- 1Institute for Cardiovascular Prevention (IPEK), University of Munich, Munich, Germany
- 2German Center for Cardiovascular Research (DZHK), Partner Site Munich Heart Alliance, Munich, Germany
- 3Department of Physiology and Pharmacology (FyFa), Karolinska Institute, Stockholm, Sweden
- 4Department of Medicine, Karolinska Institute, Stockholm, Sweden
Cardiovascular diseases, including myocardial infarction and its complications such as heart failure, are the leading cause of death worldwide. To date, basic and translational research becomes necessary to unravel the mechanisms of cardiac repair post-myocardial infarction. The local inflammatory tissue response after acute myocardial infarction determines the subsequent healing process. The diversity of leukocytes such as neutrophils, macrophages and lymphocytes contribute to the clearance of dead cells while activating reparative pathways necessary for myocardial healing. Cardiomyocyte death triggers wall thinning, ventricular dilatation, and fibrosis that can cause left ventricular dysfunction and heart failure. The ultimate goal of cardiac repair is to regenerate functionally viable myocardium after myocardial infarction to prevent cardiac death. Current therapies for heart failure after myocardial infarction are limited and non-curative. At the moment in clinic, conventional surgical interventions such as coronary artery bypass graft or percutaneous coronary interventions are only able to partially restore heart function, with a minor improvement in the left ventricular ejection fraction. The goal of this review is to provide an overview of endogenous myocardial repair mechanisms possibly transferable to future treatment strategies. Among the innovative factors identified as essential in cardiac healing, we highlight specialized pro-resolving mediators as the emerging factors that provide the key molecular signals for the activation of the reparative cells in the myocardium.
Introduction
Cardiovascular such as myocardial infarction, diseases, are the leading cause of morbidity and mortality worldwide, causing 31% of all global deaths (Benjamin et al., 2018). A history of acute myocardial infarction is associated with a 5-fold increase in the incidence of heart failure after 5 years of myocardial infarction. Therefore, there is a need to prevent cardiac failure by enhancing cardiac repair processes. Following infarction, the myocardium undergoes major changes both in its function and structure (Frangogiannis, 2012). Immediately after myocardial infarction, a robust inflammatory reaction occurs: immune cells mainly, neutrophils and monocytes, migrate into the heart, due to the release of myocardial danger-associated molecular patterns (DAMPs) derived by necrotic and stressed/injured cardiac cells (cardiomyocytes). Later, the resolution phase lasts a few days to weeks and encompasses the reparative or resolving phase. Finally, the progression phase lasts months or years depending on the resolution phase, which, if defective, leads to cardiac dysfunction, chronic heart failure, and mortality (Frangogiannis, 2012). A representative image shows the different stages of myocardial infarction (Figure 1). In the post-myocardial infarction initiation phase, numerous leukocytes travel from the splenic reservoir through the circulation to the myocardium that generates an edematous inflammatory milieu (Swirski et al., 2009). DAMPs bind to cognate pattern recognition receptors of the innate immune system on infiltrating leukocytes and activate the release of inflammatory cytokines, chemokines and activate cell adhesion molecules. The initial recruitment of immune cells can promote cardiac fibrosis and heart failure (Epelman et al., 2015). However, only recently studies show that immune cells also contribute to the repair process and the acute inflammatory response is more recently seen and described as essential and protective. Several studies in fact, report that controlling inflamed leukocytes promote cardioprotection (Nahrendorf et al., 2015). The balance between inflammation and resolution becomes crucial for the cardiac functionality, inappropriate inflammation delays the myocardial repair process. At the moment, improvement of intrinsic wound healing has emerged as a potential strategy to prevent heart failure.
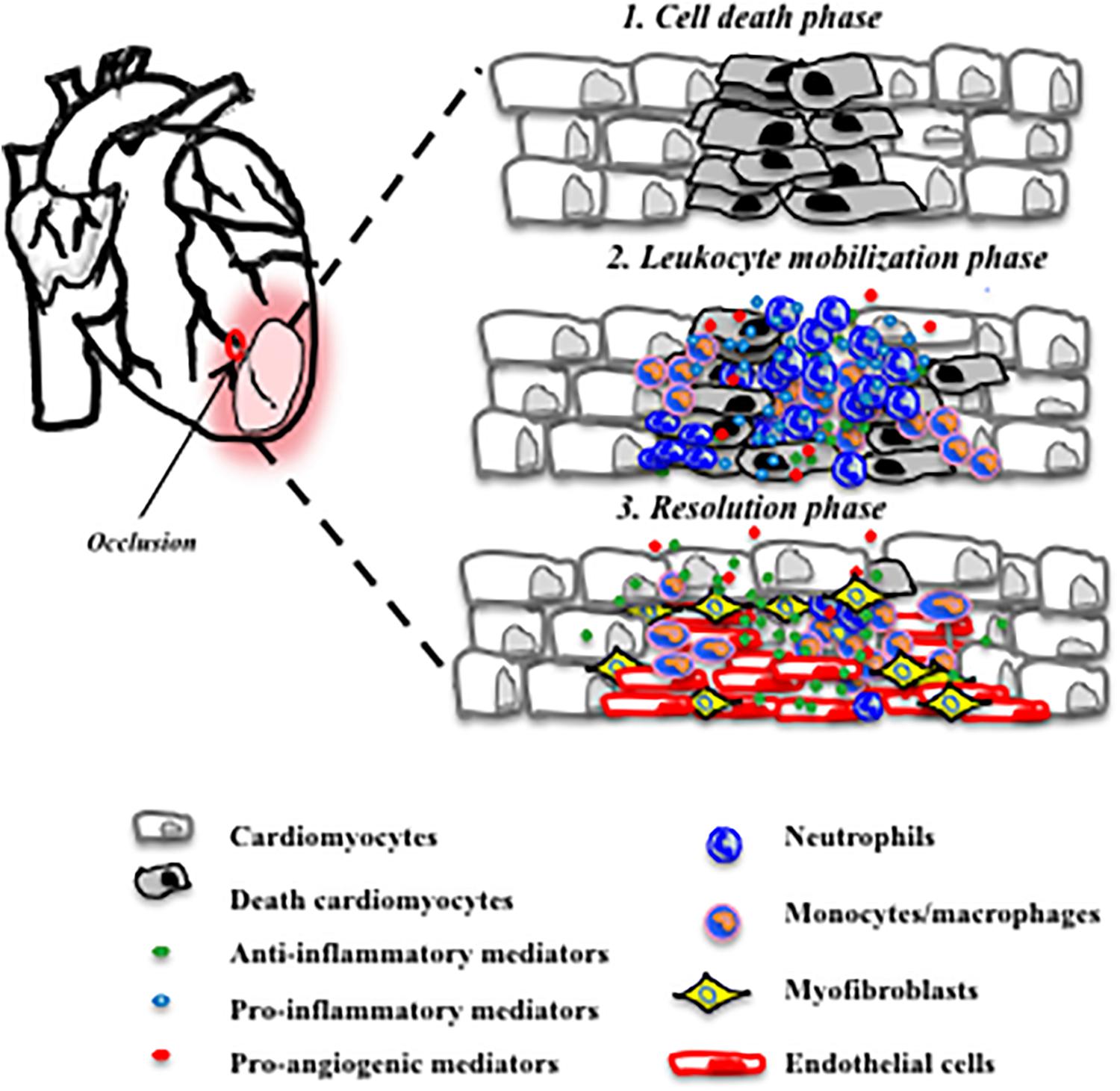
FIGURE 1. Three major phases post-myocardial infarction. Cardiomyocyte death (Benjamin et al., 2018) recruitment of neutrophils and pro-inflammatory monocytes (Frangogiannis, 2012) release of anti-inflammatory mediators and promotion of angiogenesis and repair (Swirski et al., 2009).
