
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 18 April 2019
Sec. Ethnopharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00376
 Hai-Di Li1,2,3,4
Hai-Di Li1,2,3,4 Xiao-Ming Meng1,2,3,4
Xiao-Ming Meng1,2,3,4 Cheng Huang1,2,3,4
Cheng Huang1,2,3,4 Lei Zhang1,2,3,4
Lei Zhang1,2,3,4 Xiong-Wen Lv1,2,3,4
Xiong-Wen Lv1,2,3,4 Jun Li1,2,3,4*
Jun Li1,2,3,4*Acute kidney injury (AKI) is a clinical syndrome characterized by a rapid loss of renal function, which may further develop into chronic kidney damage (CKD) or even end-stage renal disease (ESRD). AKI is a global health problem associated with high morbidity and costly treatments, and there is no specific or effective strategy to treat AKI. In recent years, Traditional Chinese Medicine (TCM) has attracted more attention, with lines of evidence showing that application of TCM improved AKI, and the mechanisms of action for some TCMs have been well illustrated. However, reviews summarizing the progress in this field are still lacking. In this paper, we reviewed TCM preparations and TCM monomers in the treatment of AKI over the last 10 years, describing their renal protective effects and mechanisms of action, including alleviating inflammation, programmed cell death, necrosis, and reactive oxygen species. By focusing on the mechanisms of TCMs to improve renal function, we provide effective complementary evidence to promote the development of TCMs to treat AKI. Moreover, we also summarized TCMs with nephrotoxicity, which provides a more comprehensive understanding of TCMs in the treatment of AKI. This review may provide a theoretical basis for the clinical application of TCMs in the future.
Acute kidney injury (AKI), characterized by an abrupt decline of renal function, can be induced by numerous causes including renal ischemia reperfusion injury (IRI), nephrotoxic insults and infection of sepsis (Waikar et al., 2008; Linkermann, 2016). Accumulating evidence shows that AKI is a global public health concern and a pivotal threat to human health, especially in hospitalized patients, as it impacts more than 13 million patients per year (Chertow et al., 2005; Lameire et al., 2013; Thomas et al., 2015; Yang et al., 2015; Allison, 2016). Excessive inflammatory responses, oxidative stress, and the imbalance of the damage and repair of renal tubules, are all highly involved in the pathological process of AKI, however, specific targets and effective therapies are still lacking (Sancho-Martinez et al., 2015; Yang et al., 2016; Zuk and Bonventre, 2016).
Traditional Chinese medicine (TCM) has been widely used for the treatment of AKI and its complications in China and neighboring countries, including Japan and Korea, for a long time. Some TCM-based therapies show good results and high efficacy in inhibiting inflammatory responses, programmed cell death and oxidative stress. In this regard, the therapeutic effects of TCMs have widely been tested in animal models of AKI and even in patients. For instance, the Xuebijing Injection is effective in improving clinical symptoms of sepsis-induced AKI patients after the Wenchuan Earthquake (Yuxi et al., 2017). Our recent study showed that wogonin not only protects cisplatin-induced AKI, but also preserves and even promotes the anti-tumor effect of cisplatin (Meng et al., 2018). However, it is noteworthy that some TCMs, such as aristolochic acids and other plant alkaloids, are nephrotoxic, (Yang et al., 2018). So, the application of TCM should be carefully evaluated.
In this paper, we reviewed the therapeutic effects of TCMs on AKI and the mechanism of action based on the assessment of evidence that supports hypotheses, additionally, TCMs with nephrotoxicity have also been discussed.
Until now, several TCM preparations have been tested in the treatment of AKI. There are shown in Table 1.
Decoctions of roots from A&A can improve renal blood flow in a murine model of acute ischemic renal injury, possibly by increasing NO production by activating eNOS and scavenging ROS, therefore accelerating renal repair after ischemic injury (Meng et al., 2007). Moreover, the therapeutic effect of A&A may be JNK-dependent (Cai et al., 2001).
Dahuang Fuzi Decoction (DFD) consists of Radix et Rhizoma Rhei, Radix Aconiti Lateralis Praeparata, and Radix et Rhizoma Asari. Emerging evidence indicates that DFD attenuates adenine-triggered renal damage and tubular epithelial apoptosis, by blocking the activation of TGF-β1-JNK pathways (Tu et al., 2014).
It consists of chuan dome, radix paeoniae rubra, safflower, Salvia miltiorrhiza, and Chinese angelica. Administration of a Xuebijing injection can suppress the production and release of high mobility group box-1 protein (HMGB1) in the kidney, thereby alleviating serious scald injury-induced AKI (Wang et al., 2007). In addition, an intravenous injection of Xuebijing attenuates the inflammatory response in AKI rats with paraquat poisoning (Xu et al., 2017). Importantly, Xuebijing improved the clinical symptoms of patients with sepsis-induced AKI after the Wenchuan Earthquake (Yuxi et al., 2017).
It is composed of Rhizoma coptidis (RC), Cortex phellodendri (CP), Radix scutellariae (RS), and Fructus gardenia by a weight ratio of 3:2:2:3. HLJDD effectively suppresses LPS-induced AKI by activating Akt/HO-1 pathway and inhibiting NF-κB and MAPK activation in mice (Li et al., 2017).
ZDW is a polyherbal formula mixed with Rehmannia glutinosa (Gaertn.) DC, baked (Radix Rehmanniae preparata), Cornus officinalis Siebold & Zucc., Dioscorea oppositifolia L., Paeonia suffruticosa Andrews, Alisma plantago-aquatica L., rhizome (Rhizoma Alismatis), and Poria cocos (Schw.) Wolf. ZDW has been used to treat chronic kidney diseases, like diabetic nephropathy, for many years. A recent study revealed that ZDW also protected against gentamicin-induced AKI both in vivo and in vitro, because it attenuated apoptosis of renal tubular epithelial cells by limiting caspase-3 activation (Hsu et al., 2014).
Compared with TCM preparations, TCM Monomers have recently attracted more attention in the treatment of diseases because they have certain molecular structures, clear mechanisms of action, predicted pharmacological effects and less drug-drug interactions. In the kidney, numerous TCM monomers have been applied in treating renal diseases including AKI caused by different stimuli. Therefore, we list TCMs that have comprehensively been studied to protect against AKI in recent years.
Astaxanthin (ATX) is a natural carotenoid extracted from marine organisms which are widely applied because of their strong antioxidant effect. Current studies demonstrate the renoprotective effects of ATX in many AKI models. ATX (5 mg/kg for 14 days via oral gavage) can improve I/R-induced AKI by exerting antioxidant activity and inhibiting tubular apoptosis/necrosis via scavenging free radicals (Qiu et al., 2015). Moreover, ATX (40 mg/kg for 5 days by intraperitoneal injection) attenuated arsenic-induced AKI by fulfilling antioxidant functions and reducing As accumulation (Wang et al., 2014), and ATX (50 mg/kg for 12 h by gavage) ameliorated HgCl2-induecd AKI by exerting anti-oxidant activity and preventing lipid and protein oxidation (Augusti et al., 2008). ATX (20 mg/kg 12 h via tail intravenous injection) consistently improved early AKI, following a severe burn, by modulating antioxidant activity and Akt/Bad/Caspases-mediated mitochondrial-apoptotic pathway (Guo et al., 2015).
Baicalin is a Scutellaria baicalensis-derived flavonoid which has been tested in multiple types of AKI models. In clinical trials, Baicalin protects against AKI in pediatric sepsis by inhibiting renal cell apoptosis (Zhu et al., 2016). In ischemia-reperfusion injured kidney, Baicalin (10 μmol/L for 24 h) exerts protective effects by inhibiting TLR2/4-mediated inflammation and mitochondrial stress-induced apoptosis of tubular epithelial cells (Ji et al., 2014). Administration of Baicalin (100 μmol/L) in HK-2 cells consistently reduced H2O2-induced cytotoxicity by activating downstream Nrf2 signaling and attenuating ER stress (Lin et al., 2014). These findings are supported by recent findings that Baicalin (50 mg/kg, i.p. for 2 weeks) prevents Lead (Pb)-induced renal injury and pediatric sepsis-induced AKI by blocking oxidative stress and apoptosis (Zhang et al., 2016; Zhu et al., 2016). Moreover, it is of note that baicalin is a novel PPAR-γ activator which may suppress NF-κB-mediated inflammation effectively (Lim et al., 2012).
