
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 27 June 2019
Sec. Ethnopharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00734
 Yiyi Cai1,2†
Yiyi Cai1,2† Claire Shuiqing Zhang2†
Claire Shuiqing Zhang2† Shaonan Liu1
Shaonan Liu1 Zehuai Wen1
Zehuai Wen1 Anthony Lin Zhang2
Anthony Lin Zhang2 Xinfeng Guo1
Xinfeng Guo1 Charlie Changli Xue1,2
Charlie Changli Xue1,2 Chuanjian Lu1,2*
Chuanjian Lu1,2*Background: Treatment for post-stroke spasticity (PSS) remains a major challenge in clinical practice. Chinese herbal medicine (CHM) is often administered to assist in routine care (RC) in the treatment of PSS, with increasing numbers of clinical research and preclinical studies suggesting that it has potential benefits. Therefore, we conducted a systematic review and meta-analysis to evaluate the add-on effects and safety of CHM for PSS.
Methods: Five English and four Chinese databases were searched from their respective inception to 28 February 2018. We included randomized controlled trials that evaluated the add-on effects of CHM for PSS, based on changes in the scores of the (Modified) Ashworth Scale (AS or MAS), Fugl-Meyer Assessment of Sensorimotor Recovery (FMA), and Barthel Index (BI).
Results: Thirty-five trials involving 2,457 patients were included. For upper-limb AS or MAS, the estimated add-on effects of CHM to RC were significantly better when using oral (SMD −1.79, 95% CI: −3.00 to −0.57) or topical CHM (SMD −1.06, 95% CI: −1.40 to −0.72). For lower-limb AS or MAS, significant add-on benefits to RC were also detected (SMD −1.01, 95% CI: −1.43 to −0.59 and SMD −1.16, 95% CI: −1.83 to −0.49) using oral and topical CHM, respectively. For FMA and BI, better results were detected when adding CHM to RC, except for the subgroup of oral CHM for upper-limb FMA. Ten of the 35 included studies reported safety information, with two of them mentioning two mild adverse events.
Conclusions: Noting the quality concerns of the included trials, this review suggests that CHM appears to be a well-tolerated therapy for patients with PSS, and the potential add-on effects of CHM in reducing spasticity and improving the daily activities of patients with PSS require further rigorous assessment.
Spasticity can adversely impact almost half of stroke survivors (Watkins et al., 2002; Kwah et al., 2012; Zorowitz et al., 2013) and may worsen other post-stroke complications, including urinary and fecal incontinence, as well as skin infection (Bravo-Esteban et al., 2013; Martin et al., 2014; Gillard et al., 2015; Milinis and Young, 2015). In particular, spasticity can be a great barrier in rehabilitation for stroke recovery (Nair and Marsden, 2014).
Although there is uncertainty about the effects of specific rehabilitation interventions targeting post-stroke spasticity (PSS) and about the timing of their initiation, control of spasticity as soon as the patient’s posture or mobility is affected is generally encouraged (European Stroke Organization (ESO) Executive Committee and ESO Writing Committee, 2008; Miller et al., 2010; Smith et al., 2010; Stroke Foundation of New Zealand and New Zealand Guidelines Group, 2010; Chinese Society of Neurology and Stroke Prevention Project Committee of National Health and Family Planning Commission in China, 2012; National Institute for Health and Care Excellence, 2013; Nair and Marsden, 2014; Australian National Stroke Foundation, 2017). In terms of spasticity management, non-pharmaceutical intervention is preferred as first-line treatment; these include position management and manual stretching (Nair and Marsden, 2014). For some alternative therapies, such as shock wave stimulation, electrical stimulation, and repetitive transcranial magnetic stimulation, comprehensive assessment is required to confirm their effectiveness (Mally and Dinya, 2008; Stein et al., 2015; Dymarek et al., 2016a; Dymarek et al., 2016b; Dymarek et al., 2016c). When these therapies do not achieve a satisfying response, oral and invasive anti-spasticity medications could be considered (Bensmail et al., 2006; Bensmail et al., 2009; Harned et al., 2011). However, more than half of patients with PSS still suffer from moderate to severe disability after using current therapies (Sze et al., 2000), since the effectiveness is limited by a relatively short maintaining period, high costs, and unwanted adverse events, such as drowsiness and muscle weakness (European Stroke Organization (ESO) Executive Committee and ESO Writing Committee, 2008; Miller et al., 2010; Smith et al., 2010; Stroke Foundation of New Zealand and New Zealand Guidelines Group, 2010; Chinese Society of Neurology and Stroke Prevention Project Committee of National Health and Family Planning Commission in China, 2012; National Institute for Health and Care Excellence, 2013; Nair and Marsden, 2014; Australian National Stroke Foundation, 2017).
From a classical Chinese medicine perspective, PSS is also considered one of the clinical manifestations of stroke. The primarily etiology of PSS is a deficiency of qi, Blood, yin, or yang, that generates internal pathological products, such as Wind, Fire, Phlegm, or Stasis, blocking the meridian and collateral channels and resulting in the failure of nourishing tendons and muscles. Eventually, spasticity, limb stiffness, and contracture occur (Zhang and Xue, 2012). Therefore, Chinese herbal medicine (CHM) that could either restore the balance of qi, Blood, yin, and yang or clean up internal pathological products would be considered in the treatment of PSS (Zhang and Xue, 2012).
Nowadays, CHM is often administered in clinical practice as an adjunct to routine care (RC) for the treatment of PSS. Clinical research has also been increasingly conducted, with a focus on both orally or topically used CHM formulas, for PSS (Liu et al., 2014b; Zhu et al., 2014). Increasing numbers of preclinical studies have suggested that CHM single herbs and formulas are related to inhibition of certain types of neurotoxicity and certain anti-spasmodic activities (Hu et al., 2013; Huang et al., 2013; Li et al., 2014; Zhu et al., 2015).
In order to provide an overall evaluation of existing clinical evidence regarding CHM for PSS, we conducted a systematic review to address whether 1) CHM (including oral and topical CHM) in combination with RC (including pharmacotherapy and/or rehabilitation therapies) is more effective than RC alone in terms of spasticity severity, motor function, and activities of daily living; and whether 2) the use of CHM is safe.
Five English databases (PubMed, Cumulative Index to Nursing and Allied Health Literature, EMBASE, Cochrane Central Register of Controlled Trials, and Allied and Complementary Medicine Database), four Chinese databases (the Wanfang Database, Chongqing VIP Database, Chinese National Knowledge Infrastructure, and Chinese Biomedical Database), and two online clinical trial registration websites (the International Clinical Trials Registry Platform and the Chinese Clinical Trial Registry) were searched from their respective inception to February 2017, with an updated search conducted in February 2018. Related trials and systematic reviews obtained by searching the references of the included studies were also researched. The detailed search strategy is presented in Table S1; three categories of search terms were used (“Chinese herbal medicine,” “post-stroke spasticity,” and “clinical trials”). Reporting details are available in File S1.
Two researchers (YC and CZ) independently screened the titles, abstracts, and full texts to remove duplicates and irrelevant trials after applying the selection criteria. Discussion with a third reviewer (SL) was used to resolve doubt or disagreement about study inclusion. Inclusion criteria were as follows: 1) randomized controlled trials (RCTs) or quasi RCTs; 2) patients with one or multiple strokes that were confirmed by computed tomography or magnetic resonance imaging; 3) Ashworth Scale (AS) or Modified Ashworth Scale (MAS) of any joint ≥1; 4) comparison of any type of RC with or without CHM (oral CHM or topical CHM, such as steaming, compression, baths, and various external application therapies of CHM; CHM injection was not regarded as topical CHM and was not included in this study), or with placebo, with co-intervention being allowed as long as it was incorporated into all arms; and 5) studies that reported at least one of the following outcome measures: AS or MAS for spasticity severity as the primary outcome measure, Fugl-Meyer Assessment of Sensorimotor Recovery (FMA) for motor function and Barthel Index (BI) for assessment of activities of daily living as secondary outcome measures, and reporting of adverse events as a safety outcome. We excluded studies of patients with stroke symptoms caused by trauma, tumor, infection, and subdural hemorrhage or where the add-on effects of CHM could not be estimated due to the involvement of other interventions (i.e., CHM plus acupuncture plus rehabilitation therapies vs. rehabilitation therapies).
