- Department of Internal Medicine, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, China
Ligustrazine (Lig) is one of the main effective components of Ligusticum Chuanxiong Hort, which possesses a variety of biological activities in the cardiovascular system. Here, we conducted a preclinical systematic review to investigate the efficacy of Lig for animal models of myocardial ischemia/reperfusion injury and its possible mechanisms. Twenty-five studies involving 556 animals were identified by searching 6 databases from inception to August 2017. The methodological quality was assessed by using Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) 10-item checklist. All the data were analyzed using Rev-Man 5.3 software. As a result, the score of study quality ranged from 2 to 6 points. Meta-analyses showed Lig can significantly decrease the myocardial infarct size, cardiac enzymes and troponin compared with control (P < 0.01). The possible mechanisms of Lig for myocardial infarction are antioxidant, anti-inflammatory, anti-apoptosis activities and improving coronary blood flow and myocardial metabolism. In conclusion, the findings indicated that Lig exerts cardio protection through multiple signaling pathways in myocardial ischemia/reperfusion injury.
Introduction
Myocardial infarction (MI) is one of the most common causes of death and disability worldwide (Mozaffarian et al., 2015). The injuries inflicted on the myocardium during acute MI are the result of two processes: ischemia and subsequent reperfusion [ischemia/reperfusion (I/R) injury] (Ibáñez et al., 2015). In patients with MI, the treatment of choice for reducing acute myocardial ischemic injury and limiting MI size is timely and effective myocardial reperfusion using either thombolytic therapy or primary percutaneous coronary intervention (PPCI) (Hausenloy and Yellon, 2013). However, abrupt restoration therapy of coronary flow can lead to possible result in adverse events (Heusch and Gersh, 2017) such as reversible impairment of myocardial contractility (myocardial stunning), ventricular arrhythmias, and microvascular dysfunction, for which there is still no effective therapy (Hausenloy and Yellon, 2013). Several strategies have been developed to attenuate and/or modulate the extent of the I/R injury associated with cardiopulmonary attack for years (Chun et al., 2011); however, results of clinical trials disappoint us, and there are some large clinical trials evaluating promising interventions from bench to bedside that have just begun (Frank et al., 2012; Schmidt et al., 2015). Thus, it is urgent to seek new cardioprotective strategies to improve myocardial salvage and cardiac function when myocardial I/R injury happen.
Chuanxiong, Rhizoma Ligustici Chuanxiong, sichuan lovage rhizome, the dried rhizomes of Ligusticum chuanxiong Hort., a perennial herbal plant of the Umbelliferae/Apiaceaefamily, has the function of activating blood and promoting Qi, first recorded in the earliest complete Pharmacopoeia of China, Shennong Bencao Jing (Shennong's Classic of Materia Medica) from Warring States Period to Han Dynasty. Qi, an important concept in Huangdi Neijing (Huangdi's Internal Classic) written in AD 206~221, is of the vital substances to comprise body and is the vital energy to maintain life (vital Qi), whereas the exogenous pathogenic factors and/or endogenous pathological changes in the body leads to varieties of diseases (pathogenic Qi) (Chen and Chen, 1998; Yuan et al., 2013). Thus, to promote Qi is a key treatment method in traditional Chinese medicine. Chuanxiong has been widely used in the treatment of cardiovacular diseases for thousands of years and is still widely used in modern time due to its extensive biological activities (Lu et al., 2015). Ligustrazine (Lig) (Figure 1) is one of the main effective components of Chuanxiong, which exerts potential cardio/cerebrovascular protective effects (Zhang et al., 2014; Xu et al., 2017). Lig have indicated that Lig and its numerous metabolites have outstanding pharmacokinetic characteristics, such as rapid metabolism, broad distribution and no accumulated toxic effect (Zou et al., 2018). In addition, animal models are invaluable tools for enriching our understanding of the mechanisms, etiology and treatment of human diseases (Sena et al., 2014). Preclinical systematic review can evaluate the efficacy of drugs more systematically, establish a test field for further animal experiments, provide reliable information for drug research, and lay a foundation for future clinical research (Sena et al., 2014; Disma et al., 2016; Zhang et al., 2017). Thus, we aim to evaluate the available preclinical evidence and possible mechanisms of Lig on cardioprotection in animal experiments of myocardial I/R injury.
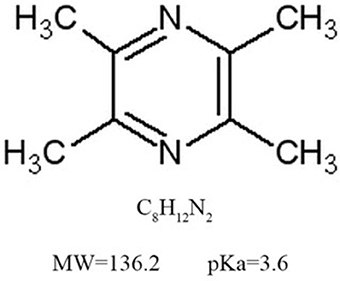
Figure 1. The chemical structure of ligustrazine. The molecular formula of ligustrazineis C8H12N2. The molecular weight of ligustrazine is 136.2, and the value of pKa is 3.6.
Methods
Data Sources and Search Strategy
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement was abided (Stewart et al., 2015). We electronically searched in PubMed, EMBASE, Science Direct, Web of Science, Wanfang data Information Site, Chinese National Knowledge Infrastructure (CNKI), and VIP information database from inception to the end of August, 2017 using the following key words: “ligustrazine (MeSH Terms) OR tetramethylpyrazine (MeSH Terms) OR Ligustrazine (Title/Abstract) OR tetramethylpyrazine (Title/Abstract)” AND “myocardial infarction OR myocardial ischemia OR myocardial ischemia/reperfusion injury OR myocardial I/R injury.” Additional studies were identified through the reference lists of relevant reports. All the studies included were limited on animals.
Study Selection and Data Extraction
Two investigators (Zheng Q and Zhu PC) independently screened the titles and/or abstracts, of the search results and assessed the remaining full-text articles for eligibility. Any uncertainty eligibility was resolved by discussion. Studies were eligible for our systematic review if they met: (1) Lig for animal models of myocardial I/R injury established by ligating of the left anterior descending coronary artery (LAD) or injecting intravenously vasoconstrictor; (2) Analyzed interventions was received Lig as monotherapy at any dose. Comparator interventions were isasteric and non-functional liquid (normal saline) or no treatment; (3) the primary outcome measures were MI size and/or cardiac enzymes and/or cardiac troponin T (cTnT) and/or the level of ST-segment depression and/or left ventricular ejection fraction (LVEF) and/or shortening fraction (FS). The secondary outcome measures were mechanisms of Lig for myocardial I/R injury. Prespecified exclusion criteria were the treatment of Lig in conjunction with other compounds or Lig-based prescriptions, non-myocardial ischemia model, no control group, not published in peer-review journals, and duplicate publications. In the case of multiple publications from one study, we chose the articles with the largest sample or the earliest publication.
Two independent investigators (Zheng Q and Huang YY) extracted the following details from the included studies: (1) name of first author and year of publication; (2) details (species, number, sex, and weight) of animals for each study; (3) methods to establish animal models of myocardial I/R, and the anesthesia methods for model preparation; (4) the information of treatment group, including therapeutic drug dosage, method of administration, duration of treatment, and the same information of control group; (5) mean value and standard deviation of outcomes. The data of highest dose was selected when the treatment group included various doses of the target drug. The result of the peak time point was included when the data were expressed at different times.