Cordyceps sinensis (CS) is used as a tonic food which is derived from an entomogenous fungus (Zhu et al., 1998). CS (5 g/kg via intragastric for 2 days) improves the outcome of I/R-induced AKI via different mechanisms including modulating SDF-1/CXCR4-signaling axis (Wang et al., 2013), up-regulating expression of HIF-1α, down-regulating the expression of NGAL (Yu et al., 2012) and reducing the expression level of TLR-4 (Zhou and Hu, 2010). Moreover, treatment of CS (1.5 mg/200 μl) significantly alleviates stress responses and tissue damage by reducing autophagy and apoptosis in LPS-induced AKI (Wu et al., 2011). Additionally, CSP, as the mycelia glycoproteins of Cordyceps sobolifera, significantly suppresses cyclosporine A (CsA)-induced apoptosis and protects against nephron loss via increasing magnesium reabsorption (Chyau et al., 2014).
As a major component of green tea, EGCG is famous for its anti-inflammatory and anti-apoptotic properties. EGCG, as a potent inducer of HO-1, can suppress renal injury by reducing oxidative stress and inflammation in several AKI models induced by contrast (EGCG 20 mg/kg intravenously) (Gao Z. et al., 2016), I/R (EGCG 50 mg/kg i.p. for 24 h) (Lv et al., 2015) and cisplatin (EGCG 100 mg/kg i.p. for 12 days) (Sahin et al., 2010), respectively. Furthermore, underlying mechanisms have extensively been explored in cisplatin nephropathy, EGCG (100 mg/kg i.p. for 2 days) prevented activation of ERK, the NF-κB pathway and caspase-12 while down-regulating the Fas-conducted extrinsic pathway and Bcl-2/Bax ratio, thereby reducing the apoptosis of tubular epithelial cells (Zou et al., 2014; Chen B. et al., 2015; Pan et al., 2015).
Ginsenoside Rd (GSRd) is isolated from the root of Panax ginseng and applied to protect cells in various types of diseases especially ischemia diseases (Ye et al., 2011). It is noteworthy that GSRd has an impact on different cell types which are involved in AKI. For instance, previous studies identified that GSRd (50 mg/kg i.p. for 2 days) prevented M1 macrophage polarization in I/R-injured kidney (Ren et al., 2016). Additionally, GSRd (5 mg/kg i.p. for 30 days) protected proximal tubule cells against I/R model-induced hypoxia-reoxygenation by inhibiting oxygen free radicals from attacking cell membranes (Yokozawa et al., 1998). The renoprotective effect of GSRd (5 mg/kg i.p. for 30 days) was further determined in cisplatin and glycerol-induced AKI models, treatment of GSRd decreased apoptosis-triggered DNA fragmentation and oxidative stress (Yokozawa and Liu, 2000; Yokozawa and Dong, 2001; Zhou et al., 2014). Other Ginsenosides, such as Rb1, Rg1 (80 mg/L for 24 h) and Rg3 also proved to be effective in the treatment of AKI. It has been identified that administration of Ginsenoside Rb1 relieves apoptosis of HK-2 cells in response to serum from I/R AKI (Zhu et al., 2009). Ginsenoside Rg1 reduces aldosterone-induced oxidative stress and abnormal autophagy correlates with AMPK/mTOR pathway. Ginsenosides 20(S)-Rg3 exerts therapeutic effects in both cisplatin (GSRd 250 μg/mL for 24 h) and LPS (GSRd 10 mg/kg i.p. for 15 days)-induced AKI by targeting JNK-p53-caspase-3 axis and NF-κB signaling pathway (Kang et al., 2007; Wang et al., 2015; Han et al., 2016).
Resveratrol (RSV), a popular natural phenolic compound which is abundant in wines and grape skins, protects against multiple types of AKI due to its low toxicity, powerful antioxidants, and anti-inflammatory properties. Resveratrol (100 mg/kg for 20 h by oral gavage) can attenuate LPS-induced AKI by suppressing inflammation and apoptosis driven by macrophages (Chen L. et al., 2015). Resveratrol (10 mg/kg i.p. for 12 h) consistently protects against sepsis-induced tubular epithelium injury by restoring the renal microcirculation and scavenging reactive nitrogen species (Holthoff et al., 2012). In addition, resveratrol (3 mg/kg for 6 days via the forearm vein) ameliorates arsenic trioxide (As2O3)-induced nephrotoxicity by antagonizing oxidative stress and facilitating arsenic metabolism (Yu et al., 2013). Moreover, resveratrol is proven to be an anti-inflammatory agent in glycerol (RSV 25 mg/kg/day for 4 days via gastric intubation)- and cisplatin (RSV 25 mg/kg/day i.p. for 2/5 days)-induced AKI (de Jesus Soares et al., 2007; Do Amaral et al., 2008). Furthermore, previous studies demonstrated that resveratrol-mediated activation of SIRT1 improved cisplatin (RSV 10 mg/kg orally once a day for 7 days)-induced AKI by deacetylating p53 and reducing apoptosis (Kim et al., 2011), and RSV also inhibited sepsis (RSV 10 mg/kg i.p. for 3 days)-induced AKI and renal inflammation through NF-κB de-acetylation (Gan et al., 2017) or SIRT3-mediated deacetylation of SOD2 (Xu et al., 2016). Resveratrol (30 mg/kg i.p. for 12 h) protected against early sepsis-induced AKI by inhibiting the endoplasmic reticulum stress (IRE1)-activated NF-κB pathway (Wang et al., 2017). A previous study showed that RSVA405 (3 mg/kg i.p. for 24 h) and RSVA314 (3 mg/kg i.p. for 24 h), two biologically active resveratrol analogs (RSVAs), attenuated I/R-induced AKI by exerting anti-oxidative and anti-inflammatory effects, indicating that RSV and its derivatives may be promising agents to prevent and/or treat AKI with high efficiency (Khader et al., 2015).
Tetramethylpyrazine (TMP) is a natural product isolated from the Chinese herb Ligusticum wallichii Franch., which is famous for its antioxidative and anti-inflammatory effects. Previous studies showed that treatment with TMP protects against arsenic (TMP 100 μM for 6 h)-induced nephrotoxicity by targeting HO-1 and ARS2, which was further evidenced by the findings that TMP (20 mg/kg/day i.p. for 7 days) relieves gentamicin-induced AKI by enhancing Hax-1 mitochondrial localization in HO-1-dependent mechanisms (Sue et al., 2009; Gong et al., 2016). Additionally, by suppressing ROS production and the consequential inflammatory response, TMP protected against cisplatin (80 mg/kg/day orally for 7 days) or arsenic (100 μM for 24 h)-induced AKI (Ali et al., 2008; Gong et al., 2015). Moreover, a recent study showed that TMP (80 mg/kg/day i.p. for 4 days) suppressed the apoptosis of renal cells by targeting FoxO1, a pro-apoptotic transcription factor, to prevent contrast-induced AKI (Gong et al., 2013). Interestingly, TMP exerted a renoprotective role by downregulating P-selectin, which has been accepted as a key modulator of neutrophil infiltration in I/R kidney injury (Chen et al., 2003).
Our group also tested the therapeutic potential of traditional Chinese medicine in the treatment of AKI. We screened 10 kinds of Chinese herbal medicine with anti-inflammatory effects and found that wogonin and protocatechuic aldehyde had significant therapeutic effects. Wogonin inhibits cisplatin-induced renal damage by inhibiting RIPK1-mediated necroptosis and attenuates inflammation (Meng et al., 2018), whereas protocatechuic aldehyde (PA) not only inhibits necroptosis, but also effectively reduces cisplatin-induced over-production of ROS (Gao L. et al., 2016). Interestingly, we all know that cisplatin is commonly used as an anti-cancer drugs in clinic, and these two TCMs could even promote anti-tumor effects of cisplatin, so wogonin and protocatechuic aldehyde may be renoprotective adjuvants for cisplatin-based anticancer therapy.
There are many other TCMs to treat AKI, and these are listed in Table 2.
As shown in Figure 1, the TGF-β receptor, Toll-like receptors (TLRs), TNF receptor, and FASL/Death receptors are stimulated by LPS, cisplatin and I/R, etc., then these receptors activate downstream pathways, further triggering ROS production and inflammatory responses, eventually leading to kidney damage. TCMs suppress cisplatin/LPS/I/R-stimulated TLRs including TLR2/4, or by activating PPAR, further inhibiting the NF-κB pathway and reducing inflammation. Moreover, TCMs reduce apoptosis by inhibiting TGF-β, PI3K/AKT and ERK/JNK/P38MARK pathways. In addition, TCMs inhibit autophagy by targeting AMPK/mTOR. In recent years, new cell death mechanisms like programmed necrosis and ferroptosis have also attracted attention. Wogonin and protocatechuic aldehyde can effectively inhibit RIPK1/RIPK3-mediated necroptosis. Breviscapine can reduce ferroptosis by increasing glutathione peroxidase levels. Additionally, TCMs can inhibit H2O2-induced endoplasmic reticulum (ER) stress and further reduce ROS production. In the AKI model, ROS, HMGB1, P53, Nrf2, HO-1, and SIRT1/3 are regarded as potential therapeutic targets of TCMs.
