
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 20 March 2020
Sec. Pharmacogenetics and Pharmacogenomics
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00267
 Minyuan Cao1,2†
Minyuan Cao1,2† Dandan Yin2†
Dandan Yin2† Yun Qin3†
Yun Qin3† Fei Liao2
Fei Liao2 Yali Su1
Yali Su1 Xuyang Xia2
Xuyang Xia2 Ju Gao1
Ju Gao1 Yiping Zhu1
Yiping Zhu1 Wei Zhang4
Wei Zhang4 Yang Shu2*
Yang Shu2* Xiaoxi Lu1*
Xiaoxi Lu1*A small proportion of patients with acute lymphoblastic leukemia (ALL) may experience severe leukopenia after treating with 6-mercaptopurine (6MP), which can be largely explained by germline variants in TPMT and NUDT15. However, a minority of patients who suffered such adverse drug reaction have NUDT15wt/wtTPMTwt/wt genotype, indicating that other genetic factors may take part in. In this study, we genotyped 539 exon-located nonsilent pharmacogenetic variants in genes involved in phase I/II of drug metabolism in 173 pediatric patients with ALL and conducted association screening for 6MP-induced leukopenia. Besides NUDT15 (rs116855232, P = 6.4 × 10−11) and TPMT (rs1142345, P = 0.003), a novel variant was identified in CYP2A7 gene (i.e., rs73032311, P = 0.0007), which is independent of NUDT15/TPMT variant. In addition, a variant (i.e., rs4680) in COMT is significantly associated with 6MP-induced hepatotoxicity (P = 0.007). In conclusion, variants in CYP2A7 and COMT may be considered as novel potential pharmacogenetic markers for 6MP-induced toxicities, but additional independent validations with large sample size and investigations on related mechanisms are further needed.
Thiopurine [e.g., 6-mercaptopurine (6MP)] is crucial for curative chemotherapy for pediatric patients with acute lymphoblastic leukemia (ALL), which is the most common childhood cancers around the world (Pui and Evans, 2006). However, 6MP may induce severe toxicities in a small proportion of patients with standard dosage because of its narrow therapeutic index, including hematotoxicity (e.g., leukopenia) and hepatotoxicity (Pui and Evans, 2006; Moriyama et al., 2015). Single-nucleotide polymorphism (SNP) in TPMT (i.e., rs1142345) was first identified as a pharmacogenetic marker through pharmacology-guided approach, explaining the majority of 6MP-induced leukopenia cases in Caucasians and Africans (Relling et al., 1999; Moriyama et al., 2015). Thereafter, genetics-guided 6MP dosage adjustment is applied clinically and largely reduced the incidence of such adverse drug reaction (ADR) (Relling et al., 2013; Relling et al., 2019). However, compared with Caucasians, allele frequency of rs1142345 is lower in East Asians (approximately 1%), which cannot explain the higher incidence of 6MP-induced leukopenia in such population. With the development of genomic technology, novel and an ethnic-specific pharmacogenetic marker in NUDT15 (i.e., rs116855232) has been identified through genome-wide association studies (Yang et al., 2015; Moriyama et al., 2016) and validated by multiple independent studies. Allele frequency of rs116855232 is approximately 10% in East Asians, accounting for a large proportion of 6MP-induced leukopenia, as well as 6MP tolerance with high sensitivity and specificity in such population (Yin et al., 2017; Zhu et al., 2018; Relling et al., 2019; Schaeffeler et al., 2019). Ethnic specificity is observed for rs116855232, because allele of this variant is close to 0% in Caucasians and Africans, which is much lower than that in East Asians. After screening the whole coding region of NUDT15 in patients, less frequent and rare variants in NUDT15 were also identified to be related to 6MP-induced leukopenia, also exhibiting ethnic-specific manner (Moriyama et al., 2017; Zhu et al., 2018). Mechanically, TPMT can anabolize 6MP into the inactive methyl-mercaptopurine (Mcleod et al., 2000), whereas NUDT15 acts as a nucleotide triphosphate diphosphatase, catalyzing the hydrolysis of nucleoside triphosphates, including dGTP and its analogs (e.g., 6MP) (Moriyama et al., 2016). Variants in both genes induce loss of function of their enzymatic activity and the subsequent accumulation of toxic metabolites (i.e., thioguanine nucleotides), resulting in leukopenia (Moriyama et al., 2016). Combination of common and rare functional variants of these two genes can increase the sensitivity for predicting ADR events without largely impacting the specificity (Zhu et al., 2018). However, a minority of ALL patients with hematotoxicity carry wild-type genotypes of both NUDT15 and TPMT, suggesting that other genetic factors may be involved in these patients.
