
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 21 August 2018
Sec. Ethnopharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.00784
This article is part of the Research Topic Ethnopharmacological Studies for the Development of Drugs with special reference to Asteraceae, Volume I View all 6 articles
 Alexsander R. Carvalho Jr.
Alexsander R. Carvalho Jr. Roseana M. Diniz
Roseana M. Diniz Mariela A. M. Suarez
Mariela A. M. Suarez Cristiane S. S. e S. Figueiredo
Cristiane S. S. e S. Figueiredo Adrielle Zagmignan
Adrielle Zagmignan Marcos A. G. Grisotto
Marcos A. G. Grisotto Elizabeth S. Fernandes
Elizabeth S. Fernandes Luís C. N. da Silva*
Luís C. N. da Silva*Severe wounds result in large lesions and/or loss of function of the affected areas. The treatment of wounds has challenged health professionals due to its complexity, especially in patients with chronic diseases (such as diabetes), and the presence of pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa. Taking this into consideration, the development of new therapies for wound healing requires immediate attention. Ethnopharmacological studies performed in different countries have shown the use of several plants from the Asteraceae family as wound-healing agents. Evidences gained from the traditional medicine have opened new ways for the development of novel and more efficient therapies based on the pharmacological properties of these plants. In this article, we discuss the literature data on the use of Asteraceae plants for the treatment of wounds, based on the ethnopharmacological relevance of each plant. Special attention was given to studies showing the mechanisms of action of Asteraceae-derived compounds and clinical trials. Ageratina pichinchensis (Kunth) R.M. King and H. Rob. and Calendula officinalis L. preparations/compounds were found to show good efficacy when assessed in clinical trials of complicated wounds, including venous leg ulcers and foot ulcers of diabetic patients. The compounds silibinin [from Silybum marianum (L.) Gaertn.] and jaceosidin (from Artemisia princeps Pamp.) were identified as promising compounds for the treatment of wounds. Overall, we suggest that Asteraceae plants represent important sources of compounds that may act as new and efficient healing products.
Wounds, especially of chronic nature, cause a serious public health concern as they negatively affect the quality of life of a large number of people, showing psychological, social, and economic impacts (Vowden and Vowden, 2016; Guest et al., 2017). When not properly treated, their associated lesions can become larger and result in the loss of function of the affected areas. Wounds are classified on the basis of the various factors such as location, borders, size, tissue type, secretion, odor, edema, and pain (Frykberg and Banks, 2015; Wernick and Stawicki, 2018). Based on these characteristics, they can be simple or complex, deep or superficial, acute or chronic, sterile or contaminated, or even defined by the type of healing (Morton and Phillips, 2016; Karthik et al., 2018).
Based on its complexity, a wound is considered as simple when it is able to spontaneously evolve to resolution or as complex when lesions are extensive and/or deep and require special resources or more specialized treatment for its complete healing (Wernick and Stawicki, 2018). Based on its depth, a wound is superficial when it is restricted to the epidermis or dermis or deep when it affects the subcutaneous tissue, muscles, and/or bones (Shin et al., 2017; Podd, 2018). Wounds can be also classified as acute or chronic, with the former achieving resolution within 3 weeks and minimal or no scar tissue formation, whereas the latter may take several weeks to heal and can often lead to the loss of function of the affected tissues (Frykberg and Banks, 2015; Jørgensen et al., 2016). The healing time is influenced by different factors such as the presence of comorbidities (diabetes, hypertension, neurological lesions, among others), infection, aging, nutritional status, personal care, and appropriate and timely treatment (Manrique et al., 2015; Kulprachakarn et al., 2017; Hou and Kim, 2018; Long et al., 2018; Yuan et al., 2018).
The treatment of chronic wounds is complex and can include the administration of vascular endothelial growth factor or erythropoietin, which may not always be efficient and present high costs and has short half-life and side effects (Hong and Park, 2014; Arslantas et al., 2015; Yu et al., 2018). Moreover, the treatment of chronic wounds often requires the use of antimicrobials due to their multifactorial nature (Karthik et al., 2018). Considering this aspect, the development of new therapies for wound healing requires immediate attention. Plant-derived products have presented protective actions in wound care, and the healing activity of several active compounds has been shown (Agar et al., 2015; Neamsuvan and Bunmee, 2016; Jaric et al., 2018). Ethnopharmacological studies performed in different countries have shown that many plants from the Asteraceae family may be useful as sources of healing agents and therefore aid in the treatment of different types of wounds. As such, their pharmacological potential has been explored in an attempt to develop novel and more efficient therapies to accelerate healing and diminish the loss of function of tissues in the wounded area (Agar et al., 2015; Neamsuvan and Bunmee, 2016; Jaric et al., 2018). This article discusses the scientific evidences supporting the use of Asteraceae plants and their derived compounds as healing therapies. The main focus was given to plants with ethnopharmacological relevance, especially to the mechanism of action of their isolated compounds and clinical trials assessing their efficacy.
Wound healing consists of a coordinated cascade of cellular and biochemical events that interact with the tissue reconstitution (Eming et al., 2014; Lee and Jang, 2018; Nagle and Wilbraham, 2018). This process consists of three distinct and superposed phases: inflammation, proliferation, and tissue remodeling (Figure 1) (Eming et al., 2014). Inflammation occurs soon after lesion. At this stage, a blood clot is formed to cease bleeding and also to make a viable matrix rich in growth factors and chemokines, which in turn contribute to the migration of leukocytes and stromal cells (Rousselle et al., 2018). After 24 h, neutrophils appear at the margins of the wounds, and the process of sterilization and waste degradation begin. This first stage of the healing process is normally completed within 3 days following surgical or acute wounds (Gurtner et al., 2008; Shaw and Martin, 2009).
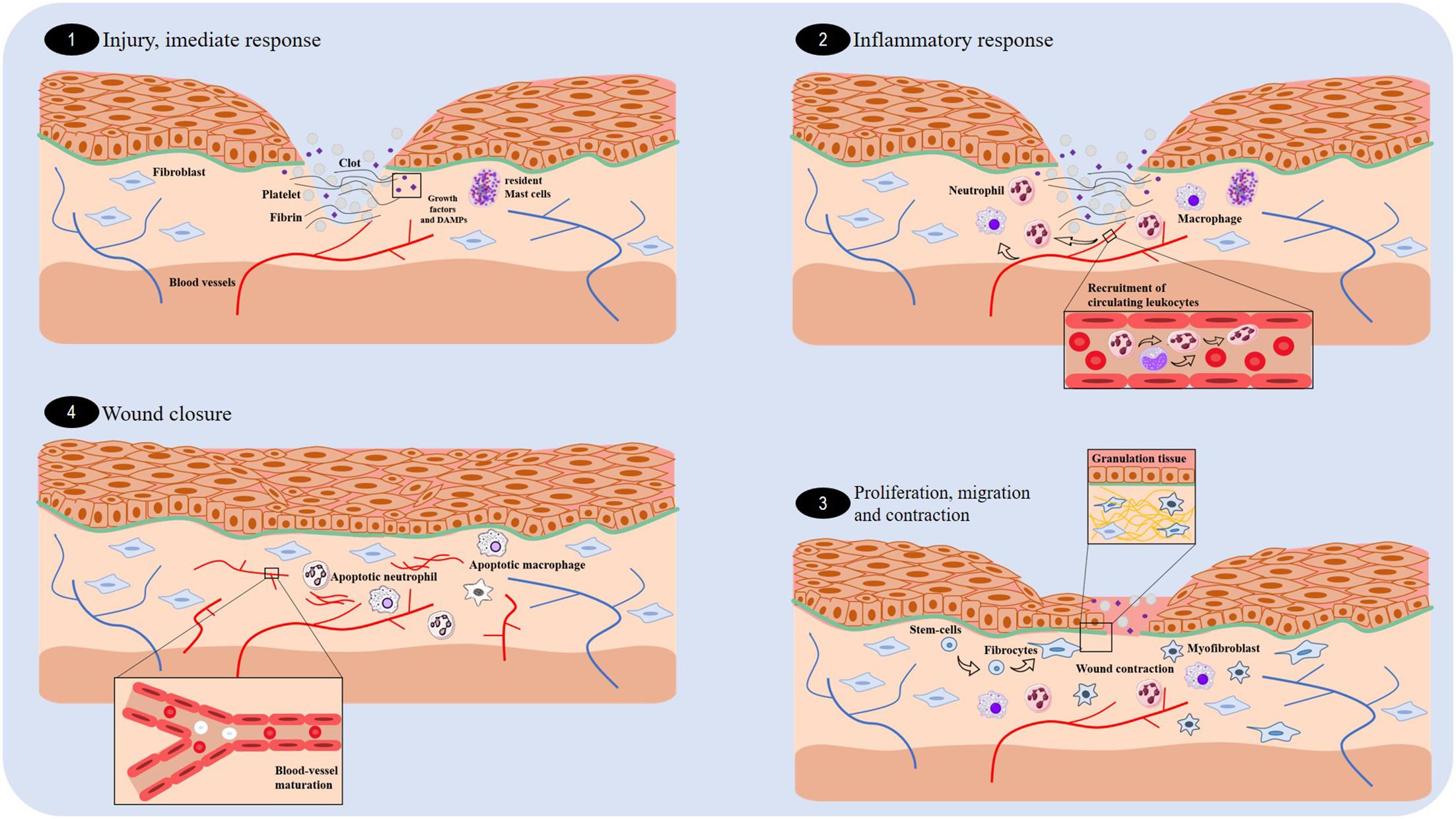
FIGURE 1. Overview of the essential stages of wound healing. There are four fundamental stages involved in wound healing: the immediate response (1), inflammation (2), proliferation (3), and wound closure (4).