Two investigators (YC and CZ) independently extracted information on the characteristics of participants, study methods, and outcomes using a pre-designed form. A third reviewer (SL) checked all extracted data and corrected inconsistencies. If important data were unclear, unavailable, or suspected of duplication, authors of the trials were contacted via phone or emails for clarification.
Two researchers (YC and CZ) assessed the methodological quality of the included studies using the Cochrane risk-of-bias tool, following the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0). Disagreement was resolved through discussion with a third investigator (SL) when necessary. Seven domains were assessed for each study: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias.
Data synthesis was conducted using the Cochrane Review Manager software (RevMan 5.3). Mean difference (MD) and 95% confidence interval (CI) was used for continuous data, whereas the standard mean difference (SMD) was applied where the same outcome was reported using different scale ranges. Changes in AS or MAS, FMA, and BI was extracted or calculated for meta-analyses. Random-effect models were used for meta-analyses.
The source of clinical heterogeneity among included trials was explored through subgroup analyses for baseline differences in terms of stroke onset (1 or >1 times), history of stroke (≤180 or >180 days), treatment duration (≤4 or >4 weeks), and preparation of herbal interventions. In consideration of the methodological quality, sensitivity analyses were performed based on the risk-of-bias judgements. In terms of herb analysis, post hoc subgroup analyses on the primary and secondary outcomes were conducted if sufficient data were available to explore the estimated effects of the individual or combination of the top five most frequently reported oral or topical herbs identified from this review.
Publication bias was assessed with a funnel plot and Egger’s linear regression test where more than 10 trials were included in a meta-analysis.
Using the comprehensive search, 46,304 studies were identified (Figure 1). A total of 2,309 possibly relevant studies were obtained for full-text screening. Thirty-five RCTs meeting our criteria were included in the systematic review, of which 24 were included in meta-analyses. The results of five RCTs (Zhang et al., 2008; Zhao, 2010; Huang et al., 2011; Chen, 2013; Zhao, 2013) could not be synthesized into meta-analyses because their reported data were incorrect or could not be pooled. Six studies (Zhu et al., 2002; Zhu et al., 2007; Zhang, 2009; Xie et al., 2011; Zhu et al., 2013; Weng, 2014) evaluated oral plus topical CHM; their results were not pooled for meta-analysis due to the diversity of interventions.
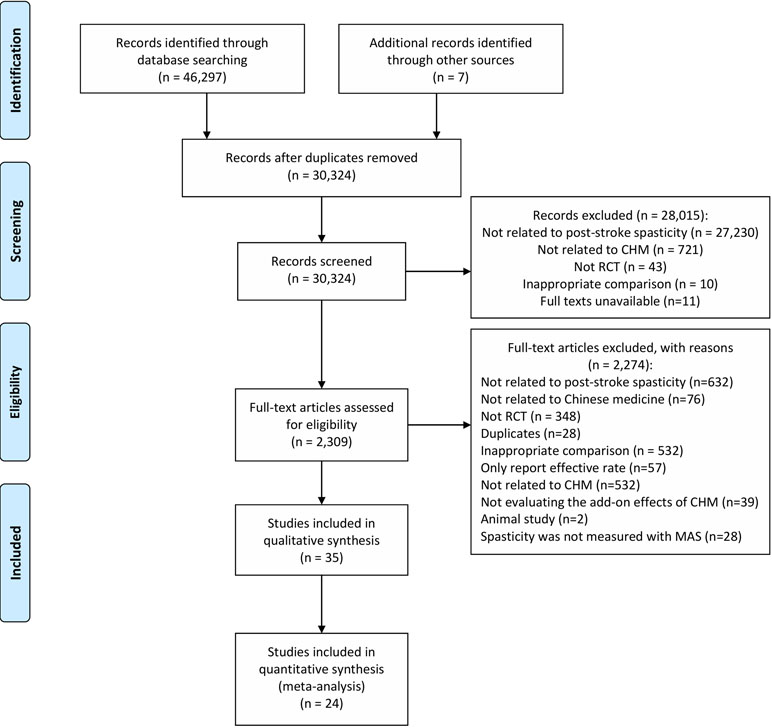
Figure 1 PRISMA flow chart. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6):e1000097. doi: 10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org.
All included RCTs were conducted in China and were published between 2002 and 2016 (Table 1). The study sample size ranged from 29 to 120. A total of 2,457 stroke patients with an average age of 61.72 years were included in these studies.
Fifteen studies (Zhang et al., 2008; Huang et al., 2011; Li and Wang, 2011; Wei et al., 2011; Zhang et al., 2012; Chen, 2013; Wang, 2013; Zhao, 2013; Chen et al., 2014; Liu et al., 2014a; Li et al., 2015; Cai, 2016; He, 2016; Murat, 2016; Zhong, 2016) investigated the add-on effects of oral CHM, 14 studies were on topical CHM (Ou et al., 2007; Shen et al., 2007; Zhang et al., 2007; Li et al., 2008; Chen et al., 2010; Zhao, 2010; Huang, 2011; Jia et al., 2012; Ou et al., 2014; Wang et al., 2014; Cao and Han, 2015; Ding, 2016; Lai, 2016; Zhang, 2016), and six studies were of oral plus topical CHM (Zhu et al., 2002; Zhu et al., 2007; Zhang, 2009; Xie et al., 2011; Zhu et al., 2013; Weng, 2014). The stroke history of the included patients was reported from 1 day to 1 year. Eight studies (Ou et al., 2007; Zhang et al., 2012; Chen, 2013; Ou et al., 2014; Weng, 2014; Ding, 2016; Murat, 2016; Zhang, 2016) only enrolled participants with a first-ever stroke. Nine trials (Zhu et al., 2002; Ou et al., 2007; Zhu et al., 2007; Zhao, 2010; Huang, 2011; Huang et al., 2011; Chen et al., 2014; Ou et al., 2014; Ding, 2016) merely included stroke patients whose AS or MAS were ≥2 at baseline. Reported CHM formulas varied greatly among the included studies, with a treatment duration ranging from 20 days to 3 months (Table 2). A variety of rehabilitation therapies were used as co-interventions (Table 3). Placebo was not used in any of the included trials. Twenty-three studies reported data on AS or MAS, 17 reported BI, and 14 reported FMA data (Table 1).
Eighteen studies (Ou et al., 2007; Shen et al., 2007; Li et al., 2008; Zhang, 2009; Chen et al., 2010; Li and Wang, 2011; Xie et al., 2011; Zhang et al., 2012; Chen, 2013; Zhu et al., 2013; Liu et al., 2014a; Ou et al., 2014; Cao and Han, 2015; Ding, 2016; Lai, 2016; Murat, 2016; Zhang, 2016; Zhong, 2016) were assessed as low risk of bias in random sequence generation with adequate methods; two trials (Zhu et al., 2007; Wang, 2013) were assessed as high risk of bias because they allocated patients based on the date of admission; others were of unclear risk due to a lack of information. Allocation was well concealed in only two studies (Chen, 2013; Lai, 2016), whereas another two (Zhu et al., 2007; Wang, 2013) were assessed as high risk of bias because participants were allocated based on their case record number. Blinding of participants and personnel was attempted in none of the included trials, but three trials (Zhang et al., 2007; Liu et al., 2014a; Weng, 2014) performed blinding in outcome assessors. All included trials were assessed as low risk of bias in incomplete outcome data. None of the included studies had prospectively registered protocols, and 10 studies (Zhang et al., 2007; Zhang et al., 2008; Li and Wang, 2011; Jia et al., 2012; Zhao, 2013; Zhu et al., 2013; Li et al., 2015; He, 2016; Murat, 2016; Zhong, 2016) did not report the results of all pre-defined outcomes mentioned in the Methods sections. Risk-of-bias assessment is summarized in Figure 2.
Results of meta-analyses are presented below for oral CHM and topical CHM, separately (Table 4).
Significant add-on effects of oral CHM were found in terms of changes in scores of AS or MAS of the upper limbs (three studies: Zhang et al., 2012; Liu et al., 2014a; Cai, 2016; SMD −1.79, 95% CI: −3.00 to −0.57, I2 = 94%) and lower limbs (three studies: Wang, 2013; Liu et al., 2014a; Cai, 2016; SMD −1.01, 95% CI: −1.43 to −0.59, I2 = 55%), although with moderate to high heterogeneity (Table 4 and Figure 3).
