- 1Vascular Pharmacology Laboratory, Cardiovascular Disease Program, Department of Pharmacology, Biomedical Discovery Institute, Monash University, Clayton, VIC, Australia
- 2Department of Medicine, Monash University, Clayton, VIC, Australia
- 3Baker Heart and Diabetes Institute, Melbourne, VIC, Australia
Fibrosis is a process of dysfunctional wound repair, described by a failure of tissue regeneration and excessive deposition of extracellular matrix, resulting in tissue scarring and subsequent organ deterioration. There are a broad range of stimuli that may trigger, and exacerbate the process of fibrosis, which can contribute to the growing rates of morbidity and mortality. Whilst the process of fibrosis is widely described and understood, there are no current standard treatments that can reduce or reverse the process effectively, likely due to the continuing knowledge gaps surrounding the cellular mechanisms involved. Several cellular targets have been implicated in the regulation of the fibrotic process including membrane domains, ion channels and more recently mechanosensors, specifically caveolae, particularly since these latter contain various signaling components, such as members of the TGFβ and MAPK/ERK signaling pathways, all of which are key players in the process of fibrosis. This review explores the anti-fibrotic influences of the caveola, and in particular the key underpinning protein, caveolin-1, and its potential as a novel therapeutic target.
Introduction
Fibrosis, a process of wound reparation, is a cellular response to tissue damage or insult, preceded by an inflammatory response. Various etiologies can act as triggers to induce inflammation and subsequent tissue damage, including infection, autoimmunity and tumors (Wynn, 2007; Wick et al., 2013). In physiological conditions, inflammation is followed by repair processes that eliminate the initial insult, through the replacement and replenishment of injured cells. The repair process progresses with the replacement of connective tissue or fibrous tissue-producing mesenchymal cells such as fibroblasts, the primary cells involved in fibrosis. Of note, this resolution step initiates the recruitment of leukocytes and subsequently promotes the process of apoptosis (Akbar and Salmon, 1997; Buckley et al., 2001). When the resolution step becomes dysregulated, observed by a transition from an acute to a chronic inflammatory process, fibrosis occurs which manifests as scar tissue, further promoting end-organ damage and ultimately death (Wynn, 2007; Wick et al., 2013). Chronic inflammation is essential for the progression of fibrosis as it promotes long-term tissue remodeling and subsequent organ dysfunction (Wynn, 2007; Wick et al., 2013; Zeisberg and Kalluri, 2013). The onset of fibrosis begins with epithelial or endothelial activation, which results in the release of inflammatory mediators, for both clot formation (hemostasis) and inflammatory cell recruitment. Innate immune cells, such as neutrophils, release pro-fibrotic factors or cytokines, including matrix metalloproteinases (MMPs), elastases, and cathepsins, to cleave connective tissue and in turn disrupt the basement membrane, promoting cell migratory capacities (Wynn, 2007; Wick et al., 2013). In addition, macrophages are vital for fibroblast activation, through their release of tumor necrosis factor-α (TNF-α) and interleukin 1 (IL-1). Further, macrophages also release transforming growth factor β (TGF-β), which is essential for the onset of processes involved in fibrosis including epithelial-mesenchymal transformation (EMT) (Wick et al., 2013). Morphologically, caveolae are described as flask-like or Ω shaped membrane invaginations with a diameter of 50–100 nm (Palade, 1953; Yamada, 1955). There are two main protein components essential for the formation and function of caveolae—caveolins and cavins (Drab et al., 2001; Galbiati et al., 2001). The primary function of caveolae is yet to be determined, although it has been shown to play a mediatory role in cholesterol homeostasis (Frank et al., 2008; Zhang et al., 2008), vascular reactivity (Drab et al., 2001; Razani et al., 2001a), mechanotransduction (Park et al., 1998; Sinha et al., 2011; Shihata et al., 2016), and cellular signaling (Yang and Rizzo, 2007). Importantly, caveolin-1 (Cav-1), originally called vesicular integral membrane protein of 21 kD (VIP-21), is generally accepted as the predominant protein involved in caveolae's key regulatory function in intracellular signaling pathways evident in fibrotic processes observed in different organs including cardiac, lung, and kidney fibrosis (Drab et al., 2001).
Cardiac Fibrosis
Cardiac fibrosis is the abnormal accumulation of extracellular matrix (ECM) in the myocardial tissue. Key to the pathogenesis of cardiac fibrosis is the limited regenerative ability of the adult mammalian heart (Soonpaa and Field, 1998). This is due to the transition phase during neonatal stages, where there is a switch from hyperplastic to hypertrophic growth, observed by an increase of myocardial mass, independent of proliferation. In the event of cardiac damage or injury, an inflammatory process is followed by the replacement of cardiomyocytes with fibrotic tissue in place of a replenished supply of cardiomyocytes (Soonpaa and Field, 1998). In the cardiac setting, Cav are present as various subtypes, however Cav-1 and Cav-3 are the predominant isoforms expressed by endothelial cells and cardiomyocytes, respectively (Tang et al., 1996).
The deletion of the gene encoding Cav-1 leads to adverse cardiac remodeling following myocardial infarction (MI). It has been demonstrated that this is due to Cav-1 knockout (Cav1−/−) mice having an impaired inflammatory reaction, where alternative or M2 macrophages, known for promoting scar formation, accumulate more in Cav1−/− mice compared to WT mice. Further adoptive transfer studies, where macrophages from Cav1−/− mice were transferred into WT mice and vice versa, showed an increase in the survival rate of Cav1−/− mice that received macrophages from WT mice and decreased survival rates for WT mice who received Cav1−/− macrophages (Shivshankar et al., 2014). Similarly, a study evaluated the degree of fibrosis in the heart of mice with overexpression of Cav-3 compared to WT mice. Following transverse aortic constriction (TAC) procedures, mice that overexpressed Cav-3 displayed lower levels of fibrosis, and improved natriuretic levels, relative to the WT mice (Horikawa et al., 2011). Further, it has been shown that reconstitution of endothelial Cav-1 into Cav-1−/− knockout mice reduces gene expression of TGF-β1, collagen I and III, ultimately decreasing fibrotic lesions in the heart and improving cardiac function (Murata et al., 2007). Importantly, several studies have highlighted the major contributory role of TGF-β in the pathogenesis and regulation of cardiac fibrosis and hypertrophy (Brooks and Conrad, 2000; Deten et al., 2001; Dewald et al., 2004).