Proliferation is the phase responsible for the wound closure, occurring 4 days following a wound. It involves re-epithelization (movement of epithelial cells), formation of granulation tissue (responsible for filling the injured tissue), and angiogenesis (Kanji and Das, 2017; Zomer and Trentin, 2018). Fibroblasts produce the new extracellular matrix necessary for the cell growth, whereas the new blood vessels carry oxygen and the nutrients necessary for the local cellular metabolism (Shaw and Martin, 2009; Rousselle et al., 2018). Two weeks after the lesion, there is a vasculature regression, and the granulation tissue is converted into an avascular scar without inflammation, which is covered by intact epithelium, as a result of collagen deposition (Broughton et al., 2006; Eming et al., 2014). Scar contraction occurs in large lesions due to the activity of myofibroblasts—fibroblast-like cells that have the contractile ability of smooth muscle cells (Wernick and Stawicki, 2018; Zomer and Trentin, 2018).
Finally, the remodeling phase begins approximately 3 weeks after the lesion. This phase is characterized by a random deposition of collagen, and then, metalloproteinases (produced by macrophages, neutrophils, fibroblasts, and epithelial cells) regulate the degradation and deposition of extracellular matrix, which are essential for wound re-epithelization (Gurtner et al., 2008; Caley et al., 2015; Kanji and Das, 2017; Rousselle et al., 2018; Zomer and Trentin, 2018). Consequently, larger collagen fibers are formed and organized according to the direction of the adjacent connective tissue. At the end of this stage, there is a limited regeneration of the skin attachments, such as hair follicles and glands, and a pale-colored scar with up to 80% of the original tensile strength present (Broughton et al., 2006; Gurtner et al., 2008; Eming et al., 2014).
Plants have been used for medicinal purposes for many years as shown in previous studies (Abd Rani et al., 2018; Ricardo et al., 2018; Tiwari et al., 2018). In this context, plants from the Asteraceae family are well known for their ethnopharmacological importance for many communities (Rodriguez-Chavez et al., 2017; Tewari et al., 2017; Saleh and Van Staden, 2018), and this family is widely distributed and is considered to be the largest family of flowering plants in the world (Gao et al., 2010). Due to their distribution and ethnopharmacological importance, several plant-derived products from this family have been studied, with some of their pharmacological activities already identified. These include anti-inflammatory (George Kallivalappil and Kuttan, 2017), antimicrobial (Ghaderi and Sonboli, 2018), antioxidant (Babota et al., 2018), anti-protozoa (Garcia et al., 2017), and healing activities (Ozbilgin et al., 2018). Some species [such as Calendula officinalis L., Achillea millefolium L., Neurolaena lobata (L.) R.Br. ex Cass.] have been specially described in the literature due to their therapeutic potential for the treatment of wounds (Parente et al., 2012; Nayak et al., 2014). Their efficacy has been suggested to be related to their ability to promote the proliferation of keratinocytes and thus the remodeling of the extracellular matrix (Speroni et al., 2002; Rosa Ados et al., 2014). Hence, the therapeutic use of these plants will now be discussed. The most relevant studies (i.e., those that provide insights into the mechanism of action) are summarized in Table 1.
Blumea balsamifera (L.) DC. is a plant used in the traditional medicine of several Asiatic countries, where it is popularly known as Ainaxiang (De Boer and Cotingting, 2014; Ong and Kim, 2014; Sujarwo et al., 2015). Its leaves are rich in volatile compounds such as L-borneol (major compound), terpenoids, fatty acids, phenols, alcohols, aldehydes, ethers, ketones, pyridines, furans, and alkanes (Pang et al., 2014a), which may contribute to the healing properties of B. balsamifera. Indeed, the topical application of the volatile oil obtained from the leaves of B. balsamifera in wounded Kun-Ming mice enhanced angiogenesis and collagen deposition, and additionally induced epithelial deposition and formation of granular tissue. This effect on the proliferation phase of healing was suggested to be associated with the increased production of the neuropeptide substance P (Pang et al., 2014b). The volatile oil also accelerated the healing of Sprague-Dawley rats with burn injuries by triggering the release of growth factors in the tissue and decreasing the plasma concentrations of pro-inflammatory cytokines (TNFα and IL-1) (Fan et al., 2015).
Pang et al. (2017) in their study evaluated the healing actions of a flavonoid-rich leaf extract from B. balsamifera on skin wounds of Sprague-Dawley rats. This extract caused wound contraction, capillary regeneration, collagen deposition, and re-epithelization 7 days following treatment. These alterations were associated with the enhanced expression of vascular endothelial growth factor, transforming growth factor-β1, and CD68 antigen in rat wound tissues. Different compounds were detected in the extract including 16 flavonoid aglucons, 5 flavonoid glycosides, 5 chlorogenic acid analogs, and 1 coumarin (Pang et al., 2017).
Silybum marianum (L.) Gaertn. is another plant of ethnopharmacological importance in wound healing (Hudaib et al., 2008; Aziz et al., 2016). Evidences have shown that silymarin, an extract from its seeds, increases epithelization and decreases inflammation in albino rats subjected to the excision wound (Sharifi et al., 2012). It was also shown that this extract protects human fibroblasts from lipopolysaccharide (LPS)-induced oxidative stress (Sharifi et al., 2013). Similarly, the silymarin-derived compound silibinin (flavonoid) accelerated the closure of skin wounds in rats by upregulating the expression of stromelysin 1 hydroxyproline, glycosaminoglycans, and collagen (important constituents of extracellular matrix) (Tabandeh et al., 2013). This compound was also found to reduce the toxic effects caused by nitrogen mustard in the mouse skin (Balszuweit et al., 2013). This action was associated with an inhibition of oxidative stress and inflammation (Jain et al., 2015). As shown in another study, the repeated topical application (14 days treatment) of a silibinin-based gel resulted in an efficient wound healing strategy, by acting on tissue re-epithelization, collagen production, and deposition of granulation tissue (as shown in Figure 2) (Samanta et al., 2016).