Risk of Bias in Individual Studies
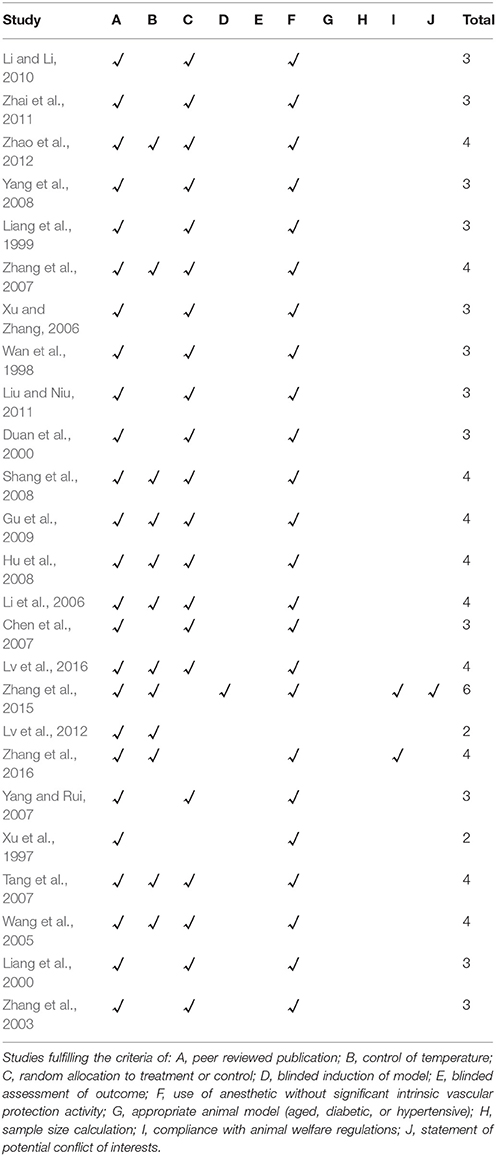
The methodological quality of each included study was evaluated by using Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) 10-item checklist (Macleod et al., 2004) with minor modification (Yu et al., 2017) as follows: A: peer-reviewed publication; B: control of temperature; C: random allocation to treatment or control; D: blinded induction of model; E: blinded assessment of outcome; F: use of anesthetic without significant intrinsic cardioprotective activity; G: appropriate animal model (aged, diabetic, or hypertensive); H: sample size calculation; I: compliance with animal welfare regulations; and J: statement of potential conflict of interests. Every item was given one point. Two investigators (P.C. Z and Y.Y. H) independently evaluated the study quality and divergences were well settled through consulting with correspondence authors.
Statistical Analysis
Meta-analyses and sub-analyses were performed using RevMan V.5.3 software. Outcome measures were all considered as continuous data and given an estimate of the combined overall effect sizes utilizing standard mean difference (SMD) or mean difference (MD) with the effects model. SMD or MD with its 95% confidence interval (CI) was used to assess the strength of efficacy of Lig for myocardial I/R injury. The I2 statistic was used for assessment of heterogeneity among individual studies. A fixed effects model (I2 < 50%) or a random effects model (I2 > 50%) was used depending on the value of I2. Probability value p < 0.05 was considered significant.
Results
Study Inclusion
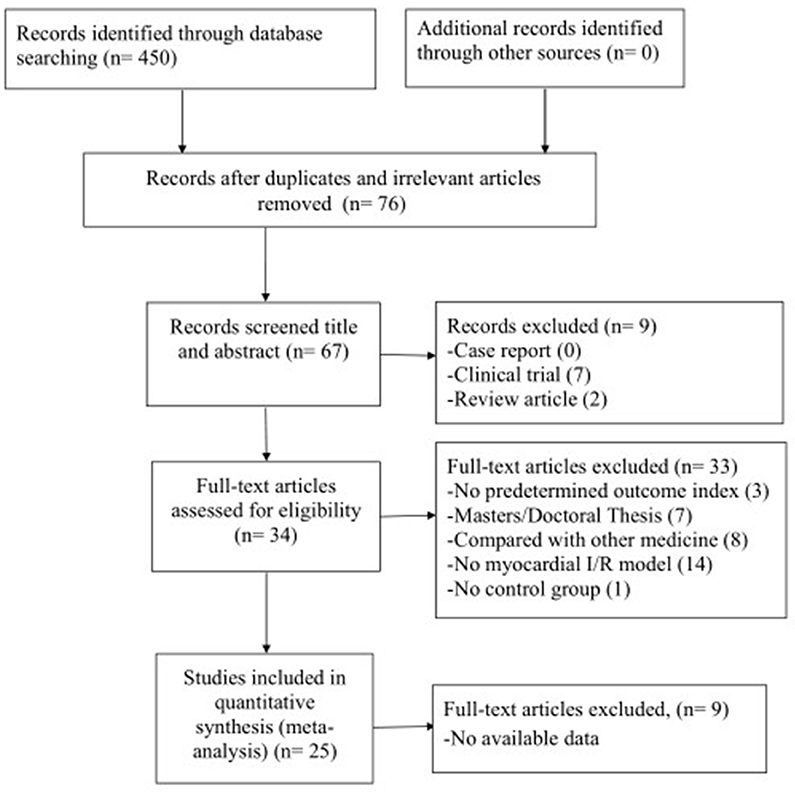
We searched 450 potential studies from the database by literature retrieval, of which 374 repetitive and irrelevant studies were excluded. After screening titles and abstracts, 9 studies were excluded because they were case reports, clinical trials or review articles. Reading the remaining full-text articles, 33 articles were excluded if: (1) not predetermined outcome index; (2) not published in peer-review journals; (3) compared with other medicine; (4) no myocardial I/R model; (5) no control group. Then, we removed 9 studies in which data of result is not available. Finally, 25 eligible studies (Xu et al., 1997; Wan et al., 1998; Liang et al., 1999, 2000; Duan et al., 2000; Zhang et al., 2003, 2007, 2015, 2016; Wang et al., 2005; Li et al., 2006; Xu and Zhang, 2006; Chen et al., 2007; Tang et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Shang et al., 2008; Yang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Zhai et al., 2011; Lv et al., 2012, 2016; Zhao et al., 2012) were included in qualitative analysis (Figure 2).