In terms of the improvement of overall motor function measured using the FMA, combining oral CHM and RC was estimated to be significantly superior to RC alone (three studies: Li and Wang, 2011; Wang, 2013; He, 2016; MD 12.14, 95% CI: 1.57 to 22.71, I2 = 89%) (Table 4 and Figure 4). Similarly, benefits of adding oral CHM to RC were seen in the FMA score changes for the lower extremities (two studies: Wei et al., 2011; Chen et al., 2014; MD 4.03, 95% CI:1.90 to 6.17, I2 = 61%), but not in that of the upper limbs (three studies: Wei et al., 2011; Chen et al., 2014; Li et al., 2015; MD 7.64, 95% CI: −1.29 to 16.57, I2 = 97%).
Seven included trials (Li and Wang, 2011; Chen et al., 2014; Liu et al., 2014; Li et al., 2015; He, 2016; Murat, 2016; Zhong, 2016) reported changes to the BI results and were pooled for meta-analysis. Results showed that the combination of oral CHM and RC yielded more improvement in the BI than RC alone (MD 13.15, 95% CI: 4.37 to 21.93), although with high heterogeneity (I2 = 98%) (Table 4 and Figure 5).
Compared to RC alone, adding topical CHM further decreased AS or MAS in the upper limbs (eight studies: Shen et al., 2007; Li et al., 2008; Chen et al., 2010; Huang, 2011; Wang et al., 2014; Ding, 2016; Lai, 2016; Zhang, 2016; SMD −1.06, 95% CI: −1.40 to −0.72, I2 = 72%) and lower limbs (five studies: Ou et al., 2007; Shen et al., 2007; Chen et al., 2010; Huang, 2011; Wang et al., 2014; SMD −1.16, 95% CI: −1.83 to −0.49, I2 = 84%) (Table 4), although with high heterogeneity detected in both analyses.
Synthesis of FMA (total motor function) changes from two trials (Zhang et al., 2007; Jia et al., 2012) showed superior effects of topical CHM combined with RC compared to RC alone (MD 5.56, 95% CI: 2.38 to 8.74, I2 = 0%) (Table 4). Similarly, meta-analysis results of another two studies (Ou et al., 2014; Lai, 2016) showed greater improvement in FMA (upper-limb motor function) with topical CHM added to RC (MD 5.88, 95% CI: 4.09 to 7.68, I2 = 0%) than with RC alone (Table 4).
Compared to RC alone, a combination of topical CHM and RC further improved BI results, as shown in a meta-analysis of six studies (Huang, 2011; Jia et al., 2012; Wang et al., 2014; Cao and Han, 2015; Lai, 2016; Zhang, 2016) (MD 12.01, 95% CI: 2.81 to 21.22, I2 = 99%) (Table 4).
In total, 10 of the included studies addressed the safety of CHM; the remaining 25 studies did not provide information on adverse events. One study (Lai, 2016) reported one case of skin allergy in the intervention group receiving topical CHM. Although the symptom was evaluated as mild by the physician and was alleviated after 3 days, the patient dropped out of the study due to this event, without further confirmation of causality. Another study reported one patient in the treatment group of topical CHM who experienced transient influenza-like symptoms after Botox injection (Ou et al., 2014), which was considered not related to the use of CHM.
Due to the limited number of included studies, there were insufficient data for subgroup analysis. In terms of sensitivity analysis of BI synthesis results, when only studies with low risk of bias in sequence generation were included, significant results remained and heterogeneity reduced to 66% (four studies: Li and Wang, 2011; Liu et al., 2014a; Murat, 2016; Zhong, 2016; MD 7.81, 95% CI: 4.31 to 11.31) (Table 5).
For AS or MAS in the upper extremity, heterogeneity reduced to 0% in the subgroup where only patients with first-stroke onset were included. Due to the limited number of studies, subgroup analysis on FMA was not possible. With regard to BI, the subgroup where patients were within 180 days after stroke (three studies: Jia et al., 2012; Wang et al., 2014; Zhang, 2016; SMD 19.14, 95% CI: 17.29 to 20.98, I2 = 43%) demonstrated a greater effect than observed in the subgroup of patients with a post-stroke period exceeding 180 days (three studies: Huang, 2011; Cao and Han, 2015; Lai, 2016; SMD 3.53, 95% CI: 0.51 to 6.54, I2 = 43%) (Table 6). In terms of the administration of CHM, an add-on effect was detected when the CHM was used as steaming therapy for the outcomes of lower-limb AS or MAS (SMD −1.22, 95% CI: −2.06 to −0.39, I2 = 82%) and BI (MD 17.12, 95% CI: 11.92 to 22.32, I2 = 82%), while there was no add-on benefit for AS or MAS of lower limb (SMD −1.09, 95% CI: −2.52 to 0.34, I2 = 91%) and BI (MD8.98, 95% CI: −2.81 to 20.76, I2 = 99%) when CHM was used for compression.
Sensitivity analysis of studies with low risk of bias for sequence generation showed that the significant treatment effects remained, while heterogeneity reduced for the score changes for upper-limb AS or MAS (six studies: Shen et al., 2007; Li et al., 2008; Chen et al., 2010; Ding, 2016; Lai, 2016; Zhang, 2016; SMD −0.86, 95% CI: −1.14 to −0.58, I2 = 50%) (Table 5), as well as that of lower-limb (three studies: Ou et al., 2007; Shen et al., 2007; Chen et al., 2010; SMD −0.61, 95% CI: −0.96 to −0.27, I2 = 0%) (Table 5).
None of the above meta-analyses included more than 10 trials; therefore, publications bias was not evaluated.
Bai Shao (Paeonia lactiflora Pall.) was the most frequently used oral herb, reported by 17 studies, followed by Gan Cao (Glycyrrhiza uralensis Fisch.) (Table 7). Shen Jin Cao (Lycopodium japonicum Thunb.) and Dang Gui [Angelica sinensis (Oliv.) Diels] were among the most frequently reported topical herbs in the included studies (Table 7). In fact, the combination of Bai Shao and Gan Cao is a traditional oral CHM formula termed Shao Yao Gan Cao Tang (SYGCT), which was reported to have anti-spasticity activity (Zhang et al., 2015).
For topical herbs, post hoc subgroup analysis was conducted to estimate the effects of individual and the combination of the top five most frequently reported herbal ingredients in the included studies. Table 6 summarizes the results with significant between-subgroup differences in post hoc analysis of herbs. Superior effects were detected in the subgroup of studies in which the formulas included Bai Shao than in subgroups of studies without Bai Shao, in terms of AS or MAS for the lower limbs (SMD −1.55, 95% CI: −2.29 to −0.82, I2 = 79%) and BI (MD 18.01, 95% CI: 14.91 to 21.12, I2 = 75%). Similarly, subgroups of studies using Dang Gui might have greater benefits than those without this herb, in terms of upper-limb AS or MAS (SMD −1.21, 95% CI: −1.58 to −0.83, I2 = 71%) and BI (MD 18.01, 95% CI: 14.91 to 21.12, I2 = 75%). It is worth noting that formulas containing three ingredients (Bai Shao, Dang Gui, and Hong Hua) demonstrated a trend for greater efficacy in terms of the three outcome measures: AS or MAS in upper limbs (SMD −1.56, 95% CI: −1.94 to −1.17, I2 = 43%), AS or MAS in lower limbs (SMD −1.93, 95% CI: −2.34 to −1.53, I2 = 0%), and BI (MD 18.01, 95% CI: 14.91 to 21.12, I2 = 75%) than the formulas without these herbs, with reduced heterogeneity.
The results of this systematic review suggested that adding oral or topical CHM to RC for PSS is beneficial for reducing muscle spasticity in the upper and lower extremities. For the overall and lower-limb motor score of FMA and BI, significant add-on effects were observed for both oral and topical CHM. In contrast, no significant effects were seen when adding oral CHM to RC for upper-limb motor function. Mild self-healing adverse events were reported in the intervention group receiving topical CHM; the connections of the CHMs to the adverse events had, however, not been explored.
