proposed clinical trials because so many studies have emerged in such a short period and some of them might lack scientific value. Therefore, we performed this survey in order to have a comprehensive understanding of the current clinical trials related to COVID-19.
Methods
This study analyzed the characteristics of the clinical studies of COVID-19 registered in ClinicalTrials.gov (https://clinicaltrials.gov/) between December 1, 2019 and February 21, 2020 (updated to May 28, 2020). All COVID-19 related studies, including etiology, risk factors, prevention, diagnosis, treatment, prognosis, and psychology were included. The search terms were: 2019-nCoV, 2019 novel coronavirus, novel coronavirus pneumonia, COVID-19, coronavirus disease 2019, SARS-CoV-2.
We extracted the following information from registered studies: registration number, registration date, registration title, primary sponsor, funding source, study type, study phase, study objectives, study design, length of the study, intervention, countries of recruitment and research settings, recruiting status, allocation, sample size, participant age, gender, masking, the time and method of sharing individual participant data (IPD), data management committee.
Descriptive statistics were used to summarize the characteristics of all included clinical studies. Categorical variables were expressed as percentages and frequencies. All data were summarized using Microsoft Excel 2019.
Results
General Characteristics of the Included Studies
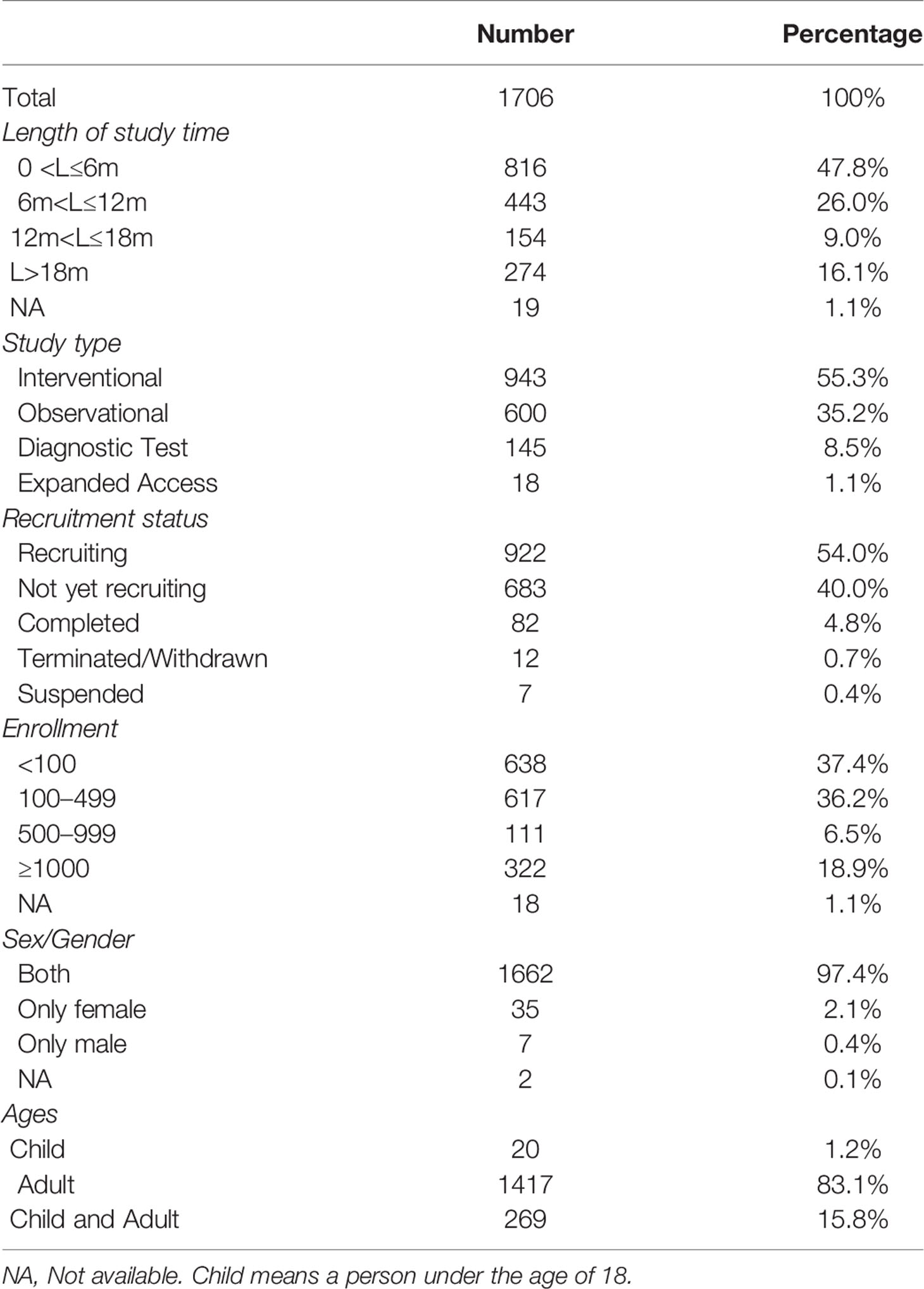
A total of 1,706 studies were included. Among these clinical studies (Table 1), the first one was registered on January 23, 2020, and the number of trials registered daily subsequently increased, peaking at 51 in a single day (Figure 1). For the total study period, 73.8% studies (n = 1259) planned to continue for less than 12 months and 25.1% more than 12 months. Of them, 943 (55.3%) used an interventional design and 600 (35.2%) used an observational design. As for the recruitment status, 82 (4.8%) studies had completed recruitment, 922 (54.0%) were recruiting, and 683 (40.0%) had not yet started recruiting, while some others were terminated/withdrawn (n = 12, 0.7%) or suspended (n = 7, 0.4%). For sample sizes, most of them (n = 1255, 73.6%) aimed to recruit less than 500 participants, 6.5% (n = 111) recruited 100 to 499 participants, 18.9% recruited more than 1,000, and 1.1% (n = 18) studies did not specify the number of participants recruited. Almost all studies recruited both males and females (n = 1662, 97.4%), 83.1% studies (n = 1417) included adults and only 16.9% (n = 289) involved children.
