- 1Dipartimento di Scienze della Terra, dell’Ambiente e della Vita, Università degli Studi di Genova, Genova, Italy
- 2Unità Operativa di Biologia Farmaceutica, Dipartimento di Scienze Fisiche, della Terra e dell’Ambiente, Università degli Studi di Siena, Siena, Italy
- 3Institute of Chinese Medical Sciences, State Key Laboratory of Quality Research in Chinese Medicine, University of Macau, Taipa, Macau
- 4Dipartimento di Farmacia, Università degli Studi di Genova, Genova, Italy
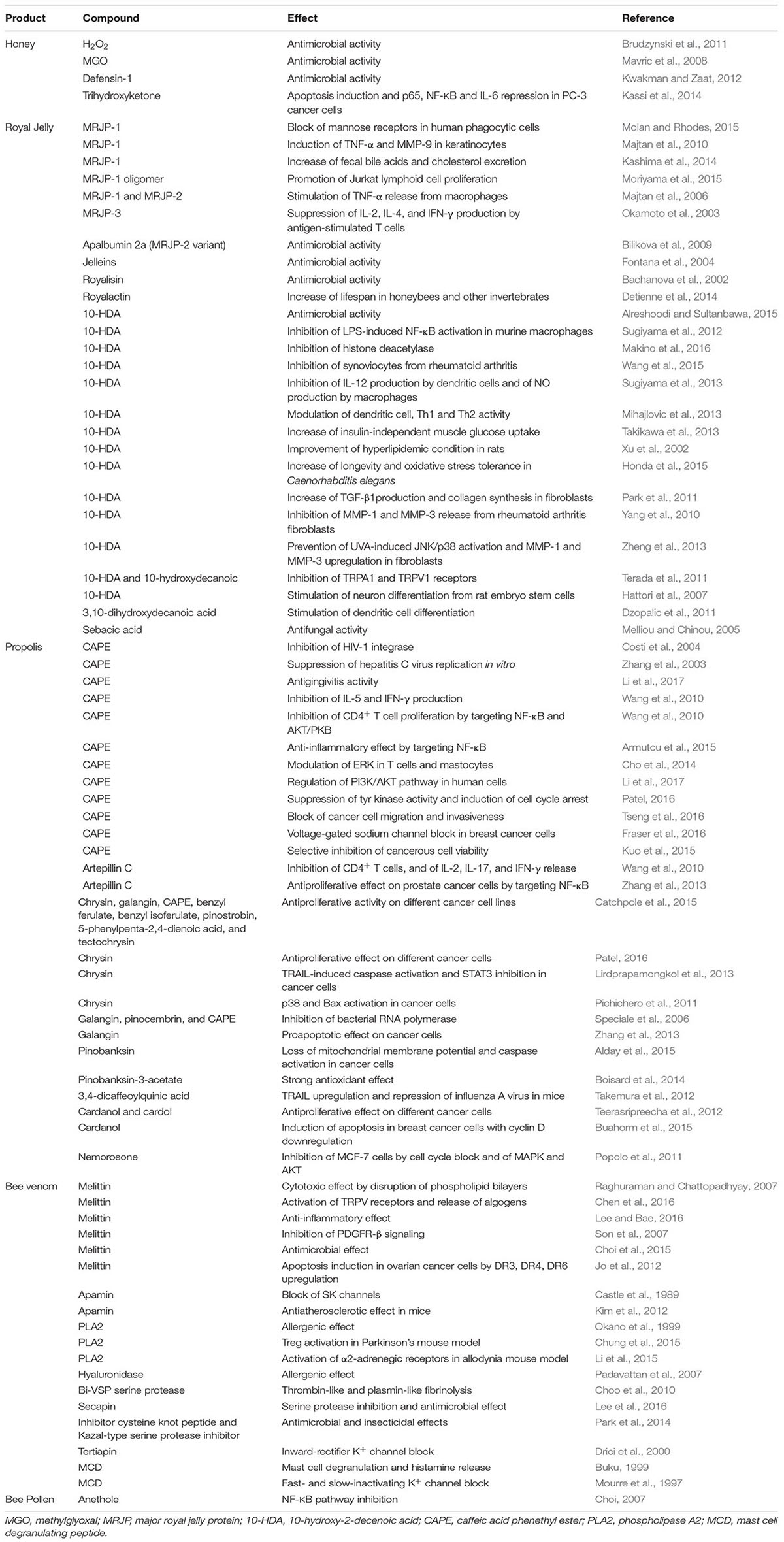
Honeybees produce honey, royal jelly, propolis, bee venom, bee pollen, and beeswax, which potentially benefit to humans due to the bioactives in them. Clinical standardization of these products is hindered by chemical variability depending on honeybee and botanical sources, but different molecules have been isolated and pharmacologically characterized. Major honey bioactives include phenolics, methylglyoxal, royal jelly proteins (MRJPs), and oligosaccharides. In royal jelly there are antimicrobial jelleins and royalisin peptides, MRJPs, and hydroxy-decenoic acid derivatives, notably 10-hydroxy-2-decenoic acid (10-HDA), with antimicrobial, anti-inflammatory, immunomodulatory, neuromodulatory, metabolic syndrome preventing, and anti-aging activities. Propolis contains caffeic acid phenethyl ester and artepillin C, specific of Brazilian propolis, with antiviral, immunomodulatory, anti-inflammatory and anticancer effects. Bee venom consists of toxic peptides like pain-inducing melittin, SK channel blocking apamin, and allergenic phospholipase A2. Bee pollen is vitaminic, contains antioxidant and anti-inflammatory plant phenolics, as well as antiatherosclerotic, antidiabetic, and hypoglycemic flavonoids, unsaturated fatty acids, and sterols. Beeswax is widely used in cosmetics and makeup. Given the importance of drug discovery from natural sources, this review is aimed at providing an exhaustive screening of the bioactive compounds detected in honeybee products and of their curative or adverse biological effects.
Introduction
Honeybees are social hymenopteran insects belonging to the genus Apis, characterized by the production and storage of honey and other substances potentially useful to humans. Two domesticated species are currently known, i.e., the western A. mellifera, native to Europe, Asia and Africa, and introduced into America, and the eastern A. cerana, distributed in southern and southeastern Asia.
Honey is the main and most widely appreciated honeybee product. It derives from digestive processing of nectar foraged from flowers, and is stored in honeycomb cells. Honey is generally marketed for its nutritive properties, but has been also used since the antiquity as a folk remedy, while in recent times it has been introduced as a pharmaceutical aid and in clinical practice (Molan, 1999). Other products derive from honeybee gland secretions and different botanical materials, either alone or variously mixed together. These substances include royal jelly, beeswax, propolis, bee pollen, and bee venom. All of them have been used by humans since ancient times for nutritional and curative purposes.
Several biological properties have been detected in honeybee products by a wide series of scientific studies, while different reviews have been dedicated to summarize therapeutic properties and uses as nutraceutical, pharmaceutical and cosmetic ingredients (Viuda-Martos et al., 2008; Burlando and Cornara, 2013). Various attempts at introducing some of these products in clinical settings have been made, but their pharmacological and medicinal standardization is made difficult by the high chemical variability, depending on honeybee varieties and botanical sources. However, different molecules or classes of compounds have been isolated and some of them have also been pharmacologically characterized, suggesting the importance of honeybee products for drug discovery from natural sources. Given the importance of this latter field in the search for new remedies against problematic diseases, this review is aimed to provide an exhaustive screening of the bioactive compounds detected in honeybee products and of their curative or adverse biological effects. Literature data were collected in Scopus, Web of Science, PubMed, Google Scholar, https://clinicaltrials.gov, and Espacenet databases. General medicinal application of honeybee products has been covered, while more in depth search on bioactivities and putative therapeutic effects has been conducted on the major constituents of these products.
Honey
Honey is produced by foraging bees that collect flower nectar and process it through repeated digestion and regurgitation. Stomach acidic pH, together with invertase, diastase and amylase enzymatic activities, give rise to a supersaturated aqueous solution composed by 80% sugars, mainly fructose and glucose, with minor amounts of sucrose, maltose, and other complex sugars.
Most nitrogen is present in amino acids and peptides. Proline is the most abundant amino acid, followed by glutamic acid, alanine, phenylalanine, tyrosine, leucine, isoleucine, and other minor ones. Honey also contains low amounts of protein, usually 0.1÷1.5% in the western honeybee A. mellifera, and 0.1÷3.0% in the Asiatic honeybee A. cerana. Most abundant peptides are defensin-1 and royal jelly protein (MRJP) isoforms, while major enzymes include glucose oxidase, diastase (amylase), α-glucosidase, catalase, and acid phosphatase (Kubota et al., 2004; Di Girolamo et al., 2012; Chua et al., 2015). Data about honey proteome are limited, but it is arguable that each honey type has its own peptide pattern, consisting of ubiquitous components and a variable set of minor elements, possibly including plant peptides.
Honey has an average pH of 3.9, mainly due to the presence of about 0.57% organic acids, predominantly gluconic acid originating from glucose oxidase activity, and citric acid. Small amounts of vitamins are also present, especially vitamin B complex due to pollen grains, and ascorbic acid. Minerals range between 0.04 and 0.2%, reflecting the mineral content of soils where source plants grow. Potassium is the major element, accounting for about one third of total mineral content.
Different plant compounds are present in honey at low concentrations and in variable amounts depending on the botanical species visited by bees and the climate of the area from which nectar is harvested. Aroma compounds are probably the most diversified fraction, as testified by the finding of over 500 volatile compounds in different types of honey. Phenolics are the most abundant phytochemicals, usually ranging from 50 to 500 mg/kg (Ramanauskiene et al., 2012; da Silva et al., 2016).
Antioxidant Activity
It is generally accepted that phenolics are important contributors to the antioxidant capacity of honey. Given that phenolic composition is greatly variable with respect to floral origin, honey is expected to show a wide range of antioxidant power (Gheldof et al., 2002; Petretto et al., 2015).
Antimicrobial Activity
Honey is known to contrast the growth of various microorganisms. This kind of effect has been a main attractive feature for honey application in clinical medicine, as testified by the development and marketing of γ-irradiated, Manuka medical grade honey. For this honey, a scale of antibacterial activity has been defined, known as Unique Manuka Factor (UMF), representing equivalents of a phenol solution yielding a certain inhibition in a radial diffusion assay on Staphylococcus aureus (Allen et al., 1991).
The role of specific honey antibacterial factors has been studied by sequential neutralization, showing that the mechanisms of action are complex and variable in different honey types. Basic antibacterial factors are low water activity and low pH. In addition, honey generally contains glucose oxidase added by bees, which by low dilution converts glucose into H2O2 and gluconic acid. However, measurements of minimum inhibitory concentration and DNA degradation on Escherichia coli and Bacillus subtilis has shown that honey H2O2 concentrations would not fully account for bacterial growth inhibition, suggesting that other honey components should be involved (Brudzynski et al., 2011). Two major non-peroxide antibacterial factors are methylglyoxal (MGO) in manuka honey and defensin-1 in Revamil source (RS) honey, which also contains MGO, but to a much lower extent than manuka honey. RS honey is the source product for Revamil® medical grade honey (Kwakman and Zaat, 2012).
Manuka honey derives from the nectar of the manuka myrtle Leptospermum scoparium, growing in southeast Australia and New Zealand. In this honey, high amounts of MGO are formed from dihydroxyacetone, present at high levels in manuka nectar, through a non-enzymatic process occurring during honey storage. The very high content of MGO, up to about 1,500 mg/kg, makes this honey able to kill various bacterial strains, including methicillin-resistant S. aureus (Mavric et al., 2008). However, other unidentified compounds besides MGO are likely to contribute to antibacterial activity, since it has been experimentally shown that MGO neutralization affects honey activity against S. aureus and B. subtilis, but not against E. coli and Pseudomonas aeruginosa (Kwakman et al., 2011).
Defensin-1 is an immunoactive peptide secreted by the honeybee hypopharyngeal gland and ultimately transferred to honey. The peptide has been detected in RS honey and is responsible for antibacterial activity against Gram-positive bacteria like Bacillus spp. Conversely, defensin-1 is not effective against S. aureus if used alone, but is essential for honey activity against this bacterial species, suggesting a possible synergistic interaction with other honey components. Peptide-dependent antibacterial activities have been found in other honey types besides RS honey, possibly depending in some cases on the presence of defensin-1. Other evidence for synergistic interactions have been detected in the effect of RS honey on E. coli and P. aeruginosa, requiring both H2O2 and MGO, and on vancomycin-resistant Enterococcus faecium, requiring H2O2 in combination with either MGO or defensin-1 (Kwakman and Zaat, 2012).
A specific study has been devoted to compare the antibacterial mechanisms of action of Manuka and RS honey, showing defensin-1 and H2O2 as major antibacterial factors of RS honey. Conversely, in manuka honey these factors have not been observed, while the antibacterial activity has been found to depend on MGO and other unidentified factors (Kwakman et al., 2011). However, another study has shown that the apparent lack of defensin-1 in manuka would be due to an inactivation of the peptide caused by MGO-induced modifications (Majtan et al., 2012).
Other antibacterial factors isolated from honey are glycoproteins with high-mannose N-glycans. These proteins have displayed agglutinating and bactericidal activity on different clinical isolates of multi drug resistant strains, such as methicillin-resistant S. aureus MRSA, P. aeruginosa, Klebsiella pneumoniae, vancomycin-resistant enterococci (VRE), and extended-spectrum β-lactamase-producing (ESBL) Proteus mirabilis, and E. coli. Q-TOF-MS analysis has shown extensive homology of these peptides with the MRJP-1 precursor, which harbors three antimicrobial jelleins typical of royal jelly. These data indicate a role for high-mannose structures in the antibacterial activity of honey glycoproteins (Brudzynski and Sjaarda, 2015; Brudzynski et al., 2015).
Fractionation of an n-hexane extract of Citrus goldcrest honey has led to the identification of a complex fraction with inhibitory activity against a clarithromycin/metronidazole-resistant Helicobacter pylori strain. This fraction contains mostly acetic acid, 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, 2-propanone, butanal, 1,3-benzenediamine, propanenitrile, 2-furanmethanol, propanoic acid, 1,3-butanediol, 1-(1-cyclopentenyl)-1-propanol, and 5-hydroxymethylfurfural (Manyi-Loh et al., 2012). However, the specific role of each single compound, or the presence of combinatory effects, has not been ascertained.
Honey is also known for antimycotic effects, e.g., against non-pathogenic Aureobasidium pullulans and Cladosporium cladosporioides, and pathogenic Candida parapsilosis, C. tropicalis, and Rhodotorula sp. (Kuncic et al., 2012; Moussa et al., 2012). Evidence has been collected that flavonoids like quercetin, kaempferol, chrysin, galangin, and apigenin may be involved in honey activity against C. albicans (Candiracci et al., 2011).
Antiparasitic Activity
Peptides from Ziziphus sp. honey with molecular masses ranging from 2 to 200 kDa, separated by size-exclusion chromatography, have shown antiprotozoal activity against the intestinal parasite Giardia lamblia (Mohammed et al., 2015). In addition, three different honeys from Plectranthus, Ziziphus, and acacia, have been found to possess nematicidal activity against Caenorhabditis elegans, which has been correlated to the presence of an unidentified glycoconjugate of about 5 kDa (Sajid and Azim, 2012).
