- 1Institute of Chinese Materia Medica, China Academy of Chinese Medical Science, Beijing, China
- 2College of Pharmacy, Henan University of Chinese Medicine, Zhengzhou, China
Angelicae Pubescentis Radix (APR) is a widely used antirheumatic Chinese medicinal herb known as “Duhuo” in China. It has the effects of dispelling wind and removing dampness, diffusing impediment, and relieving pain, and is mainly indicated for rheumatic arthritis with pain in the lower back and knees, and headache. To the best of our knowledge, an attempt is made to provide an up-to-date review on these aspects based on published materials, including ancient and modern books; Master's and doctoral theses; monographs on medicinal plants; the pharmacopoeia of different countries, websites for publication of patent and electronic databases, such as SCI finder, PubMed, Web of Science, ACS, Science Direct, Wiley, Springer, Taylor, CNKI, and Google Scholar. APR, which has a good clinical effect, has been used for traditional Chinese medicine more than 2000 years. Since 1957, a variety of chemical constituents have been reported from the medicinal plants of this herb, mostly coumarins and volatile oil. In the past 30 years, numerous studies have shown that the extracts and compounds isolated from APR showed effective analgesic and anti-inflammatory actions, also showing well effects on central nervous system, effects on cardiovascular system and deworming activity. In addition, we also present and discuss the botany, traditional medicinal use, pharmacokinetics, toxicity, quality control, future trends and prospects of APR. All this information suggest that future research of APR should be supplemented in the area of pharmacology and toxicology to provide further insight on the clinical use and quality control.
Introduction
Angelicae Pubescentis Radix (equivalent to Angelica Pubescens Root, known as “Duhuo” in China, “Dokwhal” in Korea, “Dokkatsu” in Japan) has been used as herbal medicine extensively since ancient times in Asian countries, including China, Korea and Japan. Due to its effective medicinal value, it has been included in Chinese Pharmacopoeia, British Pharmacopoeia, European Pharmacopoeia, and so on. As an antirheumatic and analgesic agent, it is commonly used as a traditional Chinese medicine (TCM) to treat rheumatic arthralgia and headache. Rheumatic arthralgia is the professional description of TCM syndrome, and includes clinical symptoms such as muscle and joint pain, joint deformities, and dysfunction, with resulting exhaustion and lack of strength. These symptoms are common in rheumatic diseases such as rheumatism and rheumatoid arthritis (RA), with modern medicine ascribing them to an immune deficiency (Wang et al., 2009). In addition, APR is also used in the field of health-care products and cosmetics.
From the Chinese Pharmacopoeia (version 1977), Angelicae Pubescentis Radix (APR) is the dried roots of Angelica biserrata (R.H.Shan & C.Q.Yuan) C.Q.Yuan & R.H.Shan (a synonym for Angelica pubescens f. biserrata R.H. Shan & C.Q. Yuan in Chinese Pharmacopoeia). Besides, Angelica pubescens Maxim used to be a plant source of APR in China, and it is still a medicinal plant in Japan. In this review, A. biserrata and A. pubescens will be reviewed collectively.
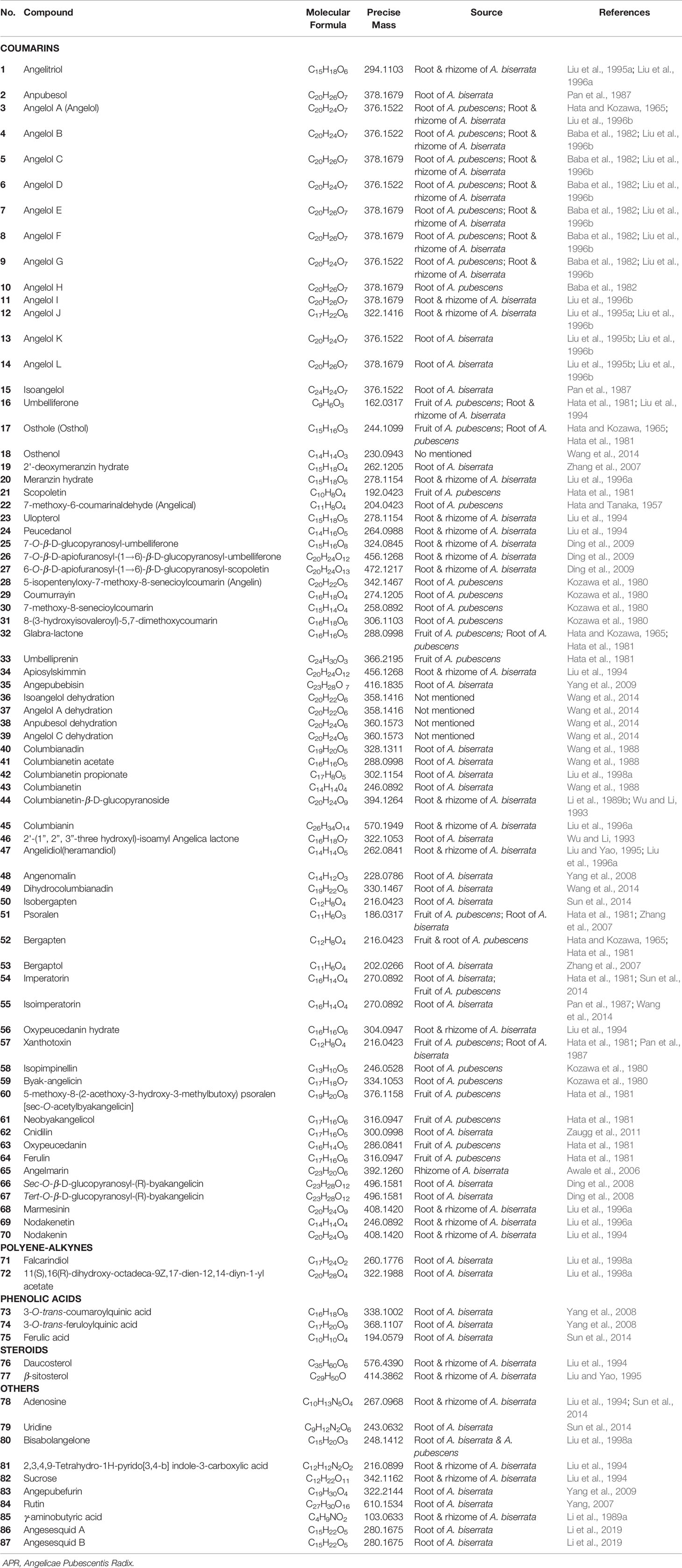
APR is mainly produced in Sichuan, Hubei, Anhui and other provinces in China. Usually, its excavation takes place in early spring or late fall. It is common to remove the fibrous roots and sediment, half dried above the heated mud. They were piled for 2 to 3 days, then heated to dry absolutely when they become soft. To date, 87 compounds, including coumarins, polyene-alkynes, phenolic acids, steroids, nucleoside elements have been isolated and identified from APR. What's more, nearly 100 volatile oil compounds have been analyzed by GC-MS. In modern clinical practice, APR plays an important role in treating RA, osteoarthritis pain, vascular dementia (VD), Alzheimer's disease (AD) and headache (Wang et al., 2011a; Jia et al., 2015; Zheng et al., 2017).
In this review, we discussed the botany, traditional medicinal use, phytochemistry, pharmacology, pharmacokinetic, toxicology and quality control of APR as comprehensively as possible, to obtain a comprehensive understanding of the effects of APR and also provide a basis for further research and development of new drugs.
Botany
Angelica biserrata (R.H.Shan & C.Q.Yuan) C.Q.Yuan & R.H.Shan (A. biserrata, Chinese name: Chongchi Maodanggui) and Angelica pubescens Maxim (A. pubescens, Chinese name: Maodanggui) used to be or still be medicinal plants of APR. At present, A. biserrata is the plant source of APR recorded in multi-national pharmacopoeia, including Chinese, British, the European. A. pubescens, which used to be a medicinal plant for APR in China, is mainly produced in Japan and is used medically in that country at present (Zhengkang, 2006; Rao et al., 1994). In the following discussion, A. biserrata and A. pubescens will be reviewed collectively.
A. biserrata and A. pubescens both belong to the genus Angelica of the family Apiaceae, and the former is a variant of the latter. Due to too much similarity of the plants and constant errors in the study of the resources, some of the A. biserrata were once mistaken for A. pubescens before the 1990s. According to the resource survey, there is no such a species as A. pubescens in China (Institute of Materia Medica, Chinese Academy of Medical Sciences, 1959; Shan et al., 2014). It has been concluded that A. biserrata is primarily distributed in China South-Central, China Southeast, and Vietnam, while A. pubescens is primarily distributed in Japan and Vietnam (http://www.plantsoftheworldonline.org/).
A. biserrata primarily grows on wet and damp slopes, under the forest grass, or in sparse shrubs in Sichuan, Hubei, Jiangxi, Anhui, and Zhejiang provinces, among other selected regions. It is also cultivated in the high mountains of the Sichuan, Hubei, and Shanxi provinces, and breeding methods include seed breeding, direct seeding, and the transplanting of seedlings. In terms of A. biserrata, the medicinal material shape of the cultivated variety is similar to the wild product, but the root of the cultivated product is large and soft, with a length of 10–20 cm, and a strong fragrance (http://frps.iplant.cn/; Xiong, 2014).
The description of A. biserrata and A. pubescens plants morphology as referenced by the Flora of China (FOC) and Flora of Japan (FOJ) is presented in Table 1. The whole plant of A. biserrata (Figure 1A), medicinal parts of APR (Figure 1B), and the processed of APR (Figure 1C) are shown in Figure 1. To further distinguish the two plants, the comparison between them were performed based on scientific synonyms included in Kew's taxonomic resources and names published in medicinal references, and the results are shown in Table 2.
Traditional Medicinal Use
APR is widely used as the important traditional Chinese medicine to treat rheumatic arthralgia and headache for nearly 2000 years. In the Chinese Pharmacopoeia (version 2015), it has been used to treat conditions such as wind-cold-dampness arthralgia, lumbar and knee pain, wind-cold dampness headache, and its recommended dosage is 3–10 g (Committee for the Pharmacopoeia of PR China., 2015).
