
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pharmacol. , 13 December 2019
Sec. Inflammation Pharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.01497
 Salvatore D'Angelo1,2*
Salvatore D'Angelo1,2* Fabrizio Cantini3
Fabrizio Cantini3 Roberta Ramonda4
Roberta Ramonda4 Luca Cantarini5
Luca Cantarini5 Antonio Carletto6
Antonio Carletto6 Maria Sole Chimenti7
Maria Sole Chimenti7 Andrea Delle Sedie8
Andrea Delle Sedie8 Rosario Foti9
Rosario Foti9 Roberto Gerli10
Roberto Gerli10 Claudia Lomater11
Claudia Lomater11 Ennio Lubrano12
Ennio Lubrano12 Antonio Marchesoni13
Antonio Marchesoni13 Alen Zabotti14
Alen Zabotti14 Carlo Salvarani15,16
Carlo Salvarani15,16 Rossana Scrivo17
Rossana Scrivo17 Raffaele Scarpa18
Raffaele Scarpa18 Giuseppina Tramontano1
Giuseppina Tramontano1 Carlotta Nannini3
Carlotta Nannini3 Mariagrazia Lorenzin4
Mariagrazia Lorenzin4 Marta Fabbroni5
Marta Fabbroni5 Federica Martinis6
Federica Martinis6 Roberto Perricone7
Roberto Perricone7 Linda Carli8
Linda Carli8 Elisa Visalli9
Elisa Visalli9 Guido Rovera11
Guido Rovera11 Fabio Massimo Perrotta12
Fabio Massimo Perrotta12 Luca Quartuccio14
Luca Quartuccio14 Alessio Altobelli17
Alessio Altobelli17 Luisa Costa18
Luisa Costa18 Laura Niccoli3
Laura Niccoli3 Augusta Ortolan4
Augusta Ortolan4 Francesco Caso18
Francesco Caso18Background: Few studies have evaluated the effectiveness of adalimumab in the real-life setting in psoriatic arthritis (PsA).
Objective: To evaluate the 2-year retention rate of adalimumab in PsA patients. Potential baseline parameters influencing persistence on treatment were also evaluated.
Methods: PsA patients from 16 Italian Rheumatology Units treated with adalimumab as first- or second-line biological therapy were retrospectively evaluated. Adalimumab retention rate was evaluated at 12 and 24 months. Logistic regression was used to evaluate the association between predictor variables and adalimumab retention rate.
Results: From 424 patients (53.5% male, aged 48.3 ± 12.8 years) who started treatment with adalimumab, 367 (86.6%) maintained treatment for 12 months and 313 (73.8%) for 2 years. At 24-months, Disease Activity in PsA (DAPSA) remission (defined as ≤4) and Low Disease Activity (LDA) (≤14) were achieved in 22.8% and 44.4% of patients, respectively. Adalimumab treatment significantly decreased the number of tender (7.0 ± 5.7 at baseline vs. 2.3 ± 3.5 at 24 months, p < 0.001) and swollen joints (2.7 ± 2.8 at baseline vs. 0.4 ± 0.9 at 24 months, p < 0.001), DAPSA (25.5 ± 10.9 at baseline vs. 11.0 ± 8.4 at 24 months, p < 0.001), PASI (5.3 ± 5.7 at baseline vs. 2.7 ± 2.8 at 24 months, p < 0.001) and CRP (3.8 ± 6.3 at baseline vs. 1.2 ± 1.7 at 24 months, p < 0.001). Among a range of laboratory and clinical variables, only female gender was associated with improved adalimumab persistence at 24 months (OR: 1.98, 95% CI: 1.2–3.2, p = 0.005).
Conclusions: Independent of a range of predictor variables, adalimumab was shown to be effective, while maintaining a high retention rate after 2 years in PsA patients.
Psoriatic arthritis (PsA) is a chronic and invalidating disease characterized by joint and entheseal inflammation affecting 0.05–0.25% of the general population and 6–41% of patients with psoriasis (Gottlieb and Dann, 2009; Laws et al., 2010; Olivieri et al., 2014; Ogdie and Weiss, 2015).