Characteristics of Included Studies
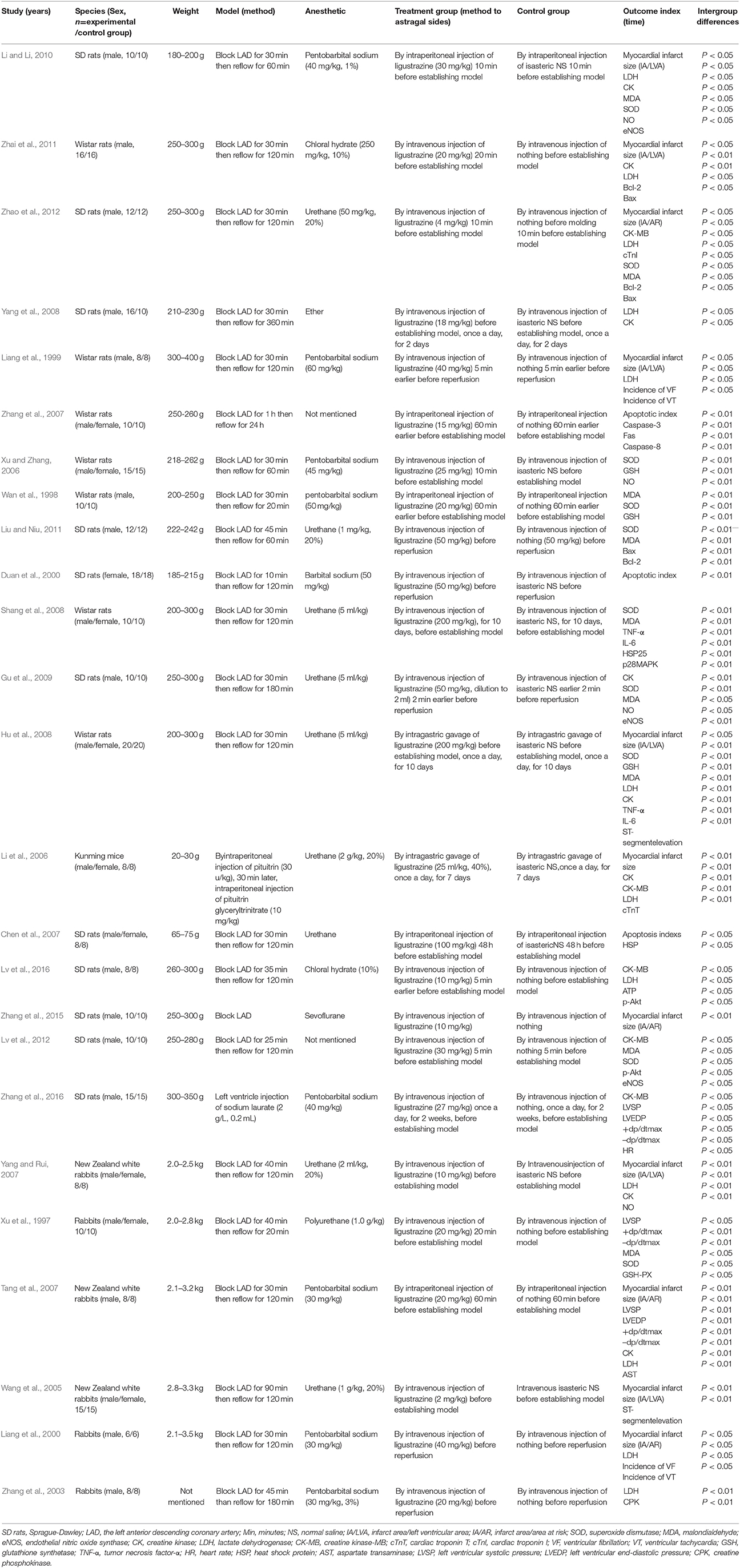
Twenty-two studies (Xu et al., 1997; Wan et al., 1998; Liang et al., 1999, 2000; Duan et al., 2000; Zhang et al., 2003, 2007; Wang et al., 2005; Li et al., 2006; Xu and Zhang, 2006; Chen et al., 2007; Tang et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Shang et al., 2008; Yang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Zhai et al., 2011; Zhao et al., 2012; Lv et al., 2016) were published in Chinese, and 3 studies (Lv et al., 2012; Zhang et al., 2015, 2016) were published in English between 1997 and 2016. Male/female Sprague Dawley rats were used in 11 studies (Duan et al., 2000; Li et al., 2006; Chen et al., 2007; Yang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Lv et al., 2012, 2016; Zhao et al., 2012; Zhang et al., 2015, 2016), male/female Wistar rats in 7 studies (Wan et al., 1998; Liang et al., 1999; Xu and Zhang, 2006; Zhang et al., 2007; Hu et al., 2008; Shang et al., 2008; Zhai et al., 2011), male/female Kunming mice in 1 study (Li et al., 2006), and male/female rabbits in 6 studies (Xu et al., 1997; Liang et al., 2000; Zhang et al., 2003; Wang et al., 2005; Tang et al., 2007; Yang and Rui, 2007). To induce anesthesia, 2 studies (Zhang et al., 2007; Zhai et al., 2011) used chloral hydrate; 1 study (Zhang et al., 2015) used sevoflurane; 8 studies (Liang et al., 1999, 2000; Zhang et al., 2003, 2007, 2016; Xu and Zhang, 2006; Tang et al., 2007; Li and Li, 2010) used pentobarbital sodium; 1 study (Duan et al., 2000) used babital sodium; 1 study (Yang et al., 2008) used ethyl ether; and 9 studies (Wang et al., 2005; Li et al., 2006; Chen et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Shang et al., 2008; Gu et al., 2009; Liu and Niu, 2011; Zhao et al., 2012) used urethane, while anaesthetic was not mentioned in the other 2 studies (Zhang et al., 2007; Lv et al., 2012). All myocardial I/R models were established by ligation of the LAD. Among the dose use of Lig, 2 studies (Hu et al., 2008; Shang et al., 2008)used 200 mg·kg−1;1 study (Li et al., 2006) used 100 mg·kg−1; 3 studies (Duan et al., 2000; Gu et al., 2009; Liu and Niu, 2011) used 50 mg·kg−1; 2 studies (Liang et al., 1999, 2000) used 40 mg·kg−1; 2 studies (Li and Li, 2010; Lv et al., 2012) used 30 mg·kg−1; 1 study (Zhang et al., 2016) used 27 mg·kg−1; 1 study (Xu and Zhang, 2006) used 25 mg·kg−1; 5 studies (Xu et al., 1997; Wan et al., 1998; Zhang et al., 2003; Tang et al., 2007; Zhai et al., 2011) used 20 mg·kg−1;1 study (Yang et al., 2008) used 18 mg·kg−1;1 study (Zhang et al., 2007) used 15 mg·kg−1; 4 studies (Li et al., 2006; Yang and Rui, 2007; Zhang et al., 2015; Lv et al., 2016) used 10 mg·kg−1;1 study (Zhao et al., 2012) used 4 mg·kg−1;the remaining 1 study (Wang et al., 2005) used 2 mg·kg−1.MI size was utilized as outcome measure in 10 studies (Liang et al., 1999, 2000; Wang et al., 2005; Tang et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Li and Li, 2010; Zhai et al., 2011; Zhao et al., 2012; Zhang et al., 2015). Incidence of ventricular fibrillation (VF) and incidence of ventricular tachycardia (VT) was reported in 2 studies (Liang et al., 1999, 2000), level of ST-segment elevation was reported in 1 study (Wang et al., 2005), but LVEF and FS was not mentioned. Lactate dehydrogenase (LDH) was reported in 11 studies (Liang et al., 1999, 2000; Zhang et al., 2003; Tang et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Yang et al., 2008; Zhai et al., 2011; Zhao et al., 2012; Lv et al., 2016), creatine kinase (CK) in 8 studies (Li et al., 2006; Tang et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Yang et al., 2008; Gu et al., 2009; Li and Li, 2010; Zhai et al., 2011), creatine kinase-MB (CK-MB) in 5 studies (Li et al., 2006; Liu and Niu, 2011; Lv et al., 2012, 2016; Zhao et al., 2012; Zhang et al., 2016), aspartate transaminase (AST) in 1 study (Tang et al., 2007), cTnT in 1 study (Liang et al., 1999), cTnI in 1 study (Zhao et al., 2012), myocardial cell apoptotic index in 3 studies (Duan et al., 2000; Chen et al., 2007; Zhang et al., 2007), superoxide dismutase (SOD) in 9 studies (Xu et al., 1997; Wan et al., 1998; Xu and Zhang, 2006; Hu et al., 2008; Shang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Lv et al., 2012), and malondialdehyde (MDA) in 9 studies (Xu et al., 1997; Wan et al., 1998; Hu et al., 2008; Shang et al., 2008; Gu et al., 2009; Li and Li, 2010; Lv et al., 2012; Zhao et al., 2012), glutathione (GSH) in 3 studies (Wan et al., 1998; Xu and Zhang, 2006; Hu et al., 2008), phosphothreonine kinase (p-Akt) in 2 studies (Lv et al., 2012, 2016), caspase-3 in 1 study (Zhang et al., 2007), NO in 4 studies (Xu and Zhang, 2006; Yang and Rui, 2007; Gu et al., 2009; Li and Li, 2010), tumor necrosis factor-α (TNF-α) in 1 study (Hu et al., 2008), heat shock protein (HSP) in 1 study (Chen et al., 2007), IL-6 in 2 studies (Hu et al., 2008; Shang et al., 2008), creatine phosphokinase (CPK) in 1 study (Zhang et al., 2003), and ATP in1 study (Lv et al., 2016). The overall characteristics of included studies are shown in Table 1.