According to TCM records, APR was initially recorded in “Shen Nong Ben Cao Jing” during the Eastern Han Dynasty (perhaps earlier), which is deemed as the earliest treatise for medicine in China. In this monograph, APR was described as a treatment for wind-cold blows, gold sore pain and hernia mass in women. In “Ben Cao Gang Mu”, another monograph of TCM, APR was described as a treatment to resist stroke, joint pain, tooth swelling and pain by wind. In ancient times, the long-term clinical application was primarily the function of expelling wind and removing dampness, thus relieving pain and exterior syndrome, acting as an antispasmodic, suppressing the hyperactive liver for calming endogenous wind, flattening the Qi, and lowering the inverse to stop vomiting, relieving itching, detoxifying, and inducing hemostasis (Zhang and Liang, 2018).
In addition, APR also has been used with other herbs to treat wind-cold exterior syndrome (TCM term: Fenghan Biaozheng, also can be interpreted as superficial syndrome due to wind-cold), and wind-cold-damp bi-syndromes (TCM term: Fenghanshi Bizheng, also can be interpreted as painful obstructions from wind, damp, and cool environments) in China since ancient times. For example, Duhuo Tang consists of APR together with Angelicae Sinensis Radix, Atractylodis Macrocephalae Rhizoma, Astragali Radix, Cinnamomi Cortex, Achyranthis Bidentatae Radix, and Glycyrrhizae Radix et Rhizoma and can be used to treat rheumatic arthralgia (Gao, 2007). In addition, it was reported that Duhuo Jisheng Tang was effective in treating arthralgia syndrome in the 1990s in Germany (Zuo, 1998). Many classic prescriptions had been created by the ancient famous doctors and have been handed down from generation to generation through repeated clinical verification for thousands of years. Due to its definite clinical effects, TCM prescriptions are the main form of TCM used in clinics. The combination of several different types of TCM can enhance efficacy and reduce adverse reactions.
The application of APR in the field of health care has a long history. Early in “Shen Nong Ben Cao Jing”, there is a record that it can make the body light and slow down your aging. There are also a variety of ways to eat APR, such as Duhuo tea recorded in “Yao Cha Zhi Bai Bing”, Duhuo Renshen wine recorded in “Tai Ping Sheng Hui Fang”, and Duhuo Danggui wine recorded in “Sheng Ji Zong Lu”. In recent years, the application of APR to the field of health care has been further developed, and there have been multiple patent authorizations for inventions, such as a types of APR to dispel wind and dehumidify hot pot material, types of production methods (Wang, 2014), treating a kind of internal injury fever, and APR health wine (Qian, 2014).
In addition to the above application areas, APR has entered the field of beauty and makeup, and has been granted a number of invention patents, such as APR grass oil emollient water (Wang, 2013), and the preparation methods for APR oil tea emulsion (Li, 2013).
Phytochemistry
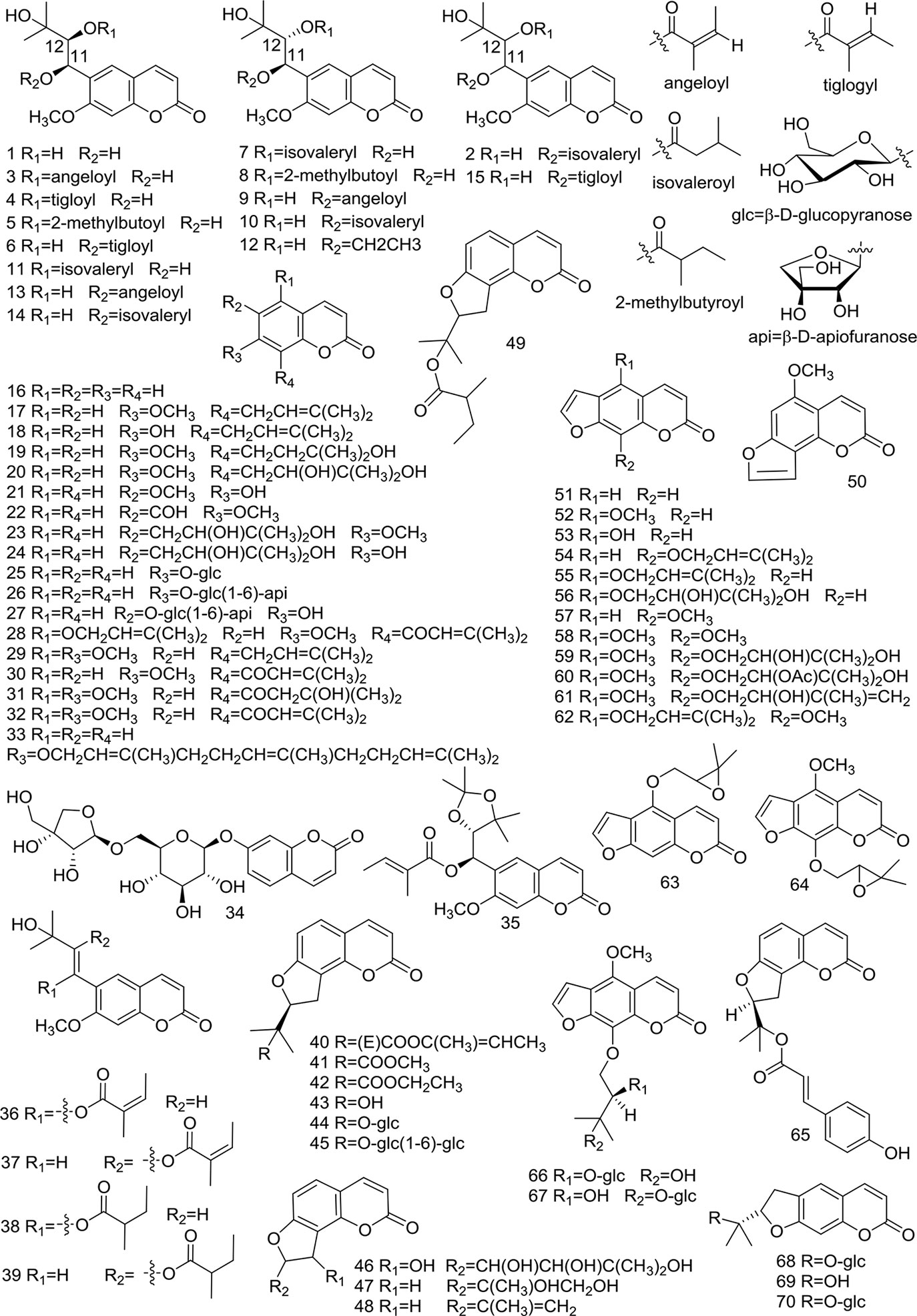
At present, many chemical compounds, including coumarins, polyene-alkynes, phenolic acids, steroids, nucleoside elements, and others, have been isolated and identified from A. biserrata and A. pubescens. Among these, Coumarins are believed to be the principle non-volatile ingredients with important biological properties. In addition, nearly 100 volatile oil compounds, consisting of terpenoids, small molecular aliphatic and aromatic compounds, have been analyzed by GC-MS (Yang et al., 2006; Wang et al., 2011b). The name, molecular formula, precise mass, and the source of these compounds are listed in Table 3. The relevant structures of these compounds are shown in Figures 2 and 3.
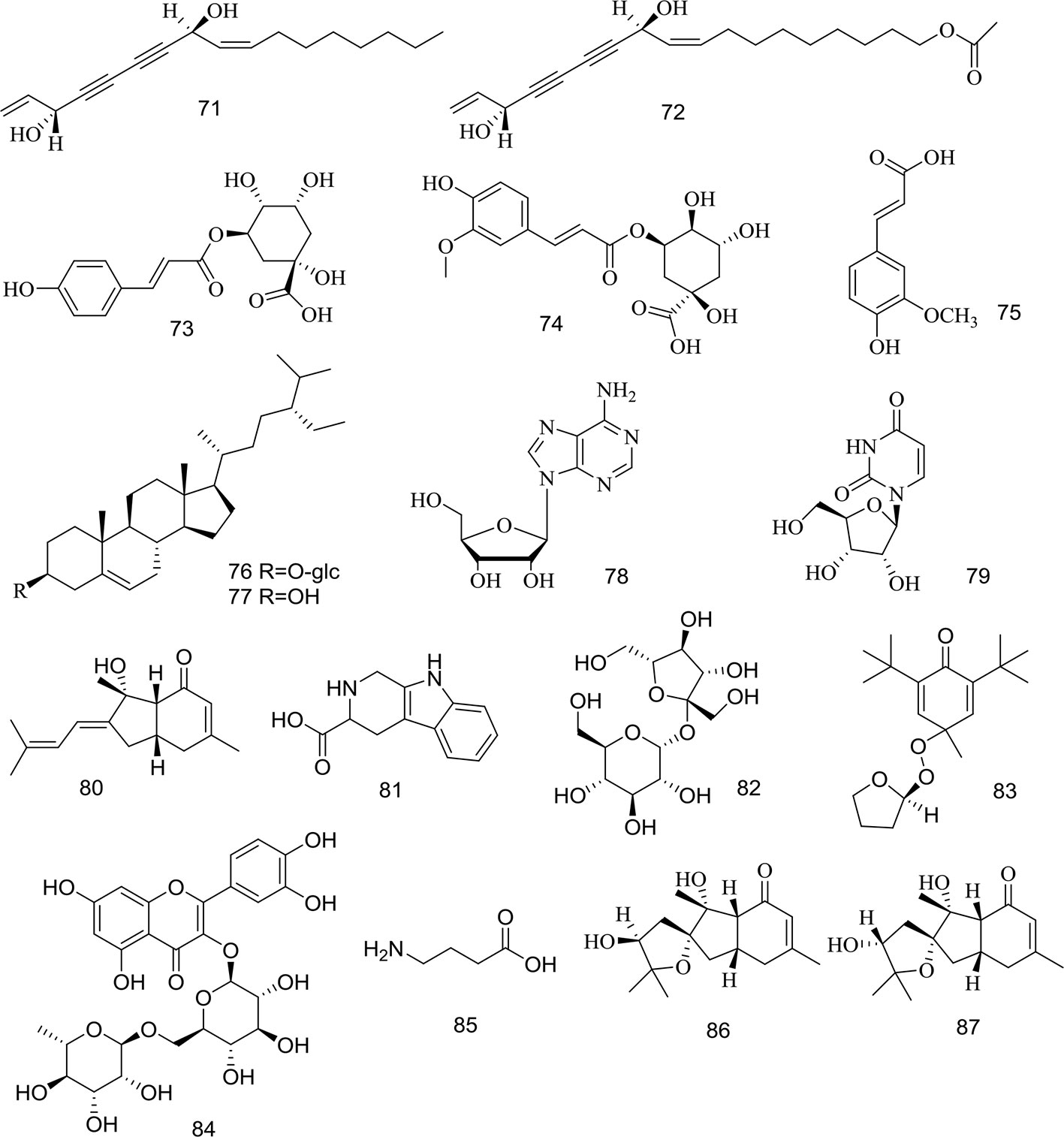
Figure 3 Structures of polyene-alkynes (71, 72), phenolic acids (73–75), steroids (76–77) and others (78–87) isolated from medicinal plants as APR.
