- 1Center for Translational Pain Medicine, Department of Anesthesiology, Duke University Medical Center, Durham, NC, United States
- 2Center for Experimental Therapeutics and Reperfusion Injury, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
- 3Department of Neurobiology, Duke University Medical Center, Durham, NC, United States
- 4Department of Cell Biology, Duke University Medical Center, Durham, NC, United States
Earlier studies have demonstrated that essential fatty acid-derived specialized pro-resolving mediators (SPMs) promote the resolution of inflammation and pain. However, the potential analgesic actions of SPMs in chemotherapy-induced peripheral neuropathy (CIPN) are not known. Recent results also showed sex dimorphism in immune cell signaling in neuropathic pain. Here, we evaluated the analgesic actions of D-series resolvins (RvD1, RvD2, RvD3, RvD4, and RvD5) on a CIPN in male and female mice. Paclitaxel (PTX, 2 mg/kg), given on days 0, 2, 4, and 6, produced robust mechanical allodynia in both sexes at 2 weeks. Intrathecal injection of RvD1 and RvD2 (100 ng, i.t.) at 2 weeks reversed PTX-induced mechanical allodynia in both sexes, whereas RvD3 and RvD4 (100 ng, i.t.) had no apparent effects on either sex. Interestingly, RvD5 (100 ng, i.t.) only reduced mechanical allodynia in male mice but not in female mice. Notably, PTX-induced mechanical allodynia was fully developed in Trpv1 or Trpa1 knockout mice, showing no sex differences. Also, intrathecal RvD5 reduced mechanical allodynia in male mice lacking Trpv1 or Trpa1, whereas female mice with Trpv1 or Trpa1 deficiency had no response to RvD5. Finally, RvD5-induced male-specific analgesia was also confirmed in an inflammatory pain condition. Formalin-induced second phase pain (licking and flinching) was reduced by intrathecal RvD5 in male but not female mice. These findings identified RvD5 as the first SPM that shows sex dimorphism in pain regulation. Moreover, these results suggest that specific resolvins may be used to treat CIPN, a rising health concern in cancer survivors.
Introduction
Pain is a cardinal feature of inflammation, as inflammation is often associated with pain (Ji et al., 2016). Mechanisms of pain induction by inflammation and inflammatory mediators have been extensively investigated in the past two decades (Hucho and Levine, 2007; Ji et al., 2014). Inflammatory mediators such as lipid mediators (prostaglandins, epoxyeicosatrienoic acids), nerve growth factor, and proinflammatory cytokines and chemokines (IL-1β, TNF, CCL2, CXCL5) sensitize nociceptors (peripheral sensitization) via modulations of ion channels such as voltage-gated sodium channels (Nav1.7, Nav1.8, Nav1.9) and TRP channels (TRPA1 and TRPV1) (Gold et al., 1996; Julius and Basbaum, 2001; White et al., 2005; Binshtok et al., 2008; Constantin et al., 2008; Dawes et al., 2011; Sisignano et al., 2012; Ji et al., 2014). However, our understanding on pain resolution is very limited. Mounting evidence indicates that resolution of acute inflammation is an active process involving the production of specialized pro-resolving mediators (SPMs), such as resolvins, protectins/neuroprotectin, and maresins (Serhan et al., 2008; Spite et al., 2009; Hong et al., 2014; Serhan, 2014; Norling et al., 2016). Resolvin D-series (e.g., RvD1, RvD2) and E-series (e.g., RvE1) are derived from omega-3 polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), respectively, and exhibit potent anti-inflammation and pro-resolution actions in various animal models of inflammation and infection (Serhan et al., 2002; Arita et al., 2005; Spite et al., 2009; Ji et al., 2011; Chiang et al., 2012; Buckley et al., 2014). For example, during infection, Resolvin D5 biosynthesis and actions in clearance of bacteria appears to be superior to other resolvins (Chiang et al., 2012) and is a major resolvin produced by human M2 macrophages (Werz et al., 2018).
Studies from different laboratories have also demonstrated potent analgesic actions of resolvins (e.g., RvE1, RvD1, and RvD2) in animal models of inflammatory pain (Bang et al., 2010a; Xu et al., 2010; Lima-Garcia et al., 2011; Park et al., 2011b), postoperative pain (Huang et al., 2011; Wang and Strichartz, 2017; Zhang et al., 2018), and nerve trauma-induced neuropathic pain (Xu et al., 2013b). Mechanistically, RvE1, RvD1, and RvD2 suppressed the processing of nociceptive information in the spinal cord pain circuit (Xu et al., 2010; Park et al., 2011b; Meesawatsom et al., 2016). The analgesic potency of resolvins is 1000 times higher than their precursors DHA and EPA (Xu et al., 2010). Resolvins inhibit pain via multiple mechanisms, including immune modulation, glial modulation, and neuronal modulation (Ji et al., 2011). Especially, resolvins (RvD1, RvD2, and RvE1) are potent inhibitors of TRP channels, such as TRPA1 and TRPV1, by acting on sensory neurons (Bang et al., 2010b; Xu et al., 2010; Park et al., 2011b). There is also an association of 17-HDHA, a precursor of D-series resolvins, with heat pain and osteoarthritis pain in humans (Valdes et al., 2017). Compared to RvD1 and RvD2, RvD3, RvD4, and RvD5 are newly established members of resolvin family and their complete stereochemical structures and total organic synthesis were recently achieved (Serhan et al., 2002; Dalli et al., 2013; Winkler et al., 2016; Norris et al., 2017; Norris et al., 2018; Winkler et al., 2018). Very recently, we found that RvD1 and RvD5, but not RvD3 and RvD4, reduced postoperative pain after bone fracture in mice (Zhang et al., 2018). Distinct roles of the DHA-derived resolvins in other pain models had not, to date, been tested. Recently, human tears and human skin blisters have shown sex differences in resolvins and SPM produced in vivo (English et al., 2017; Rathod et al., 2017).
