- 1College of Bioscience and Technology, Weifang Medical University, Weifang, China
- 2Department of Fundamental Veterinary, College of Veterinary Medicine, Shandong Agricultural University, Tai’an, China
Microcystin-leucine arginine (MC-LR) is a potent tumor initiator that can induce malignant cell transformation. Cellular mechanical characteristics are pivotal parameters that are closely related to cell invasion. The aim of this study is to determine the effect of MC-LR on mechanical parameters, microfilament, and cell invasion in DU145 and WPMY cells. Firstly, 10 μM MC-LR was selected as the appropriate concentration via cell viability assay. Subsequently, after MC-LR treatment, the cellular deformability and viscoelastic parameters were tested using the micropipette aspiration technique. The results showed that MC-LR increased the cellular deformability, reduced the cellular viscoelastic parameter values, and caused the cells to become softer. Furthermore, microfilament and microfilament-associated proteins were examined by immunofluorescence and Western blot, respectively. Our results showed that MC-LR induced microfilament reorganization and increased the expression of p-VASP and p-ezrin. Finally, the impact of MC-LR on cell invasion was evaluated. The results revealed that MC-LR promoted cell invasion. Taken together, our results suggested that mechanical changes and microfilament reorganization were involved in MC-LR-promoted cell invasion in DU145 and WPMY cells. Our data provide novel information to explain the toxicological mechanism of MC-LR.
Introduction
Microcystins (MCs) are a group of cyclic heptapeptide toxins produced by freshwater cyanobacteria (Rastogi et al., 2014), among which microcystin-leucine arginine (MC-LR) is the most abundant and toxic congener (Li Y. et al., 2016). The World Health Organization (WHO) has recommended 1 µg/L as the upper limit of MC-LR in drinking water (Wang et al., 2010). In fact, the concentration of MC-LR in the drinking water has exceeded this recommend value in many countries (Backer et al., 2010; Duong et al., 2014; Chun et al., 2018). Current studies show that MC-LR induces cytotoxicity in different ways. In detail, it has been shown that MC-LR can inhibit serine/threonine protein phosphatases (PPs) by interactions with their catalytic subunits, then affecting the cellular homeostasis (Zhou et al., 2015; Liu et al., 2016). Also, MC-LR can induce oxidative stress by increasing the reactive oxygen species (ROS) or decreasing the glutathione (GSH). Subsequently, oxidative stress caused by MC-LR might induce mitochondrial permeability transition and apoptosis (Ma et al., 2016; Ma et al., 2018; Wu et al., 2019). In addition, previous studies indicated that MC-LR is a potent tumor initiator (Zhao et al., 2016; Zhu et al., 2018), which could induce malignant cell transformation. For example, Liu et al. reported that MC-LR promoted proliferation and inhibited apoptosis in normal human liver cells (HL7702) (Liu et al., 2016). The study of Wang et al. showed that MC-LR induced cytoskeleton reorganization, resulting in increased cell migration in human laryngeal epithelial cells (Hep-2) (Wang et al., 2017). However, the effect of MC-LR on prostate cancer (PCa) cells and normal prostate cells has yet been studied.
Cellular mechanical characteristics are pivotal parameters that can reveal the properties of cells, such as their proliferation, migration, and invasion (Yin-Quan et al., 2014). It has been reported that the viscoelasticity of cancer cells decreased significantly and was closely related to their metastasis and invasion (Rother et al., 2014; Runge et al., 2014; Nguyen et al., 2016). To date, the effect of MC-LR on cellular mechanical properties is unknown. In addition, microfilaments are the main components of the cytoskeleton in the cytoplasm. Many studies have suggested that MC-LR can cause microfilament changes in different cells (Meng et al., 2011; Zeng et al., 2015; Wang et al., 2017). However, the influence of MC-LR on microfilaments is still poorly documented in PCa cells and normal prostate cells.
The aim of this study is to determine the effect of MC-LR on mechanical parameters, microfilament, and phenotype in PCa cells and normal prostate cells. To do this, we incubated PCa cells (DU145) and normal prostate cells (WPMY) with 10 μM MC-LR and then tested the cellular deformability and viscoelastic parameters by the micropipette aspiration technique. Subsequently, the changes of microfilament and microfilament-associated proteins were examined by immunofluorescence and Western blot, respectively. Moreover, invasion assay was conducted to assess the role of MC-LR in cell invasion.
Materials and Methods
Cell Culture and Treatment
DU145 and WPMY cells were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Shanghai, China) supplemented with 10% fetal bovine serum (FBS; Gibco, Shanghai, China). After 24 h of incubation, cells were treated with MC-LR (Express Technology, Beijing, China) for another 24 h. The control cells were cultured in medium without MC-LR.
CCK-8 Assay
DU145 and WPMY cells were seeded in 96-well plates, respectively. After treatment with different concentrations of MC-LR (0.1 μM, 0.5 μM, 1 μM, 2 μM, 5 μM, 10 μM, 20 μM, and 40 μM) for 24 h, relative cell viability was detected by the CCK-8 kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions (Wang et al., 2017).
Micropipette Aspiration Test and the Mechanical Behavior of Cells
The mechanical behaviors of cells were represented by the deformability and viscoelastic parameters, which were investigated using the micropipette aspiration technique combined with the Kelvin standard linear viscoelastic solid model (Xie et al., 2019) (Figure 1). A single-cell suspension was prepared for the micropipette aspiration test by micromanipulator (Olympus, Japan). A single spherical cell was captured and deformed by the negative pressure in the micropipette, and then part of the cell was aspirated into the micropipette. This process was viewed and recorded with an inversion microscope (Olympus, Japan) combined with image software. The recorded images of the micropipette aspiration test were used to measure the aspirated length of cells (Figure 1A), and then the relationships of time and aspirated length were obtained and fitted to calculate the cellular viscoelastic parameter values.
