
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 06 May 2020
Sec. Drugs Outcomes Research and Policies
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00563
 Ren-qi Yao1,2†
Ren-qi Yao1,2† De-meng Xia3†
De-meng Xia3† Li-xue Wang1†
Li-xue Wang1† Guo-sheng Wu2
Guo-sheng Wu2 Yi-bing Zhu4
Yi-bing Zhu4 Hong-qiang Zhao1
Hong-qiang Zhao1 Qi Liu1
Qi Liu1 Zhao-fan Xia2
Zhao-fan Xia2 Chao Ren1*
Chao Ren1* Yong-ming Yao1*
Yong-ming Yao1*Background: Vasopressin is an efficient remedy for septic shock patients as its great capacity in promoting hemodynamic stabilization. The aim of current systematic review and meta-analysis is to compare the clinical efficiency of vasopressin or its analogs with sole catecholamines on patients with septic shock.
Methods: A systematic search of Cochrane Library, EMBASE, and PubMed online databases was performed up to 30 Oct 2019 to identify randomized controlled trials comparing use of vasopressin or its analogs (e.g., terlipressin, selepressin) with administration of catecholamines alone.
Results: We included 23 RCTs with 4,225 patients in the current study. Compared with solely use of catecholamines, administration of vasopressin or its analogs was not associated with reduced 28-day or 30-day mortality among patients with septic shock [RR=0.94 (95% CI, 0.87–1.01), P=0.08, I2 = 0%]. The result of primary endpoint remained unchanged after conducting sensitivity analysis. Despite a significantly higher risk of digital ischemia in patients receiving vasopressin or its analogs [RR=2.65 (95% CI, 1.26–5.56), P < 0.01, I2 = 48%], there was no statistical significance in the pooled estimate for other secondary outcomes, including total adverse events, arrhythmia, acute myocardial infarction (AMI) and cardiac arrest, acute mesenteric ischemia, ICU/hospital length of stay, and mechanical ventilation (MV) duration.
Conclusions: The administration of vasopressin or its analogs was not associated with reduced 28-day or 30-day mortality among patients with septic shock, while an increased incidence of digital ischemia should be noted in patients receiving agonists for vasopressin receptors.
Sepsis is a common yet complex disorder that remains one of the major causes of death among patients admitted to intensive care units (ICUs) (Hotchkiss et al., 2016; Singer et al., 2016). Septic shock, a severe subset of sepsis, represents a lethal yet intractable condition for ICU patients (Singer et al., 2016). The mortality rate shows significant increase after identification of septic shock, from 40% to 80% due to the untenable hemodynamic status and persistent low blood perfusion (Gaieski et al., 2013). Administration of norepinephrine is the first-line choice and an effective treatment for improving survival of septic shock patients (Rhodes et al., 2017). Furthermore other vasopressor medications, such as epinephrine, dopamine, and vasopressin and its analogs, also showed noteworthy benefits in ameliorating vascular resistance, achieving target mean arterial pressure (MAP) levels, and further maintaining efficient perfusion in tissues and crucial organs during septic shock (Vincent and De Backer, 2013).
However, patients with septic shock are prone to become insensitive to catecholamines, and even develop vasopressin deficiency (Russell, 2007). Meanwhile, growing evidences have shown that catecholamine associated adverse effects might be unavoidable by exclusively using catecholamines, including myocardial ischemia and tachycardia, which also poses a great threat to the prognosis of septic shock patients (Dunser and Hasibeder, 2009; Levy et al., 2010; Schmittinger et al., 2012; Vincent and De Backer, 2013). Given that, several studies have demonstrated that combined administration of vasopressin or its analogs with catecholamines not only reduced the use of catecholamines, but also potentially attenuated catecholamine associated adverse effects (De Backer et al., 2010; Hammond et al., 2018). Thus, administration of vasopressin is recommended by recently issued Surviving Sepsis Guidelines to get the target MAP level, along with benefit of reducing NE dosage (Rhodes et al., 2017). Likewise, analogs of vasopressin, including terlipressin and selepressin, were reportedly beneficial for improving hemodynamic status, attenuating sepsis related vasodilatation and tissue edema, which were largely attributed to its selective stimulation of V1 receptors (Rehberg et al., 2010; Laporte et al., 2011). Recently, results from two large randomized controlled trials (RCTs) were published and aroused the usage and effects of vasopressin or its analogs on the prognosis of septic shock patients (Liu et al., 2018; Laterre et al., 2019). Of note, several well-designed systematic review and meta-analyses have evaluated the effects and safety of vasopressin or its analogs on the occurrence of adverse effects and short-term mortality, while the conclusions of those studies were divergent from each other (Serpa Neto et al., 2012; Avni et al., 2015; McIntyre et al., 2018; Jiang et al., 2019; Nagendran et al., 2019). This might be due to disparate inclusion criteria and methodologic selection.
Therefore, we plan to conduct an updated systematic review and meta-analysis of RCTs to test the clinical efficiency of vasopressin or its analogs versus catecholamines alone on patients-centered outcomes during septic shock. Furthermore, we aim to explore if selective V1 receptor agonists (terlipressin, selepressin) show beneficial effects on clinical outcomes of septic shock patients.
The current systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statements (Moher et al., 2009) (see Figure 1).
RCTs comparing the use of vasopressin or its analogs (e.g. terlipressin, selepressin) with catecholamines alone (e.g. norepinephrine, dopamine) or placebo among adult patients with septic shock were included in the current study.
The primary endpoint was 28-day or 30-day mortality, which was preferentially reported by majority of included trials. In case of unreported 28-day or 30-day mortality, we contacted the authors for inquiring the original data or considered the closest available mortality data. Secondary outcomes were listed as follows: total adverse events, arrhythmia, acute myocardial infarction (AMI) and cardiac arrest, acute mesenteric ischemia, digital ischemia, ICU length of stay, hospital length of stay and duration of mechanical ventilation. For studies reported length of stay in median and interquartile range, we converted those data into mean and standard deviation, respectively, by using algorithm provided by statisticians.
A systematic search was conducted by applying multiple online databases, including Cochrane Library, EMBASE, and PubMed. Relevant studies up to 30 Oct, 2019 were reviewed irrespective of languages and publication types. We conceived search strategies that involved following Medical Subject Heading (MeSH) terms: “Sepsis,” “Vasopressins,” “Arginine Vasopressin,” “Deamino Arginine Vasopressin,” “Lypressin,” “Felypressin,” “Ornipressin,” and “terlipressin.” Detailed search strategies were summarized in Supplemental File 1. Besides, unpublished trials and conference abstracts were hand-searched by the authors to obtain additional studies. We also identified references through screening the reference lists of eligible reviews and trial registries.
