
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 14 August 2018
Sec. Cancer Molecular Targets and Therapeutics
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.00781
This article is part of the Research Topic Nanoparticles in Cancer Therapy: Novel Concepts, Mechanisms and Applications View all 12 articles
 Zuojun Li1,2
Zuojun Li1,2 Jingjing Sun3
Jingjing Sun3 Yixian Huang3
Yixian Huang3 Yanhua Liu4
Yanhua Liu4 Jieni Xu3
Jieni Xu3 Yichao Chen3
Yichao Chen3 Lei Liang5
Lei Liang5 Jiang Li3
Jiang Li3 Qiongfeng Liao6
Qiongfeng Liao6 Song Li3*
Song Li3* Kechao Zhou2*
Kechao Zhou2*Ibuprofen (IBU) is a non-steroidal anti-inflammatory drug (NSAID), which is widely used to reduce fever and treat inflammation and acute pain. Recently, its application in cancer treatment is also being explored. In this work, we synthesized a well-defined IBU-based amphiphilic diblock copolymer via reversible addition fragmentation transfer (RAFT) polymerization of IBU-based vinyl monomer. The amphiphilic copolymer POEG-b-PVBIBU (denoted as POVI) was composed of a hydrophilic poly(oligo(ethylene glycol)) block and a hydrophobic IBU-bearing prodrug block, which was able to self-assemble into prodrug nanomicelles. In addition, it could serve as a carrier to co-load other drugs including doxorubicin (DOX), paclitaxel (PTX), and docetaxel (DTX). By using DOX as a model anti-cancer drug, the delivery function of POVI carrier, including the drug release, in vitro cytotoxicity, cellular uptake, and in vivo antitumor activity, was evaluated. DOX-loaded POVI micelles exhibited sustained release of DOX. Besides, DOX/POVI micelles were effectively taken up by tumor cells with an efficiency comparable to that of free DOX. Moreover, in vivo studies showed that POVI carrier itself had modest antitumor activity. After loading DOX, the antitumor activity was significantly increased, which was significantly higher than that of free DOX. Our results suggest that POVI polymer represents a simple and effective dual-functional carrier for co-delivery of IBU and DOX to improve the anticancer activity.
The clinical applications of many antitumor drugs, such as doxorubicin (DOX), paclitaxel (PTX), and docetaxel (DTX), were limited by their low water solubility, poor bioavailability, and high toxic side effects (Lee et al., 2011; Senevirathne et al., 2016). To overcome these problems, various nanocarriers including micelles, dendrimers, and liposomes have been designed and developed for delivery of these hydrophobic antitumor drugs (Cho et al., 2008; Shen et al., 2010; Wei et al., 2013; Koudelka et al., 2015; Wang et al., 2015, 2017; Miao et al., 2017). Due to the small size, good stability, and unique core–shell structure, polymeric micelles have gained tremendous interest in the drug delivery field (Gaucher et al., 2005; Rösler et al., 2012). The hydrophobic core of micelles can encapsulate hydrophobic antitumor drugs via hydrophobic interactions, and the hydrophilic shell endows the micelles colloidal stability and protects the loaded drug from premature burst release.
It is well known that the constructing material, especially the hydrophobic domain of the polymeric micelles, plays a very important role in the delivery function of carriers (Zhang et al., 2014). Recently, increasing attention has been focused on the usage of bioactive compounds as hydrophobic moieties of polymeric carriers. These carriers themselves possess biological activity, which are termed as prodrug carriers (Craparo et al., 2013; Chen et al., 2016; Sun et al., 2017a). More importantly, some bioactive compounds with special structures in the prodrug carriers could interact with the co-loaded drug via π–π stacking effect, thereby improving the drug loading capacity (DLC; Sun et al., 2017b).
Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen (IBU), indomethacin, and aspirin, are widely used to treat fever, arthritis, and rheumatic diseases. The anti-inflammatory effect of NSAIDs comes from the inhibition of the cyclooxygenase (COX) enzymes, including COX-1 and COX-2, which leads to the decrease of the production of prostaglandin, an important signaling molecule in the inflammation (Lim et al., 1999). Recently, accumulating evidences demonstrate that NSAIDs not only have anti-inflammatory effect, but also hold great potential in the prevention and treatment of several types of cancers (Ulrich et al., 2006). It has been suggested that the antitumor activity of NSAIDs can be partially explained by COX inhibition of prostaglandin synthesis (Ahnen, 1998). Other COX-independent mechanisms including inhibition of cell cycle progression (Gately and Kerbel, 2003) and induction of apoptosis (Davies et al., 2002) also contribute to the antitumor activity of NSAIDs. IBU is a commonly used NSAID that has been proven to inhibit proliferation of many tumor cells (Dial et al., 2006; Bonelli et al., 2011). Studies also demonstrate that IBU exhibited superior antitumor effect compared to other NSAIDs, mainly through the alteration of cell-cycle and induction of apoptosis (Andrews et al., 2002; Janssen et al., 2008). Moreover, compared to conventional anticancer drugs, IBU shows decreased side effect. Due to the potential of IBU in cancer treatment, IBU-conjugated prodrug polymers with various molecular structures have been developed to improve the water solubility and bioavailability of IBU. For example, Hasegawa and colleagues synthesized a series of amphiphilic diblock copolymers (PEG-PIBU) containing a hydrophilic poly(ethylene glycol) block and a hydrophobic IBU-bearing prodrug block (Hasegawa et al., 2013). By adjusting the length of hydrophobic IBU block, these polymers could self-assemble to form micelles with different morphologies. Wang et al. (2014) prepared another amphiphilic micellar carrier based on IBU-conjugated polymer for delivery of IBU. They found that in addition to the chemical conjugation, IBU could also be physically encapsulated into the micelles.
Although NSAIDs have potential antitumor activity, very high dosages of NSAIDs are needed to achieve a modest antitumor effect (Smalley and DuBois, 1997). Combination treatment of NSAIDs with other chemotherapeutic drugs is still necessary for cancer therapy (Endo et al., 2014; Lee et al., 2016). Recently, our group prepared a nanomicellar carrier that was self-assembled from PEG-Fmoc-IBU conjugate for co-delivery of IBU and PTX (Zhao et al., 2016). The IBU-based carrier showed synergistic antitumor effect with PTX, but there is only one IBU unit per carrier molecule, and thereby, large amounts of carriers were needed to deliver enough IBU dosage to tumor tissues, which might cause the unfavorable side effects.
Thus, in this work, we developed another IBU-containing polymeric carrier system with increased number of IBU units per polymer molecule via a simple synthesis method. We synthesized a POEG-b-PVBIbu diblock copolymer with a hydrophilic POEG block and a hydrophobic IBU block by reversible addition fragmentation transfer (RAFT) polymerization. The IBU-based prodrug polymer can form stable micelles with multiple IBU hydrophobic moieties in the core, which increases the solubility of IBU and allows more IBU being delivered into the tumor. Additionally, the micelle can serve as a carrier to encapsulate other hydrophobic chemotherapeutic drugs including DOX, DTX, and PTX. The size distribution and morphologies of drug-loaded micelles were evaluated. By using DOX as a model drug, the drug release, cellular uptake and antitumor activity were investigated in vitro and in vivo.
Vinylbenzyl chloride, 2, 2-Azobis(isobutyronitrile) (AIBN), 1,4-dioxane, trypsin-EDTA solution, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and Dulbecco’s Modified Eagle’s Medium (DMEM) were all purchased from Sigma-Aldrich (St. Louis, MO, United States). AIBN was purified by recrystallization in anhydrous ethanol. DOX⋅HCl and DTX were purchased from LC Laboratories (Woburn, MA, United States). PTX was purchased from AK Scientific Inc. (Union City, CA, United States). Fetal bovine serum (FBS) and penicillin-streptomycin solution were all purchased from Invitrogen (NY, United States). POEG macroCTA were prepared as previously reported (Sun et al., 2016a).
Vinylbenzyl chloride (1 eq.), IBU (1.5 eq.), and K2CO3 (2 eq.) were dissolved in DMF (5 eq.). The mixture was stirred at 50°C for 24 h, and then equal volume of water was added. The crude product was extracted by DCM for three times, and then purified by silica gel column chromatography.
Ibu-monomer (180 mg, 0.559 mmol), POEG macroCTA (280 mg, 0.0372 mmol), AIBN (2 mg, 0.0124 mmol), and 2 mL dried 1, 4-dioxane were placed in a Schlenk tube. The mixture was degassed under N2 by three freeze-pump-thaw cycles, and then immersed into an oil bath at 90°C. After 24 h, the polymerization was quenched by placing the Schlenk tube into liquid nitrogen. The polymer mixture was precipitated in petroleum ether for three times and dried in vacuum.