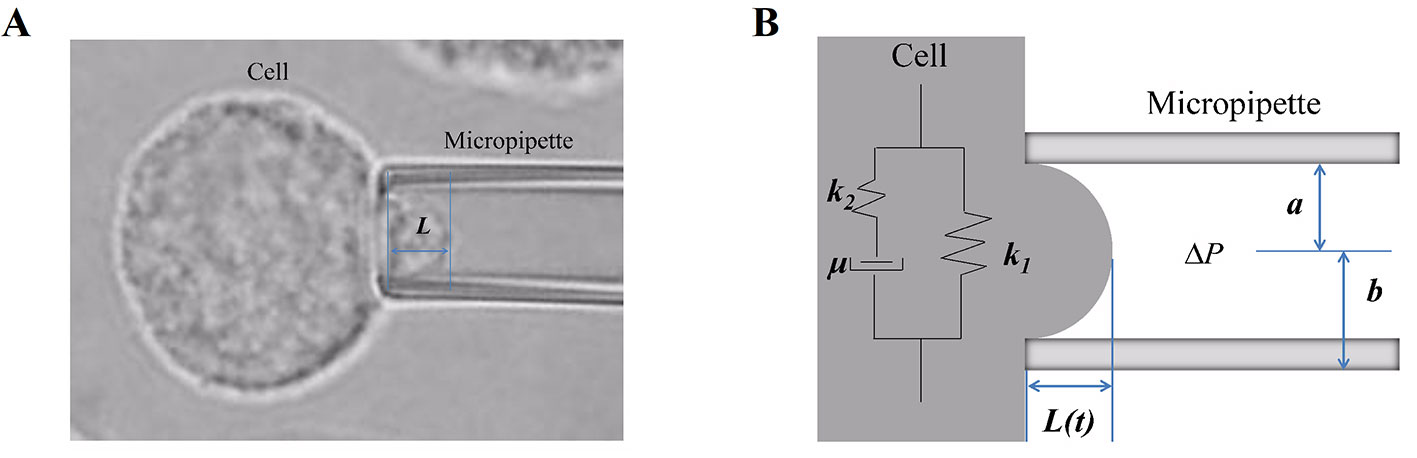
Figure 1 The micropipette aspiration technique and the mechanical behavior of cells. (A) A certain negative pressure (ranging from 294 Pa to 441 Pa) induced cell deformation with time, and then, part of the cell was aspirated into the micropipette. Images of this process were recorded, and the aspirated length L was measured. (B) Schematic representation of the Kelvin standard linear viscoelastic solid model: in the model, the cell was assumed to be a homogeneous viscoelastic spherical solid; k1, k2, and μ are the viscoelastic parameters, L is the aspirated length, and Δp is the negative pressure.
The cell was assumed to be a homogeneous viscoelastic spherical solid, and then the cellular viscoelastic parameters E0 (the instantaneous modulus), E∞ (the equilibrium modulus associated with long-term equilibrium), and μ (the apparent viscosity) were calculated by applying the Kelvin standard linear viscoelastic solid model (Figure 1B) based on the relationships of time-aspirated length, as our (Xie et al., 2019) and others’ (Zhang et al., 2008) previous studies have described. The values of the cellular viscoelastic parameters (E0, E∞, and μ) were calculated according to the following equation:
Immunofluorescence
After 24-h treatment with MC-LR, immunofluorescence was performed to detect microfilament changes in DU145 and WPMY cells as previously described (Huang et al., 2018). Briefly, the cells were fixed using 4% formaldehyde for 15 min and then washed by phosphate-buffered saline (PBS). Subsequently, phalloidin (Beyotime, Shanghai, China) and DAPI (Santa Cruz, Dallas, USA) were added and then incubated for 60 min. The images were captured using an Olympus laser-scanning confocal microscope.
Western Blot
Based on a previous study (You et al., 2017), the process of Western blot was slightly modified. After SDS-PAGE, the protein was transferred to PVDF membranes (Beyotime, Shanghai, China) and incubated with anti-VASP (CST, Boston, USA, 1:1000 dilution), anti-ezrin (CST, Boston, USA, 1:1000 dilution), anti-p-VASP (Ser157, CST, Boston, USA, 1:1000 dilution), anti-p-ezrin (Thr567, CST, Boston, USA, 1:1000 dilution), and anti-β-actin (Beyotime, Shanghai, China, 1:1000 dilution) at 4°C overnight. After incubating with secondary antibody (Proteintech, Wuhan, China, 1:2000 dilution), the bands were analyzed using the enhanced chemiluminescence reaction kit (ECL; Beyotime, Shanghai, China).
Invasion Assay
Invasion assay was carried out using the method previously described (Yan et al., 2018). DU145 and WPMY cells were seeded into transwell chambers (Sigma-Aldrich, St. Louis, USA), respectively. Meanwhile, serum-free DMEM with 10 μM MCLR was added to the upper chamber, and DMEM containing 10% FBS was added to the lower chamber. After incubating for 24 h, the cells that passed through the membranes were stained with 0.5% crystal violet solution and counted under the microscope.
Statistical Analysis
Statistical analyses were performed with SPSS 19.0 software. Independent sample t-test was used to determine the differences between groups. All experiments were performed in triplicate. Data are shown as mean ± SD, and P < 0.05 was considered to be significantly different.
Results
Screening of MC-LR Concentration
To select the appropriate concentration of MC-LR, a CCK-8 kit was used to detect the relative cell viability. As shown in Figure 2, no change was observed in the relative cell viability of DU145 and WPMY cells between the control group and the MC-LR treated group with concentrations of 0.1-10 μM. However, the relative cell viability decreased at an MC-LR concentration of 20 μM and more so at 40 μM. Therefore, 10 μM MC-LR was selected for further study.
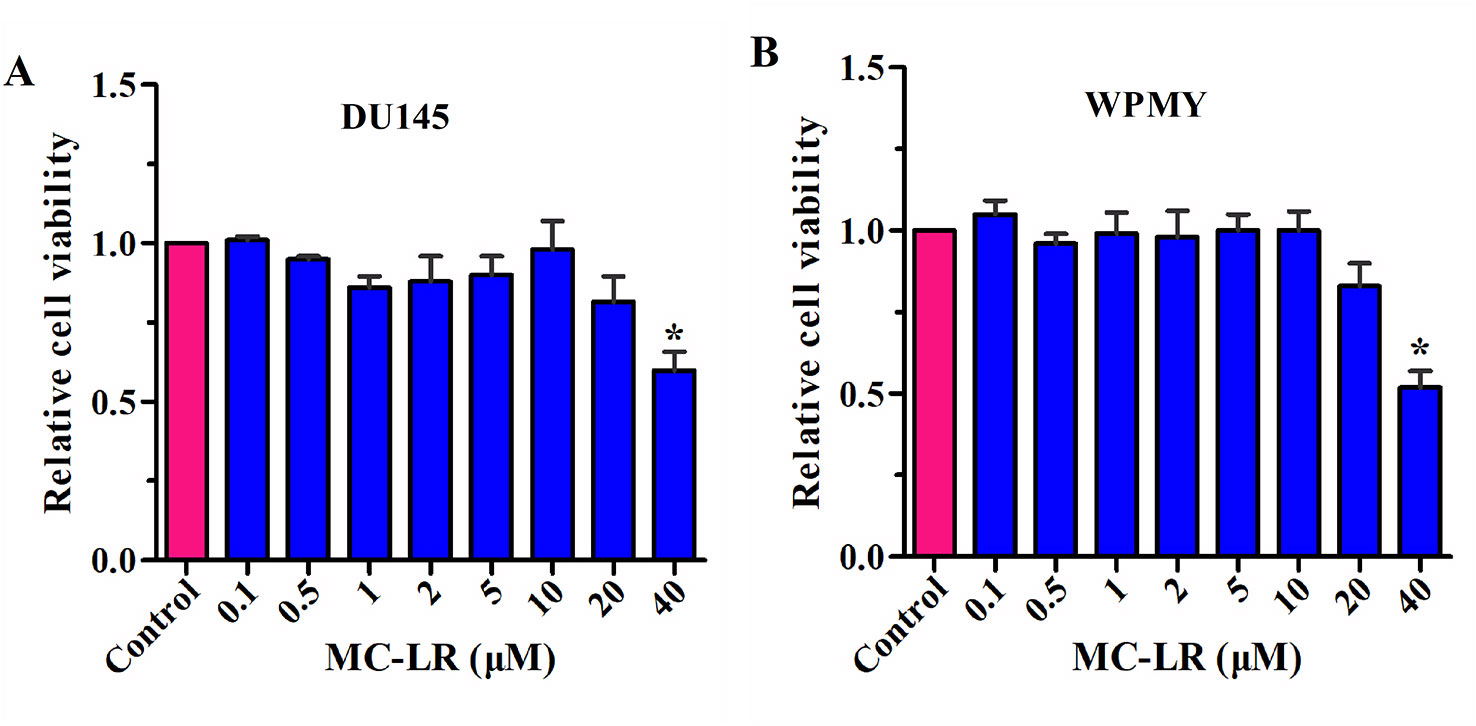
Figure 2 Screening of MC-LR concentration. DU145 cells and WPMY cells were seeded in 96-well plates, respectively. After incubation with different concentrations of MC-LR for 24 h, relative cell viability was detected with the CCK-8 kit. The results are representative of three independent experiments. *P < 0.05. Error bars indicate SD.
