- 1Research Cluster Biodiversity and Medicines, UCL School of Pharmacy, London, UK
- 2Royal Botanic Gardens, Richmond, UK
Species of Asarum are used in traditional Chinese medicine and, similar to members of the genus Aristolochia, they contain aristolochic acid analogs (AAAs). These compounds are known for their nephrotoxic and carcinogenic effects. So far, the phytochemistry and nephrotoxicity of species of Asarum is not well studied. A high-resolution LC-MS-based metabolomic approach was used to study the phytochemical variation in medicinally used Asarum species. The cytotoxicity of the samples was assessed using human kidney (HK-2) cells. The majority of samples contained potentially nephrotoxic AAAs, including 9-methoxy aristolactam (AL) IV, AL I, and AL IV. These compounds were present in methanol as well as water extracts. AAAs were detected in all parts of the plant. The majority of the extracts were not cytotoxic to HK-2 cells at the doses tested. However, other mechanisms relating to aristolochic acid nephropathy and cancer development, such as DNA adduct formation may occur. The results of this study provide a model for assessing lesser-known plant species for toxicity.
Introduction
Species of the genus Asarum are used as herbal medicines in many parts of the world, including Europe and Asia. The Chinese Pharmacopeia lists the roots and rhizomes of Asarum heteropoides f. mandshuricum (Maxim.) Kitag, and Asarum sieboldii Miq. under the Pin Yin name Xixin (Achenbach and Fischer, 1997). In Europe Asarum europaeum L. is used in homeopathic tinctures (Nitzsche et al., 2013) and in Canada and the USA Asarum canadense L. was used by Native Americans (Moermon, 2017).
Like the related genus Aristolochia (which is also listed in the Chinese Pharmacopeia), Asarum contain aristolochic acids and aristolactams (Mix et al., 1982; Kumar et al., 2003). These nitrophenanthrene derivates have nephrotoxic and carcinogenic effects (Michl et al., 2014). Species of Aristolochia have become a key concern in healthcare as they are associated with aristolochic acid nephropathy (AAN), a renal fibrosis often associated with upper urothelial cancer (UUC; Chen et al., 2012). It is estimated that in China alone 100 million people may be at risk of developing AAN (Hu et al., 2004; Grollman, 2013).
Species of Asarum are generally considered to be less toxic than species of Aristolochia. However, a few cases of Asarum-related AAN have been reported. In one case report a male patient displayed subacute renal failure after ingesting a herbal powder containing Xixin (Yang et al., 2006). A case of acute poisoning due to the intake of A. europaeum has been reported in Switzerland (Jaspersen-Schib et al., 1996). Surprisingly, only eight cases of Asarum-related AAN have been reported in the last 45 years (Kim et al., 2013). Like Aristolochia-related AAN, it is likely that health practitioners failed to identify the link between nephropathy or tumor development and the exposure to these plants.
Aristolochic acid I (AA I) and aristolochic acid II (AA II) are considered to be the cause of these nephrotoxic and carcinogenic effects (Nortier et al., 2000; Balachandran et al., 2005; Jelakovic et al., 2012). After reductive metabolic activation into aristolactams (ALs), AA I and AA II form DNA adducts, which were found in renal tissues of patients. A large number of in vitro and in vivo studies showed that AA I and AA II are toxic (Mengs, 1988; Arlt et al., 2011; Yang et al., 2011; Michl et al., 2014). However, they are not necessarily the only (or most potent) toxins present in Aristolochia and related genera (Michl et al., 2016). At least 178 aristolochic acid analogs (AAAs) exist, many of which are aristolactams. It is unclear whether these compounds are also able to form DNA adducts. Their possible implications in AAN may have been overlooked (Michl et al., 2014). Apart from AA I and AA II, other compounds may contribute to processes that lead to renal damage (Li et al., 2004; Wen et al., 2006) and carcinogenesis.
Species of Asarum generally contain lower amounts of AA I and AA II than Aristolochia species (Hashimoto et al., 1999; Chan et al., 2003, 2006; Yuan et al., 2007). Yet, high amounts of AA I (3376.9 ng/mg) were reported in Asarum crispulatum C.Y. Cheng and C.S. Yang (Jong et al., 2003). According to Zhao et al. (2008) aerial parts of Xixin herbs contained higher levels of AA I than the roots. Methanol extracts typically contained more AA I than water extracts. A second study by Hsu et al. (2009) found that the amounts of AA I in leaves were the highest followed by petioles, rhizomes and roots.
While a number of studies assessed the amounts of AA I and AA II in Asarum spp., little is known about the effects of the entire (small molecule) metabolome and specifically other AAAs. For example, although other compounds, such as AL I are often found in higher amounts in Asarum than in Aristolochia (Yuan et al., 2008), the Chinese Pharmacopoeia still lists roots and rhizomes of Asarum for medicinal use. Furthermore, only the decoction of the root portion is recommended for usage. However, it is questionable as to whether current recommendations for the medicinal uses of Asarum species are justified.
The aim of this work is to assess the metabolomic profile and in vitro toxicity of medicinally used species of Asarum and to evaluate whether current recommendations for their use are appropriate. Therefore, we utilized a systems biology approach to establish the full range of AAAs in a series of Asarum species. We carried out a LC-MS-based metabolomic study to compare the secondary metabolites of Asarum samples originating from different species, different plant parts, as well as obtained through different extraction techniques. We assessed the cytotoxicity of these extracts in HK-2 kidney cells and studied the relationship between the plants' metabolic profiles and their in vitro toxicity using statistical approaches. In a wider context, the current work can be used as a model for assessing toxicity of medicinal plant species, and for elucidating bioactive principles of medicinal plants.