Cardiac Repair Post-Myocardial Infarction Is a Superbly Orchestrated Process
During myocardial infarction, neutrophil infiltration occurs immediately peaking at day 1 (Hansen, 1995). Neutrophils are pivotal players in post-infarction healing by potentially favoring the recruitment of inflammatory monocytes (Nahrendorf et al., 2007; Soehnlein et al., 2008). These cells present at the surface chemokine receptors (CCR) such as CCR2. CCR2 expression changes in a time-of-day–dependent manner, which crucially affects cardiac monocyte recruitment during myocardial infarction (Schloss et al., 2017). Experimental evidence also suggests that neutrophils directly damage cardiomyocytes in the myocardium through the release of toxic products, such as high amount of reactive oxygen species (Vinten-Johansen, 2004). However, in addition, data also demonstrate that neutrophils can improve cardiac function and cardiac repair (Horckmans et al., 2017). Recent studies indicate that neutrophils may acquire different phenotypes and contribute to resolution of inflammation through the release of anti-inflammatory mediators. Thus, neutrophils have been proposed to shift toward, pro-resolving/N2 phenotype instead of a N1 pro-inflammatory phenotype to promote tissue repair in condition of myocardial infarction (Ma et al., 2016). Neutrophils have both beneficial and detrimental roles during myocardial infarction, depending on their phenotype: too many N1 neutrophils damage tissue and cells leading to more inflammation. Too few N2 neutrophils may not be able to promote resolution of inflammation and apoptotic cardiomyocyte clearance: the perfect balance of N1 and N2 neutrophils becomes necessary for optimal cardiac repair. Achieving this balance represents the ideal pro-resolving conditions for patients with myocardial infarction (Romson et al., 1983; Ma et al., 2013, 2016; Carbone et al., 2016; Horckmans et al., 2017).
Macrophages represent another abundant cell population after myocardial infarction. They remain predominant in the infarcted left ventricle during the late phases of myocardial infarction (Yan et al., 2013; Hilgendorf et al., 2014). Macrophages regulate multiple aspects of the cardiac healing response, such as clearance of dead cells via Tyrosine-protein kinase Mer activation during myocardial infarction (DeBerge et al., 2017). Macrophages are classified in inflammatory macrophages (M1) during the initial phase of myocardial infarction and anti-inflammatory macrophages (M2) in the later phase of myocardial infarction (Nahrendorf et al., 2007; Troidl et al., 2009). M1 macrophages display the classical M1 surface marker expressing Ly-6Chigh and CD206low and higher levels of pro-inflammatory mediators (nitric oxide synthase, IL-6 IL-1b, and IL-12a). M2 macrophages express Ly-6Clow and CD206high with pro-resolving signature genes such as IL-10, arginase-1, and TGF-b. Interestingly, M2 macrophages mediate the beneficial effects of bone marrow-derived mesenchymal stromal cells in infarct healing and repair (Ben-Mordechai et al., 2013).
Among all the cells that contribute to the cardiac functionally there are also lymphocytes, observed in patients that had myocardial infarction (Nunez et al., 2008). Lymphocytes, consisting of T cells, B cells, and natural killer (NK) cells have important roles in both innate and adaptive immune responses in myocardial infarction. However, not much attention has been paid to these cells in the context of cardiac healing. Regulatory cells also often have potent effects, despite their relative scarcity (Epelman and Mann, 2012). Proliferative T cells: Th cells (CD4), cytotoxic T cells (CD8), and Foxp3 + regulatory CD4 + T cells are present in heart draining lymph nodes (Hofmann et al., 2012). During myocardial infarction, T cells number increases, due to the recruitment in the heart, since there are no studies reporting any increase of lymphocyte proliferation. B- and T-cell levels reach the peak after 7 days of myocardial infarction (Yan et al., 2013). Studies reported that patients with myocardial infarction have lower CD4+ but higher CD8+ T lymphocytes (Blum and Yeganeh, 2003; Liu et al., 2011; Yan et al., 2015). CD4+ T lymphocytes can differentiate into Th1 and Th2 lineage in response to the local milieu of cytokines during myocardial infarction. Th2 cells show protective role during myocardial infarction (Engelbertsen et al., 2013). NK cells are cytotoxic lymphocytes critical to the acute immune system during myocardial infarction (Yan et al., 2015). Not much is known about B lymphocytes during myocardial infarction. However, several studies using for example, mice deficient in B cells, demonstrate their crucial role during ischemia/reperfusion models (Kalogeris et al., 2012; Zouggari et al., 2013).
The inflammatory response that occurs during myocardial infarction is seen as an important element for the clearance of dead cells and the stimulation of the reparative processes. If dying cells are not eliminated this can further promote permanent loss of cardiac functionality and heart failure. The process of cardiac repair involves phagocytosis/clearance of apoptotic cells in the heart, predominantly promoted by macrophages, but other non-professional phagocytes have been shown to participate in this process such as cardiomyocytes and fibroblasts. Fibroblasts during myocardial infarction become activated and differentiate into myofibroblasts (Dutta et al., 2015; Nakaya et al., 2017). Cardiomyocytes can phagocytose latex particles in vitro (Garfield et al., 1975) and potentially cardiomyocyte debris in vivo (Hurle et al., 1977, 1978). Myofibroblasts mediated clearance of dying cells after myocardial infarction via milk fat globule epidermal growth factor (Han et al., 2016). Myofibroblasts are capable of other roles, such as extracellular matrix metabolism, contractile activity, producing and secreting greater levels of extracellular matrix proteins, including several types of collagen, important to strengthen the infarct and to protect it against rupture and neovessel formation (Frangogiannis et al., 2002). During myocardial infarction injury, cardiac fibroblasts interact with cardiomyocytes and this interaction is important for the heart to heal and recover (Fu et al., 2018). Other interactions among extracellular matrix, endothelial cells, and macrophages are also important for cardiac repair and neovessel formation/angiogenesis (Carmeliet, 2000). Angiogenic agents such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are rapidly released in the ischemic myocardium and facilitate growth of blood flow vessels, heart tissue repair and prevent the onset of heart failure (Zhao et al., 2010).