Chemotherapy-induced peripheral neuropathy (CIPN) is the dose-limiting toxicity for many commonly utilized classes of anti-cancer agents, such as paclitaxel (Flatters and Bennett, 2004; Li et al., 2014). Neuropathic pain after chemotherapy results in dose reductions or discontinuation of cancer therapy (Cavaletti and Marmiroli, 2010; Sisignano et al., 2014). Given a lack of FDA-approved treatment for CIPN, it is urgent to develop safe and novel treatment for neuropathic pain after chemotherapy. In this study, we directly compared the analgesic actions of D-series resolvins (D1-D5) in a mouse model of CIPN. Our findings not only demonstrated distinct analgesic efficacy of these resolvins but also revealed striking sex dimorphism of these SPMs.
Materials and Methods
Animals
Adult CD1 mice of both sexes (25–35 g) were purchased from Charles River Laboratories. Trpv1 knockout mice (B6.129X1-Trpv1tm1Jul/J stock# 003770) and Trpa1 KO (B6;129P-Trpa1tm1Kykw/J stock# 006401) mice were purchased from Jackson Laboratory. All animals were maintained at the Duke University Animal Facility. All the animal experiments performed in this project have been approved by the Animal Care Committee of Duke University.
Chemotherapy-Induced Neuropathic Pain and Formalin-Induced Inflammatory Pain
To induce CIPN in mice, we performed four intraperitoneal injections of paclitaxel (2 mg/kg per injection, Sigma) on days 0, 2, 4 and 6. To produce inflammatory pain, formalin (5%, 20 μl, Sigma) was injected into the plantar surface of a hindpaw.
Drugs and Administration
RvD1, RvD2, RvD3, RvD4, and RvD5 were from Cayman Chemical. Ten percent ethanol in PBS was taken as vehicle treatment. For intrathecal (i.t.) injection, mice were briefly anesthetized with isoflurane (2%) and a spinal cord puncture was made between the L5 and L6 levels to deliver reagents (10 μl, 10 or 100 ng SPMs dissolved in 10% ethanol) using a 30G needle. The dose selection of resolvins was based on our previous study (Zhang et al., 2018).
Behavioral Tests
Animals were habituated for 1–2 h in plastic chambers on an elevated metal mesh floor in the testing environment for at least 2 days before testing. For testing mechanical pain threshold, the plantar surface of each hindpaw was stimulated with a series of von Frey fibers with logarithmically incrementing stiffness (0.02–2.56 g, Stoelting), presented perpendicular to the plantar surface. The 50% paw withdrawal threshold was calculated using Dixon’s up-down method (Dixon, 1980). For the formalin test, pain behavior was recorded every 5 min for 45 min following i.pl. formalin and the duration of licking and flinching in the affected paws was timed (Berta et al., 2014).
Statistics
All data were expressed as the mean ± SEM. The sample size for each experiment is indicated in the figure legends. All data were analyzed by one-way or repeated measures two-way ANOVA, followed by Bonferroni’s post hoc test. p < 0.05 was taken as statistically significant.
Results
Intrathecal Resolvins Differentially Inhibit Mechanical Allodynia in Male and Female Mice With CIPN
We first examined the potential anti-allodynic effects of DHA-derived SPMs, including RvD1, RvD2, RvD3, RvD4, and RvD5 on CIPN compared to vehicle (10% ethanol) via intrathecal administration. Intrathecal route is known to target spinal cord cells as well as DRG cells (Yaksh and Rudy, 1976; Ji et al., 2002; Alessandri-Haber et al., 2009). Also, microglial inhibitors and modulators were shown to produce sex-dependent pain inhibition following intrathecal injection (Sorge et al., 2015; Taves et al., 2016). Intrathecal injection of these SPMs (100 ng) was given 2 weeks post the first PTX injection. PTX produced robust mechanical allodynia in both sexes (Figure 1). We found that intrathecal resolvins produced different effects on PTX-evoked mechanical allodynia. RvD1 and RvD2 transiently reduced mechanical allodynia in both male and female mice: the effect was observed at 1 h but not 3 h after resolvin injection. Notably, i.t. RvD5 (100 ng) only alleviated mechanical allodynia in male but not female mice. By contrast, RvD3 and RvD4 did not alter mechanical allodynia (Figure 1A, B: F(5, 204) = 378.0 in A, F(5, 210) = 438.2 in B, p < 0.001, two-way ANOVA). Together, these results demonstrated distinct analgesic actions of DHA-derived resolvins in CIPN.
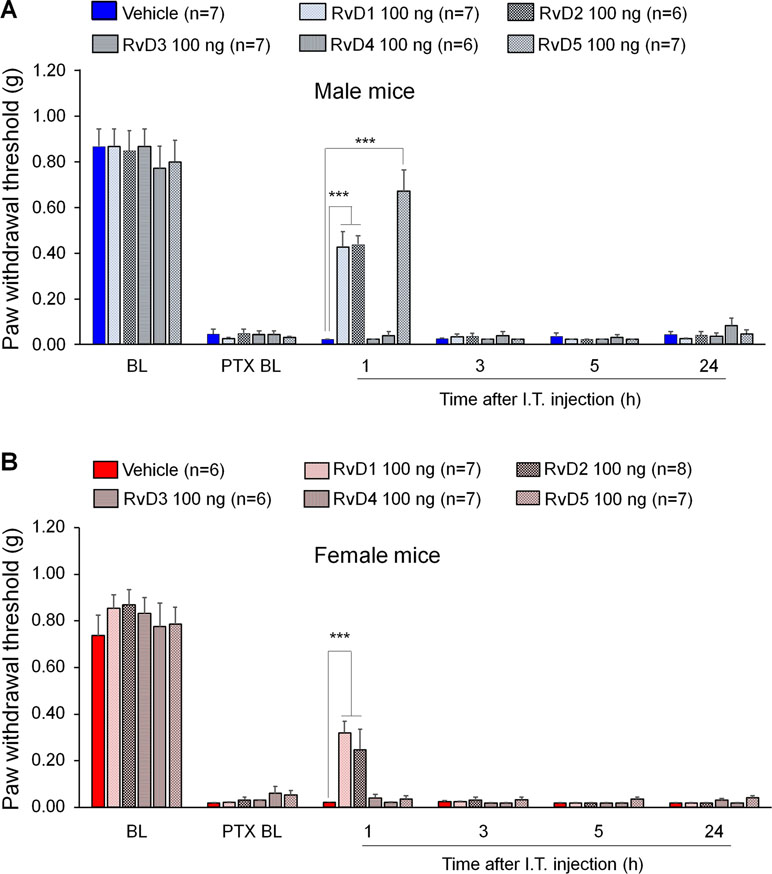
Figure 1 Effects of intrathecal RvD1-D5 on mechanical allodynia in male mice (A) and female mice (B) 2 weeks after paclitaxel (PTX) injection. Intrathecal RvD1 and RvD2 (100 ng) reduced mechanical allodynia in both sexes. However, intrathecal RvD5 only reduced mechanical allodynia in male mice. ***p < 0.001 versus the vehicle group. Two-Way ANOVA with Bonferroni’s post-hoc test, n = 6-8 mice per sex per group.
