
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 12 May 2020
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00650
This article is part of the Research TopicEthnopharmacology of Eastern European CountriesView all 14 articles
 Krystalia Lytra1
Krystalia Lytra1 Ekaterina-Michaela Tomou1
Ekaterina-Michaela Tomou1 Antonios Chrysargyris2
Antonios Chrysargyris2 Chryssoula Drouza2
Chryssoula Drouza2 Helen Skaltsa1*
Helen Skaltsa1* Nikolaos Tzortzakis2*
Nikolaos Tzortzakis2*Sideritis species are recognized as important medicinal plants and their commercial demand is continuously on the rise both in the European and in the global market. Consequently, the cultivation of Sideritis species has been occurred to successfully meet the need for mass production of high-quality plant material. The present study was undertaken in order to investigate the chemical composition of cultivated S. cypria. Infusions of flowers and leaves were prepared separately, according to the European Medicine Agency (EMA) monograph. The infusion of the flowers revealed the presence of four flavones, isoscutellarein-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside, its 4′-O-methyl-derivative, 4′-O-methyl-hypolaetin-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside, and isoscutellarein-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)]-6″-O-acetyl-β-D-glucopyranoside; four phenylethanoid glucosides, acteoside, leucosceptoside A, lamalboside, and leonoside A; one iridoid, melittoside, and one phenolic acid, chlorogenic acid, while the infusion of the leaves of the same population afforded the same first two flavones; five phenylethanoid glucosides, acteoside, leucosceptoside A, lavandulifolioside, leonoside A, and lamalboside; melittoside and chlorogenic acid. The structural elucidation of the isolated compounds was undertaken by high-field NMR spectroscopy. Moreover, the essential oils of the flowers and leaves were studied by GC-MS, separately. In addition, the mineral, bioactive compounds, protein and carbohydrate contents were evaluated for both plant materials.
Sideritis species (Lamiaceae) have been used as traditional medicine herbs for thousands of years (González-Burgos et al., 2011) and the last 30 years safe use (including 15 years in the EU) with well-defined posologies and mode of preparation (EMA, 2015). Nowadays, the infusion of Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; and Sideritis syriaca L., has been listed by European Medicine Agency (EMA) as a traditional medicine for the relief of mild gastrointestinal discomfort and against the common cold (EMA, 2015). Previous detailed studies underlie the important pharmacological activities of the genus such as the antioxidant, anti‐inflammatory, antivirus, anticancer, hepatoprotective, antispasmodic, analgesic, neuroprotective activity, as well as its great effectiveness against diseases related to the central nervous and to the urinary system (Kirimer et al., 2004; González-Burgos et al., 2011; EMA, 2015; Hofrichter et al., 2016; Deveci et al., 2017; Aneva et al., 2019). Indeed, a clinical study that carried out by Wightman et al. (2018), showed that S. scardica (Greek mountain tea) improved the aspects of cognitive function and mood in a group of healthy, older adults. Therefore, these plants have been a subject of intensive phytochemical research and are characterized mainly by the presence of terpenes, flavonoids, phenylethanoid glucosides, phenolic acids, and essential oil (González-Burgos et al., 2011; EMA, 2015).
The genus Sideritis L. comprises around 150 species (Aneva et al., 2019); among them, the endemic species S. cypria Post. is a perennial herb belonging to the section Empedoclia Rafin., 60 cm high with bright yellow flowers, growing at altitude 300–925 m in Pentadactylos Mountains in Northern Cyprus (Meikle, 1985; Yildiz and Gücel, 2006; Tsintides et al., 2007). In particular, three Sideritis species have been found in Cyprus; S. curvidens Stapf, S. perfoliata L., and the endemic S. cypria Post (Hanoğlu et al., 2020). Traditionally, the infusion of S. cypria is locally used as diaphoretic, tonic, as well as against stomach disorders, headache and common cold (Yöney et al., 2010; Karousou and Deirmentzoglou, 2011; Hanoğlu et al., 2020). Hanoğlu et al. (2016) reported the antimicrobial efficacy of essential oils derived by S. cypria, and suggested to be used as a new medicinal resource particularly against C. albicans and Gram-positive bacteria.
However, the secondary metabolites of the traditional infusion of this plant are still undershadowed. The literature survey revealed only two publications concerning the chemical characterization of wild harvested S. cypria of its essential oil (Hanoğlu et al., 2016) and extracts (Hanoğlu et al., 2020). Therefore, for the purpose of this study, we investigated and compared for the first time the chemical composition of the traditional infusions of different plant parts, leaves and flowers, of cultivated S. cypria. In parallel, the essential oils and the mineral, bioactive compounds, protein, and carbohydrate contents of the flowers and the leaves of cultivated populations have been studied.
Sideritis cypria was collected from the Cypriot National Agricultural Department, on June 2019. Species seeds are kept at the Agricultural Research Institute, national gene bank (Accession number: ARI02415), collected in 2018 (Universal Transverse Mercator-UTM with latitude 546102; longitude 3904418, altitude 640). This is the first registered time that the plant has been cultivated, and plants from the first (mother) plantation were collected at their flower stage, for this analysis. Plantation was established at 2018, frequently irrigated (~ weekly/biweekly during irrigation period) and common fertilizers applied, with 20-10-10 once a year as basic fertilizer and 19-19-19 every second month as fertigation. Representative images of the different plant parts are presented in Figure 1.

Figure 1 Sideritis cypria, (A) cultivated crop, (B) plant, (C) flower, and (D) upper and bottom leaf area.
In the present study we use the word “flowers” in a wide sense which is usual in pharmacy. In this case we refer as "flowers" the whole inflorescence, including corollas, calyces, bracts and axis. Thus, we do not use “flowers” in its strict botanical sense. Aerial parts of the plants were separated into leaves and flowers (including corollas, calyces and bracts) and dried until constant weight at 65°C, using an air-drying oven. Dried tissue was then milled to pass a 0.42 sieve and ashed in a furnace (Carbolite, AAF 1100, GERO, Germany) at 450°C for 6 h. Nitrogen was determined using the Kjeldahl method (BUCHI, Digest automat K-439 and Distillation Kjelflex K-360, Switzerland). For the determination of minerals, tissue was acidified with 2 N hydrochloric acid (HCl). Phosphorus (P) was assayed with the molybdate/vanadate method while potassium (K), calcium (Ca), sodium (Na), magnesium (Mg), copper (Cu), zinc (Zn), and iron (Fe) were determined using an atomic absorption spectrophotometer (PG Instruments AA500FG, Leicestershire, UK) as described previously (Chrysargyris et al., 2019a). The nutritional value of leaves and flowers was determined according to the methods described by AOAC (AOAC, 2016). The protein content (in dry weight; d.w.) was determined by using the Kjeldahl method (N × 6.25), petroleum ether Soxhlet extraction was used for total fat determination, and tissue incineration (600°C) for moisture. The total content of carbohydrates was determined by difference, and the energetic value was calculated using the following formula: Energy (kcal/100 g d.w.) = 4 × (g protein/100 g d.w. + g carbohydrate/100 g d.w.) + 9 × (g fat/100 g d.w.). Results were expressed in g/100 g d.w. Four replicates were analyzed for each plant part, while each replicate was a pool of three different plants (Table 1).