Resolving but Not Dampening Inflammation for Cardiac Healing
Maintaining the optimal balance of inflammation is crucial to induce myocardial healing (Kain et al., 2014). Several experimental studies have shown a better outcome in infarcted myocardium using anti-inflammatory treatment. However, some of the anti-inflammatory treatments failed in clinical practice (Silverman and Pfeifer, 1987; Saxena et al., 2016). As consequence, current guidelines recommend against the use of broad-range anti-inflammatory therapy corticosteroids and non-steroidal anti-inflammatory drugs–in patients with acute myocardial infarction (Task Force on the management of St-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) et al., 2012). In fact, the inhibition of COX-2 and TNF-α reduce cardiac functionality post-infarct in patients and COX-2 inhibitors (rofecoxib and celecoxib) in clinical settings accelerate the myocardial infarction events (Chung et al., 2003; Saito et al., 2003; Mann et al., 2004; Kimmel et al., 2005; Antman et al., 2007; Skyschally et al., 2007; Listing et al., 2008; Kleinbongard et al., 2010). Also a recombinant IL-1 receptor antagonist increase rates of recurrent myocardial infarction within 12 months, however, a larger study evaluating longer term IL-1 inhibition is active at the moment (Ridker et al., 2011; Morton et al., 2015). Possible reasons for failure of anti-inflammatory agents for myocardial infarction in clinical trials are that preclinical studies mostly involve healthy and young animals, unlike human patients (with different ages and gender) that often present chronic comorbidities. Another possible reason is that the targets were often non-specific. Diagnostic techniques such as tomography may be used to develop goal directed therapies (Herrero et al., 2007).
Promotion of cardiac repair is a key therapeutic goal against the failure of survival during ischemia. Low oxygen content that occurs during myocardial infarction is the major cause of this cardiac cell death. Re-introducing oxygen into the infarcted area represents a promising goal. A novel oxygen delivery system able to continuously release oxygen to the infarct area, protects the cardiac cells, could represent a new therapeutic approach for patients with myocardial infarction. The system was based on a thermosensitive injectable and fast gelation hydrogel, characterized by its capacity of oxygen releasing microspheres. With this technique the release of oxygen lasts 4 weeks and it significantly increases survival of cardiac cells, neovessel formation, and cardiac functionality, under the hypoxic condition that mimicked the infarcted hearts (Fan et al., 2018). Treatments with angiogenic or anti-apoptotic factors are very promising for improving cardiac functionality in condition of myocardial infarction. Despite the use of pro-angiogenic factors in clinical trials has led to good results in the improvement of cardiac function, there are still difficulties to overcome. In fact, clinical trials involving VEGF or bFGF do not have the expected beneficial effects (Hedman et al., 2003; Henry et al., 2003; Kukula et al., 2011). For example, a proper spatio-temporal delivery of multiple therapeutic proteins represents a major challenge in therapy strategies aimed to induce myocardial regeneration after myocardial infarction and on the other hand the pro-angiogenic growth factors expression has to be very tightly regulated in order to avoid side effects such as the promotion of tumor growth (Alfranca, 2009). Another important issue in therapeutic angiogenesis is that the delivery of a single growth factor might be insufficient to mimic the complex regulatory mechanisms driving neovascularization. Many of the strategies failed in the clinical setting and they rely upon using a single-targeted approach, directed to only one specific molecule or intracellular signaling pathway. Therefore, a multi-targeted approach directed to more than one intracellular signaling pathways may have more cardioprotective effects, considering also the presence of a co-morbidity in patients (Tsang et al., 2005). Several new approaches are discovered, such as VEGF enriched nanoparticles administration via local injection into the peri-infarct region is able to increase the angiogenic and therapeutic efficiency of VEGF in promoting cardiac repair (Oduk et al., 2018). VEGF-loaded microsphere patch for local protein delivery to the ischemic heart after myocardial injury in rats are even more promising: VEGF-patched hearts have better blood vessels growth, tissue repair and heart function (Rodness et al., 2016). The use of three-dimensional matrices despite the encouraging results in terms of cardiac regeneration and performance remains a relatively invasive method since they are surgically implanted over the infarct region. Drug administration in conditions of myocardial infarction at the moment includes oral or needle-based routes which can lead to patient discomfort (Suarez et al., 2015). Engineered cardiac patches are currently considered as a promising therapeutical approach for regeneration of the heart, however, their integration within the myocardium by sutures may cause further damage. A new suture-free technology for the attachment of engineered tissues positioned on the myocardium and irradiated with a laser represents an even better therapeutic approach at the moment (Malki et al., 2018). Also, inhaled calcium phosphate nanoparticles can deliver to the myocardium therapeutic compounds in a less invasive and better way (Miragoli et al., 2018). Inhalation therapy could represent a promising alternative to increase blood flow in the setting of chronic ischemia to preserve cardiac function.
Stem cells secrete high amounts of paracrine factors that can stimulate endogenous repair mechanisms (Henning, 2018). Human embryonic stem cell-derived cardiomyocytes repair the macaque monkey hearts by reducing scar tissue and improving cardiac functionality (Liu et al., 2018). Mesenchymal stem cells are at the moment under clinical investigation as a treatment for patients with advanced heart failure after myocardial infarction to improve myocardial function (Makkar et al., 2012; Malliaras et al., 2014). Injection in the myocardium of swine of human mesenchymal cell-derived extracellular vesicles (EVs) increase blood flow to ischemic myocardial tissue by stimulating capillary and arteriolar growth via activation of the protein kinase B/endothelial nitric oxide synthase and mitogen-activated protein kinase signaling pathways (Ponikowski et al., 2014). EVs significantly improve cardiac output and stroke volume (Potz et al., 2018). EVs containing anti-inflammatory proteins (e.g., Annexin A1) are also shown to activate wound repair circuits in another organ therefore they could also be beneficial for cardiac healing post-ischemia (Leoni et al., 2015). Annexin A1 during the acute phase of myocardial infarction present protective effects by controlling haematopoietic stem cell mobilization and inflammation (D’Amico et al., 2000; Qin et al., 2013; Qin et al., 2017; Ritchie et al., 2005). More studies are needed to explore its role during later cardiac repair events. In the context of myocardial infarction, members of EVs called exosomes are important for the regenerative effects in the myocardium. Cardiosphere-derived cell exosomes deliver in the myocardium decrease scarring and improve ejection fraction in a porcine myocardial infarction model (Gallet et al., 2017). Their beneficial effects have been demonstrated in multiple animal models and also in a phase 1 human study (Johnston et al., 2009; Makkar et al., 2012; Malliaras et al., 2012, 2014). To reverse injury post-myocardial infarction, cardiosphere-derived cells are currently in phase 2 clinical trials with scar reduction as the major endpoint.