1H NMR spectrum was conducted on a Varian 400 FT-NMR spectrometer at 400.0 MHz with CDCl3 as the solvent. Molecular weight (Mn and Mw) and polydispersity index (Mw/Mn) of the synthesized polymers were determined by gel permeation chromatography (GPC) equipped with a Waters 2414 refractive index detector, a Waters 515 HPLC pump, and a Waters 717 Plus Autosampler. THF was used as the eluent with a flowing rate of 1.0 mL/min at 35°C. A series of polystyrene standards with narrow molecular weight distribution were used for calibration.
The blank POVI micelles and drug-loaded micelles were prepared by dialysis method. The polymer POVI and drug (DOX, PTX, or DTX) with certain mass ratio were dissolved in DMSO, which were then transferred to dialysis bags (MWCO 3.5 kDa), and dialyzed against PBS for 24 h. The size distributions and morphologies of blank and drug-loaded micelles were measured by dynamic light scattering (DLS) and transmission election microscopy with negative staining.
Doxorubicin concentration was detected by Fluorescence Microplate Reader with excitation wavelength of 490 nm and emission wavelength of 590 nm. PTX concentration was measured by reverse phase high-performance liquid chromatography (RP-HPLC) with a mobile phase of methanol/water (70:30 v/v) at the flow rate of 1.0 mL/min, and UV detection at 227 nm. DTX concentration was detected by RP-HPLC with a mobile phase of acetonitrile/water (50:50 v/v) at the flow rate of 1.0 mL/min, and UV detection at 230 nm.
Drug loading capacity and drug loading efficiency (DLE) were calculated according to the following formula:
DLC (%) = [weight of drug loaded/(weight of polymer + drug used)] × 100%
DLE (%) = (weight of loaded drug/weight of input drug) × 100%.
Critical micelle concentration (CMC) of POVI micelle was measured using Nile red as a fluorescence probe (Klaikherd et al., 2009; Sun et al., 2016b); 10 μL Nile red in chloroform was added into each tube, and the solvent was removed by air flow and vacuum pump. Polymer solution (120 μL) with concentrations ranging from 1 × 10-4 to 0.5 mg/mL was added to each tube, and incubated overnight. The fluorescence intensity of each sample was detected by a Synergy H1 Hybrid Multi-Mode Microplate Reader (Winooski, VT, United States) at a wavelength of 480/620 nm (excitation/emission).
Doxorubicin-loaded POVI micelles (0.5 mg DOX/mL) in PBS (pH = 7.4) was placed in a dialysis bag (MWCO = 12 kDa, Spectrum Laboratories), and incubated in a 200-mL beaker with PBS containing 0.5% (w/v) Tween 80, with gentle shaking (100 rpm/min) at 37°C. DOX solution in saline with the same concentration was used as a control. The concentration of DOX outside the dialysis bag was measured by a fluorescence microplate reader at designated time points and the values were reported as the means of triplicate samples.
Mouse metastatic breast cancer cell line 4T1.2, human breast cancer cell line MCF-7, and androgen-independent human prostate cancer cell line PC-3 were cultured at 37°C in DMEM containing 10% FBS and 1% penicillin-streptomycin in a humidified environment with 5% CO2.
4T1.2 (1500 cells/well), MCF-7 (4000 cells/well), or PC-3 (2500 cells/well) were seeded in 96-well plates and incubated for 24 h. Then the cells were treated with various concentrations of drug-free POVI micelles, DOX-loaded POVI micelles, or DOX. After incubation for 72 h, 20 μL of MTT in PBS (5 mg/mL) was added into each well and further incubated for 4 h. The medium was then removed, and DMSO was added to solubilize the MTT formazan. The absorbance of each well was measured with a microplate reader at a wavelength of 550 nm and a reference wavelength of 630 nm. Untreated cells were used as a control. Cell viability was calculated as [(ODtreat - ODblank)/(ODcontrol - ODblank) × 100%].
4T1.2 cells (15,000/well) were seeded in glass bottom dishes (In Vitro Scientific, United States), and incubated overnight. The cells were treated with free DOX and DOX/POVI micelles (DOX concentration: 15.5 μg/mL) for 2 and 4 h separately. Then cells were stained with Hoechst 3342 for 15 min, and washed with cool PBS for three times. The intracellular distributions of different DOX formulations were observed under a confocal laser scanning microscope (CLSM, FluoView 1000, Olympus, Japan).
Female BALB/c mice (6–8 weeks) were purchased from Charles River (Davis, CA, United States). All animals were housed under pathogen-free conditions according to Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines. All animal-related experiments were performed in full compliance with institutional guidelines and approved by the Animal Use and Care Administrative Advisory Committee at the University of Pittsburgh.
A syngeneic murine breast cancer model (4T1.2) was used to evaluate the therapeutic efficacy of DOX-loaded POVI micelles. 4T1.2 cells (2 × 105 cells/mouse) were inoculated s.c. at the right flank of female BALB/c mice. When the tumor volume reached ∼50 mm3 (day 0), mice were randomly divided into four groups (n = 3) and received i.v. administration of saline (control), POVI micelles, free DOX, and DOX-loaded POVI micelles, respectively, on days 0, 3, 6, 9, 12, 15, and 18. The DOX dosage for free DOX and DOX-loaded POVI micelles was 5 mg DOX/kg. The dosage for POVI micelles was 73 mg POVI/kg, which was the same as that of POVI in DOX-loaded POVI micelles. Tumor volumes were measured with digital caliper and calculated as V = (L × W2)/2, where L is the longest and W is the shortest tumor diameters (mm). Each group was compared by relative tumor volume (RTV = V/V0, V0 was the tumor volume prior to first treatment). Mice were sacrificed when the tumor volume reached ∼2000 mm3. The size and weight of tumor stripped from the mice were measured. To evaluate the potential toxicity, the body weights were also monitored during the entire course of treatment.
After in vivo therapeutic study, tumor tissues were excised and preserved in 4% formaldehyde in PBS, followed by embedment in paraffin. The paraffin-embedded tumor samples were cut into thin slices of 5 μm with an HM 325 Rotary Microtome. Then the slices were stained with hematoxylin and eosin (H&E) for histopathological examination under a Zeiss Axiostar plus Microscope (PA, United States).
All results were reported as the mean ± SD unless otherwise indicated. Statistical analysis was performed with Student’s t-test for two groups, and one-way ANOVA for multiple groups, followed by Newman–Keuls test if P < 0.05. In all statistical analysis, P < 0.05 was considered statistically significant.
Reversible addition fragmentation transfer polymerization of functional monomer has become an attractive strategy to obtain well-defined functional polymers for drug/gene delivery (Sun et al., 2013a,b; Tucker et al., 2015). In this work, we synthesized a well-defined IBU-based prodrug polymer via RAFT polymerization of IBU-conjugated monomer, and investigated its function as a dual-functional carrier for co-delivery of other chemotherapeutic drugs.
As shown in Scheme 1, we first designed and synthesized a vinylbenzyl derivative of IBU (IBU-monomer) where IBU was conjugated with vinylbenzyl chloride via a hydrolyzable ester linkage. Then, the macro-chain transfer agent POEG macroCTA was synthesized by RAFT polymerization as previously reported (Sun et al., 2016a), which further initiated the polymerization of IBU-monomer to give the POEG-b-PVBIbu block copolymers. The structures of the monomer and polymer were confirmed by 1H NMR (Figure 1). As shown in Figure 1A, the average degree of polymerization (DP) of the Ibu-monomer was determined to be 12 by comparing the intensities of Ib and Ia. GPC showed unimodal peaks for the polymer with number-average molecular weight Mn of 10,700, and polydispersity of 1.20 (Figure 1B), which indicated the successful synthesis of the well-defined POEG-b-PVBIbu (POVI) block copolymers.
