- 1Key Laboratory of Molecular Target and Clinical Pharmacology, State Key Laboratory of Respiratory Disease, School of Pharmaceutical Sciences & Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
- 2Department of Pediatrics, Shenzhen Maternity and Child Health Care Hospital, Shenzhen, China
- 3Department of Biomedical Engineering, School of Basic Medical Sciences, Guangzhou Medical University, Guangzhou, China
- 4Asia-Pacific Institute of Aging Studies, Lingnan University, Tuen Mun, Hong Kong, Hong Kong
Ultrasound is one of the most commonly used methods in the diagnosis and therapy of diseases due to its safety, deep penetration into tissue, and non-invasive nature. In the drug/gene delivery systems, ultrasound shows many advantages in terms of site-specific delivery and spatial release control of drugs/genes and attracts increasing attention. Microbubbles are the most well-known ultrasound-responsive delivery materials. Recently, nanobubbles, droplets, micelles, and nanoliposomes have been developed as novel carriers in this field. Herein, we review advances of novel ultrasound-responsive materials (nanobubbles, droplets, micelles and nanoliposomes) and discuss the challenges of ultrasound-responsive materials in delivery systems to boost the development of ultrasound-responsive materials as delivery carriers.
Introduction
Drugs are important agents for combating the ailments. Drugs are mainly divided into hydrophilic and lipophilic types according to solubility. Hydrophilic drugs, in general, have difficulties entering cells through passive diffusion because cell membranes are composed mainly of lipid bilayers (Thansandote et al., 2015). However, lipophilic drugs are often difficult to dissolve in water and have unsatisfactory bioavailability (Arnott and Planey, 2012). Recently, gene drugs including DNA drugs, RNA drugs have shown promise in treating mutant gene-associated diseases (Kaufmann et al., 2013). Different from chemical drugs, these gene drugs are much larger and have difficulties entering cells. Meanwhile, gene drugs are easily degraded by nucleases in blood stream or cells.
To address the shortcomings of chemical and gene drugs in clinical practices, drug-delivery carriers are used to encapsulate drugs to improve the water solubility of lipophilic drugs, enhance the penetration of hydrophilic drugs into cells, and decrease the side-effect of drugs. For example, the nanoliposomal encapsulation improve the water solubility and bioavailability of hydrophobic polyphenol curcumin (diferuloylmethane) and enhance anticancer activity of curcumin against breast cancer (Hasan et al., 2014). Additionally, delivery systems can also protect gene drugs from degradation by extracellular and intracellular enzymes, and promote therapeutic outcome (Cavalieri et al., 2015).
Advanced drug delivery systems (DDS) require a demand of dosage, spatial, and temporal control strategy (Liu et al., 2016b). Several studies have shown that microspheres and nanoparticles can protect drugs or genes and further improve therapeutic outcomes (Nakamura and Harashima, 2017; Alkie et al., 2019; Holley et al., 2019; Yu et al., 2019). However, the uncontrolled release of drugs and genes at the disease site is the main limitation of microspheres and nanoparticles.
Since 1978, stimuli-responsive delivery systems have been widely investigated to control release of drugs and genes in targeted sites (Yatvin et al., 1978). Recently, the commonly used stimuli include microenvironment pH and enzymes in target tissues, as well as external stimuli such as photons, electromagnetic, and ultrasound waves. It supplies new perspective for the study of control release of drugs and genes in delivery system. Ultrasound wave is a promising physical stimulus for drug/gene delivery because of its safety, low cost, and portability of ultrasound instrument (Endo-Takahashi et al., 2013).
Ultrasound, including low frequency (<100 kHz) and high frequency (>100 kHz and MHz range) ultrasound (Ji et al., 2018; Matafonova and Batoev, 2019), as one of the most commonly used physical factors has been widely employed in the disease diagnosis and therapy (Witte et al., 2018). Since the mid-1990s, it has been demonstrated that ultrasound can enhance the permeability of agents into living cells (Lentacker et al., 2014). Ultrasound sonication improves the delivery efficiency of drugs/genes mainly through thermal and non-thermal effect (Husseini and Pitt, 2008a; Lentacker et al., 2010; De Temmerman et al., 2011; He et al., 2015; Tardoski et al., 2015; Endo-Takahashi et al., 2016; Liao et al., 2017). The thermal effects are produced from the absorption of acoustic energy in biological tissues. While the non-thermal effects are mainly generated from ultrasound pressure, acoustic streaming, shockwaves, liquid microjet, and ultrasound-induced oscillation or cavitation (Marin et al., 2002; Husseini and Pitt, 2008a; Mannaris et al., 2020). In particular, in the presence of cavitation nuclei, a type of particles which can lower acoustic intensity to induce cavitation, ultrasound shows higher delivery efficiency (Miller et al., 1999; Ward et al., 1999; Peruzzi et al., 2018; Mannaris et al., 2020).
In view of the advantages of cavitation nuclei in ultrasound stimuli, microbubbles as cavitation nuclei have been used widely in ultrasound-mediated drug/gene delivery (Huang et al., 2012; Yan et al., 2015; Oishi et al., 2016; Wang et al., 2016; Zullino et al., 2018). The commonly used microbubbles have gaseous cores and outer shells composed of phospholipids, polymers or proteins. The size of microbubbles (about 1–10 μm) enables them to circulate with red blood cells (Jayaweera et al., 1994; Sirsi and Borden, 2012; Mulvana et al., 2017). Microbubbles, as proven ultrasound-responsive materials, have been applied in drug delivery in clinical trials (Table 1) (Hynynen et al., 2001; Dimcevski et al., 2016; He et al., 2016). These clinical trials confirmed the controllability of delivering the cargo like drugs and gene materials with ultrasonic switch and visualization of treatment. Most noteworthy, many preclinical studies were also under study. Kuo et al. (2019) used doxorubicin-loaded microbubbles in combination with ultrasound (1 MHz) to facilitate the entering of doxorubicin into osteosarcoma cells and exhibited 3.7-fold inhibition of cancer growth compared to doxrubicin-loaded microbubbles without sonication, and simultaneously in combination with contrast-enhanced ultrasound imaging doxorubicin-loaded microbubbles were used to monitor the perfusion and volume of cancer. Lee et al. (2016) delivered miR-29b-3p to enhance fracture healing using ultrasound microbubbles system. Even in articular cartilage to which it is difficult to deliver drugs, ultrasound-responsive microbubbles can also improve the drug delivery efficiency (Nieminen et al., 2017). However, microbubbles have a short circulation time in blood because their sizes restrict their passage through the barrier between blood vessels and targeted tissues. For example, tumor tissues permit only smaller particles (<1 μm) to enter their interior (Zullino et al., 2018). In particular, nanoparticles of size 1–100 nm can have high accumulation in tumor tissues via the enhanced permeability and retention (EPR) effect (Maeda, 2001; Baghbani and Moztarzadeh, 2017).
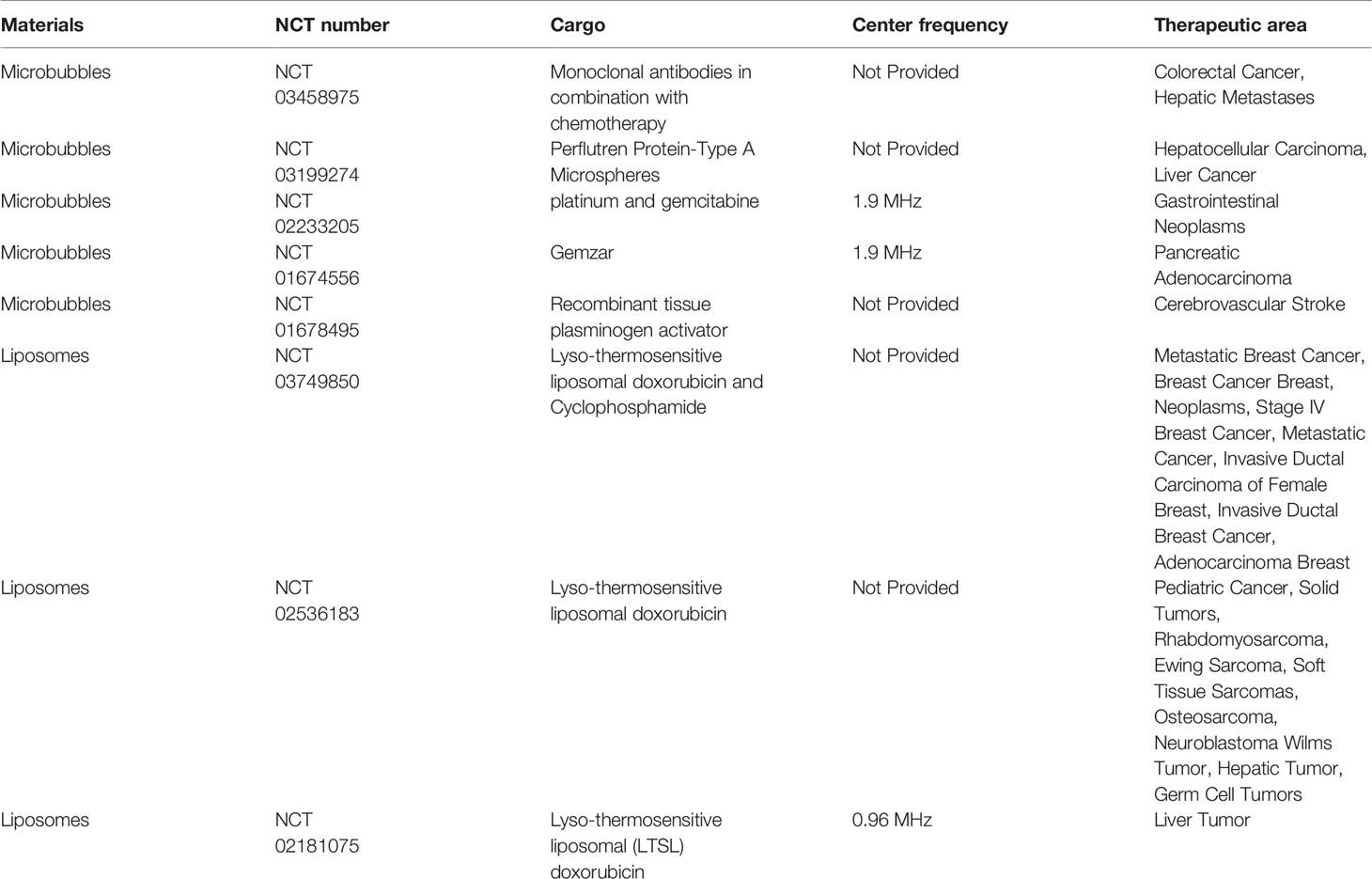
Table 1 Clinical trials of materials assisting drug delivery under sonication [the datasets for this table can be found in the (ClinicalTrials.gov) (https://clinicaltrials.gov/)].
