
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 21 August 2020
Sec. Drugs Outcomes Research and Policies
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.01307
This article is part of the Research Topic Drug Repurposing for COVID-19 Therapy View all 50 articles
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is responsible of variable clinical manifestations, ranging from no symptoms to severe pneumonia with acute respiratory distress syndrome, septic shock, and multi-organ failure resulting in death. To date no specific antiviral drug have been approved for COVID-19, so the treatment of the disease is mainly focused on symptomatic treatment and supportive care. Moreover, there are no treatments of proven efficacy to reduce the progression of the disease from mild/moderate to severe/critical. An activation of the coagulation cascade leading to severe hypercoagulability has been detected in these patients, therefore early anticoagulation may reduce coagulopathy, microthrombus formation, and the risk of organ damages. The role of heparin in COVID-19 is supported by a lot of studies describing its pleiotropic activity but it must be proven in clinical trials. Several protocols have been designed to assess the risk-benefit profile of heparin (low-molecular-weight or unfractionated heparin) in hospitalized subjects. Although prophylactic doses may be adequate in most patients, it is important to wait the results of clinical trials in order to define the appropriate effective dose able to improve disease outcome.
The clinical manifestations of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection range from asymptomatic infection to severe pneumonia with acute respiratory distress syndrome (ARDS), septic shock, and multi-organ failure resulting in death (Wang Y. et al., 2020).
A large Chinese epidemiological study showed that among 44,672 confirmed cases, 80.9% were mild, 13.8% severe, and 4.7% critical. The fatality rate for critical patients was 49%, higher in patients with comorbidities (cardiovascular disease 10.5%, diabetes 7.3%, chronic respiratory disease 6.5%, hypertension 6.0%, cancers 5.6%) than those without comorbidities (0.9%) (Wang Y. et al., 2020). Laboratory findings of Corona Virus Disease 19 (COVID-19) include lymphopenia with depletion of CD4 and CD8 lymphocytes, prolonged prothrombin time, elevated lactate dehydrogenase (LDH), D-Dimer, alanine transaminase, C-reactive protein (CRP), and creatinine kinase (Huang et al., 2020; Wang D. et al., 2020).
One of the most important mechanisms underlying the deterioration of disease is the cytokine storm (Shimabukuro-Vornhagen et al., 2018). This clinically severe phase is accompanied by high level of pro-inflammatory molecules, such as interferons α and β, and IL-6 (Mehta et al., 2020).
Severe disease is also complicated with coagulopathy and disseminated intravascular coagulation (DIC) has been reported in the majority of deaths (Tang et al., 2020a). Patients with progressive, severe COVID-19 infection with acute lung injury or ARDS have very high D-dimer and fibrinogen levels, related to a hypercoagulable state. Moreover, severe and critically ill COVID patients with prolonged immobilization are inherently at high risk of venous thromboembolism (VTE) and some patients who require mechanical ventilation may have acute pulmonary embolism (PE) or deep vein thrombosis (DVT), even without strong predisposing risk factors.
Thus, an early anticoagulation, which blocks uncontrolled blood clotting and reduce micro-thrombus formation, would lower the risk of major organ disfunction. Accordingly, even if the risk-benefit ratio has not been established, the World Health Organization (WHO) recommended in these patients thrombo-prophylaxis with either unfractionated or low molecular weight heparin (LMWH) (Driggin et al., 2020; WHO, 2020b; WHO, 2020a).
Aim of this work is to describe the link between inflammation, immune activation, and coagulopathy and the hypothetical pleiotropic role of heparin in COVID-19.
A variety of disorders (sepsis, systemic inflammatory conditions, trauma, malignant disease) lead to activation of the coagulation system, up to the most extreme form of DIC, and microvascular thrombosis is a frequent complication of critical illness conditions (Dhainaut et al., 2005; Ito, 2014).
Inflammation and coagulation are clearly linked by different molecular signals and their interactions play a major role in the pathophysiology of sepsis and DIC (Levi and Poll, 2015; Li and Ma, 2017).
Acute infections, including viral ones, induce a systemic inflammatory response and coagulation disruption (Subramaniam and Scharrer, 2018). The process is complex and multifactorial, involving cellular disruption and plasmatic elements of the hemostatic system and of the innate immune system to the pathogen (Gando et al., 2016). Thrombosis under certain circumstances plays a major physiological role in immune defense. The coagulation system and innate immunity (the so-called immunothrombosis system) play a beneficial role in early host defense against pathogens (Delvaeye and Conway, 2009; Fiusa et al., 2015), limiting microbial dissemination, protecting blood vessels, promoting recruitment and activation of leukocytes through fibrin, fibrinogen, and their degradation products, and stimulating cellular immune responses at the infection sites. Moreover, intravascular thrombi produce a distinct compartment where antimicrobial peptides can be concentrated and kept in contact with pathogens. However, aberrant or uncontrolled immunothrombosis may be harmful, determining an imbalance between pro-coagulant and anticoagulant mechanisms (Ito, 2014).
Multiple pathogenetic mechanisms have been identified in the coagulation cascade activation, and involving endothelial cells, von Willebrand factor, Toll-like receptor, and tissue-factor pathway (van Gorp et al., 1999; Ito, 2014). The effect is the deregulated thrombin generation, further worsened by the impairment of anticoagulant and fibrinolytic systems.
The pro-inflammatory mediators activate coagulation, which in turn promotes inflammatory activity (Opal, 2000; Russell, 2006; Hunt, 2014). In particular, inflammation promotes coagulation by leading to intravascular tissue factor expression, inducing the expression of leukocyte adhesion molecules on the endothelial cell, and down-regulating the fibrinolytic pathways by the up-regulation of plasminogen activator inhibitor-1 (PAI-1). On the other hand, thrombin stimulates inflammatory response in a self-propagating feedback loop.
