
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 02 July 2020
Sec. Experimental Pharmacology and Drug Discovery
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.01013
This article is part of the Research Topic Coronavirus Disease (COVID-19): Molecular Mechanisms, Translational Approaches and Therapeutics View all 118 articles
 Zhonglei Wang1,2*
Zhonglei Wang1,2* Liyan Yang3
Liyan Yang3The novel and highly pathogenic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has become a continued focus of global attention due to the serious threat it poses to public health. There are no specific drugs available to combat SARS-CoV-2 infection. Natural products (carolacton, homoharringtonine, emetine, and cepharanthine) and natural product-inspired small molecules (ivermectin, GS-5734, EIDD-2801, and ebselen) are potential anti-SARS-CoV-2 agents that have attracted significant attention due to their broad-spectrum antiviral activities. Here, we review the research on potential landmark anti-SARS-CoV-2 agents, systematically discussing the importance of natural products and natural-product-inspired small molecules in the research and development of safe and effective antiviral agents.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to be the subject of global attention due to the serious threat it poses to public health (Wang et al., 2020; Zhu et al., 2020). Currently, there are no specific drugs available to combat SARS-CoV-2 (Kupferschmidt and Cohen, 2020). The epidemic is ongoing and, as of 12 June, 2020, the World Health Organization (WHO) reported that there had been 7,410,510 confirmed cases worldwide, including 418,294 deaths (Coronavirus disease (COVID-2019)).
This means that more aggressive trials of drugs to prevent and treat SARS-CoV-2 infection should be intensely pursued across the globe (Kalil, 2020). Therefore, there is an urgent need to identify agents for treating SARS-CoV-2 infection (Li and De Clercq, 2020). As a major source for drugs and drug leads, natural products and natural-product-inspired agents have attracted significant attention, and they have played an integral role in the treatment of many different conditions (Newman and Cragg, 2020). Since the start of the multinational COVID-19 outbreak, significant progress has been made in identifying natural products and natural-product-inspired small molecules that may serve as anti-SARS-CoV-2 drugs. This review systematically discusses the current progress regarding potential anti-SARS-CoV-2 natural products and natural-product-inspired small molecules.
Natural products possess tremendous structural diversity and unique chemical diversity, and they continue to serve as excellent starting points for inspiring new drug discovery (Shen, 2015). The history of the modern pharmaceutical industry includes many stories about how natural products profoundly inspired drug discovery (Li and Weng, 2017). With the current technological advances, natural products remain potentially transformative drugs for many health conditions. The growing understanding of efficient antiviral drug development has led to the exploration of natural products as an important tactic for identifying effective COVID-19 treatments.
Carolacton, produced by the myxobacterium Sorangium cellulosum, is an antibacterial macrolide keto-carboxylic acid (Figure 1A) (Jansen et al., 2010). In vitro, it demonstrates significant bioactivity against the human pathogen Streptococcus mutans by reducing the number of viable cells in biofilms (Fu et al., 2020), and it has been found to be a methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) inhibitor (Anderson et al., 2020). SARS-CoV-2 may have originated in bats (Shi et al., 2020) (as a study has shown that its genome is similar to that of the bat coronavirus RaTG13, with 96.2% identity (Zhou et al., 2020)) and, very recently, Tan’s group demonstrated that MTHFD1 is a critical host factor for the viral RNA replication of a broad spectrum of viruses in both bats and humans (Anderson et al., 2020). Based on in-depth research, Tan’s group demonstrated that MTHFD1 is a potential target for developing anti-SARS-CoV-2 agents and that the MTHFD1 inhibitor carolacton strongly inhibited SARS-CoV-2 replication in Vero cells at a half maximal inhibitory concentration (IC50) of as low as 0.14 μM and a moderate cytotoxicity (50% cytotoxic concentration [CC50] >0.80 μM) (Anderson et al., 2020). Thus, there is hope that this antimicrobial natural product may be useful in the COVID-19 epidemic.
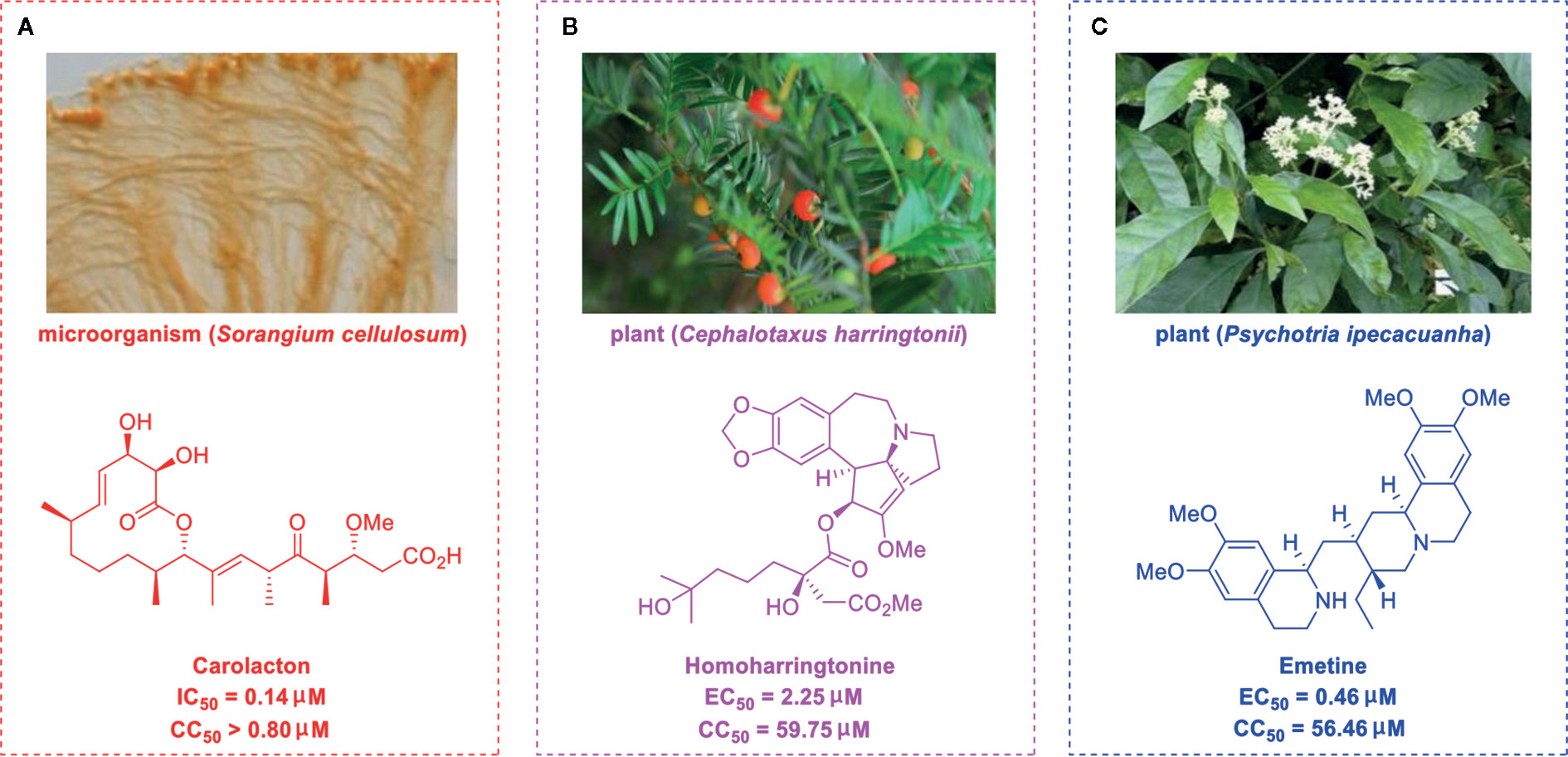
Figure 1 Promising natural products for treating COVID-19. (A) Carolacton was isolated from the myxobacterium Sorangium cellulosum. (B) Homoharringtonine was isolated from the plant Cephalotaxus harringtonii. (C) Emetine was isolated from the plant Psychotria ipecacuanha.