Along with the rapid development of nanomaterials, nanoscale bubbles, droplets, micelles and nanoliposomes have been developed as novel nanomaterials in ultrasound-responsive drug-delivery systems (Ulrich, 2002; Ahmed et al., 2015). Some liposomes have been applied for drug delivery under ultrasound in clinical trials (Table 1).
Herein, we will introduce several of the major nanoscale ultrasound-responsive materials used in drug/gene delivery. Furthermore, we will discuss the challenges and the development of ultrasound-responsive materials in drug/gene delivery.
Novel Ultrasound-Responsive Materials
Nanobubbles
Nanobubbles are a type of nanoscale bubbles (1–1,000 nm) with gaseous cores and outer shells. As a ultrasound-responsive material, nanobubbles were designed originally as contrast agents to enhance ultrasound imaging, and developed as drug-delivery carriers later (Cavalli et al., 2016).
In tumor tissues, the endothelial gaps range from 380 nm to 780 nm (Hobbs et al., 1998). Microbubbles with the size of 1–10 μm cannot generally extravasate from blood vessels to tumor tissues. However, “leaky” tumor vessels and obstructive lymphatic drainage make nanobubbles with the size of 10–780 nm extravasate through endothelial gaps and accumulate in tumor tissue via the EPR effect (Fernandes et al., 2018). Therefore, nanobubbles show great potential in drug/gene delivery for the diagnosis and therapy of cancer because they can accumulate in tumor tissues and interact with tumor cells directly. Upon ultrasound sonication, nanobubble-induced sonoporation on cells can also enhance the efficiency of drug/gene delivery (Xing et al., 2016). As early as 2009, Watanabe et al. (2010) used ultrasound-responsive nanobubbles to control the delivery of gene to skeletal muscle both in BALB/c mice. This is the first report to use isotopic imaging (PET or SPECT) to realize visualization of gene transfection and to provide an easy way to detect the transfection of gene in clinic especially in vascular diseases and muscular dystrophy. Wu et al. (2017a) used poly(lactide-co-glycolic acid) (PLGA) as the shell and octafluoropropane (C3F8) gas as core of nanobubbles to load paclitaxel, and further modified them with A10-3.2 aptamer to target prostate cell-specific membrane antigen (PSMA) for therapy of prostate cancer. Under low-frequency ultrasound stimuli, the nanobubble (PTX-A10-3.2-PLGA NB) achieved high drug release that induced significant apoptosis in vitro and significant inhibition of growth of tumor cells in BALB/c nude mice with xenograft tumors, and provided biological imaging of prostate-cancer cells. Subsequently in 2018, this research team synthesized cationic nanobubbles (CNBs) with same gas core decorated with A10-3.2 aptamer (siFoxM1-Apt-CNBs) for anti-tumor-targeted delivery of siRNA-FoxM1(Forkhead box M1) (Wu et al., 2018a). The transfection efficiency of siRNA was improved significantly, whereas FoxM1 expression was reduced significantly after siFoxM1-Apt-CNBs combined with ultrasound stimuli in xenograft tumors in nude mice as well as in PSMA-positive LNCaP cells in vivo. These actions led to significant inhibition of tumor growth and prolonged mice survival.
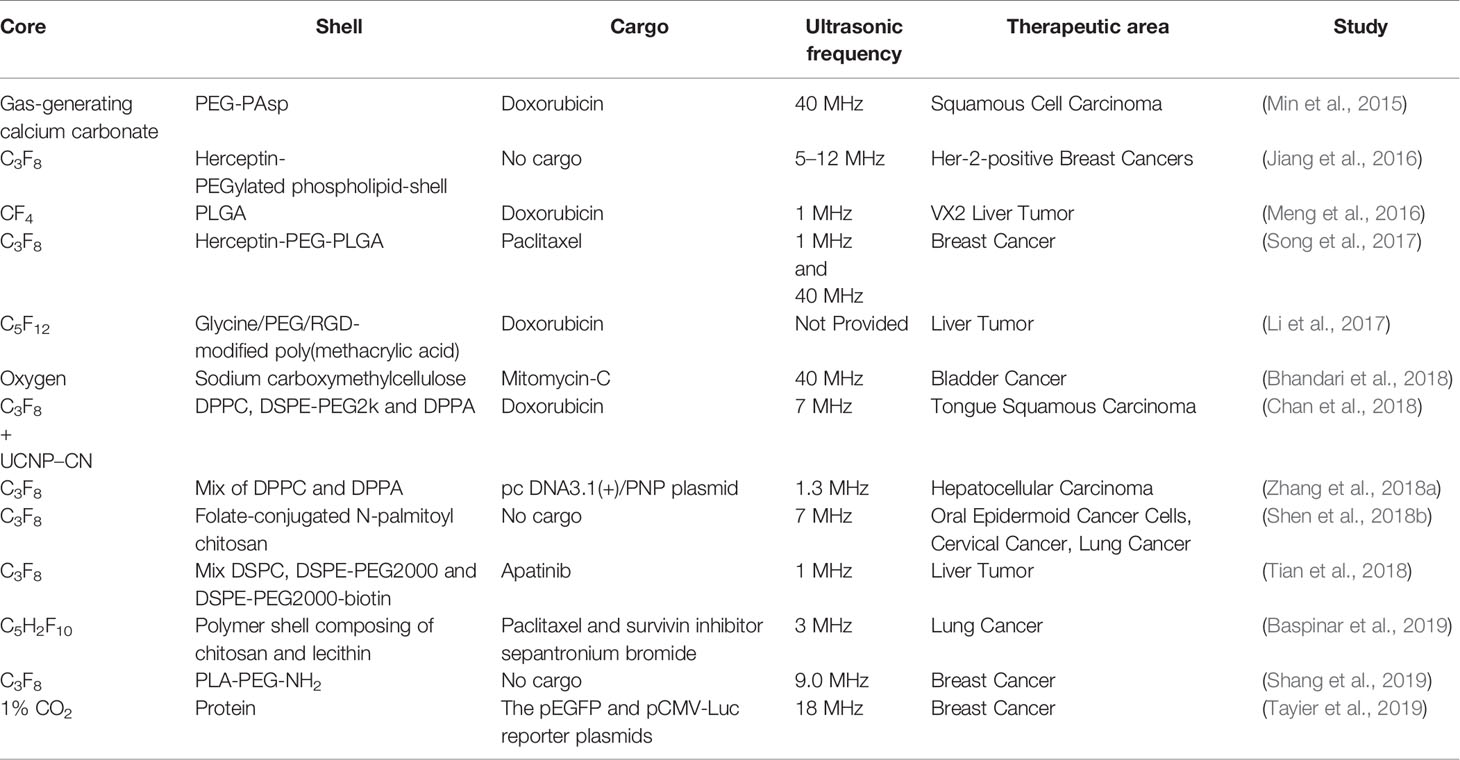
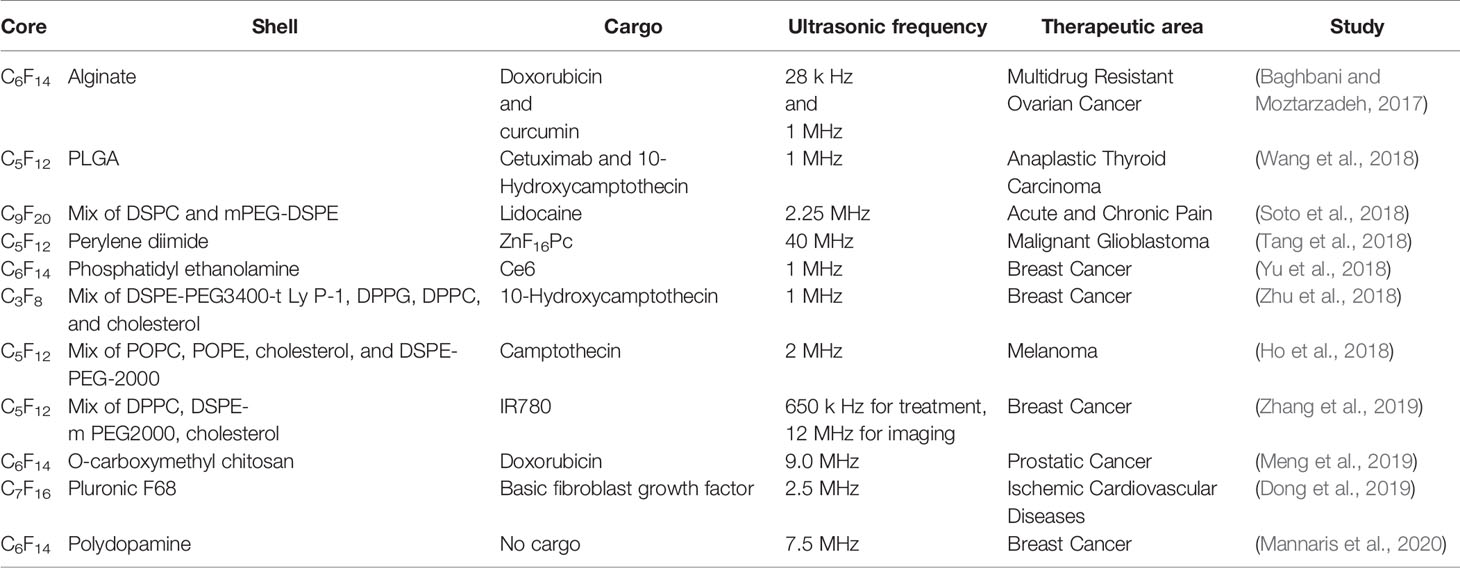
Cai et al. (2018) used C3F8 gas as the core and phospholipids as shells to prepare nanobubbles for delivering isocitrate dehydrogenase 1 (IDH1)-siRNA to gliomas. The siRNA-loaded nanobubbles interfered significantly expression of IDH1 in vitro and in vivo under ultrasound sonication. Shen et al. (2018a) modified ultrasound-mediated resveratrol-embedded nanobubbles containing C3F8 core with anti-N-cadherin 2 antibody (which is regarded as a specific binding ligand of nucleus pulposus cells in intervertebral disks) to increase the drug concentration in intervertebral disks for slowing down their degeneration in vivo. Song et al. (2018b) developed low-frequency ultrasound-responsive nanobubbles composed of C3F8 core and PEGylated lipid shell to deliver a plasmid, the expression vector of brain-derived neurotrophic factor (BDNF) for treating acute injury to the spinal cord, and microtubule-associated protein 2 (MAP-2) antibody to modify the nanobubbles to enhance the targeting. They found that combined treatment of ultrasound and nanobubbles increased BDNF expression significantly in vitro and in vivo, and improved recovery of spinal-cord injury, indicating that nanobubbles are potential ultrasound-responsive materials in drug/gene delivery. Some other studies are enumerated in Table 2.
Droplets
Droplets are especially ultrasound-responsive liquid nanomaterials consisting of volatile perfluorocarbons (PFCs). It can undergo a phase transition through ultrasound-induced acoustic droplet vaporization or heat. After ultrasound stimulation, droplets can expand and convert into nanobubbles. This characteristic feature improves the ultrasonic contrast and triggers the release of loading agents specifically. Moreover droplets are more stable than gas bubbles in blood circulation at 37°C because droplets maintain their liquid core in the circulation avoiding gas dissolution (Lanza and Wickline, 2001; Lea-Banks et al., 2019). Stable PFC emulsions, commonly used droplets, can be prepared to ~200 nm in diameter (Fabiilli et al., 2010), which is beneficial for circulating for a longer time in vivo, passing into tissues or cells, and enhancing the EPR effect (Shpak et al., 2016). More interestingly, Lattin et al. (2015) supposed that the disruption of droplets may break down the membrane of endosome to aid the escape from the endosome endocytosis pathway of macromolecules such as genes. Their findings provide a new strategy for delivering therapeutic agents especially large molecules like genes upon ultrasound sonication.
