- 1Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, VIC, Australia
- 2Department of Pharmacology, Monash University, Parkville, VIC, Australia
Adenosine is a ubiquitous molecule with key regulatory and cytoprotective mechanisms at times of metabolic imbalance in the body. Among a plethora of physiological actions, adenosine has an important role in attenuating ischaemia-reperfusion injury and modulating the ensuing fibrosis and tissue remodeling following myocardial damage. Adenosine exerts these actions through interaction with four adenosine G protein-coupled receptors expressed in the heart. The adenosine A2B receptor (A2BAR) is the most abundant adenosine receptor (AR) in cardiac fibroblasts and is largely responsible for the influence of adenosine on cardiac fibrosis. In vitro and in vivo studies demonstrate that acute A2BAR stimulation can decrease fibrosis through the inhibition of fibroblast proliferation and reduction in collagen synthesis. However, in contrast, there is also evidence that chronic A2BAR antagonism reduces tissue fibrosis. This review explores the opposing pro- and anti-fibrotic activity attributed to the activation of cardiac ARs and investigates the therapeutic potential of targeting ARs for the treatment of cardiac fibrosis.
Introduction
Cardiac fibroblasts form the largest population of interstitial cells in the adult mammalian heart (Chen and Frangogiannis, 2013). They have an essential role in the regulation of the extracellular matrix (ECM), which is crucial for maintaining the structural integrity of the myocardium and for electro-mechanical signal transduction (Camelliti et al., 2004; Souders et al., 2009). Cardiac fibroblasts are regulated by various mechanical and hormonal stimuli, in particular growth factors such as angiotensin II (ANGII) and the cytokine transforming growth factor β (TGFβ). ANGII and TGFβ can activate fibroblast cell-surface receptors to promote differentiation to myofibroblasts, the pro-fibrogenic phenotype that express the contractile protein α-smooth muscle actin (α-SMA) and exhibit enhanced secretory, migratory and proliferative properties (Schnee and Hsueh, 2000; Petrov et al., 2002; Leask, 2007; Porter and Turner, 2009; Lu and Insel, 2014). Following a myocardial infarction (MI), fibroblasts promote essential matrix deposition for proper tissue repair and scar formation to ensure structural integrity of the infarct zone. However, aberrant ECM deposition and excessive myofibroblast accumulation extending beyond the area of the original insult is responsible for maladaptive fibrosis leading to cardiac dysfunction, a hallmark feature of heart failure pathophysiology (See et al., 2005; Segura et al., 2012; Ferrari et al., 2016). Heart failure remains a major cause of mortality and morbidity in the western world with an estimated 50% 5 years survival rate after diagnosis (Mozaffarian et al., 2016). This highlights both the limitations of current therapeutic management and the crucial need for new and innovative therapies for the treatment and prevention of heart failure. Extracellular nucleotides and nucleosides have recently been implicated as important mediators of fibroblast homeostasis and as such purinergic signaling has been investigated for its role in cardiac fibrosis. AMP catabolites, including inosine and oxypurines have also been shown to contribute to cardiac fibrosis and diastolic stiffening in some animal models of heart failure (Paolocci et al., 2006). The role of nucleotide (ATP, ADP, UTP) signaling in tissue fibrosis has been comprehensively reviewed previously (Lu and Insel, 2014; Ferrari et al., 2016; Novitskaya et al., 2016), therefore the current review will focus the modulation of cardiac fibrosis mediated by the nucleoside adenosine and adenosine receptors (ARs).
Adenosine Signaling in the Heart
Adenosine is a ubiquitous purine nucleoside that is an important regulator of cardiac function. Adenosine is described as a ‘retaliatory metabolite’ owing to its enhanced local release and ability to restore energy balance during times of cellular and metabolic stress (Newby, 1984; Shyrock and Belardinelli, 1997). The well-characterized cytoprotective actions have resulted in large clinical trials for adenosine and adenosine derivatives for the treatment of ischaemia-reperfusion injury post-MI (Kopecky et al., 2003; Ross et al., 2005; Forman et al., 2006). In addition to a clear role in cardioprotection, adenosine exerts a multitude of actions on the physiological regulation of the heart, including coronary vasodilation, heart rate control and AV nodal conduction, angiogenesis, myocardial hypertrophy and remodeling and fibrosis (Auchampach and Bolli, 1999; Peart and Headrick, 2007; Headrick et al., 2013). The myriad of cardiovascular effects stimulated by adenosine occur via activation of specific cell surface ARs. The AR family is comprised of four Class A G protein-coupled receptors (GPCRs), the A1, A2A, A2B and A3ARs. They exert distinct pharmacological actions through differential coupling to intracellular G proteins; the A1AR and A3AR preferentially activate Gi/o proteins to inhibit adenylyl cyclase activity and subsequent cAMP production, while the A2AAR and A2BAR preferentially stimulate Gs proteins to activate adenylyl cyclase activity and increase cAMP accumulation (Figure 1) (Fredholm et al., 2001). The A2BAR has also been shown to stimulate robust Gq/11 protein activation in some cell types (Feoktistov and Biaggioni, 1997; Linden et al., 1999). ARs, and the A2BAR in particular, have also been shown to couple to additional transmembrane and intracellular proteins, which may influence downstream signal transduction (Mundell and Benovic, 2000; Fredholm et al., 2001; Sun and Huang, 2016). All four ARs are expressed in the heart and synchronous activation of multiple subtypes results in both complementary and opposing signal transduction for the fine-tuned regulation of cardiac function. Interestingly, both pro- and anti-fibrotic actions have been attributed to AR activation, which highlights both the complexity and ensuing challenges faced when targeting ARs for the treatment of cardiac fibrosis (Chan and Cronstein, 2009; Cronstein, 2011; Karmouty-Quintana et al., 2013). To date, the preponderance of evidence has implicated the A2BAR in cardiac fibrosis (Epperson et al., 2009; Headrick et al., 2013; Novitskaya et al., 2016). Therefore, this review will explore the current understanding of the role of AR signaling in augmenting or attenuating cardiac fibrosis, with a focus on the predominant subtype implicated, the A2BAR.
