
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 02 December 2019
Sec. Ethnopharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.01449
Red yeast rice (RYR), a Chinese traditional folk medicine produced by the fermentation of cooked rice kernels with a Monascaceae mold, Monascus purpureus, has long been used to treat blood circulation stasis, indigestion, diarrhea, and limb weakness in East Asian countries. This article provides a systematic review of the traditional uses, chemistry, biological activities, and toxicology of RYR to highlight its future prospects in the field of medicine. The literature reviewed for this article was obtained from the Web of Science, Elsevier, SciFinder, PubMed, CNKI, ScienceDirect, and Google Scholar, as well as Ph.D. and M.Sc. dissertations, published prior to July 2019. More than 101 chemical constituents have been isolated from RYR, mainly consisting of monacolins, pigments, organic acids, sterols, decalin derivatives, flavonoids, polysaccharides, and other compounds. Crude extracts of RYR, as well as its isolated compounds, possess broad pharmacological properties with hypolipidemic, anti-atherosclerotic, anti-cancer, neurocytoprotective, anti-osteoporotic, anti-fatigue, anti-diabetic, and anti-hypertensive activities. However, further studies are needed to characterize its diverse chemical constituents and the toxicological actions of the main bioactive compounds. New pharmacological trials addressing the overlooked traditional uses of RYR, such as in the treatment of indigestion and diarrhea, are required.
Red yeast rice (RYR) is a traditional Chinese medicine and food supplement popular in East Asian countries such as China, Japan, Korea, and Thailand (Patel, 2016). It is produced by the fermentation of cooked rice kernels with a Monascaceae mold, Monascus purpureus, which turns rice into reddish purple kernels due to its pigmentation capability (Kalaivani et al., 2010). As part of the Asian diet, RYR is used as a food additive to enhance the color of meat, fish, and soybean products. It is also recognized as a folk medicine for rejuvenating the body, promoting blood circulation, and restoring stomach balance (Mazzanti et al., 2017). With the increasing tendency of Western countries to use statins, a group of drugs used to treat hyperlipidemia, RYR has attracted considerable research attention (Burke, 2015). RYR has been reported to possess numerous biological properties with hypolipidemic, anti-atherosclerotic, anti-cancer, neurocytoprotective, hepatoprotective, anti-osteoporotic, anti-fatigue, anti-diabetic, anti-obesity, immunomodulatory, anti-inflammatory, anti-hypertensive, and anti-microbial activities. RYR can also improve the quality of eggs. Chemical analyses have revealed that RYR contains monacolins, pigments, organic acids, amino acids, sterols, decalin derivatives, flavonoids, lignans, coumarin, terpenoids, and polysaccharides. However, only a few of these compounds have been screened in bioactivity assays, and their structures have not been sufficiently characterized. Although RYR is effective in treating various infections, the safety of its chemical constituents has not been defined. In addition, the quality control of RYR has not been investigated, and there is a lack of pharmacological information on the traditional uses of RYR.
In this article, we provide an up-to-date and comprehensive literature analysis of RYR and address its traditional uses, chemistry, pharmacological activities, possible molecular mechanisms, and safety. A critical evaluation of pharmacological studies in terms of the ethnomedical use of RYR was also performed. This information may provide new insights on RYR or its active ingredients, and help seek effective intervention strategies for the prevention and treatment of diseases, design clinical trials of bioactive compounds in RYR in future research, and develop fungal-medicines as well as edible products containing these functional properties.
Information on studies involving RYR was gathered via the Internet using Google Scholar, Baidu Scholar, Elsevier, Web of Science, PubMed, CNKI, ScienceDirect, SciFinder, and Scopus, and a library search was performed of articles published in classic books of Chinese Herbal Medicine, local herbal encyclopedias, and Ph.D. and M.Sc. dissertations. The key words used were RYR, red mold rice, M. purpureus, secondary metabolites, chemistry, biological activity, pharmacology, medicinal use, safety, quality control, toxicology, and other related words (Zhu et al., 2018). The MycoBank database (http://mycobank.org) was used to validate the scientific names of M. purpureus.
RYR (Figures S1A, B; also known as “Hongqu” or “angkak” in China, and ‘red koji’ in Japan) is a remedy belonging to Traditional Chinese Medicine (TCM). Nowadays, it is also used as a dietary supplement in Western countries (Hong et al., 2008b; Fung et al., 2012). It is produced by the fermentation of steamed rice with a food fungus of the Monascus genus, usually M. purpureus Went. Apart from M. purpureus, other related nonpathogenic molds of the Monascus genus, such as M. ruber, M. anka, and M. pilosus, are also used for RYR production in Japan, Korea, India, and Thailand (Cheng et al., 2013; Hong et al., 2017). In TCM, however, M. purpureus is the only accepted medicinal fungus for RYR fermentation (Chinese Pharmacopoeia, 2015). According to the MycoBank database (http://mycobank.org), M. purpureus (MB#235390) is the only accepted name for the fungus, and it has six synonyms, including M. albidus var. albidus, M. anka Nakaz. and K. Satô, M. araneosus K. Satô, M. major K. Satô, M. rubiginosus K. Satô, and M. albidus var. glaber K. Satô.
M. purpureus, which was largely described by Went in 1985, belongs to family Monascaceae, class Eurotiomycetes in Ascomycota (Huang et al., 2007). It can grow rapidly at a temperature of 25°C to 30°C on Potato Dextrose Agar or Luria Bertani mediums, and it favors conditions with a humidity of 65% to 85% (Figures S1C, D) (Il Gum et al., 2017). M. purpureus mostly breeds in asexual generation style, with many botryoid conidium. In general, stromata are solitary to gregarious, colony margins are short pulvinate to filiform, the ascocarp wall pectizes to membrane-like, ascospores are oval, the surface is smooth, and the color is red to nearly gray (Figures S1E, F) (Wei, 1979).
RYR was first recorded in the Local Chronicles of Gutian (⟪古田县志⟫), which dates back to the Tang Dynasty (A.D. 618–907) (Lin, 2017). RYR has also been recorded in many ancient texts, such as Qing Yi Lu (⟪清异录⟫) (from the Five Dynasties and North Song Dynasty, A.D. 907–1127), Hai Lu Sui Shi (⟪海录碎事⟫) (Song Dynasty, A.D. 960–1279), and Tian Gong Kai Wu (⟪天工开物⟫) (Ming Dynasty, A.D. 1368–1644), and it is widely used in the Chinese culture as a food preservative, flavor enhancer, and food-coloring agent for fish sauces, rice wines, red soybean curd, pickled vegetables, and salted meats (Burke, 2015; Lin, 2017). In TCM, according to Materia Medica in Daily Use (⟪日用本草⟫) (Yuan Dynasty, A.D. 1271–1368) and the Compendium of Herbology (⟪本草纲目⟫) (A.D. 1552–1578), RYR is slightly mild and sweet, without significant toxicity, and it goes to the liver, spleen, and large intestines. It has been used to treat indigestion, diarrhea, blood circulation stasis, and limb weakness for more than 700 years (Chinese Pharmacopoeia, 2015; Patel, 2016). In some western countries, including the United States, Netherlands, and Italy, RYR has been used as an alternative to statin therapy, especially in patients who are intolerant to standardized therapy due to statin-associated myalgia or those who are opposed to taking statins (Childress et al., 2013).
RYR has been widely used in China as both food and medicine. RYR, as an ingredient, is found in more than 155 healthy foods, with purported curative powers ranging from reducing blood lipid levels and enhancing immunity to reducing blood pressure and alleviating fatigue (http://www.da.yaozh.com, last accessed on 28/07/2019). RYR-based products include Hongqu Jiaonang, Yanjitangpai Kouyiling Ruanjiaonang, and Caizhiyuan Q10 Ruanjiaonang. Moreover, RYR has been used in at least 24 TCM preparations and at least 24 prescriptions for treating various chronic diseases (http://www.da.yaozh.com, last accessed on 28/07/2019). Commonly used Chinese herbal prescriptions containing RYR are listed in Table S1. “Hong Qu Jiu,” “Huo Tui Hong Qu San,” and “Huang Lian Hong Qu Tang” are Chinese prescriptions, whose use has been recorded in many ancient books, including the Compendium of Herbology (⟪本草纲目⟫), Following Traditional Customs Medical (⟪医学从众录⟫), and Gynecological Treatment of the Zhulin Temple (⟪竹林女科证治⟫). “Xue Zhi Kang Pian,” “Xue Zhi Kang Jiao Nang,” and “Xin Huang Pian” are folk medicines that have been accredited by the China State Food and Drug Administration. These entities are manufactured and sold in China to treat hyperlipemia, fatigue, and diarrhea (Chinese Pharmacopoeia, 2015).
RYR is comprised of various chemical constituents, including monacolins (1–23), pigments (24–48), organic acids (49–55), amino acids (56, 57), sterols (58–66), decalin derivatives (67–73), flavonoids (74, 75), lignans (76, 77), coumarin (78), terpenoids (79–83), and others (84–92). In addition, polysaccharides, such as EPS-1, EPS-2, EPS-3, EPS-4, EPS-5, MPS-1, MPS-2, MPS-3, and monascan, have been isolated from RYR (Table 1). According to previously published chemical studies, monacolins and pigments are the most abundant and bioactive constituents in RYR. Among these, monacolins [e.g. monacolin K (MK), also known as lovastatin], and pigments (e.g. monascin, rubropunctatin, and rubropunctamine) have been extensively investigated and considered to have potential benefits.
Monacolins are one of the main active ingredients in RYR. In total, twenty-three monacolins have been isolated from RYR, including MK 1, monacolin L 2 (Ma et al., 2000), monacolin Q 3, monacolin R 4, monacolin S 5 (Zhang et al., 2016), mehydromonacolin J 6 (Zhu et al., 2012b), dehydromonacolin K 7, dehydromonacolin L 8, dehydromonacolin N 9, dihydromonacolin K 10, dihydromonacolin L 11 (Ma et al., 2000; Zhu et al., 2012a; Zhang et al., 2016), dihydromonacolin-MV 12, dehydromonacolin-MV2 13 (Dhale et al., 2007a; Dhale et al., 2007b), ethyl ester of MK 14, methyl ester of the hydroxyl acid form of MK 15, methyl ester of the hydroxy acid form of monacolin L 16 (Ma et al., 2000; Zhu et al., 2012b), α,β-dehydromonacolin S 17, α,β-hydromonacolin Q 18, 3α-hydroxy-3,5-dihydromonacolin L 19, 3β-hydroxy-3,5-dihydromonacolin L 20, α,β-dehydrodihydromonacolin K 21, α,β-dehydrodihydromonacolin L 22, and (1S,2S,4aR,6S,8-S,8aS,3′S,5′R,2″S)-methyl 1,2,4a,5,6,7,8,8a-octahydro-3′,5′-dihydroxy-2,6-dimethyl-8-[(2-methyl-1-oxobutyl)oxy]-1-naphthaleneheptanoate 23 (Zhu et al., 2012b; Zhang et al., 2016). Monacolins, especially RYR-derived MK, have been shown to have remarkable hypolipidemic effects (Chen, 2004) as well as anti-osteoporotic (Gutierrez et al., 2006), and anti-fatigue (Chen, 2004) activities. The chemical structures of these monacolins are shown in Figures 1 and 2.
Pigments are also important active compounds found in RYR. Twenty-five pigments have been isolated from RYR, including rubropunctamine 24, rubropunctatin 25, monascorubramine 26, monascorubrin 27, monascin 28, ankaflavin 29, xanthomonasin A 30, xanthomonasin B 31, monankarin A 32 (Wild et al., 2002b; Akihisa et al., 2005), monasfluore A 33, monasfluore B 34 (Huang et al., 2008), monapurone A 35, monapurone B 36, monapurone C 37 (Li et al., 2010), monascopyridine A 38, monascopyridine B 39, monascopyridine C 40, monascopyridine D 41, monascopyridine E 42, monascopyridine F 43 (Ferse et al., 2012), monapurfluore A 44, monapurfluore B 45 (Hsu et al., 2010), 4-[2,4-dihydroxy-6-(3-hydroxybutanethioyloxy)- 3-methylphenyl]-3,4-dihydroxy-3,6-dimethylheptanoic acid 46, 9-hexanoyl-3-(2-hydroxypropyl)-6a-methyl-9,9a-dihydro-6H-furo[2,3-h]isochromene-6,8(6aH)-dione 47 (Campoy et al., 2006), and monapilosusazaphilone 48 (Cheng et al., 2013). RYR-derived pigments possess hypolipidemic effects (Zhou et al., 2019) as well as anti-cancer (Xu et al., 2017), anti-fatigue (Chen, 2004), and anti-inflammatory (Hsu et al., 2010) activities. In addition, RYR pigments can be used to color yogurt red (Chen et al., 2012b). The chemical structures of these molecules are shown in Figures 3 and 4.
Seven organic acids, including linoleic acid 49, α-linolenic acid 50 (Zhang et al., 2018a), citrinin 51 (Wild et al., 2002b), 1-heptadecanecarboxylic acid 52, 1-pentadecanecarboxylic acid 53, 2-hydroxyoctadecanoic acid 54 (Tan, 2015), and 5-(2′-hydroxy-6′-methyl phenyl)-3-methylfuran-2-carboxylic acid 55 (Ji et al., 2018), as well as (+)-monascumic acid 56 and (−)-monascumic acid 57, two amino acids (Akihisa et al., 2004; Akihisa et al., 2005), have been isolated from RYR. However, RYR-derived citrinin has been reported to have lethal effects on the kidney, and it may also act as a teratogen (harmful to the embryo or fetus) and genotoxin at high concentrations in cultured human lymphocytes (Patel, 2016). In addition, the two amino acids have been shown to have potent inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) activation (Akihisa et al., 2005).