PTX-Evoked Mechanical Allodynia Is Fully Developed in Trpv1 and Trpa1 Knockout Mice
TRPV1 and TRPA1 are two critical pain transducers and play important roles in inflammatory pain and neuropathic pain (Caterina and Julius, 2001; Bautista et al., 2006; Constantin et al., 2008; Materazzi et al., 2012). We tested whether TRPV1 and TRPA1 are required for CIPN in a sex-dependent manner using Trpa1 knockout (KO) mice. Notably, PTX produced remarkable mechanical allodynia in both males and females in Trpv1 KO mice (Figure 2A, F(4, 90) = 140.0, p < 0.001, two-way ANOVA) as well as in Trpa1 KO (Figure 2B, F(4, 85) = 237.6, p < 0.001, two-way ANOVA) mice. Notably, there were no sex differences in PTX-induced allodynia at all the time points we tested (1 to 4 weeks, Figure 2A and B).
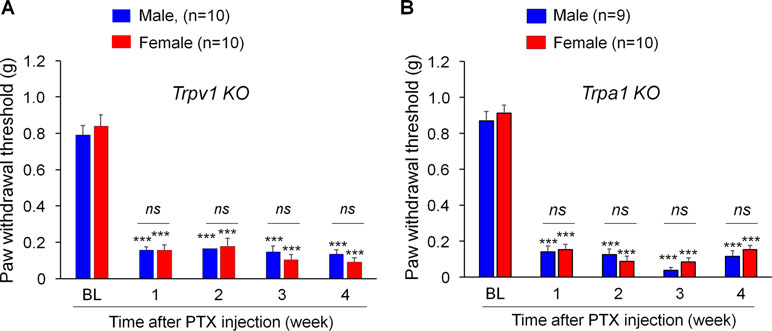
Figure 2 PTX induces sustained mechanical allodynia in Trpv1(A) and Trpa1(B) knockout (KO) mice of both sexes. Mechanical allodynia after PTX injection is fully developed in mice lacking trpv1 (A) and trpa1 (B) from week 1 and week 4, showing no sex differences. ***p < 0.001 vs. the baseline (BL) control. ns., not significant. Two-way ANOVA with Bonferroni’s post hoc test, n = 9-10 mice per sex per group.
Intrathecal RvD5 Inhibits Mechanical Allodynia After CIPN in Male But Not Female Mice Lacking TRPV1 or TRPA1
Previous studies demonstrated that SPMs are potent inhibitors of TRPV1 and/or TRPA1 (Bang et al., 2010b; Park et al., 2011a; Park et al., 2011b; Serhan et al., 2012). To test the hypothesis that RvD5 reduced PTX-induced neuropathic pain through TRPV1 or TRPA1, we tested the analgesic action of RvD5 in Trpv1 and Trpa1 KO mice. We found that i.t. RvD5 (100 ng) reduced mechanical allodynia in male but not female Trpv1 KO mice in comparison to the vehicle treatment (Figure 3A, B: F(5, 48) = 47.54 in A, F(5, 48) = 42.63 in B, p < 0.01, two-way ANOVA). Similarly, i.t. RvD5 inhibited PTX-induced pain in male but not female Trpa1 KO mice (Figure 3C, D: F(5, 42) = 76.65 in C, F(5, 48) = 121.9 in D, p < 0.001, two-way ANOVA). Taken together, these results implicated that anti-allodynic effects of RvD5 against CIPN do not require TRPV1 or TRPA1.
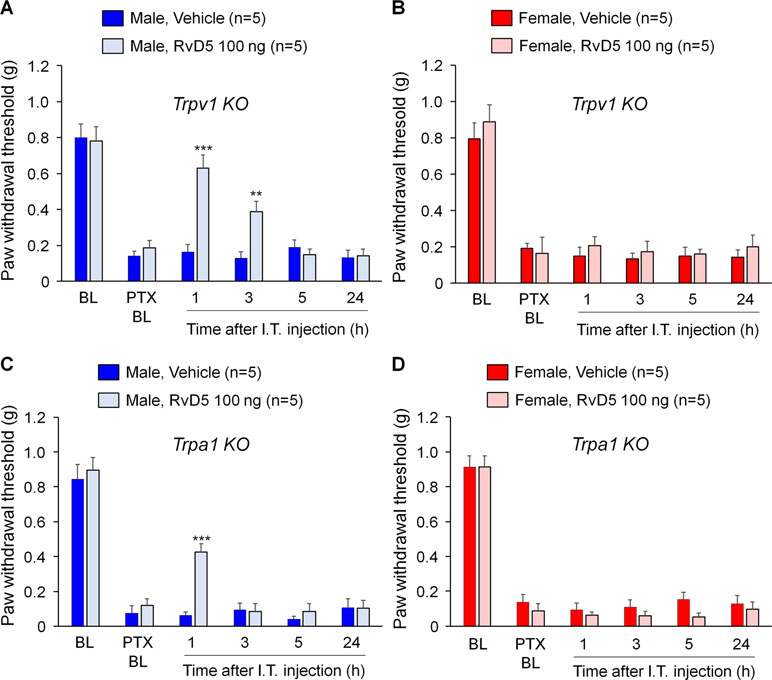
Figure 3 Effects of intrathecal RvD5 on PTX-evoked mechanical allodynia in male mice (A, C) and female mice (B, D) lacking Trpv1(A, B) and Trpa1(C, D). RvD5 (100 ng, i.t.) was able to inhibit mechanical allodynia in male TRPV1-KO mice (A) and male TRPA1-KO mice (C), showing no effects on female mice (B, D). **p < 0.01, ***p < 0.001, vs. vehicle. Two-way ANOVA with Bonferroni’s post hoc test, n = 5 mice per sex per group.