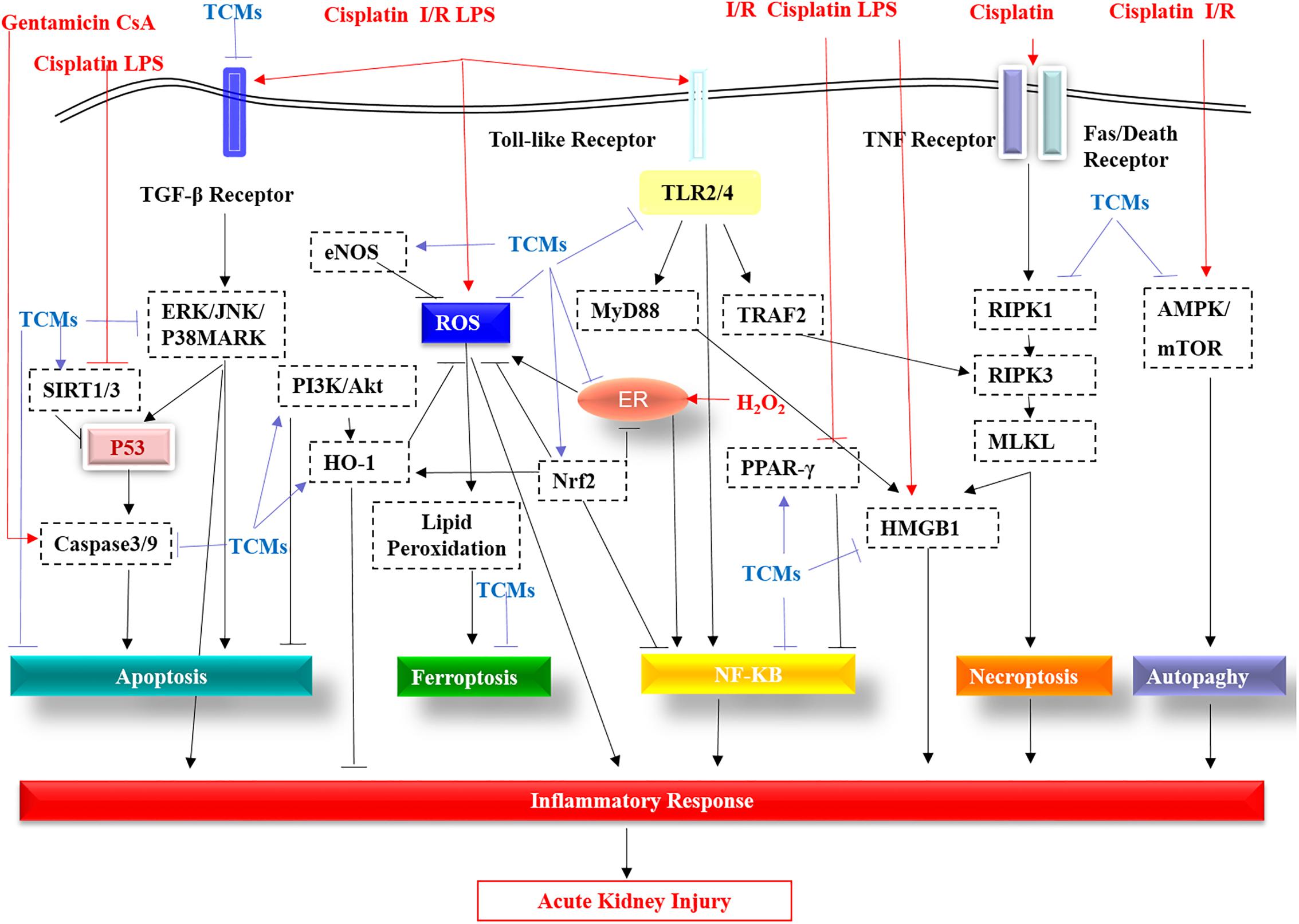
Figure 1. The molecular pathways targeted by the TCMs covered in this review are summarized. TGF-β receptor, Toll-like receptors (TLRs), TNF receptor, and FASL/Death receptors are stimulated by LPS, cisplatin, I/R, etc. These receptors are then activated by the downstream pathway, further triggering ROS production and an inflammatory response, eventually leading to kidney damage. TCMs suppress cisplatin/LPS/I/R-stimulated Toll-like receptors (TLR2/4), or by activating PPAR-γ, further inhibiting the NF-κB pathway and reducing inflammation. Additionally, apart from targeting caspase3/9, TCMs reduce apoptosis by inhibiting the TGF-β receptor, the ERK/JNK/P38MARK pathway and by promoting PI3K/AKT. In addition, TCMs inhibit autophagy by targeting inhibition of AMPK/mTOR. In addition to the traditional apoptosis, autophagy, programmed necrosis and ferroptosis are also caused during AKI. Wogonin and protocatechuic aldehyde can effectively inhibit RIPK1 in the RIPK1/RIPK3/MLKL of necroptosis. TLR2/4-mediated TRAF2 has a stimulant effect on RIPK3. Induction of HMGB2 by necroptosis and TLR2/4-regulated MyD88 aggravates the inflammatory response of acute kidney injury, while TCMs significantly improve this phenomenon via direct or indirect effects. Breviscapine can reduce ferroptosis by increasing glutathione peroxidase levels. In acute kidney injury, the production of ROS, the multiple roles of P53, the protective effects of eNOS, Nrf2, HO-1, and SIRT1/3 all become therapeutic targets of TCMs.
It has been recorded that up to 25% of all cases of AKI may be correlated to nephrotoxic medications (Bentley et al., 2010). As shown in Table 3, previous studies indicate that some TCMs, including aristolochic acid, anthraquinones, flavonoids, and glycosides from herbs, non-steroidal anti-inflammatory drugs, aminoglycosides, cytostatic drugs, osmotic agents, radiocontrast, and phosphate salts, may lead to kidney damage and induce AKI (Liangos, 2012; Yang et al., 2018). Several reasons contributing to nephrotoxicity of TCMs include the intrinsic toxicity of herb medicines, incorrect dosing, interactions between herbs and medications, adulteration, incorrect processing and storage, and contamination by heavy metals (Yang et al., 2018).
It was reported that two patients took sciadopitysin, a kind of flavonoid extracted from Taxus celebica to treat diabetes mellitus, and suffered acute tubular necrosis and acute interstitial nephritis (Lin and Ho, 1994). Andrographolide (Chuan Xin Lian) is widely used in China for the treatment of dysentery and respiratory tract infection. It is noteworthy that a systemic analysis, based on clinical cases reported in Chinese literature from January 1978 to August 2013, revealed 26 patients with AKI induced by andrographolide. The major pathologic features in these patients were acute tubular necrosis. The mechanism is still obscure (Zhang et al., 2014). Aristolochia acids (AA) is extracted from Aristolochia fangchi, within which aristolochic acid I (AAI) and aristolochic acid II (AAII) are well known. AA has widely been used as an anti-inflammatory agent. However, a series of studies demonstrated that AA could induce early and transient acute tubular necrosis and progressive tubulointerstitial injury, which finally lead to renal fibrosis (Sato et al., 2004; Debelle et al., 2008; Zhou et al., 2010; DeBroe, 2012). AA also caused nephropathy, by inducing DNA adduct formation (Allard et al., 2013). Some drugs related with AA are nephrotoxic due to the intrinsic toxicity of herbs and the misidentification of potentially toxic compounds. The root of asarum (also known as Xi Xin) contains low levels of AA and has widely been used as an analgesic for headache, toothache, and inflammatory diseases. But the whole asarum plant contains high levels of AA (Drew et al., 2002). Triptolide, isolated from Tripterygium wilfordii Hook.f (TWHf)-derived diterpenoid, has been commonly used for its immunosuppressive and anti-cancer properties (Carter et al., 2006). However, administration of triptolide may result in severe kidney injury by impairing the antioxidant system, promoting production of reactive oxygen species and inducing apoptosis of tubular epithelial cells, which may limit the application of triptolide in the clinic (Yang et al., 2011, 2012).