Up until two decades ago, treatment of PsA was often unsatisfactory. Findings based on the immunopathogenesis of the disease have led to the development of biological drugs directed against specific (pathogenetic) targets, in particular tumor necrosis factor-α (TNFα). TNFα is a pleiotropic cytokine which regulates several inflammatory reactions and immune functions through the control of cellular processes and plays a central role in the pathogenesis of PsA (Mantravadi et al., 2017). Anti-TNFα drugs have opened new therapeutic horizons in PsA, proving to be effective in the control of the signs/symptoms of inflammation, in improving the quality of life and the functional outcome, in inhibiting the progression of the structural damage in the peripheral joints and presenting a good safety profile (D'Angelo et al., 2012; Perrotta and Lubrano, 2016; D'Angelo et al., 2017). Treatment strategies of active, predominantly peripheral PsA recommended by International and National Guidelines suggest to use conventional disease-modifying drugs anti-rheumatic (DMARDs), such as methotrexate (MTX). In cases of inadequate response, contraindication or intolerance to at least one DMARD, treatment with biological drugs such as TNFα (adalimumab, infliximab, etanercept, golimumab, or certolizumab pegol) or anti-interleukin therapies (ustekinumab or secukinumab) should be considered (Gossec et al., 2016; Marchesoni et al., 2017).
Adalimumab has been shown to be effective and reasonably safe in reducing disease activity and controlling joint damage in patients with PsA, even in comorbid conditions (D'Angelo et al., 2012). However, despite its generally high efficacy, some patients with PsA may be refractory to adalimumab therapy, may lose response or develop drug intolerance over time (Perrotta and Lubrano, 2016; D'Angelo et al., 2017). The persistence in therapy in real-life clinical practice is increasingly recognised as a surrogate marker for the efficacy and safety of a drug (Saad et al., 2011). National registries provide clinical data from the real-world setting, with the aim to monitor long-term safety of a specific treatment, but they also yield other important information (difficult to achieve in clinical trials), such as drug survival and long-term effectiveness (Armuzzi et al., 2014).
The present real-life study evaluated the persistence of adalimumab in the management of PsA patients over a period of 2 years. Potential baseline clinical and laboratory parameters influencing persistence rate were also evaluated.
The present retrospective non-interventional longitudinal study included consecutive PsA patients who started a treatment with adalimumab as of 1st January 2013 in 16 Italian Rheumatology Centres. Inclusion criteria were the following: age ≥18 years; diagnosed with active PsA and having started a treatment with adalimumab in routine clinical practice, regardless of whether they were biologic naïve or whether they had previously received biologic treatment. Active PsA was defined by a rheumatologist based on clinical judgment considered peripheral arthritis, enthesitis or axial involvement. Diagnosis of PsA was clinical (D'Angelo et al., 2016) and in addition, all patients satisfied CASPAR (ClASsification criteria for Psoriatic ARthritis) criteria for the classification of PsA (Taylor et al., 2006). Patients' written consent were obtained according to the Declaration of Helsinki when patients were first entered into the database for treatment. Ethics committee approval from all participating centres and written informed consent for the anonymous use of personal data were obtained from every patient, in compliance with the Italian Legislative Decree 196/2003.
All participating Centres have a recognised expertise in the management of PsA and regularly collect data using a standardized database on the efficacy and safety of patients with PsA treated with biological drugs. For the purpose of this study, the data extracted from the database were the following: demographic features (age, sex, and time since PsA diagnosis), clinical parameters (tender and swollen joints, dactylitis, enthesitis assessed by physical examination according to the expanded Leeds index, psoriasis according to Psoriasis Area Severity Index [PASI], Disease Activity index for Psoriatic Arthritis [DAPSA]) and treatment (previous biologics, previous conventional DMARD, combined treatment, dose of adalimumab) at the time of initiation and during the follow-up of adalimumab treatment. The analysis was performed on data at three time-points: baseline, 12 and 24 months.
Drug retention was retrospectively evaluated as the number of patients (%) on treatment until definitive treatment interruption over the study period. Reasons for discontinuation were analysed and classified into the following categories: 1) lack of effectiveness (including primary and secondary); 2) adverse events (infection, skin or systemic reaction, and other adverse events, including hematologic, pulmonary, renal, cardiovascular complications, and malignancies, etc.); and 3) other reasons (patient preference, change in hospital, desire for pregnancy, disease remission, etc.). The effectiveness of adalimumab treatment was also evaluated at 24 months and was defined as the proportion of patients achieving remission, defined as a DAPSA score ≤4 and low disease activity (LDA) as >4 and ≤14 (Gossec et al., 2016 and Schoels et al., 2016) .The effect of adalimumab treatment on a range of clinical and laboratory features and disease activity variables (tender and swollen joint count, CRP, dactylitis, enthesitis, PASI and DAPSA) and extra-articular manifestations (Crohn's disease, uveitis) was also evaluated at 12 and 24 months.