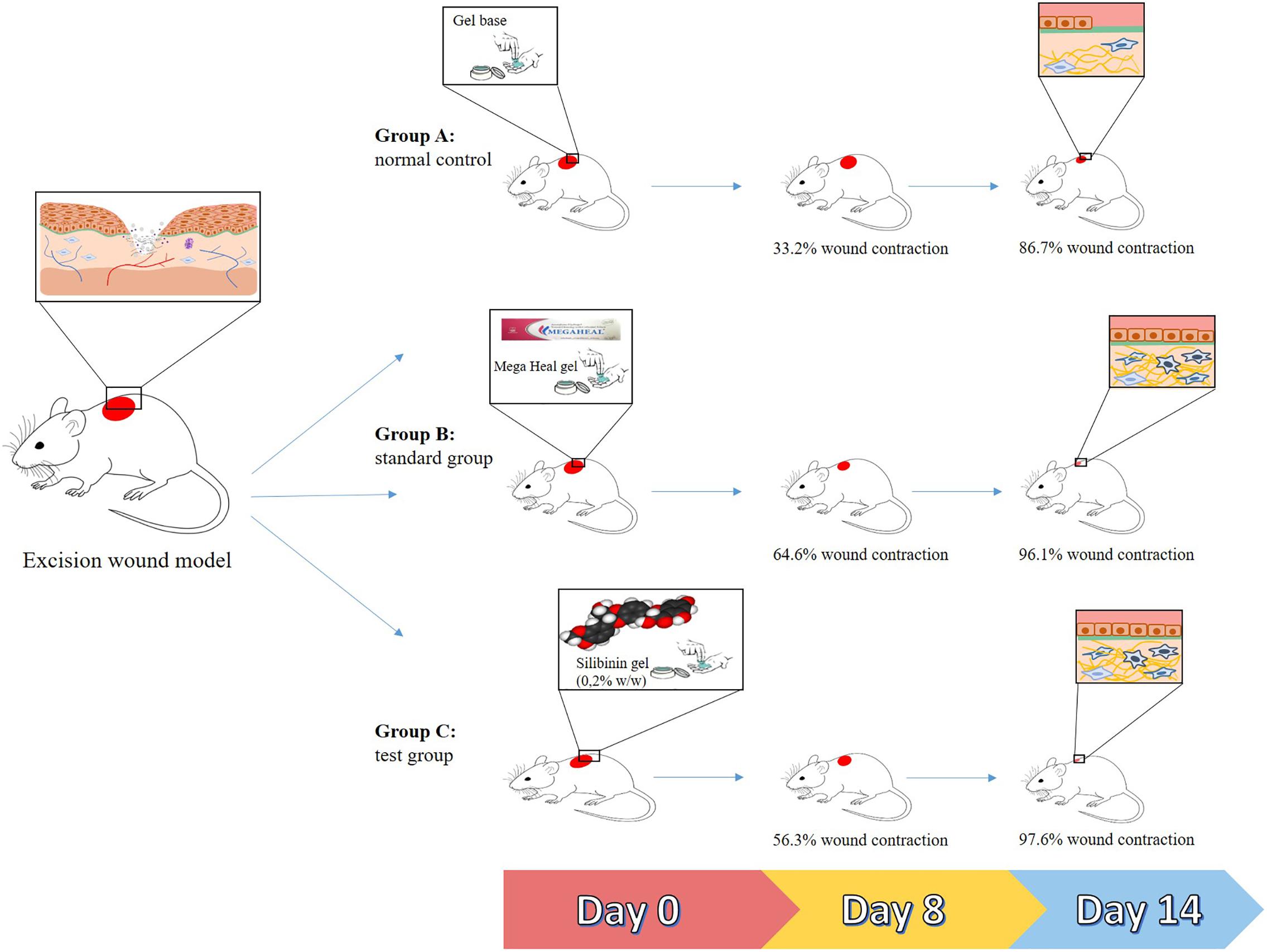
FIGURE 2. Summarized representation of the in vivo actions of silibinin (from Silybum marianum) in wound healing. Group A: mice treated with a gel base (controls). Group B: mice treated with a commercially available gel (Mega Heal gel; Aristo Pharmaceuticals Ltd.; positive control). Group C: mice treated with a silibinin-based gel (0.2% w/w). After 14 days of treatment, the mean sizes of the wounds from mice of groups B and C were smaller than those of group A.
Calendula officinalis L. (or calendula) is a species used in the treatment of wounds in Europe since 13th century, and a large number of cosmetic and personal care products have been developed using its compounds (Parente et al., 2012). Its use as a healing agent is supported by different in vivo and in vitro studies. In one of these studies, the ethanolic extract obtained from C. officinalis flowers, and its dichloromethane and hexanic fractions were found to increase angiogenesis in both chorioallantoic membranes (CAMs) of embryonated eggs and rat with skin wounds. This effect on vessels was related to discrete infiltration of inflammatory cells and increased the collagen deposition (Parente et al., 2011, 2012). Recently, a cream containing the glycolic extract from C. officinalis flowers was found to enhance collagen organization in the initial phase of the healing process, and this was correlated with an increase in the concentrations of hydroxyproline, an indicator of the collagen content in the tissue (Aro et al., 2015).
In vitro studies were performed to provide more insights into the mechanisms of action involved in the healing action of a product based on the hydroalcoholic extract of C. officinalis (approved by the European Medicines Agency (Nicolaus et al., 2017). C. officinalis tincture was able to increase the proliferation and the migration of fibroblasts in a PI3K-dependent pathway, with activation of FAK and Akt. Flavonol glycosides were the major compounds detected in this extract (Dinda et al., 2015). Human keratinocytes treated with C. officinalis flower extracts (n-hexanic and ethanolic extracts) exhibited the increased expression of IL-8 and activation of the transcription factor NF-κB, in addition to the enhanced migration ability. The ethanolic extract of this plant was also able to inhibit collagenase activity in human dermal fibroblasts. These effects were attributed to the presence of flavonoids and saponins in the extract (Nicolaus et al., 2017).
The hydroalcoholic extract and its aqueous fraction of C. officinalis (rich in rutin and quercetin-3-O-glucoside) exhibited significant in vitro effects on the proliferation and migration of human dermal fibroblasts, in addition to the increased expression of connective tissue growth factor and α-smooth muscle actin, proteins that favor healing by activating cell proliferation, migration, adhesion, and tissue repair. The topical application of hydroalcoholic extract or its aqueous fraction of C. officinalis on excisional wounds of BALB/c mice accelerated wound contraction by increasing the tissue levels of connective tissue growth factor and α-smooth muscle actin (Dinda et al., 2016).
Particularly noteworthy was the commercially available product containing the hydroglycolic extract of C. officinalis (Plenusdermax) as it promoted wound epithelization, thus decreasing the healing time in patients with venous leg ulcers (Buzzi et al., 2016). Another study showed that the use of low-intensity laser therapy associated with C. officinalis oil causes analgesia, in addition to the reduction of lesions in foot ulcers of diabetic patients (Carvalho et al., 2016). Interestingly, C. officinalis has been considered as an alternative resource by national health surveillance agencies such as the one in Brazil.
Achillea genus has been widely used in the traditional medicine as a source of healing products (Mohammadhosseini et al., 2017; Jaric et al., 2018). Achillea millefolium L. is the most studied species among others. A. millefolium is a herb, commonly known as yarrow, which is indigenous to the Northern Hemisphere of Europe and Asia, and it has been popularly used for over 3,000 years (Ali et al., 2017). Its pharmacological properties include anti-inflammatory, antioxidant, antifungal, and healing actions (Karamenderes and Apaydin, 2003; Fierascu et al., 2015), which have been attributed to several chemical constituents such as sesquiterpenes and phenolic compounds (Fierascu et al., 2015).
An in vitro study, carried out in human skin fibroblasts, showed that the hydroalcoholic extract from the aerial parts of A. millefolium induces cell proliferation (Ghobadian et al., 2015). More recently, oil extracts from aerial parts of A. millefolium were shown to reduce skin irritation in healthy individuals. Two approaches were applied to obtain the extracts: (i) the aerial parts of A. millefolium were macerated with ethanol, followed by olive oil (E1) or sunflower oil (E2) and (ii) the maceration of plant material occurred only in the presence of olive oil (E3) or sunflower oil (E4). This double-blind study enrolled 23 volunteers who had 8% sodium lauryl sulfate applied to their skin to cause irritation. After 24 h, these subjects received a topical application of the oil extracts for 7 days. All oil formulations were able to stabilize the skin pH and to increase hydration while reducing erythema. However, E1 and E2 exhibited the highest anti-inflammatory action, whereas E3 and E4 promoted highest levels of skin hydration. The presence of compounds with reported anti-inflammatory actions in both E1 and E2 (luteolin, apigenin and their glycosides, caffeic, and chlorogenic acids as well as chlorophyll derivatives) may explain these results (Tadic et al., 2017).
Evidences have also suggested a healing potential of Achillea asiatica Serg. (synonym of A. millefolium var. manshurica Kitam), popularly known as Mongolian yarrow. In vitro incubation of the ethanolic extract from the aerial parts of this plant with Hs68 fibroblasts triggered the production of collagen by these cells; this involved the activation of transforming growth factor-β-mediated pathways. The same extract also enhanced the differentiation and motility of keratinocytes through the upregulation of β-catenin, Akt, and keratinocyte differentiation markers. Compounds such as chlorogenic acid, apigenin-7-O-glucoside, and schaftoside were identified and associated with the healing effects of A. asiatica ethanolic extract (Dorjsembe et al., 2017). A comparative analysis of the in vitro healing potential of extracts obtained from A. coarctata, A. kotschyi, and A. lycaonica was performed in cultured NIH-3T3 fibroblasts. A. kotschyi extract was the most effective, presenting chlorogenic acid, hyperoside, apigenin, hesperidin, rutin, kaempferol, and luteolin in its composition (Jain et al., 2015).
Plants from the Pluchea genus have been used as healing agents by different communities (Gridling et al., 2009; Schmidt et al., 2009; Ab Rahman et al., 2014). Pluchea indica (L.) Less. healing actions have been attributed to its antioxidant and anti-inflammatory properties (Buapool et al., 2013). These evidences have been further supported by recent studies showing that nanoparticles containing P. indica leaf ethanolic extract increase the migration of oral mucosal cells in vitro. This preparation presented characteristics (size, charge, polydispersity index, increased colloidal stability) that support its use as an oral spray (Buranasukhon et al., 2017). Furthermore, the size of Leishmania amazonensis–induced cutaneous lesions in BALB/c mice was found to be reduced by the intralesional treatment with an essential oil obtained from the leaves of Pluchea carolinensis (Jacq.) D.Don. The main component of this essential oil was selin-11-en-4α-ol (Garcia et al., 2017).
