
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 12 March 2019
Sec. Translational Pharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00259
This article is part of the Research Topic Advancing Genomics for Rare Disease Diagnosis and Therapy Development View all 29 articles
 Zhimei Liu1†
Zhimei Liu1† Li Zhang2,3†
Li Zhang2,3† Danmin Shen1
Danmin Shen1 Changhong Ding1
Changhong Ding1 Xinying Yang1
Xinying Yang1 Weihua Zhang1
Weihua Zhang1 Jiuwei Li1
Jiuwei Li1 Jie Deng1
Jie Deng1 Shuai Gong1
Shuai Gong1 Jun Liu4
Jun Liu4 Suyun Qian4
Suyun Qian4 Fang Fang1*
Fang Fang1*Congenital myasthenic syndromes (CMSs) are a group of inherited disorders caused by genetic defects in neuromuscular junctions. Mutations in CHAT, encoding choline acetyltransferase, cause congenital myasthenic syndrome with episodic apnea (CMS-EA), a rare autosomal recessive disease characterized by respiratory insufficiency with cyanosis and apnea after infections, fever, vomiting, or excitement. To date, no studies have reported deletions comprised of multiple exons. Here, using next generation sequencing, we identified compound heterozygous mutations, namely a large maternally inherited deletion, including exons 4, 5, and 6, and a paternally inherited missense variant (c.914T>C [p.Ile305Thr]) in CHAT in a Chinese patient with a severe phenotype of CMS-EA. Furthermore, the large deletion was also validated by real-time fluorescence quantitative polymerase chain reaction. The patient was a 10-month-old boy, who presented with a weak cry and feeding difficulties soon after birth, ptosis at 4 months old, episodic apnea after fever at 9 months old, and respiratory insufficiency with cyanosis and apnea that required intubation after a respiratory tract infection at 10 months old. Unfortunately, he died in the Pediatric Intensive Care Unit soon after hospitalization. The patient’s elder sister had similar clinical manifestations, and she died prior to the age of 2 months old without a diagnosis. Genotype-phenotype correlation analysis revealed that loss-of-function mutations in exons 4–6 of CHAT might cause more severe CMS-EA. To our knowledge, this is the first study to show compound heterozygous CHAT mutations consisting of a large deletion and missense mutation in a patient with CMS-EA.
Congenital myasthenic syndromes (CMSs) are a group of inherited disorders caused by genetic defects in neuromuscular junctions. CMSs have been recognized as clinical entities since the 1970s (Conomy et al., 1975; Engel et al., 1977) and are classified into the presynaptic, synaptic, or postsynaptic syndrome type according to the involved mutation sites or genes. With the development of next generation sequencing (NGS) technology, over 30 CMS disease-related genes have now been reported, including CHAT, CHRNE, COLQ, RAPSN, and so on; of these, CHAT accounts for 4–5% (Abicht et al., 1993–2019; Engel, 2018; Rodriguez et al., 2018).
The CHAT gene, located on chromosome 10q11.23, encodes choline acetyltransferase (ChAT), which catalyzes the synthesis of the neurotransmitter acetylcholine from acetyl coenzyme A (AcCoA) and choline. In Ohno et al. (2001), CHAT mutations were first reported to cause congenital myasthenic syndrome with episodic apnea (CMS-EA), also named familial infantile myasthenia. Usually, CMS-EA manifests at birth or in early infancy with hypotonia, variable eyelid ptosis, severe bulbar weakness causing dysphagia, and respiratory insufficiency with cyanosis and apnea; the crises recur with infections, fever, excitement, vomiting, or overexertion, and can be prevented or mitigated by anticholinesterase drugs (Ohno et al., 2001). To date, more than 40 CHAT mutations have been identified to cause CMS-EA (Human Gene Mutation Database [HGMD®] Professional version 2018.1). Although some genetic heterogeneity regarding catalytic activity and phenotypic heterogeneity regarding onset, severity of crises, and prognosis have been described, no genotype-phenotype correlation has been identified. Here, we present the case of a 10-month-old Chinese boy with compound heterozygous CHAT variants, including a large deletion (exons 4, 5, and 6) and a missense variant c.914T>C (p.Ile305Thr), which manifested as severe CMS-EA.
The present study was approved by the Ethics Committee of Beijing Children’s Hospital, Capital Medical University, Beijing, China, and was conducted according to the principles expressed in the Declaration of Helsinki. Participants and/or their legal guardians involved in this study gave a written informed consent prior to inclusion in the study. Participants and/or their legal guardians also provided their written informed consent for the material to appear in Frontiers in Pharmacology and associated publications without limit on the duration of publication.
The present study included DNA samples from three family members, the parents and the proband. Genomic DNA was isolated using a blood DNA extraction kit according to the manufacturer’s recommendations (Beijing ComWin Biotech Co., Ltd., Beijing, China). A minimum of 3 μg DNA was used to make the indexed Illumina libraries according to the manufacturer’s protocol. The 300–400 bp library size including adapter sequences was finally selected.
Targeted sequencing was performed on the whole mitochondrial genome and 1,033 nuclear genes (Supplementary Table S1), that affect mitochondrial structure and function, or cause some disease difficult to differentiate from mitochondrial disease, such as Krabbe disease, succinic semialdehyde dehydrogenase deficiency, CMS-EA, and so on (Fang et al., 2017).
The variant prioritized through NGS was verified by Sanger sequencing in the patient and his parents. The primer sequences used were as follows: F: 5′-GCCGAGAGAAGATCAGCATAAGCA-3′, and R: 5′-GTACAGGTGGAGGTCTCGATCA-3′.
