- 1Department of Pulmonary and Critical Care Medicine, Peking University Third Hospital, Beijing, China
- 2Department of Laboratory Medicine, The First Hospital of Sanming Affiliated to Fujian Medical University, Sanming, China
- 3Department of Cardiac Surgery, Peking University Third Hospital, Beijing, China
Background: Considerable studies showed associations between chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD), we evaluated the role of endogenous hydrogen sulfide (H2S)/homocysteine (Hcy) in patients with COPD combined with CVD.
Methods: Fifty one stable patients with COPD were enrolled (25 COPD, 26 COPD + CVD). Lung function, sputum, peripheral blood samples, serum H2S, Hcy, high-sensitivity C-reactive protein (hs-CRP) and tumor necrosis factor-α (TNF-α) levels were measured. Dyspnea, symptoms and quality of life were quantified by modified Medical Research Council dyspnea scale (mMRC), COPD assessment test (CAT) and St. George’s Respiratory Questionnaire (SGRQ).
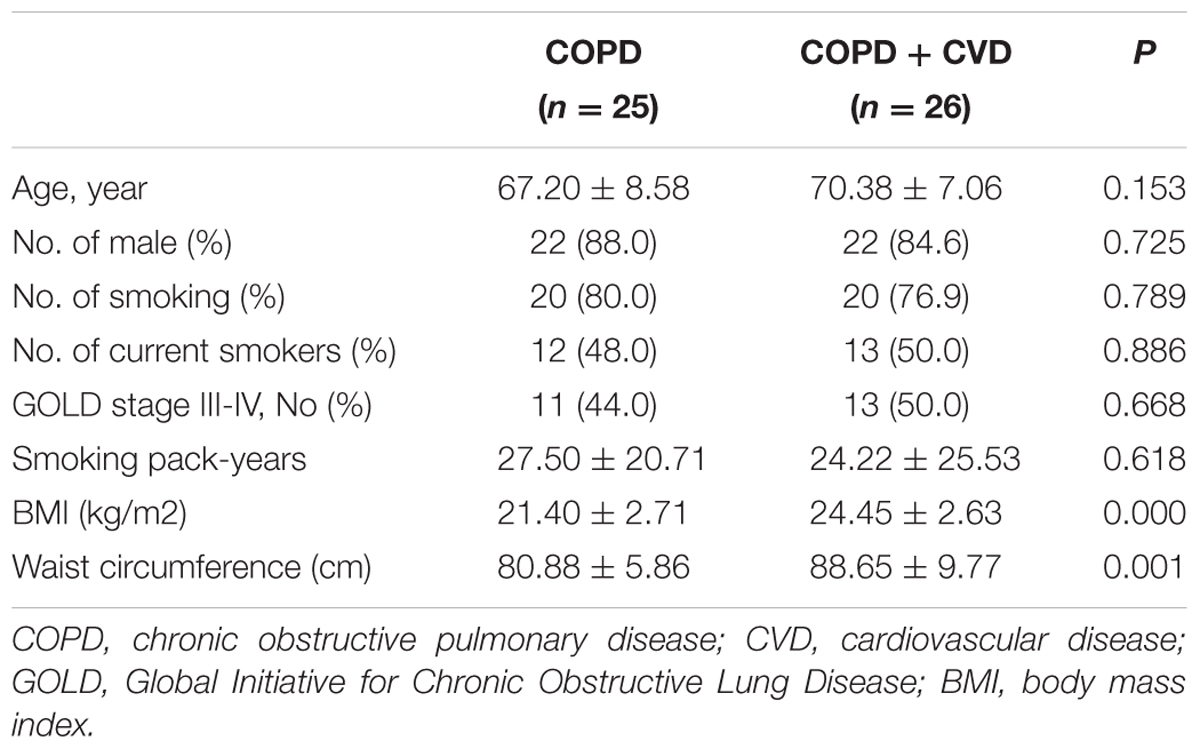
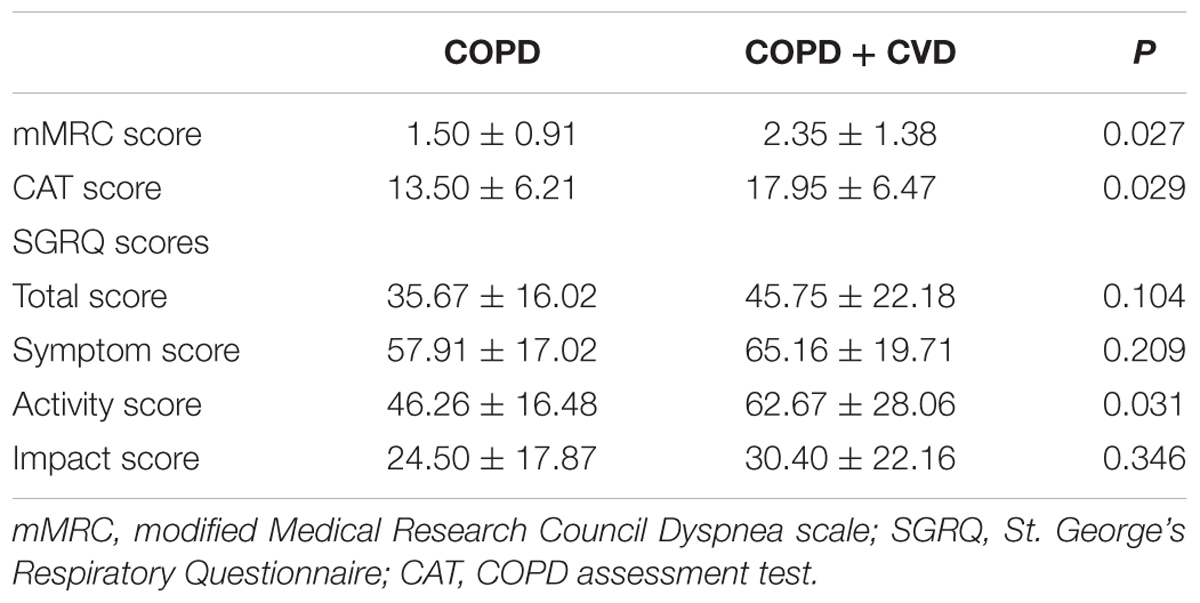
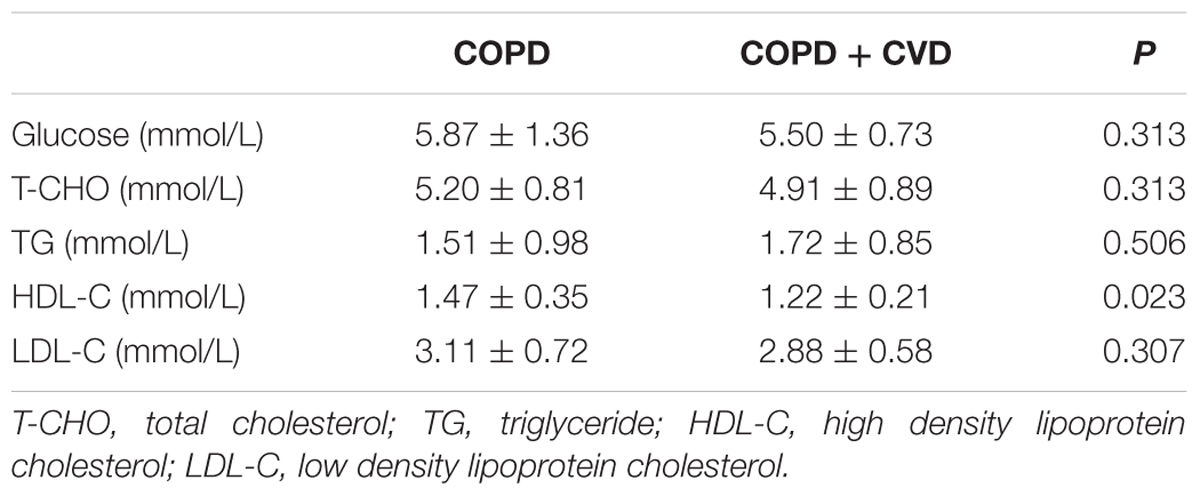
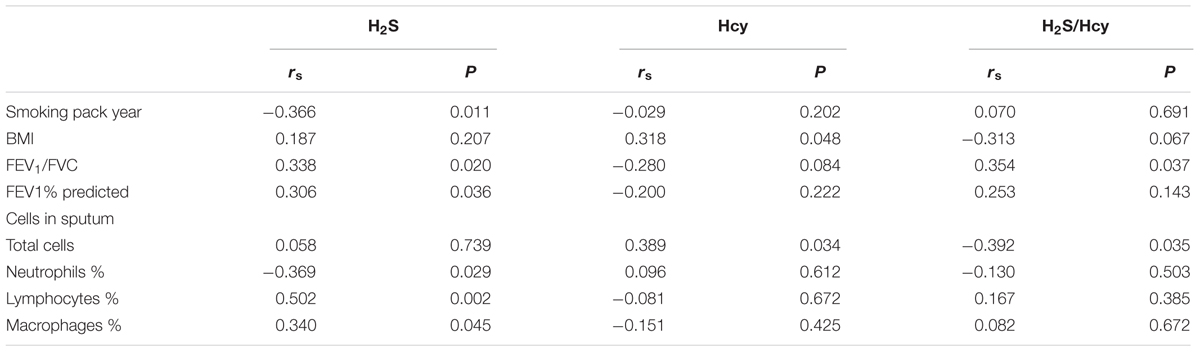
Results: Compared with COPD group, waist circumference and body mass index (BMI) were higher in COPD + CVD group, mMRC, CAT and activity scores were also higher, high density lipoprotein cholesterol (HDL-C) was lower, total cells, neutrophils (%) in sputum and serum hs-CRP level were higher, whereas macrophages (% ) in sputum was lower. H2S and Hcy levels from COPD + CVD group were higher than those from COPD group, but H2S/Hcy ratio was lower. With increasing COPD severity, H2S level was decreased, however, Hcy level was increased. H2S level was positively correlated with FEV1/FVC, FEV1% predicted, lymphocytes (%) and macrophages (%) in sputum, but negatively correlated with smoking pack-years and neutrophils (%) in sputum. Hcy level was positively correlated with BMI and total cells in sputum. The ratio of H2S/Hcy was also positively correlated with FEV1/FVC, but negatively correlated with total cells in sputum.
Conclusion: The imbalance of H2S/Hcy may be involved in the pathogenesis of COPD combined with CVD and provide novel targets for therapy.
Introduction
Chronic obstructive pulmonary disease (COPD) is one chronic inflammatory disease of the lung that is known to have systemic features, one of which is an increased risk of cardiovascular disease (CVD). There is now considerable evidence about associations between COPD and CVD. Cardiovascular events become an important cause of death in COPD. However, the mechanisms responsible for the increased risk of CVD in COPD patients are not known. Further elucidation of these mechanisms will provide novel targets for treatment of both the lung and cardiovascular complications of COPD.
