
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 16 April 2020
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00366
 Camille Keisha Mahendra1,2
Camille Keisha Mahendra1,2 Loh Teng Hern Tan2,3
Loh Teng Hern Tan2,3 Wai Leng Lee4
Wai Leng Lee4 Wei Hsum Yap5
Wei Hsum Yap5 Priyia Pusparajah6
Priyia Pusparajah6 Liang Ee Low7,8
Liang Ee Low7,8 Siah Ying Tang9,10
Siah Ying Tang9,10 Kok Gan Chan11,12*
Kok Gan Chan11,12* Learn Han Lee2*
Learn Han Lee2* Bey Hing Goh1,13,14*
Bey Hing Goh1,13,14*Angelicin, a member of the furocoumarin group, is related to psoralen which is well known for its effectiveness in phototherapy. The furocoumarins as a group have been studied since the 1950s but only recently has angelicin begun to come into its own as the subject of several biological studies. Angelicin has demonstrated anti-cancer properties against multiple cell lines, exerting effects via both the intrinsic and extrinsic apoptotic pathways, and also demonstrated an ability to inhibit tubulin polymerization to a higher degree than psoralen. Besides that, angelicin too demonstrated anti-inflammatory activity in inflammatory-related respiratory and neurodegenerative ailments via the activation of NF-κB pathway. Angelicin also showed pro-osteogenesis and pro-chondrogenic effects on osteoblasts and pre-chondrocytes respectively. The elevated expression of pro-osteogenic and chondrogenic markers and activation of TGF-β/BMP, Wnt/β-catenin pathway confirms the positive effect of angelicin bone remodeling. Angelicin also increased the expression of estrogen receptor alpha (ERα) in osteogenesis. Other bioactivities, such as anti-viral and erythroid differentiating properties of angelicin, were also reported by several researchers with the latter even displaying an even greater aptitude as compared to the commonly prescribed drug, hydroxyurea, which is currently on the market. Apart from that, recently, a new application for angelicin against periodontitis had been studied, where reduction of bone loss was indirectly caused by its anti-microbial properties. All in all, angelicin appears to be a promising compound for further studies especially on its mechanism and application in therapies for a multitude of common and debilitating ailments such as sickle cell anaemia, osteoporosis, cancer, and neurodegeneration. Future research on the drug delivery of angelicin in cancer, inflammation and erythroid differentiation models would aid in improving the bioproperties of angelicin and efficacy of delivery to the targeted site. More in-depth studies of angelicin on bone remodeling, the pro-osteogenic effect of angelicin in various bone disease models and the anti-viral implications of angelicin in periodontitis should be researched. Finally, studies on the binding of angelicin toward regulatory genes, transcription factors, and receptors can be done through experimental research supplemented with molecular docking and molecular dynamics simulation.
The use of plants as traditional medicine is common and has prevailed in many different cultures over time. Despite a lack of formal scientific evidence, the belief in the knowledge, traditions, and religious practices that endorse this is strong enough to have sustained this practice over the generations. It seems unlikely that these practices would have persisted for so long in the complete absence of beneficial effects. This suggests plants already used in traditional medicine represent an excellent start point in research to discover new, effective drugs to treat various human illnesses including cancer, bacterial infections, and cardiovascular disease, to name a few. The market trends which have shifted toward a demand for greener, cost-saving, and sustainable sources also created a drive to pursue plant bioprospecting; plants are easily obtained from the environment and are therefore regarded as a cheaper and safer source for consumers (Chandra, 2014).
Among the compounds that have emerged from plant bioprospecting studies are the furocoumarin family of compounds that have been researched since the 1950s (Bordin et al., 1991). They are a family of natural active compounds that can be found in many different plants, vegetables and fruits we consume like parsnips, celery, figs, etc (Chaudhary et al., 1985; Lohman and McConnaughay, 1998; Marrelli et al., 2012). These compounds are mainly produced under stressful conditions as a self-defence mechanism against insect predation, fungal invasion, and bacterial attack. For example, celery infected with the fungus Sclerotinia sclerotiorum before storage has been found to have increased expression of furocoumarins—such as psoralen, 5-methoxypsoralen (5-MOP) and 8-methoxypsoralen (8-MOP)—as a response toward the fungal invasion (Chaudhary et al., 1985). Other studies showed that furocoumarins also displayed anti-feeding properties against insects and inhibition toward bacterial invasion (Yajima and Munakata, 1979; Berenbaum et al., 1991; Raja et al., 2011). The main skeletal structure of furocoumarin compounds encompasses a coumarin unit fused with a furan ring. The varying derivatives of furocoumarins can be formed by the fusing of the furan ring in either 2, 3- or 3,2- arrangements on the c, f, g, or h bonds of the coumarin unit as can be seen in Figure 1 (Santana et al., 2004; Kitamura and Otsubo, 2012). Among the many isomeric derivatives of the furocoumarins, the most commonly reported furocoumarins isomeric forms are linear and angular furocoumarins, with psoralen and angelicin being the most well-known of its isomers respectively (Munakata et al., 2016).
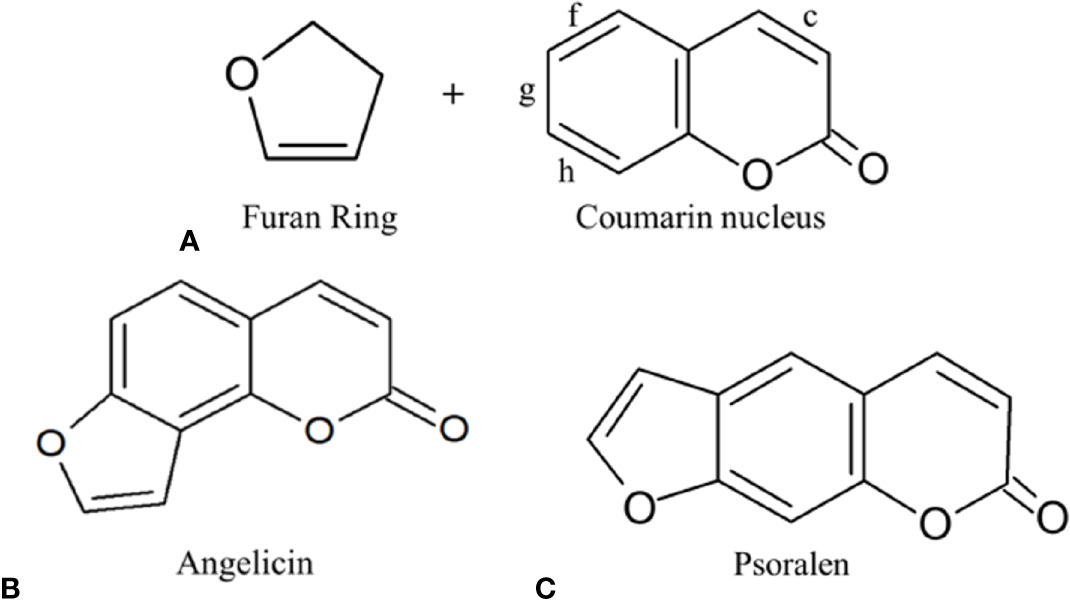
Figure 1 (A) Furocoumarin's several different possible attachments of the furan ring on the coumarin nucleus; (B) Angular furocoumarin: Angelicin; (C) Linear furocoumarin: Psoralen.
Both angelicin and psoralen are photosensitizing furocoumarins, but they interact very differently in the presence of ultraviolet radiation (UVR). With its linear structure, psoralen was discovered to form both monoadducts as well as interstrand cross-links with DNA when irradiated with UVR. When irradiated, the 3',4' or 4',5' double bonds of the molecule will covalently bind with the 5',6'-double bond of the pyrimidines from both sides of the DNA strand by absorbing photons, leading to the crosslinks that can be seen in Figure 2 (Ben-Hur and Elkind, 1973; Nagy et al., 2010). Angelicin, on the other hand, can only form monoadducts due to its steric structure (Grant et al., 1979). The monoadducts formed on DNA by angelicin are also quick to be repaired by the cells and hence, angelicin imposes lower phototoxicity as compared to psoralen (Bordin et al., 1976; Joshi and Pathak, 1983). Although both compounds are photosensitizing compounds, psoralen was favored to be used in phototherapy as it produces desirable effects such as the reduction of lesions in vitiligo, psoriasis, atopic dermatitis, etc. (Vallat et al., 1994; Lee and Jang, 1998; Petering et al., 2004). However, usage of psoralen has reduced, with other modalities of phototherapy treatments and alternative drugs being preferred as psoralen was found to increase the risk of skin cancer and other systemic side effects when consumed (Llins and Gers, 1992; Stern et al., 1997).
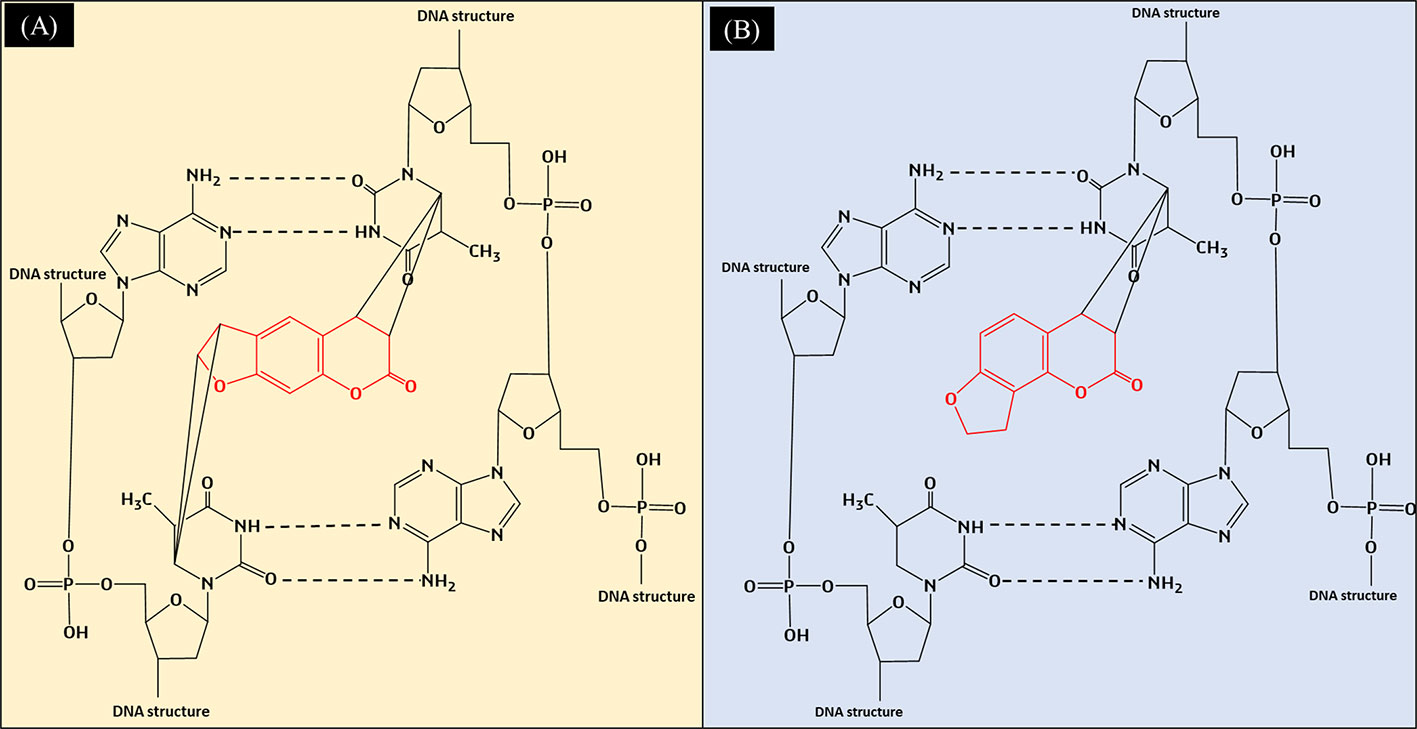
Figure 2 (A) Formation of interstrand crosslinks in the DNA by psoralen when exposed to UVR; (B) Formation of monoadducts in the DNA by angelicin when exposed to UVR.
Though psoralen has been the subject of many studies due to its phototherapeutic properties, angelicin too has demonstrated multiple effects including anti-cancer, anti-viral, anti-inflammatory, anti-microbial, pro-osteogenic and chondrogenic differentiation, and erythroid differentiating properties (Lampronti et al., 2003; Cho et al., 2013; Liu et al., 2013; Wang et al., 2017a; Li et al., 2018). Regarding its anti-viral and erythroid differentiating properties, other studies have also compared angelicin to the common drugs on the market, such as ganciclovir (GCV) and hydroxyurea, and it had was shown that angelicin had almost equal or even better effect as compared to these drugs (Lampronti et al., 2003; Cho et al., 2013). This review evaluates and summarises the findings on the bioactivities of angelicin to showcase the potential of angelicin to be used as a therapeutic agent. A summary of all the biological properties of angelicin that were reported can also be seen in Table 1 and Figure 3.
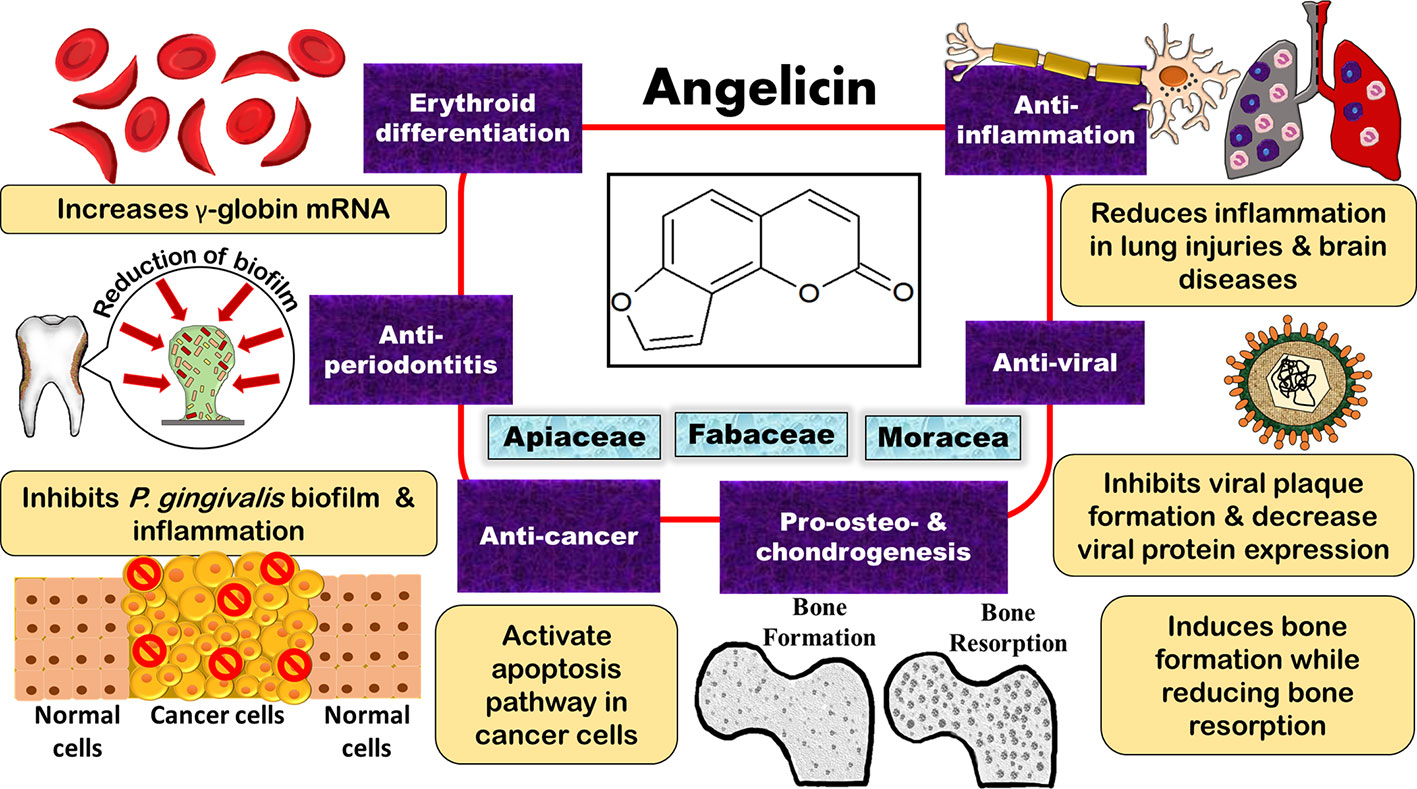
Figure 3 Potential bioproperties of the furocoumarin, angelicin, as an anti-cancer, anti-inflammation, anti-viral, anti-periodontitis, erythroid differentiating, and pro-osteo- and chondrogenic therapeutic agent.
Both psoralen and angelicin can be found in plants from the Leguminosae (Fabaceae), Apiaceae (Umbelliferae) and Moracea family. However, not all plants produce both these furocoumarins. In the Rutaceae family, which encompasses the citrus fruits, only linear isomers were produced (Pathak et al., 1962; Zobel and Brown, 1991). No plant has so far been found that produces only angular furocoumarin; the plants studied so far have always found to be either produce both isomers or linear isomers alone. This finding suggests that angular furocoumarins came later from an evolutionary viewpoint than linear furocoumarins (Dueholm et al., 2015). The existence of angular furocoumarin was hypothesized to be an evolutionary advantage over insects that can metabolize linear furocoumarins. An example is the larvae of Papilo polyxenes or the Black Swallowtail butterfly that produces microsomal cytochrome P450 monooxygenases (P450s)- an enzyme that can catalyze the metabolism of linear furocoumarins. However, angular furocomarins such as angelicin can bind to the active sites of these enzymes, inhibiting the enzyme from metabolizing the toxic linear furocoumarin (Ma et al., 1994; Wen et al., 2006). This shows that there was an evolutionary need for plants to develop angular furocoumarin, even though it is less cytotoxic than its sister isomer. In this review, the list of plants that produce angelicin is tabulated in Table 2. Other extracted compounds are also included. From the table, it can be seen that the percentage yield of angelicin and the other compounds not only changes with the type of plants but also with the part of plant and season, suggesting as mentioned above that these compounds could be produced to act as a defence system against diseases or pests. Other than that, the other listed plants were also used traditionally as medicine. However, whether the compounds contribute individually or accumulatively to the traditionally believed “medicinal” attributes of the plant is still unclear and under research.