Anti-inflammatory Activity
An extract from Italian multifloral honey, containing the flavonoids daidzein, apigenin, genistin, luteolin, kaempferol, quercetin, and chrysin, as major components, has inhibited the release of pro-inflammatory TNF-α and IL-1β from LPS-stimulated N13 microglia cells (Candiracci et al., 2012). Given a role of neuroinflammation in neurodegenerative diseases, these data confirm possible use of the honey flavonoid fraction in contrasting disorders like Alzheimer or Parkinson.
Immunomodulatory effects have been reported also for honey proteins. MRJP-3 has been found to suppress IL-2, IL-4, and IFN-γ production by antigen-stimulated T cells (Okamoto et al., 2003). Glycopeptides and glycoproteins isolated from Ziziphus honey, ranging from 2 to 450 kDa, have inhibited ROS release by zymosan-activated human neutrophils and murine macrophages, NO production and phagocytosis by LPS-activated murine macrophages, and production of TNF-α by human monocytic cells (Mesaik et al., 2015).
The honey protein apalbumin-1, aka MRJP-1, has been found to block the mannose receptors of human phagocytic cells, thereby inhibiting phagocytic activities. Such inhibitory effect seems enhanced in MGO-containing honey, due to apalbumin glycation (Molan and Rhodes, 2015).
Antidiabetic Activity
Honey has been found to reduce blood glucose in animal models and in patients with impaired glucose tolerance or diabetes, though clinical studies have not provided conclusive evidence. Fructose is a potential antidiabetic agent, while the presence in honey of a balanced mix of fructose and glucose could play an additional role in this kind of effect, since the two sugars are known to act synergistically in promoting liver glucose metabolism (Erejuwa et al., 2012).
It has been shown that non-digestible, dietary oligosaccharides, such as fructooligosaccharides, galactooligosaccharides, and lactulose, have a preventive role against obesity, insulin resistance, and diabetes mellitus, by acting as prebiotics on the intestinal flora. Given that honey contains oligosaccharides, it has been hypothesized that these compounds might contribute to honey prebiotic effects, and therefore, that they could be linked to honey antidiabetic, antihyperlipidemic and hepatoprotective virtues (Erejuwa et al., 2014). However, experimental evidence in support of such assumption is lacking.
Wound Healing
Honey has long been known for its healing capacity on wounds and burns (Molan, 2006; Vandamme et al., 2013). Revamil® medical grade honey has been specifically developed for this purpose, while another medical-grade, wound-dressing product is SurgihoneyTM, consisting of engineered honey with enhanced antimicrobial power (Al-Waili et al., 2011).
Antimicrobial activity is considered the most important factor for honey wound healing. However, various studies indicate that honey may also specifically act on skin cells involved in the wound healing process (Majtan et al., 2010; Ranzato et al., 2012). Various data have been collected on honey immunomodulatory properties, which can at least partially explain the ability of promoting wound healing. Even though some authors have argued that honey immunostimulatory effects may derive from bacterial lipopolysaccharide contamination, possible immunomodulatory honey constituents have been isolated. A 5.8 kDa constituent from manuka honey stimulates TNF-α production by macrophages via toll-like receptors (Tonks et al., 2007), while MRJP-1 induces TNF-α and metalloproteinase 9 (MMP-9) expression in keratinocytes (Majtan et al., 2010). Kanuka honey from Kunzea ericoides, a close relative of the manuka myrtle L. scoparium, contains type II arabinogalactans of plant origin that have been shown to promote TNF-α production by monocytic cell lines differentiated into macrophage by phorbol myristate acetate (Gannabathula et al., 2012). An aqueous extract rich in phenolics, obtained from fir honeydew honey, has been found to inhibit TNF-α-induced MMP-9 production by human keratinocytes, with a possible role for kaempferol and apigenin (Majtan et al., 2013). Such a result is apparently in contrast with another study reporting that different types of honey stimulate MMP-9 expression in the same cells (Ranzato et al., 2012), but in this latter case, whole honey has been used, possibly resulting in a dominant stimulatory effect due to other components, like for instance MRJPs.
Anticancer Activity
Inhibitory effects of honey on various kinds of cancer have been studied both in vitro and in animal models (Erejuwa et al., 2014). Polyphenols are known to possess chemopreventive properties, and accordingly, it has been shown that honey with higher phenolic charge is more potent in inhibiting cancer cell proliferation (Jaganathan and Mandal, 2009). Various polyphenols occurring in honey have been studied singularly for their mechanisms of action on cancer models, including caffeic acid and its phenyl esters, caffeoylquinic acid derivatives, rosmarinic acid and derivatives, ellagic acid, as well as the flavonoids chrysin, luteolin, acacetin, fisetin, myricetin, wogonin, apigenin, hesperidin, galangin, quercetin, kaempferol, pinobanksin, and pinocembrin (Jaganathan and Mandal, 2009; Abubakar et al., 2012). However, direct evidence that these compounds or their combinations are responsible for honey anticancer activity is lacking.
A unique trihydroxyketone (E-4-(1,2,4-trihydroxy-2,6,6-trimethylcyclohexyl)-but-3-en-2-one) from thyme honey, endowed with antibacterial activity, has been shown to induce apoptosis on PC-3 prostate cancer cells. Such an effect has been put in relationship with the inhibition of p65 NF-KB phosphorylation and IL-6 secretion, but these inhibitory activities have been found at concentrations ranging between 1 and 100 μM, whereas apoptosis has been observed with the 100 μM dose only (Kassi et al., 2014).
Adverse Effects
Various occurrences of toxic compounds in honey have been reported, such as polycyclic diterpene grayanotoxins in honey from rhododendron plants like R. luteum and R. ponticum. This kind of honey is known as “mad honey” since it may produce severe neural intoxication up to fatal emergency, especially in the eastern Black Sea region of Turkey. Grayanotoxins are known to affect voltage-dependent Na+ channel gating. Possibly due to this kind of action, and despite its toxicity, mad honey is used as folk medicine for hypertension, sexual dysfunction, and other ailments (Koca and Koca, 2007; Silici and Atayoglu, 2015).
Plants like Boraginaceae, Asteraceae, and Fabaceae produce pyrrolizidine alkaloids that are not toxic per se but are converted into harmful pyrrolic metabolites by liver after honey ingestion. The presence of these alkaloids in typical honey botanical sources, make these compounds a potential hazard for honey consumers (Edgar et al., 2002).
Cases of intoxication from honey consumption in New Zealand, characterized by delirium, seizures, and memory loss, have been related to honey contamination by the neurotoxic sesquiterpene lactones tutin and hyenanchin. These oxygenated sesquiterpene picrotoxanes, targeting GABAergic and glycinergic receptors, are ingested by honeybees collecting honeydew produced by passionvine hoppers (Scolypopa australis) feeding on sap of the poisonous shrub tutu (Coriaria spp.) (Fields et al., 2014; Larsen et al., 2015). Other plant secondary metabolites, which are found in honey and could induce deleterious effects to humans, include hyoscyamine and hyoscine from Solanaceae, saponins from Sapindaceae, strychnine and gelsemine from Gelsemiaceae, oleandrin and oleandrigenin from Apocynaceae (Islam et al., 2014).
Besides phytochemicals, honey can also be contaminated by environmental pollutants, like heavy metals, pesticides, and antibiotics. Moreover, prolonged honey storage or heating may give rise to Maillard reaction products, such as the furans 5-hydroxymethylfurfural from hexoses and furfural from pentoses (Islam et al., 2014).
Royal Jelly
Royal jelly is a secretion of honeybee hypopharynx and mandibular salivary glands. It is a white-yellowish, gelatinous, acidic colloid, containing about 67% water (w/w), 16% sugar, 12.5% protein and amino acids, and 5% fat, with considerable variability among different sources. Minor royal jelly constituents include enzymes, vitamins, phenolics, and minerals (Melliou and Chinou, 2005).
Proteins are the most abundant dry matter fraction, consisting for more than 80% of soluble glycoproteins named major royal jelly proteins (MRJPs), of which nine members have been described. These proteins are encoded by a family of genes arranged in tandem array, sharing a common ancestor with the Yellow protein family genes (Drapeau et al., 2006). MRJP-1 is the most abundant one and consists of monomeric and oligomeric forms. The oligomer, ranging between 350 and 420 kDa, can be separated into 55 and 5 kDa units, identified as MRJP-1 monomers and the 5 kDa protein apisimin, which is believed to act as subunit linker. MRJP-2, MRJP-3, MRJP-4 and MRJP-5 are glycoproteins ranging between 49 and 80 kDa (Tamura et al., 2009).
The lipid fraction mostly consists of medium chain fatty acids, terminally and/or internally hydroxylated, with terminal mono- or dicarboxylic acid functions, either saturated or monounsaturated at the 2-position. Major constituents are the 10-carbon atoms fatty acids trans-10-hydroxy-2-decenoic acid (10-HDA), unique to royal jelly, and 10-hydroxydecanoic acid. Sterols are also present in minor amounts (Li et al., 2013).
Royal jelly is fed until the 3rd day of life to larvae developing into female workers and male drones, or until the end of the larval period to selected individuals developing into queens. Moreover, it is an exclusive food for adult queens throughout their life (Fujita et al., 2013). The induction of larval development into queen has been ascribed to major royal jelly proteins (MRJPs) and a 57-kDa protein known as royalactin (Kamakura, 2011; Buttstedt et al., 2013), although contrary opinions have been raised against the alleged role of this latter (Buttstedt et al., 2016).
Royal jelly has been used since ancient times in traditional medicine, especially in Asiatic apitherapy, but also in the ancient Egypt. It is currently used in the pharmaceutical and cosmetic fields, and marketed as an over-the-counter functional food. Various studies have reported antimicrobial activities of royal jelly against bacteria, fungi, and viruses, while in animal models, hypotensive, antitumour, antihypercholesterolemic, and anti-inflammatory activities have been observed (Ramadan and Al-Ghamdi, 2012). Moreover, antidiabetic properties, positive effects on benign prostatic hyperplasia, and wound healing of diabetic foot ulcers have been verified in clinical trials (Siavash et al., 2015; Khoshpey et al., 2016). Most studies concern crude royal jelly or protein and lipid subfractions, but in several cases the activity of singular compounds has been ascertained.
Antioxidant Activity
Small peptides consisting of 2–4 amino acid residues have been reported to possess strong antioxidant activity. Most active ones have tyrosine residues at the C-terminal, allowing hydroxyl radical and H2O2 scavenging activities (Guo et al., 2009).
Antimicrobial Activity
The above-mentioned jelleins are four 8–9 amino acid peptides, of which jellein-I, -II, and -IV are cleavage products of MRJP-1. Antimicrobial assays conducted against the gram-positive S. aureus, S. saprophyticus, and B. subtilis, the gram negative E. coli, E. cloacae, K. pneumoniae, and P. aeruginosa, and the yeast C. albicans have shown that jellein-I and -II have broad-spectrum activity, jellein-III is less active, and jellein-IV has no antimicrobial effect (Fontana et al., 2004).
Royalisin is a 51 amino acid peptide, homologous to the haemolymph defensin-1, with antibacterial activity against various gram-positive strains, including Staphylococcus, Streptococcus, B. subtilis, Micrococcus luteus, Sarcina lutea, Clostridium, Corynebacterium, Lactobacillus helveticus, Paenibacillus larvae, and Leuconostoc, while no inhibition has been observed against the gram negative E. coli and Serratia marcescens. Antifungal activity against Botrytis cinerea has also been reported for royalisin (Fujiwara et al., 1990; Bachanova et al., 2002). In addition, a variant of MRJP-2, known as apalbumin 2a, has been found to inhibit the growth of P. larvae, B. subtilis, and E. coli (Bilikova et al., 2009).
Royal jelly carboxylic acids are known to collectively exert antimicrobial properties against gram-positive, gram-negative bacteria, and fungi. 10-HDA has been identified as a particularly strong antibacterial, especially against B. subtilis, S. aureus, and E. coli (Alreshoodi and Sultanbawa, 2015). Moreover, the compound has been shown to interfere with the adherence to cell surfaces of the oral pathogen S. mutans, by interfering with the expression of the glucosyltransferases gtfB and gtfC (Yousefi et al., 2012). Strong antifungal activity against C. albicans, C. tropicalis, and C. glabrata has been reported for sebacic acid (Melliou and Chinou, 2005).
Anti-inflammatory Activity
In a study on royal jelly potential for digestive trait diseases, 10-HDA has been found to protect rats from experimentally induced gastric ulcer (Fang et al., 1994). A mechanism putatively linked to 10-HDA anti-inflammatory effect is the inhibition of LPS-induced NF-κB activation observed in the murine macrophage cell line RAW264 (Sugiyama et al., 2012).
10-HDA and 4-hydroperoxy-2-decenoic acid ethyl ester have been shown to inhibit histone deacetylase activity, thereby enhancing the expression of extracellular SOD release by leukemia THP-1 cells, and suggesting therapeutic potential against atherosclerosis (Makino et al., 2016). Histone deacetylase inhibition by 10-HDA is thought to reactivate the expression of epigenetically silenced genes in mammalian cells, leading to the hypothesis that a similar effect could be at the basis of caste switching in bees (Spannhoff et al., 2011). Modifications of histone acetylation have also emerged from a study showing 10-HDA inhibition of fibroblast-like synoviocytes from rheumatoid arthritis patients, suggesting potential therapeutic effects against chronic inflammation degenerative disease (Wang et al., 2015).
Immunomodulatory Activity
Experimental evidence has been collected arguing for royal jelly immunomodulatory properties. A 57 kDa and 350-kDa royal jelly proteins, purportedly monomeric and oligomeric MRJP-1, have been reported to stimulate the proliferation of in vitro cultured hepatocytes and monocytes, respectively (Kamakura et al., 2001; Kimura et al., 2003). As a confirmation, an in vitro study has shown that the MRJP-1 oligomer, but not MRJP-2 or MRJP-3, induces the proliferation of the human lymphoid cell line Jurkat (Moriyama et al., 2015). MRJP-1 and MRJP-2 have been found to exert immunostimulatory and proinflammatory activities by stimulating cytokine release, such as TNF-α, from macrophages (Simuth et al., 2004; Majtan et al., 2006). In contrast, MRJP-3 has been reported to suppress interleukin production by T cells both in vitro and in vivo, reconducible to antiallergic properties (Okamoto et al., 2003).
Various immunomodulatory activities have been reported for 10-HDA, including reduced T cell proliferation, inhibition of interleukin-12 production by spleen dendritic cells, and block of LPS- and IFN-β-induced NO production in macrophages (Gasic et al., 2007; Sugiyama et al., 2013). In another study, a biphasic behavior of 10-HDA on human monocyte-derived dendritic cells has been found, resulting in Th1 response stimulation and Th2 downregulation at 50 μM, and repression of both Th1 and Th2 at 500 μM (Mihajlovic et al., 2013).
Another hydroxyl fatty acid, 3,10-dihydroxydecanoic acid, has been reported to stimulate the maturation of human monocyte-derived dendritic cells and their Th1 polarizing capability, suggesting a reinforcement of antitumour and antiviral immunity (Dzopalic et al., 2011). The immunomodulatory properties of royal jelly lipids suggest their possible use in interventions on autoimmune diseases.
Metabolic Syndrome Preventing Activity
The royal jelly proteins MRJP-1, MRJP-2, and MRJP-3 have been shown to possess bile acid-binding properties. The most active one is MRJP-1, which in rats has increased fecal bile acids and cholesterol excretion, and has powered hepatic cholesterol catabolism (Kashima et al., 2014). In a study aimed at disclosing royal jelly antihypertensive mechanisms, MRJP-1 has been transfected into vascular smooth muscle cells, leading to a reduction of contraction, migration and proliferation (Fan et al., 2016).
