- 1Department of Pharmaceutical Biology, Institute of Pharmacy and Biochemistry, Johannes Gutenberg University, Mainz, Germany
- 2Heidelberg School of Chinese Medicine, Heidelberg, Germany
- 3Abel Salazar Biomedical Sciences Institute, University of Porto, Porto, Portugal
Curcuma longa has long been used in China and India as anti-inflammatory agent to treat a wide variety of conditions and also as a spice for varied curry preparations. The chemoprofile of the Curcuma species exhibits the presence of varied phytochemicals with curcumin being present in all three species but AA only being shown in C. longa. This study explored the effect of a curcumin/AA combination on human cancer cell lines. The curcumin/AA combination was assessed by isobologram analysis using the Loewe additivity drug interaction model. The drug combination showed additive cytotoxicity toward CCRF-CEM and CEM/ADR5000 leukemia cell lines and HCT116p53+/+ and HCT116p53−/− colon cancer cell line, while the glioblastoma cell lines U87MG and U87MG.ΔEGFR showed additive to supra-additive cytotoxicity. Gene expression profiles predicting sensitivity and resistance of tumor cells to induction by curcumin and AA were determined by microarray-based mRNA expressions, COMPARE, and hierarchical cluster analyses. Numerous genes involved in transcription (TFAM, TCERG1, RGS13, C11orf31), apoptosis-regulation (CRADD, CDK7, CDK19, CD81, TOM1) signal transduction (NR1D2, HMGN1, ABCA1, DE4ND4B, TRIM27) DNA repair (TOPBP1, RPA2), mRNA metabolism (RBBP4, HNRNPR, SRSF4, NR2F2, PDK1, TGM2), and transporter genes (ABCA1) correlated with cellular responsiveness to curcumin and ascorbic acid. In conclusion, this study shows the effect of the curcumin/AA combination and identifies several candidate genes that may regulate the response of varied cancer cells to curcumin and AA.
Introduction
Curcuma longa L. belongs to the family Zingiberaceae, which is a perennial herb that measures up to 1 m height with a short stem. It is distributed throughout tropical and subtropical regions in the world, being widely cultivated in Asian countries, mainly in India and China (Kapoor, 1990). As a component of folklore medicine the use of C. longa has been documented both in Indian and Chinese cultures. The rhizomes are used as Ezhu according to the Chinese Pharmacopoeia (2010 edition; Zhao et al., 2010) and are a household remedy in Nepal (Eigner and Scholz, 1999). The long list of usages include antiseptic, analgesic, anti-inflammatory, antimalarial, and insect repellant (Chaudhri, 1950; Li et al., 1998; Niederau and Göpfert, 1999; 2001; Tawatsin et al., 2001; Duke, 2002). Traditional Indian medicine claims to use its powder against biliary disorders, anorexia, coryza, cough, diabetic wounds, hepatic disorders, rheumatism, and sinusitis (Ammon et al., 1992). In ancient Hindu medicine, C. longa is extensively used for the treatment of sprains and swelling caused by injury (Ammon and Wahl, 1991). The antioxidant activity of C. longa is well-known (Ammon and Wahl, 1991; Anand et al., 2007).
Extensive research during the past years revealed that curcumin has considerable potential against a wide variety of both malignant and non-malignant diseases. Curcumin exhibits activity against numerous inflammatory diseases, including pancreatitis (Gukovsky et al., 2003; Gülçubuk et al., 2005), arthritis (Joe et al., 1997; Liacini et al., 2002), inflammatory bowel disease (Holt et al., 2005), gastritis (Swarnakar et al., 2005), allergy (Baek et al., 2003; Ram et al., 2003), and fever (Lee et al., 2003; Shao et al., 2004), possibly through the downregulation of inflammatory markers. It is also active against autoimmune diseases, including scleroderma (Tourkina et al., 2004), psoriasis (Bosman, 1994), multiple sclerosis (Verbeek et al., 2005), and diabetes (Babu and Srinivasan, 1995, 1997; Sajithlal et al., 1998). Curcumin also exhibits a great potential against various types of cancers. Its mechanism of action involves, firstly, the suppression of tumor cell proliferation by down-regulation of anti-apoptotic gene products, activation of caspases, and induction of tumor suppressor genes (Jiang et al., 1996; Bush et al., 2001; Chan and Wu, 2004). Secondly, curcumin suppresses tumor invasion by down-regulation of matrix metalloproteinases and cell surface adhesion molecules (Lin et al., 1998; Fenton et al., 2002; Lee et al., 2006). Thirdly, curcumin inhibits angiogenic cytokines leading to suppression of angiogenesis (Shin et al., 2001; Leyon and Kuttan, 2003; Bobrovnikova-Marjon et al., 2004) and lastly the anti-inflammatory and cytotoxic effects of curcumin contribute to its antitumor activity (Srivastava, 1989; Fujiyama-Fujiwara et al., 1992; Ammon et al., 1993).
The activity of many medicinal plants results from the interaction action of several constituents, which may cooperatively act in an additive or synergistic manner. It has been repeatedly observed that extracts of medicinal plants reveal better activities than their isolated single compounds at comparable equivalent concentrations of the active components. This phenomenon is attributed to the absence of interacting substances present in crude extracts. For example, extracts were obtained from the fresh herb of Artemisa annua L. either by soaking the herb in water followed by wringing out the juice by hand or by pounding the fresh herb to a pulp followed by squeezing out the juice. The extracts were then analyzed for artemisinin concentration and tested against malaria parasites. It was found that the antiplasmodial IC50-values were 6–18-fold lower than was expected in terms of their artemisinin content suggesting that the activity of the extracts could not be entirely accounted by their artemisinin content (Wright et al., 2010). Another example are the Cinchona alkaloids. There are almost 30 alkaloids described in the bark of Cinchona officinalis L. The most well-known of these are quinine, quinidine, and cinchonine and cinchonidine. However, quinine is not the most potent of the alkaloids: quinidine, dihydroquinidine, and cinchonine all have consistently lower 50% inhibitory concentrations (IC50) in vitro. The combination of quinine with quinidine and cinchonine is 2–10 times more effective in vitro against quinine-resistant strains, and the mixture of alkaloids reveals more consistent effects than any of the alkaloids singly used (Druilhe et al., 1988; Karle and Bhattacharjee, 1999). In pharmacokinetic synergy, substances with little or no bioactivity may assist the main active principle to reach the disease target by several mechanisms, e.g., improving bioavailability, or decreasing metabolism and excretion. Comparable effects are not yet known for C. longa and needs to be elucidated. In the present investigation, we addressed this question. We have chosen ascorbic acid (vitamin C, ascorbate, C6H12O6) as phytochemical constituent of C. longa to investigate the additive or synergistic effects of its combination with curcumin.
Ascorbic acid is a ketolactone and a water-soluble antioxidant. There are two chemical forms of ascorbic acid: the reduced form (ascorbic acid; AA) and the oxidized form (dehydroascorbic acid; DHA; Mamede et al., 2011). AA is well-known for its potent antioxidant properties, as it is able to scavenge free radicals and reactive oxygen species (ROS). Thus, it has been associated with decreased oxidative stress in vivo (Carr A. and Frei, 1999; Carr A. C. and Frei, 1999). High doses of AA can reduce inflammatory biomarkers such as C-reactive protein (CRP), tumor necrosis factor (TNF-α), interferon-γ (IFN-γ), and the interleukins IL-1, IL-2, IL-6, IL-8 (Gilliam and St Clair, 2011; Mikirova et al., 2012). AA is a cofactor in vivo for enzymes involved in the biosynthesis of collagen, carnitine, neurotransmitters, and neuropeptide hormones as well as enzymes involved in regulation of epigenetic or transcription factors (Rebouche, 1991; Du et al., 2012). Furthermore, it is a cofactor in the synthesis of the neurotransmitters norepinephrine, dopamine, and serotonin and neuropeptide hormones such as oxytocin (Harrison and May, 2009; May et al., 2012). Extensive studies have been carried out on AA in the treatment of cancer and vast literature exists on AA and cancer. In 1949, AA was first proposed to be used for cancer therapy (Klenner, 1949; Mc, 1952). The first comprehensive review on AA and cancer was published in 1979 and an updated review 25 years later (Cameron et al., 1979; González et al., 2005). AA may act as a prodrug causing the formation of AA radical and hydrogen peroxide in the extracellular space (Chen et al., 2007). In a clinical trial, AA exerted antitumor activity in patients with advanced cancer as a stand-alone therapy as well as in combination with other anticancer agents (Hoffer et al., 2008). The conjugation of AA with extracts of medical herbs stimulated apoptosis and disrupted the cell cycle in different cancer cell lines. Furthermore, AA was pro-oxidant generating hydrogen peroxide-dependent cytotoxicity toward various cancer cells without adversely affecting normal cells. AA together with sodium nitrite induced genotoxicity due to oxidative DNA damage. High concentrations of AA killed tumor cells in vitro with high efficiency and inhibited angiogenesis in mice bearing sarcoma (Chen et al., 2008; Kuroiwa et al., 2008; Verrax and Calderon, 2009; Yeom et al., 2009; Rozanova Torshina et al., 2010).
In this paper, we have chosen AA because it is widely distributed in many plants and it is also synthetically available. This allows us to investigate the cytotoxicity of the combination of curcumin and AA outside of the plant at exactly defined conditions to clarify their pharmacological effects in the combination. Our strategy was to investigate the combination by isobologram analysis and to investigate the genes which are related to the cytotoxicity induced by curcumin and AA in a broad spectrum of cancers. We postulate that the identification of genes that are specifically regulated by AA and curcumin could improve the understanding of the efficacy of C. longa in cancer treatment. We have systematically studied the microarray-based mRNA expression of genes, which influence the cellular response to curcumin and AA in the tumor cell line panel of the National Cancer Institute (NCI), USA to find possible mechanistic explanations for the interaction of these two compounds.
Materials and Methods
Chemicals
All chemicals were of analytical grade. Curcumin, AA and DMSO were purchased from Sigma-Aldrich (Sigma-Aldrich Corp., St. Louis, MO, USA).