On the other hand, several candidate hypothesis studies illustrated the modest impact of variant in ITPA on 6MP-induced hepatotoxicity (Adam De Beaumais et al., 2011), which exhibits inconsistency in independent patient cohorts. With genome-wide approach, rs738409 at PNPLA3 was identified as a genetic marker to indicate 6MP-induced alanine aminotransferase increase in Caucasians (Liu et al., 2017), with independent validation only in a limited study with Spanish population (Gutierrez-Camino et al., 2017).
Multiple phases I and II enzymes are required for metabolism of most of small molecular drugs. According to the classic pharmacokinetics study, 6MP is an inactive prodrug, intracellularly activated by HPRT1, IMPDH1, and so on (Zimm et al., 1986; Kirschner-Schwabe et al., 2006), while inactivated by TPMT (a phase II enzyme). On the other hand, NUDT15, another inactivated enzyme for 6MP, was recently introduced as a phase I enzyme after identification of its involvement in 6MP-induced leukopenia. Indeed, lots of genetic variants in phase I/II genes have been reported to impact on activity their encoded enzymes and subsequent drug efficacy/safety and translated into genetics-guided precise medication clinically (Relling and Evans, 2015). Whether 6MP-induced leukopenia can be explained by these variants (besides those in NUDT15 and TPMT) is largely unknown, particularly in patients with NUDT15wt/wtTPMTwt/wt genotype.
In this study, we aim to identify independent pharmacogenetic markers of 6MP-induced toxicities through screening the reported variants in phase I/II genes with customer-designed microarray, so as to increase the predictive sensitivity of 6MP-related ADR.
This study consisted of 173 pediatric ALL patients prospectively enrolled onto the CCCG-ALL-2015 protocol-based trial (http://www.chictr.org.cn, identifier ChiCTR-IPR-14005706) from West China Second Hospital between 2015 and 2016. The same patient cohort has been described in our previous report, except that 15 patients were excluded because of the unavailability or quality control failure of their samples. 6-Mercaptopurine was used in the last 2 weeks of remission induction stage (standard dosage of 60 mg/m2), consolidation stage (25 mg/m2), and maintenance stage (50 mg/m2). Forty-seven and 36 patients experienced 6MP-induced leukopenia (decreased white blood cell/neutrophil counts) and hepatotoxicity (five folds of increased aspartate transaminase and/or alanine transaminase), respectively. This study was approved by the ethics committee of West China Second Hospital, Sichuan University, and informed consent was obtained from patients or their guardians, as appropriate.
Genotyping of ALL cases was performed by using customer-designed microarray, containing probes against 539 nonsilent exon-located SNPs in 156 phase I/II genes. Genotype calls of these SNPs were determined by the standard algorithms. Samples with more than 5% of failed genotyped SNPs, as well as SNPs with more than 5% of failed genotyped samples, were excluded from the analyses. Quality control was conducted based on genotyping quality, call rate, minor allele frequency, and Hardy–Weinberg equilibrium of all SNPs. Finally, 253 SNPs were left for association analyses (Supplementary Table 1).
The association of genotypes at each SNP with 6MP-induced leukopenia or hepatotoxicity was tested by comparing the genotype frequency between patients who experienced ADRs and those who did not by using logistic regression model. Independent association was also conducted by using rs116855232 (NUDT15) and rs1142345 (TPMT) as covariants for all patients, as well as in NUDT15wt/wtTPMTwt/wt patients (n = 133) with logistic regression.