Coumarins
Coumarins are phenolic compounds characterized by a benzene ring attached to an alpha-pyrone ring, which can be regarded as a lactone formed by the dehydration of o-hydroxy cinnamic acid. Coumarins have a fragrant smell and occur naturally in many plants. In addition, they are known to possess a myriad of pharmacological activities, including antioxidant, anti-cancer, and anticoagulant effects. They are also known to be fluorophores with their fluorescence changing drastically with varying substituents and newly introduced positions. Therefore, coumarins can be identified using fluorescence detection (Murata et al., 2005). So far, the coumarins separated from APR are mainly composed of simple coumarin as the parent nucleus, linear furocoumarin formed by substitution of seven and six positions, and flavonocoumarin formed by substitution of seven and eight positions. Simple coumarins, angular furocoumarins, psoralen furocoumarins, and nodakenetin furocoumarins have been isolated and identified, with the chemical structures of these coumarins shown in Table 3 and Figure 2.
Simple coumarins (1–39) have one or more substituents at five, six, seven, eight sites in the mother nucleus of coumarin, which are replaced by -OH, -OCH3, etc. Some (25–27) can be glycosylated with sugar. Structures of the simple coumarins are shown in Figure 2.
At present, there are 11 angular furocoumarin (40–50, 65) compounds that have been reported. They can be divided into two types, with one type (40–49, 65) having a single bond at the position of 1' and 2', while the other type (50) has a double bond. Two (44, 45) of them are glycosylated. Their structures are shown in Figure 2.
Currently, 15 psoralen furocoumarins (51–64, 66, 67) have been reported, all of which have the skeleton characteristics of psoralen. The side chains of two compounds (62, 64) have an epoxy structure, the other two (63, 64) are glycosylated, and the remaining 11 compounds are typical psoralen furocoumarins. Their structures are shown in Figure 2.
There are reported 3 nodakenetin furocoumarins (68–70) currently. The difference in these compounds from the psoralen structure is the hydrogenation of the double bonds on the furan ring. Their structures are shown in Figure 2.
Polyene-Alkynes
Two long chain polyene-alkynes (71, 72) have been isolated from the root of A. biserrata, and they have conjugated systems with multiple double bonds and alkyne bonds. Falcarindiol (71) has been reported to be an anesthetic with antifungal activity. It also exhibits prominently inhibitory effects on 5-LO with an IC50 value of 9.4 μM, and moderate inhibitory activity on COX-1 with an IC50 value of 66 μM (Liu et al., 1998a). The chemical structures of the polyene-alkynes are shown in Figure 3.
Phenolic Acids
Three phenolic acids (73–75) have been isolated from the root of A. biserrata. Since phenolic hydroxyl groups replace their structure, these compounds are unstable and thus easily transformed by water, temperature, light, enzymes, acids, and alkalis (Lin et al., 2017). The chemical structures of the phenolic acids are shown in Figure 3.
Steroids
Two steroids (76, 77) have been isolated from the root and rhizome of A. biserrata. They represent fused tetracyclic compounds composed of three hexacyclohexane (A, B, C) and a five-carbon ring (D). In addition, β-sitosterol (77) is a phytosterol that is common in higher plants. The chemical structures of the steroids are shown in Figure 3.
Other Compounds
Apart from the ingredients above, other compounds have been isolated and identified from A. biserrata and A. pubescens. Two nucleosides, including adenosine (78) and uridine (79), have been isolated from the root or rhizome of A. biserrata. Bisabolangelone (80) has been isolated from A. pubescens for the first time with strong anti-feeding properties against insects but it's been reported to be unstable in both basic and acidic media (Liu et al., 1998a). In addition, 2, 3, 4, 9-Tetrahydro-1H-pyrido [3, 4-b] indole-3-carboxylic acid (81) and Sucrose (82) were isolated from the plant for the first time in 1994 (Liu et al., 1994). A new furan, angepubefurin (83), was determined by HR-FTICR-MS and X-ray diffraction analyses (Yang et al., 2009). Rutin (84) and γ-aminobutyric acid (85) have also been isolated and identified in succession (Li et al., 1989a; Yang, 2007). Both new sesquiterpenoid derivatives (86) and (87) could moderately inhibit the inflammatory reaction. The beneficial elements Ca, Mg, and K in APR have been determined by LIBS spectra and the artificial neural network method (Wang et al., 2018a). The chemical structures of other compounds are shown in Figure 3.
Pharmacology
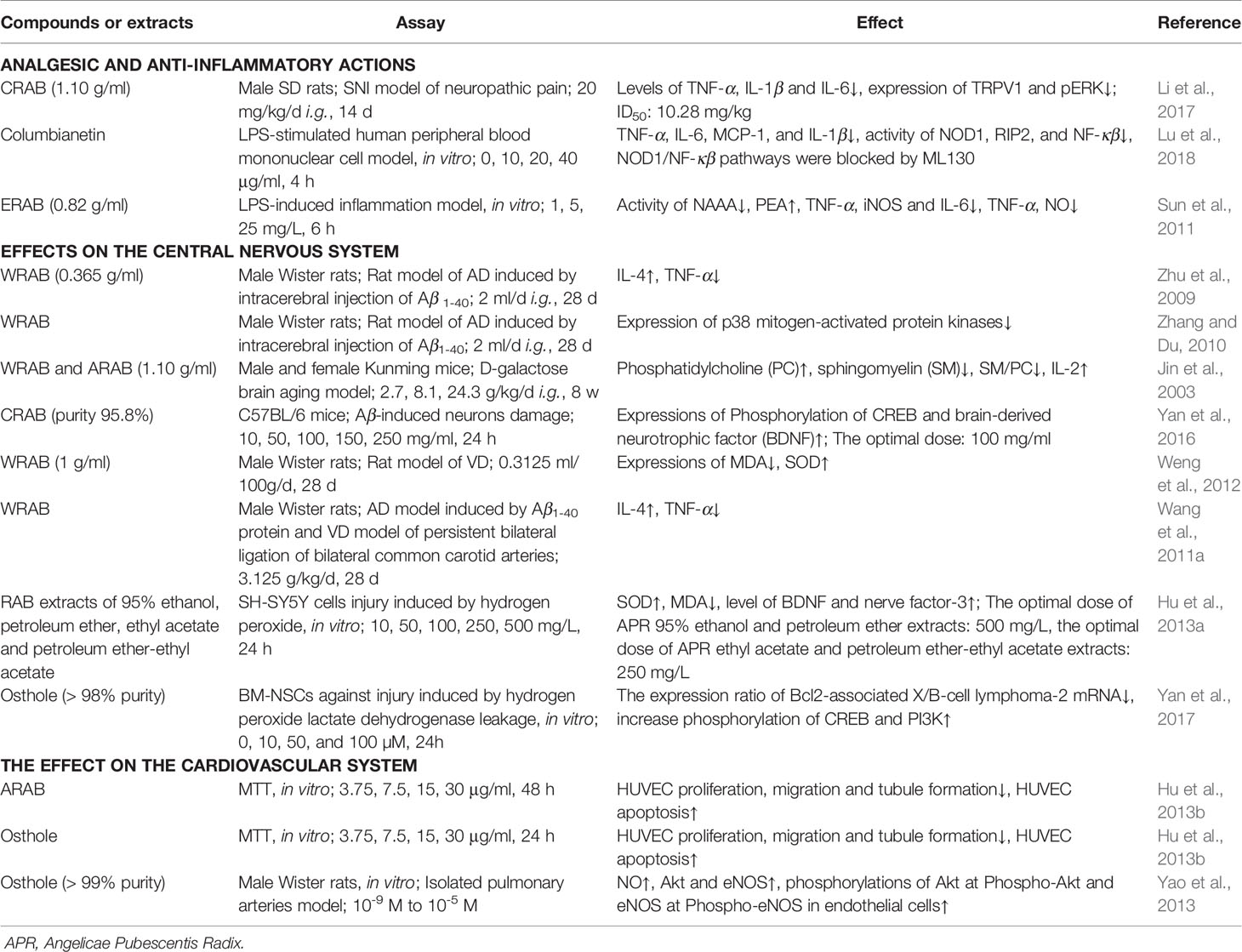
The study of pharmacological activities has indicated that APR has proven to have positive effects as an analgesic and anti-inflammatory agent, which are important for RA treatment strategies. Other reported functions of APR include effects on the central nervous system, effects on the cardiovascular system, deworming activity. The main pharmacological activities of the extracts or isolated compounds from APR that have been reported in animal and in vitro studies are summarized in Table 4.
Analgesic and Anti-Inflammatory Actions
APR is a high-frequency drug for the treatment of RA, which is an autoimmune disease requiring the use of analgesics and anti-inflammatory medicines to alleviate the associated pain. Some molecules, including extracellular signal-regulated kinase 1/2, arylhydrocarbon receptor, Histone h3, prostaglandin E receptor 2, nuclear factor κappa β (NF-κβ), programmed cell death 5, interleukin (IL)-36, IL-10, IL-4, Hypoxia Inducible Factor 1A, and arachidonate 15-lipoxygenase, are common molecular targets related to APR and RA, with the Eicosanoid signaling pathway the common pathway of APR and RA (Jia et al., 2015).
In TCM, the crude water extract of the root of A. biserrata (RAB) is considered a selective and effective herbal agent in attenuating persistent hindpaw inflammation and hyperalgesia in rats (Wei et al., 1999). RAB consisting of 60% ethanol extract (1.5 g/kg, i.g.) significantly inhibits inflammation in three models, including xylene-induced mouse ear edema test, the mice pettitoes swelling by egg white test and mouse tampon granulation swelling test (Li et al., 2013). However, the methanol-, chloroform-, and ethyl acetate extracts from the root of A. pubescens not only effectively reduced pain that was induced by 1% acetic acid and a hot plate, but also reduced the edema that was induced by 3% formalin or 1.5% carrageenan (Chen et al., 1995).