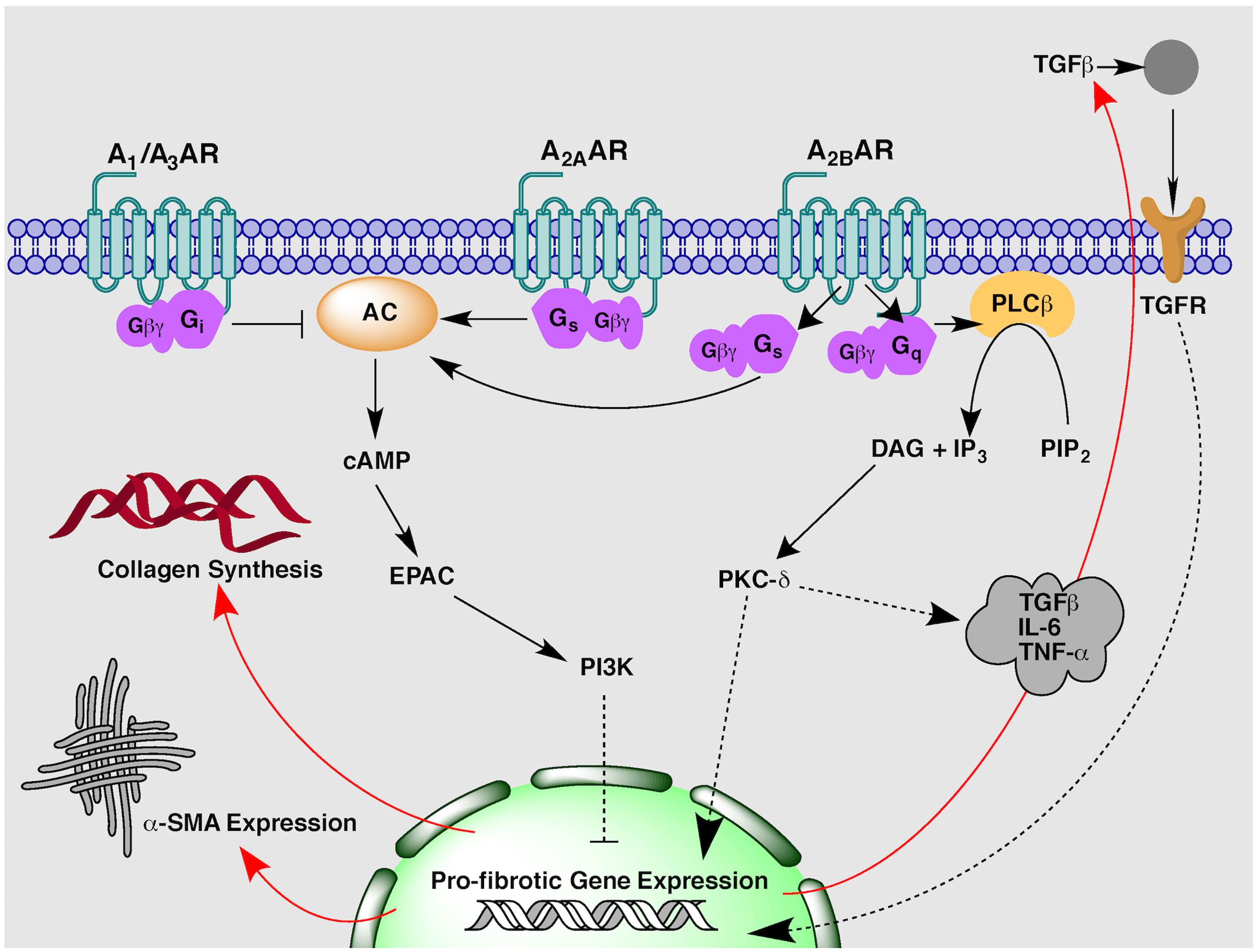
FIGURE 1. An overview of proposed adenosine receptor-mediated intracellular signaling pathways implicated in the regulation of cardiac fibrosis.