Novel treatments to resolve inflammation during myocardial infarction become necessary. Several interesting studies demonstrate the protective role of pro-resolving mediators during the resolution phase of inflammation (Keyes et al., 2010; Kain et al., 2015; Halade et al., 2016). Well-known drugs such as statins lower permeability and reduce the transit of unfavorable inflammatory leukocytes into the infarcted tissue, consequently improving left ventricular outcome (Bauersachs et al., 2001; Ramasubbu et al., 2008; Leenders et al., 2018). Statin treatment also improve endothelial barrier function during myocardial healing in ApoE−/− mice (Leenders et al., 2018). A phase III clinical trial demonstrates that a statin called rosuvastatin has beneficial effects in patients with heart failure (McMurray et al., 2009). Aspirin also, contributes to the stimulation of the generation of pro-resolving mediators (SPMs) classified as lipoxins, resolvins, protectins, maresins, and Annexin A1 (Serhan et al., 2002; Gilroy, 2005; Serhan et al., 2011; Dalli et al., 2015a; Perretti et al., 2015). Experimental models of self-resolving inflammation demonstrate their potent anti-inflammatory and pro-resolving properties in several models (Serhan, 2010). Recently, a study shows that mice treated with 15-epi-lipoxin A4 present improved ejection fraction after 5 days of myocardial infarction (Kain et al., 2017). Furthermore, resolvin D1 has similar cardioprotective effects (Kain et al., 2015). Two recent studies present a quantification of SPMs in the infarcted left ventricles and spleens, after myocardial infarction (Tourki and Halade, 2017; Halade et al., 2018b). Interestingly, the peak of neutrophils after 24 h post-myocardial infarction correlates with an increase of resolvin D series in the infarcted myocardium. Later, at day 5 post-myocardial infarction, N2 neutrophils represent the most amount population in the left ventricle and spleen. Interestingly, resolvin D1 activated its receptor (lipoxin A4 receptor/formyl peptide receptor-2) to promote clearance in the infarcted heart (Kain et al., 2015). Furthermore, resolvin D1 accelerate clearance of leukocytes from an infarcted area by the activation of the miRNA circuit (Halade et al., 2018a). Human artery segments and primary cultured human vascular cells generate D-series resolvins and maresins when the relevant fatty acid precursors are present, and in the absence of leukocytes (Chatterjee et al., 2017). Recently, novel molecules termed maresin conjugates in tissue regeneration (MCTR), protectin conjugates in tissue regeneration (PCTR), and resolvin conjugates in tissue regeneration (RCTR) are identified as other pro-repair inducers (Dalli et al., 2014, 2015b; Dalli and Serhan, 2018). These new compounds could represent new therapeutical treatments for patients with myocardial infarction. The discovery of lipid mediators will serve as a novel therapeutical approach based on endogenous mechanisms for treating inflammatory response through the stimulation of resolution instead of inhibiting the inflammation.
Another important factor that controls the severity of myocardial infarction is aging. Cardiac aging is a process characterized by increased levels of reactive oxygen species, genomic DNA damage and telomere and epigenetic modifications. Aging also disregulates the level of arachidonic acid post-myocardial infarction and lipoxins release, responsible for neutrophils infiltration inhibition (Takano et al., 1998; Serhan et al., 2000; Halade et al., 2016; Serhan and Levy, 2018). Aging has effects on the innate immune response, through dysregulation of pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α, and TGFβ, which lead to chronic inflammation, and thus contribute to the “inflammaging phenotype,” often observed in the elderly people (Ershler and Keller, 2000; Franceschi et al., 2000; Bruunsgaard et al., 2003). In the elderly, defects in dying-cell clearance could lead to a non-resolving inflammation and maladaptive cardiac repair, thereby accelerating heart failure (Chen and Frangogiannis, 2010). Since little is known about the pro-resolving mediators in aging itself more studies are needed to assess whether in human patients pro-resolving molecules are less abundant or less effective with increasing age and how these factors impact cardiac repair. The capacity of the heart to heal after a myocardial infarction is not enough to restore normal cardiac function. Resolution of inflammation can be influenced by diet. Only a good balance of omega-3 and -6 fatty acids demonstrates protective effects on cardiovascular system (Ramsden et al., 2013). High amount of omega-6 decrease specialized pro-resolving mediators (D and E-series), also increase macrophage accumulation in the myocardium and promote cardiorenal inflammation (Halade et al., 2016). Of note, omega-3 fatty acids are known for their cardiovascular benefit or to reduce elevated triglycerides using higher doses (Smith et al., 2006). Thus, omega-3 fatty acids has positive effects on controlling inflammation, including the reduction of cytokines, endothelial cell activation and platelet aggregation, heart rate and cardiac function. A clinical phase III trial demonstrates in fact that long-term administration of omega-3 result in a significant reduction in both all-cause mortality and cardiovascular readmissions in patients with heart failure (Yancy et al., 2013). Lifestyle-related post-myocardial infarction setting opens a new future perspective studies to prevent the progression of heart failure.
Clearly, there is still much unknown in the field of cardiac healing, nevertheless progress has been made, opening exciting new potential therapeutic options for patients affected by myocardial infarction, as shown in Figure 2. Several studies enhance the crucial role of endogenous pro-resolving mediators during myocardial infarction. Significant increases in resolvins, protectin, and maresin are observed after 1 and 5 days post-myocardial infarction and their increase correlate with leukocyte recruitment (Howlett et al., 2016). Therefore, the abundance of SPMs could also predict the risk of future cardiovascular events (Emami et al., 2015). The advantage of using pro-resolving mediators is that they act on specific cellular receptors to regulate leukocyte trafficking and blunt the release of inflammatory mediators, while also promoting clearance of dead cells and tissue repair. These mediators could inform the development of therapeutic strategies encompassing a novel resolution pharmacology approach.