RvD5 Reduces Formalin-Induced Spontaneous Pain Only in Male Mice
To further confirm sex dimorphism of RvD5-produced anti-allodynic effects, we tested an inflammatory pain model induced by intraplantar formalin injection. Male and female mice were pre-treated by i.t. RvD5 (10 ng) prior to formalin treatment (5% in PBS, 20 μl, i.pl). The results of formalin-induced spontaneous pain were presented as phase I (0–10 min) and phase II (10–45 min after i.pl. formalin). Intrathecal RvD5 (100 ng) significantly reduced Phase II behavior in male mice (Figure 4A, B: F(8, 72) = 21.34 in A, F(1, 16) = 71.39 in B, p < 0.01, two-way ANOVA), without effects on Phase I behavior. However, i.t. RvD5 failed to affect neither Phase I nor Phase II behavior in females (Figure 4C, D: F(8, 72) = 18.18 in C, F(1, 16) = 36.44 in D, p > 0.05, two-way ANOVA). These results confirmed a male-dominant analgesic effect of RvD5 in an inflammatory pain.
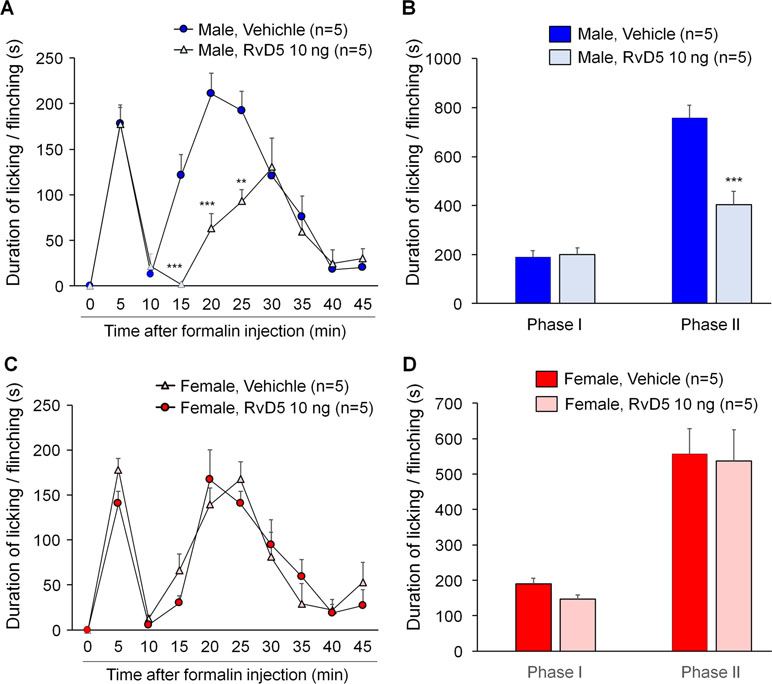
Figure 4 Intrathecal RvD5 reduces formalin-induced inflammatory pain in male mice (A, B) but not female mice (C, D). (A, C) Time course of formalin-induced pain in males (A) and females (C) with intrathecal vehicle or RvD5 (10 ng), given 30 min prior to formalin injection. (B, D) Phase I (0-10 min) and Phase II (10-45 min) responses in males (B) and females (D). Note that RvD5 only inhibits Phase II response in males but has no effect on Phase I response in both sexes. **p < 0.01, ***p < 0.001 versus vehicle group. Two-way ANOVA with Bonferroni’s post hoc test, n = 5 mice per sex per group.
To determine if RvD3 would produce a rapid and transient analgesia in inflammatory pain, we tested the actions of intrathecal RvD3 (100 ng) in both male and female mice using the formalin test. Our data indicated that intrathecal RvD3 (100 ng) did not affect Phase I or Phase II behavior in male mice (vehicle group: 135.2 ± 3.83 s in Phase I and 528.8 ± 71.38 s in Phase II; RvD3 group: 208.2 ± 21.57 s in Phase I and 653.0 ± 122.86 s in Phase II, F (1, 16) = 42.51, p > 0.05, two-way ANOVA, n = 5 mice per group). Neither did RvD3 produce any analgesic effects in female mice (vehicle group: 265.0 ± 39.37 s in Phase I and 962.8 ± 110.63 s in Phase II; RvD3 group: 307.4 ± 52.83 s in Phase I and 973.8 ± 129.47 s in Phase II, F (1, 16) = 69.77, p > 0.05, two-way ANOVA, n = 5 mice). Together, our results suggest that RvD3 has no apparent analgesic actions in inflammatory pain, neuropathic pain (Figure 1), and postoperative pain (Zhang et al., 2018).