Both the infusion and the decoction of leaves and flowers were used for the estimation of the total phenolic and flavonoid content, as well as the evaluation of bioactive compounds of the plant (Table 2). The extracts were diluted with 50% methanol to reach a concentration of 2 mg/ml. The phenolic content was determined according to the Folin-Ciocalteu method. The reaction mixture consisted of 0.5 ml of each extract (2 mg/ml), 0.5 ml of Folin Ciocalteu reagent, and 5 ml of water. The reaction was incubated for 1 h before absorbance was measured at 765 nm (Goulas et al., 2014). Results were expressed as mg of gallic acids equivalents per g of dry material. Total flavonoids content was estimated as described by Chrysargyris et al. (2016), using the aluminum chloride colorimetric method, and results were expressed as mg of rutin equivalents per g of dried tissue.
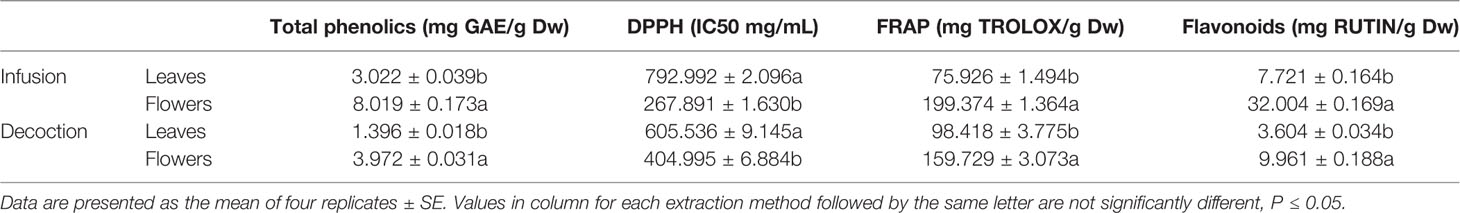
Table 2 Total phenolics and flavonoids content and bioactive compounds of infusion and decoction of S. cypria.
Extracts bioactivity was evaluated by the DPPH (2,2-diphenyl-1-picrylhydrazyl) and the ferric reducing antioxidant power (FRAP) assays. Serial dilutions of the extracts (0–1,000 μg/ml) were incubated with 0.3 mmol/L of DPPH for 30 min, and absorbance was measured at 517, as described by Goulas et al. (2014). The % of scavenging activity was determined using the formula: scavenging activity %=100-(Absorbance samples-Absorbance blank)100/Absorbance control. IC50 values are the concentration of each extract that has the 50% of the antioxidant activity. The FRAP assay was also tested, as described by Chrysargyris et al. (2016), using serial dilutions of Trolox [(±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid], as the reference standard. Results were expressed as mg Trolox per g of dried weight. All samples were tested in four replicates.
Aerial plant parts (leaves and flowers, separately) were placed in an air-drying oven at 42°C, for 48 h, until constant weight. Hydrodistillation was adopted for the extraction of the essential oils, using Clevenger apparatus. The duration of the extraction was 3 h for each sample, while four samples (pool of three plants per sample) were subjected for extraction and then for oil analysis. The essential oil yield was measured as μl of oil per 100 g of dried tissue.
The analysis of the essential oil components was carried out using a Shimadzu GC2010 gas chromatograph interfaced with a Shimadzu GC/MS QP2010plus mass spectrometer. A sample of each essential oil (1 μl) diluted in ethyl acetate (1:1,000) was injected in the autosampler, in split mode (1:20). The injector temperature was set at 230°C and the column temperature was programmed to rise from 60°C to 240°C at a rate of 5°C/min, with a 5 min hold at 240°C. Compound separation was performed on a Zebron ZB-5 (Phenomenex, USA) capillary column (0.25 μm x 30.0 m x 0.25 mm). Helium was used as the carrier gas with a flow set at 1.03 ml/min. Electron mass spectra with ionization energy of 70 eV was recorded at 35–400 m/z. The identification of oil components (Table 2) was assigned by comparison of their retention indices relative to (C8–C20) n-alkanes with those of literature or with those of authentic compounds available in our laboratory, by matching their recorded mass spectra with those stored in the NIST08 mass spectral library of the GC–MS data system and other published mass spectra (Adams, 2012).
1D- and 2D-NMR spectra were recorded in CD3OD on Bruker DRX 400 instrument at 295 K. Chemical shifts are given in ppm (δ) and were referenced to the solvent signals at 3.31 and 49.0 ppm. COSY (COrrelation SpectroscopΥ), HSQC (Heteronuclear Single Quantum Correlation), HMBC (Heteronuclear Multiple Bond Correlation), and NOESY (Nuclear Overhauser Effect SpectroscopY) (mixing time 950 ms) experiments were performed using standard Bruker microprograms. The [α]D values were obtained in CH3OH on Perkin Elmer 341 Polarimeter. UV-Vis spectra were recorded on a Shimadzu UV-160A spectrophotometer, according to Mabry et al. (1970). Column chromatography (CC): silica gel (Merck, Art. 9385, Darmstadt, Germany), gradient elution with the solvent mixtures indicated in each case. Preparative‐thin‐layer chromatography (TLC) plates pre‐coated with silica gel (Merck, Art. 5721). Fractionation was always monitored by TLC silica gel 60 F‐ 254, (Merck, Art. 5554) and cellulose (Merck, Art. 5552) with visualization under UV (254 and 365 nm) and spraying with vanillin‐sulphuric acid reagent and with Neu’s reagent (Neu, 1957) for phenolic compounds. In the whole process, all the obtained infusions, fractions, and isolated compounds were evaporated to dryness in vacuum under low temperature and then were put in activated desiccators with P2O5 until their weights had stabilized, in order to eliminate the moisture from the samples that might influence pre‐saturation performance and then lead to an intense water signal in the 1H‐NMR spectra, making it difficult to observe near signals.
Infusions of cultivated S. cypria Post were prepared based on the monograph of EMA, namely, 4.0 g of air-dried flowers and 4.0 g of air-dried leaves were separately dropped into 200 ml boiled distilled water each for 5 min, then were filtrated and concentrated to dryness. The infusion of flowers (1.1 g) was fractionated by CC over silica gel using as eluent mixtures of CH2Cl2:MeOH:H2O 9.9:0.1:0.01-0:1:0 to yield finally 27 fractions (A-Z1). Fractions J and K were combined (6.0 mg; eluted with CH2Cl2:MeOH:H2O 8:2:0.2-7.8:2.2:0.2) and afforded compound 4 (4.1 mg). Fraction N (eluted with CH2Cl2:MeOH:H2O 7.8:2.2:0.2) yielded compound 1 (3.3 mg). Fraction Q (10.2 mg; eluted with CH2Cl2:MeOH:H2O 7.5:2.5:0.2) was further purified by preparative TLC plates pre‐coated with silica gel and gave compounds 3 (1.7 mg) and 6 (3 mg). Combined fractions R, S, T, and U (108.0 mg; eluted with CH2Cl2:MeOH:H2O 7.5:2.5:0.25-6.8:3.2:0.32) were further fractionated by CC over silica gel using as eluent mixtures of CH2Cl2:MeOH:H2O 9.7:0.3:0.03-0:1:0 and afforded compound 1 (0.7 mg), a mixture of compounds 2 and 6 (0.7 mg), as well as compounds 5 (3.4 mg) and 8 in mixture with free sugars (5.2 mg). Compound 8 was further purified by preparative TLC plates pre-coated with silica gel and gave pure compound 8 (2.4 mg). Fraction Y (58.8 mg; eluted with CH2Cl2:MeOH:H2O 6:4:0.4) was subjected to CC over Sephadex (MeOH:H2O 100:0-90:10) and yielded compounds 9 (5.1 mg), 10 (15.3 mg) and 11 (1.6 mg).