Regarding the total synthesis of carolacton, three examples of milligram-scale processes have been reported. In 2012, Kirschning’s group (Schmidt and Kirschning, 2012) reported on the first total synthesis of carolacton (with an overall yield of 4.3%) on an 8.5-mg scale in 22 linear steps. The key transformations involved asymmetric Nozaki-Hiyama-Kishi cross-coupling and Negishi-Fu coupling as well as the metal-mediated Ley aldol reaction, Duthaler-Hafner aldol reaction, Marshall reaction, and Breit’s substitution. Two years later, Phillips’s group and Wuest’s group (Hallside et al., 2014) collaboratively developed an efficient synthesis of carolacton (with an overall yield of 7.9%) on a 4.0-mg scale in just 14 linear steps. This synthesis process involved ring-closing metathesis (RCM), selective reduction, Leighton crotylation, and Steglich esterification. In 2017, Goswami’s group (Kuilya and Goswami, 2017) reported a third total synthesis (with an impressive overall yield of 18.8%) on a 7.1-mg scale in just 13 linear steps. The key strategies included Urpi acetal aldol reaction, β-hydroxy elimination, intermolecular esterification, and RCM. The three elegant synthesis strategies for producing carolacton are shown in Figure 2A. However, in the current research and clinical contexts, the development of a ton-scale process to synthesize carolacton is urgently needed.
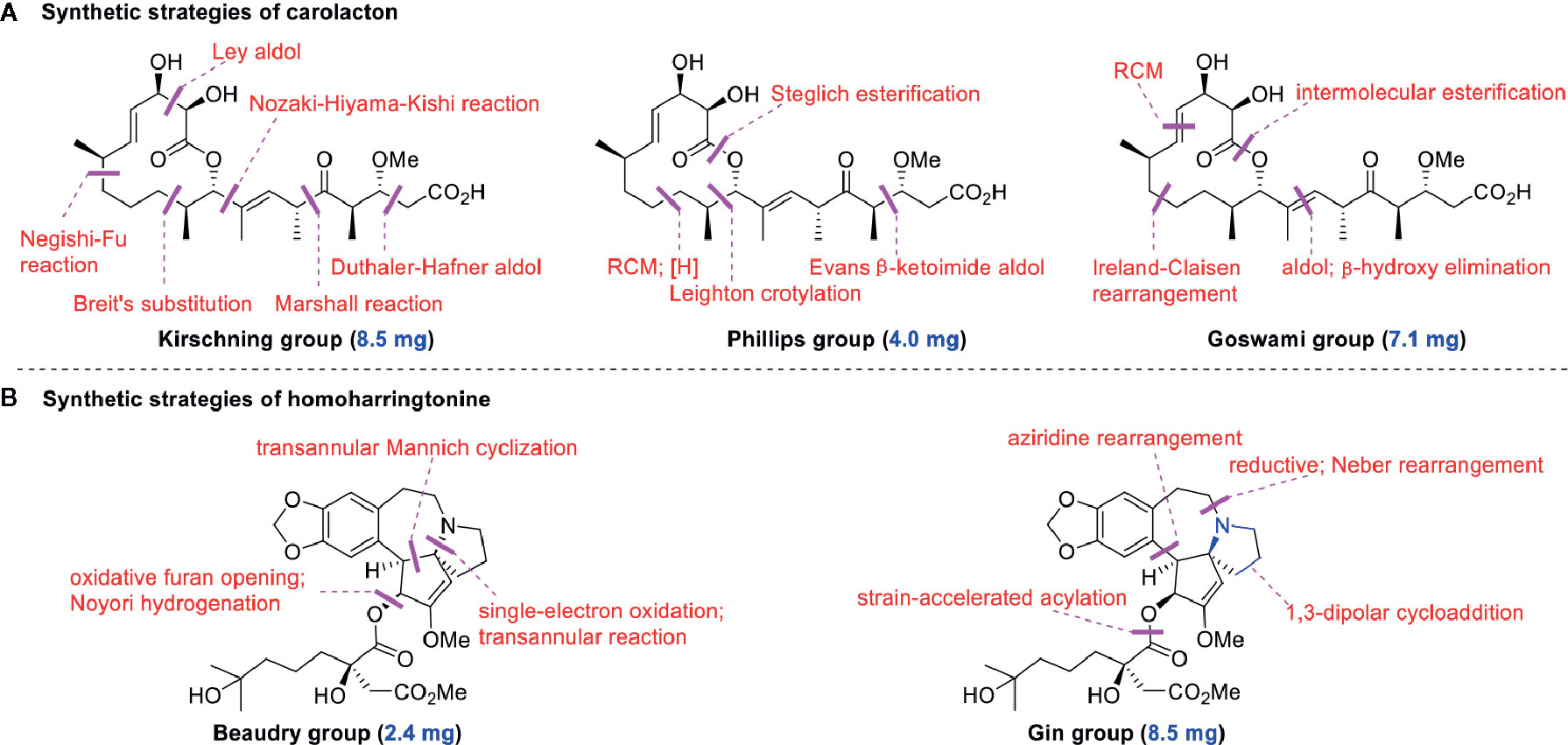
Figure 2 Key strategies in the synthesis of carolacton and homoharringtonine. (A) Synthetic strategies of carolacton by Kirschning’s group, Phillips’s group and Goswami’s group, respectively. (B) Synthetic strategies of homoharringtonine by Gin’s group and Beaudry’s group, respectively.
Homoharringtonine (omacetaxine) is a natural product that was isolated from the plant Cephalotaxus harringtonii in 1970 (Powell et al., 1970). It was approved by the US Food and Drug Administration (FDA) in 2012 as an effective anti-cancer agent to treat chronic myeloid leukemia (Figure 1B) (Mullard, 2013). Additionally, it exhibits broad-spectrum activities against viruses, such as mouse hepatitis virus (MHV) at an IC50 of 0.012 μM (Cao et al., 2015), herpes simplex virus type 1 (HSV-1) at an IC50 of 0.14 μM (Dong et al., 2018), foot and mouth disease virus (FMDV) at an IC50 of 3.05 μM (Gong et al., 2019), and echovirus 1 (EV1) at a half maximal effective concentration (EC50) of 0.12 μM (Andersen et al., 2019). Two elegant milligram-scale total syntheses of homoharringtonine have been reported by Gin’s group (Eckelbarger et al., 2008) and Beaudry’s group (Ju and Beaudry, 2019), respectively (Figure 2B). In 2008, Gin’s group reported a novel total synthesis of homoharringtonine on an 8.5-mg scale, using aziridine rearrangement and 1,3-dipolar cycloaddition as key strategies. In 2019, Beaudry’s group developed an excellent total synthesis of homoharringtonine involving 12 linear steps on a 2.4-mg scale. The key strategies were oxidative furan ring opening with spontaneous transannular Mannich reaction as well as the Noyori hydrogenation reaction.