The analgesic effect of coumarins isolated from RAB (CRAB, 20 mg/kg, i.g.) is mediated by inflammatory factors and transient receptor potential cation channel 1 (TRPV1) as evidenced by a spared nerve injury (SNI) model of neuropathic pain. Molecular profiling has revealed that CRAP reduces levels of the proinflammatory cytokines tumor necrosis factor-α (TNF-α), IL-1β, and IL-6 and significantly attenuates the expressions of TRPV1 and phosphorylated extracellular regulated protein kinases (pERK) in damaged dorsal root ganglion neurons (Li et al., 2017).
Four coumarins (10 mg/kg, i.p.), including columbianadin (40), columbianetin acetate (41), bergapten (52) and umbelliferone (16), have been demonstrated to have significant anti-inflammatory and analgesic activities. While the coumarins osthole (17) and xanthotoxin (57) appear to only have anti-inflammatory activity, isoimperatorin (55) demonstrates only an analgesic effect. Columbianetin (43) inhibits the production of inflammatory cytokines induced by lipopolysaccharide (LPS), which is involved in the downregulation of nucleotide-binding and oligomerization domain 1 (NOD1)/NF-κβ pathways (Lu et al., 2018). The methanol extract from the root of A. pubescens have inhibitory activities on rat hind paw edema induced by carrageenan and on writhing induced by acetic acid in mouse, and the active principle was isolated and identified as osthol (Kosuge et al., 1985). The anti-inflammatory and analgesic constituents from A. pubescens appear to be related to the peripheral inhibition of inflammatory substances and to have influences on the central nervous system (Chen et al., 1995).
The essential oil from RAB (ERAB) has anti-inflammatory and analgesic effects (Fan et al., 2009). The effect of EAPR on inflammation is mediated by the inhibition of N-acylethanolamine-hydrolyzing acid amidase (NAAA) activity, which increases cellular endobioactor N-palmitoylethanolamine (PEA) levels while decreasing proinflammatory factor (Sun et al., 2011).
Effects on the Central Nervous System
Brain aging is a neurodegenerative disease, which is incurable debilitating disorders characterized by structural and functional neuronal loss. The primary disease of brain aging is dementia, which includes VD, AD, and a mixed type of both (Wang et al., 2011a). Free radical damage and immune inflammatory mechanisms have been recognized in current research regarding the mechanisms of brain aging. Because of its large oxygen load, the brain becomes the most vulnerable target of oxygen free radicals, which can damage the central nervous system through a variety of processes, including causing the necrosis or apoptosis of nerve cells (Jin et al., 2003).
A water maze test on mice of a D-galactose-induced brain aging model was performed to study the ability of learning and memory (Jin et al., 2003). The authors found that RAB and its alcohol extract at 18 mg/kg/d can repair the membrane phospholipid structure in different parts of the mouse cerebral cortex and striatum, as well as improve the IL-2 content of aging model mice and resist free radicals and inflammatory damage, thereby improving the learning and memory ability of mice (Pei et al., 2005). From the perspective of inhibiting the apoptotic rate of brain tissue cells, the mechanism of RAB water decoction and its alcohol extract in retarding brain aging was revealed.
RAB and/or its water decoction (2 ml/d, i.g.) have an inhibitory effect on the inflammatory response of AD model rats induced by Human amyloid beta peptide 1–40 (Aβ1-40), thus improving the learning and memory ability of AD model rats (Zhu et al., 2009; Zhang and Du, 2010). The water decoction of RAB (1.08 mg/ml, i.g.) can improve the positioning and learning and memory ability of model rats, shortening the time to navigate the water maze (Du et al., 2009). CRAB (purity 95.8%) possess neuroprotective effects in Aβ damaging neuron (Yan et al., 2016). In addition, CRAB (purity 72.4%, 14.4 mg/kg, i.g.) can significantly protect amyloid precursor protein/presenilin-1 (APP/PS1) mice from neural damage, likely through improving Neurofilament tirplet M expression and reducing apoptotic cells in the brain of APP/PS1 transgenic mice (Jiao et al., 2014). Moreover, the water decoction of RAB (3.125 g/kg, i.g.) can inhibit the expression of malondialdehyde (MDA) in rat serum and increase the activity of Superoxide dismutase (SOD) in order to inhibit free radical damage in VD model rats (Weng et al., 2012). Finally, APR has a regulating effect on immune inflammatory injury in AD and VD rats, particularly for the treatment of VD (Wang et al., 2011a). Extracts, including 95% ethanol, petroleum ether, ethyl acetate, and petroleum ether-ethyl acetate extracts of RAB do not appear to be toxic to SH-SY5Y cells. All of these may increase cell viability and exert antioxidant activity induced by H2O2 in SH-SY5Y cells, whereas the neuroprotective effects of the petroleum ether-ethyl acetate extract have been shown to be the strongest (Hu et al., 2013a). Osthole (17) protects bone marrow-derived neural stem cells (BM-NSCs) from oxidative damage through the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt-1) pathway, and it has been shown to improve the inflammatory environment of neurodegenerative diseases and promote the survival rate of transplanted NSCs (Yan et al., 2017).
Effects on the Cardiovascular System
γ-aminobutyric acid (85) (10 mg/kg, i.v.) isolated from the water extract of RAB (WRAB) showed that it can treat a variety of empirical cardiac arrhythmias, with effects on the action potential of rat neoventricular muscle (Li et al., 1989a). The alcohol extract of RAB (ARAB) and WRAB both have anti-angiogenic effects (Zou et al., 2008; Hu et al., 2013b). Osthole (17) isolated from ARAB inhibits angiogenesis in vitro more strongly than that in the ARAP, indicating that it may be the main antiangiogenic component of ARAB, and its mechanism may be related to the inhibition of human umbilical vein endothelial cell (HUVEC) proliferation, migration and tubule formation, the induction of HUVEC apoptosis, and the arrest of HUVEC cell cycle (Hu et al., 2013b). ARAB inhibits platelet aggregation and platelet thrombosis in circulating blood (Meng et al., 1988; Li et al., 1989b). Columbianetin (43), columbianetin acetate (41), columbianadin (40), osthol and columbianetin-β-D-glucopyranoside (44) also have inhibitory effects on rat platelet aggregation induced by ADP in vitro (Li et al., 1989b).
Pulmonary arterial hypertension (PAH) is a progressive cardiovascular-disease with high mortality lacking high-efficiency drug. Excitingly, osthole (17) extracted from RAB was observed to significantly restore 98 of 315 differential proteins significantly modified by PAH progression (Yao et al., 2018). What's more, osthole (17) extracted from the root of A. pubescens has been shown to suppress tracheal smooth muscle contraction, exerting a non-specific relaxant effect on the trachealis by inhibiting cAMP and cGMP phosphodiesterases (Teng et al., 1994). However, its dilative effect is dependent on endothelial integrity and NO production, and is mediated by the endothelial PI3K/Akt- endothelial NO synthase (eNOS)-NO pathway (Yao et al., 2013). These findings may provide candidates for a new pulmonary vasodilator for the therapy of PAH.
Deworming Activity
RAB extracts using different solvents have quite different effects on treating ring worm. ARAB exhibited the best anthelmintic efficacy with 100% mortality of dactylogyrus and no death of the fish at the optimal anthelminthic concentration of 120 mg/L (Wang et al., 2011c; Xie et al., 2016). Osthole (17) can reach the 100% parasiticidal rate when the concentration was 1.6 mg/L, with no toxicity to fish at a dose up to 6.2 mg/L. As for scopoletin (21), 5 mg/L appears to represent a parasiticidal rate of 74.9% (Wang et al., 2011c; Wang et al., 2012).
Others
Osthole (17) also shows osteopromotive effects on osteoblasts both in vitro and in vivo. Osthole (17) -mediated osteogenesis is related to the activation of the cAMP/cAMP response element-binding protein (CREB) signaling pathway and downstream osterix expression (Zhang et al., 2017). N-hexane and dichloromethane extracts of RAB show inhibitory effects on COX-1 and 5-LO. Linoleic acid appears to be the most active constituent in the extract, exerting COX-1 inhibition, and also has strong inhibitory activity on 5-LO, which is similar to osthole (17) (Liu et al., 1998b).
Pharmacokinetic
So far, few pharmacokinetic studies on APR have been done, which mainly focus on coumarins. What's more, only medicinal plants A. biserrata have been studied. The main pharmacokinetic researches of the extracts or isolated compounds from APR that have been reported studies in animal are discussed below.
Four of the coumarins [columbianetin acetate (41), imperatorin (54), isoimperatorin (55), and osthole (17)] were rapidly absorbed, while the remaining three coumarins [psoralen (51), bergapten (52), and xanthotoxin (57)] were slowly absorbed in rat plasma after oral administration of APR extract (6.0 g/kg) (Chang et al., 2013). To assess the brain distributions and blood-brain barrier permeabilities of APR, a UPLC-MS/MS method was applied to the simultaneous determinations of the main coumarins in the rat cerebrospinal fluid (CSF) and brain after oral administration of APR extract (4 g/kg), including psoralen (51), xanthotoxin (57), bergapten (52), isoimperatorin (55), columbianetin (43), columbianetin acetate (41), columbianadin (40), oxypeucedanin hydrate (56), angelol B (4), osthole (17), meranzin hydrate (20), and nodakenetin (69). Most of the tested coumarins entered the rat CSF and brain quickly, and double-peak phenomena in concentration-time curves were similar to those of their plasma pharmacokinetics. Columbianetin (43) had the highest concentration in the CSF (Cmax: 485.36 ± 91.40 µg/L, AUC0→∞: 4142.82 ± 602.33 µg/L/h) and brain, while psoralen (51) and columbianetin acetate (41) had the largest percent of CSF/plasma and brain/plasma. (Yang et al., 2018). Under the administration mode and dose as above, columbianetin (43) was also easier to absorbed compound across caco-2 cell, and also had extremely highest plasma concentration (2000 ng/ml) in vivo (Yang et al., 2017). The pharmacokinetic properties of columbianetin (43) in rat after oral administration were characterized as rapid oral absorption, quick clearance and good absolute bioavailability. Columbianetin (43) showed dose proportionality over the dose range 5–20 mg/kg. The bioavailability of columbianetin (43) is independent of the doses studied (Luo et al., 2013).