The main regulatory mechanism for TGF-β signal transduction is endocytosis regulated by Cav-1-associated lipid rafts and early endosomal antigen-1 (EEA-1) non-lipid rafts. While EEA-1 increases TGF-β1 signaling, Cav-1-associated internalization reduces, and in some cases even abolishes TGF-β1 signaling (Di Guglielmo et al., 2003; Ito et al., 2004). Without downregulation from Cav-1, ligand-receptor binding of TGF-β1 initiates the assembly of a heteromeric receptor complex by transphosphorylating the TGF-β type II receptor (TβRII), which then activates TGF-β type I receptor (TβRI) (Wrana et al., 1994). Further, TβRI induces Smad signaling by phosphorylating Smads, receptor-regulated Smad (R-Smad) 2 and 3. Smad2 and Smad3 relay the signaling process from the plasma membrane to the nucleus, via R-Smads and Smad4 that translocate to the nucleus where transcriptional changes occur (Miyazono et al., 2000). Cav-1 negatively regulates Smad signaling by binding the caveolin scaffolding domain (CSD) component, the key functional component of Cav-1, to TβRI, which in turn diminishes the downstream signaling of Smads via the TGF-β receptors (Razani et al., 2001b). The interaction between Cav-1 and TβRI impairs the phosphorylation process, and in turn inhibits the heteromerization with Smad4, which is essential for the initiation of transcriptional changes (Razani et al., 2001b). In addition to Smad signaling pathways, TGF-β also exerts its actions through non-canonical, non-Smad pathways including Mitogen-activated protein kinase (MAPK) pathways, Rho-like GTPase signaling pathways, and phosphatidylinositol-3-kinase/Protein Kinase B (PI3K/AKT) pathways. Cav-1 has also been demonstrated to negatively regulate the activation state of p42/44 MAPK cascade in cardiac fibroblasts (Galbiati et al., 1998). This finding is also supported by morphological evidence demonstrating ERK-1/2 co-localization to caveolae in an in vivo model of cardiac fibrosis (Liu et al., 1997).
Lung Fibrosis
Lung fibrosis, such as idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonitis, is the excess accumulation of ECM in lung tissue. IPF is defined as a chronic, progressive lung fibrosis condition without a specific cause and a mild level of inflammation, predominantly in older populations (Raghu et al., 2011; Travis et al., 2013). The role of Cav-1 in lung fibrosis was investigated by Kasper et al. where they induced fibrosis by irradiating alveolar cell types I and II of rats and mini pigs (Kasper et al., 1998). This study revealed downregulated Cav-1 gene expression in epithelial cells following radiation, resulting in fibrosis. Morphologically, the epithelial cells showed reduced expression of caveolae, coupled with attenuated signal transductory capacities (Kasper et al., 1998). Moreover, a five-fold increase in collagen expression via Mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) activation was observed in human lung fibroblasts when Cav-1 gene expression was reduced by 70% (Tourkina et al., 2005). Similarly, a study by Wang et al. observed a two-fold decrease in Cav-1 gene expression in lung tissue from IPF patients compared to control patients (Wang et al., 2006b). Furthermore, they investigated the therapeutic effects of Cav-1 in rat lungs using bleomycin, an antineoplastic antibiotic that causes pulmonary fibrosis as a major side effect. In another injury model, mice transfected with an adenovirus vector containing the Cav-1 gene, demonstrated resolved lung fibrosis, observed by reduced fibrotic area in the lung relative to untreated mice (Wang et al., 2006b). Like cardiac fibrosis, the formation of scar tissue in lung fibrosis is also driven by TGF-β (Khalil et al., 1991, 1994, 1996; Verma and Slutsky, 2007; Del Galdo et al., 2008). In vitro models of lung fibrosis have demonstrated that the anti-fibrotic effects observed are regulated by TGF-β1 via ERK and JNK activation, which are mediated by Cav-1. Indeed, TGF-β1 induces the gene expression of collagen type I via ERK1, while it regulates the production of fibronectin via c-Jun N-terminal kinase 1 (JNK1) (Wang et al., 2006b). Of note, TGF-β signaling can also mediate Cav-1 expression via non-SMAD signaling pathways. Indeed, downregulated Cav-1 has been observed in TGF-β-treated human lung fibroblasts through the activation of p38 MAPK (MEK/ERK signaling) (Sanders et al., 2015). The anti-fibrotic therapeutic potential of Cav-1 has further been highlighted in studies utilizing pharmacologically administration of CSD peptide. Similar to knockout studies, collagen deposition was reduced by more than 95% in less than 5 h in mice treated with the CSD peptide relative to the untreated mice (Tourkina et al., 2008). CSD peptide-dependent reductions in fibrosis were accompanied by inhibitions in MEK, ERK, JNK, and AKT activity as well as altering their cellular localization, confirming the anti-fibrotic effects of Cav-1 (Tourkina et al., 2008).