Droplets, in general, are used to load lipophilic drugs, such as 10-hydroxycamptothecin (HCPT). HCPT is an efficacious anticancer drug but has limited clinical application due to its poor hydrophilicity. Encapsulation of lipophilic materials could improve the therapeutic efficacy of HCPT against cancer (Zhang et al., 2008; Li et al., 2012; Yang et al., 2013; Liu et al., 2015; Liu et al., 2016a). Based on this information, Liu et al. (2018) prepared an ultrasound-responsive droplet consisting of four parts: folic acid (FA) for overexpression of FA receptors on cancer cell membranes; superparamagnetic Fe3O4 for imaging; HCPT for cancer treatment; a PFC as the droplet core. The PFC core could undergo droplet vaporization upon sonication to cause HCPT release and enhance ultrasound imaging.
Rapoport et al. (2011) developed a novel nanoemulsion containing a perfluoro-15-crown-5-ether (PFCE) core with good stability and reversible transition from droplet to bubble. Moreover, the novel nanoemulsions could realize ultrasound and 19Flourine magnetic resonance dual-mode imaging, and enhance the inhibitory efficiency of paclitaxel-loaded nanoemulsions on the growth and metastasis of breast and pancreatic cancer cells in mice.
Doplets were also investigated in the application of brain diseases. Chen et al. (2013) compared the safety of microbubbles and droplets for drug delivery to the brain under focused ultrasound. In their studies, the same lipid compositions were used as the outer shells of microbubbles and droplets: perfluorobutane as the microbubble core and PFC as the droplet core. The cavitation induced by droplets required a higher threshold and droplets could deliver the drug more safely and more effectively than microbubbles in the brain. In 2018, another study on the delivery of biomolecules into the brain using droplets was published by colleagues in the team (Wu et al., 2018a). These findings demonstrated that ultrasound droplet-mediated delivery was a novel approach to deliver drug/gene into the brain effectively. Other up-to-date researches are listed in Table 3.
Micelles
Micelles are, in general, generated through self-assembly of polymers containing a hydrophilic group and a hydrophobic alkane (Husseini et al., 2007). Moreover, the diameters of micelles, which range from 10 nm to 100 nm, will help their application in nanoformulations (Husseini and Pitt, 2008b; Xia et al., 2016). Amphiphilic structures enable hydrophilic drugs and hydrophobic drugs to be encapsulated readily in micelles. The moderate thermal effect induced by ultrasound can increase the cell membrane penetrability resulting in enhancing extravasation in targeted cells (Rapoport, 2012). And increasing evidence has shown that micelles can be destroyed under shockwaves produced by ultrasound to release cargo loaded in micelles and deliver them to target tissues (Ahmed et al., 2015). Ultrasound-responsive micelles not only achieve the control of space release but also the quantity of release, since they can reassemble again when the ultrasound shuts off (Husseini et al., 2002; Tanbour et al., 2016). Hence, micelles are also potential materials for ultrasound-responsive delivery.
As early as in 2006, Chen et al. (2006) prepared micelles composed of three kinds of pluronics, F127, L61 and P85 as gene-delivery carriers under sonication. They found that, upon sonication, these three types of micelles enhanced the efficiency of gene transfection in 3T3-MDEI, C2C12, and CHO cell lines. Later, Wu et al. (2017b) developed a mixed micelle of pluronic P123/F127 polymers to encapsulate curcumin. They showed that curcumin was released at specific sites under ultrasound sonication, and that sonication increased cellular uptake of curcumin compared with that using free curcumin. In vitro, curcumin released from micelles increased along with increasing ultrasound intensity. Furthermore, curcumin-loaded micelles decreased the tumor weight by ~6.5-fold upon ultrasound sonication compared with the group without sonication exposure. Kang et al. (2019) studied doxorubicin (DOX) release with the help of high-intensity focused ultrasound (HIFU). The center frequency of the pre-clinical HIFU system they used was 1.5 MHz. Under high-intensity focused ultrasound, the structure of micelles loaded with DOX and hydrophobic 1,3-bis-(2,4,6-trimethylpheny l) imidazolylidene nitric oxide (IMesNO, a donor of nitric oxide, NO) was destroyed, and IMesNO was released from the micelles to produce NO. In cancer tissues, NO improved the EPR effect by expanding cancer blood vessels to increase blood blow, and subsequently enhanced the anticancer effect of DOX.