The tetrahydroisoquinoline alkaloid emetine is an older natural product that has potential cardiotoxicity and was isolated from the plant Psychotria ipecacuanha (Figure 1C) (Wiegrebe et al., 1984). As a protein synthesis inhibitor, emetine has been widely used in pharmacology (Akinboye et al., 2012). Furthermore, emetine has recently been recognized as a promising broad-spectrum antiviral drug with in vitro activity against multiple viruses, including MHV-A59 at an EC50 of 0.12 μM (Shen et al., 2019), severe acute respiratory syndrome coronavirus (SARS-CoV) at an EC50 of 0.051 μM (Dyall et al., 2014), Middle East respiratory syndrome coronavirus (MERS-CoV) at an EC50 of 0.014 μM (Dyall et al., 2014), and Ebola virus (EBOV) at an IC50 of 0.017 μM (Yang et al., 2018). Very recently, Peterson’s group showed that emetine concentrations could be 300 times higher in the lungs compared to in the blood, and emetine may achieve therapeutic concentrations at viral infection sites, especially in the lungs (Bleasel and Peterson, 2020).
Yen’s group recently revealed that both homoharringtonine and emetine could effectively inhibit the replication of SARS-CoV-2 in Vero E6 cells at an EC50 of 2.55 and 0.46 μM, respectively (Choy et al., 2020). Additionally, the combination of emetine and the C-nucleoside analog GS-5734 exhibited a synergistic inhibitory effect against SARS-CoV-2 replication (Choy et al., 2020). Multiple lines of evidence have shown the potential usefulness of homoharringtonine and emetine as treatments for viral infections, but further research is needed to explore whether they exhibit anti-SARS-CoV-2 activity in vivo.
The bisbenzylisoquinoline alkaloid cepharanthine is a natural product isolated from the plant Stephania cephalantha, which is used as a traditional herbal medicine (Figure 3) (Bailly, 2019). Specifically, this approved drug has significant bioactivity against several diseases, with anti-viral, anti-malarial, and anti-cancer effects (Fang et al., 2013). It has IC50 values of 0.026 μM and 9.5 μg/mL against HIV-1 (Okamoto et al., 1998) and SARS-CoV (Zhang et al., 2005), respectively. Furthermore, it dramatically blocked the viral replication in human coronavirus OC43 (HCoV-OC43)-infected MRC-5 human lung cells (with an IC50 of 0.83 μM) and inhibited viral S and N protein expression (Kim et al., 2019). Watashi’s group recently revealed that cepharanthine could effectively inhibit SARS-CoV-2 replication in vitro with an EC50 at 0.35 μM with minimal toxicity (selectivity index >70) (Ohashi et al., 2020). It is worth noting that cepharanthine is an approved drug with a good safety profile, highlighting a new potential role for cepharanthine regarding inhibiting SARS-CoV-2 replication.
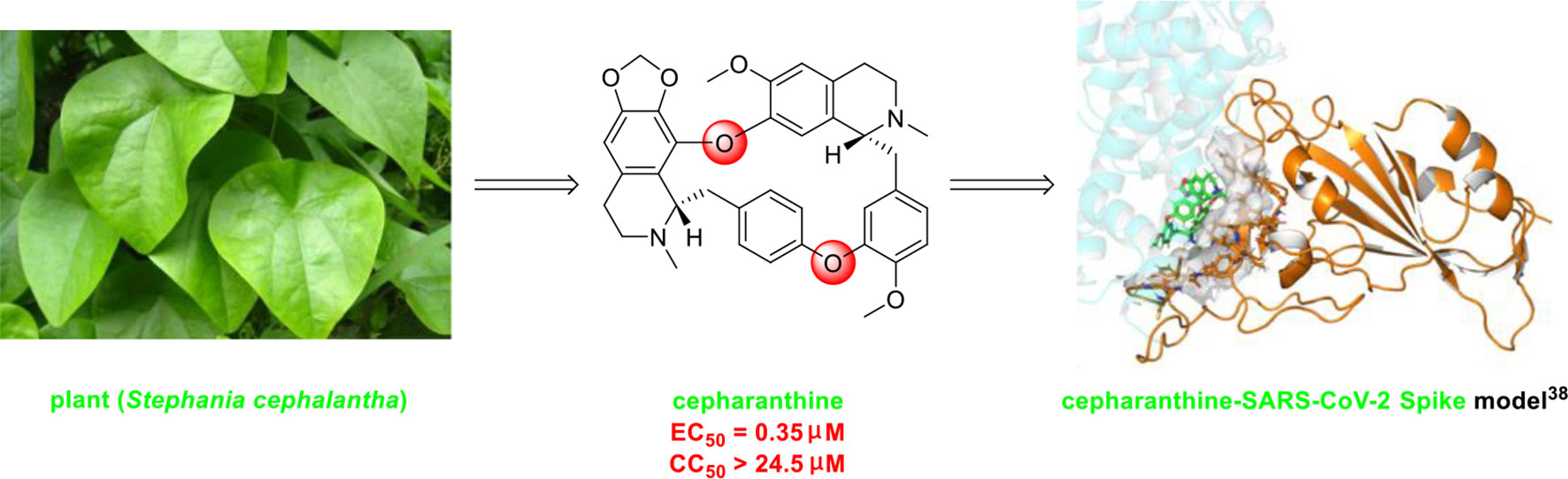
Figure 3 Promising natural product cepharanthine for treating COVID-19 (image reproduced from ref. 38, bioRxiv, doi: 10.1101/2020.04.14.039925).
There are currently no data on drug–drug interactions involving the abovementioned natural products (The liverpool drug interaction group), and close attention should be paid to these interactions during therapeutic use because drug–drug interactions will play significant roles in the safety and effectiveness of anti-COVID-19 agents.
Ivermectin (brand name: Stromectol) is a landmark broad-spectrum anti-parasitic drug that was developed by Ōmura’s group along with Merck Sharp and Dohme Research Laboratories in 1978 (Burg et al., 1979). It has been demonstrated to be highly effective (94.9% efficacy at 24 hours and 73.8% efficacy at 2 weeks) as an oral drug for the treatment of head lice (Pariser et al., 2012). Ivermectin is the semisynthetic 22,23-dihydro derivative of the natural product avermectin B1 (B1a and B1b), which is produced by Streptomyces avermitilis (Figure 4) (Campbell et al., 1983). Ivermectin is one of the most widely used antibiotics for both animals and humans, and the researchers who discovered it received the Nobel Prize in Physiology or Medicine in 2015 (Campbell, 2016).
The use of ivermectin is currently being expanded. For example, Wagstaff’s group highlighted that ivermectin is highly effective at controlling SARS-CoV-2 RNA replication in vitro, with a 5000-fold reduction in the virus within 48 hours (Caly et al., 2020). Recently, Lohmer’s group found that a single oral dose of ivermectin is unlikely to reach the IC50 (2.5 μM) in the lungs (predicted lung concentration: 0.0857 μM), and they suggested that combination therapy or inhaled treatment (to increase the concentration in the lungs) could be considered as potential solutions (Schmith et al., 2020). Nevertheless, its safety in humans has been continuously documented (Buonfrate et al., 2019), and it is hoped that it will become a key component of COVID-19 treatment regimens.
The C-nucleoside analog GS-5734 (remdesivir), a broad-spectrum antiviral agent developed by Gilead Sciences (Warren et al., 2016), exhibited promising clinical efficacy in the treatment of the first US case of SARS-CoV-2 infection (Holshue et al., 2020). Initial research on synthesizing GS-5734 (which is a prodrug) began with structural modification of tubercidin (an antibiotic and adenosine analog that is isolated from Streptomyces tubercidicus) by replacing the C-N linkage with a C-C bond to create 4-aza-7,9-dideazaadenosine (Figure 5) (Patil et al., 1994). 4-aza-7,9-dideazaadenosine has equal cytotoxicity against HL-60 cells to tubercidin (50% infectious does [ID50] = 0.82 nM) and increased hydrolytic stability (Patil et al., 1994).
