
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 03 May 2019
Sec. Predictive Toxicology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00458
This article is part of the Research Topic Mechanisms of Cell Death, Toxicity and Survival View all 9 articles
 Qihui Wu1,2,3†
Qihui Wu1,2,3† Chuipu Cai1,2†
Chuipu Cai1,2† Pengfei Guo1
Pengfei Guo1 Meiling Chen2
Meiling Chen2 Xiaoqin Wu4
Xiaoqin Wu4 Jingwei Zhou1
Jingwei Zhou1 Yunxia Luo1
Yunxia Luo1 Yidan Zou2
Yidan Zou2 Ai-lin Liu5
Ai-lin Liu5 Qi Wang1
Qi Wang1 Zaoyuan Kuang2*
Zaoyuan Kuang2* Jiansong Fang1,4*
Jiansong Fang1,4*Backgrounds and Aims: Recently, a growing number of hepatotoxicity cases aroused by Traditional Chinese Medicine (TCM) have been reported, causing increasing concern. To date, the reported predictive models for drug induced liver injury show low prediction accuracy and there are still no related reports for hepatotoxicity evaluation of TCM systematically. Additionally, the mechanism of herb induced liver injury (HILI) still remains unknown. The aim of the study was to identify potential hepatotoxic ingredients in TCM and explore the molecular mechanism of TCM against HILI.
Materials and Methods: In this study, we developed consensus models for HILI prediction by integrating the best single classifiers. The consensus model with best performance was applied to identify the potential hepatotoxic ingredients from the Traditional Chinese Medicine Systems Pharmacology database (TCMSP). Systems pharmacology analyses, including multiple network construction and KEGG pathway enrichment, were performed to further explore the hepatotoxicity mechanism of TCM.
Results: 16 single classifiers were built by combining four machine learning methods with four different sets of fingerprints. After systematic evaluation, the best four single classifiers were selected, which achieved a Matthews correlation coefficient (MCC) value of 0.702, 0.691, 0.659, and 0.717, respectively. To improve the predictive capacity of single models, consensus prediction method was used to integrate the best four single classifiers. Results showed that the consensus model C-3 (MCC = 0.78) outperformed the four single classifiers and other consensus models. Subsequently, 5,666 potential hepatotoxic compounds were identified by C-3 model. We integrated the top 10 hepatotoxic herbs and discussed the hepatotoxicity mechanism of TCM via systems pharmacology approach. Finally, Chaihu was selected as the case study for exploring the molecular mechanism of hepatotoxicity.
Conclusion: Overall, this study provides a high accurate approach to predict HILI and an in silico perspective into understanding the hepatotoxicity mechanism of TCM, which might facilitate the discovery and development of new drugs.
Liver injury induced by drug, novel foods or phytotherapy, also known as hepatotoxicity, is still a major clinical and pharmaceutical concern (Amadi and Orisakwe, 2018; Hammann et al., 2018; Kyawzaw et al., 2018; Real et al., 2018). According to the data from United States National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), hepatotoxicity accounts for 50% of all liver failure cases in the United States (Tujios and Fontana, 2011). Additionally, it is one of the leading causes of drug failure in trials and withdrawal from the market (Segall and Barber, 2014).
Over the last decades, Traditional Chinese Medicine (TCM), regarded as safe and natural, has received growing attention (Vikas and Singh, 2012). Dating back to 2,500 years ago, TCM has played an irreplaceable role in Chinese health care system to fight against various diseases and keep health for Chinese people (Cheung, 2011). Moreover, TCM is a gorgeous cradle of new active compounds in the course of drug discovery. For example, artemisinin (Qinghaosu) (Tu, 2011) is an effective anti-malaria drug, which is extracted from Chinese herb Artemisia annua L. (Qinghao). Despite its long clinical success, the most annoying problem in the herbal TCM area is the lack of proven efficacy in large-scale clinical studies and the unknown adverse reactions (ADRs). Side effects (SE) aroused by TCM, especially herb induced liver injury (HILI), have been reported widely (Teschke et al., 2015; Amadi and Orisakwe, 2018; Jing and Teschke, 2018), which also severely restricts the application of TCM (Lee et al., 2015; Kaplowitz, 2018). Thus, there is an urgent need to identify the potential hepatotoxic ingredients in TCM and explore the molecular mechanism of these compounds against HILI.
Generally, it is difficult to detect hepatotoxicity in the early phase of drug development due to its complex mechanism and lacking of scientific basis (Chatila, 1999; Williams, 2006). The traditional approach with experimental validation of drug induced hepatotoxicity on animals is costly, time-consuming and labor-intensive (Merz et al., 2014). Nowadays, due to the increasing number of hepatotoxic drugs identified in clinical or experimental studies, in silico prediction, such as machine learning (ML) approach based on ligand characteristic, provides the possibility of making predictions for HILI without knowing their underlying mechanisms. In this study, we try to identify hepatotoxic ingredients of TCM from a ligand-based ML perspective, and explore the hepatotoxicity mechanism via system pharmacology approach.
Quantitative structure-activity relationship (QSAR) are the most widely used in silico approach in absorption, distribution, metabolism, excretion and toxicity (ADMET) prediction (Cheng et al., 2013). Thus far, multiples of QSAR models have been generated for hepatotoxicity study of chemicals (Rodgers et al., 2010; Xu et al., 2015; Zhang et al., 2016; Cronin et al., 2017). For instance, Rodgers et al. (2010) reported a QSAR model with approximately 200 compounds by using the k-nearest neighbor (kNN) method, which achieved the sensitivity and specificity values of more than 73.7% and 94.4% for the prediction of liver adverse effects of drugs. Xu et al. (2015) developed hepatotoxicity prediction models by utilizing deep learning architectures, and the best model trained on 475 drugs predicted an external validation set of 198 drugs with an accuracy of 86.9%, sensitivity of 82.5%, specificity of 92.9%, and the area under the receiver operating characteristic (ROC) curve (AUC) of 0.955. Besides, Zhang et al. (2016) developed classification models using five machine learning methods based on MACCS Keys fingerprint and FingerPrint4 (FP4). However, the AUC values for the best model are only 0.656, 0.552, and 0.607 for training set, test set and external validation set, respectively. Notably, Cronin et al. (2017) proposed a new paradigm for in silico modeling by incorporating adverse outcome pathways, providing new insights into the QSAR models. Taken together, the predictive accuracies of current published QSAR models for hepatotoxicity remains to be improved due to incomplete data source. In addition, there are few consensus models reported to integrate single classifier for hepatotoxicity prediction. Furthermore, the hepatotoxicity models generated have not been applied to predict herb ingredients from TCM database yet.
In this work, we constructed a high-quality data set involving 619 hepatotoxic and 1,857 non-hepatotoxic compounds. All the hepatotoxic compounds were collected by integrating available adverse reactions databases (e.g., SIDER). Consensus models were generated to screen the Traditional Chinese Medicine systems pharmacology database and analysis platform (TCMSP) database. After identifying hepatotoxic ingredients in TCM, the molecular mechanisms of hepatotoxicity were explored. The detailed workflow could be seen in Figure 1. Firstly, data set containing hepatotoxic and non-hepatotoxic compounds were randomly assigned into training set and test set. Subsequently, four machine learning methods including artificial neural network (ANN), support vector machine (SVM), random forest (RF) and k-nearest neighbors (kNN) together with four different sets of fingerprints (EState, MACCS, PubChem, and SubFP) were utilized to develop 16 classifiers. Moreover, the consensus models by integrating the best four single classifiers were applied to screen the TCMSP database after systematic evaluation. Finally, to decipher the hepatotoxicity mechanism of TCM, systems pharmacology analyses of top 10 herbs with the largest number of potential hepatotoxic ingredients were carried out via integrating known and predicted targets.
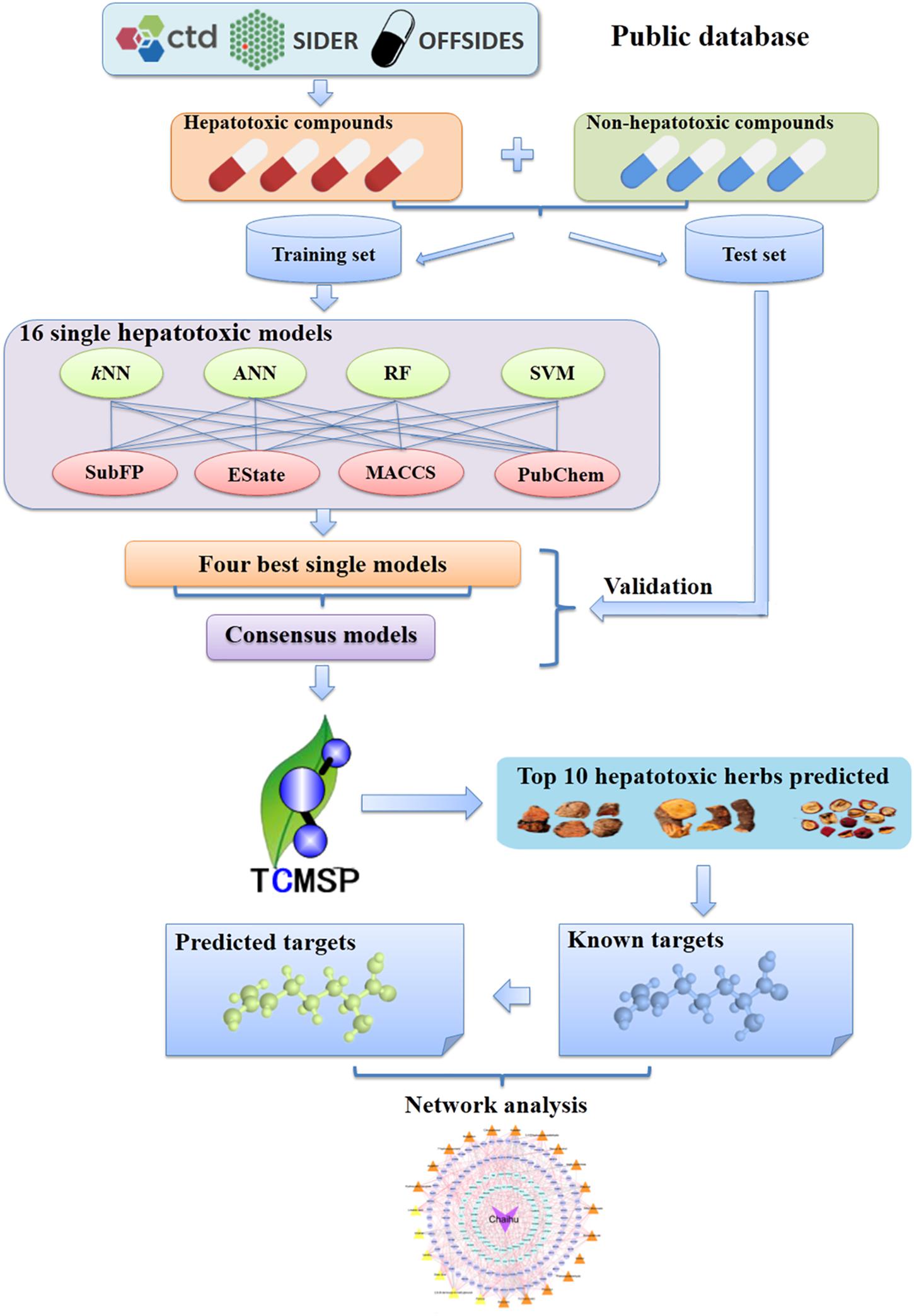
Figure 1. Schematic diagram of models building, identification of potential hepatotoxic ingredients and unraveling the hepatotoxicity mechanisms based on systems pharmacology approaches. CTD, Comparative Toxicogenomics Database; kNN, k-nearest neighbor; ANN, artificial neural network; RF, random forest; SVM, support vector machine; SubFP, Substructure fingerprint; EState, EState fingerprint; MACCS, MACCS Keys fingerprint; Pubchem, PubChem fingerprint; TCMSP, the Traditional Chinese Medicine systems pharmacology database and analysis platform.