A summary of the biosynthesis of both angelicin and psoralen is illustrated in Figure 4. Umbelliferone is the precursor compound of both linear and angular furocoumarins. Umbelliferone is an intermediate 7-hydroxycoumarin derived from p-coumaryl CoA that has undergone ortho-hydroxylation via p-coumaroyl coA2'hyroxylase (C2'H) activity. In Ruta graveolens L. 2-oxoglutarate-dependent dioxygenase (OGD) was found to synthesise 2,4-dihydroxycinnamoyl CoA which then transforms into umbelliferone after spontaneous closure of the lactone ring under acidic or neutral conditions independent of enzymes. Further investigation had also discovered that psoralen acts as negative feedback against C2'H activity to prevent excessive production of psoralen in the plant (Vialart et al., 2012). It is not known if angelicin is also involved in the negative feedback loop against C2'H activity.
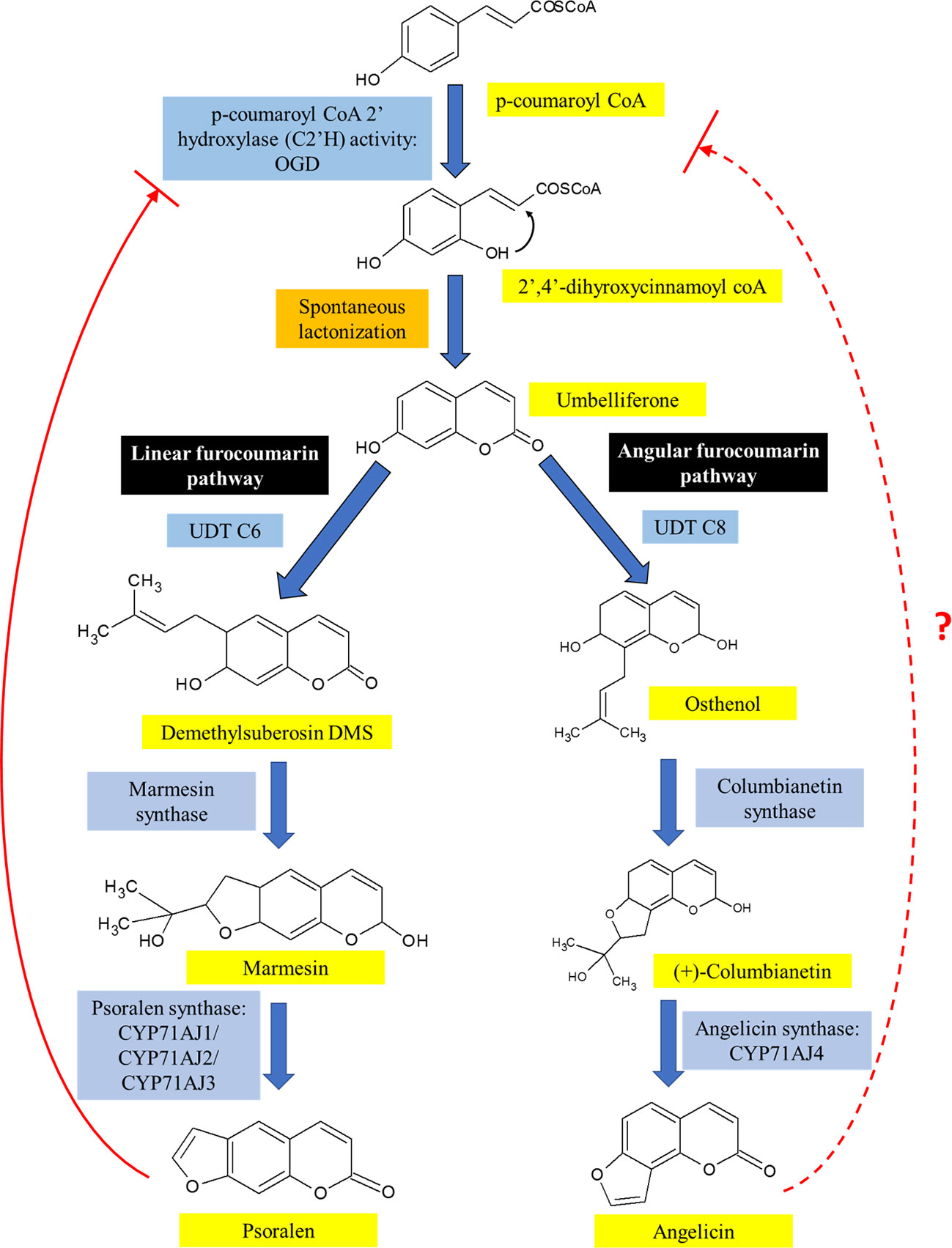
Figure 4 The biosynthesis pathway of linear and angular furocoumarin in plants. Both psoralen and angelicin come from the same precursor, umbelliferone, which had been modified differently to form both linear and angular furocoumarin pathways. Though psoralen is able to act as a negative feedback against C2'H activity, it is not known yet if angelicin is also involved in the negative feedback loop.
After umbelliferone, the distinction of both linear and angular furocoumarin biosynthesis happens based on the prenylation position of umbelliferone at C6 or C8 by umbelliferone dimethylallyltransferase (UDT). UDT is a prenyltransferase that synthesizes demethylsuberosin (DMS) at C6 position of umbelliferone and ostenol at the C8 position (Karamat et al., 2013; Munakata et al., 2016). The synthesis of DMS would lead to the formation of psoralen while osthenol is the precursor of angelicin. DMS is then catalyzed by both marmesin synthase and psoralen synthase to form psoralen itself (Hamerski and Matern, 1988; Larbat et al., 2008; Roselli et al., 2017). On the other hand, for angular furocoumarins, osthenol is catalyzed by columbianetin synthase to form (+)-columbianetin (Roselli et al., 2017). Angelicin synthase then catalyzes the conversion of (+)-columbianetin to angelicin through the abstraction of syn-C3'-hydrogen (Larbat et al., 2008). The first to discover angelicin synthase were Larbat and colleagues who isolated the parsnip variant of angelicin synthase (CYP71AJ4) together with its complementary psoralen synthase, CYP71AJ3, using the genomic sequence of psoralen synthase isolated from Ammi majus (Larbat et al., 2008; Munakata et al., 2016). They also discovered that the genes for angelicin synthase and psoralen synthase from the CYP71AJ subfamily of parsnip share 70% similarity with each other, but the portions were believed to code for active substrate sites sides only showed 40% similarity (Larbat et al., 2008). A recent journal article reported that using bacterial artificial chromosome (BAC) library, it was identified that both CYP71A3 and CYP71A4 genes were only separated by approximately 7.6 kb. Both genes also sit in a cluster on a separate chromosome from the cluster where UDT C6 and p-coumaryl CoA 2'hydroxylase genes, were located. Genes transcribing the enzymes involved upstream of the furocoumarin biosynthesis pathway were seen to cluster together with both UDT C6 and p-coumaryl CoA genes, while the other P450 genes were clustered together with CYP71A3 and CYP71A4 genes (Roselli et al., 2017). This study goes against the common idea that genes from a similar self-defence pathway would be found together as an evolutionary advantage. It could be possible that further downstream genes can be found on a separate cluster or anywhere separately on the genome. Further studies still need to be done to fully understand the biosynthesis of psoralen and angelicin including its location on the genome and the co-localization within the plant cell.
In the year 2013, cancer claimed the lives of 8.2 million people worldwide, making it the world's second leading cause of death, second only to cardiovascular diseases. In 2013 alone, there were 14.9 million new cases of cancer reported (Global Burden of Disease Cancer Collaboration, 2015). For women, breast cancer remains the most prevalent cancer, while for males, lung cancer tops the chart in developed and developing countries. Prostate cancer is also on the rise for men at the global level; and cervical cancer shows a similar trend among women (Global Burden of Disease Cancer Collaboration, 2015; Torre et al., 2015). Colorectal, stomach, and liver cancer are the next most frequently diagnosed cancers globally. Many factors are believed to contribute to the increased incidence of cancer including lifestyle behaviors which have become more common over the decades such as smoking, physical inactivity, poor diet and later dates of first births—all of which have been suggested to increase the risk of cancer (Torre et al., 2015).
The basic hallmarks of cancer include its ability to evade the immune system, resist cell death and growth suppressors, maintain cell proliferation, induce angiogenesis as well as activate invasion and metastasis (Hanahan and Weinberg, 2011). These properties make cancer a difficult disease to manage and eradicate. However, by targeting apoptotic pathways, such as the AKT pathway, MAPK pathway, and modulating the expression of pro- and anti-apoptotic proteins, anti-cancer drugs can slow the progress and even reduce the spread of cancer (Wong, 2009; Falasca, 2010). Another key factor for a good anti-cancer drug is that the drug should have as specific as possible cytotoxicity toward cancer cells only while sparing healthy cells as much as possible. Therefore, natural products have become the research focus as they are easily obtained, safer to use and have low toxicity (Pratheeshkumar et al., 2012).
Many studies have been conducted to test for anti-cancer properties in angelicin, with several of these demonstrating angelicin's ability to reduce the cell viability of human prostate cancer (PC-3), human epithelioma (Hep2), colorectal carcinoma (HCT116), rhabdomyosarcoma (RD), human cervical carcinoma (HeLa) cell line, cervical squamous cell carcinoma (SiHa) cell line and human breast adenocarcinoma (MCF7) cell lines (Mira and Shimizu, 2015; Wang et al., 2015a; Wang et al., 2019b). Historically, the first cell line tested which showed angelicin's anti-cancer properties was the neuroblastoma (SH-SY5Y) cell line, which are cells derived from a metastatic bone tumor biopsy. It was found that when treated with angelicin, upregulation of both caspase 3 and 9 could be seen. In addition, anti-apoptotic proteins were also affected by angelicin in SH-SY5Y cells. Bcl-2, Bcl-xL, and Mcl-1 proteins were seen to decrease their expression levels together with procaspase 9 (Rahman et al., 2012; Kovalevich and Langford, 2013). Irregularity in the regulation of the anti-apoptotic proteins is one of the factors that contribute to the development of cancer as overexpression of them blocks apoptosis and makes the cells resistant to anti-cancer drugs (Oltersdorf et al., 2005). When decreased in its expression, cancer cells became less resistant to anti-cancer therapies (Webb et al., 1997). Hence, the changes seen in the expression of apoptotic and anti-apoptotic proteins by angelicin is a positive indicator that angelicin has anti-cancer properties. Besides that, several different cells, such as promyelocytic leukaemia (HL-60), human lung cancer (A549), hepatoblastoma (HepG2), and hepatocellular carcinoma (Huh-7) cell lines, also yielded the same changes in the proteins mentioned above when incubated with angelicin for 24 or 48 h (Yuan et al., 2015; Li et al., 2016; Wang et al., 2017a). This shows that angelicin has the potential to be effective against multiple cancer cell lines. Besides seeing a decreased expression of anti-apoptotic Bcl-2 family proteins, increased expression of apoptosis-inducing Bcl-2 family, Bax, was also reported in the treated cell lines. Even the expression levels of cytochrome C was increased dose-dependently when tested in HepG2 and Huh-7 cells (Wang et al., 2017a). An increase in the expression of cytochrome C and Bax had been known to indicate the activation of the intrinsic apoptotic pathway. This is because Bax protein causes the permeation of the mitochondrial outer cell membrane, thus releasing cytochrome c into the cytosol, which then triggers the caspase 3 and 9 cascades, mediating apoptotic programmed cell death (Fulda and Debatin, 2006; Ashkenazi, 2008). Hence, based on what had been reported, it is possible that angelicin mainly induces cell death via the intrinsic apoptotic pathway in various cancer cell lines.
Having demonstrated possible activity in inducing apoptosis via the intrinsic pathway, the possibility of angelicin to have effects on the extrinsic pathway was also studied. In human SH-SY5Y cells no changes in the regulation of FAS receptor, FAS ligand and caspase 8 were seen, suggesting that the FAS pathway may not be activated by angelicin (Rahman et al., 2012). On the contrary, human renal carcinoma (Caki) cells displayed a different kind of result when incubated with angelicin. Caki cells have always been known to be one of those cancer cell lines that are resistant to tumor necrosis factor (TNF) apoptosis inducing ligand (TRAIL) (Han et al., 2016). When the compound was tested alone, angelicin was not able to induce apoptosis, yet when incubated together with 50 ng/ml of TRAIL the combined treatment was able to promote cell death (Min et al., 2018). Further investigation then went on to show how this combination treatment was even more effective than cycloheximide in downregulating cellular FLICE (FADD-like IL-1β- converting enzyme)-inhibiting protein (c-FLIP) post-translationally. Active caspase 3 was also upregulated and poly (ADP-ribose) polymerase was also cleaved, confirming cell apoptosis, though the mechanism was independent of endoplasmic reticulum (ER) stress and reactive oxygen species (ROS) signaling. Also, there were no changes with death receptor 5 (DR5) as well as the other intrinsic related apoptosis proteins, which suggest that angelicin does not induce apoptosis through the intrinsic pathway or DR5 in the Caki cells. There were also no changes in the inhibitor of apoptosis proteins (IAP) family, which includes cIAP1, XIAP, and survivin. Interestingly, the pro-apoptotic protein Bim was shown to increase in its expression when Caki cells which were only treated with angelicin; however, combination treatment with TRAIL attenuated the effect. The combination treatment of angelicin and TRAIL was able to induce apoptosis in other cancer cell lines, such as Sk-hep1 and MDA-MB-361 cells but normal cell lines were not affected (Min et al., 2018). This is a good therapeutic option to be exploited as this combination treatment could have the potential to be used as targeted cancer therapy toward cancer cell lines that are resistant to TRAIL.
In many cancer cell lines, the PI3K/AKT signaling pathways are antagonized by a mutation or deletion on its tumor suppressor gene, phosphatase and tensin homolog (PTEN). This mutation then leads to an attenuation of the intrinsic apoptosis pathway causing the cells to increase in cell growth and proliferation, become anti-apoptotic and increase in their angiogenesis and metastasis abilities (Downward, 2004; Roy et al., 2010). To further study the effect angelicin on the PI3K/AKT, Wang et al. (2017a) treated two liver cancer cell lines, HEPG2 and Huh-7 with angelicin. The results showed a significant reduction in the expression of PI3K and phospho-AKT proteins in both cell lines in a dose-dependent manner. However, Li et al. (2016) and Rahman et al. (2012) reported no changes in the PI3K/AKT pathway were seen in A549 lung cancer cells and SH-SY5Y neuroblastoma cells when treated with angelicin. Pro-apoptotic GSK-3β also did not experience any changes in the neuroblastoma cell line (Rahman et al., 2012). From this observation, it is possible that angelicin may be affecting the upstream processes such as the promoter or silencer genes in this pathway and not the protein expression itself. It could be a different mutation in these upstream genes in both A549 and SH-SY5Y cells that prevents angelicin from affecting the PI3K/AKT pathway in both these cell lines.
The mitogen-activated protein kinase (MAPK) signaling pathway has also been investigated as it is involved in cell proliferation or cell death. In A549 cells, an increase of phosphorylated JNK (pJNK) and phosphorylated ERK1/2 (pERK1/2) was seen but no changes in p38 MAPK was observed (Li et al., 2016). Again, this was different for SH-SY5Y cells in which the MAPK pathway (including p-38, pERK 1/2 and pJNK) was not affected (Rahman et al., 2012). The phosphorylation of JNK had been associated with the upregulation of pro-apoptotic genes while phosphorylation of ERK 1/2 has often been related to increased cell proliferation and survival. However, there had been a discussion that ERK1/2 may also be involved in apoptosis although its death mechanism has yet to be fully understood (Mebratu and Tesfaigzi, 2009; Wagner and Nebreda, 2009). In this case, inhibition of both ERK and JNK reduces the apoptotic effect which suggests the significant role of MAPKs in angelicin-mediated apoptosis in cancer cells (Li et al., 2016).
Other researchers have also investigated different aspects of angelicin in inhibiting cancer proliferation. A study on cell cycle arrest showed that angelicin downregulates cyclin B1, cyclin E1 and Cdc2 in human lung cancer A549 cells. The cell cycle assay using flow cytometry also showed an increase in cell cycle arrest at the G2/M phase and a decrease in G0/G1phase (Li et al., 2016). However, angelicin induced cell cycle arrest might differ in different cell lines. For example, HeLa and SiHa cells were significantly arrested at the G0/G1 phase while the ratio of cells was significantly decreased in the G2/M phase (Wang et al., 2019b). This could perhaps be due to the different pathways that are affected by angelicin in different cell lines. Apart from that, angelicin was also able to inhibit tubulin polymerization; in fact, it was able to inhibit this to a higher degree than psoralen according to the rank score match of both compounds with the colchicine binding residue on microtubules and histone deacetylase 8 inhibitory (HDAC8) assay (Mira and Shimizu, 2015). This is certainly an interesting area to look into as inhibition of microtubules can affect the cell proliferation of cancer cells (Jordan and Wilson, 2004). Angelicin had also been tested for its ability to reverse multidrug resistance in resistant human myelogenous leukemia (K562/A02) cell line but the results were not significant (Wang et al., 2016).
Another aspect that was looked into was the effect of angelicin on autophagy in cancer cell lines. According to Wang et al. (2019b), angelicin's anti-cancer properties on HeLa and SiHa cells could be due to its ability to inhibit autophagy. Autophagy is a process where molecular materials and organelles of cells are degraded in autophagosomes, aided by lysosomes (Maiuri et al., 2007). For cancer cells, autophagy was suggested to function in prolonging its survival by mitigating the effects of cellular and environmental stresses (Mathew et al., 2007). This catabolic process of autophagy is regulated by the mammalian target of rapamycin (mTOR) signaling pathway (Kim and Guan, 2015). It had been reported that the negative regulation of the mTOR pathway induces autophagy when cells are exposed to nitrosative and oxidative stress (Tripathi et al., 2013; Tang et al., 2014). In autophagy, the microtubule-associated protein 1 light chain 3 (LC3) was initially converted into LC3-I (a cytosolic form of LC3) and then LC3-phosphatidylethanolamine conjugate (LC3-II) when autophagosomal membranes were formed. LC3-II is then localized onto autophagosomes or autophysosomes (fusion of autophagosome and lysosome) (Tanida et al., 2005). From the study done on HeLa cells and SiHa, it can be seen that angelicin not only increased the phosphorylation of mTOR protein but also decreased the expression of LC3B-II in both cell lines (Wang et al., 2019b). Other than that, the expression of Atg3, Atg7, Atg12-5, which are essential in the formation of autophagosomes, were decreased as well (Wang et al., 2019b). Based on the data that was obtained, this indicates the involvement of angelicin in the autophagy process of cancer cells.