No formal power calculation was performed since this was a retrospective longitudinal study that included consecutive PsA patients seen in a real-life setting. Data are presented as mean ± SD or number and %. Comparisons in variables between two groups (i.e. patients discontinuing vs. those continuing adalimumab treatment at 24 months) were performed by univariate analysis using the Chi-squared test for categorical variables or the Mann-Whitney U- Test for non-parametric continuous variables. Three groups were compared (i.e. baseline, 12 and 24 months) by 1-way ANOVA followed by Bonferroni post-hoc test to account for α-inflation by type-1 error derived from multiple testing. Variables that were found to be statistically significant predictors following univariate analysis were included in multivariate regression models. A p-value of ≤0.05 was considered statistically significant. Statistical analyses were performed using SPSS statistical software, version 20.0 (SPSS, Chicago, IL, USA).
A total of 424 PsA patients were included in the present study. Baseline clinical characteristics are summarised in Table 1. The majority of patients were male (N = 227, 53.5%) and mean age was 48.3 ± 12.8 years. Three hundred and fifteen (74.3%) patients had peripheral arthritis, 148 (47.3%) enthesitis, 87 (27.8%) dactylitis, 81 (19.1%) axial involvement and 306 (72.5%) had concomitant psoriasis. Extra-articular complications such as uveitis (N = 27, 6.4%) and Crohn's disease (N = 23, 5.4%) were less frequent. Frequent comorbid diseases at baseline included hypertension (N = 130, 30.8%) and metabolic syndrome (N = 75, 17.9%). The majority of patients presented with moderately active disease, as observed by DAPSA score (25.5 ± 10.9). Prior to undertaking treatment with adalimumab, 291 (68.6%) patients were biologic naïve while almost all patients received conventional DMARDs (N = 404, 95.3%). As regards the 133 patients with a previous biologic treatment, 33 had discontinued due to primary inefficacy, 64 due to secondary inefficacy, 27 due to adverse events and 9 due to other reasons.
Adalimumab was administered as monotherapy in 190 patients (44.8%) and combined with MTX in 183 (43.2%) patients, the majority (N = 164, 89.6%) receiving MTX 10-20 mg (mean dose of 13.1 ± 3.0 mg) per week. Adalimumab was administered in combination with DMARDs other than MTX including sulfasalazine, leflunomide, cyclosporine and hydroxychloroquine in 51 (12%) patients.
The effect of adalimumab treatment was evaluated on some clinical and laboratory measures (Figure 1). Both tender joint count (TJC) and swollen joint count (SJC) were significantly decreased compared to baseline values (TJC: 7.0 ± 5.7 at baseline vs. 2.3 ± 3.5 at 24 months, p < 0.001; SJC: 2.7 ± 2.8 at baseline vs. 0.4 ± 0.9 at 24 months, p < 0.001) (Figures 1A, B). Similarly, PASI (5.3 ± 5.7 at baseline vs. 2.7 ± 2.8 at 24 months, p < 0.001) and CRP (3.8 ± 6.3 at baseline vs. 1.2 ± 1.7 at 24 months, p < 0.001) were significantly decreased in patients treated with adalimumab over the 2-year period (Figures 1C, D). Mean DAPSA score was significantly decreased compared to baseline values after 12 and 24 months (25.5 ± 10.9 at baseline vs. 11.0 ± 8.4 at 24 months, p < 0.001) of adalimumab treatment (Figure 2). Clinical remission and LDA at 24 months were achieved in 22.8% and 44.4% of patients, respectively.