The simultaneous impairment of pro-coagulant pathways and fibrinolytic systems as a result of systemic inflammation lead to platelet activation and fibrin deposition (Simmons and Pittet, 2015; Levi and van der Poll, 2017). It has been demonstrated that the most important mediators for orchestrating this imbalance during sepsis are cytokines (Levi et al., 1997), such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α), but also denatured DNA and cationic proteins, such as histones, released from damaged cells (McDonald et al., 2017)[21].
The final result of the uncontrolled activation of the coagulation system is multiple organ dysfunction (Iba and Levy, 2018; Li X. et al., 2020).
Moreover, it is relevant in the pathogenesis of specific organ damage, such as ARDS (MacLaren and Stringer, 2007; Frantzeskaki et al., 2017). The lung coagulopathy is related to a localized tissue factor-mediated thrombin generation, and depression of bronchoalveolar plasminogen activator-mediated fibrinolysis, mediated by the PAI-1 increase (Glas et al., 2013; Ozolina et al., 2016).
Thus, the involvement of the hemostatic system in severe COVID-19 is not surprising, being well documented that inflammation and sepsis are initiators of DIC (Voves et al., 2006). The most typical findings in patients with COVID-19 and coagulopathy are an increased D-dimer level, a modest decrease in platelet count, and a prolongation of the prothrombin time (Levi et al., 2020). The pattern is therefore different to that typically seen in sepsis, in which thrombocytopenia is more severe, and D-dimer not very high (Levi and Scully, 2018). In particular, markedly elevated D-dimer has been detected and associated with higher intensive care unit (ICU) admission and mortality, likely reflecting coagulation activation, cytokine storm development, and organ failure (Guan et al., 2020; Huang et al., 2020; Tang et al., 2020b; Zhou et al., 2020). Furthermore, post-mortem examinations show vascular thrombosis in small vessels of the lungs (Carsana et al., 2020; Menter et al., 2020; Wichmann et al., 2020), suggesting that the COVID-19 coagulopathy can include, besides a low-grade of DIC, a so-called “Pulmonary Intravascular Coagulopathy-PIC” (Belen-Apak and Sarialioglu, 2020; Fogarty et al., 2020; McGonagle et al., 2020), a localized pulmonary thrombotic micro-angiopathy determining organ damage (Levi et al., 2020).
It is believed that the coagulation cascade in COVID-2019 can be activated through the well-known mechanisms reported above, which lead to the deregulated thrombin generation both systemically and locally in the lungs, resulting in the deposition of fibrin with subsequent tissue damage and micro-angiopathy (Li T. et al., 2020). Moreover, SARS-CoV-2 would directly damage vascular endothelial cells through angiotensin-converting enzyme 2 (ACE2), which could represent the first injury triggering the abnormal coagulation in particular in the lung (Li H. et al., 2020). However, other studies showed that ACE2 pulmonary expression is restricted to type II pneumocytes, and is nearly absent in endothelial (McGonagle et al., 2020; Rivellese and Prediletto, 2020). In this context, the strict contact between type II pneumocytes and the pulmonary vascular network, and the severe local inflammatory reaction, is likely to drive the generalized pulmonary hypercoagulable state seen in patients with COVID-19 (Li H. et al., 2020; McGonagle et al., 2020; Rivellese and Prediletto, 2020). Nevertheless, the mechanisms contributing to coagulopathy in COVID-19 have to be comprehensively clarified yet.
To date, treatment of coagulopathy/DIC has been focused on the target of the primary associated pathology (Levi and Scully, 2018). This is limited in the case of COVID-19, due to the lack of approved antiviral drug treatment, so the management of patients is mainly focused on symptomatic and supportive care. Moreover, there are no treatments of proven efficacy to reduce the progression of the disease from mild/moderate to severe/critical, in particular counteracting the cytokine storm (Chen et al., 2020). However, reducing the release or activity of pro-inflammatory mediators can prevent or reverse the uncontrolled hyper-inflammation, thereby improving the condition of patients and a lot of drugs with this aim are under evaluation in clinical trials.
The use of anticoagulants for patients with severe COVID-19 has been recommended by expert consensus and by WHO (Driggin et al., 2020; WHO, 2020b).
The International Society of Thrombosis and Haemostasis (ISTH) introduced a new category identifying an earlier phase of sepsis-associated DIC, called “sepsis‐induced coagulopathy” (SIC) (Iba et al., 2019). In this case or in patients with markedly elevated D-dimers, LMWH at prophylactic dose should be considered (Tang et al., 2020a).
The optimal thrombo-prophylactic regimen in patients with COVID-19 is unknown (Driggin et al., 2020). Given drug-drug interaction with direct oral anticoagulants and some anti-viral regimens, heparins, either unfractionated or low molecular weight, may be preferred.
Accurate patient assessment is necessary to balance the individual risk of thrombosis and bleeding. Therapeutic anticoagulation is not required unless another indication for therapeutic anticoagulation is documented (e.g. VTE, atrial fibrillation, or mechanical valve). Moreover, evidence of coagulopathy/DIC and especially elevated D-dimer levels observed even in early phase of PIC might be useful to guide therapeutic decision (Lillicrap, 2020).
Prophylactic dose LMWH is recommended for all hospitalized COVID-19 patients in the absence of contraindications.
However, standard prophylactic regimens may be insufficient in severe and critically ill patients with variable thromboembolic/bleeding risk, and monitoring of anti-Xa activity may be considered when LMWH is used in these patients (Duranteau et al., 2018).