Hydrogen sulfide (H2S) is a gas with smell of rotten eggs, it was discovered to be the third gasotransmitter after nitric oxide (NO) and carbon monoxide (CO) (Wang, 2002). The production of H2S in mammalian cells is attributable to three endogenous enzymes: cystathionine β-synthase (CBS), cystathionineγ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST) (Kajimura et al., 2012). H2S plays an important role in respiratory diseases (Chen et al., 2005, 2011; Chung, 2014; Zhou et al., 2014; Zhang et al., 2015) and CVDs (Chen et al., 2007; Gao et al., 2015). Homocysteine (Hcy) is the main substrate to generate endogenous H2S in the body, high homocysteine is a risk factor in the cardiovascular system (Humphrey et al., 2008; Cioni et al., 2016; Liu et al., 2016; Luo et al., 2017). H2S and homocysteine are in the same metabolic pathway in the body and play a role in pathophysiological processes of diseases.
Previously we have found that serum H2S concentration was increased in patients with stable COPD and decreased in patients with acute exacerbation of COPD (AECOPD) (Chen et al., 2005); the imbalance of hydrogen and Hcy was shown in essential hypertensive children (Chen et al., 2007). It is not known whether the imbalance of endogenous H2S and Hcy is associated with CVD in patients with COPD. Therefore, this study was to explore the metabolic pathway alteration of H2S/Hcy in patients with COPD combined with CVD.
Materials and Methods
Study Design
This was a cross-sectional observational study. We collected data on demographics, smoking and medical history via interview or self-administered questionnaires. Dyspnea was quantified by application of the modified Medical Research Council Dyspnea scale (mMRC) which asked respondents to rate dyspnea on a 5-point scale from 0 (absent) to 4 (dyspnea when dressing /undressing). Symptoms were quantified by use of COPD assessment test (CAT). Quality of life in patients with COPD was evaluated by St George’s respiratory questionnaire (SGRQ). Lung function tests, sputum were performed.
Subjects
Patients were recruited from clinics at Peking University, Third Hospital between September 2010 and March 2011. We enrolled 51 stable COPD patients. The diagnosis of COPD was made according to the criteria recommended by Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline (Rabe et al., 2007). Chronic airflow limitation was defined as forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) < 70% after using an inhaled bronchodilator, bronchodilator reversibility test revealed an increase in FEV1 < 12% and/or 200 ml below the prebronchodilator FEV1 after administration of 400 μg of inhaled salbutamol. Patients with stable COPD had no acute exacerbation of symptoms and upper respiratory tract infection in the 2 months preceding the study. Subjects with concomitant respiratory diseases other than COPD were excluded. COPD subjects were categorized as non-severe (stages I and II) and severe (stages III and IV) according to GOLD stages. All selected patients were divided into COPD group (25 patients) and COPD + CVD group (26 patients). CVD referred to hypertension, coronary heart disease (angina, myocardial infarction), left ventricular failure and cardiac arrhythmia. In COPD + CVD group, 11 patients got hypertension, 5 patients got coronary heart disease, 1 patient got left ventricular failure, and 9 patients got 2 or more CVDs. For each CVD, we diagnosed according to the guidelines (Roversi et al., 2016). Protocol for this study was approved by the institutional review board of Peking University, Third Hospital (approved number IRB00006761-2012029). Written informed consents were obtained from all participants.
Pulmonary Function Test
Pulmonary function test was performed with a spirometer (Medgraphics, Elite Series DL, St. Paul, MN, United States). FEV1, FVC, FEV1/FVC, and FEV1% predicted were measured in all subjects.
Serum H2S, Hcy, hs-CRP, TNF-α, Glucose and Lipids Levels
Venous blood samples were obtained from all subjects while fasting, serum was stored at -80°C until further analysis. Serum H2S concentration was measured with use of a sulfide-sensitive electrode (Model 9616, Orion Research, Beverly) according to our published literature (Chen et al., 2005). Hcy, high sensitive C-reactive protein (hs-CRP) and Tumor necrosis factor α (TNF-α) in serum were measured using commercially ELISA kits (R&D Systems, Minneapolis, MN, United States). Glucose, and lipids (T-CHO, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol) in serum were measured using commercially reagent kits (Merit Choice, Bioengineering, Beijing; or SEKISUI.MEDICAL CO., LTD.).
Induced Sputum Analysis
Sputum was collected and processed as previous described (Chen et al., 2005). In brief, all patients inhaled 4% hypertonic saline solution for 15–30 min to induce the sputum plugs from the lower respiratory tract, before that, all patients inhaled 200 μg of salbutamol for avoiding hypertonic saline solution-induced bronchoconstriction. Sputum plugs were incubated with 0.1% dithiothreitol until completely homogenized. After filtering and centrifuging, the cell pellet was resuspended in phosphate-buffered saline solution (PBS), then placed on slides and stained with Wright-Giemsa for differential cell counts of leukocytes.
Statistical Analysis
Statistical analyses involved use of SPSS v17.0 (SPSS Inc., Chicago, IL, United States). Continuous variables were expressed as mean ± SD (standard deviation) for normal distribution. Categorical variables were expressed in number (percentage). For comparisons between two continuous variables, the independent two-sample test was used, for categorical variables, X2 test was used. Correlation analysis was performed by use of Spearman rank correlation. A two-tailed P-value < 0.05 was considered significant.
Results
Clinical Characteristics and Lung Function between Two Groups
The characteristics and lung function of subjects were shown in Tables 1–3. Body mass index (BMI) and waist circumference were higher in COPD + CVD group than in COPD group (Table 1), mMRC, CAT and activity scores were also higher (Table 2). The decrease of lung function was found in COPD + CVD group as compared to the COPD group (Table 3), but the difference was not statistically significant. So patients in COPD + CVD group had worse dyspnea, severer symptoms and worse quality of life.