In our analyses, the changes in AS or MAS scores were merged for analysis using SMD; therefore, the minimum detectable difference or minimum clinically important difference (MCID) was not applied to its clinical interpretation (Figure 3). With regard to upper-extremity FMA, both minimum detectable difference and MCID were found to be 5.2 (Wagner et al., 2008; Page et al., 2012). MCID for overall, upper-limb, and lower-limb FMA was found to be 6.0, 4.58, and 3.31, respectively, in another study (Chen et al., 2015). In fact, the changes in the total motor, upper-limb, and lower-limb FMA scores in intervention groups (oral or topical CHM plus RC) and control groups (RC alone) were all greater than the MCID (Figure 4). In terms of BI, the minimum detectable difference (4.02 points) (Hsieh et al., 2007) was established and used for interpretation of our results. The use of oral CHM with RC demonstrated clinical advantages for PSS in terms of BI when compared to RC alone (Figure 5). The reasons for inconsistent effects among different outcomes should be cautiously interpreted: first, the relatively small numbers of participants and included studies and high heterogeneity limited our confidence in these results; second, a decrease in spasticity severity might not necessarily lead to improvement in motor function (Li, 2017); third, other factors, such as muscle strength, might also contribute to changes in the results, particularly motor function and activities of daily living (Langhammer et al., 2007; Harvey, 2015; Nunes et al., 2016).
This review attempted to explore the characteristics of PSS patients who would benefit from adding CHM therapies to RC, such as the time of stroke onset and the post-stroke period. The results of subgroup analyses suggested that patients with spasticity within 180 days post-stroke might benefit more from additional topical CHM treatment. As for specific CHM treatment, further exploration of potential formulas was not applicable because of the diversity of formulas used in the included studies (Table 2). Therefore, we summarized the most frequently reported herbs and conducted subgroup analysis for individual and combinations of herbal ingredients used in the included studies. For oral CHM, Bai Shao and Gan Cao were the most frequently used herbs, although a subgroup analysis supporting the use of these two herbs was not possible. In terms of topical CHM, a combination of Bai Shao, Dang Gui, and Hong Hua demonstrated a promising therapeutic add-on effect for spasticity reduction and an improvement in activities of daily living. Specifically, for the preparation of topical CHM, steaming therapy with CHM showed a trend for better improvement than CHM compression therapy (Table 6). It is worth noting that confounding variables might also have an impact on the results, due to the complexity of the application of topical CHM. For instance, the overall treatment effects of steaming may be a combined result of CHM, steaming water, and heat. Therefore, to distinguish and confirm individual therapeutic efficacy of CHM requires further assessment. Treatment duration was reported to range from 20 days to 3 months among the included trials (Table 2). However, subgroup analysis of treatment with a pre-defined cutoff of 4 weeks’ duration was not applicable. Moreover, all participants enrolled in the included studies had already developed spasticity, with AS or MAS ≥ 1. Thus, the effects of CHM on patients at a very early post-stroke stage, when spasticity is not yet detectable with MAS, cannot be known based on the results of this review. Furthermore, all participants enrolled in the included trials were Chinese, and thus the generalizability of the results is not known; additional evidence of using CHM therapy on a non-Chinese population is therefore required.
Neuroprotective activity, exerted via activation of the adenosine A1 receptor, was observed with paeoniflorin extracted from Bai Shao (Liu et al., 2005; Zhang et al., 2009; Tang et al., 2010; Zhang et al., 2017). In terms of Gan Cao, potential neuroprotection by one of its major ingredients, glycyrrhizin, was mediated by anti-inflammatory effects via inhibition of HMGB1 secretion and inhibition of neurotoxicity by suppression of glutamate-induced apoptosis (Kim et al., 2012b). Triterpene saponins and Licochalcone E in Gan Cao were observed to have protective effects against neurotoxicity through suppression of glutamate-induced apoptosis (Cherng et al., 2006; Hwang et al., 2006) and activation of the Nrf2/antioxidant-response element signaling pathway (Kim et al., 2012a). Another bioactive component, Licochalcone A, was shown to have anti-spasmodic activity alone and when combined with paeoniflorin, potentially through inhibition of phosphodiesterases (Sato et al., 2006; Nagai et al., 2007) and by decreasing excitatory amino acid content, respectively (Kimura et al., 1984; Zhang et al., 2015). The ingredients with anti-neurotoxicity effects in Dang Gui include polysaccharides, organic acids, and phthalides. Potential mechanisms include decreased expression of nicotinic acetylcholine receptors (Gu et al., 2008) and increased brain-derived neurotrophic factor and nerve growth factor protein expression (Chen et al., 2009). Similarly, neuroprotective function could also be observed for ingredients of Hong Hua (He et al., 2012; Yu et al., 2013; Zhang et al., 2016). A combination of topically used Dang Gui, Hong Hua, and Bai Shao demonstrated a promising benefit for PSS (Table 6), but the underlying mechanism is yet to be unveiled. Representative examples of major neurological effects and potential mechanisms are summarized in Table 8.
However, various formulas with complex compounds were used in the included studies, and the potentially active ingredients isolated from CHM usually act on different mechanisms and pathways. There is no direct research evidence from studies on human skeletal muscles using these bioactive ingredients to exploring the underlying mechanisms of the effects on spasticity specifically; spasticity is characterized by a velocity-dependent increase in tonic stretch reflexes (Lance, 1980). Therefore, the causal relationship between the observed therapeutic effects on PSS and the individual components or monomolecular substance targeting at a few known cellular or molecular pathways could not be confirmed. Further mechanistic and clinical studies are needed to elucidate how the bioactive CHM ingredients work individually and interactively, to optimize and even standardize CHM components and treatment protocols in future.
Safety and long-term tolerance of therapy are a concern in the treatment of PSS (Nair and Marsden, 2014). Our systematic review suggested that oral and topical CHM were well tolerated during a treatment period as long as 3 months, with mild adverse events among 10 studies (Zhu et al., 2002; Shen et al., 2007; Zhu et al., 2007; Zhang, 2009; Chen et al., 2010; Huang, 2011; Zhao, 2013; Ou et al., 2014; Weng, 2014; Lai, 2016) (Table 1). However, the remaining 25 studies did not address the safety issue and none of the included studies covered a follow-up period, making the assessment of long-term safety inapplicable based on the results of our systematic review.
Proper randomization and allocation are essential for reducing selection bias in RCTs. However, in this systematic review, only 51.4% of the included studies applied appropriate methods for sequence generation, and only 5.7% did so for allocation concealment (Figure 2). Both of these deficits might lead to underestimation or overestimation of the treatment effects (Pildal et al., 2007). It is worth noting that none of the included trials attempted to blind participants or personnel with the use of an appropriate placebo, and outcome assessors were blinded in only three studies (Zhang et al., 2007; Liu et al., 2014a; Weng, 2014). Admittedly, there is no easy way to perform double-blinding with oral or topical CHM therapies, whose preparation, appearance, taste, and smell are so diverse that placebo control might be difficult. In the context of this challenge with decoction, other forms of oral CHM could be considered if applicable, such as granule, capsule, or dropping pills. In addition, given the improvement in the preparation of CHM and the extraction technique of active components, lipophilic compounds of herbs, such as Tanshinone, that could not be efficiently extracted through traditional decoction, might be available with supercritical carbon dioxide (Esquivel-Hernandez et al., 2016; Sulniute et al., 2017). With such techniques, the effective compounds can be extracted more efficiently, and the quality control of CHM products can be improved. Moreover, the use of more advanced CHM products may make the double-blinded, placebo-controlled trial design feasible. Furthermore, tests of blinding with placebo are needed before conducting a randomized control trial, and evaluation of addition of CHM efficacy as compared with placebo, added to rehabilitation therapies or pharmacotherapies, are required, especially for non-objective outcome assessments.
Another limitation of the synthesis results is the reporting quality of the included studies. None of the included studies reported all key items recommended by CONSORT 2010 and its Extension for Herbal Intervention and Chinese Herbal Medicine Formulas (Gagnier et al., 2006; Schulz et al., 2010; Cheng et al., 2017). Even among those reported in original studies, ambiguous terms were frequently seen (Table 2). For example, instead of specific volume, “dose” was frequently used in the reporting of oral CHM interventions. The reported oral solutions in the included studies are difficult to distinguish clearly from decoction, whose scope is yet to be specifically defined. Therefore, future trials need to improve reporting quality, and specific definitions and standardization of CHM interventions require further research and agreement.