COMPARE and Hierarchical Cluster Analyses of Microarray Data
The cancer cell lines of the Developmental Therapeutics Program of NCI consisted of a series of non-small cell lung cancer, colon cancer, renal cancer, ovarian cancer cells, leukemia, melanoma, prostate carcinoma, breast cancer, and tumor cells of the central nervous system. Their origin and processing have been previously reported (Alley et al., 1988). The cytotoxicity induced by AA, curcumin, and standard anticancer drugs of the NCI cell line panel was measured by the sulforhodamine B assay. The 50% inhibition concentrations calculated from dose-response curves and converted to logarithmic values [log10IC50 (M)] have been deposited at the NCI database (http://dtp.cancer.gov/databases_tools/default.htm). The mRNA microarray hybridization of the NCI cell lines has been reported and deposited at the NCI website (http://dtp.cancer.gov/databases_tools/default.htm). COMPARE analyses were performed to produce rank ordered lists of genes expressed in the NCI cell lines. The methodology has been previously described in detail as a tool to identify candidate genes for drug resistance and sensitivity. To derive COMPARE rankings, a scale index of correlation coefficients (R-values) was created from log10IC50 (M) values of test compounds and microarray-based mRNA expression values. Greater mRNA expression correlated with enhanced drug resistance in the standard COMPARE approach, whereas greater mRNA expression in cell lines indicated drug sensitivity in reverse COMPARE analyses. Pearson's correlation test was used to calculate significance values and rank correlation coefficients as a relative measure for the linear dependency of two variables.
For hierarchical cluster analysis, objects were classified by calculation of distances according to the closeness of between individual distances by means of hierarchical cluster analysis. All objects were assembled into cluster trees (dendrograms). Merging of objects with similar features leads to cluster formation, where the length of the branch indicates the degree of relation. Distances of subordinate cluster branches to superior cluster branches serve as criteria for the closeness of clusters. Thus, objects with tightly related features were clustered closely together, while separation of objects in the dendrogram increased with progressive dissimilarity. Hierarchical clustering and heat-map analysis were performed using Euclidean distance and ward method implemented in “dist,” “hclust,” and “heatmap” functions in R programming (Eisen et al., 1998; Gu et al., 2016). The results were further confirmed using the CIM miner software by use of the one matrix clustered image map (CIM) https://discover.nci.nih.gov/cimminer/oneMatrix.do.
Cell Culture
Drug sensitive CCRF-CEM and multidrug-resistant P-glycoprotein overexpressing CEM/ADR5000 leukemic cells were generously provided by Prof. Axel Sauerbrey (Department of Pediatrics, University of Jena, Jena, Germany). They were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin (Invitrogen, Darmstadt, Germany). Doxorubicin (5000 ng/mL) was added to maintain overexpression of P-gp (MDR1, ABCB1) in resistant cells (Kimmig et al., 1990). Human wild-type HCT116 colon cancer cells (p53+/+) and knockout clones (p53−/−) derived by homologous recombination (Waldman et al., 1995; Bunz et al., 1998) were generously provided by Dr. B. Vogelstein and H. Hermeking (Howard Hughes Medical Institute, Baltimore, MD, USA). Both colon cancer cells were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin (Invitrogen). Wild-type human U87MG glioblastoma multiform cells and cells transfected with control mock vector or an expression vector harboring EGFR cDNA with a deletion in exons 2–7 (U87MG.ΔEGFR), were kindly provided by Dr. W. K. Cavenee (Ludwig Institute for Cancer Research, San Diego, CA, USA; Huang et al., 1997).
Cell Viability Assay
Cell viability was evaluated by resazurin assay. This test is based on reduction of the indicator dye, resazurin, to the highly fluorescent resorufin by viable cells. Nonviable cells rapidly lose the metabolic capacity to reduce resazurin and thus produce no fluorescent signal. Glioblastoma and colon cancer cells were harvested with 0.25% trypsin/EDTA (Invitrogen, Germany) and diluted to a final concentration 5 × 104 cells/mL. One hundred microliters of the cell suspension were sowed into the wells of a 96-well-culture plate 1 day before treatment. However, for the leukemia cell lines 2 × 104 cells were sowed in a 96-well-culture plate in a total volume of 100 μL for each well and then the cells were immediately treated. Marginal wells were filled with 200 μL of pure medium, in order to minimize effects of evaporation. Besides, wells filled with medium served as the negative control to determine background fluorescence that may be present. Then, cells were treated with different concentrations of curcumin, vitamin C alone, or combined. After 72 h, 20 μL resazurin (Sigma-Aldrich, Germany) 0.01% w/v in ddH2O was added to each well and the plates were incubated at 37°C for 4 h. Fluorescence was measured on an Infinite M2000 Proplate reader (Tecan, Germany) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Each assay was done at least two times, with six replicates each. The cytotoxic effect of the treatment was determined as percentage of viability and compared to untreated cells. The calculated cell viability (y-axis) was plotted against the log drug concentration (x-axis) using Microsoft Excel. The obtained curve was used to determine the IC50-value, which represented the concentration of the test compound required to inhibit 50% of cell proliferation.
Statistical Analysis
The Loewe additivity model was used to calculate synergetic drug interactions between curcumin and vitamin C in inhibiting cell growth. In this model, the combination index (CI) was defined as CI = (d1/D1)/(d2/D2), where D1 and D2 were the doses of drug 1 and drug 2 that produced an response Y (e.g., 50% inhibition of CCRF-CEM growth) when used alone, d1 and d2 were the doses of drug 1 and drug 2 in combination, which can generate the same response Y. If the CI is equal, less than or more than 1, the combination dose (d1, d2) is termed as additive, synergistic, or antagonistic, respectively. The drug interaction was illustrated geometrically as isobologram.
Pearson's correlation test was used to calculate significance values and rank correlation coefficients as a relative measure for the linear dependency of two variables. This test was implemented into the WinSTAT Program (Kalmia Co.). Pearson's correlation test determined the correlation of rank positions of values. Ordinal or metric scaling of data is suited for the test and transformed into rank positions. There is no condition regarding normal distribution of the data set for the performance of this test. We used Pearson's correlation test to correlate microarray-based mRNA expression of candidate genes with the IC50-values for curcumin and ascorbic acid.
The χ2-test was applied to bivariate frequency distributions of pairs of nominal scaled variables. It was used to calculate significance values (p-values) and rank correlation coefficients (R-values) as a relative measure for the linear dependency of two variables. This test was implemented into the WinSTAT program (Kalmia Co.). The χ2-test determines the difference between each observed and theoretical frequency for each possible outcome, squaring them, dividing each by the theoretical frequency, and taking the sum of the results. Performing the χ2-test necessitated to define cell lines as being sensitive or resistant to curcumin and ascorbic acid. This has been done by taking the median IC50-value (log10 = −5.1 M) for curcumin and IC50-value (log10 = −2.7 M) for ascorbic acid as a cut-off threshold.
Results
Chemoprofiling of Different Curcuma Species
As a first step, we established chemoprofiles of three Curcuma species (C. longa, C. zedoaria, and C. xanthorrhiza) based on the chemical compositions of these species deposited at Dr. Duke's Phytochemical and Ethnobotanical Databases (http://www.arsgrin.gov/cgi-bin/duke/farmacy2.pl). We subjected the chemical composition of these plants to hierarchical cluster analysis (Figure 1). A total of 114 phytochemicals have been included in the analysis, which are listed in detail in Supplementary Table 1. Three compounds were commonly found in all three Curcuma species (curcumin, D-camphor, and desmethoxycurcumin). Ten compounds were found in two of the three species, whereas all other compounds were found in only one Curcuma species. This specific distribution of phytochemicals enables specific clustering and separation of the Curcuma species.

Figure 1. Dendrogram obtained by hierarchical cluster analysis of phytochemical constituents of Curcuma longa, C. zedoaria, and C. xanthorrhiza. The chemical compounds included in this cluster analysis are listed in detail in Supplementary Table 1.
Cytotoxicity of AA in the NCI panel of Cell Lines
We hypothesized that the cytotoxic effect of C. longa against cancer cells is not solely caused by its main compound, curcumin, but that other compounds may also contribute to this activity of the plant. To prove this hypothesis, we mined the NCI database for compounds found in C. longa and six compounds were identified (Figure 2A), i.e., AA, limonene, guaiacol, p-cymene, azulene, and curcumin. Although AA was not the most toxic compound among the six tested, we decided to continue our investigations with AA, because of its far distribution not only in C. longa but also in many other plants as well and it's enormous relevance for human health in general. Further, investigations were then carried out using AA in the NCI panel of cell lines. Leukemia and melanoma cell lines were most sensitive, while brain and lung cancer cell lines were the most resistant ones (Figure 2B). Established anticancer drugs frequently show high sensitivity toward leukemia, but resistance toward melanoma. Hence, it is interesting that AA was active against melanoma cell lines.
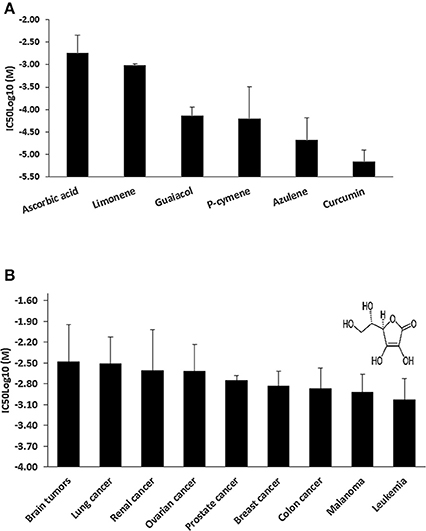
Figure 2. (A) Mean IC50log10-values of selected cytotoxic phytochemicals from Curcuma longa for the NCl tumor cell line panel as assayed by the sulforhodamine B-test. (B) Tumor-type-dependent cytotoxicity of ascorbic acid. Insert, chemical structure of ascorbic acid.