Paired-end reads of 200 bp (100 bp at each end) from the targeted sequencing were mapped to UCSC human reference genome (GRCh37/hg19) using Burrows–Wheeler Aligner (Li and Durbin, 2010) “mem” mode with default options, followed by removal of polymerase chain reaction (PCR) duplicates and low-quality reads (BaseQ <20). The binary alignment map files were then sorted, indexed, and converted into the mpileup format by SAMtools (Li et al., 2009). Variant calling was implemented in VarScan (Koboldt et al., 2012) software1 using the mpileup2snp and mpileup2indel modules.
The identified variants were annotated by ANNOVAR (Wang et al., 2010). The annotation information included minor allele frequency (MAF) in the Genome Aggregation Database (gnomAD) (Lek et al., 2016), variant pathogenicity scores by SIFT (Ng and Henikoff, 2003), PolyPhen2 (Adzhubei et al., 2013), MutationTaster (Schwarz et al., 2010), M-CAP (Jagadeesh et al., 2016), RefSeq gene and the consequences on protein, such as missense, frameshift, in-frameshift, stop-gain, and splicing. Rare variants (MAF < 0.01%) were filtered based on gnomAD (Lek et al., 2016).
The CHAT deletion was firstly identified by targeted sequencing data as the loss of heterogeneity in the proband. The read depth for each site (base) within the exons of CHAT gene was calculated. The average read depth of each exon was then calculated by averaging the read depth for the sites within the exon (Figure 3B). Using ALB gene as the internal control, copy numbers of the 4th, 5th, and 6th exons in the CHAT gene were estimated by real-time fluorescence quantitative PCR (qPCR) in the patient and his parents.
The proband, the second child of two healthy non-consanguineous parents, was a 10-month-old boy. He was born through cesarean section due to a scarred uterus after a full-term pregnancy, with a birth weight of 3.2 kg. Immediately after birth, the boy presented with a weak cry and feeding difficulties, such as slow swallowing, choking easily, and breathing difficulties with apnea if the feeding posture changed, especially when lying down. When he was 4 months old, eyelid ptosis developed. At the age of 9 months, episodic apnea occurred after fever, but improved through symptomatic treatment. The proband’s development milestones were normal.