A2BAR-Mediated Anti-Fibrotic Signal Transduction
Studies in isolated rat cardiac fibroblasts first proposed the A2BAR as the subtype responsible for mediating adenosine’s inhibitory actions on fetal calf serum-stimulated fibroblast proliferation (Dubey et al., 1997) and collagen and protein synthesis (Dubey et al., 1998). The role of the A2BAR in adenosine-mediated anti-fibrotic signal transduction was later confirmed via antisense oligonucleotide A2BAR silencing, which resulted in increased cell proliferation and basal collagen synthesis in cardiac fibroblasts (Dubey et al., 2001b). Similarly, A2BAR overexpression had the opposite effect, significantly decreasing collagen and protein synthesis (Chen et al., 2004). The second messenger cAMP, has been shown to have a central role in inhibiting fibroblast and myofibroblast activity (Swaney et al., 2005; Lu et al., 2013). Accordingly, A2BAR-mediated cAMP accumulation stimulated in fibroblasts by the non-selective AR agonist 5′-N-ethylcarboxamidoadenosine (NECA) (Epperson et al., 2009) can reduce ANGII-stimulated collagen synthesis via an exchange factor directly activated by cAMP (Epac) and phosphoinositol-3 kinase (PI3K) dependent pathway (Figure 1) (Villarreal et al., 2009). In addition to effects on collagen synthesis, A2BAR stimulation has been shown to decrease mRNA expression of pro-fibrotic gene markers including collagen I and connective tissue growth factor (CTGF) (Vecchio et al., 2016). Of specific importance to ARs, a positive feedback loop has been identified whereby β-adrenoceptor-stimulated cAMP can be secreted by fibroblasts or cardiac myocytes and metabolized in the extracellular space to adenosine to activate A2ARs, thus exerting further inhibitory effects on fibroblast growth and function (Dubey et al., 2001a; Sassi et al., 2014).
Commensurate with the in vitro findings, an in vivo study in rats demonstrated chronic administration of the stable adenosine analog, 2-chloroadenosine (CADO) or the adenosine uptake inhibitor, dipyridamole, initiated 1 week after permanent ligation of the left anterior descending (LAD) coronary artery, protected against cardiac remodeling and reduced markers of fibrosis such as collagen volume fraction and matrix metalloproteinase gene expression (Wakeno et al., 2006). The effects of CADO on fibrotic and haemodynamic parameters were abolished in the presence of the selective A2BAR antagonist MRS1754, but not selective antagonists for the other AR subtypes (Wakeno et al., 2006). Together, these studies suggest a salutary effect of A2BAR activation on cardiac fibrosis, an effect which may be lost upon A2BAR downregulation as observed in hearts taken from human patients with chronic heart failure (Asakura et al., 2007).
A2BAR-Mediated Pro-Fibrotic Signal Transduction
While the majority of in vitro studies have identified an anti-fibrotic role for the A2BAR, recent studies have demonstrated A2BAR blockade appears to be beneficial within in vivo models of cardiac remodeling and fibrosis. In an in vivo mouse model of MI involving permanent coronary artery ligation, chronic administration of a novel, highly selective A2BAR antagonist, GS-6201, significantly reduced cardiac enlargement and dysfunction compared to vehicle-treated mice (Toldo et al., 2012). Similarly in an in vivo rat myocardial ischaemia-reperfusion model, GS-6201 improved ejection fraction and decreased fibrosis in the non-infarct and border zones with the greatest effect observed when GS-6201 was given 1 week rather 1 day after MI (Zhang et al., 2014). A pro-fibrotic role for the A2BAR has been supported by a study in A2BAR knock-out (A2BAR-/-) mice that demonstrate the A2BAR contributes to post-infarction heart failure (Maas et al., 2008). A2BAR-/- mice had improved end diastolic pressure and reduced interstitial fibrosis when compared to wild-type mice 8 weeks after permanent left coronary ligation. Systolic blood pressure and infarct size remained the same between knock-out and wild-type animals suggesting the A2BAR contributes to heart failure pathology via post-infarction remodeling and reactive fibrosis rather than acute cardioprotection (Maas et al., 2008). The mechanism underlying the pro-fibrotic activity of the A2BAR may involve the pro-inflammatory effects mediated by this AR subtype. Blockade of the A2BAR inhibits caspase-1 activity and leukocyte infiltrate (Toldo et al., 2012), and attenuates secretion of pro-fibrotic and pro-inflammatory mediators such as TGFβ, tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) post-MI via a PKC-δ pathway (Figure 1) (Feng et al., 2009; Toldo et al., 2012; Zhang et al., 2014). A pro-inflammatory role of the A2BAR is reported by studies in other organ systems, in particular the lung where elevated adenosine concentrations and A2BAR activity promotes chronic fibrosis and inflammation in asthma and chronic obstructive pulmonary disease (Sun, 2006; Chan and Cronstein, 2009; Zhou et al., 2009; Karmouty-Quintana et al., 2013). Given the inflammatory response is intricately linked to the regulation of tissue fibrosis, it is perhaps unsurprising therefore, that the A2BAR has been implicated as a promoter of cardiac fibrosis in vivo (Ham and Rees, 2008; Kong et al., 2013; Stuart et al., 2016).