Two reviewers independently screened the titles and abstracts of relevant studies for enrolling eligible trials. If the abstract of a potentially eligible trial failed to provide sufficient information, the full paper was subsequently obtained to determine its eligibility. In both inclusion and exclusion processes, divergent opinions between the two authors were resolved by discussion. Otherwise, a consulting group that consisted of several experts was involved in when a consensus could not be reached.
Independently, two reviewers extracted data from included studies with a predesigned sheet. The primary and secondary outcomes were obtained across all eligible trials. In addition, detailed information about studies and participants’ characteristics were recorded accordingly, including year of publication, first author, study design, total number of enrollments, type of intervention, demographic characteristics, clinical settings and complications. Similarly, the inconsistency of extracted data and disagreement were resolved by discussion.
The methodological quality of all included studies was assessed by using the Jadad scoring system, which was comprised of three dimensions (randomization, double-blinding as well as withdrawals and dropouts). Each trial was assigned a score of 0 to 5, a study with score higher than 3 indicated high quality and low risk of bias, or else, revealing high risk of bias.
We applied risk ratios (RRs) for dichotomous outcomes, while mean differences (MDs) were used for pooling continuous data. The pooled results were calculated with 95% confidence intervals (CIs). Methodological heterogeneity of each outcome was measured by using both χ2 test and I2 statistics. In either case, I2 > 50% or P value < 0.10 (χ2 test) was regard as significant heterogeneity. We applied random effects model combined with Mantel-Haenszel method for certain outcomes when statistical heterogeneity existed, or else, fixed effects model was used. A two-sided P value < 0.05 was deemed as statistically significance. To measure publication bias across studies, the funnel plot of the primary endpoint was constructed and visually inspected by authors. Additionally, we performed Harbord and Peter tests to further evaluate the publication bias.
Subgroup analysis combined with sensitivity analysis were performed to test the robustness and consistency of our primary endpoint, as well as finding potential influencing factors. We stratified all included RCTs by administration of vasoactive agents (vasopressin or it’s analogs), clinical settings, outcome report, risk of bias, as well as publication types (full text or abstract).
All statistical analyses were performed using R software (version 3.6.1).
The quality of evidence of each outcome was evaluated in line with the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) criteria (Guyatt et al., 2008). This procedure was conducted with GRADE Pro software 3.6 (McMaster University 2014, Hamilton, Canada).
The current meta-analysis identified 2,563 relevant citations through searching online databases. After removing duplicates and subsequent screening of titles and abstracts, the full-text articles of 41 trials were reviewed, and 23 RCTs finally met the eligibility criteria (see Figure 1).
Twenty-three RCTs with 4,225 septic shock patients were enrolled in the systematic review and meta-analysis (Albanese et al., 2005; Lauzier et al., 2006; Morelli et al., 2008; Russell et al., 2008; Acevedo et al., 2009; Morelli et al., 2009; Han et al., 2012; Svoboda et al., 2012; Fonseca-Ruiz et al., 2013; Hua et al., 2013; Oliveira et al., 2014; Zambolim et al., 2014; Barzegar et al., 2016; Clem et al., 2016; Gordon et al., 2016; Xiao et al., 2016; Capoletto et al., 2017; Chen et al., 2017; Choudhury et al., 2017; Prakash et al., 2017; Russell et al., 2017; Liu et al., 2018; Laterre et al., 2019). The majority of enrolled trials were designed as single-center studies, while seven of them were multicenter studies. The administration of vasopressin was applied by 10 trials, and another 11 RCTs using terlipressin as the intervention, whereas selepressin was studied in two trials. Among them, six studies were published in conference abstract (Acevedo et al., 2009; Oliveira et al., 2014; Zambolim et al., 2014; Clem et al., 2016; Capoletto et al., 2017; Prakash et al., 2017). The detailed characteristics of all included RCTs were presented in Table 1.
Each RCT in our study was assigned a score of 0 to 5 by using Jadad scoring system (Supplemental Table S1). The majority of enrolled trials met the randomization requirements by using distribution methods. Among them, 8 RCTs (34.8%) had Jadad score of 4 or 5, while eight studies (34.8%) scored 3, indicating that 16 included RCTs (69.6%) were of low risk of bias. While seven studies (30.4%) were given Jadad score of 1 or 2, indicating a high risk of bias that was mainly due to the publication types and lack of blinding.
Mortality data were accessible in 23 trials, 17 of them have reported 28-day or 30-day mortality, while six studies reported other endpoints. Pooled data form 23 trials demonstrated no significant reduction in 28-day or 30-day mortality among patients who were given vasopressin or its analogs when comparing to those using catecholamines alone [RR=0.94 (95% CI, 0.87–1.01), P=0.08, I2 = 0%]. The forest plot was shown in Figure 2.