Another plant with the ethnopharmacological relevance is Artemisia princeps Pamp., which is traditionally used to treat inflammatory-related diseases and had its properties scientifically proven in various in vitro and in vivo models (Min et al., 2009; Chen et al., 2016). Jaceosidin is extracted from this plant, which has also been identified as the main constituent of other plants from the Artemisia genus such as A. argyi with ethnomedicinal use as a healing agent (Li et al., 2018). It has the ability to inhibit the production of pro-inflammatory mediators such as TNF-α, IL-1β, and PGE2 (Min et al., 2009). In vitro, this flavonoid induces the proliferation, migration, and differentiation of human umbilical vascular endothelial cells (Figure 3) (Lee et al., 2014). It also stimulates the formation of microvessels in rat aortic tissue, and this effect has been associated with the activation of VEGFR2/FAK/PI3K/AKT/NF-κB signaling pathways (Lee et al., 2014). Overall, all these studies suggest Jaceosidin as an interesting pro-angiogenic compound.
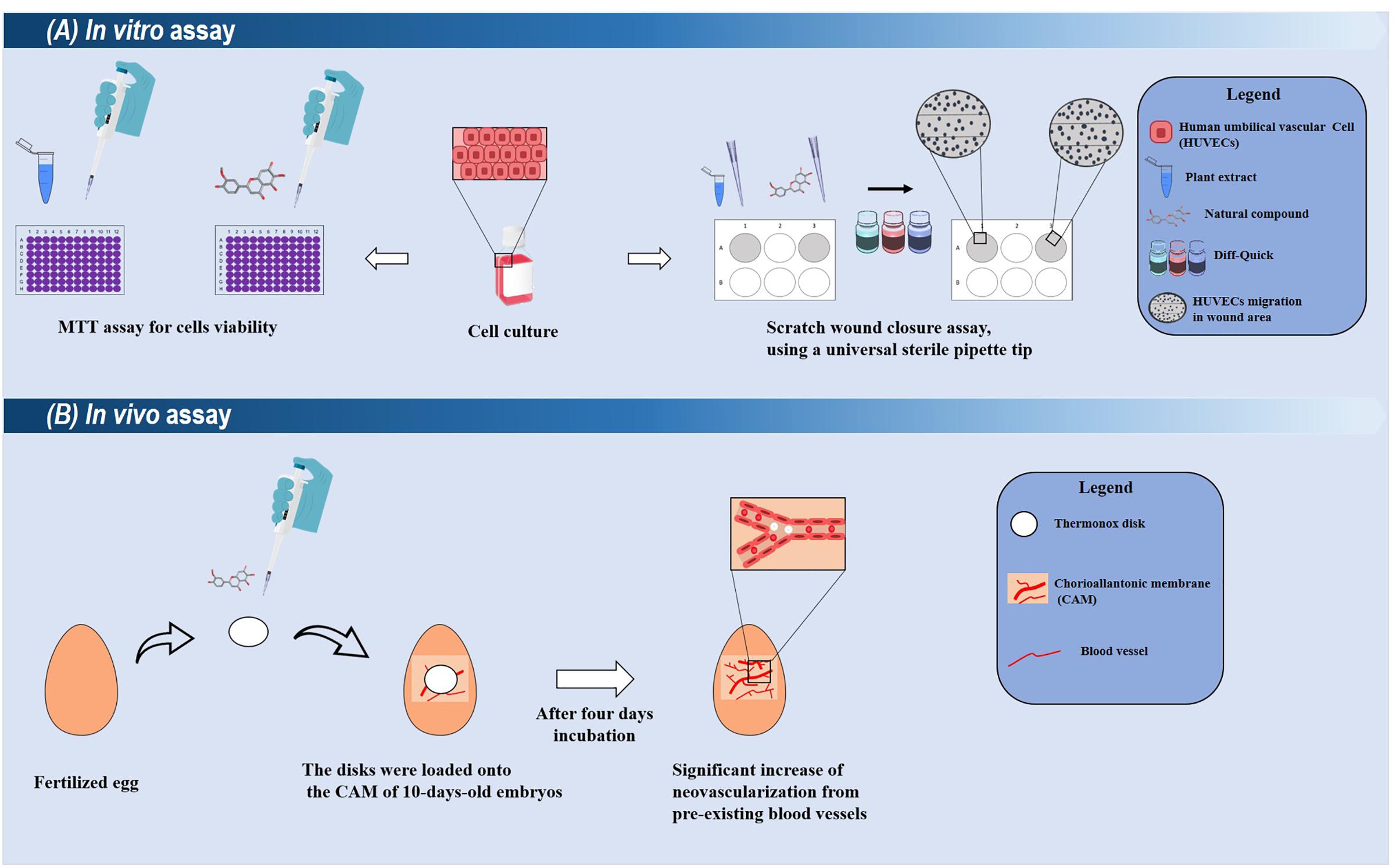
FIGURE 3. Summarized representations of in vitro and in vivo techniques used for evaluating the healing potentials of plant-derived compounds. (A) In in vitro assays, the cytotoxic and proliferative effects of a compound/extract can be evaluated toward different cell cultures (such as human umbilical vascular cells). Their proliferative effects can be analyzed through the scratch wound closure assay. Compounds with promising results can be selected for ex vivo and in vivo models. (B) A simple in vivo model using chick Chorioallantoic membrane (CAM). In this assay, the damaged CAM is treated with the selected compound (applied into a disk), and after few days, the neovascularization is measured.
Isosecotanapartholide, isolated from A. princeps, has also exhibited in vitro proliferative properties. Isosecotanapartholide (and the extract from A. princeps) inhibited the production of IL-33 by human keratinocytes (HaCaT), and this was associated with reduced levels of signaling molecules such as signal transducer and activator of transcription-1 (STAT-1), thymus and activation-regulated chemokine (TARC/CCL17), and adhesion molecule-1 (Ali et al., 2017).
Ageratina pichinchensis (Kunth) R.M. King and H. Rob. is a plant with ethnopharmacological relevance in Mexico, and its several pharmacological activities have been confirmed in murine models and clinical trials in vivo, such as onychomycosis (Romero-Cerecero et al., 2009), interdigital tinea pedis (Romero-Cerecero et al., 2012b), stomatitis (Romero-Cerecero et al., 2015b), and vulvovaginal candidiasis (Romero-Cerecero et al., 2017). Despite its use for wound healing, the first study showed that the daily topical application of an aqueous extract from the aerial parts of A. pichinchensis heals wounds in rats without inducing skin irritation (Romero-Cerecero et al., 2011). Based on these results, a bio-guided purification revealed that 7-O-(β-D-glucopyranosyl)-galactin is the major compound associated with the effects of A. pichinchensis in cell proliferation (Romero-Cerecero et al., 2013). Later, two extracts (aqueous and hexane) with standardized concentrations of 7-O-(β-D-glucopyranosyl)-galactin were shown to promote the healing of skin lesions in rats with streptozotocin-induced diabetes (Romero-Cerecero et al., 2014).
The healing properties of this plant were also assessed in human clinical trials. For instance, the effectiveness of a standardized extract of A. pichinchensis was proved to heal chronic venous leg ulcers (Romero-Cerecero et al., 2012a). In another study, a cream containing an extract of A. pichinchensis was topically used by diabetic patients with foot ulcer; the results showed this treatment decreases healing time and lesion size although no significant differences were observed. The authors attributed this fact to the sample size, but they concluded that a large clinical trial could prove the action of A. pichinchensis in this type of wound (Romero-Cerecero et al., 2015a).
Plants from the Achyrocline genus play an important role in traditional medicine and are commonly found in Latin American countries (Retta et al., 2012; Alerico et al., 2015; Bolson et al., 2015). Ethnobotanical surveys performed in the Brazilian state of Rio Grande do Sul indicated that Achyrocline satureioides (Lam.) DC. is widely used for healing. It was shown that the ethanolic extracts from the aerial parts of this plant induce the proliferation of HaCaT keratinocytes (Alerico et al., 2015). The healing activity of an essential oil of A. satureioides inflorescences incorporated into hydroxyethyl cellulose films was also demonstrated in Wistar rats (Yamane et al., 2016).
A recent study evaluated the use of the extracts from inflorescences of Achyrocline alata (Kunth) DC. and A. satureioides for the repair of cutaneous wounds in mice. Both extracts showed positive results, but only A. alata accelerated wound closure, presenting a higher probability of healing in a shorter time of treatment. The authors attributed this effect to higher concentrations of phenolic compounds in A. alata. Moreover, it was possible to observe that animals treated with A. alata extract present less mast cells at the site of inflammation, better re-epithelization and granulation of the injured tissue, and reduction of the initial inflammatory reaction (Pereira et al., 2017).