Like EMT, Endothelial-mesenchymal transition (EndoMT) has emerged as a key source of fibroblasts and myofibroblasts critical in the pathogenesis of fibrotic disease (Piera-Velazquez et al., 2011; Lin et al., 2012). EndoMT has been shown to be regulated via TGF-β (Li and Jimenez, 2011), with TGF-β being a potent inducer of EndoMT in pulmonary endothelial cells via the transcription factor, SNAIL1. In addition, the internalization of TGF-β receptors expressed on the cell membrane have been attributed to the cellular capabilities of Cav-1 (Razani et al., 2001b). Interestingly, pulmonary endothelial cells isolated from Cav-1−/− mice spontaneously underwent EndoMT. This cellular transition was further increased with the administration of TGF-β, highlighting the importance of both pathways (Li et al., 2013). Although the exact mechanisms involved in EndoMT are relatively unclear, SNAIL1 has been found to be a requirement for the induction of this process (Kokudo et al., 2008). This is supported by increased SNAIL1 gene expression observed in Cav1−/− mouse endothelial cells, which is reversed by the reconstitution of Cav-1, emphasizing the key mediatory role of Cav-1 in EndoMT (Strippoli et al., 2015).
Fibroblast proliferation is normally regulated by polymerized collagen via the inhibition of the PI3K-AKT-S6-kinase 1 (S6K1) signal pathway, by high phosphatase and tensin homolog (PTEN) (Xia et al., 2008, 2010). This negative feedback mechanism limits the proliferation of fibrotic tissue following injury in physiological conditions, however when the process is impaired, fibrotic conditions such as IPF may occur (White et al., 2006). Protein expression of both Cav-1 and PTEN was significantly reduced in the cellular membrane of myofibroblasts within fibroblast foci in lung cells of IPF patients' relative to control patients, compared to surrounding epithelial cells. In addition, a correlation in Cav-1 and PTEN levels was observed, which could be attributed to PTEN suppressing PI3K/AKT activation (Xia et al., 2008, 2010). This occurs through the translocation from the cytoplasm into the cellular membrane, where it is activated and in turn inhibits the signaling process. Cav-1 as an integral protein regulates PI3K/AKT transduction, where augmented expression of Cav-1 subsequently results in reduced levels of PTEN in the cellular membrane. This phenomenon is observed in Cav-1 null mice which demonstrate low expression of PTEN in the cellular membrane compared to wild type mice. Following reconstitution of Cav-1 using an adenovirus vector in the Cav-1 null fibroblast cells, a higher association of PTEN with cellular membrane and lower PI3K/AKT signaling was observed. This association was further supported by amino acid sequence analysis, showing a specialized binding sequence domain for PTEN which directly interacts with Cav-1 (Xia et al., 2008, 2010). Similarly in cardiac fibrosis and IPF, a downregulation in both Cav-1 and PTEN protein expression was observed in cardiac and pulmonary fibroblasts, respectively, relative to controls (Gao et al., 2014).
Further, MAPK pathways have been reported to play a mediatory role in TGF-β1 signaling, key for fibrotic processes, and have also been shown to be regulated by Cav-1 (Yue and Mulder, 2000; Fujita et al., 2004; Wang et al., 2006a). It has been demonstrated that Cav-1 regulates TGF-β1-induced ECM production via MAPK pathway in the lung. Over-expression of Cav-1 via transfection of adenovirus vector inhibited TGF-β1-induced ERK and JNK activation, consequently resulting in decreased ECM production (Wang et al., 2006b). Despite the above findings highlighting the anti-fibrotic role of Cav-1, studies have also demonstrated contrasting roles for Cav-1 in tissue fibrosis. Cav-1 deficiency has been linked with significant inhibition of premature senescence of fibroblasts (Volonte and Galbiati, 2009), whereas elsewhere it has been shown that Cav-1 is needed to induce senescence in fibroblasts (Dasari et al., 2006). Indeed, Cav-1−/− mice were found to be protected against fibrosis induced by bleomycin relative to WT mice (Shivshankar et al., 2012). When exposed to bleomycin to induce fibrosis, WT mice displayed significant collagen deposition, whereas Cav-1−/− mice who did not present a significant increase (Shivshankar et al., 2012). This blunted fibrotic response could be due to reduced epithelial cell senescence and apoptosis in the Cav-1−/− mice. However, in contrast to these findings, a study demonstrated increased fibrosis, upregulated apoptosis and cellular senescence in Cav-1−/− mice exposed to bleomycin (Linge et al., 2007). The disparity in these findings could be due to the timing of fibrotic measurements and the expression of Cav-1, where it is very likely that in the early stages of exposure to bleomycin, Cav-1 is expressed which is linked with upregulated apoptosis and senescence in epithelial cells, whereas in the later stages, Cav-1 is decreased, which may be associated with the progression of fibroblast-mediated fibrosis (Shivshankar et al., 2012).