Materials and Methods
Plant Material
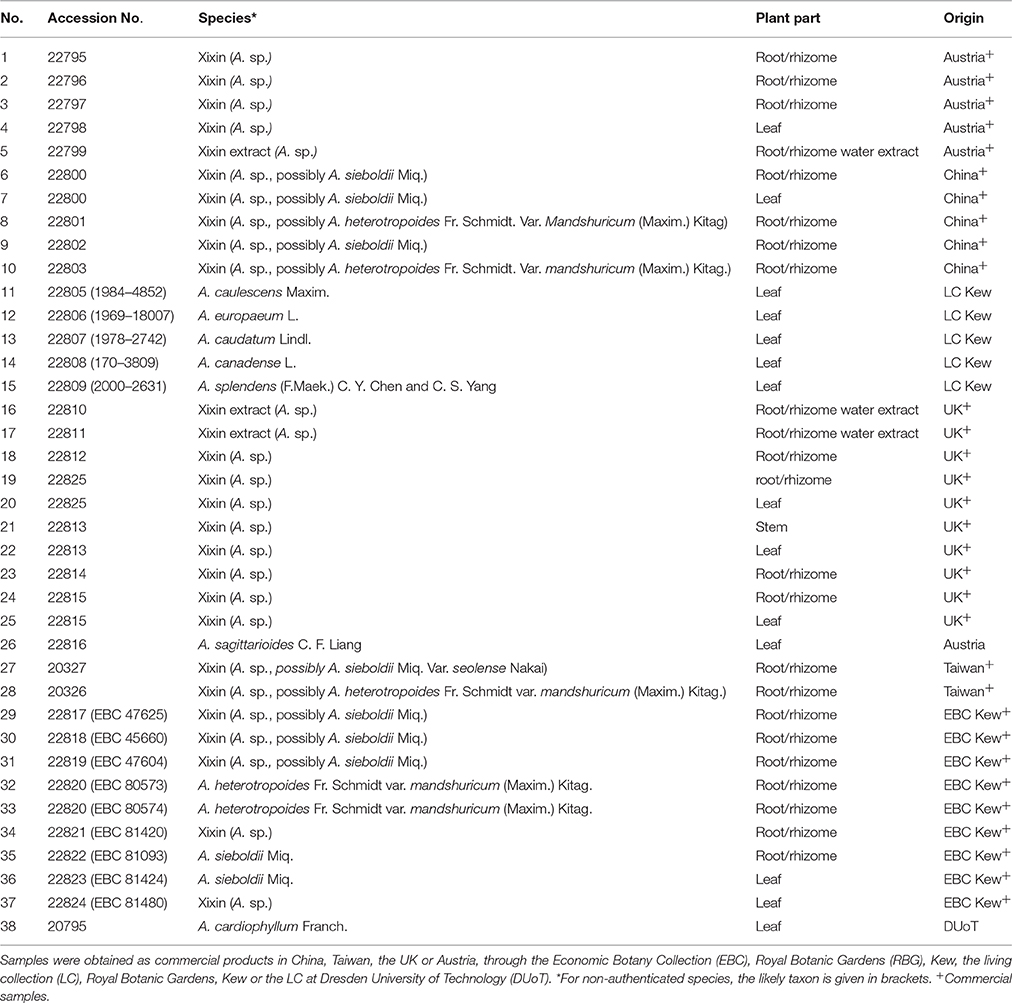
Asarum samples were mainly obtained from commercial sources in the UK, China, Taiwan or Austria (Table 1). Additional samples were obtained from the living collection at the Royal Botanic Gardens (RBG), Kew, UK and at the botanical garden at Dresden University of Technology (DUoT), Dresden, Germany. Further plant material from the Economic Botany Collection and the Chinese Medicinal Plants Authentication Centre (CMPAC) at RBG, Kew, UK was included in the analysis. When possible, plant material was identified to the species level. However, if no flowering parts were present in the sample, the species name under which the sample was traded is listed in brackets in Table 1. Fresh plant material from RBG Kew and DUoT was freeze-dried and stored at room temperature until further analysis. All other samples were stored at room temperature.
Chemicals
The chemicals used for the LC-DAD-ESI-MS analysis were methanol, acetonitrile, formic acid (all obtained from Thermo Fisher Scientific, MA, USA). Reference standards for AA I and AA II were isolated from Aristolochia repens Mill. AA C was purchased from Phytolab (Germany). AL I was isolated from A. sieboldii Miq. The purities of all reference standards were determined using HPLC.
Extraction of the Plant Material
The dried plant material was ground in a mortar and for each sample 50 mg of dried plant material was extracted with 1 mL of 70% aqueous methanol in an 1.5 mL Eppendorf microtube. After about 14 h extraction at room temperature the extracts were centrifuged at 6,600 relative centrifugal force (RCF) for 10 min. The supernatant liquid was filtered using Whatman 0.45 μm polytetrafluoroethylene (PTFE) filters and 700 μL of the sample solution were used for LC-DAD-ESI-MS measurements. The samples 3, 8, and 35 were extracted and analyzed in triplicate to demonstrate repeatability of the extraction method.