When the proband was 10 months old, respiratory insufficiency with cyanosis and apnea occurred after a respiratory tract infection, which required mechanical ventilation. A second apneic episode requiring intubation occurred soon thereafter, but the parents refused to permit endotracheal intubation, and thus he received Nasal Continuous Positive Airway Pressure respiratory support. However, repeated episodic apnea continued to occur, and eventually when intubation was deemed necessary. Intravenous immunoglobulin was administered, but was ineffective. The patient’s respiratory function decreased gradually, and he died soon after stopping treatment. Overall, the length of hospital stay was 11 days.
Upon admission to our hospital, the proband’s consciousness was clear. Fluctuating eyelid ptosis was observed, which was aggravated by fatigue. Eye movement in all directions was normal. Limb muscle strength and muscle tone decreased, while the tendon reflex was positive. Meningeal irritation signs and pathological signs were negative.
The neostigmine test was negative, and no anti-acetylcholine receptor or anti-muscle-specific kinase antibodies were detected in the serum. Biochemical examinations, including evaluations of the serum creatine kinase concentration, and serum and cerebrospinal fluid lactate levels, were normal. There were no specific changes in urinary organic acids or blood, as assessed by tandem mass spectrometry analyses. Brain magnetic resonance imaging (MRI) revealed deep sulci in the frontal and parietal lobes and a wide subarachnoid space (Figures 1A,B). Electroencephalography and echocardiography recordings were normal. Pulmonary computed tomography showed slight inflammation, and bronchoscopy did not reveal any abnormalities.
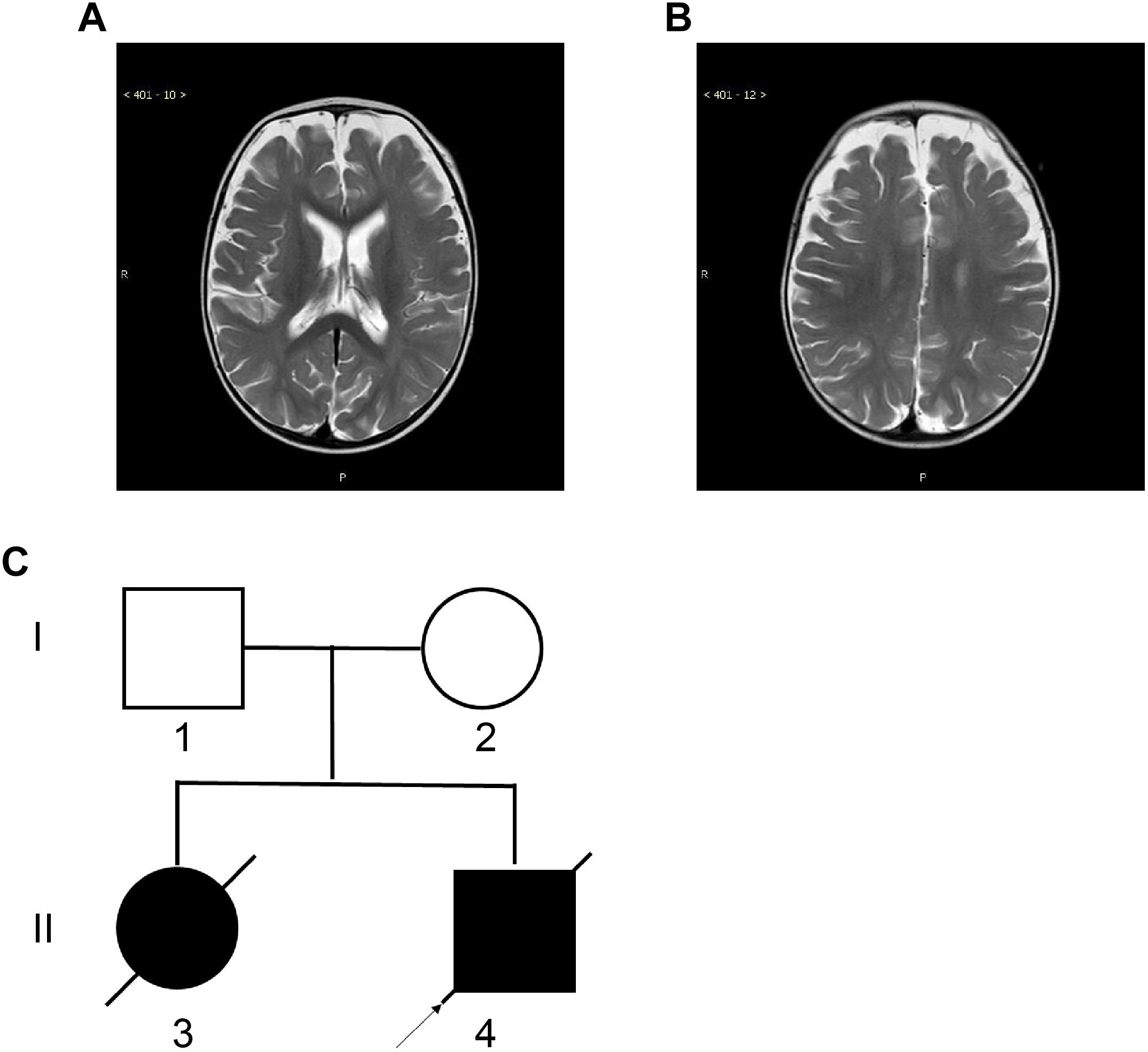
Figure 1. Magnetic Resonance Imaging (MRI) of the proband and the two-generation pedigree. (A,B) Brain MRI of the proband at the age of 10 months showed deep sulci in the frontal and parietal lobes and a wide subarachnoid space. (C) The two-generation pedigree of the family with CMS-EA. The parents are unaffected, while the two offspring are affected. The arrow indicates the proband.