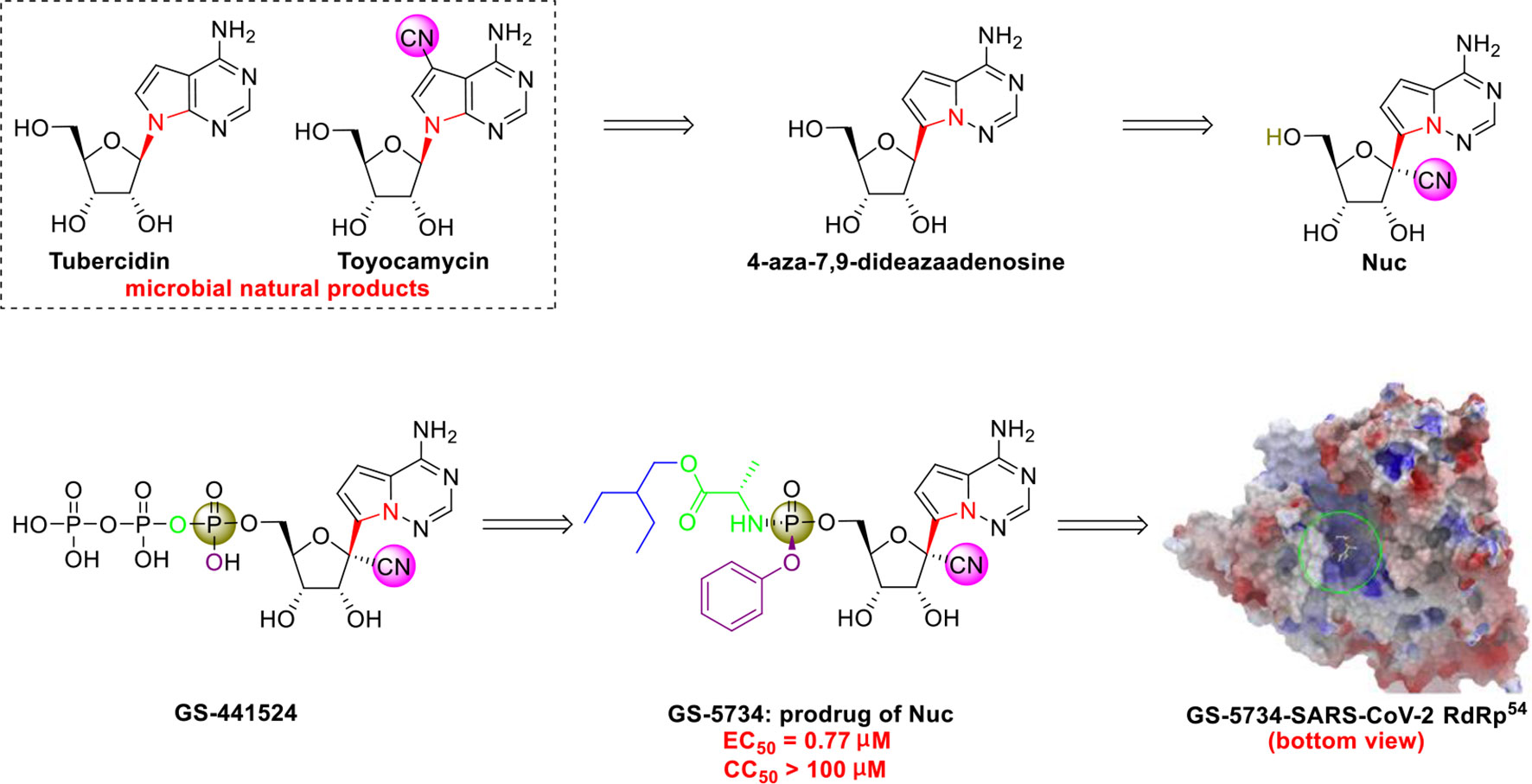
Figure 5 Promising natural-product-inspired GS-5734 for treating COVID-19 (image reproduced with permission from ref. 54, Acta Pharm. Sin. B, 2020, 10, 766-788).
However, an important contribution to antiviral drug design is 1’-CN substituted Nuc, which can be viewed as a new structure that was inspired by the natural cyanide toyocamycin (isolated from Streptomyces toyocmnsis) (Nishimura et al., 1956). Nuc strongly inhibits hepatitis C virus in vitro (EC50 = 4.1 μM) (Cho et al., 2012). The active form of Nuc in virus-infected cells has been reported to be GS-441524, but the monophosphate conversion of Nuc to GS-441524 is a rate-limiting step (Warren et al., 2016). To overcome this issue, the monophosphorylated prodrug GS-5734 was developed to achieve better cellular uptake in vivo (Warren et al., 2016).
GS-5734 has been recognized as a promising broad-spectrum antiviral drug against multiple viruses including SARS-CoV (EC50 = 69 nM) (Sheahan et al., 2017) and MERS-CoV (EC50 = 20 nM) (Sheahan et al., 2017). Furthermore, Xiao’s group (Wang et al., 2020) and Yen’s group (Choy et al., 2020) recently revealed that GS-5734 is highly effective against SARS-CoV-2 infection in vitro (EC50 = 0.77 μM, viral load fitted in linear scale; or EC50 = 23.15 μM, viral load fitted in logarithmic scale) and has low toxicity (selectivity index >130). Regarding the mechanism of action, Li’s group highlighted that GS-5734 can bind to the RNA-binding channel of the SARS-CoV-2 RNA-dependent RNA polymerase (Wu et al., 2020) (Figure 5). The 1’-ribose CN substitution observed in GS-5734 plays an important role in inhibiting the viral RNA replication of SARS-CoV-2 (Zhang et al., 2020).
Notably, since 2016, GS-5734 has been reported to be safe and exhibit clinical efficacy against EBOV infection (Jacobs et al., 2016; Dörnemann et al., 2017) and SARS-CoV-2 infection (Beigel et al., 2020; Holshue et al., 2020). On April 10, 2020, Gilead Sciences reported that 68% of patients with severe COVID-19 who were treated with GS-5734 (via the compassionate use program) exhibited clinical improvement, and no new safety issues were detected (Grein et al., 2020). In addition, in May, 2020, the US FDA issued an emergency use authorization (EUA) for GS-5734 for treating SARS-CoV-2 infection (Mullard, 2020). To increase the efficiency of pharmaceutical research on GS-5734, researchers at Gilead Sciences developed a scalable process for synthesizing GS-5734 (Warren et al., 2016) (Figure 6). Currently, there are no drug–drug interaction data on GS-5734 (Summary on compassionate use remdesivir Gilead), but the potential for clinically significant interactions is low (The liverpool drug interaction group). It is hoped that GS-5734 will be confirmed to be a safe and effective drug against SARS-CoV-2.
The N-nucleoside analog EIDD-2801, a promising orally bioavailable antiviral agent, was discovered by Plemper’s group at Emory University (Toots et al., 2019). Initial research on the synthesis of EIDD-2801 began with structurally modifying the broad-spectrum antiviral agent N4-hydroxycytidine (NHC, EIDD-1931) (Agostini et al., 2019), which was in turn derived from the essential natural product uridine found in human plasma (Figure 7) (Yamamoto et al., 2011).
More recently, researchers at Emory University reported a scalable process for synthesizing EIDD-2801 (Painter et al., 2019) (Figure 8). There is currently widespread interest in the use of EIDD-2801 as a promising anti-COVID-19 agent (Rothan and Byrareddy, 2020). Baric’s group (Cho et al., 2012) highlighted that EIDD-1931 is very effective at controlling SARS-CoV-2 replication. EIDD-1931 exhibited potent anti-SARS-CoV-2 activity in Calu-3 cells (IC50 = 0.08 μM) and Vero cells (IC50 =0.30 μM), with no observable cytotoxicity at doses of up to 5 μM (Rothan and Byrareddy, 2020). Furthermore, EIDD-2801 is an orally bioavailable prodrug that is efficiently hydrolyzed in vivo and it exhibits remarkable selectivity (therapeutic window >1713) (Sheahan et al., 2020).