As described above, many different cell types from neurological to liver cell lines have been used to study the mechanism of angelicin's anti-cancer properties. Yet, testing the effectiveness of angelicin's anti-cancer properties against cells lines could not fully emulate actual in vivo situations in the human body. Therefore, further tests on animal models would help us understand if similar effects seen on the cell lines would be applicable in an organism. One study treated UMR-106 (rat osteosarcoma) cell lines that were injected in the tuberosity region of the tibia in nude rats, with 320 and 1,600 μg/kg/d angelicin for 10 days (Lu et al., 2014). After the rats were sacrificed, the osteosarcoma volume and weight in treated rats experienced a significant decrease, as compared to the control. Besides that, there were also hallmarks of cell death such as an increased amount of necrotic tumor cells, cell debris, shrunken nuclei, condensed cells, tissue haemorrhaging, aggregation of inflammation, and infiltration of lymphocytes. On the other hand, the liver, spleen, kidney, heart, and lungs showed no changes after treatment. There were also no metastatic lesions in the abdomen (Lu et al., 2014). The decrease of alkaline phosphatase (ALP), an essential factor in bone formation, in the serum also indicated that angelicin was able to reduce osteosarcoma cell growth in vivo, showing that angelicin decreased osteosarcoma tumor growth (Lu et al., 2014). Another study by Li et al. (2016) on A549 cancer cell lines, involved implanting A549 cells in the right flanks of female nude mice. These mice were then treated with angelicin orally for 4 consecutive weeks. After the 4-week treatment, the mice were sacrificed and the tumor was retrieved for further study. It was found that the weight and the size of the tumor had been considerably reduced. In addition, the tumor also showed decreased matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9) expression levels while there were increased E-cadherin levels. This indicates that angelicin was able to have an inhibitory effect on tumor metastasis. The results on the expression levels of these three proteins correlate with those obtained from the cell line, including the experiment on wound healing that was done to show the inhibition of angelicin on cell migration (Li et al., 2016). A similar decrease in migration and invasion were also reported in in vitro model of HeLa and SiHa cells by Wang et al. (2019b), indicating that such inhibition was not limited to A549 cell lines only but also that angelicin is able to demonstrate anti-invasion and anti-migration properties in both in vitro and in vivo models. Wang et al. (2017a) discovered that angular furocoumarin decreases the growth of HepG2-induced tumor that was implanted in the back of male BALB/c mice. At the same time, the levels of proliferation marker Ki-67 and phosphorylated vascular endothelial growth factor 2 (p-VEGFR2) were significantly reduced in the tumor tissues. From this observation, it could be suggested that angelicin may be suppressing the proliferation of the tumor by disruption of vascularity, thus suppressing angiogenesis. No reduction of body weight and cases of mortality were reported, indicating the selectivity of angelicin toward cancer cells (Wang et al., 2017a).
Recently, a report depicting molecular docking analysis of angelicin on breast cancer markers was published. In breast cancer, the estrogen receptor (ERα) levels were highly expressed and its presence maintains the survival and growth of breast cancer cells (Fillmore et al., 2010). Another study on estrogen-progestin replacement therapy suggests that this regimen can be associated with an increased risk of breast cancer (Schairer et al., 2000). Epidermal growth factor receptor (EGFR) is also an important marker to look at in cancer, especially breast cancer, as it can affect the differentiation and proliferation of cancer cells (Tsutsui et al., 2002). Hence, to be able to inhibit these breast cancer marker proteins would aid in the control or decrease of breast cancer. As Acharya et al. (2019) had reported using molecular docking, angelicin displayed low binding energy toward ERα (-12.01 kcal/mol), progesterone (PR) (-11.63kcal/mol), EGFR (-12.6kcal/mol) and mTOR (-13.64kcal/mol). When compared to psoralen, the binding energy for ERα was even lower in angelicin. To confirm the ability of angelicin were able to bind to the protein of interest, in vitro analysis was done on breast cancer (MCF-7) cell line for the analysis of EGFR while the binding of angelicin to ERα was measured using ERα reporter assay system. Both the data obtained showed that angelicin was able to act as an antagonist for both proteins. For ERα, when comparing the IC50 value between angelicin and psoralen for antagonizing ERα, the IC50 value for angelicin was much lower, signifying that angelicin could antagonize ERα better than psoralen. However, both angelicin and psoralen were unable to inhibit mTOR (Acharya et al., 2019). Nevertheless, angelicin displayed potential as an antagonist against breast cancer cells.
Through the studies shown, angelicin has great anti-cancer potential due to its specific cytotoxicity toward cancer cell lines both in vitro and in vivo. This is a much better alternative compared to psoralen which was shown to be more cytotoxic toward normal cells than angelicin. Angelicin also exhibits positive cytotoxicity toward multiple cancer cell lines via the intrinsic as well as extrinsic apoptotic pathways. In some cell lines, the involvement of MAPK, mTOR, and PI3K/AKT pathways in the action of angelicin casts some light not only on its apoptotic effect but also indicates the compound's ability to interfere with the survival of cancer cells through other mechanisms. Other than that, the recent study using molecular docking to predict the inhibitory activity of angelicin against specific cancer markers is promising for a whole new avenue in producing targeted cancer drugs. A summary of the different pathways that are involved in angelicin-mediated apoptosis in cancer cells is depicted in Figure 5.
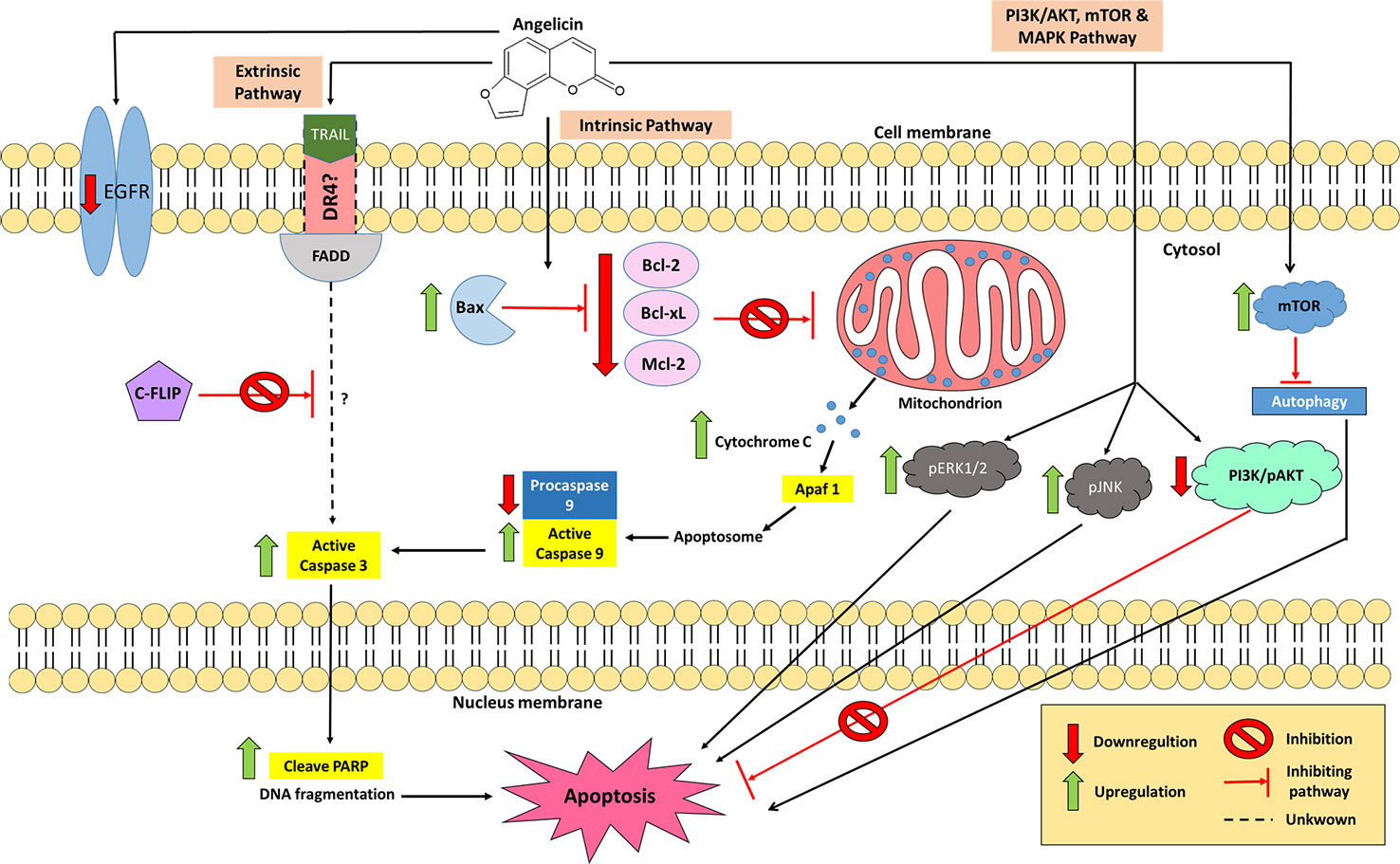
Figure 5 The involvement of angelicin in several apoptotic pathways which promote cancer cell death. In the intrinsic pathway, angelicin increases the expression of proapoptotic proteins and decreases the expression of anti-apoptotic proteins, causing a caspase cascade of caspase 3 and 9 to occur. In the extrinsic pathway, combination treatment between TRAIL and angelicin down-regulated c-FLIP which leads to an increase in active caspase 3, inducing cell apoptosis. However, the involvement of other apoptosis-related proteins with the combination treatment in the extrinsic pathway is yet to be elucidated. The PI3K/AKT and MAPK pathways are also actively involved in angelicin-mediated cancer cell death.
Inflammation is a response of the body toward tissue injury, infection or irritants. It is mainly caused by non-specific and specific immune responses to limit the spread of pathogens or injury (Watkins et al., 1995). Inflammation can be caused by many factors for example open cut or internal wounds, oxidative stress, viral and bacterial infection. These factors can cause either acute or chronic inflammation. Acute inflammation is a type of inflammation that persists for a short time and is advantageous to the host but chronic inflammation lasts for an extended period of time and when not suppressed, can cause damage to normal cells and excessive tissue damage (Rahman and MacNee, 2000; Reuter et al., 2010). The activation of NF-κB is one of the inflammatory responses toward stressors like physical stress, physiological stress, pathological invasion, and environmental stress (Liu and Malik, 2006). Once stimulated, the NF-κB dimer is released from its inhibitor IκB and it then enters the nucleus to initiate the transcription of genes that are involved in the complex regulation of inflammatory mediator networks and cytokines(Liu and Malik, 2006; Rai et al., 2015). This then initiates the recruitment of inflammatory cells like neutrophils to the inflamed site to release anti-microbial peptides and ROS to kill invading microbial pathogens and initiate cell death when necessary. However, overproduction of ROS could also lead to extensive tissue damage which is dangerous for the host (Yamamoto et al., 2008; Morgan and Liu, 2011).
Through mimicry of acute lung injury via lipopolysaccharide (LPS) induced inflammation of murine lungs, Liu et al. (2013) showed that pre-treatment with angelicin reduces the production of pro-inflammatory cytokine markers, such as IL-6 and TNF-α. Not only that, the accumulation of polymorphonuclear neutrophils and macrophages in the lungs was also reduced by the pre-treatment. Histological images of the lung tissue also displayed reduced hallmarks of lung damage such as alveolar haemorrhage, alveolar wall thickening, interstitial edema, and infiltration of inflammatory cells. A deeper look into the mechanism behind angelicin's anti-inflammatory properties revealed that angelicin actively blocked the phosphorylation of IκBα and p65 in the NF-κB pathway, blocking the translocation of NF-κB into the cell nucleus.
Additionally, angelicin also showed inhibition of phosphorylation of p38 and JNK pathways in MAPK which decreases sepsis-induced organ injury within the mice. However, the ERK pathway, which is involved in cell growth and proliferation, was not inhibited by angelicin (Liu et al., 2013). Similar findings had been discovered in other studies on inflammation in asthmatic mouse models. Treatment with angelicin in asthmatic mice inhibits the production of cytokines IL-4, 5, and 13 that are typical of inflammation in asthmatic patients. With the inhibition of these interleukins, the production of serum IgE and airway hyperresponsiveness were also inhibited. The researchers also recorded similar NF-κB pathway inhibition, as evidenced by a significant decrease in the phosphorylation of p-65 and IκB (Wei et al., 2016). This suggests that it is possible that the anti-inflammatory properties of angelicin involve the inhibition of NF-κB pathway in inflammatory- related respiratory ailments. The inhibition then, in turn, stops the production of cytokines necessary to initiate the inflammatory reaction.
Besides that, the anti-inflammatory properties of angelicin were also tested on mouse neuronal cells. Inflammation of the brain is known to be associated with many neurodegenerative diseases such as Parkinson's, Alzheimer's, Huntington's disease and stroke (Coombes et al., 2011; Nirmaladevi et al., 2014; Yang et al., 2016). For example, Alzheimer's patients have higher levels of ROS, produced partly due to inflammation, as compared to a normal person (Yang et al., 2016). Reactive nitrogen species (RNS) such as nitric oxide is also produced excessively by activated microglia and astrocytes which is deleterious toward the neurons (Liu et al., 2002). Angelicin was able to exert a neuroprotective effect on neural cells by inhibiting the occurrence of inflammation. When angelicin was applied to LPS-induced inflamed mouse BV2 microglia cells, nitric oxide (NO) production of the cells was significantly suppressed. This is a complete contrast to psoralen which showed no changes in the production of NO, making angelicin an interesting subject for further study. Other treatments involving the application of a non-radical species of ROS, hydrogen peroxide (H2O2), toward HT22 mouse hippocampal cells to initiate neuronal cell damage were also attenuated by angelicin. Again, the result is very different for psoralen, which was only able to slightly reverse the neuronal cell damage caused by H2O2 (Simon et al., 2000; Kim et al., 2016). With the results obtained from this study on neuronal cells, angelicin appears to be a good candidate for further analysis in human models to study its mechanism in neurodegenerative diseases.
On another note, a study done by Chen et al. (2017), showed that when angelicin was tested against human neutrophils obtained from the blood, the inhibitory effects of angelicin against the generation of superoxide radical anion and the release of elastase were shown to be less effective as compared to its sister isomer, psoralen. A weaker inhibitory effect was also seen from angelicin against the production of NO from RAW264.7 murine macrophages as compared to psoralen. The results obtained were the opposite of what was seen as described by Kim et al. (2016). It is possible that the pathways activated by angelicin in neuronal cells are different from those of human neutrophils. It should also be noted that in the study done by Liu et al. (2013) the NO production of RAW 264.7 mouse macrophage cell line was not tested. Hence, a deeper understanding of the pathways activated by angelicin and psoralen is still very much needed.
Nevertheless, angelicin had shown great promise in its anti-inflammatory aptitude especially as a neuroprotectant and against inflammatory-related respiratory ailments as shown in Figure 6. Perhaps with chemical modification of angelicin and a greater amount of research on the pathways involved, it is possible that angelicin can be used in developing a new anti-inflammatory drug.
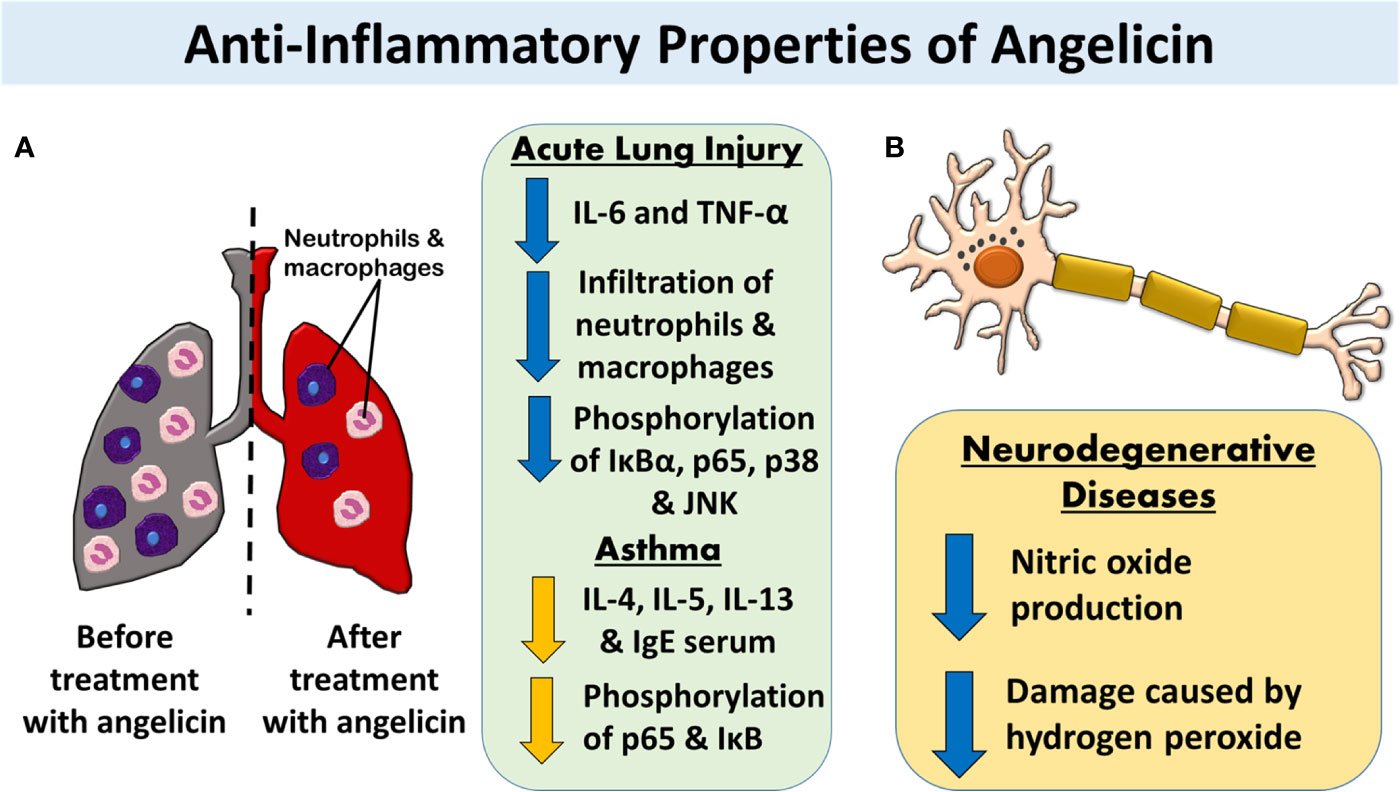
Figure 6 The anti-inflammatory properties of angelicin. (A) Angelicin attenuates inflammation-induced-damage in acute lung injuries and asthma by lowering the cytokine production and reducing the infiltration of neutrophils and macrophages. Both MAPK and NF-κB pathway was also affected by angelicin in which angelicin inhibits the phosphorylation of IκB, p65, p38, and JNK. (B) Angelicin exerts a neuroprotective effect by inhibiting the production of nitric oxide and reducing the damage caused by hydrogen peroxide in LPS-induced inflamed mouse BV2 microglia cells and HT22 mouse hippocampal cells respectively.