Study Quality
The quality score of studies ranged from 2 to 6. All studies were publications in a peer reviewed journal. Twelve studies (Wang et al., 2005; Li et al., 2006; Tang et al., 2007; Zhang et al., 2007, 2015, 2016; Hu et al., 2008; Shang et al., 2008; Gu et al., 2009; Lv et al., 2012, 2016; Zhao et al., 2012) reported control of temperature. Twenty-one studies (Wan et al., 1998; Liang et al., 1999, 2000; Duan et al., 2000; Zhang et al., 2003, 2007; Wang et al., 2005; Li et al., 2006; Xu and Zhang, 2006; Chen et al., 2007; Tang et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Shang et al., 2008; Yang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Zhai et al., 2011; Zhao et al., 2012; Lv et al., 2016) described random allocation to treatment or control. One study (Zhang et al., 2015) mentioned blinded induction of model. None of studies described blinded assessment of outcome. Twenty-four studies (Xu et al., 1997; Wan et al., 1998; Liang et al., 1999, 2000; Duan et al., 2000; Zhang et al., 2003, 2007, 2015, 2016; Wang et al., 2005; Li et al., 2006; Xu and Zhang, 2006; Chen et al., 2007; Tang et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Shang et al., 2008; Yang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Zhai et al., 2011; Zhao et al., 2012; Lv et al., 2016) used anesthetic without significant intrinsic vascular protection activity. No study describes appropriate animal model (aged, diabetic, or hypertensive) and the sample size calculation. Two studies (Zhang et al., 2015, 2016) stated compliance with animal welfare regulations and 1 study (Zhang et al., 2015) declared statement of potential conflict of interests. The methodological quality is concluded in Table 2.
Effectiveness
MI Size
Ten studies (Liang et al., 1999, 2000; Wang et al., 2005; Tang et al., 2007; Yang and Rui, 2007; Hu et al., 2008; Li and Li, 2010; Zhai et al., 2011; Zhao et al., 2012; Zhang et al., 2015) utilized MI size as outcome measure. All of them showed significant effect of Lig for decreasing the MI size (P < 0.05). Because the methods to calculate MI size are different, we divided the studies into two parts as follow: (1) Infarct area/left ventricular area (IA/LVA): Five studies (Liang et al., 1999; Hu et al., 2008; Li and Li, 2010; Zhai et al., 2011; Zhang et al., 2015) were carried out in rats and 2 studies (Wang et al., 2005; Yang and Rui, 2007) were carried out in rabbits. Four studies (Liang et al., 1999; Li and Li, 2010; Zhai et al., 2011; Zhang et al., 2015) used male rats, whereas 1 study (Hu et al., 2008) used male/female rats. Meta-analysis of above 4 studies (Liang et al., 1999; Li and Li, 2010; Zhai et al., 2011; Zhang et al., 2015) showed significant effect of Lig for decreasing the MI size compared with control group (n = 34, SMD −3.00, 95% CI [2.24 to −3.77], P < 0.01; heterogeneity: χ2 = 5.50, df = 3 (P = 0.14); I2 = 45%) (Figure 3). They failed to pool meta-analysis of 2 studies in rabbits, because the time of MI model induced was by blocking LAD for 40 min (Yang and Rui, 2007) vs. 90 min (Wang et al., 2005). (2) Infarct area/area at risk (IA/AR): Two studies (Liang et al., 2000; Tang et al., 2007) used in rabbits and one study (Zhao et al., 2012) used in rats. Meta-analysis of 2 studies in rabbits (Liang et al., 2000; Tang et al., 2007) showed significant effect of Lig for decreasing the MI size compared with control group (n = 14, SMD −2.62, 95% CI [1.52 to −3.72], P < 0.01; heterogeneity: χ2 = 0.01, df = 1 (P = 0.92); I2 = 0%; Figure 4).
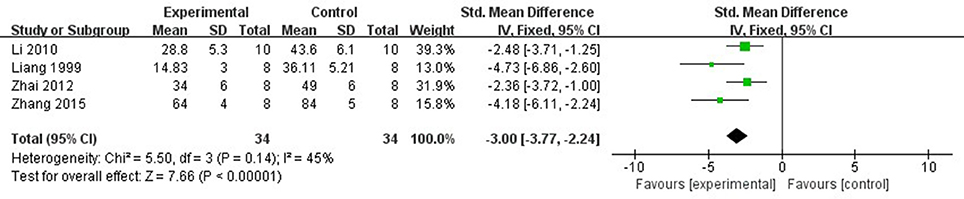
Figure 3. The forest plot: effects of ligustrazine for decreasing the myocardial infarction size (infarct area/left ventricular area) in rats compared with control group.
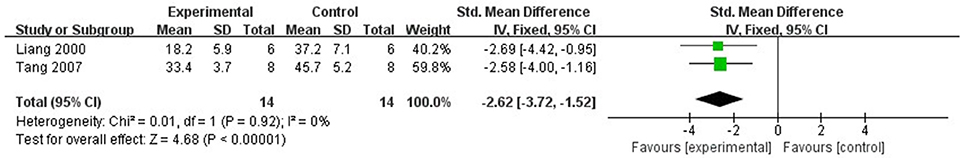
Figure 4. The forest plot: effects of ligustrazine for decreasing the myocardial infarction size (infarct area/area at risk) in rabbits compared with control group.
Cardiac Enzymes and/or Troponin
In rat studies, 7 studies used LDH as the outcome (Liang et al., 1999; Hu et al., 2008; Yang et al., 2008; Li and Li, 2010; Zhai et al., 2011; Zhao et al., 2012; Lv et al., 2016). Meta-analysis of 6 studies (Liang et al., 1999; Hu et al., 2008; Li and Li, 2010; Zhai et al., 2011; Zhao et al., 2012; Lv et al., 2016) showed significant effect of Lig for decreasing the LDH compared with control group (n = 64, SMD −3.55, 95% CI [−2.95 to −4.15], P < 0.01; heterogeneity: χ2 = 7.83, df = 5 (P = 0.17); I2 = 36%) (Figure 5). We removed 1 study (Yang et al., 2008) that used LDH as the outcome, in which time of reperfusion was 360 min vs. 90 min in above studies. Meta-analysis of 5 studies (Li et al., 2006; Hu et al., 2008; Gu et al., 2009; Li and Li, 2010; Zhai et al., 2011) in rats showed significant effect of Lig for decreasing the CK compared with control group (n = 46, SMD −1.93, 95% CI [1.40 to −2.46], P < 0.01; heterogeneity: χ2 = 7.46, df = 4 (P = 0.11); I2 = 46%; Figure 6). Meta-analysis of 4 studies in rats (Lv et al., 2012, 2016; Zhao et al., 2012; Zhang et al., 2016) showed significant effect of Lig for decreasing the CK-MB compared with control group (n = 36, SMD −2.47, 95% CI [1.82 to −3.12], P < 0.01; heterogeneity: χ2 = 1.53, df = 3 (P = 0.67); I2 = 0%) (Figure 7). Lig significantly decreased AST (Tang et al., 2007), cTnT (Liang et al., 1999) or cTnI (Zhao et al., 2012) in rats respectively compared with control (P < 0.05).