Glucose and Lipids in Blood
We determined whether the glucose or lipids in blood could be altered in COPD + CVD group. There were no significant differences in blood glucose, T-CHO, TG and LDL-C between two groups, but high density lipoprotein- cholesterol (HDL-C) was lower in COPD + CVD group (1.22 ± 0.21 mmol/L) than in COPD group(1.47 ± 0.35 mmol/L) (Table 4).
Cellular Parameters in Sputum and Inflammatory Cytokines in Serum
Total cells and neutrophils (%) were higher in sputum of COPD + CVD group than those of COPD group, while macrophages (%) was lower, there was no statistical significance in lymphocytes (%) between two groups (Table 5). hs-CRP level in serum from COPD + CVD group (21.97 ± 9.84 ng/ml) was higher than that from COPD group (16.44 ± 5.98 ng/ml), but there was no significant difference in TNF-α level (Table 5). It indicated that the airway inflammation was more obvious in COPD + CVD patients.
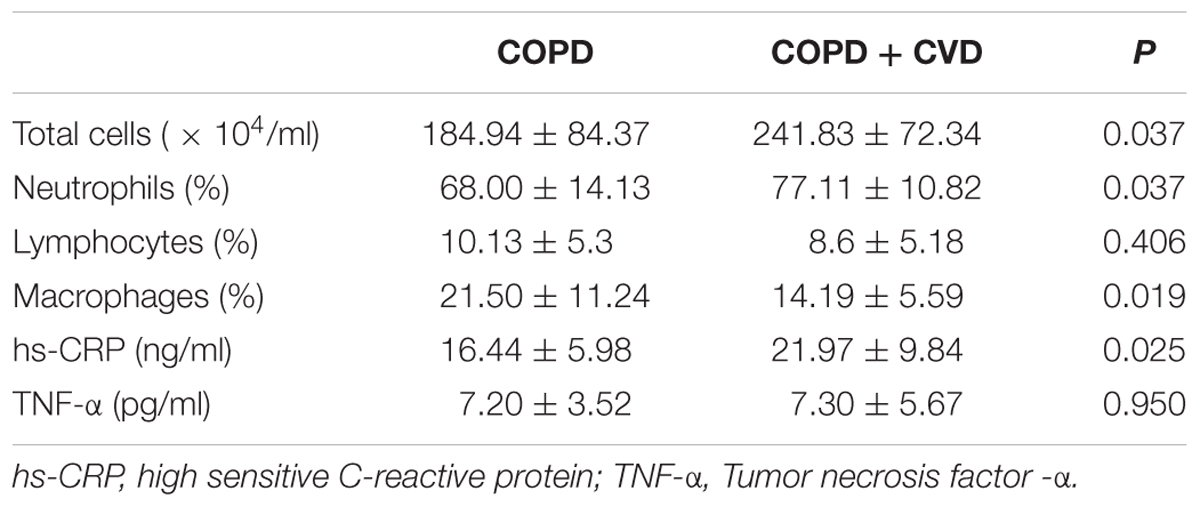
TABLE 5. The differentiated cell proportion in sputum and inflammatory cytokines in serum between two groups.
H2S, Hcy Levels in Serum and H2S/Hcy Ratio
H2S level in serum from COPD + CVD group (14.91 ± 5.72 μmol/L) was higher than that from COPD group (11.68 ± 3.14 μmol/L) (Figure 1A), and it was decreased significantly with increasing COPD severity (Figure 2A); Hcy level from COPD + CVD group (7.76 ± 7.64 