Cytotoxicity of Curcumin and AA toward Drug Resistant Cancer Cell Lines
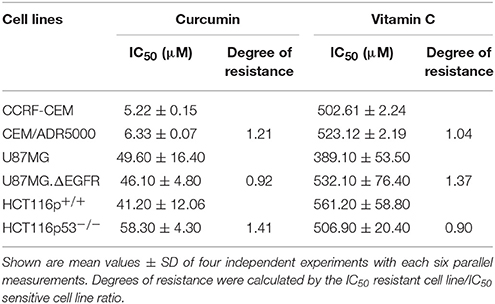
Drug-resistant cell lines with different resistance mechanisms (P-glycoprotein, EGFR, mutant p53) toward curcumin and AA were determined. All the cell lines were treated with varying concentrations of curcumin and AA for 72 h, their growth was inhibited in a dose-dependent manner, albeit at different efficacy. The IC50-values were calculated from the dose response curves and summarized in Table 1. Curcumin inhibited cell growth at lower concentrations than AA. The range of IC50-values was 5.22–58.3 μM for curcumin, while that of AA was 389.1–561.2 μM. The CCRF-CEM and CEM/ADR5000 leukemia cell lines were inhibited at concentrations of 5.22 and 6.33 μM, respectively. The U87MG.ΔEGFR-transfectant glioblastoma cells exhibited sensitivity toward curcumin with an IC50-value of 46.1 μM, which was slightly lower than the IC50-value of wild-type U87MG cells 49.6 μM. Interestingly, the HCT166p53−/− colon cancer cell line was preferentially inhibited by AA with an IC50-value of 506.9 μM compared to HCT116p53+/+ wild-type cells 561.2 μM. The degrees of resistance were calculated by dividing the IC50 of the resistant cell line by the IC50 of the sensitive cell line. Compared to the high degrees of resistance of these drug-resistant cell lines to standard drugs such as doxorubicin (Hall et al., 2009), curcumin and AA inhibited these cell lines with similar efficacies. The degrees of resistance were in a range of 0.9–1.41 (Table 1).
Cytotoxic Effects of Combination Treatments of Curcumin and AA
Next, we addressed the question, whether the combination of curcumin and AA exhibits additive or synergistic growth inhibition of cancer cells. We applied a universal reference model for evaluating the effects of drug interaction, i.e., the Loewe additivity model (isobologram analysis). The cancer cell lines were treated with varying concentrations of AA at indicated concentrations of curcumin for 72 h. In CCRF-CEM and CEM/ADR5000 cells, the IC50-values of curcumin in combination with AA were reduced by less than half of the IC50 of curcumin alone. In the glioblastoma and colon cancer cell lines, the IC50-value of two of the curcumin concentrations (20% IC50 curcumin and 40% IC50 curcumin) decreased with increasing AA concentrations less than the IC50 of curcumin alone. However, the two other concentrations of curcumin reduced the IC50 in combination with AA by less than half of the IC50-value of curcumin alone. Dose-normalized IC50 isobolograms for all cell lines were generated by plotting the combination treatment IC50-values of curcumin against AA. Additive effects were observed in CCRF-CEM and CEM/ADR5000 (Figure 3) as well as in HCT116p53+/+ and HCT116p53−/− cell lines (Figure 4), whereas supra/additive effects were visible in U87MG and U87MG.ΔEGFR cells (Figure 5).
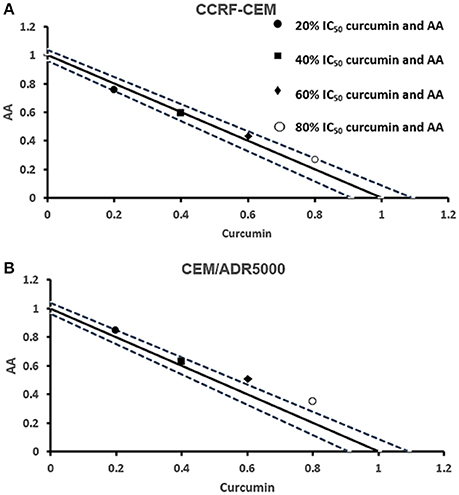
Figure 3. Isobologram analysis for the interaction of various combinations of curcumin and ascorbic acid on (A) CCRF-CEM and (B) CEM/ADR5000 leukemia cell lines.
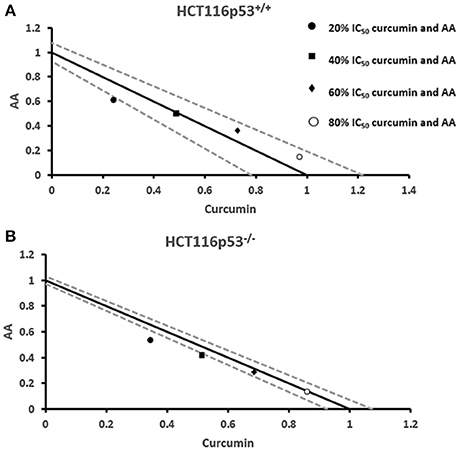
Figure 4. Isobologram analysis for the interaction of various combinations of curcumin and ascorbic acid on (A) HCT116p53+/+ and (B) HCT116p53−/− colon cancer cell lines.
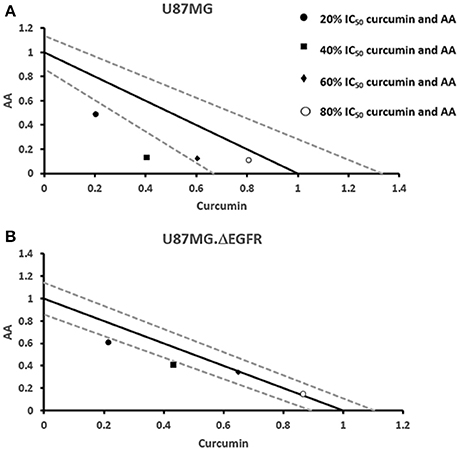
Figure 5. Isobologram analysis for the interaction of various combinations of curcumin and ascorbic acid on (A) U87MG and (B) U87MG.ΔEGFR glioblastoma cell lines.
COMPARE and Hierarchical Cluster Analyses of mRNA Expressions
The transcriptome-wide mRNA expression of the NCI cell lines based on the Novartis microarray platform was investigated by COMPARE analyses and correlated to the log10IC50 (M) values for curcumin and AA. This bioinformatical approach was performed to identify novel putative factors associated with cellular response to curcumin and AA. The top 20 genes with direct and top 20 genes with inverse correlation co-efficient are shown in Tables 2, 3. These genes were subjected to hierarchical cluster analysis to analyze, whether the expression profiles of these genes may predict sensitivity or resistance of the cells to curcumin and AA. The mRNA expression of the identified genes were subjected to hierarchical cluster analysis and cluster image mapping (Figures 6, 7). The resulting dendogram with the cell lines analyzed on the left can be divided into five major clusters for curcumin and four clusters for ascorbic acid. Using the chi-square test, we analyzed whether the distribution of cell lines being sensitive or resistant to curcumin and AA was statistically significant. As shown in Table 4, the distribution of sensitive or resistant cell lines on the dendogram was significantly different indicating that cellular response to curcumin or AA was predictable by the mRNA expression of these genes. Therefore, it is interesting to know the function of these genes. The specific functions of the proteins encoded by the genes were diverse and included signal transduction, transcription factors, proteasome deregulation, apoptosis regulating genes, proliferation-related genes, pro- as well as anti-oxidative genes (Tables 2, 3). AA induced genes that belonged to the functional groups of transcription factors (TFAM, TCERG1, RGS13, and C11orf31), apoptosis-regulating genes (CRADD, CDK7, CDK19, CD81, TOM1) and signal transduction genes (NR1D2, HMGN1, ABCA1, DE4ND4B, TRIM27). Curcumin induction resulted in DNA repair genes (TOPBP1, RPA2), mRNA metabolism genes (RBBP4, HNRNPR, SRSF4, NR2F2, PDK1, and TGM2), signal transduction genes (WWC1, DTX2, EGFR, CHRNB4, VPS41, CRIM1), proliferation-related genes (RHOD), apoptosis-regulating genes (DFFB), and transporter genes (ABCA1).
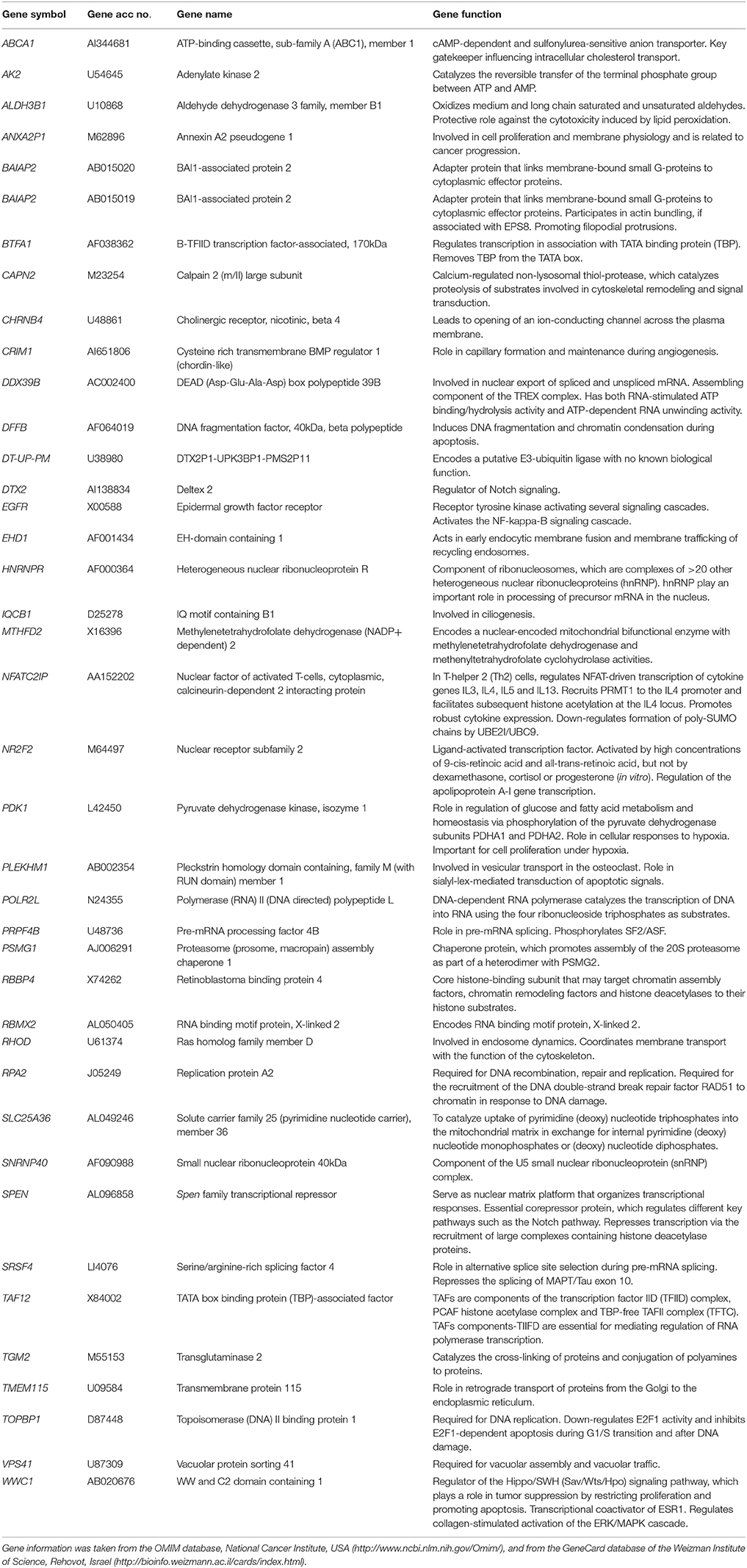
Table 2. Meta-data of genes shown in the cluster analysis whose mRNA expression correlated with the log10IC50 values of curcumin in the NCI tumor cell line panel.