MC-LR Caused Mechanical Behavior Changes in DU145 and WPMY Cells
The relationships between time and the aspirated length of the cells were plotted as curves, and the time–aspirated length curves at a negative pressure of 392 Pa are shown in Figure 3A. Under the negative pressure, the cell was deformed; meanwhile, part of the cell was aspirated into the micropipette, and the deformation rate decreased with time until it was no longer aspirated into the micropipette within 50-60 sec. The time–aspirated length curves of the cells reflected the cellular deformability. As shown in Figure 3A, MC-LR treated cells exhibited higher deformability than MC-LR untreated cells. These results suggested that MC-LR increased the deformability of the cells. In addition, DU145 cells showed higher deformability than WPMY cells, and DU145 cells without MC-LR treatment even still exhibited higher deformability than WPMY cells treated with MC-LR.
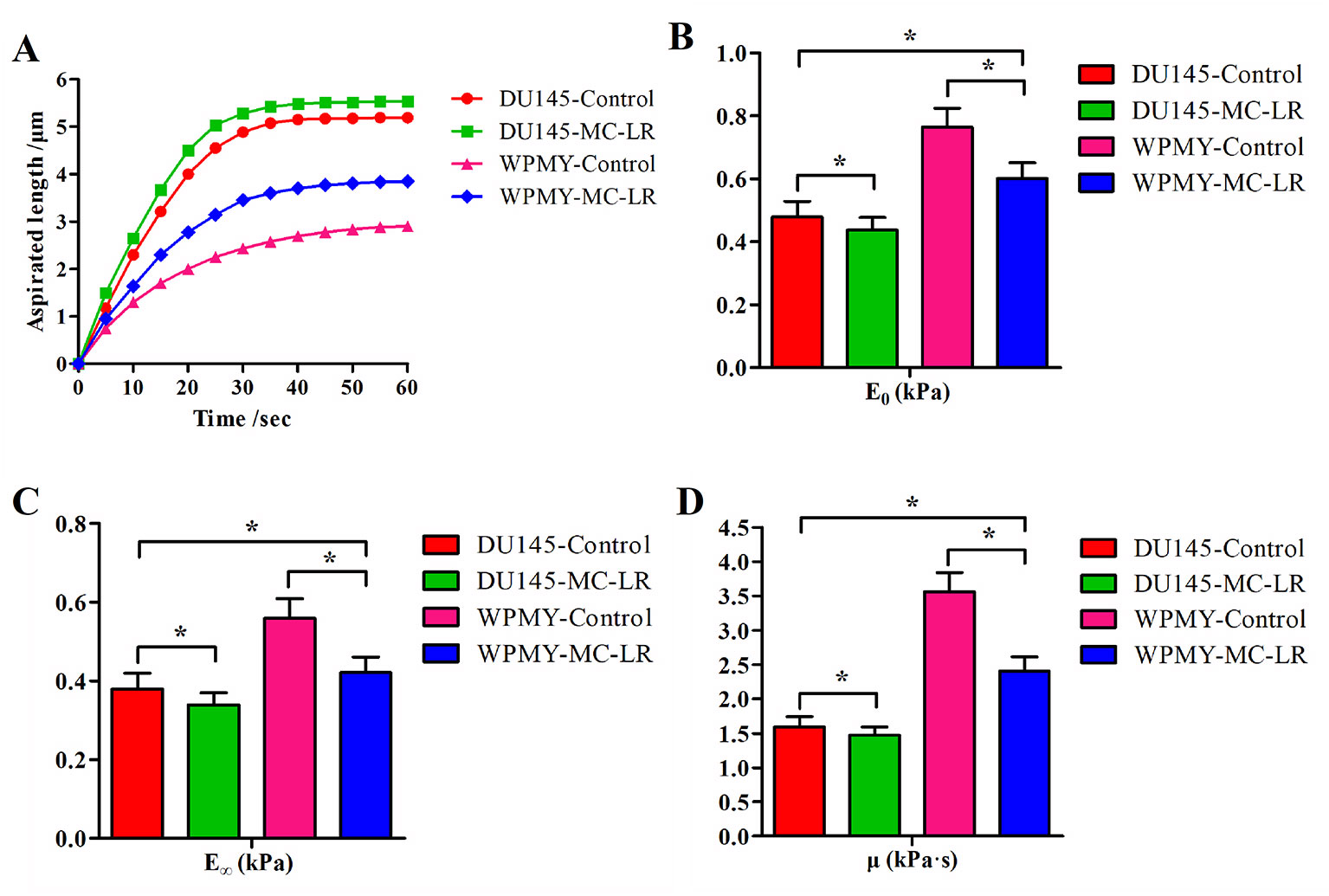
Figure 3 MC-LR caused mechanical behavior changes in DU145 cells and WPMY cells. (A) Curves of aspirated lengths L with time at a constant negative pressure of 392 Pa. (B–D) Comparison of the cellular viscoelastic parameters (E0, E∞, and μ). The viscoelastic parameter values were calculated by applying the curves of the aspirated lengths and the Kelvin standard linear viscoelastic solid model. The results are representative of three independent experiments. *P < 0.05. Error bars indicate SD.
Figures 3B–D show comparisons of the cellular viscoelastic parameters (E0, E∞, and μ), respectively. The viscoelastic parameter values (E0, E∞, and μ) of MC-LR treated cells were significantly lower than those of the untreated cells (P < 0.05). Additionally, the viscoelastic parameter values (E0, E∞, and μ) of DU145 cells were lower than those of WPMY cells (P < 0.05). These results indicated that the prostate carcinoma cells were softer than normal prostate cells and that MC-LR induced the cells to become much softer. Previous research indicated that cellular mechanical behavior is correlated with microfilament changes. Hence, we investigated the microfilament arrangement further.
MC-LR Induced Microfilament Reorganization in DU145 Cells and WPMY Cells
Changes of microfilament were detected in DU145 and WPMY cells after MC-LR treatment. As shown in Figure 4, microfilament was evenly distributed in the inner side of the cell membranes in the control group; by contrast, in the MC-LR treated group, microfilament appeared to gather to the cell surface and concentrated to form bundles. Notably, the above results were observed in both DU145 and WPMY cells. These data suggested that MC-LR induced microfilament reorganization in both DU145 and WPMY cells.