The tissues distributions research after oral administration of APR extract (6.0 g/kg) to rat, four coumarins [columbianetin (43), columbianetin acetate, (41) osthole (17), columbianadin (40)] were in the liver, followed by the ovary, uterus, kidney, lung, heart, spleen, and muscle (Ge et al., 2019). The AUC and Cmax of bisabolangelone (80) after oral administrated pure bisabolangelone (80) are higher than those after oral administrated APR extract. Among them, there are significant differences on AUC between pure bisabolangelone (80) (7.5 mg/kg i.g.) and APR extract (equivalent to bisabolangelone (80) at dose of 7.5 mg/kg, i.g.). For tissue distribution, the amount of bisabolangelone (80) mainly appeared in heart, liver and spleen. Bisabolangelone (80) appearance in the brain also revealed that bisabolangelone (80) could pass the blood-brain barrier. (Ge et al., 2018).
A sensitive LC-MS/MS method has been validated to determine the concentration of columbianadin (40) in rat plasma after intravenous administration of columbianadin (40) (1, 2.5 and 5 mg/kg). The results show that the distribution half-life (T1/2α) were 0.027 0.016, 0.060 0.065, and 0.028 0.023 h, and the elimination half-lives (T1/2β) were 0.58 0.20, 0.52 0.25, and 0.52 0.22 h (Chang et al., 2014). After oral administration of pure columbianadin (40), approximately 0.12% columbianadin (40) was transformed into columbianetin (43). Oral administration of pure columbianadin (40) may be more beneficial for the clinical efficacy of columbianadin (40) (25 mg/kg) than APR extract (3.74 g/kg). Pure columbianadin (40) group: Tmax: 3.03 ± 1.87 h, Cmax: 1.82 ± 0.64 ng/ml, AUC(0-tn): 1.05 ± 1.02 ng/ml/h; APR extract group: Tmax: 0.55 ± 0.33 h, Cmax: 13.33 ± 25.37 ng/ml, AUC(0-tn): 28.80 ± 41.46 ng/ml/h (Li et al., 2018).
Columbianetin acetate (41) and columbianetin-β-D-glucopyranoside (44, CBG) were rapidly and widely distributed in rats, and eliminated rapidly from plasma. Columbianetin acetate (41) could be metabolized into columbianetin (43) in vivo. Absolute bioavailability of pure columbianetin acetate (41) is 7.0 ± 4.3%. Other co-existing ingredients in APR extract could increase the concentration of its metabolite columbianetin (43) in plasma and this was caused by CBG (44). Cumulative excretion of columbianetin acetate (41) in urine accounted for 0.0109 ± 0.0067% of total dosage. The cumulative amounts of columbianetin acetate (41) in the feces present 9.32 ± 6.63% of the total dose. Columbianetin acetate (41) was mainly excreted in the feces (Jiao et al., 2015). Besides, CBG (44) also could be catabolized into its active metabolite columbianetin (43) in vivo. The absolute bioavailability of CBG (44) was 5.63 ± 4.42%. The other co-existing constituents from the APR ethanol extract could enhance the absorption of CBG (44). CBG (44) and columbianetin (43) were rapidly and broadly distributed in the stomach, ovary, kidney, liver, spleen, lung, muscles, heart and brain. Higher levels of accumulation of CBG (44) and columbianetin (43) were detected in the ovary and kidney tissues. Eight metabolites of CBG (44) were tentatively identified in blood, urine, bile and faeces of rats after oral administration of pure CBG (44). It was also found that CBG (44) and columbianetin (43) were mainly excreted through the faecal route (Zhang et al., 2018).
Toxicology
In ancient and modern books of traditional Chinese medicine including with APR, there was no record of toxicity. The records of toxicity also have not been reported in clinical applications. So far, only a few toxicity tests have been conducted in animals.
In acute toxicity experiments through oral administration, mice of the Kunming species appeared to have cyanosis, agitated activity, and a quickened respiration after 10 min of taking water decoction of APR, and several mice died because of respiratory failure in serious cases. The LD50 of mice (i.g.) was determined to be 7.35 ± 0.62g/kg (Duan et al., 2002). In subacute toxicity testing, WAPR has a certain nephrotoxicity, potentially inducing kidney injury through inhibiting organic anion transporters 1 (Oats1), Oat2, Oat3 (Chen et al., 2015). WAPR also can cause obvious organ toxicity to juvenile zebrafish, such as yolk sac swelling, deformation, black, pericardial edema, and bleeding, with a minimum lethal dose of 100 μg/ml at 3 days after fertilization in healthy zebrafish embryos (Chen et al., 2016). In a 90-days toxicity experiment by mouth, Wistar rats and hybrid dogs were selected. Among them, rats appeared to have cyanosis, agitated activity, and quicken respiration after 10 min of taking APR capsule; however, toxicity symptoms in the rats disappeared after 30 days of taking WAPR. At higher doses of WAPR, the rats grew slower, and pathology inspection revealed gaseous distention in the stomach and edema of mucous membrane. While there were myeline bodies in the liver cells of some rats and dogs, it appeared to be reversible. No differences in the blood and biochemistry indexes of rats and dogs were observed (Duan et al., 2002).
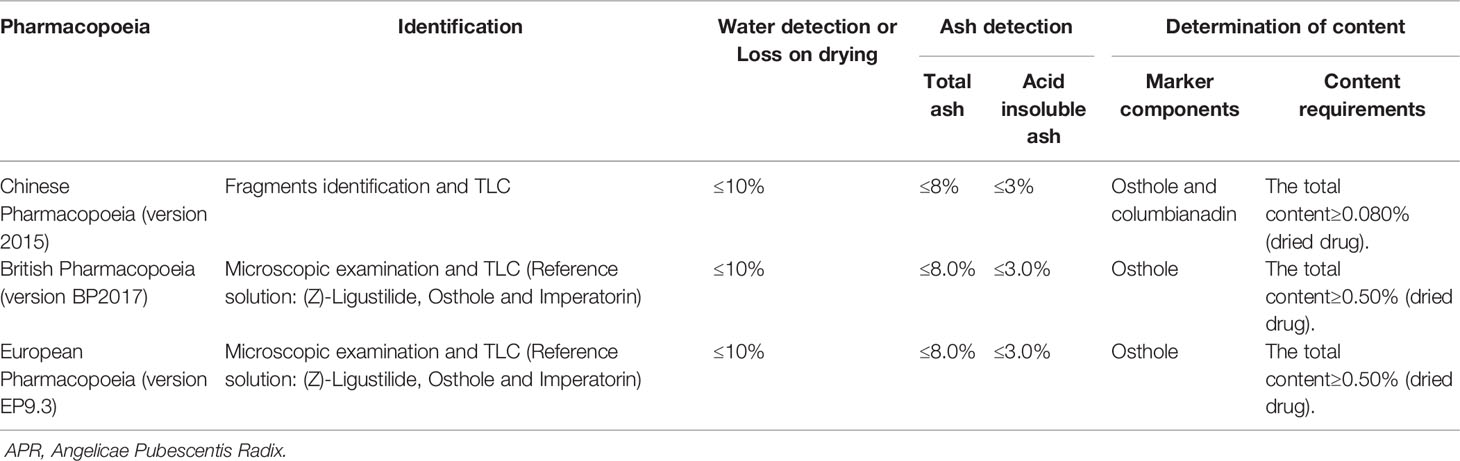
Quality Control
APR has special aromatous smell, bitter and spicy taste, and makes feeling slightly numbness of tongue. It is traditionally thought that APR with thick and fat branches, strong aroma is of good quality. From the phytochemistry and the pharmacological activity discussed above, coumarins are considered the most important constituents, demonstrating a wide range of pharmacological activities. In Chinese Pharmacopoeia (2015 edition), APR is calculated by dry product, containing osthole (17) at not less than 0.50% and columbianadin (40) at not less than 0.080%. What's more, quality control items for APR in multi-national Pharmacopoeias are listed in Table 5.
APR primarily contains coumarins and volatile oils as the active ingredients (Zhang et al., 2007). Near IR spectroscopy (NIRs) became a new analysis technique for the quantitative analysis of Chinese medicinal materials because of its rapid analysis speed and no need for sample pretreatment. NIRs also can achieve quantitative analysis for the quality control of osthole (17) and columbianadin (40) as indicators in APR, except for HPLC (Zhan et al., 2017). Modern pharmacological research has shown that ferulic acid (75) is also the main effective component, providing the effect of activating the blood. However, its content has not been used as an index in Chinese Pharmacopoeia, which is not conducive to the quality control of APR. Therefore, the method for the determination of ferulic acid (75) content in APR was established by reverse HPLC (Fang, 2013). Bisabolangelone (80), adenosine (78), and 30 coumarins in APR were identified by UPLC-PAD-Q-TOF-MS. The developed method could successfully be used to differentiate samples from different regions and could be a helpful tool for detection and confirmation of the quality of TCM (Ge et al., 2014). However, one or several chemical constituents are not the only effective parts of APR. The HPLC fingerprint of APR from different producing areas was constructed with 21 common chromatographic peaks, and the content of columbianetin (43), osthole (17), isoimperatorin (55), and columbianadin (40) were determined in APR simultaneously (Wang et al., 2018b). The GC fingerprint of APR was also established (Jin et al., 2009).
Conclusion and Future Perspectives
A significant breakthrough has been made in the last several decades in the areas of phytochemistry and pharmacology of APR. Research on phytochemistry indicated that coumarins and essential oil compounds might be the major active constituents. Crude extracts and pure compounds from the root have been shown to possess multiple biological activities, particularly analgesic and anti-inflammatory actions, as well as effects on the central nervous system. However, further study is still urgently needed to gain a better understanding of APR and its clinical use.
Firstly, 87 compounds, mainly coumarins, have been separated and identified, and more than 100 volatile components have been analyzed by GC-MS, but they were mainly separated from root of A. biserrata. Therefore, in further research on the phytochemistry of this plant, more attention should be focused on the others parts, such as rhizome, fruit. Besides, the water-soluble part of APR should be a major focus, because decoction is the main method of administration in the TCM clinic. The majority of pharmacological studies of APR have been conducted using crude and poorly characterized extracts. Thus, bioactivity-guided isolation strategies could be used to study the chemicals underlying the pharmacological activity. To illustrate the scientific significance of medicines' using, network pharmacology should be used to predict drug targets and the possible molecular mechanisms involved. Then experiments should be further conducted to prove whether the targets and signal path works.
Secondly, studies on pharmacological effects and its mechanisms mainly focus on several coumarins, and the inherent relations between them and the mechanism of treatment of disease have not been explicit enough. More relationships between the chemical composition and pharmacological activity should be established to further explain the principle of disease treatment. Moreover, the pathways of their distribution, absorption, metabolism, and excretion need to be further clarified with pharmacokinetic studies.