Nanoliposomes
Liposomes show excellent biocompatibility because they consist primarily of lipid bilayers (Schroeder et al., 2009). Liposomes can often load hydrophilic molecules and lipophilic molecules to improve their pharmacokinetics and reduce systemic toxicity (Torchilin, 2005; Allen and Cullis, 2013). Recently, accelerating evidence shows that nanoliposomes can deliver and release drugs/genes in target tissues upon ultrasound sonication (Dromi et al., 2007; Mannaris et al., 2013; Ta et al., 2014; Lyon et al., 2017). In general, nanoliposomes do not contain gas, so they are not particularly responsive to ultrasound. To achieve a particular response to ultrasound, nanoliposomes can be designed to contain vapor-phase molecules or encapsulated emulsions that can vaporize under ultrasound (Huang, 2008; Geers et al., 2012). When being exposed to ultrasound, cavitation or thermal effects can increase the release of drug/gene-loaded in nanoliposomes. Usually under sonication at high frequency, thermal effect takes the main role of delivery process. While under low frequency, cavitation plays an important role (Huang and MacDonald, 2004; Kopechek et al., 2008; Smith et al., 2010; Lattin et al., 2012).
To improve the targeting ability of ultrasound-responsive nanoliposomes, Negishi et al. (2013) used an AG73 peptide targeting syndecan (which is highly expressed in neovascular vessels) to modify liposomes with a perfluoropropane core. This AG73 peptide-modification endowed liposomes with a perfluoropropane core to have good targeting ability to tumor cells and deliver plasmids to them effectively. In 2018, a new liposome-encapsulating gas, phosphorodiamidate morpholino oligomer, was used to induce antisense oligonucleotide-mediated “exon skipping” for treating Duchenne muscular dystrophy (Negishi et al., 2018). This new liposome could deliver the antisense oligonucleotide to diseased muscles and release it upon ultrasound sonication.
Nowadays, a mixture of liposomes and microbubbles termed a “liposome–microbubble complex” (LMC) has been reported. The LMC has the high drug-loading ability of liposomes and ultrasound-responsive property of microbubbles. Zhang et al. (2018b) fabricated a LMC as a drug vehicle to deliver paclitaxel. To overcome the disadvantage that LMC was effective in vitro but not in vivo, they used iRGD peptide, a nine-unit cyclic tumor-homing and tissue-penetrating peptide, to modify the LMC to achieve better permeability into blood vessels and tissues in a tumor-specific manner. This modified LMC showed higher toxicity to 4T1 breast cancer cells and antitumor efficacy in a subcutaneous tumor model.
Challenges
Ultrasound-responsive material-based drug/gene delivery has been explored widely in treating cancer (Khokhlova et al., 2015; Qin et al., 2016; Fan et al., 2017; Yue et al., 2018; Jing et al., 2019), cardiovascular diseases (Hua et al., 2014; Dixon et al., 2015; Castle and Feinstein, 2016), orthopedic diseases (Lee et al., 2016; Pullan et al., 2017; Kuo et al., 2019), ocular diseases (Aptel and Lafon, 2012; Wan et al., 2015a; Wan et al., 2015b; Lafond et al., 2017) and brain diseases (Timbie et al., 2015; Song et al., 2018a), and also applied in vaccine immunization (Tachibana et al., 1997; Escoffre et al., 2016). However, application of ultrasound-responsive materials in drug/gene delivery faces certain challenges.
First, the prerequisite for treating diseases is a sufficient amount of drug/gene delivered and released in diseased tissues. Most ultrasound-responsive materials need an ultrasound-responsive core (gaseous, PFC, or gas-generating). These ultrasound-responsive cores consume a lot of space in ultrasound-responsive materials (microbubbles, nanobubbles, or droplets), which makes lower drug/gene-loaded contents, and decrease the amount of drug/gene delivered to diseased tissues, and eventually lead to limited therapeutic efficacy (Klibanov et al., 2010; Fabiilli et al., 2010; Shende and Jain, 2019). Second, nanoscale ultrasound-responsive materials have advantages over microbubbles with regard to targeted delivery of drugs and genes, but these nanomaterials are less responsive than microbubbles (Sirsi and Borden, 2014). So nanomaterials require higher ultrasound intensity to induce cavitation for effective release of drugs/genes from nanomaterials. But ultrasound of high intensity can cause damage to neighboring healthy tissues. High-intensity ultrasound also induces the rapid collapse of bubbles and rapid release of the drug/gene loaded in the bubbles, which may not meet the need for sustained release of some drugs (e.g., insulin).
The last but not the least, ultrasonic parameters are still noticeable issues. Low- and high-frequency ultrasound can damage biologic tissues when sonication-induced heating is too high, and the pore formation on cell membranes is irreversible (Mehier-Humbert et al., 2005). Therefore, the intensity and duration of ultrasound sonication must be controlled. Kovacs et al. (2017) found that pulsed-focused ultrasound induced the opening of the blood–brain barrier and was accompanied by increased expression of heat-shock protein 70, interleukin-1, interleukin-18, tumor necrosis factor-α, and inflammation of brain tissues, suggesting that application of ultrasound-responsive materials in drug/gene delivery to the brain system should be done with extreme caution.
Conclusions
Ultrasound-responsive materials can deliver drugs/genes to targeted tissues, and induce the release of drugs/genes in specific sites upon ultrasound sonication. However, most evidence has arisen from in vitro and in vivo animal experiments. Few clinical trials have investigated the role of ultrasound-responsive materials in drug/gene delivery. Thus, more clinical trials should be conducted to confirm the outlook of ultrasound-responsive materials in drug/gene delivery.
Recent studies have revealed that the major reason limiting application of ultrasound-responsive materials is their low drug/gene-loaded content. Enhancing the drug/gene-loaded content in ultrasound-responsive materials will be a “hotspot” for clinical translation of ultrasound-responsive materials.
In addition, sonoporation is regarded to be the main reason that ultrasound-responsive materials enhance the release of loaded drugs/genes. However, the interaction of ultrasound and ultrasound-responsive materials is complicated, and can induce mechanical forces, sonoporation, heating, and sonochemical effects. Therefore, better understanding of how ultrasound-responsive materials enhance release of loaded drugs/genes will lay a solid foundation to boost development of ultrasound-responsive materials in drug/gene delivery.
Data Availability Statement
The datasets generated for this study can be found in the ClinicalTrials.gov database (https://clinicaltrials.gov/).
Author Contributions
XC, YJ, and CX contributed to the conception and design of the study. XC wrote the first draft of the manuscript. YJ wrote sections of the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Funding
This work was supported by the Fund of Talents for High-level University in the Construction of Guangzhou (B195002009025) and the Science and Technology Project of Guangdong Province (2017B090911012).
Conflict of Interest
The authors declare that the research was conducted in the absence of commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our sincere gratitude to Dr. Qicai Xiao for the helpful assistance.
References
Ahmed, S. E., Martins, A. M., Husseini, G. A. (2015). The use of ultrasound to release chemotherapeutic drugs from micelles and liposomes. J. Drug Targeting 23, 16–42. doi: 10.3109/1061186X.2014.954119
Alkie, T. N., de Jong, J., Jenik, K., Klinger, K. M., DeWitte-Orr, S. J. (2019). Enhancing innate antiviral immune responses in rainbow trout by double stranded RNA delivered with cationic phytoglycogen nanoparticles. Sci. Rep. 9, 13619. doi: 10.1038/s41598-019-49931-2
Allen, T. M., Cullis, P. R. (2013). Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 65, 36–48. doi: 10.1016/j.addr.2012.09.037
Amani, A., Kabiri, T., Shafiee, S., Hamidi, A. (2019). Preparation and characterization of PLA-PEG-PLA/PEI/DNA nanoparticles for improvement of transfection efficiency and controlled release of DNA in gene delivery systems. Iran. J. Pharm. Res. 18, 125–141.