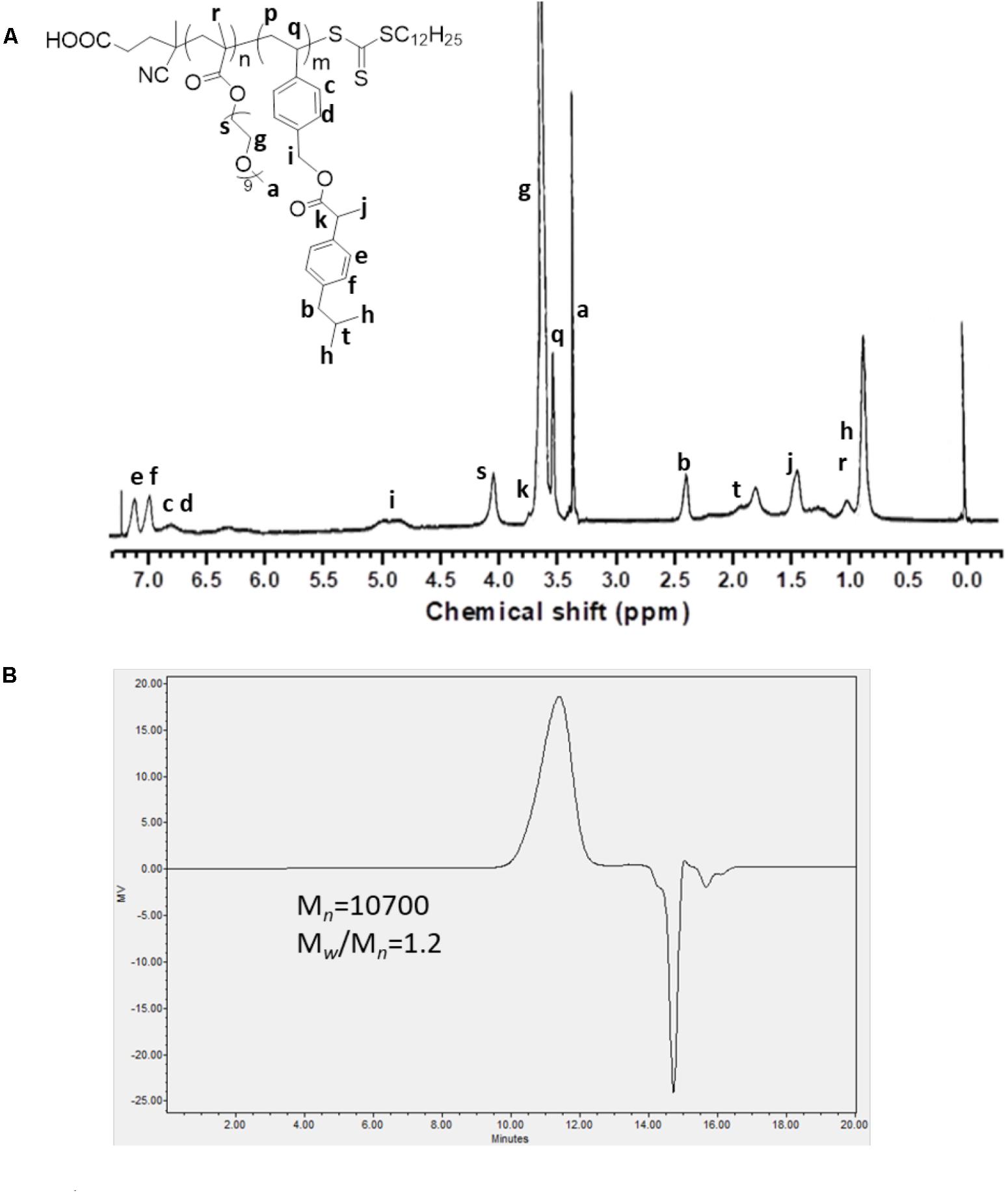
FIGURE 1. (A) 1H NMR spectrum of the POEG-b-PVBIBU (POVI) diblock copolymer in CDCl3. (B) Gel permeation chromatography curve of the POVI diblock polymer.
POVF blank micelles and drug-loaded micelles (including DOX/POVI, PTX/POVI, and DTX/POVI) were successfully prepared by dialysis method, and the physicochemical characterization is summarized in Table 1. DLS analysis showed that POVF polymers could form nano-sized micelles with a diameter of 82 nm. The sizes of micelles slightly increased to 92, 106, and 98 nm after loading of DOX, PTX, and DTX, respectively (Figure 2), which may be due to that the encapsulated hydrophobic drugs enlarged hydrophobic core of the micelles. The morphologies of the blank and drug-loaded micelles were evaluated by transmission electron microscopy (TEM). As shown in Figure 3, these micelles are spherical in morphology with uniform size. DLE and capacity were measured by a fluorescence microplate reader. As shown in the Table 1, POVI polymer was able to load DOX with a loading capacity of 6.4%. In addition, POVI polymers could also serve as a carrier to load other drugs such as PTX and DTX.
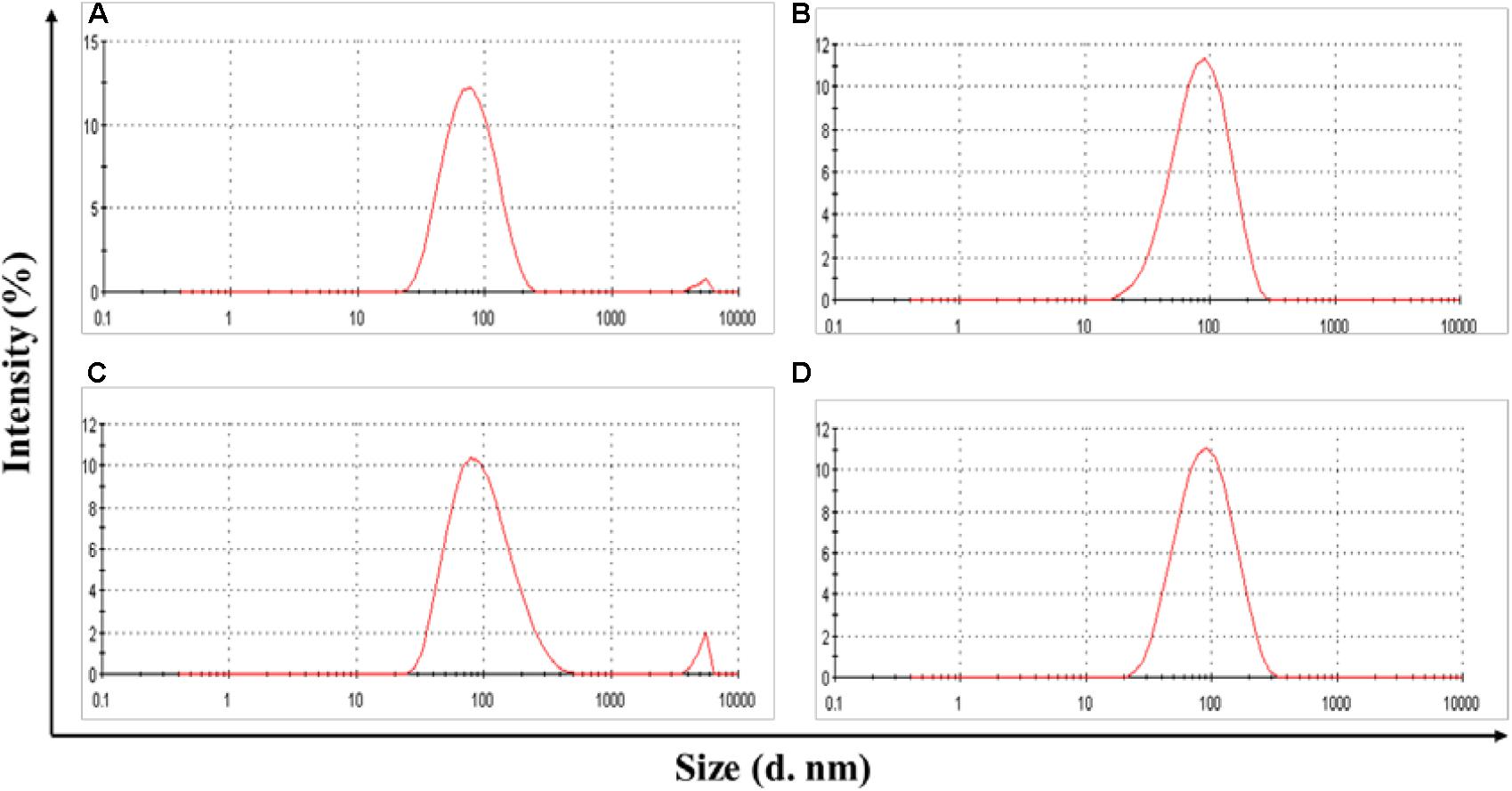
FIGURE 2. Size distribution of POVI (A), DOX/POVI (B) (mass ratio of micelle to DOX = 10:1), PTX/POVI (C), and DTX/POVI micelles (D) (mass ratio of micelle to PTX/DTX = 20:1).