Thirdly, records of toxicity were rarely recorded in ancient clinical applications; however, modern pharmacological research has related acute toxicity in animal experiments. Meanwhile, it should be noticed that most pharmacological studies on APR have only been conducted in animal models, cell models, and other in vitro experiments. Therefore, comprehensive placebo-controlled and double-blind clinical trials should be undertaken to provide remarkable evidence for these positive findings on the efficacy of APR in the future. In addition, the exact mechanisms of many medicinal properties of this herb still remain vague to date; thus, additional studies to better identify the functions and molecular targets seem to be necessary.
Next, the result of the research on plant resources found that A. biserrata had been mistaken as A. pubescens in China. At present, A. pubescens is only a medicinal plant for local use with a few researches, and the specific difference between the two medicinal plants in clinical use have not been mentioned. In order to standardize the use of traditional Chinese medicine, it is necessary to study the specific differences in the clinical use and pharmacological effect of the two medicinal plants.
Lastly, the natural resources of APR are limited, and the demand is increasing year by year. Since April 2018, the State Administration of Traditional Chinese Medicine of the People's Republic of China issued a list of Chinese classical prescriptions (first batch), in which six prescriptions contain APR. This means that the needs of APR will continue to grow. In the course of the determination of the content, our laboratory collected some unqualified cultivation products, so we considered that it might be caused by irregular planting methods. So, the safe, high quality and high efficiency planting technology of this unique living plant needs to be further studied in order to guide the production of TCM.
In summary, APR has been successfully used in clinical practice to treat rheumatic disease as an anodyne for thousands of years. Indeed, modern pharmacological studies have shown that it has effective analgesic and anti-inflammatory actions, as well as effects on the central nervous system. The linkage between traditional uses and modern scientific studies, safety, and efficacy were done on this herb ensuring its clinical use and application.
Author Contributions
YL and HW searched the literature, collected the data, and drafted the manuscript. YL and ZW contributed to analysis and manuscript preparation. XY, XZ, and HL helped in checking the chemical structures and formula. YL and LT downloaded the documents and made classification. ZW and LT contributed comments for a version of the manuscript. All authors read and approved the final manuscript.
Funding
The work was supported by grants from the National Key Research and Development Plan (2018YFC1707106), the Key Research and Development Plan of Shandong Province in 2018 (No. 2018CXGC1305) and National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2019ZX09201005). Basic scientific research project of the Institute of Chinese Materia Medica, China Academy of Chinese Medical Science (ZXKT17014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the Institute of Chinese Materia Medica, China Academy of Chinese Medical Science for providing support and assistance for this review article. And the authors are grateful to Enago for providing language assistance and grammar check.
References
Awale, S., Nakashima, E. M., Kalauni, S. K., Tezuka, Y., Kurashima, Y., Lu, J., et al. (2006). Angelmarin, a novel anti-cancer agent able to eliminate the tolerance of cancer cells to nutrient starvation. Bioorg. Med. Chem. Lett. 37, 581–583. doi: 10.1016/j.bmcl.2005.10.046
Baba, K., Matsuyama, Y., Ishida, T., Inoue, M., Kozawa, M. (1982). Studies on coumarins from the root of Angelica pubescens Maxim. V. Stereochemistry of angelols A-H. Chem. Pharm. Bull. 30, 2036–2044. doi: 10.1248/cpb.30.2036
Chang, Y., Zhang, Q., Li, J., Zhang, L., Guo, X., He, J., et al. (2013). Simultaneous determination of scopoletin, psoralen, bergapten, xanthotoxin, columbianetin acetate, imperatorin, osthole and isoimperatorin in rat plasma by LC-MS/MS for pharmacokinetic studies following oral administration of Radix Angelicae Pubescentis extract. J. Pharm. Biomed. 77, 71–75. doi: 10.1016/j.jpba.2012.12.031
Chang, Y., Wang, C., Li, J., Bai, Y., Luo, Q., He, J., et al. (2014). LC–MS/MS determination and pharmacokinetic study of columbianadin in rat plasma after intravenous administration of pure columbianadin. Chem. Cent. J. 8 (1), 64. doi: 10.1186/s13065-014-0064-1
Chen, Y. F., Tsai, H. Y., Wu, T. S. (1995). Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 61, 2–8. doi: 10.1055/s-2006-957987
Chen, S., Qi, J., Yu, W., Wang, Y., Lin, M., Zhang, N. (2015). Effects of 10 kinds of nephrotoxic TCM on kidney organic anion transporter in mice. China Pharm. 26, 2673–2676. doi: 10.6039/j.issn.1001-0408.2015.19.25
Chen, Y., Wang, J., Chen, S., Jing, L., Wei, Y. (2016). Screening on toxicity of 26 kinds of common orthopedic herbal medicine using Zebrafish model. J. Nanjing Univ. Tradit. Chin. Med. 32, 465–469. doi: 10.14148/j.issn.1672-0482.2016.0465
Committee for the Pharmacopoeia of PR China (2015). Pharmacopoeia of the People"s Republic of China, Part1 (Beijing, China: China Medical Science Press), 263.
Ding, X., Feng, X., Dong, Y., Zhao, X., Chen, Y., Wang, M. (2008). Studies on chemical constituents of the roots of Angelica pubescens. Chin. Med. Mat. 31, 516–518. doi: 10.3321/j.issn:1001-4454.2008.04.017
Ding, X., Feng, X., Dong, Y., Zhao, X., Chen, Y., Liang, Y. (2009). Studies on the constituents of coumarins from Duhuo. Chin. Tradit. Pat. Med. 31, 1102–1104. doi: 10.3969/j.issn.1001-1528.2009.07.036
Du, J., Gao, S., Wang, C., Yang, X., Liu, Y., Wang, B. (2009). Duhuo's effect on the positioning navigation ethology of the AD model rat. J. Liaoning Univ. Tradit. Chin. Med. 36, 144–145. doi: 10.13192/j.ljtcm.2009.01.148.dujg.064
Duan, Z., Zhang, Y., Li, H., Wang, J., Zhang, L., Sheng, W. (2002). The research on acute and long-time toxicity of Angelicaroot Capsule. J. Shenyang Pharm. Univ. 4, 4–9. doi: 10.3969/j.issn.1008-2344.2002.01.002
Fan, L., Li, L., He, H. (2009). Pharmacological studies on anti-inflammatory and analgesic effects of Radix Angelicae Pubeascentis' s volatile oil. Anhui Med. Pharm. J. 13, 133–134. doi: 10.3969/j.issn.1009-6469.2009.02.007
Fang, Y. (2013). Ferulic acid content of Angelica pubescens in Three Gorges Reservoir area. Med. Plant 4, 38–40.
Gao, X. M. (2007). Science of Chinese materia medica. (Beijing, China: China Press of Traditional Chinese Medicine), 651–653.
Ge, A., Ma, W., Wang, C., Li, J., He, J., Liu, E., et al. (2014). Ultra-high-performance-liquid chromatography with photodiode array detector and quadrupole time-of-flight tandem mass spectrometry coupled with discriminant analysis to evaluate Angelicae Pubescentis Radix from different regions. J. Sep. Sci. 37, 2523–2534. doi: 10.1002/jssc.201400289
Ge, Y., Li, Z., Zhang, L., Li, J., He, J., Hao, J., et al. (2018). Pharmacokinetics and tissue distribution study of bisabolangelone from Angelicae Pubescentis Radix in rat using LC–MS/MS. BioMed. Chromatogr. 33, e4433. doi: 10.1002/bmc.4433
Ge, Y., Chen, S., Luo, Q., Wang, C., Hao, J., He, J., et al. (2019). The tissue distribution of four major coumarins after oral administration of Angelicae Pubescentis Radix extract to rats using ultra-high-performance liquid chromatography. Evid.-Based Complementary Altern. Med. 2019, 8. doi: 10.1155/2019/2365697
Hata, K., Kozawa, M. (1965). The constitution of angelol, a new coumarin isolated from the root of Angelica pubescens Maxim (umbelliferae). Tetrahedron Lett. 6 (50), 4557–4562. doi: 10.1016/S0040-4039(01)89063-8
Hata, K., Tanaka, Y. (1957). Study on the chemical components of umbelliferous plants. III. Components of the root of Angelica pubescens Maxim (1). Yakugaku Zasshi J. Pharm. Soc. Japan 77, 937–940. doi: 10.1248/yakushi1947.77.9_937
Hata, K., Nishino, T., Hirai, Y., Wada, Y., Kozawa, M. (1981). On Coumarins from the fruits of Angelica pubescens Maxim. Yakugaku Zasshi J. Pharm. Soc. Japan 101, 67–71. doi: 10.1248/yakushi1947.101.1_67
Hu, Y., Zhao, D., Zhang, X., Sun, D., Hao, H., Yang, J. (2013a). Different extracts of Angelica pubescens inhibit H2 O2-induced SH-SY5Y cells injury. Chin. J. Exp. Tradit. Med. Form. 19, 184–188. doi: 10.11653/syfj2013240184
Hu, J., Lin, L., Qian, X., Liu, B., Zhang, G., Hu, W., et al. (2013b). Experimental study of Angelica pubescens and osthole isolated from Angelica pubescens inhibiting angiogenesis in vitro. J. Mod. Oncol. 21, 1945–1949. doi: 10.3969/j.issn.1672-4992.2013.09.11
Institute of Materia Medica, Chinese Academy of Medical Sciences (1959). Chinese medicinal records, Volume II (Beijing, China: People's Medical Publishing House), 345–352.