Aptel, F., Lafon, C. (2012). Therapeutic applications of ultrasound in ophthalmology. Int. J. Hyperthermia 28, 405–418. doi: 10.3109/02656736.2012.665566
Arnott, J. A., Planey, S. L. (2012). The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discovery 7, 863–875. doi: 10.1517/17460441.2012.714363
Baghbani, F., Moztarzadeh, F. (2017). Bypassing multidrug resistant ovarian cancer using ultrasound responsive doxorubicin/curcumin co-deliver alginate nanodroplets. Colloids Surf. B Biointerfaces 153, 132–140. doi: 10.1016/j.colsurfb.2017.01.051
Baspinar, Y., Erel-Akbaba, G., Kotmakci, M., Akbaba, H. (2019). Development and characterization of nanobubbles containing paclitaxel and survivin inhibitor YM155 against lung cancer. Int. J. Pharm. 566, 149–156. doi: 10.1016/j.ijpharm.2019.05.039
Bhandari, P., Novikova, G., Goergen, C. J., Irudayaraj, J. (2018). Ultrasound beam steering of oxygen nanobubbles for enhanced bladder cancer therapy. Sci. Rep. 8, 3112. doi: 10.1038/s41598-018-20363-8
Cai, W., Lv, W., Feng, Y., Yang, H., Zhang, Y., Yang, G., et al. (2018). The therapeutic effect in gliomas of nanobubbles carrying siRNA combined with ultrasound-targeted destruction. Int. J. Nanomed. 13, 6791–6807. doi: 10.2147/IJN.S164760
Castle, J., Feinstein, S. B. (2016). Drug and gene delivery using sonoporation for cardiovascular disease. Adv. Exp. Med. Biol. 880, 331. doi: 10.1007/978-3-319-22536-4_18
Cavalieri, F., Beretta, G. L., Cui, J., Braunger, J. A., Yan, Y., Richardson, J. J., et al. (2015). Redox-sensitive PEG-polypeptide nanoporous particles for survivin silencing in prostate cancer cells. Biomacromolecules 16, 2168–2178. doi: 10.1021/acs.biomac.5b00562
Cavalli, R., Soster, M., Argenziano, M. (2016). Nanobubbles: a promising efficient tool for therapeutic delivery. Ther. Deliv. 7, 117–138. doi: 10.4155/tde.15.92
Chan, M. H., Pan, Y. T., Chan, Y. C., Hsiao, M., Chen, C. H., Sun, L., et al. (2018). Nanobubble-embedded inorganic 808 nm excited upconversion nanocomposites for tumor multiple imaging and treatment. Chem. Sci. 9, 3141–3151. doi: 10.1039/c8sc00108a
Chen, Y. C., Liang, H. D., Zhang, Q. P., Blomley, M. J., Lu, Q. L. (2006). Pluronic block copolymers: novel functions in ultrasound-mediated gene transfer and against cell damage. Ultrasound Med. Biol. 32, 131–137. doi: 10.1016/j.ultrasmedbio.2005.10.002
Chen, C. C., Sheeran, P. S., Wu, S. Y., Olumolade, O. O., Dayton, P. A., Konofagou, E. E. (2013). Targeted drug delivery with focused ultrasound-induced blood-brain barrier opening using acoustically-activated nanodroplets. J. Control. Release 172, 795–804. doi: 10.1016/j.jconrel.2013.09.025
De Temmerman, M. L., Dewitte, H., Vandenbroucke, R. E., Lucas, B., Libert, C., Demeester, J., et al. (2011). mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials 32, 9128–9135. doi: 10.1016/j.biomaterials.2011.08.024
Dimcevski, G., Kotopoulis, S., Bjanes, T., Hoem, D., Schjott, J., Gjertsen, B. T., et al. (2016). A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 243, 172–181. doi: 10.1016/j.jconrel.2016.10.007
Dixon, A. J., Kilroy, J. P., Dhanaliwala, A. H., Chen, J. L., Phillips, L. C., Ragosta, M., et al. (2015). Microbubble-mediated intravascular ultrasound imaging and drug delivery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 62, 1674–1685. doi: 10.1109/TUFFC.2015.007143
Dong, X., Lu, X., Kingston, K., Brewer, E., Juliar, B. A., Kripfgans, O. D., et al. (2019). Controlled delivery of basic fibroblast growth factor (bFGF) using acoustic droplet vaporization stimulates endothelial network formation. Acta Biomater. 97, 409–419. doi: 10.1016/j.actbio.2019.08.016
Dromi, S., Frenkel, V., Luk, A., Traughber, B., Angstadt, M., Bur, M., et al. (2007). Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin. Cancer Res. 13, 2722–2727. doi: 10.1158/1078-0432.CCR-06-2443
Endo-Takahashi, Y., Negishi, Y., Nakamura, A., Suzuki, D., Ukai, S., Sugimoto, K., et al. (2013). pDNA-loaded bubble liposomes as potential ultrasound imaging and gene delivery agents. Biomaterials 34, 2807–2813. doi: 10.1016/j.biomaterials.2012.12.018
Endo-Takahashi, Y., Negishi, Y., Suzuki, R., Maruyama, K., Aramaki, Y. (2016). MicroRNA imaging in combination with diagnostic ultrasound and bubble liposomes for microRNA delivery. Methods Mol. Biol. 1372, 209–213. doi: 10.1007/978-1-4939-3148-4_16
Escoffre, J. M., Deckers, R., Bos, C., Moonen, C. (2016). Bubble-assisted ultrasound: application in immunotherapy and vaccination. Adv. Exp. Med. Biol. 880, 243–261. doi: 10.1007/978-3-319-22536-4_14
Fabiilli, M. L., Haworth, K. J., Sebastian, I. E., Kripfgans, O. D., Carson, P. L., Fowlkes, J. B. (2010). Delivery of chlorambucil using an acoustically-triggered perfluoropentane emulsion. Ultrasound Med. Biol. 36, 1364–1375. doi: 10.1016/j.ultrasmedbio.2010.04.019
Fan, P., Zhang, Y., Guo, X., Cai, C., Wang, M., Yang, D., et al. (2017). Cell-cycle-specific cellular responses to sonoporation. Theranostics 7, 4894–4908. doi: 10.7150/thno.20820
Fernandes, C., Suares, D., Yergeri, M. C. (2018). Tumor microenvironment targeted nanotherapy. Front. Pharmacol. 9, 1230. doi: 10.3389/fphar.2018.01230
Geers, B., Dewitte, H., De Smedt, S. C., Lentacker, I. (2012). Crucial factors and emerging concepts in ultrasound-triggered drug delivery. J. Control. Release 164, 248–255. doi: 10.1016/j.jconrel.2012.08.014
Hasan, M., Belhaj, N., Benachour, H., Barberi-Heyob, M., Kahn, C. J., Jabbari, E., et al. (2014). Liposome encapsulation of curcumin: physico-chemical characterizations and effects on MCF7 cancer cell proliferation. Int. J. Pharm. 461, 519–528. doi: 10.1016/j.ijpharm.2013.12.007
He, Y., Bi, Y., Ji, X. J., Wei, G. (2015). Increased efficiency of testicular tumor chemotherapy by ultrasound microbubble-mediated targeted transfection of siMDR1. Oncol. Rep. 34, 2311–2318. doi: 10.3892/or.2015.4262
He, X., Wu, D. F., Ji, J., Ling, W. P., Chen, X. L., Chen, Y. X. (2016). Ultrasound microbubble-carried PNA targeting to c-myc mRNA inhibits the proliferation of rabbit iliac arterious smooth muscle cells and intimal hyperplasia. Drug Deliv. 23, 2482–2487. doi: 10.3109/10717544.2015.1014947
Ho, Y. J., Chiang, Y. J., Kang, S. T., Fan, C. H., Yeh, C. K. (2018). Camptothecin-loaded fusogenic nanodroplets as ultrasound theranostic agent in stem cell-mediated drug-delivery system. J. Control. Release 278, 100–109. doi: 10.1016/j.jconrel.2018.04.001
Hobbs, S. K., Monsky, W. L., Yuan, F., Roberts, W. G., Griffith, L., Torchilin, V. P., et al. (1998). Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U. S. A. 95, 4607–4612. doi: 10.1073/pnas.95.8.4607
Holley, C. K., Kang, Y. J., Kuo, C. F., Abidian, M. R., Majd, S. (2019). Development and in vitro assessment of an anti-tumor nano-formulation. Colloids Surf. B Biointerfaces 184, 110481. doi: 10.1016/j.colsurfb.2019.110481
Hua, X., Zhou, L., Liu, P., He, Y., Tan, K., Chen, Q., et al. (2014). In vivo thrombolysis with targeted microbubbles loading tissue plasminogen activator in a rabbit femoral artery thrombus model. J. Thromb. Thromb. 38, 57–64. doi: 10.1007/s11239-014-1071-8
Huang, S. L., MacDonald, R. C. (2004). Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochim. Biophys. Acta 1665, 134–141. doi: 10.1016/j.bbamem.2004.07.003
Huang, Q., Deng, J., Wang, F., Chen, S., Liu, Y., Wang, Z., et al. (2012). Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp. Neurol. 233, 350–356. doi: 10.1016/j.expneurol.2011.10.027
Huang, S. L. (2008). Liposomes in ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 60, 1167–1176. doi: 10.1016/j.addr.2008.03.003
Husseini, G. A., Pitt, W. G. (2008a). Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Delivery Rev. 60, 1137–1152. doi: 10.1016/j.addr.2008.03.008
Husseini, G. A., Pitt, W. G. (2008b). The use of ultrasound and micelles in cancer treatment. J. Nanosci. Nanotechnol. 8, 2205–2215. doi: 10.1166/jnn.2008.225
Husseini, G. A., Christensen, D. A., Rapoport, N. Y., Pitt, W. G. (2002). Ultrasonic release of doxorubicin from Pluronic P105 micelles stabilized with an interpenetrating network of N,N-diethylacrylamide. J. Control. Release 83, 303–305. doi: 10.1016/s0168-3659(02)00203-1
Husseini, G. A., Diaz, D. L. R. M., Gabuji, T., Zeng, Y., Christensen, D. A., Pitt, W. G. (2007). Release of doxorubicin from unstabilized and stabilized micelles under the action of ultrasound. J. Nanosci. Nanotechnol. 7, 1028–1033. doi: 10.1166/jnn.2007.218
Hynynen, K., Pomeroy, O., Smith, D. N., Huber, P. E., McDannold, N. J., Kettenbach, J., et al. (2001). MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology 219, 176–185. doi: 10.1148/radiology.219.1.r01ap02176
Jayaweera, A. R., Edwards, N., Glasheen, W. P., Villanueva, F. S., Abbott, R. D., Kaul, S. (1994). In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ. Res. 74, 1157–1165. doi: 10.1161/01.res.74.6.1157
Ji, R., Pflieger, R., Virot, M., Nikitenko, S. I. (2018). Multibubble sonochemistry and sonoluminescence at 100 kHz: the missing link between low- and high-frequency ultrasound. . J. Phys. Chem. B 122, 6989–6994. doi: 10.1021/acs.jpcb.8b04267
Jiang, Q., Hao, S., Xiao, X., Yao, J., Ou, B., Zhao, Z., et al. (2016). Production and characterization of a novel long-acting Herceptin-targeted nanobubble contrast agent specific for Her-2-positive breast cancers. Breast Cancer-Tokyo 23, 445–455. doi: 10.1007/s12282-014-0581-8
Jing, Y., Xiu-Juan, Z., Hong-Jiao, C., Zhi-Kui, C., Qing-Fu, Q., En-Sheng, X., et al. (2019). Ultrasound-targeted microbubble destruction improved the antiangiogenic effect of Endostar in triple-negative breast carcinoma xenografts. J. Cancer Res. Clin. Oncol. 145, 1191–1200. doi: 10.1007/s00432-019-02866-7
Kang, Y., Kim, J., Park, J., Lee, Y. M., Saravanakumar, G., Park, K. M., et al. (2019). Tumor vasodilation by N-Heterocyclic carbene-based nitric oxide delivery triggered by high-intensity focused ultrasound and enhanced drug homing to tumor sites for anti-cancer therapy. Biomaterials 217, 119297. doi: 10.1016/j.biomaterials.2019.119297
Kaufmann, K. B., Buning, H., Galy, A., Schambach, A., Grez, M. (2013). Gene therapy on the move. EMBO Mol. Med. 5 (11), 1642–1661. doi: 10.1002/emmm.201202287
Khokhlova, T. D., Haider, Y., Hwang, J. H. (2015). Therapeutic potential of ultrasound microbubbles in gastrointestinal oncology: recent advances and future prospects (London, England: SAGE Publications).