Family history investigation revealed that the proband’s elder sister had similar manifestations and symptoms (Figure 1C), presenting with breathing and feeding difficulties soon after birth. She was hospitalized in the local hospital for nearly 50 days, without a diagnosis, and died of apnea after choking on milk.
The proband was suspected mitochondrial disease previously, and targeted NGS (Jia and Shi, 2017; Ni and Shi, 2017) was performed on the proband (Figure 2A). In total, we identified 4,038 variants (Figure 2B), including 3,842 single nucleotide variants (SNVs), and 196 small insertions or deletions (InDels). We then annotated these variants using ANNOVAR, and found 979 rare variants (gnomAD MAF < 0.01%). After excluding variants within non-coding regions, we identified only 11 missense variants (Figure 2C).
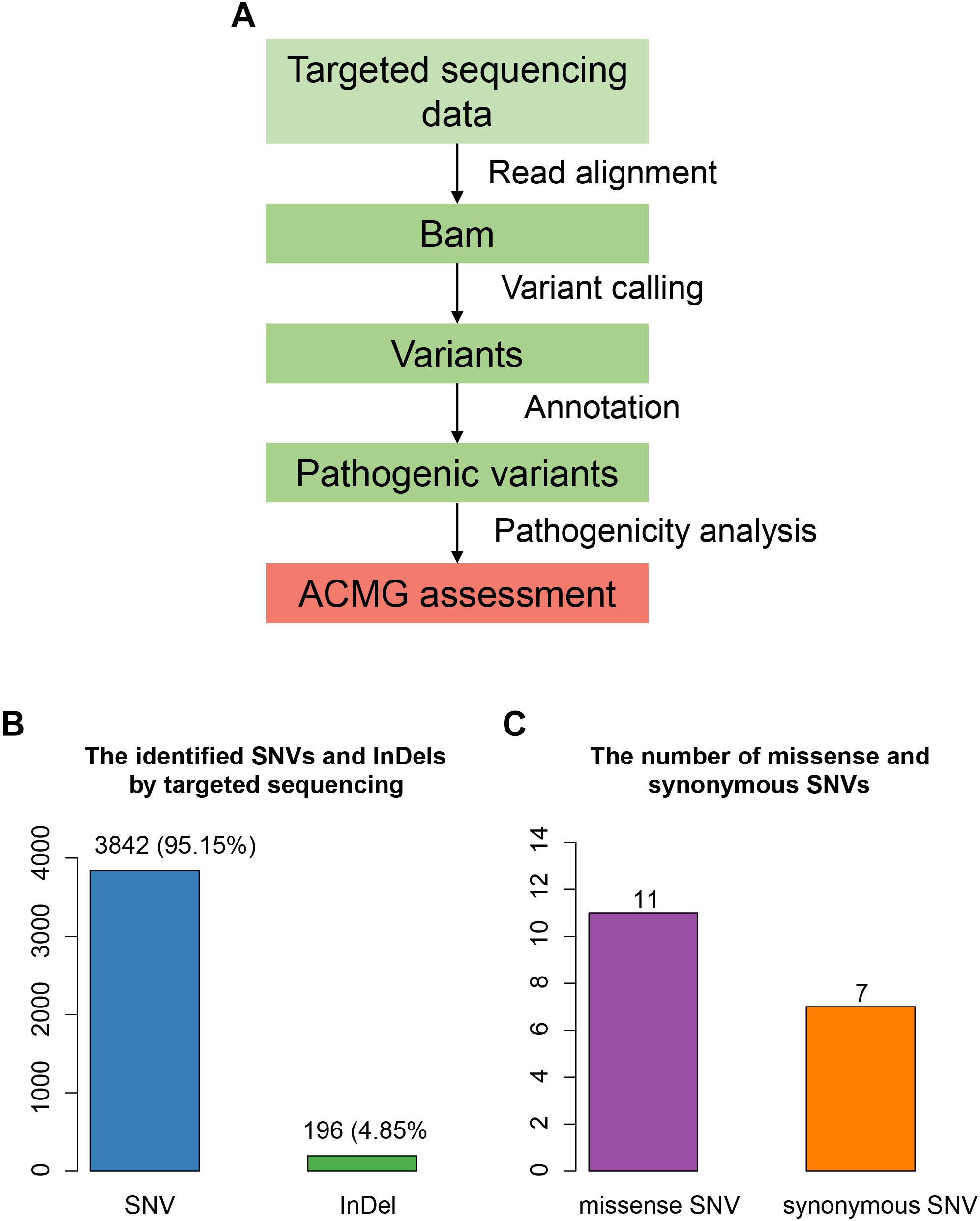
Figure 2. Targeted sequencing-based identification of pathogenic variants. (A) Workflow for the analysis of targeted sequencing data. (B) The SNVs and InDels identified by targeted sequencing. (C) The number of missense and synonymous SNVs in coding regions.
To evaluate the pathogenicity of these rare missense variants, we performed a systematic pathogenicity analysis using methods described in a previous study (Jin et al., 2018; Yu, 2018). For the missense variants, we identified a missense variant within CHAT (c.914T>C [p.Ile305Thr]) as the disease-causing variant (SIFT ≤0.05, PolyPhen ≥0.957, MutationTaster = disease causing, and M-CAP >0.025), which had a homozygous genotype in the proband.
To validate the pathogenic variants, we performed Sanger sequencing on each of the family members. The missense variant in CHAT (c.914T>C [p.Ile305Thr]) was validated in the proband, who had a homozygous genotype, and in his father, who had a heterozygous genotype (Figure 3A). However, this variant was absent in the proband’s mother, suggesting that loss of heterozygosity (LOH) may have led to the variant appearing homozygous in the proband.
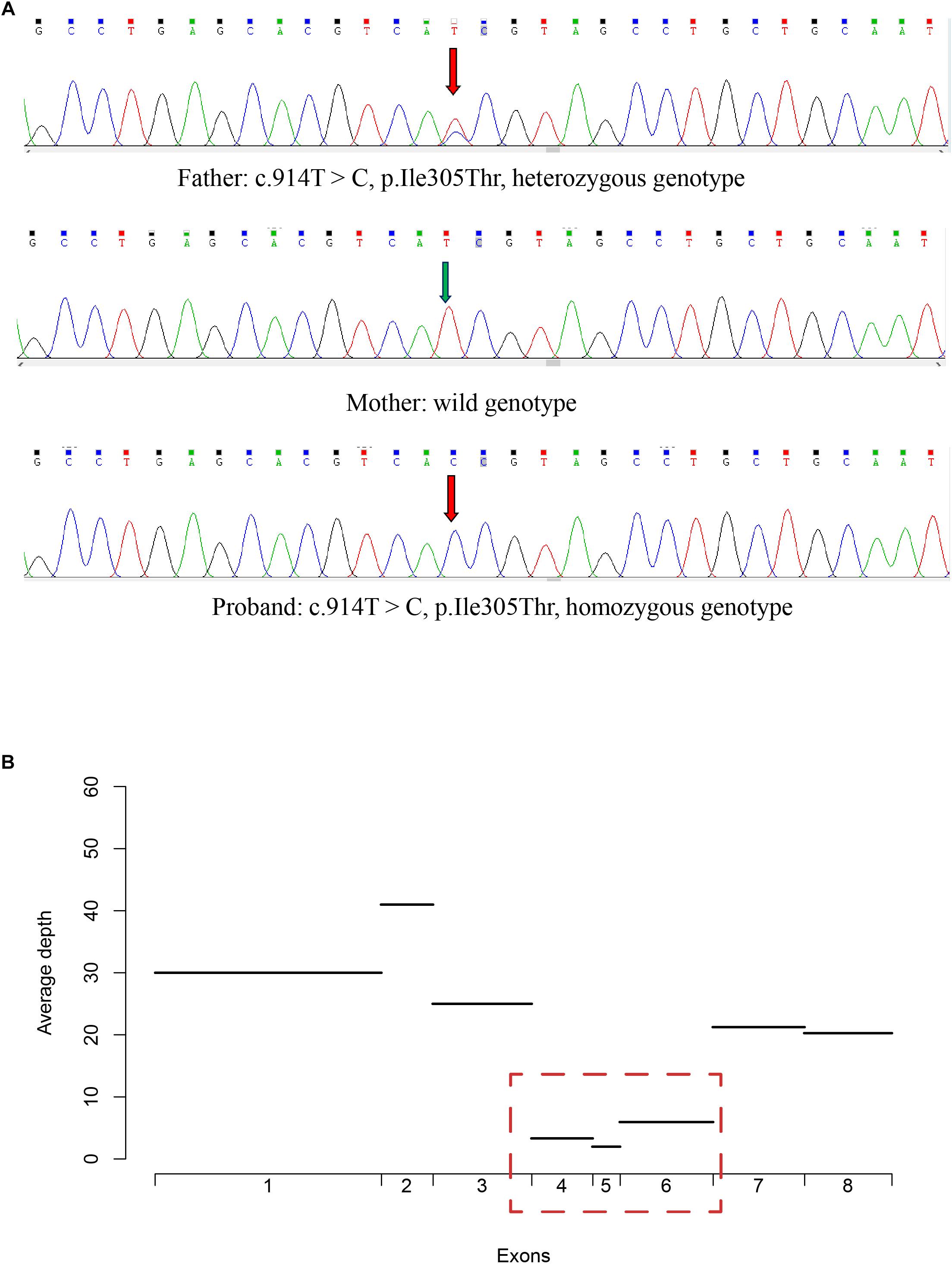
Figure 3. Validation of the missense variant and the large deletion in CHAT. (A) The genotype of the missense variant in three members of the CMS-EA family, as identified by Sanger sequencing. (B) The average read depth for the first 8 exons for the proband was calculated with the targeted sequencing data.