The infusion of leaves (0.7 g) was fractionated by CC over silica gel using as eluent mixtures of CH2Cl2:MeOH:H2O 9.9:0.1:0.01-0:1:0 to afford finally 23 fractions (A–W). Fraction I (0.9 mg; eluted with CH2Cl2:MeOH:H2O 7.8:2.2:0.2) was identified as compound 1 (0.9 mg). Fractions K and M were eluted with CH2Cl2:MeOH:H2O 7.5:2.5:0.2 and gave compounds 2 (0.7 mg) and 6 (1.2 mg), respectively. Fraction Q (56.3 mg; eluted with CH2Cl2:MeOH:H2O 7:3:0.3-6.8:3.2:0.32) was applied to CC over silica gel using as eluent mixtures of CH2Cl2:MeOH:H2O 9.9:0.1:0.01-0:1:0 and afforded compounds 5 (2.5 mg) and 8 (0.7 mg). Fraction S was eluted with CH2Cl2:MeOH:H2O 6.5:3.5:0.35 and yielded compound 7 (9.0 mg). Fraction U (61.0 mg; eluted with CH2Cl2:MeOH:H2O 6:4:0.4) was subjected to CC over Sephadex (MeOH:H2O 100:0-90:10) and afforded compounds 9 (3.4 mg), 10 (14.8 mg) and 11 (1.4 mg).
The mineral composition and the nutritional traits of both flowers and leaves of S. cypria plants are illustrated on Table 1. Flowers appeared to have significantly higher nutritional value than leaves, in terms of protein levels, total fats and carbohydrates, resulting in higher energy content. As for the minerals, flowers are richer in N, P, and Mg, while leaves have higher content of K, Ca, and Na. For the cases of K and Ca, concentration in leaves is almost two times higher than in flowers. Micronutrients as Fe and Cu were found in bigger amounts in leaves when compared to flowers but Zn remained at the same levels in leaves and flowers.
For the evaluation of the extract’s bioactivity (Table 2), two different extracts were assayed; the infusion and the decoction of both flowers and leaves. In both cases of extracts, bioactive compounds and the correlated phenolic content appeared significantly higher for the flower extracts. In all cases, the infusion values were higher than the ones obtained from the decoction extracts. Total phenolic content in the infusion of flowers appeared more than double compared to the corresponding leaf extract. The same trend was revealed for all the bioactive compounds (DPPH and FRAP) assays tested, with the most remarkable differences to be evidenced for the total flavonoid content. Here flowers had more than 4 times higher values, in terms of rutin equivalents; 32.004 mg rutin per g of dried tissue for the flowers while the leaf content was found at 7.721 mg rutin per g of dried tissue.
The essential oil yield of the two plant parts tested in this study appeared significantly different. Flowers exhibited higher yield with 0.25% (± SE 0.029) while the essential oils of leaves were in relatively low level and did not exceed 0.03% (± SE 0.003). The GC/MS analysis that followed delivered 34 compounds in the leaves’ oils and 31 compounds in the oils obtained from the flowers; 29 of these compounds appeared in the oils from both plant parts, but with great variability in terms of contribution to the oil profile (Table 3). The five and two compounds found only in leaves and flowers respectively, represent components with less than 0.350% (nonanal) each, out of the total chromatogram. In the oils from the leaves, the major terpenes were the monoterpenes hydrocarbons with 51.45%, followed by sesquiterpenes hydrocarbons (23.41%) and oxygenated sesquiterpenes (22.18%), while in flowers the total monoterpenes hydrocarbons reached 88.38%, and oxygenated compounds in total did not exceed 4.35% (1.34% and 2.99%, for mono- and sesquiterpenes, respectively). The major compounds identified in leaves were β-phellandrene (25.11%), β-caryophyllene (22.52%), α-pinene (11.92%), 14 hydroxy-β-caryophyllene (9.88%), and caryophyllene oxide (8.32%). In flowers, the dominant compound is α-pinene (37.97%), followed by β-phellandrene (25.81%), β-pinene (14.67%), β-caryophyllene (6.83%) and sabinene (5.58%). With the exception of β-phellandrene, the other major compounds differ significantly in terms of percentage (%) between the two tested oils. Comparing the total chromatographs of the two tested oils, the majority of the compounds (in terms of percentage participation), are significantly different, except eight compounds (Table 3). Considering the essential oil analysis in leaves and flowers separately, this is the first report of the analysis of the essential oils from S. cypria flowers.
The infusion of the flowers of cultivated S. cypria yielded four flavones, isoscutellarein-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (1) (Lenherr and Mabry, 1987), 4′-O-methyl-isoscutellarein-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (2) (Venditti et al., 2014), isoscutellarein-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)]-6″-O-acetyl-β-D-glucopyranoside (3) (Halfon et al., 2013), and 4′-O-methyl-hypolaetin-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (4) (Halfon et al., 2013); four phenylethanoid glucosides, acteoside (5) (Kawada et al., 1999), leucosceptoside A (6) (Miyase et al., 1982), leonoside A (8) (Çaliş et al., 1992), and lamalboside (9) (Budzianowski and Skrzypczak, 1995); one iridoid, melittoside (10) (Śawia̧tek et al., 1981), and one quinic acid derivative, chlorogenic acid (11) (Armata et al., 2008).
Moreover, the infusion of the leaves of the same population afforded two flavones, isoscutellarein-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (1) and 4′-O-methyl-isoscutellarein-7-O-[6′″-O-acetyl-β-D-allopyranosyl-(1→2)-β-D-glucopyranoside (2); five phenylethanoid glucosides, namely the four previously found in the infusion of flowers and lavandulifolioside (7) (Başaran et al., 1988), moreover the same iridoid and quinic acid derivative have been isolated (Figure 2).
Leonoside A (8) was first isolated from Stachys sieboldii in 1991 (Nishimura et al., 1991) as stachysoside B. Later, Çaliş et al. (1992) isolated it from Leonurus glaucescens and it was given the name “leonoside A”. From the genus Sideritis L., it has been previously isolated only from S. trojana Bornm. (Kirmizibekmez et al., 2012). Our study confirms the presence of leonoside A in S. cypria (Hanoğlu et al., 2020) and we report for the first time the isolation of this compound from the infusion of both, leaves and flowers. It is interesting to point out that compounds 2- 4, 9 - 11 had been mentioned previously in the genus Sideritis L., but not in S. cypria. Thus, the aforementioned compounds are reported as components of S. cypria for the first time. The structures of the isolated compounds were elucidated by spectroscopic data. Intriguingly, the NMR data of compounds 8, 9, and 10 are not fully recorded in the literature, therefore, herein, are presented (Tables S1–S3).
It is important to mention that the chemical characterization of the total infusions and the sub fractions were monitored by NMR techniques, which enabled us to identify and isolate all the main and the minor components.
The mineral content of S. cypria leaves differs from that of the flowers. As it was mentioned previously, leaves are richer in potassium and calcium while flowers in magnesium and phosphorus, and this is of great importance as reported by Bojovic et al. (2011), as water tea-infusions had large portions of K, P, Na, and Cu, and different parts of the plants can contribute in various ways on human nutrition. Comparing to other species of the genus, S. cypria leaves have similar levels of potassium (28.40 g/kg) with the leaves of S. perfoliata, ranging from 20.65 to 29.84 g/kg, depending on the cultivation method (Chrysargyris et al., 2019b). S. cypria has almost two times higher content of phosphorus and calcium, in both plant parts, than S. perfoliata (Chrysargyris et al., 2019b; Lall et al., 2019). Species S. scardica and S. raeseri appear poorer in terms of mineral content, and demonstrate almost half the quantities of the majority of nutrients as K, P, Ca, Mg (Karapandzova et al., 2013), compared to S. cypria. Trikka et al. (2019) evaluated different species of Sideritis genus from different geographical regions of Greece and plants exhibited high variability, even within species (S. perfoliata, S. scarica, S. raeseri), and had lower contents in minerals than S. cypria. These variations may be the effect of the cultivation practices applied or/and the harvesting periods (Chrysargyris et al., 2019b).