A study conducted both in vitro on L6 myotubes, and in vivo on mice, has demonstrated that 10-HDA enhances insulin-independent muscle glucose uptake via AMP-activated protein kinase activation and GLUT4 translocation to the plasma membrane (Takikawa et al., 2013). Moreover, this fatty acid has been found to improve hyperlipidemic condition in a rat model (Xu et al., 2002).
Anti-aging Activity
Royal jelly is known to extend the lifespan of honeybees. This property is at least in part due to royalactin, which has been found to induce such effect in other insect species, like Drosophila melanogaster, as well as in non-insect species, like the nematode C. elegans (Detienne et al., 2014). Moreover, also10-HDA has been found to increase longevity and confer thermal and oxidative stress tolerance to C. elegans, possibly through dietary restriction and TOR kinase signaling (Honda et al., 2015).
Royal jelly has estrogen-like effects, which in different studies have been ascribed to the ability of different lipids to act as weak activators of estrogen receptors. These constituents include 10-HDA, trans-2-decenoic, 10-hydroxydecanoic, 3,10-dihydroxydecanoic, and sebacic acids, and in addition the steroid 24-methylenecholesterol (Suzuki et al., 2008; Moutsatsou et al., 2010). These results have been proposed as a pharmacological basis for an anti-menopause use of royal jelly.
10-HDA has been shown to increase collagen synthesis and production of the collagen promoting factor, transforming growth factor β1, in human skin fibroblasts. Such an effect is thought to mediate royal jelly skin protection against UVB-induced photoaging (Koya-Miyata et al., 2004; Park et al., 2011). In addition to promoting collagen synthesis, 10-HDA has been found to inhibit the release of the MMP-1 and MMP-3 from rheumatoid arthritis synovial fibroblasts, possibly through downregulation of the pathway involving JNK/p38 MAP kinases and AP-1 transcription factor (Yang et al., 2010), and of the MMP regulator connective tissue growth factor (Wang et al., 2012). 10-HDA has also prevented UVA-induced JNK/p38 activation and MMP-1 and MMP-3 upregulation in fibroblasts (Zheng et al., 2013).
Such a complex of effects suggest skin dermal protection and antirheumatoid activity, but in most cases concentrations ranging around the millimolar level have been used, apparently with low clinical feasibility. However, a registered, synthetic 10-HDA counterpart, known as Hydroxydecine®, has been shown to activate keratinocyte differentiation in vitro, to restore skin barrier function in skin equivalents, and to improve UV-induced xerosis in human volunteers (Duplan et al., 2011).
Neuromodulatory Activity
10-HDA and 10-hydroxydecanoic acid have been shown to act as potent agonists of the human TRPA1 and TRPV1 receptors (Terada et al., 2011). 10-HDA has stimulated neuron differentiation from rat embryo neural stem cells, possibly acting like the ω-3 docosahexaenoic acid, an essential diet component that is known to promote neurogenesis in the central nervous system. Docosahexaenoic acid is reputed essential for brain development and function and has shown positive effects in a rat Parkinson’s model, suggesting similar potentials for 10-HDA, which in addition could cross more easily the blood-brain barrier due to its smaller molecule (Hattori et al., 2007). Neurogenerative potentials of royal jelly fatty acids are also suggested by a study on synthetic, medium-chain fatty acids, in which 2-decenoic acid ethyl ester, a derivative of the royal jelly 2-decenoic acid, has promoted functional recovery in a rat model of spinal cord injury (Hirakawa et al., 2010).
Adverse Effects
Similarly to honey, environmental contaminants can also be present in royal jelly. Most common ones are pesticides belonging to organochlorines, organophosphorus and carbamates, which are generally below Minimal Risk Level. However, in some cases the highly toxic, zero-tolerance chloramphenicol has been found (Bogdanov, 2006).
Royal jelly consumption can occasionally lead to contact dermatitis, asthma and anaphylaxis, while MRJP-1 and MRJP-2 have been identified as major allergens (Rosmilah et al., 2008).
Propolis
Propolis is a resinous substance that foraging bees produce by collecting resin from buds and other plant tissues and then mixing it with wax and pollen to have a malleable, compact substance that they use as hive repairing material and sanitizer (Sun et al., 2015). The chemical composition of propolis is dramatically dependent on its geographical and floral origins. Raw propolis generally contains more than 300 different compounds, mostly consisting of triterpenes (50% w/w), waxes (25–30%), volatile mono- and sesquiterpenes (8–12%), giving propolis its typical resinous odor, and phenolics (5–10%) (Huang et al., 2014). European and Asian propolis contain simple phenolic acids (Bankova et al., 2002), while lignans are main compounds in tropical propolis (Petrova et al., 2010). Caffeic acid phenethyl ester (CAPE) is a major active found in European, Asian and American propolis (Omene et al., 2013). Brazilian green propolis is characterized by the presence of the 3,5-diprenyl-4-hydroxycinnamic acid artepillin C, together with other prenylated cinnamic acids and caffeic acid derivatives (Marcucci et al., 2001). Other propolis common constituents include organic acids, ketones, aldehydes, hydrocarbons, and minerals (Wagh, 2013).
Antioxidant Activity
Propolis is the bee product containing the highest amount of phenolics and thus it has been deeply studied for antioxidant and radical scavenging activities (Viuda-Martos et al., 2008). Several of these compounds possess strong antioxidant and antiradicalic activities, including pinocembrin, chrysin, and pinobanksin (Sun et al., 2015). In DPPH and ORAC tests, pinobanksin-3-acetate has been indicated as the strongest antioxidant constituent (Boisard et al., 2014).
Antimicrobial Activity
The antimicrobial activity of propolis has been demonstrated in clinical, in vivo, and in vitro studies. Propolis possesses antibacterial properties against Gram positive and negative strains, also validated in clinical trials (Noronha et al., 2014). Sensitive strains include MRSA, VRE, Streptococcus species, and H. pylori (Kosalec et al., 2005; Coelho et al., 2007).
Propolis antibacterial effects are possibly related to the presence of such flavonoids as galangin, pinocembrin, rutin, quercetin, and naringenin, as well as of CAPE, since these compounds are known to increase bacterial membrane permeability (Stepanovic et al., 2003). Inhibition of bacterial RNA polymerase has been also considered for galangin, pinocembrin, and CAPE (Speciale et al., 2006). The antimicrobial activity of Brazilian red propolis is thought to depend on its peculiar content in isoflavones (Freires et al., 2016).
Antibacterial effectiveness has been demonstrated for different propolis volatile fractions, including β-eudesmol and δ-cadinene in Bulgarian propolis, α-pinene and trans-β-terpineol in Greek propolis, β-eudesmol and benzyl benzoate in Hungarian propolis, nerolidol, spatulenol and ledol in Canary Island propolis, and farnesol, dihydroeudesmol and guaiol in Polish propolis (Bankova et al., 2014). The antibacterial activity of Brazilian propolis has been demonstrated for its volatile fractions containing nerolidol, spatulenol, p-cimen-8-ol, ethylphenol, β-caryophyllene, acetophenone, α-pinene, β-pinene and limonene (Bankova et al., 2014).
Propolis has been shown to act as an antifungal agent against pathogenic yeasts like C. albicans, C. parapsilosis, C. tropicalis, and C. glabrata (Al-Waili et al., 2012; Mutlu Sariguzel et al., 2016). Antifungal activity has been shown for volatile compounds from Brazilian propolis, viz. α-pinene, β-pinene and δ-cadinene, and from Turkish propolis, viz. phenyl-, ethyl-, and benzyl alcohol, and decanal (Ioshida et al., 2010). The ethanolic extracts of Iranian propolis have shown strong anti-C. albicans activity imputable to inhibition of germ tube development by phenolic, aromatic, and aliphatic acids (Haghdoost et al., 2016). Another ethanolic extract containing CAPE and other caffeic acid derivatives has been effective against C. albicans, C. dubliniensis, C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis, with an MFC of 125-500 mg/L, while red Brazilian propolis rich in triterpenes and isoflavones, such as medicarpin, vestitol and formononetin, has shown the same MFC range (Freires et al., 2016).
Antiviral Activity
Propolis is well known for its antiviral activity, which in some cases can exceed that of standard drugs. For instance, an ointment containing Canadian propolis has produced better results than acyclovir or placebo in the clinical treatment of genital herpes simplex (Vynograd et al., 2000). Antiviral properties seem to depend mainly on the presence of CAPE and related compounds. CAPE has been found to inhibit the activity of HIV-1 by acting on viral integrase (Costi et al., 2004), and to suppress hepatitis C virus replication in vitro (Zhang et al., 2003). Turkish Hatay propolis containing caffeic acid derivatives has been effective on herpes simplex virus 1 and 2 (Yildirim et al., 2016). 3,4-Dicaffeoylquinic acid, a major constituent of Brazilian green propolis, has repressed influenza A virus in mice by upregulating the TNF-related, apoptosis-inducing ligand (TRAIL) (Takemura et al., 2012).
Immunomodulatory Activity
Propolis is generally known to modulate immune responses (Orsi et al., 2000), and this kind of effects could explain to some extent its antimicrobial and antiviral activities.
Brazilian green propolis standardized in 18.9% w/w polyphenols, 9.85% flavonoids and 2.3 artepillin C, administered to old mice, has enhanced phagocytosis, production of antibodies against sheep erythrocytes, and ear swelling (Gao et al., 2014). Brazilian green propolis and artepillin C have both inhibited in vitro alloreactive CD4+ T cell responses, together with the expression of IL-2, IL-17, and IFN-γ. In healthy subjects and asthmatic patients, CAPE has inhibited IL-5 and IFN-γ production, and the proliferation of CD4+ T cells stimulated by soluble anti-CD3 and anti-CD28 monoclonal antibodies, by targeting NF-κB and AKT/PKB pathways (Wang et al., 2010).
Anti-inflammatory Activity
Many studies have reported anti-inflammatory properties of propolis, possibly linked to the presence of phenolic acids. CAPE is considered a particularly strong anti-inflammatory constituent, able to specifically target NF-κB signaling (Armutcu et al., 2015). This compound has been also found to modulate ERK MAPK signaling in T cells and mastocytes (Cho et al., 2014), and to regulate PI3K/Akt pathway in different human cell lines (Li et al., 2017). Possible downstream effects of these anti-inflammatory mechanisms may include the downregulation of key inflammatory enzymes, like xanthine oxidase, cyclooxygenase, matrix metalloproteinases, and inducible nitric oxide synthase (Armutcu et al., 2015; Li et al., 2017).
Anti-inflammatory virtues of propolis are popularly exploited in mouthwash products. Antigingivitis activity has been ascribed to phenolics, especially CAPE (Li et al., 2017). Moreover, in randomized, double-blind, placebo-controlled trials, rinse products containing Brazilian green propolis rich in artepillin C have alleviated gingivitis to the same extent of a NaF/cetylpyridinium chloride rinse or a chlorhexidine solution (Bretz et al., 2014).
Propolis is also lenitive to the skin by topical application. Australian and Romanian propolis have induced photo-protective effects on animal models, possibly due to the anti-UV properties of polyphenols (Cole et al., 2010; Bolfa et al., 2013).
Wound Healing and Skin Protection
Animal models and clinical trials have shown the healing effect of propolis on diabetic foot ulcers and other problematic tissue injuries (Henshaw et al., 2014; Abu-Seida, 2015). Propolis wound healing activities are favored by the immunomodulatory, antioxidant and antiseptic effects of its rich phytocomplex (Martinotti and Ranzato, 2015). However, other mechanisms seem to play a role, since molecular studies have revealed that propolis modulates fibronectin expression and collagen I and III deposition in burns (Olczyk et al., 2013). An Indian propolis containing flavonoids, phenolic acids and terpenes, topically applied to rats with excision wounds, has upregulated hydroxyproline, hexosamine, uronic acid, nucleic acids and protein levels in wounded tissue, similar to the effect of nitrofurazone (Iyyam Pillai et al., 2010). In addition, in a study on Wistar rats, Brazilian green propolis rich in artepillin C has revealed superior wound healing activity with respect to Brazilian red propolis (Batista et al., 2012).
Anticancer Activity
A very large number of in vitro and pre-clinical studies on propolis anticancer effects are available, while only few clinical studies have been conducted and their results are controversial. Propolis from Aydin, Turkey, rich in CAPE and flavonoids, has shown a concentration-dependent apoptotic effect on CCRF-SB lymphoblastic leukemic cells involving the modulation of different miRNA expressions (Yilmaz et al., 2016). New Zealand propolis and its constituents chrysin, galangin, CAPE, benzyl ferulate, benzyl isoferulate, pinostrobin, 5-phenylpenta-2,4-dienoic acid, and tectochrysin, have exhibited antiproliferative effects on DLD-1 colon cancer, HCT-116 colon carcinoma, KYSE-30 esophageal squamous cancer, and NCI-N87 gastric carcinoma cells (Catchpole et al., 2015). Polish propolis rich in phenolic acids and flavonoids has been shown to possess dose-dependent antiproliferative and proapoptotic activities on HCT 116 colon cancer and Me45 malignant melanoma cells (Kubina et al., 2015).
Various mechanisms of action have been disclosed for CAPE, including suppression of tyrosine kinase activity and induction of cell cycle arrest in G1 or G2/M phase (Patel, 2016), block of migration and invasiveness through Wnt inhibition and ROR2 upregulation (Tseng et al., 2016), voltage-gated sodium channel block leading to reduction of breast cancer cell motility and invasiveness (Fraser et al., 2016), and selective inhibition of cancerous cell viability (Kuo et al., 2015). Moreover, CAPE seems synergistic with tamoxifen on MCF-7 breast cancer cells (Motawi et al., 2016), and has induced radiosensitivity on MDA-MB-231 (estrogen receptor negative) and T47D (estrogen receptor positive) breast cancer cell lines (Khoram et al., 2016).
The flavone chrysin has exerted an antiproliferative effect on human Hep-3B, TCC, A549, HeLa, and colorectal cancer cells (Patel, 2016), while possible mechanisms of action include TRAIL-induced caspase activation and STAT3 inhibition (Lirdprapamongkol et al., 2013), as well as p38 and Bax activation (Pichichero et al., 2011). Artepillin C has induced an antiproliferative effect on prostate cancer cells by reducing TRAIL resistance and inhibiting NF-κB, while a proapoptotic effect of galangin has been related to the induction of MAPK phosphorylation (Zhang et al., 2013).
A study on M12.C3.F6 B-cell lymphoma cancer cell line has shown that pinobanksin, pinobanksin-3-O-propanoate, pinobanksin-3-O-butyrate and pinobanksin-3-O-pentanoate exert an antiproliferative effect by inducing loss of mitochondrial membrane potential and activating caspases 3, 8 and 9 (Alday et al., 2015). Similarly, the phenolic lipids cardanol and cardol from Thai propolis have shown antiproliferative effects on several human cancer cells (Teerasripreecha et al., 2012), while cardanol has also induced apoptosis in BT-474 breast cancer cells, upregulated p21, stimulated ERK, p38 and JNK phosphorylation, and downregulated cyclin D (Buahorm et al., 2015). Finally, the polycyclic, polyisoprenylated benzophenone nemorosone from Cuban propolis has shown anticancer effects on estrogen receptor-positive MCF-7 cells by blocking cell cycle in G0/G1, and reducing MAPK and Akt phosphorylation (Popolo et al., 2011).