Peritoneal and Kidney Fibrosis
Patients with terminal stage of kidney failure require either hemodialysis or peritoneal dialysis (PD). The common adverse effects following frequent long term PD is acute inflammation, which leads to chronic inflammation and eventually fibrosis (Grassmann et al., 2005; Strippoli et al., 2015). The predominant cause of acute inflammation is the continuous exposure of the peritoneal membrane of the kidney to hyperosmotic, hyperglycaemic, and acidic dialysis solutions. This leads to a specific EMT process of the mesothelial cells, known as mesothelial-to-mesenchymal transition (MMT). Like EMT, MMT is characterized by features such as loss of E-cadherin and cytokeratin, and upregulated α-smooth muscle actin (α-SMA) and fibroblast specific protein-1 (FSP-1) expression (Yanez-Mo et al., 2003; Strippoli et al., 2015). Of note, TGF-β is one of the early pro-fibrotic factors detected during PD treatment in human patients relative to control patients (Yanez-Mo et al., 2003). Further, p38 is also implicated as a key regulator of MMT, slowing down the transition process by promoting E-cadherin gene expression. E-cadherin is important for cell-to-cell adhesion and it is preserved by p38 via its inhibition of TGF-β via TGF-β-activated kinase 1-nuclear factor kappa-light-chain-enhancer of activated B cells (TAK1-NF-κB) signaling (Strippoli et al., 2010). The main suppressor of E-cadherin is the transcription factor SNAIL1, which modulates its effects via ERK or NF-κB. SNAIL1 is known as one of the major inducers of EMT and is also implicated in the downregulation of cytokeratin gene expression (Strippoli et al., 2008). Studies using Cav-1 gene silencing in human primary mesothelial cells (HPMCs) as well as mesothelial cells from Cav-1−/− mice have shown a loss of the typical cell structure and the acquisition of a spindle-like shape, characteristic of fibroblasts. This morphological change is also accompanied by suppression of E-cadherin gene expression. In addition, Cav-1−/− mice presented with increased α-SMA and collagen type I gene expression coupled with hyper-activation of ERK1 and ERK2, even in the basal state, relative to the WT mice (Strippoli et al., 2015). This was attributed to the inhibitory nature of Cav-1 on the ERK1/2 pathway. Moreover, HPMCs deficient of Cav-1 presented with enhanced ERK1/2 activity when stimulated by TGF-β. The hyper-activation of ERK1/2 leads to a stronger repression of E-cadherin gene expression via SNAIL1, accelerating the progression of MMT to fibrosis. Lentiviral overexpression of Cav-1 in HPMCs restored and reduced E-cadherin and α-SMA gene expression, respectively (Strippoli et al., 2015). The role of Cav-1 in MMT could be ascribed to the activation of ERK1/2, which directly upregulates SNAIL1 and suppresses E-cadherin and or to the diminished Smad2/3 signaling through the inhibitory effects of Cav-1 on TGF-β receptors (Xu Y. et al., 2008).
However, similar to fibrotic conditions in the lung, the role of Cav-1 is not definitive in kidney fibrosis, with several studies suggesting that Cav-1 plays a critical role in maintaining the fibrotic condition. A study conducted by Chen et al. (2012) demonstrated increased epidermal growth factor receptor (EGFR) association with phosphorylated Cav-1 in renal proximal tubular epithelial cells exposed to angiotensin II (AngII). An increase in reactive oxygen species (ROS) production was observed following angiotensin type 1 receptor (AT1R) activation, promoting Src kinase activation, Cav-1 and EGFR phosphorylation and consequently resulting in prolonged EGFR-ERK signaling which induces prolonged EMT (Chen et al., 2012). Further, this study also observed that silencing Cav-1 gene via small interfering RNA (siRNA) or via knockdown model leads to inhibited AngII activation, suggesting that EGFR association with Cav-1 leads to prolonged activation (Chen et al., 2012). These findings are in contrast to studies which have concluded that EGFR interactions with Cav-1 in caveolae or lipid rafts result in the inactivation of the EGFR (Orth and McNiven, 2006). Moreover, Chen & colleague's findings contradict a study conducted by Forrester et al. (2017) which found worsened perivascular fibrosis and hypertrophy in kidney tissue of Cav-1+/+ compared to Cav-1−/− mice when administered with AngII, coupled with attenuated VCAM-1 expression in the endothelium and adventitia layer of Cav-1−/− mice relative to Cav-1+/+ mice (Forrester et al., 2017).
Liver Fibrosis
Liver cirrhosis, the end-stage of various liver disease, is characterized by the replacement of normal physiologic hepatocyte cells with fibrotic tissue and ultimately organ failure (Elsharkawy et al., 2005; Asrani et al., 2013). Liver injury commonly results from “sinusoidal” portal hypertension, primarily caused by intrahepatic shunts and hepatocyte swelling (Sherman et al., 1990; Yokomori et al., 2002). A key factor involved in liver cirrhosis is endothelial nitric oxide synthase (eNOS), which regulates the blood flow, a central parameter in portal hypertension, through its production of the potent vasodilator, nitric oxide (NO). Indeed, with reduced activity of eNOS, the liver is more susceptible to portal hypertension, leading to fibrosis and eventually cirrhosis (Matei et al., 2006). Cav-1 is a major negative regulator of eNOS, which impacts the enzyme through direct interaction of both C- and N- terminals of Cav with oxygenase domain of eNOS (Ju et al., 1997). Unlike in most organs discussed, Cav-1 is suggested to play a pro-fibrotic role in the liver. In fact, Cav-1 protein levels are increased in experimental liver disease as well as a murine model of Niemann-Pick disease type C (NPC), a disease characterized by impaired cholesterol homeostasis (Garver et al., 1997; Shah et al., 1999). Moreover, rats treated with dimethylnitrosamine (DMN) to induce liver cirrhosis demonstrate enhanced Cav-1-eNOS binding paired, with a positive correlation between Cav-1 protein expression and the degree of liver fibrosis (Xu B. et al., 2008). Similarly, Cav-1 was localized in liver sinusoidal endothelial cells (LSECs) of cirrhotic liver human samples, with an overexpression of Cav-1 in late-stage cirrhosis (Yamazaki et al., 2013). The role reversal of Cav-1 in liver fibrosis could be due to the fact that the cirrhotic liver contains higher levels of cholesterol, and Cav-1 is a major cholesterol binding protein, thus Cav-1 expression may be stimulated in chirrotic liver (Bist et al., 1997).