The first principle of effective therapy is to acknowledge and prevent or minimize nephrotoxicity of TCMs. In this regard, several strategies should be applied: (1) In view of the intrinsic toxicity of some herbs, researchers could modify molecular structure of TCMs to lower the toxic effects without affecting their therapeutic effects. It is essential to reveal the compound/phytochemicals present in the formulations which are correlated with the toxicity in AKI. (2) As for the incorrect identification, processing and storage, standardization of herbal products need to be emphasized. We should also ensure safe manufacturing processes to avoid contamination from heavy metals and other ingredients. (3) We should determine and limit the dosing and duration of drugs usage through adequate preclinical trials and dose conversions between animals and humans. Safe and effective dose ranges for humans as well as appropriate monitoring for adverse effects are also needed. (4) It is worth mentioning that pharmacists and doctors should clearly know the interactions between TCMs and other medications before prescribing these drugs to patients. (5) For patients with special conditions, like chronic kidney disease and liver disease, their medication needs to be carefully considered.
Collectively, previous studies showed that numerous types of TCMs protect against AKI via different mechanisms of action, including inhibiting inflammation, cell apoptosis, necroptosis, ferroptosis, and restraining oxidative stress etc. These data support the potential application of these TCMs as novel therapeutic agents in treating patients with AKI. Although some TCMs have entered preclinical trials, it is essential to initiate pre-clinical pharmacologic and toxicologic trials and clinical trials to evaluate the efficacy and safety of TCMs usage. Moreover, considering that some TCMs are deleterious to the kidney, they should be attracted more attention when utilized. In addition, it is believed that western medicines always relieve symptoms quickly while TCMs exert therapeutic effects gently and fundamentally. In this regard, the combination of TCMs and western medicines may become a promising treatment strategy for AKI by taking advantages of both and by limiting side effects. The interaction between medicines should also be considered. In conclusion, from a holistic point of view, TCM-based anti-AKI therapy should be emphasized and extensively explored, as this may help to minimize the morbidity and mortality of AKI and prolong the survival of patients.
JL, XM, and CH designed the theme and direction of the manuscript. LZ and XL critically revised the manuscript. HL drafted the manuscript.
This study was supported by the National Natural Science Foundation of China (No. 81770609), Anhui University of Science and Technology (No. 1704a0802161), and Technological Fund of Anhui Province for Outstanding Youth of China (Grant No. 1608085J07).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AKI, acute kidney injury; ER stress, endoplasmic reticulum stress; ERK, extracellular signal-regulated kinase; HMGB1, high mobility group box 1; HO-1, heme oxygenase (HO)-1; i.p., intraperitoneal; I/R, ischemic reperfusion; JNK, C-jun N-terminal kinase; LPS, lipopolysaccharide; MLKL, mixed-lineage kinase like domain; Nrf2, nuclear factor 2 correlation factor; PI3K/AKT, phosphatidylinositol 3-hydroxy kinase/protein kinase; PPAR-γ, peroxisome proliferators-activated receptor-γ; RIPK, receptor interacting serine-threonine protein kinase; ROS, reactive oxygen species; SIRT, silent information regulator; TCMs, traditional Chinese medicines.
Ali, B. H., Al-Moundhri, M., Eldin, M. T., Nemmar, A., Al-Siyabi, S., and Annamalai, K. (2008). Amelioration of cisplatin-induced nephrotoxicity in rats by tetramethylpyrazine, a major constituent of the Chinese herb Ligusticum wallichi. Exp. Biol. Med. 233, 891–896. doi: 10.3181/0711-RM-315
Allard, T., Wenner, T., Greten, H. J., and Efferth, T. (2013). Mechanisms of herb-induced nephrotoxicity. Curr. Med. Chem. 20, 2812–2819. doi: 10.2174/0929867311320220006
Allison, S. J. (2016). Acute kidney injury: AIMing to enhance debris clearance and improve outcomes in AKI. Nat. Rev. Nephrol. 12:123. doi: 10.1038/nrneph.2016.3
An, X., and Shang, F. (2018). RA-XII exerts anti-oxidant and anti-inflammatory activities on lipopolysaccharide-induced acute renal injury by suppressing NF-kappaB and MAPKs regulated by HO-1/Nrf2 pathway. Biochem. Biophys. Res. Commun. 495, 2317–2323. doi: 10.1016/j.bbrc.2017.12.131
Arjumand, W., and Sultana, S. (2011). Glycyrrhizic acid: a phytochemical with a protective role against cisplatin-induced genotoxicity and nephrotoxicity. Life Sci. 89, 422–429. doi: 10.1016/j.lfs.2011.06.016
Augusti, P. R., Conterato, G. M., Somacal, S., Sobieski, R., Spohr, P. R., Torres, J. V., et al. (2008). Effect of astaxanthin on kidney function impairment and oxidative stress induced by mercuric chloride in rats. Food Chem. Toxicol. 46, 212–219. doi: 10.1016/j.fct.2007.08.001
Bentley, M. L., Corwin, H. L., and Dasta, J. (2010). Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies. Crit. Care Med. 38, S169–S174. doi: 10.1097/CCM.0b013e3181de0c60
Cai, Q., Li, X., and Wang, H. (2001). Astragali and Angelica protect the kidney against ischemia and reperfusion injury and accelerate recovery. Chin. Med. J. 114, 119–123.
Cai, Z. Y., Sheng, Z. X., and Yao, H. (2017). Pachymic acid ameliorates sepsis-induced acute kidney injury by suppressing inflammation and activating the Nrf2/HO-1 pathway in rats. Eur. Rev. Med. Pharmacol. Sci. 21, 1924–1931.
Carter, B. Z., Mak, D. H., Schober, W. D., McQueen, T., Harris, D., Estrov, Z., et al. (2006). Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood 108, 630–637. doi: 10.1182/blood-2005-09-3898
Chao, C. S., Tsai, C. S., Chang, Y. P., Chen, J. M., Chin, H. K., and Yang, S. C. (2016). Hyperin inhibits nuclear factor kappa B and activates nuclear factor E2-related factor-2 signaling pathways in cisplatin-induced acute kidney injury in mice. Int. Immunopharmacol. 40, 517–523. doi: 10.1016/j.intimp.2016.09.020
Chen, B., Liu, G., Zou, P., Li, X., Hao, Q., Jiang, B., et al. (2015). Epigallocatechin-3-gallate protects against cisplatin-induced nephrotoxicity by inhibiting endoplasmic reticulum stress-induced apoptosis. Exp. Biol. Med. 240, 1513–1519. doi: 10.1177/1535370215573394
Chen, B. L., Wang, L. T., Huang, K. H., Wang, C. C., Chiang, C. K., and Liu, S. H. (2014). Quercetin attenuates renal ischemia/reperfusion injury via an activation of AMP-activated protein kinase-regulated autophagy pathway. J. Nutr. Biochem. 25, 1226–1234. doi: 10.1016/j.jnutbio.2014.05.013
Chen, D. Z., Chen, L. Q., Lin, M. X., Gong, Y. Q., Ying, B. Y., and Wei, D. Z. (2017). Esculentoside A inhibits LPS-induced acute kidney injury by activating PPAR-gamma. Microb. Pathog. 110, 208–213. doi: 10.1016/j.micpath.2017.06.037