In this study, all the liver toxic compounds were collected from three public databases, including side effect resource (SIDER) (Kuhn et al., 2010), OFFSIDES (Tatonetti et al., 2012) and Comparative Toxicogenomics Database (CTD) (Davis et al., 2019). Specifically, we firstly collected all the adverse SE of liver according to the criteria on “Common Terminology Criteria for Adverse Events” (CTCAE, version 4.03, 2010) released by the United States Department of Health and Human Services. Then all the liver related SE terms were annotated using Medical Subject Headings (MeSH) or Unified Medical Language System (UMLS) vocabularies (Bodenreider, 2004). Compounds that corresponded with these MeSH or UMLS IDs were extracted from the three databases. Only side effect information labeled with “frequent” or “direct evidence” were preserved. Additionally, the proteins, organic metals, compounds with molecular weight larger than 800 or smaller than 100, and duplicate structures were removed for avoiding potential noise (Wang et al., 1995). Finally, 619 liver toxic compounds were obtained for model construction.
After that, triple corresponding decoys were generated in RApid DEcoy Retriever (RADER) online database with a similarity threshold value of 0.75 between liver toxic compounds and decoys (Ling et al., 2016). Only molecules with molecular weight between 100 and 800 were preserved. All the compounds were randomly separated into training set and test set with ratio of 3:1. We considered the liver toxic compounds as hepatotoxic (labeled as “1”) during the calculation. Since decoys were randomly selected from a massive “decoy pool” containing 70,030,298 compounds (Ling et al., 2016) after eliminating hepatotoxic compounds, decoys were supposed as non-hepatotoxic (labeled as “0”) in this study. Eventually, the training set contained 453 hepatotoxic compounds and 1,359 non-hepatotoxic compounds, while the test set was consisted of 166 hepatotoxic compounds and 498 non-hepatotoxic compounds. Detailed information about the two data sets can be found in Supplementary Tables S1, S2. Meanwhile, herb ingredients covering 499 traditional Chinese herbs registered in the Chinese pharmacopeia were downloaded from TCMSP (Ru et al., 2014). Then 13,139 unique compounds were finally collected after removing duplicates.
All the data sets above were processed as the following three steps: Firstly, inorganic compounds were removed and hydrogen atoms were added. Secondly, strong acids were deprotonated and strong bases were protonated. Thirdly, three-dimensional (3D) conformers were generated by using washing and energy minimizing in Molecular Operating Environment (MOE) software (MOE, version 2010.10, Chemical Computing Group Inc., Montreal, QC, Canada, 2010).
As the chemical diversity of the datasets plays a vital role in the application domain of predictive model, we further employed the chemical space analysis to evaluate the chemical diversity within the data sets. As shown in Supplementary Figure S1, diverse chemical space distributions for all compounds as well as overlaps among the compounds within these data sets can be observed. In addition, we also proposed the chemical space analysis for TCMSP since the predictive models were applied to identify the potential hepatotoxic compounds in TCMSP. As presented in Supplementary Figure S2, the applicability domain of the predictive models covers 90.6% (11,902/13,139) chemicals of TCMSP.
Molecular fingerprints are often utilized for describing chemical structures. The main idea of fingerprints is to describe molecules based on molecular fragment. Through the segmentation of the molecular structure, a series of fragments as the characterization of the molecular structure can be obtained, and suitable fingerprints can enhance the performance of models (Fang et al., 2015a). Here, four common types of molecular fingerprints were calculated with PaDEL-Descriptor software (Yap, 2011) to represent molecular substructures and fragments information for each molecule. The four types of fingerprints are EState fingerprint (EState), MACCS Keys fingerprint (MACCS), PubChem fingerprint (PubChem) and Substructure fingerprint (SubFP).
In this part, four different machine learning methods including ANN, SVM, RF and kNN, were applied to develop predictive models and then consensus prediction was adopted to generate combined models. All the algorithms except SVM were performed using Orange Canvas (version, 2.7).
Artificial Neural Network is a powerful computational algorithm with sufficient accuracy which mimics the complex networks of neural connections in the neural brain (Dan, 1996). The network was made up of three layers: one input layer, one hidden layer and one output layer. ANN could identify complex non-linear relationship between input and output sets (Basheer and Hajmeer, 2000).
The purpose of SVM is to find a hyperplane which could discriminate molecules from different categories. Kernel function makes SVM deal with high-dimensional data effectively. The SVM algorithm was provided by the LibSVM package (Chang and Lin, 2011). And the commonly used kernel function radial bias function (RBF) was utilized to develop models after seeking the penalty parameter “C” and different kernel parameter “γ” with grid search strategy based on a 5-fold cross-validation in LibSVM.
Random Forest is also a widely used machine learning method (Biau et al., 2008). The core idea of this classification algorithm is to train the input sample vectors through constructing multitudes of decision trees in the forest. Each tree gives a classification, which means the tree “vector” for the class. Finally, the forest can select the classification which has the most vectors in all the trees of the forest.
k-Nearest Neighbors is a non-parametric method to categorize objects based on closest training examples in the feature space (Larose, 2004). The distance or similarity between each training sample was calculated by the algorithm to choose the list of its nearest neighbor, which can be classified according to the majority of the nearest neighbors (Cover and Hart, 1967). In this study, k (the number of nearest neighbors value) was set to the default (k = 5) and Hamming distance was selected for distance metric.
The main purpose of the consensus model is to combine the predicted results from various single classifiers for improving the predictive accuracy. It is generally considered that the consensus model is in a position to optimize the performance of the single classifier by improving predictive reliability (Cheng et al., 2011; Mansouri et al., 2013). Various kinds of noise from a single model can be reduced by consensus modeling (Fang et al., 2016b). In this study, four consensus models based on the best four single classifiers were generated through a “consensus prediction” procedure (Fang et al., 2015b). First, the training set and test set were screened with four single classifiers, and the compounds were considered as “hepatotoxicity” if predicted as “+1” by one of the four single classifiers. The procedure is defined as consensus prediction C1. Similarly, we obtained consensus prediction C2 (predicted as “+1” by two of the four single classifiers), C3 (predicted as “+1” by three of the four single classifiers), and C4 (predicted as “+1” by all the four single classifiers).
All classification models were evaluated by counting the numbers of true positives (TP), true negatives (TN), false positives (FP), and false negatives (FN) compounds. Additionally, sensitivity (SE), specificity (SP), overall predictive accuracy (Q) and Matthews correlation coefficient (MCC) were calculated with equations (1)–(4). Among them, SE and SP represent predictive accuracy of hepatotoxic and non-hepatotoxic compounds, respectively. Q represents predictive accuracy of total compounds, while MCC is the most significant indicator to measure the prediction performance of all the models. Usually, the higher the MCC value is, the better the model is.
Furthermore, the area under the receiver operating characteristic curve (AUC) value was also calculated. A perfect classifier can be found as AUC = 1.0 while the classifier has no discriminative power as AUC = 0.5.
The genes associated with liver diseases were collected from the previous public reference (Lu et al., 2015), which integrated the data from four public databases, including the Online Mendelian Inheritance in Man (OMIM) database (Hamosh et al., 2005), HuGE Navigator (Yu et al., 2008), PharmGKB (Hernandezboussard et al., 2008) and CTD (Davis et al., 2019). In this study, all collected genes were annotated using gene Entrez ID and official gene symbols based on the NCBI database1 and then mapped to corresponding Uniprot ID2. After removing duplicates, 627 unique genes related with liver disease were finally obtained.
The known hepatotoxic targets for compounds were acquired by two steps: (1) collecting known targets of compounds from our previous integrated natural products database (Fang et al., 2017a); (2) overlapping known targets and hepatotoxicity- related genes. The putative targets of compounds were predicted via a balanced substructure-drug-target network-based inference (bSDTNBI) (Wu et al., 2016; Fang et al., 2017c) approach, which prioritizes potential targets utilizing resource-diffusion processes for both known drugs and new chemical entities (NCEs) via substructure-drug-target network (Wu et al., 2016). In this study, the top 20 putative targets for each compound with known targets were selected. Similarly, the predicted hepatotoxic targets were acquired through overlapping predicted targets and hepatotoxicity-related genes. All the target names were subsequently normalized to the official gene name using the UniProt database (see text footnote 2).
To decipher the complex hepatotoxicity mechanisms of TCM, two types of network including herb-herb network (H-H network) and compound-target network (C-T network) were generated by Cytoscape (version 3.2.1). In the graphical network, the compounds or targets were presented by nodes, and edges encoded the interactions. The degree of nodes was calculated to measure its topological property as it characterizes the most important nodes in a network.
In this study, 16 single models were developed by four different algorithms (ANN, SVM, RF, and kNN) using four common types of fingerprints (EState, MACCS, PubChem and SubFP). The performance of each model was measured with the internal 5-fold cross validation in training set. Then, in order to further validate the predictive capability of our models, they were applied to predict the test set consisting of 664 compounds. The comprehensive performances of all the 16 classifiers are provided in Table 1.
