- 1Department of Pediatrics, Yantai Yuhuangding Hospital Affiliated to Qingdao Medical College of Qingdao University, Yantai, China
- 2Department of Integrated Chinese and Western Medicine, Yantai Yuhuangding Hospital Affiliated to Qingdao Medical College of Qingdao University, Yantai, China
- 3Department of Rheumatology and Immunology, Yantai Yuhuangding Hospital Affiliated to Qingdao Medical College of Qingdao University, Yantai, China
Background: The mainstream medications for rheumatoid arthritis (RA) include conventional disease-modifying antirheumatic drugs (cDMARDs), which mostly are methotrexate (MTX), and biologic agents such as adalimumab (ADA), certolizumab (CZP), etanercept (ETN), golimumab (GOL), infliximab (IFX), and tocilizumab (TCZ). This network meta-analysis was aimed at evaluating the efficacy and safety of the medications above and interventions combining cDMARDs and biologic agents for patients with RA.
Methods: PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov were searched systematically for eligible randomized controlled trials (RCTs). Outcomes concerning efficacy and safety were evaluated utilizing odds ratios (ORs) and 95% credible intervals (CrI). The outcomes of efficacy would be evaluated through remission and American College of Rheumatology (ACR) scores. The surface under the cumulative ranking curve (SUCRA) was calculated to rank each treatment on each index.
Results: A total of 20 RCTs with 9,047 patients were included, and the efficacy and safety of the concerning interventions for RA were evaluated. Compared with cDMARDs alone, TCZ+MTX, ETN+MTX, IFX+MTX, TCZ, and ADA+MTX showed significant statistical advantage on ACR20, ACR50, and ACR70. Apart from that, as for remission, TCZ+MTX, IFX+MTX, TCZ, and CZP+MTX performed better compared to cDMARDs alone. The SUCRA ranking also indicated that TCZ+MTX was the intervention with best ranking in the entire four efficacy indexes followed by ETX+MTX and IFX+MTX. However, there was no obvious difference among these medications compared with cDMARDs when it comes to safety, which need more specific studies on that.
Conclusion: TCZ+MTX was potentially the most recommended combination of medications for RA due to its good performance in all outcomes of efficacy. ETX+MTX and IFX+MTX, which also performed well, could be introduced as alternative treatments. However, considering the adverse events, the treatments concerning should be introduced with caution.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by its irreversible, alternating episodes and impaired joint function (Popescu et al., 1985). Patients with RA often suffered from the arthralgia caused by the synovial lining joints swelling which can result in disability and reduction of life quality (Donahue et al., 2012). Generally, patients with RA often have a shorter life expectancy compared with normal people. Thus, the primary treating target of RA patients is to maximize the quality of life associated with health through preventing structural damage, controlling the symptom of inflammation, normalizing functional, and social participation (Smolen et al., 2014; Buckley et al., 2015). Until now, there are an estimated 1.12% of adult people affected with RA in developed countries (Li et al., 2012; Stevenson et al., 2016) which leads us to find optional treatments for patients with this disease.
Recently, the potent pro-inflammatory cytokine named tumor necrosis factor-α (TNF-α) has been considered playing an important role in immune responses and inflammationincluding those involved in RA (Brennan et al., 1992), Which indicated that TNF antagonists could be an effective method for RA treatments (Lee and Bae, 2016). However, based on the American College of Rheumatology (ACR) recommendations for the treatment of RA, it should begin with the use of conventional (non-biologic) disease-modifying antirheumatic drugs (cDMARDs), mostly are methotrexate (MTX) (Singh et al., 2012). If patients were tolerant of cDMARDs or showed inadequate responses (IR), biologic agents were often applied with cDMARDs as combined therapies. On the other hand, because of cDMARDs' side effects including hepatotoxicity, primary gastrointestinal symptoms and respiratory symptoms, around one-third RA patients are treated with monotherapy of biologic agents (Listing et al., 2006; Heiberg et al., 2008; Soliman et al., 2011). Up to now, a total of five kind of biologic agents have been approved to treat patients with RA: (Popescu et al., 1985) TNF antagonists, known as anti-TNF agents (aTNF) including infliximab (IFX), certolizumab (CZP), adalimumab (ADA), golimumab (GOL), and etanercept (ETN); (Donahue et al., 2012) monoclonal antibody which could suppress B cells such as rituximab; (Buckley et al., 2015) monoclonal antibody which could suppress interleukin-6 (IL-6) receptor such as tocilizumab (TCZ); (Smolen et al., 2014) selective T-cell costimulatory modulator such as abatacept; (Stevenson et al., 2016) interleukin-1 (IL-1) receptor antagonists such as anakinra (Buckley et al., 2015).