A1AR Modulation of Cardiac Fibrosis
The protective role of A1AR activation in cardiac remodeling appears to be largely attributed to the beneficial effects on cardiomyocyte hypertrophy rather than effects on fibrosis (Liao et al., 2003; Sassi et al., 2014; Chuo et al., 2016). A study using a non-selective adenosine analog (CADO) in mice subject to 4 weeks of chronic pressure overload via transverse aortic constriction (TAC), demonstrated reduced myocardial and perivascular fibrosis and hypertrophy compared to saline-treated mice (Liao et al., 2003). Attenuation of myocardial hypertrophy was A1AR-mediated, as the anti-hypertrophic effects were reversed in the presence of an A1AR-selective antagonist. As similar antagonist studies were not reported for measures of cardiac fibrosis (Liao et al., 2003), it cannot be ruled out that the anti-fibrotic effects were mediated by another AR subtype, in particular the A2BAR. However, recent studies using more A1AR-selective agonists do suggest an involvement of the A1AR in cardiac fibrosis. A study of heart failure in dogs demonstrated capadenoson, an A1AR partial agonist, decreased interstitial fibrosis (Sabbah et al., 2013). Similarly, activation of the A1AR with a selective agonist N6-cyclopentyladenosine (CPA), attenuated left ventricular collagen content and markers of fibrosis in response to α1-adrenergic stimulation in vivo (Puhl et al., 2016).
Activation of the A1AR has been recognized as central to the acute cardioprotective actions of adenosine (McIntosh and Lasley, 2012; Headrick et al., 2013). In agreement, overexpression of the A1AR protects mice against acute ischaemic events, with cardiac infarct size markedly reduced in transgenic compared to wild-type animals (Yang et al., 2002). Paradoxically, however, chronic A1AR cardiac overexpression in older mice (20 weeks) has been associated with enhanced baseline cardiac fibrosis and dilated cardiomyopathy (Funakoshi et al., 2006). Additionally, a study investigating myocardial fibrosis secondary to chronic renal failure demonstrated that an A1AR-selective antagonist, SLV320, normalized cardiac collagen I and III content in the hearts of rats that had undergone a nephrectomy (Kalk et al., 2007). These studies may suggest chronic A1AR stimulation reduces the cardiac resistance to non-ischaemic stress and may promote fibrosis, however, the conflicting evidence highlights the need for further studies to fully elucidate the role of this AR subtype in cardiac fibrosis.
A2AAR Modulation of Cardiac Fibrosis
Separating the contribution of A2BAR-mediated fibrotic signaling from that of A2AAR activation has been difficult owing to the paucity of early subtype selective agonists and antagonists. Genetic alteration of the A2AAR demonstrated that cardiac-specific overexpression of the A2AAR in mice was protective against pressure-induced heart failure, attenuating fibrosis and improving cardiac function (Hamad et al., 2012). A more recent study demonstrated high A2AAR expression in mouse cardiac fibroblasts stimulated the accumulation of the anti-fibrotic second messenger cAMP (Sassi et al., 2014), though perhaps to a lesser extent than the A2BAR (Epperson et al., 2009). Combined with the known anti-inflammatory actions of the A2AAR in the heart (Linden, 2001; Haskó et al., 2008), there is certainly valid grounds to suggest that A2AAR signaling would attenuate cardiac fibrosis. However, further work is needed to clarify the exact role of A2AAR, as stimulation of this receptor subtype has also been demonstrated to have pro-fibrotic effects in other organs such as the liver and skin (Chan et al., 2006a,b; Perez-Aso et al., 2014).
A3AR Modulation of Cardiac Fibrosis
Comparatively few studies have investigated the role of the A3AR in cardiac fibrosis, which is unsurprising given early studies examining the A3AR (and A1AR) expressed on isolated rat cardiac fibroblasts suggested these receptors to be of lesser functional importance than the A2ARs (Chen et al., 2004). The A3AR was investigated for its involvement in protecting against maladaptive cardiac hypertrophy and fibrosis on the basis that ecto-5′-nucleotidase (CD73; catalyzes the conversion of extracellular AMP to adenosine) deficiency exacerbated myocardial hypertrophy and heart failure in TAC mice (Xu et al., 2008). Contrary to hypothesis, A3AR knock-out mice actually had reduced left ventricular hypertrophy, fibrosis and dysfunction after 5 weeks of TAC compared to wild-type animals. There was no effect of A3AR deletion on parameters in the unstressed heart, suggesting the A3AR has a deleterious role in cardiac fibrosis only in response to chronic pressure overload (Lu et al., 2008). In agreement, a recent study using a uninephrectomy and high salt-induced model of hypertension in mice, demonstrated that genetic abrogation of the A3AR resulted in significantly less cardiac hypertrophy and fibrosis compared to wild-type animals (Yang et al., 2016). These studies suggest A3AR antagonism may be a valid therapeutic approach to prevent chronic pressure overload-hypertrophy and fibrosis, however, further studies are warranted.