Acmella oleracea (L.) Spreng. (jambu) is a native plant from Brazil that is used to treat skin and gastrointestinal disorders and also as a female aphrodisiac (Neamsuvan and Bunmee, 2016; Neamsuvan and Ruangrit, 2017; Da Rocha et al., 2018). A polysaccharide extracted from A. oleracea, named rhamnogalacturonan, was found to inhibit ethanol-induced gastric ulcers in rats (Nascimento et al., 2013). This effect was better elucidated later, as this compound was shown to protect against both acute (intraperitoneal treatment) and chronic lesions (oral administration) induced by ethanol (Maria-Ferreira et al., 2014). Rhamnogalacturonan also enhanced the gastric cell proliferation and mucus content while decreasing inflammation and oxidative stress in the stomach (Maria-Ferreira et al., 2014).
Another study reported the development of hydroxyethyl cellulose (HCE) films containing an ethanolic extract from the aerial parts of A. oleracea and an essential oil obtained from the inflorescences of Achyrocline satureioides. The HCE films containing these two plant materials demonstrated wound healing activity in Wistar rats, an effect that was associated with increased levels of collagen deposition in wounds. α-Humulene and spilanthol were detected in the essential oil of A. satureioides and the extract of A. oleracea, respectively (Yamane et al., 2016).
The genus Artemisia plays an important role in the traditional medicine (Shenkman and Krivenkov, 1986; Kadioglu et al., 2017; Ota and Ulrih, 2017) and in the development of anti-inflammatory and anticancer drugs (Kadioglu et al., 2017; Coricello et al., 2018; Konstat-Korzenny et al., 2018). The pharmacological potentials of these plants have also been evaluated in healing models. For example, the extract from Artemisia asiatica (Pamp.) Nakai ex Kitam was efficient against gastric injuries induced by ethanol (Park et al., 2008), while Artemisia argyi H.Lév. and Vaniot healed oral ulcers in rats (Yin et al., 2017). Another study showed that the essential oil from Artemisia montana (Nakai) Pamp improves the proliferation of human keratinocytes and enhances their capacity to produce type IV collagen. These effects were associated with the phosphorylation of Akt and ERK 1/2. In vivo assays showed that the essential oil from A. montana promotes the healing of rats with dorsal wounds (Yoon et al., 2014). The aqueous extract from Artemisia campestris L. also reduced the number of inflammatory cells in the wounded area and presented a positive effect in the progress of wound healing (Ghlissi et al., 2016).
This review described the aspects involved in the healing properties of some Asteraceae plants. In fact, several plants from this family have ethnopharmacological relevance for the treatment of wounds due to their direct effects on healing and in some cases due to their anti-inflammatory actions. The discussed studies provided the scientific basis for the ethnopharmacological usage of these plants, since different products derived from them (isolated compounds, oils, and extracts) are effective in the models of healing in vitro and in vivo. Silibinin (from S. marianum) and jaceosidin (from A. princeps) were identified as promising compounds for the development of healing agents. Furthermore, the results obtained in clinical trials with A. pichinchensis and C. officinalis are exciting and highlight their importance for the treatment of wounds. These evidences suggest that Asteraceae plants are important sources for the development of new efficient drugs for healing.
AC, RD, MS, CF, AZ, MG, EF, and LdS contributed to conception and design and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
The authors would like to express their gratitude to Fundação de Amparo à Pesquisa e Desenvolvimento Científico do Maranhão (FAPEMA; UNIVERSAL-00998/16 and COOPI-02860/16), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; 3325/2013), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 309046/2016-5) for research funding. AC (undergraduate student), RD and MS (M.Sc. students), and CF (Ph.D. student) receive studentships from FAPEMA.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ab Rahman, M. R., Abdul Razak, F., and Mohd Bakri, M. (2014). Evaluation of wound closure activity of Nigella sativa, Melastoma malabathricum, Pluchea indica, and Piper sarmentosum extracts on scratched monolayer of human gingival fibroblasts. Evid. Based Complement. Alternat. Med. 2014:190342. doi: 10.1155/2014/190342
Abd Rani, N. Z., Husain, K., and Kumolosasi, E. (2018). Moringa genus: a review of phytochemistry and pharmacology. Front. Pharmacol. 9:108. doi: 10.3389/fphar.2018.00108
Agar, O. T., Dikmen, M., Ozturk, N., Yilmaz, M. A., Temel, H., and Turkmenoglu, F. P. (2015). Comparative studies on phenolic composition, antioxidant, wound healing and cytotoxic activities of selected Achillea L. species growing in turkey. Molecules 20, 17976–18000. doi: 10.3390/molecules201017976
Alerico, G. C., Beckenkamp, A., Vignoli-Silva, M., Buffon, A., and Von Poser, G. L. (2015). Proliferative effect of plants used for wound healing in Rio Grande do Sul state, Brazil. J. Ethnopharmacol. 176, 305–310. doi: 10.1016/j.jep.2015.11.001
Ali, S. I., Gopalakrishnan, B., and Venkatesalu, V. (2017). Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: a review. Phytother. Res. 31, 1140–1161. doi: 10.1002/ptr.5840
Aro, A. A., Perez, M. O., Vieira, C. P., Esquisatto, M. A., Rodrigues, R. A., Gomes, L., et al. (2015). Effect of Calendula officinalis cream on achilles tendon healing. Anat. Rec. 298, 428–435. doi: 10.1002/ar.23057
Arslantas, M. K., Arslantas, R., and Tozan, E. N. (2015). Effects of systemic erythropoietin on ischemic wound healing in rats. Ostomy Wound Manage. 61, 28–33.
Aziz, M. A., Adnan, M., Begum, S., Azizullah, A., Nazir, R., and Iram, S. (2016). A review on the elemental contents of Pakistani medicinal plants: implications for folk medicines. J. Ethnopharmacol. 188, 177–192. doi: 10.1016/j.jep.2016.05.011
Babota, M., Mocan, A., Vlase, L., Crisan, O., Ielciu, I., Gheldiu, A. M., et al. (2018). Phytochemical analysis, antioxidant and antimicrobial activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 23:E409. doi: 10.3390/molecules23020409
Balszuweit, F., John, H., Schmidt, A., Kehe, K., Thiermann, H., and Steinritz, D. (2013). Silibinin as a potential therapeutic for sulfur mustard injuries. Chem. Biol. Interact. 206, 496–504. doi: 10.1016/j.cbi.2013.06.010
Bolson, M., Hefler, S. M., Dall’oglio Chaves, E. I., Gasparotto Junior, A., and Cardozo Junior, E. L. (2015). Ethno-medicinal study of plants used for treatment of human ailments, with residents of the surrounding region of forest fragments of Parana, Brazil. J. Ethnopharmacol. 161, 1–10. doi: 10.1016/j.jep.2014.11.045
Broughton, G. N., Janis, J. E., and Attinger, C. E. (2006). Wound healing: an overview. Plast. Reconstr. Surg. 117(Suppl. 7), 1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9
Buapool, D., Mongkol, N., Chantimal, J., Roytrakul, S., Srisook, E., and Srisook, K. (2013). Molecular mechanism of anti-inflammatory activity of Pluchea indica leaves in macrophages RAW 264.7 and its action in animal models ofinflammation. J. Ethnopharmacol. 146, 495–504. doi: 10.1016/j.jep.2013.01.014
Buranasukhon, W., Athikomkulchai, S., Tadtong, S., and Chittasupho, C. (2017). Wound healing activity of Pluchea indica leaf extract in oral mucosal cell line and oral spray formulation containing nanoparticles of the extract. Pharm. Biol. 55, 1767–1774. doi: 10.1080/13880209.2017.1326511
Buzzi, M., De Freitas, F., and De Barros Winter, M. (2016). Therapeutic effectiveness of a Calendula officinalis extract in venous leg ulcer healing. J. Wound Care 25, 732–739. doi: 10.12968/jowc.2016.25.12.732
Caley, M. P., Martins, V. L., and O’toole, E. A. (2015). Metalloproteinases and wound healing. Adv. Wound Care 4, 225–234. doi: 10.1089/wound.2014.0581
Carvalho, A. F., Feitosa, M. C., Coelho, N. P., Rebêlo, V. C., Castro, J. G., Sousa, P. R., et al. (2016). Low-level laser therapy and Calendula officinalis in repairing diabetic foot ulcers. Rev. Esc. Enferm. USP 50, 628–634. doi: 10.1590/S0080-623420160000500013
Chen, C. C., Lin, M. W., Liang, C. J., and Wang, S. H. (2016). The anti-inflammatory effects and mechanisms of eupafolin in lipopolysaccharide-induced inflammatory responses in Raw264.7 macrophages. PLoS One 11:e0158662. doi: 10.1371/journal.pone.0158662
Coricello, A., El-Magboub, A., Luna, M., Ferrario, A., Haworth, I. S., Gomer, C. J., et al. (2018). Rational drug design and synthesis of new alpha-Santonin derivatives as potential COX-2 inhibitors. Bioorg. Med. Chem. Lett. 28, 993–996. doi: 10.1016/j.bmcl.2018.02.036
Da Rocha, C. F., De Medeiros Souza Lima, Y., Carvalho, H. O., Pinto, R. C., Ferreira, I. M., Castro, A. N., et al. (2018). Action of the hydroethanolic extract of the flowers of Acmella oleracea (L.) R. K. Jansen on the reproductive performance of Wistar females rats: a popular female aphrodisiac from the Amazon. J. Ethnopharmacol. 214, 301–308. doi: 10.1016/j.jep.2017.12.024
De Boer, H. J., and Cotingting, C. (2014). Medicinal plants for women’s healthcare in southeast Asia: a meta-analysis of their traditional use, chemical constituents, and pharmacology. J. Ethnopharmacol. 151, 747–767. doi: 10.1016/j.jep.2013.11.030
Dinda, M., Dasgupta, U., Singh, N., Bhattacharyya, D., and Karmakar, P. (2015). PI3K-mediated proliferation of fibroblasts by Calendula officinalis tincture: implication in wound healing. Phytother. Res. 29, 607–616. doi: 10.1002/ptr.5293
Dinda, M., Mazumdar, S., Das, S., Ganguly, D., Dasgupta, U. B., Dutta, A., et al. (2016). The water fraction of Calendula officinalis hydroethanol extract stimulates in vitro and in vivo proliferation of dermal fibroblasts in wound healing. Phytother. Res. 30, 1696–1707. doi: 10.1002/ptr.5678
Dorjsembe, B., Lee, H. J., Kim, M., Dulamjav, B., Jigjid, T., and Nho, C. W. (2017). Achillea asiatica extract and its active compounds induce cutaneous wound healing. J. Ethnopharmacol. 206, 306–314. doi: 10.1016/j.jep.2017.06.006
Eming, S. A., Martin, P., and Tomic-Canic, M. (2014). Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6:265sr6. doi: 10.1126/scitranslmed.3009337
Fan, Z. W., Pang, Y. X., Wang, K., Yu, F. L., Wang, D., Yang, Q., et al. (2015). Blumea balsamifera oil for the acceleration of healing of burn injuries. Molecules 20, 17166–17179. doi: 10.3390/molecules200917166
Fierascu, I., Ungureanu, C., Avramescu, S., Claudiu Fierascu, R., Ortan, A., Soare, L. C., et al. (2015). In vitro antioxidant and antifungal properties of Achillea millefolium L. Rom. Biotechnol. Lett. 20, 10626–10636.