Regarding the nutritional value, the flowers of S. cypria are richer in protein, fats, and carbohydrates than leaves. S. perfoliata aerial parts (leaves) demonstrate protein content up to 14.64%, while in S. cypria the corresponding content was 8.06% and 9.02% for leaves and flowers. Total fats and carbohydrates demonstrated almost similar values for S. cypria (1.78% and 72.90%) and S. perfoliata (1.76% and 73.53%). A research conducted to investigate the chemical profile and metabolite content of S. italica, revealed that the protein content of the plant parts were 6% and 10% for the leaves and flowers, respectively (Menghini et al., 2014), values similar to S. cypria protein content. Generally, in the Lamiaceae family, protein content may vary among different species of the same genus. As it was assayed by Tunçtürk et al. (2017), Thymus species had protein content varying from 6.85-14.34%, while Mentha species as M. longifolia and M. spicata demonstrated protein content of 13.86% and 15.13%, respectively.
The bioactive substances of the two types of aqueous extracts for both plant parts demonstrated the same trend; flowers are richer in compounds as phenolics and flavonoids, and thus, exhibiting higher bioactivity (Phatak and Hendre, 2014). Additionally, the infusions demonstrated higher bioactivity and appear to be richer than decoctions, due to the higher polarity of the solvent used (Martins et al., 2015). In general, plant’s extracts appear to be less strong compared to other species of genus. S. syriaca’s decoction demonstrated total phenolic compounds almost 18 mg GAE/g Dw (Goulas et al., 2014), while S. cypria decoction ranged between 1.39 and 3.92 mg GAE/g Dw (for leaves and flowers, respectively). Nevertheless, plant species, cultivation practices, environmental conditions, and extraction methodology are parameters, to name a few, that affect the bioactive status of plants (González-Burgos et al., 2011; Chrysargyris et al., 2019b).
According to literature, Sideritis genus species yield low essential oil quantities, compared to other Lamiaceae species (González-Burgos et al., 2011). There are reports that indicate this yield variation among species, that range from traces (< 0.01%) of essential oil in many Sideritis species (Kirimer et al., 2004; Bojovic et al., 2011) to 0.94% (S. lanata) (Koutsaviti et al., 2013). S. cypria follows the same trend, with the flower’s essential oil yield to be significantly higher (0.25%) than leaves’ yield, which was measured at considerably low numbers of 0.03%. Hanoğlu et al. (2016; 2020) recorded a yield of 0.49%, when hydrodistilled S. cypria plants, during or post-flowering that were collected from mountains in north Cyprus (500–750 m altitude). This variation with the present results might be attributed that wild harvested plant tissue in mountain areas (Hanoğlu et al., 2016; Hanoğlu et al., 2020) can differ on the essential oil yield and aromatic profile, compared with cultivated crop in lowland.
As for the compounds, of the two oils tested (leaves and flowers), analysis showed remarkable differences in composition. The major compounds of leaves are β-phellandrene (25.11%) and β-caryophyllene (22.52%), followed by α-pinene (11.92%), 14 hydroxy-β-caryophyllene (9.88%), and caryophyllene oxide (8.32%). All these components were identified as well by previous studies of the plant during flowering phase, but with different percentages, while β-phellanderene was identified again as the major compound (17.83%) (Hanoğlu et al., 2020). The same authors referenced their previous work (Hanoğlu et al., 2016) mentioning that when oils from post flowering stage were analyzed, the compounds and their amounts varied, compared to those during flowering. Plants evaluated in this study were collected during flowering stage, and leaves’ oil profile is similar to the report of Hanoğlu et al. (2020), but with differences mostly in secondary compounds, in terms of their participation in the oil profile. These differences can be ascribed to the separate analysis of leaves and flowers, and to the differences that occur due to plant’s origin and cultivation practices. Hanoğlu et al. (2020) collected plants from the mountains of north Cyprus, while plants tested in this study are plants from the first official cultivation reported in Cyprus for S. cypria. That means that plants are subjected to specific irrigation regimes and cultivation practices, while plants collected from wild exhibit great variability. Todorova and Trendafilova (2014) reviewed this variation in other Sideritis species oils as well, mentioning region as a factor of this variation. Chrysargyris et al. (2019b) also reported that cultivation practices and harvesting periods affect components of Sideritis species oils.
To our knowledge, this is the first report of S. cypria oils analysis that separates oils from different plant parts, and is the first analysis of the flower oil. The major compounds found in flower oil are α-pinene (37.97%), β-phellandrene (25.81%), β-pinene (14.67%), and β-caryophyllene (8.83%). These differences between flowers and leaves, concern not only single components, but can be examined as differences in the grouped compounds, as leaves appear richer in sesquiterpenes hydrocarbons (23.41%) and oxygenated sesquiterpenes (22.18%), while in flower the corresponding percentages are together almost 10% (7.02% and 2.99%, respectively). This is of great importance, as consumer can use either leaves or flowers or mixture of them during their usage of S. cypria, reflecting different aromatic profile and properties. Bojovic et al. (2011) reviewed the phytochemical profile of Sideritis species, indicating that essential oils are characterised by high contents of monoterpene hydrocarbons with α-pinene, β-pinene, sabinene, myrcene or limonene and of sesquiterpene hydrocarbons, particularly δ-cadinene and β-caryophyllene, as the main compounds. However, S. cypria is not on that extensive review list of species, highlighting the importance of the present study.
Monoterpenes such as pinenes and phellandrenes, among others are the main components of the odour of medicinal plants with biocidal activities. For example, β-phellandrene has been tested as a strong antimicrobial terpene while it exhibits insect repellent activities (Harrewijn et al., 2001). It has shown in vitro activity against Bacillus sp., Candida albicans, Escherichia coli, Pseudomonas aeruginosa, and S. aureus (Zhang et al., 2017). In plants, pinenes show fungicidal activity and have been used for centuries to produce flavors and fragrances, while the antimicrobial activity of some essential oils is attributed to the presence of α- and/or β-pinene (Rivas da Silva et al., 2012). α-Pinene is a monoterpene and member of hydrocarbon group of bicyclic terpenes that has not only a wide range of uses as a food additive (Yang et al., 2016), but also exhibits great biological activities as insecticidal, spasmolytic, anti-listerial, and anticholinesterase (Orhan et al., 2006). Additionally, Akutsu et al. (2002), found that α-pinene has a potential anti-stress activity. Moreover, β-pinene has also exhibited antidepressant activity, and furthermore it’s mode of action could be connected to the mechanism of the action of the most frequently used antidepressant drugs (Guzman-Guttierez et al., 2015). Orhan et al. (2006) also reported the anti-inflammatory activity of the compound, while reviewing the synergistic effect of α-pinene with β-caryophyllene, as anti- inflammatory compounds. Interestingly, β-caryophyllene alone is a compound which has been demonstrated to possess a great potential application for various pathological conditions, as central nervous system diseases (Parkinson’s disease, Alzheimer’s disease), osteoporosis, cancer, and antibacterial (Francomano et al., 2019). Importantly, it is also approved as food additive, taste enhancer, and flavoring agent by U.S. Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) (Fidyt et al., 2016; Machado et al., 2018).