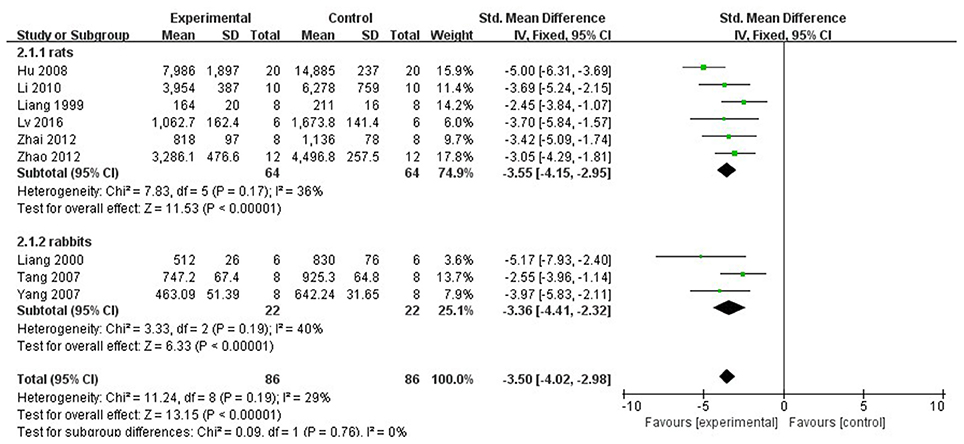
Figure 5. The forest plot: effects of ligustrazine for decreasing lactate dehydrogenase compared with control group.
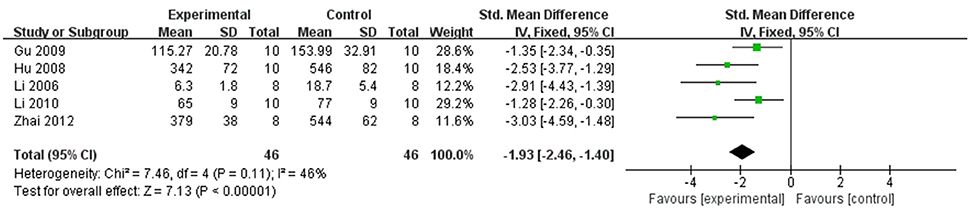
Figure 6. The forest plot: effects of ligustrazine for decreasing creatine kinase in rats compared with control group.
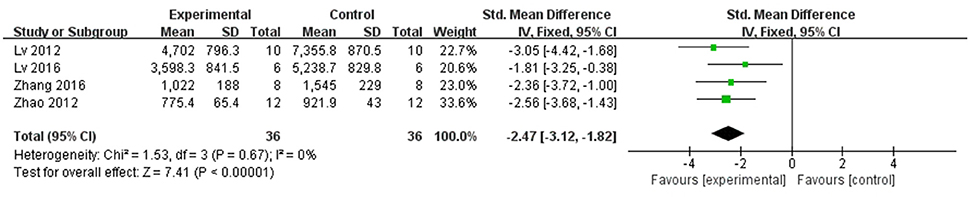
Figure 7. The forest plot: effects of ligustrazine for decreasing creatine kinase-MB in rats compared with control group.
In rabbit studies, meta-analysis of 3 studies (Liang et al., 2000; Tang et al., 2007; Yang and Rui, 2007) showed significant effect of Lig for decreasing the LDH compared with control group (n = 22, SMD −3.36, 95% CI [2.32 to −4.41], P < 0.00001; heterogeneity: χ2 = 3.33, df = 2 (P = 0.19); I2 = 40%) (Figure 5). Two studies (Tang et al., 2007; Yang and Rui, 2007) in rabbits showed significant effect of Lig for decreasing the CK compared with control group (P < 0.05). They failed to pool meta-analysis because the time of MI model induced was by blocking LAD for 40 min (Yang and Rui, 2007). One study (Wang et al., 2005) reported that Lig can improve the ST-segment depression compared with control (P < 0.05). There was no study involving LVEF and FS as outcome measure.
Cardioprotective Mechanisms
Compared with controls, meta-analysis of 6 studies (Wan et al., 1998; Xu and Zhang, 2006; Shang et al., 2008; Gu et al., 2009; Liu and Niu, 2011; Lv et al., 2012) showed that Lig significantly increased SOD (n = 67, MD 3.92, 95% CI [3.30–4.55], P < 0.01; heterogeneity: χ2 = 6.31, df = 5 (P = 0.28); I2 = 21%; Figure 8); 2 studies (Liang et al., 2000; Lv et al., 2012) for increasing GSH (n = 25, SMD 4.09, 95% CI [3.05–5.14], P < 0.01; heterogeneity: χ2 = 1.65, df = 1 (P = 0.20); I2 = 39%; Figure 9); 9 studies (Xu et al., 1997; Wan et al., 1998; Hu et al., 2008; Shang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Lv et al., 2012; Zhao et al., 2012) for reducing MDA (P < 0.05); 4 studies (Xu and Zhang, 2006; Yang and Rui, 2007; Gu et al., 2009; Li and Li, 2010) for increasing NO (P < 0.05); 1 study (Zhang et al., 2003) for increasing CPK (P < 0.05); 3 studies (Duan et al., 2000; Chen et al., 2007; Zhang et al., 2007) for decreasing apoptotic index (P < 0.05); 2 studies (Lv et al., 2012, 2016) for increasing phosphothreonine kinase (p-Akt) (P < 0.05); 1 study (Zhang et al., 2007) for decreasing caspase-3 (P < 0.05); 1 study (Hu et al., 2008) for decreasing TNF-α (P < 0.05); 1 study (Chen et al., 2007) for decreasing HSP (P < 0.05); 2 studies (Hu et al., 2008; Shang et al., 2008) for decreasing IL-6 (P < 0.05); 1 study (Lv et al., 2016) for increasing ATP (P < 0.05).
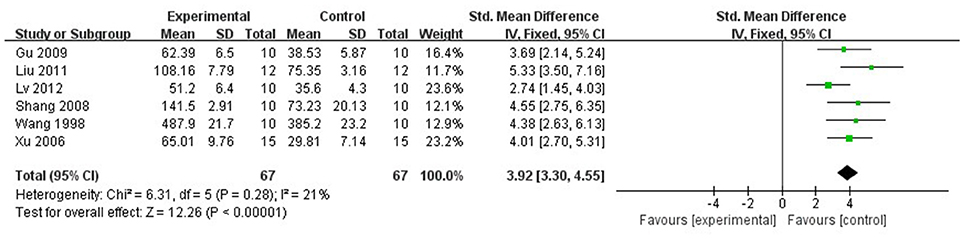
Figure 8. The forest plot: effects of ligustrazine for increasing superoxide dismutase compared with control group.
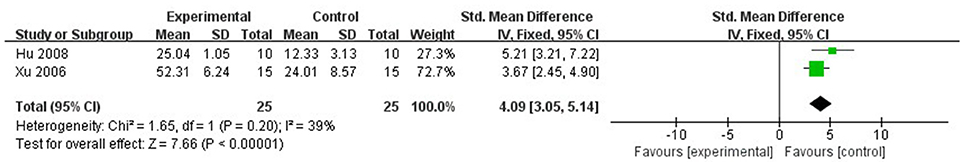
Figure 9. The forest plot: effects of ligustrazine for increasing glutathione compared with control group.