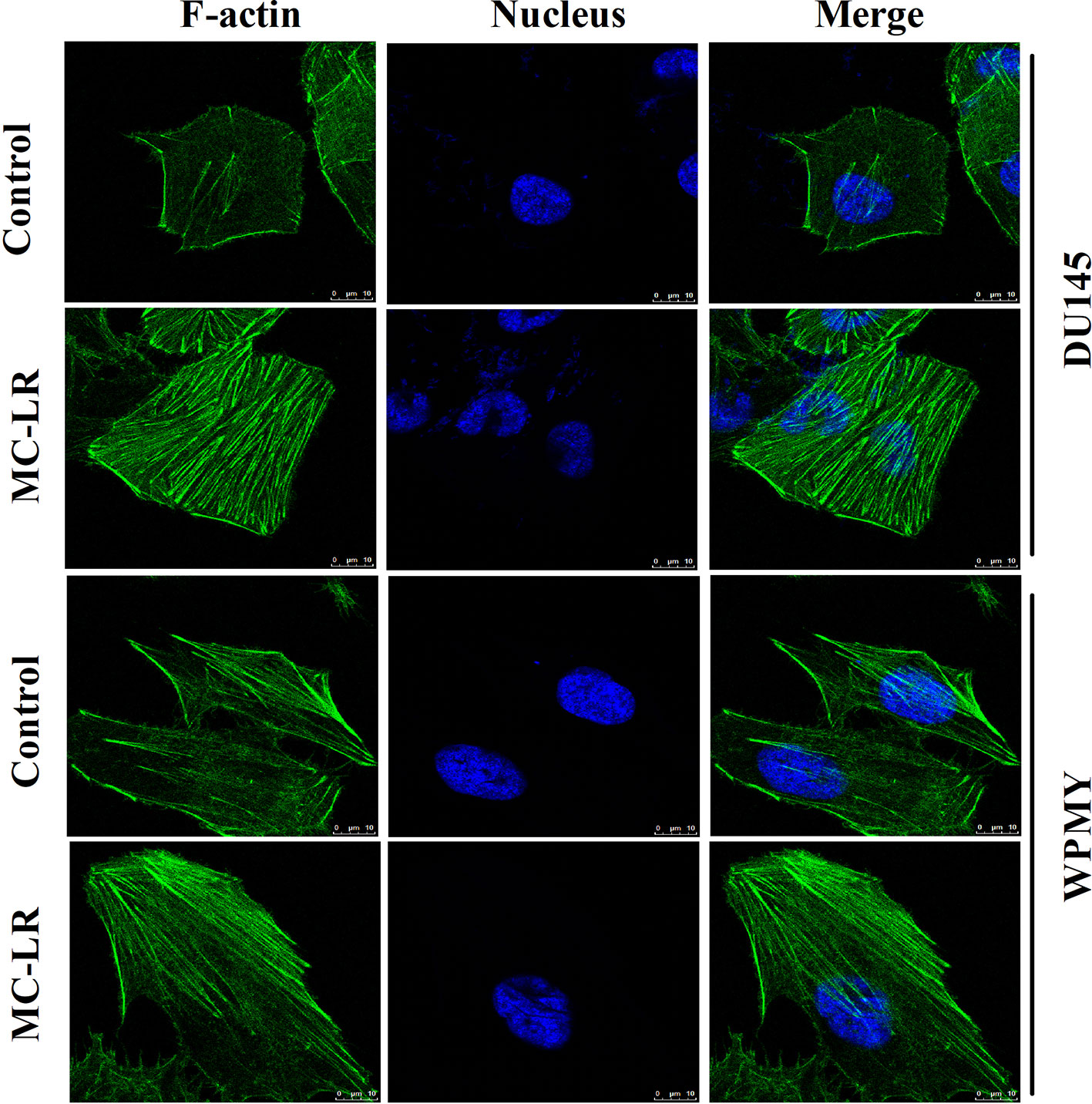
Figure 4 MC-LR induced microfilament reorganization in DU145 cells and WPMY cells. DU145 cells and WPMY cells were incubated using 10 μM MC-LR for 24 h, and then immunofluorescence was performed to label microfilament (green) and nuclei (blue), respectively. The results are representative of three independent experiments.
MC-LR Increased the Phosphorylation Level of Microfilament-Associated Proteins in DU145 and WPMY Cells
To further elucidate the mechanism of microfilament rearrangement, the expression and phosphorylation of microfilament-associated proteins were detected by Western blot. Firstly, the expression of microfilament-associated proteins (VASP and ezrin) was tested in DU145 and WPMY cells after MC-LR treatment. As shown in Figure 5A, no significant change was observed in the expression of VASP and ezrin, which implied that microfilament reorganization had no relationship with the expression of microfilament-associated proteins. Subsequently, the phosphorylation level of microfilament-associated proteins was further assessed. The results showed that the expression of p-VASP (Ser157) and p-ezrin (Thr567) was significantly higher in the MC-LR treatment group than in the untreated group (P < 0.05, Figure 5B). These results demonstrated that MC-LR increased the phosphorylation level of microfilament-associated proteins in DU145 and WPMY cells.