Continuing our study on Sideritis genus, this work reports for the first time the composition of the traditional infusions of S. cypria Post. The genus Sideritis L. is characterized by the presence of several different secondary metabolites, mainly terpenes and various phenolic derivatives. In the present study, overall, eleven compounds were isolated from both infusions of the flowers and the leaves of cultivated S. cypria. It is interesting to note that the two plant parts showed similar chemical composition. Precisely, from the sample of the flowers were isolated more flavones, compared to the sample of the leaves in which were identified more phenylethanoid glucosides.
Numerous studies over the last years have revealed the presence of a great number of glycosides of 8-hydroxy flavones in Sideritis species, such as acetylated or non-acetylated 7-O-allosyl (1→2)glucosides of isoscutellarein (8-hydroxy apigenin) and hypolaetin (8-hydroxy luteolin), as well as their 4′-methoxy derivatives (EMA, 2015; Stanoeva et al., 2015). In the current study, three isoscutellarein and one hypolaetin derivatives were found. The results are in agreement with previously well studied Sideritis species belonging to the section Empedoclia, i.e., S. scardica (Todorova and Trendafilova, 2014; EMA, 2015), S. perfoliata L. subsp. perfoliata (Charami et al., 2008; Chrysargyris et al., 2019b), S. euboea (Tomou et al., 2019), S. raeseri (Gabrieli et al., 2005; Romanucci et al., 2017), and S. syriaca (Plioukas et al., 2010; Goulas et al., 2014).
Hanoğlu et al. (2020) studied the chemical composition of non polar and polar extracts of S. cypria wild populations and reported the presence of four flavones, four phenylethanoid glucosides and one iridoid glucoside in the polar extracts. Comparing our results to the literature data, we confirm the presence of the same phenylethanoid glucosides, with the only difference the isolation of lamalboside in both infusions. It is noteworthy that lamalboside has been isolated previously only from two Sideritis species belonging to the Empedoclia section; S. germanicopolitana Bornm. and S. trojana Bornm. (Kirmizibekmez et al., 2012; Kirmizibekmez et al., 2019). Lamalboside, also known as lamiuside A, was first isolated and described from the genus Lamium L. (Budzianowski and Skrzypczak, 1995; Ito et al., 2006; Yalçın et al., 2007). Furthermore, considering the identification of the flavonoid derivatives, the two infusions were characterized by isoscutellarein derivatives. However, Hanoğlu et al. (2020) reported the isolation of four flavones; one isoscutellarein derivative and three apigenin derivatives. This distinguishment could be attributed not only in the fact that our plant material is originated from cultivation, but also to the different polarity of the investigated samples between the two studies. Importantly, our research also revealed the presence of chlorogenic acid, which has been previously found in many Sideritis spp. (Armata et al., 2008; Samanidou et al., 2012; Goulas et al., 2014; Irakli et al., 2018; Chrysargyris et al., 2019b; Tomou et al., 2019). It is interesting to note that in both infusions the main iridoid derivative was melittoside. In the specific section (Empedoclia), melittoside have been reported in the species S. euboea Heldr. (Tomou et al., 2019), S. montana L. (Koleva et al., 2003; Fraga, 2012), S. montana L. subsp. montana (Venditti et al., 2016), S. germanicopolitana Bornm (Kirmizibekmez et al., 2019), S. perfoliata L. subsp. perfoliata (Chrysargyris et al., 2019b), and S. syriaca L. (Koleva et al., 2003; Fraga, 2012). Acteoside and leucosceptoside A, found in our study, have been also isolated from S. euboea Heldr. (Tomou et al., 2019), S. perfoliata L. subsp. perfoliata (Charami et al., 2008; Chrysargyris et al., 2019b), S. scardica Gris. (Fraga, 2012; Todorova and Trendafilova, 2014), S. lysia Boiss et. Heldr. (Fraga, 2012), and S. raeseri Boiss et. Heldr. (Petreska et al., 2011), while lavandulifolioside has been isolated from S. euboea Heldr. (Tomou et al., 2019), S. perfoliata L. subsp. perfoliata (Charami et al., 2008; Chrysargyris et al., 2019b), and S. lysia Boiss et. Heldr (Fraga, 2012). Moreover, a survey of four infusions of cultivated S. raeseri subsp. raeseri was carried out and underlay the great antioxidant activity due to the high phenolic content, including chlorogenic acid, phenylethanoid glucosides and flavonoid derivatives (Pljevljakušić et al., 2011).
Chrysargyris and co-workers (2019b) have revealed the presence of three isoscutellarein derivatives, two phenylethanoids, one quinic acid derivative and six iridoids from the infusion of the cultivated S. perfoliata L. subsp. perfoliata from Cyprus. Of note, our results are in accordance with this study. It is interesting to point out that cultivated S. cypria is very poor in iridoids compared to cultivated S. perfoliata L. subsp. perfoliata, while on the other hand S. cypria consists more phenylethanoid glucosides. In the context of the presence of phenylethanoid glucosides, bearing three sugar moieties, are common group of secondary metabolites of this genus, but the trisaccharide derivatives with galactose as one of the sugars are rare.
Several studies have mentioned the antioxidant activity of isoscutellarein and hypolaetin derivatives (Gabrieli et al., 2005; Armata et al., 2008; Charami et al., 2008; Kirmizibekmez et al., 2012). It is well established that flavonoids with at least one hydroxyl-group in B ring exhibit high antioxidant activity (Charami et al., 2008; Sarian et al., 2017). However, the existence of a hydroxyl group in ring A and catechol structure or 4′-hydroxyl group in ring B improve more the antioxidant activity (Charami et al., 2008; Sarian et al., 2017). Furthermore, phenylethanoid glucosides are known for various pharmacological activities (Fu et al., 2008). Precisely, Charami et al. (2008) reported the antioxidant and anti-inflammatory activity of some isolated phenylethanoid glucosides and flavones derivatives from the species S. perfoliata subsp. perfoliata. Phenylethanoid glucosides exhibited better anti-inflammatory activity, compared to flavonoids due to their structures (caffeoyl group and to their o-dihydroxyphenyl group) (Charami et al., 2008). Moreover, a survey of four infusions of cultivated S. raeseri subsp. raeseri was carried out and underlay the great antioxidant activity due to the high phenolic content, including chlorogenic acid, phenylethanoid glucosides, and flavonoid derivatives (Pljevljakušić et al., 2011). Therefore, we could assume that the presence of flavones derivatives in combination with the abundance of phenylethanoid glucosides and chlorogenic acid in our samples could enhance the pharmacological activity, as well as it could justify the ethnopharmacological uses of S. cypria.
The present study revealed the chemical variation of the flowers and leaves essential oils (EOs) in comparison to the wild population of S. cypria previously investigated (Hanoğlu et al., 2016). The main constituents of the aerial parts of wild S. cypria EO were epi-cubebol (11.9%), trans-piperitol (8.9%), α-pinene (4.3%), and β-pinene (3.6%), while the main constituents of the flowers and leaves EOs were α-/β-pinenes in significant higher concentration, sabinene, β-phellandrene (ca. 25% in both EOs), β-caryophyllene, caryophyllene oxide, and 14-hydroxy-β-caryophyllene. In accordance with the EOs of Greek Sideritis species, where monoterpenes are the dominant components (Aligiannis et al., 2001; González-Burgos et al., 2011; Kloukina et al., 2019), the present samples are also rich in monoterpene hydrocarbons followed by sesquiterpenes. We assume that different factors (altitude, cultivation methods, and distillation methods) play an important role on the observed chemical variations.