However, no randomized controlled trial (RCT) has been conducted to evaluate all optional biologic treatments simultaneously. Clinicians now were facing increasing challenge about choosing optimal drug due to the amount of alternative biologic treatments and other DMARDs. Thus, network meta-analysis (NMA) has been applied, which could combine all the available RCTs and evaluate the potential biologic drugs through not only direct but also indirect comparison. In recent years, several NMAs of biologic treatments for patients with RA have been published (Buckley et al., 2015; Lee and Bae, 2016; Migliore et al., 2016; Stevenson et al., 2016; Choi et al., 2017). Nevertheless, those studies only focused on combined treatments such as biologic therapies with MTX. Particularly, none of the existing NMA contained all optional biologic agents. Besides, none of the existing NMA distinguished between cDMARD-naive and cDMARD-experienced. According to Egsmose et al., Tsaknoas et al., and Quinn et al., there is a period named “window of opportunity” and the underlying process of inflammatory in RA was more susceptible to biologic drugs than later time-points (Egsmose et al., 1995; Tsakonas et al., 2000; Quinn et al., 2001). In the early period of RA, the mechanical aspects and pathogenic of autoimmune prompted inflammation was not fully consistent with the current evidence of RA (Mullan and Bresnihan, 2003). Correspondingly, the RCTs on patients with RA who are cDMARD-naive should be picked out from the pool.
In this study, a comprehensive NMA was conducted to evaluate the relative efficacy and safety of 11 potential therapeutic approaches of early interventions for patients with RA. The outcomes of efficacy would be evaluated through remission and ACR scores. ACR scores that measuring changes in symptoms of RA and different degrees of improvement are referred to ACR20, ACR50, and ACR70. For example, ACR20 measures a 20% improvement on a scale of 28 intervals. As for safety, the outcomes would consist of the incidence of adverse effects (AEs) and serious adverse effects (SAEs). The purpose of this current research is to supplement the existing evidence network and select the optimal treatments for patients with RA.
Methods
Selection Strategy
We did a comprehensive research to find all relevant RCTs through Embase, PubMed, Cochrane Library and ClinicalTrials.gov with the following keywords: “rheumatoid arthritis,” “methotrexate,” “infliximab,” “etanercept,” “adalimumab,” “golimuma,” “tocilizumab,” and “randomized control trial,” etc. There is no limitation on the time of publication. All the searching process was limited to clinical trials, and no age or language restrictions were applied to literature search. Process of screening was carried out through reading titles and abstracts of eligible articles. After that, full texts of remaining articles were further read to remove articles with incomplete or irrelevant information.
Inclusion and Exclusion Criteria
All the included articles must satisfy the following criteria: (i) studies should be randomized and all involved RA patients were adults who had not treat with cDMARDs before trials; (ii) trials must include at least two of the concerning treatments for RA; (iii) trials should contain at least one of the primary outcomes of interest (as shown below). Moreover, expert opinions, editorials, letters, case reviews, reports and duplications would be excluded after title and abstract screening.
Data Extraction and Quality Assessment
All the relevant data would be extracted from the eligible studies by two independent reviewers using a standard data collection form. Any discrepancies between reviewers would be resolved by discussing with a third independent researcher. In this study, the following information would be collected: (i) baseline information including first author, publication date, sample size, blinding method, type of intervention, following time, disease durations, gender, and age; (ii) efficacy outcomes including ACR20, ACR50, ACR70, and remission; (iii) safety outcomes including AEs and SAEs.
Statistical Analysis
On the foundation of a rigorous assessment of the accuracy and authenticity of the collected data, we introduced a Bayesian framework utilizing Software R 3.2.3 and STATA 13.0 for statistical processing. One of the most significant properties of NMA is to combine all the available comparisons including the indirect evidence simultaneously. In this NMA research, the forest plots showed the results of each outcome. For binary variables (ACR20, ACR50, ACR70, remission, AEs, SAEs), odds ratios (ORs) with their 95% credible intervals (CrI) were applied for the comparison. Furthermore, relative ranking probability of each therapeutic method was calculated through surface under cumulative ranking curve (SUCRA), which is also an advantage of the Bayesian framework. Typically, a more satisfying treatment assessed under a certain outcome was indicated by a higher SUCRA value. As for the consistency analysis, the consistency between direct and indirect evidence of each outcome were conducted through node-splitting analysis and heat plots. Besides, random effects model would be implemented if significant inconsistency was found (P-value < 0.05).