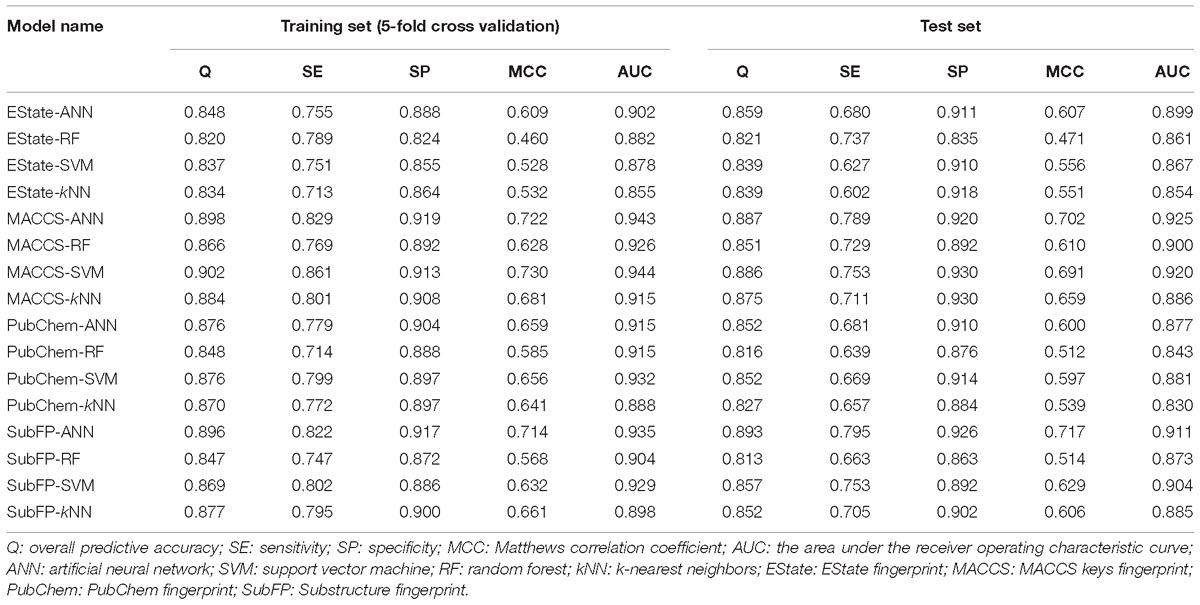
Table 1. Performance of 16 single classifiers using four sets of fingerprints and four modeling methods on 5-fold cross validation and test set.
As illustrated in Table 1, the overall predictive accuracies for 16 classifiers are acceptable. The Q-values of all single models are higher than 0.8 and the highest one is MACCS-SVM model reaching up to 0.902. The AUC value for each model is greater than 0.8, ranging from 0.855 to 0.944 on 5-fold cross validation as well as 0.830 to 0.920 on test set validation. MCC values, the most significant index to evaluate models, also have acceptable performances. The highest one (MACCS-ANN) reaches up to 0.722 on 5-fold cross validation and 0.702 on test set validation. Given the balance between SE and SP, we consider MACCS-ANN, MACCS-SVM, MACCS-kNN, and SubFP-ANN as the best four single models, which satisfy the condition that all of the MCC, SE as well as SP values are greater than 0.65 on the test set.
Consensus predictions were adopted to integrate the best four single classifiers discussed above, then four consensus models named C-1, C-2, C-3, C-4 were generated. The SE, SP, Q, and MCC values for consensus models on test set are presented in Figure 2A. The SE values of C-1 and C-2 are 0.89 and 0.83, which are higher than that of C-3 (SE = 0.77) and C-4 (SE = 0.57). Nevertheless, the SP values were opposite. The SP values for C-3 and C-4 are 0.97 and 0.99, greater than C-1 (SP = 0.81) and C-2 (SP = 0.93). The possible reason is that strict criterion for hepatotoxic prediction leads to higher SP and lower SE, and vice versa. Overall, C-3 model performs best among the four models, which keeps an equilibrium between specificity (SP = 0.97) and sensitivity (SE = 0.77), and has the highest MCC (MCC = 0.78) and Q (Q = 0.92) values.
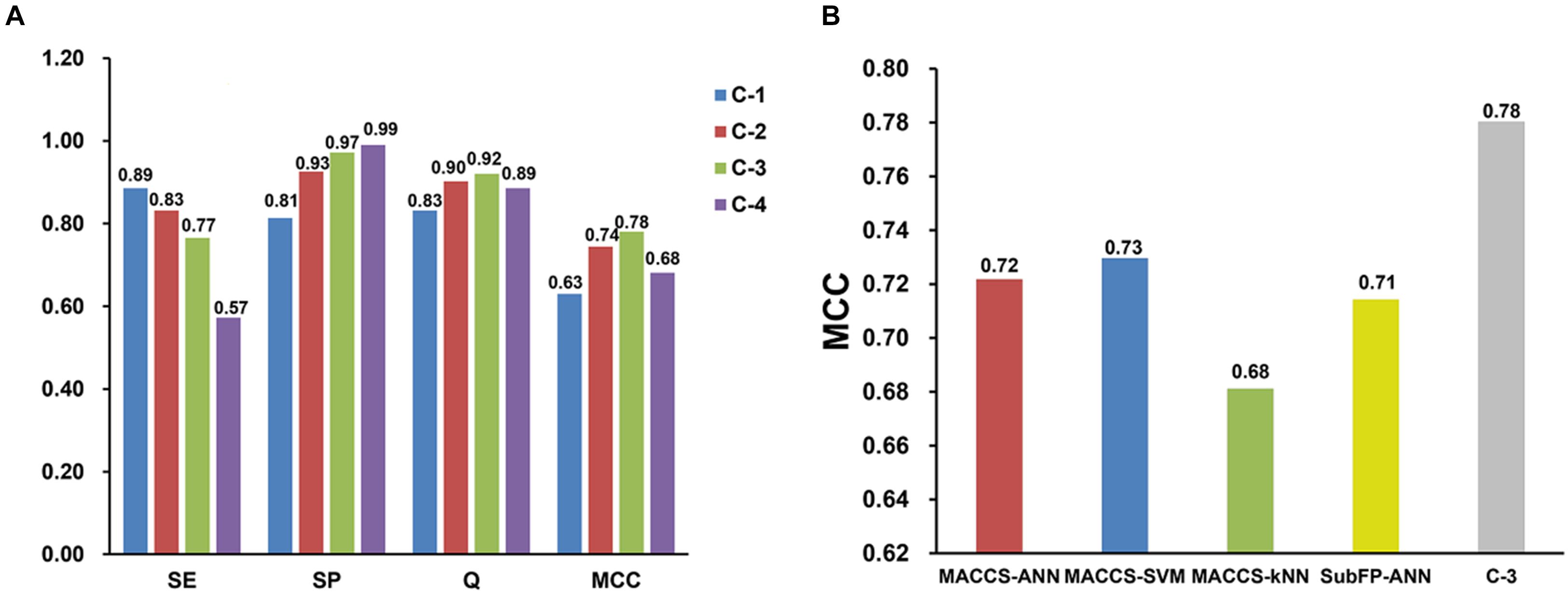
Figure 2. Performance comparisons of four consensus models (A) and four best single models with consensus model 3 (C-3) (B) on test set validation. SE, sensitivity; SP, specificity; Q, accuracy; MCC, Matthews correlation coefficient.
Moreover, the performance between the best four single models and C-3 model was compared on test set. As shown in Figure 2B, the C-3 model has a MCC value of 0.78, which is much greater than that of any other single models. This suggests that the C-3 model does enhance the predictive capabilities.
The ADME/T Descriptors is a mature protocol of commercial software Discovery Studio (version 4.0, Accelrys Inc., San Diego, CA, 2013) to estimate various pharmacokinetics features of ligands, including aqueous solubility, blood brain barrier penetration, CYP2D6 binding, intestinal absorption and hepatotoxicity. In order to further validate the predictive capability of C-3 model in practice, we compared the accuracy between C-3 model and the hepatotoxic prediction in DS software. First, 184 withdrawn drugs were downloaded from DrugBank database (Wishart et al., 2006). After browsing the detailed information about the withdrawal causes, we found that 11 of these drugs were withdrawn from the market due to clearly labeled hepatotoxicity. To evaluate if both of C-3 and DS can recognize these hepatotoxic drugs, C-3 model and DS were applied to predict these 11 drugs.
Table 2 shows that 9 out of 11 hepatotoxic compounds are predicted correctly by C-3 model, while only 7 compounds are considered hepatotoxic by DS software. However, once the positive threshold value is improved by means of setting the probability to 0.8, the correct number of DS descends to only 5, while the number of consensus model is 8. This also demonstrates that our model has higher accuracy to recognize hepatotoxic compounds.
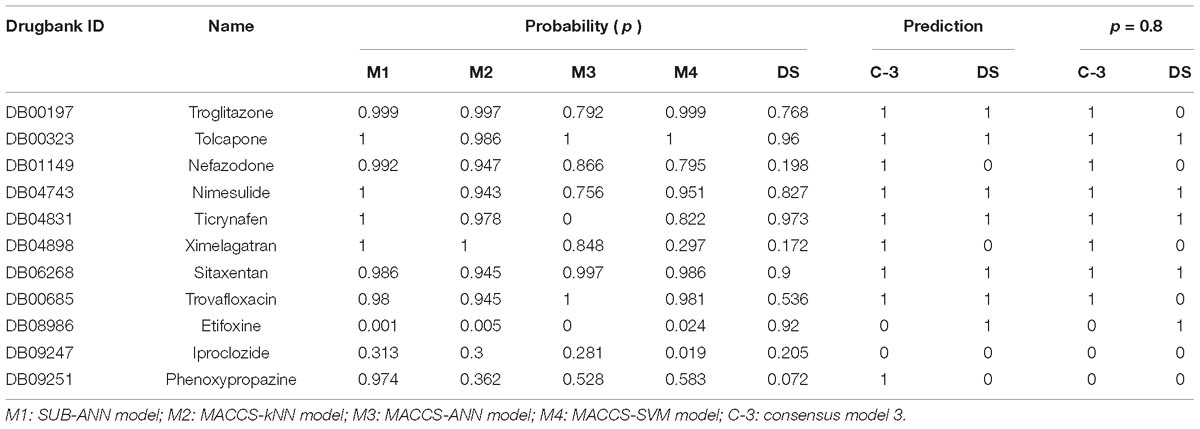
Table 2. Comparison of the hepatotoxic prediction results between consensus model 3 (C-3) and Discovery Studio (DS) for 11 withdrawn drugs due to hepatotoxicity.
The TCMSP database was built based on the framework of systems pharmacology which is aimed at accelerating the development of herbal medicines and drug discovery. It is a widely recognized database with comprehensive and authoritative data sources about the TCM (Ru et al., 2014). Therefore, the TCMSP database was selected to screen the potential hepatotoxic ingredients of TCM.