Frykberg, R. G., and Banks, J. (2015). Challenges in the treatment of chronic wounds. Adv. Wound Care 4, 560–582. doi: 10.1089/wound.2015.0635
Gao, T., Yao, H., Song, J., Zhu, Y., Liu, C., and Chen, S. (2010). Evaluating the feasibility of using candidate DNA barcodes in discriminating species of the large Asteraceae family. BMC Evol. Biol. 10:324. doi: 10.1186/1471-2148-10-324
Garcia, M., Scull, R., Satyal, P., Setzer, W. N., and Monzote, L. (2017). Chemical characterization, antileishmanial activity, and cytotoxicity effects of the essential oil from leaves of Pluchea carolinensis (Jacq.) G. Don. (Asteraceae). Phytother. Res. 31, 1419–1426. doi: 10.1002/ptr.5869
George Kallivalappil, G., and Kuttan, G. (2017). Evaluation of the anti-inflammatory and urotoxicity ameliorative effects of gamma-humulene containing active fraction of Emilia sonchifolia (L.) DC. Inflammopharmacology doi: 10.1007/s10787-017-0423-3 [Epub ahead of print].
Ghaderi, A., and Sonboli, A. (2018). Chemical composition and antimicrobial activity of the essential oil of Tanacetum walteri (Anthemideae-Asteraceae) from Iran. Nat. Prod. Res. doi: 10.1080/14786419.2018.1434640 [Epub ahead of print].
Ghlissi, Z., Sayari, N., Kallel, R., Bougatef, A., and Sahnoun, Z. (2016). Antioxidant, antibacterial, anti-inflammatory and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed. Pharmacother. 84, 115–122. doi: 10.1016/j.biopha.2016.09.018
Ghobadian, Z., Ahmadi, M. R., Rezazadeh, L., Hosseini, E., Kokhazadeh, T., and Ghavam, S. (2015). In vitro evaluation of Achillea Millefolium on the production and stimulation of human skin fibroblast cells (HFS-PI-16). Med. Arch. 69, 212–217. doi: 10.5455/medarh.2015.69.212-217
Gridling, M., Stark, N., Madlener, S., Lackner, A., Popescu, R., Benedek, B., et al. (2009). In vitro anti-cancer activity of two ethno-pharmacological healing plants from Guatemala Pluchea odorata and Phlebodium decumanum. Int. J. Oncol. 34, 1117–1128.
Guest, J. F., Ayoub, N., Mcilwraith, T., Uchegbu, I., Gerrish, A., Weidlich, D., et al. (2017). Health economic burden that different wound types impose on the UK’s National Health Service. Int. Wound J. 14, 322–330. doi: 10.1111/iwj.12603
Gurtner, G. C., Werner, S., Barrandon, Y., and Longaker, M. T. (2008). Wound repair and regeneration. Nature 453, 314–321. doi: 10.1038/nature07039
Hong, J. P., and Park, S. W. (2014). The combined effect of recombinant human epidermal growth factor and erythropoietin on full-thickness wound healing in diabetic rat model. Int. Wound J. 11, 373–378. doi: 10.1111/j.1742-481X.2012.01100.x
Hou, J., and Kim, S. (2018). Possible role of ginsenoside Rb1 in skin wound healing via regulating senescent skin dermal fibroblast. Biochem. Biophys. Res. Commun. 499, 381–388. doi: 10.1016/j.bbrc.2018.03.170
Hudaib, M., Mohammad, M., Bustanji, Y., Tayyem, R., Yousef, M., Abuirjeie, M., et al. (2008). Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J. Ethnopharmacol. 120, 63–71. doi: 10.1016/j.jep.2008.07.031
Jain, A. K., Tewari-Singh, N., Inturi, S., Kumar, D., Orlicky, D. J., Agarwal, C., et al. (2015). Flavanone silibinin treatment attenuates nitrogen mustard-induced toxic effects in mouse skin. Toxicol. Appl. Pharmacol. 285, 71–78. doi: 10.1016/j.taap.2015.03.009
Jaric, S., Kostic, O., Mataruga, Z., Pavlovic, D., Pavlovic, M., Mitrovic, M., et al. (2018). Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 211, 311–328. doi: 10.1016/j.jep.2017.09.018
Jørgensen, L. B., Sørensen, J. A., Jemec, G. B., and Yderstræde, K. B. (2016). Methods to assess area and volume of wounds–a systematic review. Int. Wound J. 13, 540–553. doi: 10.1111/iwj.12472
Kadioglu, O., Chan, A., Cong Ling Qiu, A., Wong, V. K. W., Colligs, V., Wecklein, S., et al. (2017). Artemisinin derivatives target topoisomerase 1 and cause DNA damage in silico and in vitro. Front. Pharmacol. 8:711. doi: 10.3389/fphar.2017.00711
Kanji, S., and Das, H. (2017). Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Med. Inflamm. 2017:5217967. doi: 10.1155/2017/5217967
Karamenderes, C., and Apaydin, S. (2003). Antispasmodic effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bassler on the rat isolated duodenum. J. Ethnopharmacol. 84, 175–179. doi: 10.1016/S0378-8741(02)00296-9
Karthik, N., Ward, M. C., Juloori, A., Scott, J., Mesko, N., and Shah, C. (2018). Factors associated with acute and chronic wound complications in patients with soft tissue sarcoma with long-term follow-up. Am. J. Clin. Oncol. doi: 10.1097/COC.0000000000000421 [Epub ahead of print].