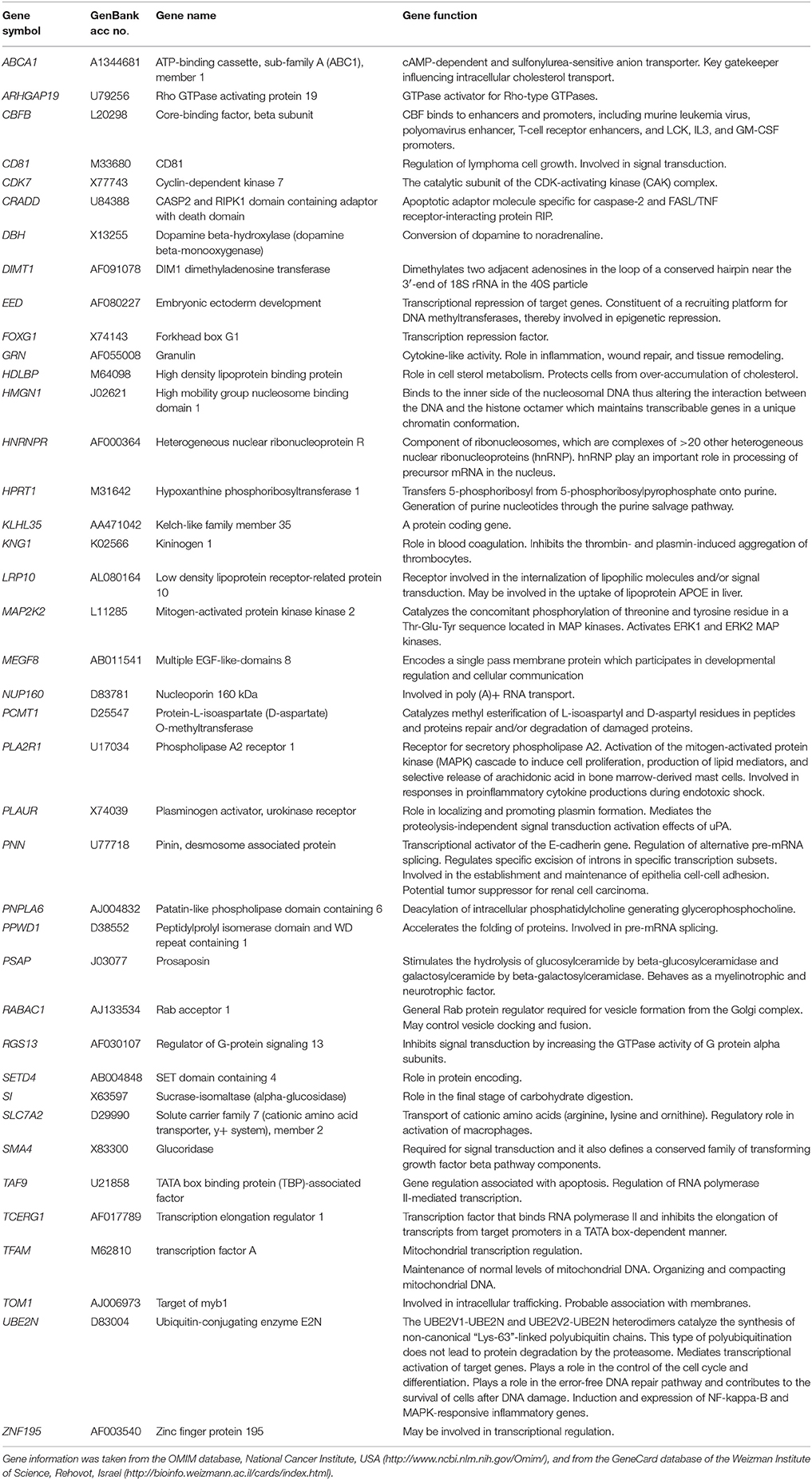
Table 3. Meta-data of genes shown in the cluster analysis of whose mRNA expression correlated with log10IC50-values of vitamin C in the NCI tumor cell line panel.
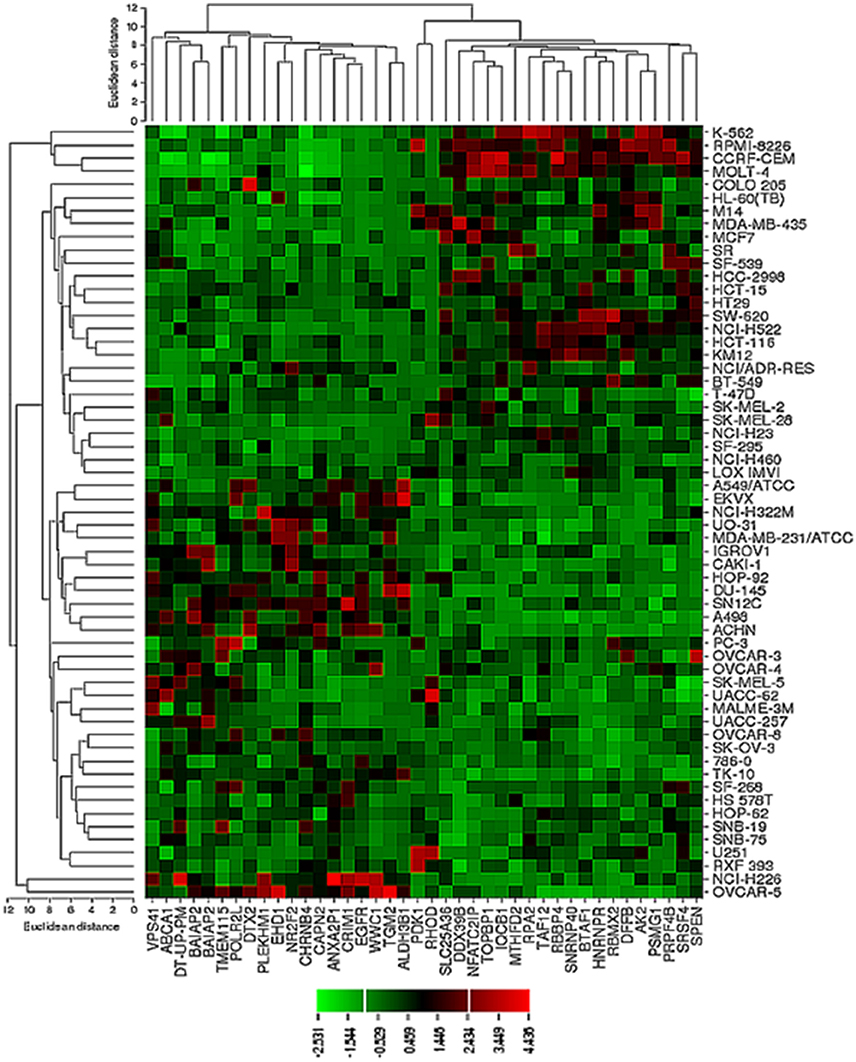
Figure 6. Dendrograms and cluster image map of curcumin obtained by hierarchical cluster analysis of mRNA expression of 40 genes in the NCI cell line panel as analyzed by the Novartis microarray platform. The dendrogram on the left shows the clustering of cell lines and the dendrogram on the top shows the clustering of genes. The cluster image map shows each single mRNA expression value obtained by microarray analysis. The expression values have been normalized and color-coded.
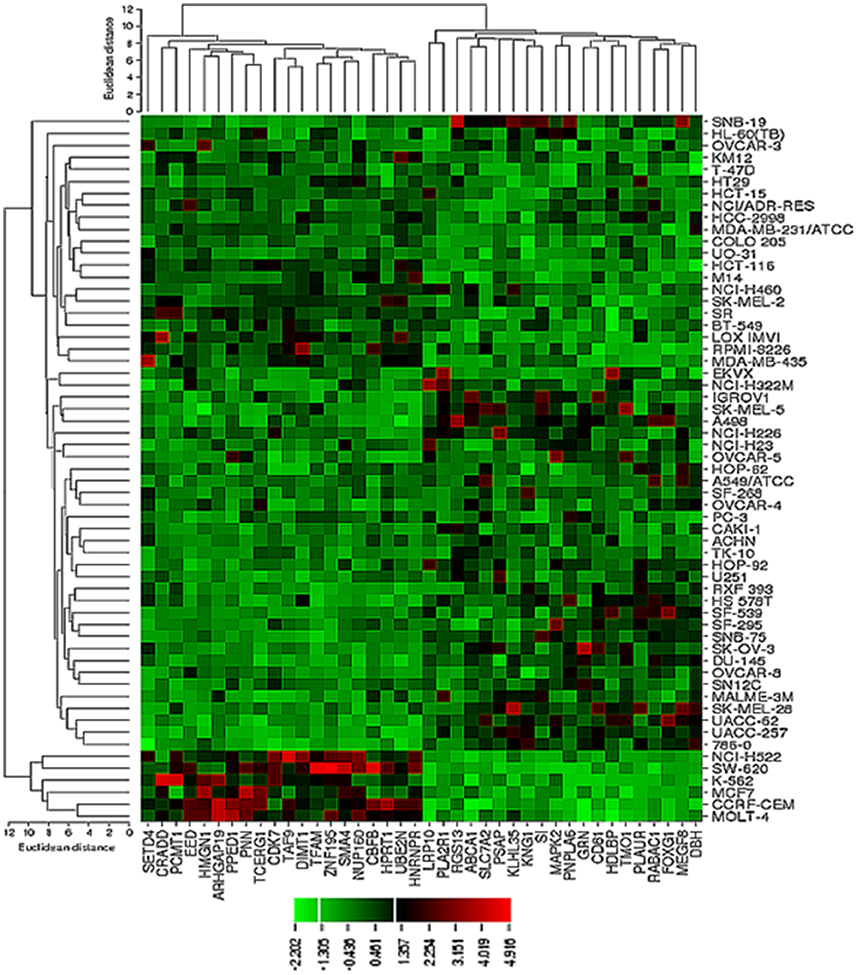
Figure 7. Dendrograms and cluster image map of vitamin C obtained by hierarchical cluster analysis of mRNA expression of 40 genes in the NCI cell line panel as analyzed by the Novartis microarray platform. The dendrogram on the left shows the clustering of cell lines and the dendrogram on the top shows the clustering of genes. The cluster image map shows each single mRNA expression value obtained by microarray analysis. The expression values have been normalized and color-coded.