Bone remodeling is a process that is coordinated by bone resorption and bone formation, mediated by osteoclast and osteoblasts respectively (Tang et al., 2009). In bone formation, transforming growth factor (TGF-β) and bone morphogenetic proteins (BMPs) are key initiators of osteogenic differentiation via the TGF-β/BMP signaling pathway (Chen et al., 2012). At the start of signaling, BMP/TGF-β interacts with either BMP or TGF-β specific type 1 and 2 serine/threonine kinase receptor to initiate BMP/TGF-β-regulated cascade through canonical (Smad dependent) and non-canonical (smad independent) pathways. In the canonical pathways, smad 1, 2, 3, and 5 are activated by BMP and TGF-β extracellular signals, while smad 4 forms complexes with the other smads and translocate into the nucleus, initiating transcription of selected genes such as Runx2/Cbfa1/Osf2/AML3. Other smads such as smad 6 and 7 act as negative regulators of BMP/TGF-β signaling pathway (Gersbach et al., 2004; Hayrapetyan et al., 2014). Runt transcription factor 2 (Runx2), is an essential transcriptional activator in osteoblastic differentiation (Gersbach et al., 2004). Runx2 can also be induced by Wnt/β-catenin signaling. In an experiment done by Gaur et al. (2005) it was seen that when Wnt proteins were upregulated in secreted fizzled -related protein-1 (SFRP1) null mice, the expression of Runx2 mRNA and protein levels were significantly increased up to 6–9 fold and 2–3 fold respectively. The increase in Runx2 was also paralleled with an increase in osteocalcin (OCN) by 1.3–3 fold higher than wild type mice. This suggests a positive involvement of Wnt/β-catenin signaling in the expression of Runx2 and its contribution to osteogenic differentiation.
In both the primary bone marrow mesenchymal stem cells (BMSC) (obtained from 4 week old C57BL/6 mice) as well as primary rat osteoblasts cells (obtained from female Winstar rats) that were treated with angelicin, significant increases in BMP-2, Runx2, OCN, and β -catenin were reported in multiple studies (Wang et al., 2017b; Ge et al., 2019). Using immunofluorescence staining, it was also revealed that not only was β-catenin upregulated by angelicin, there was also an increase in β-catenin translocation into the nucleus after treatment (Ge et al., 2019). To determine the involvement of Wnt/β-catenin signaling pathway, the cells were co-treated with both angelicin and DKK-1, an antagonist to the pathway. The results obtained revealed that DKK-1 successfully attenuated the effect angelicin had on the cells (Ge et al., 2019). Besides that, Zhang and Ta (2017) also studied the effect of angelicin on TGF-β1 reporter gene activity using transfected HEK293T cells. The cells were firstly transfected with (CAGA)12-Luc-reporter plasmid and then stimulated with various concentrations of angelicin for 12 h before luciferase activity was measured. After the measurement, the results obtained showed that angelicin significantly increased the luciferase activity and thus the activation of TGF-β1 reporter gene. However, the mechanism behind the activation of this reporter gene was not studied. Other than that, in MC3T3-E1 (mouse osteoblast precursor) cell line, the osteogenic differentiation inhibitor smad 7 protein expression were downregulated, while the expression of COL1A1 mRNA, which encodes the type I procollagen chain, and collagen type I (COL-1) protein were upregulated by angelicin as well (Zhang and Ta, 2017; Ge et al., 2018). COL-1 is one of the major organic components found in the bone extracellular matrix (Lynch et al., 1995). When rat bone marrow cells were cultured together with COL-1 matrix gel, the cells exhibited signs of osteoblastic differentiation such as the formation of nodules and expressed osteoblastic specific genes like OCN, osteopontin (OPN) and bone sialoprotein (BSP), indicating its involvement in osteogenic differentiation (Mizuno et al., 2000). Hence, the increase in expression of COL-1 protein, suggests that angelicin is able to induce osteogenic differentiation in osteoblast cells.
To demonstrate and confirm further the pro-osteogenic effect of angelicin, the ALP activity and mineralization of osteoblasts were also measured. ALP is a metalloenzyme that has an essential role in the mineralization and is often used as an indicator of early-stage osteoblastic differentiation (Golub and Boesze-Battaglia, 2007). Mineralization occurs when a calcium phosphate known as hydroxyapatite is deposited in the extracellular matrix by either chondrocytes in growth plate cartilages, osteoblasts in bones or odontoblasts in the teeth via matrix vesicles (Orimo, 2010). As hydroxyapatite crystals propagate beyond the matrix vesicle into the spaces between the collagen fibrils, the ratio of inorganic phosphate (Pi) and inorganic pyrophosphate (PPi), an inhibitor of hydroxyapatite formation, must be managed. Therefore, the role of ALP in the generation of Pi by hydrolyzing PPi is essential for osteogenic differentiation (Orimo, 2010). In the year 2014, when angelicin was tested against primary rat osteoblastic cells obtained from newborn Winstar rats, it was initially reported that angelicin had no effect on cell proliferation or alkaline phosphatase (ALP) activity (Li et al., 2014). However, in a recent paper, Ge et al. (2019) had demonstrated that angelicin was able to not only increase the proliferation of osteoblast but also to induce the expression of alkaline phosphatase (ALP) after 7 days of treatment while the matrix mineralization of the cells can be seen on the 12th day of treatment with angelicin. Similar observation of increased cell proliferation, ALP activity and mineralization of osteoblasts by angelicin were reported in MC3T3-E1 (mouse osteoblast precursor) cells and primary bone marrow mesenchymal stem cells (BMSC) with the exception that BMSC cells did not experience any changes in cell proliferation (Wang et al., 2017b; Ge et al., 2018).
Other than that, angelicin was also discovered to suppress the conversion of BMSC to adipocytes. The study by Wang et al. (2017b), reported an angelicin induced dose-dependent downregulation in PPARγ and C/EBPβ adipocyte-specific markers in adipogenesis differentiation while osteogenic markers, Runx2 and OCN were dose-dependently increased in osteogenic differentiation. In addition, two markers from the mTOR complex 1 (mTORC1) signaling pathway, p-S6 and p-4EBP1, were analyzed in both osteogenic and adipogenic conditions. When comparing the two markers, p-S6 showed marked downregulation in expression while p-4EBP1 was upregulated under both differentiation conditions for 7 and 14 days (Wang et al., 2017b). mTOR is a conserved pathway that plays a role in regulating cell growth and metabolism and it had been reported that mTORC1 participates in adipogenesis (Polak et al., 2008; Betz and Hall, 2013). Mice that had whole-body knockout of S6 were reportedly more resistant to diet-induced obesity while whole-body knockout of 4E-BP1 exhibited the opposite results (Polak et al., 2008). Hence, with similar regulation of S6 kinase and 4EBP1 expression, this suggests that angelicin was able to suppress the adipogenic differentiation in BMSC via the mTOR pathway.
To mirror what was seen in in vitro studies, mice and rats were ovariectomized or orchidectomized to induce osteoporosis and then treated with angelicin to determine if angelicin was able to reverse the symptoms. In female C57BL/6 mice, there was an increase in trabecular thickness, bone volume/total volume and trabecular number in the distal femur after treatment with angelicin. This indicates an increase in bone mass by angelicin. Runx2 protein was also upregulated and PPARγ protein downregulated in the distal femur after treatment with angelicin. It can be suggested that the cells within the distal femur were going toward osteoblastic differentiation instead of adipocyte differentiation. To reconfirm the data, a decreased number of adipocytes in the bone marrow was obtained as well (Wang et al., 2017b). Another study on ICR mice was done as well, where mice were segregated based on gender and the difference of not only between osteoporosis model group and treated group was measured but also comparison was done between the different gender groups (Yuan et al., 2016). Although some of the markers that were measured between the non-osteoporosis group (control) and osteoporosis model group were not significant, it was interesting to note that there is some difference in the expression of these markers when comparing gender groups. Nevertheless, both groups did have an increase in bone strength, trabecular number, trabecular bone area, and intramembranous ossification after treatment with angelicin. The trabecular bone gap instead was decreased by angelicin although not as small as the control group (Yuan et al., 2016). Besides that, collagen type I degradation product (CTX-1), a marker that reflects bone and cartilage breakdown, in the serum of both genders were reported to have decreased with the treatment of angelicin (Landewé et al., 2006; Yuan et al., 2016). This shows that angelicin was able to reduce collagen degradation. The interesting differences between female and male mice after treatment were the osteogenic markers such as ALP, TRACP, and OCN. The ALP levels were reportedly increased in the serum of male mice when treated with 10mg/kg of angelicin while female mice did not display any changes. On the other TRACP was seen to decrease in female mice treated with 20mg/kg angelicin (Yuan et al., 2016). Despite this difference gender-wise for ALP and TRACP, when the data were measured in terms of ALP/TRACP both genders showed an increase in the ratio of ALP/TRACP, which suggests positive bone formation (Yuan et al., 2016). As for OCN, there were no changes in female mice after being treated or even when comparing between the control and osteoporosis model groups. Yet, in the male mice, osteoporosis model showed a significant increase in OCN expression in the serum but 10 mg/kg angelicin significantly reversed the expression (Yuan et al., 2016). It could be possible that the difference in expression of these markers could be due to different physiologies between both genders. Nevertheless, angelicin still proved to be effective in reducing osteoporosis symptoms in both males and females.
During bone resorption, the generation of ROS was also found to play an essential role. Increased ROS levels stimulate the activation and differentiation of osteoclasts via receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) which then binds to receptor activator of NF-κB (RANK) that was expressed on macrophages. This then leads to a cascade of events that ultimately form osteoclasts (Ha et al., 2004; Srinivasan et al., 2010). Osteoclasts themselves too generate ROS through tartrate-resistant acid phosphatase (TRACP). This is an enzyme that is expressed by osteoclasts and has two independent enzymatic activities. It not only produces ROS using Fenton's reaction at neutral pH but also functions as a phosphatase in acidic conditions (Halleen et al., 2003; Halleen et al., 2006). The formation of ROS aids in degrading type I collagen during bone resorption (Halleen et al., 1999). By using OB-6 osteoblastic cells as a subject of study, it had been discovered that angelicin was able to attenuate H2O2 induced damage in the osteoblasts (Li et al., 2019). The cells not only showed an increase in cell viability and a decrease in the apoptotic rate but also showed suppression of ROS generation with angelicin treatment. When measuring the cells' mitochondrial function, it can be seen that angelicin reversed the H2O2 induced downregulation of mitochondrial respiratory chain complex I (MRC-1), protecting the mitochondrial function in osteoblastic OB-6 cells. Furthermore, angelicin also successfully induced the osteogenesis differentiation via Wnt/β-catenin pathway even under oxidative stress. The increase in calcium accumulation, in osteogenic differentiation marker (OCN and Runx2) and Wnt/β-catenin pathway markers (β-catenin and tankyrase), were reported when angelicin was co- treated with H2O2 in OB-6 osteoblastic cells. In addition, in vivo studies on female Sprague Dawley rats showed similar results concerning angelicin's ability to attenuate oxidative stress. Antioxidants such as superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GSH-Px) activity in the blood were increased after the treatment with 10 and 20mg/kg angelicin while lipid peroxidation marker malondialdehyde (MDA) was reportedly reduced (Del Rio et al., 2005; Wang et al., 2018). Apart from that, apoptosis markers caspase3/9 were also downregulated in the bone of the rats, suggesting a suppression of cell apoptosis in the bone. The rats also demonstrated a decrease of ALP in the blood, when compared to the group that has osteoporosis, after being treated with 10 and 20mg/kg angelicin for 12 weeks. According to Atalay et al. (2012), postmenopausal women have much higher ALP levels in the blood as compared to those who are in the premenopausal stage due to the increased bone turnover rate in postmenopausal women. Hence, the decrease of ALP in the blood of the rats indicates that there is a decrease in bone turnover rate by angelicin. Further analysis of the calcium/creatine levels in the urine, calcium and leptin levels in the serum and measurement of the bone mineral density of the rats indicates positive osteogenic differentiation in the rats (Wang et al., 2018). Wnt and β-catenin proteins were also upregulated as well in the cartilage tissue which suggests the activation of Wnt/β-catenin pathway while downregulation PPARγ protein expression indicates suppression of adipogenesis (Wang et al., 2018). Angelicin also dose-dependently inhibited the upregulation of OCN, COL-1, and osteoprotegerin (OPN)—a soluble decoy receptor that inhibits the function and differentiation of osteoclasts—in the cartilage tissues of the rats with osteoporosis, bringing them back to almost normal levels as compared with the healthy rats (Udagawa et al., 2000; Wang et al., 2018). Based on the in vitro and in vivo analysis, angelicin may potentially be exhibiting pro-osteogenesis properties by inhibiting ROS in bone resorption.
Estrogen plays an important role in the formation of bones. In early menopausal women, those that were treated with medium and high doses of natural estrogen, 17β-estradiol, and estriol, for a year had shown signs of increased bone mineral content. The ALP in the serum of these patients too were decreased (Christiansen et al., 1982). Common drugs such as bisphosphonates, denosumab, strontium ranelate, and estrogen receptor modulators, are being used to increase the estrogen levels in menopausal women. However, there have been concerns over its side effects for long term usage (Rizzoli et al., 2011). Hence, potential compounds that could upregulate the body's estrogen levels are needed. The treatment of MC3T3-E1 cells and primary rat osteoblast cells with angelicin increased the expression of ERα protein (Ge et al., 2018; Ge et al., 2019). A similar increase in ERα by angelicin in the grey matter of ventral and dorsal horns were also reported in adult male C57BL/6 mice that had suffered spinal cord injury (Li et al., 2017a). Other than that, to determine if angelicin were only upregulating ERα and not ERβ, Xin et al. (2010) incubated HeLa cells with angelicin and they confirmed that angelicin only increases ERα transcription activity. Despite previously being reported by Acharya et al. (2019) that angelicin acts as an antagonist for ERα, the in vitro analysis done on osteoblasts and HeLa cells depicted the opposite reaction. Hence, various cell lines should be analysed to confirm the effect angelicin has on estrogen.
Furthermore, angelicin also was found to inhibit the nucleocytoplasmic shuttling of the aryl hydrocarbon receptor (AhR) from the cytoplasm into the nuclei by directly binding to the receptor itself. AhR acts as a negative regulator of osteogenic differentiation and instead promoted bone resorption. In AhR knockout mice, there was a decrease in bone resorption and an increase instead in bone mass. Osteoclasts were also inhibited from differentiating in AhR knockout mice (Yu et al., 2015). Hence, the inhibition of the AhR signaling pathway by angelicin could perhaps contribute to an even higher increase in osteogenic differentiation in osteoblast cells. Angelicin also inhibited the expression of cytochrome P450 family subfamily A member 1 (CYP1A1) in MC3T3-E1 cells (Yu et al., 2015). Not only that, when C57BL/c mice were injected with 10mg/kg angelicin and the expression of CYP1A1 in the serum decreased after treatment with angelicin (Ge et al., 2018). CYP1A1 is one of the genes that is mediated by AhR via the AhR signaling pathways (Delescluse et al., 2000). The CYP1A gene encodes for microsomal cytochrome P5401A1 protein, which catalyzes the metabolism of various xenobiotics (Qiang, 2001). Furocoumarins have been known to be an interfering agent in drug metabolism, especially with cytochrome P450 and this might pose a clinical advantage to increase the absorption of poorly absorbed drugs, thereby reducing dose requirements and costs of drugs (Girennavar et al., 2007). However, recent papers had expressed concern over its hepatotoxicity. Sprague Dawley rats that were treated with 60 mg/kg of angelicin and psoralen for 7 days experienced a change in the expression of 884 and 172 genes respectively with the metabolism of xenobiotics cytochrome P450 and chemical carcinogenesis pathway were the two most upregulated enriched pathway identified from the gene expression changes. The aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bile acid (TBA), and total triglyceride (TG) levels in the serum were upregulated as well, which are signs of hepatotoxicity. In the cytochrome P450 pathway, CY1A1, CYP1A2, Gstm1, and Akr7a3 were predicted to be key genes in angelicin and psoralen hepatotoxicity. The endoplasmic reticulum too was predicted to play a role in angelicin and psoralen liver injury (Song et al., 2019). On the other hand, hepatotoxicity by angelicin was reported only in rats and not mice. A study on Winstar rats (fed with 40 and 80 mg/kg angelicin) and ICR mice (fed with 160 and 320 mg/kg) showed that the rats experienced hallmarks of cytotoxicity while the mice did not (Wang et al., 2019c). This shows that the hepatotoxicity of the liver by angelicin might occur in some species but not others. Nonetheless, care should be taken if angelicin were to be developed further into treatment.
In addition to studying osteoblasts, the effect of angelicin on chondrogenic differentiation was also analyzed. During the development of embryos, a process known as endochondral ossification forms bones of axial skeleton via a cartilage intermediate (Barry et al., 2001). It is during this process that mesenchymal cells condensate bi-potential chondro-osteoprogenitors in a pattern of future bones and chondrocytes differentiate to become cartilage templates. After proliferating and arranging themselves, the chondrocytes then differentiate into hypertrophic chondrocytes. These cells then secrete matrix vesicles which initiate cartilage calcification. Nearby bi-potential prechondrial cells also proceed to differentiate to osteoblasts and begin the formation of bones (Tsang et al., 2015). An essential regulator of chondrogenesis is SRY-box transcription factor 9 (SOX9). One of the many roles of SOX9 is to regulate the expression of collagen, such as collagen type II and X, and proteoglycan aggrecan in chondrogenic differentiation (Bell et al., 1997; Bi et al., 1999; Han and Lefebvre, 2008; Tsang et al., 2015). To determine that, pre-chondrogenic ATDC5 cells were treated with angelicin (Li et al., 2012). Despite no increase in cell growth rate being seen, the number of cartilage nodules, ALP and matrix proteoglycan were increased. The expression of chondrogenic differentiation marker genes such as SOX9, collagen II, collagen X, and BMP-2 were upregulated as well (Li et al., 2012). Even Smad 4, part of another independent pathway from SOX9, was induced (Li et al., 2012; Lim et al., 2015). Hence, it can be said that the TGF-β/BMP pathway might be involved in angelicin-induced chondrogenic differentiation via two separate paths, SOX9 and Smad4. Besides that, β-catenin and Runx2 were also reported to be upregulated together with OCN and BSP (Li et al., 2012). Although both β-catenin and Runx2 had previously been reported to be inhibited by SOX9, the expression of both these proteins might indicate the gradual shift from the initial proliferation and differentiation of chondroprogenitors toward the terminal differentiation of chondrogenesis, leading to cartilage matrix calcification and vascular invasion, and finally ossification (Goldring et al., 2006; Dy et al., 2012). According to Goldring et al. (2006), the expression of Runx2 increases at terminal differentiation and maintains its expression through the process of cartilage matrix calcification and vascular invasion, where β-catenin was then upregulated as well. This shows that the prechondrogenic ATDC5 is undergoing chondrogenic differentiation toward ossification under the influence of angelicin.