In cases where there are no contraindications, empiric therapeutic anticoagulation has been proposed by the American Society of Hematology in the following cases (Ash, 2020):
● intubated patients who develop sudden clinical and laboratory findings highly consistent with PE;
● patients with physical findings consistent with thrombosis (superficial thrombophlebitis, peripheral ischemia or cyanosis, thrombosis of dialysis filters, tubing, or catheters);
● patients with respiratory failure, particularly when D-dimer and/or fibrinogen levels are very high, in whom PE or microvascular thrombosis is highly suspected and other causes are not identified (e.g., ARDS, fluid overload).
A normal level D-dimer level provides reasonable confidence that anticoagulation should continue at prophylactic doses.
However, the efficacy and safety of anticoagulation as well as the appropriate dose regimen able to improve disease outcome in patients with COVID-19 have yet to be defined in clinical trials.
Although primarily employed for its anticoagulant properties, it is known that heparin possesses anti-inflammatory, immunomodulatory, anti-viral, and anti-complement activity which may offer benefit beyond the anti-coagulation (Davidson et al., 2002; Hoppensteadt et al., 2008; Young, 2008; Ludwig, 2009; Li et al., 2012; Li et al., 2014; Li et al., 2015; Li and Ma, 2017; Thachil, 2020).
Heparin is a member of a family of polyanionic polysaccharides called glycosaminoglycans (Young, 2008). It remains one of the most important anticoagulant drugs in clinical practice, currently used for the prevention and treatment of venous thrombosis and PE, the management of arterial thrombosis in patients with acute myocardial infarction and in the prevention of re-thrombosis after thrombolysis, and the prevention of thrombosis in extracorporeal circuits and hemodialysis.
The mechanisms behind its pleiotropic effect are complex and not completely understood.
Its polyanionic nature allows to bind sites proteins such as antithrombin III, but also cytokines, chemokines, growth factors, adhesion molecules, cytotoxic peptides, tissue destructive enzymes, involved in inflammation (Day et al., 2004). Thus, the binding of acute phase and complement proteins may contribute to the anti-inflammatory activity of heparin (Weiler et al., 1992; Young et al., 1997).
Indeed, even if the binding of released cytokines may protect them from proteolytic degradation, heparin may alter the secondary and tertiary structure of cytokines and prevent the binding to their specific receptors (Balasubramanian and Ramanathan, 2000; Mummery and Rider, 2000; Jayanthi et al., 2017), thus, influencing their biological activity, limiting accumulation of inflammatory cells and activation and subsequent tissue damage. When given in pharmacological doses, exogenous heparin and heparinoids demonstrated to attenuate tissue damage, neutralizing a variety of mediators released from inflammatory cells (Elsayed and Becker, 2003).
In line with this assumption, a large number of studies have revealed that LMWH reduce the release and the biological activity of IL-6 and IL-8 (Qian et al., 2014; Shastri et al., 2015; Li et al., 2016; Liu et al., 2019).
In addition, heparin binding to P-selectin showed to inhibit leukocyte adhesion to endothelial cells, independently by its anticoagulant activity (Lever et al., 2000).
The dysfunction of endothelial cells and the reduction of glycocalyx are key characteristics of sepsis. Heparin, as a heparan sulphate (HS) analogue, may reconstitute the protective layer of proteoglycans to restore the natural vascular barrier (Nelson et al., 2008). The protective function on the endothelial tight junctions has been demonstrated in a model of lung damage induced by lipopolysaccharide, where heparin administration decreased edema and vascular leakage (Liu et al., 2019).
Moreover, the protective responses observed with heparin in experimental models of sepsis seem to be mediated by blocking the pro-inflammatory signaling pathways regulated by MAPK, NF-κB, and STAT3 (Iba and Levy, 2018; Li X. et al., 2020). It has been demonstrated that heparin is readily bound and internalized into the cytosolic compartment, where it can prevent the NF-κB translocation to the nucleus through the binding of the positively charged nuclear localization sequence (Letourneur et al., 1995; Akimoto et al., 1996; Dudas et al., 2000). Blocking of this transcriptional factor can reduce inflammatory gene activation and regulate the production of pro-inflammatory cytokines, chemokines, and adhesion molecules.
A novel immune-modulating mechanism of heparin related to blockage of circulating histones has been studied in vitro and in septic mouse models (Wildhagen et al., 2014). It is noteworthy that extracellular histones released from dead cells play important role in cellular damage and are robustly associated with endothelial dysfunction, organ dysfunction and even death during sepsis (Xu et al., 2009; Ekaney et al., 2014; Iba et al., 2015). Heparin demonstrated a strong affinity for extracellular histones and prevents their interaction with platelets, a potential mechanism contributing to the regulation of inflammation (Fuchs et al., 2011; Alhamdi et al., 2016).
Finally, the putative antiviral role of heparin has been studied in experimental models. Thanks to its polyanionic nature, heparin can bind to several proteins, such as cell surface glycoproteins and thus inhibit herpes simplex virus attachment (Shukla and Spear, 2001). Furthermore it has been demonstrated that in zika virus infection it prevents virus-induced cell death (Ghezzi et al., 2017).
Interestingly, in vitro and in vivo experimental studies have shown that human coronaviruses utilize heparin sulfate proteoglycans for attachment to target cells (Milewska et al., 2014), and interaction between the SARS-CoV-2 Spike S1 protein receptor binding domain (SARS-CoV-2 S1 RBD) and heparin has been recently showed, supporting the role of heparin in the therapeutic armamentarium against COVID-19 beyond the anticoagulant effect (Courtney Mycroft-West et al., 2020).
However, the exact benefit and safety of heparin as anti-inflammatory and antiviral agent in clinical setting are yet to be defined and conflicting results have been reported by previous clinical trials.
According to systematic reviews and meta-analyses regarding the use of heparin as a potential treatment for patients with sepsis, treatment with low doses of heparin is associated with significantly reduced 28-day mortality in sepsis (Liu et al., 2014; Wang et al., 2014; Zarychanski et al., 2015; Fan et al., 2016).