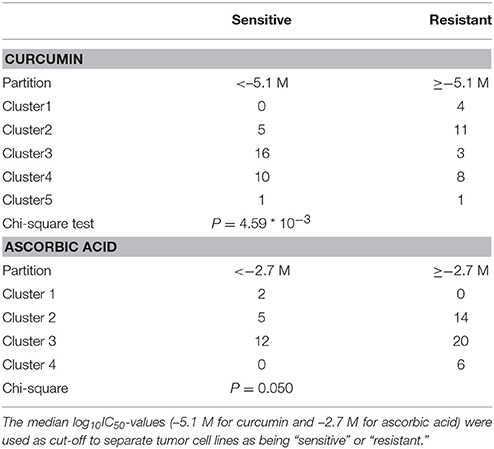
Table 4. Separation of clusters of NCI cell lines obtained by hierarchical cluster analyses for curcumin (Figure 6) or ascorbic acid (Figure 7).
Discussion
Plants are perhaps earth's most accomplished chemists. They produce thousands of specialized secondary metabolites, and during the evolution of life plants developed multi-targeted chemicals to fulfill diverse tasks. C. longa contains some 30 different phytochemicals. In the present study, we analyzed the cytotoxicity of a combination of two of these phytochemicals of C. longa, curcumin and AA. If applied alone, curcumin and AA were cytotoxic toward cell lines of different tumor types with curcumin exhibiting stronger cytotoxicity than AA. Our results are in line with other reports on the inhibitory activity of curcumin (Bimonte et al., 2016; Guzzarlamudi et al., 2016; Kasi et al., 2016; Ye et al., 2016; Yu et al., 2016; Zeng et al., 2016) and AA (Chen et al., 2015; Fukui et al., 2015; Jacobs et al., 2015; Sunil Kumar et al., 2015; Venturelli et al., 2015). The anticancer effects of curcumin in vitro and in vivo are primarily due to the activation of apoptotic pathways in cancer cells as well as the inhibition of mechanisms related to the tumor microenvironments such as inflammation, angiogenesis, invasion, and metastasis. In particular, curcumin targets numerous therapeutically important cancer signaling pathways such as p53, Ras, PI3K, AKT, Wnt, β-catenin, mTOR, and so on.
AA also reveals anticancer activity in vitro and in vivo, however at higher concentration than curcumin. A number of suggestions have been put forth on the potential mechanisms, by which AA causes death of cancer cells. The most common is that AA is a precursor for hydrogen peroxide (H2O2) generation, which is considered to be preferentially cytotoxic to cancer cells (Parrow et al., 2013). Exposure to AA concentrations of up to 5 mM for 1 h resulted in decreased survival of cancer cells and cell death was dependent on H2O2 production mediated by extracellular AA oxidation (Chen et al., 2005). Furthermore, intercellular metals contribute to the production of H2O2, with AA losing an electron to form a radical molecule. The free electron is donated to a transition metal. This reduced metal is then available to react with molecular oxygen resulting in the generation of H2O2. In the presence of AA, H2O2 reacts with another transition metal ion such as ferrous ion to generate a hydroxyl radical (Kehrer, 2000). The tumor suppressor p53 may also play a role for this activity. P53-positive cell lines were more sensitive to both AA and H2O2 treatment than p53-deficient ones (Kim et al., 2012).
Thus, having two compounds from one plant with diverse mechanisms of action, we were interested to evaluate, whether their combination would reveal synergistic, antagonistic, or additive interactions. Understanding drug-drug interactions always represents a critical issue in the drug development process, since clinically relevant changes in exposure of co-administered drugs can lead to reduced efficacy or, conversely, adverse drug reactions, depending on the therapeutic window of the drugs. The latter becomes especially important with anti-cancer medications, since they are typically administered at or close to the maximally tolerated dose (Waters, 2015). In this study, we applied isobologram analyses to evaluate the nature of interaction of curcumin and AA. Isobologram analysis is considered as gold standard to provide evidence for drug interactions. The combination of curcumin and AA exhibited additive effects in leukemia and colon cancer cell lines and supra-additive effects in the glioblastoma cell lines. Our hypothesis is that both natural products may work together by different molecular pathways to achieve their overall cytotoxicity. Unlike chemically synthesized drugs, natural products might be active at lower doses and over longer periods of incubation, which could further support the appearance of additive effects.
We further analyzed molecular determinants of sensitivity and resistance of cancer tumor cell lines toward curcumin and AA. We correlated the IC50-values expressed on induction by curcumin and AA of 60 tumor cell lines by COMPARE analysis of microarray-based transcriptome-wide mRNA expression levels of these cell lines (Scherf et al., 2000). We identified genes from diverse functional groups, which were associated with response of the tumor cells toward curcumin and AA. Under curcumin treatment, these groups of genes included DNA repair, mRNA metabolism, signal transduction, angiogenesis, proliferation, apoptosis etc. While for AA treatment, the COMPARE analysis provided genes that are involved in signal transduction, transcription factors, and apoptosis. Although the exact function of these genes for cellular responsiveness to curcumin or AA treatment is still unknown, we have some clues of explanation. On treatment with AA the following genes were downregulated: SETD4, TAF9, PNN, CDK7, TFAM, and PPWD1. SETD4 is a methyltransferase, which is involved in carcinogenesis. Its down-regulation suppressed cellular proliferation and delayed the G1/S cell cycle transition without affecting apoptosis. Furthermore, its knockdown decreased cyclin D1 (Faria et al., 2013). The TATA-binding protein associated factor 9 (TAF9) interacts with oncogenic GLI family members to form GLI-TAF9 binding, which is important for carcinogenesis activity and malignant growth (Yoon et al., 2015). PNN is a nuclear and cell adhesion-related protein participating in the regulation of gene expression and thereby, positively promoting cell-cell adhesion, and negatively affecting cell migration and cell proliferation (Shi et al., 2001). Cyclin-dependent kinase 7 (CDK7), which promotes transcription during the cell cycle, is critical for the survival of cancer cells. The inhibition of CDK7 suppressed proliferation and induced apoptotic cell death (Wang et al., 2015). Mitochondrial transcription factor A (TFAM), a member of the high mobility group (HMG) box protein family, is required for mitochondrial DNA replication and transcription. HMG proteins are often overexpressed in cancer cells and are involved in apoptosis regulation (Krynetskaia et al., 2009; Vander Heiden et al., 2009). TFAM may play a significant role in tumorigenesis (Guo et al., 2011). PPWD1 has a well-characterized peptide domain (WD40 domain for PPWD1), and this domain has also a critical role in carcinogenesis. The WD40 domain mediates signal transduction and transcriptional regulation during cell cycle and apoptosis. PPWD1 may serve as target for drug development (Davis et al., 2008; Jeon et al., 2014). From the above discussion, AA mechanism revolves around the genes affecting cell proliferation and cell cycle events; it can be assumed that these genes contribute to sensitivity of the tumor cells to AA.
On the other hand, genes potentially responsible for responsiveness on curcumin treatment were identified and curcumin downregulated, i.e., AK2, PDK1, NR2F2, DFFB, MTHFD2, and ALDH3B1. Adenylate kinases (AKs) represent enzymes that catalyze reversible high-energy phosphoryl transfer reactions between adenine nucleotides in the intermembrane space. During periods of metabolic stress, AK2 increases the amount of available adenosine monophosphate and therefore activates downstream ATP-sensing mechanisms—such as AMP-activated protein kinase (AMPK)—to regulate the cellular metabolism. Inhibition of AK2 expression significantly inhibited the proliferation of cancer cells (Dzeja and Terzic, 2009). PDK1 plays a key role in several cancer types. Alterations of PDK1 are critical for oncogenic PI3K signaling. PDK1 has an essential role in regulating cell migration, especially in the context of PTEN deficiency. Downregulation of PDK1 levels inhibits migration and metastasis. PDK1 inhibitors may be useful to prevent cancer progression and abnormal tissue dissemination (Raimondi and Falasca, 2011). The nuclear receptor subfamily 2, group F, member 2 (NR2F2) is a master regulator of angiogenesis and acts as oncogene in prostate and other human cancers. NR2F2 is robustly expressed in the stroma of healthy ovary with little or no expression in epithelia lining the ovarian surface, clefts, or crypts. The pattern of NR2F2 expression was severely disrupted in ovarian cancers, in which decreased levels of stromal expression and ectopic epithelial expression were exhibited. Targeting NR2F2 expression in ovarian cancer cell lines enhanced apoptosis and increased proliferation (Hawkins et al., 2013). DFFB contributes to both chromosomal condensation and DNA degradation during apoptosis, decreased DFFB expression favors DNA damage, which in turn may contribute to both tumorigenesis and better response to DNA damaging chemotherapy (McDonald et al., 2005). MTHFD2 mRNA and protein expression is markedly elevated in many cancers and correlated with poor survival in breast cancer. MTHFD2 is integral to mitochondrial one-carbon metabolism, a metabolic system recently implicated in rapid cancer cell proliferation. Synthesis of one-carbon units carried by the tetrahydrofolate (THF) cofactor is important for proliferating cells, required for nucleotide synthesis and methylation reactions. MTHFD2 is a bifunctional enzyme, catalyzing the NAD+ dependent CH2-THF dehydrogenase and CH+-THF cyclohydrolase reactions within the mitochondria. Within the mitochondrial folate pathway, MTHFD2 is of special interest, because MTHFD2 was one of the most consistently overexpressed mRNAs genome-wide across 19 different tumor types. The MTHFD2 protein is specifically expressed in transformed cells, but not the stroma surrounding the tumor tissues. MTHFD2 by RNAi impairs proliferation in a variety of cancer cell lines, independent of the tissue of origin, and decreases invasion and migration in breast cancer cell lines. MTHFD2 is broadly required for cancer cell proliferation and viability (Lehtinen et al., 2013; Nilsson et al., 2014). ALDH3B1 is a metabolically active enzyme with distinct specificity for various aldehyde substrates, particularly medium-, and long-chain aliphatic aldehydes. These substrates include many products that are formed during LPO, such as hexanal, 4-hydroxy-2-nonenal (4-HNE), octanal, and trans-2-nonenal. ALDH3B1 plays an important physiological role against cellular oxidative stress by detoxifying aldehydes derived from oxidative processes, such as ethanol metabolism and LPO (Marchitti et al., 2010). Our pharmacogenomics data shows curcumin suppressing cell proliferation by downregulation of anti-apoptotic genes and cell surface adhesion molecules. Curcumin is also seen to regulate cellular metabolism and inhibition of angiogenic cytokines. We can then suggest that the downregulated genes affect the sensitivity of the tumor cells to curcumin.