As discussed above, the C-3 model, which performed best, was finally chosen to identify the hepatotoxic ingredients among 13,139 unique compounds from TCMSP. Eventually, 5,666 out of them were predicted as hepatotoxic (Supplementary Table S3). To further explore the distribution of hepatotoxic compounds in herbs, the total number of compounds in each herb were obtained from TCMSP, while the number of predicted hepatotoxic compounds in each herb were also calculated. After that, each herb with specific number of hepatotoxic compounds was sorted in a descending order. Figure 3A shows the top 10 herbs possessing the highest number of hepatotoxic compounds, as well as the corresponding proportions of hepatotoxic ingredients numbers to the number of compounds in each herb. They are Bupleurum L. (Chaihu), Lonicera japonica (Jinyinhua), G. folium (the leaves of Ginkgo, Yinxingye), Commiphora myrrha (Moyao), Ligusticum chuanxiong (Chuanxiong), Ephedra sinica (Mahuang), Panax ginseng (Renshen), Micromeria biflora (Lingzhi), Capsicum annuum (Lajiao), Salvia miltiorrhiza (Danshen), respectively. The top 10 herbs contain 1,042 unique hepatotoxic compounds predicted (Supplementary Table S4). Figure 3B gives the corresponding proportions of the predicted hepatotoxic compounds for each herb to the total 1,042 hepatotoxic compounds of top 10 herbs.
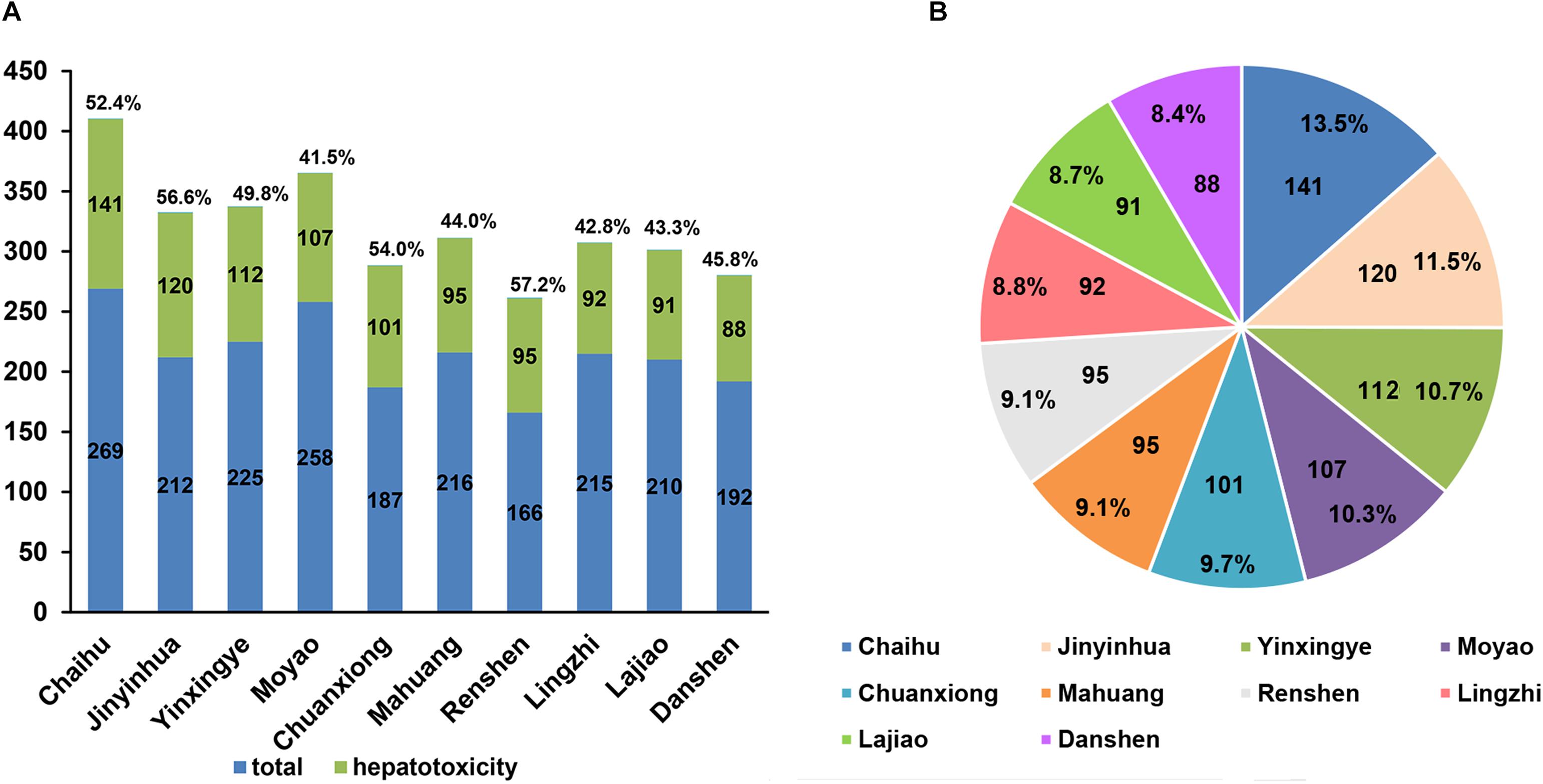
Figure 3. The top 10 herbs with the highest number of hepatotoxic compounds as well as the corresponding proportions of hepatotoxic ingredient numbers to the number of compounds in each herb (A) and the corresponding proportions of the predicted hepatotoxic compounds for each herb to the total 1,042 hepatotoxic compounds of top 10 herbs (B).
Among the top 10 herbs, Bupleurum L. (Chaihu) has the largest number (141) of potential hepatotoxic compounds, and 52% (141/269) of compounds in Chaihu were predicted to have hepatotoxicity. Interestingly, Lee et al. (2011) reported the risk of hospitalization for liver injury by using radix bupleuri (Chaihu), referring to 61 hepatotoxicity cases in 639,779 patients with chronic hepatitis B virus infection. Besides, both of Ephedra sinica (Mahuang) and Micromeria biflora (Lingzhi) also have been emphasized to be alerted for their hepatotoxicity. Estes et al. (2003) reported two cases of Ephedra sinica (Mahuang) linked acute liver failure resulting in orthotopic liver transplantation. And Yuen et al. (2004) referred to a case that in taking a formulation of Ganoderma lucidum (lingzhi) caused a significant hepatotoxicity in 2004. Collectively, these clinical reports are consistent with our predictions. It is worth noting that the hepatotoxicity level of herb are not only related to the number of potential hepatotoxic compounds in each herb, but also depended on the doses of the hepatotoxic compounds contained.
In recent years, systems pharmacology as an emerging new field, has made great contribution to unravel the nature of TCM and the actions of prescriptions (Fang et al., 2016a, 2017b; Cai et al., 2018). In our study, the systems pharmacology approach was applied to explore the hepatotoxicity mechanism of TCM.
To examine whether the top 10 herbs discussed above have similar compounds in terms of chemical structures, we explored the relationships among the top 10 herbs and constructed a herb-herb (H-H) network (Figure 4A). This network represents the relationship of different herbs based on the number of duplicate compounds between two herbs. The width of connected lines between two herbs is proportional to degree. As seen in Figure 4A, the top 10 herbs share lots of duplicate molecules in terms of structures. For example, network analysis indicates that Lonicera japonica (Jinyinhua) and Commiphora myrrha (Moyao) have the largest number of duplicate compounds (n = 29), followed by Lonicera japonica (Jinyinhua) and Panax ginseng (Renshen) (n = 27), Commiphora myrrha (Moyao) and Panax ginseng (Renshen) (n = 25). Ligusticum chuanxiong (Chuanxiong) and Salvia miltiorrhiza (Danshen), with the least number of common structures, also have 8 duplicate compounds.
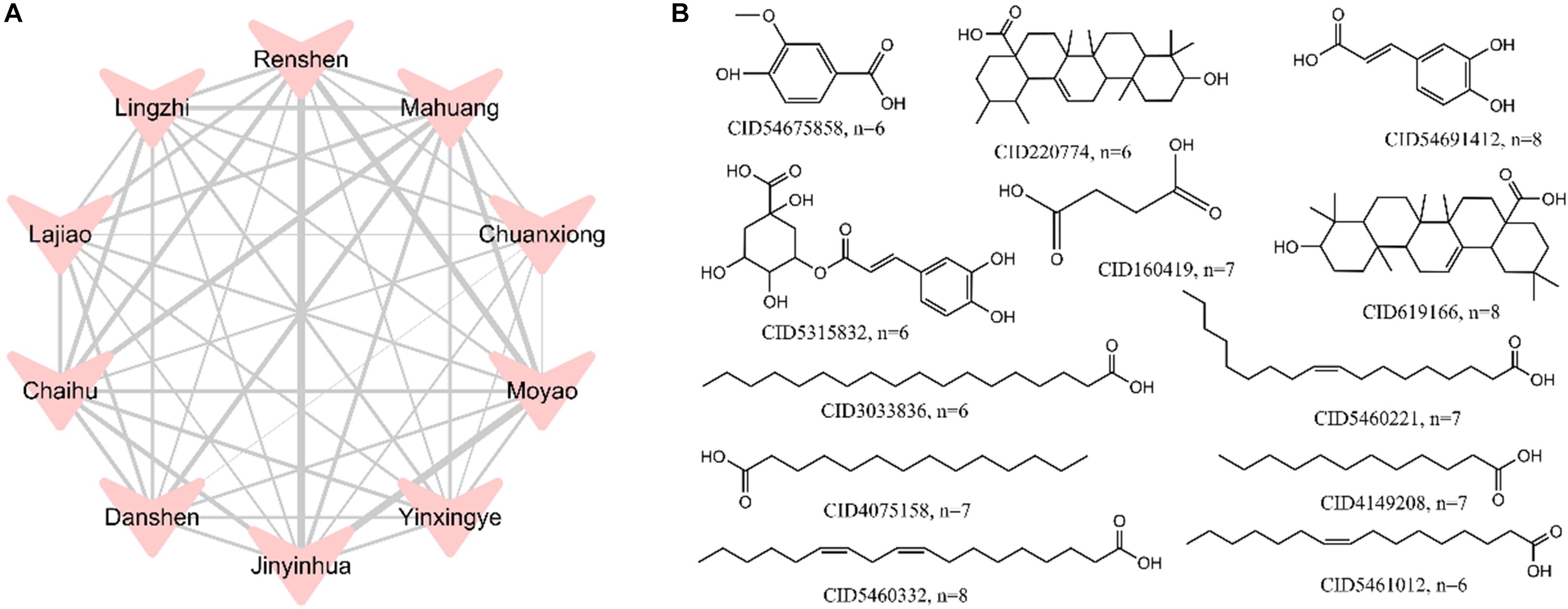
Figure 4. A herb-herb (H-H) network of the top 10 herbs (A) and the structures of 1,042 hepatotoxic compounds with frequency greater than 6 (B). The width of lines was based on the number of duplicate compounds between two herbs, which was proportional to degree.