In addition, Baric’s group showed that EIDD-2801 significantly improved the pulmonary function of mice infected with MERS-CoV or SARS-CoV (Sheahan et al., 2020). Thus, EIDD-2801 has demonstrated potential effectiveness, even though there is a lack of good evidence in humans. It is hoped that researchers will work to overcome the obstacles (such as produced on a manufacturing scale, efficacy and safety in vivo) to allow it to be assessed in clinical research.
Growing understanding of efficient antiviral drug development has led to the use of small molecules being recognized as an important potential tactic for treating COVID-19. Rao’s group (Jin et al., 2020) showed that the organoselenium compound ebselen (Table 1) and other inhibitors of the SARS-CoV-2 main protease (Mpro) exhibited potent activities, with IC50 values at micromolar or sub-micromolar levels (0.67–21.4 μM). Ebselen is highly effective at inhibiting SARS-CoV-2 infection (EC50 = 4.67 μM) and has low toxicity (median lethal dose [LD50] in rats >4,600 mg/kg). Most importantly, its safety in humans has been continuously evaluated in multiple clinical trials (Kil et al., 2020). Besides the abovementioned small molecules, several other natural products and natural product-inspired potential small molecules have also exhibited notable anti-SARS-CoV-2 activities (Table 1).
Drug development carries high risks. For example, although the approved protease inhibitors lopinavir and ritonavir (Figure 9A) were thought to be potentially effective against SARS-Cov-2 (as they have been reported to be active against SARS6), Wang’s group showed that lopinavir combined with ritonavir does not seem to be highly effective in patients with COVID-19 (Cao et al., 2020).
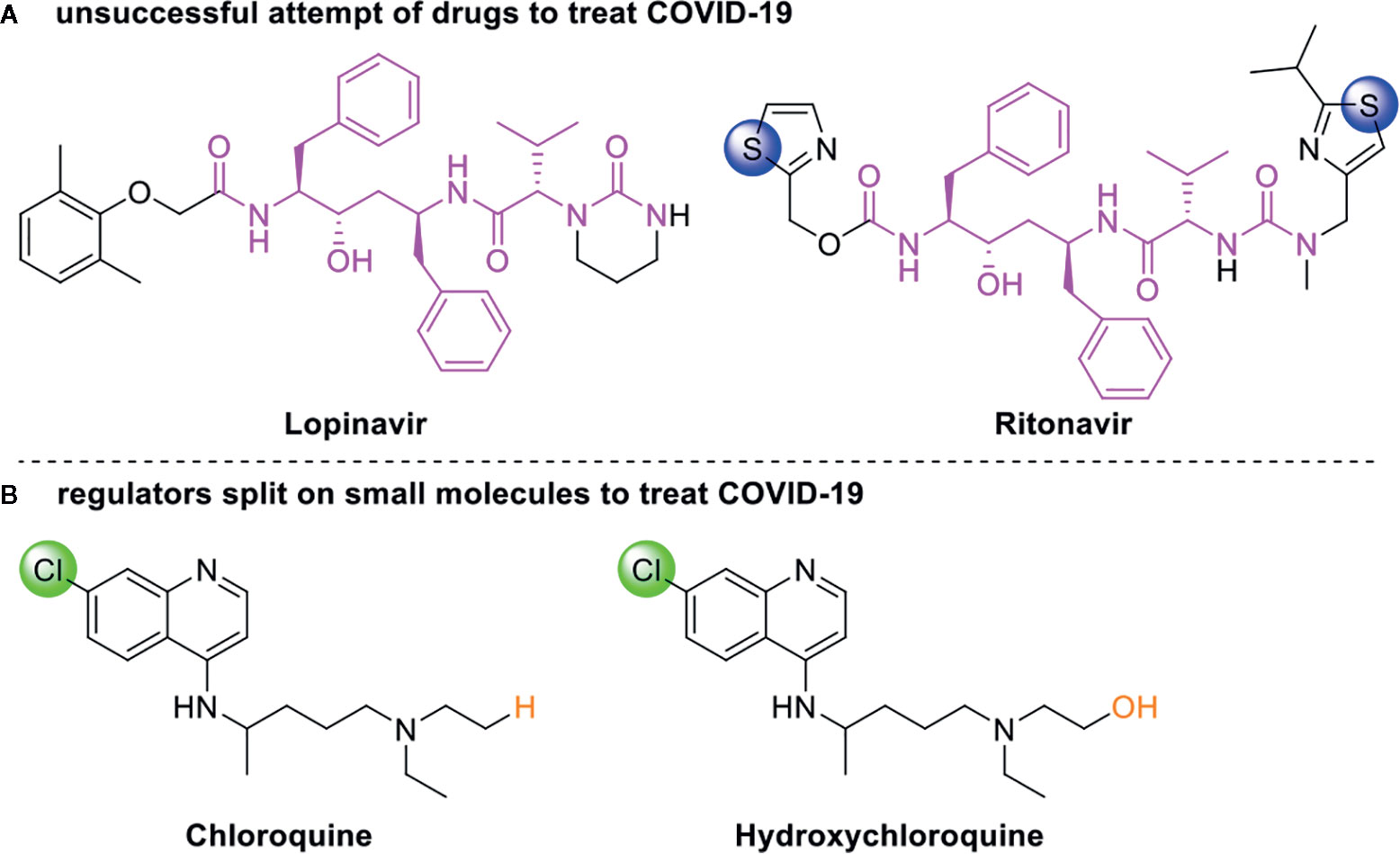
Figure 9 Other small molecules for treating COVID-19. (A) Unsuccessful attempt of lopinavir and ritonavir to treat COVID-19. (B) Regulators split on chloroquine and hydroxychloroquine to treat COVID-19.
Chloroquine and hydroxychloroquine (Figure 9B) have garnered considerable attention due to their ability to effectively inhibit SARS-CoV-2 (Yamamoto et al., 2011; Liu et al., 2020; Yao et al., 2020). For example, Xiao’s group (Wu et al., 2020) showed that chloroquine potently blocked SARS-CoV-2 infection at a low concentration in vitro (EC50 = 1.1 μM). Although the US FDA has authorized the use of chloroquine and hydroxychloroquine, the WHO has stated that the clinical data do not support their clinical use in COVID-19 patients (Jaffe, 2020; Taccone et al., 2020). In addition, chloroquine can be lethal (narrow therapeutic window) in children, and caution is warranted when it is used for critical illness (Smit et al., 2020).
Currently, global attention continues to be focused on COVID-19 due to its serious threat to public health. Scientists have discovered that SARS-CoV-2 has developed mutations (in 149 sites from the 103 sequenced SARS-Cov-2 strain) that have substantially changed its pathogenicity (Tang et al., 2020; Yao et al., 2020). Therefore, rapid discovery of safe, effective, and broad-spectrum anti-COVID-19 drugs is urgent. However, it is well-known that the development of a new drug usually takes more than 10 years (Ashburn and Thor, 2004). Very recently, potential anti-SARS-CoV-2 natural products and natural product-inspired small molecules have attracted significant attention due to their broad-spectrum antiviral activities. Here, we reviewed the research on potential landmark anti-SARS-CoV-2 natural products (carolacton, homoharringtonine, cepharanthine, and emetine) and natural product-inspired small molecules (ivermectin, GS-5734, EIDD-2801, and ebselen). In-depth research on potential anti-COVID-19 natural product-inspired small molecules has led to the development of multiple lines of evidence demonstrating their effects on SARS-CoV-2 infection (Ferner and Aronson, 2020; Pruijssers et al., 2020; Toots et al., 2020; Williamson et al., 2020; Xing et al., 2020).
