- 1Department of Biopharmaceutical Sciences, College of Pharmacy, University of Illinois at Chicago, Chicago, IL, United States
- 2Department of Biobehavioral Health Science, College of Nursing, University of Illinois at Chicago, Chicago, IL, United States
- 3Department of Biobehavioral Nursing Science, College of Nursing, University of Florida, Gainesville, FL, United States
- 4Comprehensive Sickle Cell Center, University of Illinois at Chicago, Chicago, IL, United States
- 5Jesse Brown Veteran’s Administration Medical Center, Chicago, IL, United States
- 6Division of Hematology/Oncology, College of Medicine, University of Illinois at Chicago, Chicago, IL, United States
Pain in sickle cell disease (SCD) is severe, variable, and inadequately comprehended. The β2-adrenergic receptor (ADRB2) is critical in mediating neurotransmitter response in the sympathetic nervous system. In this association study, we examined 16 single nucleotide polymorphisms (SNPs) covering 5′-UTR and coding regions of ADRB2 for pain variability in SCD. Subjects recorded their non-crisis, baseline pain experience on a computerized tool from which we obtained chronic pain measurement score- composite pain index (CPI). Regression models yielded significant associations between chronic pain and seven SNPs. Non-synonymous SNP rs1042713 A allele (Arg16) caused a 5.73-fold decrease in CPI (p = 0.002). Allele A of rs12654778 and T of rs17778257 reduced CPI by a fold of 4.52 (p = 0.019), and 4.39 (p = 0.032), respectively. Whereas, in the 5′ UTR, allele C of rs1042711, G of rs11168070, C of rs11959427, and C of rs1801704 increased CPI by a fold of 10.86 (p = 0.00049), 5.99 (p = 0.016), 5.69 (p = 0.023), and 5.26 (p = 0.031), respectively. Together, these SNPs accounted for 2–15% of CPI variance after adjusting for covariates. Moreover, these SNPs were in high linkage disequilibrium (LD) showing three LD blocks in our cohort. A 10-marker haplotype increased CPI by 11.5-fold (p = 0.000407). Thus, ADRB2 polymorphisms might contribute to chronic pain severity and heterogeneity in SCD.
Introduction
Pain is a significant problem in patients with sickle cell disease (SCD) (Ballas et al., 2012). Not only is pain severe and lifelong, it is also highly heterogeneous which presents a challenge to effective treatment for all patients (Platt et al., 1991; Rees et al., 2010; Wilkie et al., 2010). SCD pain can be characterized as both acute and chronic pain (Smith et al., 2008; Wilkie et al., 2010; Ballas et al., 2012). The acute painful crisis is an unpredictable event that leads to emergency room care and hospitalization, and causes significant morbidity and mortality (Platt et al., 1991; Aisiku et al., 2009; Rees et al., 2010). The pain episode varies in frequency and severity as documented in a study of 29,922 SCD subjects where 29.4% of patients had no pain episode, but 16.9% had three or more crises annually (Brousseau et al., 2010). Persistent chronic pain is also found in patients with SCD and is highly heterogeneous. Smith et al. (2008) reported that 29.3% of patients reported pain in greater than 95% of self-reported pain diary days. In a study of SCD subjects at a routine outpatient clinic visit, mild pain intensity was reported in 17% of subjects, moderate pain in 27%, and severe pain in 19% (Wilkie et al., 2010). A more recent study found that at baseline, most SCD subjects reported ongoing mild pain (Campbell et al., 2015).
To understand pain heterogeneity in SCD, we examined the influence of candidate gene polymorphisms on pain in SCD. In this study, we focused on single nucleotide polymorphisms (SNPs) of the beta2-adrenergic receptor gene (ADRB2). ADRB2 codes for the β2-adrenergic receptor that is a member of the seven membrane-spanning G-protein coupled receptor superfamily and is a major receptor that mediates the responses of sympathetic neurotransmitters (Litonjua et al., 2010; Reiner et al., 2010). The β2-adrenergic receptor is expressed within the nociceptive system (Hein, 2006; Yalcin et al., 2010), including in the spinal cord superficial dorsal horn neurons (Nicholson et al., 2005), which is essential to pain transmission (Peng et al., 1993; Nicholson et al., 2005; Todd, 2010). Several ADRB2 SNPs have been studied for pain in temporomandibular joint disorder (TMD) (Diatchenko et al., 2006) and chronic musculoskeletal complaints (Skouen et al., 2012). Three major haplotypes were found to be associated with ADRB2 expression, psychological traits, resting arterial pressure, and development of TMD (Diatchenko et al., 2006). In the chronic musculoskeletal study, ADRB2 rs2053044 and the H1-H1 haplotype showed an association with pain (Skouen et al., 2012). In the current study, we investigated 16 SNPs in the 5′-untranslated region (5′-UTR) and coding regions of the ADRB2 gene, including several SNPs that have not been studied for pain (e.g., 5′-UTR rs1042711), on their influence on acute and chronic pain in patients with SCD.
Materials and Methods
Subjects
The University of Illinois at Chicago (UIC) Institutional Review Board approved the study. Blood and/or buccal swab samples were collected at the University of Illinois (UI) Hospital and Health Sciences System in Chicago, Chicago, IL, United States from patients during their regular clinic visits. All participants gave written informed consent. Analysis was conducted on 115 to 136 subjects with SCD where both clinical data and genetic samples were available. A power analysis was not performed a priori for this exploratory study.
Genotyping and Pain Assessment
DNA was extracted from blood and buccal samples using a modified phenol/chloroform method, or a modified salting-out procedure, or the QuickGene DNA whole blood extraction kit as previously described (Jhun et al., 2014; Sadhu et al., 2018). This was followed by genotyping on the MassARRAY iPLEX Platform (Sequenom, San Diego, CA, United States) to generate genotype data. Chronic pain assessment utilized composite pain index (CPI) as a measure of the multidimensional pain experience. The reported CPI value has a range of 0 to 100 for each subject as previously reported for patients with SCD (Ezenwa et al., 2014). Briefly, the subjects recorded their baseline pain information on a computerized tool called PAINReportIt® which is based on the well-established McGill Pain Questionnaire for pain assessment (Melzack, 1975). Self-reported baseline raw pain scores were converted to a scale of 0–100 and then averaged (Wilkie et al., 2003, 2010, 2015).
Alternatively, acute health care utilization served as the surrogate marker for acute crisis pain as reported by us previously (Ezenwa et al., 2014; Jhun et al., 2014; Sadhu et al., 2018). Utilization is defined as the number of admissions to the emergency department and/or acute care center resulting from a sickle cell pain crisis for the subsequent 12 months after the patient completed the baseline pain assessment. In short, data was collected by medical record review (for UI utilization) or biweekly telephones calls (for non-UI utilization).
Statistical Analysis
Single nucleotide polymorphisms were selected based on literature as discussed in the introduction. Hardy–Weinberg equilibrium was evaluated by a χχ goodness-of-fit test. The effect of SNPs on CPI value was analyzed by additive (allele effects), dominant (major allele homozygous genotypes versus combined heterozygous and minor allele homozygous), and recessive (combined major allele homozygous genotype and heterozygous versus minor allele homozygous) multiple linear regression models (Lettre et al., 2007; Clarke et al., 2011) adjusted for age, sex, ethnicity, and sickle cell type with major alleles as reference genotypes in the analysis. SNP effects on utilization data was analyzed by additive, dominant, and recessive negative binomial regression models (Cameron and Trivedi, 1998) adjusted for age, sex, ethnicity, and sickle cell type. SNP effects on three different utilization groups were analyzed by an additive, dominant, and recessive ordinal logistic regression model adjusted for the same covariates (Ezenwa et al., 2014). The regression models used to fit the data were driven by the nature and distribution of the dependent variables- CPI and utilization. Zero, low, and high utilization categories for logistic regression were based on previous work by us and others (Epstein et al., 2006; Lanzkron et al., 2006; Carroll et al., 2011). The recessive model for the following SNPs was not performed since the minor allele frequency was too low: rs11168070, rs11959427, rs1042711, and rs1801704. Analysis was performed on SPSS software (version 20; IBM, Armonk, NY, United States) or on R (version 3.4.0; R Foundation for Statistical Computing, Vienna, Austria) for these analyses. Linkage disequilibrium (LD) plot was generated from Haploview version 4.2 (Broad Institute, Cambridge, MA, United States) (Barrett et al., 2005). Haplotype association analyses were performed with PLINK (Massachusetts General Hospital and the Broad Institute of Harvard and MIT, Cambridge) (Purcell et al., 2007; Purcell, 2010) Hap-linear options were used on PLINK to include covariates (age, sex, ethnicity, and sickle cell type).