Conclusion
The pathophysiology of organ fibrosis has been studied extensively, and the anti-fibrotic role of caveolae and caveolins has been explored. Indeed, novel studies using both animal and pharmacological models have identified Cav-1 as a potential anti-fibrotic target in a number of fibrotic settings including cardiac, lung, and kidney fibrosis. Although the relationship between Cav-1 and key regulatory molecules involved in fibrotic processes such as TGF-β, ERK1/2, and Smad is understood extensively, it is still relatively unclear how they interact at the intracellular level and whether these Cav-1-associated signaling pathways can be targeted as potential therapies in fibrosis. Moreover, the tissue-specific effects of Cav-1 highlights the gap in knowledge regarding its role in fibrotic conditions. Indeed, contrasting studies highlight the dynamic role of Cav-1 in organ fibrosis, specifically in the kidney and lungs (Figure 1). Thus, further studies are essential to completely understand possible therapeutic effects of caveolae/caveolin-1 in fibrosis and whether its therapeutic potential is limited between varying human fibrotic conditions.
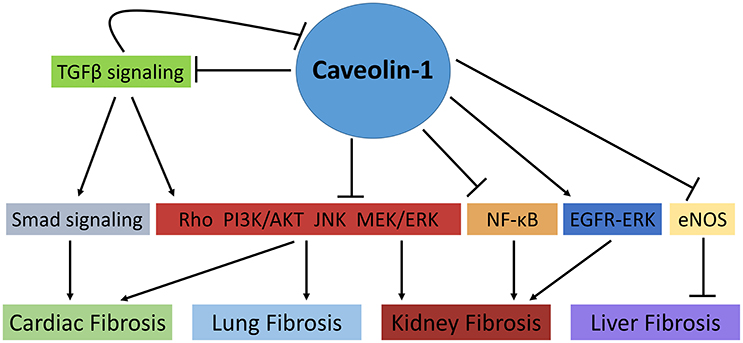
Figure 1. The role of Caveolin-1 in cellular signaling mechanisms involved in fibrosis. Caveolin-1 (Cav-1) directly and indirectly regulates fibrotic processes in various tissues. In cardiac and lung fibrosis, Cav-1 prevents collagen deposition, fibroblast proliferation and TGFβ signaling through its negative regulation of Smad and non-Smad signaling pathways such as Rho-like GTPase, PI3K/AKT, MAPK (MEK/ERK), and JNK signaling pathways. Similarly, in kidney fibrosis, Cav-1 modulates fibrotic processes via the aforementioned pathways as well as NF-κB signaling. Of note, TGFβ signaling can also mediate Cav-1 expression via the activation of non-SMAD signaling pathways. Conversely, Cav-1 has been shown to promote kidney fibrosis by prolonging EGFR-ERK signaling. Moreover, in liver fibrosis, Cav-1 promotes liver cirrhosis through its negative regulation of eNOS ( , activation;
, activation;  , inhibition).
, inhibition).
Author Contributions
WS and MP drafted the manuscript and contributed to the interpretation of data in the review. WS prepared the manuscript. WS, MP, and JC reviewed, edited, and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
−/−, knockout; AKT, Protein Kinase B; AngII, angiotensin II; AT1R, angiotensin type 1 receptor; Cav-, caveolin-; CSD, caveolin scaffolding domain; ECM, extracellular matrix; EEA-1, endosomal antigen-1; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transformation; EndoMT, Endothelial-mesenchymal transition; ERK, extracellular signal-regulated kinase; FSP-1, fibroblast specific protein-1; HPMCs, human primary mesothelial cells; IL-, Interleukin-; IPF, Idiopathic pulmonary fibrosis; JNK, c-Jun N-terminal kinase; MAPK, Mitogen-activated protein kinase; MEK, Mitogen-activated protein kinase kinase; MI, myocardial infarction; MMP, matrix metalloproteinase; MMT, Mesothelial-mesenchymal transition; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PD, Peritoneal dialysis; PI3K, phosphatidylinositol-3-kinase; PTEN, phosphatase and tensin homolog; R-Smad, Receptor-regulated Smad; S6K1, S6 kinase 1; siRNA, Small interfering RNA; TAK1, TGF-β-activated kinase 1; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor-α; TβRI, TGF-β type I receptor; TβRII, TGF-β type II receptor; α-SMA, α-smooth muscle actin.
References
Akbar, A. N., and Salmon, M. (1997). Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol. Today 18, 72–76. doi: 10.1016/S0167-5699(97)01003-7
Asrani, S. K., Larson, J. J., Yawn, B., Therneau, T. M., and Kim, W. R. (2013). Underestimation of liver-related mortality in the United States. Gastroenterology 145, 375–382.e371–e372. doi: 10.1053/j.gastro.2013.04.005
Bist, A., Fielding, P. E., and Fielding, C. J. (1997). Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proc. Natl. Acad. Sci. U.S.A. 94, 10693–10698. doi: 10.1073/pnas.94.20.10693
Brooks, W. W., and Conrad, C. H. (2000). Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J. Mol. Cell. Cardiol. 32, 187–195. doi: 10.1006/jmcc.1999.1065
Buckley, C. D., Pilling, D., Lord, J. M., Akbar, A. N., Scheel-Toellner, D., and Salmon, M. (2001). Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 22, 199–204. doi: 10.1016/S1471-4906(01)01863-4
Chen, J., Chen, J. K., and Harris, R. C. (2012). Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol. Cell. Biol. 32, 981–991. doi: 10.1128/MCB.06410-11
Dasari, A., Bartholomew, J. N., Volonte, D., and Galbiati, F. (2006). Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 66, 10805–10814. doi: 10.1158/0008-5472.CAN-06-1236
Del Galdo, F., Lisanti, M. P., and Jimenez, S. A. (2008). Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr. Opin. Rheumatol. 20, 713–719. doi: 10.1097/BOR.0b013e3283103d27
Deten, A., Holzl, A., Leicht, M., Barth, W., and Zimmer, H. G. (2001). Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J. Mol. Cell. Cardiol. 33, 1191–1207. doi: 10.1006/jmcc.2001.1383
Dewald, O., Ren, G., Duerr, G. D., Zoerlein, M., Klemm, C., Gersch, C., et al. (2004). Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am. J. Pathol. 164, 665–677. doi: 10.1016/S0002-9440(10)63154-9
Di Guglielmo, G. M., Le Roy, C., Goodfellow, A. F., and Wrana, J. L. (2003). Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5, 410–421. doi: 10.1038/ncb975
Drab, M., Verkade, P., Elger, M., Kasper, M., Lohn, M., Lauterbach, B., et al. (2001). Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449–2452. doi: 10.1126/science.1062688