μmol/L) was higher than that from COPD group (3.08 ± 3.21 μmol/L) (Figure 1B), and it was increased significantly with increasing COPD severity (Figure 2B). We also measured the H2S/Hcy ratio and found that H2S/Hcy ratio in serum from COPD + CVD group was lower than that from COPD group (Figure 1C).
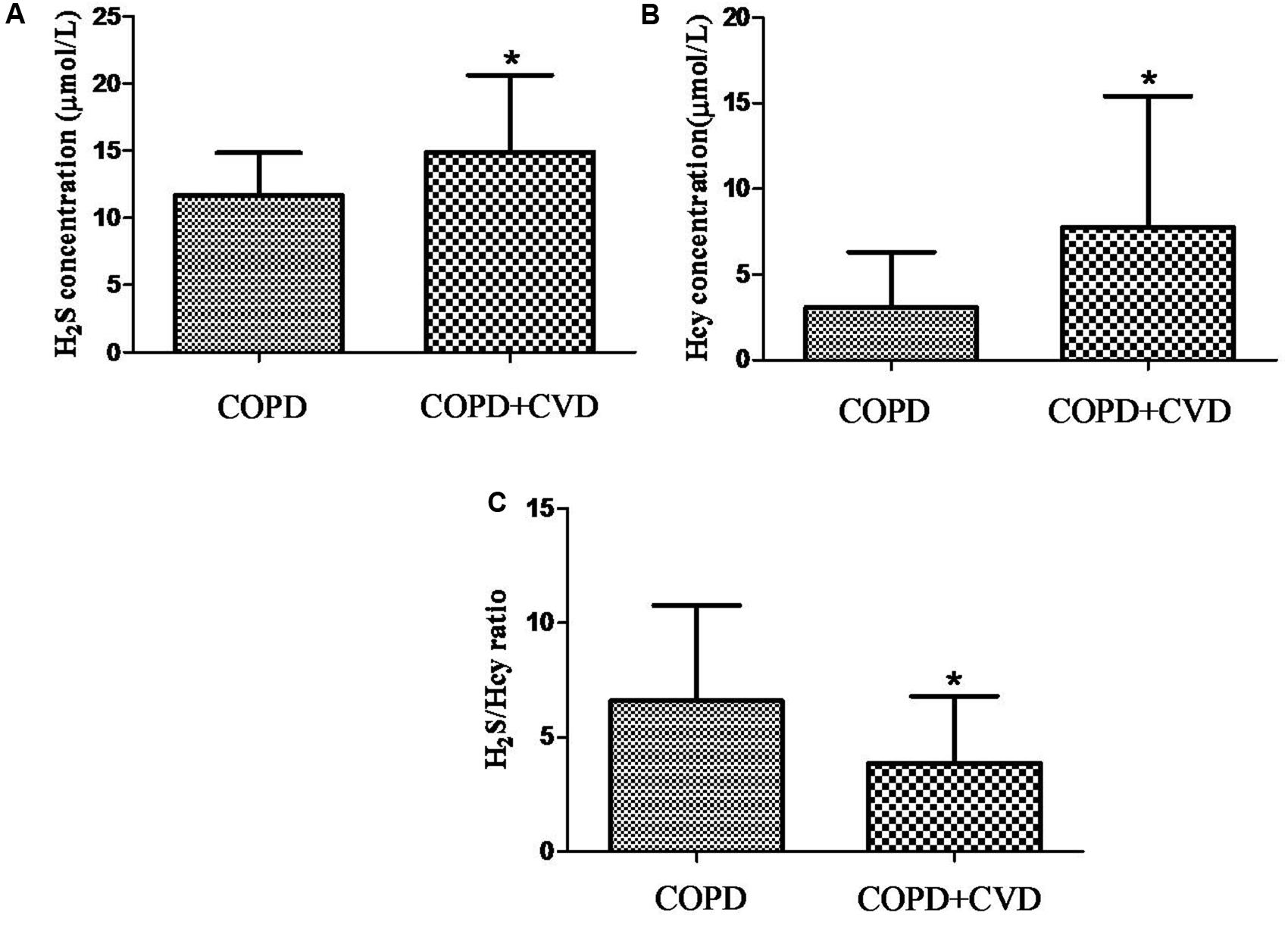
FIGURE 1. The serum H2S, Hcy and H2S/Hcy ratio in COPD and COPD + CVD groups. (A) Serum H2S concentration in both groups; (B) Serum Hcy concentration in both groups, (C) The ratio of H2S/Hcy in both groups. The data are described as mean ± SD. ∗P < 0.05 versus COPD.
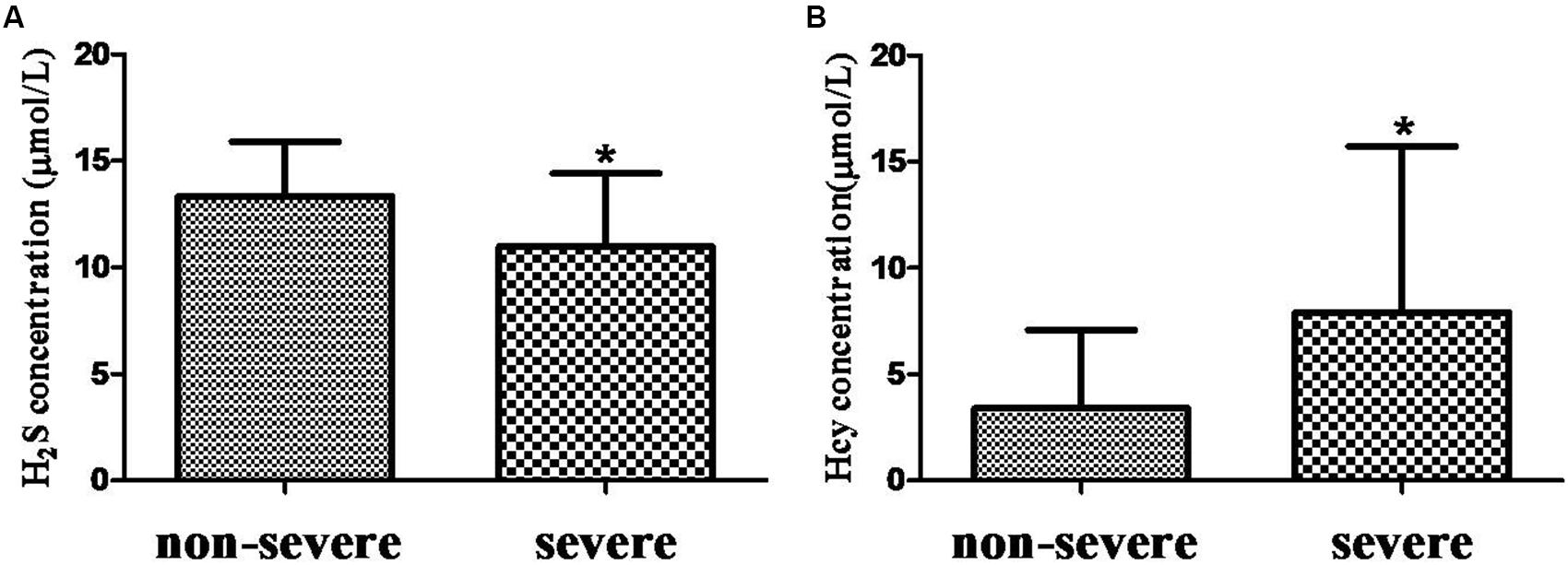
FIGURE 2. The H2S and Hcy in non-severe and severe groups. (A) Serum H2S concentration in both groups; (B) Serum Hcy concentration in both groups. The data are described as mean ± SD. ∗P < 0.05 versus COPD.
Correlations among H2S, Hcy, H2S/Hcy Ratio and Other Variables
Table 6 showed the correlations among serum H2S, Hcy levels, H2S/Hcy ratio and other variables in all subjects. Serum H2S level was positively correlated with FEV1/FVC, FEV1% predicted, lymphocytes (%) and macrophages (%) in sputum, while it was negatively correlated with smoking pack-years and neutrophils (%) in sputum. Serum Hcy level was positively correlated with BMI and total cells in sputum. H2S/Hcy ratio was positively correlated with FEV1/FVC.