Klibanov, A. L., Shevchenko, T. I., Raju, B. I., Seip, R., Chin, C. T. (2010). Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: a tool for targeted drug delivery. J. Control. Release 148, 13–17. doi: 10.1016/j.jconrel.2010.07.115
Kopechek, J. A., Abruzzo, T. M., Wang, B., Chrzanowski, S. M., Smith, D. A., Kee, P. H., et al. (2008). Ultrasound-mediated release of hydrophilic and lipophilic agents from echogenic liposomes. J. Ultrasound Med. 27, 1597–1606. doi: 10.7863/jum.2008.27.11.1597
Kovacs, Z. I., Kim, S., Jikaria, N., Qureshi, F., Milo, B., Lewis, B. K., et al. (2017). Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. U. S. A. 114, E75–E84. doi: 10.1073/pnas.1614777114
Kuo, T. T., Wang, C. H., Wang, J. Y., Chiou, H. J., Fan, C. H., Yeh, C. K. (2019). Concurrent osteosarcoma theranostic strategy using contrast-enhanced ultrasound and drug-loaded bubbles. Pharmaceutics 11, 223. doi: 10.3390/parmaceutics11050223
Lafond, M., Aptel, F., Mestas, J. L., Lafon, C. (2017). Ultrasound-mediated ocular delivery of therapeutic agents: a review. Expert Opin. Drug Deliv. 14, 539–550. doi: 10.1080/17425247.2016.1198766
Lanza, G. M., Wickline, S. A. (2001). Targeted ultrasonic contrast agents for molecular imaging and therapy. Prog. Cardiovasc. Dis. 44, 13–31. doi: 10.1053/pcad.2001.26440
Lattin, J. R., Pitt, W. G., Belnap, D. M., Husseini, G. A. (2012). Ultrasound-induced calcein release from eLiposomes. Ultrasound Med. Biol. 38, 2163–2173. doi: 10.1016/j.ultrasmedbio.2012.08.001
Lattin, J. R., Javadi, M., McRae, M., Pitt, W. G. (2015). Cytosolic delivery via escape from the endosome using emulsion droplets and ultrasound. J. Drug Targeting 23, 469–479. doi: 10.3109/1061186X.2015.1009074
Lea-Banks, H., O'Reilly, M. A., Hynynen, K. (2019). Ultrasound-responsive droplets for therapy: a review. J. Control. Release 293, 144–154. doi: 10.1016/j.jconrel.2018.11.028
Lee, W. Y., Li, N., Lin, S., Wang, B., Lan, H. Y., Li, G. (2016). miRNA-29b improves bone healing in mouse fracture model. Mol. Cell. Endocrinol. 430, 97–107. doi: 10.1016/j.mce.2016.04.014
Lentacker, I., Geers, B., Demeester, J., De Smedt, S. C., Sanders, N. N. (2010). Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol. Ther. 18, 101–108. doi: 10.1038/mt.2009.160
Lentacker, I., De Cock, I., Deckers, R., De Smedt, S. C., Moonen, C. T. (2014). Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 72, 49–64. doi: 10.1016/j.addr.2013.11.008
Li, P., Zheng, Y., Ran, H., Tan, J., Lin, Y., Zhang, Q., et al. (2012). Ultrasound triggered drug release from 10-hydroxycamptothecin-loaded phospholipid microbubbles for targeted tumor therapy in mice. J. Control. Release 162, 349–354. doi: 10.1016/j.jconrel.2012.07.009
Li, Y., Wan, J., Zhang, Z., Guo, J., Wang, C. (2017). Targeted soft biodegradable glycine/PEG/RGD-modified poly(methacrylic acid) nanobubbles as intelligent theranostic vehicles for drug delivery. ACS Appl. Mater. Interfaces 9, 35604–35612. doi: 10.1021/acsami.7b11392
Liao, W. H., Hsiao, M. Y., Lo, C. W., Yang, H. S., Sun, M. K., Lin, F. H., et al. (2017). Intracellular triggered release of DNA-quaternary ammonium polyplex by ultrasound. Ultrason. Sonochem. 36, 70–77. doi: 10.1016/j.ultsonch.2016.11.002
Liu, M., Chen, D., Wang, C., Chen, X., Wen, Z., Cao, Y., et al. (2015). Intracellular target delivery of 10-hydroxycamptothecin with solid lipid nanoparticles against multidrug resistance. J. Drug Targeting 23, 800–805. doi: 10.3109/1061186X.2015.1020427
Liu, M., Chen, D., Mukerabigwi, J. F., Chen, S., Zhang, Y., Lei, S., et al. (2016a). Intracellular delivery of 10-hydroxycamptothecin with targeted nanostructured lipid carriers against multidrug resistance. J. Drug Targeting 24, 433–440. doi: 10.3109/1061186X.2015.1086358
Liu, D., Yang, F., Xiong, F., Gu, N. (2016b). The smart drug delivery system and its clinical potential. Theranostics 6 (9), 1306–1323. doi: 10.7150/thno.14858
Liu, J., Xu, F., Huang, J., Xu, J., Liu, Y., Yao, Y., et al. (2018). Low-intensity focused ultrasound (LIFU)-activated nanodroplets as a theranostic agent for noninvasive cancer molecular imaging and drug delivery. Biomater. Sci. 6, 2838–2849. doi: 10.1039/c8bm00726h
Lyon, P. C., Griffiths, L. F., Lee, J., Chung, D., Carlisle, R., Wu, F., et al. (2017). Clinical trial protocol for TARDOX: a phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox(R)) using focused ultrasound in patients with liver tumours. J. Ther. Ultrasound 5, 28. doi: 10.1186/s40349-017-0104-0
Maeda, H. (2001). The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 41, 189–207. doi: 10.1016/s0065-2571(00)00013-3
Mannaris, C., Efthymiou, E., Meyre, M. E., Averkiou, M. A. (2013). In vitro localized release of thermosensitive liposomes with ultrasound-induced hyperthermia. Ultrasound Med. Biol. 39, 2011–2020. doi: 10.1016/j.ultrasmedbio.2013.06.001
Mannaris, C., Yang, C., Carugo, D., Owen, J., Lee, J. Y., Nwokeoha, S., et al. (2020). Acoustically responsive polydopamine nanodroplets: a novel theranostic agent. Ultrason. Sonochem. 60, 104782. doi: 10.1016/j.ultsonch.2019.104782
Marin, A., Sun, H., Husseini, G. A., Pitt, W. G., Christensen, D. A., Rapoport, N. Y. (2002). Drug delivery in pluronic micelles: effect of high-frequency ultrasound on drug release from micelles and intracellular uptake. J. Control. Release 84, 39–47. doi: 10.1016/s0168-3659(02)00262-6
Matafonova, G., Batoev, V. (2019). Review on low- and high-frequency sonolytic, sonophotolytic and sonophotochemical processes for inactivating pathogenic microorganisms in aqueous media. Water Res. 166, 115085. doi: 10.1016/j.watres.2019.115085
Mehier-Humbert, S., Bettinger, T., Yan, F., Guy, R. H. (2005). Plasma membrane poration induced by ultrasound exposure: implication for drug delivery. J. Control. Release 104, 213–222. doi: 10.1016/j.jconrel.2005.01.007
Meng, M., Gao, J., Wu, C., Zhou, X., Zang, X., Lin, X., et al. (2016). Doxorubicin nanobubble for combining ultrasonography and targeted chemotherapy of rabbit with VX2 liver tumor. Tumour Biol. 37, 8673–8680. doi: 10.1007/s13277-015-4525-5
Meng, D., Guo, L., Shi, D., Sun, X., Shang, M., Zhou, X., et al. (2019). Charge-conversion and ultrasound-responsive O-carboxymethyl chitosan nanodroplets for controlled drug delivery. Nanomed. (Lond.) 14, 2549–2565. doi: 10.2217/nnm-2019-0217
Miller, D. L., Bao, S., Morris, J. E. (1999). Sonoporation of cultured cells in the rotating tube exposure system. Ultrasound Med. Biol. 25, 143–149. doi: 10.1016/s0301-5629(98)00137-9
Min, K. H., Min, H. S., Lee, H. J., Park, D. J., Yhee, J. Y., Kim, K., et al. (2015). pH-controlled gas-generating mineralized nanoparticles: a theranostic agent for ultrasound imaging and therapy of cancers. ACS Nano 9, 134–145. doi: 10.1021/nn506210a
Mulvana, H., Browning, R. J., Luan, Y., de Jong, N., Tang, M. X., Eckersley, R. J., et al. (2017). Characterization of contrast agent microbubbles for ultrasound imaging and therapy research. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 64, 232–251. doi: 10.1109/TUFFC.2016.2613991