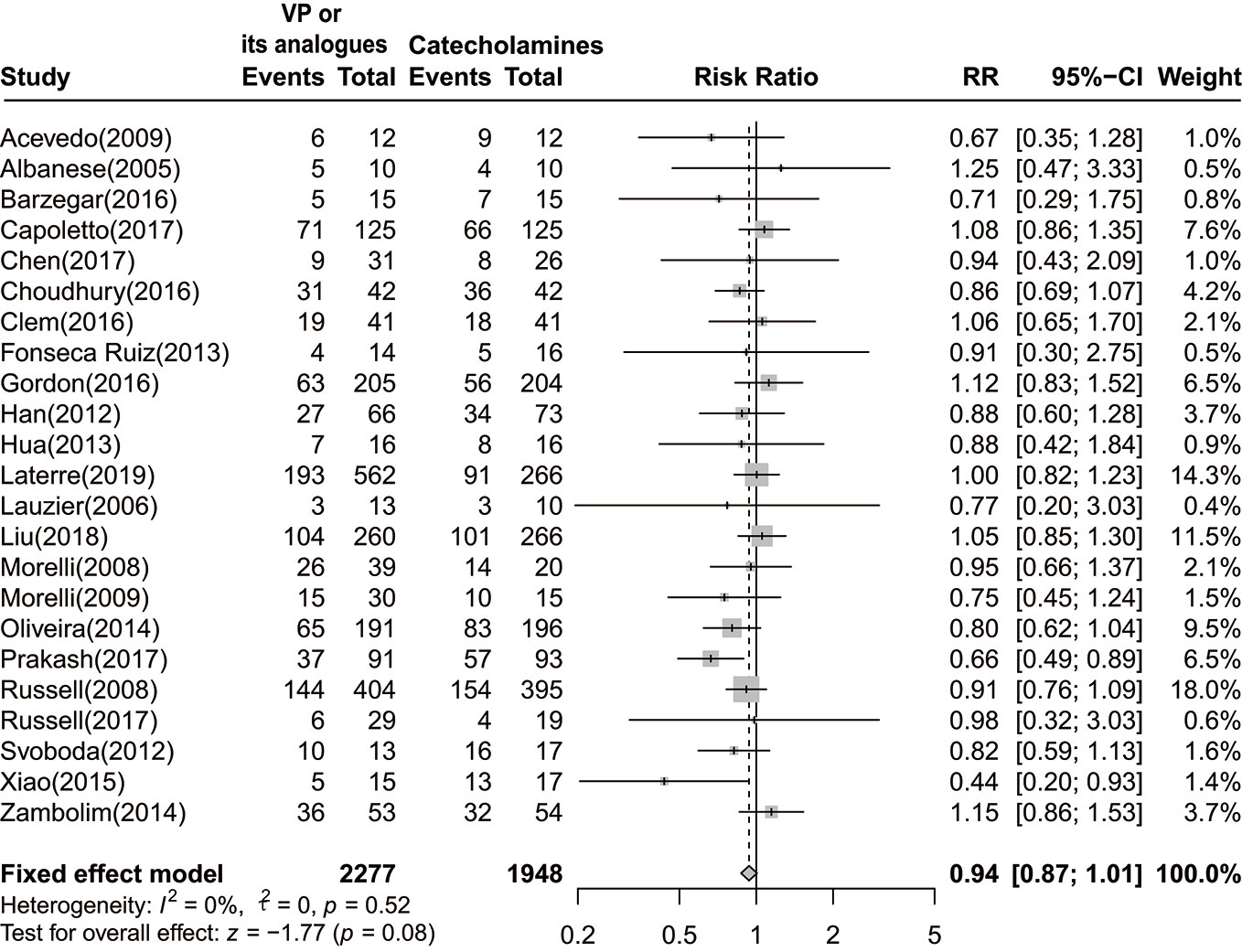
Figure 2 Forest plot of 28-day or 30-day mortality comparing vasopressin or its analogs to catecholamines alone among septic shock patients. VP, vasopressin; RR, risk ratio; CI, confidence intervals.
As summarized in Table 2, the consequence remained unchanged when conducting sensitivity analysis. As stratifying by interventions, RR of the vasopressin group was 0.96 [(95% CI, 0.87–1.06), P=0.44, I2 = 0%]. For studies incorporated septic shock patients without other primary complications, the combined RR was 0.94 [(95% CI, 0.87–1.03), P=0.20, I2 = 0%]. After removing trials reported mortality in other phases, there was no significant difference between two groups [RR=0.95 (95% CI, 0.88–1.02), P=0.18, I2 = 0%]. Besides, combined RR of pooling data from studies with low risk of bias was 0.95 [(95% CI, 0.88–1.03), P=0.22, I2 = 0%]. Of note, combined RR for studies published in full text and abstract were 0.95 [(95% CI, 0.87–1.04), P=0.30, I2 = 0%], and 0.90 [(95% CI, 0.79–1.02), P=0.09, I2 = 55%], respectively.
Meanwhile, we performed a subgroup analysis based on disparate administration of vasoactive agents. As no statistical significances were observed in both vasopressin and selepressin subgroups, we found a significant lower 28-day or 30-day mortality rate among septic shock patients who received terlipressin [RR=0.87 (95% CI, 0.77–0.98), P=0.02, I2 = 12%] (Figure 3).
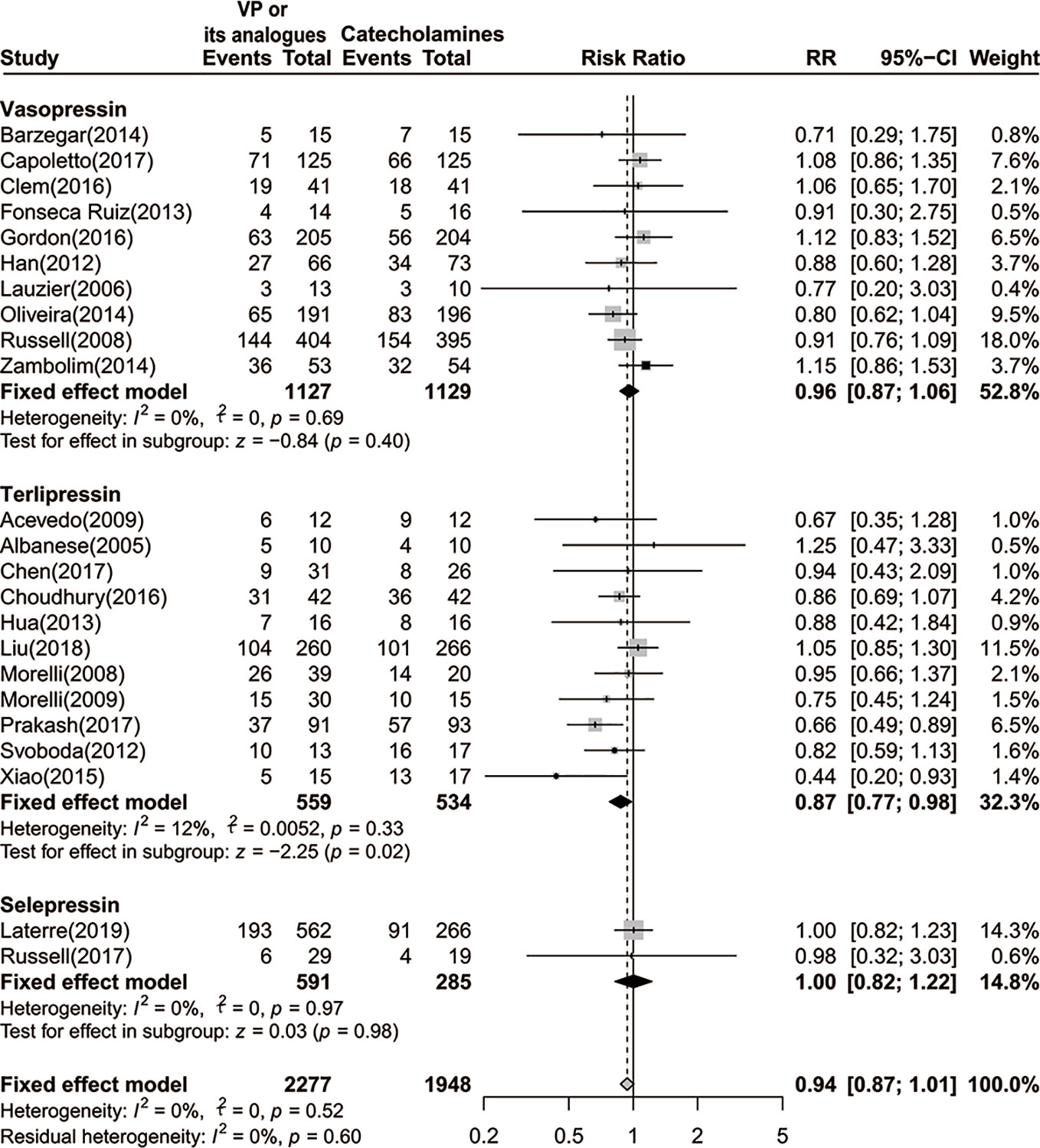
Figure 3 Forest plot of 28-day or 30-day mortality comparing vasopressin or its analogs to catecholamines alone stratified by disparate vasopressors. VP, vasopressin; RR, risk ratio; CI, confidence intervals.