Results
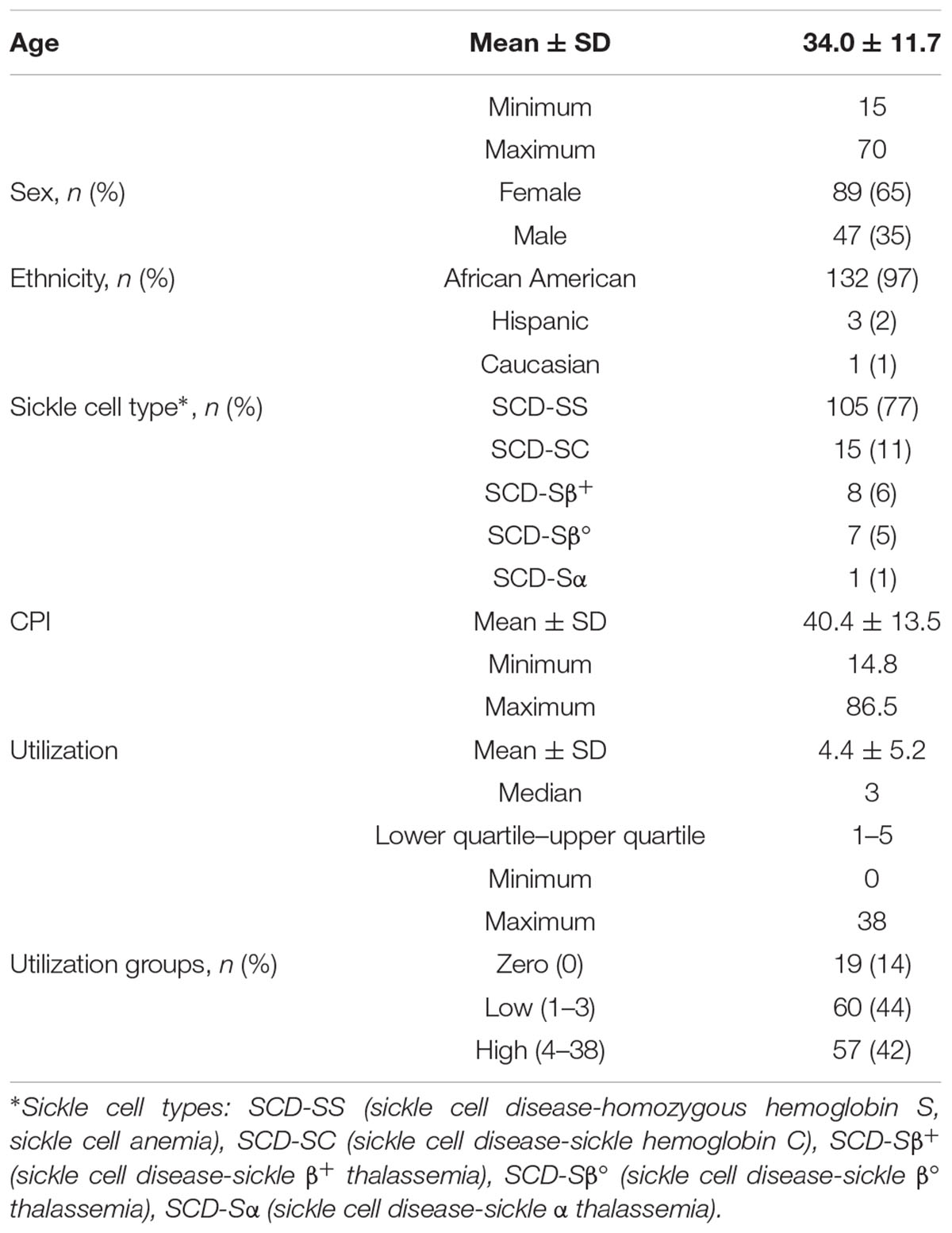
Patient demographics for the 136 subjects are provided in Table 1. The average age of the SCD subjects in our cohort was 34 years with a range from 15 to 70 years and a median of 32 years. More females than males were enrolled in the study; however, no known preference was given toward females during recruitment and the prevalence of SCD is not known to be gender biased. In SCD, males have been reported to have more frequent admissions for pain crises (Ballas, 2005), and higher mortality than females (Shankar et al., 2005). Sickle cell types and self-reported ethnicity are also given in Table 1. The mean CPI (Wilkie et al., 2015), a measurement for chronic pain, was 40.4 with a large range of 14.8 to 86.5, reaffirming pain heterogeneity. Utilization within a period of 12 months, another highly variable pain phenotype, ranged from 0 to 38. The number of subjects within each utilization group is also given and separated into no, low (Platt et al., 1991; Wilkie et al., 2010; Ballas et al., 2012) and high (>3) groups based on previous studies (Platt et al., 1991; Brousseau et al., 2010; Ezenwa et al., 2014).
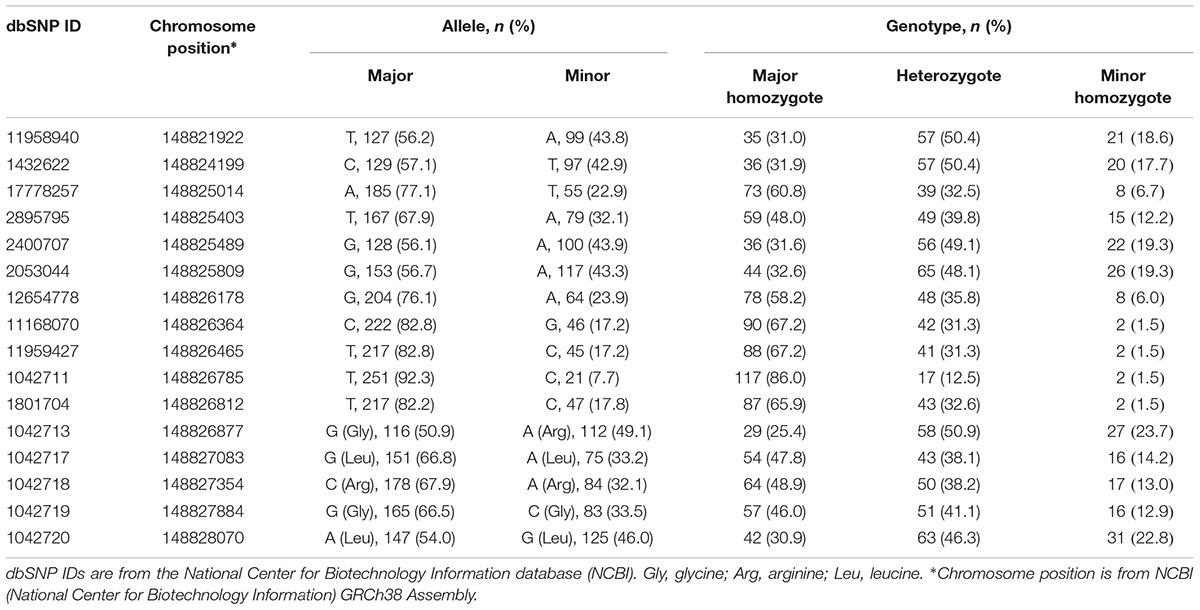
Allele and genotype frequencies for all 16 SNPs are listed in Table 2. SNPs are listed in order of chromosomal location from 5′→3′ starting from 5′-UTR. No significant deviations from Hardy–Weinberg equilibrium were observed for any of the 16 SNPs (p > 0.05).