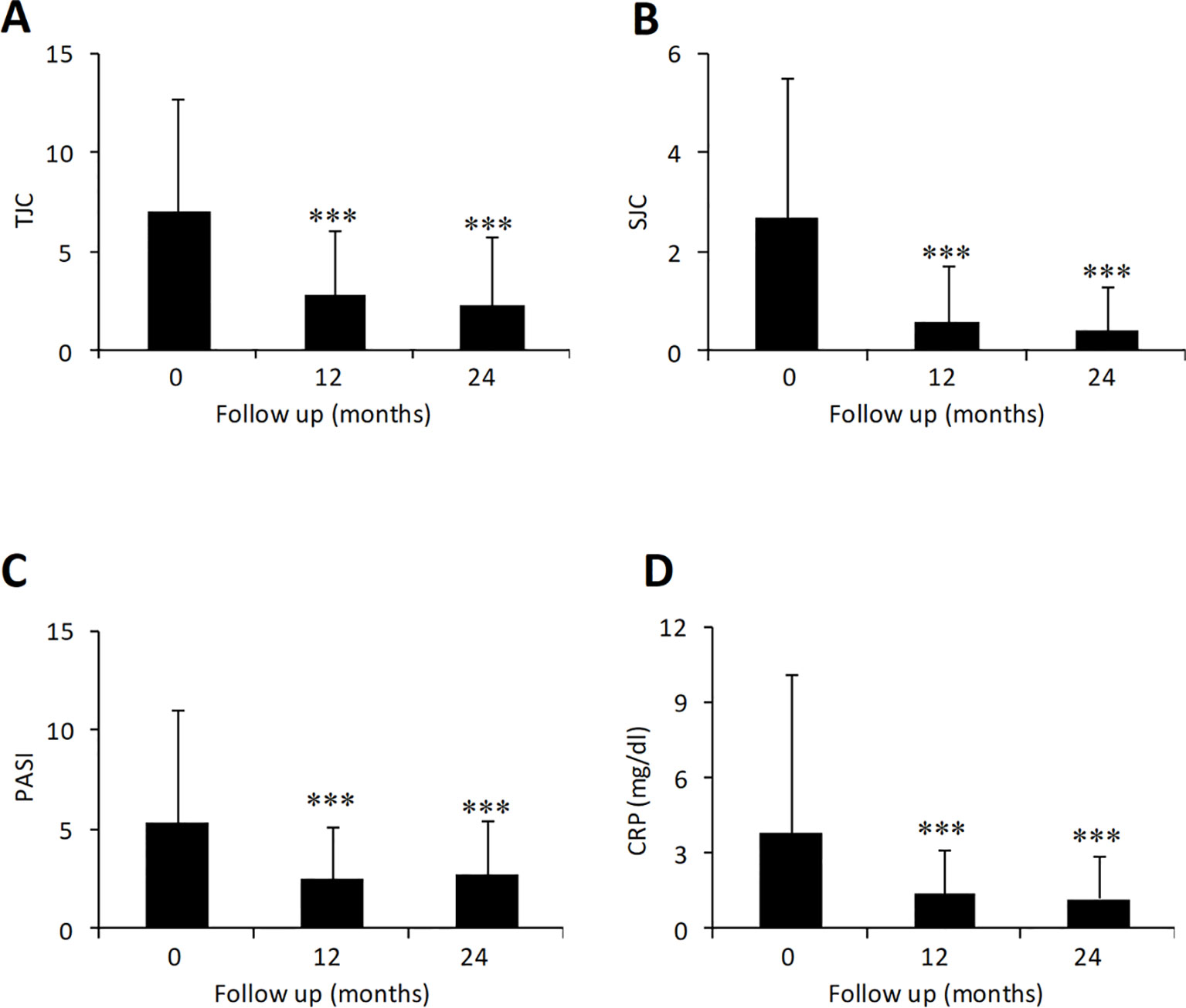
Figure 1 Effect of adalimumab treatment in PsA patients on tender [(A) N = 303, 271, 275 at 0, 12, and 24 months] and swollen joints [(B) N = 303, 271, 275 at 0, 12, and 24 months], CRP [(C) N = 298, 269, 272 at 0, 12, and 24 months] and PASI score [(D) N = 99, 73, 39 at 0, 12, and 24 months]. CRP, C-reactive protein; PASI, psoriasis area severity index; SJC, swollen joint count; TJC, tender joint count. Data presented as mean ± SD. Asterisks denote statistically significant differences compared to baseline values after 1-way ANOVA followed by Bonferroni post-hoc test.