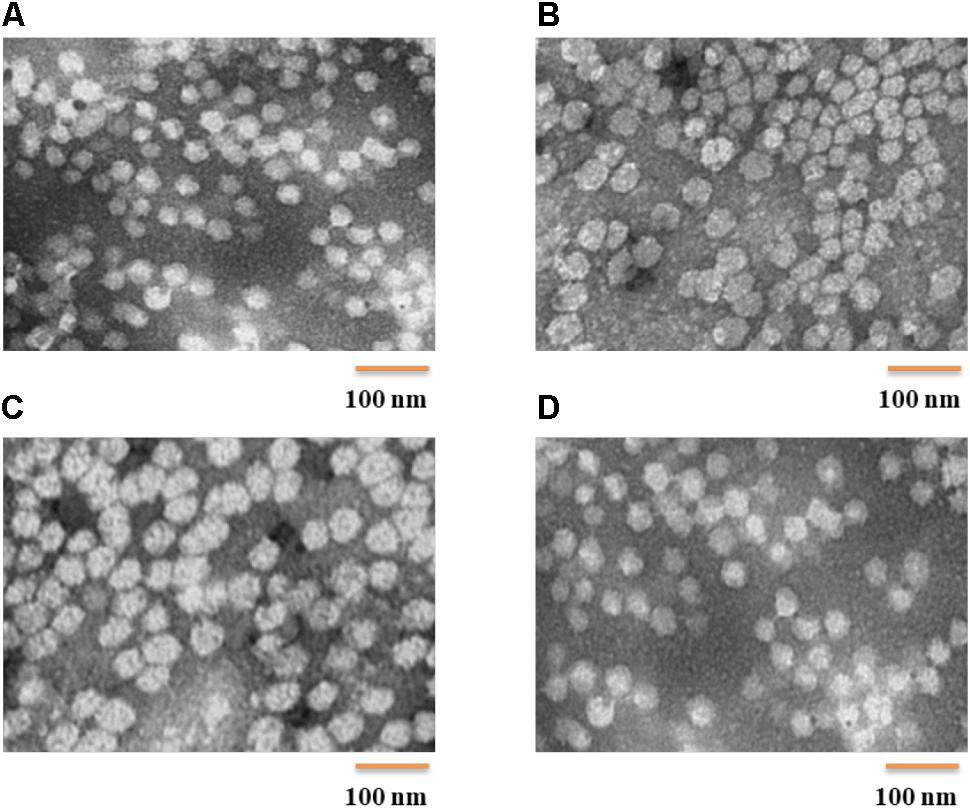
FIGURE 3. TEM images (direct mag: 150,000X) of POVI (A), DOX/POVI (B) PTX/POVI (C), and DTX/POVI (D) micelles.
The CMC value of POVF micelles was evaluated with Nile red as a fluorescence probe. As shown in Figure 4, the CMC of POVI micelles was measured to be 9.8 mg/L. The low CMC suggests a good stability of our micelles following dilution in the blood after i.v. administration.
The release kinetics of DOX from DOX/POVI micelles was evaluated using a dialysis method, and free DOX solution was used as a control. As shown in Figure 5, free DOX solution showed a burst DOX release of almost 80% within the first 12 h, while DOX/POVI micelles showed a slow DOX release of about 43%. Even after 72 h, less than 50% of DOX was released from DOX/POVI micelles.
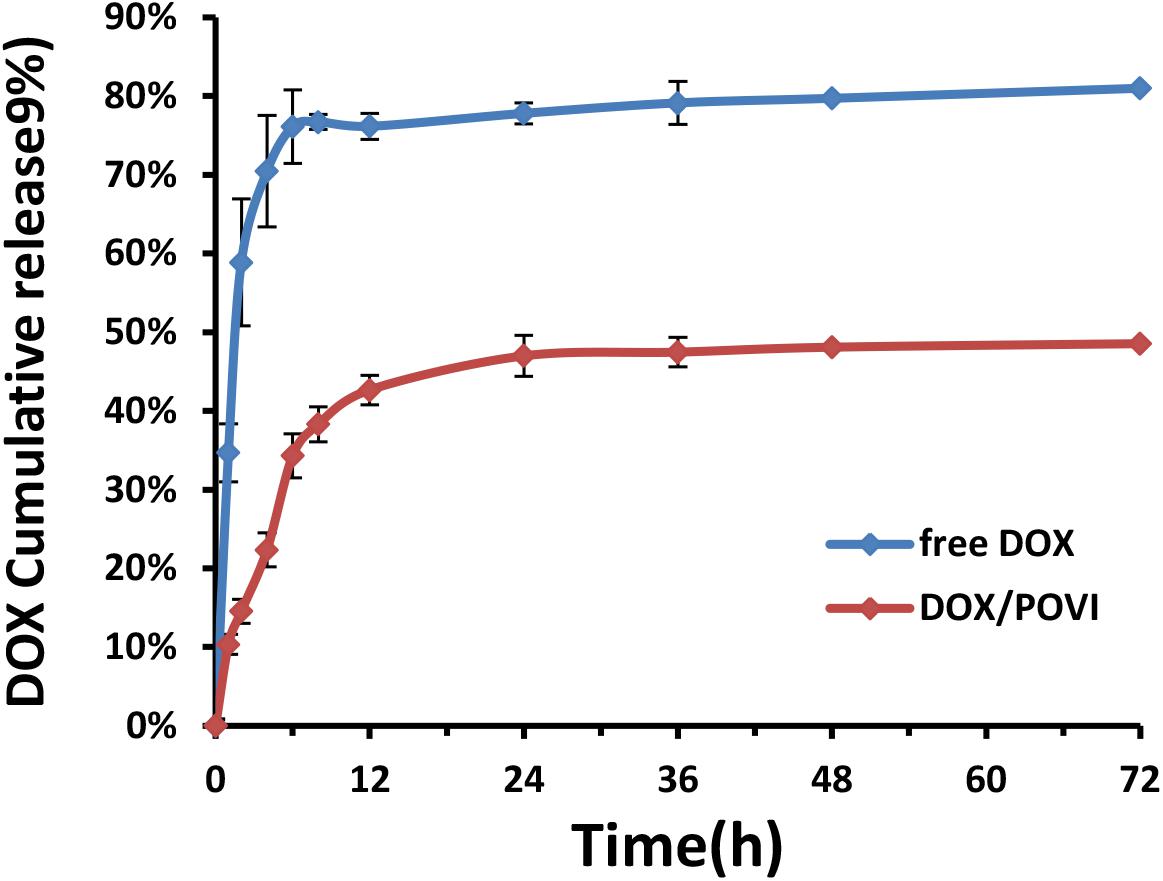
FIGURE 5. DOX release from DOX-loaded POVI micelles via a dialysis method. PBS solution was used as release medium, and DOX concentrations were analyzed at 0, 1, 2, 4, 6, 8, 12, 24, 36, 48, and 72 h.
In vitro cytotoxicity of DOX/POVI micelles was evaluated in 4T1.2, MCF7, and PC3 cell lines. As shown in Figure 6, POVI carrier did not show any remarkable cytotoxicity among all cell lines tested. DOX/POVI micelles and DOX solution exhibited potent cytotoxicity in a concentration-dependent manner. DOX/POVI micelles exhibited lower cytotoxicity than free DOX in the three cell lines, which is likely due to the slow release of DOX from DOX/POVI micelles.