Consistent with our previous report, no significant association of clinical characteristic with 6MP-induced leukopenia/hepatotoxicity was found (Table 1). Next, we conducted screening for pharmacogenetics markers from all exon-located SNPs in reported genes involved in phases I and II drug metabolism. In total, 3 SNPs exhibit significant association with 6MP-induced leukopenia (P < 0.01, Table 1), including two well-reported variants at NUDT15 (rs116855232, P = 6.37 × 10−11) and TPMT (rs1142345, P = 0.003), respectively. Interestingly, a novel variant (rs73032311) at CYP2A7 was also identified (P = 0.0007), with risk allele frequency (RAF) of 26.1% versus 10.7% in patients with/without such ADR, respectively. Moreover, rs73032311 exhibits significance after adjusting for rs116855232 and rs1142345 (Padj = 0.027), indicating its independence of predicting 6MP-induced leukopenia. Next, we estimated the impact of rs73032311 in patients who carry no risk allele of NUDT15 and TPMT variants (n = 133) and established the significance in such subgroup of patients with P = 0.04. Risk allele frequency of rs73032311 in NUDT15wt/wtTPMTwt/wt patient with 6MP-induced leukopenia is 27.3% compared to 10.3% in patients without such ADR (Table 1). Additionally, we noticed the P value of rs73032311 increased after adjusting for NUDT15 and TPMT variants (P = 0.0007 vs. 0.02), suggesting that rs73032311 may interact with these two variants. We therefore check the RAF of rs73032311 in patients with heterozygous genotype at either rs116855232 or rs1142345. Interestingly, patients with heterozygous genotype of NUDT15 but not the TPMT variant may have a higher risk to experience 6MP-induced leukopenia when they also carry risk allele of rs73032311. In our study, 100% of patients with NUDT15wt/mutCYP2A7mut/mut (n = 1) and 80% with NUDT15wt/mutCYP2A7wt/mut (n = 15) genotypes experience 6MP-induced leukopenia, compared to 50% NUDT15wt/mutCYP2A7wt/wt (n = 14). Finally, the combination of NUDT15, TPMT, and CYP2A7 variants improves predictive sensitivity of 6MP-induced leukopenia from 70.7% to 85.4%, but decreases the specificity (91.7% vs. 70.7%).
First, we estimated the association of the reported variants with 6MP-induced hepatotoxicity in our patients, including rs1127354 in ITPA and rs738409 in PNPLA3. However, no significance was observed for either of these two variants, with P = 0.89 and 0.55, respectively. Therefore, screening was performed for 6MP-induced hepatotoxicity through the similar strategy described above. Only 1 of 253 variants integrated in our microarray has P < 0.01, located in COMT gene (i.e., rs4680, P = 0.006) (Table 2). Allele frequency of this SNP is 27.54%, with 40.3% of patients experiencing hepatotoxicity compared to 24.2% in those who do not. Taking patients with either heterozygous or homozygous of the risk allele into account, sensitivity and specificity are 52.9% and 66.7%, respectively.
Receiver operating characteristic curves were used to demonstrate the prediction accuracies for 6MP-induced leukopenia and hepatotoxicity in single variant as well as combination. Not surprisingly, rs116855232 in NUDT15 exhibited the best prediction efficacy in a single gene prediction model. Area under the curve (AUC) for 6MP-induced leukopenia with this variant reached 0.82 [95% confidence interval (CI), 0.75–0.90], compared with 0.58 (95% CI, 0.52–0.63) for rs1142345 in TPMT and 0.63 (95% CI, 0.55–0.72) for rs73032311 in CYP2A7. After combining these three SNPs, the prediction accuracy increased with AUC of 0.87 (95% CI, 0.81–0.93) (Figure 1A). In another hand, AUC for 6MP-induced hepatotoxicity with rs4680 in COMT reached 0.62 (95% CI, 0.52–0.71) (Figure 1B).