Chen, J. L., Zhou, T., Chen, W. X., Zhu, J. S., Chen, N. W., Zhang, M. J., et al. (2003). Effect of tetramethylpyrazine on P-selectin and hepatic/renal ischemia and reperfusion injury in rats. World J. Gastroenterol. 9, 1563–1566. doi: 10.3748/wjg.v9.i7.1563
Chen, L., Yang, S., Zumbrun, E. E., Guan, H., Nagarkatti, P. S., and Nagarkatti, M. (2015). Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 59, 853–864. doi: 10.1002/mnfr.201400819
Chen, Y., Du, Y., Li, Y., Wang, X., Gao, P., Yang, G., et al. (2015). Panaxadiol saponin and dexamethasone improve renal function in lipopolysaccharide-induced mouse model of acute kidney injury. PLoS One 10:e0134653. doi: 10.1371/journal.pone.0134653
Chertow, G. M., Burdick, E., Honour, M., Bonventre, J. V., and Bates, D. W. (2005). Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 16, 3365–3370. doi: 10.1681/ASN.2004090740
Chyau, C. C., Chen, C. C., Chen, J. C., Yang, T. C., Shu, K. H., and Cheng, C. H. (2014). Mycelia glycoproteins from Cordyceps sobolifera ameliorate cyclosporine-induced renal tubule dysfunction in rats. J. Ethnopharmacol. 153, 650–658. doi: 10.1016/j.jep.2014.03.020
Debelle, F. D., Vanherweghem, J. L., and Nortier, J. L. (2008). Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 74, 158–169. doi: 10.1038/ki.2008.129
DeBroe, M. E. (2012). Chinese herbs nephropathy and Balkan endemic nephropathy: toward a single entity, aristolochic acid nephropathy. Kidney Int. 81, 513–515. doi: 10.1038/ki.2011.428
de Jesus Soares, T., Volpini, R. A., Francescato, H. D., Costa, R. S., da Silva, C. G., and Coimbra, T. M. (2007). Effects of resveratrol on glycerol-induced renal injury. Life Sci. 81, 647–656. doi: 10.1016/j.lfs.2007.06.032
Do Amaral, C. L., Francescato, H. D., Coimbra, T. M., Costa, R. S., Darin, J. D., Antunes, L. M., et al. (2008). Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch. Toxicol. 82, 363–370. doi: 10.1007/s00204-007-0262-x
Domitrovic, R., Cvijanovic, O., Pugel, E. P., Zagorac, G. B., Mahmutefendic, H., and Skoda, M. (2013). Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology 310, 115–123. doi: 10.1016/j.tox.2013.05.015
Drew, A. K., Whyte, I. M., Bensoussan, A., Dawson, A. H., Zhu, X., and Myers, S. P. (2002). Chinese herbal medicine toxicology database: monograph on Herba Asari, “xi xin”. J. Toxicol. Clin. Toxicol. 40, 169–172. doi: 10.1081/CLT-120004405
Fan, H. Y., Qi, D., Yu, C., Zhao, F., Liu, T., Zhang, Z. K., et al. (2016). Paeonol protects endotoxin-induced acute kidney injury: potential mechanism of inhibiting TLR4-NF-kappaB signal pathway. Oncotarget 7, 39497–39510. doi: 10.18632/oncotarget.8347
Feng, C., Xie, X., Wu, M., Li, C., Gao, M., Liu, M., et al. (2013). Tanshinone I protects mice from aristolochic acid I-induced kidney injury by induction of CYP1A. Environ. Toxicol. Pharmacol. 36, 850–857. doi: 10.1016/j.etap.2013.07.017
Feng, L., Ke, N., Cheng, F., Guo, Y., Li, S., Li, Q., et al. (2011). The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. J. Surg. Res. 166, 298–305. doi: 10.1016/j.jss.2009.04.005
Francescato, H. D., Coimbra, T. M., Costa, R. S., and Bianchi Mde, L. (2004). Protective effect of quercetin on the evolution of cisplatin-induced acute tubular necrosis. Kidney Blood Press. Res. 27, 148–158. doi: 10.1159/000078309
Fu, H., Hu, Z., Di, X., Zhang, Q., Zhou, R., and Du, H. (2016). Tenuigenin exhibits protective effects against LPS-induced acute kidney injury via inhibiting TLR4/NF-kappaB signaling pathway. Eur. J. Pharmacol. 791, 229–234. doi: 10.1016/j.ejphar.2016.08.013
Gan, Y., Tao, S., Cao, D., Xie, H., and Zeng, Q. (2017). Protection of resveratrol on acute kidney injury in septic rats. Hum. Exp. Toxicol. 36, 1015–1022. doi: 10.1177/0960327116678298
Gao, H., Cui, Y., Kang, N., Liu, X., Liu, Y., Zou, Y., et al. (2017). Isoacteoside, a dihydroxyphenylethyl glycoside, exhibits anti-inflammatory effects through blocking toll-like receptor 4 dimerization. Br. J. Pharmacol. 174, 2880–2896. doi: 10.1111/bph.13912
Gao, L., Wu, W. F., Dong, L., Ren, G. L., Li, H. D., Yang, Q., et al. (2016). Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Front. Pharmacol. 7:479. doi: 10.3389/fphar.2016.00479
Gao, Z., Han, Y., Hu, Y., Wu, X., Wang, Y., Zhang, X., et al. (2016). Targeting HO-1 by epigallocatechin-3-gallate reduces contrast-induced renal injury via anti-oxidative stress and anti-inflammation pathways. PLoS One 11:e0149032. doi: 10.1371/journal.pone.0149032
Gong, X., Ivanov, V. N., Davidson, M. M., and Hei, T. K. (2015). Tetramethylpyrazine (TMP) protects against sodium arsenite-induced nephrotoxicity by suppressing ROS production, mitochondrial dysfunction, pro-inflammatory signaling pathways and programed cell death. Arch. Toxicol. 89, 1057–1070. doi: 10.1007/s00204-014-1302-y
Gong, X., Ivanov, V. N., and Hei, T. K. (2016). 2,3,5,6-Tetramethylpyrazine (TMP) down-regulated arsenic-induced heme oxygenase-1 and ARS2 expression by inhibiting Nrf2, NF-kappaB, AP-1 and MAPK pathways in human proximal tubular cells. Arch. Toxicol. 90, 2187–2200. doi: 10.1007/s00204-015-1600-z
Gong, X., Wang, Q., Tang, X., Wang, Y., Fu, D., Lu, H., et al. (2013). Tetramethylpyrazine prevents contrast-induced nephropathy by inhibiting p38 MAPK and FoxO1 signaling pathways. Am. J. Nephrol. 37, 199–207. doi: 10.1159/000347033
Guo, S. X., Zhou, H. L., Huang, C. L., You, C. G., Fang, Q., Wu, P., et al. (2015). Astaxanthin attenuates early acute kidney injury following severe burns in rats by ameliorating oxidative stress and mitochondrial-related apoptosis. Mar. Drugs 13, 2105–2123. doi: 10.3390/md13042105
Han, M. S., Han, I. H., Lee, D., An, J. M., Kim, S. N., Shin, M. S., et al. (2016). Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J. Ginseng Res. 40, 135–140. doi: 10.1016/j.jgr.2015.06.006
Holthoff, J. H., Wang, Z., Seely, K. A., Gokden, N., and Mayeux, P. R. (2012). Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 81, 370–378. doi: 10.1038/ki.2011.347
Hsu, Y. H., Chen, T. H., Wu, M. Y., Lin, Y. F., Chen, W. L., Cheng, T. H., et al. (2014). Protective effects of Zhibai Dihuang Wan on renal tubular cells affected with gentamicin-induced apoptosis. J. Ethnopharmacol. 151, 635–642. doi: 10.1016/j.jep.2013.11.031
Huang, Y., Zhou, L. S., Yan, L., Ren, J., Zhou, D. X., and Li, S. S. (2015). Alpinetin inhibits lipopolysaccharide-induced acute kidney injury in mice. Int. Immunopharmacol. 28, 1003–1008. doi: 10.1016/j.intimp.2015.08.002
Huang, Y. C., Tsai, M. S., Hsieh, P. C., Shih, J. H., Wang, T. S., Wang, Y. C., et al. (2017). Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol. Appl. Pharmacol. 329, 128–139. doi: 10.1016/j.taap.2017.05.034
Ji, H. L., Tong, L. G., Bai, C. Z., Song, M. Q., Chen, N. H., and Feng, M. L. (2014). [Protective effect of baicalin against rotenone induced injury on PC12 cells]. Zhongguo Zhong Yao Za Zhi 39, 2947–2951.