We identified one published systematic review and meta-analysis investigating the effects of the oral CHM formula SYGCT for PSS (Chen and Tan, 2016). Ten RCTs involving 732 participants were included in that meta-analysis through a database search from January 1990 to November 2015. The Jadad scale was used to assess the methodological quality of the included studies. Based on the synthesis results of FMA and BI, the review concluded that the decoction SYGCT had potential benefits for patients with PSS. However, because different comparisons, such as SYGCT vs. RC, and SYGCT add-on to RC vs. RC, were pooled into one meta-analysis, this conclusion was not confirmed. Moreover, that review did not evaluate the outcome related to the severity of spasticity. Our systematic review and meta-analysis differed from this previous systematic review in the following ways: First, our review focused specifically on the add-on effects of CHM, including oral and topical CHM for PSS; second, comprehensive outcome measures were evaluated in terms of spasticity severity, motor function, and activities of daily living; third, our research provided up-to-date evidence by performing a search from database inception to February 2018; fourth, the Cochrane risk-of-bias tool was used for methodological quality assessment, since the validity of the total score of the Jadad scale has increasingly been challenged (Emerson et al., 1990; Schulz et al., 1995; Juni et al., 1999).
Within the limitations of the quality concerns of the included trials, this review suggested that CHM is a well-tolerated potential add-on therapy for patients with PSS. Future trials of high methodological quality with prospectively registered protocols and valid placebo control are needed to confirm the add-on effectiveness of CHM in reducing spasticity and improving daily activities.
Protocol registration information: PROSPERO 2016: CRD42016043281. Available: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=43281
CL and CX initiated the research. YC, CZ, and SL conducted the database search, study screening, data extraction, and data analyses. AZ, ZW, and XG were involved in data analysis and interpretation and in resolving disagreements. YC and CZ drafted the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
This work was supported by 1) “Construction of High-level University” Public Projects from Guangzhou University of Chinese Medicine [no. (2016) 64]; 2) “Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (2016)” (no. YN2016QL01); 3) “National Key Technology R&D Program for the 12th Five-year Plan of Ministry of Science and Technology, China” (no. 2013BAI02B10); and 4) China–Australia International Research Centre for Chinese Medicine, funded by the Guangdong Provincial Academy of Chinese Medical Sciences and Guangdong Provincial Hospital of Chinese Medicine, Guangdong, China, and RMIT University, Australia. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Authors’ positions are partially supported by the above funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AS, Ashworth Scale; BI, Barthel Index; CHM, Chinese herbal medicine; CI, confidence interval; FMA, Fugl-Meyer Assessment; MAS, Modified Ashworth Scale; MCID, minimum clinically important difference; MD, mean difference; PSS, post-stroke spasticity; RC, routine care; RCT(s), randomized controlled trial(s); SD, standard difference; SMD, standard mean difference; SYGCT, Shao Yao Gan Cao Tang.
We appreciate the assistance of the research students involved in database searches and data extraction. We would like to thank Editage (www.editage.com) for English language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00734/full#supplementary-material
Australian National Stroke Foundation. 2017. Clinical guidelines for stroke management. Available: https://www.magicapp.org/app#/guideline/2148 [Accessed March 28, 2018].
Bensmail, D., Quera Salva, M. A., Roche, N., Benyahia, S., Bohic, M., Denys, P., et al. (2006). Effect of intrathecal baclofen on sleep and respiratory function in patients with spasticity. Neurology 67 (8), 1432–1436. doi: 10.1212/01.wnl.0000239827.38036.23
Bensmail, D., Ward, A. B., Wissel, J., Motta, F., Saltuari, L., Lissens, J., et al. (2009). Cost-effectiveness modeling of intrathecal baclofen therapy versus other interventions for disabling spasticity. Neurorehabil. Neural Repair 23 (6), 546–552. doi: 10.1177/1545968308328724
Bravo-Esteban, E., Taylor, J., Abian-Vicen, J., Albu, S., Simon-Martinez, C., Torricelli, D., et al. (2013). Impact of specific symptoms of spasticity on voluntary lower limb muscle function, gait and daily activities during subacute and chronic spinal cord injury. NeuroRehabilitation 33 (4), 531–543. doi: 10.3233/nre-131000
Cai, Y. F. (2016). Effects of combined therapy of traditional Chinese medicine and western medicine on spasticity of hemiparalysis after stroke [Article in Chinese]. China Health Standard Management 17 (22), 118–121. doi: 10.3969/j.issn.1674-9316.2016.22.071
Cao, Y. L., Han, Z. C. (2015). A study of hot compression therapy with Chinese herbal medicine for post-stroke spasticity [Article in Chinese]. Shaanxi J Tradit Chin Med. 36 (10), 1344–1345. doi: 10.3969/j.issn.1000-7369.2015.10.040
Chen, J., Zhang, G. Q., Zhou, X. M., Yang, C. Q. (2010). A study of fumigation of Chinese herbal Medicine combined with Baclofen for post-stroke spasticity [Article in Chinese]. Chin. J. Rehabil. Theory Pract. 16 (2), 170–171. doi: 10.3969/j.issn.1006-9771.2010.02.024
Chen, L. G., Zhu, J. D., Chen, J., Sun, F., Huang, F. F. (2014). Clinical observation on Jiawei Buyang Huanwu decoction combined with rehabilitation training on cerebral infarction of spastic period [Article in Chinese]. Chin. Manipulation Rehabil. Med. 5 (5), 21–22.
Chen, R. Q., Wu, J. X., Shen, X. S. (2015). A research on the minimal clinically important diferences of Chinese version of the Fugl-Meyer Motor Scale [Article in Chiense]. Acta Universitatis Medicinalis Anhui 50 (4), 4. doi: 10.19405/j.cnki.issn1000-1492.2015.04.025
Chen, Y., Cao, B., Mei, X. M., Cao, K., Yu, H. (2009). The influence of intrauterine hypoxia in different time on rat embryo neural stem cells and the protective effect of Chinese Angelica [Article in Chinese]. Lishizhen Med. Mater. Med. Res. 20 (7), 1642–1644. doi: 10.3969/j.issn.1008-0805.2009.07.037
Chen, Y. L. 2013. [A clinical study of Gua Lou Gui Zhi decoction for lower-limb spasticity after stroke] [Article in Chinese]. [master’s thesis]. [Fuzhou (Fujian)]: Fujian University of Traditional Chinese Medicine. Available: http://cdmd.cnki.com.cn/Article/CDMD-10393-1013186325.htm [Accessed March 28 2018].