Other than studying the chondrogenic differentiation markers, the activation of well-known MAPK kinases (JNK, ERK, and p38) were studied as well. After treatment of the cells with angelicin, there was a marked increase in the phosphorylation of ERK and p38. However, there were no changes in the expression of pJNK. This shows that there is an activation of ERK and p38 by angelicin. Then to confirm that both these kinases are involved in angelicin induced chondrogenic differentiation, the kinases were inhibited using MEK/ERK and p38 inhibitors. With the inhibition of both these kinases separately, the number of cartilage nodules and ALP activity decreased even though the cells were co-treated with angelicin (Li et al., 2012). Hence, this indicates that angelicin mediates chondrogenic differentiation via the MAPK pathway.
In summary, it can be suggested that angelicin not only induce osteogenic differentiation but also able to initiate chondrogenic differentiation. In this process, several pathways such as TGF-β/BMP, Wnt/β-catenin, AhR, and MAPK pathway were reported to be activated by angelicin. On the other hand, the suppression of adipogenesis, bone resorption gene markers and ROS by angelicin also aid in the increase osteogenic differentiation. A summary of the various pathways involved can be seen in Figure 7.
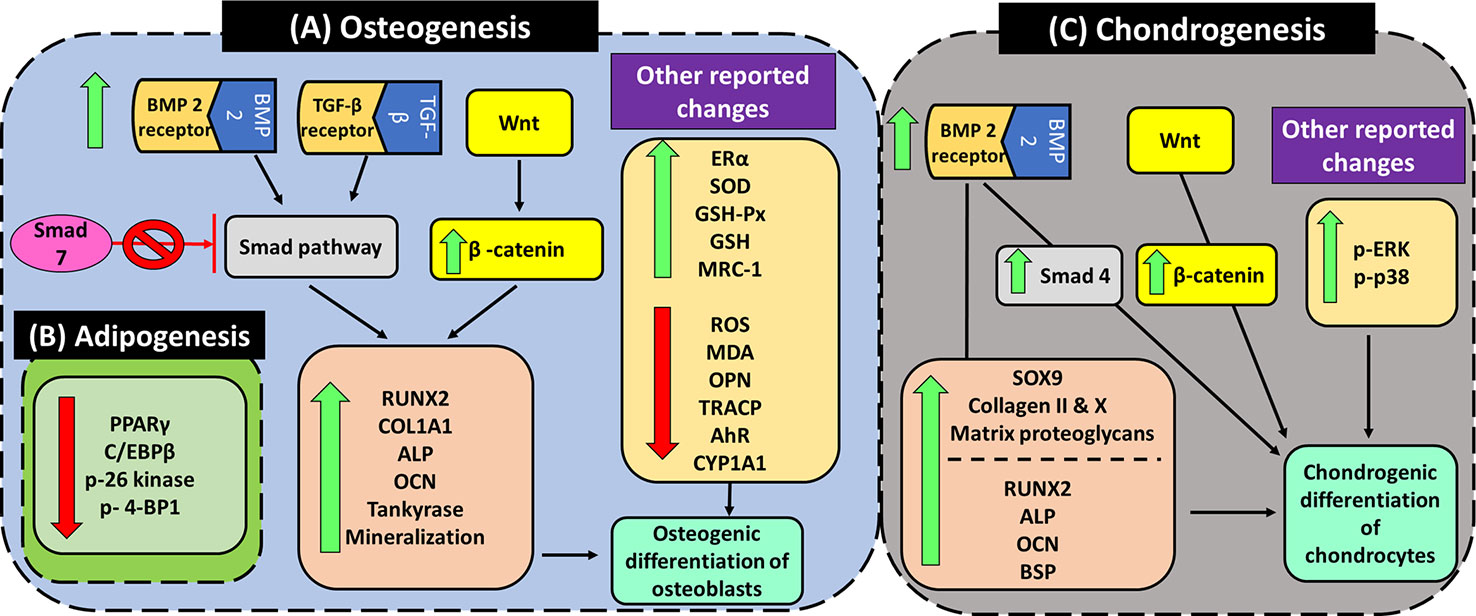
Figure 7 Angelicin exhibits pro-osteogenic, chondrogenic differentiating properties while suppressing adipogenic differentiation. (A) Several genes and proteins were upregulated by angelicin in the osteogenic differentiation via TGF-β/BMP, Wnt/β-catenin pathway and AhR pathway. It is also able to attenuate oxidative stress by upregulating antioxidant enzymes, inhibiting bone resorption. Other markers like ERα are upregulated as well by angelicin. (B) The suppression of adipogenesis by angelicin through the regulation of the mTOR pathway. (C) The induction of angelicin in chondrogenic differentiation via the Wnt/β-catenin, MAPK, and TGF-β/BMP pathway.
In 2018, a new potential application for angelicin on periodontitis was investigated. This study not only involved the anti-inflammatory studies of angelicin but also explored its anti-microbial properties on Porphyromonas gingivalis (P. gingivalis). P. gingivalis is a gram-negative oral anaerobe that can induce inflammation in the periodontal tissue. This causes gum retrogression, bone weakness, and damage to the soft tissue. Advanced periodontitis can even cause tooth loss. What makes P. gingivalis unique is its ability to elude the immune response (Rafiei et al., 2017). In a study done by Li et al. (2018), angelicin was seen to be able to inhibit, reduce the formation and decrease the thickening of P. gingivalis biofilm. The minimal inhibitory and reduction concentration of angelicin against P. gingivalis biofilm was also surprisingly lower than psoralen. Besides that, angelicin was also able to decrease the viable counts of the bacteria either alone or in biofilm, while remaining non-cytotoxic toward human periodontal ligament cells (hPDLCs) and monocyte -like THP-1 cells. Angelicin was also able to significantly reduce the mRNA transcription and protein translation of inflammatory markers IL-1β and IL-8 in THP-1 cells while increasing osteogenic differentiation and the expression of osteogenic genes in hPDLCs cells (Li et al., 2018). This increase in the osteogenic differentiation and osteogenic genes expression in hPDLCs cells might also be due to angelicin itself as an osteogenesis promoter. In vivo tests on mice also mirrored results of the in vitro tests with histological images showing reduction of alveolar bone loss (Li et al., 2018).
Moreover, angelicin was reported to have anti-viral properties. In the research done by Coppey et al. (1979); Altamirano-Dimas et al. (1986) and Hudson and Towers (1988), angelicin had shown anti-viral activity against murine cytomegalovirus (MCMV),Sindbis virus (SV), bacteriophage T4, bacteriophage M13, and herpes simplex virus (HSV) under UVA exposure. Although its anti-viral activity level is not as strong as 8-MOP, it was suggested by Altamirano-Dimas et al. (1986) that the formation of monoadducts could still cause the viral genome to become non-infectious. Following these initial findings, angelicin was tested against the murine gammaherpesvirus 68 (MHV-68), which is biologically and genetically related to the human version. In this study, the replication of viral genome was decreased dose-dependently by angelicin as the compound inhibits the expression of replication and transcription (RTA) mRNA, which is responsible for the induction of the virus's lytic cycle, at the early stages of virus infection (Ragoczy and Miller, 1999; Cho et al., 2013). This, in turn, downregulates the expression of both tegument protein ORF45 and small capsid protein ORF65, both of which are late gene protein expression known to be trans-activated by RTA (Cho et al., 2013; Purushothaman et al., 2015; Wang et al., 2015b). When tested on plaque formation, angelicin was found to deter the formation of plaques after infection (post-treatment) but not before virus adoption. Although angelicin is not as effective as the positive control ganciclovir (GCV), which is a common anti-viral drug used against herpesviruses, it was much more effective as compared to psoralen. Further tests were also done on two famous strains of gammaherpesvirus; Kaposi's sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV). An inhibition by angelicin on the early stages of lytic replication was also experienced by both strains, similar to MHV-68. However, psoralen shows a better inhibition of KSHV than angelicin (Cho et al., 2013).
Besides that, the erythroid differentiation of angelicin was also investigated. Globally, 2 in 1000 individuals are afflicted with haemoglobinopathies and there is a frequency of over 4.5% of the human population who are carriers of this disease. Beta-thalassemia and sickle cell anaemia (SCA) are the most common blood disorders and are autosomal recessive diseases (De Rycke et al., 2001). To counter these blood disorders, the production of fetal haemoglobin (HbF) must be increased as it had been found that individuals with higher levels of HbF showed milder or alleviated symptoms (Stamatoyannopoulos et al., 1975; Uda et al., 2008; Xu et al., 2011). In an attempt to increase the HbF levels in SCA and thalassemia patients, drugs such as hydroxyurea, mithramycin and psoralen were used. These drugs were able to increase the HbF production by inducing an increase in γ-globin mRNA production with hydroxyurea being the most common drug to be used in high-income countries (Fibach et al., 2003; Dixit et al., 2005; Viola et al., 2008; Mulaku et al., 2013). In a study, angelicin displayed an ability to induce erythroid differentiation in K562 cells which was associated with the increase in the production of γ-globin mRNA. It was also noted that the ability of angelicin to stimulate γ-globin mRNA was higher than hydroxyurea, in the two-phase liquid cultural model, though it is not as strong as compared to mithamycin and cytosine arabinoside (Ara-C) (Lampronti et al., 2003). Another interesting study was also done on the photoproducts of angelicin which was obtained through pre-irradiation of angelicin before its application on K562 cells had shown that under anaerobic conditions, the photoproducts are also able to induce erythroid differentiation. Interestingly, in the two-phase liquid culture model, angelicin photoproducts showed even higher fold increases of adult haemoglobin (HbA) and HbF as compared to both 8-MOP and 5-MOP (Viola et al., 2008). This study is certainly intriguing and warrants further in vitro and in vivo studies not only on angelicin itself but also on its photoproducts.
To sum it up, more studies including in vivo studies are needed to be done on the anti-viral and erythroid differentiating properties of angelicin, hence to help us further understand their underlying mechanisms. Nevertheless, the study of angelicin on periodontitis also uncovered a new potential bioactivity, which is its anti-microbial properties. To date, only Li and colleagues had reported its anti-microbial activity. As angelicin is developed as part of a plant's self-defence mechanism, angelicin may possess anti-microbial activity not just only against P. gingivalis but also other pathogenic bacteria as well. Thus, research on its anti-microbial activity should also be looked into. A summary of angelicin's anti-periodontitis, anti-viral, and erythroid differentiating properties can be seen in Figure 8.
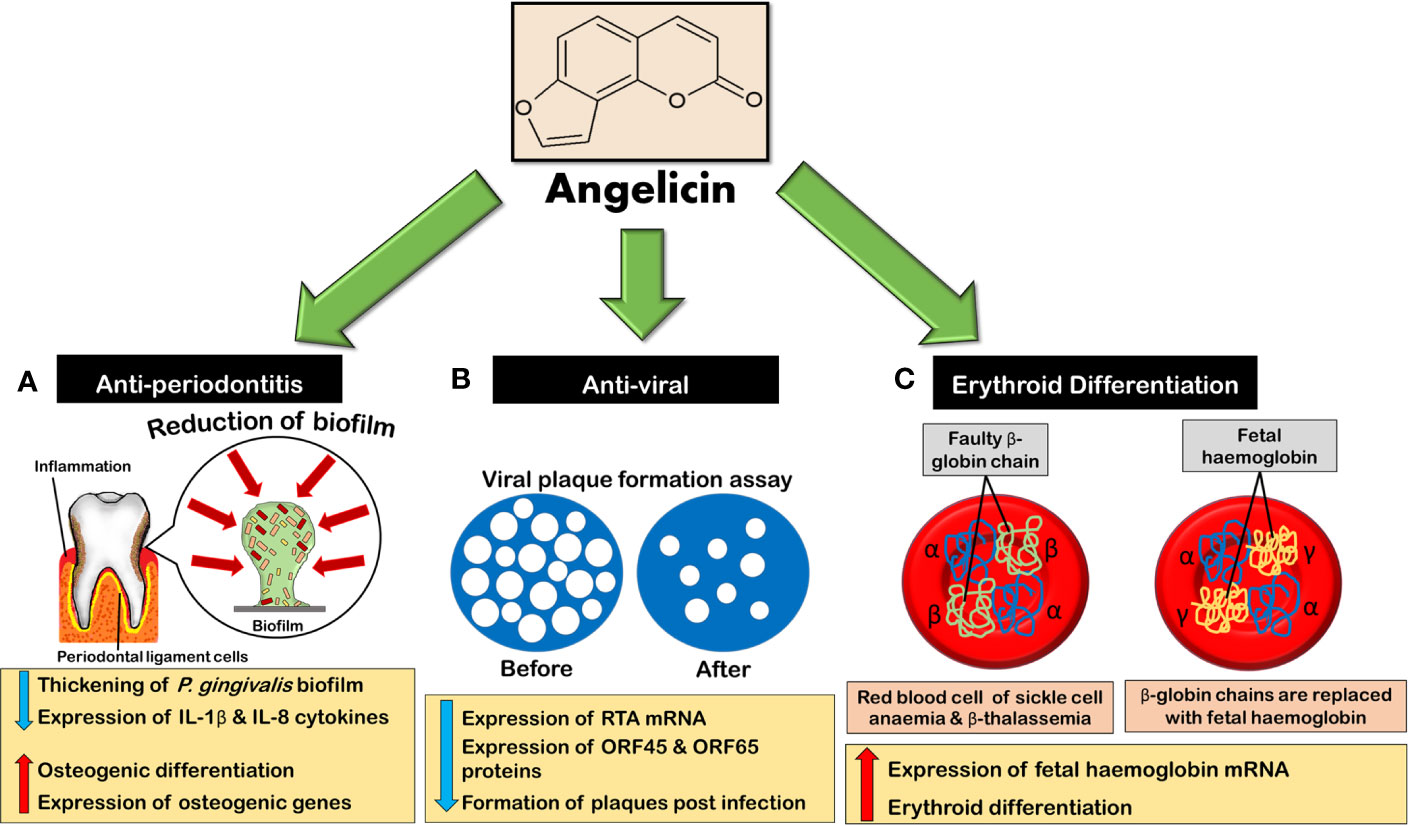
Figure 8 Angelicin exhibits anti-periodontitis, anti-viral and erythroid differentiating properties. (A) Angelicin reduces and inhibits the thickening of P. gingivalis biofilm while inhibiting the expression of pro-inflammatory cytokines. Besides that, angelicin also promotes osteogenic differentiation and an increase in the expression of osteogenic genes in hPDLCs. (B) Angelicin inhibits murine gammaherpesvirus 68 lytic cycle at early stages of infection by affecting the expression of RTA mRNA and thus indirectly the expression of ORF45 and ORF65 proteins, decreasing the formation of plaques. (C) Erythroid differentiation increases as angelicin and its photoproduct increase the expression of fetal haemoglobin mRNA.
According to “Scifinder”, not many patents for angelicin had been made. Out of 11,663 hits on “Scifinder” for the word “Angelicin”, only 59 were classified as patents. But even so, not more than 10 contain angelicin as the main ingredient for commercial use. Through compilation, the main use of angelicin described in patents for pharmaceutical drugs are for thalassemia and sickle cell treatment, vaccines for herpes virus and preparation material for hyperlipemia medicine (Bianchi et al., 2003; Bianchi et al., 2008; Song et al., 2010; Spector et al., 2012; Li et al., 2017b). Through the brief description of the patents, angelicin was able to decrease the content of total cholesterol, low-density lipoprotein cholesterol, and triglycerides in rats. It can also increase high-density lipoprotein cholesterol (Li et al., 2017b). Angelicin has also been patented as an active ingredient for a herbicide component (Kim et al., 2009). Through these patents, it can be seen how under-researched angelicin is even though it demonstrates great potential in in vitro and in vivo studies.
For future prospects, studies on improving angelicin drug delivery, such as the use of nanoparticles should be done to increase its delivery efficacy while reducing unwanted side effects. For example, angelicin can be encapsulated in nanoparticles to improve its anti-cancer properties. In a study done by Boondireke et al. (2019), hydrophobic monomyristin encapsulated in dextran-covered polylactide nanoparticle, which had a superficial layer of conjugated protein transferrin, had showed increased cytotoxicity against HeLa cells in comparison to non-encapsulated monomyristin. Another method was utilizing photosensitive nanoparticles to change the microenvironment of tumors, making it more susceptible to anti-cancer treatments. To combat tumor hypoxia, Lan et al. (2018) developed novel nanoscale metal-organic based nanophotosensitizers, Fe-TBP. Fe-TBP was made from 5,10,15,20-tetra(p-benzoato)porphyrin (TBP) and Fe3O clusters, which catalyzes intracellular H2O2 to O2 via Fenton-like reaction when irradiated. The O2 was then converted by photoexcited porphyrins to cytotoxic singlet oxygen (1O2). The end results were significant improvement of anti-programmed death-ligand 1 (α-PD-L1) treatment efficacy via the increasing tumor infiltration by cytotoxic T cells, prompting abscopal effects in mouse colorectal cancer. In a recent study, psoralen that had been encapsulated in polymer -lipid hybrid nanocarrier had not only displayed improved water solubility but also displayed an increased inhibitory effect against MCF-7 tumors in vivo as compared to non-encapsulated psoralen. This is due to the passive accumulation of encapsulated psoralen in the tumor and extended circulation time, which brings about a lengthened exposure of the psoralen toward the tumor (Du et al., 2019). Gut microbes also metabolize the consumed psoralen and angelicin, therefore encapsulation might improve the transfer of angelicin to the targeted site (Liu et al., 2019). Similar usage of nanoformulations containing arsenic sulphide had also been used on chronic myeloid leukemia cells to induce erythroid differentiation (Wang et al., 2019a), while Pabari et al. (2019) had utilized biodegradable polyesterurethane and PEGylated polymers to form nanocarriers of infliximab in the treatment against inflammatory cytokines in monocytes for 24 h. As can be seen, nanoparticles or nanoformulations can mediate a more targeted, effective and controlled release of drugs. It can also be used to improve the microenvironment in deep parts of tumors to increase the efficacy of drugs. Hence, its incorporation with anti-cancer, anti-inflammatory and erythroid differentiation angelicin studies should be considered.