Results
Study Selection and Characteristics of Included Studies
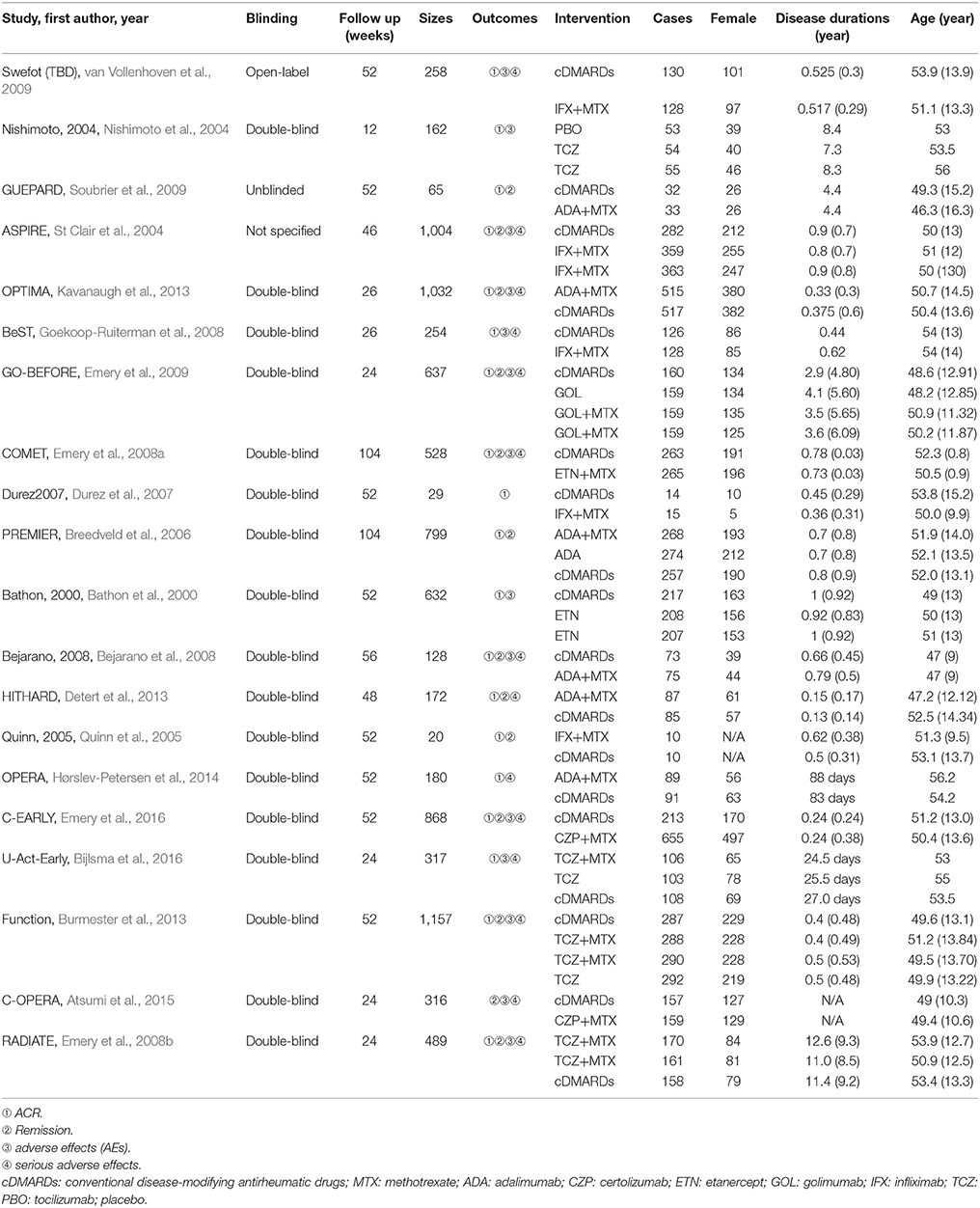
We identified 2,527 published articles according to the searching strategy which has been mentioned before. Then 864 articles were removed for duplicated, and after scanning title and abstract 1,427 articles were also removed for lack of relevance. 216 studies were eventually excluded. At the end, a total of 20 studies published from 2000 to 2016 met our selection criteria and has been involved in this NMA (Bathon et al., 2000; Nishimoto et al., 2004; St Clair et al., 2004; Quinn et al., 2005; Breedveld et al., 2006; Durez et al., 2007; Bejarano et al., 2008; Emery et al., 2008a,b, 2009, 2016; Goekoop-Ruiterman et al., 2008; Soubrier et al., 2009; van Vollenhoven et al., 2009; Burmester et al., 2013; Detert et al., 2013; Kavanaugh et al., 2013; Hørslev-Petersen et al., 2014; Atsumi et al., 2015; Bijlsma et al., 2016), among which 12 were two-arm trials, 6 were three-arm trials and 2 were four-arm trials. The baseline characteristics of included studies were presented in Table 1. Overall, 9,047 patients with RA were contained and the average age of them was 52.4 ± 10 years. The network structures of ACR scores, remission and safety were shown in Figure 1 and in the diagram, each circle represents an individual treatment and the thickness of lines represents the number of trials.
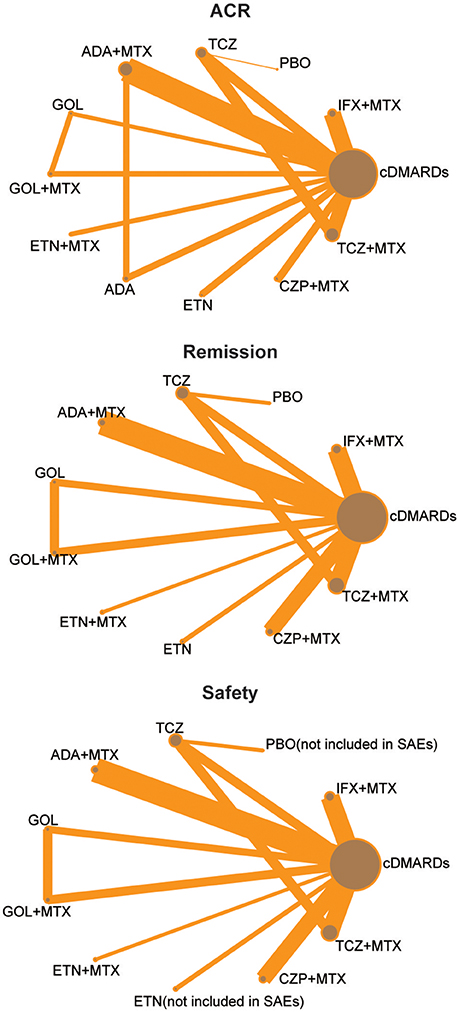
Figure 1. Full network of comparisons of efficacy and safety outcomes (The width of the lines is proportional to the number of trials comparing each pair of treatments; the area of circles represents the cumulative number of patients for each intervention).