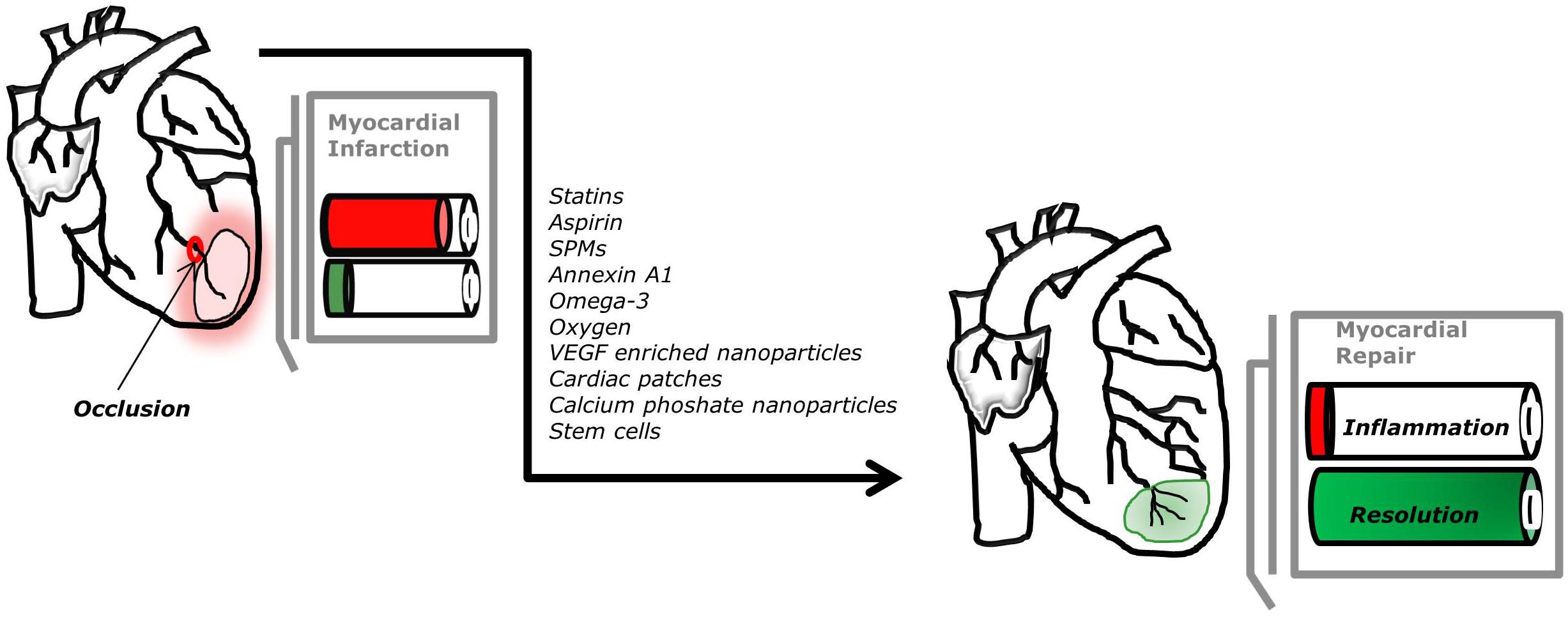
FIGURE 2. Potential new therapeutic approaches to promote myocardial repair. The optimal process of repair after myocardial infarction, arising from occlusion of the coronary circulation, requires timely induction and resolution of inflammation.
In future, nanocarriers engineered to recognize pro-resolving specific receptors at the cellular levels and to deliver pro-resolving mediators into the diseased sites to subpopulations of immune cells represents a highly appealing approach to specifically improve cardiac repair.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alfranca, A. (2009). VEGF therapy: a timely retreat. Cardiovasc. Res. 83, 611–612. doi: 10.1093/cvr/cvp228
Antman, E. M., Bennett, J. S., Daugherty, A., Furberg, C., Roberts, H., Taubert, K. A., et al. (2007). Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation 115, 1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424
Bauersachs, J., Galuppo, P., Fraccarollo, D., Christ, M., and Ertl, G. (2001). Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation 104, 982–985. doi: 10.1161/hc3401.095946
Benjamin, E. J., Virani, S. S., Callaway, C. W., Chamberlain, A. M., Chang, A. R., Cheng, S., et al. (2018). Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation 137, e67–e492. doi: 10.1161/CIR.0000000000000558
Ben-Mordechai, T., Holbova, R., Landa-Rouben, N., Harel-Adar, T., Feinberg, M. S., Abd Elrahman, I., et al. (2013). Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J. Am. Coll. Cardiol. 62, 1890–1901. doi: 10.1016/j.jacc.2013.07.057
Blum, A., and Yeganeh, S. (2003). The role of T-lymphocyte subpopulations in acute myocardial infarction. Eur. J. Intern. Med. 14, 407–410. doi: 10.1016/j.ejim.2003.09.002
Bruunsgaard, H., Andersen-Ranberg, K., Hjelmborg, J., Pedersen, B. K., and Jeune, B. (2003). Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 115, 278–283. doi: 10.1016/S0002-9343(03)00329-2
Carbone, F., Crowe, L. A., Roth, A., Burger, F., Lenglet, S., Braunersreuther, V., et al. (2016). Treatment with anti-RANKL antibody reduces infarct size and attenuates dysfunction impacting on neutrophil-mediated injury. J. Mol. Cell. Cardiol. 94, 82–94. doi: 10.1016/j.yjmcc.2016.03.013
Carmeliet, P. (2000). Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6, 389–395. doi: 10.1038/74651
Chatterjee, A., Komshian, S., Sansbury, B. E., Wu, B., Mottola, G., Chen, M., et al. (2017). Biosynthesis of proresolving lipid mediators by vascular cells and tissues. FASEB J. 31, 3393–3402. doi: 10.1096/fj.201700082R
Chen, W., and Frangogiannis, N. G. (2010). The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail. Rev. 15, 415–422. doi: 10.1007/s10741-010-9161-y
Chung, E. S., Packer, M., Lo, K. H., Fasanmade, A. A., Willerson, J. T., and Anti-TNF Therapy Against Congestive Heart Failure Investigators (2003). Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107, 3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2
Dalli, J., Chiang, N., and Serhan, C. N. (2014). Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. U.S.A. 111, E4753–E4761. doi: 10.1073/pnas.1415006111
Dalli, J., Chiang, N., and Serhan, C. N. (2015a). Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 21, 1071–1075. doi: 10.1038/nm.3911
Dalli, J., Ramon, S., Norris, P. C., Colas, R. A., and Serhan, C. N. (2015b). Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways. FASEB J. 29, 2120–2136. doi: 10.1096/fj.14-268441
Dalli, J., and Serhan, C. N. (2018). Identification and structure elucidation of the proresolving mediators provides novel leads for resolution pharmacology. Br. J. Pharmacol. doi: 10.1111/bph.14336 [Epub ahead of print].
D’Amico, M., Di Filippo, C., La, M., Solito, E., McLean, P. G., Flower, R. J., et al. (2000). Lipocortin 1 reduces myocardial ischemia-reperfusion injury by affecting local leukocyte recruitment. FASEB J. 14, 1867–1869. doi: 10.1096/fj.99-0602fje
DeBerge, M., Yeap, X. Y., Dehn, S., Zhang, S., Grigoryeva, L., Misener, S., et al. (2017). MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ. Res. 121, 930–940. doi: 10.1161/CIRCRESAHA.117.311327
Dutta, P., Sager, H. B., Stengel, K. R., Naxerova, K., Courties, G., Saez, B., et al. (2015). Myocardial infarction activates CCR2+ hematopoietic stem, and progenitor cells. Cell Stem Cell 16, 477–487. doi: 10.1016/j.stem.2015.04.008
Emami, H., Singh, P., MacNabb, M., Vucic, E., Lavender, Z., Rudd, J. H., et al. (2015). Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc. Imaging 8, 121–130. doi: 10.1016/j.jcmg.2014.10.009
Engelbertsen, D., Andersson, L., Ljungcrantz, I., Wigren, M., Hedblad, B., Nilsson, J., et al. (2013). T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler. Thromb. Vasc. Biol. 33, 637–644. doi: 10.1161/ATVBAHA.112.300871
Epelman, S., Liu, P. P., and Mann, D. L. (2015). Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 15, 117–129. doi: 10.1038/nri3800
Epelman, S., and Mann, D. L. (2012). Communication in the heart: the role of the innate immune system in coordinating cellular responses to ischemic injury. J. Cardiovasc. Transl. Res. 5, 827–836. doi: 10.1007/s12265-012-9410-7
Ershler, W. B., and Keller, E. T. (2000). Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 51, 245–270. doi: 10.1146/annurev.med.51.1.245
Fan, Z., Xu, Z., Niu, H., Gao, N., Guan, Y., Li, C., et al. (2018). An injectable oxygen release system to augment cell survival and promote cardiac repair following myocardial infarction. Sci. Rep. 8:1371. doi: 10.1038/s41598-018-19906-w
Franceschi, C., Bonafe, M., Valensin, S., Olivieri, F., De Luca, M., Ottaviani, E., et al. (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x
Frangogiannis, N. G. (2012). Regulation of the inflammatory response in cardiac repair. Circ. Res. 110, 159–173. doi: 10.1161/CIRCRESAHA.111.243162
Frangogiannis, N. G., Smith, C. W., and Entman, M. L. (2002). The inflammatory response in myocardial infarction. Cardiovasc. Res. 53, 31–47. doi: 10.1016/S0008-6363(01)00434-5
Fu, X., Khalil, H., Kanisicak, O., Boyer, J. G., Vagnozzi, R. J., Maliken, B. D., et al. (2018). Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Invest. 128, 2127–2143. doi: 10.1172/JCI98215
Gallet, R., Dawkins, J., Valle, J., Simsolo, E., de Couto, G., Middleton, R., et al. (2017). Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38, 201–211. doi: 10.1093/eurheartj/ehw240
Garfield, R. E., Chacko, S., and Blose, S. (1975). Phagocytosis by muscle cells. Lab. Invest. 33, 418–427.