Conclusion and Future Directions
Cardiac fibrosis is an important determinant of left ventricular dysfunction and remodeling following MI and is a hallmark of heart failure pathology, which is associated with an extremely high rate of mortality (See et al., 2005; Segura et al., 2012). It is therefore crucial to find new therapeutic approaches to prevent and ideally reverse underlying cardiac fibrosis in order to modify the disease progression of heart failure. Purinergic signaling downstream of AR activation represents one such novel strategy to influence fibrosis homeostasis, however, much work is still needed to clarify the exact role of the receptor subtypes involved. A central question that remains is how the same receptor subtype can have both pro- and anti-fibrotic activity. The opposing effects as outlined in this review, may reflect differences in underlying disease pathology due to the type and duration of cardiac insult; whereby AR activation appears to be largely anti-fibrotic in acute ischaemic events but potentially pro-fibrotic under conditions of chronic myocardial stress. This supposition is supported by studies of adenosine’s involvement in fibrosis of other organ systems (Karmouty-Quintana et al., 2013). In the lung, A2BAR stimulation is protective in acute-bleomycin-induced lung injury but actually promotes fibrosis in chronic models of lung disease (Zhou et al., 2009, 2011). Similarly in the kidney, A2BAR activation is beneficial in attenuating acute kidney injury (Grenz et al., 2012) but prolonged A2BAR signaling increases interstitial fibrosis and collagen deposition in renal tissue (Roberts et al., 2014a,b). The exact mechanism behind these paradoxical effects requires further elucidation, but may reflect changes in differential receptor coupling with changes in cellular background as the disease progresses. Certainly, this idea is readily foreseeable for the A2BAR with its high degree of plasticity and ability to couple to multiple G proteins and intracellular signaling cascades (Figure 1) (Cohen et al., 2010). In addition, it should be noted a great deal of our understanding of adenosine’s role in cardiac fibrosis, in particular downstream of A2BAR, has come from in vitro studies. This may not reflect the true course of disease progression in vivo due to the exclusion of the inflammatory response and loss of organ complexity including cross-talk with other cell types. Therefore, while AR signaling appears to be a promising target in cardiac fibrosis, further studies are needed to fully appreciate the potential of AR therapeutics in heart failure and underlying fibrosis.
Author Contributions
EV drafted the manuscript. PW and LM made substantial contribution to the writing. EV, PW, and LM provided critical revision of the manuscript and approved it for publication.
Funding
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (Project Grant ID APP1084487) and the Australian Research Council (ARC; ID DE130100117). EAV holds an Australian Government Research Training Program Scholarship and an Australian Cancer Therapeutics scholarship.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Asakura, M., Asanuma, H., Kim, J., Liao, Y., Nakamaru, K., Fujita, M., et al. (2007). Impact of adenosine receptor signaling and metabolism on pathophysiology in patients with chronic heart failure. Hypertens. Res. 30, 781–787. doi: 10.1291/hypres.30.781
Auchampach, J. A., and Bolli, R. (1999). Adenosine receptor subtypes in the heart: therapeutic opportunities and challenges. Am. J. Physiol. 276, H1113–H1116.
Camelliti, P., Green, C. R., LeGrice, I., and Kohl, P. (2004). Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ. Res. 94, 828–835. doi: 10.1161/01.RES.0000122382.19400.14
Chan, E. S. L., and Cronstein, B. N. (2009). Adenosine in fibrosis. Mod. Rheumatol. 20, 114–122. doi: 10.1007/s10165-009-0251-4
Chan, E. S. L., Fernandez, P., Merchant, A. A., Montesinos, M. C., Trzaska, S., Desai, A., et al. (2006a). Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 54, 2632–2642. doi: 10.1002/art.21974
Chan, E. S. L., Montesinos, M. C., Fernandez, P., Desai, A., Delano, D. L., Yee, H., et al. (2006b). Adenosine A2A receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 148, 1144–1155. doi: 10.1038/sj.bjp.0706812
Chen, W., and Frangogiannis, N. G. (2013). Fibroblasts in post-infarction inflammation and cardiac repair. Biochim. Biophys. Acta 1833, 945–953. doi: 10.1016/j.bbamcr.2012.08.023
Chen, Y., Epperson, S., Makhsudova, L., Ito, B., Suarez, J., Dillmann, W., et al. (2004). Functional effects of enhancing or silencing adenosine A2B receptors in cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 287, H2478–H2486. doi: 10.1152/ajpheart.00217.2004
Chuo, C. H., Devine, S. M., Scammells, P. J., Krum, H., Christopoulos, A., May, L. T., et al. (2016). VCP746, a novel A1 adenosine receptor biased agonist, reduces hypertrophy in a rat neonatal cardiac myocyte model. Clin. Exp. Pharmacol. Physiol. 43, 976–982. doi: 10.1111/1440-1681.12616
Cohen, M. V., Yang, X., and Downey, J. M. (2010). A2B adenosine receptors can change their spots. Br. J. Pharmacol. 159, 1595–1597. doi: 10.1111/j.1476-5381.2010.00668.x
Cronstein, B. N. (2011). Adenosine receptors and fibrosis: a translational review. F1000 Biol. Rep. 3:21. doi: 10.3410/B3-21
Dubey, R. K., Gillespie, D. G., and Jackson, E. K. (1998). Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension 31, 943–948. doi: 10.1161/01.HYP.31.4.943
Dubey, R. K., Gillespie, D. G., Mi, Z., and Jackson, E. K. (1997). Exogenous and endogenous adenosine inhibits fetal calf serum–induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation 96, 2656–2666. doi: 10.1161/01.CIR.96.8.2656
Dubey, R. K., Gillespie, D. G., Mi, Z., and Jackson, E. K. (2001a). Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension 37, 1095–1100. doi: 10.1161/01.HYP.37.4.1095
Dubey, R. K., Gillespie, D. G., Zacharia, L. C., Mi, Z., and Jackson, E. K. (2001b). A2B receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension 37, 716–721. doi: 10.1161/01.HYP.37.2.716
Epperson, S. A., Brunton, L. L., Ramirez-Sanchez, I., and Villarreal, F. (2009). Adenosine receptors and second messenger signaling pathways in rat cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 296, C1171–C1177. doi: 10.1152/ajpcell.00290.2008
Feng, W., Song, Y., Chen, C., Lu, Z. Z., and Zhang, Y. (2009). Stimulation of adenosine A2B receptors induces interleukin-6 secretion in cardiac fibroblasts via the PKC-δ-P38 signalling pathway. Br. J. Pharmacol. 159, 1598–1607. doi: 10.1111/j.1476-5381.2009.00558.x
Ferrari, D., Gambari, R., Idzko, M., Muller, T., Albanesi, C., Pastore, S., et al. (2016). Purinergic signaling in scarring. FASEB J. 30, 3–12. doi: 10.1096/fj.15-274563
Forman, M. B., Stone, G. W., and Jackson, E. K. (2006). Role of adenosine as adjunctive therapy in acute myocardial infarction. Cardiovasc. Drug Rev. 24, 116–147. doi: 10.1111/j.1527-3466.2006.00116.x
Fredholm, B. B., IJzerman, A. P., Jacobson, K. A., Klotz, K. N., and Linden, J. (2001). International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53, 527–552.