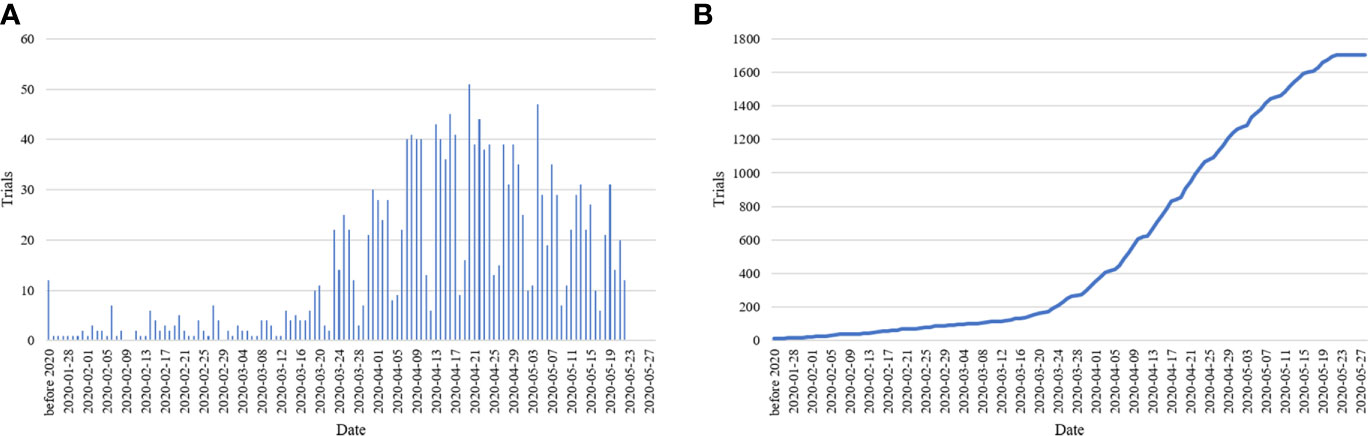
Figure 1 Bar chart of registered studies per day (A) and cumulative sum chart of registered studies (B).
Methodological Quality of the Included Studies
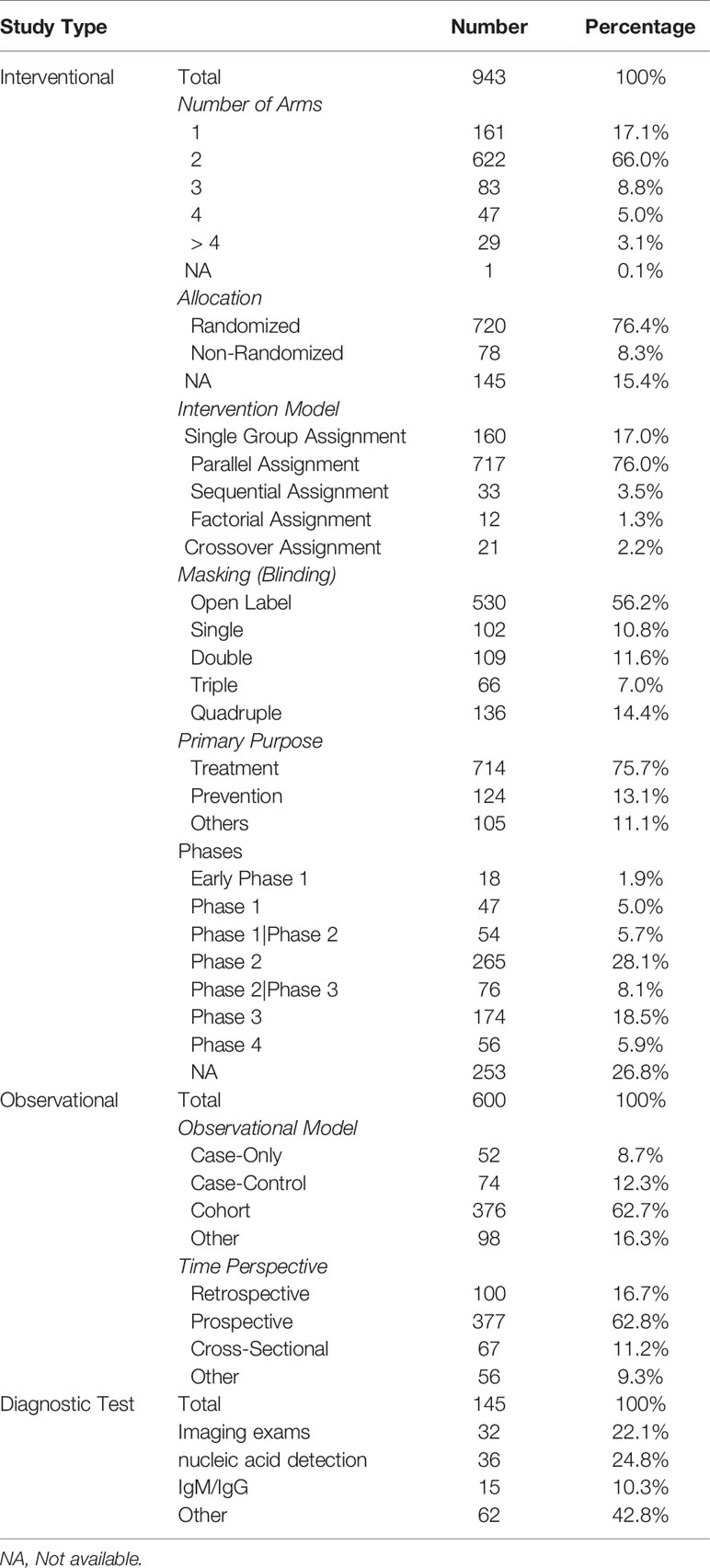
Among the 943 interventional studies, the primary purpose was treatment of the disease (n = 714, 75.7%). Seven hundred eighty-one (82.8%) were designed with at least two groups, most commonly parallel assignment (n = 717, 76.0%). Seven hundred twenty (76.4%) were randomized and 78 (8.3%) were non-randomized. More than 56.2% studies (n = 530) were open label, and only 33.0% being double, triple, or quad-masked. As for the 600 observational studies, 376 (62.7%) were cohort studies, and 377 (62.8%) were prospective design. For the 145 diagnostic studies, 32 studies (22.1%) focused on imaging studies, 36 studies (24.8%) focused on nucleic acid detection, and 15 studies (10.3%) focused on specific antibody. Details are shown in Table 2.