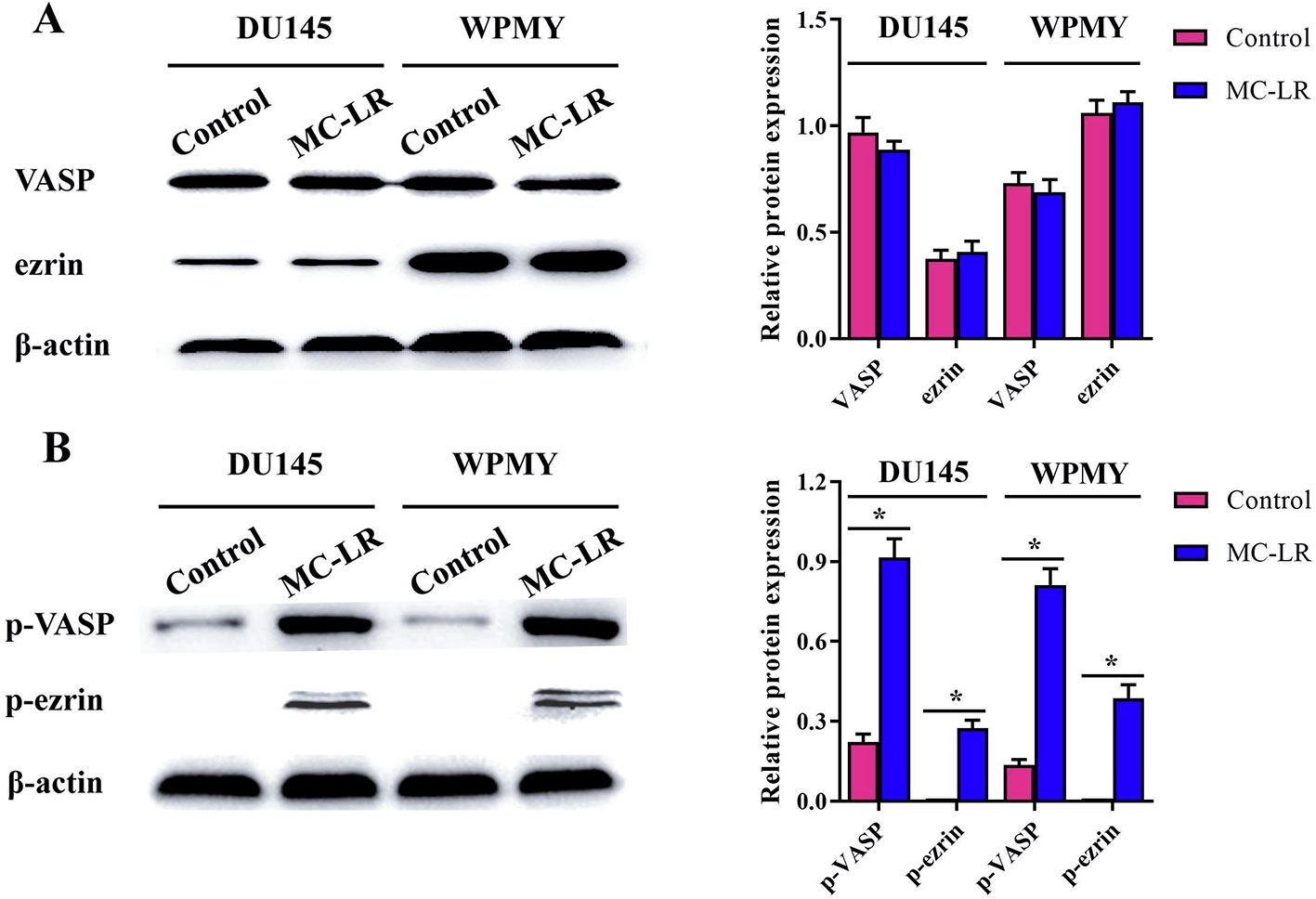
Figure 5 MC-LR increased the phosphorylation level of microfilament-associated proteins in DU145 cells and WPMY cells. DU145 cells and WPMY cells were incubated with 10 μM MC-LR for 24 h, and then Western blot was carried out to detect the expression and phosphorylation of VASP and ezrin. The results are representative of three independent experiments. * P < 0.05. Error bars indicate SD.
MC-LR Promoted Cell Invasion in DU145 and WPMY Cells
It has been reported that MC-LR has potential carcinogenicity; therefore, transwell assay was performed to determine the effect of MC-LR on cell invasion. The results revealed that the invasion ability of the MC-LR treatment group was substantially reinforced compared with the untreated group in DU145 and WPMY cells (P < 0.05, Figure 6). Thus, these data demonstrated that MC-LR promoted cell invasion in both DU145 and WPMY cells.
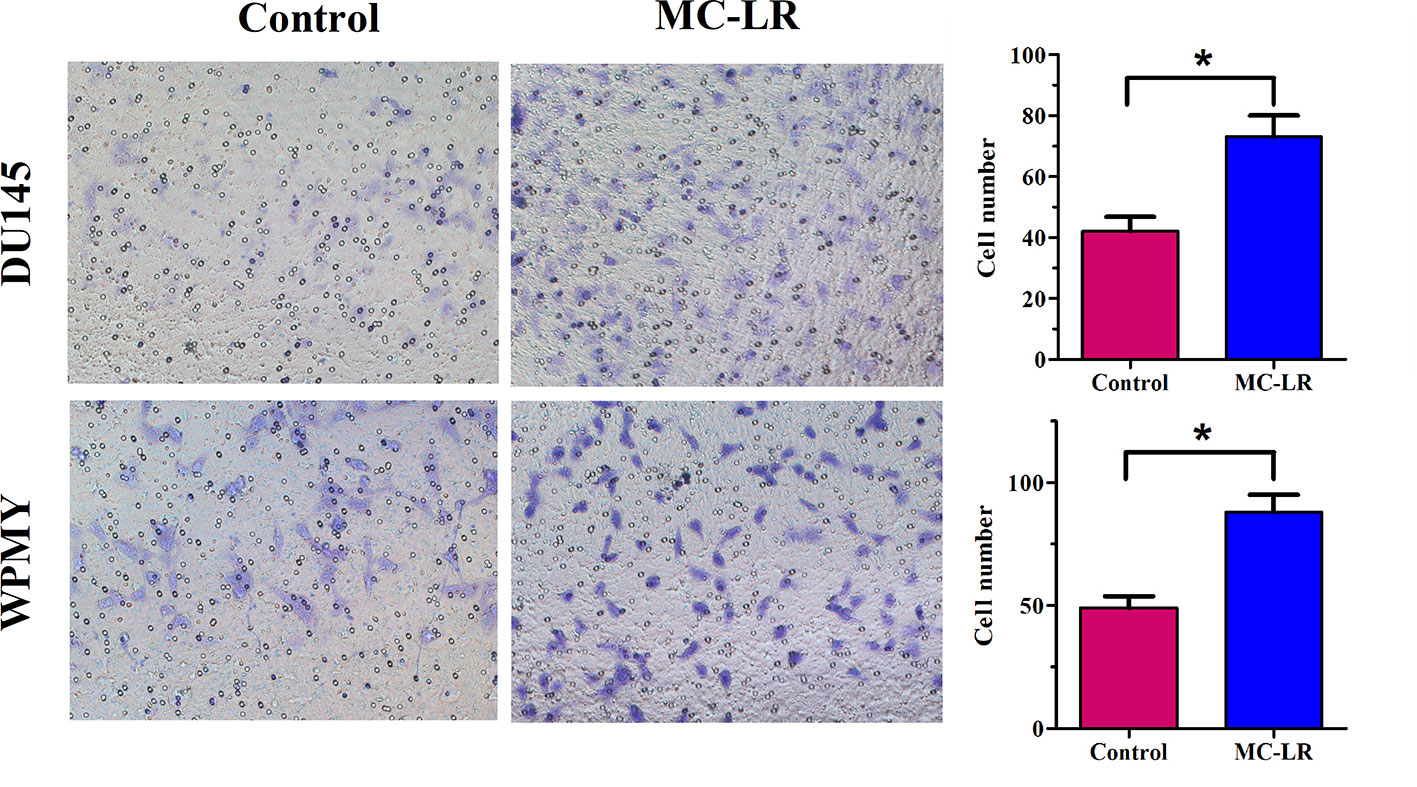
Figure 6 MC-LR promoted cell invasion in DU145 cells and WPMY cells. DU145 cells and WPMY cells were treated with 10 μM MC-LR for 24 h. The invasion ability was determined by transwell assay. The results are representative of three independent experiments. * P < 0.05. Error bars indicate SD.
Discussion
In the present study, we investigated the influence of MC-LR on mechanical parameters, microfilament, and cell invasion in DU145 and WPMY cells. DU145 and WPMY cells were treated with 10 μM MC-LR, and then the cellular deformability and viscoelastic parameters were tested by the micropipette aspiration technique. The results showed that MC-LR increased the cellular deformability, reduced the cellular viscoelastic parameter values, and caused the cells to become softer. Moreover, the immunofluorescence of microfilament was performed, and then Western blot of microfilament-associated proteins was carried out. The results demonstrated that MC-LR induced microfilament reorganization and increased the phosphorylation level of microfilament-associated proteins. Finally, the effect of MC-LR on cell invasion was examined. The results revealed that MC-LR promoted cell invasion. Collectively, our results suggested that mechanical changes and microfilament reorganization were involved in MC-LR-promoted cell invasion in DU145 and WPMY cells.