Discussion
SPMs have been shown to produce pain relief in inflammatory pain (Svensson et al., 2007; Bang et al., 2010a; Xu et al., 2010; Lima-Garcia et al., 2011; Park et al., 2011b), postoperative pain (Huang et al., 2011; Wang and Strichartz, 2017; Zhang et al., 2018), and nerve trauma-induced neuropathic pain (Xu et al., 2013a; Xu et al., 2013b). CIPN is a unique type of neuropathic pain, as there is very limited activation of microglia in the spinal cord after CIPN by PTX (Zheng et al., 2011; Robinson et al., 2014). In contrast, nerve injury/nerve trauma produces remarkable microglial activation and microgliosis in the spinal cord (Echeverry et al., 2008; Suter et al., 2009; Clark et al., 2012). Furthermore, spinal blockade of p38 MAP kinase reduced nerve trauma-induced mechanical allodynia but failed to reduce PTX-induced mechanical allodynia (Taves et al., 2016; Luo et al., 2018). In this study, we tested the analgesic actions of DHA-derived SPMs (resolvin D1-D5) in a mouse CIPN model induced by paclitaxel and had two interesting findings. First, D-series resolvins showed different analgesic efficacy in male mice: intrathecal injection of RvD1, RvD2, and RvD5 (100 ng) reduced PTX-induced mechanical allodynia, whereas RvD3 and RvD4 treatment had no effect. RvD3 and RvD4 (100 ng, i.t.) also failed to inhibit postoperative pain after bone fracture (Zhang et al., 2018). However, inflammatory pain is much more sensitive to SPMs. For example, 10 ng (i.t.) of RvE1 is sufficient to reduce formalin-induced inflammatory pain (Xu et al., 2010) but 100 ng RvE1 is needed to inhibit neuropathic pain (Xu et al., 2013b). Also, intrathecal RvD5 at a lower dose (10 ng) was able to reduce formalin-induced inflammatory pain (Figure 4), as compared to higher dose (100 ng) for treating neuropathic pain. Second, the analgesic action of RvD5 is sex-dependent: RvD5 only reduced mechanical allodynia after CIPN in male mice, showing no pain relief in female mice. This sex dimorphism of RvD5’s analgesia was also demonstrated in another pain model: formalin-induced inflammatory pain was reduced by i.t. RvD5 in male but not female mice. This is the first report of sex dimorphism in SPM-induced analgesia in any pain model.
Despite higher incidence of chronic pain in women, majority of preclinical studies on pain used male animals (Mogil, 2012; Chen et al., 2018). To our knowledge, previous studies on SPM regulation of pain were all conducted in male mice and male rats. Mechanisms underlying sex dimorphism in chronic pain are unclear. Multiple social, psychological, and biological factors have been proposed (Riley et al., 1998; Mogil, 2012; Fillingim et al., 2009). Of note, the neuro-immune interactions are considered an important contributor to sex dimorphism in chronic pain (Sorge et al., 2015). Recent evidence suggests that spinal microglia regulate neuropathic pain and inflammatory pain only in male mice (Sorge et al., 2015). For example, spinal injection of microglial inhibitor minocycline reduced neuropathic pain only in male mice (Sorge et al., 2015; Chen et al., 2018). Blockade of microglial signaling with inhibitors of P2X4, TLR4, and p38 MAP kinase via intrathecal injection also inhibited neuropathic pain in male but not female mice (Sorge et al., 2011; Sorge et al., 2015; Taves et al., 2016; Luo et al., 2018). Sex dimorphism in the pathogenesis of CIPN is largely unknown. Given the limited role of spinal microglia in PTX-induced CIPN (Zheng et al., 2011; Robinson et al., 2014; Luo et al., 2018), other immune cell types such as macrophages and T cells should play an important role in CIPN. For example, macrophage infiltration and activation in DRG and sciatic nerve promotes the development of CIPN (Liu et al., 2014; Liu et al., 2016; Montague and Malcangio, 2017; Montague et al., 2018). T cells appear to regulate both the induction and resolution of CIPN (Liu et al., 2014; Krukowski et al., 2016). Although resolvins have been shown to inhibit TRPA1 and TRPV1 in inflammatory pain (Park et al., 2011b), TRPA1 and TRPV1 are not required for the development of CIPN (Figure 2). Consistently, mechanical allodynia after CIPN requires large Aβ-fibers but not C-fibers (Xu et al., 2015). Furthermore, the anti-allodynic effect of RvD5 retained in mice lacking TRPA1 and TRPV1 (Figure 3). Thus, intrathecal injection of RvD5 may target immune cells in DRGs for producing sex-dependent analgesic actions. Notably, macrophage signaling in DRGs appears to regulate CIPN in a sex-dependent manner (Luo and Ji, In press).
The D-series resolvins produce their beneficial actions via specific G protein coupled receptors, such as GPR32 for RvD1 and RvD5, and GPR18 for RvD2 (Krishnamoorthy et al., 2010; Chiang et al., 2012; Chiang et al., 2015), and specific unique receptors for RvD3 and RvD4 remain to be identified. Nonetheless, RvD3 has been found to activate human GPR32 in vitro (Dalli et al., 2013). It is surprising that only RvD5 but not RvD1 showed sex-dependent analgesia in CIPN, since both RvD1 and RvD5 share the same receptor (GPR32). It is possible that RvD1 and RvD5 may differently activate GPR32 in resolving pain. Moreover, additional specific receptors for RvD5 might exist in immune cells (e.g., macrophages) that mediate RvD5-induced pain reduction in males. Notably, RvD5 is a major resolvin produced by human M2 macrophages (Werz et al., 2018). Intrathecal RvD5 may also act on M2 macrophages to produce anti-inflammatory cytokines (e.g., IL-10) for pain relief (Bang et al., 2018). Sex dimorphism in SPMs was also revealed in recent human studies. For example, human emotional tears and human skin blisters have shown sex differences in resolvins and SPM produced in vivo, as well as the rate of resolution of inflammation (English et al., 2017; Rathod et al., 2017). Hence, it is not unexpected that sex differences are present in the actions of SPMs, which may also reflect sex differences in the expression patterns of their specific receptors. Future studies are warranted to investigate the analgesic actions of RvD5 in GPR32 knockout mice and identify additional receptors of RvD5 in different sexes of mice with CIPN. Future studies are also needed to investigate how sex hormones regulate the production of resolvins and the actions of resolvins.