Jiang, C., Zhu, W., Shao, Q., Yan, X., Jin, B., Zhang, M., et al. (2016). Tanshinone IIA protects against folic acid-induced acute kidney injury. Am. J. Chin. Med. 44, 737–753. doi: 10.1142/S0192415X16500403
Kang, K. P., Park, S. K., Kim, D. H., Sung, M. J., Jung, Y. J., Lee, A. S., et al. (2011). Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol. Dial. Transplant. 26, 814–822. doi: 10.1093/ndt/gfq528
Kang, K. S., Kim, H. Y., Yamabe, N., Park, J. H., and Yokozawa, T. (2007). Preventive effect of 20(S)-ginsenoside Rg3 against lipopolysaccharide-induced hepatic and renal injury in rats. Free Radic. Res. 41, 1181–1188. doi: 10.1080/10715760701581740
Kaur, A., Kaur, T., Singh, B., Pathak, D., Singh, H., Buttar, et al. (2016). Curcumin alleviates ischemia reperfusion-induced acute kidney injury through NMDA receptor antagonism in rats. Ren. Fail. 38, 1462–1467. doi: 10.1080/0886022X.2016.1214892
Khader, A., Yang, W. L., Kuncewitch, M., Prince, J. M., Marambaud, P., Nicastro, J., et al. (2015). Novel resveratrol analogues attenuate renal ischemic injury in rats. J. Surg. Res. 193, 807–815. doi: 10.1016/j.jss.2014.08.015
Kim, D. H., Jung, Y. J., Lee, J. E., Lee, A. S., Kang, K. P., Lee, S., et al. (2011). SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am. J. Physiol. Renal Physiol. 301, F427–F435. doi: 10.1152/ajprenal.00258.2010
Kim, H. J., Ravichandran, K., Ozkok, A., Wang, Q., He, Z., Jani, A., et al. (2014). The water-soluble triptolide derivative PG490-88 protects against cisplatin-induced acute kidney injury. J. Pharmacol. Exp. Ther. 349, 518–525. doi: 10.1124/jpet.114.213769
Lameire, N. H., Bagga, A., Cruz, D., De Maeseneer, J., Endre, Z., Kellum, J. A., et al. (2013). Acute kidney injury: an increasing global concern. Lancet 382, 170–179. doi: 10.1016/S0140-6736(13)60647-9
Lau, A., Wang, S., Liu, W., Haig, A., Zhang, Z. X., and Jevnikar, A. M. (2014). Glycyrrhizic acid ameliorates HMGB1-mediated cell death and inflammation after renal ischemia reperfusion injury. Am. J. Nephrol. 40, 84–95. doi: 10.1159/000364908
Li, J., Tan, Y. J., Wang, M. Z., Sun, Y., Li, G. Y., Wang, Q. L., et al. (2019). Loganetin protects against rhabdomyolysis-induced acute kidney injury by modulating the Toll-like receptor 4 signalling pathway. Br. J. Pharmacol. doi: 10.1111/bph.14595 [Epub ahead of print].
Li, P., Liao, S. T., Wang, J. S., Zhang, Q., Xu, D. Q., Lv, Y., et al. (2017). Protection by Huang-Lian-Jie-Du decoction and its constituent herbs of lipopolysaccharide-induced acute kidney injury. FEBS Open Bio 7, 221–236. doi: 10.1002/2211-5463.12178
Li, Y., Xiong, W., Yang, J., Zhong, J., Zhang, L., Zheng, J., et al. (2015). Attenuation of inflammation by emodin in lipopolysaccharide-induced acute kidney injury via inhibition of toll-like receptor 2 signal pathway. Iran. J. Kidney Dis. 9, 202–208.
Lim, H. A., Lee, E. K., Kim, J. M., Park, M. H., Kim, D. H., Choi, Y. J., et al. (2012). PPARgamma activation by baicalin suppresses NF-kappaB-mediated inflammation in aged rat kidney. Biogerontology 13, 133–145. doi: 10.1007/s10522-011-9361-4
Lin, J. L., and Ho, Y. S. (1994). Flavonoid-induced acute nephropathy. Am. J. Kidney Dis. 23, 433–440. doi: 10.1016/S0272-6386(12)81008-0
Lin, M., Li, L., Zhang, Y., Zheng, L., Xu, M., Rong, R., et al. (2014). Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int. J. Mol. Sci. 15, 12507–12522. doi: 10.3390/ijms150712507
Linkermann, A. (2016). Nonapoptotic cell death in acute kidney injury and transplantation. Kidney Int. 89, 46–57. doi: 10.1016/j.kint.2015.10.008
Liu, C., Cheng, Z., Wang, Y., Dai, X., Zhang, J., and Xue, D. (2015). Paeoniflorin exerts a nephroprotective effect on concanavalin A-induced damage through inhibition of macrophage infiltration. Diagn. Pathol. 10:120. doi: 10.1186/s13000-015-0347-4
Liu, H., Gu, L. B., Tu, Y., Hu, H., Huang, Y. R., and Sun, W. (2016). Emodin ameliorates cisplatin-induced apoptosis of rat renal tubular cells in vitro by activating autophagy. Acta Pharmacol. Sin. 37, 235–245. doi: 10.1038/aps.2015.114
Liu, H. B., Meng, Q. H., Huang, C., Wang, J. B., and Liu, X. W. (2015). Nephroprotective effects of polydatin against ischemia/reperfusion injury: a role for the PI3K/Akt signal pathway. Oxid. Med. Cell. Longev. 2015:362158. doi: 10.1155/2015/362158
Liu, W. J., Tang, H. T., Jia, Y. T., Ma, B., Fu, J. F., Wang, Y., et al. (2010). Notoginsenoside R1 attenuates renal ischemia-reperfusion injury in rats. Shock 34, 314–320. doi: 10.1097/SHK.0b013e3181ceede4
Liu, X., Huang, Z., Zou, X., Yang, Y., Qiu, Y., and Wen, Y. (2014). Panax notoginseng saponins attenuates cisplatin-induced nephrotoxicity via inhibiting the mitochondrial pathway of apoptosis. Int. J. Clin. Exp. Pathol. 7, 8391–8400.
Liu, X., Huang, Z., Zou, X., Yang, Y., Qiu, Y., and Wen, Y. (2015). Possible mechanism of PNS protection against cisplatin-induced nephrotoxicity in rat models. Toxicol. Mech. Methods 25, 347–354. doi: 10.3109/15376516.2015.1006492
Liu, X. H., Li, J., Li, Q. X., Ai, Y. X., and Zhang, L. (2008). Protective effects of ligustrazine on cisplatin-induced oxidative stress, apoptosis and nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 26, 49–55. doi: 10.1016/j.etap.2008.01.006
Lou, X. Y., Cheng, J. L., and Zhang, B. (2015). Therapeutic effect and mechanism of breviscapine on cisplatin-induced nephrotoxicity in mice. Asian Pac. J. Trop. Med. 8, 873–877. doi: 10.1016/j.apjtm.2015.09.017
Luo, L. N., Xie, Q., Zhang, X. G., and Jiang, R. (2016). Osthole decreases renal ischemia-reperfusion injury by suppressing JAK2/STAT3 signaling activation. Exp. Ther. Med. 12, 2009–2014. doi: 10.3892/etm.2016.3603
Lv, J., Feng, M., Zhang, L., Wan, X., Zeng, Y. C., Liang, P. F., et al. (2015). Protective effect of epigallocatechin gallate, a major constituent of green tea, against renal ischemia-reperfusion injury in rats. Int. Urol. Nephrol. 47, 1429–1435. doi: 10.1007/s11255-015-1030-0
Ma, T., Huang, C., Meng, X., Li, X., Zhang, Y., Ji, S., et al. (2016). A potential adjuvant chemotherapeutics, 18beta-glycyrrhetinic acid, inhibits renal tubular epithelial cells apoptosis via enhancing BMP-7 epigenetically through targeting HDAC2. Sci. Rep. 6:25396. doi: 10.1038/srep25396