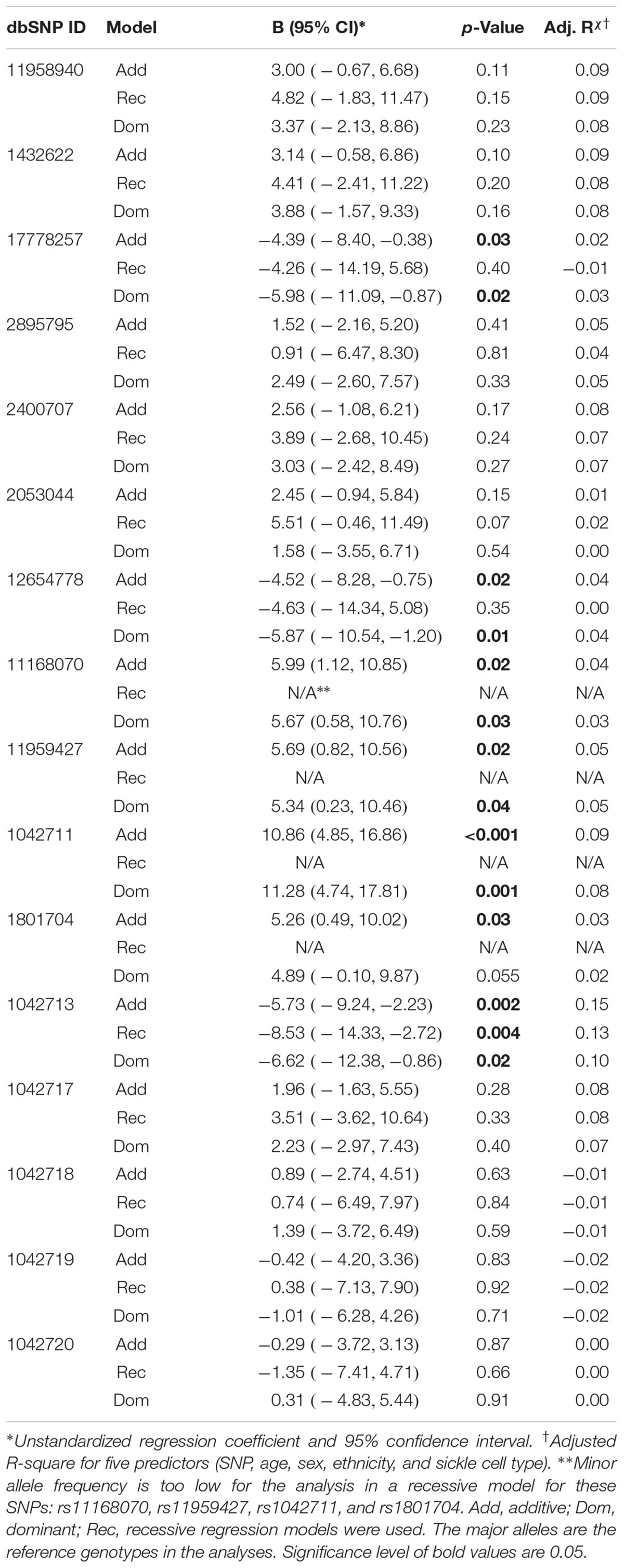
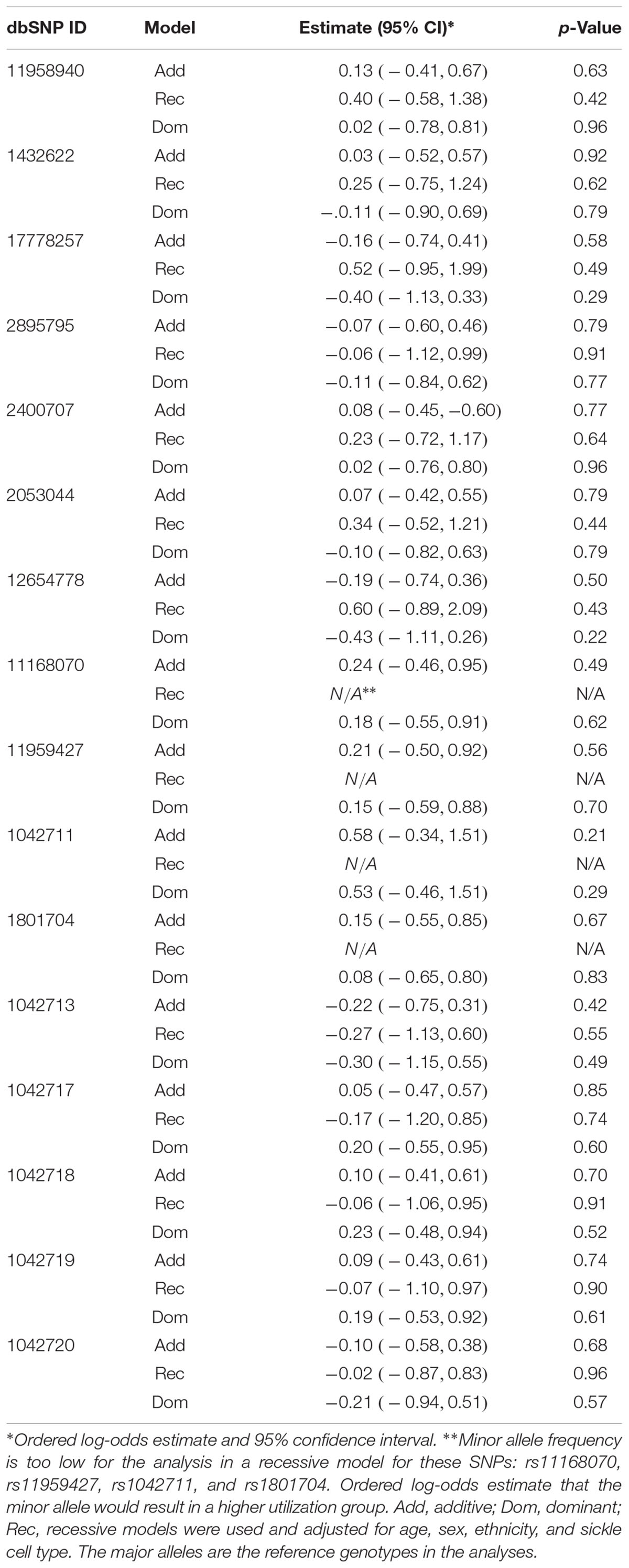
Seven of the 16 ADRB2 SNPs were found to be significantly associated with CPI in the additive multiple linear regression model adjusted for age, sex, ethnicity, and sickle cell type (Table 3). 5′-UTR rs1042711 C allele, rs11168070 G allele, rs11959427 C allele, and rs1801704 C allele were associated with increased CPI of 10.86 (p = 0.00049), 5.99 (p = 0.016), 5.69 (p = 0.023), and 5.26 (p = 0.031), respectively (Table 3). On the other hand, coding SNP rs1042713 A allele (Arg16), rs17778257 T allele, and rs12654778 A allele were associated with CPI reduction of 5.73 (p = 0.002), 4.39 (p = 0.032), and 4.52 (p = 0.019), respectively.
In the dominant regression models, rs17778257 TT/TA, rs12654778 AA/AG, rs1042713 AA/AG genotypes were associated with CPI reduction of 5.98 (p = 0.022), 5.87 (p = 0.014), and 6.62 (p = 0.025), respectively. SNPs rs11168070 GG/GC, rs11959427 CC/CT, and rs1042711 CC/CT genotypes, on the other hand, associated with CPI increase of 5.67 (p = 0.029), 5.34 (p = 0.041), and 11.28 (p = 0.001), respectively. Rs1801704 did not show statistical significance in the dominant model (B = 4.89, 95%CI [-0.10, 9.87], p = 0.055).
Coding SNP rs1042713 was the only SNP to show significance in all three models including the recessive regression model where the AA genotype caused 8.53-fold decrease in CPI (p = 0.004). These models explained 2–15% of the variance in CPI (adjusted r-square). A figure with unstandardized regression coefficients for each SNP and model is shown in Figure 1.