The present study illustrates the phytochemical investigation of leaves and flowers of cultivated S. cypria, is a very rare and vulnerable species endemic to Cyprus with a small distribution area restricted to the Pentadaktylos Range (Tsintides et al., 2007; Bilz, 2011). The present study reveals that the cultivation of S. cypria is feasible without affecting its chemical profile. Cyprus ironwort has a chemical profile rich in bioactive secondary metabolites and can be used as culinary herb, but the plant material sold in local markets should not be collected from the field and should come from crops. S. cypria has a high phosphorus and calcium levels in both leaves and flowers. The nutritional value is mainly oriented on flowers which are richer in protein, fats, and carbohydrates than leaves. Flowers exhibited higher essential oil yield comparing to the leaves. The five major constituents identified in leaves were β-phellandrene, β-caryophyllene, α-pinene, 14 hydroxy-β-caryophyllene, and caryophyllene oxide, while in flowers the dominant compound was α-pinene, β-phellandrene, β-pinene, β-caryophyllene, and sabinene. Moreover, the infusion of the flowers was more abundant in flavones, though the infusion of the leaves was mainly consisted by phenylethanoid glucosides, based on the isolated compounds and NMR data.
All datasets generated for this study are included in the article/Supplementary Material.
KL carried out the chemical analyses of the infusions. E-MT contributed to the chemical analyses of the infusions and to the writing of the article. HS supervised the chemical analyses of the infusions and contributed to the writing of the article. AC and CD carried out the analyses of EOs, of mineral and nutritional contents of the leaves and flowers infusions/decoctions. NT supervised the latter analyses and contributed to the writing of the article.
The research performed at the Department of Pharmacognosy & Chemistry of Natural Products and at the Department of Agricultural Sciences, Biotechnology and Food Science, were funded by the National & Kapodistrian University of Athens and by the Cyprus University of Technology, respectively. Funding for publication was granted by the Cyprus University of Technology Open Access Author Fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to the Cyprus University of Technology Open Access Author Fund.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00650/full#supplementary-material
Çaliş, İ., Ersöz, T., Taşdemir, D., Rüedi, P. (1992). Two phenylpropanoid glycosides from Leonurus glaucescens. Phytochemistry 31, 357–359. doi: 10.1016/0031-9422(91)83078-Y
Śawia̧tek, L., Lehmann, D., Chaudhuri, R. K., Sticher, O. (1981). Occurrence of melittoside in the seeds of Plantago media. Phytochemistry 20, 2023–2024. doi: 10.1016/0031-9422(81)84058-7
Adams, R. P. (2012). Identification of essential oil components by gas chromatography and mass spectroscopy. 4rth edition (Carol Stream, IL, USA: Allured Publishing Corp.).
Akutsu, H., Kikusuia, T., Takeuchia, Y., Sanob, K., Hatanakac, A., Moria, Y. (2002). Alleviating effects of plant-derived fragrances on stress-induced hyperthermia in rats. Physiol. Behav. 75, 355–360. doi: 10.1016/S0031-9384(01)00670-9
Aligiannis, N., Kalpoutzakis, E., Chinou, I. B., Mitakou, S., Gikas, E., Tsarbopoulos, A. (2001). Composition and antimicrobial activity of the essential oils of five taxa of Sideritis from Greece. J. Agr. Food Chem. 49, 811–815. doi: 10.1021/jf001018w
Aneva, I., Zhelev, P., Kozuharova, E., Danova, K., Nabavi, S. F., Behzad, S. (2019). Genus Sideritis, section Empedoclia in southeastern Europe and Turkey – studies in ethnopharmacology and recent progress of biological activities. DARU J. Pharm. Sci. 27, 407–421. doi: 10.1007/s40199-019-00261-8
AOAC (2016). “Official methods of analysis of AOAC International,” in Official Methods of Analysis of AOAC International, 20th ed.). Eds. Horwitz, W., Latimer, G. (Gaithersburg: MD: AOAC International).
Armata, M., Gabrieli, C., Termentzi, A., Zervou, M., Kokkalou, E. (2008). Constituents of Sideritis syriaca ssp. syriaca (Lamiaceae) and their antioxidant activity. Food Chem. 111, 179–186. doi: 10.1016/j.foodchem.2008.03.061
Başaran, A. A., Çaliş, İ., Anklin, C., Nishibe, S., Sticher, O. (1988). Lavandulifolioside: A New Phenylpropanoid Glycoside from Stachys lavandulifolia. HCA 71, 1483–1490. doi: 10.1002/hlca.19880710612
Bilz, M. (2011). The IUCN Red List of Threatened Species 2011. Sideritis Cypria, e.T162296A5570289. doi: 10.2305/IUCN.UK.2011-1.RLTS.T162296A5570289.en
Bojovic, D., Jankovic, S., Potpara, Z., Tadic, V. (2011). Summary of the phytochemical research performed to date on Sideritis species. Ser. J. Exp. Clin. Res. 12, 109–122. doi: 10.5937/sjecr1103109B
Budzianowski, J., Skrzypczak, L. (1995). Phenylpropanoid esters from Lamium album flowers. Phytochemistry 38, 997–1001. doi: 10.1016/0031-9422(94)00727-B
Charami, M.-T., Lazari, D., Karioti, A., Skaltsa, H., Hadjipavlou-Litina, D., Souleles, C. (2008). Antioxidant and antiinflammatory activities of Sideritis perfoliata subsp. perfoliata (Lamiaceae). Phytother. Res. 22, 450–454. doi: 10.1002/ptr.2333
Chrysargyris, A., Panayiotou, C., Tzortzakis, N. (2016). Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crop Prod. 83, 577–586. doi: 10.1016/j.indcrop.2015.12.067
Chrysargyris, A., Antoniou, O., Athinodorou, F., Vassiliou, R., Papadaki, A., Tzortzakis, N. (2019a). Deployment of olive-stone waste as a substitute growing medium component for Brassica seedlings production in nurseries. Environ. Sci. Pollut. Res. 26, 35461–35472. doi: 10.1007/s11356-019-04261-8
Chrysargyris, A., Kloukina, C., Vassiliou, R., Tomou, E.-M., Skaltsa, H., Tzortzakis, N. (2019b). Cultivation strategy to improve chemical profile and anti-oxidant activity of Sideritis perfoliata L. subsp. perfoliata. Ind. Crop Prod. 140, 111694. doi: 10.1016/j.indcrop.2019.111694
Deveci, E., Tel-Çayan, G., Yıldırım, H., Duru, M. E. (2017). Chemical composition, antioxidant, anticholinesterase and anti-urease activities of Sideritis pisidica Boiss. & Heldr. endemic to Turkey. Marmara Pharm. J. 21, 898–905. doi: 10.12991/mpj.2017.13
EMA, /HMPC/39455/ (2015). Assessment report on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba.