Detailed Characteristics of Included Studies
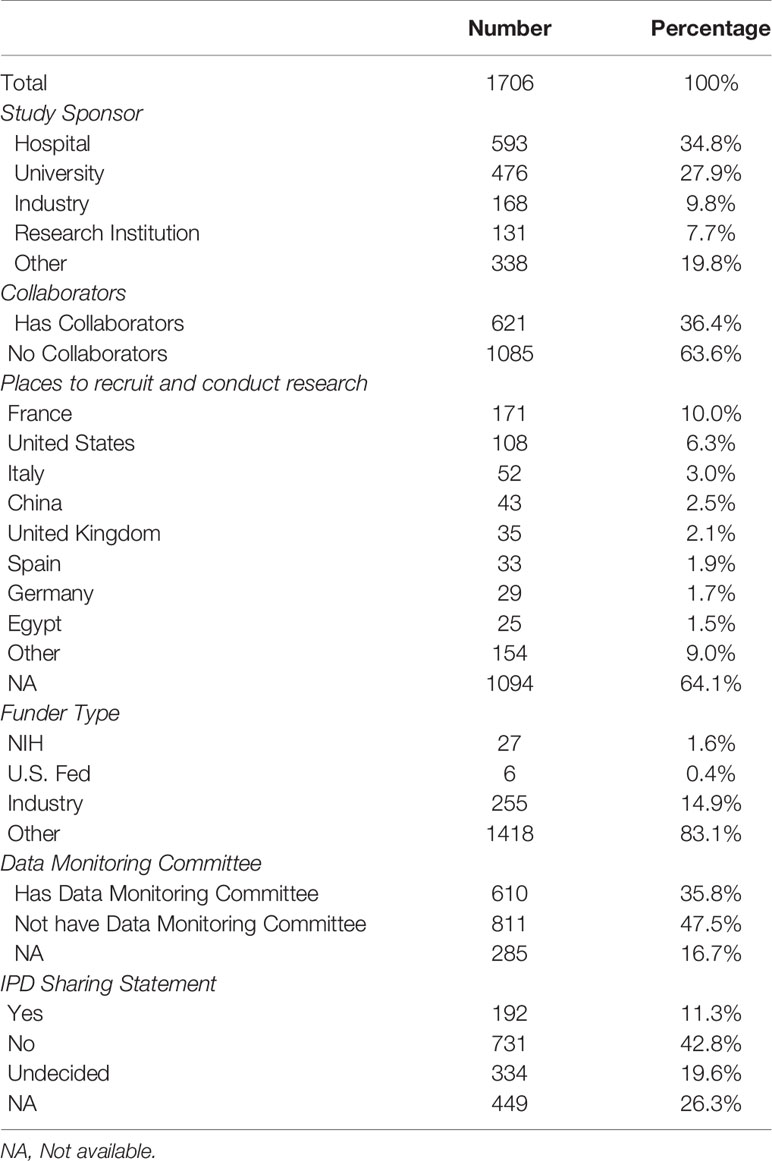
Of the 1,706 studies, 1,200 (70.3%) were initiated by researchers from hospitals, universities, or scientific research institutions; whereas a few (9.8%) were initiated by companies, and 338 (19.8%) were funded by others, such as individuals or community-based organizations. The highest number of studies were conducted in France (n = 171, 10.0%) and the second highest in the United States (n = 108, 6.3%). Of the 1,706 studies, only 33 studies (1.9%) were funded by National Institutes of Health (NIH) or U.S. Federal agencies, 255 (14.9%) were funded by pharmaceutical or device companies, and 83.1% were funded by others, such as individuals, universities, or community-based organizations. Six hundred ten (35.8%) clearly reported the existence of a data monitoring committee, and 192 (11.3%) had IPD sharing statement. Details are shown in Table 3.