Given that 14 trials with a total of 3,206 patients reported total adverse events (980 events) (Lauzier et al., 2006; Russell et al., 2008; Morelli et al., 2009; Fonseca-Ruiz et al., 2013; Zambolim et al., 2014; Barzegar et al., 2016; Clem et al., 2016; Gordon et al., 2016; Xiao et al., 2016; Choudhury et al., 2017; Prakash et al., 2017; Russell et al., 2017; Liu et al., 2018; Laterre et al., 2019), administration of vasopressin or its analogs showed no significant association with the incidence of adverse effects [RR=1.21 (95% CI, 0.88–1.68), P=0.24, I2 = 73%] (Supplemental Figure S1). Although this outcome displayed a relatively high heterogeneity, the conclusion was proven to be stable after performing sensitivity analysis via excluding each study one at a time from the pooled estimate. Meanwhile, we found that study by Liu et al. was the main source of heterogeneity, which resulted in remarkable reduction in heterogeneity after exclusion (I2 from 73% to 41%).
The development of arrhythmia was documented in nine RCTs with 358 events (Russell et al., 2008; Morelli et al., 2009; Barzegar et al., 2016; Clem et al., 2016; Gordon et al., 2016; Choudhury et al., 2017; Russell et al., 2017; Liu et al., 2018; Laterre et al., 2019). We observed no significant difference in the occurrence of arrhythmia between two groups [RR=1.05 (95% CI, 0.87–1.27), P=0.61, I2 = 25%] (Supplemental Figure S2). Of note, trials by Laterre and colleagues accounted for over 76.4% weight. Even so, the conclusion was not altered after removing this study.
By pooling data from nine studies with 2,929 participants (a total of 105 events) (Russell et al., 2008; Svoboda et al., 2012; Fonseca-Ruiz et al., 2013; Barzegar et al., 2016; Gordon et al., 2016; Capoletto et al., 2017; Russell et al., 2017; Liu et al., 2018; Laterre et al., 2019), we demonstrated that vasopressin or its analogs could lead to approximately two times risk of digital ischemia when compared to sole use of catecholamine in septic shock patients [RR=2.65 (95% CI, 1.26–5.56), P < 0.01, I2 = 48%] (Figure 4). We further confirmed that the result was robust through implementing sensitivity analysis, and excluding trial by Liu et al. could completely eliminate the heterogeneity (I2=0%).
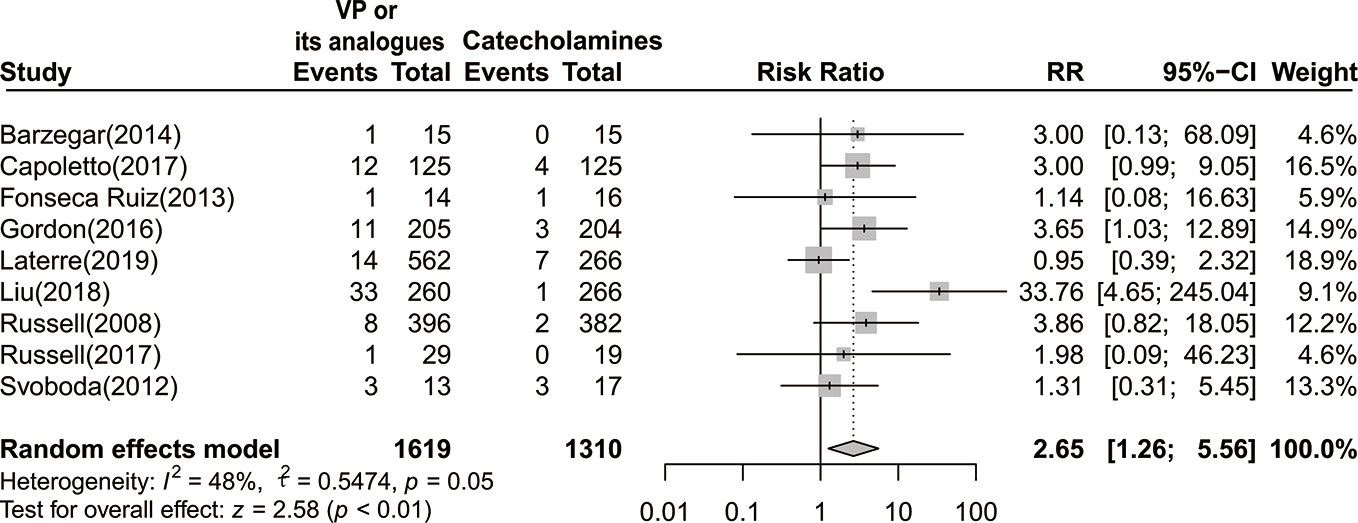
Figure 4 Forest plot of digital ischemia comparing vasopressin or its analogs to catecholamines alone among septic shock patients. VP, vasopressin; RR, risk ratio; CI, confidence interval.