LC-DAD-ESI-MS Analysis
The analysis was carried out using a Thermo Scientific HRAM LC-MS system (Thermo Scientific, MA, USA) consisting of an “Accela” liquid chromatograph with diode array detector and an “LTQ-Orbitrap XL” hybrid linear ion trap-orbitrap mass spectrometer. Chromatography was performed on a 150 × 3 mm, 3 μm Phenomenex Luna C18 column using a 400 μL/min flow rate. The separation was obtained using the following linear mobile phase gradient: 0 min: 90% A, 0% B, 10% C; 50 min: 0% A, 90% B, 10% C, 55 min: 0% A, 90% B, 10% C, 57 min: 90% A, 0% B, 10% C, 60 min 90% A, 0% B, 10% C (A: water, B: methanol, C: acetontirile containing 1% formic acid). The injection volume was 5 μL. Ammonia solution was added post column at a flow rate of 0.1 μL/min. The mass spectrometer was fitted with an electrospray source (Thermo “Ion Max”) operated in positive mode at a source voltage of 3.5 kV using sheath and auxiliary nitrogen flow rates of 60 and 20 units, respectively, and a capillary temperature of 300°C. High resolution MS1 scanning was performed in the orbitrap (m/z 250–2000; resolution 30,000). Low resolution data dependent MS2 scans were undertaken in the linear ion trap (isolation width 4 m/z units; collision energy 35%).
Data Reduction and Multivariate Data Analysis
Filtering, peak extraction, chromatogram de-convolution and peak alignment of the high-resolution LC-MS data were performed automatically using Mzmine 2 (http://mzmine.sourceforge.net). Peaks between 0 and 50 min retention time with a minimum peak intensity of 50,000 and m/z between 200 and 650 were extracted. Only the 1,000 metabolites with the highest average peak areas across all samples were used for further data analysis. This process resulted in a data set consisting of 38 observations (samples) and 1000 variables (metabolites). Before PCA, the resulting data set was normalized to the total raw signal. PCA and OPLS-DA was carried out on the normalized and pareto scaled dataset using the software SIMCA P+ (v. 12, Umetrics, Umea, Sweden).
Metabolite Identification Assignment
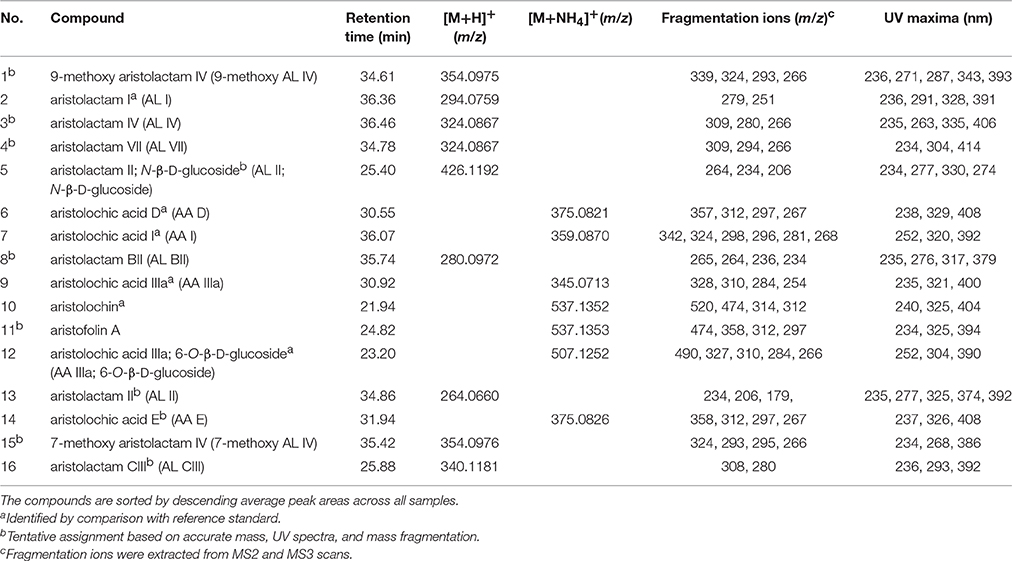
For the peaks extracted by Mzmine 2, the ion species (i.e., adducts) were determined so as to obtain an experimental accurate molecular mass. This was then searched against the CRC Dictionary of Natural Products to identify candidate compounds having molecular masses within 5 ppm of the experimental value. AA I, AA II, AA III, AA IIIa, AA IV, AA D, AA IIIa-6-β-D-glucoside, aristolochin, and AL I were identified by comparison to reference standards. Further, compounds were identified by comparing accurate mass data to an in-house database (UCL School of Pharmacy, London, UK) consisting of 178 AAAs, which have previously been isolated from natural sources. Putative assignments for those AAAs were made based on their accurate mass, retention time, mass fragmentation, and UV spectra.
Cell Culture Conditions
HK-2 cells (American Type Culture Collection, Manassas, VA, USA) were maintained in Dulbecco's modified eagle medium (DMEM, Gibco Invitrogen) supplemented with 10% fetal bovine serum (FBS, Gibco Invitrogen), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco Invitrogen). All cells were cultured in a humidified atmosphere with 5% CO2 at 37°C. For routine cultivation, cells were maintained up to 80% of confluency and the medium was replaced every 3 days.
Sulphorhodamine B (SRB) Assay
Cytotoxicity assessment of the plant extracts was carried out using the SRB assay (Houghton et al., 2007). HK-2 cells (104 cells/well) were plated in 96-well plates overnight. Afterwards the medium was replaced by medium containing the plant extracts dissolved in dimethyl sulfoxide (DMSO, VWR, Poole, UK). The cells were treated with plant extracts for 72 h at concentrations of 0, 12.5, 25, 50 100, and 200 μg/mL. The final concentration of DMSO in the medium did not exceed 1% v/v. Afterwards the cells were fixed with 40% trichloroacetic acid (TCA, Sigma, St. Louis, MO, USA) for at least 1 h. The fixed cells were washed with deionized water and stained with 0.4% SRB (Sigma, St. Louis, MO, USA) in 1% acetic acid (Fisher Scientific, Loughborough, UK) for 30 min. Afterwards the cells were washed with 1% acetic acid to remove excess color. Plates were dried and then dissolved in 30 mM Tris base (Sigma, St. Louis, MO, USA). Cell growth was quantitated based on the amount of SRB measured by a spectrophotometer (Tecan Infinite M200 plate reader, Tecan, Maennedorf, Switzerland) at 490 nm. Three independent experiments were carried out, each carried out in technical triplicates. IC50 values were obtained using the curve fit through non-linear regression function in GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). Colchicine (10 μg/mL, Sigma, St. Louis, MO, USA) was used as a positive control to ensure the performance of the assay.