Fidyt, K., Fiedorowicz, A., Strządała, L., Szumny, A. (2016). β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 5 (10), 3007–3017. doi: 10.1002/cam4.816
Fraga, B. M. (2012). Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 76, 7–24. doi: 10.1016/j.phytochem.2012.01.018
Francomano, F., Caruso, A., Barbarossa, A., Fazio, A., LaTorre, C., Ceramella, J., et al. (2019). β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 9, 5420. doi: 10.3390/app9245420
Fu, G., Pang, H., Wong, Y. H. (2008). Naturally Occurring Phenylethanoid Glycosides: Potential Leads for New Therapeutics. Curr. Med. Chem. 15 (25), 2592–2613. doi: 10.2174/092986708785908996
Gabrieli, C. N., Kefalas, P. G., Kokkalou, E. L. (2005). Antioxidant activity of flavonoids from Sideritis raeseri. J. Ethnopharmacol. 96, 423–428. doi: 10.1016/j.jep.2004.09.031
González-Burgos, E., Carretero, M. E., Gómez-Serranillos, M. P. (2011). Sideritis spp.: uses, chemical composition and pharmacological activities-a review. J. Ethnopharmacol. 135, 209–225. doi: 10.1016/j.jep.2011.03.014
Goulas, V., Exarchou, V., Kanetis, L., Gerothanassis, I. P. (2014). Evaluation of the phytochemical content, antioxidant activity and antimicrobial properties of mountain tea (Sideritis syriaca) decoction. J. Funct. Foods 6, 248–258. doi: 10.1016/j.jff.2013.10.014
Guzman-Guttierez, S. L., Bonilla-Jaime, H., Gomez-Cansino, R., Reyes-Chilpa, R. (2015). Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 128, 24–29. doi: 10.1016/j.lfs.2015.02.021
Halfon, B., Çiftçi, E., Topçu, G. (2013). Flavonoid constituents of Sideritis caesarea. Turk. J. Chem. 37, 464–472. doi: 10.3906/kim-1206-45
Hanoğlu, D. Y., Hanoğlu, A., Güvenir, M., Süer, K., Demirci, B., Başer, K. H. C., et al. (2016). Chemical composition and antimicrobial activity of the essential oil of Sideritis cypria Post endemic in Northern Cyprus. J. Essent. Oil Res. 29, 228–232. doi: 10.1080/10412905.2016.1251503
Hanoğlu, D. Y., Hanoğlu, A., Yusufoğlu, H., Demirci, B., Başer, K. H. C., Çalış, İ., et al. (2020). Phytochemical Investigation of Endemic Sideritis cypria Post. Rec. Nat. Prod. 14, 105–115. doi: 10.25135/rnp.140.18.11.1079
Harrewijn, P., Van Oosten, A. M., Piron, P. G. M. (2001). “Natural Terpenoids as Messengers,” in A multidisciplinary study of their production, biological functions and practical applications (Dordrecht: Kluwer Academic Publishers), 440.
Hofrichter, J., Krohn, M., Schumacher, T., Lange, C., Feistel, B., Walbroel, B., et al. (2016). Sideritis spp. Extracts Enhance Memory and Learning in Alzheimer’s β-Amyloidosis Mouse Models and Aged C57Bl/6 Mice. J. Alzheimers Dis. 53, 967–980. doi: 10.3233/JAD-160301
Irakli, M., Tsifodimou, K., Sarrou, E., Chatzopoulou, P. (2018). Optimization infusions conditions for improving phenolic content and antioxidant activity in Sideritis scardica tea using response surface methodology. J. Appl. Res. Med. Aromat. Plants 8, 67–74. doi: 10.1016/j.jarmap.2017.12.001
Ito, N., Nihei, T., Kakuda, R., Yaoita, Y., Kikuchi, M. (2006). Five new Phenylethanoid Glycosides from the whole plants of Lamium purpureum L. Chem. Pharm. Bull. 54, 1705–1708. doi: 10.1248/cpb.54.1705
Karapandzova, M., Qazimia, B., Stefkova, G., Bačevac, K., Stafilovc, T., Kadifkova Panovskab, T., et al. (2013). Chemical characterization, mineral content and radical scavenging activity of Sideritis scardica and S. raeseri from R. Macedonia, and R. Albania. Nat. Prod. Commun. 8 (5), 639–644.
Karousou, R., Deirmentzoglou, S. (2011). The herbal market of Cyprus: Traditional links and cultural exchanges. J. Ethnopharmacol. 133, 191–203. doi: 10.1016/j.jep.2010.09.034
Kawada, T., Asano, R., Hayashida, S., Sakuno, T. (1999). Total synthesis of the Phenylpropanoid Glycoside, Acteoside. J. Org. Chem. 64, 9268–9271. doi: 10.1021/jo9906983
Kirimer, N., Baser, K. H. C., Demirci, B., Duman, H. (2004). Essential oils of Sideritis species of Turkey belonging to the section Empedoclia. Chem. Nat. Compd. 40, 19–23. doi: 10.1023/B:CONC.0000025458.00475.cf
Kirmizibekmez, H., Ariburnu, E., Masullo, M., Festa, M., Capasso, A., Yesilada, E., et al. (2012). Iridoid, phenylethanoid and flavonoid glycosides from Sideritis trojana. Fitoterapia 83, 130–136. doi: 10.1016/j.fitote.2011.10.003
Kirmizibekmez, H., Erdoğan, M., Kúsz, N., Karaca, N., Erdem, U., Demirci, F., et al. (2019). Secondary metabolites from the aerial parts of Sideritis germanicopolitana and their in vitro enzyme inhibitory activities. Nat. Prod. Res., 1–4. doi: 10.1080/14786419.2019.1586700
Kloukina, C., Tomou, E.-M., Skaltsa, H. (2019). Essential oil composition of two Greek cultivated. Sideritis Spp. Nat. Volatiles Essent. Oils 6 (3), 16–23.
Koleva, I. I., Linssen, J. P., van Beek, T. A., Evstatieva, L. N., Kortenska, V., Handjieva, N. (2003). Antioxidant activity screening of extracts from Sideritis species (Labiatae) grown in Bulgaria. J. Sci. Food Agric. 83, 809–819. doi: 10.1002/jsfa.1415
Koutsaviti, A., Bazon, I., Milenkovic, M., Pavlovic-Drobac, M., Tzakou, O. (2013). Antimicrobial activity and essential oil composition of five Sideritis taxa of Empedoclia and Hesiodia Sect. from Greece. Rec. Nat. Prod. 7 (1), 6–14.
Lall, N., Chrysargyris, A., Lambrechts, I., Fibrich, B., van Staden, A. B., Twilley, D., et al. (2019). Sideritis perfoliata (subsp. perfoliata) nutritive value and its potential medicinal properties. Antioxidants 8 (11), 521. doi: 10.3390/antiox8110521
Lenherr, A., Mabry, T. J. (1987). Acetylated allose-containing flavonoid glucosides from Stachys anisochila. Phytochemistry 26, 1185–1188. doi: 10.1016/S0031-9422(00)82375-4
Mabry, T. G., Markham, K. R., Thomas, M. B. (1970). The Systematic Identification of Flavonoids. Springer-Verlag New Y., 354. doi: 10.1007/978-3-642-88458-0
Machado, K., da, C., Islam, M. T., Ali, E. S., Rouf, R., Uddin, S. J., et al. (2018). A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 32, 2376–2388. doi: 10.1002/ptr.6199
Martins, N., Barros, L., Santos-Buelga, C., Silva, S., Henriques, M., Ferreira, I. C. F. R. (2015). Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterization. Ind. Crop Prod. 167, 131–137. doi: 10.1016/j.foodchem.2014.06.094
Menghini, L., Pintore, G., Tirillini, B., Leporini, L. (2014). Chemical composition, antioxidant activities and protective effects of Sideritis italica extract on C2C12 oxidative stress. Eur. J. Med. Plant 4 (4), 365–382. doi: 10.9734/EJMP/2014/7090
Miyase, T., Koizumi, A., Ueno, A., Noro, T., Kuroyanagi, M., Fukushima, S., et al. (1982). Studies on the acyl glycosides from Leucoseptrum japonicum (Miq.) Kitamura et Murata. Chem. Pharm. Bull. 30, 2732–2737. doi: 10.1248/cpb.30.2732
Neu, R. (1957). Chelate von Diarylborsäuren mit aliphatischen Oxyalkylaminen als Reagenzien für den Nachweis von Oxyphenyl-benzo-γ-pyronen. Naturwissenschaften 44, 181–183. doi: 10.1007/BF00599857
Nishimura, H., Sasaki, H., Nobuyuki, I., Chin, M., Mitsuhashi, H. (1991). Nine phenethyl alcohol glycosides from Stachys sieboldii. Phytochemistry 30 (3), 965–969. doi: 10.1016/0031-9422(91)85288-b
Orhan, I., Küpeli, E., Aslan, M., Kartal, M., Yesilada, E. (2006). Bioassay-guided evaluation of anti-inflammatory and antinociceptive activities of pistachio, Pistacia vera L. J. Ethnopharmacol. 105, 235–240. doi: 10.1016/j.jep.2005.10.023
Petreska, J., Stefova, M., Ferreres, F., Moreno, D. A., Tomás-Barberán, F. A., Stefkov, G., et al. (2011). Potential bioactive phenolics of Macedonian Sideritis species used for medicinal “mountain tea”. Food Chem. 125, 13–20. doi: 10.1016/j.foodchem.2010.08.019
Phatak, R. S., Hendre, A. S. (2014). Total antioxidant capacity (TAC) of fresh leaves of Kalanchoe pinnata. J.Pharmacogn. Phytochem. 2, 32–35.