Twelve included RCTs with 3,203 patients were eligible for the analysis of ICU length of stay (Morelli et al., 2008; Russell et al., 2008; Morelli et al., 2009; Han et al., 2012; Hua et al., 2013; Barzegar et al., 2016; Gordon et al., 2016; Capoletto et al., 2017; Chen et al., 2017; Choudhury et al., 2017; Liu et al., 2018; Laterre et al., 2019). Pooled effects revealed that administration of vasopressin or its analogs did not impact the length of stay in ICU [MD=–0.21 (95% CI, −0.75–0.33), P =0.44, I2 = 0%] (Supplemental Figure S3).
The occurrence of AMI and cardiac arrest, acute mesenteric ischemia, and hospital length of stay as well as MV duration were reported in 7, 5, 6, 6 studies, respectively. None of those outcomes showed significant differences between two groups (Supplemental Figure S4–S7). Accordingly, the pooled effects and detailed information for those outcomes were presented in Table 3.
The results of GRADE evaluation for all outcomes were summarized in Table 3 and Supplemental Table S2. The quality of evidence for the primary endpoint and several secondary outcomes were ranked as moderate, including arrhythmia, AMI and cardiac arrest, digital ischemia, and acute mesenteric ischemia. Whereas, total adverse effects, hospital/ICU lengths of stay as well as MV duration were assessed as outcomes with low quality of evidence.
Publication bias was evaluated through visually inspecting the funnel plot, which revealed no evidence of publication bias. Furthermore, we applied Harbord test (P=0.47), and Peters test (P=0.25) to validate the funnel plot symmetry, which were both noted with no statistical significance (Supplemental Figure S8).
In the current systematic review and meta-analysis, we have evaluated the clinical efficiency of vasopressin or its analogs among patients with septic shock. We found that administration of vasopressin or selective V1 receptor agonists showed no benefits in reducing 28-day or 30-day mortality when compared with solely using NE, which was further validated by sensitivity analysis. Intriguingly, terlipressin might have potential prosurvival effects on septic shock patients independent of NE administration, as reported in subgroup analysis. Further large RCTs were required to strengthen the clinical efficiency of terlipressin among patients with septic shock. Meanwhile, no significant differences were observed in the incidence of most adverse effects between two groups, including total adverse events, arrhythmia, AMI and cardiac arrest, acute mesenteric ischemia. Of note, the occurrence of digital ischemia was more frequent among patients who received vasopressin or its analogs than those solely using catecholamines. Additionally, there were no significant improvements on ICU or hospital length of stay, as well as MV duration after administration of vasopressin or its analogs.
NE has long been recommended as the first-line vasoactive agent among patients with septic shock (Rhodes et al., 2017), and was broadly applied in restoring blood perfusion in tissue and vital organs and enhancing efficacy of fluid resuscitation (Avni et al., 2015; Hessler et al., 2016; Rhodes et al., 2017). However, high dose of NE reportedly caused severe side-effects, such as myocardial injury and immunological dysfunction, which made its long-lasting usage impossible (Andreis and Singer, 2016; Ukor and Walley, 2019). Vasopressin, an alternative non-catecholamine agent, was capable of increasing vascular resistance and restoring blood pressure via promoting arterioles constriction, which was also in favor of reducing the requirement of catecholamines among septic shock patients (Dunser et al., 2002; Holmes and Walley, 2004; McIntyre et al., 2018; Ukor and Walley, 2019). Meanwhile, it has been manifested that vasopressin could promote water resorption in renal tubules through its affinity to V2 receptors, thereby maintaining circulation blood volume (Ukor and Walley, 2019). Given that, recently issued Surviving Sepsis Guidelines has recommended adding vasopressin as the second-line vasoactive agent for the treatment of septic shock patients (Rhodes et al., 2017). Up to now, the Vasopressin or Septic Shock Trial (VASST) that was carried out by Russell and colleagues was the largest RCT for addressing this topic (Russell et al., 2008). However, they failed to reveal a reduced 28-day mortality among septic shock patients receiving NE combined with low-dose vasopressin (0.01 to 0.03 U/min) compared to those with sole use of NE, while a higher survival rate for vasopressin plus NE group was observed merely in the subset of patients with less severe septic shock (Russell et al., 2008). Meanwhile, other researchers have reported that administration of vasopressin in relatively late phases (after 12 h) and at high lactate levels might result in low response to vasopressin (Sacha et al., 2018). Additionally, growing evidences also suggested that vasopressin might be associated with decreased urine and cardiac outputs, as well as prothrombotic state due to its nonselective effects on V1 and V2 receptors (Torgersen et al., 2010; Salazar et al., 2015; Zhu et al., 2019). Therefore, the effects and safety of vasopressin should be taken cautiously.
In the current meta-analysis, we found that the use of vasopressin or its analogs had no effect on reducing 28-day or 30-day mortality among patients with septic shock. Whereas, it was disparate from many previous published systematic review and meta-analyses (McIntyre et al., 2018; Jiang et al., 2019). A recent study by Jiang et al. has concluded that the use of vasopressin might lead to reduced mortality among patients with septic shock (Jiang et al., 2019). Although the study was well-performed and comprehensive, some flaws might interfere the interpretation of their findings. They incorporated Malay’s study in the analysis of 28-day or 30-day mortality, but that study merely reported 24-h mortality, which might render an unsolvable bias. Besides, they failed to conduct sensitivity analysis for primary endpoint which was clearly unstable. Neto et al. performed a systematic review and meta-analysis by enrolling nine RCTs and demonstrated a reduced all-cause mortality in patients with vasodilatory shock, which showed statistical significance in septic shock subgroup (Serpa Neto et al., 2012). Of note, an updated study by McIntyre et al. showed that vasopressin in addition to catecholamines was associated lower mortality rate and incidence of atrial fibrillation among patients with distributive shock (McIntyre et al., 2018). The reason for the divergent results was mainly due to stricter inclusion criteria and incorporation of newly published RCTs. However, the current meta-analysis initially revealed a reduced 28-day or 30-day mortality among patients receiving terlipressin compared to those with catecholamines alone, which haven’t been reported by any other existing systematic review and meta-analyses.