In conclusion, CIPN is a rising health concern in the world due to increasing number of cancer survivors. There is a lack of approved treatment for CIPN. Our findings suggest that different resolvins may be used to treat CIPN in cancer patients and improve the life quality of millions of cancer survivors in different sexes.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal studies were approved by Duke IACUC.
Author Contributions
XL, YG, and XT did the experiments and analyzed the data. R-RJ, XL, and CS wrote the paper.
Funding
The work was supported by US National Institutes of Health grants R01DE17794, R01NS87988, and R21NS91779 to R-RJ. CS is supported by National Institutes of Health grant R01GM038765.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alessandri-Haber, N., Dina, O. A., Chen, X., Levine, J. D. (2009). TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J. Neurosci. 29, 6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009
Arita, M., Bianchini, F., Aliberti, J., Sher, A., Chiang, N., Hong, S., et al. (2005). Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201, 713–722. doi: 10.1084/jem.20042031
Bang, S., Xie, Y. K., Zhang, Z. J., Wang, Z., Xu, Z. Z., Ji, R. R. (2018). GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Invest. 128, 3568–3582. doi: 10.1172/JCI99888
Bang, S., Yoo, S., Oh, U., Hwang, S. W. (2010a). Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch. Pharm. Res. 33, 1509–1520. doi: 10.1007/s12272-010-1004-9
Bang, S., Yoo, S., Yang, T. J., Cho, H., Kim, Y. G., Hwang, S. W. (2010b). Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br. J. Pharmacol. 161, 707–720. doi: 10.1111/j.1476-5381.2010.00909.x
Bautista, D. M., Jordt, S. E., Nikai, T., Tsuruda, P. R., Read, A. J., Poblete, J., et al. (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. doi: 10.1016/j.cell.2006.02.023
Berta, T., Park, C. K., Xu, Z. Z., Xie, R. G., Liu, T., Lu, N., et al. (2014). Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J. Clin. Invest. 124, 1173–1186. doi: 10.1172/JCI72230
Binshtok, A. M., Wang, H., Zimmermann, K., Amaya, F., Vardeh, D., Shi, L., et al. (2008). Nociceptors are interleukin-1beta sensors. J. Neurosci. 28, 14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008
Buckley, C. D., Gilroy, D. W., Serhan, C. N. (2014). Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327. doi: 10.1016/j.immuni.2014.02.009
Caterina, M. J., Julius, D. (2001). The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517. doi: 10.1146/annurev.neuro.24.1.487
Cavaletti, G., Marmiroli, P. (2010). Chemotherapy-induced peripheral neurotoxicity. Nat. Rev. Neurol. 6, 657–66. doi: 10.1038/nrneurol.2010.160
Chen, G., Luo, X., Qadri, M. Y., Berta, T., Ji, R. R. (2018). Sex-dependent glial signaling in pathological pain: distinct roles of spinal microglia and astrocytes. Neurosci. Bull. 34, 98–108. doi: 10.1007/s12264-017-0145-y
Chiang, N., Dalli, J., Colas, R. A., Serhan, C. N. (2015). Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 212, 1203–1217. doi: 10.1084/jem.20150225
Chiang, N., Fredman, G., Backhed, F., Oh, S. F., Vickery, T., Schmidt, B. A., et al. (2012). Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–28. doi: 10.1038/nature11042
Clark, A. K., Grist, J., Al-Kashi, A., Perretti, M., Malcangio, M. (2012). Spinal cathepsin S and fractalkine contribute to chronic pain in the collagen-induced arthritis model. Arthritis Rheum. 64, 2038–2047. doi: 10.1002/art.34351
Constantin, C. E., Mair, N., Sailer, C. A., Andratsch, M., Xu, Z. Z., Blumer, M. J., et al. (2008). Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J. Neurosci. 28, 5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008
Dalli, J., Winkler, J. W., Colas, R. A., Arnardottir, H., Cheng, C. Y., Chiang, N., et al. (2013). Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 20, 188–201. doi: 10.1016/j.chembiol.2012.11.010
Dawes, J. M., Calvo, M., Perkins, J. R., Paterson, K. J., Kiesewetter, H., Hobbs, C., et al. (2011). CXCL5 Mediates UVB irradiation-induced pain. Sci. Transl. Med. 3, 90ra60. doi: 10.1126/scitranslmed.3002193
Dixon, W. J. (1980). Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 20, 441–462. doi: 10.1146/annurev.pa.20.040180.002301
Echeverry, S., Shi, X. Q., Zhang, J. (2008). Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain 135, 37–47. doi: 10.1016/j.pain.2007.05.002
English, J. T., Norris, P. C., Hodges, R. R., Dartt, D. A., Serhan, C. N. (2017). Identification and profiling of specialized pro-resolving mediators in human tears by lipid mediator metabolomics. Prostaglandins Leukot. Essent. Fatty Acids 117, 17–27. doi: 10.1016/j.plefa.2017.01.004
Fillingim, R. B., King, C. D., Ribeiro-Dasilva, M. C., Rahim-Williams, B., Riley, J. L., 3rd (2009). Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain 10, 447–485. doi: 10.1016/j.jpain.2008.12.001
Flatters, S. J., Bennett, G. J. (2004). Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 109, 150–161. doi: 10.1016/j.pain.2004.01.029
Gold, M. S., Shuster, M. J., Levine, J. D. (1996). Role of a Ca(2+)-dependent slow afterhyperpolarization in prostaglandin E2-induced sensitization of cultured rat sensory neurons. Neurosci. Lett. 205, 161–164. doi: 10.1016/0304-3940(96)12401-0
Hong, S., Tian, H., Lu, Y., Laborde, J. M., Muhale, F. A., Wang, Q., et al. (2014). Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am. J. Physiol., Cell Physiol. 307, C1058–C1067. doi: 10.1152/ajpcell.00270.2014