While the current COVID-19 pandemic has led to more rapid natural product-based drug discovery and development, it is also worth noting that ton-scale total synthesis strategies for the abovementioned potential anti-SARS-CoV-2 natural products (such as carolacton and homoharringtonine) are urgently needed. However, significant challenges (for example, attainment of clinical evidence regarding the anti-SARS-CoV-2 effects of the agents in patients, and traditional drug development approach in SARS-CoV-2 is becoming untenable) will need to be overcome in order for successful clinical research to be completed. We hope that natural products and natural product-inspired small molecules will be shown to be safe and effective for treating SARS-CoV-2 infection.
Figures were reproduced with permission through Copyright Clearance Center’s RightsLink® service.
ZW conceived the review. LY collected the literatures. ZW, and LY wrote the manuscript. ZW edited the manuscript. All authors read and approved the final version of the manuscript.
Project supported by the PhD research start-up funds of Qufu Normal University, China (Grant Nos. bsqd20190164, and bsqd20190060) and the project of introduction and cultivation for young innovation talents in colleges and universities of Shandong Province.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Agostini, M. L., Pruijssers, A. J., Chappell, J. D., Gribble, J., Lu, X. T., Andres, E. L., et al. (2019). Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 93, e01348–e01319. doi: 10.1128/JVI.01348-19
Akinboye, E. S., Rosen, M. D., Denmeade, S. R., Kwabi-Addo, B., Bakare, O. (2012). Design, synthesis, and evaluation of pH-dependent hydrolyzable emetine analogues as treatment for prostate cancer. J. Med. Chem. 55, 7450–7459. doi: 10.1021/jm300426q
Andersen, P., Krpina, K., Ianevski, A., Shtaida, N., Jo, E., Yang, J., et al. (2019). Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses 11, 964. doi: 10.3390/v11100964
Anderson, D. E., Cui, J., Ye, Q., Huang, B. Y., Zu, W. H., Gong, J., et al. (2020). Orthogonal genome-wide screenings in bat cells identify MTHFD1 as a target of broad antiviral therapy. bioRxiv. doi: 10.1101/2020.03.29.014209
Ashburn, T. T., Thor, K. B. (2004). Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discovery 3, 673–683. doi: 10.1038/nrd1468
Bailly, C. (2019). Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine 62, 152956. doi: 10.1016/j.phymed.2019.152956
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020). Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. doi: 10.1056/NEJMoa2007764
Bleasel, M. D., Peterson, G. M. (2020). Emetine, ipecac, ipecac alkaloids and analogues as potential antiviral agents for coronaviruses. Pharmaceuticals 13, 51. doi: 10.3390/ph13030051
Buonfrate, D., Salas-Coronas, J., Muñoz, J., Maruri, B. T., Rodari, P., Castelli, F., et al. (2019). Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): a multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect. Dis. 19, 1181–1190. doi: 10.1016/S1473-3099(19)30289-0
Burg, R. W., Miller, B. M., Baker, E. E., Birnbaum, J., Currie, S. A., Hartman, R., et al. (1979). Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob. Agents Chemother. 15, 361–367. doi: 10.1128/AAC.15.3.361
Caly, L., Druce, J. D., Catton, M. G., Jans, D. A., Wagstaff, K. M. (2020). The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 178, 104787. doi: 10.1016/j.antiviral.2020.104787
Campbell, W. C., Fisher, M. H., Stapley, E. O., Albers-Schonberg, G., Jacob, T. A. (1983). Ivermectin: a potent new antiparasitic agent. Science 221, 823–828. doi: 10.1126/science.6308762
Campbell, W. C. (2016). Ivermectin: a potent new antiparasitic agent. Angew. Chem. Int. Ed. 55, 10184–10189. doi: 10.1002/anie.201601492
Cao, J., Forrest, J. C., Zhang, X. (2015). A screen of the NIH clinical collection small molecule library identifies potential anti-coronavirus drugs. Antiviral Res. 114, 1–10. doi: 10.1016/j.antiviral.2014.11.010
Cao, B., Wang, Y. M., Wen, D. N., Liu, W., Wang, J. L., Fan, G. H., et al. (2020). A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. 382, 1787–1799. doi: 10.1056/NEJMoa2001282
Cho, A., Saunders, O. L., Butler, T., Zhang, L. J., Xu, J., Vela, J. E., et al. (2012). Synthesis and antiviral activity of a series of 1’-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 22, 2705–2707. doi: 10.1016/j.bmcl.2012.02.105
Choy, K. T., Wong, A. Y. L., Kaewpreedee, P., Sia, S. F., Chen, D. D., Hui, K. P. Y., et al. (2020). Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 178, 104786. doi: 10.1016/j.antiviral.2020.104786
Coronavirus disease (COVID-2019). situation reports 1-144; https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200612-covid-19-sitrep-144.pdf?sfvrsn=66ff9f4f_2
Dörnemann, J., Burzio, C., Ronsse, A., Sprecher, A., De Clerck, H., Van Herp, M., et al. (2017). First newborn baby to receive experimental therapies survives Ebola virus disease. J. Infect. Dis. 215, 171–174. doi: 10.1093/infdis/jiw493
Dong, H. J., Wang, Z. H., Meng, W., Li, C. C., Hu, Y. X., Zhou, L., et al. (2018). The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses 10, 10. doi: 10.3390/v10110601
Dyall, J., Coleman, C. M., Hart, B. J., Venkataraman, T., Holbrook, M. R., Kindrachuk, J., et al. (2014). Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 58, 4885–4893. doi: 10.1128/AAC.03036-14
Eckelbarger, J. D., Wilmot, J. T., Epperson, M. T., Thakur, C. S., Shum, D., Antczak, C., et al. (2008). Synthesis of antiproliferative Cephalotaxus esters and their evaluation against several human hematopoietic and solid tumor cell lines: uncovering differential susceptibilities to multidrug resistance. Chem. Eur. J. 14, 4293–4306. doi: 10.1002/chem.200701998
Fang, Z. H., Li, Y. J., Chen, Z., Wang, J. J., Zhu, L. H. (2013). Inhibition of signal transducer and activator of transcription 3 and cyclooxygenase-2 is involved in radiosensitization of cepharanthine in HeLa cells. Int. J. Gynecol. Cancer 23, 608–614. doi: 10.1097/IGC.0b013e31828a05fd
Ferner, R. E., Aronson, J. K. (2020). Remdesivir in covid-19: A drug with potential-don’t waste time on uncontrolled observations. BMJ 369, m1610. doi: 10.1136/bmj.m1610
Fu, C. Z., Sikandar, A., Donner, J., Zaburannyi, N., Herrmann, J., Reck, M., et al. (2020). The natural product carolacton inhibits folatedependent C1 metabolism by targeting FolD/MTHFD. Nat. Commu. 8, 1529. doi: 10.1038/s41467-017-01671-5
Ge, Y. Y., Tian, T. Z., Huang, S. L., Wan, F. P., Li, J. X., Li, S. Y., et al. (2020). A data-driven drug repositioning framework discovered a potential therapeutic agent targeting COVID-19. bioRxiv. doi: 10.1101/2020.03.11.986836
Gong, M. J., Li, S. F., Xie, Y. L., Zhao, F. R., Shao, J. J., Zhang, Y. G., et al. (2019). Inhibitory effects of homoharringtonine on foot and mouth disease virus in vitro. J. Med. Virol. 91, 1595–1601. doi: 10.1002/jmv.25494
Grein, J., Ohmagari, N., Shin, D., Diaz, G., Asperges, E., Castagna, A., et al. (2020). Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 382, 2327–2336. doi: 10.1056/NEJMoa2007016
Hallside, M. S., Brzozowski, R. S., Wuest, W. M., Phillips, A. J. (2014). A concise synthesis of carolacton. Org. Lett. 16, 1148–1151. doi: 10.1021/ol500004k
Holshue, M. L., DeBolt, C., Lindquist, S., Lofy, K. H., Wiesman, J., Bruce, H., et al. (2020). First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382, 929–936. doi: 10.1056/NEJMoa2001191