Network Meta-Analysis
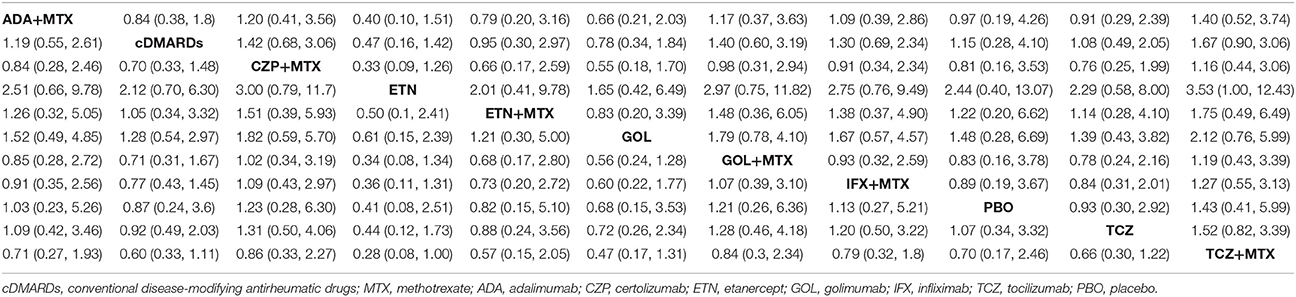
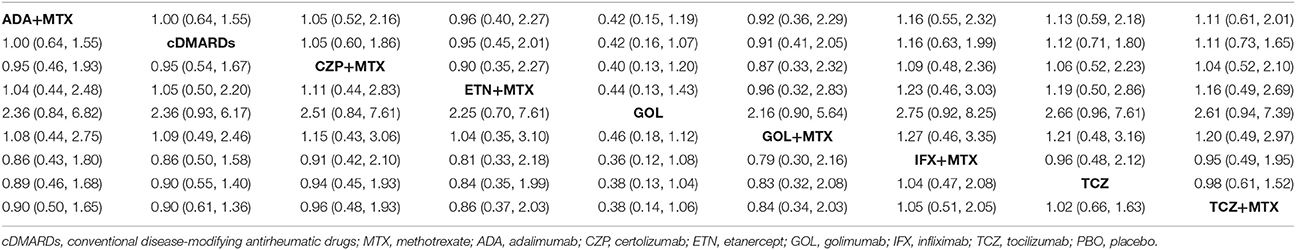
ACR20, ACR50, ACR70, and remission were used to estimate the effectiveness of each therapeutic method and the results were shown in Figure 2. According to the results of ACR scores, TCZ+MTX, IFX+MTX, and ETN+MTX showed statistical difference compared with cDMARDs alone on ACR20, ACR50, and ACR70, which indicated the superior efficacy of these drug combinations. Apart from that, as for remission, CZP+MTX (OR = 5.20, 95% CrI: 1.20–21.0), TCZ (OR = 3.20, 95% CrI: 1.10–10.0), TCZ+MTX (OR = 4.20, 95% CrI: 1.80–12.0) were statistically significant compared to cDMARDs. Tables 2, 3 showed the results of safety outcomes on AEs and SAEs, and according to that, there is no significant difference of the concerning treatments compared with cDMARDs and PBO.
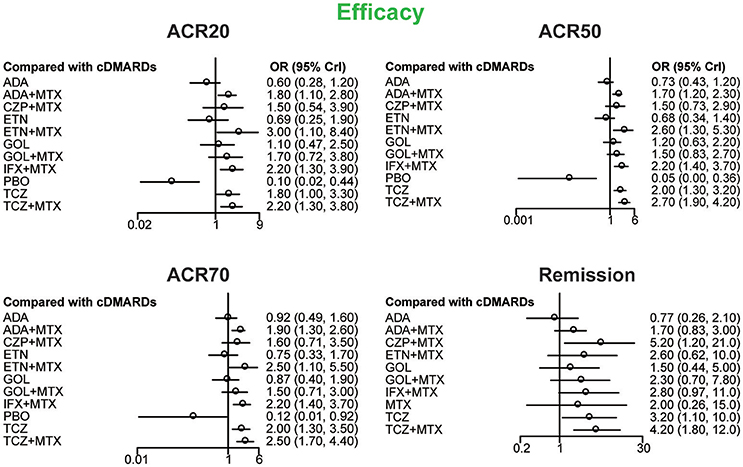
Figure 2. The Odds ratio estimate with 95% credible intervals of efficacy endpoints compared to DMARDs.