Huang, L., Wang, C. F., Serhan, C. N., Strichartz, G. (2011). Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain 152, 557–565. doi: 10.1016/j.pain.2010.11.021
Hucho, T., Levine, J. D. (2007). Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55, 365–376. doi: 10.1016/j.neuron.2007.07.008
Ji, R. R., Chamessian, A., Zhang, Y. Q. (2016). Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577. doi: 10.1126/science.aaf8924
Ji, R. R., Samad, T. A., Jin, S. X., Schmoll, R., Woolf, C. J. (2002). p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36, 57–68. doi: 10.1016/S0896-6273(02)00908-X
Ji, R. R., Xu, Z. Z., Gao, Y. J. (2014). Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 13, 533–548. doi: 10.1038/nrd4334
Ji, R. R., Xu, Z. Z., Strichartz, G., Serhan, C. N. (2011). Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 34, 599–609. doi: 10.1016/j.tins.2011.08.005
Julius, D., Basbaum, A. I. (2001). Molecular mechanisms of nociception. Nature 413, 203–210. doi: 10.1038/35093019
Krishnamoorthy, S., Recchiuti, A., Chiang, N., Yacoubian, S., Lee, C. H., Yang, R., et al. (2010). Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. U.S.A. 107, 1660–1665. doi: 10.1073/pnas.0907342107
Krukowski, K., Eijkelkamp, N., Laumet, G., Hack, C. E., Li, Y., Dougherty, P. M., et al. (2016). CD8+ T cells and endogenous il-10 are required for resolution of chemotherapy-induced neuropathic pain. J. Neurosci. 36, 11074–11083. doi: 10.1523/JNEUROSCI.3708-15.2016
Li, Y., Zhang, H., Zhang, H., Kosturakis, A. K., Jawad, A. B., Dougherty, P. M. (2014). Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J. Pain 15, 712–725. doi: 10.1016/j.jpain.2014.04.001
Lima-Garcia, J. F., Dutra, R. C., Silva, K., Motta, E. M., Campos, M. M., Calixto, J. B. (2011). The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 164, 278–293. doi: 10.1111/j.1476-5381.2011.01345.x
Liu, X. J., Liu, T., Chen, G., Wang, B., Yu, X. L., Yin, C., et al. (2016). TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci. Rep. 6, 28188. doi: 10.1038/srep28188
Liu, X. J., Zhang, Y., Liu, T., Xu, Z. Z., Park, C. K., Berta, T., et al. (2014). Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 24, 1374–1377. doi: 10.1038/cr.2014.106
Luo, X., Fitzsimmons, B., Mohan, A., Zhang, L., Terrando, N., Kordasiewicz, H., et al. (2018). Intrathecal administration of antisense oligonucleotide against p38alpha but not p38beta MAP kinase isoform reduces neuropathic and postoperative pain and TLR4-induced pain in male mice. Brain Behav. Immun. 72, 34–44. doi: 10.1016/j.bbi.2017.11.007
Luo, X., Huh, Y., Bang, S., He, Q., Zhang, L., Matsuda, M., et al. (In press). Macrophage toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J. Neurosci.
Materazzi, S., Fusi, C., Benemei, S., Pedretti, P., Patacchini, R., Nilius, B., et al. (2012). TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. 463, 561–569. doi: 10.1007/s00424-011-1071-x
Meesawatsom, P., Burston, J., Hathway, G., Bennett, A., Chapman, V. (2016). Inhibitory effects of aspirin-triggered resolvin D1 on spinal nociceptive processing in rat pain models. J. Neuroinflammation 13, 233. doi: 10.1186/s12974-016-0676-6
Mogil, J. S. (2012). Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 13, 859–866. doi: 10.1038/nrn3360
Montague, K., Malcangio, M. (2017). The therapeutic potential of monocyte/macrophage manipulation in the treatment of chemotherapy-induced painful neuropathy. Front. Mol. Neurosci. 10, 397. doi: 10.3389/fnmol.2017.00397
Montague, K., Simeoli, R., Valente, J., Malcangio, M. (2018). A novel interaction between CX3CR1 and CCR2 signalling in monocytes constitutes an underlying mechanism for persistent vincristine-induced pain. J. Neuroinflammation 15, 101. doi: 10.1186/s12974-018-1116-6
Norling, L. V., Headland, S. E., Dalli, J., Arnardottir, H. H., Haworth, O., Jones, H. R., et al. (2016). Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight 1, e85922. doi: 10.1172/jci.insight.85922
Norris, P. C., Arnardottir, H., Sanger, J. M., Fichtner, D., Keyes, G. S., Serhan, C. N. (2018). Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot. Essent. Fatty Acids. 138, 81–89. doi: 10.1016/j.plefa.2016.01.001
Norris, P. C., Libreros, S., Chiang, N., Serhan, C. N. (2017). A cluster of immunoresolvents links coagulation to innate host defense in human blood. Sci. Signal. 10, eaan1471. doi: 10.1126/scisignal.aan1471
Park, C. K., Lu, N., Xu, Z. Z., Liu, T., Serhan, C. N., Ji, R. R. (2011a). Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J. Neurosci. 31, 15072–15085. doi: 10.1523/JNEUROSCI.2443-11.2011
Park, C. K., Xu, Z. Z., Liu, T., Lu, N., Serhan, C. N., Ji, R. R. (2011b). Resolvin d2 is a potent endogenous inhibitor for transient receptor potential subtype v1/a1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin d1, d2, and e1. J. Neurosci. 31, 18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011
Rathod, K. S., Kapil, V., Velmurugan, S., Khambata, R. S., Siddique, U., Khan, S., et al. (2017). Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Invest. 127, 169–182. doi: 10.1172/JCI89429
Riley, J. L., 3rd, Robinson, M. E., Wise, E. A., Myers, C. D., Fillingim, R. B. (1998). Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain 74, 181–187. doi: 10.1016/S0304-3959(97)00199-1
Robinson, C. R., Zhang, H., Dougherty, P. M. (2014). Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 274, 308–17. doi: 10.1016/j.neuroscience.2014.05.051