Results
Phytochemical Variation in Xixin Samples Using Non-targeted LD-DAD-ESI-MS
We obtained chromatographic information from the non-targeted metabolomic analysis of 38 Asarum samples (Table 1) in positive-ionization mode of high resolution accurate mass LC-MS (HRAM LC-MS). We preprocessed the information to extract chromatographic features. Matching features from different samples were aligned. Normalization and pareto scaling resulted in the final data set for subsequent statistical analysis.
To study the variability within the data set, principal component analysis (PCA) was carried out. A 2-component PCA model explained 23.6% of the total variance. The PCA scores plot in Figure 1 shows a clear separation between Asarum leaf and root material. However, different modes of preparation (methanolic vs. water extracts) were not differentiated. All leaf samples, as well as sample 21 [Xixin, A. sp. (stem)] were clearly separated (left hand side of the plot) from the root samples. This included the commercial root extracts, which are generally extracted using water decoction (right hand side of the plot). Thus, the metabolic profile of Asarum extracts is largely dependent on the part of the plant used, rather than genetic variability (species) or extraction method. Samples 11–14 originate from leaves of different species of Asarum (A. caulescens, A. europaeum, A. caudatum, and A. canadense) and have similar metabolite profiles. Since many samples used in traditional Chinese medicine are only available in the form of botanical drugs, these were not identified to species level (Table 1). However, since most of these samples were traded under the Pin Yin name Xixin, it is likely that they originate from either A. sieboldii or A. heterotropoides. From the PCA scores plot, it is evident that the phytochemistries of A. sieboldii and A. heterotropoides are similar (Figure 1). For example, samples 33 and 35 (A. heterotropoides (root) and A. sieboldii (root), respectively) are located in close proximity in the scores plot.
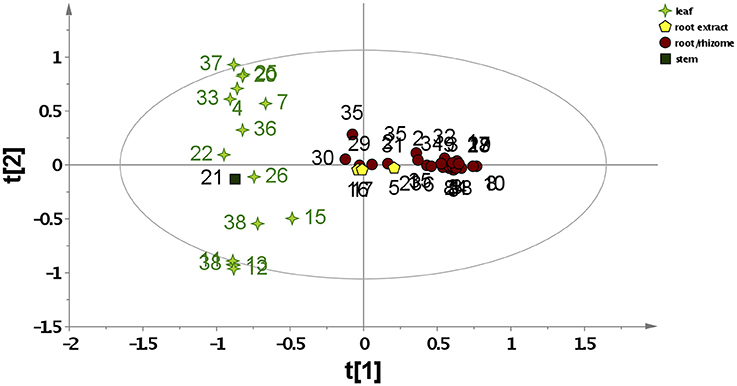
Figure 1. PCA scores plots based on LC-MS data for all Asarum samples. The samples are labeled according to their sample number (Table 1) and different symbols were assigned for different plant parts or extraction methods (data normalized to the total raw signal, mean-centered, and pareto scaled).
The PCA loadings plots were used to identify metabolites responsible for the differentiation in the PCA analysis (Figure S1). Samples with positive PC1 scores (such as the majority of root/rhizome samples) are characterized by a major metabolite giving m/z at 248.2012 (tR = 40.63 min), assigned as a protonated molecule from the presence of a confirmatory sodiated molecule at m/z 270.1831, both of which suggest a molecular formula of C16H25NO (e.g., calc. for C16H26NO+ = m/z 248.2009; 1.2 ppm error). A possible candidate is a mixture of (2E,4E,8Z,10E)-N-isobutyl-2,4,8,10-dodecatetraenamide and (2E,4E,8Z,10Z)-N-isobutyl-2,4,8,10-dodecatetraenamide, which was previously isolated from A. sieboldii (Quang et al., 2012; Wen et al., 2014), Asarum heterotropoides (Quang et al., 2012) and Asarum forbesii Maxim (Zhang et al., 2005). These candidates are supported by major fragments in the MS/MS spectrum of the protonated molecule at m/z 175.119 (C12H15O+), 149.1326 (C11) and 142.1227 (C8H16NO+), which can be rationalized against the structures. Samples with negative PC1 scores (such as all leaf samples) contain higher amounts of a compound giving m/z at 277.2164 (tR = 42.76 min). This signal can be assigned to the [M+H]+ ion from a confirmatory [M+NH4]+ ion at m/z 294.2430, both suggesting a molecular formula of C18H28O2 (e.g., calc for C18H29 277.2162; 0.6 ppm error). Possible candidates for this compound are various octadecatetraenoic acids. The differentiation of the Asarum samples by PC2 is more complex (Figure S2). Negative PC2 scores correspond to a metabolite giving a protonated molecule at m/z 597.1815 (tR = 16.45 min). This is likely to be 2′,4,4′,6′-tetrahydroxychalcone-2′,4′-di-O-β-D-glucopyranoside (chalcononaringenin 2′,4′-di-O-glucoside), a flavonoid previously isolated from Asarum canadense L. (Iwashina and Kitajima, 2000). This identification was supported by a match of the MS3 (m/z 597 → 273) spectrum with the MS2 (m/z 273) spectrum of chalcononaringenin an in-house library. Further, supporting this identification is the failure of the sodiated molecule to lose an aglycone moiety following MS/MS (suggesting the sugars were substituted at two positions). In contrast, positive PC2 scores correspond to m/z 277.2164 (tR = 42.76 min; an octadecatetraenoic acid as discussed above) and m/z 295.2269 (tR = 44.21 min; the protonated molecule of a hydroxyoctadecatrienoic acid).