To examine whether a large deletion was located within CHAT, we calculated the read depth for each exon of CHAT. As expectedly, the read depth was significantly reduced from the 4th to 6th exon of the transcript with RefSeq accession number NM_020549 (Figure 3B), indicating that a large deletion was located within these exons. To further validate the large deletion, we performed qPCR to estimate the copy number for exons 4, 5, and 6. In accordance with the read depth analysis, loss of heterozygosity was also observed within regions of the 4th to 6th exon in both the proband and his mother (Figure 4). Hence, the large deletion combined with the missense variant (c.914T>C [p.Ile305Thr]) led to the occurrence of CMS-EA in the proband.
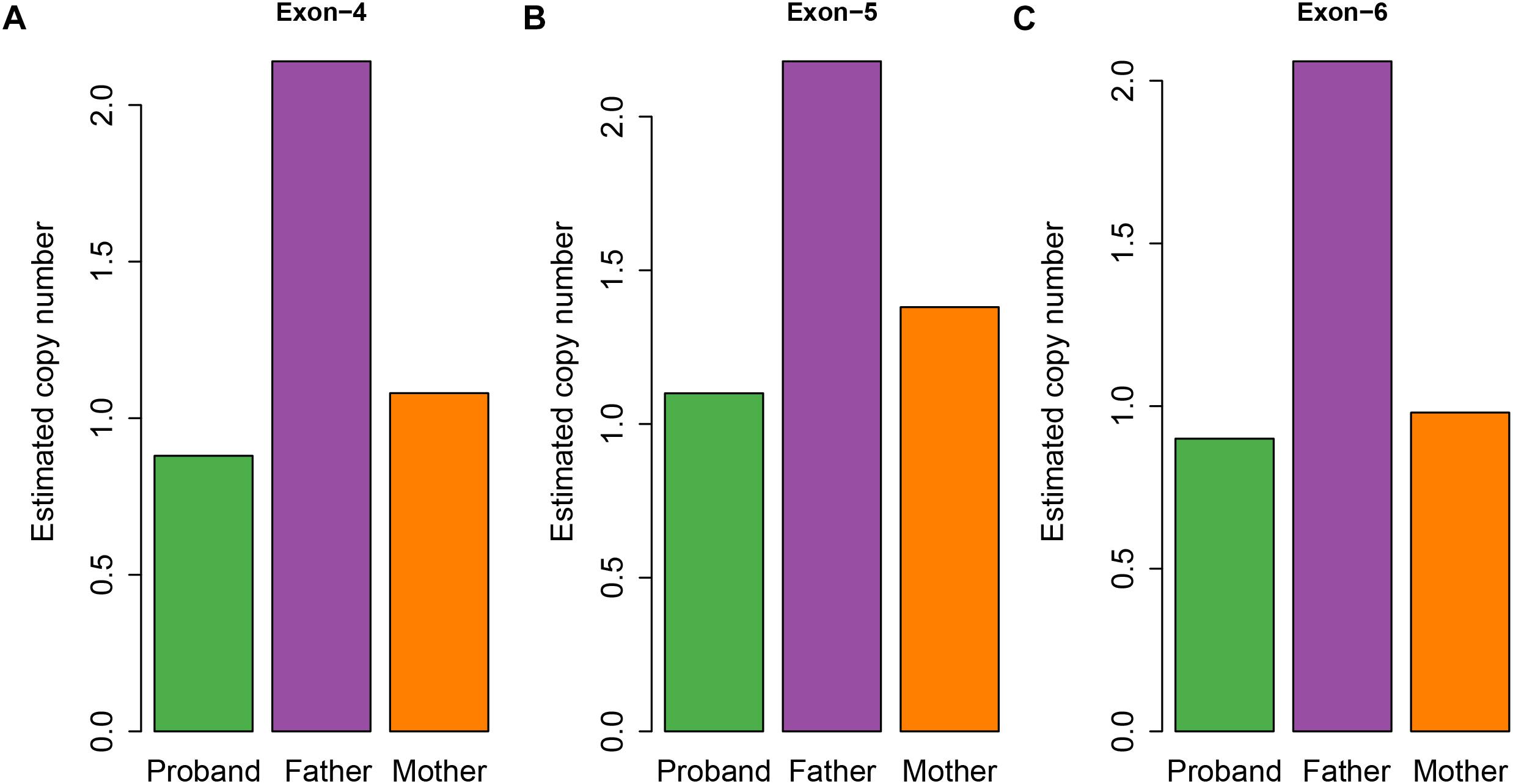
Figure 4. Estimated copy number of the exons for the CMS family members. The estimated copy number of the 4th (A), 5th (B), and 6th (C) exons were estimated by qPCR.