Description of Drugs in the Included Interventional Studies
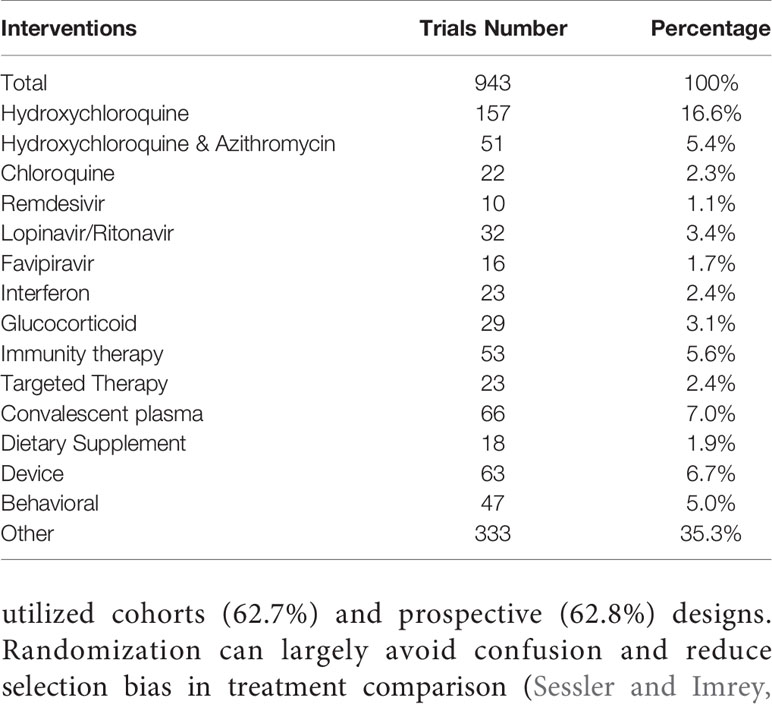
Among the 943 interventional studies, 416 studies (44.1%) explored the effectiveness and/or safety of drugs commonly used in preventing and treating COVID-19, such as hydroxychloroquine (HCQ), chloroquine (CQ), immunotherapy (including stem cell therapy, monoclonal antibody, immunoregulation), lopinavir/ritonavir, glucocorticoids, interferon, targeted therapy (Baricitinib, Ruxolitinib, Imatinib), favipiravir, and Remdesivir. In addition, 66 studies (7.0%) focused on convalescent plasma. Other interventions, such as dietary supplements, devices and behavioral programs, accounted for 48.9%. Details are shown in Table 4.
Discussion
The COVID-19 epidemic is still raging around the world. Exploring the characteristics of registered clinical studies related to COVID-19 and clarifying the direction of further research can help reduce the potential disease burden of COVID-19 (Gupta et al., 2020). There was a cross-sectional study that reviewed the drug and plasma registration trials in March 2020, characterizing the scope, objectives and content of clinical studies (Mehta et al., 2020). With the rapid increase in registration research, the status of registration studies may also change. This survey conducted a comprehensive summary of COVID-19 related studies registered in the ClinicalTrials.gov as of May 28, 2020. Results showed that most studies with an interventional design were aimed at adult participants, and were conducted using multicenter, randomized, parallel assignments, and open-label methods. A systematic review showed that compared with adults, children with COVID-19 have a milder disease course, with better prognosis and extremely low mortality (Ludvigsson, 2020). As a result, only 16.9% of registered studies involved children. As a factor of disease outcomes (Hou et al., 2019), only 2.5% studies focus on the participants’ gender. The included clinical studies involved disease prevention, diagnostic accuracy, drug treatment, medical devices, prognosis, as well as treatment of critical COVID-19. A number of these studies (n=638, 37.4%) may have had insufficient sample size.
Registration of COVID-19 related clinical studies is ongoing. The underlying methodological quality limitations of these clinical studies should be noted, such as lack of control group, insufficient sample size, or non-randomization, which might preclude drawing concrete conclusions (Bauchner and Fontanarosa, 2020; Ma et al., 2020). Our results found that nearly half of the registered trials did not exceed 6 months, and 37.4% of the registered trials recruited less than 100 people. The inclusion of less than 100 people does not automatically indicate that the study results are unreliable. Different studies need to estimate sample size according to outcomes. More studies are needed which use samples based on the estimated sample size. Insufficient or under-estimated sample size is a major shortcoming of the current clinical trials, which can cause false negative or false positive results, reduce credibility, and even have catastrophic consequences (Ruberg and Akacha, 2017). Therefore, although some studies had reported that some interventions may shorten intubation time, hospitalization time or reduce mortality; these findings did not represent the actual therapeutic effect of the drug (Gautret et al., 2020). The outbreak of the epidemic may pressurize researchers to quickly find targeted therapeutic drugs which are effective in the short term. However, if the length of the study was too short, it might preclude carrying out multiple follow-ups on the patients, and the long-term effect index of drug treatment cannot be obtained.
Our results found that of the intervention study 82.8% of the registered trials were designed for at least two groups, 76.4% were assigned randomly, 56.2% were open label, and 75.7% were mainly for treatment. Of the observational studies, most utilized cohorts (62.7%) and prospective (62.8%) designs. Randomization can largely avoid confusion and reduce selection bias in treatment comparison (Sessler and Imrey, 2015). However, RCTs often require large sample size, long research duration, incur high costs and may also be difficult to implement. At this time, adaptive trial design can usually be adopted (Bhatt and Mehta, 2016). However, it should be noted that observational research will be accompanied by some biases and limitations, and it is necessary to interpret the test results carefully (Shang et al., 2020). Besides, some of these studies did not have a control group or lack a real “control”, which will limit the effective inferences that can be drawn. There is a need for rigorous design and attention to trial protocols for research drug management to discover the true efficacy of interventions (Bauchner and Fontanarosa, 2020).