Previously, we reported the mRNA expression profile induced by curcumin in the NCI cell line panel, which was merged from four different microarray platforms (Novartis, Stanford, Chiron, and Genelogic; Sertel et al., 2012). In the present investigation, we focused only on the Novartis microarray platform for the comparison of curcumin and vitamin C and to reduce the degree of complexity. If we compared the top ranked genes in the previous analysis with those of the present investigation, we found some genes in common (MTHFD2, AK2, NFATC21P, BTAF1, RBBP4), although the majority of genes were different in both analyses. This result points to an observation that was frequently made by many investigators: different microarray platforms deliver different results. Nevertheless, several biological functional groups were found to be common in our previous and the present paper, e.g. cell cycle, DNA damage response, cell migration, inflammation, signaling pathways, and apoptosis-regulating genes. To our opinion, microarray data are reliable, if they are used for the generation of testable hypotheses. In this respect, both of our microarray analyses were useful. Microarray data represents the starting point for the elucidation of modes of action of cytotoxic compounds rather than completed end results.
Cluster analyses were applied in the present investigation under the assumption that responsiveness of cancer cells might be predicted by using gene expression patterns and that appropriate gene expression profiles might be sufficient to predict whether a cancer cell line is sensitive or resistant to a cytotoxic compound (Sertel et al., 2010). Curcumin revealed two clusters with predominantly sensitive and three with predominantly resistant cell lines. For AA cluster analysis revealed two clusters containing mainly resistant and two clusters containing mainly sensitive cell lines in a comparable fashion to curcumin. The prediction of sensitivity or resistance to cytotoxic drugs by mRNA expression profiles is interesting in the context of individualized or precision medicine, because it may open the possibility to determine prior to treatment, whether or not a tumor will respond to specific drugs. Our data demonstrate that this may not only be feasible to established anticancer drugs, but also to investigative natural products such as curcumin or AA.
The fact that the mRNA expression profiles induced by curcumin and AA related to different gene expression patterns may be related to different modes of actions of both compounds. Medicinal herbs generally contain mixtures of active compounds, which may interact in an additive or synergistic manner. Synergistic interactions may need common mechanisms e.g., a common specific pathway that they inhibit. From an evolutionary point of view, synergistic interactions need co-evolutionary selection pressures to evolve. Hence, it can be speculated that synergisms are less likely to occur than additive effects. Therefore, additive drug interactions can be more frequently found in medicinal herbs. Compounds with different modes of action can efficiently and sufficiently fulfill the requirements for plants to survive under specific evolutionary selection pressure. This may explain that we found additive rather than synergistic interactions in isobologram analyses between curcumin and AA in the panel of cell lines tested. This observation is in accordance with previous data with several cytotoxic compounds from Artemisia annua L., which also showed additive rather than synergistic interactions (Efferth et al., 2011).
In summary, we have identified some genes which were downregulated by AA and curcumin. The genes may be responsible for cellular responsiveness of the varied cancer cells to AA and curcumin treatments. The cellular activities tackled by the downregulated genes include inhibition of cell proliferation and cell cycle activities, reduction in cellular metabolism, downregulation of anti-apoptotic gene products and inhibition of angiogenic cytokines. The two natural products seem to induce cytotoxicity by different mechanisms and this may lead to achieve tumor eradication in vivo. The varied cellular functionalities represented by the genes downregulated may support additive effects observed in the isobologram analyses.
Author Contributions
Title selection and research design: TE, EO. Laboratory experiments, results generation, data analysis, and interpretation: EO, OK. Manuscript writing and submission: EO. Manuscript writing: HG. Advising in research design, results interpretation, and manuscript writing: TE.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the German Academic Exchange Service (DAAD) for a Ph.D. stipend to EO.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphar.2017.00038/full#supplementary-material
Abbreviations
AA, ascorbic acid; IC50, 50% inhibitory concentration; NCI, National Cancer Institute.
References
(2001). Alley, M. C., Scudiero, D. A., Monks, A., Hursey, M. L., Czerwinski, M. J., Fine, D. L., et al. Curcuma longa (turmeric). Altern. Med. Rev. 6(Suppl.), S62–S66.
Alley, M. C., Scudiero, D. A., Monks, A., Hursey, M. L., Czerwinski, M. J., Fine, D. L., et al. (1988). Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 48, 589–601.
Ammon, H. P., Anazodo, M. I., Safayhi, H., Dhawan, B. N., and Srimal, R. C. (1992). Curcumin: a potent inhibitor of leukotriene B4 formation in rat peritoneal polymorphonuclear neutrophils (PMNL). Planta Med. 58, 226. doi: 10.1055/s-2006-961438
Ammon, H. P., Safayhi, H., Mack, T., and Sabieraj, J. (1993). Mechanism of antiinflammatory actions of curcumine and boswellic acids. J. Ethnopharmacol. 38, 113–119. doi: 10.1016/0378-8741(93)90005-P
Ammon, H. P., and Wahl, M. A. (1991). Pharmacology of Curcuma longa. Planta Med. 57, 1–7. doi: 10.1055/s-2006-960004
Anand, P., Kunnumakkara, A. B., Newman, R. A., and Aggarwal, B. B. (2007). Bioavailability of curcumin: problems and promises. Mol. Pharm. 4, 807–818. doi: 10.1021/mp700113r
Babu, P. S., and Srinivasan, K. (1995). Influence of dietary curcumin and cholesterol on the progression of experimentally induced diabetes in albino rat. Mol. Cell. Biochem. 152, 13–21.
Babu, P. S., and Srinivasan, K. (1997). Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol. Cell. Biochem. 166, 169–175. doi: 10.1023/A:1006819605211
Baek, O. S., Kang, O. H., Choi, Y. A., Choi, S. C., Kim, T. H., Nah, Y. H., et al. (2003). Curcumin inhibits protease-activated receptor-2 and -4-mediated mast cell activation. Clin. Chim. Acta 338, 135–141. doi: 10.1016/j.cccn.2003.08.015
Bimonte, S., Barbieri, A., Leongito, M., Piccirillo, M., Giudice, A., Pivonello, C., et al. (2016). Curcumin anticancer studies in pancreatic cancer. Nutrients 8:E433. doi: 10.3390/nu8070433
Bobrovnikova-Marjon, E. V., Marjon, P. L., Barbash, O., Vander Jagt, D. L., and Abcouwer, S. F. (2004). Expression of angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 is highly responsive to ambient glutamine availability: role of nuclear factor-kappaB and activating protein-1. Cancer Res. 64, 4858–4869. doi: 10.1158/0008-5472.CAN-04-0682
Bosman, B. (1994). Testing of lipoxygenase inhibitors, cyclooxygenase inhibitors, drugs with immunomodulating properties and some reference antipsoriatic drugs in the modified mouse tail test, an animal model of psoriasis. Skin Pharmacol. 7, 324–334. doi: 10.1159/000211314
Bunz, F., Dutriaux, A., Lengauer, C., Waldman, T., Zhou, S., Brown, J. P., et al. (1998). Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282, 1497–1501. doi: 10.1126/science.282.5393.1497
Bush, J. A., Cheung, K. J. Jr., and Li, G. (2001). Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 271, 305–314. doi: 10.1006/excr.2001.5381
Cameron, E., Pauling, L., and Leibovitz, B. (1979). Ascorbic acid and cancer: a review. Cancer Res. 39, 663–681.
Carr, A. C., and Frei, B. (1999). Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 69, 1086–1107.
Carr, A., and Frei, B. (1999). Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 13, 1007–1024.
