
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Pharmacol. , 21 December 2018
Sec. Predictive Toxicology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.01489
This article is a correction to:
The Reproductive Toxicity of Mequindox in a Two-Generation Study in Wistar Rats
 Qianying Liu1
Qianying Liu1 Zhixin Lei2
Zhixin Lei2 Qin Wu2
Qin Wu2 Ihsan Awais2
Ihsan Awais2 Muhammad A. B. Shabbir2
Muhammad A. B. Shabbir2 Saeed Ahmed2
Saeed Ahmed2 Zainab Fatima2
Zainab Fatima2 Xu Wang2
Xu Wang2 Yuanhu Pan2
Yuanhu Pan2 Shuyu Xie2*
Shuyu Xie2* Zonghui Yuan1,2,3*
Zonghui Yuan1,2,3*A Corrigendum on
The Reproductive Toxicity of Mequindox in a Two-Generation Study in Wistar Rats
by Liu, Q., Lei, Z., Wu, Q., Awais, I., Shabbir, M. A. B., Ahmed, S., et al. (2018). Front. Pharmacol. 9:870. doi: 10.3389/fphar.2018.00870
In the original article, there was a mistake in Figure 4 as published. Figure 4B was not displayed at the correct magnitude than that described in the figure legend. The corrected Figure 4 appears below.
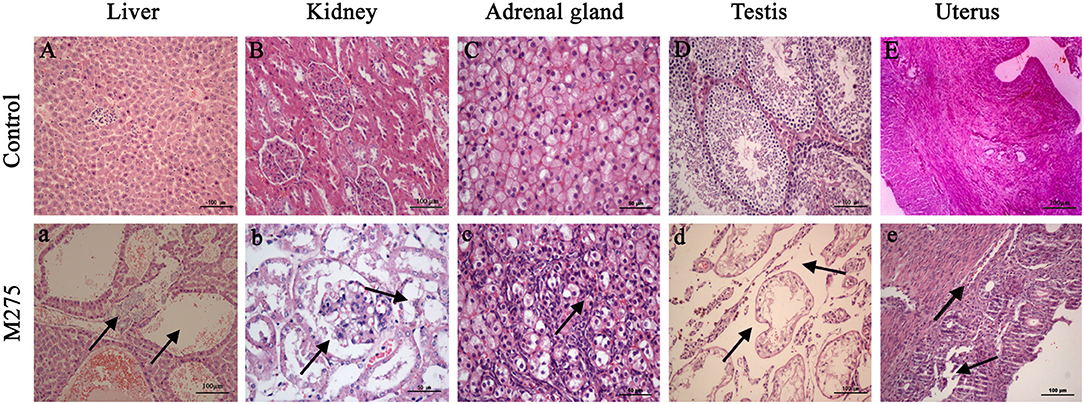
Figure 4. Selected microphotographs of liver, kidney, adrenal gland, testis and uterus (200X and 400X). M275, 275 mg/kg diet. (A) Liver (200X), (B) kidney (200X), (C) adrenal (400X), (D) testis (200X), and (E) uterus (200X) of F0 and F1 from the control group; (a) Liver in the 275 mg/kg MEQ group (200X). The vacuoles with a large number of blood cells, and hyperplasia of the epithelioid cells of the bile duct were marked with arrows; (b) Kidney in the 275 mg/kg MEQ group (400X). The swelling and hyperplasia of renal vesicle wall cell, and degeneration and necrosis of renal tubular epithelium were marked with arrows; (c) Adrenal gland in the 275 mg/kg MEQ group (400X). The proliferation of fascicular zone cell, increased binuclear cell and adrenocortical tumor were marked with arrows; (d) Testis in the 275 mg/kg MEQ group (200X). The broadening of interstitial, necrosis and dissolution of spermatogonial cells and spermatocytes in the lumen were marked with arrows; (e) Uterus in the 275 mg/kg MEQ group (200X). The incomplete structure and neutrophil infiltration in submucosal glands were marked with arrows.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: reproductive toxicity, teratogenicity, mequindox, Wistar rats, developmental toxicity
Citation: Liu Q, Lei Z, Wu Q, Awais I, Shabbir MAB, Ahmed S, Fatima Z, Wang X, Pan Y, Xie S and Yuan Z (2018) Corrigendum: The Reproductive Toxicity of Mequindox in a Two-Generation Study in Wistar Rats. Front. Pharmacol. 9:1489. doi: 10.3389/fphar.2018.01489
Received: 28 October 2018; Accepted: 05 December 2018;
Published: 21 December 2018.
Edited and reviewed by: Eleonore Fröhlich, Medical University of Graz, Austria
Copyright © 2018 Liu, Lei, Wu, Awais, Shabbir, Ahmed, Fatima, Wang, Pan, Xie and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyu Xie, c254c3kxQDEyNi5jb20=
Zonghui Yuan, eXVhbjU4MDJAbWFpbC5oemF1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.