Adverse Effects
Clinical and in vivo studies on animal models have reported that propolis is well tolerated and non-toxic. The No Observed Adverse Effect Level (NOAEL) on mice and rats is over 1,470 mg/Kg/day at 60 days, and over 2,470 mg/Kg/day at 90 days (Burdock, 1998). In humans, toxic effects occur at dosages as high as 15 g/die (Castaldo and Capasso, 2002). However, despite its favorable safety profile, propolis is a common cause of allergic reactions. It has been reported that 1.2–6.6% of patients with dermatitis are sensitive to propolis (Walgrave et al., 2005), while major sensitizers are 3-methyl-2-butenyl caffeate, phenylethyl caffeate, benzyl salicylate, benzyl cinnamate, and 1,1-dimethylallylcaffeic acid (Burdock, 1998; Walgrave et al., 2005).
Bee Venom
Bee venom, also known as apitoxin, is a complex fluid secreted by the bee venom gland located in the abdominal cavity and injected into victims by a stinger, causing local inflammation, anticoagulant effect, and immune response. Bee venom constituents include amphipathic polycationic peptides, of which major ones are melittin and apamin, enzymes such as phospholipase A2, and low-molecular weight compounds including active bioamines such as histamine and catecholamines (Lee et al., 2016).
The venom has been traditionally, used in acupuncture and apitherapy, consisting in its injection to the patient as analgesic, against chronic pain and inflammation, and for other purposes such as immunotherapy and Parkinson’s treatment. A number of anticancer effects have been reported, together with antimutagenic, antinociceptive, and radioprotective properties. Approved pharmaceutical use has been introduced, while attempts at validating clinical treatment for chronic pain have been made (Moreno and Giralt, 2015; Sobral et al., 2016). However, different bee venom constituents are allergenic and in hypersensitive people bee sting can arrive to produce fatal outcome (Gelder et al., 1996).
Melittin
Melittin is a peptide of 26 amino acid residues, with a prevalently hydrophobic N-terminus and a hydrophilic C-terminus. It has distinct biological activities and has attracted much interest from pharmacological and biotechnological points of view.
The toxicity mechanism consists in the disruption of phospholipid bilayers, leading to cell lysis and the release of tissue injurious compounds such as lysosomal enzymes, serotonin, and histamine, triggering inflammation and pain (Raghuraman and Chattopadhyay, 2007). Together with hyaluronidase and phospholipase A2, melittin is responsible for venom allergenic properties. It seems also a major cause of pain induction by the bee venom, through activation of TRPV receptors and release of algogens from injured cells (Chen et al., 2016). In contrast to its toxicity, melittin is known as a traditional anti-inflammatory remedy for various diseases, such as dermatitis, neuritis, liver inflammation, atherosclerosis, and arthritis, but the mechanism of action at the cellular level has not been clarified (Lee and Bae, 2016). A possible coherent mechanism of antiatherosclerotic effects of melittin consists in the inhibition of vascular smooth muscle proliferation through the hindrance of platelet-derived growth factor beta-receptor signaling (Son et al., 2007).
The ability of interacting with biological membranes confers strong antimicrobial properties to melittin, which have attracted interest for fighting human pathogens, such as methicillin-resistant S. aureus (Choi et al., 2015), as well as plant pathogens (Stockwell and Duffy, 2012). Anticancer activities of melittin have been reported by different sources, while attempts at clarifying molecular mechanisms have been made by in vitro studies (Gajski and Garaj-Vrhovac, 2013). It has been shown for instance that melittin induces apoptosis in human ovarian cancer cells, SKOV3 and PA-1, by increasing the expression levels of the DR3, DR4, and DR6 death receptors (Jo et al., 2012).
Despite many indications of possible therapeutic applications of melittin, in vivo injection is known to entails side effects like hemolysis and liver injury, which have stimulated studies for the development of non-toxic hybrid derivatives. Engineered melittin peptides have also been developed for different biotechnological applications, e.g., to enhance antimicrobial properties or promote siRNA release from endosomes into target cells. (Moreno and Giralt, 2015).
Apamin
Apamin is a peptide of 18 amino acids, tightly cross-linked by the presence of two disulphide bonds (Habermann, 1984). It exerts a highly specific toxicity mechanism, consisting in a block of small conductance Ca2+-dependent K+ channels (SK channels) expressed in the central nervous system and in other districts, like the cardiovascular system and smooth muscle (Adelman et al., 2012).
Due to its ability of selectively targeting SK channels, apamin has been used as a tool for the physiological characterizations of this kind of K+ conductance (Castle et al., 1989). On a pharmacological ground, such a property has been adopted as an explanatory paradigm for accumulating evidence that apamin facilitates learning and memory. Apamin can cross the blood-brain barrier, and its administration to animals improves cognitive deficits, suggesting that SK channels would be appropriate apamin targets in the treatment of these neural disorders (Deschaux and Bizot, 2005; Brennan et al., 2008). In addition, the possibility of using apamin or less toxic analogs as blood-brain barrier, drug-delivery shuttles has been explored (Oller-Salvia et al., 2013).
SK channels are known to be involved in the pathogenesis of Parkinson’s disease. Consistent with this premise, another important perspective for neuro-therapeutic uses of apamin derives from its ability of protecting dopaminergic neurons from degeneration in experimental models of Parkinson’s (Alvarez-Fischer et al., 2013; Thomas and Justin, 2013). Among other possible uses, experimental work has shown antiatherosclerotic effects of apamin administered to mice (Kim et al., 2012), while as a K+ channel blocker, apamin can be useful for long-term whole blood storage (Delgado and Pitt, 2008).
Phospholipase A2
Phospholipase A2 (PLA2) hydrolyzes complex lipids to produce a fatty acid and various reaction products, including lysophosphatidic acid, lysophosphatidylcholine, and sphingosine phosphate. These latters exert cytotoxic and immunostimulatory effects on various cell types, eventually triggering immune responses and inflammation.
Phospholipase A2 is the major allergen of bee venom, containing three peptide and one glycopeptide T cell epitopes recognized by allergic and non-allergic subjects (Dhillon et al., 1992; Okano et al., 1999). However, PLA2 has also properties translatable into therapeutic treatments. It has exerted neuroprotective effects in a mouse model of Parkinson’s disease by activating regulatory T lymphocytes (Treg) that are known to mediate peripheral immune tolerance (Chung et al., 2015). Systemic PLA2 administration to a mouse model of neuropathic pain has alleviated cold and mechanical allodynia through the activation of α2-adrenegic receptors (Li et al., 2015). It has also been shown that PLA2 acts cooperatively with phosphatidylinositol-(3,4)-bisphosphate in inducing in vitro lysis of different tumor cell lines (Putz et al., 2006).
Minor Peptides and Enzymes
The second major allergen of honeybee venom is hyaluronidase (Padavattan et al., 2007), while other allergenic peptides include icarapin isolated from A. cerana (Wong et al., 2012), and two serine proteases named Api SI and Api SII belonging to the prophenoloxidase activating factor II family (Georgieva et al., 2011). Another serine protease of this family, named Bi-VSP, has a dual behavior, since in arthropods it triggers the phenoloxidase cascade inducing a lethal immune response, while in mammals it acts as a toxic thrombin-like and plasmin-like fibrinolytic protease (Choo et al., 2010).
Secapin is a serine protease inhibitor-like peptide exerting anti-fibrinolytic and anti-elastolytic activities, and also displaying antimicrobial properties by binding to the surfaces of fungi and bacteria (Lee et al., 2016). Two peptides isolated from A. cerana venom, viz. an inhibitor cysteine knot (ICK) peptide and a Kazal-type serine protease inhibitor, have been shown to act as antibacterial, antifungal and insecticidal venom toxins (Kim et al., 2013; Park et al., 2014).
Tertiapin is a 21 amino acid neurotoxin blocking inward-rectifier K+ channels expressed in epithelial cells, heart, and central nervous system. In the heart, tertiapin contrasts G-protein-gated, acetylcholine-activated K+ current that mediate parasympathetic heart rate decrease. This toxin could be allegedly useful as a drug for treating disorders in atrio-ventricular transmission, but at present it is used solely as a tool for K+ channel modulation (Drici et al., 2000).
Mast cell degranulating (MCD) peptide is a 22-amino acid peptide with two disulfide bridges, structurally similar to apamin but with different mechanisms of action. At low concentrations, MCD induces mast cell degranulation through histamine release, while at higher concentrations it can produce anti-inflammatory effects (Buku, 1999). Moreover, MCD also acts as a neurotoxin by blocking fast-inactivating (A-type) and slow-inactivating (delayed rectifier) K+ channels, thereby increasing neuronal excitability. Long term potentiation in the hippocampus CA1 region has been experimentally observed, while direct brain injection leads to convulsions and neurodegeneration (Mourre et al., 1997).
Bee Pollen
Foraging bees bring pollen back to the hive where it is packed into pellets and stored. During this process, the pollen mixed with nectar and bee salivary secretions becomes the “bee bread,” representing a main food reserve for the hive colony (Almeida-Muradian et al., 2005).
Main chemical compounds of bee pollen include carbohydrates, proteins and amino acids, lipids and fatty acids, phenolics, enzymes and coenzymes, vitamins and minerals (Komosinska-Vassev et al., 2015). However, the chemical composition of bee pollen is highly variable, depending on plant source, geographical region, and climatic conditions, thus deeply affecting biological properties and therapeutic virtues (Denisow and Denisow-Pietrzyk, 2016).
Bee pollen is an energy food used by humans as a diet supplement and for the conditioning of athletes. The high content of protein, fat, and minerals (particularly Ca, Mg, Fe, and P) gives bee pollen a nutritional value similar to, or higher than, that of dried legumes. Among vitamins, the levels of pantothenic and nicotinic acids are close to those of beef, ascorbic acid is similar to that of vegetables, such as lettuce and tomatoes, and riboflavin is comparable to that of skimmed milk power (Linskens and Jorde, 1997).
Bee pollen is used in complementary and alternative medicine to cure prostatitis, stomach ulcers, infectious diseases, and for the prevention and treatment of high-altitude-sickness syndrome (Linskens and Jorde, 1997). A wide range of therapeutic properties have been suggested, including antimicrobic, antioxidant, hepatoprotective, chemopreventive and anticarcinogenic, antiatherosclerotic, anti-inflammatory, antiallergenic, and immunomodulatory activities (Komosinska-Vassev et al., 2015; Denisow and Denisow-Pietrzyk, 2016).
Antioxidant Activity
The antioxidant activity of bee pollen seems to be mainly due to phenolic acids, like vanillic, protocatechuic, gallic, and p-coumaric acids, and to flavonoids like hesperidin, rutin, kaempferol, apigenin, luteolin, quercetin, and isorhamnetin. These compounds are thought to inactivate electrophiles and scavenge free radicals and reactive oxygen species (Bonvehí et al., 2001; Pascoal et al., 2014).
Antimicrobial Activity
Antimicrobial effects of bee pollen are well known, possibly mediated by glucose oxidase activity, deriving from honeybee secretion, while plant phenolics and flavonoids could also be involved (Denisow and Denisow-Pietrzyk, 2016; Fatrcova-Sramkova et al., 2016). Evidence about the activity of phenolic compounds from bee pollen extracts against Gram-positive and Gram-negative pathogenic bacteria, microscopic fungi and yeasts, has been reported (Baltrušaitytė et al., 2007; Kacániová et al., 2012).
Anti-inflammatory Activity
Bee pollen exerts anti-inflammatory effects that have been compared to those of common non-steroidal anti-inflammatory drugs, possibly depending on the activity of flavonoids, phenolic acids, phytosterols, and flavoring substances like anethole, an inhibitor of the NF-KB pathway (Middleton, 1998; Choi, 2007). Specific effects include the capability of removing swellings caused by cardiovascular and renal pathologies (Yakusheva, 2010), of protecting the liver from carbon tetrachloride-induced damages (Yildiz et al., 2013), and of alleviating prostate inflammation and hyperplasia (Yakusheva, 2010). Positive effects on prostatic conditions have been also ascribed to antiandrogen actions (Rzepecka-Stojko et al., 2012).
Anticancer Activity
Different studies have shown potential anticancer activity of bee pollen, probably associated with antioxidant and antimutagenic potentials (Denisow and Denisow-Pietrzyk, 2016). The steroid fraction of a chloroform extract from Brassica campestris bee pollen has shown strong cytotoxicity on human prostate cancer PC-3 cells, associated to stimulation of TNF-α secretion and apoptosis induction (Wu and Lou, 2007).
Antiatherosclerotic and Antidiabetic Activities
Bee pollen has shown antiatherosclerotic and cardioprotective effects and has been successfully applied to patients who did not respond to classical drugs (Polanski et al., 1998). Hypolipidemic activity, confirmed by pharmacological studies conducted on rats and rabbits, has been ascribed to the presence of unsaturated fatty acids, especially the ω-3, α-linolenic acid, and to phospholipids and phytosterols (Komosinska-Vassev et al., 2015). α-Linolenic acid is a precursor of prostaglandin-3a that is considered a major inhibitor of platelet aggregation (Denisow and Denisow-Pietrzyk, 2016).
Ghoshal and Saoji (2013) have found the presence of antidiabetic compounds in pollen grains, such as steroids and alkaloids in the pollen of C. roseus, saponins, flavonoids, sugars, and tannins in M. charantia, sugars, flavonoids and sterols in B. monosperma, and alkaloids and tannins in S. cuminii, suggesting therapeutic possibilities for bee pollen as a hypoglycemic agent.
Immunomodulatory Activity
Evidence of antiallergic activity of bee pollen has been reported, including prevention of IgE binding to their high-affinity receptor FcεRI, inhibition of histamine release from mast cells, and basophil degranulation (Ishikawa et al., 2008; Moita et al., 2014). Flavonoids, steroids, and volatile oil compounds seem to be involved in immunosuppressive activities.
Nutritional Properties
Bee pollen has been used as a diet supplement in recovery periods, in cases of malnutrition, asthenia and apathy, and to increase physical and mental ability or strengthen the immune system. Experiments on animals have shown that the administration of bee pollen prolongs life span, promotes weight gain, increases plasma hemoglobin levels, and provides tissues with vitamin C and Mg (Khalil and El-Sheikh, 2010; Attia et al., 2011). These virtues may be related to a complex of active substances, including amino acids, vitamins like tocopherol, niacin, thiamine, biotin, and folic acid, polyphenols, carotenoids, phytosterols, and minerals (Denisow and Denisow-Pietrzyk, 2016).
Adverse Effects
Health risks linked to the use of bee pollen may derive from the occasional presence of contaminants such as heavy metals, pesticides, mycotoxins (e.g., ochratoxin A), and bacteria (Denisow and Denisow-Pietrzyk, 2016). Moreover, bee pollen derived from Echium vulgare, Symphytum officinale, and Senecio jacobaea may contain dangerous levels of pyrrolizidine alkaloids with hepatotoxic properties (Kempf et al., 2010).
As known, pollen is highly allergenic, and consequently, complications or anaphylaxis due to bee pollen use have been reported, making tests for individual sensitivity highly recommendable before use (Jagdis and Sussman, 2012).
Beeswax
Worker bees secrete beeswax by wax glands located in abdominal segments. This substance is generally produced in greatest amount during colony growth phase in late spring, and is used for making combs. Beeswax is synthesized starting from honey sugars, and has a crystalline structure suitable for hive construction.