Ma, Z. N., Li, Y. Z., Li, W., Yan, X. T., Yang, G., Zhang, J., et al. (2017). Nephroprotective effects of saponins from leaves of Panax quinquefolius against cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 18:E1407. doi: 10.3390/ijms18071407
Meng, L., Qu, L., Tang, J., Cai, S. Q., Wang, H., and Li, X. (2007). A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vascul. Pharmacol. 47, 174–183. doi: 10.1016/j.vph.2007.06.002
Meng, X. M., Li, H. D., Wu, W. F., Ming-Kuen Tang, P., Ren, G. L., Gao, L., et al. (2018). Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab. Invest. 98, 79–94. doi: 10.1038/labinvest.2017.115
Ortega-Dominguez, B., Aparicio-Trejo, O. E., Garcia-Arroyo, F. E., Leon-Contreras, J. C., Tapia, E., Molina-Jijon, E., et al. (2017). Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 107, 373–385. doi: 10.1016/j.fct.2017.07.018
Pan, H., Chen, J., Shen, K., Wang, X., Wang, P., Fu, G., et al. (2015). Mitochondrial modulation by Epigallocatechin 3-Gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and NF-kB in mice. PLoS One 10:e0124775. doi: 10.1371/journal.pone.0124775
Qiu, X., Fu, K., Zhao, X., Zhang, Y., Yuan, Y., Zhang, S., et al. (2015). Protective effects of astaxanthin against ischemia/reperfusion induced renal injury in mice. J. Transl. Med. 13:28. doi: 10.1186/s12967-015-0388-1
Ren, K., Jin, C., Ma, P., Ren, Q., Jia, Z., and Zhu, D. (2016). Ginsenoside Rd alleviates mouse acute renal ischemia/reperfusion injury by modulating macrophage phenotype. J. Ginseng Res. 40, 196–202. doi: 10.1016/j.jgr.2015.12.003
Sahin, K., Tuzcu, M., Gencoglu, H., Dogukan, A., Timurkan, M., Sahin, N., et al. (2010). Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 87, 240–245. doi: 10.1016/j.lfs.2010.06.014
Sancho-Martinez, S. M., Lopez-Novoa, J. M., and Lopez-Hernandez, F. J. (2015). Pathophysiological role of different tubular epithelial cell death modes in acute kidney injury. Clin. Kidney J. 8, 548–559. doi: 10.1093/ckj/sfv069
Sato, N., Takahashi, D., Chen, S. M., Tsuchiya, R., Mukoyama, T., Yamagata, S., et al. (2004). Acute nephrotoxicity of aristolochic acids in mice. J. Pharm. Pharmacol. 56, 221–229. doi: 10.1211/0022357023051
Shin, Y. J., Kim, J. J., Kim, Y. J., Kim, W. H., Park, E. Y., Kim, I. Y., et al. (2015). Protective effects of quercetin against HgCl(2)-induced nephrotoxicity in sprague-dawley rats. J. Med. Food 18, 524–534. doi: 10.1089/jmf.2014.3242
Sue, Y. M., Cheng, C. F., Chang, C. C., Chou, Y., Chen, C. H., and Juan, S. H. (2009). Antioxidation and anti-inflammation by haem oxygenase-1 contribute to protection by tetramethylpyrazine against gentamicin-induced apoptosis in murine renal tubular cells. Nephrol. Dial. Transplant. 24, 769–777. doi: 10.1093/ndt/gfn545
Sun, G., Yang, W., Zhang, Y., and Zhao, M. (2017). Esculentoside A ameliorates cecal ligation and puncture-induced acute kidney injury in rats. Exp. Anim. 66, 303–312. doi: 10.1538/expanim.16-0102
Tan, R. Z., Liu, J., Zhang, Y. Y., Wang, H. L., Li, J. C., Liu, Y. H., et al. (2019). Curcumin relieved cisplatin-induced kidney inflammation through inhibiting Mincle-maintained M1 macrophage phenotype. Phytomedicine 52, 284–294. doi: 10.1016/j.phymed.2018.09.210
Thomas, M. E., Blaine, C., Dawnay, A., Devonald, M. A., Ftouh, S., Laing, C., et al. (2015). The definition of acute kidney injury and its use in practice. Kidney Int. 87, 62–73. doi: 10.1038/ki.2014.328
Tu, Y., Sun, W., Wan, Y. G., Gao, K., Liu, H., Yu, B. Y., et al. (2014). Dahuang Fuzi Decoction ameliorates tubular epithelial apoptosis and renal damage via inhibiting TGF-beta1-JNK signaling pathway activation in vivo. J. Ethnopharmacol. 156, 115–124. doi: 10.1016/j.jep.2014.08.035
Waikar, S. S., Liu, K. D., and Chertow, G. M. (2008). Diagnosis, epidemiology and outcomes of acute kidney injury. Clin. J. Am. Soc. Nephrol. 3, 844–861. doi: 10.2215/CJN.05191107
Wang, H. P., Liu, C. W., Chang, H. W., Tsai, J. W., Sung, Y. Z., and Chang, L. C. (2013). Cordyceps sinensis protects against renal ischemia/reperfusion injury in rats. Mol. Biol. Rep. 40, 2347–2355. doi: 10.1007/s11033-012-2316-2
Wang, L., Mao, N., Tan, R. Z., Wang, H. L., Wen, J., Liu, Y. H., et al. (2015). Ginsenoside Rg1 reduces aldosterone-induced autophagy via the AMPK/mTOR pathway in NRK-52E cells. Int. J. Mol. Med. 36, 518–526. doi: 10.3892/ijmm.2015.2242
Wang, N., Mao, L., Yang, L., Zou, J., Liu, K., Liu, M., et al. (2017). Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-kappaB pathway. Oncotarget 8, 36449–36461. doi: 10.18632/oncotarget.16860
Wang, P., Wang, W., Shi, Q., Zhao, L., Mei, F., Li, C., et al. (2016). Paeoniflorin ameliorates acute necrotizing pancreatitis and pancreatitis induced acute renal injury. Mol. Med. Rep. 14, 1123–1131. doi: 10.3892/mmr.2016.5351
Wang, Q., Yao, Y. M., Wang, W. J., Xian, L. M., Dong, N., Xu, S., et al. (2007). [Effect of Xuebijing injection on renal high mobility group box-1 protein expression and acute kidney injury in rats after scald injury]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 29, 478–483.
Wang, X., Zhao, H., Shao, Y., Wang, P., Wei, Y., Zhang, W., et al. (2014). Nephroprotective effect of astaxanthin against trivalent inorganic arsenic-induced renal injury in wistar rats. Nutr. Res. Pract. 8, 46–53. doi: 10.4162/nrp.2014.8.1.46
Wang, Y., Feng, F., Liu, M., Xue, J., and Huang, H. (2018). Resveratrol ameliorates sepsis-induced acute kidney injury in a pediatric rat model via Nrf2 signaling pathway. Exp. Ther. Med. 16, 3233–3240. doi: 10.3892/etm.2018.6533
Wu, C. H., Chen, A. Z., and Yen, G. C. (2015). Protective effects of glycyrrhizic acid and 18beta-glycyrrhetinic acid against cisplatin-induced nephrotoxicity in BALB/c mice. J. Agric. Food Chem. 63, 1200–1209. doi: 10.1021/jf505471a
Wu, J., Pan, X., Fu, H., Zheng, Y., Dai, Y., Yin, Y., et al. (2017). Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci. Rep. 7:10114. doi: 10.1038/s41598-017-10693-4
Wu, M. F., Li, P. C., Chen, C. C., Ye, S. S., Chien, C. T., and Yu, C. C. (2011). Cordyceps sobolifera extract ameliorates lipopolysaccharide-induced renal dysfunction in the rat. Am. J. Chin. Med. 39, 523–535. doi: 10.1142/S0192415X11009007
Xia, S., Lin, H., Liu, H., Lu, Z., Wang, H., Fan, S., et al. (2019). Honokiol attenuates sepsis-associated acute kidney injury via the inhibition of oxidative stress and inflammation. Inflammation doi: 10.1007/s10753-018-0937-x [Epub ahead of print].