Nine sterols (ergosterol 58, stigmasterol 59, β-sitosterol 60, 3β-hydroxylstigmast-5-en-7-one 61, 3β-hydroxystigmasta-5,22-dien-7-one 62, 6β-hydroxystigmast-4-en-3-one 63, 6β-hydroxystigmasta-4,22-dien-3-one 64, daucosterol 65, and β-sitosteryl palmitate 66) were isolated from RYR (Cheng et al., 2010b; Wang, 2010; Cheng et al., 2013; Tan, 2015). Among these, stigmasterol has been reported to possess hypolipidemic effects (Wang, 2010).
Seven decalin derivatives, including monascusic acid A 67, monascusic acid B 68, monascusic acid C 69, monascusic acid D 70, monascusic acid E 71, monascusic lactone A 72, and heptaketide 73 were isolated from RYR (Zhang et al., 2009; Zhu et al., 2012a; Zhang et al., 2016). These decalin derivatives could suppress human T cell proliferation in a dose-dependent manner from 10 µmol/L to 100 µmol/L (Zhu et al., 2012a). The chemical structures of these decalin derivatives are shown in Figure 5.
Two flavonoids, including daidzein 74 and genistein 75 (Ji et al., 2018), two lignans, including 5,5′-dimethoxylariciresinol 76 and lariciresinol 77, and one coumarin (scopoletin 78) (Cheng et al., 2013), as well as five terpenoids, including 3-epi-betulinic acid 79, 3-epi-betulinic acid acetate 80, Friedelan-3-one 81, α-cadinol 82, and anticopalol 83 (Cheng et al., 2010a), have been isolated from RYR. Presently, there are few studies investigating the pharmacological activities of these RYR-derived compounds.
Nine polysaccharides, including EPS-1, EPS-2, EPS-3, EPS-4, EPS-5, MPS-1, MPS-2, MPS-3, and monascan, were isolated from RYR (Tian et al., 1998; Jiang, 2016). These polysaccharides are all composed of mannose, glucose, and galactose. EPS-1, EPS-2, EPS-3, EPS-4, and EPS-5 are composed of mannose, glucose, and galactose at a molar ratio of 0.364:0.415:0.221, whereas MPS-1, MPS-2, and MPS-3 are composed of mannose, glucose, and galactose at a molar ratio of 0.500:0.318:0.192 (Jiang, 2016). Monacan, a homogeneous polysaccharide with a molecular weight of ∼400,000 Da, is composed of mannose, glucose, and galactose at a molar ratio of 1:2:4, and its backbone is comprised of 1→3, 1→2, and 1→6 glucosidic bonds (Tian et al., 1998). Moreover, RYR-derived polysaccharides have been reported to possess anti-cancer (Ding, 2007) and immunomodulatory (Zhang et al., 2008) activities.
Nine other compounds, including peroxymonascuspyrone 84, α-tocospiro A 85, spathulenol 86 (Cheng et al., 2010a), monascodilone 87 (Wild et al., 2002a; Wild et al., 2002b), monascustin 88 (Wei et al., 2017), N-cis-feruloylmethoxytyramine 89 (Cheng et al., 2013), monaspurpurone 90, p-nitrophenol 91 (Cheng et al., 2010b), and 1-dotriacontanol 92 (Tan, 2015), were isolated from RYR. The chemical structures of these compounds are shown in Figure 6.
RYR has been the subject of several pharmacological investigations due to its various ethnomedicinal uses. Studies have demonstrated that RYR exhibits a wide range of biological properties with hypolipidemic, anti-atherosclerotic, anti-cancer, neurocytoprotective, hepatoprotective, anti-osteoporotic, anti-fatigue, anti-diabetic, anti-obesity, immunomodulatory, anti-inflammatory, and anti-hypertensive activities. Among these, hypolipidemic and anti-atherosclerotic activities are most pronounced given that RYR is thought to play curative roles in resolving turbidity, invigorating blood circulation, and resolving blood stasis. These effects are summarized in Table 2 and discussed in greater detail in the following sections. The relationships between the doses of RYR and its medicinal properties are shown in Figure 7.
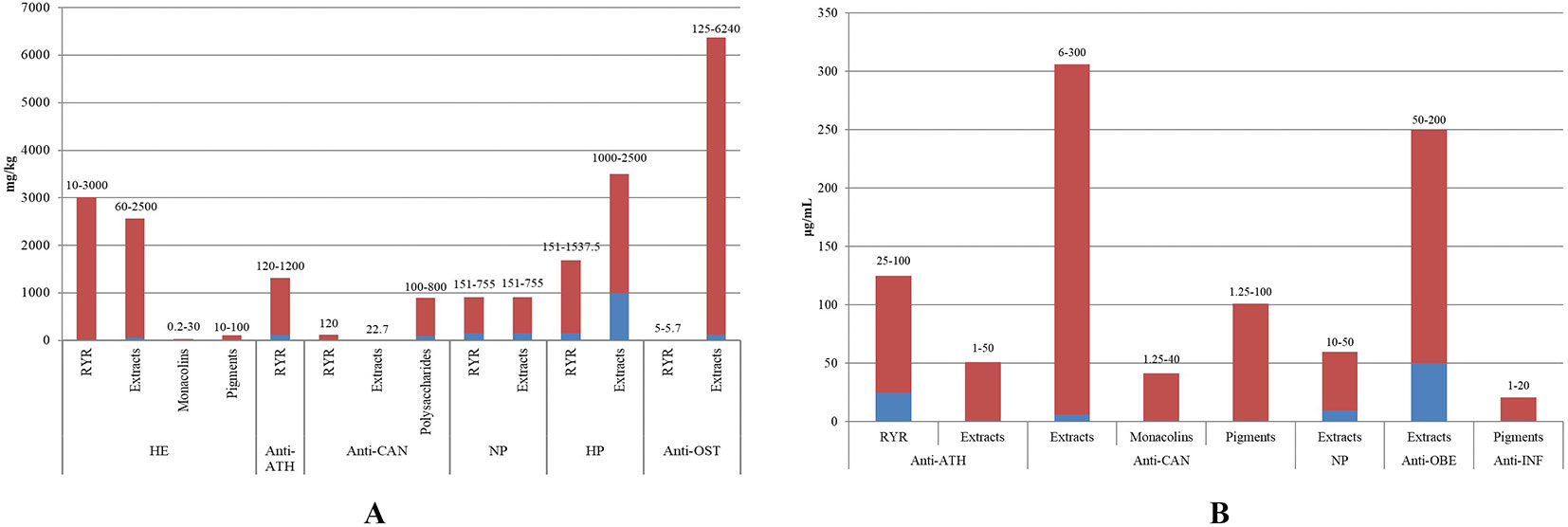
Figure 7 Dose-related effects of RYR extracts against various disorders in vivo (A) and in vitro (B) (HE, hypolipidemic effect; anti-ATH, anti-atherosclerotic activity; anti-CAN, anti-cancer activity; NP, neurocytoprotective activity; HP, hepatoprotective effect; anti-OST, anti-osteoporotic activity; anti-OBE, anti-obesity activity; anti-INF, anti-inflammatory activity.
Hyperlipidemia is the bane of current dietary patterns and rather torpid lifestyles. As a result, the search for supplements that can lower the triglyceride (TG) and cholesterol levels has gained significant momentum. Statins have been used to eliminate vascular occlusions, but with increasing reports of side effects, alternatives are being pursued (Patel, 2016). In this regard, the hypolipidemic potential of RYR has been consistently proved with reliable experimental results (Gerards et al., 2015). In dyslipidemia patients, RYR, which was administered as a dietary supplement (4–48 mg/kg), significantly decreased TG, total cholesterol (TC), and low density lipoprotein cholesterol (LDL-C) levels, and increased the high density lipoprotein cholesterol (HDL-C) level (Heber et al., 1999; Becker et al., 2009; Bruno et al., 2018). However, further studies that follow patients for longer periods of time are needed to investigate the effects RYR on the risk factors and treatment of various chronic diseases. In animals fed a high-fat diet, the administration of RYR, as a capsule, or RYR aqueous extracts significantly down-regulated TG, TC, and LDL-C levels (Wei et al., 2003). These hypolipidemic activities of RYR were mediated at least partially by enhanced acidic sterol excretion and reduced expression of inflammatory transcription factors such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (Ma et al., 2009; Ding et al., 2014). However, the mechanisms of action remain to be defined.
The putative hypolipidemic effects of RYR have been mostly ascribed to the rich contents of monacolins and pigments (Zhou et al., 2019). In a crossover, double-blind, placebo-controlled randomized clinical trial, short-term treatment (4 weeks) with monacolins (10 mg) could significantly reduce TC, LDL-C, and non-HDL levels (Cicero et al., 2013). However, these results have yet to be confirmed in studies following a larger cohort for a longer period of time. In another study that utilized a high-fat diet rat model, the administration of MK (5–30 mg/kg) decreased serum TC, TG, and LDL-C levels by up-regulating lipoprotein lipase and low density lipoprotein receptor mRNA expression in the liver (Chen, 2004). The oral administration of RYR yellow, red, and orange pigments also markedly alleviated disturbances in lipid metabolism; ameliorated serum lipid levels; suppressed hepatic lipid accumulation and steatosis; and promoted fecal cholesterol, triacylglycerol, and bile acid excretion. The mechanisms of action involve an up-regulation of the mRNA levels of farnesoid X receptor and peroxisome-proliferator-activated receptor-gamma, the main receptors for the metabolism of cholesterol and homeostasis of bile acids (Zhou et al., 2019). However, some of these results did not show a dose-effective relationship, and some of these studies lacked positive controls.
RYR is often combined with other products that can effectively reduce lipid levels, such as berberine (Millan et al., 2016), policosanols (Guardamagna et al., 2009), coenzyme Q10 (Cicero et al., 2016), plant stanols and sterols (Feuerstein and Bjerke, 2012), olive extracts (Verhoeven et al., 2015), and curcumin (Derosa et al., 2018). Among these, berberine and policosanols have been extensively investigated due to their lipid-lowering properties, in which an increase in LDL receptor half-life and expression and an increase in insulin sensitivity via the activation of AMP-activated protein kinase (AMPK) (Spigoni et al., 2017) were observed. Bifidobacterium longum, a probiotic strain showing potent hypolipidemic effects, has also been combined with RYR in nutraceutical formulations given that alterations in gut microbiota were found to be involved in the pathogenesis of systemic diseases related to hypercholesterolemia (Weis, 2018; Ruscica et al., 2019). Additionally, several Chinese medicines have been used with RYR to treat hyperlipidemia, such as Tao He Cheng Qi Tang consisting of Prunus persica semen, Cinnamomum cassia ramulus, Rheum palmatum rhizome, natrii sulfas, and Glycyrrhiza uralensis rhizome (Yang, 2017). However, the possible interactions, synergistic effects, and underlying mechanisms of RYR in combination with other ingredients from poly-herbal preparations remain unknown and should be further investigated.
The inhibition of cholesterol synthesis is effective for the primary and secondary prevention of atherosclerotic diseases (Li et al., 2005b). Experimental studies in animal and cell models have demonstrated that RYR, as well as Xuezhikang (XZK, its pure extract), has anti-atherosclerotic activity. An eight-week administration of XZK (600–1,200 mg/kg) to C57BL/6 mice was found to significantly and dose-dependently inhibit vulnerable plaque progression, decrease the plaque area, and suppress lesional endoplasmic reticulum stress (Shen et al., 2017). In another study involving atherosclerotic rats fed a high-cholesterol diet, the oral administration of RYR at 120 mg/kg significantly reduced the plaque size, stabilized the plaque, protected the endothelium, and decreased the number of lipid droplets and cholesterol calculi, and lowered the levels of high sensitivity C-reaction protein (Hs-CRP), IL-6, and TNF-α, suggesting that the anti-atherosclerotic activity of RYR may be related to inflammatory signaling pathways (Wu et al., 2017). The administration of aqueous and ethanol extracts of RYR to homocysteine-treated human aortic smooth muscle cells (HASMCs) (1–160 µg/ml) for 24 h reduced TNF-α-induced metalloproteinase (MMP)-2 and MMP-9 expression. It also reduced nuclear factor-κB (NF-κB) activation and intracellular reactive oxygen species (ROS) formation, supporting the notion that RYR may have a potential application in the treatment of atherosclerosis (Lin et al., 2011). Unfortunately, the chemical analyses of XZK and the aqueous and ethanol extracts of RYR were not performed in these studies. Based on the significant decrease in the viability of HASMCs treated with relatively high concentrations of RYR (160 µg/ml), attention should be paid to the possible side-effects in clinical applications.
Cancer, a major disease, is the leading cause of death throughout the world, and developing effective anti-cancer therapies remains a challenge for those in medical research (Zhu et al., 2018). Previous studies have reported that RYR supplements have anti-cancer activity. The oral administration of RYR (120 mg/kg, qd) to breast cancer patients for 12 weeks significantly decreased serum tumor biomarker (CA125, CA153) levels and improved their life quality and immune function after chemotherapy and resection surgery (Zhong, 2017). Although this study showed that RYR possesses anti-cancer activity, only a single dose was used, thereby limiting information on the dose-dependent effect. In SCID tumor mice induced by human prostate cancer cells, the administration of powdered RYR significantly reduced the oral tumor volume and decreased the serum prostate-specific antigen level, as well as the gene expression of enzymes involved in androgen synthesis (HSD3B2, AKR1C3, and SRD5A1) (Hong et al., 2011). Thus, clinical studies of RYR use for the prevention of prostate cancer in men undergoing active surveillance of the disease should be considered.