Variation in AAAs
In order to study the variation in AAAs content, targeted metabolite profiling was carried out. Several AAAs (AA I, AL I, AA IIIa, AA D, AA IIIa 6-O-β-D-glucoside, and aristolochin) could be identified by comparison to reference standards. However, tentative assignments based on accurate mass, retention times, UV maxima, and fragmentation ions obtained through LC-DAD-ESI-MS analysis were made for other AAAs (Table 2, Figure 2). Table 2 shows the 16 most commonly occurring AAAs, which could be identified within the set of analyzed samples. 9-Methoxy AA IV (or one of its isomers) was found in the highest average amount across all samples. However, while this compound was found in large amounts in species such as A. cardiophyllum, and A. saggitaroides, it was detected in only a few of the commercial Xixin samples (A. sp. A. sieboldii and A. heterotropoides). Within the analyzed Xixin samples, AL I was the most common aristolochic acid analog. However, a variety of other aristolactams such as AL IV and its isomer AL VII, as well as AL II and N-β-D-glucoside were detected. While AA I was only detected in seven samples (4, 14, 15, 21, 23, 32, and 36), AA II was not detected at all. AA I was found in high amounts in A. canadense and A. splendends. AA I occurred only as a minor compound within Xixin samples, with the average amount being higher in aerial parts compared to roots; but in contrast to previous reports22 these differences were not statistically significant (p = 0.11). On the other hand, the amounts of AL I in Xixin root samples were significantly higher, compared to aerial parts (p = 0.03). This finding has not previously reported, since AL I was only quantified in a few studies. Although AL I is a rather non-polar compound and therefore poorly soluble in water, it was also detected in water based commercial root decoctions. This suggests that AL I can be extracted into water when heat is used during the extraction process, or it could be mediated through the formation of ion-pairs with other substances in the extracts. Although AA I is more water soluble than ALI, AA I was not detected in any of the three root decoction samples.
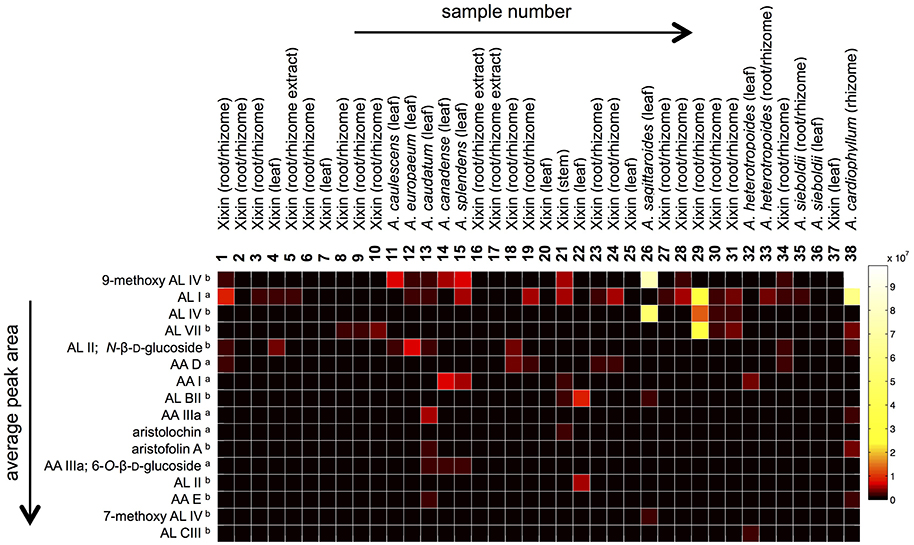
Figure 2. Heat map comparing relative LC-MS peak areas of identified aristolochic acid analogs. Information about the origin of the samples is given in Table 1. The compounds (rows) are sorted by descending average peak areas across all samples (aidentified by comparison with reference standard; bTentative assignment based on accurate mass, UV spectra and mass fragmentation).
In vitro Toxicity of Asarum Extracts
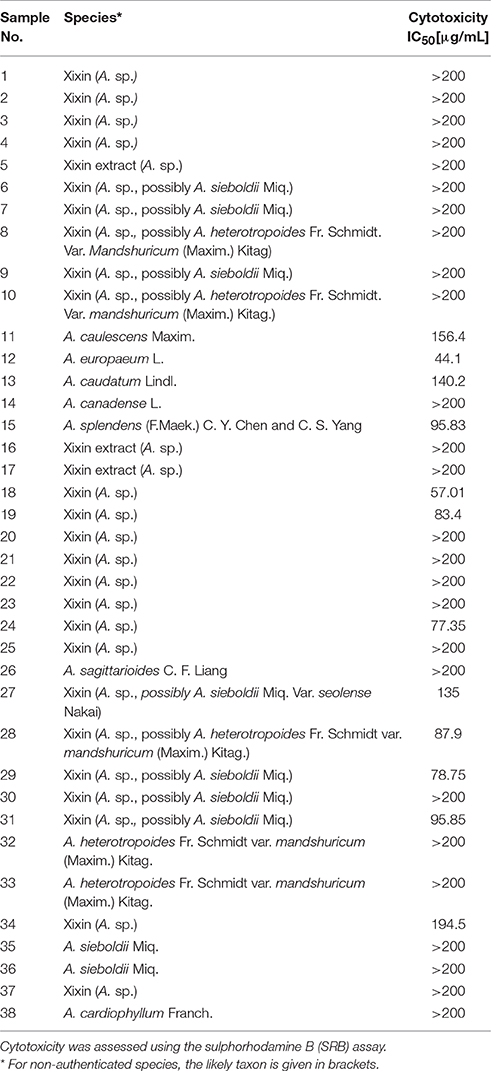
The cytotoxicity of the Asarum samples in HK-2 cells was assessed after treatment for 72 h using the sulphorhodamine B (SRB) assay (Houghton et al., 2007). Several Asarum extracts showed cytotoxic effects in HK-2 cells (Table 3). For example, sample 12 [A. europaeum (leaf)] and sample 18 [Xixin, A. sp. (root)], exhibited IC50 values of 44.10 and 57.01 μg/mL, respectively. This is comparable to the cytotoxicity of species of Aristolochia, which was previously assessed in HK-2 cells. However, the majority of the tested samples did not reduce cell viability to fewer than 50% of the solvent control at a concentration of 200 μg/mL.