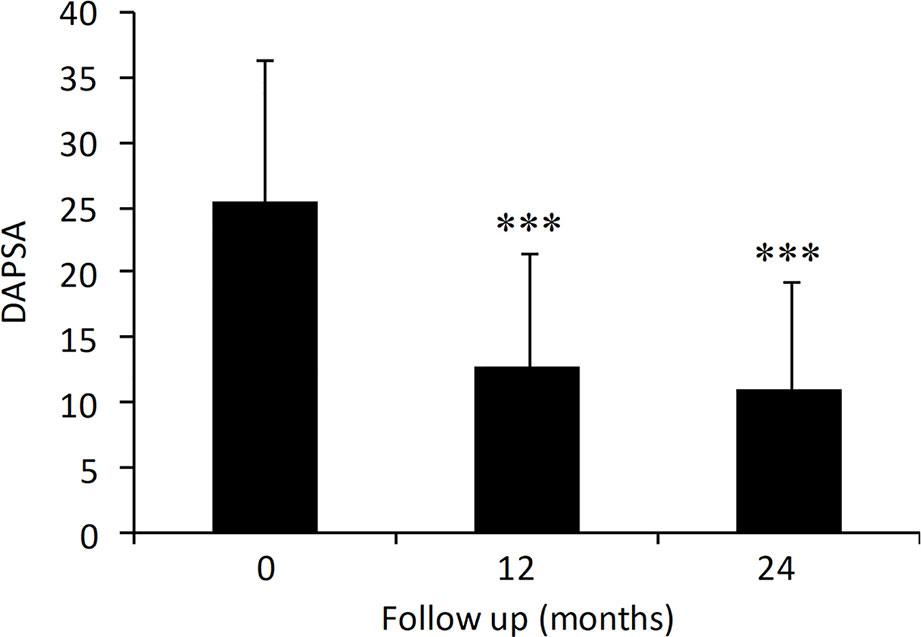
Figure 2 Effect of adalimumab treatment in PsA patients on DAPSA (N = 209, 183, 189 at 0, 12 and 24 months). DAPSA, disease activity in psoriatic arthritis. Data are presented as mean ± SD. Asterisks denote statistically significant differences compared to baseline values after 1-way ANOVA followed by Bonferroni post-hoc test.
In addition, patients with enthesitis (47.3% at baseline vs. 15.1% at 24 months, p < 0.001) and dactylitis (27.8% at baseline vs. 3.2% at 24 months, p < 0.001) were also significantly decreased over the follow-up period.
Of the 424 patients who started treatment with adalimumab, 367 (86.6%) maintained treatment for 12 months and 313 (73.8%) for 24 months.
Univariate and multivariate analyses were used to examine predictors of 24-month adalimumab persistence in PsA patients. Considering all potential variables compared using univariate analyses (Table 2), only high baseline CRP levels (3.2 ± 5.3 mg/dl discontinuing vs. 3.8 ± 6.3 mg/dl in adalimumab continuing patients, p = 0.047) and female gender (34.2% in discontinuing vs. 50.8% in adalimumab continuing patients, p = 0.004) emerged as being significantly associated with improved adalimumab persistence at 24 months. Stratification of patients based on concomitant treatment (e.g. adalimumab monotherapy vs. adalimumab plus MTX or adalimumab plus other DMARD) did not reveal any significant difference in adalimumab retention (p = 0.46) (Table 2). Furthermore, the presence of comorbid diseases or exposure to previous biologic were not associated with adalimumab retention. In a multivariate regression model (including only gender and high baseline CRP levels), only female sex emerged as a significant predictor of improved adalimumab retention at 24 months (OR 1.98, 95% CI 1.2–3.2, p = 0.005).
Over the 24-month treatment period, adalimumab was suspended in a total of 111/424 (26.2%) patients. Reasons for discontinuation were primary inefficacy (N = 30, 7.1%), secondary inefficacy (N = 15, 3.5%), adverse events (N = 26, 6.1%; subjective intolerance, allergic reaction, biliary colic, diplopia, and paresthesia in limbs or other side effects) and other reasons (N = 40, 9.4%; lost during follow up, pregnancy, paternity leave, not reported, or not recorded).
Efficacy and safety data currently available for anti-TNFα drugs for the treatment of PsA are mainly derived from randomised clinical trials (RCTs). Although RCTs still represent the most powerful research tool to confirm the efficacy of a treatment, results emerging from these studies are based on a selected population, with the exclusion of co-morbidities, treated and observed for a limited period of time. In routine clinical practice (real life), the decision to choose a specific biologic needs to take into consideration that patients may often be affected by multiple comorbidities, receive concomitant medication, and necessitate treatment for a greater duration, characteristics that are profoundly different from a RCT. It is increasingly recognised that the persistence in treatment is a good surrogate of both effectiveness (efficacy in the real-life setting) and tolerability of a drug (Saad et al., 2011).
The short- and long-term benefit of adalimumab for the treatment of PsA is already documented from several clinical trials and meta-analyses (Gladman et al., 2007; Mease et al., 2009; Burmester et al., 2013). However, little evidence is available on the persistence and effectiveness of adalimumab administered as first- or second-line biologic treatment in the real-life setting.