Studies have reported that polysaccharides, monacolins, and pigments from RYR are responsible for the anti-cancer activity of RYR. The administration of RYR polysaccharides (at a very high dose of 800 mg/kg) to replanted S180 tumor mice for 2 weeks remarkably inhibited tumor growth and improved body weight (Ding, 2007). However, this trial lacked a positive control as well as an evaluation of serum biochemical indicators of the tumor. In K-562, SK-OV-3, and SNU-1 cells, the administration of rubropunctatin, a red pigment, and RYR-derived monacolin L (1.25–40 µg/ml) for 48 h exhibited very strong inhibitory effects against cancer cell proliferation, and the effect of rubropunctatin was comparable with that of anti-cancer drugs cis-platinum, taxol, and 10-hydroxy-camptothecin in their IC50 values. In this study, the authors demonstrated that RYR at least partially exerted its anti-cancer effects through telomerase-mediated inhibition of rubropunctatin and monacolin L-induced apoptosis (Xu et al., 2017). However, both studies failed to discuss the effectiveness of RYR-derived monacolin and pigments using animal models, and more in-depth studies should be carried out to further develop these compounds into anti-cancer drugs.
RYR extracts have also been shown to possess neurocytoprotective activity. In rats treated with amyloid β (Aβ), an amino acid associated with Alzheimer’s disease, the oral administration of RYR (151–755 mg/kg) for 4 weeks could markedly reverse the memory deficits in the water maze and passive avoidance tasks, as well as prevent Aβ40 infusion and damage in the hippocampus and cortex, which are known involve in the increase of thiobarbituric acid reactive substances and ROS. Using human neuroblastoma IMR32 cells exposed to a high concentration of cholesterol, the authors also reported that RYR down-regulated Aβ40 formation and deposition by suppressing cholesterol-induced β-secretase activity and apolipoprotein E expression. RYR also mediated the proteolysis of amyloid precursor protein (APP) into a soluble APP α-fragment with neuroprotective activity in the hippocampus (Lee et al., 2010). However, further studies are needed to confirm the neurocytoprotective effect using low doses of RYR suitable for human administration. Lovastatin derivatives are also responsible for the neurocytoprotective effect of RYR. The administration of one lovastatin-derived compound, designated 3f (12.5–100 µmol/L), for 24 h significantly reduced 6-hydroxydopamine (6-OHDA)-induced apoptosis in PC12 cells, reduced caspase-3, -8, and -9 activities, and lowered intracellular calcium concentrations elevated by 6-OHDA in a concentration-dependent manner, without inhibiting the production of ROS (Lin et al., 2015). Studies on the mechanism of action of compound 3f are being conducted.
RYR extracts exhibit a strong hepatoprotective effect both in alcoholic fatty liver and non-alcoholic fatty liver disease (NAFLD) mice models. In chronic alcohol-induced mice, the oral administration of powdered RYR (307.5–1,537.5 mg/kg) for 5 weeks significantly attenuated the increased levels of serum transaminases (aspartate aminotransferase and alanine aminotransferase), hepatic TGs, and TC. Furthermore, RYR elevated the hepatic antioxidant activity that reduced hepatic cell damage (steatosis) and decreased tissue inflammatory cytokine levels (Cheng and Pan, 2011). These findings suggested that RYR may represent a novel, protective strategy against alcoholic liver disease by attenuating oxidative stress, inflammatory responses, and steatosis. However, the authors of this study did not perform comparisons using a positive control. The administration of RYR extracts (XZK at 300 mg/kg) to NAFLD mice for 6 weeks significantly ameliorated dyslipidemia and fat accumulation in the liver; improved insulin resistance; ameliorated oxidative stress; lessened hepatic steatosis, necro-inflammation, and collagen deposition; and reversed abnormalities in the aminotransferase level. The mechanism of action of RYR likely involves inhibition of the hepatic expression of TNF-α (Hong et al., 2007). These results suggested that RYR may have a potential clinical application in the treatment of NAFLD. In this study, however, the optimal dose, constituents, and side effects of RYR were not assessed.
Osteoporosis is a common health problem characterized by low bone mass and structural deterioration of the bone, resulting in an increased susceptibility to fractures (Zhang et al., 2018b). Several studies have reported the protective role of RYR in osteoporosis. In rats with ovariectomy-induced bone loss and osteoblast cells, the administration of ethanol extracts from RYR (at a very high dose of 1.56–6.24 g/kg) for 20 weeks significantly increased the bone mineral density and decreased the levels of bone turnover markers in vivo, including osteocalcin and tartrate-resistant acid phosphatase. Moreover, its administration improved the viability of osteoblasts and enhanced the mRNA and protein expression of bone morphogenetic protein (Bmp) 2 and Bmp4, suggesting that RYR may be useful in preventing and treating osteoporosis (Wang et al., 2015). However, future studies should confirm this anti-osteoporotic effect under clinical conditions with low doses of RYR that are suitable for human administration. In rabbits with bone defects and UMR 106 cells, the administration of RYR extracts significantly promoted new bone formation in vivo, enhanced bone optical density, and increased alkaline phosphatase activity in vitro, suggesting that RYR is a natural product that can potentially treat bone defects and osteoporosis (Wong and Rabie, 2008). However, additional evidence from randomized controlled trials is required to identify other regulatory mechanisms that may be responsible for the anti-osteoporotic effects. The bioactive constituents of these extracts also remain unknown.
RYR extracts exhibit strong anti-fatigue capability. The oral administration of RYR (24 mg/kg) to dyslipidemia patients for 4 weeks was found to significantly reduce muscle fatigue and preserve physical activity (Xue et al., 2017). Nevertheless, only a single dose of RYR was used in this study. In a Wistar rat model, the administration of powdered RYR (at a very high dose of 1–5 g/kg) for 4 weeks significantly extended the swimming time for the rats, effectively delayed the lowering of the glucose level in the blood, prevented the increase in lactate and blood urea nitrogen concentrations, and decreased the contribution of exercise-induced oxidative stress, suggesting that RYR has anti-fatigue activity and may potentially be a useful pharmacological agent (Wang et al., 2006). However, the main shortcomings of this study were that the dose of RYR was too high and no mechanism of action was provided. RYR extracts are abundant monacolins and pigments. The administration of MK (10–90 mg/kg) or ethanol-soluble red pigments (50–200 mg/kg) to mice for 30 days significantly improved the hepatic glycogen level, decreased the serum urea nitrogen level, and increased the swimming time in an endurance test, indicating that MK and red pigments are responsible for the anti-fatigue activity of RYR (Chen, 2004). However, future studies are needed to identify the mechanism of action of these constituents.
Diabetes is assuming epidemic proportions across the world (Lam and LeRoith, 2012). The best approach to control hyperglycemia and mitigate diabetes is dietary intervention, rather than a reliance on drugs (Patel, 2016). In this regard, the possible role of RYR was investigated. The administration of RYR (50–350 mg/kg) to streptozotocin-induced diabetic rats for 2 weeks markedly decreased the plasma glucose level, reversed hyperphagia, and decreased the mRNA level of phosphoenolpyruvate carboxykinase in the liver in a dose-dependent manner, indicating that RYR decreased hepatic gluconeogenesis and lowered the plasma glucose level in diabetic rats (Chang et al., 2006). A similar study has reported that RYR could promote the release of acetylcholine from nerve terminals, which in turn stimulated muscarinic M3 receptors in pancreatic cells and augmented insulin release in order to lower the plasma glucose level (Chen and Liu, 2006). In another study, the administration of RYR (300 mg/kg) to diabetic mice for 8 weeks significantly decreased the blood glucose level by improving glucose tolerance and insulin secretion, protected islets from hyperglycemic injury, and inhibited the expression of key factors in oxidative stress, including 8-OHdG, 4-HNE, and gp91phox, indicating that the effects of RYR on oxidative stress may at least partly account for the improved insulin secretion of pancreatic islets in diabetes (Wang et al., 2014). However, this evidence is still tenuous; no detailed clinical trials involving RYR supplementation have been performed, and no chemical constituents of RYR responsible for the anti-diabetic activity have been presented. Furthermore, several studies also failed to include positive controls and dose-dependent effect analyses.
Obesity is defined as an excess of white adipose tissue, which is associated with a higher risk of developing diabetes and cardiovascular disease (Marques et al., 1998). RYR has been shown to possess anti-obesity activity. In mice fed a high-fat diet, the administration of RYR (at a very high dose of 4–20 g/kg) significantly lowered weight gain and fat pad mass, which was accompanied by smaller fat cells. The anti-obesity effects of RYR were mainly derived from the lipolytic activity and mild anti-appetite potency of RYR. In 3T3-L1 cells, the investigators demonstrated that RYR extracts suppressed the proliferation and differentiation of preadipocytes, which may have inhibited new adipocyte formation or hyperplasia in adipose tissue (Chen et al., 2008). The main shortcoming in this study was the very high dose of RYR, which will restrict the clinical application of RYR in the treatment of obesity.
Inflammation signifies an agitated metabolic and immunological system (Odegaard and Chawla, 2013), and anti-inflammatory drugs that can restore the homeostasis of a perturbed system are in great demand. Various anti-inflammatory drugs exist, although many associate with adverse effects (Patel, 2016). RYR has been reported to possess an inflammation-quenching effect. In RAW264.7 cells induced by lipopolysaccharide, the administration of azaphilonoid derivatives isolated from RYR (5–20 µg/ml), including monapurfluores A and B, monascopyridines C and D, and monasfluores A and B, significantly inhibited the release of the inflammatory mediator nitric oxide (NO) from macrophages (Hsu et al., 2010). NO has been reported to be a signaling agent for various inflammatory diseases; therefore, its suppression is likely to ameliorate irritation (Patel, 2016). However, this study failed to include a positive control, and other inflammatory indicators, such as IL-1β, TNF-α, and IL-6, should be studied in the future. In mice fed a high-cholesterol diet, the administration of XZK (300 mg/kg) for 6 weeks significantly reduced the levels of the inflammatory transcription factors TNF-α and IL-6, and attenuated renal injury by managing the abnormal lipid levels and the consequent stress (Ding et al., 2014). This study also lacked a positive control, and the effects of the various doses were not assessed.
Hypertension, commonly recognized as a silent killer, is the most common cardiovascular disease and a major risk factor for atherosclerosis, metabolic syndrome, renal dysfunction, myocardial infarction, heart attack, and stroke, which are the most prevalent causes of death in industrialized countries (Williams, 2009; Wang et al., 2010). As many drugs have side effects, healthy diets are expected to prevent or alleviate the complications. The potential of RYR, in combination with other bioactive components, to manage the problem is discussed below. In grade 1 essential hypertension patients, the administration of a nutraceutical formulation containing RYR, folic acid, coenzyme Q10, Orthosiphon stamineus (a tropical herb from the Lamiaceae family), policosanol, and berberine significantly reduced the mean 24-h systolic and diastolic blood pressure levels (Trimarco et al., 2012). However, the ratios between RYR and the other constituents are not optimized based on the anti-hypertensive activity. In spontaneously hypertensive rats, the intravenous bolus administration of RYR ethanol extracts (10–50 mg/kg) elicited biphasic and potent hypotensive and cardiac inhibitory effects, as well as a significant decrease in sympathetic vasomotor activity (Wang et al., 2010). Further studies are needed to identify the chemical constituents in RYR ethanol extracts responsible for the anti-hypertensive effects. Meta-analysis of the existing literature showed that RYR, together with conventional therapy and statins, can lower the systolic blood pressure, without any significant adverse side effects (Patel, 2016; Xiong et al., 2017). Taken collectively, the results are encouraging but limited. Although meaningful reductions in blood pressure have been observed, evidence for the use RYR in the treatment of hypertension is weak based on the available data. Thus, more rigorous, high-quality trials are required to provide stronger evidence.
Polysaccharides from RYR have been shown to possess immunomodulatory activity. In a mice model, the administration of RYR-derived polysaccharides (50–300 mg/kg) significantly improved the phagocytic activity of abdominal cavity macrophages, prompted the formation of the peripheral blood E-rose loop, increased the transformation rate of lymphocytes, and enhanced nonspecific immunity (Zhang et al., 2008). However, this study failed to include a positive control, and the relationship between polysaccharide structure and immunomodulatory activity should be further investigated. In laying hens, the administration of RYR significantly increased the laying rate, Haugh units, and albumen height, as well as decreased the yolk cholesterol content, suggesting that RYR plays important roles in improving the production and quality of eggs (Sun et al., 2015). Further studies are required to determine the level of citrinin, a nephrotoxin in RYR, in the serum and eggs of hens in order to meet a lower citrinin level with regard to RYR production.
Methane (CH4), a greenhouse gas that is 25 times more effective than carbon dioxide in global warming, is emitted during enteric fermentation in ruminants, accounting for approximately 10% of the total anthropogenic CH4 (Holter and Young, 1992; Hristov et al., 2013). In a castrated Boer crossbred goat model, the administration of RYR significantly reduced enteric CH4 emissions, serum lipid levels, and the growth of several archaeal species (e.g. Methanobrevibacter) in the rumen. Therefore, as a natural fermentation product, RYR can reduce enteric methane emissions in goats (Wang et al., 2016). However, caution should be taken when considering the digestibility of protein and organic matter, and further studies are needed to evaluate its effects in different animals with various diets as well as its effects on animal health and food safety.