Another meta-analysis shows a reduction of the risk of 7-day and of 28-day mortality, and a significant improvement of PaO2/FiO2 ratio in patients with ARDS treated with high-dose LMWH (Li et al., 2018), demonstrating that treatment with heparin may be helpful in mitigating the pulmonary coagulopathy found in ARDS.
The existing evidence on the use of heparin to prevent or treat thrombotic complications in COVID-19 derives from retrospective and observational data.
Recently, a retrospective cohort study analyzed the relieving effect of LMWH in patients with COVID-19, to investigate the anti-inflammatory effects of heparin and the delay of disease progression (Shi C et al., 2020). Compared to the control group, patients treated with heparin had an improvement of hypercoagulability, a reduction of IL-6 and neutralization of its biological activity, and an increase in the percentage of lymphocytes. A large retrospective cohort showed lower mortality in COVID-19 patients treated with heparin, even after adjustment for age and gender (OR 95% CI 0.55, 0.37–0.82; p = 0.003), saturation of oxygen <90%, and temperature >37°C (OR 0.54, 0.36–0.82; p = 0.003), and use of concomitant medications (OR 0.42, 0.26–0.66; p < 0.001) (Ayerbe et al., 2020). Moreover, a recent observational study conducted in US found a reduced risk of mortality among patients (n = 786) hospitalized with COVID-19 who received anticoagulation (Paranjpe et al., 2020).
Randomized controlled trials are necessary to confirm these preliminary observations.
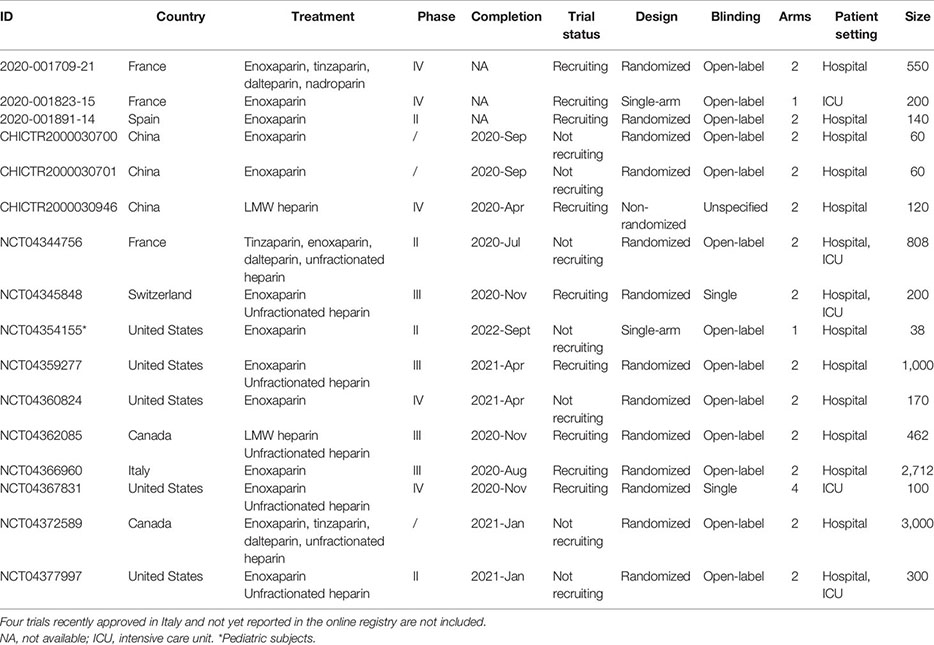
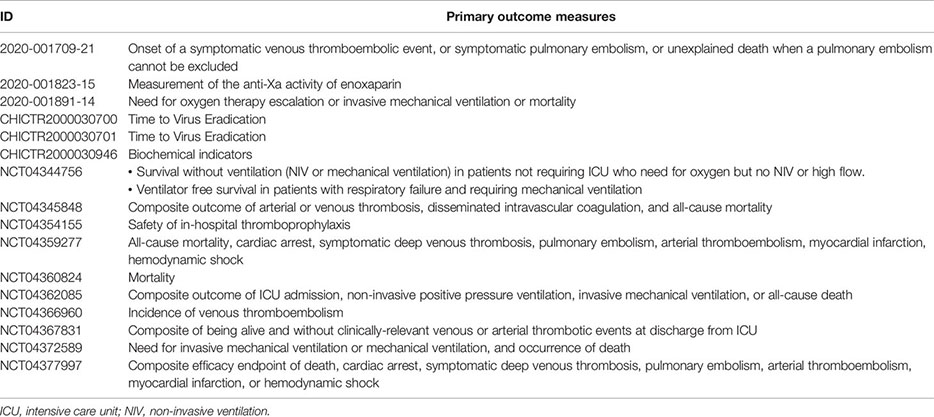
As reported on the COVID-19 clinical trials registry (http://www.covid-trials.org, 2020) which collects all trials from International Clinical Trials Registry Platform (Chinese Clinical Trial Registry, ClinicalTrials.gov, Clinical Research Information Service—Republic of Korea, EU Clinical Trials Register, ISRCTN, Iranian Registry of Clinical Trials, Japan Primary Registries Network, and German Clinical Trials Register), 16 clinical trials are ongoing (9/16 recruiting and 7/16 not-recruiting) to evaluate the effect of anticoagulation with heparin (low-molecular-weight—mainly enoxaparin—or unfractionated heparin) in hospitalized patients with COVID-19 (Appendix 1). More than 80% of these studies are open-label, randomized, two-arm trials, and at least 75% of protocols include a comparison between therapeutic anticoagulation (investigational arm) and thromboprophylaxis (control arm), in line with the uncertainty about the benefit/risk ratio of the two treatment strategies. As reported in Appendix 2, the primary outcome measures of heparin clinical trials are hard endpoints such as mortality or composite measure of clinical events and/or survival, as recommended by the WHO guidelines (WHO, 2020d).