The AAAs AA I and AA II are considered to be the main compounds responsible for the nephrotoxic effects of species of Aristolochia and Asarum. In order to investigate, whether there is a link between the amounts of AA I in the samples and their cytotoxicity in HK-2 cells, univariate regression analysis was carried out. However, no correlation was found between AA I peak areas and the extracts IC50 values (R2 = 0.000). This can be explained by the fact that only one of the samples containing AA I [A. splendends (leaf)] exhibited cytotoxicity, while no cytotoxicity was observed for all other samples with doses up to 200 μg/mL.
To elucidate the possible bioactive principle behind the cytotoxic effects of the extracts, an untargeted approach was used, where all detected metabolites were taken into account. Therefore, we used orthogonal projection to latent structures (OPLS) regression analysis. In contrast to univariate regression analysis, where the correlation between only one X and one Y variable is assessed, OPLS is a method for relating two data matrices (X and Y) within a linear multivariate model. In this study, the X matrix consisted of 38 observations (samples) and 1,000 variables (the 1,000 metabolites with the highest average LC-MS peak areas across all samples). However, the Y matrix consisted of only one qualitative variable (0 for non-toxic vs. 1 for toxic extracts). In this case, since the Y matrix consists of discrete values, the method is called OPLS-discriminant analysis (OPLS-DA). The OPLS-DA loadings plots were used to identify which metabolites correlate directly or indirectly, significantly or non-significantly with the Y variable (toxic vs. non-toxic). OPLS-DA showed that toxic samples contain higher amounts of metabolites giving m/z at 274.2167, 276.2325, and 252.2324. The molecular formulas and MS/MS fragmentations of these ions are in accordance with them being the protonated molecules of various alkylamides. Aristolochic acids and ALs were not found to be important discriminators between toxic vs. non-toxic samples.
Discussion
Asarum species have been used as medicinal plants in China and in other parts of the world. However, so far little is known about their content in aristolochic acids and aristolactams or their potential kidney toxicity. A comprehensive metabolomic approach has shown that the majority of Asarum samples contain AAAs considered to be nephrotoxic.
In TCM, plants known to contain toxic compounds are often regarded as safer after preparation of the plant material in a specific way, or only those plant parts known not to contain the toxins are used. Furthermore, monographs on species in national pharmacopeias require time to change and often do not take into account recent advances in the knowledge about the chemistry of the species. This could result in misleading recommendations about the use of some species with potential hazards for public health. The plant genus Asarum is one example of this, especially since its true level of usage is poorly known (e.g., in China and Southern Europe). In addition to changing guidelines in national pharmacopeias, more awareness needs to be raised about potential health risks associated with the use of herbal medicine. This will in time lead to changes in the traditional practices.
Previous reports have shown that levels of aristolochic acids were low in decoction of roots and rhizomes of Herba Asari (Zhao et al., 2008) thus it was retained in the Chinese Pharmacopoeia. However, in this study, no significant difference in the levels of AA I was detected in aerial parts of Asarum spp. compared to root samples. Relative levels of AL I were found to be even higher in root samples compared to aerial parts, and AL I was also detected in root decoctions. Little is known about the toxicity and carcinogenicity of AL I.
Although most Xixin samples included in this study contained potentially nephrotoxic AAAs, some samples did not contain detectable amounts of these compounds. This could indicate that Xixin samples purchased from markets may not originate from Asarum sp., and were either misidentified or replaced with other medicinal plants. The lack of detectable AAAs could also be due to natural variation (genetic, seasonal or ecological) and requires further investigation. Most samples were non-cytotoxic to kidney cells in vitro. Interestingly, no correlation was found between the amounts of AA I and their toxicity. However, other mechanisms relating to aristolochic acid nephropathy, such as DNA adduct formation may occur and deserve further research.
The present study has important implications on whether and how the genus Asarum should be used medicinally in the future. In contrast to previous studies, where only AA I and AA II were taken into account, we detected other AAAs in root and rhizome samples of Asarum. Since little is known about the toxicity of these compounds, we cannot conclude that root and rhizome samples are safe to use. The study also demonstrates that systematic assessment of a group of species' metabolomic profile can provide a basis for a broader assessment of associated risks. Not only are there other compounds than aristolochic acids that need to be taken into consideration, but the entire composition of some of the most active extracts needs to be understood. The study thus serves as a model for assessing closely related species used as traditional medicines. More broadly, the strategy presented here can also be used in identifying new drug leads from medicinal plants (and fungi).
Author Contributions
JM, OB, and GK carried out the experiments. JM and OB obtained sample materials. JM and MH wrote the manuscript. JM, MS, and MH designed the study.