In addition, research on the effect of angelicin on osteoclasts and other disease models, such as bone fractures and osteoarthritis, should be done as well. Psoralen had been reported to speed up the healing of fractures on the tibia of rats by activating osteoclasts and osteoblasts through ERK signaling (Zhang et al., 2019). As psoralen and angelicin are isomers of each other, it would prove interesting to see the effect of angelicin on bone fractures. Lately, there was a publication reporting that neural EGFL like 1 (NELL-1)-haploinsufficient mice displayed increased inflammatory markers and symptoms of aggravated and accelerated osteoarthritis (Li et al., 2020). Li et al. (2020) had also reported that NELL-1 mediates a decrease of inflammatory marker IL1β by increasing the Runx1 gene in chondrocytes. Therefore, there is a possibility that angelicin might be able to reverse the effect of osteoarthritis as previous studies had shown that angelicin increases Runx2 transcription and also has anti-inflammatory properties. To confirm this hypothesis, further studies of angelicin on osteoarthritis model needs to be done. On another note, periodontitis is not only caused by persistent microbial infections but also viral infections by the Herpesviridae family of viruses (Chen et al., 2020). Saliva samples of patients with apical periodontitis had detected the presence of EBV and HCMV (Jakovljevic et al., 2018). With angelicin having anti-viral properties, the effect of angelicin on the virus population found in periodontitis can be explored. Different virus strains too should be tested against angelicin to see if angelicin is able to inhibit a wide range of viruses.
As previously reported, angelicin forms monoadducts in the DNA under UV irradiation. Studies have shown modulation of transcription factors such as NF-κB and Runx after the application of angelicin. It is possible that these changes in expression could be due to the interaction of angelicin with DNA or RNA molecules. However, this has yet to be elucidated and more in-depth studies are needed. Interestingly, a study on 4,6,4-trimethylangelicin (4,6,4-TMA), a derivative of angelicin, showed inhibitory effects on NF-κB/DNA interaction. Using electrophoretic mobility shift assay, the angelicin analogues successfully suppressed the interaction of NF-κB p50 and double-stranded oligonucleotides, which was designed to mimic the NF-κB consensus sequence (Lampronti et al., 2017). To verify if angelicin can also directly inhibit the binding of NF-κB to DNA, experimental research, molecular docking, and molecular dynamics simulation studies can be performed to understand their interaction. Similarly, the interaction of angelicin with other regulatory genes, transcription factors and receptors could be explored in future research.
In summary, angelicin shows great promise in various aspects especially in its anti-cancer, anti-inflammatory, and pro-osteogenic properties. Besides that, further studies involving the drug delivery of angelicin may improve the effect of angelicin toward the targeted sites in cancer, inflammation and erythroid differentiation models. A deeper understanding of the effect of angelicin in the mechanism of bone modeling and other bone-related models are also needed. Other than that, knowledge on the anti-viral implications of angelicin in periodontitis and the interaction of angelicin with regulatory genes can be expanded.
The writing was performed by CM, LT, PP, LHL, and BG. While WY, WL, LHL, ST, KC, LEL and BG provided vital guidance and insight to the work. The project was conceptualized by BG.
This work was inspired by Monash Pharmacy Degree Course, Unit PAC3512 which entitled “Current aspects of pharmaceutical research” and financially supported by Taylor's University Emerging Grant (TRGS/ERFS/2/2018/SBS/016), University of Malaya Research Grant (FRGS grant to KGC grant no: FP022-2018A), External Industry Grant from Biotek Abadi Sdn Bhd (vote no. GBA-81811A), Monash Global Asia in the 21st Century (GA21) research grants (GA-HW-19-L01 & GA-HW-19-S02), and Fundamental Research Grant Scheme (FRGS/1/2019/WAB09/MUSM/02/1 & FRGS/1/2019/SKK08/MUSM/02/7).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acharya, R., Chacko, S., Bose, P., Lapenna, A., Pattanayak, S. P. (2019). Structure based multitargeted molecular docking analysis of selected furanocoumarins against breast cancer. Sci. Rep. 9 (1), 15743. doi: 10.1038/s41598-019-52162-0
Altamirano-Dimas, M., Hudson, J. B., Towers, G. H. N. (1986). Induction of cross-links in viral DNA by naturally occurring photosensitizers. Photochem. Photobiol. 44 (2), 187–192. doi: 10.1111/j.1751-1097.1986.tb03584.x
Ashkenazi, A. (2008). Targeting the extrinsic apoptosis pathway in cancer. Cytokine. Growth Factor. Rev. 19 (3), 325–331. doi: 10.1016/j.cytogfr.2008.04.001
Atalay, S., Elci, A., Kayadibi, H., Onder, C. B., Aka, N. (2012). Diagnostic utility of osteocalcin, undercarboxylated osteocalcin, and alkaline phosphatase for osteoporosis in premenopausal and postmenopausal women. Ann. Lab. Med. 32 (1), 23–30. doi: 10.3343/alm.2012.32.1.23
Azzouzi, S., Zaabat, N., Medjroubi, K., Akkal, S., Benlabed, K., Smati, F., et al. (2014). Phytochemical and biological activities of Bituminaria bituminosa L. (Fabaceae). Asian Pac. J. Trop. Med. 7, S481–S484. doi: 10.1016/S1995-7645(14)60278-9
Bahadori, M. B., Dinparast, L., Zengin, G. (2016). The genus Heracleum: a comprehensive review on its phytochemistry, pharmacology, and ethnobotanical values as a useful herb. Compr. Rev. Food Sci. Food Saf. 15 (6), 1018–1039. doi: 10.1111/1541-4337.12222
Bandeira Reidel, R. V., Giovanelli, S., Pipitone, A., Minissale, P., Pistelli, L. (2017). Phytochemical study of Bituminaria basaltica aerial parts, an Italian endemism. Nat. Prod. Res. 31 (20), 2375–2380. doi: 10.1080/14786419.2017.1308368
Barry, F., Boynton, R. E., Liu, B., Murphy, J. M. (2001). Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp. Cell Res. 268 (2), 189–200. doi: 10.1006/excr.2001.5278
Baskin, J. M., Baskin, C. M. (1979). Studies on the autecology and population biology of the weedy monocarpic perennial, Pastinaca sativa. J. Ecol. 67 (2), 601–610. doi: 10.2307/2259115
Bell, D. M., Leung, K. K. H., Wheatley, S. C., Ng, L. J., Zhou, S., Ling, K. W., et al. (1997). SOX9 directly regulates the type-ll collagen gene. Nat. Gen. 16 (2), 174–178. doi: 10.1038/ng0697-174
Ben-Hur, E., Elkind, M. M. (1973). Psoralen plus near ultraviolet light inactivation of cultured chinese hamster cells and its relation to DNA cross-links. Mutat. Res. 18 (3), 315–324. doi: 10.1016/0027-5107(73)90216-9
Berenbaum, M. R., Nitao, J. K., Zangerl, A. R. (1991). Adaptive significance of furanocoumarin diversity in Pastinaca sativa (Apiaceae). J. Chem. Ecol. 17, 207–215. doi: 10.1007/bf00994434
Betz, C., Hall, M. N. (2013). Where is mTOR and what is it doing there? J. Cell Biol. 203 (4), 563–574. doi: 10.1083/jcb.201306041
Bi, W., Deng, J. M., Zhang, Z., Behringer, R. R., de Crombrugghe, B. (1999). Sox9 is required for cartilage formation. Nat. Gen. 22 (1), 85–89. doi: 10.1038/8792
Bianchi, N., Borgatti, M., Gambari, R., Lampronti, I. (2003). Use of angelicin and its structural analogs for the treatment of thalassemia, Italy patent application WO 2003-IB3462.
Bianchi, N., Borgatti, M., Gambari, R., Lampronti, I., Dall'acqua, F., Vedaldi, D., et al. (2008). Drug for treating thalassemia, falciform anemia and all other forms of anemia treatable therewith, Italy patent application EP 2008-100891.
Boondireke, S., Léonard, M., Durand, A., Thanomsub Wongsatayanon, B. (2019). Encapsulation of monomyristin into polymeric nanoparticles improved its in vitro antiproliferative activity against cervical cancer cells. Colloids. Surf. B: Biointerfaces 176, 9–17. doi: 10.1016/j.colsurfb.2018.12.062
Bordin, F., Carlassare, F., R. Baccichetti, F., Anselmo, L. (1976). DNA repair and recovery in Escherichia coli after psoralen and angelicin photosensitization. Biochim. Biophys. Acta 447 (3), 249–259. doi: 10.1016/0005-2787(76)90048-4
Bordin, F., Dall'acqua, F., Guiotto, A. (1991). Angelicins, angular analogs of psoralens: chemistry, photochemical, photobiological and phototherapeutic properties. Pharmacol. Ther. 52 (3), 331–363. doi: 10.1016/0163-7258(91)90031-G
Chandra, S. (2014). Importance of pharmacognostic study of medicinal plants: an overview. J. Pharmacogn. Phytochem. 2 (5), 69–73.
Chaudhary, S. K., Ceska, O., Warrington, P. J., Ashwood-Smith, M. J. (1985). Increased furocoumarin content of celery during storage. J. Agric. Food Chem. 33 (6), 1153–1157. doi: 10.1021/jf00066a032
Chen, G., Deng, C., Li, Y.-P. (2012). TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 8 (2), 272–288. doi: 10.7150/ijbs.2929
Chen, C.-H., Hwang, T.-L., Chen, L.-C., Chang, T.-H., Wei, C.-S., Chen, J.-J. (2017). Isoflavones and anti-inflammatory constituents from the fruits of Psoralea corylifolia. Phytochemistry 143, 186–193. doi: 10.1016/j.phytochem.2017.08.004
Chen, C., Feng, P., Slots, J. (2020). Herpesvirus-bacteria synergistic interaction in periodontitis. Periodontol. 2000 82 (1), 42–64. doi: 10.1111/prd.12311
Cho, H.-J., Jeong, S.-G., Park, J.-E., Han, J.-A., Kang, H.-R., Lee, D., et al. (2013). Antiviral activity of angelicin against gammaherpesviruses. Antiviral Res. 100 (1), 75–83. doi: 10.1016/j.antiviral.2013.07.009
Choi, J.-G., Jin, Y.-H., Kim, J.-H., Oh, T. W., Yim, N.-H., Cho, W.-K., et al. (2016). In vitro anti-viral activity of Psoraleae semen water extract against Influenza A viruses. Front. Pharmacol. 7, 460. doi: 10.3389/fphar.2016.00460
Christiansen, C., Christensen, M. S., Larsen, N.-E., Transbøl, I. B. (1982). Pathophysiological mechanisms of estrogen effect on bone metabolism. Dose-response relationships in early postmenopausal women. J. Clin. Endocrinol. Metab. 55 (6), 1124–1130. doi: 10.1210/jcem-55-6-1124
Coombes, E., Jiang, J., Chu, X.-P., Inoue, K., Seeds, J., Branigan, D., et al. (2011). Pathophysiologically Relevant Levels of Hydrogen Peroxide Induce Glutamate-Independent Neurodegeneration That Involves Activation of Transient Receptor Potential Melastatin 7 Channels. Antioxid. Redox Signal. 14 (10), 1815–1827. doi: 10.1089/ars.2010.3549.
Coppey, J., Averbeck, D., Moreno, G. (1979). Herpes virus production in monkey kidney and human skin cells treated with angelicin or 8-methoxypsoralen plus 365 nm light. Photochem. Photobiol. 29 (4), 797–801. doi: 10.1111/j.1751-1097.1979.tb07769.x
De Rycke, M., Van De Velde, H., Sermon, K., Lissens, W., De Vos, A., Vandervorst, M., et al. (2001). Preimplantation genetic diagnosis for sickle-cell anemia and for β-thalassemia. Prenat. Diagn. 21 (3), 214–222. doi: 10.1002/1097-0223(200103)21:3<214::aid-pd51>3.0.co;2-4
Dehghan, H., Sarrafi, Y., Salehi, P., Ebrahimi, S. N. (2017). α-Glucosidase inhibitory and antioxidant activity of furanocoumarins from Heracleum persicum. Med. Chem. Res. 26 (4), 849–855. doi: 10.1007/s00044-017-1796-y
Del Rio, D., Stewart, A. J., Pellegrini, N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15 (4), 316–328. doi: 10.1016/j.numecd.2005.05.003
Delescluse, C., Lemaire, G., de Sousa, G., Rahmani, R. (2000). Is CYP1A1 induction always related to AHR signaling pathway? Toxicology 153 (1-3), 73–82. doi: 10.1016/s0300-483x(00)00305-x
Dincel, D., Hatipoğlu, S. D., Gören, A. C., Topçu, G. (2013). Anticholinesterase furocoumarins from Heracleum platytaenium, a species endemic to the Ida Mountains. Turk. J. Chem. 37, 675– 683. doi: 10.3906/kim-1303-55
Dixit, A., Chatterjee, T. C., Mishra, P., Choudhry, D. R., Mahapatra, M., Tyagi, S., et al. (2005). Hydroxyurea in thalassemia intermedia—a promising therapy. Ann. Hematol. 84 (7), 441–446. doi: 10.1007/s00277-005-1026-4
Downward, J. (2004). PI 3-kinase, Akt and cell survival. Sem. Cell Dev. Biol. 15 (3), 177–182. doi: 10.1016/j.semcdb.2004.01.002
Du, M., Ouyang, Y., Meng, F., Zhang, X., Ma, Q., Zhuang, Y., et al. (2019). Polymer-lipid hybrid nanoparticles: a novel drug delivery system for enhancing the activity of Psoralen against breast cancer. Int. J. Pharm. 561, 274–282. doi: 10.1016/j.ijpharm.2019.03.006
Dueholm, B., Krieger, C., Drew, D., Olry, A., Kamo, T., Taboureau, O., et al. (2015). Evolution of substrate recognition sites (SRSs) in cytochromes P450 from Apiaceae exemplified by the CYP71AJ subfamily. BMC Evol. Biol. 15, 122. doi: 10.1186/s12862-015-0396-z
Dy, P., Wang, W., Bhattaram, P., Wang, Q., Wang, L., Ballock, R. T., et al. (2012). Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev. Cell 22 (3), 597–609. doi: 10.1016/j.devcel.2011.12.024
Falasca, M. (2010). PI3K/Akt signalling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr. Pharm. Des. 16 (12), 1410–1416. doi: 10.2174/138161210791033950
Fibach, E., Bianchi, N., Borgatti, M., Prus, E., Gambari, R. (2003). Mithramycin induces fetal hemoglobin production in normal and thalassemic human erythroid precursor cells. Blood 102 (4), 1276–1281. doi: 10.1182/blood-2002-10-3096
Fillmore, C. M., Gupta, P. B., Rudnick, J. A., Caballero, S., Keller, P. J., Lander, E. S., et al. (2010). Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc. Natl. Acad. Sci. U.S.A. 107 (50), 21737–21742. doi: 10.1073/pnas.1007863107
Fulda, S., Debatin, K. M. (2006). Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25 (34), 4798–4811. doi: 10.1038/sj.onc.1209608
Gaur, T., Lengner, C. J., Hovhannisyan, H., Bhat, R. A., Bodine, P. V. N., Komm, B. S., et al. (2005). Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280 (39), 33132–33140. doi: 10.1074/jbc.M500608200
Ge, L., Cui, Y., Cheng, K., Han, J. (2018). Isopsoralen enhanced osteogenesis by targeting AhR/ERα. Molecules 23 (10), 2600. doi: 10.3390/molecules23102600
Ge, L., Cui, Y., Liu, B., Yin, X., Pang, J., Han, J. (2019). ERα and Wnt/β−catenin signaling pathways are involved in angelicin−dependent promotion of osteogenesis. Mol. Med. Rep. 19 (5), 3469–3476. doi: 10.3892/mmr.2019.9999
Gersbach, C. A., Byers, B. A., Pavlath, G. K., García, A. J. (2004). Runx2/Cbfa1 stimulates transdifferentiation of primary skeletal myoblasts into a mineralizing osteoblastic phenotype. Exp. Cell Res. 300 (2), 406–417. doi: 10.1016/j.yexcr.2004.07.031
Gibernau, M., Buser, H. R., Frey, J. E., Hossaert-Mckey, M. (1997). Volatile compounds from extracts of figs of Ficus carica. Phytochemistry 46 (2), 241–244. doi: 10.1016/S0031-9422(97)00292-6
Girennavar, B., Jayaprakasha, G. K., Patil, B. S. (2007). Potent inhibition of human cytochrome P450 3A4, 2D6, and 2C9 isoenzymes by grapefruit juice and its furocoumarins. J. Food Sci. 72 (8), C417–C421. doi: 10.1111/j.1750-3841.2007.00483.x
Global Burden of Disease Cancer Collaboration (2015). The global burden of cancer 2013. JAMA Oncol. 1 (4), 505–527. doi: 10.1001/jamaoncol.2015.0735
Goldring, M. B., Tsuchimochi, K., Ijiri, K. (2006). The control of chondrogenesis. J. Cell. Biochem. 97 (1), 33–44. doi: 10.1002/jcb.20652
Golub, E. E., Boesze-Battaglia, K. (2007). The role of alkaline phosphatase in mineralization. Cur. Opin. Orthop. 18 (5), 444–448. doi: 10.1097/BCO.0b013e3282630851
Grant, E. L., Von Borstel, R. C., Ashwood-Smith, M. J. (1979). Mutagenicity of cross-links and monoadducts of furocoumarins (psoralen and angelicin) induced by 360-nm radiation in excision-repair-defective and radiation-insensitive strains of saccharomyces cerevisiae. Environ. Mol. Mutagen. 1 (1), 55–63. doi: 10.1002/em.2860010112
Ha, H., Kwak, H. B., Lee, S. W., Jin, H. M., Kim, H.-M., Kim, H.-H., et al. (2004). Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 301 (2), 119–127. doi: 10.1016/j.yexcr.2004.07.035
Halleen, J. M., Räisänen, S., Salo, J. J., Reddy, S. V., Roodman, G. D., Hentunen, T. A., et al. (1999). Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J. Biol. Chem. 274 (33), 22907–22910. doi: 10.1074/jbc.274.33.22907
Halleen, J. M., Räisänen, S. R., Alatalo, S. L., Väänänen, H. K. (2003). Potential function for the ROS-generating activity of TRACP. J. Bone Miner. Res. 18 (10), 1908–1911. doi: 10.1359/jbmr.2003.18.10.1908
Halleen, J. M., Tiitinen, S. L., Ylipahkala, H., Fagerlund, K. M., Väänänen, H. K. (2006). Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin. Lab. 52 (9-10), 499–509.