Discussion
In our study, we have identified the imbalance of endogenous H2S and Hcy in COPD + CVD. Compared with patients with COPD, patients with COPD + CVD had severer dyspnea, worse symptoms and quality of life, the ratio of endogenous H2S/Hcy was lower, they also had obvious local and systemic inflammation reaction.
Chronic obstructive pulmonary disease is an inflammatory disease, there is growing evidence that the inflammatory state associated with COPD is not only confined to the lung but also to the circulation system and non-pulmonary organs. Epidemiological studies indicate that COPD is associated with high frequencies of coronary artery disease, congestive heart failure and cardiac arrhythmia, independent of shared risk factors (Roversi et al., 2016). Whether they have distinct clinical characteristics is not clear.
In our study, we found that COPD + CVD patients had higher waist circumference and BMI values. BMI is an indicator reflecting the overall obesity; Waist circumference, which reflects abdominal fat content, is an indicator of abdominal obesity. Studies have shown that visceral fat produced more angiotensin, interleukin-6 and plasminogen activator inhibitor than subcutaneous fat did, (Wajchenberg et al., 2001) it had closer correlation with hypertension, diabetes, metabolic syndrome, (Liu et al., 2010) and was more important than BMI as a cardiovascular risk. So higher waist circumference is an obvious risk factor for COPD + CVD patients.
We also found patients with COPD and cardiac comorbidities had severer dyspnea, worse symptoms and quality of life. SGRQ is the most widely used questionnaire in COPD to assess the quality of life, the SGRQ score is related to the prognosis of COPD and considered as an independent risk factor for mortality (Fan et al., 2002). mMRC is a commonly used questionnaire to evaluate dyspnea of patients. CAT is a new test questionnaire which covers all aspects such as symptoms, activity and sleep, psychological and social impacts. Induced sputum analysis showed COPD + CVD patients had higher total cells, neutrophils (%) and lower macrophages (%) in sputum, which indicated that the airway inflammation was more obvious in COPD + CVD patients. These results showed that COPD + CVD patients had obvious clinical characteristics and airway inflammation.
Researchers have proposed multiple mechanisms that link coronary heart disease with COPD. Possible pathways include associations with chronic low-grade systemic inflammation, oxidative stress and shared risk factors, such as older age, cigarette smoking and environment pollution. H2S is found to be a protective factor in CVDs, (Yang et al., 2008; Calvert et al., 2009; Lu et al., 2017) while high Hcy is a risk factor in cardiovascular system (Humphrey et al., 2008; Cioni et al., 2016; Liu et al., 2016; Luo et al., 2017). We speculate that the imbalance of endogenous H2S/Hcy metabolic pathway plays an important role in the pathogenesis of COPD + CVD. It was reported that the plasma H2S/Hcy ratio in children with hypertension was lower than that of control (Chen et al., 2007). Plasma H2S level was significantly lower in patients with acute coronary syndrome than patients with stable angina pectoris or non-coronary artery disease (Gao et al., 2015). We found that serum H2S level was significantly increased in COPD + CVD patients and decreased with the severity of COPD. H2S level was positively correlated with FEV1, FEV1/FVC, and FEV1% predicted, which was consistent with our previous findings (Chen et al., 2005). It was shown that plasma homocysteine was elevated in COPD patients compared to healthy controls, (Seemungal et al., 2007; Fimognari et al., 2009) and elevated with COPD severity (Seemungal et al., 2007). In this study, serum Hcy level was elevated in patients with COPD + CVD and also increased with severity of COPD, which was consistent with previous findings, (Seemungal et al., 2007), however, we found that the ratio of H2S/Hcy in COPD + CVD patients was lower, and it was positively correlated with FEV1/FVC. These results suggested that the imbalance of H2S/Hcy may be a risk factor for COPD + CVD patients. In COPD + CVD patients, whether protein expression or activity of CBS/CSE decreasing need to be explored further.
On the other hand, many studies have shown that H2S played a protective role through anti-inflammation and/or antioxidant stress, for example, exerting protective effects in rats of pulmonary fibrosis or acute lung injury (Cao et al., 2014; Zhou et al., 2014; Tang et al., 2017). In contrast, high Hcy triggered an inflammatory reaction in vascular muscle cells by promoting CRP production, (Pang et al., 2014) induced inflammatory injury in endothelial cells (Han et al., 2015). In COPD + CVD patients, there was higher serum hs-CRP, total cells and neutrophils% in sputum, this indicated more obvious inflammation reaction. Both H2S and Hcy levels were increased in patients with COPD and cardiac comorbidities, but H2S/Hcy ratio was lower, maybe the higher level of H2S is a consequence of increased Hcy, it is a kind of compensation reaction, however, the increased H2S was not in proportion to the increased Hcy, the beneficial compensation of H2S in body may not inhibit the adverse effect of increased Hcy.
This study also showed that HDL-C was lower in COPD + CVD patients. Hcy level was positively correlated with BMI, which was consistent with the reports of and Ikkruthi et al. (2013) and Yakub et al. (2014) Hcy is also associated with lipid metabolism. In a cohort survey based in Chinese population of a community, it showed that hyperhomocysteinemia was related to increasing risk of low HDL-C and high TG, it predicted that Hcy levels might influence lipid metabolism (Momin et al., 2017). Dyslipidemia and overweight are recognized risk factors for CVD. The associations among H2S/Hcy, dyslipidemia and overweight may provide other targets for treatments in COPD + CVD patients.