Gilroy, D. W. (2005). The role of aspirin-triggered lipoxins in the mechanism of action of aspirin. Prostaglandins Leukot. Essent. Fatty Acids 73, 203–210. doi: 10.1016/j.plefa.2005.05.007
Halade, G. V., Kain, V., Black, L. M., Prabhu, S. D., and Ingle, K. A. (2016). Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging 8, 2611–2634. doi: 10.18632/aging.101077
Halade, G. V., Kain, V., and Serhan, C. N. (2018a). Immune responsive resolvin D1 programs myocardial infarction-induced cardiorenal syndrome in heart failure. FASEB J. 32, 3717–3729. doi: 10.1096/fj.201701173RR
Halade, G. V., Norris, P. C., Kain, V., Serhan, C. N., and Ingle, K. A. (2018b). Splenic leukocytes define the resolution of inflammation in heart failure. Sci. Signal. 11:eaao1818.
Han, C. Z., Juncadella, I. J., Kinchen, J. M., Buckley, M. W., Klibanov, A. L., Dryden, K., et al. (2016). Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature 539, 570–574. doi: 10.1038/nature20141
Hansen, P. R. (1995). Role of neutrophils in myocardial ischemia and reperfusion. Circulation 91, 1872–1885. doi: 10.1161/01.CIR.91.6.1872
Hedman, M., Hartikainen, J., Syvanne, M., Stjernvall, J., Hedman, A., Kivela, A., et al. (2003). Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 107, 2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92
Henning, R. J. (2018). Current status of stem cells in cardiac repair. Future Cardiol. 14, 181–192. doi: 10.2217/fca-2017-0072
Henry, T. D., Annex, B. H., McKendall, G. R., Azrin, M. A., Lopez, J. J., Giordano, F. J., et al. (2003). The VIVA trial: vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation 107, 1359–1365. doi: 10.1161/01.CIR.0000061911.47710.8A
Herrero, P., Kisrieva-Ware, Z., Dence, C. S., Patterson, B., Coggan, A. R., Han, D. H., et al. (2007). PET measurements of myocardial glucose metabolism with 1-11C-glucose and kinetic modeling. J. Nucl. Med. 48, 955–964. doi: 10.2967/jnumed.106.037598
Hilgendorf, I., Gerhardt, L. M., Tan, T. C., Winter, C., Holderried, T. A., Chousterman, B. G., et al. (2014). Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 114, 1611–1622. doi: 10.1161/CIRCRESAHA.114.303204
Hofmann, U., Beyersdorf, N., Weirather, J., Podolskaya, A., Bauersachs, J., Ertl, G., et al. (2012). Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125, 1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164
Horckmans, M., Ring, L., Duchene, J., Santovito, D., Schloss, M. J., Drechsler, M., et al. (2017). Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 38, 187–197. doi: 10.1093/eurheartj/ehw002
Howlett, J. G., Chan, M., Ezekowitz, J. A., Harkness, K., Heckman, G. A., Kouz, S., et al. (2016). The Canadian cardiovascular society heart failure companion: bridging guidelines to your practice. Can. J. Cardiol. 32, 296–310. doi: 10.1016/j.cjca.2015.06.019
Hurle, J. M., Lafarga, M., and Ojeda, J. L. (1977). Cytological and cytochemical studies of the necrotic area of the bulbus of the chick embryo heart: phagocytosis by developing myocardial cells. J. Embryol. Exp. Morphol. 41, 161–173.
Hurle, J. M., Lafarga, M., and Ojeda, J. L. (1978). In vivo phagocytosis by developing myocardial cells: an ultrastructural study. J. Cell Sci. 33, 363–369.