Elsharkawy, A. M., Oakley, F., and Mann, D. A. (2005). The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis 10, 927–939. doi: 10.1007/s10495-005-1055-4
Forrester, S. J., Elliott, K. J., Kawai, T., Obama, T., Boyer, M. J., Preston, K. J., et al. (2017). Caveolin-1 deletion prevents hypertensive vascular remodeling induced by angiotensin II. Hypertension 69, 79–86. doi: 10.1161/HYPERTENSIONAHA.116.08278
Frank, P. G., Pavlides, S., Cheung, M. W., Daumer, K., and Lisanti, M. P. (2008). Role of caveolin-1 in the regulation of lipoprotein metabolism. Am. J. Physiol. Cell Physiol. 295, C242–C248. doi: 10.1152/ajpcell.00185.2008
Fujita, Y., Maruyama, S., Kogo, H., Matsuo, S., and Fujimoto, T. (2004). Caveolin-1 in mesangial cells suppresses MAP kinase activation and cell proliferation induced by bFGF and PDGF. Kidney Int. 66, 1794–1804. doi: 10.1111/j.1523-1755.2004.00954.x
Galbiati, F., Engelman, J. A., Volonte, D., Zhang, X. L., Minetti, C., Li, M., et al. (2001). Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J. Biol. Chem. 276, 21425–21433. doi: 10.1074/jbc.M100828200
Galbiati, F., Volonte, D., Engelman, J. A., Watanabe, G., Burk, R., Pestell, R. G., et al. (1998). Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 17, 6633–6648. doi: 10.1093/emboj/17.22.6633
Gao, Y., Chu, M., Hong, J., Shang, J., and Xu, D. (2014). Hypoxia induces cardiac fibroblast proliferation and phenotypic switch: a role for caveolae and caveolin-1/PTEN mediated pathway. J. Thorac. Dis. 6, 1458–1468. doi: 10.3978/j.issn.2072-1439.2014.08.31
Garver, W. S., Erickson, R. P., Wilson, J. M., Colton, T. L., Hossain, G. S., Kozloski, M. A., et al. (1997). Altered expression of caveolin-1 and increased cholesterol in detergent insoluble membrane fractions from liver in mice with Niemann–Pick disease type C. Biochim. Biophys. Acta 1361, 272–280. doi: 10.1016/S0925-4439(97)00047-1
Grassmann, A., Gioberge, S., Moeller, S., and Brown, G. (2005). ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol. Dial. Transplant 20, 2587–2593. doi: 10.1093/ndt/gfi159
Horikawa, Y. T., Panneerselvam, M., Kawaraguchi, Y., Tsutsumi, Y. M., Ali, S. S., Balijepalli, R. C., et al. (2011). Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J. Am. Coll. Cardiol. 57, 2273–2283. doi: 10.1016/j.jacc.2010.12.032
Ito, T., Williams, J. D., Fraser, D. J., and Phillips, A. O. (2004). Hyaluronan regulates transforming growth factor-beta1 receptor compartmentalization. J. Biol. Chem. 279, 25326–25332. doi: 10.1074/jbc.M403135200
Ju, H., Zou, R., Venema, V. J., and Venema, R. C. (1997). Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 272, 18522–18525. doi: 10.1074/jbc.272.30.18522
Kasper, M., Reimann, T., Hempel, U., Wenzel, K. W., Bierhaus, A., Schuh, D., et al. (1998). Loss of caveolin expression in type I pneumocytes as an indicator of subcellular alterations during lung fibrogenesis. Histochem. Cell Biol. 109, 41–48. doi: 10.1007/s004180050200
Khalil, N., O'Connor, R. N., Flanders, K. C., Shing, W., and Whitman, C. I. (1994). Regulation of type II alveolar epithelial cell proliferation by TGF-beta during bleomycin-induced lung injury in rats. Am. J. Physiol. 267, L498–L507.
Khalil, N., O'Connor, R. N., Flanders, K. C., and Unruh, H. (1996). TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am. J. Respir. Cell Mol. Biol. 14, 131–138. doi: 10.1165/ajrcmb.14.2.8630262
Khalil, N., O'Connor, R. N., Unruh, H. W., Warren, P. W., Flanders, K. C., Kemp, A., et al. (1991). Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 5, 155–162. doi: 10.1165/ajrcmb/5.2.155
Kokudo, T., Suzuki, Y., Yoshimatsu, Y., Yamazaki, T., Watabe, T., and Miyazono, K. (2008). Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J. Cell Sci. 121, 3317–3324. doi: 10.1242/jcs.028282
Li, Z., and Jimenez, S. A. (2011). Protein kinase Cdelta and c-Abl kinase are required for transforming growth factor beta induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum. 63, 2473–2483. doi: 10.1002/art.30317
Li, Z., Wermuth, P. J., Benn, B. S., Lisanti, M. P., and Jimenez, S. A. (2013). Caveolin-1 deficiency induces spontaneous endothelial-to-mesenchymal transition in murine pulmonary endothelial cells in vitro. Am. J. Pathol. 182, 325–331. doi: 10.1016/j.ajpath.2012.10.022
Lin, F., Wang, N., and Zhang, T. C. (2012). The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life 64, 717–723. doi: 10.1002/iub.1059
Linge, A., Weinhold, K., Blasche, R., Kasper, M., and Barth, K. (2007). Downregulation of caveolin-1 affects bleomycin-induced growth arrest and cellular senescence in A549 cells. Int. J. Biochem. Cell Biol. 39, 1964–1974. doi: 10.1016/j.biocel.2007.05.018
Liu, P., Ying, Y., and Anderson, R. G. (1997). Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc. Natl. Acad. Sci. U.S.A. 94, 13666–13670. doi: 10.1073/pnas.94.25.13666
Matei, V., Rodriguez-Vilarrupla, A., Deulofeu, R., Colomer, D., Fernandez, M., Bosch, J., et al. (2006). The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology 44, 44–52. doi: 10.1002/hep.21228
Miyazono, K., ten Dijke, P., and Heldin, C. H. (2000). TGF-β signaling by Smad proteins. Adv. Immunol. 75, 115–157. doi: 10.1016/S0065-2776(00)75003-6
Murata, T., Lin, M. I., Huang, Y., Yu, J., Bauer, P. M., Giordano, F. J., et al. (2007). Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J. Exp. Med. 204, 2373–2382. doi: 10.1084/jem.20062340
Orth, J. D., and McNiven, M. A. (2006). Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 66, 11094–11096. doi: 10.1158/0008-5472.CAN-06-3397
Palade, G. E. (1953). The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1, 455–458.