Discussion
Summary of Evidence
Twenty-five studies involving 556 animals were identified. The findings from present study demonstrated that Lig exerts cardioprotection inmyocardial I/R injury, largely through antioxidant, anti-inflammatory, anti-apoptotic activities, and improving coronary blood flow and myocardial metabolism via multiple signaling pathways. Despite significant positive results, we should treat the results consciously because of the flaw of methodological quality.
Limitations
Some limitations should be considered while interpreting this study. First, we only included studies from Chinese and English databases. The absence of studies written in other languages may lead to certain degree of selective bias (Nolting et al., 2012). Second, study quality was considered as moderate, which ranged from 2 to 6 points, may affecting the accuracy of the results (Landis et al., 2012). Third, none of the included studies used animals with relevant comorbidities, which are not in conformity with pathophysiology in patients with MI (Landis et al., 2012). Finally, although the heart protective effect of estrogen has been reported both in clinical and preclinical studies (Menazza et al., 2017), 10 including studies (Duan et al., 2000; Wang et al., 2005; Li et al., 2006; Xu and Zhang, 2006; Chen et al., 2007; Tang et al., 2007; Yang and Rui, 2007; Zhang et al., 2007; Hu et al., 2008; Shang et al., 2008)adopted female animals in this study.
Implications
Poor design of animal research is considered as a roadblock to translate animal research into promising preclinical drug treatments for human disease (Baginskaite, 2012). In the present study, many domains had flaws in aspects of randomization, allocation concealment, and blinding and sample size calculation, which are the core standards of study design (Moher et al., 2015). A lower-quality study trends toward better outcomes, leading to the global estimated effect overstated (García et al., 2012). Thus, in the future study, we recommended that the experimental research of Lig for MI need be promoted by means of incorporating the ARRIVE guidelines (Kilkenny et al., 2012).
The possible mechanisms of Lig for cardioprotective function are summarized as follows: (1) antioxidant through increasing glutathione (GSH) (Wan et al., 1998; Xu and Zhang, 2006; Hu et al., 2008), and enhancing SOD-induced (Xu et al., 1997; Wan et al., 1998; Xu and Zhang, 2006; Hu et al., 2008; Shang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Lv et al., 2012) antioxidant via attenuating chondriokinesis to reduce the release of malondialdehyde (MDA) (Xu et al., 1997; Wan et al., 1998; Xu and Zhang, 2006; Hu et al., 2008; Shang et al., 2008; Gu et al., 2009; Li and Li, 2010; Liu and Niu, 2011; Lv et al., 2012; Zhao et al., 2012); (2) the main anti-inflammatory mechanisms: Lig can inhibit P38MAPK pathway (Qian et al., 2014) and enhance nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/hypoxia-inducible factor 1-alpha (HIF-1α) pathway, and they further inhibited the expression of NF-κB (Lu et al., 2017). Simultaneously, Lig can inhibit the expression of the endothelial cell adhesion molecule of E-selectin, P-selectin and intercellular adhesion molecule-1 (Yang et al., 2008). Ultimately, they can alleviate inflammatory of myocardial I/R injury through inhibiting the expression of tumor necrosis factor-α (Hu et al., 2008), IL- 6 (Hu et al., 2008; Shang et al., 2008; Wang et al., 2017), and/or promoting the expression of HSP (Chen et al., 2007; Shang et al., 2008); (3) inhibition of apoptosis (Duan et al., 2000; Chen et al., 2007; Zhang et al., 2007) through increasing the expression of Bcl-2 (Liu and Niu, 2011; Zhai et al., 2011; Zhao et al., 2012), reducing the expression of Bax protein (Liu and Niu, 2011; Zhai et al., 2011; Zhao et al., 2012) in the myocardium, and down-regulating the expression of caspase and Fas (Zhang et al., 2007); (4) metabolism mechanism through increasing the expression of the protein of p-Akt (Lv et al., 2012, 2016) and content of ATP in myocardium (Lv et al., 2016) with activating PI3K/Akt signal pathway (Lv et al., 2012, 2016); (5) improvement of the circulation by enhancing the expression of NO (Wan et al., 1998; Yang and Rui, 2007; Gu et al., 2009; Li and Li, 2010) via up-regulating the expression of NOS (Li et al., 2006; Xu and Zhang, 2006; Yang and Rui, 2007; Gu et al., 2009). A schematic representation of cardioprotective mechanism of Lig for myocardial I/R injury was summarized in Figure 10. Thus, Ligexerts cardioprotection inmyocardial I/R injury through multiple signaling pathways. Further studies should clarify the exact mechanisms of Lig for MI.
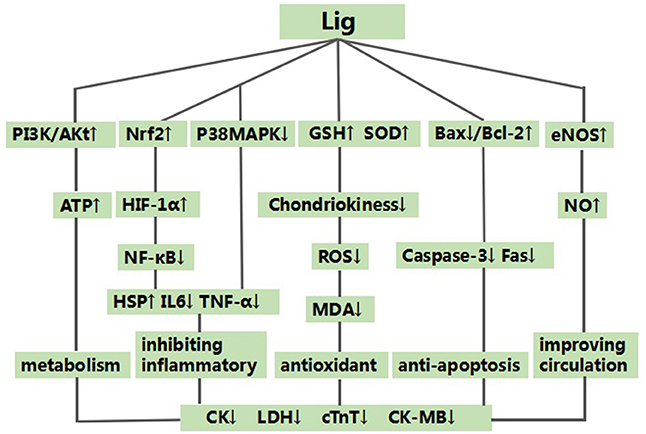
Figure 10. A schematic representation of cardioprotective mechanisms of Ligustrazine for myocardial ischemia/reperfusion injury. ↑ means enhance the expression of relevant protein or pathway. ↓ means inhibit the expression of relevant protein or pathway.
Conclusion
Our findings indicate that Ligexerted cardioprotective function for myocardial I/R injury largely through antioxidant, anti-inflammatory, anti-apoptosis activities and improving coronary blood flow and myocardial metabolism.
Author Contributions
QZ, YH, and PZ contributed equally to this work. QZ, YH, PZ, QT, XB, YW and GZ designed the study; QZ, YH and PZ collected the data; QZ and YH performed all analyses; All authors contributed to writing of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by the grant of National Natural Science Foundation of China (81573750/81473491/81173395/H2902).
References
Baginskaite, J. (2012). Scientific Quality Issues in the Design and Reporting of Bioscience Research: A Systematic Study of Randomly Selected Original in vitro, in vivo and Clinical Study Articles listed in the PubMed database. Available online at: http://www.dcn.ed.ac.uk/camarades/files/Camarades%20Monograph%20201201.pdf (Accessed July 5, 2017).
Chen, T. S., and Chen, P. S. (1998). The liver in traditional Chinese medicine. J. Gastroenterol. Hepatol. 13, 437–442.