Meanwhile, to reveal the most common hepatotoxic ingredients in top 10 herbs, frequency analysis of 1,042 potential hepatotoxic compounds was performed. Figure 4B displays the structures of compounds which have frequency greater than 6. Among them, CID5460332 (Linoleic acid), CID54691412 (trans-caffeic acid) and CID619166 (Oleanoic acid) show the highest frequency (n = 8), which indicates these compounds should be highly alerted for hepatotoxicity.
To further decipher the hepatotoxicity mechanism of action between herb ingredients and targets, a compound-target (C-T) network of the top 10 herbs was constructed, which contains 1,056 known compound-target interactions (CTIs) and 1,245 predicted CTIs. This network connects 91 hepatotoxic compounds to 675 target nodes (211 hepatotoxic targets and 464 non-hepatotoxic proteins). Figure 5 suggests that most compounds are connected to multiple targets with the average degree of 7.4 for each compound. Among the 91 compounds, quercetin (CID5280343, D = 240) has the highest number of target connections (D), followed by apigenin (CID5280443, D = 134) and genistein (CID5280961, D = 131). For the 675 targets, 211 out of them are associated with liver toxicity. Network analysis shows 9 targets with the degree (K) larger than 40, including LMNA, CYP3A4, MAPT, ALDH1A1, HSD17B10, TP53, ALOX15, HPGD, and CYP2C19. Among them, LMNA (K = 59) exhibits the largest number of connected compounds, followed by CYP3A4 (K = 56) and MAPT (K = 55). According to in vitro studies, CYP3A4 may play an important role in liver toxicity (Kostrubsky et al., 1995, 1997a,b; Zhang et al., 2010; Zhou et al., 2017, Li et al., 2018). For instance, Dictamnine (DTN), the main alkaloid from a herb called Dictamni Cortex (DC), has been reported to induce liver injury through the regulation of CYP3A4 (Li et al., 2018). The inhibition of CYP3A4 could alleviate the toxicity both in vitro and in vivo while induction of CYP3A4 was able to aggravate the toxicity effects. Accordingly, it is likely that the hepatotoxic compounds take effects through the regulation of these targets with higher degree, which deserves to be validated by in vivo or in vitro experiments. The detailed information for the C-T network is provided in Supplementary Table S5.
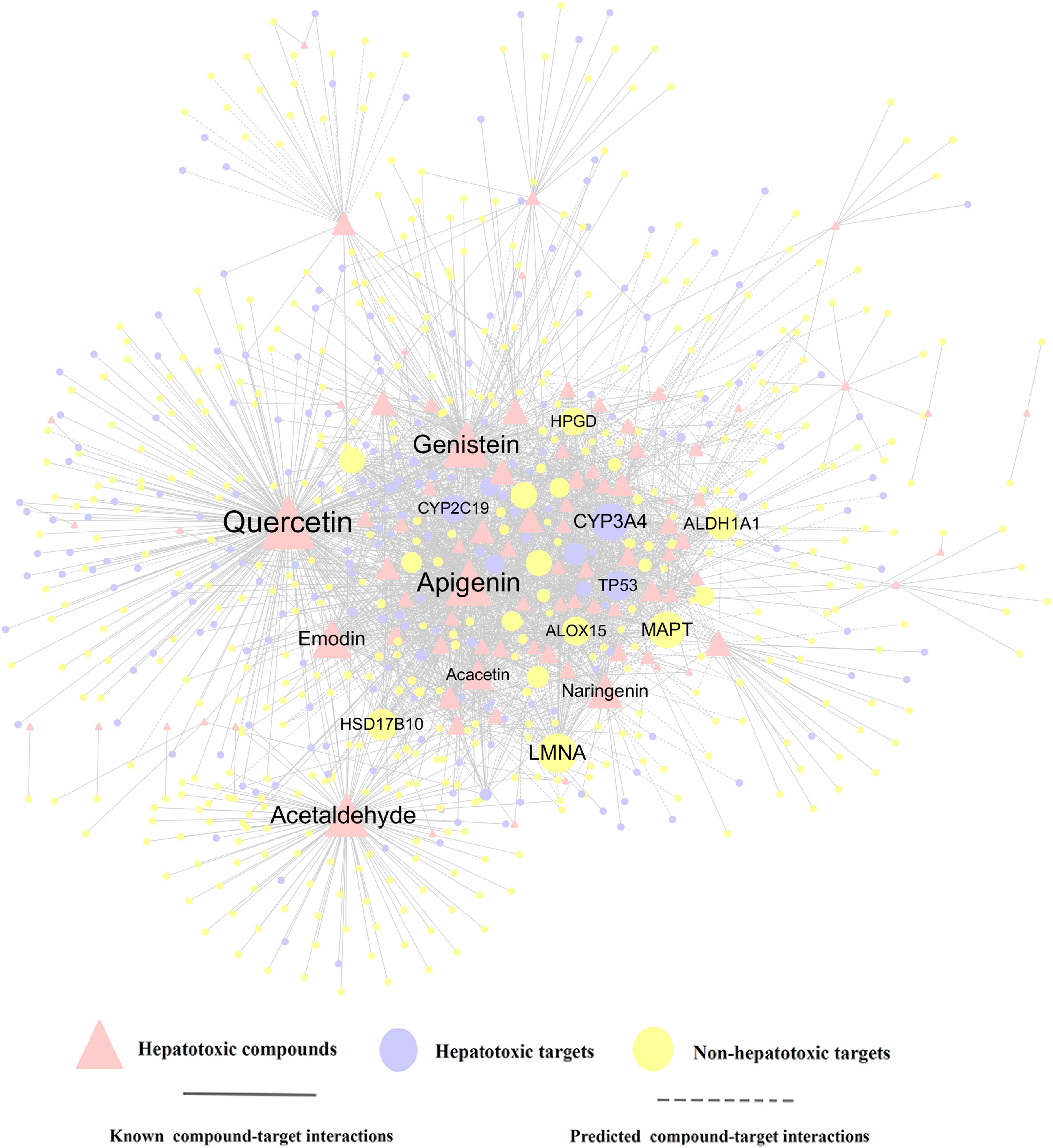
Figure 5. A compound-target (C-T) network consists of 1,056 known interactions as well as 1,245 predicted interactions, including 91 compounds, 211 hepatotoxic targets and 464 non-hepatotoxic targets. The label font size and node size are proportional to degree.
In addition, KEGG pathway enrichment analysis by utilizing Cluego (a plugin in Cytoscape) was proposed. Here, we choose the top 20 targets (D ≥ 28) in the C-T network. As shown in Table 3, the top 20 targets are enriched in 7 pathways. Among them, 5 out of 7 pathways have been experimentally validated involved with liver injury, including arachidonic acid metabolism pathway (Holownia et al., 2014) (P = 4.5 × 10−4), linoleic acid metabolism pathway (Lu et al., 2014) (P = 6.3 × 10−7), retinol metabolism pathway (Freund and Gotthardt, 2017) (P = 5.2 × 10−4), metabolism of xenobiotics by cytochrome P450 pathway (Gonzalez, 2005) (P = 7.6 × 10−4), and drug metabolism-cytochrome P450 pathway (Gabbia et al., 2017) (P = 2.3 × 10−7). Taking retinol metabolism pathway as an example, the pathway has been demonstrated to regulate hepatic immunological response to cholestatic injury and alleviate hepatic fibrosis in different rodent models (Freund and Gotthardt, 2017). Interestingly, there are two pathways (metabolism of xenobiotics by cytochrome P450 pathway and drug metabolism-cytochrome P450 pathway) both relevant with cytochrome P450 (CYP450), which indicates CYP450 may play a key role in HILI. According to experimental studies, lots of CYP450 related enzymes have been validated to involve with the hepatotoxicity of TCM, such as the CYP2E1 enzyme (Gonzalez, 2005), CYP2A6 enzyme (Yamaguchi et al., 2013) and CYP2A8 enzyme (Albassam et al., 2015). Therefore, these pathways are more likely to be regulated by hepatotoxic ingredients of TCM and thus induce liver injury, which merits further investigation by experimental assays.
Bupleurum L. (Chaihu) was selected as the case study for exploring the molecular mechanism of hepatotoxicity. Through integration of the known and putative targets of the potential hepatotoxic compounds, we found that 23 compounds of Bupleurum L. possessed compound-target interactions and selected them to construct the compound-target (C-T) network of Bupleurum L. Figure 6 shows a specific compound-target (C-T) network of Bupleurum L., which contains 133 known and 380 predicted C-T interactions connecting 23 compounds and 172 targets. Among the 23 compounds, the average number of non-hepatotoxic and hepatotoxic targets for each compound is 4.6 and 2.8, respectively. The network indicates that CID5280442 (Acacetin, D = 44) has the largest number of target connections, followed by CID5280378 (Formononetin, D = 36) and CID11092 (Paeonol, D = 35).