MK (also known as lovastatin), categorized as a class II compound according to the Biopharmaceutics Classification System (BCS), is mainly responsible for the blood cholesterol lowering effect of RYR (Wu and Benet, 2005). MK exhibits poor oral bioavailability (<5%) because of its low solubility (1.3 µg/mL in water), extensive metabolism in the gut and liver, and transmembrane efflux via the drug transporter P-glycoprotein (Serajuddin et al., 1991; Chen et al., 2005). Its bioavailability can be improved by increasing the dissolution rate and/or decreasing pre-systemic clearance (Neuvonen et al., 2008; Wu et al., 2011). In Caco-2 cells, extracts of RYR (LipoCol Forte, XZK, and Cholestin) were more effective in inhibiting the activities of CYP450 enzymes and P-glycoprotein, and showed higher lovastatin absorption and dissolution rates than that of lovastatin alone (Chen et al., 2012a; Chen et al., 2013). Moreover, in human studies, the authors demonstrated that volunteers receiving LipoCol Forte capsules or powder exhibited higher area under the plasma concentration-time curve and Cmax (maximum plasma concentration) values for both lovastatin and its active metabolite, lovastatin acid, and lower Tmax (time to reach the peak concentration) values than those receiving lovastatin tablets, suggesting that the oral bioavailability of lovastatin is significantly improved when combined with RYR as a result of the higher dissolution rate (Chen et al., 2013). In this regard, the increased dissolution rate seen with RYR products might enable lovastatin (a BCS class II drug) to function as a BCS class I drug. Additionally, Leone and colleagues (Leone et al., 2016) prepared a new formulation that combined 60% gelatin with 40% alginate. They observed a delayed release of lovastatin from RYR, a prolonged inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, and decreased cholesterol synthesis. However, further studies are needed to explore the underlying mechanism of actions, as well as the pharmacokinetics of the other bioactive compounds present in RYR, including monacolins and pigments such as rubropunctamine, monascorubramine, and ankaflavin.
To assess and control the quality of RYR, the Chinese Pharmacopoeia suggests using morphology, ultraviolet-visible spectrophotometry, thin-layer chromatography, and high-performance liquid chromatography (HPLC). MK can be used as a marker of quality in the official monograph of the RYR as well as in the label claim of several commercial RYR-related products. The MK content of RYR should be more than 0.22% by HPLC (Chinese Pharmacopoeia, 2015). However, the European Commission has not legislated on the limits for RYR supplements and there is no standardization in European countries, while the Food and Drug Administration (FDA) has stated in 2007 that RYR products containing MK are identical to a drug, and therefore, subject to regulation (FDA, U.S, 2007). With regard to therapeutic efficacy, a 2011 European Food Safety Authority (EFSA) report confirmed that the daily intake of 10 mg of MK was beneficial in maintaining normal cholesterol levels (EFSA, 2011). However, manufactures of RYR products rarely disclose the levels of MK and the active ingredients are not standardized.
Various methods are available for the determination of the MK level in RYR products, including HPLC (Theunis et al., 2017), ultra-high-performance liquid chromatographic with diode array detection and/or with mass spectrometry (UHPLC–DAD–QToF-MS) (Avula et al., 2014), matrix effect-free MISPE-UHPLC-MS/MS (Svoboda et al., 2017), LC-DAD/FLD (fluorescence detection)-MSn (Mornar et al., 2013), UHPLC hyphenated with triple quadrupole tandem MS (UHPLC–QQQ-MS) (Zhu et al., 2013), flow injection FI-MS/MS (Song et al., 2012), square-wave voltammetry (Nigovic et al., 2015), and contact angle, atomic force microscopy with Fourier transform infrared spectroscopy (Eren et al., 2015). However, the use of only one quantitative marker may be insufficient to properly assess the quality of RYR extracts. There is evidence to indicate that the pigments found in RYR have hypolipidemic (Zhou et al., 2019), anti-cancer (Xu et al., 2017), anti-fatigue (Chen, 2004), and anti-inflammatory (Hsu et al., 2010) activities, and therefore, the presence of pigments may be a critical indicator of extract quality. Although several pigments, such as rubropunctatin, monascorubrin, monascin, monasfluore A, and monasfluore B, have been identified in RYR by liquid chromatography–mass spectrometry (LC-MS) (Jin et al., 2016), HPLC-FLD (Huang et al., 2011), and HPLC-UV (Huang et al., 2016), more rapid, sensitive, and selective technologies should be developed for the detection of pigments.
In RYR extracts, the content of active ingredients, which includes MK, pigments, phenolic compounds, and flavonoids, varies in quality with the fermentation process, extraction procedures used, strain of M. purpureus, and storage conditions. The MK concentration in RYR can be increased by the mixed-culture fermentation of rice with two different Monascus species (M. purpureus and M. ruber), with the optimized fermentation process parameters including a pH of 6.03 at a temperature of 29.46°C for 13.89 days. This process predicts 2.83 mg/g and yields 2.80 mg/g of MK/gram of fermented rice with 98.93% accuracy (Panda et al., 2010). Moreover, the supplementation of the fermentation substrate with nitrogen sources influences the pigment production efficiency of RYR. For instance, the addition of orange peels and sunflower meal to solid-state fermentation (SSF) cultures of M. purpureus can enhance pigment production in RYR, yielding 9 absorbance units (AU)/per g of dry fermented substrate (gdfs) (Kantifedaki et al., 2018). Babitha and colleagues (Babitha et al., 2007) reported that the addition of monosodium glutamate to jackfruit seed SSF of M. purpureus improved the production of red (30.8 AU/gdfs) and yellow (25.5 AU/gdfs) pigments, followed by the addition of soybean meal, peptone, and chitin powder. In addition, a combination of light and bacteria can be used to enhance secondary metabolite production during RYR fermentation. The fermentation of RYR with Bacillus subtilis, a rod-shaped, Gram-positive endospore-forming bacterium, under blue light-emitting diodes enhanced the production of phenolic compounds (68.4 ± 1 mg GAE/g DW) and flavonoids (51.7 ± 1 mg QE/g DW) compared to white light and darkness (Elumalai et al., 2019). Further studies should focus on the molecular mechanisms and optimization of conditions for the commercial-scale production of these secondary metabolites. Under optimal extraction conditions with 45% ethanol, 1.5% phosphate, and an extraction time of 70 min, 91.6% of citrinin was removed and 79.5% of MK was retained in the final RYR extract (Lee et al., 2007a). M. purpureus strains are commonly used to enhance the production of MK and pigments in RYR. Using rigorous physicochemical mutagenesis technologies involving ultraviolet-lithium chloride, microwave heating, and genome shuffling mutations, two mutant M. purpureus strains, strain 183-3 with a highly stable capacity to produce pigment and strain R”-30 with a highly stable capacity to produce MK, were obtained (Xu, 2014; Li, 2016). In addition, Li et al. reported that the MK content in RYR significantly decreased under conditions of high humidity, high temperature (75% RH, 60°C), and sunlight, indicating that MK in RYR is light- and heat-sensitive, and RYR should be stored in the cool and dark place (Li et al., 2005a).
The area in which RYR is produced also influences its quality. RYR is now produced in more than 120 cities in 15 countries in Asia, Europe, and North America, such as China, Japan, the United States, Germany, Italy, Thailand, India, Korea, and Belgium. The general geographical distribution of RYR is shown in Figure S2. In studies that used UHPLC–DAD–QToF-MS to determine the MK content in 26 brands of RYR from four large retail chains in the United States (i.e. GNC, Walgreens, Walmart, and Whole Foods), it was reported that the MK content was highly variable, ranging more than 60-fold from 0.09 to 5.48 mg per 1,200 mg (Cohen et al., 2017). In another study that involved LC-PDA-MS, Huang and colleagues found that RYR from cities in Fujian Province, especially Gutian, Nanping, and Pinnan, possessed a higher MK content compared to six other habitats in China (Huang et al., 2006). These results indicate that the MK content in RYR extracts significantly differs between samples from different habitats. Thus, the MK content should be compared across RYR products, uniform legislation should be proposed, and strict quality control of RYR extracts should be implemented.
Citrinin is a polyketide secondary metabolite found in food and feed that is produced by several fungi, including M. purpureus (Bezeria da Rocha et al., 2014; Kiebooms et al., 2016). It is a mycotoxin known to cause kidney damage as well as disrupt metabolic processes in the liver (Mornar et al., 2013). Citrinin, as a component, has been involved in several controversies related to the quality and safety of RYR products, because up to 80% of RYR products may contain this mycotoxin (Heber et al., 2001). The EFSA has set a level of no concern for citrinin-caused nephrotoxicity at 0.2 µg/kg body weight per day, but it has acknowledged the need for more reliable data on the citrinin level in food and feed before it can undertake exposure studies and risk assessment (Marley et al., 2016). Different analytical methods have been developed for the quantification of citrinin in a variety of samples in the last few years, including LC-FDA (Marley et al., 2016), UHPLC-MS/MS (Kiebooms et al., 2016), UHPLC–DAD–QToF-MS (Avula et al., 2014), LC-DAD/FLD-MSn (Mornar et al., 2013), microsphere-based flow cytometric immunoassays (MFCI) (Li et al., 2012), and a multi-commutated fluorometric optosensor technology (Jimenez Lopez et al., 2014). The maximum allowed level of citrinin as a contaminant in RYR is 200 ppb in Japan, but the European Union has recommended a limit of 100 ppb (Mornar et al., 2013; Nigovic et al., 2013). However, many commercial RYR products do not meet the criteria, and the highest citrinin concentration detected by HPLC was 140 mg/kg (Ji et al., 2015). More in-depth studies are necessary to arrive at more accurate conclusions and to extrapolate data for risk assessment with respect to the prevalence of citrinin in our food chain and its potential impact on human health.
According to the currently available results of in vitro and in vivo studies, RYR does not seem to be no mutagenic or toxic. In an albino rat model, the administration of acute doses of RYR at 0.5–5.0 g/kg body weight did not cause toxicity or mortality. Similarly, the administration of RYR at a level of 2.0–12.0% (w/w) for 14 weeks did not produce any significant changes in the food intake or body weight of the treated rats compared to control rats (Kumari et al., 2009). In an Ames test, the equivalent of up to 1 mg of ethanol extract from RYR per plate exhibited no genotoxicity toward Salmonella typhimurium strains TA 98, TA 100, and TA 102. Moreover, it has been reported that high levels of RYR exhibited no toxicity, following subchronic administration at levels up to 1,000 mg/kg of body weight for 28 and 90 consecutive days in the rats (Yu et al., 2008).
However, RYR does have adverse effects because MK and citrinin associate with an increased risk of myopathy (Lapi et al., 2008; Polsani et al., 2008; Dobremez et al., 2018), symptomatic hepatitis (Roselle et al., 2008), peripheral neuropathy (Kumari et al., 2013), erectile dysfunction (Liu and Chen, 2018), and anaphylactic reactions (Hipler et al., 2002). Among these, the prevalence rates of myopathy and hepatitis are the highest. Using Italy’s WHO-UMC system, CIOMS/RUCAM score, and WHO-Vigibase, 52 out of 1,261 studies reported 55 adverse reactions to RYR dietary supplements from April 2002 to September 2015, and myopathy and hepatitis accounted for 52.73% of the total adverse reactions (Mazzanti et al., 2017). Although RYR supplementation appears promising for patients with hypercholesterolemia or hyperlipidemia or those at high risk for cardiovascular events, there is insufficient regulation of RYR-containing supplements in China, the United States, and European countries. Until there is a standardized system in place for product formulation and manufacturing, and the amount of MK in a given RYR supplement is clearly stated, the effectiveness and safety of these products will remain in question (Dujovne, 2017; Peng et al., 2017). Thus, real-world vigilance should be used, and it should include consumers, clinicians, and policymakers to promote the proper use of RYR. Furthermore, randomized controlled trials should be carried out to test the safety and efficacy of each RYR preparation.
The safety profile of RYR supplements is highly similar to that of statins (Mazzanti et al., 2017). Some statins, including lovastatin (MK in RYR), are metabolized by the cytochrome P450 enzyme CYP3A4 and it is known that coadministration of drugs that inhibit CYP3A4 can increase the plasma half-lives of these statins and the risk of myotoxicity (Mastaglia and Needham, 2012). CYP3A4 inhibitors reported to interact with statins and to cause rhabdomyolysis include clarithromycin (Grunden and Fisher, 1997), verapamil (Choi et al., 2010), cyclosporine (Olbricht et al., 1997), diltiazem (Azie et al., 1998), itraconazole (Neuvonen and Jalava, 1996), and azithromycin (Grunden and Fisher, 1997). Statin-induced rhabdomyolysis may result from increased exposure to the drug, plausible mechanisms for the interaction include increased absorption of lovastatin by competition at gut P-glycoprotein or decreased excretion of statin due to competition for renal tubular secretion or interaction at hepatic CYP3A4 sites (DiGregorio and Pasikhova, 2009). Co-administration of a statin and a fibrate drug also increases the rhabdomyolysis risk, and gemfibrozil in particular should be avoided in patients receiving lovastatin (Franssen et al., 2009). Therefore, the healthcare providers need to heighten their awareness and screening for potential drug-drug interaction in patients consuming RYR products, and consider early monitoring of liver function and signs of muscle injury. In parallel, consumers should be informed that MK contained in RYR is identical to lovastatin, and need to be discouraged from using RYR preparations as self-medication, particularly if they have experienced previous adverse reactions to statins.
RYR is widely used in prescriptions, as well as an alternative medicine and a food supplement, in Asia, the United States, and European countries. This review summarized the current information on the traditional uses, chemistry, pharmacology, pharmacokinetics, quality control, and toxicity of RYR. In classical Chinese herbal textbooks and the Chinese Pharmacopoeia, RYR is commonly used for resolving turbidity, invigorating blood circulation, and resolving blood stasis. Pharmacological studies have revealed that RYR possesses many biological properties with hypolipidemic, anti-atherosclerotic, anti-cancer, neurocytoprotective, hepatoprotective, anti-osteoporotic, anti-fatigue, anti-diabetic, anti-obesity, immunomodulatory, anti-inflammatory, anti-hypertensive, and anti-microbial activities. The results of these pharmacological investigations support the traditional use of RYR. Furthermore, more than 101 compounds have been isolated from RYR. Among these, monacolins and pigments are the most abundant and the major bioactive constituents in RYR.