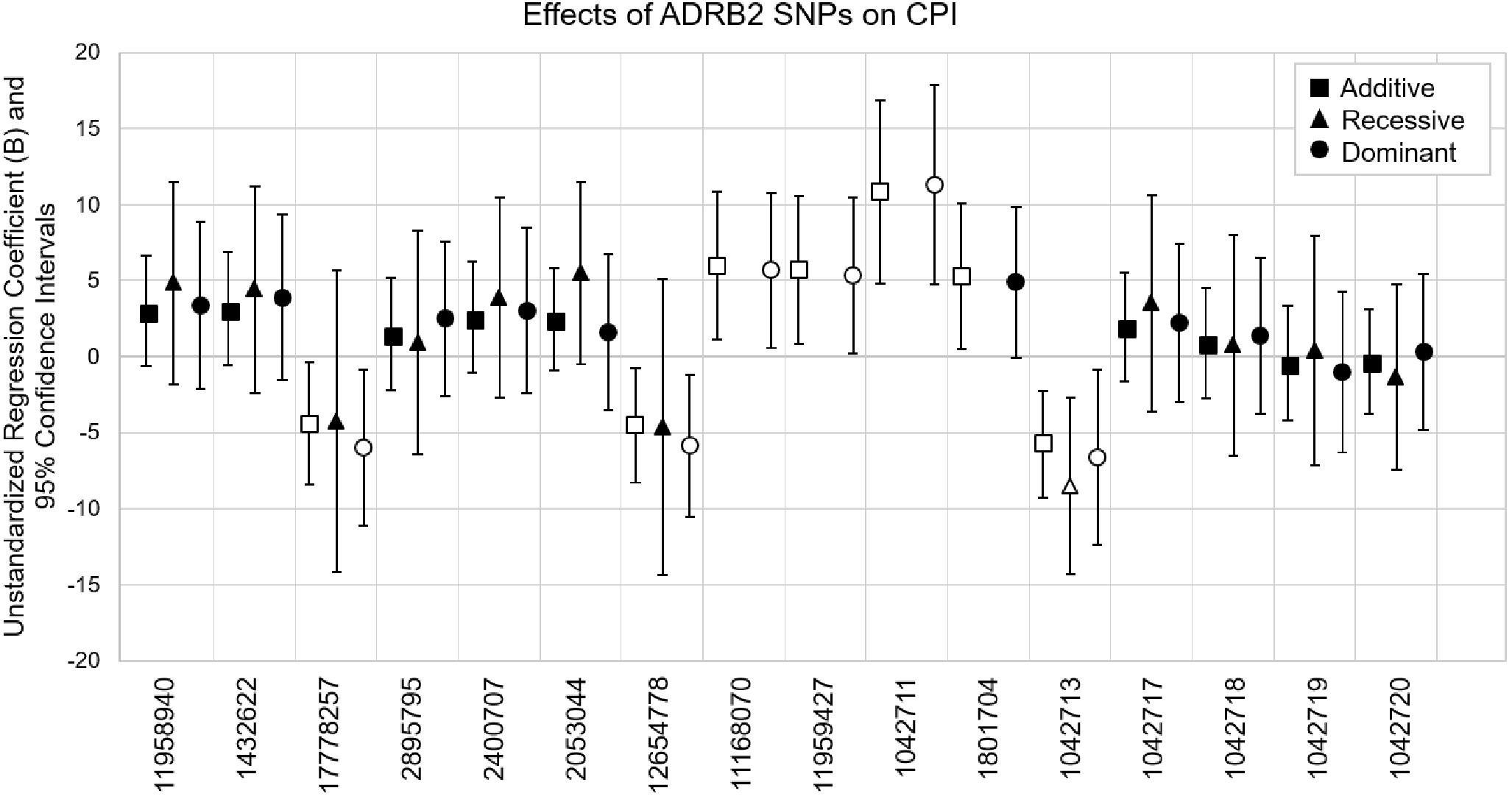
Figure 1. Multiple linear regression models evaluating the effects of ADRB2 SNPs on CPI. SNPs are in the order of ADRB2 5′→3′. Unstandardized regression coefficients with 95% confidence intervals for additive, recessive and dominant models are shown for each SNP. The major alleles are the reference genotypes in the analyses. Open symbols are significant associations (p < 0.05).
We performed association analyses in African American only cohort (n = 132). Findings were consistent. In the additive model risk alleles of rs1042711, rs11168070, rs11959427, and rs1801704 associated with increased CPI and that of rs1042713, rs17778257, and rs12654778 with decreased CPI. Similarly, in the dominant model, risk alleles of rs17778257, rs12654778, and rs1042713 associated with CPI reduction and that of SNPs rs11168070, rs11959427, and rs1042711 with increase in CPI. SNP rs1042713 too exhibited significant association with decreased CPI in recessive model. Additionally, minor alleles of SNPs rs1432622 (p = 0.037) and rs11958940 (p = 0.043) in the additive model, rs1801704 (p = 0.039) in the dominant model, and rs2053044 (p = 0.048) in the recessive also showed significant association with increase in CPI.
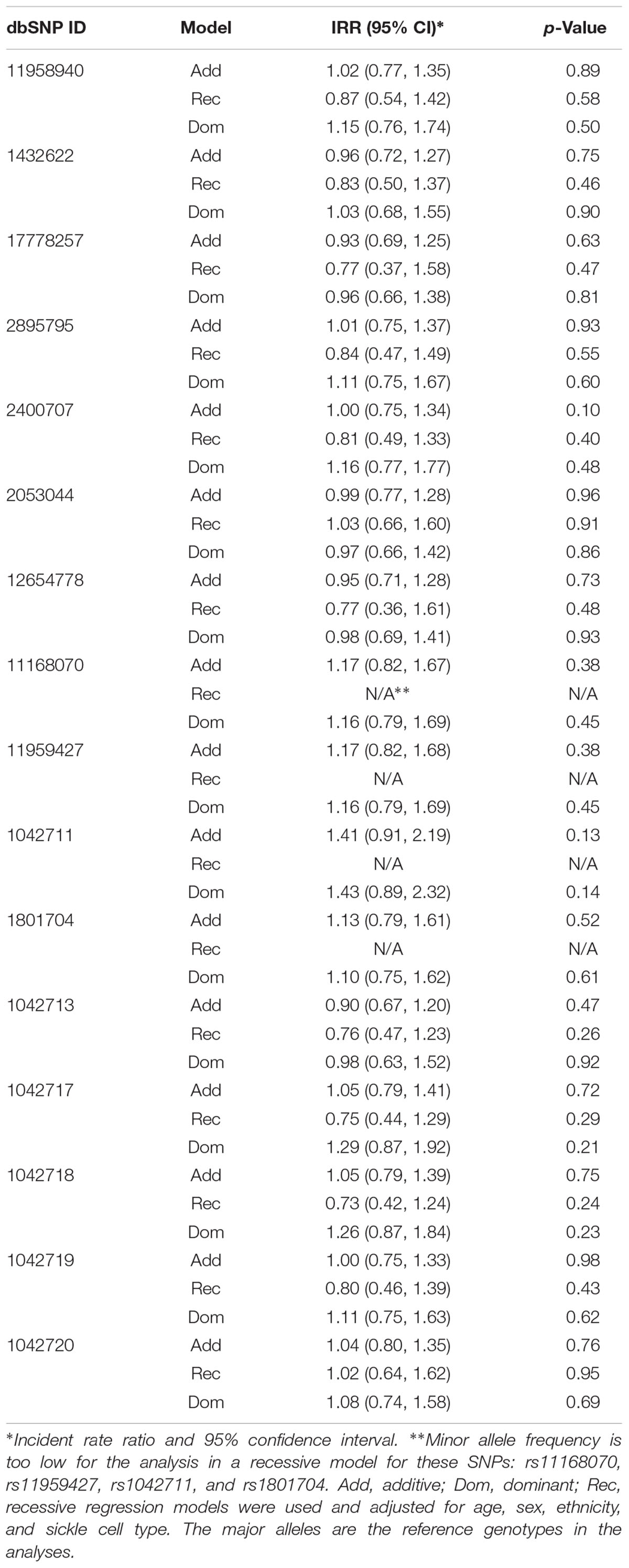
We did not find any significant influences of ADRB2 SNPs on the number of utilization or acute pain in the 136 SCD subjects (Table 4) or in the African American only cohort.
Previously, we found CPI scores predicted subsequent 1-year acute care utilization that can be divided into three groups: none (zero), 1 to 3 (low), or > 3 events (high) (Ezenwa et al., 2014). We took a similar approach and separated our data into three groups: none (zero), 1 to 3 (low), or > 3 events (high) for analyses with covariates that included age, sex, ethnicity, and sickle cell type in order to identify independent effects of SNPs on utilization. Again, ordinal logistic regression models did not reveal significant influence of ADRB2 SNPs on utilization (Table 5). These data suggested that ADRB2 SNPs influence chronic, but not acute, pain in SCD.