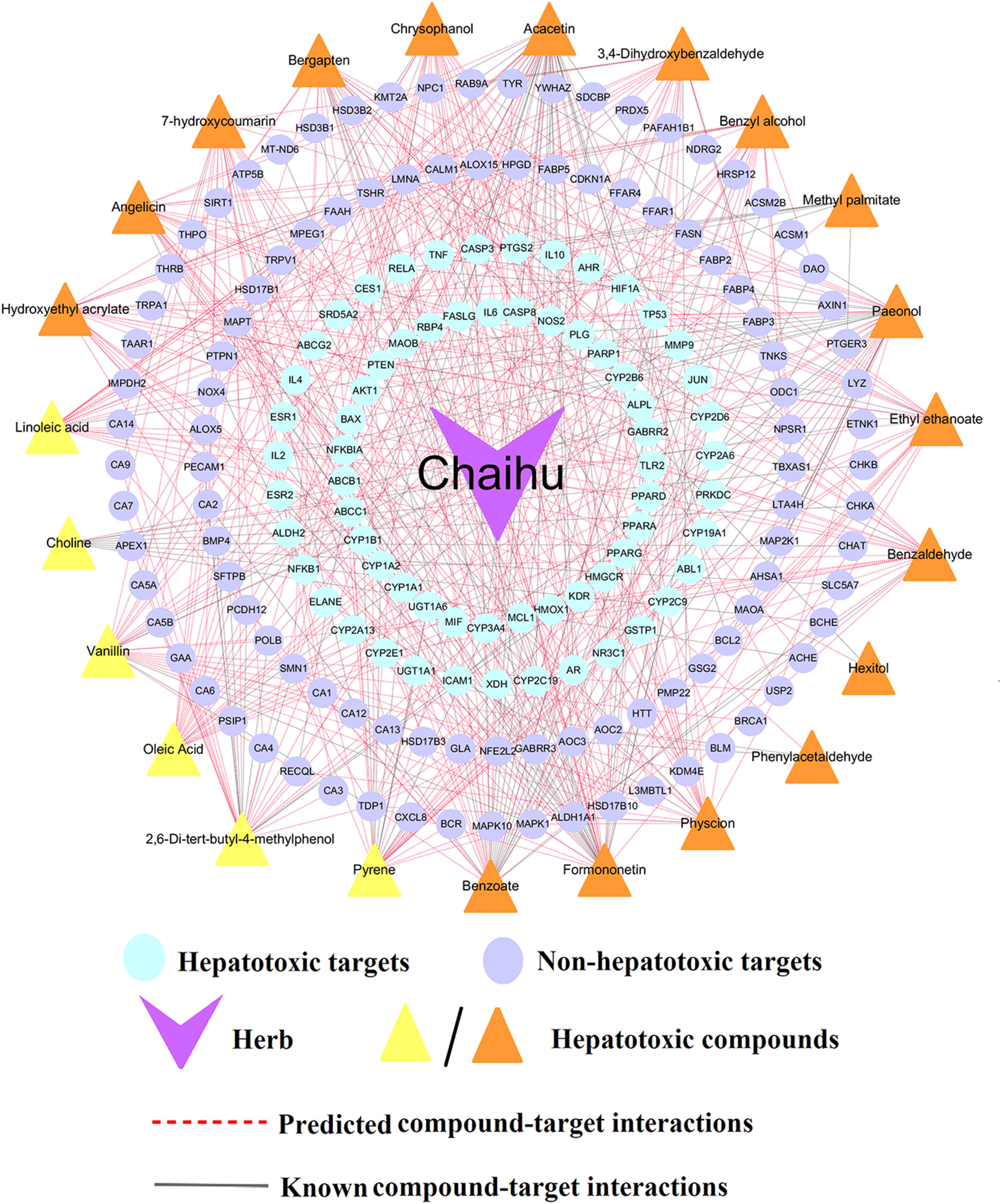
Figure 6. A compound-target (C-T) network of Bupleurum L. (Chaihu) contains 133 known and 380 predicted C-T interactions connecting 23 compounds and 172 targets (including 66 hepatotoxic targets and 106 non-hepatotoxic targets). The yellow triangles represent the predicted hepatotoxic compounds validated by literatures, while the orange triangles stand for the predicted hepatotoxic compounds without literature evidences.
Further literature analyses suggested that 6 out of 23 compounds, including Linoleic acid, Vanillin, Oleic acid, 2,6-Di-tert-butyl-4-methylphenol, Pyrene and Choline, had been reported with confirmed hepatotoxicity (Supplementary Table S6). For instance, Linoleic acid (CID5460332), a polyunsaturated omega-6 fatty acid, could significantly increase the protein levels of hepatic ALOX15 in Hepa-1c1c7 cells, which contributed to the pathogenesis of alcohol-induced liver injury (Zhang et al., 2017). Additionally, Linoleic acid has been reported to cause oxidative damage (Tong et al., 2016; Li et al., 2019; Qu et al., 2019; Zhang et al., 2019) and mediate selective loss of intrahepatic CD4(+) T lymphocytes, which leads to accelerated hepatocarcinogenesis (Ma et al., 2016). Several clinical case reports also indicate that linoleic acid may induce hepatic disease, such as acute hepatitis (Ramos et al., 2009; Rita and José, 2012; Mohammad et al., 2015). These results were also consistent with its highest frequency (n = 8) in top 10 herbs aforementioned. Another study showed high choline (CID305) caused oxidative damage, significant dyslipidemia and liver injury in mice (Ren et al., 2016). Additionally, 2,6-Di-tert-butyl-4-methylphenol (Butylated hydroxytoluene, CID31404), a lung toxicant, was reported to produce liver injury in mice with depressed hepatic GSH levels (Mizutani et al., 1987) and a report indicated that a single dose of BHT (1000 mg/kg) to rats could induce a hepatic injury accompanying centrilobular necrosis (Nakagawa et al., 1984). Taken together, these investigations demonstrate the high prediction accuracy of our consensus model C-3, showing promise for the identification of hepatotoxic compounds.
To further clarify the detailed molecular mechanism of 6 validated hepatotoxic compounds against HILI, we constructed a specific C-T network for these 6 compounds. As illustrated in Figure 7, the specific C-T network is composed of 33 known CTIs and 97 predicted CTIs connecting 6 compounds and 89 targets (including 32 hepatotoxic targets and 57 non-hepatotoxic targets). For instance, Linoleic acid (LA) interacts with 1 known targets and 20 computationally predicted targets. Among the 21 targets, 5 out of them (TLR2, PPARD, PPARA, PPARG, HMGCR) are hepatotoxic targets, providing potential hepatotoxicity mechanism of LA. Similarly, Pyrene binds with 27 targets, including 7 known targets and 20 predicted targets. Among the 27 targets, 19 out of them are associated with liver toxicity, including 13 hepatotoxic targets predicted as well as 6 known hepatotoxic targets. For example, CYP2C19, one of the 13 hepatotoxic targets predicted, has been reported to mediate the hepatotoxicity of rhein as well as be implicated in the mechanism of clopidogrel-induced hepatotoxicity (He et al., 2015; Zhai et al., 2016). It is likely that Pyrene induces liver injury through regulation of these targets that deserves to be validated by experiments, which indicates new liver toxicity mechanism of Pyrene.
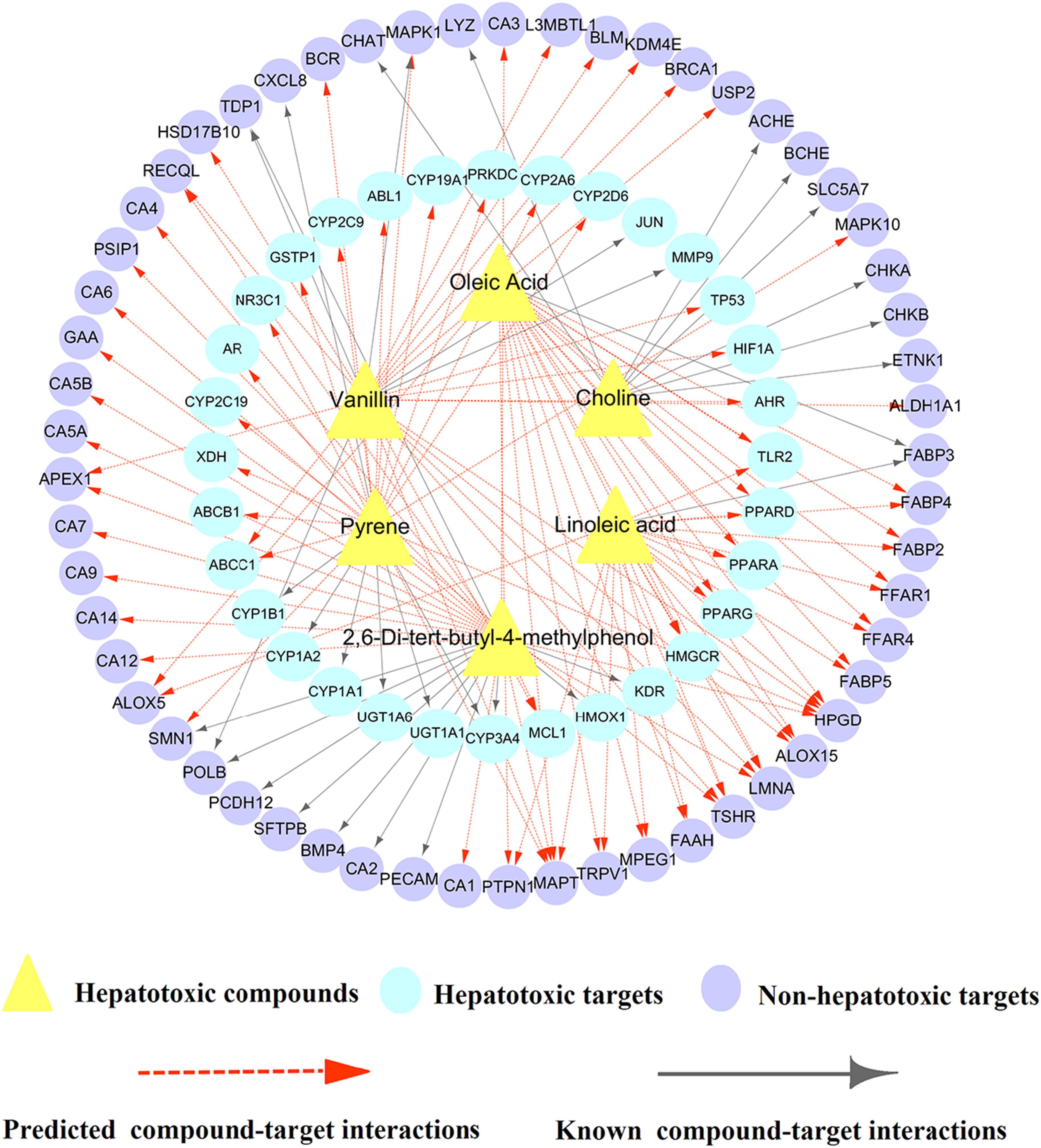
Figure 7. A biqartite compound-target (C-T) network for 6 hepatotoxic compounds including 33 known and 97 predicted CTIs connecting 6 hepatotoxic compounds and 89 targets (including 32 hepatotoxic targets and 57 non-hepatotoxic targets).
Overall, aforementioned examples indicate that systems pharmacology-based network analyses provide a new perspective for exploring the hepatotoxicity mechanisms of TCM. In the future, experimental validation combined with epidemiologic studies will be introduced to further validate our discovery of HILI and its underlining mechanisms.
In this study, consensus models were developed by integrating the best four single classifiers to improve the predictive capability of HILI. Based on the evaluated results of withdrawn drugs, we found that C-3 exhibited excellent performance in contrast to the hepatotoxic prediction of ADME/T Descriptor module in DS. Subsequently, C-3 model was utilized to identify potential hepatotoxic ingredients from TCMSP. Furthermore, systems pharmacology analyses and KEGG pathway enrichment were proposed to decipher the hepatotoxicity mechanisms of hepatotoxic ingredients among the top 10 herbs. Finally, we exemplified molecular mechanism of HILI by a case study of Bupleurum L. (Chaihu).