Xu, D., Chen, M., Ren, X., and Wu, Y. (2014). Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-kappaB signaling pathway. Fitoterapia 97, 148–155. doi: 10.1016/j.fitote.2014.06.005
Xu, J. J., Zhen, J. T., Tang, L., and Lin, Q. M. (2017). Intravenous injection of Xuebijing attenuates acute kidney injury in rats with paraquat intoxication. World J. Emerg. Med. 8, 61–64. doi: 10.5847/wjem.j.1920-8642.2017.01.011
Xu, Y., Zhang, J., Liu, J., Li, S., Li, C., Wang, W., et al. (2015). Luteolin attenuate the D-galactose-induced renal damage by attenuation of oxidative stress and inflammation. Nat. Prod. Res. 29, 1078–1082. doi: 10.1080/14786419.2014.981181
Xu, S., Gao, Y., Zhang, Q., Wei, S., Chen, Z., Dai, X., et al. (2016). SIRT1/3 activation by resveratrol attenuates acute kidney injury in a septic rat model. Oxid. Med. Cell. Longev. 2016:7296092. doi: 10.1155/2016/7296092
Yan, W., Xu, Y., Yuan, Y., Tian, L., Wang, Q., Xie, Y., et al. (2017). Renoprotective mechanisms of Astragaloside IV in cisplatin-induced acute kidney injury. Free Radic. Res. 51, 669–683. doi: 10.1080/10715762.2017.1361532
Yang, B., Xie, Y., Guo, M., Rosner, M. H., Yang, H., and Ronco, C. (2018). Nephrotoxicity and Chinese herbal medicine. Clin. J. Am. Soc. Nephrol. 13, 1605–1611. doi: 10.2215/CJN.11571017
Yang, F., Ren, L., Zhuo, L., Ananda, S., and Liu, L. (2012). Involvement of oxidative stress in the mechanism of triptolide-induced acute nephrotoxicity in rats. Exp. Toxicol. Pathol. 64, 905–911. doi: 10.1016/j.etp.2011.03.013
Yang, F., Zhuo, L., Ananda, S., Sun, T., Li, S., and Liu, L. (2011). Role of reactive oxygen species in triptolide-induced apoptosis of renal tubular cells and renal injury in rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 31, 335–341. doi: 10.1007/s11596-011-0377-4
Yang, L., Xing, G., Wang, L., Wu, Y., Li, S., Xu, G., et al. (2015). Acute kidney injury in China: a cross-sectional survey. Lancet 386, 1465–1471. doi: 10.1016/S0140-6736(15)00344-X
Yang, Y., Song, M., Liu, Y., Liu, H., Sun, L., Peng, Y., et al. (2016). Renoprotective approaches and strategies in acute kidney injury. Pharmacol. Ther. 163, 58–73. doi: 10.1016/j.pharmthera.2016.03.015
Ye, H. Y., Jin, J., Jin, L. W., Chen, Y., Zhou, Z. H., and Li, Z. Y. (2017). Chlorogenic acid attenuates lipopolysaccharide-induced acute kidney injury by inhibiting TLR4/NF-kappaB signal pathway. Inflammation 40, 523–529. doi: 10.1007/s10753-016-0498-9
Ye, Q., Zhu, Y. I., Ye, S., Liu, H., She, X., Niu, Y., et al. (2016). Gypenoside attenuates renal ischemia/reperfusion injury in mice by inhibition of ERK signaling. Exp. Ther. Med. 11, 1499–1505. doi: 10.3892/etm.2016.3034
Ye, R., Yang, Q., Kong, X., Han, J., Zhang, X., Zhang, Y., et al. (2011). Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem. Int. 58, 391–398. doi: 10.1016/j.neuint.2010.12.015
Yokozawa, T., and Dong, E. (2001). Role of ginsenoside-Rd in cisplatin-induced renal injury: special reference to DNA fragmentation. Nephron 89, 433–438. doi: 10.1159/000046116
Yokozawa, T., and Liu, Z. W. (2000). The role of ginsenoside-Rd in cisplatin-induced acute renal failure. Ren. Fail. 22, 115–127. doi: 10.1081/JDI-100100858
Yokozawa, T., Liu, Z. W., and Dong, E. (1998). A study of ginsenoside-Rd in a renal ischemia-reperfusion model. Nephron 78, 201–206. doi: 10.1159/000044911
Yu, C., Li, P., Qi, D., Wang, L., Qu, H. L., Zhang, Y. J., et al. (2017). Osthole protects sepsis-induced acute kidney injury via down-regulating NF-kappaB signal pathway. Oncotarget 8, 4796–4813. doi: 10.18632/oncotarget.13592
Yu, H., Zhou, Q., Huang, R., Yuan, M., Ao, X., and Yang, J. (2012). [Effect of Cordyceps sinensis on the expression of HIF-1alpha and NGAL in rats with renal ischemia-reperfusion injury]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 37, 57–66. doi: 10.3969/j.issn.1672-7347.2012.01.011
Yu, M., Xue, J., Li, Y., Zhang, W., Ma, D., Liu, L., et al. (2013). Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch. Toxicol. 87, 1025–1035. doi: 10.1007/s00204-013-1026-4
Yuxi, Q., Zhang, H., Baili, Y., and Shi, S. (2017). Effects of xuebijing injection for patients with sepsis-induced acute kidney injury after Wenchuan earthquake. Altern. Ther. Health Med. 23, 36–42.
Zhang, J., Yang, S., Chen, F., Li, H., and Chen, B. (2017). Ginkgetin aglycone ameliorates LPS-induced acute kidney injury by activating SIRT1 via inhibiting the NF-kappaB signaling pathway. Cell Biosci. 7:44. doi: 10.1186/s13578-017-0173-3
Zhang, J. X., Dang, S. C., Qu, J. G., and Wang, X. Q. (2006). Ligustrazine alleviates acute renal injury in a rat model of acute necrotizing pancreatitis. World J. Gastroenterol. 12, 7705–7709. doi: 10.3748/wjg.v12.i47.7705
Zhang, L., Sun, D., Bao, Y., Shi, Y., Cui, Y., and Guo, M. (2017). Nerolidol protects against LPS-induced acute kidney injury via inhibiting TLR4/NF-kappaB signaling. Phytother. Res. 31, 459–465. doi: 10.1002/ptr.5770
Zhang, W. X., Zhang, Z. M., Zhang, Z. Q., Wang, Y., and Zhou, W. (2014). Andrographolide induced acute kidney injury: analysis of 26 cases reported in Chinese Literature. Nephrology 19, 21–26. doi: 10.1111/nep.12172
Zhang, Z., Gao, X., Guo, M., Jiang, H., Cao, Y., and Zhang, N. (2016). The protective effect of baicalin against lead-induced renal oxidative damage in mice. Biol. Trace Elem. Res. 175, 129–135. doi: 10.1007/s12011-016-0731-2
Zhang, Z., Gao, X., Guo, M., Jiang, H., Cao, Y., and Zhang, N. (2017). The protective effect of baicalin against lead-induced renal oxidative damage in mice. Biol. Trace Elem. Res. 175, 129–135. doi: 10.1007/s12011-016-0731-2
Zhao, H., Liu, Z., Shen, H., Jin, S., and Zhang, S. (2016). Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur. J. Pharmacol. 781, 92–99. doi: 10.1016/j.ejphar.2016.04.006
Zhou, J., Zhang, H. A., Lin, Y., Liu, H. M., Cui, Y. M., Xu, Y., et al. (2014). Protective effect of ginsenoside against acute renal failure via reduction of renal oxidative stress and enhanced expression of ChAT in the proximal convoluted tubule and ERK1/2 in the paraventricular nuclei. Physiol. Res. 63, 597–604.
Zhou, L., Fu, P., Huang, X. R., Liu, F., Lai, K. N., and Lan, H. Y. (2010). Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J. Am. Soc. Nephrol. 21, 31–41. doi: 10.1681/ASN.2008111133
Zhou, Q., and Hu, S. (2010). [Effect of Cordyceps Cinensis extractant on apoptosis and expression of Toll-like receptor 4 mRNA in the ischemia-reperfusion injured NRK-52E cells]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 35, 77–84. doi: 10.3969/j.issn.1672-7347.2010.01.011
Zhu, J. S., Halpern, G. M., and Jones, K. (1998). The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part I. J. Altern. Complement. Med. 4, 289–303. doi: 10.1089/acm.1998.4.3-289
Zhu, M. X., Ran, B., Feng, Z. Q., and Pan, Q. W. (2009). [Effects of Rb1 and Rg1 on the expression of Bcl-2, Bax in apoptosis of HK-2 cells induced by the serum of kidney ischemia/reperfusion]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 25, 496–499.
Zhu, X. L., Wang, Y. J., Yang, Y., Yang, R. C., Zhu, B., Zhang, Y., et al. (2012). Suppression of lipopolysaccharide-induced upregulation of toll-like receptor 4 by emodin in mouse proximal tubular epithelial cells. Mol. Med. Rep. 6, 493–500. doi: 10.3892/mmr.2012.960
Zhu, Y., Fu, Y., and Lin, H. (2016). Baicalin inhibits renal cell apoptosis and protects against acute kidney injury in pediatric sepsis. Med. Sci. Monit. 22, 5109–5115. doi: 10.12659/MSM.899061
Zou, P., Song, J., Jiang, B., Pei, F., Chen, B., Yang, X., et al. (2014). Epigallocatechin-3-gallate protects against cisplatin nephrotoxicity by inhibiting the apoptosis in mouse. Int. J. Clin. Exp. Pathol. 7, 4607–4616.
Keywords: acute kidney injury (AKI), traditional Chinese medicine (TCM), inflammation, apoptosis, nephrotoxicity
Citation: Li H-D, Meng X-M, Huang C, Zhang L, Lv X-W and Li J (2019) Application of Herbal Traditional Chinese Medicine in the Treatment of Acute Kidney Injury. Front. Pharmacol. 10:376. doi: 10.3389/fphar.2019.00376
Received: 02 August 2018; Accepted: 26 March 2019;
Published: 18 April 2019.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaReviewed by:
Subhalakshmi Ghosh, Independent Researcher, Kolkata, IndiaCopyright © 2019 Li, Meng, Huang, Zhang, Lv and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, MTE2NTc5NjE1NEBxcS5jb20=; bGpAYWhtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.