Ianevski, A., Yao, R. A., Fenstad, M. H., Biza, S., Zusinaite, E., Lysvand, H., et al. (2020). Antiviral options against SARS-CoV-2 infection. bioRxiv. doi: 10.1101/2020.05.12.091165
Jacobs, M., Rodger, A., Bell, D. J., Bhagani, S., Cropley, I., Filipe, A., et al. (2016). Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 388, 498–503. doi: 10.1016/S0140-6736(16)30386-5
Jaffe, S. (2020). Regulators split on antimalarials for COVID-19. Lancet. 395, 1179–1179. doi: 10.1016/s0140-6736(20)30817-5
Jansen, R., Irschik, H., Huch, V., Schummer, D., Steinmetz, H., Bock, M., et al. (2010). Carolacton-a macrolide ketocarbonic acid that reduces biofilm formation by the caries- and endocarditis-associated bacterium Streptococcus mutans. Eur. J. Org. Chem. 2010, 1284–1289. doi: 10.1002/ejoc.200901126
Jeon, S., Ko, M., Lee, J., Choi, I., Byun, S. Y., Park, S., et al. (2020). Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Ch. 64, e00819–20. doi: 10.1128/AAC.00819-20
Jin, Z. M., Du, X. Y., Xu, Y. C., Deng, Y. Q., Liu, M. Q., Zhao, Y., et al. (2020). Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 582, 289–293. doi: 10.1038/s41586-020-2223-y
Jin, Z. M., Zhao, Y., Sun, Y., Zhang, B., Wang, H. F., Wu, Y., et al. (2020). Structural basis for the inhibition of COVID-19 virus main protease by carmofur, an antineoplastic drug. bioRxiv. doi: 10.1101/2020.04.09.033233
Ju, X., Beaudry, C. M. (2019). Total synthesis of (-)-cephalotaxine and (-)-homoharringtonine via furan oxidation-transannular mannich cyclization. Angew. Chem. Int. Ed. 58, 6752–6755. doi: 10.1002/anie.201902174
Kalil, A. C. (2020). Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 323, 1897–1898. doi: 10.1001/jama.2020.4742
Kil, J., Lobarinas, E., Spankovich, C., Griffiths, S. K., Antonelli, P. J., Lynch, E. D., et al. (2020). Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 390, 969–979. doi: 10.1016/S0140-6736(17)31791-9
Kim, D. E., Min, J. S., Jang, M. S., Lee, J. Y., Shin, Y. S., Song, J. H., et al. (2019). Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules 9, 696. doi: 10.3390/biom9110696
Ko, M., Jeon, S., Ryu, W. S., Kim, S. (2020). Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells: Nafamostat is the most potent antiviral drug candidate. bioRxiv. doi: 10.1101/2020.05.12.090035
Kuilya, T. K., Goswami, R. K. (2017). Stereoselective total synthesis of carolacton. Org. Lett. 19, 2366–2369. doi: 10.1021/acs.orglett.7b00903
Kupferschmidt, K., Cohen, J. (2020). Race to find COVID-19 treatments accelerates. Science 367, 1412–1413. doi: 10.1126/science.367.6485.1412
Li, G. D., De Clercq, E. (2020). Therapeutic options for the 2019 novel coronavirus, (2019-nCoV). Nat. Rev. Drug Discovery 19, 149–150. doi: 10.1038/d41573-020-00016-0
Li, F. S., Weng, J. K. (2017). Demystifying traditional herbal medicine with modern approaches. Nat. Plants 3, 17109. doi: 10.1038/nplants.2017.109
Liu, H. B., Ye, F., Sun, Q., Liang, H., Li, C. M., Lu, R. J., et al. (2020). Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. bioRxiv. doi: 10.1101/2020.04.10.035824
Liu, J., Cao, R. Y., Xu, M. Y., Wang, X., Zhang, H. Y., Hu, H. R., et al. (2020). Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery 6, 16. doi: 10.1038/s41421-020-0156-0
Ma, C. L., Hurst, B., Hu, Y. M., Szeto, T., Tarbet, B., Wang, J. (2020). Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. bioRxiv. doi: 10.1101/2020.04.20.051581
Mullard, A. (2013). 2012 FDA drug approvals. Nat. Rev. Drug Discovery 12, 87–90. doi: 10.1038/nrd3946
Newman, D. J., Cragg, G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803. doi: 10.1021/acs.jnatprod.9b01285
Nishimura, H., Katagiri, K., Sato, K., Mayama, M., Shimaoka, N. (1956). Toyocamycin, a new anti-candida antibiotic. J. Antibiot. 9, 60–62.
Ohashi, H., Watashi, K., Saso, W., Shionoya, K., Iwanami, S., Hirokawa, T., et al. (2020). Multidrug treatment with nelfinavir and cepharanthine against COVID-19. bioRxiv. doi: 10.1101/2020.04.14.039925
Okamoto, M., Ono, M., Baba, M. (1998). Potent inhibition of HIV type 1 replication by an antiinflammatory alkaloid, cepharanthine, in chronically infected monocytic cells. AIDS Res. Hum. Retrovir. 14, 1239–1245. doi: 10.1089/aid.1998.14.1239
Painter, G. R., Guthrie, D. B., Bluemling, G. R., Natchus, M. R. (2019). N4-hydroxy cytidine and derivatives and anti-viral uses related thereto. WO 2019/113462 A1.
Pariser, D. M., Meinking, T. L., Bell, M., Ryan, W. G. (2012). Topical 0.5% ivermectin lotion for treatment of head lice. N. Engl. J. Med. 367, 1687–1693. doi: 10.1056/nejmoa1200107
Patil, S. A., Otter, B. A., Klein, S. (1994). 4-Aza-7,9-dideazaadenosine, a new cytotoxic synthetic C-nucleoside analogue of adenosine. Tetrahedron Lett. 35, 5339–5342. doi: 10.1016/S0040-4039(00)73494-0
Powell, R. G., Weisleder, D., Smith, C. R., Rohwedder, W. K. (1970). Structures of harringtonine, isoharringtonine, and homoharringtonine. Tetrahedron Lett. 11, 815–818. doi: 10.1016/S0040-4039(01)97839-6
Pruijssers, A. J., George, A. S., Schäfer, A., Leist, S. R., Gralinski, L. E., Dinnon III, K. H., et al. (2020). Remdesivir potently inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. bioRxiv. doi: 10.1101/2020.04.27.064279
Rothan, H. A., Byrareddy, S. N. (2020). The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 109, 102433. doi: 10.1016/j.jaut.2020.102433
Rothan, H. A., Stone, S., Natekar, J., Kumari, P., Arora, K., Kumar, M. (2020). The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology 547, 7–11. doi: 10.1016/j.virol.2020.05.002
Schmidt, T., Kirschning, A. (2012). Total synthesis of carolacton, a highly iotent biofilm inhibitor. Angew. Chem. Int. Ed. 51, 1063–1066. doi: 10.1002/anie.201106762
Schmith, V. D., Zhou, J., Lohmer, L. R. L. (2020). The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin. Pharmacol. Ther. doi: 10.1002/CPT.1889
Sheahan, T. P., Sims, A. C., Graham, R. L., Menachery, V. D., Gralinski, L. E., Case, J. B., et al. (2017). Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 9, eaal3653. doi: 10.1126/scitranslmed.aal3653
Sheahan, T. P., Sims, A. C., Zhou, S. T., Graham, R. L., Pruijssers, A. J., Agostini, M. L., et al. (2020). An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 12, eabb5883. doi: 10.1126/scitranslmed.abb5883
Shen, L., Niu, J. W., Wang, C. H., Huang, B. Y., Wang, W. L., Zhu, N., et al. (2019). High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J. Virol. 93, e00023–e00019. doi: 10.1128/JVI.00023-19
Shen, B. (2015). A new golden age of natural products drug discovery. Cell 163, 1297–1300. doi: 10.1016/j.cell.2015.11.031
Shi, J. Z., Wen, Z. Y., Zhong, G. X., Yang, H. L., Wang, C., Huang, B. Y., et al. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368, 1016–1020. doi: 10.1126/science.abb7015
Smit, C., Peeters, M. Y. M., Anker, J. N., Knibbe, C. A. J. (2020). Chloroquine for SARS−CoV−2: implications of its unique pharmacokinetic and safety properties. Clin. Pharmacokinet. 59, 659–669. doi: 10.1007/s40262-020-00891-1
Su, H. X., Yao, S., Zhao, W. F., Li, M. J., Liu, J., Shang, W. J., et al. (2020). Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. doi: 10.1101/2020.04.13.038687
Summary on compassionate use remdesivir Gilead. https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf. (Accessed May 5, 2020).