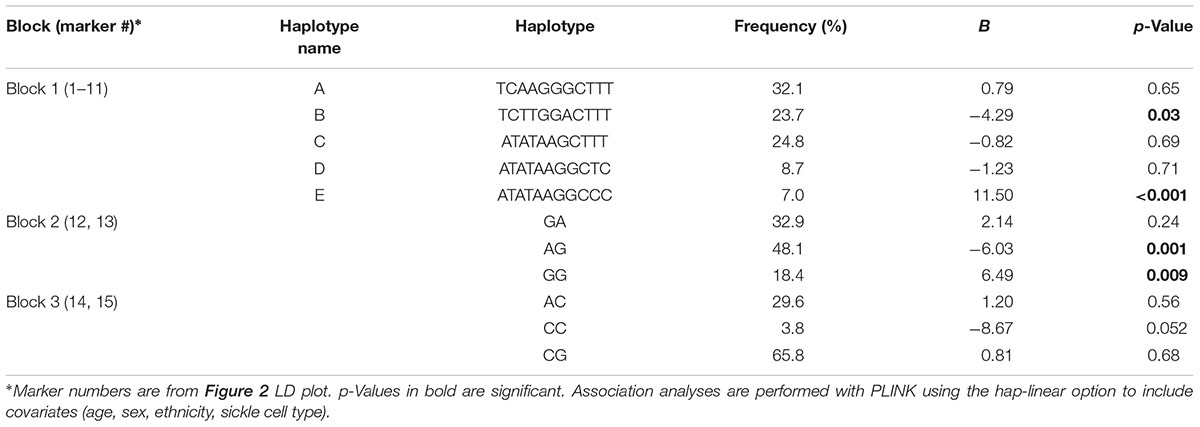
ADRB2 SNPs have previously been reported to be a part of a nine-marker haplotype where 2–4 markers were sufficient to maximize haplotype diversity (Belfer et al., 2005). We analyzed our SNPs for linkage disequilibrium (LD) using Haploview (Figure 2). Three haplotype blocks were formed from 15 SNPs (block 1: 11958940, 1432622, 17778257, 2895795, 2400707, 2053044, 12654778, 11168070, 11959427, 1042711, 1801704; block 2: 1042713, 1042717; and block 3: 1042718, 1042719).
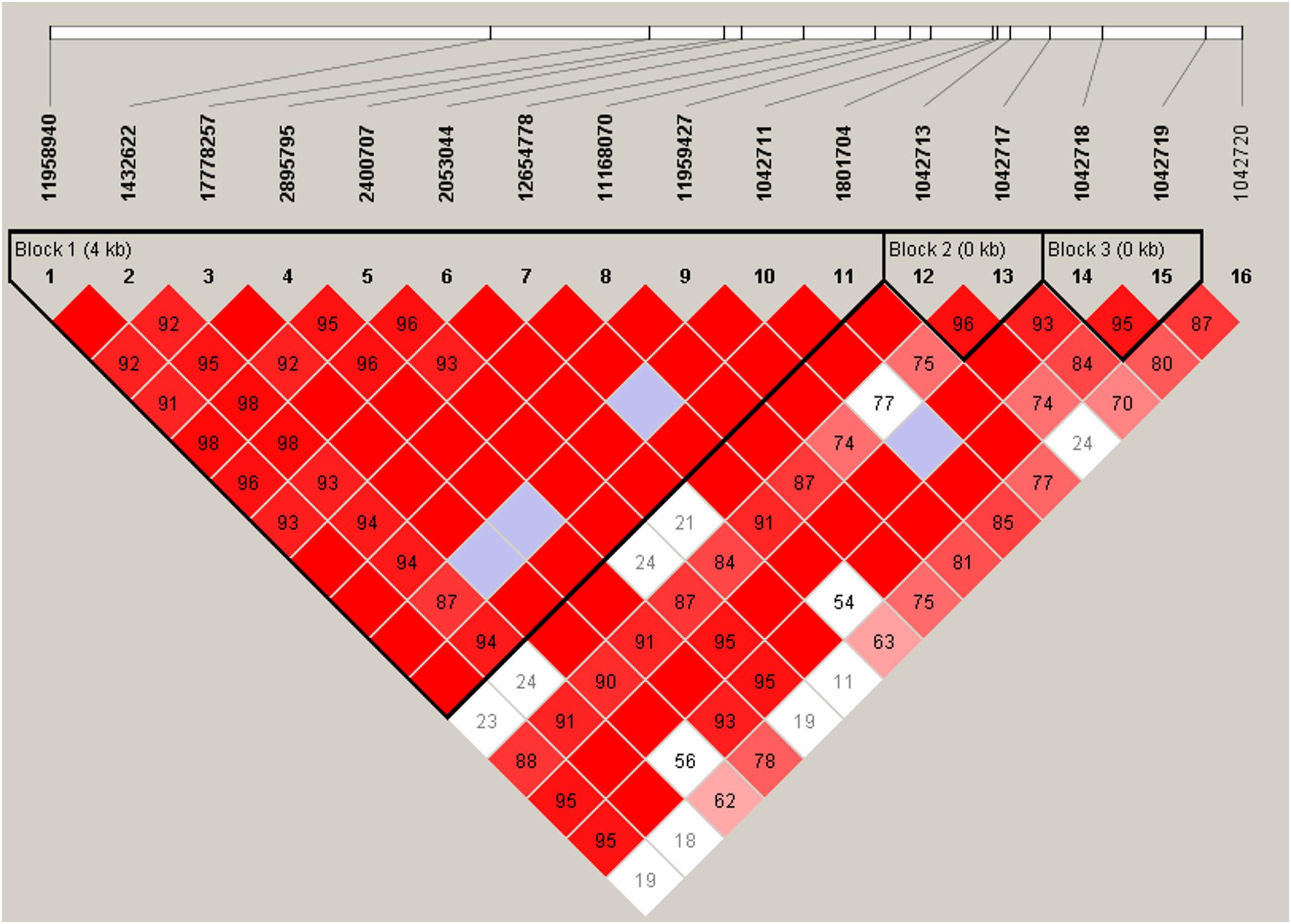
Figure 2. Linkage disequilibrium plot of ADRB2 SNPs. Haplotype block organization of 16 ADRB2 SNPs generated from Haploview 4.2 using the standard color scheme with the plot displaying D′ values, i.e., the linkage disequilibrium coefficients. Red = high D′ and high LOD, white = low D′, and low LOD; blue = high D′ and low LOD; shades of pink/red = low D′ and high LOD. Haplotype blocks did not change when four non-African American subjects were excluded.
These haplotypes (Table 6) were able to capture > 96% of the haplotypes from block 1, >99% from block 2, and >99% from block 3. Haplotype E consisting of markers 1–11 from block 1 (annotated in Figure 2) associated with an 11.5-fold increase in CPI score (p = 0.0004, Table 6). Haplotype E differs from D by rs1042711 that contains the C allele that increased CPI by 10.86 (Table 3, p = 0.0004). Other haplotypes containing the T allele, which decreased CPI by 4.29 in haplotype B (p = 0.033). Block 2 haplotypes AG caused 6.03 decrease in CPI score (p = 0.001) and GG increase CPI by 6.49 (p = 0.009). GA haplotype did not significantly associate with CPI. Rs1042713 may have a major role in influencing CPI score in this block, however, rs1042717 cannot be discounted as GA haplotype was not significant. Haplotype CC in block 3 approached statistical significance with a reduced CPI of 8.67 (p = 0.052). Haplotype analyses with African American only subjects yielded same haploblocks and pain-associated haplotypes.
Discussion
The beta2-adrenergic receptor (β2-AR) is a major receptor that mediates the action of sympathetic neurotransmitters NE and E. The ADRB2 gene polymorphisms had not been studied for pain in SCD. Here, we showed that ADRB2 SNPs and haplotypes have an important role in influencing chronic, but not acute, pain in SCD. Seven of the 16 SNPs examined (rs1042711, rs1042713, rs12654778, rs17778257 C, rs11168070, rs11959427, and rs1801704) were found to be associated with the severity of chronic pain, accounting for 2–15% of the variance in CPI after adjusting for variables including age, sex, ethnicity, and sickle cell type. Post hoc power analyses using statistical tool, G∗Power, indicated that our study has approximately 85% power to detect the observed effect sizes at a significance level of α = 0.05 (Faul et al., 2007). Furthermore, haplotype analysis found that several of these SNPs are in LD. We found the 10-marker haplotype caused 11.5 points increase in CPI (p = 0.0004). The coding region SNP rs1042711 alone was associated with an increase of more than 10 points in CPI. Although we found that CPI and age were significant predictors of utilization events (Ezenwa et al., 2014), none of the ADRB2 SNPs showed significant associations with utilization.