Chemical composition varies among bee species and geographical zones, and includes hydrocarbons, of which major ones are heptacosane, nonacosane, hentriacontane, pentacosane and tricosane, free fatty acids and free fatty alcohols, linear wax monoesters, hydroxymonoesters deriving from palmitic, 15-hydroxypalmitic, and oleic acids, and complex wax esters containing 15-hydroxypalmitic acid and diols (Münstedt and Bogdanov, 2009). A total of about 50 aroma components has also been reported (Ferber and Nursten, 1977). The ester/acid ratio is important for beeswax characterization by different Pharmacopeias, being generally lower (3–4) in European, and higher (8–9) in Asian beeswax (Münstedt and Bogdanov, 2009).
Beeswax is used as an additive in a variety of industrial products and processes, such as food industry, candles, and cosmetics. In pharmaceutical preparations it plays a role as a thickener, binder, drug carrier and release retardant.
Antimicrobial Activity
Beeswax has been used since ancient times for its antimicrobial properties in European and Asian traditional medicines. Preservative effects are possibly at the basis of its use in embalming and mummification practices by old Egyptians and Persian, or to model death masks by ancient Romans.
A beeswax crude extract has shown inhibitory effects against S. aureus, Salmonella enterica, C. albicans and Aspergillus niger (Ghanem, 2011), while effects against pathogenic bacteria and microscopic fungi have been reported for methanol and ethanol extracts (Kacániová et al., 2012). This kind of effects could depend at least in part on beeswax compounds of plant origin (Puleo and Keunen, 1991).
Dermatological and Cosmetic Properties
Beeswax has been known as a major Ayurvedic remedy for inflammation, bruises, burns, and cracked heels (Gokani, 2014). Ointments based on beeswax useful for joint pain, wounds and burns, are reported in the Ebers Papyrus (about 3500 B.P.), by the Greek-Roman physician Galen (about 2150 B.P.), and in old texts of traditional Chinese medicine, such as the “Shen Nong Book of Herbs” (about 2100–2200 B.P.) (Rit and Behrer, 1999).
Thanks to its very low irritant and comedogenic effects, beeswax is widely used in modern cosmetics and makeup as a thickener, emollient and emulsifier (Münstedt and Bogdanov, 2009).
Future Perspectives
Pharmaceutical and clinical uses of honeybee products are attracting increasing interest. Research and development in this field generally concern whole materials or subfractions rather than single compounds. Clinical trials have been conducted, among others, with honey for cicatrization problems and diabetes, with royal jelly for diabetes and rheumatoid arthritis, with propolis for disinfection and gingivitis, and with bee venom for Parkinsons’ and rheumatoid arthritis1. However, the complexity and variability in composition of these products raise the need of their standardization before safe and predictable clinical uses can be achieved.
Conversely, the use of specific compounds from honeybee products as curative drugs has not been implemented yet. As shown in the present review, various of these compounds have been in-depth characterized for their effects on biochemical pathways, cells, and organs, suggesting a series of possible uses as therapeutic drugs (Table 1). Moreover, preclinical studies indicate that some of these agents may be competitive with standard drugs, possibly including MGO, MRJPs, jelleins, royalisin, 10-HDA, CAPE, artepillin C, melittin, and apamin. However, none of them is reported at the online clinical trial database https://clinicaltrials.gov/, with the exception of CAPE2, suggesting that their medicinal use as specific drugs is not forthcoming.
Different reasons may explain the gap between the clinical exploitation of whole honeybee products and their single constituents. A role may be played in some cases by the toxicity of the bioactive agent, or alternatively, by an economically inefficient scale-up of pharmaceutical manufacturing, even though some compounds can be obtained by synthetic way too. Procedures for the chemical synthesis of MGO and CAPE have been patented, while synthetic MGO has been added in some cases to medicinal honey for strengthening wound healing properties (Wardell and Sabacinski, 2016). Methods for 10-HDA purification from royal jelly, artepillin C from propolis, melittin and apamin from bee venom, and their uses in pharmaceutical preparations have been also patented (Iinuma et al., 2014). Regardless whether the pharmaceutical exploitation of these bioactives is going to take off or not, studies aimed at refining the knowledge of their mechanisms of action remain of pivotal importance for developing applications of honeybee products to medicinal uses.
Author Contributions
LC contributed with ethnobotanical and pharmacognosy aspects; MB contributed with pharmaceutical aspects; JX contributed with chemical aspects; BB contributed with aspects on mechanisms of actions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work was granted by University of Genova, n. 100022-2015 FRA.
Footnotes
- ^https://clinicaltrials.gov
- ^https://clinicaltrials.gov/ct2/show/NCT02744703?term=caffeic+acid\&rank=3
References
Abubakar, M. B., Abdullah, W. Z., Sulaiman, S. A., and Suen, A. B. (2012). A review of molecular mechanisms of the anti-leukemic effects of phenolic compounds in honey. Int. J. Mol. Sci. 13, 15054–15073. doi: 10.3390/ijms131115054
Abu-Seida, A. M. (2015). Effect of propolis on experimental cutaneous wound healing in dogs. Vet. Med. Int. 2015:672643. doi: 10.1155/2015/672643
Adelman, J. P., Maylie, J., and Sah, P. (2012). Small-conductance Ca2+-activated K+ channels: form and function. Annu. Rev. Physiol. 74, 245–269. doi: 10.1146/annurev-physiol-020911-153336
Alday, E., Valencia, D., Carreno, A. L., Picerno, P., Piccinelli, A. L., Rastrelli, L., et al. (2015). Apoptotic induction by pinobanksin and some of its ester derivatives from Sonoran propolis in a B-cell lymphoma cell line. Chem. Biol. Interact. 242, 35–44. doi: 10.1016/j.cbi.2015.09.013
Allen, K. L., Molan, P. C., and Reid, G. M. (1991). A survey of the antibacterial activity of some New Zealand honeys. J. Pharm. Pharmacol. 43, 817–822. doi: 10.1111/j.2042-7158.1991.tb03186.x
Almeida-Muradian, L. B., Pamplona, L. C., Coimbra, S., and Barth, O. M. (2005). Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compost. Anal. 18, 105–111. doi: 10.1016/j.jfca.2003.10.008
Alreshoodi, F. M., and Sultanbawa, Y. (2015). Antimicrobial activity of royal jelly. Antiinfect. Agents 13, 50–59. doi: 10.2174/2211352513666150318234430
Alvarez-Fischer, D., Noelker, C., Vulinovic, F., Grunewald, A., Chevarin, C., Klein, C., et al. (2013). Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS ONE 8:e61700. doi: 10.1371/journal.pone.0061700
Al-Waili, N., Al-Ghamdi, A., Ansari, M. J., Al-Attal, Y., and Salom, K. (2012). Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 9, 793–800. doi: 10.7150/ijms.4722
Al-Waili, N., Salom, K., and Al-Ghamdi, A. A. (2011). Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. ScientificWorldJournal 11, 766–787. doi: 10.1100/tsw.2011.78
Armutcu, F., Akyol, S., Ustunsoy, S., and Turan, F. F. (2015). Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Exp. Ther. Med. 9, 1582–1588. doi: 10.3892/etm.2015.2346
Attia, Y. A., Al-Hanoun, A., El-Din, A. E., Bovera, F., and Shewika, Y. E. (2011). Effect of bee pollen levels on productive, reproductive and blood traits of NZW rabbits. J. Anim. Physiol. Anim. Nutr. (Berl) 95, 294–303. doi: 10.1111/j.1439-0396.2010.01054.x
Bachanova, K., Klaudiny, J., Kopernicky, J., and Simuth, J. (2002). Identification of honeybee peptide active against Paenibacillus larvae larvae through bacterial growth-inhibition assay on polyacrylamide gel. Apidologie 33, 259–269. doi: 10.1051/apido:2002015
Baltrušaitytė, V., Venskutonis, P. R., and Čeksterytë, V. (2007). Antibacterial activity of honey and beebread of different origin against S. aureus and S. epidermidis. Food Technol. Biotechnol. 45, 201–208.
Bankova, V., Popova, M., Bogdanov, S., and Sabatini, A. G. (2002). Chemical composition of European propolis: expected and unexpected results. Z. Naturforsch. C 57, 530–533. doi: 10.1515/znc-2002-5-622
Bankova, V., Popova, M., and Trusheva, B. (2014). Propolis volatile compounds: chemical diversity and biological activity: a review. Chem. Cent. J. 8:28. doi: 10.1186/1752-153X-8-28
Batista, L. L., Campesatto, E. A., Assis, M. L., Barbosa, A. P., Grillo, L. A., and Dornelas, C. B. (2012). Comparative study of topical green and red propolis in the repair of wounds induced in rats. Rev. Col. Bras. Cir. 39, 515–520. doi: 10.1590/S0100-69912012000600012
Bilikova, K., Mirgorodskaya, E., Bukovska, G., Gobom, J., Lehrach, H., and Simuth, J. (2009). Towards functional proteomics of minority component of honeybee royal jelly: the effect of post-translational modifications on the antimicrobial activity of apalbumin2. Proteomics 9, 2131–2138. doi: 10.1002/pmic.200800705
Boisard, S., Le Ray, A. M., Gatto, J., Aumond, M. C., Blanchard, P., Derbre, S., et al. (2014). Chemical composition, antioxidant and anti-AGEs activities of a French poplar type propolis. J. Agric. Food Chem. 62, 1344–1351. doi: 10.1021/jf4053397
Bolfa, P., Vidrighinescu, R., Petruta, A., Dezmirean, D., Stan, L., Vlase, L., et al. (2013). Photoprotective effects of Romanian propolis on skin of mice exposed to UVB irradiation. Food Chem. Toxicol. 62, 329–342. doi: 10.1016/j.fct.2013.08.078
Bonvehí, J. S., Torrentó, M. S., and Lorente, E. C. (2001). Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J. Agric. Food Chem. 49, 1848–1853. doi: 10.1021/jf0012300
Brennan, A. R., Dolinsky, B., Vu, M. A., Stanley, M., Yeckel, M. F., and Arnsten, A. F. (2008). Blockade of IP3-mediated SK channel signaling in the rat medial prefrontal cortex improves spatial working memory. Learn. Mem. 15, 93–96. doi: 10.1101/lm.767408
Bretz, W. A., Paulino, N., Nor, J. E., and Moreira, A. (2014). The effectiveness of propolis on gingivitis: a randomized controlled trial. J. Altern. Complement. Med. 20, 943–948. doi: 10.1089/acm.2013.0431
Brudzynski, K., Abubaker, K., St-Martin, L., and Castle, A. (2011). Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2:213. doi: 10.3389/fmicb.2011.00213
Brudzynski, K., and Sjaarda, C. (2015). Honey glycoproteins containing antimicrobial peptides, jelleins of the major royal jelly protein 1, are responsible for the cell wall lytic and bactericidal activities of honey. PLoS ONE 10:e0120238. doi: 10.1371/journal.pone.0120238
Brudzynski, K., Sjaarda, C., and Lannigan, R. (2015). MRJP1-containing glycoproteins isolated from honey, a novel antibacterial drug candidate with broad spectrum activity against multi-drug resistant clinical isolates. Front. Microbiol. 6:711. doi: 10.3389/fmicb.2015.00711
Buahorm, S., Puthong, S., Palaga, T., Lirdprapamongkol, K., Phuwapraisirisan, P., Svasti, J., et al. (2015). Cardanol isolated from Thai Apis mellifera propolis induces cell cycle arrest and apoptosis of BT-474 breast cancer cells via p21 upregulation. Daru 23, 55. doi: 10.1186/s40199-015-0138-1
Buku, A. (1999). Mast cell degranulating (MCD) peptide: a prototypic peptide in allergy and inflammation. Peptides 20, 415–420. doi: 10.1016/S0196-9781(98)00167-3
Burdock, G. A. (1998). Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 36, 347–363. doi: 10.1016/S0278-6915(97)00145-2
Burlando, B., and Cornara, L. (2013). Honey in dermatology and skin care: a review. J. Cosmet. Dermatol. 12, 306–313. doi: 10.1111/jocd.12058
Buttstedt, A., Ihling, C. H., Pietzsch, M., and Moritz, R. F. (2016). Royalactin is not a royal making of a queen. Nature 537, E10–E12. doi: 10.1038/nature19349
Buttstedt, A., Moritz, R. F., and Erler, S. (2013). More than royal food - Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 10:72. doi: 10.1186/1742-9994-10-72
Candiracci, M., Citterio, B., Diamantini, G., Blasa, M., Accorsi, A., and Piatti, E. (2011). Honey flavonoids, natural antifungal agents against Candida albicans. Int. J. Food Prop. 14, 799–808. doi: 10.1080/10942910903453355
Candiracci, M., Piatti, E., Dominguez-Barragan, M., Garcia-Antras, D., Morgado, B., Ruano, D., et al. (2012). Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J. Agric. Food Chem. 60, 12304–12311. doi: 10.1021/jf302468h
Castaldo, S., and Capasso, F. (2002). Propolis, an old remedy used in modern medicine. Fitoterapia 73(Suppl. 1), S1–S6. doi: 10.1016/S0367-326X(02)00185-5
Castle, N. A., Haylett, D. G., and Jenkinson, D. H. (1989). Toxins in the characterization of potassium channels. Trends Neurosci. 12, 59–65. doi: 10.1016/0166-2236(89)90137-9
Catchpole, O., Mitchell, K., Bloor, S., Davis, P., and Suddes, A. (2015). Antiproliferative activity of New Zealand propolis and phenolic compounds vs human colorectal adenocarcinoma cells. Fitoterapia 106, 167–174. doi: 10.1016/j.fitote.2015.09.004
Chen, J., Guan, S. M., Sun, W., and Fu, H. (2016). Melittin, the major pain-producing substance of bee venom. Neurosci. Bull. 32, 265–272. doi: 10.1007/s12264-016-0024-y
Cho, M. S., Park, W. S., Jung, W. K., Qian, Z. J., Lee, D. S., Choi, J. S., et al. (2014). Caffeic acid phenethyl ester promotes anti-inflammatory effects by inhibiting MAPK and NF-kappaB signaling in activated HMC-1 human mast cells. Pharm. Biol. 52, 926–932. doi: 10.3109/13880209.2013.865243
Choi, E. M. (2007). Antinociceptive and antiinflammatory activities of pine (Pinus densiflora) pollen extract. Phytother. Res. 21, 471–475. doi: 10.1002/ptr.2103
Choi, J. H., Jang, A. Y., Lin, S., Lim, S., Kim, D., Park, K., et al. (2015). Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 12, 6483–6490. doi: 10.3892/mmr.2015.4275
Choo, Y. M., Lee, K. S., Yoon, H. J., Kim, B. Y., Sohn, M. R., Roh, J. Y., et al. (2010). Dual function of a bee venom serine protease: prophenoloxidase-activating factor in arthropods and fibrin(ogen)olytic enzyme in mammals. PLoS ONE 5:e10393. doi: 10.1371/journal.pone.0010393
Chua, L. S., Lee, J. Y., and Chan, G. F. (2015). Characterization of the proteins in honey. Anal. Lett. 48, 697–709. doi: 10.1080/00032719.2014.952374
Chung, E. S., Lee, G., Lee, C., Ye, M., Chung, H. S., Kim, H., et al. (2015). Bee venom phospholipase A2, a novel Foxp3+ regulatory T cell inducer, protects dopaminergic neurons by modulating neuroinflammatory responses in a mouse model of Parkinson’s Disease. J. Immunol. 195, 4853–4860. doi: 10.4049/jimmunol.1500386
Coelho, L. G., Bastos, E. M., Resende, C. C., Paula e Silva, C. M., Sanches, B. S., de Castro, F. J., et al. (2007). Brazilian green propolis on Helicobacter pylori infection. a pilot clinical study. Helicobacter 12, 572–574. doi: 10.1111/j.1523-5378.2007.00525.x
Cole, N., Sou, P. W., Ngo, A., Tsang, K. H., Severino, J. A., Arun, S. J., et al. (2010). Topical ‘Sydney’ propolis protects against UV-radiation-induced inflammation, lipid peroxidation and immune suppression in mouse skin. Int. Arch. Allergy Immunol. 152, 87–97. doi: 10.1159/000265530
Costi, R., Santo, R. D., Artico, M., Massa, S., Ragno, R., Loddo, R., et al. (2004). 2,6-Bis(3,4,5-trihydroxybenzylydene) derivatives of cyclohexanone: novel potent HIV-1 integrase inhibitors that prevent HIV-1 multiplication in cell-based assays. Bioorg. Med. Chem. 12, 199–215. doi: 10.1016/j.bmc.2003.10.005
da Silva, P. M., Gauche, C., Gonzaga, L. V., Costa, A. C., and Fett, R. (2016). Honey: chemical composition, stability and authenticity. Food Chem. 196, 309–323. doi: 10.1016/j.foodchem.2015.09.051
Delgado, M. C., and Pitt, B. (2008). Composition and methods for preserving red blood cells. U.S. Patent No WO2008089337 A1. Washington, DC: U.S. Patent and Trademark Office.