Jia, D., He, X., Jiang, M., Yi, X., Zhao, N., Tan, Y., et al. (2015). Prediction of molecular mechanism of Radix Angelicae Pubescentis in treatment of rheumatoid arthritis by network pharmacology. J. Liaoning Univ. Tradit. Chin. Med. 42, 1838–1841. doi: 10.13192/j.issn.1000-1719.2015.10.005
Jiao, Y., Hu, Y., Zhao, D., Sun, D., Hao, H., Yang, J. (2014). The neuroprotective effect of total coumarins of Angelica Pubescentis on APP/PS1 double transgenic AD model mouse. Chin. Med. Pharmaco Clinic. 30, 67–70. doi: 10.13412/j.cnki.zyyl.2014.05.022
Jiao, X., Li, J., Yu, X., Liu, W., Tian, J., He, J., et al. (2015). The pharmacokinetics, bioavailability and excretion of columbianetin acetate and its metabolite columbianetin were analysed in rat plasma by LC-MS/MS after administration of columbianetin acetate and Angelicae Pubescentis Radix extract. RSC Adv. 5, 95882–95893. doi: 10.1039/C5RA13961A
Jin, H., Li, D., Sun, S. (2003). Experimental research into the mechanism in delaying brain aging process by Angelica pubescens and its alcohol extractive. Shanghai J. Tradit. Chin. Med. 37, 54–56. doi: 10.3969/j.issn.1007-1334.2003.11.025
Jin, H., Chen, X., Zhang, S., Li, Q., Bi, K. (2009). Establishment of chromatographic fingerprint and dentification of Rhizoma et Radix Notopterygii and Radix Angelicae Pubescentis by GC. J. Shenyang Pharm. Univ. 26, 369–375. doi: 10.14066/j.cnki.cn21-1349/r.2009.05.007
Kosuge, T., Yokota, M., Sugiyama, K., Yamamoto, T., Mure, T., Yamazawa, H. (1985). Studies on Bioactive Substances in Crude Drugs Used for Arthritic Diseases in Traditional Chinese Medicine. II. Isolation and Identification of an Anti-inflammatory and Analgesic Principle from the Root of Angelica Pubescens Maxim. Chem. Pharm. Bull. 33, 5351–5354. doi: 10.1248/cpb.33.5351
Kozawa, M., Baba, K., Matsuyama, Y., Hata, K. (1980). Studies on coumarins from the root of Angelica pubescens Maxim. III. Structures of various coumarins including angelin, a new prenylcoumarin. Chem. Pharm. Bull. 28, 1782–1787. doi: 10.1248/cpb.28.1782
Li, R., He, Y., Zhang, Q., Meng, J., Wang, L., Gu, Y. (1989a). Study on γ-aminobutyric acid, an antiarrhythmic active component of traditional Chinese medicine Duhuo. J. Beijing Med. Univ. 21, 376.
Li, R., He, Y., Qiao, M., Xu, Y., Zhang, Q., Meng, J., et al. (1989b). Studies of the active constituents of the Chinese drug Duhuo Angelica pubescens. Acta Pharm. Sin. 24, 546–551.
Li, X., Wang, J., Gao, L. (2013). Anti-inflammatory and analgesic activity of R.A.P. (Radix Angelicae Pubescentis) ethanol extracts. Afr J. Tradit. Complem. 10, 422–426. doi: 10.4314/ajtcam.v10i3.6
Li, R., Zhao, C., Yao, M., Wu, Y., Song, Y., Wen, A. (2017). Analgesic effect of coumarins from Radix Angelicae Pubescentis is mediated by inflammatory factors and TRPV1 in a spared nerve injury model of neuropathic pain. J. Ethnopharmacol. 195, 81–88. doi: 10.1016/j.jep.2016.11.046
Li, J., Li, Z., Luo, Q., Wang, C., He, J., Pang, X., et al. (2018). Simultaneous determination of columbianadin and its metabolite columbianetin in rat plasma by LC-MS/MS: application to pharmacokinetics of columbianadin after oral administration. Evid.-Based Complementary Altern. Med. 2018, 8. doi: 10.1155/2018/8568303
Li, M., Wen, J., Ni, F., Xue, X., Wu, Y., Wang, Z., et al. (2019). Anti-inflammatory activity of two new sesquiterpenoids from Radix Angelicae Pubescentis. Acta Pharm. Sin. 54 (02), 173–177. doi: 10.16438/j.0513-4870.2018-0960
Li, J. (2013). Preparation method of oil tea emulsion from Angelica pubescens Maxim. (China, CN103271846A: State Intellectual Property Office).
Lin, P., Jia, X., Qi, Y., Liao, S. (2017). Advances in study on phenolic acids. Guangdong Chem. Ind. 44, 50–52. doi: 10.3969/j.issn.1007-1865.2017.01.025
Liu, J., Yao, X. (1995). Studies on the chemical constituents of Angelica pubescens f. biserrata and its Anti-human platelet and Anti-inflammatory effects. J. Shenyang Pharm. Univ. 12, 116. doi: 10.14066/j.cnki.cn21-1349/r.1995.02.010
Liu, J., Tan, Y., Chen, Y., Xu, S., Yao, X. (1994). Studies on the chemical constituents of Angelica pubescence f. biserrata. Chin. Tradit. Herbal Drugs 25, 288–291.
Liu, J., Xu, S., Yao, X., Kobayashi, H. (1995a). Two new 6-Alkylcoumarins from Angelica pubescens f. biserrata. Planta Med. 61, 482–484. doi: 10.1055/s-2006-958145
Liu, J., Xu, S., Yao, X., Kobayashi, H. (1995b). Angelol-type coumarins from Angelica pubescence f. biserrata and their inhibitory effect on platelet aggergation. Phytochemistry. 39, 1099–1101. doi: 10.1016/0031-9422(95)00045-9
Liu, J., Xu, S., Yao, X., Kobayashi, H. (1996a). Studies of NMR and MS on 6- or 8-alkyl-7-oxycoumarins and dihydrofuranocoumarins. Chin. J. Magn. Reson. 13, 35–46. doi: 10.1007/BF02943147
Liu, J., Xu, S., Yao, X., Wu, Y. (1996b). Further studies on chemical constituents of Angelica pubescens f. biserrata. Acta Pharm. Sin. 31, 63–67. doi: 10.16438/j.0513-4870.1996.01.014
Liu, J., Zschocke, S., Bauer, R. (1998a). A polyacetylenic acetate and a coumarin from Angelica pubescens f. biserrata. Phytochemistry. 49, 211–213. doi: 10.1016/S0031-9422(97)00951-5
Liu, J. H., Zschocke, S., Reininger, E., Bauer, R. (1998b). Comparison of Radix Angelicae Pubescentis and substitutes. constituents and inhibitory effect on 5-lipoxygenase and cyclooxygenase. Pharm. Biol. (Lisse Netherlands) 36, 207–216. doi: 10.1076/phbi.36.3.207.6343
Lu, J., Fang, K., Xiong, L., Zhang, C., Guan, X., Zheng, R., et al. (2018). Anti-Inflammatory effect of columbianetin on Lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Mediat. Inflamm. 2018, 1–8. doi: 10.1155/2018/9191743
Luo, Q., Wang, C., Li, J., Ma, W., Bai, Y., Ma, L., et al. (2013). The pharmacokinetics and oral bioavailability studies of columbianetin in rats after oral and intravenous administration. J. Ethnopharmacol. 150, 175–180. doi: 10.1016/j.jep.2013.08.030
Meng, J., Gu, Y., Ge, L., Li, R., He, Y. (1988). Effect of alcohol extract (H6F4) on platelet aggregation and experimental thrombosis. Chin. Tradit. Herbal Drugs 19, 23–25.
Murata, C., Masuda, T., Kamochi, Y., Todoroki, K., Yoshida, H., Nohta, H., et al. (2005). Improvement of fluorescence characteristics of coumarins: Syntheses and fluorescence properties of 6-methoxycoumarin and benzocoumarin derivatives as novel fluorophores emitting in the longer wavelength region and their application to analytical reagents. Chem. Pharm. Bull. 53, 750–758. doi: 10.1002/chin.200549022
Pan, J., Lamy, K., Arison, B., Smith, J., Han, G. (1987). Isolation and identification of isoangelol, anpubesol and other coumarins from Angelica Pubescens Maxin. Acta Pharm. Sin. 22, 380–384. doi: 10.16438/j.0513-4870.1987.05.013
Pei, Y., Li, D., Sun, S. (2005). Effects of Duhuo and its alcohol extracts on apoptosis of brain tissue in natural aging mice. Chin. J. Gerontol. 25 (8), 959. doi: 10.3969/j.issn.1005-9202.2005.08.044
Qian, B. (2014). A kind of internal injury fever and Angelica pubescens Maxim health wine. (China, CN103720991A: State Intellectual Property Office).
Rao, G., Yang, Q., Dai, W. (1994). Herb evolution and plant origin of Chinese medicine Duhuo and Qianghuo. J. Yunnan Univ. Tradit. Chin. Med. 17 (4), 11–16. doi: 10.19288/j.cnki.issn.1000-2723.1994.04.003
Shan, F., Yuan, Y., Hao, J., Huang, L. (2014). Herbal textual research on origin and development of traditional Chinese medicine Duhuo and Qianghuo. Chin. J. Chin. Mater. Med. 39, 3399–3403. doi: 10.4268/cjcmm20141740
Sun, W., Yang, L., Qiu, Y., Ren, J., Huang, R., Fu, J. (2011). Identify nature N-acylethanolamide-hydrolyzing acid amide (NAAA) inhibitor: effect of Angelicae Pubescentis Radix on anti-inflammation. Chin. J. Chin. Mater. Med. 36, 3161–3166. doi: 10.4268/cjcmm20112221
Sun, D., Xu, X., Yan, S., Song, X., Li, X. (2014). Analysis on chemical components from water extract of Angelicae Pubescentis Radix by high performance liquid chromatography-electrospray Ionization-quadrupole-time of flight-mass spectrometry. Nat. Prod. Res. Dev. 23, 69–76. doi: 10.16333/j.1001-6880.2014.01.019
Teng, C., Lin, C., Ko, F., Wu, T., Huang, T. (1994). The relaxant action of osthole isolated from Angelica pubescens in guinea-pig trachea. Naunyn-Schmiedeberg's Arch. Pharmacol. 349, 202–208. doi: 10.1007/BF00169838
Wang, C., Shen, P., Hu, Y. (2009). Symptoms of rheumatism. (Beijing, China: People's Medical Publishing House), 22–33.
Wang, Z., Shen, Y., Chen, Y., Yao, X. (1988). Studies on the active principles of Angelica pubescens Maxim. J. Shenyang Pharm. Univ. 5 (3), 183–188. doi: 10.14066/j.cnki.cn21-1349/r. 1988.03.005.