Chen, Z. W., Tan, Z. H. (2016). Systematic review of stroke spastic hemiplegia patients treated by Paeoniae-Glycyrrhizae decoction [Article in Chinese]. Yunnan J Tradit Chin Med. Mater. Med. 37 (2), 23–27. doi: 10.16254/j.cnki.53-1120/r.2016.02.008
Cheng, C. W., Wu, T. X., Shang, H. C., Li, Y. P., Altman, D. G., Moher, D., et al. (2017). CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann. Intern. Med. 167 (2), 112–121. doi: 10.7326/M16-2977
Cherng, J. M., Lin, H. J., Hung, M. S., Lin, Y. R., Chan, M. H., Lin, J. C. (2006). Inhibition of nuclear factor kappaB is associated with neuroprotective effects of glycyrrhizic acid on glutamate-induced excitotoxicity in primary neurons. Eur. J. Pharmacol. 547 (1–3), 10–21. doi: 10.1016/j.ejphar.2006.06.080
Chinese Society of Neurology and Stroke Prevention Project Committee of National Health and Family Planning Commission in China (2012). China post-stroke rehabilitation guideline [Article in Chinese]. Chin. J. Rehabil. Theory Pract. 18 (18), 55–76. doi: 10.3969/j.issn.1006-9771.2012.04.001
Ding, X. (2016). A study of hot compression therapy with Chinese herbal medicine for post-stroke spasticity in upper extremity [Article in Chinese]. Heber J. Tradit. Chin. Med. 28 (3), 367–402. doi: 10.3969/j.issn.1002-2619.2016.03.012
Dymarek, R., Ptaszkowski, K., Slupska, L., Halski, T., Taradaj, J., Rosinczuk, J. (2016a). Effects of extracorporeal shock wave on upper and lower limb spasticity in post-stroke patients: a narrative review. Top Stroke Rehabil. 23 (4), 293–303. doi: 10.1080/10749357.2016.1141492
Dymarek, R., Taradaj, J., Rosinczuk, J. (2016b). The effect of radial extracorporeal shock wave stimulation on upper limb spasticity in chronic stroke patients: a single-blind, randomized, placebo-controlled study. Ultrasound Med. Biol. 42 (8), 1862–1875. doi: 10.1016/j.ultrasmedbio.2016.03.006
Dymarek, R., Taradaj, J., Rosinczuk, J. (2016c). Extracorporeal shock wave stimulation as alternative treatment modality for wrist and fingers spasticity in poststroke patients: a prospective, open-label, preliminary clinical trial. Evid. Based Complement. Alternat. Med. 2016, 4648101. doi: 10.1155/2016/4648101
Emerson, J. D., Burdick, E., Hoaglin, D. C., Mosteller, F., Chalmers, T. C. (1990). An empirical study of the possible relation of treatment differences to quality scores in controlled randomized clinical trials. Control Clin. Trials 11 (5), 339–352. doi: 10.1016/0197-2456(90)90175-2
Esquivel-Hernandez, D. A., Lopez, V. H., Rodriguez-Rodriguez, J., Aleman-Nava, G. S., Cuellar-Bermudez, S. P., Rostro-Alanis, M., et al. (2016). Supercritical carbon dioxide and microwave-assisted extraction of functional lipophilic compounds from Arthrospira platensis. Int. J. Mol. Sci. 17 (5), 658. doi: 10.3390/ijms17050658
European Stroke Organisation (ESO) Executive Committee, and ESO Writing Committee (2008). Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 25 (5), 457–507. doi: 10.1159/000131083
Gagnier, J. J., Boon, H., Rochon, P., Moher, D., Barnes, J., Bombardier, C. (2006). Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann. Intern. Med. 144 (5), 364–367. doi: 10.7326/0003-4819-144-5-200603070-00013
Gillard, P. J., Sucharew, H., Kleindorfer, D., Belagaje, S., Varon, S., Alwell, K., et al. (2015). The negative impact of spasticity on the health-related quality of life of stroke survivors: a longitudinal cohort study. Health Qual. Life Outcomes 13, 159. doi: 10.1186/s12955-015-0340-3
Gu, R., Liu, R. Y., Zhang, L. J., Hao, X. Y., Xiao, Y., Qi, X. L., et al. (2008). Antagonist effect of Angelica on neurotoxicity induced by β-amyloid pepitide in SH-SY5Y cells [Article in Chinese]. Lishizhen Med. Mater. Med. Res. 19 (7), 1554–1556. doi: 10.3969/j.issn.1008-0805.2008.07.004
Harned, M. E., Salles, S. S., Grider, J. S. (2011). An introduction to trialing intrathecal baclofen in patients with hemiparetic spasticity: a description of 3 cases. Pain Physician 14 (5), 483–489.
Harvey, R. L. (2015). Predictors of functional outcome following stroke. Phys. Med. Rehabil. Clin. N. Am. 26 (4), 583–598. doi: 10.1016/j.pmr.2015.07.002
He, Y., Wan, H., Du, Y., Bie, X., Zhao, T., Fu, W., et al. (2012). Protective effect of Danhong injection on cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 144 (2), 387–394. doi: 10.1016/j.jep.2012.09.025
He, Y. C. (2016). A study of Tong Luo Jie Jing decoction combined with rehabilitation therapies for post-stroke spasticity [Article in Chinese]. Chin. J. Clin. Ration. Drug Use 9 (7), 42–43. doi: 10.15887/j.cnki.13-1389/r.2016.20.027
Hsieh, Y. W., Wang, C. H., Wu, S. C., Chen, P. C., Sheu, C. F., Hsieh, C. L. (2007). Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil. Neural Repair 21 (3), 233–238. doi: 10.1177/1545968306294729
Hu, H., Li, Z., Zhu, X., Lin, R., Peng, J., Tao, J., et al. (2013). GuaLou GuiZhi decoction inhibits LPS-induced microglial cell motility through the MAPK signaling pathway. Int. J. Mol. Med. 32 (6), 1281–1286. doi: 10.3892/ijmm.2013.1522
Huang, J., Tao, J., Xue, X., Yang, S., Han, P., Lin, Z., et al. (2013). Gua Lou Gui Zhi decoction exerts neuroprotective effects on post-stroke spasticity via the modulation of glutamate levels and AMPA receptor expression. Int. J. Mol. Med. 31 (4), 841–848. doi: 10.3892/ijmm.2013.1262
Huang, J. X. (2011). The clinical research of Shujin Tongluo Fang fumigating and laundering with rehabilitation training to improve the limb spasm after stroke [Article in Chinese]. China J. Chin. Med. 26 (7), 859–860.
Huang, W., Lv, W. H., Pang, L., Jiang, Y. J. (2011). A study of Wen Jing Shu Jin capsule for post-stroke spasticity [Article in Chinese]. Shandong J. Tradit. Chin. Med. 30 (2), 91–92.
Hwang, I. K., Lim, S. S., Choi, K. H., Yoo, K. Y., Shin, H. K., Kim, E. J., et al. (2006). Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacol. Sin. 27 (8), 959–965. doi: 10.1111/j.1745-7254.2006.00346.x
Jia, A. M., Hu, W. M., Zhang, H., Liu, Y., Diao, F. S., Bi, W. L., et al. (2012). Clinical research of Jiejingzhitongfang herbal fumigating to relieve post-stroke limb spasm with pain [Article in Chinese]. Chin. J. Difficult Complicated Cases 11 (5), 346–348. doi: 10.3969/j.issn.1671-6450.2012.05.008
Juni, P., Witschi, A., Bloch, R., Egger, M. (1999). The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282 (11), 1054–1060. doi: 10.1001/jama.282.11.1054
Kim, S. S., Lim, J., Bang, Y., Gal, J., Lee, S. U., Cho, Y. C., et al. (2012a). Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem. 23 (10), 1314–1323. doi: 10.1016/j.jnutbio.2011.07.012
Kim, S. W., Jin, Y., Shin, J. H., Kim, I. D., Lee, H. K., Park, S., et al. (2012b). Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol. Dis. 46 (1), 147–156. doi: 10.1016/j.nbd.2011.12.056
Kimura, M., Kimura, I., Takahashi, K., Muroi, M., Yoshizaki, M., Kanaoka, M., et al. (1984). Blocking effects of blended paeoniflorin or its related compounds with glycyrrhizin on neuromuscular junctions in frog and mouse. Jpn. J. Pharmacol. 36 (3), 275–282. doi: 10.1254/jjp.36.275
Kwah, L. K., Harvey, L. A., Diong, J. H., Herbert, R. D. (2012). Half of the adults who present to hospital with stroke develop at least one contracture within six months: an observational study. J. Physiother. 58 (1), 41–47. doi: 10.1016/S1836-9553(12)70071-1
Lai, M. X. (2016). Effect of the Ke Jing Fang moist heat on the spasticity of the upper limb in patients with stroke [Article in Chinese]. [master’s thesis]. [Fuzhou (Fujian)]: University of Traditional Chinese Medicine. Available: http://cdmd.cnki.com.cn/Article/CDMD-10393-1017005696.htm [Accessed March 28 2018].
Lance, J. (1980). “Spasticity: disorders motor control,” in Symposium Synopsis. Eds. Feldman, R. G., Young, R. P., Koella, W. P. (Miami, FL: Year Book Medical Publishers).