Denisow, B., and Denisow-Pietrzyk, M. (2016). Biological and therapeutic properties of bee pollen: a review. J. Sci. Food Agric. 96, 4303–4309. doi: 10.1002/jsfa.7729
Deschaux, O., and Bizot, J. C. (2005). Apamin produces selective improvements of learning in rats. Neurosci. Lett. 386, 5–8. doi: 10.1016/j.neulet.2005.05.050
Detienne, G., De Haes, W., Ernst, U. R., Schoofs, L., and Temmerman, L. (2014). Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp. Gerontol. 60, 129–135. doi: 10.1016/j.exger.2014.09.021
Dhillon, M., Roberts, C., Nunn, T., and Kuo, M. (1992). Mapping human T cell epitopes on phospholipase A2: the major bee-venom allergen. J. Allergy Clin. Immunol. 90, 42–51. doi: 10.1016/S0091-6749(06)80009-6
Di Girolamo, F., D’Amato, A., and Righetti, P. G. (2012). Assessment of the floral origin of honey via proteomic tools. J. Proteomics 75, 3688–3693. doi: 10.1016/j.jprot.2012.04.029
Drapeau, M. D., Albert, S., Kucharski, R., Prusko, C., and Maleszka, R. (2006). Evolution of the yellow/major royal jelly protein family and the emergence of social behavior in honey bees. Genome Res. 16, 1385–1394. doi: 10.1101/gr.5012006
Drici, M. D., Diochot, S., Terrenoire, C., Romey, G., and Lazdunski, M. (2000). The bee venom peptide tertiapin underlines the role of I(KACh) in acetylcholine-induced atrioventricular blocks. Br. J. Pharmacol. 131, 569–577. doi: 10.1038/sj.bjp.0703611
Duplan, H., Questel, E., Hernandez-Pigeon, H., Galliano, M. F., Caruana, A., Ceruti, I., et al. (2011). Effects of Hydroxydecine((R)) (10-hydroxy-2-decenoic acid) on skin barrier structure and function in vitro and clinical efficacy in thetreatment of UV-induced xerosis. Eur. J. Dermatol. 21, 906–915. doi: 10.1684/ejd.2011.1531
Dzopalic, T., Vucevic, D., Tomic, S., Djokic, J., Chinou, I., and Colic, M. (2011). 3,10-Dihydroxy-decanoic acid, isolated from royal jelly, stimulates Th1 polarising capability of human monocyte-derived dendritic cells. Food Chem. 126, 1211–1217. doi: 10.1016/j.foodchem.2010.12.004
Edgar, J. A., Roeder, E., and Molyneux, R. J. (2002). Honey from plants containing pyrrolizidine alkaloids: a potential threat to health. J. Agric. Food Chem. 50, 2719–2730. doi: 10.1021/jf0114482
Erejuwa, O. O., Sulaiman, S. A., and Wahab, M. S. (2012). Honey–a novel antidiabetic agent. Int. J. Biol. Sci. 8, 913–934. doi: 10.7150/ijbs.3697
Erejuwa, O. O., Sulaiman, S. A., and Wahab, M. S. (2014). Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 19, 2497–2522. doi: 10.3390/molecules19022497
Fan, P., Han, B., Feng, M., Fang, Y., Zhang, L., Hu, H., et al. (2016). Functional and proteomic investigations reveal major royal jelly protein 1 associated with anti-hypertension activity in mouse vascular smooth muscle cells. Sci. Rep. 6:30230. doi: 10.1038/srep30230
Fang, E.-L., Zhou, H.-Z., Xu, H.-L., and Xing, M.-J. (1994). Antiulcer effects of 10-hydroxy-2-decenoic in rats. Chin. Pharmacol. Bull. 10, 139–142.
Fatrcova-Sramkova, K., Nozkova, J., Mariassyova, M., and Kacaniova, M. (2016). Biologically active antimicrobial and antioxidant substances in the Helianthus annuus L. bee pollen. J. Environ. Sci. Health B 51, 176–181. doi: 10.1080/03601234.2015.1108811
Ferber, C. E. M., and Nursten, H. E. (1977). The aroma of wax. J. Sci. Food Agric. 28, 511–518. doi: 10.1002/jsfa.2740280608
Fields, B. A., Reeve, J., Bartholomaeus, A., and Mueller, U. (2014). Human pharmacokinetic study of tutin in honey; a plant-derived neurotoxin. Food Chem. Toxicol. 72, 234–241. doi: 10.1016/j.fct.2014.07.032
Fontana, R., Mendes, M. A., de Souza, B. M., Konno, K., Cesar, L. M., Malaspina, O., et al. (2004). Jelleines: a family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 25, 919–928. doi: 10.1016/j.peptides.2004.03.016
Fraser, S. P., Hemsley, F., and Djamgoz, M. B. (2016). Caffeic acid phenethyl ester: inhibition of metastatic cell behaviours via voltage-gated sodium channel in human breast cancer in vitro. Int. J. Biochem. Cell Biol. 71, 111–118. doi: 10.1016/j.biocel.2015.12.012
Freires, I. A., Queiroz, V. C., Furletti, V. F., Ikegaki, M., de Alencar, S. M., Duarte, M. C., et al. (2016). Chemical composition and antifungal potential of Brazilian propolis against Candida spp. J. Mycol. Med. 26, 122–132. doi: 10.1016/j.mycmed.2016.01.003
Fujita, T., Kozuka-Hata, H., Ao-Kondo, H., Kunieda, T., Oyama, M., and Kubo, T. (2013). Proteomic analysis of the royal jelly and characterization of the functions of its derivation glands in the honeybee. J. Proteome Res. 12, 404–411. doi: 10.1021/pr300700e
Fujiwara, S., Imai, J., Fujiwara, M., Yaeshima, T., Kawashima, T., and Kobayashi, K. (1990). A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 265, 11333–11337.
Gajski, G., and Garaj-Vrhovac, V. (2013). Melittin: a lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 36, 697–705. doi: 10.1016/j.etap.2013.06.009
Gannabathula, S., Skinner, M. A., Rosendale, D., Greenwood, J. M., Mutukumira, A. N., Steinhorn, G., et al. (2012). Arabinogalactan proteins contribute to the immunostimulatory properties of New Zealand honeys. Immunopharmacol. Immunotoxicol. 34, 598–607. doi: 10.3109/08923973.2011.641974
Gao, W., Wu, J., Wei, J., Pu, L., Guo, C., Yang, J., et al. (2014). Brazilian green propolis improves immune function in aged mice. J. Clin. Biochem. Nutr. 55, 7–10. doi: 10.3164/jcbn.13-70
Gasic, S., Vucevic, D., Vasilijic, S., Antunovic, M., Chinou, I., and Colic, M. (2007). Evaluation of the immunomodulatory activities of royal jelly components in vitro. Immunopharmacol. Immunotoxicol. 29, 521–536. doi: 10.1080/08923970701690977
Gelder, C., Harris, J., and Williams, D. (1996). Allergy to bee and wasp venom. Br. J. Hosp. Med. 55, 349–352.
Georgieva, D., Greunke, K., Arni, R. K., and Betzel, C. (2011). Three-dimensional modelling of honeybee venom allergenic proteases: relation to allergenicity. Z. Naturforsch. C 66, 305–312. doi: 10.5560/ZNC.2011.66c0305
Ghanem, N. (2011). Study on the antimicrobial activity of honey products and some Saudi folkloric substances. Res. J. Biotechnol. 6, 38–43.
Gheldof, N., Wang, X. H., and Engeseth, N. J. (2002). Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 50, 5870–5877. doi: 10.1021/jf0256135
Ghoshal, K. P., and Saoji, A. A. (2013). Phytochemical screening of the pollen of some selected plants with antidiabetic properties. Aust. J. Basic Appl. Sci. 7, 105–109.
Guo, H., Kouzuma, Y., and Yonekura, M. (2009). Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 113, 238–245. doi: 10.1016/j.foodchem.2008.06.081
Haghdoost, N. S., Salehi, T. Z., Khosravi, A., and Sharifzadeh, A. (2016). Antifungal activity and influence of propolis against germ tube formation as a critical virulence attribute by clinical isolates of Candida albicans. J. Mycol. Med. 26, 298–305. doi: 10.1016/j.mycmed.2015.11.004
Hattori, N., Nomoto, H., Fukumitsu, H., Mishima, S., and Furukawa, S. (2007). Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed. Res. 28, 261–266. doi: 10.2220/biomedres.28.261
Henshaw, F. R., Bolton, T., Nube, V., Hood, A., Veldhoen, D., Pfrunder, L., et al. (2014). Topical application of the bee hive protectant propolis is well tolerated and improves human diabetic foot ulcer healing in a prospective feasibility study. J. Diabetes Complications 28, 850–857. doi: 10.1016/j.jdiacomp.2014.07.012
Hirakawa, A., Shimizu, K., Fukumitsu, H., Soumiya, H., Iinuma, M., and Furukawa, S. (2010). 2-Decenoic acid ethyl ester, a derivative of unsaturated medium-chain fatty acids, facilitates functional recovery of locomotor activity after spinal cord injury. Neuroscience 171, 1377–1385. doi: 10.1016/j.neuroscience.2010.10.004
Honda, Y., Araki, Y., Hata, T., Ichihara, K., Ito, M., Tanaka, M., et al. (2015). 10-hydroxy-2-decenoic acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015, 7. doi: 10.1155/2015/425261
Huang, S., Zhang, C. P., Wang, K., Li, G. Q., and Hu, F. L. (2014). Recent advances in the chemical composition of propolis. Molecules 19, 19610–19632. doi: 10.3390/molecules191219610
Iinuma, M., Furukawa, S., Naiki, M., Matsumoto, T., and Higashiura, K. (2014). Trans-2-decenoic acid derivative and drug containing same. U.S. Patent No SG11201406942S. Washington, DC: U.S. Patent and Trademark Office.
Ioshida, M. D. M., Young, M. C. M., and Lago, J. H. G. (2010). Chemical composition and antifungal activity of essential oil from Brazilian propolis. J. Essent. Oil Bearing Plants 13, 633–637. doi: 10.1080/0972060X.2010.10643873
Ishikawa, Y., Tokura, T., Nakano, N., Hara, M., Niyonsaba, F., Ushio, H., et al. (2008). Inhibitory effect of honeybee-collected pollen on mast cell degranulation in vivo and in vitro. J. Med. Food 11, 14–20. doi: 10.1089/jmf.2006.163
Islam, M. N., Khalil, M. I., Islam, M. A., and Gan, S. H. (2014). Toxic compounds in honey. J. Appl. Toxicol. 34, 733–742. doi: 10.1002/jat.2952
Iyyam Pillai, S., Palsamy, P., Subramanian, S., and Kandaswamy, M. (2010). Wound healing properties of Indian propolis studied on excision wound-induced rats. Pharm. Biol. 48, 1198–1206. doi: 10.3109/13880200903578754
Jaganathan, S. K., and Mandal, M. (2009). Antiproliferative effects of honey and of its polyphenols: a review. J. Biomed. Biotechnol. 2009:830616. doi: 10.1155/2009/830616
Jagdis, A., and Sussman, G. (2012). Anaphylaxis from bee pollen supplement. CMAJ 184, 1167–1169. doi: 10.1503/cmaj.112181
Jo, M., Park, M. H., Kollipara, P. S., An, B. J., Song, H. S., Han, S. B., et al. (2012). Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 258, 72–81. doi: 10.1016/j.taap.2011.10.009
Kacániová, M., Vuković, N., Chlebo, R., Haščík, P., Rovná, K., Cubon, J., et al. (2012). The antimicrobial activity of honey bee pollen, loads and bees wax from Slovakia. Arch. Biol. Sci. Belgrade 64, 927–934. doi: 10.2298/ABS1203927K
Kamakura, M. (2011). Royalactin induces queen differentiation in honeybees. Nature 473, 478–483. doi: 10.1038/nature10093
Kamakura, M., Suenobu, N., and Fukushima, M. (2001). Fifty-seven-kDa protein in royal jelly enhances proliferation of primary cultured rat hepatocytes and increases albumin production in the absence of serum. Biochem. Biophys. Res. Commun. 282, 865–874. doi: 10.1006/bbrc.2001.4656
Kashima, Y., Kanematsu, S., Asai, S., Kusada, M., Watanabe, S., Kawashima, T., et al. (2014). Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 9:e105073. doi: 10.1371/journal.pone.0105073
Kassi, E., Chinou, I., Spilioti, E., Tsiapara, A., Graikou, K., Karabournioti, S., et al. (2014). A monoterpene, unique component of thyme honeys, induces apoptosis in prostate cancer cells via inhibition of NF-kappaB activity and IL-6 secretion. Phytomedicine 21, 1483–1489. doi: 10.1016/j.phymed.2014.04.032
Kempf, M., Heil, S., Hasslauer, I., Schmidt, L., von der Ohe, K., Theuring, C., et al. (2010). Pyrrolizidine alkaloids in pollen and pollen products. Mol. Nutr. Food Res. 54, 292–300. doi: 10.1002/mnfr.200900289
Khalil, F. A., and El-Sheikh, N. M. (2010). The effects of dietary Egyptian propolis and bee pollen supplementation against toxicity of sodium fluoride in rats. J. Am. Sci. 11, 310–316.