Outstanding progress has been made in the chemistry and pharmacology of RYR. However, several gaps in knowledge will require additional studies. Firstly, the systematic data on the toxicology and drug interactions of RYR extracts are limited, and there are only a few studies documenting the toxic effects of RYR. Presently, the evidence is insufficient for RYR to be interpreted as toxic. Thus, a comprehensive toxicological study of both the bioactive extracts and isolated constituents from RYR is urgently needed. Furthermore, due to its potential in vitro and in vivo toxic effects, the clinical application of RYR should be restricted until more definitive studies demonstrate its safety, quality, and efficacy. Secondly, although monacolins in RYR have been found to possess pharmacological activities that are similar to those in pigments, such as hypolipidemic activity, the mechanisms underlying the absorption, distribution, metabolism, and excretion, as well as the synergistic or antagonistic effects between the two constituents, are unknown; thus, they should be further studied. Moreover, other bioactive constituents of RYR, such as organic acids, sterols, decalin derivatives, and flavonoids, should also be examined for their potential use in the development of drugs and as a food supplement. Thirdly, well-designed pharmacodynamic and pharmacological studies should be designed, and comprehensive investigations should be conducted to identify the relevant compounds responsible for the pharmacological effects and the potential mechanisms. Fourthly, although the pharmacological properties support the traditional uses of RYR in the treatment of blood circulation stasis, bone defects, and limb weakness, additional pharmacological studies are needed to address its use in the remediation of indigestion and diarrhea. Finally, the possible interactions, safety profile, synergistic or antagonistic effects, and underlying mechanisms between the bioactive compounds present in RYR and other combined used products remain unknown and should be further studied.
In conclusion, future studies should focus on the absorption, distribution, metabolism, and excretion of monacolins and their interactions with pigments, which would promote our understanding of the underlying mechanisms of the various biological activities of RYR. Further research should also consider the pharmacological activities that were overlooked with regard to the traditional uses of RYR, especially in the treatment of gastrointestinal diseases, such as indigestion and diarrhea. Finally, more efforts are needed in order to gain new insights into the toxicological effects of the main active compounds and the quality control of RYR based on its diverse chemical constituents.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
BZ, JH, and LQ conceived the review. BZ, GY, and FQ wrote the manuscript. JW collected the literatures. QZ edited the manuscript. All the authors have seen and agreed on the finally submitted version of the manuscript.
This work was financed by the National Natural Science Foundation of China (81673528 and 81872953).
Author GY was employed by company Hangzhou Twin-horse Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
RYR, red yeast rice; TCM, Traditional Chinese Medicine; MK, monacolin K; EBV-EA, Epstein-Barr virus early antigen; TG, triglyceride; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TNF-α, tumor necrosis factor-α; IL, interleukin; LPL, lipoprotein lipase; LDLR, low density lipoprotein receptor; PPARγ, peroxisome-proliferator-activated receptor-gamma; AMPK, AMP-activated protein kinase; XZK, Xuezhikang; Hs-CRP, high sensitivity C-reaction protein; HASMCs, human aortic smooth muscle cells; MMP, metalloproteinase; NF-κB, nuclear factor-κB; ROS, reactive oxygen species; PSA, prostate-specific antigen; Aβ, amyloid; APP, amyloid precursor protein; 6-OHDA, 6-hydroxydopamine; NAFLD, non-alcoholic fatty liver disease; Bmp, bone morphogenetic protein; ALP, alkaline phosphatase; Ach, acetylcholine; NO, nitric oxide; CH4, methane; BCS, Biopharmaceutics Classification System; Cmax, maximum plasma concentration; Tmax, time to reach the peak concentration; HPLC, high-performance liquid chromatography; FDA, Food and Drug Administration; EFSA, European Food Safety Authority; UHPLC, ultra-high-performance liquid chromatographic; DAD, diode array detection; MS, mass spectrometry; FLD, fluorescence detection; SSF, solid-state fermentation; AU, absorbance units; gdfs, g of dry fermented substrate; MFCI, microsphere-based flow cytometric immunoassays; ACC, acetyl-CoA carboxylase.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01449/full#supplementary-material
Figure S1 | The fermentation (A) and commercial product (B) of RYR; Morphological characteristics of M. purpureus on Potato Dextrose Agar medium, front (C) and back (D); Microstructure of M. purpureus under the optical (E) and scanning electron microscopes (F), the arrows denote stromata of M. purpureus.
Figure S2 | The general geographical distribution of RYR.
Tablel S1 | Examples of traditional Chinese medicine prescriptions containing red yeast rice.
Akihisa, T., Mafune, S., Ukiya, M., Kimura, Y., Yasukawa, K., Suzuki, T., et al. (2004). (+)- and (-)-syn-2-isobutyl-4-methylazetidine-2,4-dicarboxylic acids from the extract of Monascus pilosus-fermented rice (red-mold rice). J. Nat. Prod. 67 (3), 479–480. doi: 10.1021/np030394i
Akihisa, T., Tokuda, H., Yasukawa, K., Ukiya, M., Kiyota, A., Sakamoto, N., et al. (2005). Azaphilones, furanoisophthalides, and amino acids from the extracts of Monascus pilosus-fermented rice (red-mold rice) and their chemopreventive effects. J. Agric. Food Chem. 53 (3), 562–565. doi: 10.1021/jf040199p
Avula, B., Cohen, P. A., Wang, Y. H., Sagi, S., Feng, W., Wang, M., et al. (2014). Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw materials and dietary supplements using liquid chromatography-accurate QToF mass spectrometry: Chemometrics application. J. Pharm. Biomed. Anal. 100, 243–253. doi: 10.1016/j.jpba.2014.07.039
Azie, N. E., Brater, D. C., Becker, P. A., Jones, D. R., Hall, S. D. (1998). The interaction of diltiazem with lovastatin and pravastatin. Clin. Pharmacol. Ther. 64 (4), 369–377. doi: 10.1016/s0009-9236(98)90067-4
Babitha, S., Soccol, C. R., Pandey, A. (2007). Solid-state fermentation for the production of Monascus pigments from jackfruit seed. Bioresour. Technol. 98 (8), 1554–1560. doi: 10.1016/j.biortech.2006.06.005
Becker, D. J., Gordon, R. Y., Halbert, S. C., French, B., Morris, P. B., Rader, D. J. (2009). Red yeast rice for dyslipidemia in statin-intolerant patients a randomized trial. Ann. Intern. Med. 150 (12), 830–839. doi: 10.7326/0003-4819-150-12-200906160-00006
Bezeria da Rocha, M. E.Oliveira Freire, F.d.C. Feitosa Maia, F. B.Florindo Guedes, M. I.Rondina, D. (2014). Mycotoxins and their effects on human and animal health. Food Control 36 (1), 159–165. doi: 10.1016/j.foodcont.2013.08.021
Bogsrud, M. P., Ose, L., Langslet, G., Ottestad, I., Strom, E. C., Hagve, T.-A., et al. (2010). HypoCol (red yeast rice) lowers plasma cholesterol - a randomized placebo controlled study. Scand. Cardiovasc. J. 44 (4), 197–200. doi: 10.3109/14017431003624123
Bruno, A., Pandolfo, G., Crucitti, M., Troilia, G. M., Spina, E., Zoccali, R. A., et al. (2018). Red Yeast Rice (RYR) supplementation in patients treated with second generation antipsychotics. Complement. Ther. Med. 37, 167–171. doi: 10.1016/j.ctim.2018.03.007
Burke, F. M. (2015). Red yeast rice for the treatment of dyslipidemia. Curr. Atheroscler. Rep. 17 (4), 22. doi: 10.1007/s11883-015-0495-8
Campoy, S., Rumbero, A., Martin, J. F., Liras, P. (2006). Characterization of an hyperpigmenting mutant of Monascus purpureus IB1: identification of two novel pigment chemical structures. Appl. Microbiol. Biotechnol. 70 (4), 488–496. doi: 10.1007/s00253-005-0090-y
Chang, J. C., Wu, M. C., Liu, I. M., Cheng, J. T. (2006). Plasma glucose-lowering action of Hon-Chi in streptozotocin-induced diabetic rats. Horm. Metab. Res. 38 (2), 76–81. doi: 10.1055/s-2006-925116
Chen, C. C., Liu, I. M. (2006). Release of acetylcholine by Hon-Chi to raise insulin secretion in Wistar rats. Neurosci. Lett. 404 (1-2), 117–121. doi: 10.1016/j.neulet.2006.05.024
Chen, C., Mireles, R. J., Campbell, S. D., Lin, J., Mills, J. B., Xu, J. J., et al. (2005). Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab. Dispos. 33 (4), 537–546. doi: 10.1124/dmd.104.002477
Chen, W. P., Ho, B. Y., Lee, C. L., Lee, C. H., Pan, T. M. (2008). Red mold rice prevents the development of obesity, dyslipidemia and hyperinsulinemia induced by high-fat diet. Int. J. Obes. 32 (11), 1694–1704. doi: 10.1038/ijo.2008.156
Chen, C. H., Uang, Y. S., Wang, S. T., Yang, J. C., Lin, C. J. (2012a). Interaction between Red Yeast Rice and CYP450 Enzymes/P-Glycoprotein and its implication for the clinical pharmacokinetics of lovastatin. Evid. Based Complement. Alternat. Med. 2012, 127043. doi: 10.1155/2012/127043
Chen, S., Lv, B., Du, X. Z., Chen, F. S. (2012b). Pigment from red fermented rice as colouring agent for stirred skimmed milk yoghurts. Int. J. Dairy Technol. 65 (2), 287–292. doi: 10.1111/j.1471-0307.2012.00831.x
Chen, C. H., Yang, J. C., Uang, Y. S., Lin, C. J. (2013). Improved dissolution rate and oral bioavailability of lovastatin in red yeast rice products. Int. J. Pharm. 444 (1-2), 18–24. doi: 10.1016/j.ijpharm.2013.01.028
Chen, Y. Z. (2004). The structure of bioactive components of Red Kojic and its health-care function evaluation–expression of LPL mRNA and LDL-receptor mRNA, lipid metabolism adjusting and anti-fatigue activity. Doctor, Huazhong Agricultural University.
Cheng, C. F., Pan, T. M. (2011). Protective effect of Monascus-fermented Red Mold Rice against alcoholic liver disease by attenuating oxidative stress and inflammatory response. J. Agric. Food. Chem. 59 (18), 9950–9957. doi: 10.1021/jf202577t
Cheng, M. J., Chen, J. J., Wu, M. D., Yang, P. S., Yuan, G. F. (2010a). Isolation and structure determination of one new metabolite isolated from the red fermented rice of Monascus purpureus. Nat. Prod. Res. 24 (10), 979–988. doi: 10.1080/14786410903368290
Cheng, M. J., Wu, M. D., Chen, I. S., Chen, C. Y., Lo, W. L., Yuan, G. F. (2010b). Secondary metabolites from the red mould rice of Monascus purpureus BCRC 38113. Nat. Prod. Res. 24 (18), 1719–1725. doi: 10.1080/14786410902941477
Cheng, M. J., Wu, M. D., Chen, Y. L., Chen, I. S., Su, Y. S., Yuan, G. F. (2013). Chemical constituents of red yeast rice fermented with the fungus Monascus pilosus. Chem. Nat. Compd. 49 (2), 249–252. doi: 10.1007/s10600-013-0573-5
Childress, L., Gay, A., Zargar, A., Ito, M. K. (2013). Review of red yeast rice content and current Food and Drug Administration oversight. J. Clin. Lipidol. 7 (2), 117–122. doi: 10.1016/j.jacl.2012.09.003
Chinese Pharmacopoeia. (2015). Editorial Committee of Chinese Pharmacopoeia, 2015 (Bei jing: China Medical Science and Technology Press), 860–861.
Cho, Y. E., Alcantara, E., Kumaran, S., Son, K. H., Sohn, H. Y., Lee, J. H., et al. (2010). Red yeast rice stimulates osteoblast proliferation and increases alkaline phosphatase activity in MC3T3-E1 cells. Nutr. Res. 30 (7), 501–510. doi: 10.1016/j.nutres.2010.06.011
Choi, D. H., Chung, J. H., Choi, J. S. (2010). Pharmacokinetic interaction between oral lovastatin and verapamil in healthy subjects: role of P-glycoprotein inhibition by lovastatin. Eur. J. Clin. Pharmacol. 66 (3), 285–290. doi: 10.1007/s00228-009-0757-x
Cicero, A. F. G., Derosa, G., Parini, A., Maffioli, P., D’Addato, S., Reggi, A., et al. (2013). Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr. Res. 33 (8), 622–628. doi: 10.1016/j.nutres.2013.05.015
Cicero, A. F. G., Morbini, M., Rosticci, M., D’Addato, S., Grandi, E., Borghi, C. (2016). Middle-term dietary supplementation with Red Yeast Rice plus Coenzyme Q10 improves lipid pattern, endothelial reactivity and arterial stiffness in moderately hypercholesterolemic subjects. Ann. Nutr. Metab. 68 (3), 213–219. doi: 10.1159/000445359
Cohen, P. A., Avula, B., Khan, I. A. (2017). Variability in strength of red yeast rice supplements purchased from mainstream retailers. Eur. J. Prev. Cardiol. 24 (13), 1431–1434. doi: 10.1177/2047487317715714
Derosa, G., Catena, G., Raddino, R., Gaudio, G., Maggi, A., D’Angelo, A., et al. (2018). Effects on oral fat load of a nutraceutical combination of fermented red rice, sterol esters and stanols, curcumin, and olive polyphenols: a randomized, placebo controlled trial. Phytomedicine 42, 75–82. doi: 10.1016/j.phymed.2018.01.014
Dhale, M. A., Divakar, S., Kumar, S. U., Vijayalakshmi, G. (2007a). Isolation and characterization of dihydromonacolin-MV from Monascus purpureus for antioxidant properties. Appl. Microbiol. Biotechnol. 73 (5), 1197–1202. doi: 10.1007/s00253-006-0578-0
Dhale, M. A., Divakar, S., Umesh-Kumar, S., Vijayalakshmi, G. (2007b). Characterization of dehydromonacolin-MV2 from Monascus purpureus mutant. J. Appl. Microbiol. 103 (6), 2168–2173. doi: 10.1111/j.1365-2672.2007.03457.x
DiGregorio, R. V., Pasikhova, Y. (2009). Rhabdomyolysis caused by a potential sitagliptin-lovastatin interaction. Pharmacotherapy 29 (3), 352–356. doi: 10.1592/phco.29.3.352
Ding, M., Si, D. Y., Zhang, W. Q., Feng, Z. H., He, M., Yang, P. (2014). Red yeast rice repairs kidney damage and reduces inflammatory transcription factors in rat models of hyperlipidemia. Exp. Ther. Med. 8 (6), 1737–1744. doi: 10.3892/etm.2014.2035
Ding, H. M. (2007). Preliminary study on antineoplastic effect of polysaccharide from red yeast rice. J. Fungal Res. 5 (3), 171–173.