More and more researchers realize that clinical trials need to be registered before the recruitment, and registration is beneficial for sharing clinical trial information and reducing publication bias (Aslam et al., 2013). It is understandable that clinical trials must be launched and implemented quickly due to the sudden COVID-19 epidemic; however, a properly designed clinical trial is still the core to provide scientific evidence and achieve clinical conclusions. Randomized controlled trials are considered to be the highest quality clinical research methods, and random sequence generation, blinding, and allocation concealment during the implementation of the study are critical to the success of the study (Schulz and Grimes, 2002b; Schulz and Grimes, 2002a; Sessler and Imrey, 2015). It is thus essential for clinical trials to be designed by a professional team to meet the requirements of a successful study before registration. An appropriate research design should be selected according to the research purpose, with sample size being estimated in advance, and timely submission of the research plan to the ethical committee to avoid deficiencies. At the time of registration, the person responsible for the registration should have a comprehensive understanding of the characteristics of the study protocol and clinical trial, so as not to cause confusion to other researchers due to the ambiguity of registration content, such as countries of location, presence or absence of data monitoring committee. We found some registered clinical trials have incomplete content. Therefore, clinical trial registration agencies should strengthen supervision of trial registration. After the study completion, collation and strict statistical analysis the researchers should upload the resulting data to the registration agency in a timely manner (Goldacre, 2017). IPD sharing helps to accelerate the conversion of clinical resources and promote scientific breakthroughs. Hence, we call on researchers to share IPD to promote transparency, so that effective conclusions drawn from trials can be quickly applied to control the epidemic, and to provide a basis for COVID-19 prevention and treatment.
COVID-19 is a new infectious disease, which has affected health insurance (Gheorghe et al., 2019), and its underlying mechanisms of transmission and pathogenesis are still being explored. High quality clinical studies are the basis of clinical practice guidelines, especially WHO’s emergency guidelines (Norris et al., 2019). Some clinical trials focus on the prevention of COVID-19. It is widely believed that SARS-CoV-2 is transmitted through respiratory droplets and by close contact (Jin et al., 2020). Earlier studies have shown that masks are very effective for filtering influenza viruses (Zhou et al., 2018). However, there are no clinical study results that can prove that wearing masks can prevent COVID-19. A study has analyzed the pandemic trends and mitigation measures of COVID-19 in Wuhan, China, Italy, and New York City. Results showed that the difference between with and without facial masks represents the determinant of pandemic trends in the three epicenters. The authors thought that wearing a mask is the most effective way to prevent interpersonal transmission in public places (Zhang R. et al., 2020). In hospitals, healthcare professionals are at greater risk of exposure to SARS-CoV-2 than public. A multi-center RCT (Registration number: NCT04296643) from Canada is expected to recruit 576 nurses to compare and analyze the preventive effects of medical masks with N95 respirators on COVID-19. In addition, some clinical studies have focused on the preventive effects of drugs for COVID-19, such as CQ and HCQ. Chloroquine and hydroxychloroquine are both antimalarial drugs, and the mechanism of preventing and treating COVID-19 is not yet clear. Some researchers thought CQ and HCQ may confer antiviral effect at the pre-infection stages (Zhou D. et al., 2020). However, the possible cardiac side effects caused by the combination of CQ or HCQ and AZ, such as prolonged QT interval must be considered. Hence, clinical studies are needed to confirm the preventive effect of CQ or HCQ on COVID-19 (Registration number: NCT04303507, NCT04334148).
An accurate diagnosis is the fundamental prerequisite for efficient control of COVID-19. We included 145 clinical studies exploring the diagnosis of COVID-19. These diagnostic accuracy tests mainly focus on imaging examination, nucleic acid detection, and IgM/IgG. Detection of SARS-CoV-2 RNA by reverse-transcription polymerase chain reaction (RT-PCR) is the most commonly used to diagnose COVID-19. Early studies have shown that RT-PCR has relatively poor sensitivity, and false negative test results will miss some potential infected persons, which has a huge impact (Fang et al., 2020). Furthermore, the standard RT-PCR test takes about 3 h to complete. The cost of each test is about $10. The high cost per test may limit the number of tests (Esbin et al., 2020). Hence, researchers wanted to design some test kits in order to detect SARS-CoV-2 quickly and conveniently (Chu et al., 2020; Shirato et al., 2020; To et al., 2020; Yu et al., 2020). COVID-19 patients also have some typical computed tomography (CT) manifestations, such as ground glass opacities (Fang et al., 2020; Lu et al., 2020; Zhang J. J. et al., 2020). As a fast and effective method, CT can be used for auxiliary diagnosis. However, it should be noted that some patients may have atypical CT imaging manifestations (Jin et al., 2020; Lu et al., 2020; Wang W. G. et al., 2020). In addition, as the product of human immune system reaction to SARS-CoV-2, IgM/IgG can provide information about the course of the virus infection over time and provide the basis for the diagnosis of COVID-19. Some researchers have developed an IgM-IgG combined antibody test kit with a sensitivity of 88.66% and a specificity of 90.63%, but there were still false negative and false positive results (Li et al., 2020). The sensitivity and specificity of the IgM/IgG rapid diagnostic kit are currently being evaluated in some studies (Registration number: NCT04346186, NCT04348864).