Serhan, C. N. (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. doi: 10.1038/nature13479
Serhan, C. N., Chiang, N., Van Dyke, T. E. (2008). Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–61. doi: 10.1038/nri2294
Serhan, C. N., Dalli, J., Karamnov, S., Choi, A., Park, C. K., Xu, Z. Z., et al. (2012). Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26, 1755–1765. doi: 10.1096/fj.11-201442
Serhan, C. N., Hong, S., Gronert, K., Colgan, S. P., Devchand, P. R., Mirick, G., et al. (2002). Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196, 1025–1037. doi: 10.1084/jem.20020760
Sisignano, M., Baron, R., Scholich, K., Geisslinger, G. (2014). Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 10, 694–707. doi: 10.1038/nrneurol.2014.211
Sisignano, M., Park, C. K., Angioni, C., Zhang, D. D., von Hehn, C., Cobos, E. J., et al. (2012). 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J. Neurosci. 32, 6364–6372. doi: 10.1523/JNEUROSCI.5793-11.2012
Sorge, R. E., Lacroix-Fralish, M. L., Tuttle, A. H., Sotocinal, S. G., Austin, J. S., Ritchie, J., et al. (2011). Spinal cord toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 31, 15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011
Sorge, R. E., Mapplebeck, J. C., Rosen, S., Beggs, S., Taves, S., Alexander, J. K., et al. (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081–1083. doi: 10.1038/nn.4053
Spite, M., Norling, L. V., Summers, L., Yang, R., Cooper, D., Petasis, N. A., et al. (2009). Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291. doi: 10.1038/nature08541
Suter, M. R., Berta, T., Gao, Y. J., Decosterd, I., Ji, R. R. (2009). Large a-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol. Pain. 5, 53. doi: 10.1186/1744-8069-5-53
Svensson, C. I., Zattoni, M., Serhan, C. N. (2007). Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J. Exp. Med. 204, 245–252. doi: 10.1084/jem.20061826
Taves, S., Berta, T., Liu, D. L., Gan, S., Chen, G., Kim, Y. H., et al. (2016). Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 55, 70–81. doi: 10.1016/j.bbi.2015.10.006
Valdes, A. M., Ravipati, S., Menni, C., Abhishek, A., Metrustry, S., Harris, J., et al. (2017). Association of the resolvin precursor 17-HDHA, but not D- or E- series resolvins, with heat pain sensitivity and osteoarthritis pain in humans. Sci. Rep. 7, 10748. doi: 10.1038/s41598-017-09516-3
Wang, J. C., Strichartz, G. R. (2017). Prevention of chronic post-thoracotomy pain in rats by intrathecal resolvin d1 and d2: effectiveness of perioperative and delayed drug delivery. J. Pain. 18 (5), 535–545. doi: 10.1016/j.jpain.2016.12.012
Werz, O., Gerstmeier, J., Libreros, S., De la Rosa, X., Werner, M., Norris, P. C., et al. (2018). Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 9, 59. doi: 10.1038/s41467-017-02538-5
White, F. A., Sun, J., Waters, S. M., Ma, C., Ren, D., Ripsch, M., et al. (2005). Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc. Natl. Acad. Sci. U.S.A. 102, 14092–14097. doi: 10.1073/pnas.0503496102
Winkler, J. W., Libreros, S., De La Rosa, X., Sansbury, B. E., Norris, P. C., Chiang, N., et al. (2018). Structural insights into resolvin D4 actions and further metabolites via a new total organic synthesis and validation. J. Leukoc. Biol. 103, 995–1010. doi: 10.1002/JLB.3MI0617-254R
Winkler, J. W., Orr, S. K., Dalli, J., Cheng, C. Y., Sanger, J. M., Chiang, N., et al. (2016). Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci. Rep. 6, 18972. doi: 10.1038/srep18972
Xu, Z. Z., Kim, Y. H., Bang, S., Zhang, Y., Berta, T., Wang, F., et al. (2015). Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med. 21, 1326–1331. doi: 10.1038/nm.3978
Xu, Z. Z., Liu, X. J., Berta, T., Park, C. K., Lu, N., Serhan, C. N., et al. (2013a). Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann. Neurol. 74, 490–495. doi: 10.1002/ana.23928
Xu, Z. Z., Berta, T., Ji, R. R. (2013b). Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J. Neuroimmune Pharmacol. 8, 37–41. doi: 10.1007/s11481-012-9394-8
Xu, Z. Z., Zhang, L., Liu, T., Park, J. Y., Berta, T., Yang, R., et al. (2010). Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 16, 592–597, 1p. doi: 10.1038/nm.2123
Yaksh, T. L., Rudy, T. A. (1976). Analgesia mediated by a direct spinal action of narcotics. Science 192, 1357–1358. doi: 10.1126/science.1273597
Zhang, L., Terrando, N., Xu, Z. Z., Bang, S., Jordt, S. E., Maixner, W., et al. (2018). Distinct analgesic actions of dha and dha-derived specialized pro-resolving mediators on post-operative pain after bone fracture in mice. Front. Pharmacol. 9, 412. doi: 10.3389/fphar.2018.00412
Keywords: chemotherapy-induced peripheral neuropathy (CIPN), docosahexaenoic acid (DHA), intrathecal injection, neuropathic pain, resolvin D-series (RvDs), sex dimorphism, specialized pro-resolving mediators (SPMs)
Citation: Luo X, Gu Y, Tao X, Serhan CN and Ji R-R (2019) Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Front. Pharmacol. 10:745. doi: 10.3389/fphar.2019.00745
Received: 16 March 2019; Accepted: 11 June 2019;
Published: 05 July 2019.
Edited by:
Peregrine B. Osborne, The University of Melbourne, AustraliaReviewed by:
Jon David Levine, University of California, United StatesChristopher W. Vaughan, University of Sydney, Australia
Copyright © 2019 Luo, Gu, Tao, Serhan and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ru-Rong Ji, cnUtcm9uZy5qaUBkdWtlLmVkdQ==
 Xin Luo
Xin Luo Yun Gu1
Yun Gu1 Charles Nicholas Serhan
Charles Nicholas Serhan Ru-Rong Ji
Ru-Rong Ji