Langhammer, B., Lindmark, B., Stanghelle, J. K. (2007). Stroke patients and long-term training: is it worthwhile? A randomized comparison of two different training strategies after rehabilitation. Clin. Rehabil. 21 (6), 495–510. doi: 10.1177/0269215507075207
Li, S. (2017). Spasticity, motor recovery, and neural plasticity after stroke. Front. Neurol. 8, 120. doi: 10.3389/fneur.2017.00120
Li, X., Zhong, G. L., Wang, L. H. (2008). A study of fumigation therapy with Chinese herbal medicine for post-stroke spasticity [Article in Chinese]. Chin. J. Misdiagnostics 8 (31), 7626–7627. doi: 10.3969/j.issn.1009-6647.2008.31.058
Li, Y., Wang, S. L. (2011). A study of Tong Luo Jie Jing decoction combined with rehabilitation therapies for post-stroke spasticity in forty cases [Article in Chinese]. Tradit. Chin. Med. Res. 24 (4), 36–37. doi: 10.3969/j.issn.1001-6910.2011.04.018
Li, Y., Yue, J. J., Zheng, X. L., Wang, B. S. (2015). A study of Yi Qi Rou Jin decoction combined with occupational therapies for post-stroke upper-limb spasticity [Article in Chinese]. Mongol J. Tradit. Chin. Med. 34 (10), 23. doi: 10.16040/j.cnki.cn15-1101.2015.10.024
Li, Z., Hu, H., Lin, R., Mao, J., Zhu, X., Hong, Z., et al. (2014). Neuroprotective effects of Gua Lou Gui Zhi decoction against glutamate-induced apoptosis in BV-2 cells. Int. J. Mol. Med. 33 (3), 597–604. doi: 10.3892/ijmm.2013.1612
Liu, D. Z., Xie, K. Q., Ji, X. Q., Ye, Y., Jiang, C. L., Zhu, X. Z. (2005). Neuroprotective effect of paeoniflorin on cerebral ischemic rat by activating adenosine A1 receptor in a manner different from its classical agonists. Br. J. Pharmacol. 146 (4), 604–611. doi: 10.1038/sj.bjp.0706335
Liu, S. Y., Lin, B., Jin, C. J., Xi, Z. Z., Ding, H. M. (2014a). A study of Chinese herbal medicine combined with rehabilitation therapies for post-stroke spasticity [Article in Chinese]. Zhejiang J. Integr Tradit. Chin. West. Med. 24 (9), 796–797. doi: 10.3969/j.issn.1005-4561.2014.09.021
Liu, X., Meng, Q., Yu, D., Zhao, X., Zhao, T. (2014b). Novel medical bathing with traditional Chinese herb formula alleviates paraplegia spasticity. Int. J. Nurs. Pract. 20 (3), 227–232. doi: 10.1111/ijn.12145
Mally, J., Dinya, E. (2008). Recovery of motor disability and spasticity in post-stroke after repetitive transcranial magnetic stimulation (rTMS). Brain Res. Bull. 76 (4), 388–395. doi: 10.1016/j.brainresbull.2007.11.019
Martin, A., Abogunrin, S., Kurth, H., Dinet, J. (2014). Epidemiological, humanistic, and economic burden of illness of lower limb spasticity in adults: a systematic review. Neuropsychiatr. Dis. Treat. 10, 111–122. doi: 10.2147/NDT.S53913
Milinis, K., Young, C. A. (2016). Systematic review of the influence of spasticity on quality of life in adults with chronic neurological conditions. Disabil. Rehabil. 38 (15), 1431–1441. doi: 10.3109/09638288.2015.1106592
Miller, E. L., Murray, L., Richards, L., Zorowitz, R. D., Bakas, T., Clark, P., et al. (2010). Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke 41 (10), 2402–2448. doi: 10.1161/STR.0b013e3181e7512b
Murat, U. (2016). The observation on the clinic treatment effect of nourishment venation spasm relief method for spastic hemiplegia after stroke [Article in Chinese]. [master’s thesis]. [Urumchi (Xinjiang)]: Xinjiang Medical University. Available: http://cdmd.cnki.com.cn/Article/CDMD-10760-1016115449.htm [Accessed March 28 2018].
Nagai, H., He, J. X., Tani, T., Akao, T. (2007). Antispasmodic activity of licochalcone A, a species-specific ingredient of Glycyrrhiza inflata roots. J. Pharm. Pharmacol. 59 (10), 1421–1426. doi: 10.1211/jpp.59.10.0013
Nair, K. P., Marsden, J. (2014). The management of spasticity in adults. BMJ 349, g4737. doi: 10.1136/bmj.g4737
National Institute for Health and Care Excellence. 2013. Stroke rehabilitation in adults clinical guideline. Available: https://www.nice.org.uk/guidance/cg162 [Accessed 20 January 2016].
Nunes, M. F., Hukuda, M. E., Favero, F. M., Oliveira, A. B., Voos, M. C., Caromano, F. A. (2016). Relationship between muscle strength and motor function in Duchenne muscular dystrophy. Arq. Neuropsiquiatr. 74 (7), 530–535. doi: 10.1590/0004-282X20160085
Ou, H. N., Lu, W. Y., Zhao, S. H., Liu, Y. F., Nong, W. H. (2014). Shujin Huoluo lotion and botulinum toxin A injection in the treatment of upper extremity spasitcity after stroke [Article in Chinese]. China Med. Herald 11 (20), 98–102.
Ou, H. N., Zhan, L. C., Nong, W. H., Chen, H. X. (2007). Impact of channel-soothing collateral-activating lotion plus type A Botulinus toxin injection on spasticity in ankle-planter flexors following spoplexy [Article in Chinese]. World Chin. Med. 2 (4), 214–216. doi: 10.3969/j.issn.1673-7202.2007.04.007
Page, S. J., Fulk, G. D., Boyne, P. (2012). Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 92 (6), 791–798. doi: 10.2522/ptj.20110009
Pildal, J., Hrobjartsson, A., Jorgensen, K. J., Hilden, J., Altman, D. G., Gotzsche, P. C. (2007). Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int. J. Epidemiol. 36 (4), 847–857. doi: 10.1093/ije/dym087
Sato, Y., Akao, T., He, J. X., Nojima, H., Kuraishi, Y., Morota, T., et al. (2006). Glycycoumarin from Glycyrrhizae radix acts as a potent antispasmodic through inhibition of phosphodiesterase 3. J. Ethnopharmacol. 105 (3), 409–414. doi: 10.1016/j.jep.2005.11.017
Schulz, K. F., Altman, D. G., Moher, D. (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340, c332. doi: 10.1136/bmj.c332
Schulz, K. F., Chalmers, I., Hayes, R. J., Altman, D. G. (1995). Empirical evidence of bias. JAMA 273 (5), 408–412. doi: 10.1001/jama.1995.03520290060030
Shen, J. H., Wang, H. M., Zhu, F. L. (2007). A study of compression therapy of Chinese herbal medicine combined with rehabilitation therapies for post-stroke spasticity [Article in Chinese]. Chin. J. Integr. Tradit. West. Med. 27 (9), 834. doi: 10.3321/j.issn:1003-5370.2007.09.034
Smith, L. N., James, R., Barber, M., Ramsay, S., Gillespie, D., Chung, C. (2010). Rehabilitation of patients with stroke: summary of SIGN guidance. BMJ 340, c2845. doi: 10.1136/bmj.c2845
Stein, C., Fritsch, C. G., Robinson, C., Sbruzzi, G., Plentz, R. D. (2015). Effects of electrical stimulation in spastic muscles after stroke: systematic review and meta-analysis of randomized controlled trials. Stroke 46 (8), 2197–2205. doi: 10.1161/STROKEAHA.115.009633
Stroke Foundation of New Zealand, and New Zealand Guidelines Group, (2010). Clinical guidelines for stroke management. Wellington: Stroke Foundation of New Zealand.
Sulniute, V., Pukalskas, A., Venskutonis, P. R. (2017). Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 224, 37–47. doi: 10.1016/j.foodchem.2016.12.047
Sze, K. H., Wong, E., Or, K. H., Lum, C. M., Woo, J. (2000). Factors predicting stroke disability at discharge: a study of 793 Chinese. Arch. Phys. Med. Rehabil. 81 (7), 876–880. doi: 10.1053/apmr.2000.6279
Tang, N. Y., Liu, C. H., Hsieh, C. T., Hsieh, C. L. (2010). The anti-inflammatory effect of paeoniflorin on cerebral infarction induced by ischemia-reperfusion injury in Sprague-Dawley rats. Am. J. Chin. Med. 38 (1), 51–64. doi: 10.1142/S0192415X10007786
Wagner, J. M., Rhodes, J. A., Patten, C. (2008). Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Phys. Ther. 88 (5), 652–663. doi: 10.2522/ptj.20070255
Wang, L., Fu, M. C., Huang, K. Y., Luo, X. L. (2014). A study of Tazi therapy of Chinese herbal medicine combined with Bobath therapy for increased muscle tone after stroke [Article in Chinese]. Chin. Manipulation Rehabil. Med. 5 (10), 104–106.