Park, H., Go, Y. M., St. John, P. L., Maland, M. C., Lisanti, M. P., Abrahamson, D. R., et al. (1998). Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J. Biol. Chem. 273, 32304–32311. doi: 10.1074/jbc.273.48.32304
Piera-Velazquez, S., Li, Z., and Jimenez, S. A. (2011). Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am. J. Pathol. 179, 1074–1080. doi: 10.1016/j.ajpath.2011.06.001
Raghu, G., Collard, H. R., Egan, J. J., Martinez, F. J., Behr, J., Brown, K. K., et al. (2011). An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183, 788–824. doi: 10.1164/rccm.2009-040GL
Razani, B., Engelman, J. A., Wang, X. B., Schubert, W., Zhang, X. L., Marks, C. B., et al. (2001a). Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 276, 38121–38138. doi: 10.1074/jbc.M105408200
Razani, B., Zhang, X. L., Bitzer, M., von Gersdorff, G., Bottinger, E. P., and Lisanti, M. P. (2001b). Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-β type I receptor. J. Biol. Chem. 276, 6727–6738. doi: 10.1074/jbc.M008340200
Sanders, Y. Y., Cui, Z., Le Saux, C. J., Horowitz, J. C., Rangarajan, S., Kurundkar, A., et al. (2015). SMAD-Independent down-regulation of caveolin-1 by TGF-β: effects on proliferation and survival of myofibroblasts. PLoS ONE 10:e0116995. doi: 10.1371/journal.pone.0116995
Shah, V., Toruner, M., Haddad, F., Cadelina, G., Papapetropoulos, A., Choo, K., et al. (1999). Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 117, 1222–1228. doi: 10.1016/S0016-5085(99)70408-7
Sherman, I. A., Pappas, S. C., and Fisher, M. M. (1990). Hepatic microvascular changes associated with development of liver fibrosis and cirrhosis. Am. J. Physiol. 258, H460–H465.
Shihata, W. A., Michell, D. L., Andrews, K. L., and Chin-Dusting, J. P. (2016). Caveolae: a role in endothelial inflammation and mechanotransduction? Front. Physiol. 7:628. doi: 10.3389/fphys.2016.00628
Shivshankar, P., Brampton, C., Miyasato, S., Kasper, M., Thannickal, V. J., and Le Saux, C. J. (2012). Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am. J. Respir. Cell Mol. Biol. 47, 28–36. doi: 10.1165/rcmb.2011-0349OC
Shivshankar, P., Halade, G. V., Calhoun, C., Escobar, G. P., Mehr, A. J., Jimenez, F., et al. (2014). Caveolin-1 deletion exacerbates cardiac interstitial fibrosis by promoting M2 macrophage activation in mice after myocardial infarction. J. Mol. Cell. Cardiol. 76, 84–93. doi: 10.1016/j.yjmcc.2014.07.020
Sinha, B., Koster, D., Ruez, R., Gonnord, P., Bastiani, M., Abankwa, D., et al. (2011). Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413. doi: 10.1016/j.cell.2010.12.031
Soonpaa, M. H., and Field, L. J. (1998). Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ. Res. 83, 15–26. doi: 10.1161/01.RES.83.1.15
Strippoli, R., Benedicto, I., Foronda, M., Perez-Lozano, M. L., Sanchez-Perales, S., Lopez-Cabrera, M., et al. (2010). p38 maintains E-cadherin expression by modulating TAK1-NF-κB during epithelial-to-mesenchymal transition. J. Cell Sci. 123, 4321–4331. doi: 10.1242/jcs.071647
Strippoli, R., Benedicto, I., Perez Lozano, M. L., Cerezo, A., Lopez-Cabrera, M., and del Pozo, M. A. (2008). Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-κB/Snail1 pathway. Dis. Model. Mech. 1, 264–274. doi: 10.1242/dmm.001321
Strippoli, R., Loureiro, J., Moreno, V., Benedicto, I., Perez Lozano, M. L., Barreiro, O., et al. (2015). Caveolin-1 deficiency induces a MEK-ERK1/2-Snail-1-dependent epithelial-mesenchymal transition and fibrosis during peritoneal dialysis. EMBO Mol. Med. 7, 102–123. doi: 10.15252/emmm.201404127
Tang, Z., Scherer, P. E., Okamoto, T., Song, K., Chu, C., Kohtz, D. S., et al. (1996). Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 271, 2255–2261. doi: 10.1074/jbc.271.4.2255
Tourkina, E., Gooz, P., Pannu, J., Bonner, M., Scholz, D., Hacker, S., et al. (2005). Opposing effects of protein kinase calpha and protein kinase cepsilon on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J. Biol. Chem. 280, 13879–13887. doi: 10.1074/jbc.M412551200
Tourkina, E., Richard, M., Gooz, P., Bonner, M., Pannu, J., Harley, R., et al. (2008). Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L843–L861. doi: 10.1152/ajplung.00295.2007
Travis, W. D., Costabel, U., Hansell, D. M., King, T. E. Jr., Lynch, D. A., Nicholson, A. G., et al. (2013). An official american thoracic society/european respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188, 733–748. doi: 10.1164/rccm.201308-1483ST