Funakoshi, H., Chan, T. O., Good, J. C., Libonati, J. R., Piuhola, J., Chen, X., et al. (2006). Regulated overexpression of the A1-adenosine receptor in mice results in adverse but reversible changes in cardiac morphology and function. Circulation 114, 2240–2250. doi: 10.1161/CIRCULATIONAHA.106.620211
Grenz, A., Kim, J. H., Bauerle, J. D., Tak, E., Eltzschig, H. K., and Clambey, E. T. (2012). Adora2B adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF-α release. J. Immunol. 189, 4566–4573. doi: 10.4049/jimmunol.1201651
Ham, J., and Rees, D. A. (2008). The adenosine A2B receptor: its role in inflammation. Endocr. Metab. Immune Disord. Drug Targets 8, 244–254. doi: 10.2174/187153008786848303
Hamad, E. A., Zhu, W., Chan, T. O., Myers, V., Gao, E., Li, X., et al. (2012). Cardioprotection of controlled and cardiac-specific over-expression of A2A-adenosine receptor in the pressure overload. PLoS ONE 7:e39919. doi: 10.1371/journal.pone.0039919.t002
Haskó, G., Linden, J., Cronstein, B., and Pacher, P. (2008). Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7, 759–770. doi: 10.1038/nrd2638
Headrick, J. P., Ashton, K. J., Rose’Meyer, R. B., and Peart, J. N. (2013). Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacol. Ther. 140, 92–111. doi: 10.1016/j.pharmthera.2013.06.002
Kalk, P., Eggert, B., Relle, K., Godes, M., Heiden, S., Sharkovska, Y., et al. (2007). The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br. J. Pharmacol. 151, 1025–1032. doi: 10.1038/sj.bjp.0707319
Karmouty-Quintana, H., Xia, Y., and Blackburn, M. R. (2013). Adenosine signaling during acute and chronic disease states. J Mol. Med. 91, 173–181. doi: 10.1007/s00109-013-0997-1
Kong, P., Christia, P., and Frangogiannis, N. G. (2013). The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 71, 549–574. doi: 10.1007/s00018-013-1349-6
Kopecky, S. L., Aviles, R. J., Bell, M. R., Lobl, J. K., Tipping, D., Frommell, G., et al. (2003). A randomized, double-blinded, placebo-controlled, dose-ranging study measuring the effect of an adenosine agonist on infarct size reduction in patients undergoing primary percutaneous transluminal coronary angioplasty: the ADMIRE (AmP579 delivery for myocardial infarction REduction) study. Am. Heart J. 146, 146–152. doi: 10.1016/S0002-8703(03)00172-8
Leask, A. (2007). TGFβ, cardiac fibroblasts, and the fibrotic response. Cardiovasc. Res. 74, 207–212. doi: 10.1016/j.cardiores.2006.07.012
Liao, Y., Liao, Y., Takashima, S., Asano, Y., Asakura, M., Ogai, A., et al. (2003). Activation of adenosine A1 receptor attenuates cardiac hypertrophy and prevents heart failure in murine left ventricular pressure-overload model. Circ. Res. 93, 759–766. doi: 10.1161/01.RES.0000094744.88220.62
Linden, J. (2001). Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 41, 775–787. doi: 10.1146/annurev.pharmtox.41.1.775
Linden, J., Thai, T., Figler, H., Jin, X., and Robeva, A. S. (1999). Characterization of human A2B adenosine receptors: radioligand binding, western blotting, and coupling to Gq in human embryonic kidney 293 cells and HMC-1 mast cells. Mol. Pharmacol. 56, 705–713.