The results from this real-life study indicate that adalimumab can be considered as a therapeutic option for the long-term treatment in PsA patients, regardless of their prior exposure to biologics or DMARDs or the presence of comorbid diseases. The majority of patients (86.6%) retained treatment with adalimumab for up to 1 year with only a slight reduction observed at 2 years (73.8% persistence), corroborating with findings from European registry studies (70–88%) for 1 year persistence rates (Heiberg et al., 2008; Saad et al., 2009; Glintborg et al., 2011; Aaltonen et al., 2017; Stober et al., 2018). However, our results showed higher rates than another real-life registry performed in Italy, with 2-year retention rate of 48% in PsA patients treated with golimumab (Manara et al., 2017). Furthermore, in that study, no difference was observed in retention rate between first- and second-line treatment in patients with rheumatoid arthritis or PsA (Manara et al., 2017). Although clinical characteristics and disease severity of patients treated in our study were similar to European registries, it is important to note that 68.6% were naïve to biologics and 55.2% were receiving concomitant DMARDs (43.2% in combination with MTX). These features may favour drug response and persistence, since evidence suggests that response to adalimumab is lower after previous TNF inhibitor (Merola et al., 2017) and concomitant MTX can improve anti-TNF drug survival (Glintborg et al., 2011), although other studies dispute the benefit of combined use of DMARDs and anti-TNF agents on drug survival (Mease et al., 2015; Aaltonen et al., 2017). In addition, we did not observe any advantage in drug persistence in patients treated with adalimumab as monotherapy compared to those receiving MTX and/or other DMARDs. Recently, a retrospective single-centre cohort study based in the UK was performed in patients with PsA who initiated anti-TNF therapy (adalimumab in 42% of cases) (Stober et al., 2018). Retention rates were similar to those observed in our study at 12 (79%) and 24 months (73%). Interestingly, the presence of metabolic syndrome and female sex were identified as predictors of lower drug persistence (Stober et al., 2018), findings that have been reported by other groups (Heiberg et al., 2008; Glintborg et al., 2011), but have not been confirmed by our study. The small number of patients with metabolic syndrome and BMI value ≥30 in our cohort might account for the lack of association between obesity and worse adalimumab performance. Why female sex emerged as a predictor of better adalimumab survival rate does not seem to have a logical explanation, although this has also been observed in psoriasis patients (Verma et al., 2018). However, published data on this topic are based on study populations of PsA patients taking any TNF inhibitors and do not investigate drugs individually. Differences in patient characteristics across studies such as age, baseline disease severity and the presence of underlying fibromyalgia may also play a role in gender related differences in drug persistence. Further studies well help to clarify this result in more detail.
High levels of CRP at baseline also predicted improved adalimumab persistence at 24 months in our hands, a finding that has also been observed previously in ankylosing spondylitis (Glintborg et al., 2010) as well as PsA patients (Glintborg et al., 2011; Aaltonen et al., 2017). High CRP levels at baseline are associated with systemic inflammation and, therefore, may help identify patients with more active disease who are more likely to benefit from adalimumab treatment than patients with less inflammatory active disease.
Given the lack of association observed in our analysis between a range of laboratory and clinical variables with persistence rate, adalimumab may be considered as a viable therapeutic option in a heterogeneous population of patients in the real-world PsA setting, without the need to restrict treatment to specific subgroups or special patient populations.
High persistence rate was paralleled with a marked improvement in arthritis measures such as TJC, SJC, DAPSA, enthesitis, and dactylitis. PASI and CRP were also significantly improved as early as 12 months and remained stable up to 2 years. We also observed a good safety profile with adalimumab in PsA patients. Of 26.5% PsA patients who discontinued treatment with adalimumab after 2 years, only 6.1% were actually due to adverse events.
While the main strength of the present study lies in the large sample size (N = 424) and 2-year follow-up period, subgroup analysis for some specific clinical (e.g. tender and swollen joint count, PASI and DAPSA) and laboratory measures (e.g. CRP) were hampered by missing data for some patients. The retrospective design was another limitation. However, as this study involved 16 rheumatological centres, evaluating the real-life use of adalimumab as first or second-line treatment in biologic naïve or previous biologic failure in the PsA setting, results can be generalised to the larger Italian territory.
In this large real-life cohort, the use of adalimumab was found to be highly effective in PsA patients. High retention was achieved at 1 (86.6%) and 2 years (73.8%) and given the lack of association between several laboratory and clinical variables with persistence rate, adalimumab may be considered as a viable therapeutic option in a heterogeneous population of patients in the real-world PsA setting.
The datasets generated for this study are available upon request from the corresponding author.
Ethics committee approval from all participating centres and written informed consent for the anonymous use of personal data were obtained from every patient, in compliance with the Italian Legislative Decree 196/2003. The patients/participants provided their written informed consent to participate in this study.