In addition, our previous study showed that smoking can cause the decline of serum H2S level, (Chen et al., 2005) current study showed that serum H2S was negatively correlated with smoking pack-years. It is well known that smoking is a common risk factor for COPD and CVD, leading to oxidative/antioxidant imbalance and systemic inflammation. Decreased H2S level may reduce its anti-inflammatory and anti-oxidation capability, resulting in an increased risk for COPD and CVD.
However, our study had some limitations. First, it was a cross-sectional study, without the possibility to properly infer causality. Therefore, we need prospective studies to investigate the role of H2S/Hcy in metabolic pathway of COPD + CVD patients for providing some directions in diagnosis and treatment. Second, this study was a pilot study, the samples of each group were a little small, and study subjects were restricted to stable COPD patients without acute exacerbation, so our results cannot be generalized. Last but not least, We didn’t include healthy controls in this study, but in our previous study, (Chen et al., 2005) we have observed the difference of plasma H2S between COPD patients and healthy controls; Also, in other studies, (Seemungal et al., 2007; Fimognari et al., 2009) the difference of Hcy between COPD patients and healthy controls was observed. The objects of this study were to explore the differences of serum H2S and Hcy between COPD patients and COPD + CVD patients. Nevertheless, it is a foundation for future large cohort study.
In summary, a plethora of factors may be associated with the increased risks of CVD in COPD. The role of H2S/Hcy metabolic pathway and its clinical significance need further exploration. Future exploration of these mechanisms shall provide novel targets for treatment of cardiovascular complications in COPD.
Author Contributions
YH, SL, YC, ZZ, and WY contributed to study concept and design. YH, SL, CL, and FL contributed to data collection. YH and SL contributed to data analysis, interpretation, and drafting. YC, ZZ, and WY contributed to manuscript revise. All authors contributed to the final approval of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the National Nature Science Foundation of China (81170012 and 81370141), the Research Special Fund for Public Welfare Industry of Health (201002008), Capital Medicine Development Fund (2011-1004-01), and Health Committee Youth Research Project Foundation of Fujian Province (2014-1-89). The authors thank Dr. Dexiang Zhuo (The Affiliated Sanming First Hospital of Fujian Medical University), Rong Liu and Yuzhu Wang (Peking University Third Hospital) for technical assistance. The authors also thank ATS International Conference 2012 for presenting this initial idea.
References
Calvert, J. W., Jha, S., Gundewar, S., Elrod, J. W., Ramachandran, A., Pattillo, C. B., et al. (2009). Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 105, 365–374. doi: 10.1161/CIRCRESAHA.109.199919
Cao, H., Zhou, X., Zhang, J., Huang, X., Zhai, Y., Zhang, X., et al. (2014). Hydrogen sulfide protects against bleomycin-induced pulmonary fibrosis in rats by inhibiting NF-κB expression and regulating Th1/Th2 balance. Toxicol. Lett. 224, 387–394. doi: 10.1016/j.toxlet.2013.11.008
Chen, L., Ingrid, S., Ding, Y. G., Liu, Y., Qi, J. G., Tang, C. S., et al. (2007). Imbalance of endogenous homocysteine and hydrogen sulfide metabolic pathway in essential hypertensive children. Chin. Med. J. 120, 389–393.
Chen, Y. H., Wang, P. P., Wang, X. M., He, Y. J., Yao, W. Z., Qi, Y. F., et al. (2011). Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine 53, 334–341. doi: 10.1016/j.cyto.2010.12.006
Chen, Y. H., Yao, W. Z., Geng, B., Ding, Y. L., Lu, M., Zhao, M. W., et al. (2005). Endogenous hydrogen sulfide in patients with COPD. Chest 128, 3205–3211. doi: 10.1378/chest.128.5.3205
Chung, K. F. (2014). Hydrogen sulfide as a potential biomarker of asthma. Expert Rev. Respir. Med. 8, 5–13. doi: 10.1586/17476348.2014.856267
Cioni, G., Marcucci, R., Gori, A. M., Valente, S., Giglioli, C., Gensini, G. F., et al. (2016). Increased homocysteine and lipoprotein(a) levels highlight systemic atherosclerotic burden in patients with a history of acute coronary syndromes. J. Vasc. Surg. 64, 163–170. doi: 10.1016/j.jvs.2016.01.056
Fan, V. S., Curtis, J. R., Tu, S.-P., McDonell, M. B., and Fihn, S. D. (2002). Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest 122, 429–436. doi: 10.1378/chest.122.2.429
Fimognari, F. L., Loffredo, L., Di Simone, S., Sampietro, F., Pastorelli, R., Monaldo, M., et al. (2009). Hyperhomocysteinaemia and poor vitamin B status in chronic obstructive pulmonary disease. Nutr. Metab. Cardiovasc. Dis. 19, 654–659. doi: 10.1016/j.numecd.2008.12.006
Gao, L., Xu, Z., Yin, Z., Chen, K., Wang, C., and Zhang, H. (2015). Association of hydrogen sulfide with alterations of monocyte chemokine receptors, CCR2 and CX3CR1 in patients with coronary artery disease. Inflamm. Res. 64, 627–635. doi: 10.1007/s00011-015-0844-7
Han, S., Wu, H., Li, W., and Gao, P. (2015). Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol. Cell. Biochem. 403, 43–49. doi: 10.1007/s11010-015-2335-0