However, several shortcomings should be recognized in the presented study. First, due to lacking of sufficient quantitative data from clinical and non-clinical (in vitro and in vivo) studies, the current predictive models are qualitative rather than quantitative models and mainly aimed at the prediction of intrinsic HILI type. As the increasing number of hepatotoxic compounds with quantitative pharmacological data are reported, we intend to develop the quantitative predictive models in the future. Second, as the intrinsic interactions on different ingredients are complicated and unobtainable from public resources, thus it is difficult to explore the specific mixture activity and toxicity of plant ingredients just through this research. Moreover, integration of biological descriptors from drug-target networks (Cheng and Zhao, 2014) and clinical data from multiple sources, such as Drug Induced Liver Injury Rank (DILIrank) dataset (Chen et al., 2016) and Roussel Uclaf Causality Assessment Method (RUCAM) (Danan and Teschke, 2015), will be utilized to further analyze the causal relationship of HILI and improve the application domain of current study. Last, current study have not considered antagonistic or agonistic effects of compound–targets pairs. In the future, specific biological functions (upregulation or downregulation) will be integrated into current network by fetching specific information (upregulation or downregulation) from Connectivity Map (CMap) and LINCs databases.
In summary, this study provides a high accurate approach to predict the herb-induced liver toxicity and systematically explore the hepatotoxicity mechanism of TCM, which facilitates the discovery and development of new drugs.
JF and ZK conceived and designed the experiments. QWu and CC wrote the manuscript. PG, MC, JZ, YL, and YZ data acquired and interpreted. XW, A-lL, QWa, and JF reviewed and revised the manuscript.
This work was supported by the National Natural Science Foundation of China (Nos. 81603318, 81473740, and 81673627), Guangdong Provincial Major Science and Technology for Special Program of China (No. 2015A030302072), CAMS Innovation Fund for Medical Science (2017-I2M-1-016), and the Guangzhou Science Technology and Innovation Commission Technology Research Projects (No. 201805010005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00458/full#supplementary-material
Albassam, A. A., Mohamed, M. E., and Frye, R. F. (2015). Inhibitory effect of six herbal extracts on CYP2C8 enzyme activity in human liver microsomes. Xenobiotica 45, 406–412. doi: 10.3109/00498254.2014.989935
Amadi, C. N., and Orisakwe, O. E. (2018). Herb-induced liver injuries in developing nations: an update. Toxics 6:E24. doi: 10.3390/toxics6020024
Basheer, I. A., and Hajmeer, M. (2000). Artificial neural networks: fundamentals, computing, design, and application. J. Microbiol. Methods 43, 3–31.
Biau, G., Devroye, L., and Lugosi, G. B. (2008). Consistency of random forests and other averaging classifiers. J. Mach. Learn. Res. 9, 2015–2033.
Bodenreider, O. (2004). The unified medical language system (UMLS): integrating biomedical terminology. Nucleic Acids Res. 32, 267–270.
Cai, H., Luo, Y., Yan, X., Ding, P., Huang, Y., Fang, J., et al. (2018). The mechanisms of Bushen-Yizhi formula as a therapeutic agent against Alzheimer’s disease. Sci. Rep. 8:3104.
Chang, C. C., and Lin, C. J. (2011). LIBSVM: a library for support vector machines. Intell. Syst. Technol. 2, 1–27.
Chatila, R. (1999). Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. Philadelphia, PA: Lippincott Williams &Wilkins.
Chen, M., Suzuki, A., Thakkar, S., Yu, K., Hu, C. C., and Tong, W. (2016). Dilirank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov. Today 21, 648–653.
Cheng, F., Li, W., Liu, G., and Tang, Y. (2013). In silico ADMET prediction: recent advances, current challenges and future trends. Curr. Topics Med. Chem. 13, 1273–1289.
Cheng, F., Yu, Y., Shen, J., Yang, L., Li, W., Liu, G., et al. (2011). Classification of cytochrome P450 inhibitors and noninhibitors using combined classifiers. J. Chem. Inform. Model. 51, 996–1011. doi: 10.1021/ci200028n
Cheng, F., and Zhao, Z. (2014). Machine learning-based prediction of drug–drug interactions by integrating drug phenotypic, therapeutic, chemical, and genomic properties. J. Am. Med. Inform. Assoc. Jamia 21, 278–286. doi: 10.1136/amiajnl-2013-002512
Cover, T. M., and Hart, P. E. (1967). Nearest neighbor pattern classification. IEEE Trans. Inform. Theory 13, 21–27.
Cronin, M. T. D., Enoch, S. J., Mellor, C. L., Przybylak, K. R., Richarz, A.-N., and Madden, J. C. (2017). In silico prediction of organ level toxicity: linking chemistry to adverse effects. Toxicol. Res. 33, 173–182. doi: 10.5487/TR.2017.33.3.173
Dan, W. P. (1996). Artificial Neural Networks: Theory and Applications. Upper Saddle River, NJ: Prentice Hall.
Danan, G., and Teschke, R. (2015). RUCAM in drug and herb induced liver injury: the update. Int. J. Mol. Sci. 17:E14. doi: 10.3390/ijms17010014
Davis, A. P., Murphy, C. G., Johnson, R., Sciaky, D., McMorran, R., Wiegers, J., et al. (2019). The comparative toxicogenomics database: update 2019. Nucleic Acids Res. 47, D948–D954. doi: 10.1093/nar/gky868
Estes, J. D., Stolpman, D., Olyaei, A., Corless, C. L., Ham, J. M., Schwartz, J. M., et al. (2003). High prevalence of potentially hepatotoxic herbal supplement use in patients with fulminant hepatic failure. Arch. Surg. 138, 852–858.
Fang, J., Cai, C., Wang, Q., Lin, P., Zhao, Z., and Cheng, F. (2017a). Systems pharmacology-based discovery of natural products for precision oncology through targeting cancer mutated genes. CPT Pharmacometr. Syst. Pharmacol. 6, 177–187. doi: 10.1002/psp4.12172
Fang, J., Liu, C., Wang, Q., Lin, P., and Cheng, F. (2017b). In silico polypharmacology of natural products. Brief. Bioinform. 19, 1153–1171.
Fang, J., Wu, Z., Cai, C., Wang, Q., Tang, Y., and Cheng, F. (2017c). Quantitative and systems pharmacology. 1. In silico prediction of drug-target interaction of natural products to enable of new targeted cancer therapy. J. Chem. Inform. Model. 57, 2657–2671. doi: 10.1021/acs.jcim.7b00216
Fang, J., Li, Y., Liu, R., Pang, X., Li, C., Yang, R., et al. (2015a). Discovery of multitarget-directed ligands against Alzheimer’s disease through systematic prediction of chemical-protein interactions. J. Chem. Inform. Model. 55, 149–164. doi: 10.1021/ci500574n
Fang, J., Yang, R., Gao, L., Yang, S., Pang, X., Li, C., et al. (2015b). Consensus models for CDK5 inhibitors in silico and their application to inhibitor discovery. Mol. Divers. 19, 149–162. doi: 10.1007/s11030-014-9561-3
Fang, J., Ling, W., Tian, W., Cong, Y., Li, G., Cai, H., et al. (2016a). Network pharmacology-based study on the mechanism of action for herbal medicines in Alzheimer treatment. J. Ethnopharmacol. 196, 281–292. doi: 10.1016/j.jep.2016.11.034
Fang, J., Pang, X., Yan, R., Lian, W., Li, C., Wang, Q., et al. (2016b). Discovery of neuroprotective compounds by machine learning approaches. Rsc Adv. 6, 9857–9871.
Freund, C., and Gotthardt, D. N. (2017). Vitamin A deficiency in chronic cholestatic liver disease — is vitamin A therapy beneficial? Liver Int. 37, 1752–1758. doi: 10.1111/liv.13433
Gabbia, D., Pozza, A. D., Albertoni, L., Lazzari, R., Zigiotto, G., Carrara, M., et al. (2017). Pregnane X receptor and constitutive androstane receptor modulate differently CYP3A-mediated metabolism in early- and late-stage cholestasis. World J. Gastroenterol. 23, 7519–7530. doi: 10.3748/wjg.v23.i42.7519
Gonzalez, F. J. (2005). Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mut. Res. 569, 101–110.
Hammann, F., Schöning, V., and Drewe, J. (2018). Prediction of clinically relevant drug-induced liver injury from structure using machine. J. Appl. Toxicol. 39, 412–419. doi: 10.1002/jat.3741
Hamosh, A., Scott, A. F., Amberger, J., Bocchini, C., Valle, D., and Mckusick, V. A. (2005). Online mendelian inheritance in man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 33, 514–517.
He, L., Yang, A., Cui, T., Zhai, Y., Zhang, F., Chen, J., et al. (2015). Reactive metabolite activation by cyp2c19-mediated rhein hepatotoxicity. Xenobiotica 45, 361–372. doi: 10.3109/00498254.2014.984794
Hernandezboussard, T., Whirlcarrillo, M., Hebert, J. M., Gong, L., Owen, R., Gong, M., et al. (2008). The pharmacogenetics and pharmacogenomics knowledge base: accentuating the knowledge. Nucleic Acids Res. 36, 913–918.
Holownia, A., Mroz, R. M., Wielgat, P., Jakubow, P., Jablonski, J., Sulek, J., et al. (2014). Histone acetylation and arachidonic acid cytotoxicity in HepG2 cells overexpressing CYP2E1. Naunyn Schmiedeberg’s Arch. Pharmacol. 387, 271–280. doi: 10.1007/s00210-013-0942-4
Jing, J., and Teschke, R. (2018). Traditional Chinese medicine and herb-induced liver injury: comparison with drug-induced liver injury. J. Clin. Transl. Hepatol. 6, 57–68. doi: 10.14218/JCTH.2017.00033
Kaplowitz, N. (2018). Herb-induced liver injury: a global concern. Chin. J. Integr. Med. 24, 643–644. doi: 10.1007/s11655-018-3004-4
Kostrubsky, V. E., Szakacs, J. G., Jeffery, E. H., Wood, S. G., Bement, W. J., Wrighton, S. A., et al. (1997a). Role of CYP3A in ethanol-mediated increases in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 143, 315–323.
Kostrubsky, V. E., Szakacs, J. G., Jeffery, E. H., Woods, S. G., Bement, W. J., Wrighton, S. A., et al. (1997b). Protection of ethanol-mediated acetaminophen hepatotoxicity by triacetyloleandomycin, a specific inhibitor of CYP3A. Ann. Clin. Lab. Sci. 27, 57–62.
Xu, Y., Dai, Z., Chen, F., Gao, S., Pei, J., and Lai, L. (2015). Deep learning for drug-induced liver injury. J. Chem. Inform. Model. 55, 2085–2093. doi: 10.1021/acs.jcim.5b00238
Kostrubsky, V. E., Wood, S. G., Bush, M. D., Szakacs, J., Bement, W. J., Sinclair, P. R., et al. (1995). Acute hepatotoxicity of acetaminophen in rats treated with ethanol plus isopentanol. Biochem. Pharmacol. 50, 1743–1748.