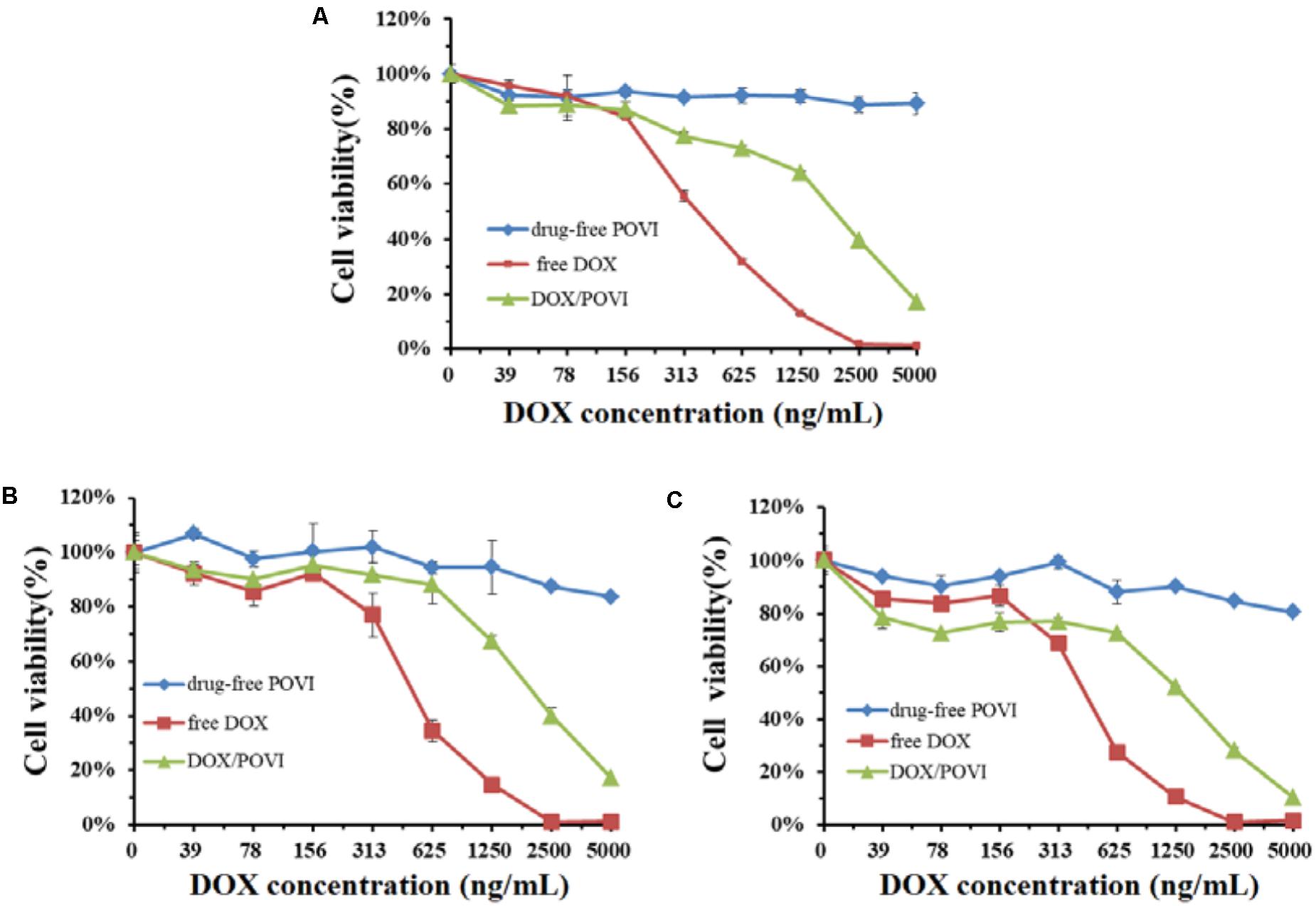
FIGURE 6. In vitro cytotoxicity of DOX/POVI micelles. 4T1.2 (A), MCF7 (B), and PC3 (C) cells were treated with DOX-free POVI, DOX/POVI micelles, and free DOX for 72 h, and the cytotoxicity was determined by MTT assay, respectively.
Intracellular trafficking and distribution of DOX/POVF micelles were studied by confocal laser scanning microcopy (CLSM). 4T1.2 cells were incubated with free DOX and DOX/POVF micelles for 2 and 4 h, respectively. At 2 h, cells treated with DOX/POVF micelles exhibited strong DOX fluorescence signals around the nucleus, similar to the cells treated with free DOX (Figure 7A). At 4 h, increased signals of red fluorescence appeared in nuclei, both for DOX/POVF micelle group and free DOX group (Figure 7B).
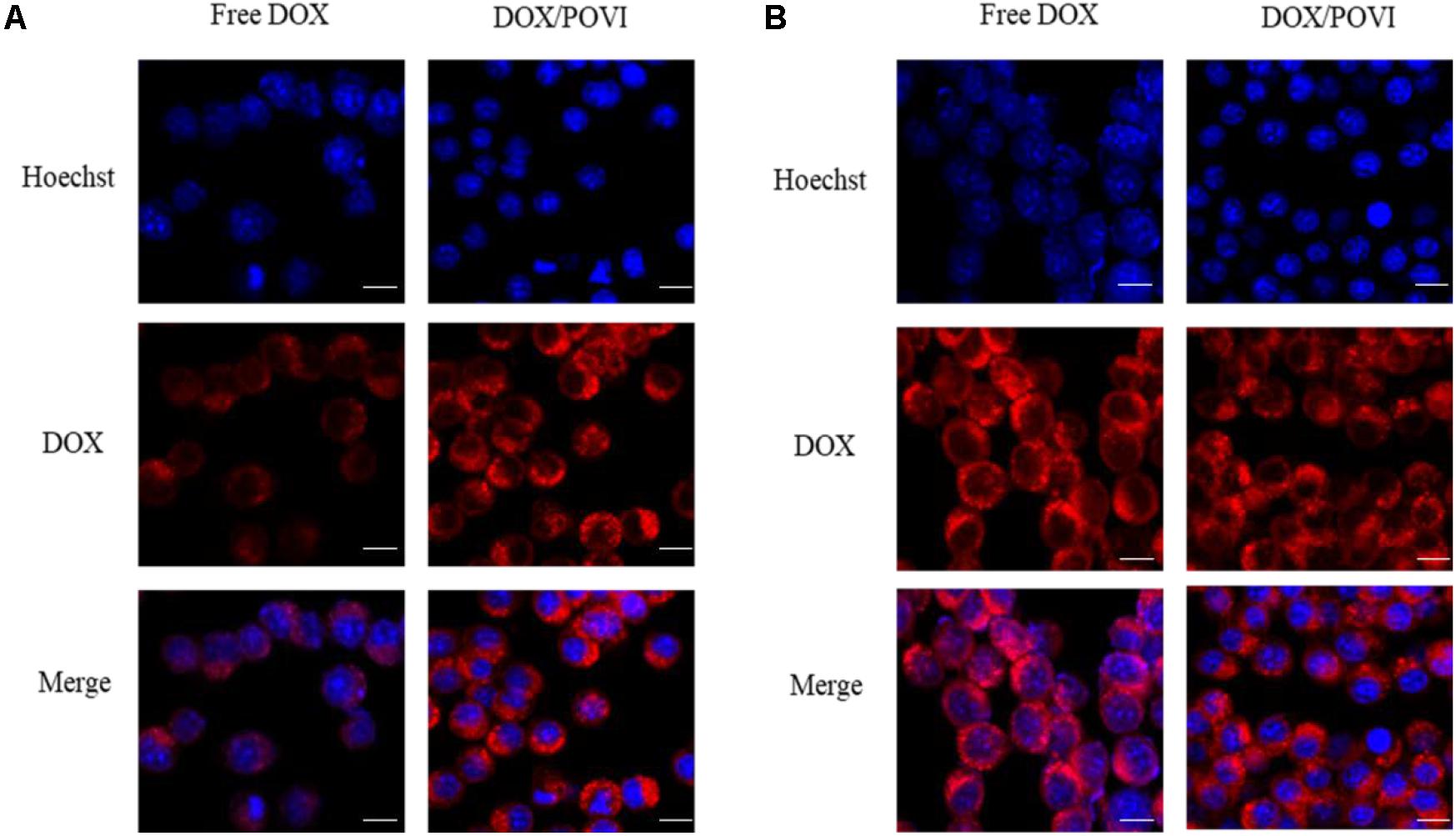
FIGURE 7. Intracellular trafficking of DOX in 4T1.Cells were treated with free DOX, DOX/POVI micelle at 15.5 μg/mL for 2 h (A) and 4 h (B). The nuclei were stained with Hoechst 33342. Scale bar: 20 μm.
The in vivo therapeutic efficacy of DOX/POVI micelles was evaluated in the 4T1.2 breast tumor-bearing BALB/c mice. The mice were treated with saline, POVI carrier, free DOX, and DOX/POVI micelles by i.v. injection, respectively. As shown in Figure 8A, DOX exhibited a modest tumor growth inhibition at a dose of 5 mg DOX/kg. DOX/POVI micelles were more effective than DOX in inhibiting tumor growth (p < 0.05) at the same dose. Interestingly, POVI carrier alone also showed tumor growth inhibition effect, although it was less effective than DOX.