Drug treatment is a very important part of the registration studies. Few drugs were used to treat COVID-19, such as CQ, HCQ, IFN, lopinavir/ritonavir, Oseltamivir, Umifenovir, dexamethasone. There is currently no clear evidence that these drugs are specific drugs for the treatment of COVID-19 other than dexamethasone (Gautret et al., 2020; RECOVERY Collaborative Group, 2020; Tang et al., 2020). The RECOVERY trial claims that dexamethasone can reduce the risk of death for patients on ventilators (RR 0.64; 95% CI, 0.51 to 0.81) and patients on oxygen (RR 0.82; 95% CI, 0.72 to 0.94) (RECOVERY Collaborative Group, 2020). The National Institutes of Health recommends the use of dexamethasone to treat COVID-19 patients who require supplemental oxygen in its guidelines (COVID-19 Treatment Guidelines Panel, 2020). As a new experimental broad-spectrum antiviral medication, Remdesivir is considered to be effective in inhibiting the replication of SARS coronavirus and MERS coronavirus. Two RCT studies showed that compared with placebo, the use of Remdesivir could shorten the recovery time of patients with COVID-19 (Beigel et al., 2020; Wang Y. et al., 2020). As of June 2020, it has been authorized for emergency treatment of COVID-19 in the US, Singapore, Japan, and the UK. CQ was first used to treat malaria, HCQ as its analogue is less toxic than CQ. CQ/HCQ is other drugs under consideration for treating COVID-19. So far, the drugs have been controversial. Some studies have shown that the drugs have significant efficacy in alleviating symptoms (Sarma et al., 2020; Tang et al., 2020), but some studies have reported that CQ/HCQ has potential cardiac side effects, such as prolonging QT interval (Borba et al., 2020). In June 2020, the U.S. Food and Drug Administration revoked the emergency use authorization for HCQ. A clinical trial to evaluate the safety and effectiveness of HCQ for the treatment of COVID-19 has been stopped by the NIH. After its fourth interim analysis, the data and safety monitoring board concluded that while there was no harm, HCQ was unlikely to be beneficial to hospitalized COVID-19 patients (NIH, 2020). In July 2020, WHO discontinued the Solidarity Trial’s HCQ and lopinavir/ritonavir arms. Although lopinavir/ritonavir can reduce SARS-CoV-2 viral loads (Lim et al., 2020), the Solidarity Trial’s interim results showed that compared with standard treatment, HCQ and lopinavir/ritonavir produce little or no reduction in the mortality of hospitalized COVID-19 patients (WHO, 2020). IFN has been used to treat SARS and MERS, and can improve patient survival (Haagmans et al., 2004; Mustafa et al., 2018); Liu et al. (Liu et al., 2020) reported that the efficacy is not clear for the treatment of COVID-19 using IFN. Hence, a clinical trial has been investigating the efficacy of IFN for the treatment COVID-19 (Registration number: NCT04254874). A study (Tian et al., 2020) reported a new coronavirus-specific human monoclonal antibody—CR3022, which can bind SARS-CoV-2 receptor-binding domain, and has potential function to prevent and treat SARS-CoV-2 infections. In addition, there have been some clinical studies investigating the convalescent plasma for the treatment of COVID-19.
A few limitations should be noted in this study. Because COVID-19 is a new disease, its name as well as the name of virus changed many times, so there may be a small number of studies using other names for the registration, which may not have been retrieved. Additionally, due to the worldwide spread of COVID-19, studies will continue to be registered every day and the number of clinical studies is growing, which may also cause some bias. In addition, this study only retrieved trials registered in ClinicalTrials.gov. Although ClinicalTrials.gov includes more than 3.4 million research studies in 214 countries, some studies may not have been registered on this platform.
In conclusion, the number of registered COVID-19 related clinical studies has increased rapidly since the outbreak, involving epidemiology, risk factors, prevention, diagnosis, treatment, rehabilitation, and psychological aspects. However, some registration parameters are not complete, so it is necessary to strengthen the registration monitoring and supervision for providing high-quality clinical evidence.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.
Author Contributions
X-TZ and JJ take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: X-TZ, X-QR, and JJ. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: L-LM, B-HL, and DH. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: XY, Y-YW, and J-YY. Administrative, technical, or material support: Y-YW, B-HL, and XY. Supervision and review: X-TZ, X-QR, and JJ.
Funding
This work was supported (in part) by the National Key Research and Development Program of China (2020YFC0845500).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our gratitude to Jean Glover from Tianjin Golden Framework Consulting Company for English editing. We also express our gratitude for the contribution of all authors of included clinical studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.540187/full#supplementary-material
References
Aslam, A., Imanullah, S., Asim, M., El-Menyar, A. (2013). Registration of Clinical Trials: Is it Really Needed? N. Am. J. Med. Sci. 5 (12), 713–715. doi: 10.4103/1947-2714.123266
Bauchner, H., Fontanarosa, P. B. (2020). Randomized Clinical Trials and COVID-19: Managing Expectations. JAMA 323 (22), 2262–2263. doi: 10.1001/jama.2020.8115
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020). Remdesivir for the Treatment of Covid-19 — Preliminary Report. N. Engl. J. Med. NEJMoa2007764. doi: 10.1056/NEJMoa2007764
Bhatt, D. L., Mehta, C. (2016). Adaptive Designs for Clinical Trials. N. Engl. J. Med. 375 (1), 65–74. doi: 10.1056/NEJMra1510061
Borba, M. G. S., Val, F. F. A., Sampaio, V. S., Alexandre, M. A. A., Melo, G. C., Brito, M., et al. (2020). Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Network Open 3 (4), e208857. doi: 10.1001/jamanetworkopen.2020.8857
Chu, D. K. W., Pan, Y., Cheng, S. M. S., Hui, K. P. Y., Krishnan, P., Liu, Y., et al. (2020). Molecular Diagnosis of a Novel Coronavirus, (2019-nCoV) Causing an Outbreak of Pneumonia. Clin. Chem. 66 (4), 549–555. doi: 10.1093/clinchem/hvaa029
COVID-19 Treatment Guidelines Panel (2020). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines (National Institutes of Health). Available at: https://www.covid19treatmentguidelines.nih.gov/ (Accessed July 30, 2020).