Khoram, N. M., Bigdeli, B., Nikoofar, A., and Goliaei, B. (2016). Caffeic Acid Phenethyl ester increases radiosensitivity of estrogen receptor-positive and -negative breast cancer cells by prolonging radiation-induced DNA damage. J. Breast. Cancer 19, 18–25. doi: 10.4048/jbc.2016.19.1.18
Khoshpey, B., Djazayeri, S., Amiri, F., Malek, M., Hosseini, A. F., Hosseini, S., et al. (2016). Effect of royal jelly intake on serum glucose, Apolipoprotein A-I (ApoA-I), Apolipoprotein B (ApoB) and ApoB/ApoA-I ratios in patients with type 2 Diabetes: a randomized, double-blind clinical trial study. Can. J. Diabetes 40, 324–328. doi: 10.1016/j.jcjd.2016.01.003
Kim, B. Y., Lee, K. S., Zou, F. M., Wan, H., Choi, Y. S., Yoon, H. J., et al. (2013). Antimicrobial activity of a honeybee (Apis cerana) venom Kazal-type serine protease inhibitor. Toxicon 76, 110–117. doi: 10.1016/j.toxicon.2013.09.017
Kim, S. J., Park, J. H., Kim, K. H., Lee, W. R., Pak, S. C., Han, S. M., et al. (2012). The protective effect of apamin on LPS/fat-induced atherosclerotic mice. Evid. Based Complement. Alternat. Med. 2012, 305454. doi: 10.1155/2012/305454
Kimura, M., Kimura, Y., Tsumura, K., Okihara, K., Sugimoto, H., Yamada, H., et al. (2003). 350-kDa royal jelly glycoprotein (apisin), which stimulates proliferation of human monocytes, bears the beta1-3galactosylated N-glycan: analysis of the N-glycosylation site. Biosci. Biotechnol. Biochem. 67, 2055–2058. doi: 10.1271/bbb.67.2055
Koca, I., and Koca, A. F. (2007). Poisoning by mad honey: a brief review. Food Chem. Toxicol. 45, 1315–1318. doi: 10.1016/j.fct.2007.04.006
Komosinska-Vassev, K., Olczyk, P., Kazmierczak, J., Mencner, L., and Olczyk, K. (2015). Bee pollen: chemical composition and therapeutic application. Evid. Based Complement. Alternat. Med. 2015:297425. doi: 10.1155/2015/297425
Kosalec, I., Pepeljnjak, S., Bakmaz, M., and Vladimir-Knezevic, S. (2005). Flavonoid analysis and antimicrobial activity of commercially available propolis products. Acta Pharm. 55, 423–430.
Koya-Miyata, S., Okamoto, I., Ushio, S., Iwaki, K., Ikeda, M., and Kurimoto, M. (2004). Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci. Biotechnol. Biochem. 68, 767–773. doi: 10.1271/bbb.68.767
Kubina, R., Kabala-Dzik, A., Dziedzic, A., Bielec, B., Wojtyczka, R. D., Buldak, R. J., et al. (2015). The ethanol extract of polish propolis exhibits anti-proliferative and/or pro-apoptotic effect on HCT 116 colon cancer and Me45 malignant melanoma cells In Vitro conditions. Adv. Clin. Exp. Med. 24, 203–212. doi: 10.17219/acem/31792
Kubota, M., Tsuji, M., Nishimoto, M., Wongchawalit, J., Okuyama, M., Mori, H., et al. (2004). Localization of alpha-glucosidases I, II, and III in organs of European honeybees, Apis mellifera L., and the origin of alpha-glucosidase in honey. Biosci. Biotechnol. Biochem. 68, 2346–2352. doi: 10.1271/bbb.68.2346
Kuncic, M. K., Jaklic, D., Lapanje, A., and Gunde-Cimerman, N. (2012). Antibacterial and antimycotic activities of Slovenian honeys. Br. J. Biomed. Sci. 69, 154–158.
Kuo, Y. Y., Jim, W. T., Su, L. C., Chung, C. J., Lin, C. Y., Huo, C., et al. (2015). Caffeic Acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 16, 10748–10766. doi: 10.3390/ijms160510748
Kwakman, P. H., Te Velde, A. A., de Boer, L., Vandenbroucke-Grauls, C. M., and Zaat, S. A. (2011). Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS ONE 6:e17709. doi: 10.1371/journal.pone.0017709
Kwakman, P. H., and Zaat, S. A. (2012). Antibacterial components of honey. IUBMB Life 64, 48–55. doi: 10.1002/iub.578
Larsen, L., Joyce, N. I., Sansom, C. E., Cooney, J. M., Jensen, D. J., and Perry, N. B. (2015). Sweet poisons: honeys contaminated with glycosides of the neurotoxin tutin. J. Nat. Prod. 78, 1363–1369. doi: 10.1021/acs.jnatprod.5b00241
Lee, G., and Bae, H. (2016). Anti-inflammatory applications of melittin, a major component of bee venom: detailed mechanism of action and adverse effects. Molecules 21, E616. doi: 10.3390/molecules21050616
Lee, K. S., Kim, B. Y., Yoon, H. J., Choi, Y. S., and Jin, B. R. (2016). Secapin, a bee venom peptide, exhibits anti-fibrinolytic, anti-elastolytic, and anti-microbial activities. Dev. Comp. Immunol. 63, 27–35. doi: 10.1016/j.dci.2016.05.011
Li, D., Lee, Y., Kim, W., Lee, K., Bae, H., and Kim, S. K. (2015). Analgesic effects of bee venom derived phospholipase A(2) in a mouse model of oxaliplatin-induced neuropathic pain. Toxins (Basel) 7, 2422–2434. doi: 10.3390/toxins7072422
Li, L., Sun, W., Wu, T., Lu, R., and Shi, B. (2017). Caffeic acid phenethyl ester attenuates lipopolysaccharide-stimulated proinflammatory responses in human gingival fibroblasts via NF-kappaB and PI3K/Akt signaling pathway. Eur. J. Pharmacol. 794, 61–68. doi: 10.1016/j.ejphar.2016.11.003
Li, X., Huang, C., and Xue, Y. (2013). Contribution of lipids in honeybee (Apis mellifera) royal jelly to health. J. Med. Food 16, 96–102. doi: 10.1089/jmf.2012.2425
Linskens, H. F., and Jorde, W. (1997). Pollen as food and medicine: a review. Econ. Bot. 51, 78–86. doi: 10.1007/BF02910407
Lirdprapamongkol, K., Sakurai, H., Abdelhamed, S., Yokoyama, S., Athikomkulchai, S., Viriyaroj, A., et al. (2013). Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation. Int. J. Oncol. 43, 329–337. doi: 10.3892/ijo.2013.1926
Majtan, J., Bohova, J., Garcia-Villalba, R., Tomas-Barberan, F. A., Madakova, Z., Majtan, T., et al. (2013). Fir honeydew honey flavonoids inhibit TNF-alpha-induced MMP-9 expression in human keratinocytes: a new action of honey in wound healing. Arch. Dermatol. Res. 305, 619–627. doi: 10.1007/s00403-013-1385-y
Majtan, J., Klaudiny, J., Bohova, J., Kohutova, L., Dzurova, M., Sediva, M., et al. (2012). Methylglyoxal-induced modifications of significant honeybee proteinous components in manuka honey: Possible therapeutic implications. Fitoterapia 83, 671–677. doi: 10.1016/j.fitote.2012.02.002
Majtan, J., Kovacova, E., Bilikova, K., and Simuth, J. (2006). The immunostimulatory effect of the recombinant apalbumin 1-major honeybee royal jelly protein-on TNFalpha release. Int. Immunopharmacol. 6, 269–278. doi: 10.1016/j.intimp.2005.08.014
Majtan, J., Kumar, P., Majtan, T., Walls, A. F., and Klaudiny, J. (2010). Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp. Dermatol. 19, e73–e79. doi: 10.1111/j.1600-0625.2009.00994.x
Makino, J., Ogasawara, R., Kamiya, T., Hara, H., Mitsugi, Y., Yamaguchi, E., et al. (2016). Royal jelly constituents increase the expression of extracellular superoxide dismutase through histone acetylation in monocytic THP-1 cells. J. Nat. Prod. 79, 1137–1143. doi: 10.1021/acs.jnatprod.6b00037
Manyi-Loh, C. E., Clarke, A. M., and Ndip, R. N. (2012). Detection of phytoconstituents in column fractions of n-hexane extract of Goldcrest honey exhibiting anti-Helicobacter pylori activity. Arch. Med. Res. 43, 197–204. doi: 10.1016/j.arcmed.2012.04.006
Marcucci, M. C., Ferreres, F., Garcia-Viguera, C., Bankova, V. S., De Castro, S. L., Dantas, A. P., et al. (2001). Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 74, 105–112. doi: 10.1016/S0378-8741(00)00326-3
Martinotti, S., and Ranzato, E. (2015). Propolis: a new frontier for wound healing? Burns Trauma 3, 9. doi: 10.1186/s41038-015-0010-z
Mavric, E., Wittmann, S., Barth, G., and Henle, T. (2008). Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 52, 483–489. doi: 10.1002/mnfr.200700282
Melliou, E., and Chinou, I. (2005). Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 53, 8987–8992. doi: 10.1021/jf051550p
Mesaik, M. A., Dastagir, N., Uddin, N., Rehman, K., and Azim, M. K. (2015). Characterization of immunomodulatory activities of honey glycoproteins and glycopeptides. J. Agric. Food Chem. 63, 177–184. doi: 10.1021/jf505131p
Middleton, E. Jr. (1998). Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 439, 175–182. doi: 10.1007/978-1-4615-5335-9_13
Mihajlovic, D., Rajkovic, I., Chinou, I., and Colic, M. (2013). Dose-dependent immunomodulatory effects of 10-hydroxy-2-decenoic acid on human monocyte-derived dendritic cells. J. Funct. Foods 5, 838–846. doi: 10.1016/j.jff.2013.01.031
Mohammed, S. E., Kabashi, A. S., Koko, W. S., and Azim, M. K. (2015). Antigiardial activity of glycoproteins and glycopeptides from Ziziphus honey. Nat. Prod. Res. 29, 2100–2102. doi: 10.1080/14786419.2014.986659
Moita, E., Sousa, C., Andrade, P. B., Fernandes, F., Pinho, B. R., Silva, L. R., et al. (2014). Effects of Echium plantagineum L. bee pollen on basophil degranulation: relationship with metabolic profile. Molecules 19, 10635–10649. doi: 10.3390/molecules190710635
Molan, P. C. (1999). Why honey is effective as a medicine. I. Its use in modern medicine. Bee World 80, 80–92.
Molan, P. C. (2006). The evidence supporting the use of honey as a wound dressing. Int. J. Low Extrem. Wounds 5, 40–54. doi: 10.1177/1534734605286014
Moreno, M., and Giralt, E. (2015). Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins (Basel) 7, 1126–1150. doi: 10.3390/toxins7041126
Moriyama, T., Ito, A., Omote, S., Miura, Y., and Tsumoto, H. (2015). Heat resistant characteristics of major royal jelly protein 1 (MRJP1) oligomer. PLoS ONE 10:e0119169. doi: 10.1371/journal.pone.0119169
Motawi, T. K., Abdelazim, S. A., Darwish, H. A., Elbaz, E. M., and Shouman, S. A. (2016). Modulation of tamoxifen cytotoxicity by caffeic acid phenethyl ester in MCF-7 breast cancer cells. Oxid. Med. Cell Longev. 2016, 3017108. doi: 10.1155/2016/3017108
Mourre, C., Lazdunski, M., and Jarrard, L. E. (1997). Behaviors and neurodegeneration induced by two blockers of K+ channels, the mast cell degranulating peptide and Dendrotoxin I. Brain Res. 762, 223–227. doi: 10.1016/S0006-8993(97)00481-2
Moussa, A., Noureddine, D., Saad, A., Abdelmelek, M., and Abdelkader, B. (2012). Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2, 554–557. doi: 10.1016/S2221-1691(12)60096-3
Moutsatsou, P., Papoutsi, Z., Kassi, E., Heldring, N., Zhao, C., Tsiapara, A., et al. (2010). Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 5:e15594. doi: 10.1371/journal.pone.0015594
Münstedt, K., and Bogdanov, S. (2009). Bee products and their potential use in modern medicine. J. ApiProduct ApiMedical Sci. 1, 57–63. doi: 10.3896/IBRA.4.01.3.01
Mutlu Sariguzel, F., Berk, E., Koc, A. N., Sav, H., and Demir, G. (2016). Antifungal activity of propolis against yeasts isolated from blood culture: In Vitro evaluation. J. Clin. Lab. Anal. 30, 513–516. doi: 10.1002/jcla.21889
Noronha, V. R., Araujo, G. S., Gomes, R. T., Iwanaga, S. H., Barbosa, M. C., Abdo, E. N., et al. (2014). Mucoadhesive propolis gel for prevention of radiation-induced oral mucositis. Curr. Clin. Pharmacol. 9, 359–364. doi: 10.2174/1574884709666140205210051
Okamoto, I., Taniguchi, Y., Kunikata, T., Kohno, K., Iwaki, K., Ikeda, M., et al. (2003). Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 73, 2029–2045. doi: 10.1016/S0024-3205(03)00562-9
Okano, M., Nishizaki, K., Satoskar, A. R., Yoshino, T., Masuda, Y., and Harn, D. A. Jr. (1999). Involvement of carbohydrate on phospholipase A2, a bee-venom allergen, in in vivo antigen-specific IgE synthesis in mice. Allergy 54, 811–818. doi: 10.1034/j.1398-9995.1999.00096.x
Olczyk, P., Wisowski, G., Komosinska-Vassev, K., Stojko, J., Klimek, K., Olczyk, M., et al. (2013). Propolis modifies collagen types I and III accumulation in the matrix of burnt tissue. Evid. Based Complement. Alternat. Med. 2013, 423809. doi: 10.1155/2013/423809
Oller-Salvia, B., Teixido, M., and Giralt, E. (2013). From venoms to BBB shuttles: synthesis and blood-brain barrier transport assessment of apamin and a nontoxic analog. Biopolymers 100, 675–686. doi: 10.1002/bip.22257
Omene, C., Kalac, M., Wu, J., Marchi, E., Frenkel, K., and O’Connor, O. A. (2013). propolis and its active component, Caffeic Acid Phenethyl Ester (CAPE), modulate breast cancer therapeutic targets via an epigenetically mediated mechanism of action. J. Cancer Sci. Ther. 5, 334–342.
Orsi, R. O., Funari, S. R. C., Soares, A. M. V. C., Calvi, S. A., Oliveira, S. L., Sforcin, J. M., et al. (2000). Immunomodulatory action of propolis on macrophage activation. J. Venom. Anim. Toxins Incl. Trop. Dis. 6, 205–219. doi: 10.1590/S0104-79302000000200006
Padavattan, S., Schirmer, T., Schmidt, M., Akdis, C., Valenta, R., Mittermann, I., et al. (2007). Identification of a B-cell epitope of hyaluronidase, a major bee venom allergen, from its crystal structure in complex with a specific Fab. J. Mol. Biol. 368, 742–752. doi: 10.1016/j.jmb.2007.02.036
Park, H. G., Kyung, S. S., Lee, K. S., Kim, B. Y., Choi, Y. S., Yoon, H. J., et al. (2014). Dual function of a bee (Apis cerana) inhibitor cysteine knot peptide that acts as an antifungal peptide and insecticidal venom toxin. Dev. Comp. Immunol. 47, 247–253. doi: 10.1016/j.dci.2014.08.001
Park, H. M., Hwang, E., Lee, K. G., Han, S. M., Cho, Y., and Kim, S. Y. (2011). Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. J. Med. Food 14, 899–906. doi: 10.1089/jmf.2010.1363
Pascoal, A., Rodrigues, S., Teixeira, A., Feas, X., and Estevinho, L. M. (2014). Biological activities of commercial bee pollens: antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 63, 233–239. doi: 10.1016/j.fct.2013.11.010
Patel, S. (2016). Emerging adjuvant therapy for cancer: propolis and its constituents. J. Diet. Suppl. 13, 245–268. doi: 10.3109/19390211.2015.1008614
Petretto, G. L., Cossu, M., and Alamanni, M. C. (2015). Phenolic content, antioxidant and physico-chemical properties of Sardinian monofloral honeys. Int. J. Food Sci. Technol. 50, 482–491. doi: 10.1111/ijfs.12652
Petrova, A., Popova, M., Kuzmanova, C., Tsvetkova, I., Naydenski, H., Muli, E., et al. (2010). New biologically active compounds from Kenyan propolis. Fitoterapia 81, 509–514. doi: 10.1016/j.fitote.2010.01.007
Pichichero, E., Cicconi, R., Mattei, M., and Canini, A. (2011). Chrysin-induced apoptosis is mediated through p38 and Bax activation in B16-F1 and A375 melanoma cells. Int. J. Oncol. 38, 473–483. doi: 10.3892/ijo.2010.876
Polanski, M., Okon, K., Przybylo, R., and Frasik, W. (1998). Cardioprotective properties of hydrophilic pollen extract (HPE). Pol. J. Pathol. 49, 109–112.