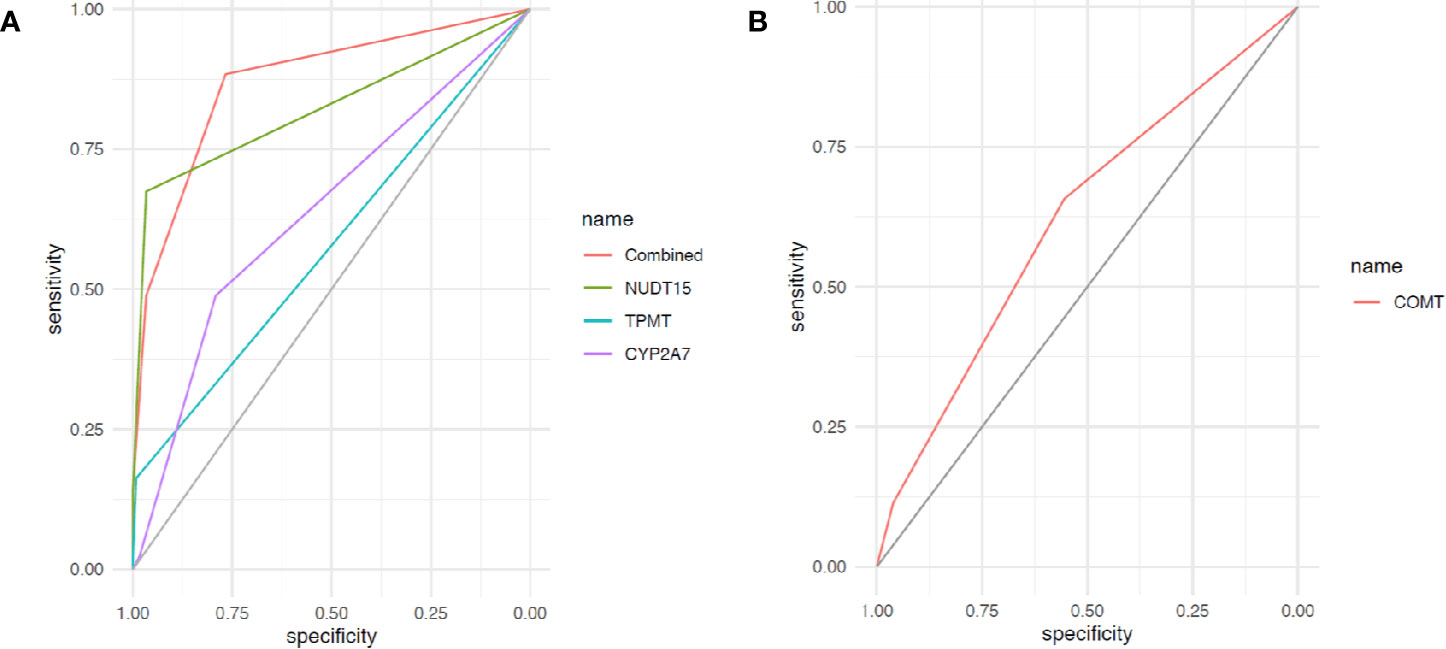
Figure 1 Receiver operating characteristic (ROC) curves of gene variants for 6-mercaptopurine (6MP)–induced ADRs. (A) Receiver operating characteristic curve of variants in NUDT15, TPMT, CYP2A7, and combinations for 6MP-induced leukopenia; (B) ROC curve of variants in NUDT15, TPMT, CYP2A7, and combinations for 6MP-induced hepatotoxicity.
With the rapid development of genomic techniques, strategies to screen pharmacogenetic markers have changed from pharmacology-based to genomics-based approaches, which is also considered as from candidate hypothesis to zero hypothesis. For instance, multiple novel pharmacogenetic biomarkers have been found by conducting genome-wide association approaches (Mccormack et al., 2011; Zhou et al., 2011; Xu et al., 2015). Recently, common variant in NUDT15 (i.e., rs116855232) was identified to largely explain thiopurine-induced leukopenia in ALL and immune diseases (e.g., inflammatory bowel disease) in East Asians (Yang et al., 2014; Yang et al., 2015; Moriyama et al., 2016). Subsequent pharmacological analyses revealed the mechanism of how NUDT15 works in thiopurine metabolism (Moriyama et al., 2016). Additionally, rare variants that affect enzyme the activity of NUDT15 will also result in the 6MP-induced leukopenia (Moriyama et al., 2016; Moriyama et al., 2017; Zhu et al., 2018). Importantly, 6MP-induced leukopenia can also be validated in the mouse model (Nishii et al., 2018), indicating that it is practically to conduct this zero-hypotheses approach to identify pharmacogenetic markers. In this study, we focused on patients with NUDT15wt/wtTPMTwt/wt genotype, in order to identify the novel variants in drug metabolism–related genes that can explain the rest of patients’ experience of 6MP-induced leukopenia. Finally, we found that rs73032311 is not only independently associated with such ADR, but also interacted with the variant in NUDT15 but not TPMT. Statistically, variant in CYP2A7 has a smaller P value as well as odds ratio than the variant in TPMT, probably because of its higher RAF. In addition, rs73032311 is a common SNP with varied RAF among different ethnicities, indicating that it may be served as a general pharmacogenetic marker. Importantly, variant in NUDT15 exhibits the strongest prediction accuracy, and rs73032311 in CYP2A7 has a higher accuracy than that of rs1142345 in TPMT, indicating the importance of the novel variant identified in this study for Chinese population. However, the limitation of this study is obvious due to the lack of validation cohort; independent replication studies are urgently needed, especially in patient cohorts with a large sample size. On the other hand, the clinical strategy should be carefully designed given that the significance of the CYP2A7 variant was validated, because the specificity was greatly decreased after introducing the novel variant. It will be more practical than genotyping rs73032311 in the first place, following up the risk allele carriers without reducing the dosage immediately. Functionally, amino acid alteration induced by rs73032311 of CYP2A7 gene is predicted to be damaged according to CADD analysis, with the estimated PHRED score of 12.48 (rank as top 10%), suggesting its potential as a causal variant. Functionally, CYP2A7 is considered to encode a member of the cytochrome P450 enzymes, which are well-reported to be involved in phase I of drug metabolism. Compared to the well-described P450 enzymes (e.g., CYP2D6), substrate to CYP2A7 has not yet been clearly determined. On the other hand, no p450 enzyme has been reported to be involved in thiopurine metabolism according to the current pharmacologic records. Considering CYP2A7 is highly expressed in the liver, which is the main organ in which thiopurine is metabolized, the details on the mechanism of how the variant in CYP2A7 impacts on 6MP-induced leukopenia are needed to be further elucidated.