Plioukas, M., Termentzi, A., Gabrieli, C., Zervou, M., Kefalas, P., Kokkalou, E. (2010). Novel acylflavones from Sideritis syriaca ssp. syriaca. Food Chem. 123, 1136–1141. doi: 10.1016/j.foodchem.2010.05.076
Pljevljakušić, D., Šavikin, K., Janković, T., Zdunić, G., Ristić, M., Godjevac, D., et al. (2011). Chemical properties of the cultivated Sideritis raeseri Boiss. & Heldr. subsp. raeseri. Food Chem. 124, 226–233. doi: 10.1016/j.foodchem.2010.06.023
Rivas da Silva, A. C., Lopes, P. M., Barros de Azevedo, M. M., Machado Costa, D. C., Alviano, C. S., Alviano, D. S. (2012). Biological Activities of α-Pinene and β-Pinene enantiomers. Molecules 17, 6305–6316. doi: 10.3390/molecules17066305
Romanucci, V., Di Fabio, G., D’Alonzo, D., Guaragna, A., Scapagnini, G., Zarrelli, A. (2017). Traditional uses, chemical composition and biological activities of Sideritis raeseri Boiss. & Heldr.: Uses, composition and activities of S. raeseri. J. Sci. Food Agric. 97, 373–383. doi: 10.1002/jsfa.7867
Samanidou, V., Tsagiannidis, A., Sarakatsianos, I. (2012). Simultaneous determination of polyphenols and major purine alkaloids in Greek Sideritis species, herbal extracts, green tea, black tea, and coffee by high-performance liquid chromatography-diode array detection. J. Sep. Sci. 35, 608–615. doi: 10.1002/jssc.201100894
Sarian, M. N., Ahmed, Q. U., Mat So’ad, S. Z., Alhassan, A. M., Murugesu, S., Perumal, V., et al. (2017). Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. BioMed. Res. Int. 2017, 1–14. doi: 10.1155/2017/8386065
Stanoeva, J. P., Stefova, M., Stefkov, G., Kulevanova, S., Alipieva, K., Bankova, V., et al. (2015). Chemotaxonomic contribution to the Sideritis species dilemma on the Balkans. Biochem. Syst. Ecol. 61, 477–487. doi: 10.1016/j.bse.2015.07.008
Todorova, M., Trendafilova, A. (2014). Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 152, 256–265. doi: 10.1016/j.jep.2014.01.022
Tomou, E. M., Chatzopoulou, P., Skaltsa, H. (2019). NMR analysis of cultivated Sideritis euboea Heldr. Phytochem. Anal. 31, 1–9. doi: 10.1002/pca.2874
Trikka, F., Michailidou, S., Makris, A. M., Argiriou, A. (2019). Biochemical fingerprint of Greek Sideritis spp.: Implications for potential drug discovery and advanced breeding strategies. Med. Aromat. Plants. 8, 335. doi: 10.35248/2167-0412.19.8.335
Tsintides, T., Christodoulou, C. S., Delipetrou, P., Georghiou, K. (2007). The red data book of the flora of Cyprus, Lefkosia, CY : Cyprus Forestry Association.
Tunçtürk, M., Eryiğit, T., Rahmi Kaya, A. (2017). Nutritional properties, minerals, and selected heavy metal contents in herby cheese plants of Lamiaceae. Appl. Biol. Chem. 60 (1), 41–44. doi: 10.1007/s13765-016-0245
Venditti, A., Bianco, A., Nicoletti, M., Quassinti, L., Bramucci, M., Lupidi, G., et al. (2014). Characterization of secondary metabolites, biological activity and glandular trichomes of Stachys tymphaea Hausskn. from the Monti Sibillini National Park (Central Apennines, Italy). Chem. Biodiver. 11, 245–261. doi: 10.1002/cbdv.201300253
Venditti, A., Bianco, A., Frezza, C., Serafini, M., Giacomello, G., Giuliani, C., et al. (2016). Secondary metabolites, glandular trichomes and biological activity of Sideritis montana L. subsp. montana from Central Italy. Chem. Biodiver. 13, 1380–1390. doi: 10.1002/cbdv.201600082
Wightman, E. L., Jackson, P. A., Khan, J., Forster, J., Heiner, F., Feistel, B., et al. (2018). The acute and chronic cognitive and cerebral blood flow effects of a Sideritis scardica (Greek mountain tea) extract: A double blind, randomized, placebo controlled, parallel groups study in healthy humans. Nutrients 10, 955. doi: 10.3390/nu10080955
Yöney, A., Prieto, J. M., Lardos, A., Heinrich, M. (2010). Ethnopharmacy of Turkish-speaking Cypriots in greater London. Phytother. Res. 24, 731–740. doi: 10.1002/ptr.3012
Yalçın, F. N., Kaya, D., Kılıç, E. (2007). Antimicrobial and free radical scavenging activities of some Lamium species from Turkey. Hacettepe Univ. Eczacı. Fak. Derg. 27, 11–22.
Yang, H., Woo, J., Nim Pae, A., Young Um, M., Cho, N. C., Park, K. D., et al. (2016). a-Pinene, a major constituent of pine tree oils, enhances non-rapid eye movement sleep in mice through GABAA-benzodiazepine receptors. Mol. Pharmacol. 90, 530–539. doi: 10.1124/mol.116.105080
Yildiz, K., Gücel, S. (2006). Chromosome numbers of 16 endemic plant taxa from Northern Cyprus. Turk. J. Bot. 30, 181–192.
Keywords: Sideritis cypria, cultivation, infusions, flowers, leaves, melittoside, leonoside A, lamalboside
Citation: Lytra K, Tomou E-M, Chrysargyris A, Drouza C, Skaltsa H and Tzortzakis N (2020) Traditionally Used Sideritis cypria Post.: Phytochemistry, Nutritional Content, Bioactive Compounds of Cultivated Populations. Front. Pharmacol. 11:650. doi: 10.3389/fphar.2020.00650
Received: 24 January 2020; Accepted: 22 April 2020;
Published: 12 May 2020.
Edited by:
Andrei Mocan, Iuliu Hațieganu University of Medicine and Pharmacy, RomaniaReviewed by:
Lillian Barros, Polytechnic Institute of Bragança, PortugalCopyright © 2020 Lytra, Tomou, Chrysargyris, Drouza, Skaltsa and Tzortzakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helen Skaltsa, c2thbHRzYUBwaGFybS51b2EuZ3I=; Nikolaos Tzortzakis, bmlrb2xhb3MudHpvcnR6YWtpc0BjdXQuYWMuY3k=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.