Ranking with SUCRA Value
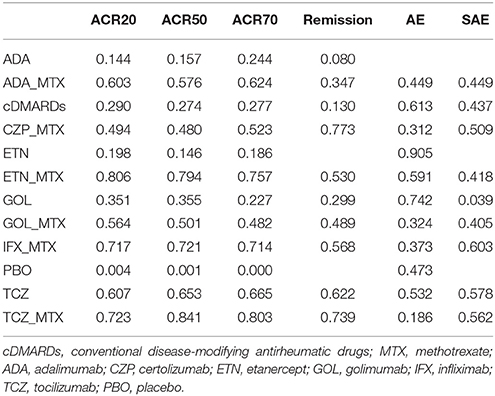
Table 4 showed the results of ranking probabilities in terms of each outcome. As for the efficacy outcomes, TCZ+MTX ranked first on ACR50, ACR70 and ranked second on ACR20 and remission, which indicated its best performance among all treatments. With respect to alternative treatment options, ETN+MTX and IFX+MTX also performed well due to their higher SUCRA values compared with other treatments. And PBO ranked last on all efficacy outcomes as expected. Regarding the safety outcomes, though ETN ranked first followed by GOL for AEs and IFX+MTX, TCZ+MTX had the similar highest values for SAEs according to the SUCRA ranking, the conclusion about the relative safety of each concerning treatment seemed not so credible if we combined the results of forest plots. What's more, due to the lack of data, some SUCRA results were missing which may also reduce the reliability of the outcomes.
Consistency Analysis
Figures S1–S6 showed the results of node-splitting analysis and their corresponding heat plots. All the P-value was larger than 0.05 which revealed that there was no statistical inconsistency between direct and indirect comparisons among all outcomes. The same results came from the heat plots, which also contributed to the reliability of this NMA.
Discussion
RA was a type of chronic inflammatory arthritis, which would have negative effects on patients' living quality. Moreover it can lead to functional limitations and employment obstacle (Singh et al., 2009). Many kinds of medications have been introduced to cure RA, including conventional DMARDs (like MTX), biologics (like IFX, ETN, ADA, and PCZ), and other concerning medicines (Jansen et al., 2014). The mechanism of biologic agents is that they can target TNF-α, IL-1, IL-6, T cells, or B cells, and significantly inhibit the damage of joint. In our NMA, we collected data from 20 eligible trials of 9,047 patients with RA who were cDRAMDs-naïve. Eleven interventions along with PBO were compared simultaneously on both efficacy and safety.
Four outcomes concerning efficacy were measured, including ACR20, ACR50, ACR70, and remission. As was shown in this NMA, all of the 11 therapies worked notably better than PBO. Moreover, we can also find that the combination of biological agents with MTX might be superior to monotherapy of cDMARDs, particularly TCZ+MTX, which performed well in all four outcomes with respect to efficacy. In addition, the monotherapy of TCZ also ranked roundly well in all outcomes despite that it was inferior to combination of TCZ and MTX. The results above were consistent to former trials. For instance, a double-blind, 2-year study (Kremer et al., 2011) containing 1196 RA patients indicated that TCZ+MTX had better efficacy on helping patients slow down the joint damage and improving their body function than MTX alone. IL-6 is a pleiotropic cytokine that can regulate the immune response, hematopoiesis, inflammation, and bone metabolism through combining with IL-6 receptor. The constitutive overproduction of IL-6 is considered to play a pathological role in RA (Nishimoto et al., 2007). Correspondingly, TCZ is a humanized monoclonal antibody that can suppress the bindling of IL-6 to membrane expressed IL-6 soluble receptors, thus preventing the pro-inflammatory activities of IL-6 or IL-6 receptor signaling (Kremer et al., 2011), which might explain TCZ as an effective medication for RA.
Apart from that, ETN+MTX and IFX+MTX also performed pretty well as alternative treatment options. According to previous studies, TNF is a kind of soluble protein playing an important role in RA. It is believed that TNF can lead to continuous occurrence of inflammatory response and progressive destruction of cartilage and bone (Brennan et al., 1992). Both ETN and IFX are TNF antagonists, which can be specifically combined with TNF-α and then break the activity of it to achieve the goal of controlling inflammation and continuing to alleviate the symptom of RA (Emery et al., 2008a; Lee and Bae, 2016).
What more, when comparing the combinations of cDMARDs and biological agents with monotherapy of biologic agents, according to the results, the combination therapies, showed the much higher efficacy than the corresponding biologic medicine alone. While as for the safety outcomes, the results didn't show the significant difference, which also indicated the superiority of combination therapies.
When choosing an appropriate treatment, not only the efficacy, but also the safety ought to be considered. When it came to safety, the results of AEs together with SAEs were measured in this NMA. Though ETN ranked first with respect to avoiding adverse events and those medications which performed well in the outcomes of efficacy did not rank well in safety according to the SUCRA ranking, the result could not be so responsible due to the outcomes of forest plots which indicated that there is no statistical difference between those combinations of drugs and cDRAMDs alone.