On the other hand, no widely accepted pharmacogenetic biomarker has been recorded for 6MP-induced hepatotoxicity. Variants in ITPA and PNPLA3 were reported to be associated with such ADR through candidate- or zero-hypothesis approaches, respectively (Adam De Beaumais et al., 2011; Liu et al., 2017). However, more replication studies are needed in additional patient cohorts with larger samples because of the inconsistency or limited validation, especially in other ethnicities (e.g., East Asians) (Steponaitiene et al., 2016; Gutierrez-Camino et al., 2017; Khera et al., 2019). In our study, neither rs1127354 in ITPA nor rs738409 in PNPLA3 can be validated possibly due to the difference in patient cohort and ethnicities. Sample size may not be the major cause because no trend was observed with odds ratio of 1.32 and 1.18, respectively, which is much lower than that of rs4680 in COMT. Inconsistent association of these variants in our patients may be induced by their ethnic specificity; that is, the reported variants themselves may not be the functional cause for 6MP-induced hepatotoxicity, but only tag the causal variants through linkage disequilibrium, which is different among ethnicities. We thus performed screening variants in genes involved in phase I or phase II drug metabolism to find the potential pharmacogenetic markers for 6MP-induced hepatotoxicity in Chinese population. This is the first systematic screening in such ethnicity to the best of our knowledge. However, the accuracy for the novel variant is modest to predict 6MP-induced hepatotoxicity and thus is not efficient enough as a strong pharmacogenetic marker. Therefore, screening with a large sample size is needed, not only validating the significance of COMT variant, but also finding additional variants to further increase the predictive accuracy for 6MP-induced hepatotoxicity. Functionally, rs4680 in COMT has been widely reported to be associated with a variety of different drug reactions, labeling as level 2 pharmacogenetic biomarker in PharmGKB data resource (https://www.pharmgkb.org/) for efficacy of multiple drugs, including nicotine and morphine, without well-described mechanism (Sun et al., 2012; Ahlers et al., 2013). Functionally, COMT is universally expressed in multiple organs, including the liver; the details of how this gene is involved in 6MP-induced hepatotoxicity are also needed to be investigated after validation of the association of rs4680 with such ADR in independent patient cohorts.
The datasets generated for this study can be found in Array Express in the Genome Variation Map (GVM) database (http://bigd.big.ac.cn/gvm) with the accession number of GVM000060, and EBI database (https://www.ebi.ac.uk/arrayexpress/) with the accession number of E-MTAB-8613.
This study was approved by the Ethics Committee of West China Second Hospital, Sichuan University, and informed consent was obtained from patients or their guardians, as appropriate.
XL and YS conceived the research project, wrote the manuscript, and gave final approval. MC, DY, YQ, FL, and XX analyzed and interpreted the data. MC, YLS, YZ, JG, and XL evaluated the patients and collected the clinical samples and data. WZ critically reviewed the manuscript.