Konstat-Korzenny, E., Ascencio-Aragon, J. A., Niezen-Lugo, S., and Vazquez-Lopez, R. (2018). Artemisinin and its synthetic derivatives as a possible therapy for cancer. Med. Sci. 6:E19. doi: 10.3390/medsci6010019
Kulprachakarn, K., Ounjaijean, S., Wungrath, J., Mani, R., and Rerkasem, K. (2017). Micronutrients and natural compounds status and their effects on wound healing in the diabetic foot ulcer. Int. J. Low. Extrem. Wounds 16, 244–250. doi: 10.1177/1534734617737659
Lee, H. J., and Jang, Y. J. (2018). Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int. J. Mol. Sci. 19:E711. doi: 10.3390/ijms19030711
Lee, T. H., Jung, H., Park, K. H., Bang, M. H., Baek, N. I., and Kim, J. (2014). Jaceosidin, a natural flavone, promotes angiogenesis via activation of VEGFR2/FAK/PI3K/AKT/NF-kappaB signaling pathways in endothelial cells. Exp. Biol. Med. 239, 1325–1334. doi: 10.1177/1535370214533883
Li, S., Zhou, S., Yang, W., and Meng, D. (2018). Gastro-protective effect of edible plant Artemisia argyi in ethanol-induced rats via normalizing inflammatory responses and oxidative stress. J. Ethnopharmacol. 214, 207–217. doi: 10.1016/j.jep.2017.12.023
Long, M., Cai, L., Li, W., Zhang, L., Guo, S., Zhang, R., et al. (2018). DPP-4 inhibitors improve diabetic wound healing via direct and indirect promotion of epithelial-mesenchymal transition and reduction of scarring. Diabetes Metab. Res. Rev. 67, 518–531. doi: 10.2337/db17-0934
Manrique, N., Pereira, C. C., Luvizuto, E. R., Sanchez Mdel, P., Okamoto, T., Okamoto, R., et al. (2015). Hypertension modifies OPG, RANK, and RANKL expression during the dental socket bone healing process in spontaneously hypertensive rats. Clin. Oral Investig. 19, 1319–1327. doi: 10.1007/s00784-014-1369-0
Maria-Ferreira, D., Da Silva, L. M., Mendes, D. A., Cabrini Dde, A., Nascimento, A. M., Iacomini, M., et al. (2014). Rhamnogalacturonan from Acmella oleracea (L.) R. K. Jansen: gastroprotective and ulcer healing properties in rats. PLoS One 9:e84762. doi: 10.1371/journal.pone.0084762
Min, S. W., Kim, N. J., Baek, N. I., and Kim, D. H. (2009). Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J. Ethnopharmacol. 125, 497–500. doi: 10.1016/j.jep.2009.06.001
Mohammadhosseini, M., Sarker, S. D., and Akbarzadeh, A. (2017). Chemical composition of the essential oils and extracts of Achillea species and their biological activities: a review. J. Ethnopharmacol. 199, 257–315. doi: 10.1016/j.jep.2017.02.010
Morton, L. M., and Phillips, T. J. (2016). Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 74, 589–605. doi: 10.1016/j.jaad.2015.08.068
Nascimento, A. M., De Souza, L. M., Baggio, C. H., Werner, M. F., Maria-Ferreira, D., Da Silva, L. M., et al. (2013). Gastroprotective effect and structure of a rhamnogalacturonan from Acmella oleracea. Phytochemistry 85, 137–142. doi: 10.1016/j.phytochem.2012.08.024
Nayak, B. S., Ramlogan, S., Chalapathi Rao, A., and Maharaj, S. (2014). Neurolaena lobata L. promotes wound healing in Sprague Dawley rats. Int. J. Appl. Basic Med. Res. 4, 106–110. doi: 10.4103/2229-516X.136791
Neamsuvan, O., and Bunmee, P. (2016). A survey of herbal weeds for treating skin disorders from Southern Thailand: Songkhla and Krabi Province. J. Ethnopharmacol. 193, 574–585. doi: 10.1016/j.jep.2016.09.048
Neamsuvan, O., and Ruangrit, T. (2017). A survey of herbal weeds that are used to treat gastrointestinal disorders from southern Thailand: Krabi and Songkhla provinces. J. Ethnopharmacol. 196, 84–93. doi: 10.1016/j.jep.2016.11.033
Nicolaus, C., Junghanns, S., Hartmann, A., Murillo, R., Ganzera, M., and Merfort, I. (2017). In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 196, 94–103. doi: 10.1016/j.jep.2016.12.006
Ong, H. G., and Kim, Y. D. (2014). Quantitative ethnobotanical study of the medicinal plants used by the Ati Negrito indigenous group in Guimaras island, Philippines. J. Ethnopharmacol. 157, 228–242. doi: 10.1016/j.jep.2014.09.015
Ota, A., and Ulrih, N. P. (2017). An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 8:436. doi: 10.3389/fphar.2017.00436
Ozbilgin, S., Akkol, E. K., Ergene Oz, B., Ilhan, M., Saltan, G., Acikara, O. B., et al. (2018). In vivo activity assessment of some Tanacetum species used as traditional wound healer along with identification of the phytochemical profile by a new validated HPLC method. Iran. J. Basic Med. Sci. 21, 145–152. doi: 10.22038/IJBMS.2018.24258.6055
Pang, Y., Wang, D., Fan, Z., Chen, X., Yu, F., Hu, X., et al. (2014a). Blumea balsamifera–a phytochemical and pharmacological review. Molecules 19, 9453–9477. doi: 10.3390/molecules19079453
Pang, Y., Wang, D., Hu, X., Wang, H., Fu, W., Fan, Z., et al. (2014b). Effect of volatile oil from Blumea Balsamifera (L.) DC. leaves on wound healing in mice. J. Tradit. Chin. Med. 34, 716–724.
Pang, Y., Zhang, Y., Huang, L., Xu, L., Wang, K., Wang, D., et al. (2017). Effects and mechanisms of total flavonoids from Blumea balsamifera (L.) DC. on skin wound in rats. Int. J. Mol. Sci. 18:E2766. doi: 10.3390/ijms18122766
Parente, L. M., Andrade, M. A., Brito, L. A., Moura, V. M., Miguel, M. P., Lino-Junior Rde, S., et al. (2011). Angiogenic activity of Calendula officinalis flowers L. in rats. Acta Cir. Bras. 26, 19–24. doi: 10.1590/S0102-86502011000100005
Parente, L. M., Lino Junior Rde, S., Tresvenzol, L. M., Vinaud, M. C., De Paula, J. R., and Paulo, N. M. (2012). Wound healing and anti-inflammatory effect in animal models of Calendula officinalis L. growing in Brazil. Evid. Based Complement. Alternat. Med. 2012:375671. doi: 10.1155/2012/375671
Park, S. W., Oh, T. Y., Kim, Y. S., Sim, H., Park, S. J., Jang, E. J., et al. (2008). Artemisia asiatica extracts protect against ethanol-induced injury in gastric mucosa of rats. J. Gastroenterol. Hepatol. 23, 976–984. doi: 10.1111/j.1440-1746.2008.05333.x
Pereira, L. X., Silva, H. K. C., Longatti, T. R., Silva, P. P., Di Lorenzo Oliveira, C., De Freitas Carneiro Proietti, A. B., et al. (2017). Achyrocline alata potentiates repair of skin full thickness excision in mice. J. Tissue Viability 26, 289–299. doi: 10.1016/j.jtv.2017.09.005
Podd, D. (2018). Beyond skin deep: managing pressure injuries. JAAPA doi: 10.1097/01.JAA.0000531043.87845.9e [Epub ahead of print].