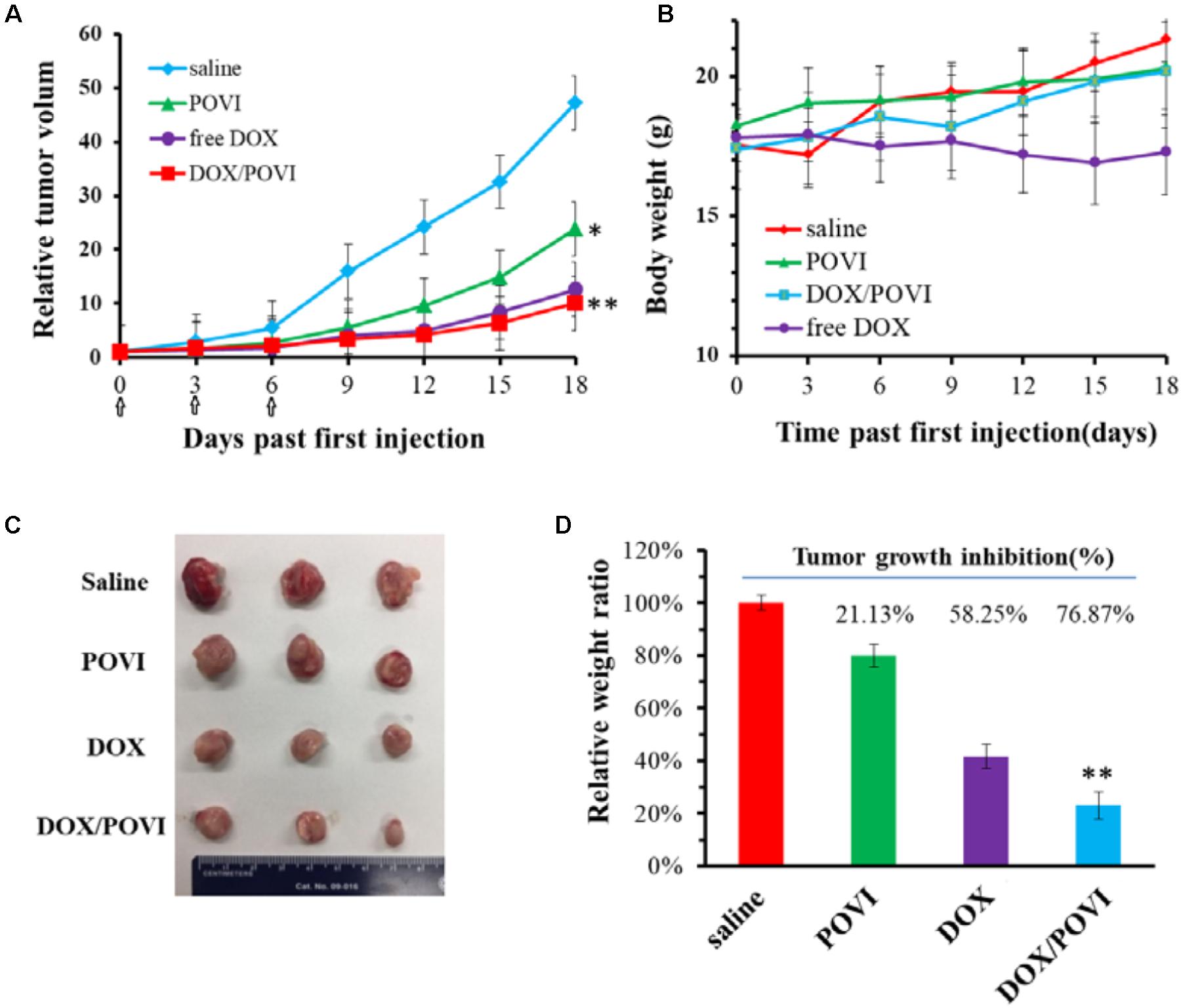
FIGURE 8. In vivo therapeutic efficacy of DOX/POVI micelles. BALB/c mice in 4T1.2 murine breast cancer model were injected with saline, POVI, free DOX, and DOX/POVI (5 mg DOX/kg body weight) on days 0, 3, and 6, separately. ∗P < 0.05; ∗∗P < 0.01 (vs saline). (A) Relative tumor volume curves of mice receiving different treatments. (B) Changes of body weight in mice were monitored. (C) Image of tumors stripped from mice on the day 18. (D) Tumor weights of mice were measured on day 21 and tumor growth inhibition (%) was calculated. ∗∗P < 0.05 (vs free DOX).
No significant changes were found in the body weights of mice following various treatments (Figure 8B), indicating that all treatments were well tolerated. Figure 8C shows the photographs of tumors removed from the mice after various treatments. Compared to saline treatment group, DOX/POVI treatment led to an obvious decrease in tumor size. The tumor growth inhibition rate of DOX/POVI micelles is calculated to be significantly higher than those of free DOX and POVI blank micelles (P < 0.05; Figure 8D).
Figure 9 shows the images of H&E-stained slices of tumors collected after the completion of in vivo therapy study. Tumors treated with saline showed normal tumor morphology with large nuclei. In contrast, tumors in other treatment groups showed shrunk nuclei. Among them, DOX/POVF treatment resulted in the most significant tumor necrosis.
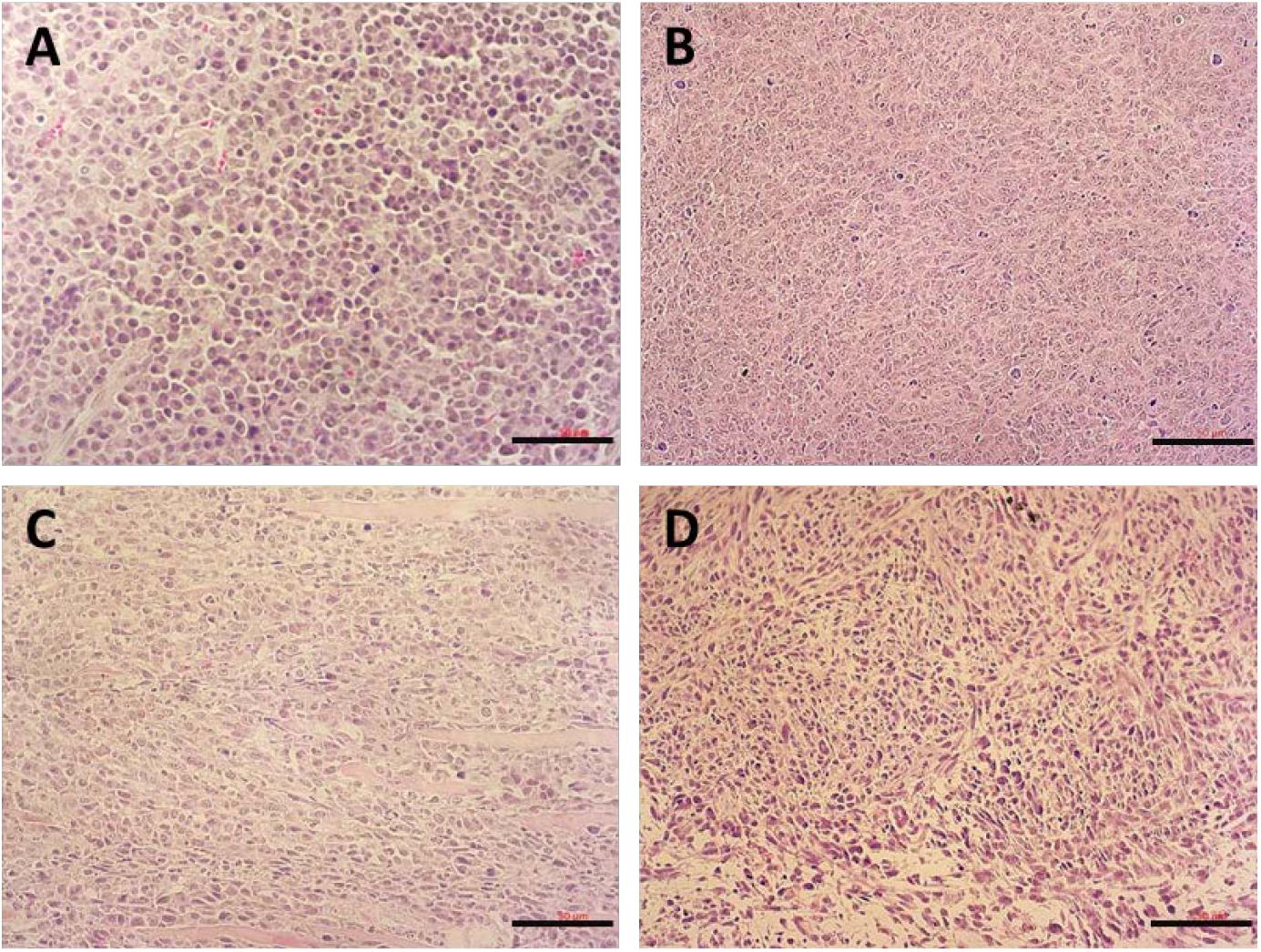
FIGURE 9. H&E stained tumor tissues from mice received different treatments: (A) saline, (B) POVI, (C) free DOX, and (D) DOX/POVI at a dosage of 5 mg DOX/kg. Three injections were made into BALB/c mice bearing 4T1.2 tumor on days 0, 3, and 6, separately. Tumor tissues were harvested on day 18. Scale bar: 50 μm.