The β2-AR has been known to play a pivotal role in pain perception and transmission. Agonists of β2-AR have been shown to attenuate chronic pain in rodent models of experimental mononeuropathy (Choucair-Jaafar et al., 2009, 2011), and diabetic peripheral neuropathy (Choucair-Jaafar et al., 2014; Baraka et al., 2015). Reductions in β2-ARs are associated with psychiatric disorders and comorbidities (Diatchenko et al., 2006) and stimulation of central β2-ARs produced antidepressant-like effects (Zhang et al., 2003). The converse was true where antidepressants recruitment of noradrenaline and its stimulation of β2-AR resulted in the relief of allodynia (Bohren et al., 2013).
The β2-AR also interacts with other receptors in modulating pain. For example, pancreatic hyperalgesia induced by sensitization of purinergic receptors was found to be mediated by adrenergic signaling in primary sensory neurons and attenuated by blocking the purinergic receptor or β2-AR (Wang et al., 2015).
In addition, β2-AR downstream signaling pathways of the beta2-adrenergic receptor have also been associated with pain. Hyperalgesia induced by epinephrine was prolonged by low levels of G protein-coupled receptor kinase 2 (GRK2) (Wang et al., 2011). GRK2 phosphorylates epinephrine activated β2-ARs. Furthermore, heterotypical intermittent stress-treated rats showed visceral hyperalgesia that was alleviated by β2-AR antagonist but not β1- or β3-AR antagonists (Zhang et al., 2014). β2-AR antagonists also block the hyperalgesic effects of opioids (Samoshkin et al., 2015). Moreover, β2-AR modulates opioid tolerance and physical dependence as shown in a study where morphine failed to cause tolerance in β2-AR knockout mice and physical dependence was reduced (Liang et al., 2007). Endogenous agonists of β2-AR show distinct conformational changes that can lead to different downstream signaling effects (Reiner et al., 2010).
The coding region rs1042713 is a non-synonymous SNP (Gly16Arg) that has been previously studied in affecting expression and function of β2-AR (Green et al., 1994, 1995; Small et al., 2003). In vitro studies found that rs1042713 Gly16 enhanced agonist-induced receptor downregulation (Green et al., 1994; Small et al., 2003). Haplotypes containing homozygous Arg16 allele were associated with the highest temporomandibular disorder incidence rate (Diatchenko et al., 2006). Another haplotype combination including Arg16 allele was found to be a risk factor for fibromyalgia syndrome (Vargas-Alarcon et al., 2009). In chronic widespread pain, however, Gly16 allele was associated with an increased risk for the disorder (Hocking et al., 2010). On the other hand, another study did not find any association of migraine with rs1042713 genotype, allele or haplotype (Schurks et al., 2009).
In our studies, we found that rs1042713 allele and genotype were associated with chronic pain in SCD using three different regression models. The Arg16 allele associated with lower chronic pain. Our finding is in agreement with attenuation of (chronic) pain by β2-AR agonists (Green et al., 1994). Patients with 16Arg would have less agonist-induced downregulation of the receptor. Moreover, Gly16 may directly affect SCD. In a study examining sickle red cell adhesion to laminin, Gly16 homozygotes showed significantly higher measurements of adhesion than the heterozygous and Arg16 homozygous genotypes combined (Eyler et al., 2008). This increased adhesion in sickle red blood cells may lead to increased disease severity in SCD. This is in line with our finding that Arg16 allele had strong association with lower baseline pain in our SCD population. These results suggest that rs1042713 plays a major role in SCD pain reported during a routine outpatient clinic visit. Of the 16 SNPs investigated rs1042717, rs1042718, rs1042719, and rs1042720 lie in the exon region of the gene along with rs1042713. They are reported to be synonymous variants and functional roles have not been established. It is thereby important to understand their contribution to linkage disequilibrium.
Although rs1042713 plays a significant role in baseline pain, other SNPs in linkage disequilibrium with this haploblock could be contributing to baseline pain in SCD as well (Belfer et al., 2005; Diatchenko et al., 2006). LD plot and haplotype analysis with rs1042717 revealed that rs1042713 risk allele was not always significantly associated with CPI. The GA haplotype in block 2 (rs1042713–rs1042717) consisting of rs1042713 Gly16 allele and A (Leu84) allele of rs1042717 did not associate with CPI. GG haplotype, however, caused a 5.88 increase in CPI score (p = 0.013). AG haplotype caused a decrease in CPI score. On the other hand, though rs1042717 is not independently associated with CPI scores as seen in Table 3, but as part of the rs1042713-rs1042717 haploblock we find that certain haplotypes associate with CPI. Conflicting conclusions from other studies also suggest the influence of haplotypes in different pain phenotypes. ADRB2 rs11958940, rs1432622, rs2400707, rs1042717, and rs1042719 were found to be in linkage disequilibrium with rs1042713 in African Americans forming one haploblock (Belfer et al., 2005). Our LD plots however show two separate blocks with the lesser number of SNPs.
ADRB2 rs1042711 lies in the 5′-untranslated region and has been studied in haplotype analysis for a malaria and asthma study where the T allele was shown to be both protective and associated with risk for malaria and asthma (Saadi et al., 2013). The other SNPs in this study included rs1801704, rs1042713, rs1042714, rs1042717, and rs1042718. Perhaps the SNPs that were not genotyped for our study would have had an influence over the effects of rs1042711 C allele in our study as associations seen in the malaria and asthma study show significant influences by other SNPs. A study on childhood lung function and ADRB2 haplotypes show that a haplotype with the rs1042711 C allele was possibly showing a protective effect on reduced FEV1 (forced expiratory volume at 1-s) in 10-year old children (Torjussen et al., 2013). The other SNPs included in this study were rs1042713, rs1042714, and rs1800888. The latter two lie in the exon region as well with rs1042714 being a stop-gained variant and rs1800888 being a non-synonymous variant. ADRB2 rs1042711 has not been previously studied for pain. The C allele in our study increases CPI score and the SNP is also in high LD with other SNPs but may confer a greater influence on CPI over the effect of the whole haplotype.
The findings of this study are limited by the small sample size and need to be replicated in larger studies that also evaluate the potential effect of population structure and admixture on the association. It would have also been of interest to examine if hydroxyurea has any effect on the observed associations. Unfortunately, we do not have information on hydroxyurea use at the time of sample acquisition. However, our center had contributed a large number of subjects to the hydroxyurea study and patients who tolerated therapy continued on it. While there has been some speculation on the role of hydroxyurea in decreasing acute care utilization (Lanzkron et al., 2018; Zhou et al., 2018), we were not able to add to these findings.
Our study identifies β2-AR as a potential target in the development of pharmacological agents for alleviating chronic baseline pain in SCD.
Ethics Statement
This study was carried out in accordance with the recommendations of the University of Illinois at Chicago (UIC) Institutional Review Board, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the University of Illinois at Chicago (UIC) Institutional Review Board.
Author Contributions
EJ performed research, analyzed and interpreted data, and wrote and edited manuscript. NS analyzed and interpreted data and revised and edited manuscript. XH interpreted data and wrote manuscript. YY analyzed data and revised manuscript. YH performed research and revised manuscript. DW and RM designed study and revised manuscript. ZW designed study, interpreted data, wrote, revised, and edited manuscript.
Funding
The study was supported in part by funds from the Illinois Department of Public Health (IDPH) and grants from the National Heart, Lung, and Blood Institute (NHLBI) (Grant Nos. R01HL078536, R01HL124945, and R35HL140031). EJ was supported by a predoctoral fellowship from National Institute of Dental and Craniofacial Research (NIDCR) (Grant No. T32DE018381). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the IDPH, NIH, NHLBI, NIDCR, or Veteran’s Administration. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Some preliminary findings from this study were presented at the 35th Annual Scientific Meeting of the American Pain Society, Austin, Texas on 11–14 May 2016 (Jhun et al., 2016).