To further understand the role of the two CHAT variants in CMS, we investigated their potential consequences on the ChAT protein. The missense variant in CHAT (c.914T>C [p.Ile305Thr]) was located within the CoA-dependent acyltransferase domain (the amino acid sites from 128 to 508) based on the SuperFamily (Pandurangan et al., 2019) annotation (Figure 5A). Moreover, the large deletion located within the 4th to 6th exons also overlapped with the CoA-dependent acyltransferase domain (Figure 5B). As reported in a previous study, the missense mutation markedly reduced ChAT expression in COS cells and had significantly impaired catalytic efficiencies in kinetic studies (Ohno et al., 2001). These results indicated that the CoA-dependent acyltransferase domain might have been disrupted by the two variants in the patient of this study.
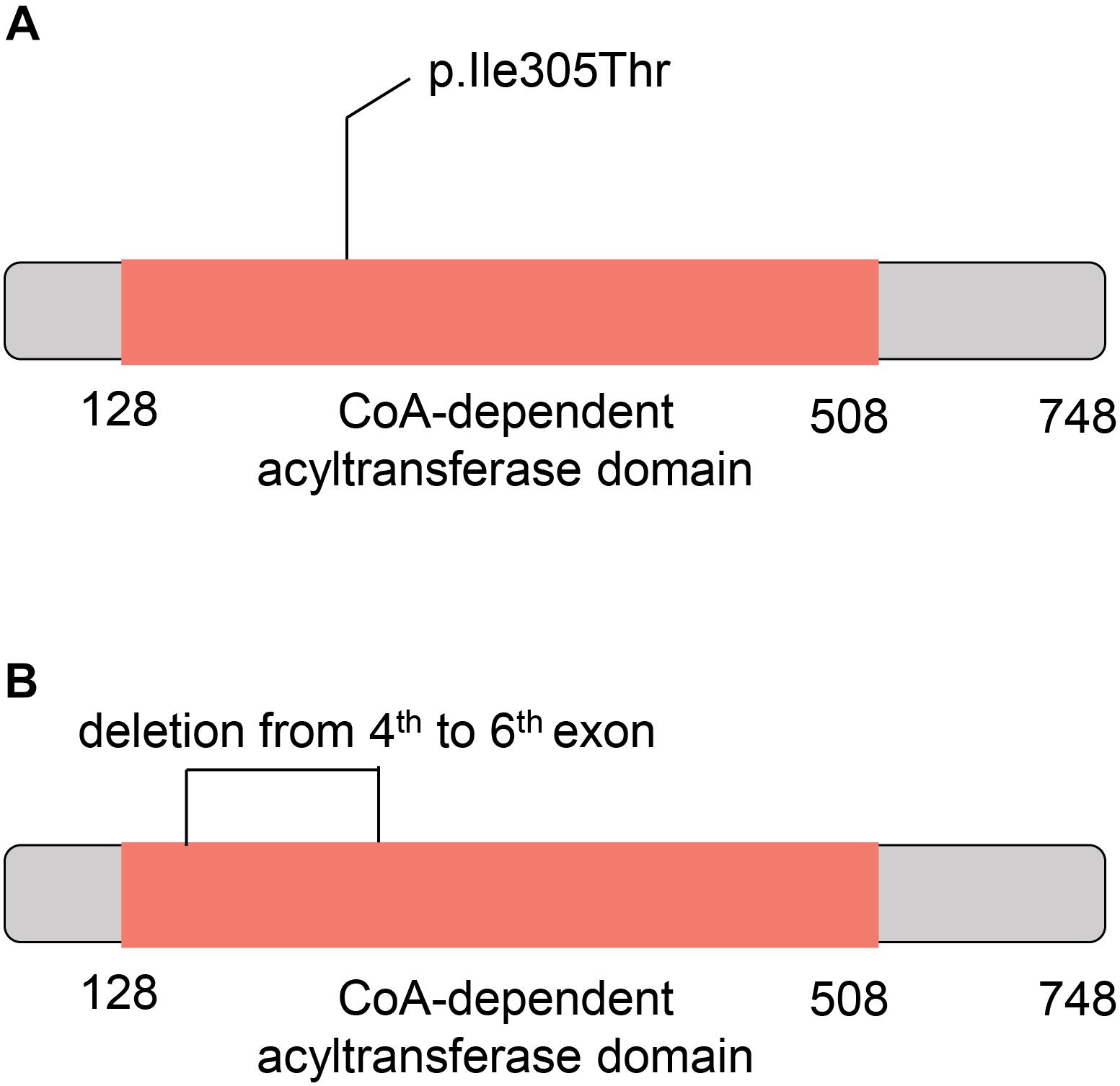
Figure 5. Schematic diagram for the mutant CHAT proteins. (A) The ChAT protein with c.914T>C (p.Ile305Thr) variant. (B) The ChAT protein with the large deletion from the 4th to 6th exons. The red box indicates the acetyl coenzyme -dependent acyltransferase domain.