This work was supported by the National Natural Science Foundation of China (No. 81673452, No. 81903735, No. 81973408, and No. 81902872) and the National Key Research and Development Program of China (No. 2016YFC0905000 [2016YFC0905001]).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00267/full#supplementary-material
Supplementary Table 1 | Information of the raw genotype and p values for each SNP.
Adam De Beaumais, T., Fakhoury, M., Medard, Y., Azougagh, S., Zhang, D., Yakouben, K., et al. (2011). Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br. J. Clin. Pharmacol. 71, 575–584. doi: 10.1111/j.1365-2125.2010.03867.x
Ahlers, S. J., Elens, L. L., Van Gulik, L., Van Schaik, R. H., Van Dongen, E. P., Bruins, P., et al. (2013). The Val158Met polymorphism of the COMT gene is associated with increased pain sensitivity in morphine-treated patients undergoing a painful procedure after cardiac surgery. Br. J. Clin. Pharmacol. 75, 1506–1515. doi: 10.1111/bcp.12052
Gutierrez-Camino, A., Martin-Guerrero, I., Garcia-Orad, A. (2017). PNPLA3 rs738409 and Hepatotoxicity in Children With B-cell Acute Lymphoblastic Leukemia: A Validation Study in a Spanish Cohort. Clin. Pharmacol. Ther. 102, 906. doi: 10.1002/cpt.756
Khera, S., Trehan, A., Bhatia, P., Singh, M., Bansal, D., Varma, N. (2019). Prevalence of TPMT, ITPA and NUDT 15 genetic polymorphisms and their relation to 6MP toxicity in north Indian children with acute lymphoblastic leukemia. Cancer Chemother. Pharmacol. 83, 341–348. doi: 10.1007/s00280-018-3732-3
Kirschner-Schwabe, R., Lottaz, C., Todling, J., Rhein, P., Karawajew, L., Eckert, C., et al. (2006). Expression of late cell cycle genes and an increased proliferative capacity characterize very early relapse of childhood acute lymphoblastic leukemia. Clin. Cancer Res. 12, 4553–4561. doi: 10.1158/1078-0432.CCR-06-0235
Liu, Y., Fernandez, C. A., Smith, C., Yang, W., Cheng, C., Panetta, J. C., et al. (2017). Genome-Wide Study Links PNPLA3 Variant With Elevated Hepatic Transaminase After Acute Lymphoblastic Leukemia Therapy. Clin. Pharmacol. Ther. 102, 131–140. doi: 10.1002/cpt.629
Mccormack, M., Alfirevic, A., Bourgeois, S., Farrell, J. J., Kasperaviciute, D., Carrington, M., et al. (2011). HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 364, 1134–1143. doi: 10.1056/NEJMoa1013297
Mcleod, H., Krynetski, E., Relling, M., Evans, W. J. L. (2000). Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia 14, 567. doi: 10.1038/sj.leu.2401723
Moriyama, T., Relling, M. V., Yang, J. J. (2015). Inherited genetic variation in childhood acute lymphoblastic leukemia. Blood 125, 3988–3995. doi: 10.1182/blood-2014-12-580001
Moriyama, T., Nishii, R., Perez-Andreu, V., Yang, W., Klussmann, F. A., Zhao, X., et al. (2016). NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat. Genet. 48, 367–373. doi: 10.1038/ng.3508
Moriyama, T., Yang, Y. L., Nishii, R., Ariffin, H., Liu, C., Lin, T. N., et al. (2017). Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood 130, 1209–1212. doi: 10.1182/blood-2017-05-782383
Nishii, R., Moriyama, T., Janke, L. J., Yang, W., Suiter, C. C., Lin, T. N., et al. (2018). Preclinical evaluation of NUDT15-guided thiopurine therapy and its effects on toxicity and antileukemic efficacy. Blood 131, 2466–2474. doi: 10.1182/blood-2017-11-815506
Pui, C. H., Evans, W. E. (2006). Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 354, 166–178. doi: 10.1056/NEJMra052603
Relling, M. V., Evans, W. E. (2015). Pharmacogenomics in the clinic. Nature 526, 343–350. doi: 10.1038/nature15817