Lu, D., Aroonsakool, N., Yokoyama, U., Patel, H. H., and Insel, P. A. (2013). Increase in cellular cyclic AMP concentrations reverses the profibrogenic phenotype of cardiac myofibroblasts: a novel therapeutic approach for cardiac fibrosis. Mol. Pharmacol. 84, 787–793. doi: 10.1124/mol.113.087742
Lu, D., and Insel, P. A. (2014). Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis. Am. J. Physiol. Cell Physiol. 306, C779–C788. doi: 10.1152/ajpcell.00381.2013
Lu, Z., Fassett, J., Xu, X., Hu, X., Zhu, G., French, J., et al. (2008). Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle. Circulation 118, 1713–1721. doi: 10.1161/CIRCULATIONAHA.108.788307
Maas, J. E., Koupenova, M., Ravid, K., and Auchampach, J. A. (2008). Abstract 4831: the A2B adenosine receptor contributes to post-infarction heart failure. Circulation 118, S_946.
McIntosh, V. J., and Lasley, R. D. (2012). Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant? J. Cardiovasc. Pharmacol. Ther. 17, 21–33. doi: 10.1177/1074248410396877
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2016). Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation 133, e38–360. doi: 10.1161/CIR.0000000000000350
Mundell, S. J., and Benovic, J. L. (2000). Selective regulation of endogenous G protein-coupled receptors by arrestins in HEK293 cells. J. Biol. Chem. 275, 12900–12908. doi: 10.1074/jbc.275.17.12900
Newby, A. C. (1984). Adenosine and the concept of “retaliatory metabolites.”. Trends Biochem. Sci. 9, 42–44. doi: 10.1016/0968-0004(84)90176-2
Novitskaya, T., Chepurko, E., Covarrubias, R., Novitskiy, S., Ryzhov, S. V., Feoktistov, I., et al. (2016). Extracellular nucleotide regulation and signaling in cardiac fibrosis. J. Mol. Cell. Cardiol. 93, 47–56. doi: 10.1016/j.yjmcc.2016.02.010
Paolocci, N., Tavazzi, B., Biondi, R., Gluzband, Y. A., Amorini, A. M., Tocchetti, C. G., et al. (2006). Metalloproteinase inhibitor counters high-energy phosphate depletion and AMP deaminase activity enhancing ventricular diastolic compliance in subacute heart failure. J. Pharmacol. Exp. Ther. 317, 506–513. doi: 10.1124/jpet.105.099168
Peart, J. N., and Headrick, J. P. (2007). Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol. Ther. 114, 208–221. doi: 10.1016/j.pharmthera.2007.02.004
Perez-Aso, M., Fernandez, P., Mediero, A., Chan, E. S., and Cronstein, B. N. (2014). Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 28, 802–812. doi: 10.1096/fj.13-241646
Petrov, V. V., Fagard, R. H., and Lijnen, P. J. (2002). Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 39, 258–263. doi: 10.1161/hy0202.103268
Porter, K. E., and Turner, N. A. (2009). Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther. 123, 255–278. doi: 10.1016/j.pharmthera.2009.05.002
Puhl, S.-L., Kazakov, A., Müller, A., Fries, P., Wagner, D. R., Böhm, M., et al. (2016). A1 receptor activation attenuates cardiac hypertrophy and fibrosis in response to α1-adrenergic stimulation in vivo. Br. J. Pharmacol. 173, 88–102. doi: 10.1111/bph.13339
Roberts, V. S., Cowan, P. J., Alexander, S. I., Robson, S. C., and Dwyer, K. M. (2014a). The role of adenosine receptors A2A and A2B signaling in renal fibrosis. Kidney Int. 86, 685–692. doi: 10.1038/ki.2014.244
Roberts, V., Lu, B., Dwyer, K. M., and Cowan, P. J. (2014b). Adenosine receptor expression in the development of renal fibrosis following ischemic injury. Transplant. Proc. 46, 3257–3261. doi: 10.1016/j.transproceed.2014.09.151
Ross, A. M., Gibbons, R. J., Stone, G. W., Kloner, R. A., and Alexander, R. W. (2005). A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J. Am. Coll. Cardiol. 45, 1775–1780. doi: 10.1016/j.jacc.2005.02.061
Sabbah, H. N., Gupta, R. C., Kohli, S., Wang, M., Rastogi, S., Zhang, K., et al. (2013). Chronic therapy with a partial adenosine A1-receptor agonist improves left ventricular function and remodeling in dogs with advanced heart failure. Circ. Heart Fail. 6, 563–571. doi: 10.1161/CIRCHEARTFAILURE.112.000208
Sassi, Y., Ahles, A., Truong, D.-J. J., Baqi, Y., Lee, S.-Y., Husse, B., et al. (2014). Cardiac myocyte-secreted cAMP exerts paracrine action via adenosine receptor activation. J. Clin. Invest. 124, 5385–5397. doi: 10.1172/JCI74349DS1
Schnee, J. M., and Hsueh, W. A. (2000). Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc. Res. 46, 264–268. doi: 10.1016/S0008-6363(00)00044-4
See, F., Kompa, A., Martin, J., Lewis, D. A., and Krum, H. (2005). Fibrosis as a therapeutic target post-myocardial infarction. Curr. Pharm. Des. 11, 477–487. doi: 10.2174/1381612053382098