References
Aisiku, P., Smith, W. R., McClish, D. K., Levenson, J. L., Penberthy, L. T., Roseff, S. D., et al. (2009). Comparisons of high versus low emergency department utilizers in sickle cell disease. Ann. Emerg. Med. 53, 587–593. doi: 10.1016/j.annemergmed.2008.07.050
Ballas, S. K. (2005). Pain management of sickle cell disease. Hematol. Oncol. Clin. North Am. 19, 785–802. doi: 10.1016/j.hoc.2005.07.008
Ballas, S. K., Gupta, K., and Adams-Graves, P. (2012). Sickle cell pain: a critical reappraisal. Blood 120, 3647–3656. doi: 10.1182/blood-2012-04-383430
Baraka, M., Darwish, I. E., Ghoneim, M. T., and Korayem, H. K. (2015). beta2-adrenoceptor agonists as potential therapeutic drugs in diabetic peripheral neuropathy. Eur. J. Pharmacol 746, 89–95. doi: 10.1016/j.ejphar.2014.11.004
Barrett, J. C., Fry, B., Maller, J., and Daly, M. J. (2005). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265. doi: 10.1093/bioinformatics/bth457
Belfer, I., Buzas, B., Evans, C., Hipp, H., Phillips, G., Taubman, J., et al. (2005). Haplotype structure of the beta adrenergic receptor genes in US Caucasians and African Americans. Eur. J. Hum. Genet. 13, 341–351. doi: 10.1038/sj.ejhg.5201313
Bohren, Y., Tessier, L. H., Megat, S., Petitjean, H., Hugel, S., Daniel, D., et al. (2013). Antidepressants suppress neuropathic pain by a peripheral beta2-adrenoceptor mediated anti-TNFalpha mechanism. Neurobiol. Dis. 60, 39–50. doi: 10.1016/j.nbd.2013.08.012
Brousseau, D. C., Owens, P. L., Mosso, A. L., Panepinto, J. A., and Steiner, C. A. (2010). Acute care utilization and rehospitalizations for sickle cell disease. JAMA 303, 1288–1294. doi: 10.1001/jama.2010.378
Cameron, A. C., and Trivedi, P. K. (1998). Regression Analysis of Count Data. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511814365
Campbell, C. M., Carroll, C. P., Kiley, K., Han, D., Haywood, C. Jr., Lanzkron, S., et al. (2015). Quantitative sensory testing and pain-evoked cytokine reactivity: comparison of patients with sickle cell disease to healthy matched controls. Pain 157, 949–956. doi: 10.1097/j.pain.0000000000000473
Carroll, P., Haywood, C., and Lanzkron, S. (2011). Prediction of onset and course of high hospital utilization in sickle cell disease. J. Hosp. Med. 6, 248–255. doi: 10.1002/jhm.850
Choucair-Jaafar, N., Beetz, N., Gilsbach, R., Yalcin, I., Waltisperger, E., Freund-Mercier, M. J., et al. (2011). Cardiovascular effects of chronic treatment with a beta2-adrenoceptor agonist relieving neuropathic pain in mice. Neuropharmacology 61, 51–60. doi: 10.1016/j.neuropharm.2011.02.015
Choucair-Jaafar, N., Salvat, E., Freund-Mercier, M. J., and Barrot, M. (2014). The antiallodynic action of nortriptyline and terbutaline is mediated by beta(2) adrenoceptors and delta opioid receptors in the ob/ob model of diabetic polyneuropathy. Brain Res. 1546, 18–26. doi: 10.1016/j.brainres.2013.12.016
Choucair-Jaafar, N., Yalcin, I., Rodeau, J. L., Waltisperger, E., Freund-Mercier, M. J., and Barrot, M. (2009). Beta2-adrenoceptor agonists alleviate neuropathic allodynia in mice after chronic treatment. Br. J. Pharmacol. 158, 1683–1694. doi: 10.1111/j.1476-5381.2009.00510.x
Clarke, G. M., Anderson, C. A., Pettersson, F. H., Cardon, L. R., Morris, A. P., and Zondervan, K. T. (2011). Basic statistical analysis in genetic case-control studies. Nat. Protoc. 6, 121–133. doi: 10.1038/nprot.2010.182
Diatchenko, L., Anderson, A. D., Slade, G. D., Fillingim, R. B., Shabalina, S. A., Higgins, T. J., et al. (2006). Three major haplotypes of the beta2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 141B, 449–462. doi: 10.1002/ajmg.b.30324
Epstein, K., Yuen, E., Riggio, J. M., Ballas, S. K., and Moleski, S. M. (2006). Utilization of the office, hospital and emergency department for adult sickle cell patients: a five-year study. J. Natl. Med. Assoc. 98, 1109–1113.
Eyler, C. E., Jackson, T., Elliott, L. E., De Castro, L. M., Jonassaint, J., Ashley-Koch, A., et al. (2008). beta(2)-Adrenergic receptor and adenylate cyclase gene polymorphisms affect sickle red cell adhesion. Br. J. Haematol. 141, 105–108. doi: 10.1111/j.1365-2141.2008.07008.x
Ezenwa, M. O., Molokie, R. E., Wang, Z. J., Yao, Y., Suarez, M. L., Angulo, V., et al. (2014). Outpatient pain predicts subsequent one-year acute health care utilization among adults with sickle cell disease. J. Pain Symptom Manage. 48, 65–74. doi: 10.1016/j.jpainsymman.2013.08.020
Faul, F., Erdfelder, E., Lang, A. G., and Buchner, A. (2007). G∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/BF03193146
Green, S. A., Turki, J., Bejarano, P., Hall, I. P., and Liggett, S. B. (1995). Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 13, 25–33. doi: 10.1165/ajrcmb.13.1.7598936
Green, S. A., Turki, J., Innis, M., and Liggett, S. B. (1994). Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry 33, 9414–9419. doi: 10.1021/bi00198a006
Hein, L. (2006). Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 326, 541–551. doi: 10.1007/s00441-006-0285-2
Hocking, L. J., Smith, B. H., Jones, G. T., Reid, D. M., Strachan, D. P., and Macfarlane, G. J. (2010). Genetic variation in the beta2-adrenergic receptor but not catecholamine-O-methyltransferase predisposes to chronic pain: results from the 1958 british birth cohort study. Pain 149, 143–151. doi: 10.1016/j.pain.2010.01.023
Jhun, E., He, Y., Yao, Y., Molokie, R. E., Wilkie, D. J., and Wang, Z. J. (2014). Dopamine D3 receptor Ser9Gly and catechol-o-methyltransferase Val158Met polymorphisms and acute pain in sickle cell disease. Anesth. Analg. 119, 1201–1207. doi: 10.1213/ANE.0000000000000382
Jhun, E., He, Y., Yao, Y., Wilkie, D., Molokie, R., and Wang, J. (2016). (283) Beta2-adrenergic receptor gene polymorphisms and haplotypes associate with chronic pain in sickle cell disease. J. Pain 17, S46–S47. doi: 10.1016/j.jpain.2016.01.189
Lanzkron, S., Haywood, C., Segal, J. B., and Dover, G. J. (2006). Hospitalization rates and costs of care of patients with sickle-cell anemia in the state of Maryland in the era of hydroxyurea. Am. J. Hematol. 81, 927–932. doi: 10.1002/ajh.20703
Lanzkron, S., Little, J., Field, J., Shows, J. R., Wang, H., Seufert, R., et al. (2018). Increased acute care utilization in a prospective cohort of adults with sickle cell disease. Blood Adv. 2, 2412–2417. doi: 10.1182/bloodadvances.2018018382
Lettre, G., Lange, C., and Hirschhorn, J. N. (2007). Genetic model testing and statistical power in population-based association studies of quantitative traits. Genet. Epidemiol. 31, 358–362. doi: 10.1002/gepi.20217
Liang, D. Y., Shi, X., Li, X., Li, J., and Clark, J. D. (2007). The beta2 adrenergic receptor regulates morphine tolerance and physical dependence. Behav. Brain Res. 181, 118–126. doi: 10.1016/j.bbr.2007.03.037
Litonjua, A., Gong, L., Duan, Q. L., Shin, J., Moore, M. J., Weiss, S. T., et al. (2010). Very important pharmacogene summary ADRB2. Pharmacogenet. Genom. 20, 64–69. doi: 10.1097/FPC.0b013e328333dae6
Melzack, R. (1975). The McGill pain questionnaire: major properties and scoring methods. Pain 1, 277–299. doi: 10.1016/0304-3959(75)90044-5
Nicholson, R., Dixon, A. K., Spanswick, D., and Lee, K. (2005). Noradrenergic receptor mRNA expression in adult rat superficial dorsal horn and dorsal root ganglion neurons. Neurosci. Lett. 380, 316–321. doi: 10.1016/j.neulet.2005.01.079
Peng, Y. I., Liu, H. J., and Fu, T. C. (1993). Involvement of alpha- and beta-adrenoceptors in antinociception at the lumber spinal level in mice. Chin. J. Physiol. 36, 177–180.