SD'A conceived and designed the study. All authors were responsible for data collection/acquisition and have critically reviewed and approved the final version of the manuscript prior to submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supported by the Italian Society of Rheumatology (SIR) “Spondyloartritis and Psoriatic Arthritis study group—A. Spadaro.” The authors are grateful to Dr Colin Gerard Egan (CE Medical Writing, Pisa, Italy) for the preparation of the manuscript.
Aaltonen, K., Heinonen, A., Joensuu, J., Parmanne, P., Karjalainen, A., Varjolahti-Lehtinen, T., et al. (2017). Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin. Arthritis Rheumatol. 46, 732–739. doi: 10.1016/j.semarthrit.2016.09.005
Armuzzi, A., Lionetti, P., Blandizzi, C., Caporali, R., Chimenti, S., Cimino, L., et al. (2014). anti-TNF agents as therapeutic choice in immune-mediated inflammatory diseases: focus on adalimumab. Int. J. Immunopathol. Pharmacol. 27, 11–32. doi: 10.1177/03946320140270S102
Burmester, G. R., Panaccione, R., Gordon, K. B., McIlraith, M. J., Lacerda, A. P. M. (2013). Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann. Rheumatol. Dis. 72, 517–524. doi: 10.1136/annrheumdis-2011-201244
D'Angelo, S., Palazzi, C., Olivieri, I. (2012). Psoriatic arthritis: treatment strategies using biologic agents. Reumatismo 64(2), 113–121. doi: 10.4081/reumatismo.2012.113
D'Angelo, S., Palazzi, C., Gilio, M., Leccese, P., Padula, A., Olivieri, I. (2016). Improvements in diagnostic tools for early detection of psoriatic arthritis. Expert Rev. Clin. Immunol. 12, 1209–1215. doi: 10.1080/1744666X.2016.1193436
D'Angelo, S., Tramontano, G., Gilio, M., Leccese, P., Olivieri, I. (2017). Review of the treatment of psoriatic arthritis with biological agents: choice of drug for initial therapy and switch therapy for non-responders. Open Access Rheumatol. Res. Rev. 9, 21–28. doi: 10.2147/OARRR.S56073
Gladman, D. D., Mease, P. J., Ritchlin, C. T., Choy, E. H. S., Sharp, J. T., Ory, P. A., et al. (2007). Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheumatol. 56, 476–488. doi: 10.1002/art.22379
Glintborg, B., Ostergaard, M., Krogh, N. S., Dreyer, L., Kristensen, H. L., Hetland, M. L. (2010). Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years' surveillance in the Danish nationwide DANBIO registry. Ann. Rheumatol. Dis. 69, 2002–2008. doi: 10.1136/ard.2009.124446
Glintborg, B., Østergaard, M., Dreyer, L., Krogh, N. S., Tarp, U., Hansen, M. S., et al. (2011). Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheumatol. 63, 382–390. doi: 10.1002/art.30117
Gossec, L., Smolen, J. S., Ramiro, S., de Wit, M., Cutolo, M., Dougados, M., et al. (2016). European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann. Rheumatol. Dis. 75, 499–510. doi: 10.1136/annrheumdis-2015-208337
Gottlieb, A. B., Dann, F. (2009). Comorbidities in patients with psoriasis. Am. J. Med. 122, 1150.e1–9. doi: 10.1016/j.amjmed.2009.06.021
Heiberg, M. S., Koldingsnes, W., Mikkelsen, K., Rødevand, E., Kaufmann, C., Mowinckel, P., et al. (2008). The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheumatol. 59, 234–240. doi: 10.1002/art.23333
Laws, P., Barton, A., Warren, R. B. (2010). Psoriatic arthritis–what the dermatologist needs to know. J. Eur. Acad. Dermatol. Venereol. JEADV 24, 1270–1277. doi: 10.1111/j.1468-3083.2010.03654.x
Manara, M., Caporali, R., Favalli, E. G., Grosso, V., Atzeni, F., Sarzi Puttini, P., et al. (2017). Two-year retention rate of golimumab in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: data from the LORHEN registry. Clin. Exp. Rheumatol. 35(5), 804–809.
Mantravadi, S., Ogdie, A., Kraft, W. K. (2017). Tumor necrosis factor inhibitors in psoriatic arthritis. Expert Rev. Clin. Pharmacol. 10, 899–910. doi: 10.1080/17512433.2017.1329009
Marchesoni, A., Olivieri, I., Salvarani, C., Pipitone, N., D'Angelo, S., Mathieu, A., et al. (2017). Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian Society of Rheumatology. Clin. Exp. Rheumatol. 35(6), 991–1010.