Chan, W. H., and Wu, H. J. (2004). Anti-apoptotic effects of curcumin on photosensitized human epidermal carcinoma A431 cells. J. Cell. Biochem. 92, 200–212. doi: 10.1002/jcb.20059
Chen, N., Yin, S., Song, X., Fan, L., and Hu, H. (2015). Vitamin B(2) sensitizes cancer cells to vitamin-c-induced cell death via modulation of akt and bad phosphorylation. J. Agric. Food Chem. 63, 6739–6748. doi: 10.1021/acs.jafc.5b01909
Chen, Q., Espey, M. G., Krishna, M. C., Mitchell, J. B., Corpe, C. P., Buettner, G. R., et al. (2005). Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U.S.A. 102, 13604–13609. doi: 10.1073/pnas.0506390102
Chen, Q., Espey, M. G., Sun, A. Y., Lee, J. H., Krishna, M. C., Shacter, E., et al. (2007). Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. U.S.A. 104, 8749–8754. doi: 10.1073/pnas.0702854104
Chen, Q., Espey, M. G., Sun, A. Y., Pooput, C., Kirk, K. L., Krishna, M. C., et al. (2008). Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. U.S.A. 105, 11105–11109. doi: 10.1073/pnas.0804226105
Davis, T. L., Walker, J. R., Ouyang, H., Mackenzie, F., Butler-Cole, C., Newman, E. M., et al. (2008). The crystal structure of human WD40 repeat-containing peptidylprolyl isomerase (PPWD1). FEBS J. 275, 2283–2295. doi: 10.1111/j.1742-4658.2008.06381.x
Druilhe, P., Brandicourt, O., Chongsuphajaisiddhi, T., and Berthe, J. (1988). Activity of a combination of three cinchona bark alkaloids against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 32, 250–254. doi: 10.1128/AAC.32.2.250
Du, J., Cullen, J. J., and Buettner, G. R. (2012). Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 1826, 443–457. doi: 10.1016/j.bbcan.2012.06.003
Dzeja, P., and Terzic, A. (2009). Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int. J. Mol. Sci. 10, 1729–1772. doi: 10.3390/ijms10041729
Efferth, T., Herrmann, F., Tahrani, A., and Wink, M. (2011). Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine 18, 959–969. doi: 10.1016/j.phymed.2011.06.008
Eigner, D., and Scholz, D. (1999). Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J. Ethnopharmacol. 67, 1–6. doi: 10.1016/S0378-8741(98)00234-7
Eisen, M. B., Spellman, P. T., Brown, P. O., and Botstein, D. (1998). Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U.S.A. 95, 14863–14868. doi: 10.1073/pnas.95.25.14863
Faria, J. A., Corrêa, N. C., de Andrade, C., de Angelis Campos, A. C., Dos Santos Samuel De Almeida, R., Rodrigues, T. S., et al. (2013). SET domain-containing Protein 4 (SETD4) is a newly identified cytosolic and nuclear lysine methyltransferase involved in breast cancer cell proliferation. J. Cancer Sci. Ther. 5, 58–65.
Fenton, J. I., Wolff, M. S., Orth, M. W., and Hord, N. G. (2002). Membrane-type matrix metalloproteinases mediate curcumin-induced cell migration in non-tumorigenic colon epithelial cells differing in Apc genotype. Carcinogenesis 23, 1065–1070. doi: 10.1093/carcin/23.6.1065
Fujiyama-Fujiwara, Y., Umeda, R., and Igarashi, O. (1992). Effects of sesamin and curcumin on delta 5-desaturation and chain elongation of polyunsaturated fatty acid metabolism in primary cultured rat hepatocytes. J. Nutr. Sci. Vitaminol. 38, 353–363. doi: 10.3177/jnsv.38.353
Fukui, M., Yamabe, N., Choi, H. J., Polireddy, K., Chen, Q., and Zhu, B. T. (2015). Mechanism of ascorbate-Induced cell death in human pancreatic cancer cells: role of Bcl-2, beclin 1 and autophagy. Planta Med. 81, 838–846. doi: 10.1055/s-0035-1546132
Gilliam, L. A., and St Clair, D. K. (2011). Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid. Redox Signal. 15, 2543–2563. doi: 10.1089/ars.2011.3965
González, M. J., Miranda-Massari, J. R., Mora, E. M., Guzman, A., Riordan, N. H., Riordan, H. D., et al. (2005). Orthomolecular oncology review: ascorbic acid and cancer 25 years later. Integr. Cancer Ther. 4, 32–44. doi: 10.1177/1534735404273861
Gu, Z., Eils, R., and Schlesner, M. (2016). Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849. doi: 10.1093/bioinformatics/btw313
Gukovsky, I., Reyes, C. N., Vaquero, E. C., Gukovskaya, A. S., and Pandol, S. J. (2003). Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G85–G95. doi: 10.1152/ajpgi.00138.2002
Gülçubuk, A., Sönmez, K., Gürel, A., Altunatmaz, K., Gürler, N., Aydin, S., et al. (2005). Pathologic alterations detected in acute pancreatitis induced by sodium taurocholate in rats and therapeutic effects of curcumin, ciprofloxacin and metronidazole combination. Pancreatology 5, 345–353. doi: 10.1159/000086534
Guo, J., Zheng, L., Liu, W., Wang, X., Wang, Z., Wang, Z., et al. (2011). Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 71, 2978–2987. doi: 10.1158/0008-5472.CAN-10-3482
Guzzarlamudi, S., Singh, P. K., Pawar, V. K., Singh, Y., Sharma, K., Paliwal, S. K., et al. (2016). Synergistic chemotherapeutic activity of curcumin bearing methoxypolyethylene glycol-g-linoleic acid based micelles on breast cancer cells. J. Nanosci. Nanotechnol. 16, 4180–4190. doi: 10.1166/jnn.2016.11699
Hall, M. D., Handley, M. D., and Gottesman, M. M. (2009). Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol. Sci. 30, 546–556. doi: 10.1016/j.tips.2009.07.003
Harrison, F. E., and May, J. M. (2009). Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 46, 719–730. doi: 10.1016/j.freeradbiomed.2008.12.018
Hawkins, S. M., Loomans, H. A., Wan, Y. W., Ghosh-Choudhury, T., Coffey, D., Xiao, W., et al. (2013). Expression and functional pathway analysis of nuclear receptor NR2F2 in ovarian cancer. J. Clin. Endocrinol. Metab. 98, E1152–E1162. doi: 10.1210/jc.2013-1081
Hoffer, L. J., Levine, M., Assouline, S., Melnychuk, D., Padayatty, S. J., Rosadiuk, K., et al. (2008). Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 19, 1969–1974. doi: 10.1093/annonc/mdn377
Holt, P. R., Katz, S., and Kirshoff, R. (2005). Curcumin therapy in inflammatory bowel disease: a pilot study. Dig. Dis. Sci. 50, 2191–2193. doi: 10.1007/s10620-005-3032-8
Huang, H. S., Nagane, M., Klingbeil, C. K., Lin, H., Nishikawa, R., Ji, X. D., et al. (1997). The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J. Biol. Chem. 272, 2927–2935. doi: 10.1074/jbc.272.5.2927
Jacobs, C., Hutton, B., Ng, T., Shorr, R., and Clemons, M. (2015). Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist 20, 210–223. doi: 10.1634/theoncologist.2014-0381
Jeon, J., Nim, S., Teyra, J., Datti, A., Wrana, J. L., Sidhu, S. S., et al. (2014). A systematic approach to identify novel cancer drug targets using machine learning, inhibitor design and high-throughput screening. Genome Med. 6, 57. doi: 10.1186/s13073-014-0057-7
Jiang, M. C., Yang-Yen, H. F., Yen, J. J., and Lin, J. K. (1996). Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr. Cancer 26, 111–120. doi: 10.1080/01635589609514468
Joe, B., Rao, U. J., and Lokesh, B. R. (1997). Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Mol. Cell. Biochem. 169, 125–134. doi: 10.1023/A:1006877928703
Karle, J. M., and Bhattacharjee, A. K. (1999). Stereoelectronic features of the cinchona alkaloids determine their differential antimalarial activity. Bioorg. Med. Chem. 7, 1769–1774. doi: 10.1016/S0968-0896(99)00120-0
Kasi, P. D., Tamilselvam, R., Skalicka-Wozniak, K., Nabavi, S. F., Daglia, M., Bishayee, A., et al. (2016). Molecular targets of curcumin for cancer therapy: an updated review. Tumour Biol. 37, 13017–13028. doi: 10.1007/s13277-016-5183-y
Kehrer, J. P. (2000). The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149, 43–50. doi: 10.1016/S0300-483X(00)00231-6
Kim, J., Lee, S. D., Chang, B., Jin, D. H., Jung, S. I., Park, M. Y., et al. (2012). Enhanced antitumor activity of vitamin C via p53 in cancer cells. Free Radic. Biol. Med. 53, 1607–1615. doi: 10.1016/j.freeradbiomed.2012.07.079
Kimmig, A., Gekeler, V., Neumann, M., Frese, G., Handgretinger, R., Kardos, G., et al. (1990). Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 50, 6793–6799.
Klenner, F. R. (1949). The treatment of poliomyelitis and other virus diseases with vitamin C. South. Med. Surg. 111, 209–214.
Krynetskaia, N. F., Phadke, M. S., Jadhav, S. H., and Krynetskiy, E. Y. (2009). Chromatin-associated proteins HMGB1/2 and PDIA3 trigger cellular response to chemotherapy-induced DNA damage. Mol. Cancer Ther. 8, 864–872. doi: 10.1158/1535-7163.MCT-08-0695
Kuroiwa, Y., Yamada, M., Matsui, K., Okamura, T., Ishii, Y., Masumura, K., et al. (2008). Combined ascorbic acid and sodium nitrite treatment induces oxidative DNA damage-associated mutagenicity in vitro, but lacks initiation activity in rat forestomach epithelium. Toxicol. Sci. 104, 274–282. doi: 10.1093/toxsci/kfn081
Lee, C. W., Lin, W. N., Lin, C. C., Luo, S. F., Wang, J. S., Pouyssegur, J., et al. (2006). Transcriptional regulation of VCAM-1 expression by tumor necrosis factor-alpha in human tracheal smooth muscle cells: involvement of MAPKs, NF-kappaB, p300, and histone acetylation. J. Cell. Physiol. 207, 174–186. doi: 10.1002/jcp.20549
Lee, J. J., Huang, W. T., Shao, D. Z., Liao, J. F., and Lin, M. T. (2003). Blocking NF-kappaB activation may be an effective strategy in the fever therapy. Jpn. J. Physiol. 53, 367–375. doi: 10.2170/jjphysiol.53.367
Lehtinen, L., Ketola, K., Mäkelä, R., Mpindi, J. P., Viitala, M., Kallioniemi, O., et al. (2013). High-throughput RNAi screening for novel modulators of vimentin expression identifies MTHFD2 as a regulator of breast cancer cell migration and invasion. Oncotarget 4, 48–63. doi: 10.18632/oncotarget.756
Leyon, P. V., and Kuttan, G. (2003). Studies on the role of some synthetic curcuminoid derivatives in the inhibition of tumour specific angiogenesis. J. Exp. Clin. Cancer Res. 22, 77–83.
Li, C., Li, L., Luo, J., and Huang, N. (1998). [Effect of turmeric volatile oil on the respiratory tract]. Zhongguo Zhong Yao Za Zhi 23, 624–625 [article in Chinese].