Overall, almost 10,000 patients are expected to be enrolled. However, the completion of some studies (expected in the second half of 2020 and in 2021) would be difficult at least in European countries and China due to the reduction in the number of new cases and hospitalizations (WHO, 2020c).
Coagulation activation has been reported in COVID-19, determining pathological changes specifically involving the lung microvasculature, and an increased risk of DVT, PE, and DIC in severe phase. The use of anticoagulants, in particular heparin, is recommended by expert consensus for patients with severe COVID-19, although a final guidance cannot be implemented yet.
There are several ways in which probably heparin administration can benefit patients with COVID-19, beyond the anticoagulant effect.
Although prophylactic doses may be adequate in most patients, it would be important to administer therapeutic dosage based on the individual risk of coagulopathy and thrombosis. To assess the efficacy and safety in patients with COVID-19 in clinical trials is crucial in order to find the appropriate effective dose of LMWH/UFH and improve disease outcomes. Different well-designed clinical trials (randomized, controlled, with appropriate outcome measures, even if not-blinded) are ongoing. However, the completion of trials and the consequent definition of risk/benefit profile of drugs candidate for COVID-19 would be complicated by the reduced (albeit strongly awaited) spread of the virus.
LG wrote the first draft of the manuscript. PV and FD checked and revised the draft manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akimoto, H., Ito, H., Tanaka, M., Adachi, S., Hata, M., Lin, M., et al. (1996). Heparin and heparan sulfate block angiotensin II-induced hypertrophy in cultured neonatal rat cardiomyocytes. A possible role of intrinsic heparin-like molecules in regulation of cardiomyocyte hypertrophy. Circulation 93, 810–816. doi: 10.1161/01.CIR.93.4.810
Alhamdi, Y., Abrams, S. T., Lane, S., Wang, G., Toh, C. H. (2016). Histone-Associated Thrombocytopenia in Patients Who Are Critically Ill. JAMA 315, 817–819. doi: 10.1001/jama.2016.0136
Ash (2020). COVID-19 and Pulmonary Embolism: Frequently Asked Questions. Version 2.0. Available at: https://http://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism (Accessed May 2020).
Ayerbe, L., Risco, C., Ayis, S. (2020). The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. Thrombolysis 50, 298–301. doi: 10.1007/s11239-020-02162-z
Balasubramanian, V., Ramanathan, M. (2000). Glycosaminoglycans alter the conformation of interferon-gamma. Cytokine 12, 466–471. doi: 10.1006/cyto.1999.0592
Belen-Apak, F. B., Sarialioglu, F. (2020). Pulmonary intravascular coagulation in COVID-19: possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J. Thromb. Thrombolysis 50, 278–280. doi: 10.1007/s11239-020-02129-0
Carsana, L., Sonzogni, A., Nasr, A., Rossi, R. S., Pellegrinelli, P., Zerbi, P., et al. (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study [published online ahead of print, 2020 Jun 8]. Lancet. Infect. Dis. S1473-3099 (20), 30434–5. doi: 10.1016/S1473-3099(20)30434-5
Chen, C., Zhang, X. R., Ju, Z. Y., He, W. F. (2020). Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi 36 (6), 471–475. doi: 10.3760/cma.j.cn501120-20200224-00088
Courtney Mycroft-West, Su, D., Elli, S., Guimond, S., Miller, G., Turnbull, V., et al. (2020). The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. doi: 10.1101/2020.02.29.971093
Davidson, B. L., Geerts, W. H., Lensing, A. W. (2002). Low-dose heparin for severe sepsis. N Engl. J. Med. 347, 1036–1037. doi: 10.1056/NEJM200209263471316
Day, J. R., Landis, R. C., Taylor, K. M. (2004). Heparin is much more than just an anticoagulant. J. Cardiothorac Vasc. Anesth. 18, 93–100. doi: 10.1053/j.jvca.2003.10.021
Delvaeye, M., Conway, E. M. (2009). Coagulation and innate immune responses: can we view them separately? Blood 114, 2367–2374. doi: 10.1182/blood-2009-05-199208
Dhainaut, J. F., Shorr, A. F., Macias, W. L., Kollef, M. J., Levi, M., Reinhart, K., et al. (2005). Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit. Care Med. 33, 341–348. doi: 10.1097/01.CCM.0000153520.31562.48
Driggin, E., Madhavan, M. V., Bikdeli, B., Chuich, T., Laracy, J., Bondi-Zoccai, G., et al. (2020). Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the Coronavirus Disease 2019 (COVID-19) Pandemic. J. Am. Coll. Cardiol. 75 (18), 2352–2371. doi: 10.1016/j.jacc.2020.03.031
Dudas, J., Ramadori, G., Knittel, T., Neubauer, K., Raddatz, D., Egedy, K., et al. (2000). Effect of heparin and liver heparan sulphate on interaction of HepG2-derived transcription factors and their cis-acting elements: altered potential of hepatocellular carcinoma heparan sulphate. Biochem. J. 350(Pt 1), 245–251. doi: 10.1042/bj3500245
Duranteau, J., Taccone, F. S., Verhamme, P., Ageno, W., Force, E. V. G. T. (2018). European guidelines on perioperative venous thromboembolism prophylaxis: Intensive care. Eur. J. Anaesthesiol 35, 142–146. doi: 10.1097/EJA.0000000000000707