Mease, P. J., Ory, P., Sharp, J. T., Ritchlin, C. T., Van den Bosch, F., Wellborne, F., et al. (2009). Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann. Rheumatol. Dis. 68, 702–709. doi: 10.1136/ard.2008.092767
Mease, P. J., Collier, D. H., Saunders, K. C., Li, G., Kremer, J. M., Greenberg, J. D. (2015). Comparative effectiveness of biologic monotherapy versus combination therapy for patients with psoriatic arthritis: results from the Corrona registry. RMD Open 1, e000181. doi: 10.1136/rmdopen-2015-000181
Merola, J. F., Lockshin, B., Mody, E. A. (2017). Switching biologics in the treatment of psoriatic arthritis. Semin. Arthritis Rheumatol. 47, 29–37. doi: 10.1016/j.semarthrit.2017.02.001
Ogdie, A., Weiss, P. (2015). The epidemiology of psoriatic arthritis. Rheumatol. Dis. Clin. North Am. 41, 545–568. doi: 10.1016/j.rdc.2015.07.001
Olivieri, I., D'Angelo, S., Palazzi, C., Padula, A. (2014). Advances in the management of psoriatic arthritis. Nat. Rev. Rheumatol. 10, 531–542. doi: 10.1038/nrrheum.2014.106
Perrotta, F. M., Lubrano, E. (2016). New approved drugs for psoriatic arthritis. Reumatismo 68, 57–64. doi: 10.4081/reumatismo.2016.873
Saad, A. A., Ashcroft, D. M., Watson, K. D., Hyrich, K. L., Noyce, P. R., Symmons, D. P. M., et al. (2009). Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res. Ther. 11, R52. doi: 10.1186/ar2670
Saad, A. A., Hyrich, K. L., Ashcroft, D. M. (2011). Drug persistence, effectiveness and safety assessment of anti-TNF therapies in psoriatic arthritis. Expert Opin. Drug Saf. 10, 219–226. doi: 10.1517/14740338.2010.516250
Schoels, M. M., Aletaha, D., Alasti, F., Smolen, J. S. (2016). Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann. Rheumatol. Dis. 75, 811–818. doi: 10.1136/annrheumdis-2015-207507
Stober, C., Ye, W., Guruparan, T., Htut, E., Clunie, G., Jadon, D. (2018). Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatol. Oxf. Engl. 57, 158–163. doi: 10.1093/rheumatology/kex387
Taylor, W., Gladman, D., Helliwell, P., Marchesoni, A., Mease, P., Mielants, H., et al. (2006). Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheumatol. 54, 2665–2673. doi: 10.1002/art.21972
Keywords: psoriatic arthritis, biological drugs, adalimumab, retention rate, real-life
Citation: D'Angelo S, Cantini F, Ramonda R, Cantarini L, Carletto A, Chimenti MS, Delle Sedie A, Foti R, Gerli R, Lomater C, Lubrano E, Marchesoni A, Zabotti A, Salvarani C, Scrivo R, Scarpa R, Tramontano G, Nannini C, Lorenzin M, Fabbroni M, Martinis F, Perricone R, Carli L, Visalli E, Rovera G, Perrotta FM, Quartuccio L, Altobelli A, Costa L, Niccoli L, Ortolan A and Caso F (2019) Effectiveness of Adalimumab for the Treatment of Psoriatic Arthritis: An Italian Real-Life Retrospective Study. Front. Pharmacol. 10:1497. doi: 10.3389/fphar.2019.01497
Received: 07 August 2019; Accepted: 19 November 2019;
Published: 13 December 2019.
Edited by:
Daniel Merk, Goethe University Frankfurt, GermanyReviewed by:
Simona Gabriela Bungau, University of Oradea, RomaniaCopyright © 2019 D'Angelo, Cantini, Ramonda, Cantarini, Carletto, Chimenti, Delle Sedie, Foti, Gerli, Lomater, Lubrano, Marchesoni, Zabotti, Salvarani, Scrivo, Scarpa, Tramontano, Nannini, Lorenzin, Fabbroni, Martinis, Perricone, Carli, Visalli, Rovera, Perrotta, Quartuccio, Altobelli, Costa, Niccoli, Ortolan and Caso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salvatore D'Angelo, c2FsZGFuZ2Vsb0BrYXRhbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.