Funding
We thank the Bloomsbury Colleges (University of London) for funding this project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Christine Leon, Mark Nesbitt (Economic Botany Collection, Kew, Richmond, UK) as well as. S. Wanke and Chr. Neinhuis from the Technical University of Dresden (Germany) for providing some of the Asarum samples used in this study. We are also thankful to Tony Booker and Vafa Amirika (UCL School of Pharmacy) and Erich Stöger (Plantasia, Austria) for commercial Xixin samples.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphar.2017.00215/full#supplementary-material
References
Achenbach, H., and Fischer, A. (1997). Aristolochic acids and aristolactams from the seeds of Aristolochia baetica. Planta Med. 63:387. doi: 10.1055/s-2006-957716
Arlt, V. M., Zuo, J., Trenz, K., Roufosse, C. A., Lord, G. M., Nortier, J. L., et al. (2011). Gene expression changes induced by the human carcinogen aristolochic acid I in renal and hepatic tissue of mice. Int. J. Cancer 128, 21–32. doi: 10.1002/ijc.25324
Balachandran, P., Wei, F., Lin, R. C., Khan, I. A., and Pasco, D. S. (2005). Structure activity relationships of aristolochic acid analogues: toxicity in cultured renal epithelial cells. Kidney Int. 67, 1797–1805. doi: 10.1111/j.1523-1755.2005.00277.x
Chan, S. A., Chen, M. J., Liu, T. Y., Fuh, M. R., Deng, J. F., Wu, M. L., et al. (2003). Determination of aristolochic acids in medicinal plant and herbal product by liquid chromatography-electrospray-ion trap mass spectrometry. Talanta 60, 679–685. doi: 10.1016/S0039-9140(03)00142-5
Chan, W., Hui, K. M., Poon, W. T., Lee, K. C., and Cai, Z. (2006). Differentiation of herbs linked to Chinese herb nephropathy from the liquid chromatographic determination of aristolochic acids. Anal. Chim. Acta 576, 112–116. doi: 10.1016/j.aca.2006.03.008
Chen, C. H., Dickman, K. G., Moriya, M., Zavadil, J., Sidorenko, V. S., Edwards, K. L., et al. (2012). Aristolochic acid-associated urothelial cancer in Taiwan. Proc. Natl. Acad. Sci. U.S.A. 109, 8241–8246. doi: 10.1073/pnas.1119920109
Grollman, A. P. (2013). Aristolochic acid nephropathy: harbinger of a global iatrogenic disease. Environ. Mol. Mutagen. 54, 1–7. doi: 10.1002/em.21756
Hashimoto, K., Higuchi, M., Makino, B., Sakakibara, I., Kubo, M., Komatsu, Y., et al. (1999). Quantitative analysis of aristolochic acids, toxic compounds, contained in some medicinal plants. J. Ethnopharmacol. 64, 185–189. doi: 10.1016/S0378-8741(98)00123-8
Houghton, P., Fang, R., Techatanawat, I., Steventon, G., Hylands, P. J., and Lee, C. C. (2007). The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 42, 377–387. doi: 10.1016/j.ymeth.2007.01.003
Hsu, Y.-H., Lo, C.-F., Liu, F.-S., and Lin, J.-H. (2009). Analysis of aristolochic acid in Asarum (Xixin) and its preparations by liquid chromatography/tandem mass spectrometry. J. Food Drug Anal. 17, 274–281. Available online at: http://web.a.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=10219498&AN=46981093&h=s11ZNjpsLF3VcGPa%2bs1QeW3eiJ3G9YvzLRqNrDiYIhyBhKPqiQRBBWdhknJdVzq%2fgHVqwWYj0tXOOR1vGHws4Q%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d10219498%26AN%3d46981093
Hu, S. L., Zhang, H. Q., Chan, K., and Mei, Q. X. (2004). Studies on the toxicity of Aristolochia manshuriensis (Guanmuton). Toxicology 198, 195–201. doi: 10.1016/j.tox.2004.01.026
Iwashina, T., and Kitajima, J. (2000). Chalcone and flavonol glycosides from Asarum canadense (Aristolochiaceae). Phytochemistry 55, 971–974. doi: 10.1016/S0031-9422(00)00216-8
Jaspersen-Schib, R., Theus, L., Guirguis-Oeschger, M., Gossweiler, B., and Meier-Abt, P. J. (1996). Serious plant poisonings in Switzerland 1966-1994. Case analysis from the Swiss toxicology information center. Schweiz. Med. Wochenschr. 126, 1085–1098.
Jelakovic, B., Karanovic, S., Vukovic-Lela, I., Miller, F., Edwards, K. L., Nikolic, J., et al. (2012). Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int. 81, 559–567. doi: 10.1038/ki.2011.371
Jong, T. T., Lee, M. R., Hsiao, S. S., Hsai, J. L., Wu, T. S., Chiang, S. T., et al. (2003). Analysis of aristolochic acid in nine sources of Xixin, a traditional Chinese medicine, by liquid chromatography/atmospheric pressure chemical ionization/tandem mass spectrometry. J. Pharm. Biomed. Anal. 33, 831–837. doi: 10.1016/S0731-7085(03)00310-8
Kim, E. J., Chen, Y., Huang, J. Q., Li, K. M., Razmovski-Naumovski, V., Poon, J., et al. (2013). Evidence-based toxicity evaluation and scheduling of Chinese herbal medicines. J. Ethnopharmacol. 146, 40–61. doi: 10.1016/j.jep.2012.12.027
Kumar, V., Poonam, Prasad, A. K., and Parmar, V. S. (2003). Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat. Prod. Rep. 20, 565–583. doi: 10.1039/b303648k
Li, B., Li, X. M., Zhang, C. Y., Wang, X., and Cai, S. Q. (2004). Cellular mechanism of renal proximal tubular empithelial cell injury induced by aristolochic acid I and aristololactam I. Beijing Da Xue Xue Bao 36, 36–40.