Ibuprofen is one type of NSAID that not only possesses anti-inflammatory effect, but also demonstrates great potential in inhibiting proliferation of various types of tumor cells (Dial et al., 2006; Bonelli et al., 2011). Currently, combination of IBU with chemotherapeutic drugs is being evaluated as a new regimen for clinical cancer treatment. However, free drugs are readily eliminated in the blood and they lack tumor targeting. Thus, co-delivery of IBU and a chemotherapeutic drug into tumors using a delivery vehicle is highly needed. Previously, we reported a nanomicellar carrier based on PEG-Fmoc-IBU conjugate for co-delivery of IBU and PTX (Zhao et al., 2016). Although PEG-Fmoc-IBU carrier was effective in loading and delivering PTX into tumors, the efficiency of delivery of IBU itself was relatively low because there was only one IBU unit per polymer molecule. In the present work, we developed a new IBU-conjugated polymeric carrier POVI with increased units of IBU per carrier molecule via a facile RAFT polymerization method. Compared to the previous system, the IBU loading capacity in the new POVI system was increased from 8.2 to 20.9%, indicating that more IBU could be delivered into the tumor tissues when injecting the same amount of carriers.
First, we designed and synthesized a novel IBU-conjugated vinylbenzyl monomer, which was further polymerized to yield the IBU-containing block copolymers. Compared with the post-conjugation method in which a drug was conjugated to the polymerized backbone, our method has an obvious advantage of simplicity. In addition, it avoids the low drug conjugation efficiency caused by the steric hindrance.
The synthesized POVI polymer could serve as a carrier that is effective in loading various types of chemotherapeutic drugs, including DOX, PTX, and DTX. We found that the monomer structure is critical for the loading capacity of the carrier. In the preliminary study, we also synthesized another IBU-based polymer using IBU-conjugated hydroxyethyl methacrylate monomer. However, the polymer obtained performed poorly in encapsulating other chemotherapeutic drugs (data not shown). When we introduced vinylbenzyl group into the polymers, the DLC was significantly improved, suggesting that, in addition to hydrophobic interaction, the π–π stacking effect between carrier and the chemotherapeutic drug may also contribute to the overall DLC. Moreover, the structures of chemotherapeutic drugs also affected the loading capacity. The POVI carrier was more effective in encapsulating DOX compared to other chemotherapeutic drugs, which might be due to the stronger carrier/DOX interaction.
The DOX-loaded micelles showed small size with a diameter of ∼90 nm (Figure 2), which was optimal for a long blood circulation time and passive targeting to solid tumor sites through the enhanced permeability and retention (EPR) effect (Mitra et al., 2001; Meng et al., 2011; Kobayashi et al., 2014). DOX-loaded micelles showed slow and sustained drug release (Figure 5), which could prevent the premature release of encapsulated DOX before entering into tumors. The slow release of DOX from the micelles may be due to the strong interaction (e.g., hydrophobic interaction and π–π stacking effect) between DOX and POVI carrier (Sun et al., 2016a).
In vitro cytotoxicity showed that DOX formulated in the carrier was less effective in inhibiting the proliferation of tumor cells compared to free DOX. The in vitro cytotoxicity comes from the overall outcome of intracellular uptake and drug release. The DOX/POVI micelles showed similar cellular uptake compared to free DOX (Figure 7); the lower in vitro cytotoxicity of DOX/POVI micelles might be attributed to the slow release of DOX from the micelles.
POVI carrier was not effective in inhibiting the proliferation of cultured tumor cells in vitro (Figure 6) at the concentrations that were used for DOX delivery in vitro. This may be due to the slow release of IBU during a short period of culture and its relatively low potency. However, in vivo data showed that the POVI carrier itself showed moderate antitumor activity (Figure 8). In our previous study, PEG-IBU conjugate could also inhibit tumor growth compared with control group (Zhao et al., 2016). So the antitumor activity of POVI carrier in vivo may come from the released IBU mediated by various kinds of enzymes in the tumor tissues over a relatively long period of time. Moreover, DOX-loaded micelles showed a much more pronounced antitumor activity compared with free DOX, which is different from the in vitro cytotoxicity assay. The improved therapeutic efficacy of DOX/POVI micelles is likely attributed to the combination effect of the carrier with co-delivered DOX. The improved delivery of DOX by the POVI carrier may also play a role. More studies are warranted to further investigate the underlying mechanisms.
We have developed a new IBU-based prodrug di-block polymer POVI via facile RAFT polymerization. POVI polymer could load various hydrophobic drugs including DOX, PTX, and DTX with high loading capacity. DOX/POVI micelles showed similar cytotoxicity and cellular uptake compared to free DOX. More importantly, DOX/POVI micelles were more effective in inhibiting the tumor growth than free DOX in vivo. Our results suggest that POVI polymer can be employed as a safe and effective dual-functional carrier for co-delivery of other chemotherapeutic drugs.
SL, JS, and YH conceived and designed the study. JS and ZL contributed to chemical synthesis and micelle characterization. ZL, YL, JX, YC, LL, JL, and QL contributed to biological study. ZL, JS, YL, and JX analyzed the data. ZL, JS, and SL drafted the manuscript. All authors approved the final version of the manuscript.
This work was supported by NIH Grant Nos. RO1CA174305, RO1CA219399, and RO1CA223788.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ahnen, D. J. (1998). Colon cancer prevention by NSAIDs: what is the mechanism of action? Eur. J. Surg. 164, 111–114. doi: 10.1080/11024159850191544
Andrews, J., Djakiew, D., Krygier, S., and Andrews, P. (2002). Superior effectiveness of ibuprofen compared with other NSAIDs for reducing the survival of human prostate cancer cells. Cancer Chemother. Pharmacol. 50, 277–284. doi: 10.1007/s00280-002-0485-8
Bonelli, P., Tuccillo, F., Calemma, R., Pezzetti, F., Borrelli, A., Martinelli, R., et al. (2011). Changes in the gene expression profile of gastric cancer cells in response to ibuprofen: a gene pathway analysis. Pharmacogenomics J. 11, 412–428. doi: 10.1038/tpj.2010.55
Chen, Y., Xia, R., Huang, Y., Zhao, W., Li, J., Zhang, X., et al. (2016). An immunostimulatory dual-functional nanocarrier that improves cancer immunochemotherapy. Nat. Commun. 7:13443. doi: 10.1038/ncomms13443
Cho, K., Wang, X., Nie, S., and Shin, D. M. (2008). Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 14, 1310–1316. doi: 10.1158/1078-0432.CCR-07-1441
Craparo, E. F., Teresi, G., Licciardi, M., Bondí, M. L., and Cavallaro, G. (2013). Novel composed galactosylated nanodevices containing a ribavirin prodrug as hepatic cell-targeted carriers for HCV treatment. J. Biomed. Nanotechnol. 9, 1107–1122. doi: 10.1166/jbn.2013.1608
Davies, G., Martin, L.-A., Sacks, N., and Dowsett, M. (2002). Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann. Oncol. 13, 669–678. doi: 10.1093/annonc/mdf125
Dial, E. J., Doyen, J. R., and Lichtenberger, L. M. (2006). Phosphatidylcholine-associated nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit DNA synthesis and the growth of colon cancer cells in vitro. Cancer Chemother. Pharmacol. 57, 295–300. doi: 10.1007/s00280-005-0048-x