Relling, M. V., Hancock, M. L., Rivera, G. K., Sandlund, J. T., Ribeiro, R. C., Krynetski, E. Y., et al. (1999). Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J. Natl. Cancer Inst. 91, 2001–2008. doi: 10.1093/jnci/91.23.2001
Relling, M. V., Gardner, E. E., Sandborn, W. J., Schmiegelow, K., Pui, C. H., Yee, S. W., et al. (2013). Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin. Pharmacol. Ther. 93, 324–325. doi: 10.1038/clpt.2013.4
Relling, M. V., Schwab, M., Whirl-Carrillo, M., Suarez-Kurtz, G., Pui, C. H., Stein, C. M., et al. (2019). Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 105, 1095–1105. doi: 10.1002/cpt.1304
Schaeffeler, E., Jaeger, S. U., Klumpp, V., Yang, J. J., Igel, S., Hinze, L., et al. (2019). Impact of NUDT15 genetics on severe thiopurine-related hematotoxicity in patients with European ancestry. Genet. Med. 21, 2145–2150. doi: 10.1038/s41436-019-0448-7
Steponaitiene, R., Kupcinskas, J., Survilaite, S., Varkalaite, G., Jonaitis, L., Kiudelis, G., et al. (2016). TPMT and ITPA genetic variants in Lithuanian inflammatory bowel disease patients: Prevalence and azathioprine-related side effects. Adv. Med. Sci. 61, 135–140. doi: 10.1016/j.advms.2015.09.008
Sun, H., Guo, S., Chen, D., Yang, F., Zou, Y., Di, X., et al. (2012). Association of functional COMT Val108/Met polymorphism with smoking cessation in a nicotine replacement therapy. J. Neural Transm. (Vienna) 119, 1491–1498. doi: 10.1007/s00702-012-0841-8
Xu, H., Robinson, G. W., Huang, J., Lim, J. Y., Zhang, H., Bass, J. K., et al. (2015). Common variants in ACYP2 influence susceptibility to cisplatin-induced hearing loss. Nat. Genet. 47, 263–266. doi: 10.1038/ng.3217
Yang, S. K., Hong, M., Baek, J., Choi, H., Zhao, W., Jung, Y., et al. (2014). A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat. Genet. 46, 1017–1020. doi: 10.1038/ng.3060
Yang, J. J., Landier, W., Yang, W., Liu, C., Hageman, L., Cheng, C., et al. (2015). Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J. Clin. Oncol. 33, 1235–1242. doi: 10.1200/JCO.2014.59.4671
Yin, D., Xia, X., Zhang, J., Zhang, S., Liao, F., Zhang, G., et al. (2017). Impact of NUDT15 polymorphisms on thiopurines-induced myelotoxicity and thiopurines tolerance dose. Oncotarget. 8 (8), 13575–13585. doi: 10.18632/oncotarget.14594
Zhou, K., Bellenguez, C., Spencer, C. C., Bennett, A. J., Coleman, R. L., Tavendale, R., et al. (2011). Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat. Genet. 43, 117–120. doi: 10.1038/ng.735
Zhu, Y., Yin, D., Su, Y., Xia, X., Moriyama, T., Nishii, R., et al. (2018). Combination of common and novel rare NUDT15 variants improves predictive sensitivity of thiopurine-induced leukopenia in children with acute lymphoblastic leukemia. Haematologica 103, e293–e295. doi: 10.3324/haematol.2018.187658
Keywords: mercaptopurine, leukopenia, hepatoxicity, adverse drug reaction, pharmacogenetics, CYP2A7, NUDT15/TPMT widetype, COMT
Citation: Cao M, Yin D, Qin Y, Liao F, Su Y, Xia X, Gao J, Zhu Y, Zhang W, Shu Y and Lu X (2020) Screening of Novel Pharmacogenetic Candidates for Mercaptopurine-Induced Toxicity in Patients With Acute Lymphoblastic Leukemia. Front. Pharmacol. 11:267. doi: 10.3389/fphar.2020.00267
Received: 24 November 2019; Accepted: 25 February 2020;
Published: 20 March 2020.
Edited by:
Chonlaphat Sukasem, Mahidol University, ThailandReviewed by:
Maoxiang Qian, Fudan University, ChinaCopyright © 2020 Cao, Yin, Qin, Liao, Su, Xia, Gao, Zhu, Zhang, Shu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Shu, c2h1eWFuZzE5ODZAZ21haWwuY29t; Xiaoxi Lu, bHVfaGVsZW5hQHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.