Wang, C., Cui, J., Zhu, M., Weng, J., Yu, M. (2011a). Experimental study on the regulation of IL-4 and TNF-α in rats with AD and VD by Chinese medicine Duhuo. J. Liaoning Univ. Tradit. Chin. Med. 38, 1701–1702. doi: 10.13192/j.ljtcm.2011.09.8.wangcx.070
Wang, X., Qiu, J., Li, S. (2011b). Rapid GC-MS analysis of essential oils in Radix Angelicae Biseratae. J. Yanbian Univ. (Nat. Sci.) 37, 128–131. doi: 10.3969/j.issn.1004-4353.2011.02.008
Wang, K., Yao, L., Du, Y., Xie, J., Huang, J., Yin, Z. (2011c). Anthelmintic activity of the crude extracts, fractions, and osthole from Radix Angelicae Pubescentis against Dactylogyrus intermedius in goldfish (Carassius auratus) in vivo. Parasitol. Res. 108, 195–200. doi: 10.1007/s00436-010-2058-9
Wang, K., Yao, L., Xie, J., Huang, J., Miao, C. (2012). Effect of active monomer from Radix Angelicae Pubescentis on killing Dactylogyrus intermedius and its active component identification. Acta Hydrobiol. Sinica 36, 93–101. doi: 10.3724/SP.J.1035.2012.00093
Wang, B., Liu, X., Zhou, A., Meng, M., Li, Q. (2014). Simultaneous analysis of coumarin derivatives in extracts of Radix Angelicae Pubescentis (Duhuo) by HPLC-DAD-ESI-MSn technique. Anali. Methods 6, 7996–8002. doi: 10.1039/C4AY01468E
Wang, J., Shi, M., Zheng, P., Xue, S., Peng, R. (2018a). Quantitative Analysis of Ca, Mg, and K in the roots of Angelica pubescens f. biserrata by Laser-induced breakdown spectroscopy combined with artificial neural networks. J. Appl. Spectrosc. 85, 190–196. doi: 10.1007/s10812-018-0631-7
Wang, J., Tan, J., Li, L., Liu, J., Zhang, J. (2018b). HPLC fingerprint of Angelica Pubescens Radix and determination of four kinds of coumarin. Chin. J. Pharm. Anal. 6, 955–963. doi: 10.16155/j.0254-1793.2018.06.06
Wang, F. (2013). Grass oil emollient water from Angelica pubescens Maxim. (China, CN103340784A: State Intellectual Property Office).
Wang, L. (2014). A kind of material for removing wind and dehumidifying hot pot and its production method of Angelica pubescens Maxim. (China, CN103798715A: State Intellectual Property Office).
Wei, F., Zou, S., Young, A., Dubner, R., Ren, K. (1999). Effects of four herbal extracts on adjuvant-induced inflammation and hyperalgesia in rats. J. Altern. Complem. Med. (New York N.Y.) 5, 429–436. doi: 10.1089/acm.1999.5.429
Weng, J., Wang, C., Yang, W. (2012). Experiment research on protective effect of Duhuo on free radical of rats with Vascular Dementia. Chin. Arch. Tradit. Chin. Med. 30, 899–900. doi: 10.13193/j.archtcm.2012.04.229.wengj.065
Wu, Q., Li, R. (1993). Studies on the chemical constituents of Duhuo. Chin. Tradit. Herbal Drugs 24, 3. 48.
Xie, J., Yao, L., Li, C., Chen, D., Liang, L. (2016). Study on the active site and extraction method of Duhuo in killing Dioscorea japonicus. Veter. Orientat. 39 (12), 211–212. doi: 10.3969/j.issn.1673-8586.2016.12.202
Xiong, F. (2014). High-yield cultivation techniques for Angelicae Pubescentis Radix seedling transplanting. Sci. Breed. 4, 17–18. doi: 10.3969/j.issn.1673-3339.2014.04.011
Yan, Y., Li, S., Kong, L., Jiao, Y., Yao, Y., Tao, Z., et al. (2016). Neuroprotective effects of total coumarins in Angelica pubescens against Aβ-Induced neurons damage. J. Liaoning Univ. Tradit. Chin. Med. 43, 1714–1717. doi: 10.13192/j.issn.1000-1719.2016.08.052
Yan, Y., Li, S., Li, H., Lin, Y., Yang, J. (2017). Osthole protects bone Marrow-Derived neural stem cells from oxidative damage through PI3K/Akt-1 pathway. Neurochem. Res. 42, 398–405. doi: 10.1007/s11064-016-2082-y
Yang, X., Liu, Y., Tao, H., Yang, Z., Xiao, S. (2006). GC-MS analysis of essential oils from Radix Angelicae Pubescentis. Chin. J. Chin. Mater. Med. 31, 663–666. doi: 10.3321/j.issn:1001-5302.2006.08.012
Yang, X., Guo, Q., Zhang, C., Zhang, B. (2008). Further studies on the chemical constituents of the root of Angelica pubescens f. biserrata. Pharm. J. Chin. Pla. 24, 389–392. doi: CNKI:SUN:JFJN.0.2008-05-005
Yang, X. W., Zhang, C. Y., Zhang, B. G., Lu, Y., Luan, J. W., Zheng, Q. T. (2009). Novel coumarin and furan from the roots of Angelica pubescens f. biserrata. J. Asian Nat. Prod. Res. 11, 698–703. doi: 10.1080/10286020802619140
Yang, Y., Zhang, L., Zhang, Y., Yang, X. (2017). Simultaneous assessment of absorption characteristics of coumarins from Angelicae Pubescentis Radix: In vitro transport across Caco-2 cell and in vivo pharmacokinetics in rats after oral administration. J. Chromatogr. B. 1060, 308–315. doi: 10.1016/j.jchromb.2017.06.020
Yang, Y., Zhang, L., Yang, X. (2018). Distribution assessments of coumarins from Angelicae Pubescentis Radix in rat cerebrospinal fluid and brain by Liquid Chromatography Tandem Mass Spectrometry analysis. Molecules. 23, 225–236. doi: 10.3390/molecules23010225
Yang, W. (2007). Divide the light intensity of light method to measurese Radix Angelcae Pubesentis inside the flavonoid's research. Sci. Technol. Inf. 23 (36), 391. 200. doi: CNKI:SUN:KJXX.0.2007-36-299
Yao, L., Lu, P., Li, Y., Yang, L., Feng, H., Huang, Y., et al. (2013). Osthole relaxes pulmonary arteries through endothelial phosphatidylinositol 3-kinase/Akt-eNOS-NO signaling pathway in rats. Eur. J. Pharmacol. 699, 23–32. doi: 10.1016/j.ejphar.2012.11.056
Yao, L., Yang, Y., He, G., Ou, C., Wang, L., Liu, K. (2018). Global Proteomics Deciphered Novel-Function of Osthole Against Pulmonary Arterial Hypertension. Sci. Rep.-UK 8 (1), 5556. doi: 10.1038/s41598-018-23775-8
Zaugg, J., Eickmeier, E., Rueda, D. C., Hering, S., Hamburger, M. (2011). HPLC-based activity profiling of Angelica pubescens roots for new positive GABA A receptor modulators in Xenopus oocytes. Fitoterapia 82, 434–440. doi: 10.1016/j.fitote.2010.12.001
Zhan, H., Fang, J., Yang, B., Fu, M., Liu, M., Li, H., et al. (2017). Determination of osthole and columbianadin in Radix Angelicae Pubescentis with near infrared spectroscopy. Spectrosc. Spect. Anal. (Beijing) 37, 1110–1113. doi: 10.3964/j.issn.1000-0593(2017)04-1110-04
Zhang, J., Du, W. (2010). Effect of Duhuo on p38MAPK signal transduction pathway in brain of Dementia rats. Chin. J. Gerontol. 30, 1514–1515. doi: 10.3969/j.issn.1005-9202.2010.11.020
Zhang, L., Liang, M. (2018). Exploration and analysis on the potential function of Radix Angelicae Pubescentis. Chin. J. Tradit. Chin. Med. Pharm. 33, 46–49. doi: 10.1016/S0254-6272(13)60099-0
Zhang, C., Zhang, B., Yang, X. (2007). Studies on chemical consituents of the root of Angelica pubescens f. biserrata. Med. Pharm. J. Chin. Pla. 23, 241–245. doi: 10.3969/j.issn.1008-9926.2007.04.001
Zhang, Z., Leung, W., Li, G., Kong, S., Lu, X., Wong, Y., et al. (2017). Osthole enhances osteogenesis in osteoblasts by elevating transcription factor osterix via cAMP/CREB signaling in vitro and in vivo. Nutrients. 9, 588. doi: 10.3390/nu9060588
Zhang, L., Ge, Y., Li, J., Hao, J., Wang, H., He, J., et al. (2018). Simultaneous determination of columbianetin-β-D-glucopyranoside and columbianetin in a biological sample by High-performance liquid chromatography with fluorescence detection and identification of other columbianetin-β-D-glucopyranoside metabolites by ultra-high-performance-liquid-chromatography coupled with quadrupole-time of flight mass spectrometry. J. Pharm. Biomed. 153, 221–231. doi: 10.1016/j.jpba.2018.02.055
Zheng, C., Fu, C., Ye, J., Wu, G., Li, X., Ye, H., et al. (2017). A computer simulation study of heracleum for treatment of osteoarthritis pain. J. Trad. Chin. Orthop. Trauma 29, 1–4. doi: 10.3969/j.issn.1001-6015.2017.07.001
Zhu, M., Cui, J., Wang, C. (2009). The experimental study of the intervention effect of Duhuo on immune damage of model rats suffering from Alzheimer. J. Liaoning Univ. Tradit. Chin. Med. 38, 2085–2086. doi: 10.13192/j.ljtcm.2011.10.168.zhumd.063
Zou, X., Wang, R., Dai, H., Hu, Y. (2008). Experimental study on antiangiogenic effect of Duhuo. J. Nanjing Univ. Tradit. Chin. Med. 24, 194–196. doi: 10.14148/j.issn.1672-0482.2008.03.020
Keywords: Angelicae Pubescentis Radix, phytochemistry, pharmacology, pharmacokinetics, botany, traditional medicinal use, toxicity, quality
Citation: Lu Y, Wu H, Yu X, Zhang X, Luo H, Tang L and Wang Z (2020) Traditional Chinese Medicine of Angelicae Pubescentis Radix: A Review of Phytochemistry, Pharmacology and Pharmacokinetics. Front. Pharmacol. 11:335. doi: 10.3389/fphar.2020.00335
Received: 02 August 2019; Accepted: 06 March 2020;
Published: 18 March 2020.
Edited by:
Wei Zhou, Hospital of Shenzhen University, ChinaReviewed by:
Yi Ding, Fourth Military Medical University, ChinaTao Yi, Hong Kong Baptist University, Hong Kong
Copyright © 2020 Lu, Wu, Yu, Zhang, Luo, Tang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liying Tang, Ymp0YW5nbGl5aW5nQDE2My5jb20=; Zhuju Wang, d2FuZ3podWp1QHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Yaqi Lu
Yaqi Lu Hongwei Wu1†
Hongwei Wu1†