Terlipressin, which is a synthetic long-acting analog of vasopressin, has high affinity toward V1 receptors on vascular smooth muscle (Leone et al., 2004). Theoretically, the use of terlipressin may result in less adverse effects than vasopressin does in treating septic shock, including thrombocytopenia, decreased cardiac output and hyponatremia (Rehberg et al., 2010; Salazar et al., 2015). Indeed, several pre-clinical studies have demonstrated that administration of terlipressin could reduce the requirement of NE, restore hemodynamic status and promote creatinine clearance among septic shock patients (Morelli et al., 2008; Morelli et al., 2009; Liu et al., 2018). Previous studies reported that terlipressin could improve renal function in patients diagnosed with hepatorenal syndrome, two trials further identified a reduced 28-day or 30-day mortality among septi shock patients complicated with liver cirrhosis after administration of terlipressin. Their findings were in accordance with the results of our analysis (Choudhury et al., 2017; Prakash et al., 2017). However, study by Zhu et al. didn’t reveal any pro-survival benefits of terlipressin among septic shock patients compared with sole administration of catecholamines (Zhu et al., 2019). They enrolled a total of 10 studies in their analysis, but one trial was conducted in pediatric ICU, which was largely disparate from other included trials and might potentially introduce bias. Besides, study by Liu et al. took over more than half of the total sample size, which might lead to significant publication bias and impaired robustness.
Selepressin is a novel vasopressin analog, which selectively stimulates V1a receptors (Laporte et al., 2011; Russell et al., 2017). As demonstrated in pre-clinical studies, selepressin was capable of reducing fluid requirements and attenuating edema (Maybauer et al., 2014; He et al., 2016; Saad and Maybauer, 2017). In a recently published phase 2b/3 trial in septic shock patients, researchers didn’t find any favorable improvements in vasopressor- and ventilator-free days as well as 30-day mortality after administration of selepressin (Laterre et al., 2019).
As for secondary endpoints of interest, we found that the incidence of digital ischemia was significantly higher in patients receiving vasopressin or its analogs when compared to those solely using catecholamines. This finding was in line with many other systematic review and meta-analyses, which could be partially explained by vasopressin related cardiac output reduction and prothrombotic state. Meanwhile, we did not reveal any statistical differences between two groups in analysis of remaining secondary outcomes, including total adverse events, arrhythmia, AMI and cardiac arrest, acute mesenteric ischemia, ICU/hospital length of stay, and MV duration.
Several limitations should be taken into consideration when interpreting our findings. Firstly, the reported endpoints varied across included studies. Although the majority of trials provided 28-day or 30-day mortality, there were still a few studies that only presented data on ICU mortality, hospital mortality or 7-day mortality. However, we enrolled survival data of all included trials in the analysis of primary outcomes, which might potentially introduce bias. Given that, we performed sensitivity analysis and excluded studies that reported mortality in other phases. Correspondingly, the conclusions remained unchanged. Secondly, heterogeneity of some secondary endpoints was relatively high. We further conducted sensitivity analyses in order to unravel the source of heterogeneity. Thirdly, as the timing, duration, precise dose, delivery methods as well as weaning of vasopressin or its analogs were disparate and uncontrollable among included trials, it might render bias and affect the robustness of our conclusions. However, the detailed information was insufficient in many trials, which restricted us from performing further subgroup analyses. Finally, we failed to compare the clinical efficacy between vasopressin and its analogs. Thus, network meta-analyses were required to address this issue in the future.
In this study, administration of vasopressin or its analogs showed no significant improvement in reducing 28-day or 30-day mortality among patients with septic shock when compared with those solely using catecholamines, while administration of terlipressin might be benefit for survival of septic shock patients comparing to catecholamines alone. Besides, septic shock patients receiving vasopressin or its analogs did present an increased risk of digital ischemia in comparison with those solely using catecholamines.
Y-MY, CR, and Z-FX conceived the meta-analysis. R-QY and D-MX extracted all data. L-XW, G-SW, Y-BZ, H-QZ, and QL undertook and refined the searches. R-QY, D-MX, and L-XW cowrote the paper. R-QY undertook the statistical analyses. All authors contributed to and revised the final manuscript.
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81730057, 81842025, 81801935, 81901957), the National Key Research and Development Program of China (No. 2017YFC1103302), and the Key Project of Military Medical Innovation Program of Chinese PLA (No. 18CXZ026).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00563/full#supplementary-material.
AMI, acute myocardial infarction; CI, confidence intervals; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; ICU, intensive care units; MAP, mean arterial pressure; MDs, mean differences; MeSH, Medical Subject Heading; NE, norepinephrine; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; RCTs, randomized controlled trials; RRs, risk ratios.
Acevedo, J., Fernández, J., Escorsell, A., Mas, A., Gines, P., Arroyo, V. (2009). 174 clinical efficacy and safety of terlipressin administration in cirrhotic patients with septic shock. J. Hepatol. 50, S73. doi: 10.1016/S0168-8278(09)60176-8
Albanese, J., Leone, M., Delmas, A., Martin, C. (2005). Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Crit. Care Med. 33, 1897–1902. doi: 10.1097/01.ccm.0000178182.37639.d6
Andreis, D. T., Singer, M. (2016). Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med. 42, 1387–1397. doi: 10.1007/s00134-016-4249-z
Avni, T., Lador, A., Lev, S., Leibovici, L., Paul, M., Grossman, A. (2015). Vasopressors for the Treatment of Septic Shock: Systematic Review and Meta-Analysis. PloS One 10, e0129305. doi: 10.1371/journal.pone.0129305
Barzegar, E., Ahmadi, A., Mousavi, S., Nouri, M., Mojtahedzadeh, M. (2016). The Therapeutic Role of Vasopressin on Improving Lactate Clearance During and After Vasogenic Shock: Microcirculation, Is It The Black Box? Acta Med. Iran 54, 15–23.
Capoletto, C., Almeida, J., Ferrari, G., Fukushima, J., Nakamura, R., Risk, S., et al. (2017). Vasopressin versus norepinephrine for the management of septic shock in cancer patients (vancs ii). Crit. Care 21, 168.
Chen, Z., Zhou, P., Lu, Y., Yang, C. (2017). [Comparison of effect of norepinephrine and terlipressin on patients with ARDS combined with septic shock: a prospective single-blind randomized controlled trial]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 29, 111–116. doi: 10.3760/cma.j.issn.2095-4352.2017.02.004
Choudhury, A., Kedarisetty, C. K., Vashishtha, C., Saini, D., Kumar, S., Maiwall, R., et al. (2017). A randomized trial comparing terlipressin and noradrenaline in patients with cirrhosis and septic shock. Liver Int. 37, 552–561. doi: 10.1111/liv.13252
Clem, O., Painter, J., Cullen, J., McCain, K., Kakkera, K., Meena, N., et al. (2016). 1350: Norepinephrine and vasopressin vs norepinephrine alone for septic shock: randomized controlled trial. Crit. Care Med. 44, 413. doi: 10.1097/01.ccm.0000510024.07609.07
De Backer, D., Biston, P., Devriendt, J., Madl, C., Chochrad, D., Aldecoa, C., et al. (2010). Comparison of dopamine and norepinephrine in the treatment of shock. N Engl. J. Med. 362, 779–789. doi: 10.1056/NEJMoa0907118
Dunser, M. W., Hasibeder, W. R. (2009). Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J. Intensive Care Med. 24, 293–316. doi: 10.1177/0885066609340519
Dunser, M. W., Mayr, A. J., Stallinger, A., Ulmer, H., Ritsch, N., Knotzer, H., et al. (2002). Cardiac performance during vasopressin infusion in postcardiotomy shock. Intensive Care Med. 28, 746–751. doi: 10.1007/s00134-002-1265-y
Fonseca-Ruiz, N., Cano, L. A., Ortiz Carmona, P. D., Correa Aguirre, M., Gómez Hernández, P. M., Osorio García, C., et al. (2013). Uso de vasopresina en pacientes con choque séptico refractario a catecolaminas. Acta Colombiana Cuidado Intensivo 13, 114–123.