Chen, Z. Q., Wu, P. Y., Li, Z. Q., and Zhang, Y. (2007). HSP70 expression, apoptosis and the effect of Ligustrazine on immature myocardial ischemia reperfusion injury. Shan Dong Med. J. 47, 36–37. doi: 10.3969/j.issn.1002-266X.2007.11.015
Chun, K. J., Park, Y. H., Kim, J. S., Jang, Y., Kim, J. H., Kim, J., et al. (2011). Comparison of 5 different remifentanil strategies against myocardial ischemia-reperfusion injury. J. Cardiothorac. Vasc. Anesth. 25, 926–930. doi: 10.1053/j.jvca.2011.02.019
Disma, N., Mondardini, M. C., Terrando, N., Absalom, A. R., and Bilotta, F. (2016). A systematic review of methodology applied during preclinical anesthetic neurotoxicity studies: important issues and lessons relevant to the design of future clinical research. Paediatr. Anaesth. 26, 6–36. doi: 10.1111/pan.12786
Duan, H., Li, Y., Wang, X. P., and Yan, P. S. (2000). Effects of tetramethylpyrazine on apoptosis of myocardial cells in myocardial ischemic reperfusion in rats. Med. J. Chin. People' s Armed Police Forces 11, 70–73. doi: 10.14010/j.cnki.wjyx.2000.02.002
Frank, A., Bonney, M., Bonney, S., Weitzel, L., Koeppen, M., and Eckle, T. (2012). Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin. Cardiothorac. Vasc. Anesth. 16, 123–132. doi: 10.1177/1089253211436350
García, B. L., Campos, M., Giralt, D., Salat, D., Chacón, P., Hernández, G. M., et al. (2012). Evidence for the efficacy of statins in animal stroke models: a meta-analysis. J. Neurochem. 122, 233–243. doi: 10.1111/j.1471-4159.2012.07773.x
Gu, Y. C., Yu, X. L., and Guo, W. J. (2009). Protective effect of tetramethylpyrazine postconditioning on ischemia-reperfusion injured myocardium in rats. Int. J. Cardiovasc. Dis. 36, 304–306. doi: 10.3969/j.issn.1673-6583.2009.05.014
Hausenloy, D. J., and Yellon, D. M. (2013). Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100. doi: 10.1172/JCI62874
Heusch, G., and Gersh, B. J. (2017). The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur. Heart J. 38, 774–784. doi: 10.1093/eurheartj/ehw224
Hu, M. F., Shang, L. Z., and Wei, D. W. (2008). Protective effects of ligustr azitine on myocardial ischemia reper fusion injury in rats. SH.J.TCM 42, 66–68. doi: 10.3969/j.issn.1007-1334.2008.04.028
Ibáñez, B., Heusch, G., Ovize, M., and Van, D. W. F. (2015). Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 65, 1454–1471. doi: 10.1016/j.jacc.2015.02.032
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., and Altman, D. G. (2012). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarth. Cartil. 20, 256–260. doi: 10.1016/j.joca.2012.02.010
Landis, S. C., Amara, S. G., Asadullah, K., Austin, C. P., Blumenstein, R., Bradley, E. W., et al. (2012). A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490, 187–191. doi: 10.1038/nature11556
Li, C. Q., and Li, Y. X. (2010). Adiponectin participates in the protective effect of Ligustrazine on myocardial ischemia reperfusion injury. Guide China Med. 8, 215–216. doi: 10.15912/j.cnki.gocm.2010.36.112
Li, P., Li, H., He, D. L., Zhao, Y., Xiong, F., and Fu, Q. (2006). Protective effect of ligustrazine injection on the ischemia and reperfusion myocardium. Chin. Hosp. Pharm. J. 26, 32–34. doi: 10.3321/j.issn:1001-5213.2006.01.015
Liang, R. L., Li, W., Liu, J. Y., and Yin, X. J. (1999). Protection of pharmacological preconditioning by litustrazini on myocardial ischemia and reperfusion injury in the anesthetized rat. Pharmacol. Clin. Chin. Mater. Med. 15, 13–14. doi: 10.3969/j.issn.1001-859X.1999.05.00
Liang, R. X., Liao, F. L., and Han, D. (2000). Protection of pharmacological preconditioning by ligustrazini on myocardial ischemia and reperfusion injury in the anesthetized rabbit. Pharmacol. Clin. Chin. Mater. Med. 16, 11–13. doi: 10.3969/j.issn.1001-859X.2000.02.006
Liu, J., and Niu, P. W. (2011). Protective effects of chuan xiong qin against ischemia /reperfusion injury in rats. China Prac. Med. 6, 29–30. doi: 10.14163/j.cnki.11-5547/r.2011.02.021
Lu, C. F., Xu, W., Zhang, F., Jin, H., Chen, Q., Chen, L., et al. (2015). Ligustrazine prevents alcohol-induced liver injury by attenuating hepatic steatosis and oxidative stress. Int. Immunopharmacol. 29, 613–621. doi: 10.1016/j.intimp.2015.09.020
Lu, C., Xu, W., Shao, J., Zhang, F., Chen, A., and Zheng, S. (2017). Nrf2 activation is required for ligustrazine to inhibit hepatic steatosis in alcohol-preferring mice and hepatocytes. Toxicol. Sci. 155, 432–443. doi: 10.1093/toxsci/kfw228
Lv, L., Guo, H., Zhang, J., and Xu, J. (2016). The role of glycogen synthase kinase 3β in the protective effect of ligustrazine on myocardial ischemia reperfusion injury in rats. J. Southeast Univ. 35, 836–840. doi: 10.3969/j.issn.1671-6264.2016.06.003
Lv, L., Meng, Q. X., Xu, J., Gong, J. B., Cheng, Y., and Jiang, S. S. (2012). Ligustrazine attenuates myocardial ischemia reperfusioninjury in rats by activating the phosphatidylinositol3-kinase/Akt pathway. Ann. Clin. Lab. Sci. 42, 198–202.
Macleod, M. R., O'Collins, T., Howells, D. W., and Donnan, G. A. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35, 1203–1208. doi: 10.1161/01.STR.0000125719.25853.20
Menazza, S., Sun, J., Appachi, S., Chambliss, K. L., Kim, S. H., Aponte, A., et al. (2017). Non-nuclear estrogen receptor alpha activation in endothelium reduces cardiac ischemia-reperfusion injury in mice. J. Mol. Cell. Cardiol. 107, 41–51. doi: 10.1016/j.yjmcc.2017.04.004
Moher, D., Avey, M., Antes, G., and Altman, D. G. (2015). Erratum: the national institutes of health and guidance for reporting preclinical research. BMC Med. 13, 1741–7015. doi: 10.1186/s12916-015-0321-8
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2015). Heart disease and stroke statistics−2015 update: a report from the American Heart Association. Circulation 131, e29–322. doi: 10.1161/CIR.0000000000000152
Nolting, A., Perleth, M., Langer, G., Meerpohl, J. J., Gartlehner, G., Kaminski, H. A., et al. (2012). Schünemann, H. J. GRADE guidelines: 5. Rating the quality of evidence: publication bias. Z. Evid. Fortbild. Qual. Gesundhwes. 106, 670–676. doi: 10.1016/j.zefq.2012.10.015
Qian, W., Xiong, X., Fang, Z., Lu, H., and Wang, Z. (2014). Protective effect of tetramethylpyrazine on myocardial ischemia-reperfusion injury. Evid. Based Complement Alternat. Med. 2014:107501. doi: 10.1155/2014/107501
Schmidt, M. R., Redington, A., and Botker, H. E. (2015). Remote conditioning the heart overview: translatability and mechanism. Br. J. Pharmacol. 172, 1947–1960. doi: 10.1111/bph.12933
Sena, E. S., Currie, G. L., McCann, S. K., Macleod, M. R., and Howells, D. W. (2014). Systematic reviews and meta-analysis of preclinical studies: why perform them and how to appraise them critically. J. Cereb. Blood Flow Metab. 34, 737–742. doi: 10.1038/jcbfm.2014.28
Shang, L. Z., Wei, D. W., and Wang, F. (2008). Effects of ligustrazine on expression of HSP25 and p38MAPK proteins in rats withmyocardial ischemia reperfusion injury. China J. Trad. Chin. Med. Pharm. 23, 882–884.