Esbin, M. N., Whitney, O. N., Chong, S., Maurer, A., Darzacq, X., Tjian, R. (2020). Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA 26 (7), 771–783. doi: 10.1261/rna.076232.120
Fang, Y., Zhang, H., Xie, J., Lin, M., Ying, L., Pang, P., et al. (2020). Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 296 (2), E115–E117. doi: 10.1148/radiol.2020200432
Gautret, P., Lagier, J. C., Parola, P., Hoang, V. T., Meddeb, L., Mailhe, M., et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 56 (1), 105949. doi: 10.1016/j.ijantimicag.2020.105949
Gheorghe, A., Straehler-Pohl, K., Nkhoma, D., Mughandira, W., Garand, D., Malema, D., et al. (2019). Assessing the feasibility and appropriateness of introducing a national health insurance scheme in Malawi. Glob. Health Res. Policy 4, 13. doi: 10.1186/s41256-019-0103-5
Goldacre, B. (2017). The WHO joint statement from funders on trials transparency. BMJ 357:j2816. doi: 10.1136/bmj.j2816
Gupta, M., Wahl, B., Adhikari, B., Bar-Zeev, N., Bhandari, S., Coria, A., et al. (2020). The need for COVID-19 research in low- and middle-income countries. Global Health Res. Policy 5 (1), 33. doi: 10.1186/s41256-020-00159-y
Haagmans, B. L., Kuiken, T., Martina, B. E., Fouchier, R. A., Rimmelzwaan, G. F., van Amerongen, G., et al. (2004). Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 10 (3), 290–293. doi: 10.1038/nm1001
Hou, Y. P., Wu, J. L., Tan, C., Chen, Y., Guo, R., Luo, Y. J. (2019). Sex-based differences in the prevalence of acute mountain sickness: a meta-analysis. Mil. Med. Res. 6 (1), 38. doi: 10.1186/s40779-019-0228-3
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. doi: 10.1016/s0140-6736(20)30183-5
Jin, Y. H., Cai, L., Cheng, Z. S., Cheng, H., Deng, T., Fan, Y. P., et al. (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus, (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 7 (1):4. doi: 10.1186/s40779-020-0233-6
Johns Hopkins University (2020). COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) (Accessed 2020/7/13 2020).
Li, Z., Yi, Y., Luo, X., Xiong, N., Liu, Y., Li, S., et al. (2020). Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 92, 1518–1524. doi: 10.1002/jmv.25727
Lim, J., Jeon, S., Shin, H. Y., Kim, M. J., Seong, Y. M., Lee, W. J., et al. (2020). Case of the Index Patient Who Caused Tertiary Transmission of COVID-19 Infection in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. J. Korean Med. Sci. 35 (6), e79. doi: 10.3346/jkms.2020.35.e79
Liu, Y., Yang, Y., Zhang, C., Huang, F., Wang, F., Yuan, J., et al. (2020). Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 63 (3), 364–374. doi: 10.1007/s11427-020-1643-8
Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H., et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395 (10224), 565–574. doi: 10.1016/s0140-6736(20)30251-8
Ludvigsson, J. F. (2020). Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 109 (6), 1088–1095. doi: 10.1111/apa.15270
Ma, L. L., Wang, Y. Y., Yang, Z. H., Huang, D., Weng, H., Zeng, X. T. (2020). Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil. Med. Res. 7 (1), 7. doi: 10.1186/s40779-020-00238-8
Mehta, H. B., Ehrhardt, S., Moore, T. J., Segal, J. B., Alexander, G. C. (2020). Characteristics of registered clinical trials assessing treatments for COVID-19: a cross-sectional analysis. BMJ Open 10 (6), e039978. doi: 10.1136/bmjopen-2020-039978
Mustafa, S., Balkhy, H., Gabere, M. N. (2018). Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): A review. J. Infect. Public Health 11 (1), 9–17. doi: 10.1016/j.jiph.2017.08.009
NIH (2020). NIH halts clinical trial of hydroxychloroquine: Study shows treatment does no harm, but provides no benefit (National Institutes of Health). Available at: https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine (Accessed June 20, 2020).