Johnston, P. V., Sasano, T., Mills, K., Evers, R., Lee, S. T., Smith, R. R., et al. (2009). Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 120, 1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058
Kain, V., Ingle, K. A., Colas, R. A., Dalli, J., Prabhu, S. D., Serhan, C. N., et al. (2015). Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 84, 24–35. doi: 10.1016/j.yjmcc.2015.04.003
Kain, V., Liu, F., Kozlovskaya, V., Ingle, K. A., Bolisetty, S., Agarwal, A., et al. (2017). Resolution agonist 15-epi-lipoxin A4 programs early activation of resolving phase in post-myocardial infarction healing. Sci. Rep. 7:9999. doi: 10.1038/s41598-017-10441-8
Kain, V., Prabhu, S. D., and Halade, G. V. (2014). Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res. Cardiol. 109:444. doi: 10.1007/s00395-014-0444-7
Kalogeris, T., Baines, C. P., Krenz, M., and Korthuis, R. J. (2012). Cell biology of ischemia/reperfusion injury. Int. Rev. Cell. Mol. Biol. 298, 229–317. doi: 10.1016/B978-0-12-394309-5.00006-7
Keyes, K. T., Ye, Y., Lin, Y., Zhang, C., Perez-Polo, J. R., Gjorstrup, P., et al. (2010). Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 299, H153–H164. doi: 10.1152/ajpheart.01057.2009
Kimmel, S. E., Berlin, J. A., Reilly, M., Jaskowiak, J., Kishel, L., Chittams, J., et al. (2005). Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann. Intern. Med. 142, 157–164. doi: 10.7326/0003-4819-142-3-200502010-00005
Kleinbongard, P., Heusch, G., and Schulz, R. (2010). TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 127, 295–314. doi: 10.1016/j.pharmthera.2010.05.002
Kukula, K., Chojnowska, L., Dabrowski, M., Witkowski, A., Chmielak, Z., Skwarek, M., et al. (2011). Intramyocardial plasmid-encoding human vascular endothelial growth factor A165/basic fibroblast growth factor therapy using percutaneous transcatheter approach in patients with refractory coronary artery disease (VIF-CAD). Am. Heart J. 161, 581–589. doi: 10.1016/j.ahj.2010.11.023
Leenders, G. J., Smeets, M. B., van den Boomen, M., Berben, M., Nabben, M., van Strijp, D., et al. (2018). Statins promote cardiac infarct healing by modulating endothelial barrier function revealed by contrast-enhanced magnetic resonance imaging. Arterioscler. Thromb. Vasc. Biol. 38, 186–194. doi: 10.1161/ATVBAHA.117.310339
Leoni, G., Neumann, P. A., Kamaly, N., Quiros, M., Nishio, H., Jones, H. R., et al. (2015). Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Invest. 125, 1215–1227. doi: 10.1172/JCI76693
Listing, J., Strangfeld, A., Kekow, J., Schneider, M., Kapelle, A., Wassenberg, S., et al. (2008). Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 58, 667–677. doi: 10.1002/art.23281
Liu, L. L., Lu, J. L., Chao, P. L., Lin, L. R., Zhang, Z. Y., and Yang, T. C. (2011). Lower prevalence of circulating invariant natural killer T (iNKT) cells in patients with acute myocardial infarction undergoing primary coronary stenting. Int. Immunopharmacol. 11, 480–484. doi: 10.1016/j.intimp.2010.12.019
Liu, Y. W., Chen, B., Yang, X., Fugate, J. A., Kalucki, F. A., Futakuchi-Tsuchida, A., et al. (2018). Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 36, 597–605. doi: 10.1038/nbt.4162
Ma, Y., Yabluchanskiy, A., Iyer, R. P., Cannon, P. L., Flynn, E. R., Jung, M., et al. (2016). Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 110, 51–61. doi: 10.1093/cvr/cvw024
Ma, Y., Yabluchanskiy, A., and Lindsey, M. L. (2013). Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 6:11. doi: 10.1186/1755-1536-6-11
Makkar, R. R., Smith, R. R., Cheng, K., Malliaras, K., Thomson, L. E., Berman, D., et al. (2012). Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904. doi: 10.1016/S0140-6736(12)60195-0
Malki, M., Fleischer, S., Shapira, A., and Dvir, T. (2018). Gold nanorod-based engineered cardiac patch for suture-free engraftment by near IR. Nano Lett. 18, 4069–4073. doi: 10.1021/acs.nanolett.7b04924
Malliaras, K., Li, T. S., Luthringer, D., Terrovitis, J., Cheng, K., Chakravarty, T., et al. (2012). Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 125, 100–112. doi: 10.1161/CIRCULATIONAHA.111.042598
Malliaras, K., Makkar, R. R., Smith, R. R., Cheng, K., Wu, E., Bonow, R. O., et al. (2014). Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 63, 110–122. doi: 10.1016/j.jacc.2013.08.724
Mann, D. L., McMurray, J. J., Packer, M., Swedberg, K., Borer, J. S., Colucci, W. S., et al. (2004). Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109, 1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2
McMurray, J. J., Kjekshus, J., Gullestad, L., Dunselman, P., Hjalmarson, A., Wedel, H., et al. (2009). Effects of statin therapy according to plasma high-sensitivity C-reactive protein concentration in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): a retrospective analysis. Circulation 120, 2188–2196. doi: 10.1161/CIRCULATIONAHA.109.849117
Miragoli, M., Ceriotti, P., Iafisco, M., Vacchiano, M., Salvarani, N., Alogna, A., et al. (2018). Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 10:eaan6205.
Morton, A. C., Rothman, A. M., Greenwood, J. P., Gunn, J., Chase, A., Clarke, B., et al. (2015). The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA heart study. Eur. Heart J. 36, 377–384. doi: 10.1093/eurheartj/ehu272
Nahrendorf, M., Frantz, S., Swirski, F. K., Mulder, W. J., Randolph, G., Ertl, G., et al. (2015). Imaging systemic inflammatory networks in ischemic heart disease. J. Am. Coll. Cardiol. 65, 1583–1591. doi: 10.1016/j.jacc.2015.02.034
Nahrendorf, M., Swirski, F. K., Aikawa, E., Stangenberg, L., Wurdinger, T., Figueiredo, J. L., et al. (2007). The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047. doi: 10.1084/jem.20070885
Nakaya, M., Watari, K., Tajima, M., Nakaya, T., Matsuda, S., Ohara, H., et al. (2017). Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J. Clin. Invest. 127, 383–401. doi: 10.1172/JCI83822
Nunez, J., Nunez, E., Bodi, V., Sanchis, J., Minana, G., Mainar, L., et al. (2008). Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am. J. Cardiol. 101, 747–752. doi: 10.1016/j.amjcard.2007.11.004
Oduk, Y., Zhu, W., Kannappan, R., Zhao, M., Borovjagin, A. V., Oparil, S., et al. (2018). VEGF nanoparticles repair the heart after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 314, H278–H284. doi: 10.1152/ajpheart.00471.2017
Perretti, M., Leroy, X., Bland, E. J., and Montero-Melendez, T. (2015). Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 36, 737–755. doi: 10.1016/j.tips.2015.07.007
Ponikowski, P., Mitrovic, V., Ruda, M., Fernandez, A., Voors, A. A., Vishnevsky, A., et al. (2014). A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur. Heart J. 35, 431–441. doi: 10.1093/eurheartj/eht459
Potz, B. A., Scrimgeour, L. A., Pavlov, V. I., Sodha, N. R., Abid, M. R., and Sellke, F. W. (2018). Extracellular vesicle injection improves myocardial function and increases angiogenesis in a swine model of chronic ischemia. J. Am. Heart Assoc. 7:e008344. doi: 10.1161/JAHA.117.008344
Qin, C., Buxton, K. D., Pepe, S., Cao, A. H., Venardos, K., Love, J. E., et al. (2013). Reperfusion-induced myocardial dysfunction is prevented by endogenous annexin-A1 and its N-terminal-derived peptide Ac-ANX-A1(2-26). Br. J. Pharmacol. 168, 238–252. doi: 10.1111/j.1476-5381.2012.02176.x