Moreover, the main adverse events of those therapies included rhinopharyngitis, respiratory tract disorder, pathology of skin and soft tissue, gastrointestinal side effects and so on (Campbell et al., 2011). A study conducted among 48,676 participants (Singh et al., 2011) concluded that biologics were connected with significantly higher rates of tuberculosis reactivation, serious infections, total AEs and withdrawals resulted by AEs, which is worthy noticing. Therefore, due to the potential adverse effects, the use of effective interventions still needs further evaluation.
However, there are inevitably some limitations in our research. Firstly, the sample size of some outcomes was relatively small and duration time was relatively short, thus we could not obtain sufficient evidence to get general results. Secondly, most of the RCTs included in our NMA compared medications with cDMARDs, as a consequence, some direct comparisons between other treatments could not been achieved and we could not get a further understanding in the inconsistency among these RCTs. For instance, we were not able to compare ETN+MTX and TCZ+MTX directly. Besides, there were some confounding factors while analyzing the outcomes, which may have some effects on study. For example, doses across treatments had not been accounted for in this analysis. In addition, the different stages of RA might also affect the result of study since patients in different stages appeared varied symptoms and responded differently to medications.
In summary, based on the studies we selected, our NMA provided a systematic evaluation on the efficacy and safety of therapies on rheumatoid arthritis. Our NMA concluded that TCZ+MTX was potentially the most preferable treatment for RA, with ETN+MTX and IFX+MTX as alternative treatment options. However, considering the adverse effects, it should be introduced with caution and more advanced studies should be carried out to find out the most appropriate way for treating RA.
Author Contributions
WC, YG, and HC: Substantial contribution to the conception and design of the work; YC: Analysis and interpretation of the data; WC, YG, XW, and YY: Drafting the manuscript; MW: Revising the work critically for important intellectual content; Final approval of the work: all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00138/full#supplementary-material
Figure S1. The results of consistency analysis by note-splitting plot and heat plot of ACR20.
Figure S2. The results of consistency analysis by note-splitting plot and heat plot of ACR50.
Figure S3. The results of consistency analysis by note-splitting plot and heat plot of ACR70.
Figure S4. The results of consistency analysis by note-splitting plot and heat plot of remission.
Figure S5. The results of consistency analysis by note-splitting plot and heat plot of adverse events.
Figure S6. The results of consistency analysis by note-splitting plot and heat plot of serious adverse events.
References
Atsumi, T., Yamamoto, K., Takeuchi, T., Yamanaka, H., Ishiguro, N., Tanaka, Y., et al. (2015). Baseline parameters identified in early, methotrexate-naïve rheumatoid arthritis patients with better outcomes with certolizumab pegol+methotrexate compared to placebo+methotrexate: post-hoc analyses of c-opera, a randomized, controlled, phase 3 study. Ann. Rheum. Dis. 74, 716–717. doi: 10.1136/annrheumdis-2015-eular.1501
Bathon, J. M., Martin, R. W., Fleischmann, R. M., Tesser, J. R., Schiff, M. H., Keystone, E. C., et al. (2000). A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. New Engl. J. Med. 343, 1586–1593. doi: 10.1056/NEJM200011303432201
Bejarano, V., Quinn, M., Conaghan, P. G., Reece, R., Keenan, A. M., Walker, D., et al. (2008). Effect of the early use of the anti-tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis Care Res. 59, 1467–1474. doi: 10.1002/art.24106
Bijlsma, J. W. J., Welsing, P. M. J., Woodworth, T. G., Middelink, L. M., Pethö-Schramm, A., Bernasconi, C., et al. (2016). Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 388, 345–355. doi: 10.1016/S0140-6736(16)30363-4
Breedveld, F. C., Weisman, M. H., Kavanaugh, A. F., Cohen, S. B., Pavelka, K., Van Vollenhoven, R., et al. (2006). The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 54, 26–37. doi: 10.1002/art.21519
Brennan, F. M., Maini, R. N., and Feldmann, M. (1992). TNF alpha–a pivotal role in rheumatoid arthritis? Br. J. Rheumatol. 31, 293–298. doi: 10.1093/rheumatology/31.5.293
Buckley, F., Finckh, A., Huizinga, T. W., Dejonckheere, F., and Jansen, J. P. (2015). Comparative efficacy of novel DMARDs as monotherapy and in combination with methotrexate in rheumatoid arthritis patients with inadequate response to conventional DMARDs: a network meta-analysis. J. Manag. Care Spec. Pharm. 21, 409–423. doi: 10.18553/jmcp.2015.21.5.409
Burmester, G. R., Blanco, R., Charles-Schoeman, C., Wollenhaupt, J., Zerbini, C., Benda, B., et al. (2013). Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381, 451–460. doi: 10.1016/S0140-6736(12)61424-X
Campbell, L., Chen, C., Bhagat, S. S., Parker, R. A., and Östör, A. J. (2011). Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology 50, 552–562. doi: 10.1093/rheumatology/keq343
Choi, M., Hyun, M. K., Choi, S., Tchoe, H. J., Lee, S. Y., Son, K. M., et al. (2017). Comparative efficacy of biological agents in methotrexate-refractory rheumatoid arthritis patients: a Bayesian mixed treatment comparison. Korean J. Intern. Med. 32, 536–547. doi: 10.3904/kjim.2015.135
Detert, J., Bastian, H., Listing, J., Weiß, A., Wassenberg, S., Liebhaber, A., et al. (2013). Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naïve patients with early rheumatoid arthritis: HIT HARD, aninvestigator-initiated study. Ann. Rheum. Dis. 72, 844–850. doi: 10.1136/annrheumdis-2012-201612
Donahue, K. E., Jonas, D. E., Hansen, R. A., Roubey, R., Jonas, B., Lux, L. J., et al. (2012). “Drug therapy for rheumatoid arthritis in adults: an update,” in AHRQ Comparative Effectiveness Reviews, Vol. 12 (Rockville, MD: Edward Elgar), 1.