Popolo, A., Piccinelli, A. L., Morello, S., Sorrentino, R., Osmany, C. R., Rastrelli, L., et al. (2011). Cytotoxic activity of nemorosone in human MCF-7 breast cancer cells. Can. J. Physiol. Pharmacol. 89, 50–57. doi: 10.1139/y10-100
Puleo, S. L., and Keunen, K. (1991). Beeswax minor components a new approach. Cosmet. Toiletries 106, 83.
Putz, T., Ramoner, R., Gander, H., Rahm, A., Bartsch, G., and Thurnher, M. (2006). Antitumor action and immune activation through cooperation of bee venom secretory phospholipase A2 and phosphatidylinositol-(3,4)-bisphosphate. Cancer Immunol. Immunother. 55, 1374–1383. doi: 10.1007/s00262-006-0143-9
Raghuraman, H., and Chattopadhyay, A. (2007). Melittin: a membrane-active peptide with diverse functions. Biosci. Rep. 27, 189–223. doi: 10.1007/s10540-006-9030-z
Ramadan, M. F., and Al-Ghamdi, A. (2012). Bioactive compounds and health-promoting properties of royal jelly: a review. J. Funct. Foods 4, 39–52. doi: 10.1016/j.jff.2011.12.007
Ramanauskiene, K., Stelmakiene, A., Briedis, V., Ivanauskas, L., and Jakstas, V. (2012). The quantitative analysis of biologically active compounds in Lithuanian honey. Food Chem. 132, 1544–1548. doi: 10.1016/j.foodchem.2011.12.007
Ranzato, E., Martinotti, S., and Burlando, B. (2012). Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: comparison among different honeys. Wound Repair Regen. 20, 778–785. doi: 10.1111/j.1524-475X.2012.00825.x
Rosmilah, M., Shahnaz, M., Patel, G., Lock, J., Rahman, D., Masita, A., et al. (2008). Characterization of major allergens of royal jelly Apis mellifera. Trop. Biomed. 25, 243–251.
Rzepecka-Stojko, A., Pilawa, B., Ramos, P., and Stojko, J. (2012). Antioxidative properties of bee pollen extracts examined by EPR spectroscopy. J. Apicult. Sci. 56, 23–31. doi: 10.2478/v10289-012-0003-0
Sajid, M., and Azim, M. K. (2012). Characterization of the nematicidal activity of natural honey. J. Agric. Food Chem. 60, 7428–7434. doi: 10.1021/jf301653n
Siavash, M., Shokri, S., Haghighi, S., Shahtalebi, M. A., and Farajzadehgan, Z. (2015). The efficacy of topical royal jelly on healing of diabetic foot ulcers: a double-blind placebo-controlled clinical trial. Int. Wound J. 12, 137–142. doi: 10.1111/iwj.12063
Silici, S., and Atayoglu, A. T. (2015). Mad honey intoxication: a systematic review on the 1199 cases. Food Chem. Toxicol. 86, 282–290. doi: 10.1016/j.fct.2015.10.018
Simuth, J., Bilikova, K., Kovacova, E., Kuzmova, Z., and Schroder, W. (2004). Immunochemical approach to detection of adulteration in honey: physiologically active royal jelly protein stimulating TNF-alpha release is a regular component of honey. J. Agric. Food Chem. 52, 2154–2158. doi: 10.1021/jf034777y
Sobral, F., Sampaio, A., Falcao, S., Queiroz, M. J., Calhelha, R. C., Vilas-Boas, M., et al. (2016). Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 94, 172–177. doi: 10.1016/j.fct.2016.06.008
Son, D. J., Kang, J., Kim, T. J., Song, H. S., Sung, K. J., Yun, D. Y., et al. (2007). Melittin, a major bioactive component of bee venom toxin, inhibits PDGF receptor beta-tyrosine phosphorylation and downstream intracellular signal transduction in rat aortic vascular smooth muscle cells. J. Toxicol. Environ. Health A 70, 1350–1355. doi: 10.1080/15287390701428689
Spannhoff, A., Kim, Y. K., Raynal, N. J., Gharibyan, V., Su, M. B., Zhou, Y. Y., et al. (2011). Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep. 12, 238–243. doi: 10.1038/embor.2011.9
Speciale, A., Costanzo, R., Puglisi, S., Musumeci, R., Catania, M. R., Caccamo, F., et al. (2006). Antibacterial activity of propolis and its active principles alone and in combination with macrolides, beta-lactams and fluoroquinolones against microorganisms responsible for respiratory infections. J. Chemother. 18, 164–171. doi: 10.1179/joc.2006.18.2.164
Stepanovic, S., Antic, N., Dakic, I., and Svabic-Vlahovic, M. (2003). In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 158, 353–357. doi: 10.1078/0944-5013-00215
Stockwell, V. O., and Duffy, B. (2012). Use of antibiotics in plant agriculture. Rev. Sci. Tech. 31, 199–210. doi: 10.20506/rst.31.1.2104
Sugiyama, T., Takahashi, K., Kuzumaki, A., Tokoro, S., Neri, P., and Mori, H. (2013). Inhibitory mechanism of 10-hydroxy-trans-2-decenoic acid (royal jelly acid) against lipopolysaccharide- and interferon-beta-induced nitric oxide production. Inflammation 36, 372–378. doi: 10.1007/s10753-012-9556-0
Sugiyama, T., Takahashi, K., Tokoro, S., Gotou, T., Neri, P., and Mori, H. (2012). Inhibitory effect of 10-hydroxy-trans-2-decenoic acid on LPS-induced IL-6 production via reducing IkappaB-zeta expression. Innate Immun. 18, 429–437. doi: 10.1177/1753425911416022
Sun, C., Wu, Z., Wang, Z., and Zhang, H. (2015). Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of beijing propolis extracts. Evid. Based Complement. Alternat. Med. 2015, 595393. doi: 10.1155/2015/595393
Suzuki, K. M., Isohama, Y., Maruyama, H., Yamada, Y., Narita, Y., Ohta, S., et al. (2008). Estrogenic activities of Fatty acids and a sterol isolated from royal jelly. Evid. Based Complement. Alternat. Med. 5, 295–302. doi: 10.1093/ecam/nem036
Takemura, T., Urushisaki, T., Fukuoka, M., Hosokawa-Muto, J., Hata, T., Okuda, Y., et al. (2012). 3,4-Dicaffeoylquinic acid, a major constituent of brazilian propolis, increases TRAIL expression and extends the lifetimes of mice infected with the influenza A Virus. Evid. Based Complement. Alternat. Med. 2012, 946867. doi: 10.1155/2012/946867
Takikawa, M., Kumagai, A., Hirata, H., Soga, M., Yamashita, Y., Ueda, M., et al. (2013). 10-Hydroxy-2-decenoic acid, a unique medium-chain fatty acid, activates 5’-AMP-activated protein kinase in L6 myotubes and mice. Mol. Nutr. Food Res. 57, 1794–1802. doi: 10.1002/mnfr.201300041
Tamura, S., Amano, S., Kono, T., Kondoh, J., Yamaguchi, K., Kobayashi, S., et al. (2009). Molecular characteristics and physiological functions of major royal jelly protein 1 oligomer. Proteomics 9, 5534–5543. doi: 10.1002/pmic.200900541
Teerasripreecha, D., Phuwapraisirisan, P., Puthong, S., Kimura, K., Okuyama, M., Mori, H., et al. (2012). In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from Thai Apis mellifera propolis. BMC Complement. Altern. Med. 12:27. doi: 10.1186/1472-6882-12-27
Terada, Y., Narukawa, M., and Watanabe, T. (2011). Specific hydroxy fatty acids in royal jelly activate TRPA1. J. Agric. Food Chem. 59, 2627–2635. doi: 10.1021/jf1041646
Thomas, N. C., and Justin, D. O. L. (2013). Composition for treating parkinson’s disease. U.S. Patent No 20070004639 A1. Washington, DC: U.S. Patent and Trademark Office.
Tonks, A. J., Dudley, E., Porter, N. G., Parton, J., Brazier, J., Smith, E. L., et al. (2007). A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J. Leukoc. Biol. 82, 1147–1155. doi: 10.1189/jlb.1106683
Tseng, J. C., Lin, C. Y., Su, L. C., Fu, H. H., Yang, S. D., and Chuu, C. P. (2016). CAPE suppresses migration and invasion of prostate cancer cells via activation of non-canonical Wnt signaling. Oncotarget 7, 38010–38024. doi: 10.18632/oncotarget.9380
Vandamme, L., Heyneman, A., Hoeksema, H., Verbelen, J., and Monstrey, S. (2013). Honey in modern wound care: a systematic review. Burns 39, 1514–1525. doi: 10.1016/j.burns.2013.06.014
Viuda-Martos, M., Ruiz-Navajas, Y., Fernandez-Lopez, J., and Perez-Alvarez, J. A. (2008). Functional properties of honey, propolis, and royal jelly. J. Food Sci. 73, R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x
Vynograd, N., Vynograd, I., and Sosnowski, Z. (2000). A comparative multi-centre study of the efficacy of propolis, acyclovir and placebo in the treatment of genital herpes (HSV). Phytomedicine 7, 1–6. doi: 10.1016/S0944-7113(00)80014-8
Wagh, V. D. (2013). Propolis: a wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 308249. doi: 10.1155/2013/308249
Walgrave, S. E., Warshaw, E. M., and Glesne, L. A. (2005). Allergic contact dermatitis from propolis. Dermatitis 16, 209–215.
Wang, J., Zhang, W., Zou, H., Lin, Y., Lin, K., Zhou, Z., et al. (2015). 10-Hydroxy-2-decenoic acid inhibiting the proliferation of fibroblast-like synoviocytes by PI3K-AKT pathway. Int. Immunopharmacol. 28, 97–104. doi: 10.1016/j.intimp.2015.05.036
Wang, J. G., Ruan, J., Li, C. Y., Wang, J. M., Li, Y., Zhai, W. T., et al. (2012). Connective tissue growth factor, a regulator related with 10-hydroxy-2-decenoic acid down-regulate MMPs in rheumatoid arthritis. Rheumatol. Int. 32, 2791–2799. doi: 10.1007/s00296-011-1960-5
Wang, L. C., Chu, K. H., Liang, Y. C., Lin, Y. L., and Chiang, B. L. (2010). Caffeic acid phenethyl ester inhibits nuclear factor-kappaB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells. Clin. Exp. Immunol. 160, 223–232. doi: 10.1111/j.1365-2249.2009.04067.x
Wardell, M., and Sabacinski, K. (2016). Wound healing compositions involving medicinal honey, mineral ions, and methylglyoxal, 1and methods of use. U.S. Patent No WO 2016123539 A1. Washington, DC: U.S. Patent and Trademark Office.
Wong, K. L., Li, H., Wong, K. K., Jiang, T., and Shaw, P. C. (2012). Location and reduction of icarapin antigenicity by site specific coupling to polyethylene glycol. Protein Pept. Lett. 19, 238–243. doi: 10.2174/092986612799080211
Wu, Y. D., and Lou, Y. J. (2007). A steroid fraction of chloroform extract from bee pollen of Brassica campestris induces apoptosis in human prostate cancer PC-3 cells. Phytother. Res. 21, 1087–1091. doi: 10.1002/ptr.2235
Xu, D., Mei, X., and Xu, S. (2002). [The research of 10-hydroxy-2-decenoic acid on experiment hyperlipoidemic rat]. Zhong Yao Cai 25, 346–347.
Yakusheva, E. (2010). “Pollen and bee bread: physico-chemical properties. Biological and pharmacological effects. Use in medical practice,” in Theoretical and Practical Basics of Apitherapy, eds D. Rakita, N. Krivtsov, and D. G. Uzbekova (Ryazan: Roszdrav), 84–97.
Yang, X. Y., Yang, D. S., Wei, Z., Wang, J. M., Li, C. Y., Hui, Y., et al. (2010). 10-Hydroxy-2-decenoic acid from Royal jelly: a potential medicine for RA. J. Ethnopharmacol. 128, 314–321. doi: 10.1016/j.jep.2010.01.055
Yildirim, A., Duran, G. G., Duran, N., Jenedi, K., Bolgul, B. S., Miraloglu, M., et al. (2016). Antiviral activity of hatay propolis against replication of herpes simplex virus type 1 and type 2. Med. Sci. Monit. 22, 422–430. doi: 10.12659/MSM.897282
Yildiz, O., Can, Z., Saral, O., Yulug, E., Ozturk, F., Aliyazicioglu, R., et al. (2013). Hepatoprotective potential of chestnut bee pollen on carbon tetrachloride-induced hepatic damages in rats. Evid. Based Complement. Alternat. Med. 2013:461478. doi: 10.1155/2013/461478
Yilmaz, U. C., Bagca, B. G., Karaca, E., Durmaz, A., Durmaz, B., Aykut, A., et al. (2016). Evaluation of the miRNA profiling and effectiveness of the propolis on B-cell acute lymphoblastic leukemia cell line. Biomed. Pharmacother. 84, 1266–1273. doi: 10.1016/j.biopha.2016.10.056
Yousefi, B., Ghaderi, S., Rezapoor-Lactooyi, A., Amiri, N., Verdi, J., and Shoae-Hassani, A. (2012). Hydroxy decenoic acid down regulates gtfB and gtfC expression and prevents Streptococcus mutans adherence to the cell surfaces. Ann. Clin. Microbiol. Antimicrob. 11:21. doi: 10.1186/1476-0711-11-21
Zhang, T., Li, Y., Lai, J. P., Douglas, S. D., Metzger, D. S., O’Brien, C. P., et al. (2003). Alcohol potentiates hepatitis C virus replicon expression. Hepatology 38, 57–65. doi: 10.1053/jhep.2003.50295
Zhang, W., Lan, Y., Huang, Q., and Hua, Z. (2013). Galangin induces B16F10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 MAPK. Cytotechnology 65, 447–455. doi: 10.1007/s10616-012-9499-1
Keywords: bee pollen, bee venom, beeswax, honey, propolis, royal jelly
Citation: Cornara L, Biagi M, Xiao J and Burlando B (2017) Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 8:412. doi: 10.3389/fphar.2017.00412
Received: 16 February 2017; Accepted: 12 June 2017;
Published: 28 June 2017.
Edited by:
Monique S. J. Simmonds, Royal Botanic Gardens, Kew, United KingdomReviewed by:
Parimal C. Sen, Bose Institute, IndiaLetizia Angiolella, Sapienza Università di Roma, Italy
Copyright © 2017 Cornara, Biagi, Xiao and Burlando. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Cornara, Y29ybmFyYUBkaXB0ZXJpcy51bmlnZS5pdA==
 Laura Cornara
Laura Cornara Marco Biagi
Marco Biagi Jianbo Xiao
Jianbo Xiao Bruno Burlando4
Bruno Burlando4