Qin, C. X., Finlayson, S. B., Al-Sharea, A., Tate, M., De Blasio, M. J., Deo, M., et al. (2017). Endogenous annexin-A1 regulates haematopoietic stem cell mobilisation and inflammatory response post myocardial infarction in mice in vivo. Sci. Rep. 7:16615. doi: 10.1038/s41598-017-16317-1
Ramasubbu, K., Estep, J., White, D. L., Deswal, A., and Mann, D. L. (2008). Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 51, 415–426. doi: 10.1016/j.jacc.2007.10.009
Ramsden, C. E., Zamora, D., Leelarthaepin, B., Majchrzak-Hong, S. F., Faurot, K. R., Suchindran, C. M., et al. (2013). Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 346:e8707. doi: 10.1136/bmj.e8707
Ridker, P. M., Thuren, T., Zalewski, A., and Libby, P. (2011). Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 162, 597–605. doi: 10.1016/j.ahj.2011.06.012
Ritchie, R. H., Gordon, J. M., Woodman, O. L., Cao, A. H., and Dusting, G. J. (2005). Annexin-1 peptide Anx-1(2-26) protects adult rat cardiac myocytes from cellular injury induced by simulated ischaemia. Br. J. Pharmacol. 145, 495–502. doi: 10.1038/sj.bjp.0706211
Rodness, J., Mihic, A., Miyagi, Y., Wu, J., Weisel, R. D., and Li, R. K. (2016). VEGF-loaded microsphere patch for local protein delivery to the ischemic heart. Acta Biomater. 45, 169–181. doi: 10.1016/j.actbio.2016.09.009
Romson, J. L., Hook, B. G., Kunkel, S. L., Abrams, G. D., Schork, M. A., and Lucchesi, B. R. (1983). Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 67, 1016–1023. doi: 10.1161/01.CIR.67.5.1016
Saito, T., Rodger, I. W., Shennib, H., Hu, F., Tayara, L., and Giaid, A. (2003). Cyclooxygenase-2 (COX-2) in acute myocardial infarction: cellular expression and use of selective COX-2 inhibitor. Can. J. Physiol. Pharmacol. 81, 114–119. doi: 10.1139/y03-023
Saxena, A., Russo, I., and Frangogiannis, N. G. (2016). Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl. Res. 167, 152–166. doi: 10.1016/j.trsl.2015.07.002
Schloss, M. J., Hilby, M., Nitz, K., Guillamat Prats, R., Ferraro, B., Leoni, G., et al. (2017). Ly6Chigh monocytes oscillate in the heart during homeostasis and after myocardial infarction-brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1640–1645. doi: 10.1161/ATVBAHA.117.309259
Serhan, C. N. (2010). Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177, 1576–1591. doi: 10.2353/ajpath.2010.100322
Serhan, C. N., Clish, C. B., Brannon, J., Colgan, S. P., Chiang, N., and Gronert, K. (2000). Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204. doi: 10.1084/jem.192.8.1197
Serhan, C. N., Fredman, G., Yang, R., Karamnov, S., Belayev, L. S., Bazan, N. G., et al. (2011). Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 18, 976–987. doi: 10.1016/j.chembiol.2011.06.008
Serhan, C. N., Hong, S., Gronert, K., Colgan, S. P., Devchand, P. R., Mirick, G., et al. (2002). Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196, 1025–1037. doi: 10.1084/jem.20020760
Serhan, C. N., and Levy, B. D. (2018). Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669. doi: 10.1172/JCI97943
Silverman, H. S., and Pfeifer, M. P. (1987). Relation between use of anti-inflammatory agents and left ventricular free wall rupture during acute myocardial infarction. Am. J. Cardiol. 59, 363–364. doi: 10.1016/0002-9149(87)90817-4
Skyschally, A., Gres, P., Hoffmann, S., Haude, M., Erbel, R., Schulz, R., et al. (2007). Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ. Res. 100, 140–146. doi: 10.1161/01.RES.0000255031.15793.86
Smith, S. C. Jr., Allen, J., Blair, S. N., Bonow, R. O., Brass, L. M., Fonarow, G. C., et al. (2006). AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 113, 2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516
Soehnlein, O., Zernecke, A., Eriksson, E. E., Rothfuchs, A. G., Pham, C. T., Herwald, H., et al. (2008). Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112, 1461–1471. doi: 10.1182/blood-2008-02-139634
Suarez, S., Almutairi, A., and Christman, K. L. (2015). Micro- and nanoparticles for treating cardiovascular disease. Biomater. Sci. 3, 564–580. doi: 10.1039/C4BM00441H
Swirski, F. K., Nahrendorf, M., Etzrodt, M., Wildgruber, M., Cortez-Retamozo, V., Panizzi, P., et al. (2009). Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616. doi: 10.1126/science.1175202
Takano, T., Clish, C. B., Gronert, K., Petasis, N., and Serhan, C. N. (1998). Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J. Clin. Invest. 101, 819–826. doi: 10.1172/JCI1578
Task Force on the management of St-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg, P. G., James, S. K., Atar, D., Badano, L. P., Blomstrom-Lundqvist, C., et al. (2012)., ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 33, 2569–2619.
Tourki, B., and Halade, G. (2017). Leukocyte diversity in resolving, and nonresolving mechanisms of cardiac remodeling. FASEB J. 31, 4226–4239. doi: 10.1096/fj.201700109R
Troidl, C., Mollmann, H., Nef, H., Masseli, F., Voss, S., Szardien, S., et al. (2009). Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J. Cell. Mol. Med. 13, 3485–3496. doi: 10.1111/j.1582-4934.2009.00707.x
Tsang, A., Hausenloy, D. J., Mocanu, M. M., Carr, R. D., and Yellon, D. M. (2005). Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes Metab. Res. Rev. 54, 2360–2364. doi: 10.2337/diabetes.54.8.2360
Vinten-Johansen, J. (2004). Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 61, 481–497. doi: 10.1016/j.cardiores.2003.10.011
Yan, W., Zhou, L., Wen, S., Duan, Q., Huang, F., Tang, Y., et al. (2015). Differential loss of natural killer cell activity in patients with acute myocardial infarction and stable angina pectoris. Int. J. Clin. Exp. Pathol. 8, 14667–14675.
Yan, X., Anzai, A., Katsumata, Y., Matsuhashi, T., Ito, K., Endo, J., et al. (2013). Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 62, 24–35. doi: 10.1016/j.yjmcc.2013.04.023
Yancy, C. W., Jessup, M., Bozkurt, B., Butler, J., Casey, D. E. Jr., Drazner, M. H., et al. (2013). ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128, e240–e327.
Zhao, T., Zhao, W., Chen, Y., Ahokas, R. A., and Sun, Y. (2010). Vascular endothelial growth factor (VEGF)-A: role on cardiac angiogenesis following myocardial infarction. Microvasc. Res. 80, 188–194. doi: 10.1016/j.mvr.2010.03.014
Keywords: cardiac, ischemia, inflammation, resolution, repair
Citation: Leoni G and Soehnlein O (2018) (Re) Solving Repair After Myocardial Infarction. Front. Pharmacol. 9:1342. doi: 10.3389/fphar.2018.01342
Received: 12 July 2018; Accepted: 31 October 2018;
Published: 26 November 2018.
Edited by:
Mauro Perretti, Queen Mary University of London, United KingdomReviewed by:
Asma Nusrat, University of Michigan, United StatesRaffaele Marfella, Università degli Studi della Campania “Luigi Vanvitelli”, Italy
Copyright © 2018 Leoni and Soehnlein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanna Leoni, Z2lvbGVvYmlAZ21haWwuY29t; R2lvdmFubmEuTGVvbmlAbWVkLnVuaS1tdWVuY2hlbi5kZQ==
 Giovanna Leoni
Giovanna Leoni Oliver Soehnlein
Oliver Soehnlein