Hamerski, D., Matern, U. (1988). Elicitor-induced biosynthesis of psoralens in Ammi majus L. suspension cultures. Eur. J.Biochem. 171 (1-2), 369–375. doi: 10.1111/j.1432-1033.1988.tb13800.x
Han, Y., Lefebvre, V. (2008). L-Sox5 and Sox6 drive expression of the aggrecan gene in cartilage by securing binding of Sox9 to a far-upstream enhancer. Mol. Cell. Biol. 28 (16), 4999–5013. doi: 10.1128/MCB.00695-08
Han, M. A., Lee, D. H., Woo, S. M., Seo, B. R., Min, K.-J., Kim, S., et al. (2016). Galangin sensitizes TRAIL-induced apoptosis through down-regulation of anti-apoptotic proteins in renal carcinoma Caki cells. Sci. Rep. 6, 18642. doi: 10.1038/srep18642
Hanahan, D., Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144 (5), 646–674. doi: 10.1016/j.cell.2011.02.013
Hayrapetyan, A., Jansen, J. A., van den Beucken, J. J. J. P. (2014). Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng. Part B Rev. 21 (1), 75–87. doi: 10.1089/ten.teb.2014.0119
Hendrix, S. (1984). Variation in seed weight and its effects on germination in Pastinaca sativa L. (Umbelliferae). Am. J. Bot. 71 (6), 795–802. doi: 10.2307/2443470
Hudson, J. B., Towers, G. H. N. (1988). Antiviral properties of photosensitizers. Photochem. Photobiol. 48 (3), 289–296. doi: 10.1111/j.1751-1097.1988.tb02823.x
Ikram, A., Zahra, N. B., Shinwari, Z. K., Qaisar, M. (2015). Ethnomedicinal review of folklore medicinal plants belonging to family Apiaceae of Pakistan. Pak. J. Bot. 47 (3), 1007–1014.
Jakovljevic, A., Knezevic, A., Nikolic, N., Soldatovic, I., Jovanovic, T., Milasin, J., et al. (2018). Herpesviruses viral loads and levels of proinflammatory cytokines in apical periodontitis. Dis. 24 (5), 840–846. doi: 10.1111/odi.12829
Jordan, M. A., Wilson, L. (2004). Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4 (4), 253–265. doi: 10.1038/nrc1317
Joshi, P. C., Pathak, M. A. (1983). Production of singlet oxygen and superoxide radicals by psoralens and their biological significance. Biochem. Biophys. Res. Commun. 112 (2), 638–646. doi: 10.1016/0006-291x(83)91511-5
Karamat, F., Olry, A., Munakata, R., Koeduka, T., Sugiyama, A., Paris, C., et al. (2013). A coumarin-specific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley. Plant J. 77 (4), 627–638. doi: 10.1111/tpj.12409
Kim, Y. C., Guan, K.-L. (2015). mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 125 (1), 25–32. doi: 10.1172/JCI73939
Kim, S. M., Cho, N. G., Lee, S. E., Yoo, J. M. (2009). Angelicin isolated from Rumex crispus and having weeding activity. South Korea patent application KR 2009-62536.
Kim, Y. J., Lim, H.-S., Lee, J., Jeong, S.-J. (2016). Quantitative analysis of Psoralea corylifolia Linne and its neuroprotective and anti-neuroinflammatory effects in HT22 hippocampal cells and BV-2 microglia. Molecules 21 (8), 1076. doi: 10.3390/molecules21081076
Kitamura, T., Otsubo, K. (2012). Palladium-catalyzed intramolecular hydroarylation of 4-benzofuranyl alkynoates. Approach to angelicin derivatives. J. Org. Chem. 77 (6), 2978–2982. doi: 10.1021/jo300021a
Kovalevich, J., Langford, D. (2013). Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 1078, 9–21. doi: 10.1007/978-1-62703-640-5_2
Lampronti, I., Bianchi, N., Borgatti, M., Fibach, E., Prus, E., Gambari, R. (2003). Accumulation of gamma-globin mRNA in human erythroid cells treated with angelicin. Eur. J. Haematol. 71 (3), 189–198. doi: 10.1034/j.1600-0609.2003.00113.x
Lampronti, I., Manzione, M. G., Sacchetti, G., Ferrari, D., Spisani, S., Bezzerri, V., et al. (2017). Differential effects of angelicin analogues on NF-κB activity and IL-8 gene expression in cystic fibrosis IB3-1 cells. Mediators Inflamm. 2017, 1–11. doi: 10.1155/2017/2389487. 2389487.
Lan, G., Ni, K., Xu, Z., Veroneau, S. S., Song, Y., Lin, W. (2018). Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc 140 (17), 5670–5673. doi: 10.1021/jacs.8b01072
Landewé, R. B. M., Geusens, P., van der Heijde, D. M. F. M., Boers, M., van der Linden, S. J., Garnero, P. (2006). Arthritis instantaneously causes collagen type I and type II degradation in patients with early rheumatoid arthritis: a longitudinal analysis. Ann. Rheumatol. Dis. 65 (1), 40–44. doi: 10.1136/ard.2004.035196
Larbat, R., Hehn, A., Hans, J., Scheider, S., Jugdé, H., Schneider, B., et al. (2008). Isolation and functional characterization of CYP71AJ4 encoding for the first P450 monooxygenase of angular furanocoumarin biosynthesis. J. Biol. Chem. 284 (8), 4776–4785. doi: 10.1074/jbc.M807351200
Lee, A.-Y., Jang, J.-H. (1998). Autologous epidermal grafting with PUVA-irradiated donor skin for the treatment of vitiligo. Int. J. Dermatol. 37 (7), 551–554. doi: 10.1046/j.1365-4362.1998.00437.x
Leonti, M., Casu, L., Gertsch, J., Bonsignore, L., Floris, C., Casu, M., et al. (2010). A pterocarpan from the seeds of Bituminaria morisiana. J. Nat. Med. 64 (3), 354–357. doi: 10.1007/s11418-010-0408-7
Li, L., Eun, J.-S., Nepal, M., Ryu, J.-H., Cho, H.-K., Choi, B.-Y., et al. (2012). Isopsoralen induces differentiation of prechondrogenic ATDC5 cells via activation of MAP kinases and BMP-2 signaling pathways. Biomol. Ther. 20, 299–305. doi: 10.4062/biomolther.2012.20.3.299
Li, W. D., Yan, C. P., Wu, Y., Weng, Z. B., Yin, F. Z., Yang, G. M., et al. (2014). Osteoblasts proliferation and differentiation stimulating activities of the main components of Fructus Psoraleae corylifoliae. Phytomedicine 21 (4), 400–405. doi: 10.1016/j.phymed.2013.09.015
Li, G., He, Y., Yao, J., Huang, C., Song, X., Deng, Y., et al. (2016). Angelicin inhibits human lung carcinoma A549 cell growth and migration through regulating JNK and ERK pathways. Oncol. Rep. 36 (6), 3504–3512. doi: 10.3892/or.2016.5166
Li, X.-M., Yang, Q., Li, X.-B., Cheng, Q., Zhang, K., Han, J., et al. (2017a). Estrogen-like neuroprotection of isopsoralen against spinal cord injury through estrogen receptor ERα. Metab. Brain Dis. 32 (1), 259–265. doi: 10.1007/s11011-016-9913-z
Li, Z., Aibai, S., Dou, Q., Maimaiti, Y. (2017b). Application of angelicin in preparing medicine for treating hyperlipemia, People Republic of China patent application CN 2017-10074278.
Li, X., Yu, C., Hu, Y., Xia, X., Liao, Y., Zhang, J., et al. (2018). New application of psoralen and angelicin on periodontitis with anti-bacterial, anti-inflammatory, and osteogenesis effects. Front. Cell. Infect. Microbiol. 8, 178. doi: 10.3389/fcimb.2018.00178
Li, Y.-P., Wu, B., Liang, J., Li, F. (2019). Isopsoralen ameliorates H2O2-induced damage in osteoblasts via activating the Wnt/β-catenin pathway. Exp. Ther. Med. 18 (3), 1899–1906. doi: 10.3892/etm.2019.7741
Li, C., Zheng, Z., Ha, P., Jiang, W., Berthiaume, E. A., Lee, S., et al. (2020). Neural EGFL like 1 as a potential pro-chondrogenic, anti-inflammatory dual-functional disease-modifying osteoarthritis drug. Biomaterials 226, 1–18. doi: 10.1016/j.biomaterials.2019.119541. 119541.
Lim, J., Tu, X., Choi, K., Akiyama, H., Mishina, Y., Long, F. (2015). BMP–Smad4 signaling is required for precartilaginous mesenchymal condensation independent of Sox9 in the mouse. Dev. Biol. 400 (1), 132–138. doi: 10.1016/j.ydbio.2015.01.022
Lin, C.-F., Huang, Y. L., Chien, M. Y., Sheu, S.-J., Chen, C.-C. (2007). Analysis of bakuchiol, psoralen and angelicin in crude drugs and commercial concentrated products of Fructus Psoraleae. J. Food Drug Anal. 15 (4), 433–437.
Liu, S. F., Malik, A. B. (2006). NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 290 (4), L622–L645. doi: 10.1152/ajplung.00477.2005
Liu, B., Gao, H.-M., Wang, J.-Y., Jeohn, G.-H., Cooper, C. L., Hong, J.-S. (2002). Role of nitric oxide in inflammation-mediated neurodegeneration. Ann. N. Y. Acad. Sci. 962 (1), 318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x
Liu, F., Sun, G.-Q., Gao, H.-Y., Li, R.-S., Soromou, L.-W., Chen, N., et al. (2013). Angelicin regulates LPS-induced inflammation via inhibiting MAPK/NF-κB pathways. J. Surg. Res. 185 (1), 300–309. doi: 10.1016/j.jss.2013.05.083
Liu, L., Zhang, L., Cui, Z.-X., Liu, X.-Y., Xu, W., Yang, X.-W. (2019). Transformation of psoralen and isopsoralen by human intestinal microbial in vitro, and the biological activities of its metabolites. Molecules 24 (22), 4080. doi: 10.3390/molecules24224080
Llins, P. C., Gers, S. R. (1992). Bath-water compared with oral delivery of 8-methoxypsoralen PUVA therapy for chronic plaque psoriasis. Br. J. Dermatol. 127 (4), 392–395. doi: 10.1111/j.1365-2133.1992.tb00460.x
Lohman, D. J., McConnaughay, K. D. (1998). Patterns of defensive chemical production in wild parsnip seedlings (Apiaceae: Pastinaca sativa L.). Chemoecology 8, 195–200. doi: 10.1007/s000490050025
Lu, H., Zhang, L., Liu, D., Tang, P., Song, F. (2014). Isolation and purification of psoralen and isopsoralen and their efficacy and safety in the treatment of osteosarcoma in nude rats. Afr. Health Sci. 14 (3), 641–647. doi: 10.4314/ahs.v14i3.20
Lynch, M. P., Stein, J. L., Stein, G. S., Lian, J. B. (1995). The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp. Cell Res. 216 (1), 35–45. doi: 10.1006/excr.1995.1005
Ma, R. L., Cohen, M. B., Berenbaum, M. R., Schuler, M. A. (1994). Black swallowtail (Papilio polyxenes) alleles encode cytochrome P450S that selectively metabolize linear furanocoumarins. Arch. Biochem. Biophys. 310 (2), 332–340. doi: 10.1006/abbi.1994.1175
Mahmoodi Kordi, F., Valizadeh, H., Hosseinzade, Z., Bahadori, M. B. (2015). Furocoumarins from Heracleum rawianum in Iran. Iran. Chem. Commun. 3 (1), 1–5.
Maiuri, M. C., Zalckvar, E., Kimchi, A., Kroemer, G. (2007). Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8 (9), 741–752. doi: 10.1038/nrm2239
Marrelli, M., Menichini, F., Statti, G. A., Bonesi, M., Duez, P., Menichini, F., et al. (2012). Changes in the phenolic and lipophilic composition, in the enzyme inhibition and antiproliferative activity of Ficus carica L. cultivar Dottato fruits during maturation. Food Chem. Toxicol. 50 (3-4), 726–733. doi: 10.1016/j.fct.2011.12.025
Mathew, R., Karantza-Wadsworth, V., White, E. (2007). Role of autophagy in cancer. Nat. Rev. Cancer 7 (12), 961–967. doi: 10.1038/nrc2254
Mebratu, Y., Tesfaigzi, Y. (2009). How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 8 (8), 1168–1175. doi: 10.4161/cc.8.8.8147
Min, K. J., Um, H. J., Seo, S. U., Woo, S. M., Kim, S., Park, J. W., et al. (2018). Angelicin potentiates TRAIL- induced apoptosis in renal carcinoma Caki cells through activation of caspase 3 and down- regulation of c-FLIP expression. Drug Dev. Res. 79 (1), 3–10. doi: 10.1002/ddr.21414
Minissale, P., Brullo, C., Brullo, S., Giusso Del Galdo, G., Sciandrello, S. (2013). Bituminaria basaltica (Fabaceae), a new species from Italy. Phytotaxa 98 (1), 1–15. doi: 10.11646/phytotaxa.98.1.1
Mira, A., Shimizu, K. (2015). In vitro cytotoxic activities and molecular mechanisms of Angelica shikokiana extract and its isolated compounds. Pharmacogn. Mag. 11 (Suppl 4), S564–S569. doi: 10.4103/0973-1296.172962
Mira, A., Shimizu, K. (2016). “An update on antitumor activity of Angelica species,” in Medicinal Plants - Recent Advances in Research and Development. Eds. Tsay, H.-S., Shyur, L.-F., Agrawal, D. C., Wu, Y.-C., Wang, S.-Y. (Singapore: Springer Singapore), 363–371.
Mizuno, M., Fujisawa, R., Kuboki, Y. (2000). Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J. Cell. Physiol. 184 (2), 207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U
Morgan, M. J., Liu, Z.-G. (2011). Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21 (1), 103–115. doi: 10.1038/cr.2010.178
Mulaku, M., Opiyo, N., Karumbi, J., Kitonyi, G., Thoithi, G., English, M. (2013). Evidence review of hydroxyurea for the prevention of sickle cell complications in low-income countries. Arch. Dis. Child. 98 (11), 908–914. doi: 10.1136/archdischild-2012-302387
Munakata, R., Olry, A., Karamat, F., Courdavault, V., Sugiyama, A., Date, Y., et al. (2016). Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis. New Phytol. 211 (1), 332–344. doi: 10.1111/nph.13899
Nagy, E. M., Dalla Via, L., Ronconi, L., Fregona, D. (2010). Recent advances in PUVA photochemotherapy and PDT for the treatment of cancer. Curr. Pharm. Des. 16 (16), 1863–1876. doi: 10.2174/138161210791209018
Naylor, E. C., Watson, R. E. B., Sherratt, M. J. (2011). Molecular aspects of skin ageing. Maturitas 69 (3), 249–256. doi: 10.1016/j.maturitas.2011.04.011
Nirmaladevi, D., Venkataramana, M., Chandranayaka, S., Ramesha, A., Jameel, N. M., Srinivas, C. (2014). Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 34 (7), 973–985. doi: 10.1007/s10571-014-0073-6
O׳Neill, T., Johnson, J. A., Webster, D., Gray, C. A. (2013). The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins. J. Ethnopharmacol. 147 (1), 232–237. doi: 10.1016/j.jep.2013.03.009
Oltersdorf, T., Elmore, S. W., Shoemaker, A. R., Armstrong, R. C., Augeri, D. J., Belli, B. A., et al. (2005). An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681. doi: 10.1038/nature03579
Orhan, I. E., Tosun, F., Skalicka-Woźniak, K. (2016). Cholinesterase and tyrosinase inhibitory, and antioxidant potential of randomly selected Umbelliferous plant species and the chromatographic profile of Heracleum platytaenium Boiss. and Angelica sylvestris L. var. sylvestris. J. Serb. Chem. Soc 81 (4), 357–368. doi: 10.2298/JSC150902017O
Orimo, H. (2010). The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 77 (1), 4–12. doi: 10.1272/jnms.77.4
Pabari, R. M., Mattu, C., Partheeban, S., Almarhoon, A., Boffito, M., Ciardelli, G., et al. (2019). Novel polyurethane-based nanoparticles of infliximab to reduce inflammation in an in-vitro intestinal epithelial barrier model. Int. J. Pharm. 565, 533–542. doi: 10.1016/j.ijpharm.2019.05.025
Pathak, M. A., Daniels, F., Fitzpatrick, T. B. (1962). The presently known distribution of furocoumarins (Psoralens) in plants. J. Invest.Dermatol. 39 (3), 225–239. doi: 10.1038/jid.1962.106
Patnaik, G. K., Banaudha, K. K., Khan, K. A., Shoeb, A., Dhawan, B. N. (1988). Spasmolytic activity of angelicin: a coumarin from Heracleum thomsoni. Planta Med. 53 (6), 517–520. doi: 10.1055/s-2006-962799
Pecetti, L., Mella, M., Tava, A. (2016). Variation in herbage biochemical composition among pitch trefoil (Bituminaria bituminosa) populations from Elba Island, Italy. J. Agric.Food Chem. 64 (1), 195–203. doi: 10.1021/acs.jafc.5b05050
Petering, H., Breuer, C., Herbst, R., Kapp, A., Werfel, T. (2004). Comparison of localized high-dose UVA1 irradiation versus topical cream psoralen-UVA for treatment of chronic vesicular dyshidrotic eczema. J. Am. Acad. Dermatol. 50 (1), 68–72. doi: 10.1016/S0190-9622(03)00782-5
Pistelli, L., Noccioli, C., Appendino, G., Bianchi, F., Sterner, O., Ballero, M. (2003). Pterocarpans from Bituminaria morisiana and Bituminaria bituminosa. Phytochemistry 64 (2), 595–598. doi: 10.1016/s0031-9422(03)00190-0
Polak, P., Cybulski, N., Feige, J. N., Auwerx, J., Rüegg, M. A., Hall, M. N. (2008). Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 8 (5), 399–410. doi: 10.1016/j.cmet.2008.09.003
Prasad, N. R., Anandi, C., Balasubramanian, S., Pugalendi, K. V. (2004). Antidermatophytic activity of extracts from Psoralea corylifolia (Fabaceae) correlated with the presence of a flavonoid compound. J. Ethnopharmacol. 91 (1), 21–24. doi: 10.1016/j.jep.2003.11.010
Pratheeshkumar, P., Sreekala, C., Zhang, Z., Budhraja, A., Ding, S., Son, Y.-O., et al. (2012). Cancer prevention with promising natural products: mechanisms of action and molecular targets. Anticancer Agents Med. Chem. 12 (10), 1159–1184. doi: 10.2174/187152012803833035
Purushothaman, P., Uppal, T., Verma, S. C. (2015). Molecular biology of KSHV lytic reactivation. Viruses 7 (1), 116–153. doi: 10.3390/v7010116
Qiang, M. (2001). Induction of CYP1A1. The AhR/DRE paradigm transcription, receptor regulation, and expanding biological roles. Curr. Drug Metab. 2 (2), 149–164. doi: 10.2174/1389200013338603
Rafiei, M., Kiani, F., Sayehmiri, F., Sayehmiri, K., Sheikhi, A., Zamanian Azodi, M. (2017). Study of Porphyromonas gingivalis in periodontal diseases: a systematic review and meta-analysis. Med. J. Islam Repub Iran. 31, 62. doi: 10.18869/mjiri.31.62
Ragoczy, T., Miller, G. (1999). Role of the Epstein-Barr virus Rta protein in activation of distinct classes of viral lytic cycle genes. J. Virol. 73 (12), 9858–9866. doi: 10.1128/JVI.73.12.9858-9866.1999
Rahman, I., MacNee, W. (2000). Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 16 (3), 534–554. doi: 10.1034/j.1399-3003.2000.016003534.x
Rahman, M. A., Kim, N.-H., Yang, H., Huh, S.-O. (2012). Angelicin induces apoptosis through intrinsic caspase-dependent pathway in human SH-SY5Y neuroblastoma cells. Mol. Cell. Biochem. 369, 95–104. doi: 10.1007/s11010-012-1372-1
Rai, A., Kapoor, S., Singh, S., Chatterji, B. P., Panda, D. (2015). Transcription factor NF-κB associates with microtubules and stimulates apoptosis in response to suppression of microtubule dynamics in MCF-7 cells. Biochem. Pharmacol. 93 (3), 277–289. doi: 10.1016/j.bcp.2014.12.007
Raja, S. B., Murali, M. R., Roopa, K., Devaraj, S. N. (2011). Imperatorin a furocoumarin inhibits periplasmic Cu-Zn SOD of Shigella dysenteriae their by modulates its resistance towards phagocytosis during host pathogen interaction. Biomed. Pharmacother. 65 (8), 560–568. doi: 10.1016/j.biopha.2010.10.010
Rather, M. A., Pandey, D. P., Singh, R. P., Singh, Y., Nautiyal, D. N. (2017). A new furocoumarin glycoside from aerial parts of Pleurospermum brunonis. Asian J. Chem. 29 (1), 233–234. doi: 10.14233/ajchem.2017.20366
Reuter, S., Gupta, S. C., Chaturvedi, M. M., Aggarwal, B. B. (2010). Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 49 (11), 1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006
Rizzoli, R., Reginster, J.-Y., Boonen, S., Bréart, G., Diez-Perez, A., Felsenberg, D., et al. (2011). Adverse reactions and drug–drug interactions in the management of women with postmenopausal osteoporosis. Calcif. Tissue Int. 89 (2), 91–104. doi: 10.1007/s00223-011-9499-8
Roselli, S., Olry, A., Vautrin, S., Coriton, O., Ritchie, D., Galati, G., et al. (2017). A bacterial artificial chromosome (BAC) genomic approach reveals partial clustering of the furanocoumarin pathway genes in parsnip. Plant J. 89 (6), 1119–1132. doi: 10.1111/tpj.13450
Roy, S. K., Srivastava, R. K., Shankar, S. (2010). Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J. Mol. Signal. 5, 10. doi: 10.1186/1750-2187-5-10
Santana, L., Uriarte, E., Roleira, F., Millhazes, N., Borges, F. (2004). Furocoumarins in medicinal chemistry. Synthesis, natural occurance and biological activity. Curr. Med. Chem. 11 (24), 3239–3261. doi: 10.2174/0929867043363721
Sarker, S. D., Eynon, E., Fok, K., Kumarasamy, Y., Murphy, E. M., Nahar, L., et al. (2003). Screening the extracts of the seeds of Achillea millefolium, Angelica sylvestris and Phleum pratense for antibacterial, antioxidant activities and general toxicity. Orient. Pharm. Exp. Med. 3 (3), 157–162. doi: 10.3742/OPEM.2003.3.3.157
Schairer, C., Lubin, J., Troisi, R., Sturgeon, S., Brinton, L., Hoover, R. (2000). Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 283 (4), 485–491. doi: 10.1001/jama.283.4.485
Sharma, P. K., Chauhan, N. S., Lal, B. (2005). Studies on plant associated indigenous knowledge among the Malanis of Kullu district, Himachal Pradesh. Indian J. Tradit. Knowl. 4 (4), 403–408.