Segura, A. M., Frazier, O. H., and Buja, L. M. (2012). Fibrosis and heart failure. Heart Fail. Rev. 19, 173–185. doi: 10.1007/s10741-012-9365-4
Shyrock, J. C., and Belardinelli, L. (1997). Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am. J. Cardiol. 79, 2–10. doi: 10.1016/S0002-9149(97)00256-7
Souders, C. A., Bowers, S. L. K., and Baudino, T. A. (2009). Cardiac fibroblast: the renaissance cell. Circ. Res. 105, 1164–1176. doi: 10.1161/CIRCRESAHA.109.209809
Stuart, S. D. F., De Jesus, N. M., Lindsey, M. L., and Ripplinger, C. M. (2016). The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J. Mol. Cell. Cardiol. 91, 114–122. doi: 10.1016/j.yjmcc.2015.12.024
Sun, C. X. (2006). Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Invest. 116, 2173–2182. doi: 10.1172/JCI27303
Sun, Y., and Huang, P. (2016). Adenosine A2B receptor: from cell biology to human diseases. Front. Chem. 4:1329. doi: 10.4049/jimmunol.0900515
Swaney, J. S., Roth, D. M., Olson, E. R., Naugle, J. E., Meszaros, J. G., and Insel, P. A. (2005). Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 102, 437–442. doi: 10.1073/pnas.0408704102
Toldo, S., Zhong, H., Mezzaroma, E., Van Tassell, B. W., Kannan, H., Zeng, D., et al. (2012). GS-6201, a selective blocker of the A2B adenosine receptor, attenuates cardiac remodeling after acute myocardial infarction in the mouse. J. Pharmacol. Exp. Ther. 343, 587–595. doi: 10.1124/jpet.111.191288
Vecchio, E. A., Chuo, C. H., Baltos, J.-A., Ford, L., Scammells, P. J., Wang, B. H., et al. (2016). The hybrid molecule, VCP746, is a potent adenosine A2B receptor agonist that stimulates anti-fibrotic signalling. Biochem. Pharmacol. 117, 46–56. doi: 10.1016/j.bcp.2016.08.007
Villarreal, F., Epperson, S. A., Ramirez-Sanchez, I., Yamazaki, K. G., and Brunton, L. L. (2009). Regulation of cardiac fibroblast collagen synthesis by adenosine: roles for Epac and PI3K. Am. J. Physiol. Cell Physiol. 296, C1178–C1184. doi: 10.1152/ajpcell.00291.2008
Wakeno, M., Minamino, T., Seguchi, O., Okazaki, H., Tsukamoto, O., Okada, K. I., et al. (2006). Long-term stimulation of adenosine A2B receptors begun after myocardial infarction prevents cardiac remodeling in rats. Circulation 114, 1923–1932. doi: 10.1161/CIRCULATIONAHA.106.630087
Xu, X., Fassett, J., Hu, X., Zhu, G., Lu, Z., Li, Y., et al. (2008). Ecto-5′-nucleotidase deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction. Hypertension 51, 1557–1564. doi: 10.1161/HYPERTENSIONAHA.108.110833
Yang, T., Zollbrecht, C., Winerdal, M. E., Zhuge, Z., Zhang, X. M., Terrando, N., et al. (2016). Genetic abrogation of adenosine A3 receptor prevents uninephrectomy and high salt–induced hypertension. J. Am. Heart Assoc. 5, e003868. doi: 10.1161/JAHA.116.003868
Yang, Z., Cerniway, R. J., Byford, A. M., Berr, S. S., French, B. A., and Matherne, G. P. (2002). Cardiac overexpression of A1-adenosine receptor protects intact mice against myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 282, H949–H955. doi: 10.1152/ajpheart.00741.2001
Zhang, H., Zhong, H., Everett, T. H., Wilson, E., Chang, R., Zeng, D., et al. (2014). Blockade of A2B adenosine receptor reduces left ventricular dysfunction and ventricular arrhythmias 1 week after myocardial infarction in the rat model. Heart Rhythm 11, 101–109. doi: 10.1016/j.hrthm.2013.10.023
Zhou, Y., Schneider, D. J., and Blackburn, M. R. (2009). Adenosine signaling and the regulation of chronic lung disease. Pharmacol. Ther. 123, 105–116. doi: 10.1016/j.pharmthera.2009.04.003
Keywords: adenosine, adenosine A2B receptor, cardiac fibrosis, fibroblast, collagen synthesis, cAMP, myocardial infarction, heart failure
Citation: Vecchio EA, White PJ and May LT (2017) Targeting Adenosine Receptors for the Treatment of Cardiac Fibrosis. Front. Pharmacol. 8:243. doi: 10.3389/fphar.2017.00243
Received: 28 February 2017; Accepted: 18 April 2017;
Published: 05 May 2017.
Edited by:
Tim David Hewitson, Royal Melbourne Hospital, AustraliaReviewed by:
Jason N. Peart, Griffith University, AustraliaNazareno Paolocci, Johns Hopkins University, USA
Copyright © 2017 Vecchio, White and May. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren T. May, bGF1cmVuLm1heUBtb25hc2guZWR1 Paul J. White, cGF1bC53aGl0ZUBtb25hc2guZWR1
 Elizabeth A. Vecchio
Elizabeth A. Vecchio Paul J. White
Paul J. White Lauren T. May
Lauren T. May