Taccone, F. S., Gorham, J., Vincent, J. L. (2020). Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir. Med. 8, 539–541. doi: 10.1016/S2213-2600(20)30172-7
Tang, X. L., Wu, C. C., Li, X., Song, Y. H., Yao, X. M., Wu, X. K., et al. (2020). On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 7, 1012–1023. doi: 10.1093/nsr/nwaa036
The liverpool drug interaction group. Evaluating the interaction risk of experimental COVID-19 therapies. https://undark.org/2020/05/07/drug-interaction-covid-19/ (Accessed May 28, 2020).
Toots, M., Yoon, J. J., Cox, R. M., Hart, M., Sticher, Z. M., Makhsous, N., et al. (2019). Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 11, eaax5866. doi: 10.1126/scitranslmed.aax5866
Toots, M., Yoon, J. J., Hart, M., Natchus, M. G., Painter, G. R., Plemper, R. K. (2020). Quantitative efficacy paradigms of the influenza clinical drug candidate. Transl. Res. 218, 16–28. doi: 10.1016/j.trsl.2019.12.002
Touret, F., Gilles, M., Barral, K., Nougairède, A., Decroly, E., de Lamballerie, X., et al. (2020). In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. bioRxiv. doi: 10.1101/2020.04.03.023846
Wang, C., Horby, P. W., Hayden, F. G., Gao, G. F. (2020). A novel coronavirus outbreak of global health concern. Lancet 395, 470–473. doi: 10.1016/S0140-6736(20)30185-9
Wang, M. L., Cao, R. Y., Zhang, L. K., Yang, X. L., Liu, J., Xu, M. Y., et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus, (2019-nCoV) in vitro. Cell Res. 30, 269–271. doi: 10.1038/s41422-020-0282-0
Warren, T. K., Jordan, R., Lo, M. K., Ray, A. S., Mackman, R. L., Soloveva, V., et al. (2016). Therapeutic Efficacy of The Small Molecule GS-5734 Against Ebola virus in rhesus monkeys. Nature 531, 381–385. doi: 10.1038/nature17180
Weston, S., Coleman, C. M., Haupt, R., Logue, J., Matthews, K., Frieman, M. B. (2020). Broad anti-coronaviral activity of FDA approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. bioRxiv. doi: 10.1101/2020.03.25.008482
Wiegrebe, W., Kramer, W. J., Shamma, M. (1984). The emetine alkaloids. J. Nat. Prod. 47, 397–408. doi: 10.1021/np50033a001
Williamson, B. N., Feldmann, F., Schwarz, B., Meade-White, K., Porter, D. P., Schulz, J. (2020). Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. doi: 10.1101/2020.04.15.043166
Wu, C. R., Liu, Y., Yang, Y. Y., Zhang, P., Zhong, W., Wang, Y. L., et al. (2020). Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 10, 766–788. doi: 10.1016/j.apsb.2020.02.008
Xing, J., Shankar, R., Drelich, A., Paithankar, S., Chekalin, E., Dexheimer, T., et al. (2020). Reversal of infected host gene expression identifies repurposed drug candidates for COVID-19. bioRxiv. doi: 10.1101/2020.04.07.030734
Xiong, R., Zhang, L. K., Li, S. L., Sun, Y., Ding, M. Y., Wang, Y., et al. (2020). Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. bioRxiv. doi: 10.1101/2020.03.11.983056
Yamamoto, T., Koyama, H., Kurajoh, M., Shoji, T., Tsutsumi, Z., Moriwaki, Y. (2011). Biochemistry of uridine in plasma. Clin. Chim. Acta 412, 1712–1724. doi: 10.1016/j.cca.2011.06.006
Yang, S., Xu, M., Lee, E. M., Gorshkov, K., Shiryaev, S. A., He, S. H., et al. (2018). Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discovery 4, 31. doi: 10.1038/s41421-018-0034-1
Yao, X. T., Ye, F., Zhang, M., Cui, C., Huang, B. Y., Niu, P. H., et al. (2020). In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. doi: 10.1093/cid/ciaa237
Yao, H. P., Lu, X. Y., Chen, Q., Xu, K. J., Chen, Y., Cheng, L. F., et al. (2020). Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. doi: 10.1101/2020.04.14.20060160
Yuan, S. F., Chan, J. F. W., Chik, K. K. H., Chan, C. C. Y., Tsang, J. O. L., Liang, R. H., et al. (2020). Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system. Pharmacol. Res. 159, 104960. doi: 10.1016/j.phrs.2020.104960
Zhang, C. H., Wang, Y. F., Liu, X. J., Lu, J. H., Qian, C. W., Wan, Z. Y., et al. (2005). Antiviral activity of cepharanthine against severe acute respiratory syndrome coronavirus in vitro. Chin. Med. J. 118, 493–496.
Zhang, L., Zhang, D., Yuan, C., Wang, X. W., Li, Y. F., Jia, X. L., et al. (2020). Role of 1’-ribose cyano substitution for remdesivir to effectively inhibit both nucleotide addition and proofreading in SARS-CoV-2 viral RNA replication. bioRxiv. doi: 10.1101/2020.04.27.063859
Zhou, P., Yang, X. L., Wang, X. G., Hu, B., Zhang, L., Zhang, W., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273. doi: 10.1038/s41586-020-2012-7
Keywords: COVID-19, SARS-Cov-2, natural products, natural-product-inspired, potential anti-SARSCoV-2 agents
Citation: Wang Z and Yang L (2020) Turning the Tide: Natural Products and Natural-Product-Inspired Chemicals as Potential Counters to SARS-CoV-2 Infection. Front. Pharmacol. 11:1013. doi: 10.3389/fphar.2020.01013
Received: 29 April 2020; Accepted: 23 June 2020;
Published: 02 July 2020.
Edited by:
Xian-Tao Zeng, Wuhan University, ChinaReviewed by:
Sina Bavari, United States Army Medical Research Institute of Infectious Diseases (USAMRIID), United StatesCopyright © 2020 Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhonglei Wang, d2FuZ3psMTZAdHNpbmdodWEub3JnLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.