Ekaney, M. L., Otto, G. P., Sossdorf, M., Sponholz, C., Boehringer, M., Loesche, W., et al. (2014). Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit. Care 18, 543. doi: 10.1186/s13054-014-0543-8
Elsayed, E., Becker, R. C. (2003). The impact of heparin compounds on cellular inflammatory responses: a construct for future investigation and pharmaceutical development. J. Thromb. Thrombolysis 15, 11–18. doi: 10.1023/A:1026184100030
Fan, Y., Jiang, M., Gong, D., Zou, C. (2016). Efficacy and safety of low-molecular-weight heparin in patients with sepsis: a meta-analysis of randomized controlled trials. Sci. Rep. 6, 25984. doi: 10.1038/srep25984
Fiusa, M. M., Carvalho-Filho, M. A., Annichino-Bizzacchi, J. M., De Paula, E. V. (2015). Causes and consequences of coagulation activation in sepsis: an evolutionary medicine perspective. BMC Med. 13, 105. doi: 10.1186/s12916-015-0327-2
Fogarty, H., Townsend, L., Ni Cheallaigh, C., Bergin, C., Martin-Loeches, I., Browne, P., et al. (2020). COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 189 (6), 1044–1049. doi: 10.1111/bjh.16749
Frantzeskaki, F., Armaganidis, A., Orfanos, S. E. (2017). Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respiration 93, 212–225. doi: 10.1159/000453002
Fuchs, T. A., Bhandari, A. A., Wagner, D. D. (2011). Histones induce rapid and profound thrombocytopenia in mice. Blood 118, 3708–3714. doi: 10.1182/blood-2011-01-332676
Gando, S., Levi, M., Toh, C. H. (2016). Disseminated intravascular coagulation. Nat. Rev. Dis. Primers 2, 16037. doi: 10.1007/978-3-319-28308-1_13
Ghezzi, S., Cooper, L., Rubio, A., Pagani, I., Capobianchi, M. R., Ippolito, G., et al. (2017). Heparin prevents Zika virus induced-cytopathic effects in human neural progenitor cells. Antiviral Res. 140, 13–17. doi: 10.1016/j.antiviral.2016.12.023
Glas, G. J., Van Der Sluijs, K. F., Schultz, M. J., Hofstra, J. J., Van Der Poll, T., Levi, M. (2013). Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J. Thromb. Haemost. 11, 17–25. doi: 10.1111/jth.12047
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., He, J. X., et al. (2020). Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl. J. Med. 382, 1708–1720. doi: 10.1056/NEJMoa2002032
Hoppensteadt, D., Fareed, J., Klein, A. L., Jasper, S. E., Apperson-Hansen, C., Lieber, E. A., et al. (2008). Comparison of anticoagulant and anti-inflammatory responses using enoxaparin versus unfractionated heparin for transesophageal echocardiography-guided cardioversion of atrial fibrillation. Am. J. Cardiol. 102, 842–846. doi: 10.1016/j.amjcard.2008.05.025
Http://Www.Covid-Trials.Org (2020). Global Coronavirus COVID-19 Clinical Trial Tracker. Available at: http://www.covid-trials.org (Accessed May 2020).
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi: 10.1016/S0140-6736(20)30183-5
Hunt, B. J. (2014). Bleeding and coagulopathies in critical care. N Engl. J. Med. 370, 2153. doi: 10.1056/NEJMra1208626
Iba, T., Levy, J. H. (2018). Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 16, 231–241. doi: 10.1111/jth.13911
Iba, T., Hashiguchi, N., Nagaoka, I., Tabe, Y., Kadota, K., Sato, K. (2015). Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intensive Care Med. Exp. 3, 36. doi: 10.1186/s40635-015-0072-z
Iba, T., Levy, J. H., Warkentin, T. E., Thachil, J., Van Der Poll, T., Levi, M., et al. (2019). Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 17, 1989–1994. doi: 10.1111/jth.14578
Ito, T. (2014). PAMPs and DAMPs as triggers for DIC. J. Intensive Care 2, 67. doi: 10.1186/s40560-014-0065-0
Jayanthi, S., Koppolu, B. P., Nguyen, K. G., Smith, S. G., Felber, B. K., Kumar, T. K. S., et al. (2017). Modulation of Interleukin-12 activity in the presence of heparin. Sci. Rep. 7, 5360. doi: 10.1038/s41598-017-05382-1
Letourneur, D., Caleb, B. L., Castellot, J. J., Jr. (1995). Heparin binding, internalization, and metabolism in vascular smooth muscle cells: I. Upregulation of heparin binding correlates with antiproliferative activity. J. Cell Physiol. 165, 676–686. doi: 10.1002/jcp.1041650327
Lever, R., Hoult, J. R., Page, C. P. (2000). The effects of heparin and related molecules upon the adhesion of human polymorphonuclear leucocytes to vascular endothelium in vitro. Br. J. Pharmacol. 129, 533–540. doi: 10.1038/sj.bjp.0703099
Levi, M., Poll, T. (2015). Coagulation in patients with severe sepsis. Semin. Thromb. Hemost. 41, 9–15. doi: 10.1055/s-0034-1398376
Levi, M., Scully, M. (2018). How I treat disseminated intravascular coagulation. Blood 131, 845–854. doi: 10.1182/blood-2017-10-804096
Levi, M., van der Poll, T. (2017). Coagulation and sepsis. Thromb. Res. 149, 38–44. doi: 10.1016/j.thromres.2016.11.007
Levi, M., van Der Poll, T., Ten Cate, H., Van Deventer, S. J. (1997). The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur. J. Clin. Invest. 27, 3–9. doi: 10.1046/j.1365-2362.1997.570614.x
Levi, M., Thachil, J., Iba, T., Levy, J. H. (2020). Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 7, e438–e440. doi: 10.1016/S2352-3026(20)30145-9
Li, X., Ma, X. (2017). The role of heparin in sepsis: much more than just an anticoagulant. Br. J. Haematol. 179, 389–398. doi: 10.1111/bjh.14885