Simon, H.-U., Haj-Yehia, A., Levi-Schaffer, F. (2000). Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5 (5), 415–418. doi: 10.1023/a:1009616228304
Song, M. J., Han, J. A., Lee, D. H. (2010). Angelicin for inhibiting herpesvirus, South Korea patent application KR 2010-138318.
Song, L., Yu, B., Yang, L., Wang, Z. X., Zhang, Y., Yu, Y. L., et al. (2019). The mechanism of psoralen and isopsoralen hepatotoxicity as revealed by hepatic gene expression profiling in SD rats. Basic Clin. Pharmacol. Toxicol. 125 (6), 527–535. doi: 10.1111/bcpt.13287
Spector, D. H., Morello, C. S., Kraynyak, K. A. (2012). Herpes virus vaccines, USA patent application WO 2012-US45290.
Srinivasan, S., Koenigstein, A., Joseph, J., Sun, L., Kalyanaraman, B., Zaidi, M., et al. (2010). Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann. N. Y. Acad. Sci. 1192 (1), 245–252. doi: 10.1111/j.1749-6632.2009.05377.x
Stamatoyannopoulos, G., Wood, W. G., Papayannopoulou, T., Nute, P. E. (1975). A new form of hereditary persistence of fetal hemoglobin in blacks and its association with sickle cell trait. Blood 46 (5), 683–692. doi: 10.1182/blood.V46.5.683.683
Stern, R. S., Nichols, K. T., Väkevä, L. H. (1997). Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). N. Engl. J. Med. 336 (15), 1041–1045. doi: 10.1056/NEJM199704103361501
Tang, Y., Wu, X., Lei, W., Pang, L., Wan, C., Shi, Z., et al. (2009). TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 15 (7), 757–765. doi: 10.1038/nm.1979
Tang, B., Cai, J., Sun, L., Li, Y., Qu, J., Snider, B. J., et al. (2014). Proteasome inhibitors activate autophagy involving inhibition of PI3K-Akt-mTOR pathway as an anti-oxidation defense in human RPE cells. PloS One 9 (7), e103364. doi: 10.1371/journal.pone.0103364
Tanida, I., Minematsu-Ikeguchi, N., Ueno, T., Kominami, E. (2005). Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1 (2), 84–91. doi: 10.4161/auto.1.2.1697
Tian, J., Ban, X., Zeng, H., He, J., Huang, B., Wang, Y. (2011). Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int. J. Food Microbiol. 145 (2-3), 464–470. doi: 10.1016/j.ijfoodmicro.2011.01.023
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., Jemal, A. (2015). Global cancer statistics 2012. CA Cancer J. Clin. 65 (2), 87–108. doi: 10.3322/caac.21262
Tripathi, D. N., Chowdhury, R., Trudel, L. J., Tee, A. R., Slack, R. S., Walker, C. L., et al. (2013). Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc. Natl. Acad. Sci. U.S.A. 110 (32), E2950–E2957. doi: 10.1073/pnas.1307736110
Tsang, K. Y., Chan, D., Cheah, K. S. E. (2015). Fate of growth plate hypertrophic chondrocytes: death or lineage extension? Dev. Growth Differ. 57 (2), 179–192. doi: 10.1111/dgd.12203
Tsutsui, S., Ohno, S., Murakami, S., Hachitanda, Y., Oda, S. (2002). Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res. Treat 71 (1), 67–75. doi: 10.1023/a:1013397232011
Uda, M., Galanello, R., Sanna, S., Lettre, G., Sankaran, V. G., Chen, W., et al. (2008). Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of β-thalassemia. Proc. Natl. Acad. Sci. U.S.A. 105 (5), 1620–1625. doi: 10.1073/pnas.0711566105
Udagawa, N., Takahashi, N., Yasuda, H., Mizuno, A., Itoh, K., Ueno, Y., et al. (2000). Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141 (9), 3478–3484. doi: 10.1210/endo.141.9.7634
Uwai, K., Ohashi, K., Takaya, Y., Ohta, T., Tadano, T., Kisara, K., et al. (2000). Exploring the structural basis of neurotoxicity in C17 -polyacetylenes isolated from water hemlock. J. Med. Chem. 43 (23), 4508–4515. doi: 10.1021/jm000185k
Vallat, V. P., Gilleaudeau, P., Battat, L., Wolfe, J., Nabeya, R., Heftler, N., et al. (1994). PUVA bath therapy strongly suppresses immunological and epidermal activation in psoriasis: a possible cellular basis for remittive therapy. J. Exp. Med. 180 (1), 283–296. doi: 10.1084/jem.180.1.283
Vialart, G., Hehn, A., Olry, A., Ito, K., Krieger, C., Larbat, R., et al. (2012). A 2-oxoglutarate-dependent dioxygenase from Ruta graveolens L. exhibits p-coumaroyl CoA 2'-hydroxylase activity (C2'H): a missing step in the synthesis of umbelliferone in plants. Plant J. 70 (3), 460–470. doi: 10.1111/j.1365-313X.2011.04879.x
Viola, G., Vedaldi, D., Dall'Acqua, F., Lampronti, I., Bianchi, N., Zuccato, C., et al. (2008). Furocoumarins photolysis products induce differentiation of human erythroid cells. J. Photochem. Photobiol. B. 92 (1), 24–28. doi: 10.1016/j.jphotobiol.2008.04.001
Wagner, E. F., Nebreda, Á.R. (2009). Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549. doi: 10.1038/nrc2694
Waksmundzka-Hajnos, M., Petruczynik, A., Dragan, A., Wianowska, D., Dawidowicz, A. L., Sowa, I. (2004). Influence of the extraction mode on the yield of some furanocoumarins from Pastinaca sativa fruits. J. Chromatogr. B 800 (1-2), 181–187. doi: 10.1016/j.jchromb.2003.07.006
Wang, S.-Q., Li, X., Wang, X.-N., Wei, N.-N., Lou, H.-X. (2011). Coumarins from Cicuta virosa and their modulating effects on multidrug-resistant (MDR) tumors. Phytochem. Lett. 4 (2), 97–100. doi: 10.1016/j.phytol.2010.11.007
Wang, Q., Wang, Y. P., Lin, H., Zhang, L. Q., Wu, L. J., Pang, L. X. (2015a). Antiproliferative and apoptotic effects of angelicin in highly invasive prostate cancer cells. Trop. J. Pharm. Res. 14 (8), 1405–1411. doi: 10.4314/tjpr.v14i8.12
Wang, Y., Yu, K., Pei, X., Zhang, T., Guo, Y., Wood, C., et al. (2015b). Activation and degradation of open reading frame 45 by the replication and transcription activator of Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 96 (7), 1883–1889. doi: 10.1099/vir.0.000125
Wang, S.-Q., Zhang, Q.-W., Wang, X.-L., Di, X.-X., Wang, X.-N., Lou, H.-X. (2016). Chemical constituents from Cicuta virosa Linnaeus and their reversal effects on doxorubicin-resistant human myelogenous leukemia (K562/A02) cells. Chin. Chem. Lett. 27 (7), 1013–1016. doi: 10.1016/j.cclet.2016.03.039
Wang, F., Li, J., Li, R., Pan, G., Bai, M., Huang, Q. (2017a). Angelicin inhibits liver cancer growth in vitro and in vivo. Mol. Med. Rep. 16 (4), 5441–5449. doi: 10.3892/mmr.2017.7219
Wang, J., Li, S.-F., Wang, T., Sun, C.-H., Wang, L., Huang, M.-J., et al. (2017b). Isopsoralen-mediated suppression of bone marrow adiposity and attenuation of the adipogenic commitment of bone marrow-derived mesenchymal stem cells. Int. J. Mol. Med. 39 (3), 527–538. doi: 10.3892/ijmm.2017.2880
Wang, J., Wang, G., Gong, L., Sun, G., Shi, B., Bao, H., et al. (2018). Isopsoralen regulates PPAR−γ/WNT to inhibit oxidative stress in osteoporosis. Mol. Med. Rep. 17 (1), 1125–1131. doi: 10.3892/mmr.2017.7954
Wang, T., Wen, T., Li, H., Han, B., Hao, S., Wang, C., et al. (2019a). Arsenic sulfide nanoformulation induces erythroid differentiation in chronic myeloid leukemia cells through degradation of BCR-ABL. Int. J. Nanomed. 14, 5581–5594. doi: 10.2147/IJN.S207298
Wang, Y., Chen, Y., Chen, X., Liang, Y., Yang, D., Dong, J., et al. (2019b). Angelicin inhibits the malignant behaviours of human cervical cancer potentially via inhibiting autophagy. Exp. Ther. Med. 18 (5), 3365–3374. doi: 10.3892/etm.2019.7985
Wang, Y., Zhang, H., Jiang, J.-M., Zheng, D., Chen, Y.-Y., Wan, S.-J., et al. (2019c). Hepatotoxicity induced by psoralen and isopsoralen from Fructus Psoraleae: wistar rats are more vulnerable than ICR mice. Food Chem. Toxicol. 125, 133–140. doi: 10.1016/j.fct.2018.12.047
Watkins, L. R., Maier, S. F., Goehler, L. E. (1995). Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 63 (3), 289–302. doi: 10.1016/0304-3959(95)00186-7
Webb, A., Cunningham, D., Cotter, F., Clarke, P. A., di Stefano, F., Ross, P., et al. (1997). BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet 349 (9059), 1137–1141. doi: 10.1016/s0140-6736(96)11103-x
Wei, D.-Z., Guo, X.-Y., Lin, L.-N., Lin, M.-X., Gong, Y.-Q., Ying, B.-Y., et al. (2016). Effects of angelicin on ovalbumin (OVA)-induced airway inflammation in a mouse model of asthma. Inflammation 39 (6), 1876–1882. doi: 10.1007/s10753-016-0423-2
Wen, Z., Berenbaum, ,. M. R., Schuler, ,. M. A. (2006). Inhibition of CYP6B1-mediated detoxification of xanthotoxin by plant allelochemicals in the black swallowtail (Papilio polyxenes). J. Chem. Ecol. 32 (3), 507–522. doi: 10.1007/s10886-005-9014-7
Wong, K.-K. (2009). Recent developments in anti-cancer agents targeting the Ras/Raf/ MEK/ERK pathway. Recent Pat. Anticancer Drug Discovery 4 (1), 28–35. doi: 10.2174/157489209787002461
Xin, D., Wang, H., Yang, J., Su, Y. F., Fan, G. W., Wang, Y. F., et al. (2010). Phytoestrogens from Psoralea corylifolia reveal estrogen receptor-subtype selectivity. Phytomedicine 17 (2), 126–131. doi: 10.1016/j.phymed.2009.05.015
Xu, J., Peng, C., Sankaran, V. G., Shao, Z., Esrick, E. B., Chong, B. G., et al. (2011). Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 334 (6058), 993–996. doi: 10.1126/science.1211053
Xu, G.-M., Wang, Z.-M., Liu, N.-Z., He, W.-J., Peng, S., Zhou, X.-J. (2015). Study on chemical constituents from ethyl acetate extraction of Sanicula lamelligera. Zhong Yao Cai 38 (8), 1661–1664.
Yajima, T., Munakata, K. (1979). Phloroglucinol-type furocoumarins, a group of potent naturally-occurring insect antifeedants. Agric. Biol. Chem. 43 (8), 1701–1706. doi: 10.1271/bbb1961.43.1701
Yamamoto, S., Shimizu, S., Kiyonaka, S., Takahashi, N., Wajima, T., Hara, Y., et al. (2008). TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 14 (7), 738–747. doi: 10.1038/nm1758
Yang, J., Yang, J., Liang, S. H., Xu, Y., Moore, A., Ran, C. (2016). Imaging hydrogen peroxide in Alzheimer's disease via cascade signal amplification. Sci. Rep. 6, 35613. doi: 10.1038/srep35613
Yeol Park, S., Lee, N., Lee, S., Jo Kim, M., Chun, W., Pyo Kim, H., et al. (2017). A new 3, 4-epoxyfurocoumarin from Heracleum moellendorffii roots. Nat. Prod. Sci. 23 (3), 213–216. doi: 10.20307/nps.2017.23.3.213
Yu, T.-Y., Pang, W.-J., Yang, G.-S. (2015). Aryl hydrocarbon receptors in osteoclast lineage cells are a negative regulator of bone mass. PloS One 10 (1), e0117112. doi: 10.1371/journal.pone.0117112
Yuan, X. F., Sun, Y. M., Zhang, Z. Y., Li, C. L., Guo, Q., Song, G. H., et al. (2015). Molecular mechanism of apoptosis of HL-60 cells induced by angelicin. Chin. J. Cancer Prev. Treat. 22 (21), 1675–1679 and 1687. doi: 10.16073/j.cnki.cjcpt.2015.21.007
Yuan, X., Bi, Y., Yan, Z., Pu, W., Li, Y., Zhou, K. (2016). Psoralen and isopsoralen ameliorate sex hormone deficiency-induced osteoporosis in female and male mice. Bio Med. Res.Int. 2016, 1–8. doi: 10.1155/2016/6869452
Zhang, H., Ta, N. (2017). Effect of isopsoralen on Smad7 in osteoblastic MC3T3-E1 cells. Exp. Ther. Med. 14 (2), 1561–1567. doi: 10.3892/etm.2017.4688
Zhang, T., Han, W., Zhao, K., Yang, W., Lu, X., Jia, Y., et al. (2019). Psoralen accelerates bone fracture healing by activating both osteoclasts and osteoblasts. FASEB J. 33 (4), 5399–5410. doi: 10.1096/fj.201801797R
Zhou, L.-Y., Liu, H.-Y., Xie, B.-B., Liu, Z.-H., Chen, C.-X. (2014). Two new glycosides from Sanicula lamelligera. J. Chem. Sci. 61, 607–610. doi: 10.1515/znb-2006-0517
Keywords: angelicin, psolaren, furocoumarin, biological activities, biological potential
Citation: Mahendra CK, Tan LTH, Lee WL, Yap WH, Pusparajah P, Low LE, Tang SY, Chan KG, Lee LH and Goh BH (2020) Angelicin—A Furocoumarin Compound With Vast Biological Potential. Front. Pharmacol. 11:366. doi: 10.3389/fphar.2020.00366
Received: 13 February 2019; Accepted: 10 March 2020;
Published: 16 April 2020.
Edited by:
Alejandro Urzua, Universidad de Santiago de Chile, ChileReviewed by:
Nicoletta Bianchi, University of Ferrara, ItalyCopyright © 2020 Mahendra, Tan, Lee, Yap, Pusparajah, Low, Tang, Chan, Lee and Goh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kok Gan Chan, a29rZ2FuQHVtLmVkdS5teQ==; Learn Han Lee, bGVlLmxlYXJuLmhhbkBtb25hc2guZWR1; Bey Hing Goh, Z29oLmJleS5oaW5nQG1vbmFzaC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.