Since the effect of MC-LR on DU145 and WPMY cells has not been reported, the appropriate concentration of MC-LR was screened. The results showed that MC-LR did not affect the relative cell viability of DU145 and WPMY cells at concentrations of 0.1–10 μM. Consistent with our results, Wang et al. treated Hep-2 cells with 0.5–10 μM MC-LR for 24 h. Their results suggested that the relative cell viability remained unchanged in the treated group compared with the untreated group (Wang et al., 2017). Also, MC-LR at 10 μM was used in several other studies (Zeng et al., 2015; Liu et al., 2016; Wang et al., 2017). Therefore, 10 μM MC-LR was selected for the subsequent experiments to obtain a more obvious effect in this study.
Micropipette aspiration is a useful technique for detecting the viscoelasticity of cells, such as colon cancer cells (Taranejoo et al., 2016), scleral fibroblasts (Wang and Chen, 2012), and chondrocytes (Zhang et al., 2008). Therefore, in the present study, the micropipette aspiration technique was used to investigate the effect of MC-LR on the mechanical properties of DU145 and WPMY cells. The results showed that MC-LR increased the deformability of the cells, reduced the cellular viscoelastic parameter values, and caused the cells to become softer. Additionally, DU145 cells showed higher deformability and weaker viscoelastic behavior than WPMY cells, respectively. Previous studies indicated that mechanical properties are the key parameters that are correlated with metastasis and invasion of cancer cells (Rother et al., 2014; Runge et al., 2014; Nguyen et al., 2016). Specifically, Wang et al. reported that the viscoelastic characteristics of breast cancer cells were substantially decreased, and the cancer cells became softer and more fluid than normal cells (Wang et al., 2016). Xie et al. analyzed different cancer cell lines, including human skin cancer cells (A2058), lung cancer cells (MSTO-211H), and hepatocellular carcinoma cells (Hep G2), and their results suggested that all cancer cells exhibited higher deformability and weaker viscoelastic behavior than the normal cells, respectively (Xie et al., 2019). Based on our results and others’ studies, we speculated that MC-LR could induce cells to alter their structures and functions and become softer, easier to deform, and potentially easier to metastasize. To our knowledge, this is the first time that the influence of MC-LR on cellular mechanical properties has been investigated, providing novel information to explain the carcinogenic mechanism of MC-LR.
Previous research reported that cellular mechanical behavior was associated with microfilament changes. As a result, cellular microfilament arrangement was further tested in this study. The results of immunofluorescence showed that MC-LR induced microfilament reorganization, leading to microfilament distribution on the cell surface and aggregation into bundles in DU145 and WPMY cells. Consistent with our study, Zeng et al. found that MC-LR caused aggregation of microfilament around the cell periphery in HL7702 cells (Zeng et al., 2015). In addition, Wang et al. observed that microfilaments were reconstructed, gathering at the cell edge and forming bundles, in MC-LR-treated Hep-2 cells (Wang et al., 2017). Other studies also demonstrated that MC-LR could cause microfilament reorganization in human liver cancer cells (SMMC-7721) (Wang et al., 2014) and neuroendocrine cells (PC12) (Meng et al., 2011). Additionally, it has been reported that cellular mechanical behavior is closely related to the components of the cytoskeleton, such as microfilament and microtubule (Chen et al., 2013; Pachenari et al., 2014). Further studies suggested that chemical drugs could cause cytoskeleton reorganization and then induce mechanical changes (Seyedpour et al., 2015; Li M. et al., 2016). Therefore, our results indicated that MC-LR could induce mechanical changes by microfilament reorganization in DU145 and WPMY cells.
Several studies have suggested that microfilament-associated proteins play key roles in maintaining the structure of microfilament (Wang et al., 2017). Therefore, microfilament-associated proteins need to be further investigated. Several relevant studies have been documented. For example, Zeng et al. reported that MC-LR induced hyperphosphorylation of VASP and ezrin in HL7702 cells but had no significant effect on their expression level (Zeng et al., 2015). The study of Wang et al. showed that the expression of p-VASP was up-regulated remarkably in SMMC-7721 cells treated with MC-LR (Wang et al., 2014). In addition, other studies found that ezrin and VASP were regulated by phosphorylation in MC-LR-treated cells, which could lead to microfilament rearrangement (Neisch and Fehon, 2011; Thomson et al., 2011; Zhou et al., 2015). Likewise, we detected the expression and phosphorylation of microfilament-associated proteins, and the results confirmed that the expression of p-VASP and p-ezrin was significantly upregulated in MC-LR treatment cells. However, the expression of VASP and ezrin remained unchanged. It is well known that VASP and ezrin are important microfilament-associated proteins, belonging to Ena/VASP family of adaptor proteins (Yu et al., 2015) and the ERM (Ezrin/Radixin/Moesin) protein family (Mcclatchey, 2014), respectively. Previous studies have demonstrated that the phosphorylation changes in VASP and ezrin induced microfilament rearrangement (Zhou et al., 2015; Wang et al., 2017). As a result, the MC-LR-induced increase in the phosphorylation level of microfilament-associated proteins that we found in our study could contribute to microfilament reorganization in DU145 and WPMY cells.
It has been reported that MC-LR can promote cell migration and metastasis (Pengfei et al., 2013; Wang et al., 2017). In more detail, Wang et al. showed that MC-LR accelerated Hep-2 cell migration (Wang et al., 2017). Xu et al. reported that MC-LR promoted MDA-MB-435 cell invasion and metastasis via the PI3-K/AKT signaling pathway (Pengfei et al., 2013). In this study, we found that MC-LR promoted cell invasion in DU145 and WPMY cells. Previous studies indicated that mechanical properties are correlated with the metastasis and invasion of cancer cells (Rother et al., 2014; Runge et al., 2014; Nguyen et al., 2016). In addition, many studies also suggested that microfilaments were functionally associated with cell invasion (Moon and Wynshaw-Boris, 2013; Du et al., 2015; Wang et al., 2017). In the present study, we demonstrated that MC-LR induced cellular mechanical changes and microfilament reorganization and promoted cell invasion. Based on our results and previous studies, we speculate that MC-LR promoted cell invasion by microfilament reorganization and mechanical changes in DU145 and WPMY cells. Relevant further studies will be performed to elucidate the molecular mechanism of MC-LR-promoted cell invasion.
Conclusion
In conclusion, we demonstrated that mechanical changes and microfilament reorganization were involved in MC-LR-promoted cell invasion in DU145 and WPMY cells. Our results provided novel and useful information to explain the toxicological mechanism of MC-LR.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
WF, ZP, and GuoW designed the experiments and wrote the paper. QZ, GuiW, and YX performed the experiments. ZG and ZL analyzed the data. All of the authors read and approved the final manuscript.