Nakamura, T., Harashima, H. (2017). Integration of nano drug-delivery system with cancer immunotherapy. Ther. Delivery 8, 987–1000. doi: 10.4155/tde-2017-0071
Negishi, Y., Tsunoda, Y., Hamano, N., Omata, D., Endo-Takahashi, Y., Suzuki, R., et al. (2013). Ultrasound-mediated gene delivery systems by AG73-modified Bubble liposomes. Biopolymers 100, 402–407. doi: 10.1002/bip.22246
Negishi, Y., Ishii, Y., Nirasawa, K., Sasaki, E., Endo-Takahashi, Y., Suzuki, R., et al. (2018). PMO delivery system using bubble liposomes and ultrasound exposure for duchenne muscular dystrophy treatment. Methods Mol. Biol. 1687, 185–192. doi: 10.1007/978-1-4939-7374-3_13
Nieminen, H. J., Barreto, G., Finnila, M. A., Garcia-Perez, A., Salmi, A., Ranjan, S., et al. (2017). Laser-ultrasonic delivery of agents into articular cartilage. Sci. Rep. 7 (1), 3991. doi: 10.1038/s41598-017-04293-5
Oishi, Y., Kakimoto, T., Yuan, W., Kuno, S., Yamashita, H., Chiba, T. (2016). Fetal gene therapy for ornithine transcarbamylase deficiency by intrahepatic plasmid DNA-micro-bubble injection combined with hepatic ultrasound insonation. Ultrasound Med. Biol. 42, 1357–1361. doi: 10.1016/j.ultrasmedbio.2015.10.007
Peruzzi, G., Sinibaldi, G., Silvani, G., Ruocco, G., Casciola, C. M. (2018). Perspectives on cavitation enhanced endothelial layer permeability. Colloids Surf. B Biointerfaces 168, 83–93. doi: 10.1016/j.colsurfb.2018.02.027
Pullan, J. E., Bullan, A. T., Taylor, V. B., Brooks, B. B., Ewert, D., Brooks, A. E. (2017). Energy-triggering drug release from polymer nanoparticles for orthopedic applications. Ther. Deliv. 8, 5–14. doi: 10.4155/tde-2016-0066
Qin, J., Wang, T. Y., Willmann, J. K. (2016). Sonoporation: applications for cancer therapy. Adv. Exp. Med. Biol. 880, 263–291. doi: 10.1007/978-3-319-22536-4_15
Rapoport, N., Nam, K. H., Gupta, R., Gao, Z., Mohan, P., Payne, A., et al. (2011). Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J. Control. Release 153, 4–15. doi: 10.1016/j.jconrel.2011.01.022
Rapoport, N. (2012). Ultrasound-mediated micellar drug delivery. Int. J. Hyperthermia 28, 374–385. doi: 10.3109/02656736.2012.665567
Schroeder, A., Kost, J., Barenholz, Y. (2009). Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem. Phys. Lipids 162, 1–16. doi: 10.1016/j.chemphyslip.2009.08.003
Shang, M., Wang, K., Guo, L., Duan, S., Lu, Z., Li, J. (2019). Development of novel ST68/PLA-PEG stabilized ultrasound nanobubbles for potential tumor imaging and theranostic. Ultrasonics 99, 105947. doi: 10.1016/j.ultras.2019.105947
Shen, J., Zhuo, N., Xu, S., Song, Z., Hu, Z., Hao, J., et al. (2018a). Resveratrol delivery by ultrasound-mediated nanobubbles targeting nucleus pulposus cells. Nanomed. (Lond) 13, 1433–1446. doi: 10.2217/nnm-2018-0019
Shen, S., Li, Y., Xiao, Y., Zhao, Z., Zhang, C., Wang, J., et al. (2018b). Folate-conjugated nanobubbles selectively target and kill cancer cells via ultrasound-triggered intracellular explosion. Biomaterials 181, 293–306. doi: 10.1016/j.biomaterials.2018.07.030
Shende, P., Jain, S. (2019). Polymeric nanodroplets: an emerging trend in gaseous delivery system. J. Drug Targeting 27, 1035–1045. doi: 10.1080/1061186X.2019.1588281
Shpak, O., Verweij, M., de Jong, N., Versluis, M. (2016). Droplets, bubbles and ultrasound interactions. Adv. Exp. Med. Biol. 880, 157–174. doi: 10.1007/978-3-319-22536-4_9
Sirsi, S. R., Borden, M. A. (2012). Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics 2, 1208–1222. doi: 10.7150/thno.4306
Sirsi, S. R., Borden, M. A. (2014). State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 72, 3–14. doi: 10.1016/j.addr.2013.12.010
Smith, D. A., Vaidya, S. S., Kopechek, J. A., Huang, S. L., Klegerman, M. E., McPherson, D. D., et al. (2010). Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med. Biol. 36, 145–157. doi: 10.1016/j.ultrasmedbio.2009.08.009
Song, W., Luo, Y., Zhao, Y., Liu, X., Zhao, J., Luo, J., et al. (2017). Magnetic nanobubbles with potential for targeted drug delivery and trimodal imaging in breast cancer: an in vitro study. Nanomed. (Lond.) 12, 991–1009. doi: 10.2217/nnm-2017-0027
Song, K. H., Harvey, B. K., Borden, M. A. (2018a). State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 8, 4393–4408. doi: 10.7150/thno.26869
Song, Z., Ye, Y., Zhang, Z., Shen, J., Hu, Z., Wang, Z., et al. (2018b). Noninvasive, targeted gene therapy for acute spinal cord injury using LIFU-mediated BDNF-loaded cationic nanobubble destruction. Biochem. Biophys. Res. Commun. 496, 911–920. doi: 10.1016/j.bbrc.2018.01.123
Soto, F., Jeerapan, I., Silva-Lopez, C., Lopez-Ramirez, M. A., Chai, I., Xiaolong, L., et al. (2018). Noninvasive transdermal delivery system of lidocaine using an acoustic droplet-vaporization based wearable patch. Small 14, e1803266. doi: 10.1002/smll.201803266
Ta, T., Bartolak-Suki, E., Park, E. J., Karrobi, K., McDannold, N. J., Porter, T. M. (2014). Localized delivery of doxorubicin in vivo from polymer-modified thermosensitive liposomes with MR-guided focused ultrasound-mediated heating. J. Control. Release 194, 71–81. doi: 10.1016/j.jconrel.2014.08.013
Tachibana, K., Uchida, T., Hisano, S., Morioka, E. (1997). Eliminating adult T-cell leukaemia cells with ultrasound. Lancet 349, 325. doi: 10.1016/S0140-6736(97)24005-5
Tanbour, R., Martins, A. M., Pitt, W. G., Husseini, G. A. (2016). Drug delivery systems based on polymeric micelles and ultrasound: a review. Curr. Pharm. Des. 22, 2796–2807. doi: 10.2174/1381612822666160217125215
Tang, W., Yang, Z., Wang, S., Wang, Z., Song, J., Yu, G., et al. (2018). Organic semiconducting photoacoustic nanodroplets for laser-activatable ultrasound imaging and combinational cancer therapy. ACS Nano 12, 2610–2622. doi: 10.1021/acsnano.7b08628
Tardoski, S., Gineyts, E., Ngo, J., Kocot, A., Clezardin, P., Melodelima, D. (2015). Low-intensity ultrasound promotes clathrin-dependent endocytosis for drug penetration into tumor cells. Ultrasound Med. Biol. 41, 2740–2754. doi: 10.1016/j.ultrasmedbio.2015.06.006
Tayier, B., Deng, Z., Wang, Y., Wang, W., Mu, Y., Yan, F. (2019). Biosynthetic nanobubbles for targeted gene delivery by focused ultrasound. Nanoscale 11, 14757–14768. doi: 10.1039/c9nr03402a
Thansandote, P., Harris, R. M., Dexter, H. L., Simpson, G. L., Pal, S., Upton, R. J., et al. (2015). Improving the passive permeability of macrocyclic peptides: Balancing permeability with other physicochemical properties. Bioorg. Med. Chem. 23, 322–327. doi: 10.1016/j.bmc.2014.11.034
Tian, Y., Liu, Z., Zhang, L., Zhang, J., Han, X., Wang, Q., et al. (2018). Apatinib-loaded lipid nanobubbles combined with ultrasound-targeted nanobubble destruction for synergistic treatment of HepG2 cells in vitro. Oncol. Targets Ther. 11, 4785–4795. doi: 10.2147/OTT.S170786
Timbie, K. F., Mead, B. P., Price, R. J. (2015). Drug and gene delivery across the blood-brain barrier with focused ultrasound. J. Control. Release 219, 61–75. doi: 10.1016/j.jconrel.2015.08.059
Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discovery 4, 145–160. doi: 10.1038/nrd1632
Ulrich, A. S. (2002). Biophysical aspects of using liposomes as delivery vehicles. Biosci. Rep. 22, 129–150. doi: 10.1023/a:1020178304031
Wan, C., Li, F., Li, H. (2015a). Gene therapy for ocular diseases meditated by ultrasound and microbubbles (Review). Mol. Med. Rep. 12, 4803–4814. doi: 10.3892/mmr.2015.4054
Wan, C., Qian, J., Li, F., Li, H. (2015b). Ultrasound-targeted microbubble destruction enhances polyethylenimine-mediated gene transfection in vitro in human retinal pigment epithelial cells and in vivo in rat retina. Mol. Med. Rep. 12, 2835–2841. doi: 10.3892/mmr.2015.3703
Wang, P., Yin, T., Li, J., Zheng, B., Wang, X., Wang, Y., et al. (2016). Ultrasound-responsive microbubbles for sonography-guided siRNA delivery. Nanomedicine-UK 12, 1139–1149. doi: 10.1016/j.nano.2015.12.361
Wang, Y., Sui, G., Teng, D., Wang, Q., Qu, J., Zhu, L., et al. (2018). Low intensity focused ultrasound (LIFU) triggered drug release from cetuximab-conjugated phase-changeable nanoparticles for precision theranostics against anaplastic thyroid carcinoma. Biomater. Sci. 7, 196–210. doi: 10.1039/c8bm00970h
Ward, M., Wu, J., Chiu, J. F. (1999). Ultrasound-induced cell lysis and sonoporation enhanced by contrast agents. J. Acoust. Soc. Am. 105, 2951–2957. doi: 10.1121/1.426908
Watanabe, Y., Horie, S., Funaki, Y., Kikuchi, Y., Yamazaki, H., Ishii, K., et al. (2010). Delivery of Na/I symporter gene into skeletal muscle using nanobubbles and ultrasound: visualization of gene expression by PET. J. Nucl. Med. 51 (6), 951–958. doi: 10.2967/jnumed.109.074443
Witte, R. S., Karunakaran, C., Zuniga, A. N., Schmitz, H., Arif, H. (2018). Frontiers of cancer imaging and guided therapy using ultrasound, light, and microwaves. Clin. Exp. Metastasis 35, 413–418. doi: 10.1007/s10585-018-9923-9
Wu, M., Wang, Y., Wang, Y., Zhang, M., Luo, Y., Tang, J., et al. (2017a). Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. Int. J. Nanomed. 12, 5313–5330. doi: 10.2147/IJN.S136032
Wu, P., Jia, Y., Qu, F., Sun, Y., Wang, P., Zhang, K., et al. (2017b). Ultrasound-responsive polymeric micelles for sonoporation-assisted site-specific therapeutic action. ACS Appl. Mater. Interfaces 9, 25706–25716. doi: 10.1021/acsami.7b05469
Wu, M., Zhao, H., Guo, L., Wang, Y., Song, J., Zhao, X., et al. (2018a). Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of A10-3.2 aptamer targeted and siRNA-loaded cationic nanobubbles for therapy of prostate cancer. Drug Deliv. 25, 226–240. doi: 10.1080/10717544.2017.1422300
Wu, S. Y., Fix, S. M., Arena, C. B., Chen, C. C., Zheng, W., Olumolade, O. O., et al. (2018b). Focused ultrasound-facilitated brain drug delivery using optimized nanodroplets: vaporization efficiency dictates large molecular delivery. Phys. Med. Biol. 63, 035002. doi: 10.1088/1361-6560/aaa30d
Xia, H., Zhao, Y., Tong, R. (2016). Ultrasound-mediated polymeric micelle drug delivery. Adv. Exp. Med. Biol. 880, 365–384. doi: 10.1007/978-3-319-22536-4_20
Xing, L., Shi, Q., Zheng, K., Shen, M., Ma, J., Li, F., et al. (2016). Ultrasound-mediated microbubble destruction (UMMD) facilitates the delivery of CA19-9 targeted and paclitaxel loaded mPEG-PLGA-PLL nanoparticles in pancreatic cancer. Theranostics 6, 1573–1587. doi: 10.7150/thno.15164
Yan, C., Zhu, D., Huang, D., Xia, G. (2015). Role of ultrasound and microbubble-mediated heat shock protein 72 siRNA on ischemia-reperfusion liver injury in rat. Int. J. Clin. Exp. Med. 8, 5746–5752.
Yang, Z., Luo, X., Zhang, X., Liu, J., Jiang, Q. (2013). Targeted delivery of 10-hydroxycamptothecin to human breast cancers by cyclic RGD-modified lipid-polymer hybrid nanoparticles. Biomed. Mater. 8, 025012. doi: 10.1088/1748-6041/8/2/025012
Yatvin, M. B., Weinstein, J. N., Dennis, W. H., Blumenthal, R. (1978). Design of liposomes for enhanced local release of drugs by hyperthermia. Science 202, 1290–1293. doi: 10.1126/science.364652
Yu, M., Xu, X., Cai, Y., Zou, L., Shuai, X. (2018). Perfluorohexane-cored nanodroplets for stimulations-responsive ultrasonography and O2-potentiated photodynamic therapy. Biomaterials 175, 61–71. doi: 10.1016/j.biomaterials.2018.05.019
Yu, Y., Wang, B., Guo, C., Zhao, F., Chen, D. (2019). Protoporphyrin IX-loaded laminarin nanoparticles for anticancer treatment, their cellular behavior, ROS detection, and animal studies. Nanoscale Res. Lett. 14, 316. doi: 10.1186/s11671-019-3138-0
Yue, P., Gao, L., Wang, X., Ding, X., Teng, J. (2018). Ultrasound-triggered effects of the microbubbles coupled to GDNF- and Nurr1-loaded PEGylated liposomes in a rat model of Parkinson's disease. J. Cell. Biochem. 119, 4581–4591. doi: 10.1002/jcb.26608
Zhang, X., Pan, W., Gan, L., Zhu, C., Gan, Y., Nie, S. (2008). Preparation of a dispersible PEGylate nanostructured lipid carriers (NLC) loaded with 10-hydroxycamptothecin by spray-drying. Chem. Pharm. Bull. (Tokyo) 56, 1645–1650. doi: 10.1248/cpb.56.1645
Zhang, B., Chen, M., Zhang, Y., Chen, W., Zhang, L., Chen, L. (2018a). An ultrasonic nanobubble-mediated PNP/fludarabine suicide gene system: a new approach for the treatment of hepatocellular carcinoma. PloS One 13, e0196686. doi: 10.1371/journal.pone.0196686
Zhang, J., Wang, S., Deng, Z., Li, L., Tan, G., Liu, X., et al. (2018b). Ultrasound-triggered drug delivery for breast tumor therapy through iRGD-targeted paclitaxel-loaded liposome-microbubble complexes. J. Biomed. Nanotechnol. 14, 1384–1395. doi: 10.1166/jbn.2018.2594
Zhang, L., Yi, H., Song, J., Huang, J., Yang, K., Tan, B., et al. (2019). Mitochondria-targeted and ultrasound-activated nanodroplets for enhanced deep-penetration sonodynamic cancer therapy. ACS Appl. Mater. Interfaces 11, 9355–9366. doi: 10.1021/acsami.8b21968
Zhu, L., Zhao, H., Zhou, Z., Xia, Y., Wang, Z., Ran, H., et al. (2018). Peptide-functionalized phase-transformation nanoparticles for low intensity focused ultrasound-assisted tumor imaging and therapy. Nano Lett. 18, 1831–1841. doi: 10.1021/acs.nanolett.7b05087
Keywords: ultrasound-responsive materials, drug, gene, delivery, microbubbles
Citation: Cai X, Jiang Y, Lin M, Zhang J, Guo H, Yang F, Leung W and Xu C (2020) Ultrasound-Responsive Materials for Drug/Gene Delivery. Front. Pharmacol. 10:1650. doi: 10.3389/fphar.2019.01650
Received: 31 July 2019; Accepted: 16 December 2019;
Published: 31 January 2020.
Edited by:
Marc Derieppe, Princess Maxima Center for Pediatric Oncology, NetherlandsReviewed by:
Christoph Eugen Hagemeyer, Monash University, AustraliaAbdallah El-Sayed Allam, Tanta University, Egypt
Aditi Jhaveri, AnTolRx Inc., United States
Copyright © 2020 Cai, Jiang, Lin, Zhang, Guo, Yang, Leung and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanshan Xu, eGNzaGFuQDE2My5jb20=; Wingnang Leung, YXdubGV1bmdAZ21haWwuY29t
 Xiaowen Cai
Xiaowen Cai Yuan Jiang1
Yuan Jiang1 Chuanshan Xu
Chuanshan Xu