Mengs, U. (1988). Tumour induction in mice following exposure to aristolochic acid. Arch. Toxicol. 61, 504–505. doi: 10.1007/BF00293699
Michl, J., Ingrouille, M. J., Simmonds, M. S., and Heinrich, M. (2014). Naturally occurring aristolochic acid analogues and their toxicities. Nat. Prod. Rep. 31, 676–693. doi: 10.1039/c3np70114j
Michl, J., Kite, G. C., Wanke, S., Zierau, O., Vollmer, G., Neinhuis, C., et al. (2016). LC-MS- and 1H NMR-based metabolomic analysis and in vitro toxicological assessment of 43 Aristolochia species. J. Nat. Prod. 79, 30–37. doi: 10.1021/acs.jnatprod.5b00556
Mix, D. B., Guinaudeau, H., and Shamma, M. (1982). The aristolochic acids and aristolactams. J. Nat. Prod. 45, 657–666. doi: 10.1021/np50024a001
Moermon, D. (2017). Native American Ethnobotany DB. Available online at: http://naeb.brit.org
Nitzsche, D., Melzig, M. F., and Arlt, V. M. (2013). Evaluation of the cytotoxicity and genotoxicity of aristolochic acid I-a component of Aristolochiaceae plant extracts used in homeopathy. Environ. Toxicol. Pharmacol. 35, 325–334. doi: 10.1016/j.etap.2013.01.007
Nortier, J. L., Martinez, M. C., Schmeiser, H. H., Arlt, V. M., Bieler, C. A., Petein, M., et al. (2000). Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). New Engl. J. Med. 342, 1686–1692. doi: 10.1056/NEJM200006083422301
Quang, T. H., Ngan, N. T., Minh, C. V., Kiem, P. V., Tai, B. H., Thao, N. P., et al. (2012). Anti-inflammatory and PPAR transactivational effects of secondary metabolites from the roots of Asarum sieboldii. Bioorg. Med. Chem. Lett. 22, 2527–2533. doi: 10.1016/j.bmcl.2012.01.136
Wen, H., Gao, H. Y., Qi, W., Xiao, F., Wang, L. L., Wang, D., et al. (2014). Simultaneous determination of twenty-two components in Asari Radix et Rhizoma by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Planta Med. 80, 1753–1762. doi: 10.1055/s-0034-1383296
Wen, Y. J., Su, T., Tang, J. W., Zhang, C. Y., Wang, X., Cai, S. Q., et al. (2006). Cytotoxicity of phenanthrenes extracted from Aristolochia contorta in human proximal tubular epithelial cell line. Nephron. Exp. Nephrol. 103, e95–e102. doi: 10.1159/000092194
Yang, H., Dou, Y., Zheng, X., Tan, Y., Cheng, J., Li, L., et al. (2011). Cysteinyl leukotrienes synthesis is involved in aristolochic acid I-induced apoptosis in renal proximal tubular epithelial cells. Toxicology 287, 38–45. doi: 10.1016/j.tox.2011.05.014
Yang, H. Y., Lin, J. L., Chen, K. H., Yu, C. C., Hsu, P. Y., and Lin, C. L. (2006). Aristolochic acid-related nephropathy associated with the popular Chinese herb Xi Xin. J. Nephrol. 19, 111–114.
Yuan, J., Liu, Q., Wei, G., Tang, F., Ding, L., and Yao, S. (2007). Characterization and determination of six aristolochic acids and three aristololactams in medicinal plants and their preparations by high-performance liquid chromatography-photodiode array detection/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 21, 2332–2342. doi: 10.1002/rcm.3097
Yuan, J., Liu, Q., Zhu, W., Ding, L., Tang, F., and Yao, S. (2008). Simultaneous analysis of six aristolochic acids and five aristolactams in herbal plants and their preparations by high-performance liquid chromatography-diode array detection-fluorescence detection. J. Chromatogr. A 1182, 85–92. doi: 10.1016/j.chroma.2007.12.076
Zhang, F., Chu, C. H., Xu, Q., Fu, S. P., Hu, J. H., Xiao, H. B., et al. (2005). A new amide from Asarum forbesii Maxim. J. Asian Nat. Prod. Res. 7, 1–5. doi: 10.1080/10286020310001596015
Keywords: metabolomics, LC-MS, nephrotoxicity, Asarum, aristolactam
Citation: Michl J, Bello O, Kite GC, Simmonds MSJ and Heinrich M (2017) Medicinally Used Asarum Species: High-Resolution LC-MS Analysis of Aristolochic Acid Analogs and In vitro Toxicity Screening in HK-2 Cells. Front. Pharmacol. 8:215. doi: 10.3389/fphar.2017.00215
Received: 28 September 2016; Accepted: 06 April 2017;
Published: 22 May 2017.
Edited by:
Thomas Efferth, Johannes Gutenberg-Universität Mainz, GermanyReviewed by:
Anna Rita Bilia, University of Florence, ItalyJoelle L. Nortier, Université libre de Bruxelles, Belgium
Valérian Bunel, Institut Scientifique de Santé Publique, Belgium
Copyright © 2017 Michl, Bello, Kite, Simmonds and Heinrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Heinrich, bS5oZWlucmljaEB1Y2wuYWMudWs=
 Johanna Michl
Johanna Michl Olusheyi Bello1
Olusheyi Bello1 Monique S. J. Simmonds
Monique S. J. Simmonds Michael Heinrich
Michael Heinrich