Dobremez, V., Serra, A., Grosset-Janin, D., Dopter, A., Pineau-Blondel, E., Ruel, J. H. (2018). Myasthenia gravis exacerbation after red yeast rice use. Rev. Neurol. 174 (7-8), 577–578. doi: 10.1016/j.neurol.2017.08.006
Dujovne, C. A. (2017). Red Yeast Rice preparations: Are they suitable substitutions for statins? Am. J. Med. 130 (10), 1148–1150. doi: 10.1016/j.amjmed.2017.05.013
EFSA. (2011). Scientific opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL cholesterol concentrations. EFSA J. 13 (1), 1–16. doi: 10.2903/j.efsa.2011.2304
Elumalai, P., Park, Y. J., Cho, M., Shea, P. J., Oh, B. T. (2019). Red yeast rice fermentation with Bacillus subtilis B2 under blue light-emitting diodes increases antioxidant secondary products. Bioprocess Biosyst. Eng. 42 (4), 529–539. doi: 10.1007/s00449-018-2056-3
Eren, T., Atar, N., Yola, M. L., Karimi Maleh, H. (2015). A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 185, 430–436. doi: 10.1016/j.foodchem.2015.03.153
FDA, U.S. (2007). "Warns Consumers to Avoid Red Yeast Rice Products Promoted on Internet as Treatments for High Cholesterol".).
Ferse, I., Langlitz, M., Kleigrewe, K., Rzeppa, S., Lenczyk, M., Harrer, H., et al. (2012). Isolation and structure elucidation of two new cytotoxic metabolites from red yeast rice. Nat. Prod. Res. 26 (20), 1914–1921. doi: 10.1080/14786419.2011.639074
Feuerstein, J. S., Bjerke, W. S. (2012). Powdered red yeast rice and plant stanols and sterols to lower cholesterol. J. Diet. 9 (2), 110–115. doi: 10.3109/19390211.2012.682645
Franssen, R., Vergeer, M., Stroes, E. S. G., Kastelein, J. J. P. (2009). Combination statin-fibrate therapy: safety aspects. Diabetes Obes. Metab. 11 (2), 89–94. doi: 10.1111/j.1463-1326.2008.00917.x
Fung, W. T., Subramaniam, G., Lee, J., Loh, H. M., Leung, P. H. H. (2012). Assessment of extracts from red yeast rice for herb-drug interaction by in-vitro and in-vivo assays. Sci. Rep. 2, 298. doi: 10.1038/srep00298
Ge, F., Wang, Y., Wang, J. P., Yu, H., Li, Z. H. (2012). Research on main bioactive components in red fermented rice. J. Kunming Univ. Sci. Technol. 37 (2), 61–64.
Gerards, M. C., Terlou, R. J., Yu, H., Koks, C. H. W., Gerdes, V. E. A. (2015). Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain - A systematic review and meta-analysis. Atherosclerosis 240 (2), 415–423. doi: 10.1016/j.atherosclerosis.2015.04.004
Grunden, J. W., Fisher, K. A. (1997). Lovastatin-induced rhabdomyolysis possibly associated with clarithromycin and azithromycin. Ann. Pharmacother. 31 (7-8), 859–863. doi: 10.1177/106002809703100710
Guardamagna, O., Abello, F., Baracco, V., Stasiowska, B., Martino, F. (2009). The treatment of hypercholesterolemic children: efficacy and safety of a combination of red yeast rice extract and policosanols. Nutr. Metab. Cardiovasc. Dis. 21 (6), 424–429. doi: 10.1016/j.numecd.2009.10.015
Gutierrez, G. E., Mundy, B., Rossini, G., Garrett, I. R., Chen, S. T., Mundy, G. R. (2006). Red yeast rice stimulates bone formation in rats. Nutr. Res. 26 (2006), 124–129. doi: 10.1016/j.nutres.2006.02.006
Heber, D., Yip, I., Ashley, J. M., Elashoff, D. A., Elashoff, R. M., Go, V. L. (1999). Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 69 (2), 231–236. doi: 10.1093/ajcn/69.2.231
Heber, D., Lembertas, A., Lu, Q. Y., Bowerman, S., Go, V. L. (2001). An analysis of nine proprietary Chinese red yeast rice dietary supplements: implications of variability in chemical profile and contents. J. Altern. Complement. Med. 7 (2), 133–139. doi: 10.1089/107555301750164181
Hipler, U. C., Wigger Alberti, W., Bauer, A., Elsner, P. (2002). Case report. Monascus purpureus–A new fungus of allergologic relevance. Mycoses 45 (1-2), 58–60. doi: 10.1046/j.1439-0507.2002.d01-119.x
Holter, J. B., Young, A. J. (1992). Methane prediction in dry and lactating Holstein cows. J. Dairy Sci. 75 (8), 2165–2175. doi: 10.3168/jds.S0022-0302(92)77976-4
Hong, X. Z., Li, L. D., Wu, L. M. (2007). Effects of fenofibrate and xuezhikang on high-fat diet-induced non-alcoholic fatty liver disease. Clin. Exp. Pharmacol. Physiol. 34 (1-2), 27–35. doi: 10.1111/j.1440-1681.2007.04547.x
Hong, M. Y., Seeram, N. P., Zhang, Y., Heber, D. (2008a). Chinese Red Yeast Rice versus lovastatin effects on prostate cancer cells with and without androgen receptor overexpression. J. Med. Food 11 (4), 657–666. doi: 10.1089/jmf.2007.0702
Hong, M. Y., Seerarn, N. P., Zhang, Y., Heber, D. (2008b). Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J. Nutr. Biochem. 19 (7), 448–458. doi: 10.1016/j.jnutbio.2007.05.012
Hong, M. Y., Henning, S., Moro, A., Seeram, N. P., Zhang, Y., Heber, D. (2011). Chinese Red Yeast Rice inhibition of prostate tumor growth in SCID mice. Cancer Prev. Res. 4 (4), 608–615. doi: 10.1158/1940-6207.Capr-10-0219
Hong, H., Park, J., Lumbera, W. L., Hwang, S. G. (2017). Monascus ruber-fermented buckwheat (Red Yeast Buckwheat) suppresses adipogenesis in 3T3-L1 cells. J. Med. Food 20 (4), 352–359. doi: 10.1089/jmf.2016.3761
Hossain, C. F., Okuyama, E., Yamazaki, M. (1996). A new series of coumarin derivatives having monoamine oxidase inhibitory activity from Monascus anka. Chem. Pharm. Bull. 44 (8), 1535–1539. doi: 10.1248/cpb.44.1535
Hristov, A. N., Oh, J., Firkins, J. L., Dijkstra, J., Kebreab, E., Waghorn, G., et al. (2013). SPECIAL TOPICS-Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 91 (11), 5045–5069. doi: 10.2527/jas.2013-6583
Hsu, Y. W., Hsu, L. C., Chang, C. L., Liang, Y. H., Kuo, Y. H., Pan, T. M. (2010). New anti-inflammatory and anti-proliferative constituents from fermented Red Mold Rice Monascus purpureus NTU 568. Molecules 15 (11), 7815–7824. doi: 10.3390/molecules15117815
Huang, H. N., Hua, Y. Y., Bao, G. R., Xie, L. H. (2006). The quantification of monacolin K in some red yeast rice from Fujian province and the comparison of the other product. Chem. Pharm. Bull. 54 (5), 687–689. doi: 10.1248/cpb.54.687
Huang, C. F., Li, T. C., Lin, C. C., Liu, C. S., Shih, H. C., Lai, M. M. (2007). Efficacy of Monascus purpureus Went rice on lowering lipid ratios in hypercholesterolemic patients. Eur. J. Cardiovasc. Prev. Rehabil. 14 (3), 438–440. doi: 10.1097/HJR.0b013e32801da137
Huang, Z. B., Xu, Y., Li, L. S., Li, Y. P. (2008). Two new Monascus metabolites with strong blue fluorescence isolated from red yeast rice. J. Agric. Food Chem. 56 (1), 112–118. doi: 10.1021/jf072985a
Huang, Z. B., Xu, Y., Zhang, H., Li, L. S., He, Q. H., Li, Y. P. (2011). Simultaneous determination of two Monascus metabolites in red yeast rice by HPLC using fluorescence detection. Food Chem. 127 (4), 1837–1841. doi: 10.1016/j.foodchem.2011.01.004
Huang, Z. B., Zhang, S. Y., Xu, Y., Li, L. S., Li, Y. P. (2016). Development of an HPLC-UV detection method for the simultaneous determination of two Monascus orange pigments in Red Yeast Rice. Food Anal. Methods 9 (1), 148–155. doi: 10.1007/s12161-015-0185-8
Il Gum, S., Nguyen, P. A., Lee, J. R., Han, Y. H., Cho, M. K. (2017). The physico-chemical alteration of lovastatin and enhanced antioxidant effect of Bacillus subtilis fermented-red yeast rice product. Food Chem. 232, 203–209. doi: 10.1016/j.foodchem.2017.04.023
Ji, X. F., Xu, J. F., Wang, X. F., Qi, P. P., Wei, W., Chen, X. Y., et al. (2015). Citrinin Determination in Red Fermented Rice Products by Optimized Extraction Method Coupled to Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS). J. Food Sci. 80 (6), T1438–T1444. doi: 10.1111/1750-3841.12900
Ji, Y. B., Xu, F., Liu, B. Y., Wei, Q., Guo, Y. Z., Dong, Y., et al. (2018). Trace phenolic compounds from Red Yeast Rice. China J. Chin. Mater. Med. 43 (4), 755–759. doi: 10.19540/j.cnki.cjcmm.20180104.014
Jiang, W. (2016). Preliminary study on the fermentation of Monacus polysaccharide and its properties of Monacus polysaccharide (Master, Anhui Polytechnic University, Wuhu).