Verma, S., and Slutsky, A. S. (2007). Idiopathic pulmonary fibrosis–new insights. N. Engl. J. Med. 356, 1370–1372. doi: 10.1056/NEJMcibr070490
Volonte, D., and Galbiati, F. (2009). Caveolin-1, cellular senescence and pulmonary emphysema. Aging 1, 831–835. doi: 10.18632/aging.100079
Wang, X. M., Kim, H. P., Song, R., and Choi, A. M. (2006a). Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am. J. Respir. Cell Mol. Biol. 34, 434–442. doi: 10.1165/rcmb.2005-0376OC
Wang, X. M., Zhang, Y., Kim, H. P., Zhou, Z., Feghali-Bostwick, C. A., Liu, F., et al. (2006b). Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J. Exp. Med. 203, 2895–2906. doi: 10.1084/jem.20061536
White, E. S., Atrasz, R. G., Hu, B., Phan, S. H., Stambolic, V., Mak, T. W., et al. (2006). Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10). Am. J. Respir. Crit. Care Med. 173, 112–121. doi: 10.1164/rccm.200507-1058OC
Wick, G., Grundtman, C., Mayerl, C., Wimpissinger, T. F., Feichtinger, J., Zelger, B., et al. (2013). The immunology of fibrosis. Annu. Rev. Immunol. 31, 107–135. doi: 10.1146/annurev-immunol-032712-095937
Wrana, J. L., Attisano, L., Wieser, R., Ventura, F., and Massague, J. (1994). Mechanism of activation of the TGF-beta receptor. Nature 370, 341–347. doi: 10.1038/370341a0
Wynn, T. A. (2007). Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529. doi: 10.1172/JCI31487
Xia, H., Diebold, D., Nho, R., Perlman, D., Kleidon, J., Kahm, J., et al. (2008). Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J. Exp. Med. 205, 1659–1672. doi: 10.1084/jem.20080001
Xia, H., Khalil, W., Kahm, J., Jessurun, J., Kleidon, J., and Henke, C. A. (2010). Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am. J. Pathol. 176, 2626–2637. doi: 10.2353/ajpath.2010.091117
Xu, B., Zhu, G. H., Weng, J. F., Cai, W. S., Xia, J. T., and Li, S. H. (2008). The roles of caveolin-1 and endothelial nitric oxide synthase in the development of portal hypertension in rats with liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi 16, 184–187.
Xu, Y., Buikema, H., van Gilst, W. H., and Henning, R. H. (2008). Caveolae and endothelial dysfunction: filling the caves in cardiovascular disease. Eur. J. Pharmacol. 585, 256–260. doi: 10.1016/j.ejphar.2008.02.086
Yamada, E. (1955). The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1, 445–458. doi: 10.1083/jcb.1.5.445
Yamazaki, H., Oda, M., Takahashi, Y., Iguchi, H., Yoshimura, K., Okada, N., et al. (2013). Relation between ultrastructural localization, changes in caveolin-1, and capillarization of liver sinusoidal endothelial cells in human hepatitis C-related cirrhotic liver. J. Histochem. Cytochem. 61, 169–176. doi: 10.1369/0022155412468590
Yanez-Mo, M., Lara-Pezzi, E., Selgas, R., Ramirez-Huesca, M., Dominguez-Jimenez, C., Jimenez-Heffernan, J. A., et al. (2003). Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 348, 403–413. doi: 10.1056/NEJMoa020809
Yang, B., and Rizzo, V. (2007). TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 292, H954–H962. doi: 10.1152/ajpheart.00758.2006
Yokomori, H., Oda, M., Ogi, M., Sakai, K., and Ishii, H. (2002). Enhanced expression of endothelial nitric oxide synthase and caveolin-1 in human cirrhosis. Liver 22, 150–158. doi: 10.1034/j.1600-0676.2002.01588.x
Yue, J., and Mulder, K. M. (2000). Activation of the mitogen-activated protein kinase pathway by transforming growth factor-beta. Methods Mol. Biol. 142, 125–131. doi: 10.1385/1-59259-053-5:125
Zeisberg, M., and Kalluri, R. (2013). Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 304, C216–C225. doi: 10.1152/ajpcell.00328.2012
Keywords: caveolae, caveolin-1, cardiac fibrosis, lung fibrosis, kidney fibrosis
Citation: Shihata WA, Putra MRA and Chin-Dusting JPF (2017) Is There a Potential Therapeutic Role for Caveolin-1 in Fibrosis? Front. Pharmacol. 8:567. doi: 10.3389/fphar.2017.00567
Received: 24 April 2017; Accepted: 09 August 2017;
Published: 24 August 2017.
Edited by:
Pedro D'Orléans-Juste, Université de Sherbrooke, CanadaReviewed by:
Beate Rassler, Leipzig University, GermanyJoan Krepinsky, McMaster University, Canada
Yan Sanders, University of Alabama at Birmingham, United States
Copyright © 2017 Shihata, Putra and Chin-Dusting. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waled A. Shihata, d2FsZWQuc2hpaGF0YUBtb25hc2guZWR1
 Waled A. Shihata
Waled A. Shihata Mohammad R. A. Putra1
Mohammad R. A. Putra1 Jaye P. F. Chin-Dusting
Jaye P. F. Chin-Dusting