Kuhn, M., Campillos, M., Letunic, I., Jensen, L. J., and Bork, P. (2010). A side effect resource to capture phenotypic effects of drugs. Mol. Syst. Biol. 6:343. doi: 10.1038/msb.2009.98
Kyawzaw, L., Aung Naing, L., Sandar, L., Pwint Phyu, H., Viswanath, V., and Madhavi, R. (2018). Ginseng-related drug-induced liver injury. Case Rep. Gastroenterol. 12, 439–446. doi: 10.1159/000490525
Larose, D. T. (2004). Discovering Knowledge in Data: An Introduction to Data Mining. Hoboken, NJ: Wiley-Interscience.
Lee, C. H., Wang, J. D., and Chen, P. C. (2011). Risk of liver injury associated with Chinese herbal products containing radix bupleuri in 639,779 patients with hepatitis B virus infection. Plos One 6:e16064. doi: 10.1371/journal.pone.0016064
Lee, W. J., Kim, H. W., Lee, H. Y., and Son, C. G. (2015). Systematic review on herb-induced liver injury in korea. Food Chem. Toxicol. 84, 47–54. doi: 10.1016/j.fct.2015.06.004
Li, M., Wang, S., Li, X., Kou, R., Wang, Q., Wang, X., et al. (2019). Diallyl sulfide treatment protects against acetaminophen-/carbon tetrachloride-induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. Toxicol. Res. 8, 67–76. doi: 10.1039/c8tx00185e
Li, Z., Jiang, L., Zhao, D., Zhou, J., Wang, L., Wu, Z., et al. (2018). The modulatory role of cyp3a4 in dictamnine-induced hepatotoxicity. Front. Pharmacol. 9:1033. doi: 10.3389/fphar.2018.01033
Ling, W., Pang, X., Li, Y., Zhang, Z., and Wen, T. (2016). RADER: a RApid DEcoy Retriever to facilitate decoy based assessment of virtual screening. Bioinformatics 33, 1235–1237. doi: 10.1093/bioinformatics/btw783
Lu, W., Cheng, F., Jiang, J., Zhang, C., Deng, X., Xu, Z., et al. (2015). FXR antagonism of NSAIDs contributes to drug-induced liver injury identified by systems pharmacology approach. Sci. Rep. 5:8114. doi: 10.1038/srep08114
Lu, X., Tian, Y., Lian, X., Jin, Y., Jin, T., Zhao, Q., et al. (2014). Integrated systems toxicology approaches identified the possible involvement of ABC transporters pathway in erythromycin estolate-induced liver injury in rat. Food Chem. Toxicol. 65, 343–355. doi: 10.1016/j.fct.2013.12.050
Ma, C., Kesarwala, A. H., Eggert, T., Medina-Echeverz, J., Kleiner, D. E., Jin, P., et al. (2016). NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257. doi: 10.1038/nature16969
Mansouri, K., Ringsted, T., Ballabio, D., Todeschini, R., and Consonni, V. (2013). Quantitative structure–activity relationship models for ready biodegradability of chemicals. J. Chem. Inform. Model. 53, 867–878.
Merz, M., Lee, K. R., Kullak-Ublick, G. A., Brueckner, A., and Watkins, P. B. (2014). Methodology to assess clinical liver safety data. Drug Saf. 37, 33–45.
Mizutani, T., Nomura, H., Nakanishi, K., and Fujita, S. (1987). Hepatotoxicity of butylated hydroxytoluene and its analogs in mice depleted of hepatic glutathione. Toxicol. Appl. Pharmacol. 87, 166–176.
Mohammad, B., Yogesh, P., and Micheal, B. (2015). Linoleic acid induced acute hepatitis: a case report and review of the literature. Case Rep. Hepatol. 2015, 1–3. doi: 10.1155/2015/807354
Nakagawa, Y., Tayama, K., Nakao, T., and Hiraga, K. (1984). On the mechanism of butylated hydroxytoluene-induced hepatic toxicity in rats. Biochem. Pharmacol. 33, 2669–2674.
Qu, L., Zhu, Y., Liu, Y., Yang, H., Zhu, C., Ma, P., et al. (2019). Protective effects of ginsenoside Rk3 against chronic alcohol-induced liver injury in mice through inhibition of inflammation, oxidative stress, and apoptosis. Food Chem. Toxicol. 126, 277–284. doi: 10.1016/j.fct.2019.02.032
Ramos, R., Mascarenhas, J., Duarte, P., Vicente, C., and Casteleiro, C. (2009). Conjugated linoleic acid-induced toxic hepatitis: first case report. Dig. Dis. Sci. 54, 1141–1143. doi: 10.1007/s10620-008-0461-1
Real, M., Barnhill, M. S., Higley, C., Rosenberg, J., and Lewis, J. H. (2018). Drug-induced liver injury: highlights of the recent literature. Drug Saf. 42, 1–23.
Ren, D., Liu, Y., Zhao, Y., and Yang, X. (2016). Hepatotoxicity and endothelial dysfunction induced by high choline diet and the protective effects of phloretin in mice. Food Chem. Toxicol. 94, 203–212. doi: 10.1016/j.fct.2016.06.004
Rita, N., and José, B. (2012). Fulminant hepatitis during self-medication with conjugated linoleic acid. Annals of Hepatology 11, 265–267.
Rodgers, A. D., Zhu, H., Fourches, D., Rusyn, I., and Tropsha, A. (2010). Modeling liver-related adverse effects of drugs using knearest neighbor quantitative structure-activity relationship method. Chem. Res. Toxicol. 23, 724–732. doi: 10.1021/tx900451r
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6:13. doi: 10.1186/1758-2946-6-13
Segall, M. D., and Barber, C. (2014). Addressing toxicity risk when designing and selecting compounds in early drug discovery. Drug Discov. Today 19, 688–693. doi: 10.1016/j.drudis.2014.01.006
Tatonetti, N. P., Ye, P. P., Daneshjou, R., and Altman, R. B. (2012). Data-driven prediction of drug effects and interactions. Sci. Transl. Med. 4:125ra31. doi: 10.1126/scitranslmed.3003377
Teschke, R., Wolff, A., Frenzel, C., and Schulze, J. (2015). Letter: herbal hepatotoxicity–an update on traditional Chinese medicine preparations. Aliment. Pharmacol. Ther. 6, 737–738.
Tong, L., Xia, Y., Li, J., Li, S., Jiao, F., Wu, L., et al. (2016). Shikonin attenuates concanavalin a-induced acute liver injury in mice via inhibition of the jnk pathway. Med. Inflamm. 2016, 1–14. doi: 10.1155/2016/2748367
Tu, Y. (2011). The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17, 1217–1220.
Tujios, S., and Fontana, R. J. (2011). Mechanisms of drug-induced liver injury: from bedside to bench. Nat. Rev. Gastroenterol. Hepatol. 8, 202–211. doi: 10.1038/nrgastro.2011.22
Vikas, D., and Singh, A. K. (2012). Complementary and alternative medicine usage among Alzheimer’s disease patients. Int. Psychogeriatr. 24, 1361–1362.
Wang, R. Y., Storey, V. C., and Firth, C. P. (1995). A framework for analysis of data quality research. IEEE Trans. Knowl. Data Eng. 7, 623–640.
Wishart, D. S., Knox, C., Guo, A. C., Shrivastava, S., Hassanali, M., Stothard, P., et al. (2006). DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 34, 668–672.
Wu, Z., Cheng, F., Li, J., Li, W., Liu, G., and Tang, Y. (2016). SDTNBI: an integrated network and chemoinformatics tool for systematic prediction of drug-target interactions and drug repositioning. Brief. Bioinform. 18, 333–347. doi: 10.1093/bib/bbw012
Yamaguchi, Y., Akimoto, I., Motegi, K., Yoshimura, T., Wada, K., Nishizono, N., et al. (2013). Synthetic models related to methoxalen and menthofuran-cytochrome p450 (cyp) 2a6 interactions. benzofuran and coumarin derivatives as potent and selective inhibitors of cyp2a6. Chem. Pharm. Bull. 61, 997–1001.
Yap, C. W. (2011). PaDEL-descriptor: an open source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 32, 1466–1474. doi: 10.1002/jcc.21707
Yu, W., Gwinn, M., Clyne, M., Yesupriya, A., and Khoury, M. J. (2008). A navigator for human genome epidemiology. Nat. Genet. 40, 124–125.
Yuen, M. F., Ip, P., Ng, W. K., and Lai, C. L. (2004). Hepatotoxicity due to a formulation of Ganoderma lucidum (lingzhi). J. Hepatol. 41, 686–687.
Zhai, Y., Wang, L., Yang, F., Feng, G., Feng, S., Cui, T., et al. (2016). The mechanism and risk factors of clopidogrel-induced liver injury. Drug Chem. Toxicol. 39, 367–374. doi: 10.3109/01480545.2015.1122606
Zhang, C., Cheng, F., Li, W., Liu, G., Lee, P. W., and Tang, Y. (2016). In silico prediction of drug induced liver toxicity using substructure pattern recognition method. Qsar Comb. Sci. 35, 136–144. doi: 10.1002/minf.201500055
Zhang, Q. X., Melnikov, Z., and Feierman, D. E. (2010). Characterization of the acetaminophen-induced degradation of cytochrome P450-3A4 and the proteolytic pathway. Basic Clin. Pharmacol. Toxicol. 94, 191–200.
Zhang, W., Zhong, W., Sun, Q., Sun, X., and Zhou, Z. (2017). Hepatic overproduction of 13-HODE due to ALOX15 upregulation contributes to alcohol-induced liver injury in mice. Sci. Rep. 7:8976. doi: 10.1038/s41598-017-02759-0
Zhang, Y., Lu, Y., Ji, H., and Li, Y. (2019). Anti-inflammatory, anti-oxidative stress and novel therapeutic targets for cholestatic liver injury. BioSci. Trends 13, 23–31. doi: 10.5582/bst.2018.01247
Keywords: herb induced liver injury, consensus model, traditional chinese medicine, hepatotoxicity mechanism, in silico
Citation: Wu Q, Cai C, Guo P, Chen M, Wu X, Zhou J, Luo Y, Zou Y, Liu A-l, Wang Q, Kuang Z and Fang J (2019) In silico Identification and Mechanism Exploration of Hepatotoxic Ingredients in Traditional Chinese Medicine. Front. Pharmacol. 10:458. doi: 10.3389/fphar.2019.00458
Received: 30 October 2018; Accepted: 11 April 2019;
Published: 03 May 2019.
Edited by:
Eleonore Fröhlich, Medical University of Graz, AustriaReviewed by:
Falko Partosch, University Medical Center Göttingen, GermanyCopyright © 2019 Wu, Cai, Guo, Chen, Wu, Zhou, Luo, Zou, Liu, Wang, Kuang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaoyuan Kuang, enlrdWFuZ0BnenVjbS5lZHUuY24= Jiansong Fang, ZmFuZ2pzQGd6dWNtLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.