Platt, O. S., Thorington, B. D., Brambilla, D. J., Milner, P. F., Rosse, W. F., Vichinsky, E., et al. (1991). Pain in sickle cell disease. Rates and risk factors. N. Engl. J. Med. 325, 11–16. doi: 10.1056/NEJM199107043250103
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi: 10.1086/519795
Rees, D. C., Williams, T. N., and Gladwin, M. T. (2010). Sickle-cell disease. Lancet 376, 2018–2031. doi: 10.1016/S0140-6736(10)61029-X
Reiner, S., Ambrosio, M., Hoffmann, C., and Lohse, M. J. (2010). Differential signaling of the endogenous agonists at the beta2-adrenergic receptor. J. Biol. Chem. 285, 36188–36198. doi: 10.1074/jbc.M110.175604
Saadi, V., Gupta, H., Angural, A., Dhanya, S. K., Mony, S., Oberoi, D., et al. (2013). Single nucleotide polymorphisms of ADRB2 gene and their association with susceptibility for Plasmodium falciparum malaria and asthma in an Indian population. Infect. Genet. Evol. 20, 140–147. doi: 10.1016/j.meegid.2013.08.026
Sadhu, N., Jhun, E. H., Yao, Y., He, Y., Molokie, R. E., Wilkie, D. J., et al. (2018). Genetic variants of GCH1 associate with chronic and acute crisis pain in African Americans with sickle cell disease. Exp. Hematol. 66, 42–49. doi: 10.1016/j.exphem.2018.07.004
Samoshkin, A., Convertino, M., Viet, C. T., Wieskopf, J. S., Kambur, O., Marcovitz, J., et al. (2015). Structural and functional interactions between six-transmembrane mu-opioid receptors and beta2-adrenoreceptors modulate opioid signaling. Sci. Rep. 5:18198. doi: 10.1038/srep18198
Schurks, M., Kurth, T., Ridker, P. M., Buring, J. E., and Zee, R. Y. (2009). Association between polymorphisms in the beta2-adrenoceptor gene and migraine in women. Headache 49, 235–244. doi: 10.1111/j.1526-4610.2008.01207.x
Shankar, S. M., Arbogast, P. G., Mitchel, E., Cooper, W. O., Wang, W. C., and Griffin, M. R. (2005). Medical care utilization and mortality in sickle cell disease: a population-based study. Am. J. Hematol. 80, 262–270. doi: 10.1002/ajh.20485
Skouen, J. S., Smith, A. J., Warrington, N. M., O’ Sullivan, P. B., McKenzie, L., Pennell, C. E., et al. (2012). Genetic variation in the beta-2 adrenergic receptor is associated with chronic musculoskeletal complaints in adolescents. Eur. J. Pain 16, 1232–1242. doi: 10.1002/j.1532-2149.2012.00131.x
Small, K. M., McGraw, D. W., and Liggett, S. B. (2003). Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu. Rev. Pharmacol. Toxicol. 43, 381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823
Smith, W. R., Penberthy, L. T., Bovbjerg, V. E., McClish, D. K., Roberts, J. D., Dahman, B., et al. (2008). Daily assessment of pain in adults with sickle cell disease. Ann. Intern. Med. 148, 94–101. doi: 10.7326/0003-4819-148-2-200801150-00004
Todd, J. (2010). Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 11, 823–836. doi: 10.1038/nrn2947
Torjussen, T. M., Munthe-Kaas, M. C., Mowinckel, P., Carlsen, K. H., Undlien, D. E., and Lodrup Carlsen, K. C. (2013). Childhood lung function and the association with beta2-adrenergic receptor haplotypes. Acta Paediatr. 102, 727–731. doi: 10.1111/apa.12221
Vargas-Alarcon, G., Fragoso, J. M., Cruz-Robles, D., Vargas, A., Martinez, A., Lao-Villadoniga, J. I., et al. (2009). Association of adrenergic receptor gene polymorphisms with different fibromyalgia syndrome domains. Arthritis Rheum 60, 2169–2173. doi: 10.1002/art.24655
Wang, H., Heijnen, C. J., Eijkelkamp, N., Garza Carbajal, A., Schedlowski, M., Kelley, K. W., et al. (2011). GRK2 in sensory neurons regulates epinephrine-induced signalling and duration of mechanical hyperalgesia. Pain 152,1649–1658. doi: 10.1016/j.pain.2011.03.010
Wang, S., Zhu, H. Y., Jin, Y., Zhou, Y., Hu, S., Liu, T., et al. (2015). Adrenergic signaling mediates mechanical hyperalgesia through activation of P2X3 receptors in primary sensory neurons of rats with chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G710–G719. doi: 10.1152/ajpgi.00395.2014
Wilkie, D. J., Judge, M. K., Berry, D. L., Dell, J., Zong, S., and Gilespie, R. (2003). Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J. Pain Symptom Manage. 25, 213–224. doi: 10.1016/S0885-3924(02)00638-3
Wilkie, D. J., Molokie, R., Boyd-Seal, D., Suarez, M. L., Kim, Y. O., Zong, S., et al. (2010). Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J. Natl. Med. Assoc. 102, 18–27. doi: 10.1016/S0027-9684(15)30471-5
Wilkie, D. J., Molokie, R. E., Suarez, M. L., Ezenwa, M. O., and Wang, Z. J. (2015). Composite pain index: reliability, validity, and sensitivity of a patient-reported outcome for research. Pain Med. 16, 1341–1348. doi: 10.1111/pme.12703
Yalcin, I., Tessier, L. H., Petit-Demouliere, N., Waltisperger, E., Hein, L., Freund-Mercier, M. J., et al. (2010). Chronic treatment with agonists of beta(2)-adrenergic receptors in neuropathic pain. Exp. Neurol. 221, 115–121. doi: 10.1016/j.expneurol.2009.10.008
Zhang, C., Rui, Y. Y., Zhou, Y. Y., Ju, Z., Zhang, H. H., Hu, C. Y., et al. (2014). Adrenergic beta2-receptors mediates visceral hypersensitivity induced by heterotypic intermittent stress in rats. PLoS One 9:e94726. doi: 10.1371/journal.pone.0094726
Zhang, H. T., Huang, Y., and O’Donnell, J. M. (2003). Antagonism of the antidepressant-like effects of clenbuterol by central administration of beta-adrenergic antagonists in rats. Psychopharmacology 170, 102–107. doi: 10.1007/s00213-003-1512-0
Keywords: single nucleotide polymorphism, haplotype, sickle cell disease, beta2-adrenergic receptor, chronic pain
Citation: Jhun EH, Sadhu N, Hu X, Yao Y, He Y, Wilkie DJ, Molokie RE and Wang ZJ (2019) Beta2-Adrenergic Receptor Polymorphisms and Haplotypes Associate With Chronic Pain in Sickle Cell Disease. Front. Pharmacol. 10:84. doi: 10.3389/fphar.2019.00084
Received: 27 October 2018; Accepted: 21 January 2019;
Published: 07 February 2019.
Edited by:
Rick Kittles, City of Hope National Medical Center, United StatesReviewed by:
Ken Batai, The University of Arizona, United StatesMelissa B. Davis, Cornell University, United States
Copyright © 2019 Jhun, Sadhu, Hu, Yao, He, Wilkie, Molokie and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaijie Jim Wang, emp3YW5nQHVpYy5lZHU=
†These authors have contributed equally to this work
 Ellie H. Jhun1†
Ellie H. Jhun1† Diana J. Wilkie
Diana J. Wilkie Zaijie Jim Wang
Zaijie Jim Wang