Stewart, L. A., Clarke, M., Rovers, M., Riley, R. D., Simmonds, M., Stewart, G., et al. (2015). Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 313, 1657–1665. doi: 10.1001/jama.2015.3656
Tang, B. E., Chen, X. M., and Zhang, N. (2007). Study of ligustrazine in protecting myocardial ischemia reperfusion injury. J. Jinhua Polytech. 7, 63–65. doi: 10.3969/j.issn.1671-3699.2007.04.016
Wan, F. S., Zhao, X. M., Liu, B., and Li, J. S. (1998). Protective effects of liqustrazin on myocardial ischemia_reperfusion injury in rats. Chin. J. Clin. Pharmacol. Ther. 3, 184–186.
Wang, H. T., Yang, J., He, Y. S., Mao, Q. M., Fang, Y. M., Wang, X. M., et al. (2005). The effect of Ligustrazine on the No-reflow phenomenon after restoration of coronary blood flow in rabbits with experimental acute myocardial infarction. J. Chin. Microcirc. 9, 82–84.
Wang, L., Lu, W. G., Shi, J., Zhang, H. Y., Xu, X. L., Gao, B., et al. (2017). Anti-osteoporotic effects of tetramethylpyrazine via promoting osteogenic differentiation and inhibiting osteoclast formation. Mol. Med. Rep. 16, 8307–8314. doi: 10.3892/mmr.2017.7610
Xu, B., Xu, X., Zhang, C., Zhang, Y., Wu, G., Yan, M., et al. (2017). Synthesis and protective effect of new ligustrazine-vanillic acid derivatives against CoCl(2)-induced neurotoxicity in differentiated PC12 cells. Chem. Cent. J. 11, 1–22. doi: 10.1186/s13065-017-0250-z
Xu, C. H., and Zhang, W. D. (2006). Protective effect of pharmacological preconditioning of ligustrazine on myocardial ischemical reperfusion injury in rats in relation to nitrogen monoxide. Chin. J. Clin. Rehabil. 10, 74–76. doi: 10.3321/j.issn:1673-8225.2006.03.030
Xu, Z. J., Wang, W. T., and Li, D. (1997). Protective effect of Liqustrazin on myocardial ischemia-reperfusion injury in rabbits and its mechanism. Basic Med. Sci. Clin. 17, 68–71.
Yang, F., and Rui, J. (2007). Study on the protective effect of Ligustrazine on myocardial ischemia preconditioning in rabbits. J. Pract. Trad. Chin. Med. 23, 418–419. doi: 10.3969/j.issn.1004-2814.2007.07.005
Yang, J. R., Zhang, M. X., Chang, L. T., Zhuang, P. W., Li, H. Y., Feng, Y., et al. (2008). Effects of ligustrazine, ferulic acid, and their compatibility on influence and expression of adhesion molecules in rats with myocardial ischemia reperfusion model. Chinese Traditional and Herbal Drugs, 39, 1054–1056. doi: 10.3321/j.issn:0253-2670.2008.07.032
Yu, L. J., Zhang, K. J., Zhu, J. Z., Zheng, Q., Bao, X. Y., Thapa, S., et al. (2017). Salvianolic acid exerts cardioprotection through promoting angiogenesis in animal models of acute myocardial infarction: preclinical evidence. Oxid. Med. Cell Longev. 2017:8192383. doi: 10.1155/2017/8192383
Yuan, H. W., Ma, L. X., Qi, D. D., Zhang, P., Li, C. H., and Zhu, J. (2013). The historical development of deqi concept from classics of traditional chinese medicine to modern research:exploitation of the connotation of deqi in chinese medicine. Evid. Based Complement Alternat. Med. 2013:639302. doi: 10.1155/2013/639302
Zhai, Z. Y., Yang, J. H., Zhang, S. T., Wu, B. H., Xin, D., and Zhou, L. H. (2011). Role of Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway in attenuation of myocardial ischemia-reperfusion injury by teramethylpyrazine in rats. Chin. J. Anesthesiol. 31, 1005–1008. doi: 10.3760/cma.j.issn.0254-1416.2011.08.029
Zhang, J., Liu, L. Q., Yu, L. H., Zhao, X. M., Li, H., and Wan, F. S. (2007). Effects of liqustrazin on the apoptosis in rats with myocardial ischemia/reperfusion injury. Acta Acad. Med. Jiangxi 47, 20–22. doi: 10.3969/j.issn.1000-2294.2007.06.007
Zhang, C., Teng, F., Tu, J., and Zhang, D. (2014). Ultrasound-enhanced protective effect of tetramethylpyrazine against cerebral ischemia/reperfusion injury. PLoS ONE 9:e113673. doi: 10.1371/journal.pone.0113673
Zhang, K. J., Zhu, J. Z., Bao, X. Y., Zheng, Q., Zheng, G. Q., and Wang, Y. (2017). Shexiang baoxin pills for coronary heart disease in animal models: preclinical evidence and promoting angiogenesis mechanism. Front. Pharmacol. 8:404. doi: 10.3389/fphar.2017.00404
Zhang, Y., Ma, X. J., Guo, C. Y., Wang, M. M., Kou, N., Qu, H., et al. (2016). Pretreatment with a combination of ligustrazineand berberine improves cardiac function in rats withcoronary microembolization. Acta Pharmacol. Sin. 37, 463–472. doi: 10.1038/aps.2015.147
Zhang, Y., Tian, Y. K., Wang, P., and Wu, Z. (2003). Myocardial protective effects of hemodilution and Ligustrazine against ischemia/reperfusion injury in rabbits. Chin. J. Anesthesiol. 23, 504–507. doi: 10.3760/j.issn:0254-1416.2003.07.006
Zhang, Z. G., Zhang, X. L., Wang, X. Y., Luo, Z. R., and Song, J. C. (2015). Inhibition of acid sensing ion channel by ligustrazine onangina model in rat. Am. J. Transl. Res. 7, 1798–1811.
Zhao, R. Y., Hao, W., Meng, X. J., Zhao, L. N., Li, Z., Wang, J. P., et al. (2012). Protective effects and molecular mechanism of ligustrazine and ferulate on myocardial ischemia-reperfusion injury in rats. Chin. J. Exp. Trad. Med. Formulae 18, 230–234. doi: 10.13422/j.cnki.syfjx.2012.19.066
Keywords: ligustrazine, myocardial ischemia/reperfusion injury, efficacy, mechanisms, meta-analysis
Citation: Zheng Q, Huang Y, Zhu P, Tong Q, Bao X, Zhang Q, Zheng G and Wang Y (2018) Ligustrazine Exerts Cardioprotection in Animal Models of Myocardial Ischemia/Reperfusion Injury: Preclinical Evidence and Possible Mechanisms. Front. Pharmacol. 9:729. doi: 10.3389/fphar.2018.00729
Received: 28 February 2018; Accepted: 18 June 2018;
Published: 25 July 2018.
Edited by:
Li-Long Pan, Fudan University, ChinaReviewed by:
Vishal Diwan, The University of Queensland, AustraliaGiustino Orlando, Università degli Studi G. d'Annunzio Chieti e Pescara, Italy
Copyright © 2018 Zheng, Huang, Zhu, Tong, Bao, Zhang, Zheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-qing Zheng, Z3FfemhlbmdAc29odS5jb20=
Yan Wang, d3l3emNoaW5hQHNpbmEuY29t
†These authors have contributed equally to this work.
 Qun Zheng
Qun Zheng Yue-yue Huang†
Yue-yue Huang† Qi-hao Zhang
Qi-hao Zhang Guo-qing Zheng
Guo-qing Zheng Yan Wang
Yan Wang