Gaieski, D. F., Edwards, J. M., Kallan, M. J., Carr, B. G. (2013). Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 41, 1167–1174. doi: 10.1097/CCM.0b013e31827c09f8
Gordon, A. C., Mason, A. J., Thirunavukkarasu, N., Perkins, G. D., Cecconi, M., Cepkova, M., et al. (2016). Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA 316, 509–518. doi: 10.1001/jama.2016.10485
Guyatt, G. H., Oxman, A. D., Kunz, R., Vist, G. E., Falck-Ytter, Y., Schunemann, H. J. (2008). What is “quality of evidence” and why is it important to clinicians? BMJ 336, 995–998. doi: 10.1136/bmj.39490.551019.BE
Hammond, D. A., Ficek, O. A., Painter, J. T., McCain, K., Cullen, J., Brotherton, A. L., et al. (2018). Prospective Open-label Trial of Early Concomitant Vasopressin and Norepinephrine Therapy versus Initial Norepinephrine Monotherapy in Septic Shock. Pharmacotherapy 38, 531–538. doi: 10.1002/phar.2105
Han, X. D., Sun, H., Huang, X. Y., Zhang, S. Y., Wang, Y. D., Ren, K., et al. (2012). [A clinical study of pituitrin versus norepinephrine in the treatment of patients with septic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 24, 33–37. doi: 10.3760/cma.j.issn.1003-0603.2012.01.008
He, X., Su, F., Taccone, F. S., Laporte, R., Kjolbye, A. L., Zhang, J., et al. (2016). A Selective V(1A) Receptor Agonist, Selepressin, Is Superior to Arginine Vasopressin and to Norepinephrine in Ovine Septic Shock. Crit. Care Med. 44, 23–31. doi: 10.1097/ccm.0000000000001380
Hessler, M., Kampmeier, T. G., Rehberg, S. (2016). Effect of non-adrenergic vasopressors on macro- and microvascular coupling in distributive shock. Best Pract. Res. Clin. Anaesthesiol. 30, 465–477. doi: 10.1016/j.bpa.2016.10.010
Holmes, C. L., Walley, K. R. (2004). Vasopressin in the ICU. Curr. Opin. Crit. Care 10 (6), 442–448. doi: 10.1097/01.ccx.0000144769.19213.0c
Hotchkiss, R. S., Moldawer, L. L., Opal, S. M., Reinhart, K., Turnbull, I. R., Vincent, J. L. (2016). Sepsis and septic shock. Nat. Rev. Dis. Primers 2, 16045. doi: 10.1038/nrdp.2016.45
Hua, F., Wang, X., Zhu, L. (2013). Terlipressin decreases vascular endothelial growth factor expression and improves oxygenation in patients with acute respiratory distress syndrome and shock. J. Emerg. Med. 44, 434–439. doi: 10.1016/j.jemermed.2012.02.073
Jiang, L., Sheng, Y., Feng, X., Wu, J. (2019). The effects and safety of vasopressin receptor agonists in patients with septic shock: a meta-analysis and trial sequential analysis. Crit. Care 23, 91. doi: 10.1186/s13054-019-2362-4
Laporte, R., Kohan, A., Heitzmann, J., Wisniewska, H., Toy, J., La, E., et al. (2011). Pharmacological characterization of FE 202158, a novel, potent, selective, and short-acting peptidic vasopressin V1a receptor full agonist for the treatment of vasodilatory hypotension. J. Pharmacol. Exp. Ther. 337, 786–796. doi: 10.1124/jpet.111.178848
Laterre, P. F., Berry, S. M., Blemings, A., Carlsen, J. E., Francois, B., Graves, T., et al. (2019). Effect of Selepressin vs Placebo on Ventilator- and Vasopressor-Free Days in Patients With Septic Shock: The SEPSIS-ACT Randomized Clinical Trial. JAMA 322, 1476–1485. doi: 10.1001/jama.2019.14607
Lauzier, F., Levy, B., Lamarre, P., Lesur, O. (2006). Vasopressin or norepinephrine in early hyperdynamic septic shock: a randomized clinical trial. Intensive Care Med. 32, 1782–1789. doi: 10.1007/s00134-006-0378-0
Leone, M., Albanese, J., Delmas, A., Chaabane, W., Garnier, F., Martin, C. (2004). Terlipressin in catecholamine-resistant septic shock patients. Shock 22 (4), 314–319. doi: 10.1097/01.shk.0000136097.42048.bd
Levy, B., Collin, S., Sennoun, N., Ducrocq, N., Kimmoun, A., Asfar, P., et al. (2010). Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med. 36, 2019–2029. doi: 10.1007/s00134-010-2045-8
Liu, Z. M., Chen, J., Kou, Q., Lin, Q., Huang, X., Tang, Z., et al. (2018). Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med. 44, 1816–1825. doi: 10.1007/s00134-018-5267-9
Maybauer, M. O., Maybauer, D. M., Enkhbaatar, P., Laporte, R., Wisniewska, H., Traber, L. D., et al. (2014). The selective vasopressin type 1a receptor agonist selepressin (FE 202158) blocks vascular leak in ovine severe sepsis*. Crit. Care Med. 42, e525–e533. doi: 10.1097/ccm.0000000000000300
McIntyre, W. F., Um, K. J., Alhazzani, W., Lengyel, A. P., Hajjar, L., Gordon, A. C., et al. (2018). Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis. JAMA 319, 1889–1900. doi: 10.1001/jama.2018.4528
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269, w264. doi: 10.7326/0003-4819-151-4-200908180-00135
Morelli, A., Ertmer, C., Lange, M., Dunser, M., Rehberg, S., Van Aken, H., et al. (2008). Effects of short-term simultaneous infusion of dobutamine and terlipressin in patients with septic shock: the DOBUPRESS study. Br. J. Anaesth. 100, 494–503. doi: 10.1093/bja/aen017
Morelli, A., Ertmer, C., Rehberg, S., Lange, M., Orecchioni, A., Cecchini, V., et al. (2009). Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): a randomized, controlled pilot study. Crit. Care 13, R130. doi: 10.1186/cc7990
Nagendran, M., Russell, J. A., Walley, K. R., Brett, S. J., Perkins, G. D., Hajjar, L., et al. (2019). Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive Care Med. 45, 844–855. doi: 10.1007/s00134-019-05620-2
Oliveira, S., Dessa, F., Rocha, C., Oliveira, F. (2014). Early Vasopressin Application in Shock study. Crit. Care 18, P158. doi: 10.1186/cc13348
Prakash, V., Choudhury, A. K., Sarin, S. K. (2017). Early introduction of a combination of low dose terlipressin and noradrenaline as vasopressors is superior to high dose noradrenaline alone in patients of cirrhosis with septic shock. Hepatology 66, 243.