Jimenez Lopez, J., Llorent Martinez, E. J., Ortega Barrales, P., Ruiz Medina, A. (2014). Multi-commutated fluorometric optosensor for the determination of citrinin in rice and red yeast rice supplements. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 31 (10), 1744–1750. doi: 10.1080/19440049.2014.949874
Jin, Y., Cheng, X., Jiang, F., Guo, Z., Xie, J., Fu, L. (2016). Application of the ultrafiltration-based LC-MS approach for screening PTP1B inhibitors from Chinese red yeast rice. Anal. Methods 8 (2), 353–361. doi: 10.1039/c5ay01767j
Kalaivani, M., Sabitha, R., Kalaiselvan, V., Rajasekaran, A. (2010). Health benefits and clinical impact of major nutrient, red yeast rice: A review. Food Bioprocess Tech. 3 (3), 333–339. doi: 10.1007/s11947-009-0197-8
Kantifedaki, A., Kachrimanidou, V., Mallouchos, A., Papanikolaou, S., Koutinas, A. A. (2018). Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Clean Prod. 185, 882–890. doi: 10.1016/j.jclepro.2018.03.032
Kiebooms, J. A. L., Huybrechts, B., Thiry, C., Tangni, E. K., Callebaut, A. (2016). A quantitative UHPLC-MS/MS method for citrinin and ochratoxin A detection in food, feed and red yeast rice food supplements. World Mycotoxin J. 9 (3), 343–352. doi: 10.3920/wmj2015.1971
Kiem, P. V., Minh, C. V., Huong, H. T., Lee, J. J., Kim, Y. H. (2005). Caesaldecan, a cassane diterpenoid from the leaves of Caesalpinia decapetala. Chem. Pharm. Bull. 53 (4), 428–430. doi: 10.1248/cpb.53.428
Knecht, A., Cramer, B., Humpf, H.-U. (2006). New Monascus metabolites: structure elucidation and toxicological properties studied with immortalized human kidney epithelial cells. Mol. Nutr. Food Res. 50 (3), 314–321. doi: 10.1002/mnfr.200500229
Kumari, H. P. M., Naidu, K. A., Vishwanatha, S., Narasimhamurthy, K., Vijayalakshmi, G. (2009). Safety evaluation of Monascus purpureus red mould rice in albino rats. Food Chem. Toxicol. 47 (8), 1739–1746. doi: 10.1016/j.fct.2009.04.038
Kumari, S., Sherriff, J. M., Spooner, D., Beckett, R. (2013). Peripheral neuropathy induced by red yeast rice in a patient with a known small bowel gastrointestinal tumour. BMJ Case Rep. 2013, bcr2013009060. doi: 10.1136/bcr-2013-009060
Lam, D. W., LeRoith, D. (2012). The worldwide diabetes epidemic. Curr. Opin. Endocrinol. Diabetes Obes. 19 (2), 93–96. doi: 10.1097/MED.0b013e328350583a
Lapi, F., Gallo, E., Bernasconi, S., Vietri, M., Menniti-Ippolito, F., Raschetti, R., et al. (2008). Myopathies associated with red yeast rice and liquorice: spontaneous reports from the Italian surveillance system of natural health products. Br. J. Clin. Pharmacol. 66 (4), 572–574. doi: 10.1111/j.1365-2125.2008.03224.x
Lee, C. L., Chen, W. P., Wang, J. J., Pan, T. M. (2007a). A simple and rapid approach for removing citrinin while retaining monacolin K in red mold rice. J. Agric. Food Chem. 55 (26), 11101–11108. doi: 10.1021/jf071640p
Lee, C. L., Kuo, T. F., Wang, J. J., Pan, T. M. (2007b). Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid beta-infused rat by repressing amyloid beta accumulation. J. Neurosci. Res. 85 (14), 3171–3182. doi: 10.1002/jnr.21428
Lee, B. H., Ho, B. Y., Wang, C. T., Pan, T. M. (2009). Red mold rice promoted antioxidase activity against oxidative injury and improved the memory ability of Zinc-deficient rats. J. Agric. Food. Chem. 57 (22), 10600–10607. doi: 10.1021/jf902046s
Lee, C. L., Kuo, T. F., Wu, C. L., Wang, J. J., Pan, T. M. (2010). Red Mold Rice promotes neuroprotective sAPPalpha secretion instead of Alzheimer’s risk factors and Amyloid beta expression in hyperlipidemic A beta 40-infused rats. J. Agric. Food Chem. 58 (4), 2230–2238. doi: 10.1021/jf904027y
Lee, B. H., Hsu, W. H., Pan, T. M. (2012). Red Mold Rice against hepatic inflammatory damage in Zn-deficient rats. J. Tradit. Complement. Med. 2 (1), 52–60. doi: 10.1016/s2225-4110(16)30071-2
Lee, C. I., Lee, C. L., Hwang, J. F., Lee, Y. H., Wang, J. J. (2013a). Monascus-fermented red mold rice exhibits cytotoxic effect and induces apoptosis on human breast cancer cells. Appl. Microbiol. Biotechnol. 97 (3), 1269–1278. doi: 10.1007/s00253-012-4279-6
Lee, C. Y., Jan, M. S., Yu, M. C., Lin, C. C., Wei, J. C. C., Shih, H. C. (2013b). Relationship between adiponectin and leptin, and blood lipids in hyperlipidemia patients treated with Red Yeast Rice. Forsch. Komplementarmed. 20 (3), 197–203. doi: 10.1159/000351455
Lee, H. S., Lee, Y. J., Chung, Y. H., Nam, Y., Kim, S. T., Park, E. S., et al. (2015). Beneficial effects of Red Yeast Rice on high-fat diet-induced obesity, hyperlipidemia, and fatty liver in mice. J. Med. Food 18 (10), 1095–1102. doi: 10.1089/jmf.2014.3259
Leone, G., Consumi, M., Pepi, S., Lamponi, S., Bonechi, C., Tamasi, G., et al. (2016). New formulations to enhance lovastatin release from red yeast rice (RYR). J. Drug Deliv. Sci. Technol. 36, 110–119. doi: 10.1016/j.jddst.2016.10.001
Li, Y. G., Liu, H., Wang, Z. T. (2005a). A validated stability-indicating HPLC with photodiode array detector (PDA) method for the stress tests of Monascus purpureus-fermented rice, red yeast rice. J. Pharm. Biomed. Anal. 39 (1-2), 82–90. doi: 10.1016/j.jpba.2005.03.005
Li, Z. P., Seeram, N. P., Lee, R., Thames, G., Minutti, C., Wang, H. J., et al. (2005b). Plasma clearance of lovastatin versus chinese red yeast rice in healthy volunteers. J. Altern. Complement. Med. 11 (6), 1031–1038. doi: 10.1089/acm.2005.11.1031
Li, J. J., Shang, X. Y., Li, L. L., Liu, M. T., Zheng, J. Q., Jin, Z. L. (2010). New cytotoxic azaphilones from Monascus purpureus-fermented rice (Red Yeast Rice). Molecules 15 (3), 1958–1966. doi: 10.3390/molecules15031958
Li, P., Yang, Y., Liu, M. (2011). Xuezhikang, extract of Red Yeast Rice, inhibited tissue factor and hypercoagulable state through suppressing nicotinamide adenine dinucleotide phosphate oxidase and extracellular signal-regulated kinase activation. J. Cardiovasc. Pharmacol. 58 (3), 307–318. doi: 10.1097/FJC.0b013e3182244a2d
Li, Y. N., Wu, H. Y., Guo, L. Q., Zheng, Y. Q., Guo, Y. H. (2012). Microsphere-based flow cytometric immunoassay for the determination of citrinin in red yeast rice. Food Chem. 134 (4), 2540–2545. doi: 10.1016/j.foodchem.2012.04.072
Li, L. (2016). Research of breeding and fermentation conditions of high-lovastatin Monascus strains (Master, Fuzhou Universtiy, Fuzhou).
Lin, W. Y., Hsu, W. Y., Hish, C. H., Pan, T. M. (2007). Proteome changes in Caco-2 cells treated with Monascus-fermented red mold rice extract. J. Agric. Food Chem. 55 (22), 8987–8994. doi: 10.1021/jf072197l
Lin, C. P., Chen, Y. H., Chen, J. W., Leu, H. B., Liu, T. Z., Liu, P. L., et al. (2008). Cholestin (Monascus purpureus rice) inhibits homocysteine-induced reactive oxygen species generation, nuclear factor-kappa B activation, and vascular cell adhesion molecule-1 expression in human aortic endothelial cells. J. Biomed. Sci. 15 (2), 183–196. doi: 10.1007/s11373-007-9212-0
Lin, C. P., Huang, P. H., Tsai, H. S., Wu, T. C., Leu, H. B., Liu, P. L., et al. (2011). Monascus purpureus-fermented rice inhibits tumor necrosis factor-alpha-induced upregulation of matrix metalloproteinase 2 and 9 in human aortic smooth muscle cells. J. Pharm. Pharmacol. 63 (12), 1587–1594. doi: 10.1111/j.2042-7158.2011.01364.x
Lin, C. M., Lin, Y. T., Lin, R. D., Huang, W. J., Lee, M. H. (2015). Neurocytoprotective effects of aliphatic hydroxamates from lovastatin, a secondary metabolite from Monascus-fermented Red Mold Rice, in 6-hydroxydopamine (6-OHDA)-treated nerve growth factor (NGF)-differentiated PC12 cells. ACS Chem. Neurosci. 6 (5), 716–724. doi: 10.1021/cn500275k
Lin, F. (2017). Textual research on Gutian as place where red yeast rice originated. Chin. Tradit. Herb. Drug 48 (13), 2793–2800.
Lithander, F. E., Mahmud, A. (2015). The effect of red yeast rice on serum and haemodynamic risk factors for cardiovascular disease (E77-E77: P. Nutr. Soc. 74(OCE1)). doi: 10.1017/s0029665115000920
Liu, Z. G., Chen, P. J. (2018). A case of erectile dysfunction induced by red yeast rice in lipid-lowering therapy. Phytother. Res. 32 (5), 953–954. doi: 10.1002/ptr.6025
Liu, Y., Guo, X., Duan, W., Wang, X., Du, J. (2010). Accelerated solvent extraction of monacolin K from red yeast rice and purification by high-speed counter-current chromatography. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 878 (28), 2881–2885. doi: 10.1016/j.jchromb.2010.08.028
Liu, J. T., Chen, H. Y., Chen, W. C., Man, K. M., Chen, Y. H. (2017). Red Yeast Rice protects circulating bone marrow-derived proangiogenic cells against high-glucose-induced senescence and oxidative stress: The role of heme oxygenase-1. Oxid. Med. Cell. Longev. 2017, 3831750. doi: 10.1155/2017/3831750
Luo, W. Z. (2009). The experimental study on the molecular mechanism of monascus on preventing NAFLD rat models induced by high-fatty diet (Doctor, Southern Medical University, Guangzhou).
Ma, J., Li, Y., Ye, Q., Li, J., Hua, Y., Ju, D., et al. (2000). Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 48 (11), 5220–5225. doi: 10.1021/jf000338c
Ma, K. Y., Zhang, Z. S., Zhao, S. X., Chang, Q., Wong, Y. M., Yeung, S. Y. V., et al. (2009). Red Yeast Rice increases excretion of bile acids in hamsters. Biomed. Environ. Sci. 22 (4), 269–277. doi: 10.1016/s0895-3988(09)60056-8
Maisarah, M. N., Samat, F. D., Kavanagh, P. V., Walsh, J. J. (2012). Nature’s cholesterol-lowering drug: Isolation and structure elucidation of lovastatin from Red Yeast Rice-Containing dietary supplements. J. Chem. Educ. 89 (1), 138–140. doi: 10.1021/ed1004195
Marley, E., Brown, P., Leeman, D., Donnelly, C. (2016). Analysis of citrinin in cereals, red yeast rice dietary supplement, and animal feed by immunoaffinity column cleanup and LC with fluorescence detection. J. AOAC Int. 99 (4), 1025–1031. doi: 10.5740/jaoacint.16-0060
Marques, B. G., Hausman, D. B., Martin, R. J. (1998). Association of fat cell size and paracrine growth factors in development of hyperplastic obesity. Am. J. Physiol. 275 (6), R1898–R1908. doi: 10.1152/ajpregu.1998.275.6.R1898
Mastaglia, F. L., Needham, M. (2012). Update on toxic myopathies. Curr. Neurol. Neurosci. Rep. 12 (1), 54–61. doi: 10.1007/s11910-011-0232-9
Mazzanti, G., Moro, P. A., Raschi, E., Da Cas, R., Menniti Ippolito, F. (2017). Adverse reactions to dietary supplements containing red yeast rice: assessment of cases from the Italian surveillance system. Br. J. Clin. Pharmacol. 83 (4), 894–908. doi: 10.1111/bcp.13171
Millan, J., Cicero, A. F. G., Torres, F., Anguera, A. (2016). Effects of a nutraceutical combination containing berberine (BRB), policosanol, and red yeast rice (RYR), on lipid profile in hypercholesterolemic patients: A meta-analysis of randomised controlled trials. Clin. Investig. Arterioscler. 28 (4), 178–187. doi: 10.1016/j.arteri.2016.03.002
Mornar, A., Sertic, M., Nigovic, B. (2013). Development of a rapid LC/DAD/FLD/MSn method for the simultaneous determination of monacolins and citrinin in red fermented rice products. J. Agric. Food Chem. 61 (5), 1072–1080. doi: 10.1021/jf304881g
Neuvonen, P. J., Jalava, K. M. (1996). Itraconazole drastically increases plasma concentrations of lovastatin and lovastatin acid. Clin. Pharmacol. Ther. 60 (1), 54–61. doi: 10.1016/s0009-9236(96)90167-8
Neuvonen, P. J., Backman, J. T., Niemi, M. (2008). Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin. Clin. Pharmacokinet. 47 (7), 463–474. doi: 10.2165/00003088-200847070-00003
Nigovic, B., Sertic, M., Mornar, A. (2013). Simultaneous determination of lovastatin and citrinin in red yeast rice supplements by micellar electrokinetic capillary chromatography. Food Chem. 138 (1), 531–538. doi: 10.1016/j.foodchem.2012.10.104
Nigovic, B., Zavrski, M., Sertic, M., Mornar, A. (2015). Simple and fast voltammetric method for assaying monacolin K in red yeast rice formulated products. Food Anal. Methods 8 (1), 180–188. doi: 10.1007/s12161-014-9890-y
Odegaard, J. I., Chawla, A. (2013). The immune system as a sensor of the metabolic state. Immunity 38 (4), 644–654. doi: 10.1016/j.immuni.2013.04.001
Olbricht, C., Wanner, C., Eisenhauer, T., Kliem, V., Doll, R., Boddaert, M., et al. (1997). Accumulation of lovastatin, but not pravastatin, in the blood of cyclosporine-treated kidney graft patients after multiple doses. Clin. Pharmacol. Ther. 62 (3), 311–321. doi: 10.1016/s0009-9236(97)90034-5
Panda, B. P., Javed, S., Ali, M. (2010). Optimization of fermentation parameters for higher lovastatin production in Red Mold Rice through co-culture of Monascus purpureus and Monascus ruber. Food Bioproc. Tech. 3 (3), 373–378. doi: 10.1007/s11947-008-0072-z
Patel, S. (2016). Functional food red yeast rice (RYR) for metabolic syndrome amelioration: a review on pros and cons. World J. Microb. Biot. 32 (5), 87. doi: 10.1007/s11274-016-2035-2
Peng, D., Fong, A., van Pelt, A. (2017). Original research: The effects of Red Yeast Rice supplementation on cholesterol levels in adults. Am. J. Nurs. 117 (8), 46–54. doi: 10.1097/01.NAJ.0000521973.38717.2e
Pengnoi, P., Kumla, J., Khanongnuch, C., Lumyong, S. (2018). Evaluation of Red Mold Rice for cholesterol reduction in the serum and yolks of Japanese quail eggs and its effect on growth performance. Chiang Mai J. Sci. 45 (4), 1667–1679.