Durez, P., Malghem, J., Nzeusseu Toukap, A., Depresseux, G., Lauwerys, B. R., Westhovens, R., et al. (2007). Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum. 56, 3919–3927. doi: 10.1002/art.23055
Egsmose, C., Lund, B., Borg, G., Pettersson, H., Berg, E., Brodin, U., et al. (1995). Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year followup of a prospective double blind placebo controlled study. J. Rheumatol. 22, 2208–2213.
Emery, P., Bingham, C. O., Burmester, G. R., Bykerk, V. P., Furst, D. E., Mariette, X., et al. (2016). Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naïve patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from C-EARLY, a randomised, double-blind, placebo-controlled phase III study. Ann. Rheum. Dis. 76, 96–104. doi: 10.1136/annrheumdis-2015-209057
Emery, P., Breedveld, F. C., Hall, S., Durez, P., Chang, D. J., Robertson, D., et al. (2008a). Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 372, 375–382. doi: 10.1016/S0140-6736(08)61000-4
Emery, P., Fleischmann, R. M., Moreland, L. W., Hsia, E. C., Strusberg, I., Durez, P., et al. (2009). Golimumab, a human anti-tumor necrosis factor α monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 60, 2272–2283. doi: 10.1002/art.24638
Emery, P., Keystone, E., Tony, H. P., Cantagrel, A., Van Vollenhoven, R., Sanchez, A., et al. (2008b). IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: Results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 67, 1516–1523. doi: 10.1136/ard.2008.092932
Goekoop-Ruiterman, Y. P., de Vries-Bouwstra, J. K., Allaart, C. F., van Zeben, D., Kerstens, P. J., Hazes, J. M., et al. (2008). Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 58(Suppl. 2), S126–S135. doi: 10.1002/art.23364
Heiberg, M. S., Koldingsnes, W., Mikkelsen, K., Rodevand, E., Kaufmann, C., Mowinckel, P., et al. (2008). The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 59, 234–240. doi: 10.1002/art.23333
Hørslev-Petersen, K., Hetland, M. L., Junker, P., Pødenphant, J., Ellingsen, T., Ahlquist, P., et al. (2014). Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled Trial. Ann. Rheum. Dis. 73, 654–661. doi: 10.1136/annrheumdis-2012-202735
Jansen, J. P., Buckley, F., Dejonckheere, F., and Ogale, S. (2014). Comparative efficacy of biologics as monotherapy and in combination with methotrexate on patient reported outcomes (PROs) in rheumatoid arthritis patients with an inadequate response to conventional DMARDs–a systematic review and network meta-analysis. Health Qual. Life Outcomes 12:102. doi: 10.1186/1477-7525-12-102
Kavanaugh, A., Fleischmann, R. M., Emery, P., Kupper, H., Redden, L., Guerette, B., et al. (2013). Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-Week results from the randomised, controlled OPTIMA study. Ann. Rheum. Dis. 72, 64–71. doi: 10.1136/annrheumdis-2011-201247
Kremer, J. M., Blanco, R., Brzosko, M., Burgos-Vargas, R., Halland, A. M., Vernon, E., et al. (2011). Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 63, 609–621. doi: 10.1002/art.30158
Lee, Y. H., and Bae, S. C. (2016). Comparative efficacy and safety of tocilizumab, rituximab, abatacept and tofacitinib in patients with active rheumatoid arthritis that inadequately responds to tumor necrosis factor inhibitors: a Bayesian network meta-analysis of randomized controlled trials. Int. J. Rheum. Dis. 19, 1103–1111. doi: 10.1111/1756-185X.12822
Li, R., Sun, J., Ren, L. M., Wang, H. Y., Liu, W. H., Zhang, X. W., et al. (2012). Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology 51, 721–729. doi: 10.1093/rheumatology/ker370
Listing, J., Strangfeld, A., Rau, R., Kekow, J., Gromnica-Ihle, E., Klopsch, T., et al. (2006). Clinical and functional remission: even though biologics are superior to conventional DMARDs overall success rates remain low–results from RABBIT, the German biologics register. Arthritis Res. Ther. 8:R66. doi: 10.1186/ar1933
Migliore, A., Bizzi, E., Petrella, L., Bruzzese, V., Cassol, M., and Integlia, D. (2016). The challenge of treating early-stage rheumatoid arthritis: the contribution of mixed treatment comparison to choosing appropriate biologic agents. BioDrugs 30, 105–115. doi: 10.1007/s40259-016-0164-7
Mullan, R. H., and Bresnihan, B. (2003). Disease-modifying anti-rheumatic drug therapy and structural damage in early rheumatoid arthritis. Clin. Exp. Rheumatol. 21(5 Suppl. 31), S158–S164.