Retta, D., Dellacassa, E., Villamil, J., Suárez, S. A., and Bandoni, A. L. (2012). Marcela, a promising medicinal and aromatic plant from Latin America: a review. Ind. Crops Prod. 38, 27–38. doi: 10.1016/j.indcrop.2012.01.006
Ricardo, L. M., Dias, B. M., Mugge, F. L. B., Leite, V. V., and Brandao, M. G. L. (2018). Evidence of traditionality of Brazilian medicinal plants: the case studies of Stryphnodendron adstringens (Mart.) Coville (barbatimao) barks and Copaifera spp. (copaiba) oleoresin in wound healing. J. Ethnopharmacol. 219, 319–336. doi: 10.1016/j.jep.2018.02.042
Rodriguez-Chavez, J. L., Egas, V., Linares, E., Bye, R., Hernandez, T., Espinosa-Garcia, F. J., et al. (2017). Mexican arnica (Heterotheca inuloides Cass. Asteraceae: Astereae): ethnomedical uses, chemical constituents and biological properties. J. Ethnopharmacol. 195, 39–63. doi: 10.1016/j.jep.2016.11.021
Romero-Cerecero, O., Islas-Garduno, A. L., Zamilpa, A., and Tortoriello, J. (2017). Effectiveness of Ageratina pichinchensis extract in patients with vulvovaginal candidiasis. a randomized, double-blind, and controlled pilot study. Phytother. Res. 31, 885–890. doi: 10.1002/ptr.5802
Romero-Cerecero, O., Roman-Ramos, R., Zamilpa, A., Jimenez-Ferrer, J. E., Rojas-Bribiesca, G., and Tortoriello, J. (2009). Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J. Ethnopharmacol. 126, 74–78. doi: 10.1016/j.jep.2009.08.007
Romero-Cerecero, O., Zamilpa, A., Diaz-Garcia, E. R., and Tortoriello, J. (2014). Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. J. Ethnopharmacol. 156, 222–227. doi: 10.1016/j.jep.2014.09.002
Romero-Cerecero, O., Zamilpa, A., Gonzalez-Cortazar, M., Alonso-Cortes, D., Jimenez-Ferrer, E., Nicasio-Torres, P., et al. (2013). Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 79, 622–627. doi: 10.1055/s-0032-1328462
Romero-Cerecero, O., Zamilpa, A., and Tortoriello, J. (2015a). Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis in patients with diabetic foot ulcer: a randomized, controlled pilot study. Planta Med. 81, 272–278. doi: 10.1055/s-0034-1396315
Romero-Cerecero, O., Zamilpa, A., and Tortoriello, J. (2015b). Pilot study that evaluated the clinical effectiveness and safety of a phytopharmaceutical elaborated with an extract of Ageratina pichinchensis in patients with minor recurrent aphthous stomatitis. J. Ethnopharmacol. 173, 225–230. doi: 10.1016/j.jep.2015.06.021
Romero-Cerecero, O., Zamilpa-Alvarez, A., Jimenez-Ferrer, E., and Tortoriello, J. (2012a). Exploratory study on the effectiveness of a standardized extract from Ageratina pichinchensis in patients with chronic venous leg ulcers. Planta Med. 78, 304–310. doi: 10.1055/s-0031-1280448
Romero-Cerecero, O., Zamilpa, A., Jimenez-Ferrer, E., and Tortoriello, J. (2012b). Therapeutic effectiveness of Ageratina pichinchensis on the treatment of chronic interdigital tinea pedis: a randomized, double-blind clinical trial. J. Altern. Complement. Med. 18, 607–611. doi: 10.1089/acm.2011.0319
Romero-Cerecero, O., Zamilpa-Alvarez, A., Ramos-Mora, A., Alonso-Cortes, D., Jimenez-Ferrer, J. E., Huerta-Reyes, M. E., et al. (2011). Effect on the wound healing process and in vitro cell proliferation by the medicinal Mexican plant Ageratina pichinchensis. Planta Med. 77, 979–983. doi: 10.1055/s-0030-1250743
Rosa Ados, S., Bandeira, L. G., Monte-Alto-Costa, A., and Romana-Souza, B. (2014). Supplementation with olive oil, but not fish oil, improves cutaneous wound healing in stressed mice. Wound Repair Regen. 22, 537–547. doi: 10.1111/wrr.12191
Rousselle, P., Montmasson, M., and Garnier, C. (2018). Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. [Epub ahead of print]. doi: 10.1016/j.matbio.2018.01.002
Saleh, E. I. M. M., and Van Staden, J. (2018). Ethnobotany, phytochemistry and pharmacology of Arctotis arctotoides (L.f.) O. Hoffm.: a review. J. Ethnopharmacol. 220, 294–320. doi: 10.1016/j.jep.2018.01.011
Samanta, R., Pattnaik, A. K., Pradhan, K. K., Mehta, B. K., Pattanayak, S. P., and Banerjee, S. (2016). Wound healing activity of silibinin in mice. Pharmacognosy Res. 8, 298–302. doi: 10.4103/0974-8490.188880
Schmidt, C., Fronza, M., Goettert, M., Geller, F., Luik, S., Flores, E. M., et al. (2009). Biological studies on Brazilian plants used in wound healing. J. Ethnopharmacol. 122, 523–532. doi: 10.1016/j.jep.2009.01.022
Sharifi, R., Pasalar, P., Kamalinejad, M., Dehpour, A. R., Tavangar, S. M., Paknejad, M., et al. (2013). The effect of silymarin (Silybum marianum) on human skin fibroblasts in an in vitro wound healing model. Pharm. Biol. 51, 298–303. doi: 10.3109/13880209.2012.721789
Sharifi, R., Rastegar, H., Kamalinejad, M., Dehpour, A. R., Tavangar, S. M., Paknejad, M., et al. (2012). Effect of topical application of silymarin (Silybum marianum) on excision wound healing in albino rats. Acta Med. Iran. 50, 583–588.
Shaw, T. J., and Martin, P. (2009). Wound repair at a glance. J. Cell Sci. 122, 3209–3213. doi: 10.1242/jcs.031187
Shenkman, G. S., and Krivenkov, S. G. (1986). Hygienic recommendations with regard to the rational employment of persons with limited work capacity in dairy cattle breeding. Gig. Sanit. 8, 47–49.
Shin, J. M., Choi, D. K., Sohn, K. C., Lee, Y., Kim, C. D., Lee, J. H., et al. (2017). The effect of FK 506 on the reepithelialization of superficial skin wound. Ann. Dermatol. 29, 635–637. doi: 10.5021/ad.2017.29.5.635
Speroni, E., Govoni, P., Guizzardi, S., Renzulli, C., and Guerra, M. C. (2002). Anti-inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. J. Ethnopharmacol. 79, 265–272. doi: 10.1016/S0378-8741(01)00391-9
Sujarwo, W., Keim, A. P., Savo, V., Guarrera, P. M., and Caneva, G. (2015). Ethnobotanical study of Loloh: traditional herbal drinks from Bali (Indonesia). J. Ethnopharmacol. 169, 34–48. doi: 10.1016/j.jep.2015.03.079
Tabandeh, M. R., Oryan, A., Mohhammad-Alipour, A., and Tabatabaei-Naieni, A. (2013). Silibinin regulates matrix metalloproteinase 3 (stromelysine1) gene expression, hexoseamines and collagen production during rat skin wound healing. Phytother. Res. 27, 1149–1153. doi: 10.1002/ptr.4839
Tadic, V., Arsic, I., Zvezdanovic, J., Zugic, A., Cvetkovic, D., and Pavkov, S. (2017). The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J. Ethnopharmacol. 199, 138–148. doi: 10.1016/j.jep.2017.02.002
Tewari, D., Mocan, A., Parvanov, E. D., Sah, A. N., Nabavi, S. M., Huminiecki, L., et al. (2017). Ethnopharmacological approaches for therapy of jaundice: part II. Highly used plant species from Acanthaceae, Euphorbiaceae, Asteraceae, Combretaceae, and Fabaceae families. Front. Pharmacol. 8:519. doi: 10.3389/fphar.2017.00519
Tiwari, R., Latheef, S. K., Ahmed, I., Iqbal, H. M. N., Bule, M. H., Dhama, K., et al. (2018). Herbal immunomodulators, a remedial panacea for the designing and developing effective drugs and medicines: current scenario and future prospects. Curr. Drug Metab. 19, 264–301. doi: 10.2174/1389200219666180129125436
Vowden, P., and Vowden, K. (2016). The economic impact of hard-to-heal wounds: promoting practice change to address passivity in wound management. Wounds Int. 7, 10–15.
Yamane, L. T., De Paula, E., Jorge, M. P., De Freitas-Blanco, V. S., Junior, I. M., Figueira, G. M., et al. (2016). Acmella oleracea and Achyrocline satureioides as sources of natural products in topical wound care. Evid. Based Complement. Alternat. Med. 2016:3606820. doi: 10.1155/2016/3606820
Yin, S., Yan, Y., Huang, T., Guan, J., Wu, L., and Li, K. (2017). Therapeutic effect of Artemisia argyi on oral ulcer in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 42, 824–830. doi: 10.11817/j.issn.1672-7347.2017.07.014
Yoon, M. S., Won, K. J., Kim, D. Y., Hwang, D. I., Yoon, S. W., Kim, B., et al. (2014). Skin regeneration effect and chemical composition of essential oil from Artemisia Montana. Nat. Prod. Commun. 9, 1619–1622.
Yu, Y., Chen, R., Sun, Y., Pan, Y., Tang, W., Zhang, S., et al. (2018). Manipulation of VEGF-induced angiogenesis by 2-N, 6-O-sulfated chitosan. Acta Biomater. 71, 510–521. doi: 10.1016/j.actbio.2018.02.031
Yuan, Y. F., Das, S. K., and Li, M. Q. (2018). Vitamin D ameliorates impaired wound healing in streptozotocin-induced diabetic mice by suppressing endoplasmic reticulum stress. J. Diabetes Res. 2018:1757925. doi: 10.1155/2018/1757925
Keywords: Ageratina pichinchensis, Calendula officinalis, silibinin, jaceosidin, drug development, ethnomedicine
Citation: Carvalho AR Jr., Diniz RM, Suarez MAM, Figueiredo CSSS, Zagmignan A, Grisotto MAG, Fernandes ES and da Silva LCN (2018) Use of Some Asteraceae Plants for the Treatment of Wounds: From Ethnopharmacological Studies to Scientific Evidences. Front. Pharmacol. 9:784. doi: 10.3389/fphar.2018.00784
Received: 24 March 2018; Accepted: 27 June 2018;
Published: 21 August 2018.
Edited by:
Adolfo Andrade-Cetto, Universidad Nacional Autónoma de México, MexicoReviewed by:
István Zupkó, University of Szeged, HungaryCopyright © 2018 Carvalho, Diniz, Suarez, Figueiredo, Zagmignan, Grisotto, Fernandes and da Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luís C. N. da Silva, bHVpc2NuLnNpbHZhQGNldW1hLmJy; bHVpc2NsYXVkaW9uc2lsdmFAeWFob28uY29tLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.