Li, X., Zheng, Z., Li, X., Ma, X. (2012). Unfractionated heparin inhibits lipopolysaccharide-induced inflammatory response through blocking p38 MAPK and NF-kappaB activation on endothelial cell. Cytokine 60, 114–121. doi: 10.1016/j.cyto.2012.06.008
Li, X., Li, X., Zheng, Z., Liu, Y., Ma, X. (2014). Unfractionated heparin suppresses lipopolysaccharide-induced monocyte chemoattractant protein-1 expression in human microvascular endothelial cells by blocking Kruppel-like factor 5 and nuclear factor-kappaB pathway. Immunobiology 219, 778–785. doi: 10.1016/j.imbio.2014.06.005
Li, X., Liu, Y., Wang, L., Li, Z., Ma, X. (2015). Unfractionated heparin attenuates LPS-induced IL-8 secretion via PI3K/Akt/NF-kappaB signaling pathway in human endothelial cells. Immunobiology 220, 399–405. doi: 10.1016/j.imbio.2014.10.008
Li, X., Ma, Y., Chen, T., Tang, J., Ma, X. (2016). Unfractionated heparin inhibits lipopolysaccharide-induced expression of chemokines in human endothelial cells through nuclear factor-KappaB signaling pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 28, 117–121. doi: 10.3760/cma.j.issn.2095-4352.2016.02.007
Li, H., Zhang, L. L. D., Xu, J., Dai, H., Tang, N., Su, X., et al. (2020). SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 395 (10235), 1517–1520. doi: 10.1016/S0140-6736(20)30920-X
Li, T., Lu, H., Zhang, W. (2020). Clinical observation and management of COVID-19 patients. Emerg. Microbes Infect. 9, 687–690. doi: 10.1080/22221751.2020.1741327
Li, X., Li, L., Shi, Y., Yu, S., Ma, X. (2020). Different signaling pathways involved in the anti-inflammatory effects of unfractionated heparin on lipopolysaccharide-stimulated human endothelial cells. J. Inflammation (Lond.) 17, 5. doi: 10.1186/s12950-020-0238-7
Li, J., Li, Y., Yang, B., Wang, H., Li, L. (2018). Low-molecular-weight heparin treatment for acute lung injury/acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 11, 414–422.
Lillicrap, D. (2020) Disseminated intravascular coagulation in patients with 2019- nCoV pneumonia. J. Thromb. Haemost. 00, 1–2. doi: 10.1111/jth.14781
Liu, Z., Zhu, H., Ma, X. (2014). Heparin for treatment of sepsis: a systemic review. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 26, 135–141. doi: 10.3760/cma.j.issn.2095-4352.2014.03.003
Liu, Y., Mu, S., Li, X., Liang, Y., Wang, L., Ma, X. (2019). Unfractionated Heparin Alleviates Sepsis-Induced Acute Lung Injury by Protecting Tight Junctions. J. Surg. Res. 238, 175–185. doi: 10.1016/j.jss.2019.01.020
Ludwig, R. J. (2009). Therapeutic use of heparin beyond anticoagulation. Curr. Drug Discovery Technol. 6, 281–289. doi: 10.2174/157016309789869001
MacLaren, R., Stringer, K. A. (2007). Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy 27, 860–873. doi: 10.1592/phco.27.6.860
McDonald, B., Davis, R. P., Kim, S. J., Tse, M., Esmon, C. T., Kolaczkowska, E., et al. (2017). Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129, 1357–1367. doi: 10.1182/blood-2016-09-741298
McGonagle, D., O'Donnell, J. S. ,., Sharif, K., Emery, P., Bridgewood, C. (2020). Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2, e437–45. doi: 10.1016/S2665-9913(20)30121-1
Mehta, P., Mcauley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., Manson, J. J., et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034. doi: 10.1016/S0140-6736(20)30628-0
Menter, T., Haslbauer, J. D., Nienhold, R., Savic, S., Hopfer, H., Deigendesch, N., et al. (2020). Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 77 (2), 198–209. doi: 10.1111/his.14134
Milewska, A., Zarebski, M., Nowak, P., Stozek, K., Potempa, J., Pyrc, K. (2014). Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 88, 13221–13230. doi: 10.1128/JVI.02078-14
Mummery, R. S., Rider, C. C. (2000). Characterization of the heparin-binding properties of IL-6. J. Immunol. 165, 5671–5679. doi: 10.4049/jimmunol.165.10.5671
Nelson, A., Berkestedt, I., Schmidtchen, A., Ljunggren, L., Bodelsson, M. (2008). Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock 30, 623–627. doi: 10.1097/SHK.0b013e3181777da3
Opal, S. M. (2000). Therapeutic rationale for antithrombin III in sepsis. Crit. Care Med. 28, S34–S37. doi: 10.1097/00003246-200009001-00008
Ozolina, A., Sarkele, M., Sabelnikovs, O., Skesters, A., Jaunalksne, I., Serova, J., et al. (2016). Activation of Coagulation and Fibrinolysis in Acute Respiratory Distress Syndrome: A Prospective Pilot Study. Front. Med. (Lausanne) 3:64. doi: 10.3389/fmed.2016.00064
Paranjpe, I., Fuster, V., Lala, A., Russak, A. J., Glicksberg, B. S., Levin, M. A., et al. (2020). Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 76 (1), 122–124. doi: 10.1016/j.jacc.2020.05.001
Qian, Y., Xie, H., Tian, R., Yu, K., Wang, R. (2014). Efficacy of low molecular weight heparin in patients with acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. COPD 11, 171–176. doi: 10.3109/15412555.2013.831062
Rivellese, F., Prediletto, E. (2020). ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun. Rev. 19, 102536. doi: 10.1016/j.autrev.2020.102536