Norris, S. L., Louis, H., Sawin, V. I., Porgo, T. V., Lau, Y. H. A., Wang, Q., et al. (2019). An evaluation of WHO emergency guidelines for Zika virus disease. J. Evid. Based Med. 12 (3), 218–224. doi: 10.1111/jebm.12347
RECOVERY Collaborative Group (2020). Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N. Engl. J. Med. NEJMoa2021436. doi: 10.1056/NEJMoa2021436
Ruberg, S. J., Akacha, M. (2017). Considerations for Evaluating Treatment Effects From Randomized Clinical Trials. Clin. Pharmacol. Ther. 102 (6), 917–923. doi: 10.1002/cpt.869
Sarma, P., Kaur, H., Kumar, H., Mahendru, D., Avti, P., Bhattacharyya, A., et al. (2020). Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis. J. Med. Virol. 92 (7), 776–785. doi: 10.1002/jmv.25898
Schulz, K. F., Grimes, D. A. (2002a). Allocation concealment in randomised trials: defending against deciphering. Lancet 359 (9306), 614–618. doi: 10.1016/s0140-6736(02)07750-4
Schulz, K. F., Grimes, D. A. (2002b). Blinding in randomised trials: hiding who got what. Lancet 359 (9307), 696–700. doi: 10.1016/s0140-6736(02)07816-9
Sessler, D. I., Imrey, P. B. (2015). Clinical Research Methodology 3: Randomized Controlled Trials. Anesth. Analg. 121 (4), 1052–1064. doi: 10.1213/ane.0000000000000862
Shang, L., Zhao, J., Hu, Y., Du, R., Cao, B. (2020). On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395 (10225), 683–684. doi: 10.1016/s0140-6736(20)30361-5
Shirato, K., Nao, N., Katano, H., Takayama, I., Saito, S., Kato, F., et al. (2020). Development of Genetic Diagnostic Methods for Novel Coronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis. 73 (4), 304–307. doi: 10.7883/yoken.JJID.2020.061
Tang, W., Cao, Z., Han, M., Wang, Z., Chen, J., Sun, W., et al. (2020). Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 369, m1849. doi: 10.1136/bmj.m1849
Tian, X., Li, C., Huang, A., Xia, S., Lu, S., Shi, Z., et al. (2020). Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 9 (1), 382–385. doi: 10.1080/22221751.2020.1729069
To, K. K., Tsang, O. T., Chik-Yan Yip, C., Chan, K. H., Wu, T. C., Chan, J. M. C., et al. (2020). Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 71 (15), 841–843. doi: 10.1093/cid/ciaa149
Wang, W. G., Hu, H., Song, L., Gong, X. M., Qu, Y. J., Lu, Z. Y. (2020). Image of pulmonary and diagnosis of atypical novel coronavirus, (2019-nCoV) infected pneumonia: case series of 14 patients. Yixue Xinzhi Zazhi 30 (1), 7–9. doi: 10.12173/j.issn.1004-5511.2020.01.04
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., et al. (2020). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395 (10236), 1569–1578. doi: 10.1016/S0140-6736(20)31022-9
WHO (2020). WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19 (World Health Organization). Available at: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 (Accessed July 4, 2020).
Yu, F., Du, L., Ojcius, D. M., Pan, C., Jiang, S. (2020). Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 22 (2), 74–79. doi: 10.1016/j.micinf.2020.01.003
Zhang, J. J., Dong, X., Cao, Y. Y., Yuan, Y. D., Yang, Y. B., Yan, Y. Q., et al. (2020). Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy 75 (7), 1730–1741. doi: 10.1111/all.14238
Zhang, R., Li, Y., Zhang, A. L., Wang, Y., Molina, M. J. (2020). Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. 117 (26), 14857–14863. doi: 10.1073/pnas.2009637117
Zhou, S. S., Lukula, S., Chiossone, C., Nims, R. W., Suchmann, D. B., Ijaz, M. K. (2018). Assessment of a respiratory face mask for capturing air pollutants and pathogens including human influenza and rhinoviruses. J. Thorac. Dis. 10 (3), 2059–2069. doi: 10.21037/jtd.2018.03.103
Zhou, D., Dai, S.-M., Tong, Q. (2020). COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 75 (7), 1667–1670. doi: 10.1093/jac/dkaa114
Keywords: coronavirus disease 2019, SARS-CoV-2, clinical trial, registration, ClinicalTrials.gov
Citation: Ma L-L, Yin X, Li B-H, Yang J-Y, Jin Y-H, Huang D, Deng T, Wang Y-Y, Ren X-Q, Ji J and Zeng X-T (2020) Coronavirus Disease 2019 Related Clinical Studies: A Cross-Sectional Analysis. Front. Pharmacol. 11:540187. doi: 10.3389/fphar.2020.540187
Received: 04 March 2020; Accepted: 14 August 2020;
Published: 02 September 2020.
Edited by:
Min Yang, Anhui Medical University, ChinaReviewed by:
Suyash Prasad, Independent Researcher, San Francisco, CA, United StatesJanet Sultana, University of Messina, Italy
Marc Henri De Longueville, UCB Pharma, Belgium
Copyright © 2020 Ma, Yin, Li, Yang, Jin, Huang, Deng, Wang, Ren, Ji and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-Qun Ren, cmVueHVlcXVuMDAxQDE2My5jb20=; Jianguang Ji, amlhbmd1YW5nLmppQG1lZC5sdS5zZQ==; Xian-Tao Zeng, emVuZ3hpYW50YW8xMTI4QDE2My5jb20=
†These authors share first authorship
 Lin-Lu Ma
Lin-Lu Ma Xuan Yin2†
Xuan Yin2† Bing-Hui Li
Bing-Hui Li Ying-Hui Jin
Ying-Hui Jin Di Huang
Di Huang Yun-Yun Wang
Yun-Yun Wang Xue-Qun Ren
Xue-Qun Ren Jianguang Ji
Jianguang Ji Xian-Tao Zeng
Xian-Tao Zeng