Polsani, V. R., Jones, P. H., Ballantyne, C. M., Nambi, V. (2008). A case report of myopathy from consumption of red yeast rice. J. Clin. Lipidol. 2 (1), 60–62. doi: 10.1016/j.jacl.2007.12.005
Roselle, H., Ekatan, A., Tzeng, J., Sapienza, M., Kocher, J. (2008). Symptomatic hepatitis associated with the use of herbal red yeast rice. Ann. Intern. Med. 149 (7), 516–517. doi: 10.7326/0003-4819-149-7-200810070-00021
Ruscica, M., Pavanello, C., Gandini, S., Macchi, C., Botta, M., Dall’Orto, D., et al. (2019). Nutraceutical approach for the management of cardiovascular risk - a combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: results from a randomized, double-blind, placebo-controlled study. Nutr. J. 18, 13. doi: 10.1186/s12937-019-0438-2
Serajuddin, A. T., Ranadive, S. A., Mahoney, E. M. (1991). Relative lipophilicities, solubilities, and structure-pharmacological considerations of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors pravastatin, lovastatin, mevastatin, and simvastatin. J. Pharm. Sci. 80 (9), 830–834. doi: 10.1002/jps.2600800905
Shen, L., Sun, Z., Chu, S., Cai, Z., Nie, P., Wu, C., et al. (2017). Xuezhikang, an extract from red yeast rice, attenuates vulnerable plaque progression by suppressing endoplasmic reticulum stress-mediated apoptosis and inflammation. PloS One 12 (11), e0188841. doi: 10.1371/journal.pone.0188841
Song, F. H., El-Demerdash, A., Lee, S.-J.S.H., Smith, R. E. (2012). Fast screening of lovastatin in red yeast rice products by flow injection tandem mass spectrometry. J. Pharm. Biomed. Anal. 57, 76–81. doi: 10.1016/j.jpba.2011.08.039
Spigoni, V., Aldigeri, R., Antonini, M., Micheli, M. M., Fantuzzi, F., Fratter, A., et al. (2017). Effects of a new nutraceutical formulation (Berberine, Red Yeast Rice and Chitosan) on non-HDL cholesterol levels in individuals with dyslipidemia: Results from a randomized, double blind, placebo-controlled study. Int. J. Mol. Sci. 18 (7), 1498. doi: 10.3390/ijms18071498
Su, N. W., Lin, Y. L., Lee, M. H., Ho, C. Y. (2005). Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J. Agric. Food Chem. 53 (6), 1949–1954. doi: 10.1021/jf048310e
Sun, H., Wu, Y. F., Wang, X., Liu, Y., Yao, X. H., Tang, J. W. (2015). Effects of dietary supplementation with red yeast rice on laying performance, egg quality and serum traits of laying hens. Ital. J. Anim. Sci. 14 (3), 4059. doi: 10.4081/ijas.2015.4059
Svoboda, P., Sander, D., Plachka, K., Novakova, L. (2017). Development of matrix effect-free MISPE-UHPLC-MS/MS method for determination of lovastatin in Pu-erh tea, oyster mushroom, and red yeast rice. J. Pharm. Biomed. Anal. 140, 367–376. doi: 10.1016/j.jpba.2017.03.058
Tan, Y. L. (2015). Preliminary study of chemical components and quality control in high barley Monascus (Master, Chengdu Chinese Medical University, Chengdu).
Theunis, M., Naessens, T., Verhoeven, V., Hermans, N., Apers, S. (2017). Development and validation of a robust high-performance liquid chromatographic method for the analysis of monacolins in red yeast rice. Food Chem. 234, 33–37. doi: 10.1016/j.foodchem.2017.04.136
Tian, J., Lin, Z. L., Shen, X. Y., Wei, G. (1998). Chemical structure of Monascan. J. Nanjing Univ. TCM 14 (4), 217–218.
Trimarco, V., Cimmino, C. S., Santoro, M., Pagnano, G., Manzi, M. V., Piglia, A., et al. (2012). Nutraceuticals for blood pressure control in patients with high-normal or grade 1 hypertension. High Blood Press Cardiovasc. Prev. 19 (3), 117–122. doi: 10.2165/11632160-000000000-00000
Verhoeven, V., Van der Auwera, A., Van Gaal, L., Remmen, R., Apers, S., Stalpaert, M., et al. (2015). Can red yeast rice and olive extract improve lipid profile and cardiovascular risk in metabolic syndrome?: a double blind, placebo controlled randomized trial. BMC Complement Altern. Med. 15, 52. doi: 10.1186/s12906-015-0576-9
Wang, J. J., Shieh, M. J., Kuo, S. L., Lee, C. L., Pan, T. M. (2006). Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl. Microbiol. Biotechnol. 70 (2), 247–253. doi: 10.1007/s00253-005-0051-5
Wang, J. J., Wang, H. Y., Shih, C. D. (2010). Autonomic nervous system and nitric oxide in antihypertensive and cardiac inhibitory effects induced by Red Mold Rice in spontaneously hypertensive rats. J. Agric. Food Chem. 58 (13), 7940–7948. doi: 10.1021/jf100339p
Wang, J., Jiang, W., Zhong, Y., Lu, B., Shao, J., Jiang, S., et al. (2014). Xuezhikang attenuated the functional and morphological impairment of pancreatic islets in diabetic mice via the inhibition of oxidative stress. J. Cardiovasc. Pharmacol. 63 (3), 282–289. doi: 10.1097/fjc.0000000000000047
Wang, Y. F., Liu, W. T., Chen, C. Y., Ke, H. P., Jiang, H. L., Chen, X. L., et al. (2015). Anti-osteoporosis activity of red yeast rice extract on ovariectomy-induced bone loss in rats. Genet. Mol. Res. 14 (3), 8137–8146. doi: 10.4238/2015.July.27.2
Wang, L. Z., Zhou, M. L., Wang, J. W., Wu, D., Yan, T. (2016). The effect of dietary replacement of ordinary rice with Red Yeast Rice on nutrient utilization, enteric methane emission and rumen archaeal diversity in goats. PloS One 11 (7), e0160198. doi: 10.1371/journal.pone.0160198
Wang, J. P. (2010). Research on main active components in Red Fermented Rice from Yunnan province (Master, Kunming University of Science and Technology, Kumming).
Wei, W., Li, C., Wang, Y., Su, H., Zhu, J., Kritchevsky, D. (2003). Hypolipidemic and anti-atherogenic effects of long-term Cholestin (Monascus purpureus-fermented rice, red yeast rice) in cholesterol fed rabbits. J. Nutr. Biochem. 14 (6), 314–318. doi: 10.1016/s0955-2863(03)00051-2
Wei, W. D., Lin, S., Chen, M. H., Liu, T. X., Wang, A., Li, J. J., et al. (2017). Monascustin, an unusual gamma-lactam from Red Yeast Rice. J. Nat. Prod. 80 (1), 201–204. doi: 10.1021/acs.jnatprod.6b00493
Wei, J. C. (1979). Manual of Determinative Mycology (Shanghai: Shanghai Science and Technology Press).
Weis, M. (2018). Impact of the gut microbiome in cardiovascular and autoimmune diseases. Clin. Sci. 132 (22), 2387–2389. doi: 10.1042/cs20180410
Wild, D., Gareis, M., Humpf, H. U. (2002a). Analyses of red fermented rice (angkak) and report of a new Monascus metabolite. Mycotoxin Res. 18 (Suppl 2), 212–216. doi: 10.1007/bf02946098
Wild, D., Toth, G., Humpf, H.-U. (2002b). New monascus metabolite isolated from red yeast rice (angkak, red koji). J. Agric. Food. Chem. 50 (14), 3999–4002. doi: 10.1021/jf020023s
Wild, D., Toth, G., Humpf, H.-U. (2003). New monascus metabolites with a pyridine structure in red fermented rice. J. Agric. Food Chem. 51 (18), 5493–5496. doi: 10.1021/jf030213i
Williams, B. (2009). The year in hypertension. J. Am. Coll. Cardiol. 55 (1), 65–73. doi: 10.1016/j.jacc.2009.08.037
Wong, R. W. K., Rabie, B. (2008). Chinese red yeast rice (Monascus purpureus-fermented rice) promotes bone formation. Chin. Med. 3, 4. doi: 10.1186/1749-8546-3-4
Wu, C. Y., Benet, L. Z. (2005). Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharma. Res. 22 (1), 11–23. doi: 10.1007/s11095-004-9004-4
Wu, C., Wang, Z., Zhi, Z., Jiang, T., Zhang, J., Wang, S. (2011). Development of biodegradable porous starch foam for improving oral delivery of poorly water soluble drugs. Int. J. Pharm. 403 (1-2), 162–169. doi: 10.1016/j.ijpharm.2010.09.040
Wu, M. D., Cheng, M. J., Yech, Y. J., Chen, Y. L., Chen, K. P., Yang, P. H., et al. (2013). Monascusazaphilones A-C, three new azaphilone analogues isolated from the fungus Monascus purpureus BCRC 38108. Nat. Prod. Res. 27 (13), 1145–1152. doi: 10.1080/14786419.2012.715289
Wu, M., Zhang, W. G., Liu, L. T. (2017). Red yeast rice prevents atherosclerosis through regulating inflammatory signaling pathways. Chin. J. Integr. Med. 23 (9), 689–695. doi: 10.1007/s11655-017-2416-x
Xiong, X. J., Wang, P. Q., Li, X. K., Zhang, Y. Q., Li, S. J. (2017). The effects of red yeast rice dietary supplement on blood pressure, lipid profile, and C-reactive protein in hypertension: a systematic review. Crit. Rev. Food Sci. Nutr. 57 (9), 1831–1851. doi: 10.1080/10408398.2015.1018987
Xu, B. J., Wang, Q. J., Sung, C. K. (2017). Telomerase inhibitory effects of red pigment rubropunctatin and statin Monacolin L isolated from Red Yeast Rice. Genes 8 (5), 129. doi: 10.3390/genes8050129
Xu, M. A. (2014). Breeding and fermentation study of strains with Monascus pigment and glucoamylase production (Master, Fuzhou University, Fuzhou).
Xue, Y. J., Tao, L. Y., Wu, S. Z., Wang, G. Q., Qian, L., Li, J. W., et al. (2017). Red yeast rice induces less muscle fatigue symptom than simvastatin in dyslipidemic patients: a single center randomized pilot trial. BMC Cardiovasc. Disord. 2017 (17), 127. doi: 10.1186/s12872-017-0560-z
Yang, J. L. (2017). Clinical research on Taohe Chengqi Decoction and Red Rice for hyperlipemia in Taiwan (Doctor, Nanjing University of Chinese Medicine, Nanjing).
Yu, C. C., Wang, J. J., Lee, C. L., Lee, S. H., Pan, T. M. (2008). Safety and mutagenicity evaluation of nanoparticulate Red Mold Rice. J. Agric. Food Chem. 56 (22), 11038–11048. doi: 10.1021/jf801335u
Zhang, J. F., Chang, Y. Q., Chen, G., Zhang, T. Y. (2008). Study on immunocompetence of Monascus polysaccharides. Food Sci. 29 (2), 391–393.
Zhang, Y. T., Wang, Y., Zhang, X. T., Wu, D. L., Zhang, X. Q., Ye, W. C. (2009). A new decalin derivative from red yeast rice. J. Asian Nat. Prod. Res. 11 (9), 792–795. doi: 10.1080/10286020903164269
Zhang, Z. H., Ali, Z., Khan, S. I., Khan, I. A. (2016). Cytotoxic monacolins from red yeast rice, a Chinese medicine and food. Food Chem. 202, 262–268. doi: 10.1016/j.foodchem.2015.12.039
Zhang, H. L., Li, G. L., Su, G. C., Liu, J. W., Li, J. (2018a). Separation and purification of the antioxidant compounds from Gutian Red Yeast Rice. Mod. Food Sci. Technol. 34 (5), 136–143.
Zhang, J. H., Xin, H. L., Xu, Y. M., Shen, Y., He, Y. Q., Hsien, Y., et al. (2018b). Morinda officinalis How. - A comprehensive review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 213 (2018), 230–255. doi: 10.1016/j.jep.2017.10.028
Zhong, H. (2017). The effect and mechanism study about the red yeast rice inhibiting the angiogenesis of breast cancer (Doctor, Shandong University of Traditional Chinese Medicine, Ji'nan).
Zhou, W. B., Guo, R., Guo, W. L., Hong, J. L., Li, L., Ni, L., et al. (2019). Monascus yellow, red and orange pigments from red yeast rice ameliorate lipid metabolic disorders and gut microbiota dysbiosis in Wistar rats fed on a high- fat diet. Food Funct. 10 (2), 1073–1084. doi: 10.1039/c8fo02192a
Zhu, L., Lu, J. G., Li, T., Zhu, G. Y., Han, Q. B., Hsiao, W. L., et al. (2012a). Immunosuppressive decalin derivatives from Red Yeast Rice. J. Nat. Prod. 75 (4), 567–571. doi: 10.1021/np2007236
Zhu, L., Yau, L. F., Lu, J. G., Zhu, G. Y., Wang, J. R., Han, Q. B., et al. (2012b). Cytotoxic dehydromonacolins from Red Yeast Rice. J. Agric. Food Chem. 60 (4), 934–939. doi: 10.1021/jf203579f
Zhu, L., Han, Q. B., Ho, A., Hsiao, W. L., Jiang, Z. H. (2013). Characterization and simultaneous determination of immunosuppressive decalins in red yeast rice by ultra-high-performance liquid chromatography hyphenated with mass spectrometry. J. Chromatogr. 1303, 54–61. doi: 10.1016/j.chroma.2013.06.045
Keywords: red yeast rice, Monascus purpureus, traditional use, chemical constituent, biological activity, quality control
Citation: Zhu B, Qi F, Wu J, Yin G, Hua J, Zhang Q and Qin L (2019) Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology, and Quality Control of an Important Chinese Folk Medicine. Front. Pharmacol. 10:1449. doi: 10.3389/fphar.2019.01449
Received: 16 August 2019; Accepted: 12 November 2019;
Published: 02 December 2019.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaReviewed by:
Ligia Salgueiro, University of Coimbra, PortugalCopyright © 2019 Zhu, Qi, Wu, Yin, Hua, Zhang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinwei Hua, aGp3bHNAMTI2LmNvbQ==; Qiaoyan Zhang, enF5MTk2NUAxNjMuY29t; Luping Qin, bHBxaW5AemNtdS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.