Humphrey, L. L., Fu, R., Rogers, K., Freeman, M., and Helfand, M. (2008). Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin. Proc. 83, 1203–1212. doi: 10.1016/S0025-6196(11)60637-X
Ikkruthi, S., Rajappa, M., Nandeesha, H., Satheesh, S., Sundar, I., Ananthanarayanan, P., et al. (2013). Hyperhomocysteinemia and hyperlipoproteinemia (a) in obese south Indian men: an indication for increased cardiovascular risk. Acta Physiol. Hung. 101, 13–20. doi: 10.1556/APhysiol.100.2013.017
Kajimura, M., Nakanishi, T., Takenouchi, T., Morikawa, T., Hishiki, T., Yukutake, Y., et al. (2012). Gas biology: tiny molecules controlling metabolic systems. Respir. Physiol. Neurobiol. 184, 139–148. doi: 10.1016/j.resp.2012.03.016
Liu, J., Fox, C. S., Hickson, D. A., May, W. D., Hairston, K. G., Carr, J. J., et al. (2010). Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J. Clin. Endocrinol. Metab. 95, 5419–5426. doi: 10.1210/jc.2010-1378
Liu, J., Wei Zuo, S., Li, Y., Jia, X., Jia, S. H., Zhang, T., et al. (2016). Hyperhomocysteinaemia is an independent risk factor of abdominal aortic aneurysm in a Chinese Han population. Sci. Rep. 6:17966. doi: 10.1038/srep17966
Lu, G., Xu, C., Tang, K., Zhang, J., Li, Q., Peng, L., et al. (2017). H2S inhibits angiotensin II-induced atrial Kv1.5 upregulation by attenuating Nox4-mediated ROS generation during atrial fibrillation. Biochem. Biophys. Res. Commun. 483, 534–540. doi: 10.1016/j.bbrc.2016.12.110
Luo, J.-L., Chien, K.-L., Hsu, H.-C., Su, T.-C., Lin, H.-J., Chen, P.-C., et al. (2017). Association between plasma homocysteine concentration and the risk of all-cause death in adults with diastolic dysfunction in a community: a 13-year cohort study. Medicine 96:e6716. doi: 10.1097/MD.0000000000006716
Momin, M., Jia, J., Fan, F., Li, J., Dou, J., Chen, D., et al. (2017). Relationship between plasma homocysteine level and lipid profiles in a community-based Chinese population. Lipids Health Dis. 16, 54. doi: 10.1186/s12944-017-0441-6
Pang, X., Liu, J., Zhao, J., Mao, J., Zhang, X., Feng, L., et al. (2014). Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 236, 73–81. doi: 10.1016/j.atherosclerosis.2014.06.021
Rabe, K. F., Hurd, S., Anzueto, A., Barnes, P. J., Buist, S. A., Calverley, P., et al. (2007). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 176, 532–555. doi: 10.1164/rccm.200703-456SO
Roversi, S., Fabbri, L. M., Sin, D. D., Hawkins, N. M., Agustí, A., et al. (2016). Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am. J. Respir. Crit. Care Med. 94, 1319–1336. doi: 10.1164/rccm.201604-0690SO
Seemungal, T. A., Lun, J. C. F., Davis, G., Neblett, C., Chinyepi, N., Dookhan, C., et al. (2007). Plasma homocysteine is elevated in COPD patients and is related to COPD severity. Int. J. Chron. Obstruct. Pulmon. Dis. 2, 313–321. doi: 10.2147/COPD.S2147
Tang, B., Ma, L., Yao, X., Tan, G., Han, P., Yu, T., et al. (2017). Hydrogen sulfide ameliorates acute lung injury induced by infrarenal aortic cross-clamping by inhibiting inflammation and angiopoietin 2 release. J. Vasc. Surg. 65, 501.e1–508.e1. doi: 10.1016/j.jvs.2015.10.010
Wajchenberg, B., Giannella-Neto, D., Da Silva, M., and Santos, R. (2001). Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm. Metab. Res. 34, 616–621. doi: 10.1055/s-2002-38256
Wang, R. (2002). Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16, 1792–1798. doi: 10.1096/fj.02-0211hyp
Yakub, M., Schulze, K. J., Khatry, S. K., Stewart, C. P., Christian, P., and West, K. P. (2014). High plasma homocysteine increases risk of metabolic syndrome in 6 to 8 year old children in rural Nepal. Nutrients 6, 1649–1661. doi: 10.3390/nu6041649
Yang, G., Wu, L., Jiang, B., Yang, W., Qi, J., Cao, K., et al. (2008). H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322, 587–590. doi: 10.1126/science.1162667
Zhang, J., Wang, X., Chen, Y., and Yao, W. (2015). Exhaled hydrogen sulfide predicts airway inflammation phenotype in COPD. Respir. Care 60, 251–258. doi: 10.4187/respcare.03519
Keywords: chronic obstructive pulmonary disease, cardiovascular disease, hydrogen sulfide, homocysteine, quality of life
Citation: He Y, Liu S, Zhang Z, Liao C, Lin F, Yao W and Chen Y (2017) Imbalance of Endogenous Hydrogen Sulfide and Homocysteine in Chronic Obstructive Pulmonary Disease Combined with Cardiovascular Disease. Front. Pharmacol. 8:624. doi: 10.3389/fphar.2017.00624
Received: 02 July 2017; Accepted: 25 August 2017;
Published: 12 September 2017.
Edited by:
Jinsong Bian, National University of Singapore, SingaporeReviewed by:
Hongfang Jin, Peking University First Hospital, ChinaYi Zhun Zhu, Macau University of Science and Technology, China
Copyright © 2017 He, Liu, Zhang, Liao, Lin, Yao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yahong Chen, Y2hlbnlhaG9uZ0B2aXAuc2luYS5jb20= Zhe Zhang, emhhbmd6aGVAYmptdS5lZHUuY24=
†These authors have contributed equally to this work.
 Yanjing He
Yanjing He Shaoyu Liu
Shaoyu Liu Zhe Zhang
Zhe Zhang Chengcheng Liao
Chengcheng Liao Fan Lin
Fan Lin Wanzhen Yao1
Wanzhen Yao1 Yahong Chen
Yahong Chen