Nishimoto, N., Hashimoto, J., Miyasaka, N., Yamamoto, K., Kawai, S., Takeuchi, T., et al. (2007). Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann. Rheum. Dis. 66, 1162–1167. doi: 10.1136/ard.2006.068064
Nishimoto, N., Yoshizaki, K., Miyasaka, N., Yamamoto, K., Kawai, S., Takeuchi, T., et al. (2004). Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 50, 1761–1769. doi: 10.1002/art.20303
Popescu, V., Arion, C., Dragomir, D., and Balasoiu, D. (1985). [Prolonged febrile syndrome in children]. Rev. Pediatr. Obstet. Ginecol. Pediatr. 34, 193–215.
Quinn, M. A., Conaghan, P. G., and Emery, P. (2001). The therapeutic approach of early intervention for rheumatoid arthritis: what is the evidence? Rheumatology 40, 1211–1220. doi: 10.1093/rheumatology/40.11.1211
Quinn, M. A., Conaghan, P. G., O'Connor, P. J., Karim, Z., Greenstein, A., Brown, A., et al. (2005). Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 52, 27–35. doi: 10.1002/art.20712
Singh, J. A., Christensen, R., Wells, G. A., Suarez-Almazor, M. E., Buchbinder, R., Lopez-Olivo, M. A., et al. (2009). A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ 181, 787–796. doi: 10.1503/cmaj.091391
Singh, J. A., Furst, D. E., Bharat, A., Curtis, J. R., Kavanaugh, A. F., Kremer, J. M., et al. (2012). 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 64, 625–639. doi: 10.1002/acr.21641
Singh, J. A., Wells, G. A., Christensen, R., Tanjong Ghogomu, E., Maxwell, L., Macdonald, J. K., et al. (2011). Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst. Rev. 16:CD008794. doi: 10.1002/14651858.CD008794.pub2
Smolen, J. S., Braun, J., Dougados, M., Emery, P., Fitzgerald, O., Helliwell, P., et al. (2014). Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann. Rheum. Dis. 73, 6–16. doi: 10.1136/annrheumdis-2013-203419
Soliman, M. M., Ashcroft, D. M., Watson, K. D., Lunt, M., Symmons, D. P., Hyrich, K. L., et al. (2011). Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 70, 583–589. doi: 10.1136/ard.2010.139774
Soubrier, M., Puéchal, X., Sibilia, J., Mariette, X., Meyer, O., Combe, B., et al. (2009). Evaluation of two strategies (initial methotrexate monotherapy vs. its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology 48, 1429–1434. doi: 10.1093/rheumatology/kep261
St Clair, E. W., Van Der Heijde, D. M. F. M., Smolen, J. S., Maini, R. N., Bathon, J. M., Emery, P., et al. (2004). Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 50, 3432–3443. doi: 10.1002/art.20568
Stevenson, M., Archer, R., Tosh, J., Simpson, E., Everson-Hock, E., Stevens, J., et al. (2016). Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol. Assess. 20, 1–610. doi: 10.3310/hta20350
Tsakonas, E., Fitzgerald, A. A., Fitzcharles, M. A., Cividino, A., Thorne, J. C., M'Seffar, A., et al. (2000). Consequences of delayed therapy with second-line agents in rheumatoid arthritis: a 3 year followup on the hydroxychloroquine in early rheumatoid arthritis (HERA) study. J. Rheumatol. 27, 623–629.
van Vollenhoven, R. F., Ernestam, S., Geborek, P., Petersson, I. F., Cöster, L., Waltbrand, E., et al. (2009). Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 374, 459–466. doi: 10.1016/S0140-6736(09)60944-2
Keywords: rheumatoid arthritis, DMARDs, safety, efficacy, network meta-analysis
Citation: Cai W, Gu Y, Cui H, Cao Y, Wang X, Yao Y and Wang M (2018) The Efficacy and Safety of Mainstream Medications for Patients With cDMARD-Naïve Rheumatoid Arthritis: A Network Meta-Analysis. Front. Pharmacol. 9:138. doi: 10.3389/fphar.2018.00138
Received: 25 November 2017; Accepted: 08 February 2018;
Published: 21 March 2018.
Edited by:
Gerfried Karl Hans Nell, NPC Nell Pharma Connect Ltd, AustriaReviewed by:
Domenico Criscuolo, Genovax S.r.l., ItalyFathi M. Sherif, University of Tripoli, Libya
Copyright © 2018 Cai, Gu, Cui, Cao, Wang, Yao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyu Wang, emhhbmd6aGl5dTcyQDE2My5jb20=
†These authors have contributed equally to this work.
 Weiyan Cai
Weiyan Cai Youyi Gu2†
Youyi Gu2† Mingyu Wang
Mingyu Wang