Since the first report in Ohno et al. (2001) showing that CHAT mutations cause CMS-EA, more than 40 mutations have been found to cause CMS-EA; of these, bi-allelic point mutations are the most frequent (HGMD® Professional version 2018.1). To our knowledge, the present study is the first study to report the case of a patient with CMS-EA caused by compound heterozygous exons deletion and missense mutation in the CHAT gene. Our functional characterization results indicated that the CoA-dependent acyltransferase domain might have been disrupted by the two variants in this study. The missense variant has previously been demonstrated to markedly reduce ChAT expression in COS cells and significantly impair catalytic efficiencies in kinetic studies (Ohno et al., 2001). In accordance with this previous report, the severe phenotype of the patient in our study may have been caused by the missense variant combined with the large deletion.
As mentioned earlier, CMS-EA usually presents at birth or in early infancy with hypotonia, ptosis, dysphagia due to severe bulbar weakness, and respiratory insufficiency with cyanosis and apnea. Occasionally, apnea crises may be mistaken for seizures and thus anticonvulsive drugs may be initiated, without positive effects (Schara et al., 2010); as such, EEG is essential for distinguishing apneic crises from seizures, especially video EEG. Several reported patients presented with mental retardation, and MRI showed brain atrophy. There are two possible explanations for this. First, the apnea may have led to brain hypoxemia. Second, mental retardation may be a symptom of ChAT deficiency in the brain (Schara et al., 2010). Indeed, ChAT deficiency has been reported in various developmental and neurodegenerative disorders, including Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis, Schizophrenia, Rett syndrome, and Sudden Infant Death Syndrome (SIDS) (Oda, 1999). The cause of brain atrophy is undetermined, but normal development milestone in the present study support more that may be induced by brain hypoxemia.
Electrophysiological assessments are important for diagnosing CMS-EA, as repetitive nerve stimulation testing at a low frequency (10 stimuli at 2–3 Hz) might be normal, but prolonged subtetanic stimulation (10 Hz for 5 min) decreased the amplitude of the compound muscle action potential (CMAP) and endplate potential to 50% below baseline (normal decrease is <30%), followed by slow recovery. This slow recovery suggests the presence of a defect in acetylcholine resynthesis and previously aided in the discovery of mutations in CHAT (Ohno et al., 2001; Dilena et al., 2014; Engel et al., 2015). Prolonged subtetanic nerve stimulation is rarely used in infants, and thus performing genetic testing as soon as possible is helpful for ensuring early diagnosis and treatment. Because of the severe condition of our patient, electrophysiological assessments were not conducted.
In general, CMS-EA is a treatable rare disease that responds positively to acetylcholinesterase (AchE) inhibitors, and thus early treatment with pyridostigmine is helpful for improving the clinical symptoms and prognosis (Engel, 2018). Although it was reported that midazolam may mitigate the severity of apnea episodes (Mallory et al., 2009; Barisic et al., 2011), further studies are needed. Unfortunately, the symptoms in our patient were so severe that he died before he could receive AchE inhibitor therapy. We think that the findings of this study will help clinicians identify such cases early, perhaps avoiding a negative outcome.
In addition, there are still some limitations in the present study. First, the association between CHAT large deletion and poor prognosis requires more cases to support. Moreover, the breakpoint of CHAT deletion was not accurately determined by Sanger sequencing due to lack of more blood sample. To conclude, we first reported compound heterozygous CHAT mutations consisting of a large deletion (exons 4, 5, and 6) and missense mutation (c.914T>C [p.Ile305Thr]) in a patient with severe CMS-EA.
All the clinical data and identified genetic variations have been deposited into the rare disease database, eRAM (Jia et al., 2018), at http://www.pediascape.org/eram/.
FF, CD, SQ designed the study. DS, XY, WZ, JwL, JD, SG, and JL collected the data and performed the research, ZL and LZ analyzed the data and wrote the manuscript. All authors reviewed and approved the final manuscript.
This work was supported by Beijing Municipal Science and Technology Plan Projects (Z161100002616004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Editage (www.editage.com) for English language editing. We also acknowledge the financial support of the Open Access Publication Fund of Beijing Children’s Hospital.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00259/full#supplementary-material
TABLE S1 | Nuclear gene list of targeted NGS used in the present study.
Abicht, A., Müller, J. S., and Lochmüller, H. (1993–2019). “Congenital myasthenic syndromes,” in Source® registered GeneReviews, eds M. P. Adam, H. H. Ardinger, R. A. Pagon, S. E. Wallace, L. J. H. Bean, K. Stephens, et al. (Seattle, WA: University of Washington).