Liacini, A., Sylvester, J., Li, W. Q., and Zafarullah, M. (2002). Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 21, 251–262. doi: 10.1016/S0945-053X(02)00007-0
Lin, L. I., Ke, Y. F., Ko, Y. C., and Lin, J. K. (1998). Curcumin inhibits SK-Hep-1 hepatocellular carcinoma cell invasion in vitro and suppresses matrix metalloproteinase-9 secretion. Oncology 55, 349–353. doi: 10.1159/000011876
Mamede, A. C., Tavares, S. D., Abrantes, A. M., Trindade, J., Maia, J. M., and Botelho, M. F. (2011). The role of vitamins in cancer: a review. Nutr. Cancer 63, 479–494. doi: 10.1080/01635581.2011.539315
Marchitti, S. A., Orlicky, D. J., Brocker, C., and Vasiliou, V. (2010). Aldehyde dehydrogenase 3B1 (ALDH3B1): immunohistochemical tissue distribution and cellular-specific localization in normal and cancerous human tissues. J. Histochem. Cytochem. 58, 765–783. doi: 10.1369/jhc.2010.955773
May, J. M., Qu, Z. C., and Meredith, M. E. (2012). Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem. Biophys. Res. Commun. 426, 148–152. doi: 10.1016/j.bbrc.2012.08.054
McDonald, J. M., Dunmire, V., Taylor, E., Sawaya, R., Bruner, J., Fuller, G. N., et al. (2005). Attenuated expression of DFFB is a hallmark of oligodendrogliomas with 1p-allelic loss. Mol. Cancer 4:35. doi: 10.1186/1476-4598-4-35
Mikirova, N., Casciari, J., Rogers, A., and Taylor, P. (2012). Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J. Transl. Med. 10:189. doi: 10.1186/1479-5876-10-189
Niederau, C., and Göpfert, E. (1999). [The effect of chelidonium- and turmeric root extract on upper abdominal pain due to functional disorders of the biliary system. Results from a placebo-controlled double-blind study]. Med. Klin. (Munich) 94, 425–430. [article in German]. doi: 10.1007/BF03044726
Nilsson, R., Jain, M., Madhusudhan, N., Sheppard, N. G., Strittmatter, L., Kampf, C., et al. (2014). Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat. Commun. 5, 3128. doi: 10.1038/ncomms4128
Parrow, N. L., Leshin, J. A., and Levine, M. (2013). Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics. Antioxid. Redox Signal. 19, 2141–2156. doi: 10.1089/ars.2013.5372
Raimondi, C., and Falasca, M. (2011). Targeting PDK1 in cancer. Curr. Med. Chem. 18, 2763–2769. doi: 10.2174/092986711796011238
Ram, A., Das, M., and Ghosh, B. (2003). Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol. Pharm. Bull. 26, 1021–1024. doi: 10.1248/bpb.26.1021
Rebouche, C. J. (1991). Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 54, 1147S–1152S.
Rozanova Torshina, N., Zhang, J. Z., and Heck, D. E. (2010). Catalytic therapy of cancer with ascorbate and extracts of medicinal herbs. Evid. Based Complement. Alternat. Med. 7, 203–212. doi: 10.1093/ecam/nem159
Sajithlal, G. B., Chithra, P., and Chandrakasan, G. (1998). Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem. Pharmacol. 56, 1607–1614. doi: 10.1016/S0006-2952(98)00237-8
Scherf, U., Ross, D. T., Waltham, M., Smith, L. H., Lee, J. K., Tanabe, L., et al. (2000). A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 24, 236–244. doi: 10.1038/73439
Sertel, S., Eichhorn, T., Bauer, J., Hock, K., Plinkert, P. K., and Efferth, T. (2012). Pharmacogenomic determination of genes associated with sensitivity or resistance of tumor cells to curcumin and curcumin derivatives. J. Nutr. Biochem. 23, 875–884. doi: 10.1016/j.jnutbio.2011.04.012
Sertel, S., Eichhorn, T., Sieber, S., Sauer, A., Weiss, J., Plinkert, P. K., et al. (2010). Factors determining sensitivity or resistance of tumor cell lines towards artesunate. Chem. Biol. Interact. 185, 42–52. doi: 10.1016/j.cbi.2010.02.002
Shao, D. Z., Lee, J. J., Huang, W. T., Liao, J. F., and Lin, M. T. (2004). Inhibition of nuclear factor-kappa B prevents staphylococcal enterotoxin A-induced fever. Mol. Cell. Biochem. 262, 177–185. doi: 10.1023/B:MCBI.0000038233.20276.e0
Shi, Y., Simmons, M. N., Seki, T., Oh, S. P., and Sugrue, S. P. (2001). Change in gene expression subsequent to induction of Pnn/DRS/memA: increase in p21(cip1/waf1). Oncogene 20, 4007–4018. doi: 10.1038/sj.onc.1204507
Shin, E. Y., Kim, S. Y., and Kim, E. G. (2001). c-Jun N-terminal kinase is involved in motility of endothelial cell. Exp. Mol. Med. 33, 276–283. doi: 10.1038/emm.2001.45
Srivastava, R. (1989). Inhibition of neutrophil response by curcumin. Agents Actions 28, 298–303. doi: 10.1007/BF01967418
Sunil Kumar, B. V., Singh, S., and Verma, R. (2015). Anticancer potential of dietary vitamin D and ascorbic acid: a review. Crit. Rev. Food Sci. Nutr. doi: 10.1080/10408398.2015.1064086. [Epub ahead of print].
Swarnakar, S., Ganguly, K., Kundu, P., Banerjee, A., Maity, P., and Sharma, A. V. (2005). Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J. Biol. Chem. 280, 9409–9415. doi: 10.1074/jbc.M413398200
Tawatsin, A., Wratten, S. D., Scott, R. R., Thavara, U., and Techadamrongsin, Y. (2001). Repellency of volatile oils from plants against three mosquito vectors. J. Vector Ecol. 26, 76–82.
Tourkina, E., Gooz, P., Oates, J. C., Ludwicka-Bradley, A., Silver, R. M., and Hoffman, S. (2004). Curcumin-induced apoptosis in scleroderma lung fibroblasts: role of protein kinase cepsilon. Am. J. Respir. Cell Mol. Biol. 31, 28–35. doi: 10.1165/rcmb.2003-0354OC
Vander Heiden, M. G., Cantley, L. C., and Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033. doi: 10.1126/science.1160809
Venturelli, S., Sinnberg, T. W., Niessner, H., and Busch, C. (2015). Molecular mechanisms of pharmacological doses of ascorbate on cancer cells. Wien. Med. Wochenschr. 165, 251–257. doi: 10.1007/s10354-015-0356-7
Verbeek, R., van Tol, E. A., and van Noort, J. M. (2005). Oral flavonoids delay recovery from experimental autoimmune encephalomyelitis in SJL mice. Biochem. Pharmacol. 70, 220–228. doi: 10.1016/j.bcp.2005.04.041
Verrax, J., and Calderon, P. B. (2009). Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 47, 32–40. doi: 10.1016/j.freeradbiomed.2009.02.016
Waldman, T., Kinzler, K. W., and Vogelstein, B. (1995). p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 55, 5187–5190.
Wang, Y., Zhang, T., Kwiatkowski, N., Abraham, B. J., Lee, T. I., Xie, S., et al. (2015). CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell 163, 174–186. doi: 10.1016/j.cell.2015.08.063
Waters, N. J. (2015). Evaluation of drug-drug interactions for oncology therapies: in vitro-in vivo extrapolation model-based risk assessment. Br. J. Clin. Pharmacol. 79, 946–958. doi: 10.1111/bcp.12563
Wright, C. W., Linley, P. A., Brun, R., Wittlin, S., and Hsu, E. (2010). Ancient Chinese methods are remarkably effective for the preparation of artemisinin-rich extracts of Qing Hao with potent antimalarial activity. Molecules 15, 804–812. doi: 10.3390/molecules15020804
Ye, H., Wei, X., Wang, Z., Zhang, S., Ren, J., Yao, S., et al. (2016). A novel double carbonyl analog of curcumin induces the apoptosis of human lung cancer H460 cells via the activation of the endoplasmic reticulum stress signaling pathway. Oncol. Rep. 36, 1640–1648. doi: 10.3892/or.2016.4911
Yeom, C. H., Lee, G., Park, J. H., Yu, J., Park, S., Yi, S. Y., et al. (2009). High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J. Transl. Med. 7:70. doi: 10.1186/1479-5876-7-70
Yoon, J. W., Lamm, M., Iannaccone, S., Higashiyama, N., Leong, K. F., Iannaccone, P., et al. (2015). p53 modulates the activity of the GLI1 oncogene through interactions with the shared coactivator TAF9. DNA Repair (Amst). 34, 9–17. doi: 10.1016/j.dnarep.2015.06.006
Yu, X., Zhong, J., Yan, L., Li, J., Wang, H., Wen, Y., et al. (2016). Curcumin exerts antitumor effects in retinoblastoma cells by regulating the JNK and p38 MAPK pathways. Int. J. Mol. Med. 38, 861–868. doi: 10.3892/ijmm.2016.2676
Zeng, Y., Weng, G., Fan, J., Li, Z., Wu, J., Li, Y., et al. (2016). Curcumin reduces the expression of survivin, leading to enhancement of arsenic trioxide-induced apoptosis in myelodysplastic syndrome and leukemia stem-like cells. Oncol. Rep. 36, 1233–1242. doi: 10.3892/or.2016.4944
Keywords: drug interaction, isobologram analysis, pharmacogenomics, phytotherapy, synergism
Citation: Ooko E, Kadioglu O, Greten HJ and Efferth T (2017) Pharmacogenomic Characterization and Isobologram Analysis of the Combination of Ascorbic Acid and Curcumin—Two Main Metabolites of Curcuma longa—in Cancer Cells. Front. Pharmacol. 8:38. doi: 10.3389/fphar.2017.00038
Received: 07 September 2016; Accepted: 18 January 2017;
Published: 02 February 2017.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Subhalakshmi Ghosh, Jadavpur University, IndiaFumiaki Uchiumi, Tokyo University of Science, Japan
Copyright © 2017 Ooko, Kadioglu, Greten and Efferth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Efferth, ZWZmZXJ0aEB1bmktbWFpbnouZGU=
 Edna Ooko1
Edna Ooko1 Onat Kadioglu
Onat Kadioglu Thomas Efferth
Thomas Efferth