Wang, X. X. (2013). The Buchang Naoxintong Jiaonang combined with rehabilitation therapy stroke spastic hemiplegia efficacy study [Article in Chinese]. Chin. J. Mod. Drug Appl. 7 (13), 36–37. doi: 10.3969/j.issn.1673-9523.2013.13.019
Watkins, C. L., Leathley, M. J., Gregson, J. M., Moore, A. P., Smith, T. L., Sharma, A. K. (2002). Prevalence of spasticity post stroke. Clin. Rehabil. 16 (5), 515–522. doi: 10.1191/0269215502cr512oa
Wei, Y. B., Hao, J. J., Huang, B. T., Hong, S. D. (2011). A study of Rou Jin decoction for convalescent-phase stroke patients with spasticity [Article in Chinese]. Chin Med. Mod. Distance Educ. China 9 (7), 17–18.
Weng, X. J. (2014). A study of Jiejing mixture and tizaniding hydrochloride for post-stroke spastic hemiplegia [Article in Chinese]. [master’s thesis]. [Hangzhou (Zhejiang)]: Zhejiang Chinese Medical University. Available: http://xuewen.cnki.net/CMFD-1015546965.nh.html [Accessed March 28 2018].
Xie, R. M., Chen, H. X., Wang, Z. F., Zhu, L. Y. (2011). A study of Chinese herbal medicine combined with rehabilitation therapies for post-stroke spasticity in sixty cases [Article in Chinese]. Shandong J. Tradit. Chin. Med. 30 (1), 18–20.
Yu, L., Chen, C., Wang, L. F., Kuang, X., Liu, K., Zhang, H., et al. (2013). Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-kappaB and STAT3 in transient focal stroke. PLoS One 8 (2), e55839. doi: 10.1371/journal.pone.0055839
Zhang, B., Xue, B., (2012). Chinese internal medicine [book in Chinese]. Beijing: Chaoyang: People’s Medical Publishing House.
Zhang, C. Z. (2016). A study of fumigation with Jie Jing Shu Jin decoction combined with rehabilitation therapies for post-stroke upper-limb spasticity [Article in Chinese]. Chin. J. Tradit. Med. Sci. Technol. 23 (5), 582–583.
Zhang, L. L., Tian, K., Tang, Z. H., Chen, X. J., Bian, Z. X., Wang, Y. T., et al. (2016). Phytochemistry and pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 44 (2), 197–226. doi: 10.1142/S0192415X16500130
Zhang, M. W. (2009). The clinical stochastic antitheses diquisition of therapy of cerebral infarction spastic paralysis (Yinxufengdong Style) [Article in Chinese]. [master’s thesis]. [Zhengzhou (Henan)]: Henan University of Chinese Medicine. Available: http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D442593 [Accessed March 28 2018].
Zhang, X. J., Chen, H. L., Li, Z., Zhang, H. Q., Xu, H. X., Sung, J. J., et al. (2009). Analgesic effect of paeoniflorin in rats with neonatal maternal separation-induced visceral hyperalgesia is mediated through adenosine A(1) receptor by inhibiting the extracellular signal-regulated protein kinase (ERK) pathway. Pharmacol. Biochem. Behav. 94 (1), 88–97. doi: 10.1016/j.pbb.2009.07.013
Zhang, Y., Gao, N. Q., Chen, H. X. (2007). Clinical study on herbal steaming plus kinetotherapy in treating post-stroke hemiplegic spasticity [Article in Chinese]. Shanghai J. Tradit. Chin. Med. 41 (11), 26–28. doi: 10.3969/j.issn.1007-1334.2007.11.011
Zhang, Y., Qiao, L., Chen, H. X. (2008). Chinese herbal medicine and therapeutic exercise for relieving spasticity in hemiplegic patients [Article in Chinese]. Chin. J. Phys. Med. Rehabil. 30 (6), 390–393.
Zhang, Y., Qiao, L., Xu, W., Wang, X., Li, H., Xu, W., et al. (2017). Paeoniflorin attenuates cerebral ischemia-induced injury by regulating Ca2+/CaMKII/CREB signaling pathway. Molecules 22 (3), 359. doi: 10.3390/molecules22030359
Zhang, Y., Yang, J., Gao, N. Q. (2012). Clinical study on ‘Shaoyao Gancao decoction’ and physical therapy in treating poststroke elbow spasticity [Article in Chinese]. Shanghai J. Tradit. Chin. Med. 46 (11), 47–50.
Zhang, Y., Yang, J., Liu, G. Y., Jia, X. L., Yan, G. F., Liu, H. M., et al. (2015). Effects of “Shaoyao Gancao decoction” on spastic hemiplegia after cerebral ischemia-reperfusion and level of excitatory amino acids in rats[Article in Chinese]. Shanghai J. Tradit. Chin. Med. 49 (5), 85–89. doi: 10.16305/j.1007-1334.2015.05.028
Zhao, F. C. (2013). The warming Yiqihuoxue the treatment of patients with ischemic stroke a spastic hemiplegia clinical research [Article in Chinese]. [master’s thesis]. [Jinan (Shandong)]: University of Traditional Chinese Medicine. Available: http://cdmd.cnki.com.cn/Article/CDMD-10441-1014115595.htm [Accessed March 28 2018].
Zhao, N. (2010). A study of foot bath therapy with Chinese herbal medicine for post-stroke spasticity [Article in Chinese]. Chin. J. Tradit. Med. Sci. Technol. 17 (6), 504. doi: 10.3969/j.issn.1005-7072.2010.06.025
Zhong, H. Z. (2016). A study of Shao Yao Gan Cao decoction combined with rehabiltation therapies for post-stroke muscle spasticity [Article in Chinese]. J. N. Pharm 13 (5), 40–41.
Zhu, W., Zheng, G., Gu, Y., Chen, X., Jin, Y., Zhang, G., et al. (2014). Clinical efficacy and sEMG analysis of a new traditional Chinese medicine therapy in the treatment of spasticity following apoplectic hemiparalysis. Acta Neurol. Belg. 114 (2), 125–129. doi: 10.1007/s13760-014-0279-x
Zhu, W. Z., Hu, W. H., Zheng, G. Q., Zhou, L. S. (2007). Randomized and controlled clinical research of Jiejing mixture on spasticity after apoplectic hemparalysis [Article in Chinese]. Chin. Arch. Tradit. Chin. Med. 25 (8), 1648–1649. doi: 10.3969/j.issn.1673-7717.2007.08.047
Zhu, W. Z., Hu, W. H., Zhou, L. S. (2002). [Effect of Jie-jing mixture on spasticity of apoplectic hemiparalysis [Article in Chinese]. Chin. J. Rehabil. Theory Pract. 8 (1), 26–27. doi: 10.3969/j.issn.1006-9771.2002.01.011
Zhu, W. Z., Jin, Y. X., Chen, X., Huang, J. P., Zhang, G. W., Wu, H. Z., et al. (2013). A study on the clinical effects and sEMG analysis of Shao Yao Gan Cao decoction combined with rehabilitation therapies for post-stroke spasticity [Article in Chinese]. Chin. J. Phys. Med. Rehabil. 35 (6), 488–491. doi: 10.3760/cma.J.issn.0254-1424.2013.06.017
Zhu, X., Hu, H., Li, Z., Lin, R., Mao, J., Chen, L. (2015). Gua Lou Gui Zhi decoction attenuates poststroke spasticity via the modulation of GABAB receptors. Mol. Med. Rep. 12 (4), 5957–5962. doi: 10.3892/mmr.2015.4207
Keywords: herbal medicine, meta-analysis, muscle spasticity, randomized controlled trial, stroke
Citation: Cai Y, Zhang CS, Liu S, Wen Z, Zhang AL, Guo X, Xue CC and Lu C (2019) Add-On Effects of Chinese Herbal Medicine for Post-Stroke Spasticity: A Systematic Review and Meta-Analysis. Front. Pharmacol. 10:734. doi: 10.3389/fphar.2019.00734
Received: 01 February 2019; Accepted: 07 June 2019;
Published: 27 June 2019.
Edited by:
Alexander N. Shikov, St-Petersburg Institite of Pharmacy, RussiaReviewed by:
Anthony Booker, University of Westminster, United KingdomCopyright © 2019 Cai, Zhang, Liu, Wen, Zhang, Guo, Xue and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanjian Lu, bHVjaHVhbmppYW44ODhAdmlwLnNpbmEuY29t
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.