Funding
The study was supported by the Natural Science Foundation of Shandong Province (ZR2014CL034, ZR2018MC015, ZR2019MA018), Medical and Health Development Plan of Shandong Province (2017WS058), Research and Development Plan of University in Shandong Province (J18KA120), National Natural Science Foundation of China (11802209), and Funds of the Shandong “Double Tops” Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00089/full#supplementary-material
Data Sheet 1 | MC-LR increased the phosphorylation level of microfilament-associated proteins in DU145 and WPMY cells. These photos (including ezrin, vasp and β-actin-1) were the original image files for the Western blot of Figure 5A. Other photos (including p-ezrin, p-vasp and β-actin-2) were the original image files for the Western blot of Figure 5B.
References
Backer, L. C., Mcneel, S. V., Terry, B., Barbara, K., Christopher, W., Mitch, I., et al. (2010). Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon Off. J. Int. Soc. Toxinol. 55 (5), 909–921. doi: 10.1016/j.toxicon.2009.07.006
Chen, Y.-Q., Kuo, C.-Y., Wei, M.-T., Wu, K., Su, P.-T., Huang, C.-S., et al. (2013). Intracellular viscoelasticity of HeLa cells during cell division studied by video particle-tracking microrheology. J. Biomed. Optics 19 (1), 011008. doi: 10.1117/1.JBO.19.1.011008
Chun, P., Yabing, C., Tianchi, X., Jing, W., Dongmei, L., Xiaodong, H. (2018). Chronic exposure to microcystin-leucine-arginine promoted proliferation of prostate epithelial cells resulting in benign prostatic hyperplasia. Environ. Pollut. 242 (2), 1535–1545. doi: 10.1016/j.envpol.2018.08.024
Du, M., Wang, G., Barraclough, R., Rudland, P. (2015). P0017 S100P regulates cytoskeleton dynamics to promote cell migration and metastasis. Eur. J. Cancer 51 (2), e6–e7. doi: 10.1016/j.ejca.2015.06.022
Duong, T. T., Jähnichen, S., Le, T. P. Q., Ho, C. T., Hoang, T. K., Nguyen, T. K., et al. (2014). The occurrence of cyanobacteria and microcystins in the Hoan Kiem Lake and the Nui Coc reservoir (North Vietnam). Environ. Earth Sci. 71 (5), 2419–2427. doi: 10.1007/s12665-013-2642-2
Huang, P., Wang, S., Weng, D., Xu, L. (2018). Alpha4-overexpressing HL7702 cells can counteract microcystin-LR effects on cytoskeletal structure. Environ. Toxicol. 33 (9), 978–987. doi: 10.1002/tox.22585
Li, M., Liu, L., Xiao, X., Xi, N., Wang, Y. (2016). Effects of methotrexate on the viscoelastic properties of single cells probed by atomic force microscopy. J. Biol. Phys. 42 (4), 551–569. doi: 10.1007/s10867-016-9423-6
Li, Y., Li, J., Huang, H., Yang, M., Zhuang, D., Cheng, X., et al. (2016). Microcystin-LR induces mitochondria-mediated apoptosis in human bronchial epithelial cells. Exp. Ther. Med. 12 (2), 633–640. doi: 10.3892/etm.2016.3423
Liu, J., Wang, H., Wang, B., Chen, T., Wang, X., Huang, P., et al. (2016). Microcystin-LR promotes proliferation by activating Akt/S6K1 pathway and disordering apoptosis and cell cycle associated proteins phosphorylation in HL7702 cells. Toxicol. Lett. 240 (1), 214. doi: 10.1016/j.toxlet.2015.10.015
Ma, J., Feng, Y., Liu, Y., Li, X. (2016). PUMA and survivin are involved in the apoptosis of HepG2 cells induced by microcystin-LR via mitochondria-mediated pathway. Chemosphere 157, 241–249. doi: 10.1016/j.chemosphere.2016.05.051
Ma, J., Li, Y., Duan, H., Sivakumar, R., Li, X. (2018). Chronic exposure of nanomolar MC-LR caused oxidative stress and inflammatory responses in HepG2 cells. Chemosphere 192, 305–317. doi: 10.1016/j.chemosphere.2017.10.158
Mcclatchey, A. I. (2014). ERM proteins at a glance. J. Cell Sci. 127 (15), 3199–3204. doi: 10.1242/jcs.098343
Meng, G., Sun, Y., Fu, W., Guo, Z., Xu, L. (2011). Microcystin-LR induces cytoskeleton system reorganization through hyperphosphorylation of tau and HSP27 via PP2A inhibition and subsequent activation of the p38 MAPK signaling pathway in neuroendocrine (PC12) cells. Toxicology 290 (2), 218–229. doi: 10.1016/j.tox.2011.09.085
Moon, H. M., Wynshaw-Boris, A. (2013). Cytoskeleton in action: lissencephaly, a neuronal migration disorder. Wiley Interdiscip. Rev. Dev. Biol. 2 (2), 229–245. doi: 10.1002/wdev.67
Neisch, A. L., Fehon, R. G. (2011). Ezrin, radixin and moesin: key regulators of membrane–cortex interactions and signaling. Curr. Opin. In Cell Biol. 23 (4), 377–382. doi: 10.1016/j.ceb.2011.04.011
Nguyen, N., Shao, Y., Wineman, A., Fu, J., Waas, A. (2016). Atomic force microscopy indentation and inverse analysis for non-linear viscoelastic identification of breast cancer cells. Math. Biosci. 277, 77–88. doi: 10.1016/j.mbs.2016.03.015
Pachenari, M., Seyedpour, S. M., Janmaleki, M., Shayan, S. B., Taranejoo, S., Hosseinkhani, H. (2014). Mechanical properties of cancer cytoskeleton depend on actin filaments to microtubules content: investigating different grades of colon cancer cell lines. J. Biomechanics 47 (2), 373–379. doi: 10.1016/j.jbiomech.2013.11.020
Pengfei, X., Xu-Xiang, Z., Chen, M., Ziyi, F., Zhengrong, L., Gen, Z., et al. (2013). Promotion of melanoma cell invasion and tumor metastasis by microcystin-LR via phosphatidylinositol 3-kinase/AKT pathway. Environ. Sci. Technol. 47 (15), 8801–8808. doi: 10.1021/es4007228
Rastogi, R. P., Sinha, R. P., Incharoensakdi, A. (2014). The cyanotoxin-microcystins: current overview. Rev. In Environ. Sci. Bio/Technol. 13 (2), 215–249. doi: 10.1007/s11157-014-9334-6
Rother, J., Nöding, H., Mey, I., Janshoff, A. (2014). Atomic force microscopy-based microrheology reveals significant differences in the viscoelastic response between malign and benign cell lines. Open Biol. 4 (5), 140046–140046. doi: 10.1098/rsob.140046
Runge, J., Reichert, T. E., Fritsch, A., Käs, J., Bertolini, J., Remmerbach, T. W. (2014). Evaluation of single-cell biomechanics as potential marker for oral squamous cell carcinomas: a pilot study. Dis. 20 (3), e120–e127. doi: 10.1111/odi.12171