Russell, J. A. (2006). Management of sepsis. N Engl. J. Med. 355, 1699–1713. doi: 10.1056/NEJMra043632
Shastri, M. D., Stewart, N., Horne, J., Peterson, G. M., Gueven, N., Sohal, S. S., et al. (2015). In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One 10, e0126763. doi: 10.1371/journal.pone.0126763
Shi C, W. C., Wang, H., Yang, C., Cai, F., Zeng, F., Cheng, F., et al. (2020). The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective clinical study. doi: 10.1101/2020.03.28.20046144
Shimabukuro-Vornhagen, A., Godel, P., Subklewe, M., Stemmler, H. J., Schlosser, H. A., Schlaak, M., et al. (2018). Cytokine release syndrome. J. Immunother. Cancer 6, 56. doi: 10.1186/s40425-018-0343-9
Shukla, D., Spear, P. G. (2001). Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Invest. 108, 503–510. doi: 10.1172/JCI200113799
Simmons, J., Pittet, J. F. (2015). The coagulopathy of acute sepsis. Curr. Opin. Anaesthesiol 28, 227–236. doi: 10.1097/ACO.0000000000000163
Subramaniam, S., Scharrer, I. (2018). Procoagulant activity during viral infections. Front. Biosci. (Landmark Ed) 23, 1060–1081. doi: 10.2741/4633
Tang, N., Bai, H., Chen, X., Gong, J., Li, D., Sun, Z. (2020a). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 18 (5), 1094–1099. doi: 10.1111/jth.14817
Tang, N., Li, D., Wang, X., Sun, Z. (2020b). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 18, 844–847. doi: 10.1111/jth.14768
Thachil, J. (2020). The versatile heparin in COVID-19. J. Thromb. Haemost. 18 (5), 1020–1022. doi: 10.1111/jth.14821
van Gorp, E. C., Suharti, C., Ten Cate, H., Dolmans, W. M., Van Der Meer, J. W., Ten Cate, J. W., et al. (1999). Review: infectious diseases and coagulation disorders. J. Infect. Dis. 180, 176–186. doi: 10.1086/314829
Voves, C., Wuillemin, W. A., Zeerleder, S. (2006). International Society on Thrombosis and Haemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patients. Blood Coagul. Fibrinolysis 17, 445–451. doi: 10.1097/01.mbc.0000240916.63521.2e
Wang, C., Chi, C., Guo, L., Wang, X., Guo, L., Sun, J., et al. (2014). Heparin therapy reduces 28-day mortality in adult severe sepsis patients: a systematic review and meta-analysis. Crit. Care 18, 563. doi: 10.1186/s13054-014-0563-4
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., et al. (2020). Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323 (11), 1061–1069. doi: 10.1001/jama.2020.1585
Wang, Y., Wang, Y., Chen, Y., Qin, Q. (2020). Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 92 (6), 568–576. doi: 10.1002/jmv.25748
Weiler, J. M., Edens, R. E., Linhardt, R. J., Kapelanski, D. P. (1992). Heparin and modified heparin inhibit complement activation in vivo. J. Immunol. 148, 3210–3215.
WHO (2020a). Clinical Care for Severe Acute Respiratory Infection. Available at: https://www.who.int/publications/i/item/clinical-care-of-severe-acute-respiratory-infections-tool-kit (Accessed July 2020).
WHO (2020b). Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance V 1.2. Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (Accessed July 2020).
WHO (2020c). Available at: https://covid19.who.int (Accessed July 2020).
Wichmann, D., Sperhake, J. P., Lutgehetmann, M., Steurer, S., Edler, C., Heinemann, A., et al. (2020). Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. M20–2003. doi: 10.7326/M20-2003
Wildhagen, K. C., Garcia De Frutos, P., Reutelingsperger, C. P., Schrijver, R., Areste, C., Ortega-Gomez, A., et al. (2014). Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 123, 1098–1101. doi: 10.1182/blood-2013-07-514984
Xu, J., Zhang, X., Pelayo, R., Monestier, M., Ammollo, C. T., Semeraro, F., et al. (2009). Extracellular histones are major mediators of death in sepsis. Nat. Med. 15, 1318–1321. doi: 10.1038/nm.2053
Young, E., Podor, T. J., Venner, T., Hirsh, J. (1997). Induction of the acute-phase reaction increases heparin-binding proteins in plasma. Arterioscler. Thromb. Vasc. Biol. 17, 1568–1574. doi: 10.1161/01.ATV.17.8.1568
Young, E. (2008). The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 122, 743–752. doi: 10.1016/j.thromres.2006.10.026
Zarychanski, R., Abou-Setta, A. M., Kanji, S., Turgeon, A. F., Kumar, A., Houston, D. S., et al. (2015). The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit. Care Med. 43, 511–518. doi: 10.1097/CCM.0000000000000763
Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062. doi: 10.1016/S0140-6736(20)30566-3
Keywords: COVID-19, coagulopathy, heparin, pleiotropic activity, clinical trials
Citation: Gozzo L, Viale P, Longo L, Vitale DC and Drago F (2020) The Potential Role of Heparin in Patients With COVID-19: Beyond the Anticoagulant Effect. A Review. Front. Pharmacol. 11:1307. doi: 10.3389/fphar.2020.01307
Received: 15 June 2020; Accepted: 07 August 2020;
Published: 21 August 2020.
Edited by:
Brian Godman, Karolinska Institutet (KI), SwedenReviewed by:
Amos Yared Massele, University of Botswana, BotswanaCopyright © 2020 Gozzo, Viale, Longo, Vitale and Drago. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filippo Drago, Zi5kcmFnb0B1bmljdC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.