Adzhubei, I., Jordan, D. M., and Sunyaev, S. R. (2013). Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 76, 7.20.1–7.20.41. doi: 10.1002/0471142905.hg0270s76
Barisic, N., Chaouch, A., Muller, J. S., and Lochmuller, H. (2011). Genetic heterogeneity and pathophysiological mechanisms in congenital myasthenic syndromes. Eur. J. Paediatr. Neurol. 15, 189–196. doi: 10.1016/j.ejpn.2011.03.006
Conomy, J. P., Levinsohn, M., and Fanaroff, A. (1975). Familial infantile myasthenia gravis: a cause of sudden death in young children. J. Pediatr. 87, 428–430. doi: 10.1016/S0022-3476(75)80651-2
Dilena, R., Abicht, A., Sergi, P., Comi, G. P., Di Fonzo, A., Chidini, G., et al. (2014). Congenital myasthenic syndrome due to choline acetyltransferase mutations in infants: clinical suspicion and comprehensive electrophysiological assessment are important for early diagnosis. J. Child Neurol. 29, 389–393. doi: 10.1177/0883073812470000
Engel, A. G. (2018). Genetic basis and phenotypic features of congenital myasthenic syndromes. Handb. Clin. Neurol. 148, 565–589. doi: 10.1016/B978-0-444-64076-5.00037-5
Engel, A. G., Lambert, E. H., and Gomez, M. R. (1977). A new myasthenic syndrome with end-plate acetylcholinesterase deficiency, small nerve terminals, and reduced acetylcholine release. Ann. Neurol. 1, 315–330. doi: 10.1002/ana.410010403
Engel, A. G., Shen, X. M., Selcen, D., and Sine, S. M. (2015). Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol. 14, 420–434. doi: 10.1016/S1474-4422(14)70201-7
Fang, F., Liu, Z., Fang, H., Wu, J., Shen, D., Sun, S., et al. (2017). The clinical and genetic characteristics in children with mitochondrial disease in China. Sci. China Life Sci. 60, 746–757. doi: 10.1007/s11427-017-9080-y
Jagadeesh, K. A., Wenger, A. M., Berger, M. J., Guturu, H., Stenson, P. D., Cooper, D. N., et al. (2016). M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat. Genet. 48, 1581–1586. doi: 10.1038/ng.3703
Jia, J., An, Z., Ming, Y., Guo, Y., Li, W., Liang, Y., et al. (2018). eRAM: encyclopedia of rare disease annotations for precision medicine. Nucleic Acids Res. 46, D937–D943. doi: 10.1093/nar/gkx1062
Jia, J., and Shi, T. (2017). Towards efficiency in rare disease research: what is distinctive and important? Sci. China Life Sci. 60, 686–691. doi: 10.1007/s11427-017-9099-3
Jin, Y., Zhang, L., Ning, B., Hong, H., Xiao, W., Tong, W., et al. (2018). Application of genome analysis strategies in the clinical testing for pediatric diseases. Pediatr. Investig. 2, 72–81. doi: 10.1002/ped4.12044
Koboldt, D. C., Zhang, Q., Larson, D. E., Shen, D., McLellan, M. D., Lin, L., et al. (2012). VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576. doi: 10.1101/gr.129684.111
Lek, M., Karczewski, K. J., Minikel, E. V., Samocha, K. E., Banks, E., Fennell, T., et al. (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291. doi: 10.1038/nature19057
Li, H., and Durbin, R. (2010). Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595. doi: 10.1093/bioinformatics/btp698
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Mallory, L. A., Shaw, J. G., Burgess, S. L., Estrella, E., Nurko, S., Burpee, T. M., et al. (2009). Congenital myasthenic syndrome with episodic apnea. Pediatr. Neurol. 41, 42–45. doi: 10.1016/j.pediatrneurol.2009.02.017
Ng, P. C., and Henikoff, S. (2003). SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814. doi: 10.1093/nar/gkg509
Ni, X., and Shi, T. (2017). The challenge and promise of rare disease diagnosis in China. Sci. China Life Sci. 60, 681–685. doi: 10.1007/s11427-017-9100-1
Oda, Y. (1999). Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol. Int. 49, 921–937. doi: 10.1046/j.1440-1827.1999.00977.x
Ohno, K., Tsujino, A., Brengman, J. M., Harper, C. M., Bajzer, Z., Udd, B., et al. (2001). Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc. Natl. Acad. Sci. U.S.A. 98, 2017–2022. doi: 10.1073/pnas.98.4.2017
Pandurangan, A. P., Stahlhacke, J., Oates, M. E., Smithers, B., and Gough, J. (2019). The SUPERFAMILY 2.0 database: a significant proteome update and a new webserver. Nucleic Acids Res. 47, D490–D494. doi: 10.1093/nar/gky1130
Rodriguez, C. P., Palace, J., and Beeson, D. (2018). The neuromuscular junction and wide heterogeneity of congenital myasthenic syndromes. Int. J. Mol. Sci. 19:E1677. doi: 10.3390/ijms19061677
Schara, U., Christen, H. J., Durmus, H., Hietala, M., Krabetz, K., Rodolico, C., et al. (2010). Long-term follow-up in patients with congenital myasthenic syndrome due to CHAT mutations. Eur. J. Paediatr. Neurol. 14, 326–333. doi: 10.1016/j.ejpn.2009.09.009
Schwarz, J. M., Rodelsperger, C., Schuelke, M., and Seelow, D. (2010). MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575–576. doi: 10.1038/nmeth0810-575
Wang, K., Li, M., and Hakonarson, H. (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38:e164. doi: 10.1093/nar/gkq603
Keywords: CHAT, congenital myasthenic syndromes, episodic apnea, large deletion, severe
Citation: Liu Z, Zhang L, Shen D, Ding C, Yang X, Zhang W, Li J, Deng J, Gong S, Liu J, Qian S and Fang F (2019) Compound Heterozygous CHAT Gene Mutations of a Large Deletion and a Missense Variant in a Chinese Patient With Severe Congenital Myasthenic Syndrome With Episodic Apnea. Front. Pharmacol. 10:259. doi: 10.3389/fphar.2019.00259
Received: 16 November 2018; Accepted: 28 February 2019;
Published: 12 March 2019.
Edited by:
Tieliu Shi, East China Normal University, ChinaReviewed by:
Judith Cossins, University of Oxford, United KingdomCopyright © 2019 Liu, Zhang, Shen, Ding, Yang, Zhang, Li, Deng, Gong, Liu, Qian and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Fang, MTM5MTAxNTAzODlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.