Rehberg, S., Ertmer, C., Lange, M., Morelli, A., Whorton, E., Dunser, M., et al. (2010). Role of selective V2-receptor-antagonism in septic shock: a randomized, controlled, experimental study. Crit. Care 14, R200. doi: 10.1186/cc9320
Rhodes, A., Evans, L. E., Alhazzani, W., Levy, M. M., Antonelli, M., Ferrer, R., et al. (2017). Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 43, 304–377. doi: 10.1007/s00134-017-4683-6
Russell, J. A., Walley, K. R., Singer, J., Gordon, A. C., Hebert, P. C., Cooper, D. J., et al. (2008). Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl. J. Med. 358, 877–887. doi: 10.1056/NEJMoa067373
Russell, J. A., Vincent, J. L., Kjolbye, A. L., Olsson, H., Blemings, A., Spapen, H., et al. (2017). Selepressin, a novel selective vasopressin V1A agonist, is an effective substitute for norepinephrine in a phase IIa randomized, placebo-controlled trial in septic shock patients. Crit. Care 21, 213. doi: 10.1186/s13054-017-1798-7
Russell, J. A. (2007). Vasopressin in vasodilatory and septic shock. Curr. Opin. Crit. Care 13, 383–391. doi: 10.1097/MCC.0b013e328263885e
Saad, A. F., Maybauer, M. O. (2017). The role of vasopressin and the vasopressin type V1a receptor agonist selepressin in septic shock. J. Crit. Care 40, 41–45. doi: 10.1016/j.jcrc.2017.03.008
Sacha, G. L., Lam, S. W., Duggal, A., Torbic, H., Bass, S. N., Welch, S. C., et al. (2018). Predictors of response to fixed-dose vasopressin in adult patients with septic shock. Ann. Intensive Care 8, 35. doi: 10.1186/s13613-018-0379-5
Salazar, M., Hu, B. B., Vazquez, J., Wintz, R. L., Varon, J. (2015). Exogenous Vasopressin-Induced Hyponatremia in Patients With Vasodilatory Shock: Two Case Reports and Literature Review. J. Intensive Care Med. 30, 253–258. doi: 10.1177/0885066613507410
Schmittinger, C. A., Torgersen, C., Luckner, G., Schroder, D. C., Lorenz, I., Dunser, M. W. (2012). Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. 38, 950–958. doi: 10.1007/s00134-012-2531-2
Serpa Neto, A., Nassar, A. P., Cardoso, S. O., Manetta, J. A., Pereira, V. G., Esposito, D. C., et al. (2012). Vasopressin and terlipressin in adult vasodilatory shock: a systematic review and meta-analysis of nine randomized controlled trials. Crit. Care 16, R154. doi: 10.1186/cc11469
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810. doi: 10.1001/jama.2016.0287
Svoboda, P., Scheer, P., Kantorova, I., Doubek, J., Dudra, J., Radvan, M., et al. (2012). Terlipressin in the treatment of late phase catecholamine-resistant septic shock. Hepatogastroenterology 59, 1043–1047. doi: 10.5754/hge10550
Torgersen, C., Dunser, M. W., Wenzel, V., Jochberger, S., Mayr, V., Schmittinger, C. A., et al. (2010). Comparing two different arginine vasopressin doses in advanced vasodilatory shock: a randomized, controlled, open-label trial. Intensive Care Med. 36, 57–65. doi: 10.1007/s00134-009-1630-1
Ukor, I. F., Walley, K. R. (2019). Vasopressin in Vasodilatory Shock. Crit. Care Clin. 35, 247–261. doi: 10.1016/j.ccc.2018.11.004
Vincent, J. L., De Backer, D. (2013). Circulatory shock. N Engl. J. Med. 369, 1726–1734. doi: 10.1056/NEJMra1208943
Xiao, X., Zhang, J., Wang, Y., Zhou, J., Zhu, Y., Jiang, D., et al. (2016). Effects of terlipressin on patients with sepsis via improving tissue blood flow. J. Surg. Res. 200, 274–282. doi: 10.1016/j.jss.2015.07.016
Zambolim, C., Nagaoka, D., Fukushima, J., Park, C., Carneiro, J., Osawa, E., et al. (2014). Vasopressin versus norepinephrine for the management of septic shock in cancer patients. Crit. Care 18, P161. doi: 10.1186/cc13351
Keywords: vasopressin, terlipressin, selepressin, norepinephrine, septic shock
Citation: Yao R-q, Xia D-m, Wang L-x, Wu G-s, Zhu Y-b, Zhao H-q, Liu Q, Xia Z-f, Ren C and Yao Y-m (2020) Clinical Efficiency of Vasopressin or Its Analogs in Comparison With Catecholamines Alone on Patients With Septic Shock: A Systematic Review and Meta-Analysis. Front. Pharmacol. 11:563. doi: 10.3389/fphar.2020.00563
Received: 28 February 2020; Accepted: 14 April 2020;
Published: 06 May 2020.
Edited by:
Tahir Mehmood Khan, University of Veterinary and Animal Sciences, PakistanReviewed by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyCopyright © 2020 Yao, Xia, Wang, Wu, Zhu, Zhao, Liu, Xia, Ren and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Ren, cmMxOThAc2luYS5jb20=; Yong-ming Yao, Y19mZkBzaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.