Endo, H., Yano, M., Okumura, Y., and Kido, H. (2014). Ibuprofen enhances the anticancer activity of cisplatin in lung cancer cells by inhibiting the heat shock protein 70. Cell Death Dis. 5:e1027. doi: 10.1038/cddis.2013.550
Gately, S., and Kerbel, R. (2003). Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis. Prog. Exp. Tumor. Res. 37, 179–192. doi: 10.1159/000071373
Gaucher, G., Dufresne, M.-H., Sant, V. P., Kang, N., Maysinger, D., and Leroux, J.-C. (2005). Block copolymer micelles: preparation, characterization and application in drug delivery. J. Control Release 109, 169–188. doi: 10.1016/j.jconrel.2005.09.034
Hasegawa, U., Van Der Vlies, A. J., Wandrey, C., and Hubbell, J. A. (2013). Preparation of well-defined ibuprofen prodrug micelles by RAFT polymerization. Biomacromolecules 14, 3314–3320. doi: 10.1021/bm4009149
Janssen, A., Schiffmann, S., Birod, K., Maier, T. J., Wobst, I., Geisslinger, G., et al. (2008). p53 is important for the anti-proliferative effect of ibuprofen in colon carcinoma cells. Biochem. Biophys. Res. Commun. 365, 698–703. doi: 10.1016/j.bbrc.2007.11.051
Klaikherd, A., Nagamani, C., and Thayumanavan, S. (2009). Multi-stimuli sensitive amphiphilic block copolymer assemblies. J. Am. Chem. Soc. 131, 4830–4838. doi: 10.1021/ja809475a
Kobayashi, H., Watanabe, R., and Choyke, P. L. (2014). Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 4, 81–89. doi: 10.7150/thno.7193
Koudelka, S., Knotigova, P. T., Masek, J., Prochazka, L., Lukac, R., Miller, A. D., et al. (2015). Liposomal delivery systems for anti-cancer analogues of vitamin E. J. Control Release 207, 59–69. doi: 10.1016/j.jconrel.2015.04.003
Lee, A., Di Mascolo, D., Francardi, M., Piccardi, F., Bandiera, T., and Decuzzi, P. (2016). Spherical polymeric nanoconstructs for combined chemotherapeutic and anti-inflammatory therapies. Nanomedicine 12, 2139–2147. doi: 10.1016/j.nano.2016.05.012
Lee, H., Lee, Y. S., Lee, K. D., and Park, S. Y. (2011). Development of disulfide core-crosslinked pluronic nanoparticles as an effective anticancer-drug-delivery system. Macromol. Biosci. 11, 1264–1271. doi: 10.1002/mabi.201100083
Lim, J. T., Piazza, G. A., Han, E. K.-H., Delohery, T. M., Li, H., Finn, T. S., et al. (1999). Sulindac derivatives inhibit growth and induce apoptosis in human prostate cancer cell lines. Biochem. Pharmacol. 58, 1097–1107. doi: 10.1016/S0006-2952(99)00200-2
Meng, H., Xue, M., Xia, T., Ji, Z., Tarn, D. Y., Zink, J. I., et al. (2011). Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano 5, 4131–4144. doi: 10.1021/nn200809t
Miao, L., Guo, S., Lin, C. M., Liu, Q., and Huang, L. (2017). Nanoformulations for combination or cascade anticancer therapy. Adv. Drug Deliv. Rev. 115, 3–22. doi: 10.1016/j.addr.2017.06.003
Mitra, S., Gaur, U., Ghosh, P., and Maitra, A. (2001). Tumour targeted delivery of encapsulated dextran–doxorubicin conjugate using chitosan nanoparticles as carrier. J. Control Release 74, 317–323. doi: 10.1016/S0168-3659(01)00342-X
Rösler, A., Vandermeulen, G. W., and Klok, H.-A. (2012). Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 64, 270–279. doi: 10.1016/j.addr.2012.09.026
Senevirathne, S. A., Washington, K. E., Biewer, M. C., and Stefan, M. C. (2016). PEG based anti-cancer drug conjugated prodrug micelles for the delivery of anti-cancer agents. J. Mater. Chem. B 4, 360–370. doi: 10.1039/C5TB02053K
Shen, Y., Jin, E., Zhang, B., Murphy, C. J., Sui, M., Zhao, J., et al. (2010). Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J. Am. Chem. Soc. 132, 4259–4265. doi: 10.1021/ja909475m
Smalley, W. E., and DuBois, R. N. (1997). Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv. Pharmacol. 39, 1–20. doi: 10.1016/S1054-3589(08)60067-8
Sun, J., Chen, Y., Huang, Y., Zhao, W., Liu, Y., Venkataramanan, R., et al. (2017a). Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemotherapeutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier. Acta Pharmacol. Sin. 38, 823–834. doi: 10.1038/aps.2017.44
Sun, J., Liu, Y., Chen, Y., Zhao, W., Zhai, Q., Rathod, S., et al. (2017b). Doxorubicin delivered by a redox-responsive dasatinib-containing polymeric prodrug carrier for combination therapy. J. Control Release 258, 43–55. doi: 10.1016/j.jconrel.2017.05.006
Sun, J., Chen, Y., Li, K., Huang, Y., Fu, X., Zhang, X., et al. (2016a). A prodrug micellar carrier assembled from polymers with pendant farnesyl thiosalicylic acid moieties for improved delivery of paclitaxel. Acta Biomater. 43, 282–291. doi: 10.1016/j.actbio.2016.07.014
Sun, J., Luo, T., Sheng, R., Li, H., Wang, Z., and Cao, A. (2016b). Intracellular plasmid DNA delivery by self-assembled nanoparticles of amphiphilic PHML-b-PLLA-b-PHML copolymers and the endocytosis pathway analysis. J. Biomater. Appl. 31, 606–621.
Sun, J., Luo, T., Sheng, R., Li, H., and Cao, A. (2013a). Preparation of cationic l-lysine conjugated poly (2-hydroxyethyl methacrylate) s and their potential application as low cytotoxic efficient gene delivery vectors. J. Control Release 172:e108. doi: 10.1016/j.jconrel.2013.08.263
Sun, J., Luo, T., Sheng, R., Li, H., Chen, S., Hu, F., et al. (2013b). Preparation of functional water-soluble low-cytotoxic poly(methacrylate)s with pendant cationic l-lysines for efficient gene delivery. Macromol. Biosci. 13, 35–47. doi: 10.1002/mabi.201200304
Tucker, B. S., Getchell, S. G., Hill, M. R., and Sumerlin, B. S. (2015). Facile synthesis of drug-conjugated PHPMA core-crosslinked star polymers. Polym. Chem. 6, 4258–4263. doi: 10.1039/C5PY00497G
Ulrich, C. M., Bigler, J., and Potter, J. D. (2006). Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat. Rev. Cancer 6, 130–140. doi: 10.1038/nrc1801
Wang, Y., Luo, Q., Gao, L., Gao, C., Du, H., Zha, G., et al. (2014). A facile strategy to prepare redox-responsive amphiphilic PEGylated prodrug with high drug loading content and low critical micelle concentration. Biomater. Sci. 2, 1367–1376. doi: 10.1039/C4BM00065J
Wang, Z., Luo, T., Sheng, R., Li, H., Sun, J., and Cao, A. (2015). Amphiphilic diblock terpolymer PMAgala-b-P (MAA-co-MAChol) s with attached galactose and cholesterol grafts and their intracellular pH-responsive doxorubicin delivery. Biomacromolecules 17, 98–110. doi: 10.1021/acs.biomac.5b01227
Wang, Z., Sheng, R., Luo, T., Sun, J., and Cao, A. (2017). Synthesis and self-assembly of diblock glycopolypeptide analogues PMAgala-b-PBLG as multifunctional biomaterials for protein recognition, drug delivery and hepatoma cell targeting. Polym. Chem. 8, 472–484. doi: 10.1039/C6PY01526C
Wei, H., Zhuo, R.-X., and Zhang, X.-Z. (2013). Design and development of polymeric micelles with cleavable links for intracellular drug delivery. Prog. Polym. Sci. 38, 503–535. doi: 10.1016/j.progpolymsci.2012.07.002
Zhang, X., Huang, Y., Zhao, W., Chen, Y., Zhang, P., Li, J., et al. (2014). PEG-farnesyl thiosalicylic acid telodendrimer micelles as an improved formulation for targeted delivery of paclitaxel. Mol. Pharm. 11, 2807–2814. doi: 10.1021/mp500181x
Keywords: ibuprofen, nanomicellar, prodrug, carrier, doxorubicin, co-delivery
Citation: Li Z, Sun J, Huang Y, Liu Y, Xu J, Chen Y, Liang L, Li J, Liao Q, Li S and Zhou K (2018) A Nanomicellar Prodrug Carrier Based on Ibuprofen-Conjugated Polymer for Co-delivery of Doxorubicin. Front. Pharmacol. 9:781. doi: 10.3389/fphar.2018.00781
Received: 30 April 2018; Accepted: 27 June 2018;
Published: 14 August 2018.
Edited by:
Qingxin Mu, University of Washington, United StatesCopyright © 2018 Li, Sun, Huang, Liu, Xu, Chen, Liang, Li, Liao, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Li, c29sNEBwaXR0LmVkdQ== Kechao Zhou, emhvdWtlY2hhb0Bjc3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.