Seyedpour, S., Pachenari, M., Janmaleki, M., Alizadeh, M., Hosseinkhani, H. (2015). Effects of an antimitotic drug on mechanical behaviours of the cytoskeleton in distinct grades of colon cancer cells. J. Biomechanics 48 (6), 1172–1178. doi: 10.1016/j.jbiomech.2014.11.026
Taranejoo, S., Janmaleki, M., Pachenari, M., Seyedpour, S. M., Chandrasekaran, R., Cheng, W., et al. (2016). Dual effect of F-actin targeted carrier combined with antimitotic drug on aggressive colorectal cancer cytoskeleton: Allying dissimilar cell cytoskeleton disrupting mechanisms. Int. J. Pharmaceut. 513 (1-2), 464–472. doi: 10.1016/j.ijpharm.2016.09.056
Thomson, D. M., Ascione, M. P., Grange, J., Nelson, C., Hansen, M. D. (2011). Phosphorylation of VASP by AMPK alters actin binding and occurs at a novel site. Biochem. Biophys. Res. Commun. 414 (1), 215–219. doi: 10.1016/j.bbrc.2011.09.059
Wang, G., Chen, W. (2012). Effects of mechanical stimulation on viscoelasticity of rabbit scleral fibroblasts after posterior scleral reinforcement. Exp. Biol. Med. 237 (10), 1150–1154. doi: 10.1258/ebm.2012.012196
Wang, Q., Niu, Y., Xie, P., Chen, J., Ma, Z., Tao, M., et al. (2010). Factors affecting temporal and spatial variations of microcystins in Gonghu Bay of Lake Taihu, with potential risk of microcystin contamination to human health. Thescientificworldjournal 10 (1), 1795–1809. doi: 10.1100/tsw.2010.172
Wang, H., Liu, J., Lin, S., Wang, B., Xing, M., Guo, Z., et al. (2014). MCLR-induced PP2A inhibition and subsequent Rac1 inactivation and hyperphosphorylation of cytoskeleton-associated proteins are involved in cytoskeleton rearrangement in SMMC-7721 human liver cancer cell line. Chemosphere 112, 141–153. doi: 10.1016/j.chemosphere.2014.03.130
Wang, Y., Xu, C., Jiang, N., Zheng, L., Zeng, J., Qiu, C., et al. (2016). Quantitative analysis of the cell-surface roughness and viscoelasticity for breast cancer cells discrimination using atomic force microscopy. Scanning 38 (6), 558–563. doi: 10.1002/sca.21300
Wang, B., Liu, J., Pu, H., Xu, K., Wang, H., Wang, X., et al. (2017). Protein phosphatase 2A inhibition and subsequent cytoskeleton reorganization contributes to cell migration caused by microcystin-LR in human laryngeal epithelial cells (Hep-2). Environ. Toxicol. 32 (3), 890–903. doi: 10.1002/tox.22289
Wu, Y.-L., He, Y., Shi, J.-J., Zheng, T.-X., Lin, X.-J., Lin, X. (2019). Microcystin-LR promotes necroptosis in primary mouse hepatocytes by overproducing reactive oxygen species. Toxicol. Appl. Pharmacol. 377, 114626. doi: 10.1016/j.taap.2019.114626
Xie, Y., Wang, M., Cheng, M., Gao, Z., Wang, G. (2019). The viscoelastic behaviors of several kinds of cancer cells and normal cells. J. Mechanical Behav. Biomed. Mater. 91, 54–58. doi: 10.1016/j.jmbbm.2018.11.029
Yan, G., Ru, Y., Wu, K., Yan, F., Wang, Q., Wang, J., et al. (2018). GOLM1 promotes prostate cancer progression through activating PI3K-AKT-mTOR signaling. Prostate 78 (3), 166–177. doi: 10.1002/pros.23461
Yin-Quan, C., Chia-Yu, K., Ming-Tzo, W., Kelly, W., Pin-Tzu, S., Chien-Shiou, H., et al. (2014). Intracellular viscoelasticity of HeLa cells during cell division studied by video particle-tracking microrheology. J. Biomed. Optics 19 (1), 011008. doi: 10.1117/1.JBO.19.1.011008
You, X., Wei, L., Fan, S., Yang, W., Liu, X., Wang, G., et al. (2017). Expression pattern of Zinc finger protein 185 in mouse testis and its role in regulation of testosterone secretion. Mol. Med. Rep. 16 (2), 2101–2106. doi: 10.3892/mmr.2017.6797
Yu, S., Jing-Hui, L., Pu, H., Zong-Lou, G., Li-Hong, X. (2015). Alterations of tau and VASP during microcystin-LR-induced cytoskeletal reorganization in a human liver cell line. Environ. Toxicol. 30 (1), 92–100. doi: 10.1002/tox.21898
Zeng, J., Tu, W. W., Lazar, L., Chen, D. N., Zhao, J. S., Xu, J. (2015). Hyperphosphorylation of microfilament-associated proteins is involved in microcystin-LR-induced toxicity in HL7702 cells. Environ. Toxicol. 30 (8), 981–988. doi: 10.1002/tox.21974
Zhang, Q., Wang, X., Wei, X., Chen, W. (2008). Characterization of viscoelastic properties of normal and osteoarthritic chondrocytes in experimental rabbit model. Osteoarthritis Cartilage 16 (7), 837–840. doi: 10.1016/j.joca.2007.09.016
Zhao, S., Xie, P., Chen, J., Liu, L., Fan, H., Zhao, S., et al. (2016). A proteomic study on liver impairment in rat pups induced by maternal microcystin-LR exposure. Environ. Pollut. 212, 197–207. doi: 10.1016/j.envpol.2015.12.055
Zhou, M., Tu, W. W., Xu, J. (2015). Mechanisms of microcystin-LR-induced cytoskeletal disruption in animal cells. Toxicon 101, 92–100. doi: 10.1016/j.toxicon.2015.05.005
Keywords: microcystin-LR, mechanical parameters, invasion, microfilament, microfilament-associated protein
Citation: Zhang Q, Wang G, Xie Y, Gao Z, Liang Z, Pan Z, Wang G and Feng W (2020) Mechanical Changes and Microfilament Reorganization Involved in Microcystin-LR-Promoted Cell Invasion in DU145 and WPMY Cells. Front. Pharmacol. 11:89. doi: 10.3389/fphar.2020.00089
Received: 12 November 2019; Accepted: 27 January 2020;
Published: 26 February 2020.
Edited by:
Zhe-Sheng Chen, St. John’s University, United StatesReviewed by:
Wenjie Liu, Xiamen University, ChinaWentao Li, University of North Carolina at Chapel Hill, United States
Copyright © 2020 Zhang, Wang, Xie, Gao, Liang, Pan, Wang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhifang Pan, c2R3ZnB6ZkAxMjYuY29t; Guohui Wang, d2FuZ2doQHdmbWMuZWR1LmNu; Weiguo Feng, ZmVuZ3dnQHdmbWMuZWR1LmNu
†These authors have contributed equally to this work
 Qiang Zhang1†
Qiang Zhang1† Guihua Wang
Guihua Wang Zhiqin Gao
Zhiqin Gao Weiguo Feng
Weiguo Feng