
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol., 16 December 2016
Sec. Experimental Pharmacology and Drug Discovery
Volume 7 - 2016 | https://doi.org/10.3389/fphar.2016.00491
This article is part of the Research TopicPrecision Medicine: an Approach of Multiple Targeted Therapy in CancerView all 15 articles
 Stefania Trino1*†
Stefania Trino1*† Luciana De Luca1†
Luciana De Luca1† Ilaria Laurenzana1
Ilaria Laurenzana1 Antonella Caivano1
Antonella Caivano1 Luigi Del Vecchio2,3
Luigi Del Vecchio2,3 Giovanni Martinelli4
Giovanni Martinelli4 Pellegrino Musto5
Pellegrino Musto5The tumor suppressor p53 is a canonical regulator of different biological functions, like apoptosis, cell cycle arrest, DNA repair, and genomic stability. This gene is frequently altered in human tumors generally by point mutations or deletions. Conversely, in acute lymphoblastic leukemia (ALL) genomic alterations of TP53 are rather uncommon, and prevalently occur in patients at relapse or with poor prognosis. On the other hand, p53 pathway is often compromised by the inactivation of its regulatory proteins, as MDM2 and ARF. MDM2 inhibitor molecules are able to antagonize p53-MDM2 interaction allowing p53 to exert tumor suppressor transcriptional regulation and to induce apoptotic pathways. Recent preclinical and clinical studies propose that MDM2 targeted therapy represents a promising anticancer strategy restoring p53 dependent mechanisms in ALL disease. Here, we discussed the use of new small molecule targeting p53 pathways as a promising drug target therapy in ALL.
TP53 is a tumor suppressor gene, located on chromosome 17p13.1, with the main function to prevent cancer transformation (Brady and Attardi, 2010). P53 is a transcription factor that activates or represses a series of target genes exerting different biological functions (Shi and Gu, 2012; Leenders and Tuszynski, 2013). Consequently to a plethora of multiple stress signals, p53 determines cell fate activating apoptosis or maintaining cells at the G1/S regulation point in a reversible cell cycle arrest process; furthermore, it can induce cellular senescence characterized by an irreversible loss of proliferative potential (Demidenko et al., 2010; Timofeev et al., 2013; Burgess et al., 2016). P53 dysfunction can promote the initiation or progression of different human tumors and confer malignant characteristics, such as altered cellular differentiation, genetic instability, and increased metastatic potential (Muller and Vousden, 2013; Bieging et al., 2014). Generally, TP53 is inactivated in the majority of human solid tumors by missense mutations and deletions impairing transcriptional function of the protein (Olivier et al., 2010; Naccarati et al., 2012; Gibbons et al., 2014). Conversely, in hematological malignancies, where p53 mutations are less recurrent, its activity may be likewise compromised by the alterations of MDM2 (Table 1) and ARF (Richmond et al., 2015; Kojima et al., 2016), two regulators of p53. MDM2 (mouse double minute-2) binds p53 regulating its stability and cellular localization. This interaction inhibits p53 mediated transcriptional activity and induces p53 proteasomal degradation (Eischen and Lozano, 2009; Van Maerken et al., 2014). ARF (alternative reading frame), instead, is a tumor suppressor encoded by CDKN2A gene, that participates to the regulation of p53, by interacting with MDM2. This binding blocks MDM2 shuttling between the nucleus and cytoplasm avoiding p53 degradation (Maggi et al., 2014; Vivo et al., 2015).
In acute lymphoblastic leukemia (ALL) MDM2 is overexpressed (Zhou et al., 1995, 2000; Gu et al., 2008) and CDKN2A gene is frequently deleted (Usvasalo et al., 2008; Iacobucci et al., 2011).
In this review, we summarized the current knowledge about p53-MDM2 axis in ALL focusing our attention on a new potential therapeutic agent restoring p53 dependent mechanisms in this hematological disease.
TP53 mutations were considered infrequent in ALL (Hof et al., 2011; Chiaretti et al., 2013; Saha et al., 2013) and were correlated with cytogenetic alterations, like low hypodiploidy, or MYC-rearrangements (Holmfeldt et al., 2013; Stengel et al., 2014). Moreover, the disruption of both TP53 alleles was associated with adverse prognosis (Stengel et al., 2014). Also the aberrant methylation could contribute to TP53 gene inactivation; in particular, Agirre et al. (2003) showed that TP53 promoter resulted methylated in 8 of out 25 ALL patients and its expression was decreased in all the methylated samples. Other literature data found 13 genes, involved in the TP53 dependent pathway, down-regulated by hypermethylation in a large cohort of ALL patients at diagnosis. Methylation of at least 1 of the 13 genes was observed in 78% of the patients, which significantly correlated with a higher relapse and mortality rate predicting the clinical outcome of patients (Vilas-Zornoza et al., 2011).
On the other hand, also deregulation of microRNAs was found to be correlated with p53 alteration. In particular, Nucera et al. (2016) focused their attention of miRNA-126, a regulator of hematopoietic stem cell quiescence. They found that mir-126 was highly expressed in human B-ALL and target p53 response genes orchestrating an oncogenic program by down-regulation of p53-dependent pathway. Another microRNA found to have a role as onco-miRNA in ALL was mir-181a that down-regulated the expression of tumor suppressor gene EGR1 (Verduci et al., 2015).
Finally, p53 was also inactivated by the frequent deletion of CDNK2A (Usvasalo et al., 2008; Iacobucci et al., 2011) and the overexpression of MDM2 in ALL patients (Zhou et al., 1995, 2000; Gu et al., 2008).
B-ALL is a heterogeneous disease on biological and clinical point of view, affecting pediatric, adolescent, adult, and older patients. It prevalently occurs, however, in childhood, in whom the prognosis is more favorable respect to adult patients, reaching a cure rate of 80–90% thanks to multi-agent and intensive combination chemotherapy regimens that have significantly improved the outcome in the pediatric setting (Hunger and Mullighan, 2015; Pui et al., 2015), as well as in that of adolescent and younger adults (Curran and Stock, 2015). In other patients, instead, “conventional” treatments remain unsatisfactory (Marks, 2015; Al Ustwani et al., 2016; Fedorov et al., 2016), due to pharmacologic resistance (Ronson et al., 2016; Seiter, 2016) or toxicity events, above all when aggressive “pediatric-like” protocols are applied (Dias et al., 2016).
A subset of B-ALL shows t(9:22) translocation that generates “Philadelphia” chromosome (Ph) encoding a specific BCR-ABL1 tyrosine kinase fusion protein. This alteration occurs in 3–4% of pediatric ALL and about 25% of adult patients, increasing with age: these patients strongly benefit of the BCR–ABL1 tyrosine kinase inhibitors (TKI) as first-line treatment (Malagola et al., 2016). However, although TKI monotherapy induces complete remission rates of 90–100% with low toxicity profile even in older patients (Vignetti et al., 2007; Foa et al., 2011), the combination of TKI with standard chemotherapy is generally required to obtain higher long-term disease free survival in both adults (Fielding et al., 2014; Fielding, 2015) and children (Biondi et al., 2012; Bleckmann and Schrappe, 2016) with Ph positive ALL.
More recently, new therapies seem to be appealing for treatment of refractory/relapsed patients. They are based on monoclonal antibodies targeting antigens, including CD19, CD20, CD22, and CD52, expressed on leukemic blast cell surface (Jabbour et al., 2015). Rituximab, an anti-CD20 antibody, in combination with conventional chemotherapy, has been shown to improve survival in newly diagnosed CD20+ ALL (Maury et al., 2016). Blinatumomab, a T-cell engaging bi-specific single-chain antibody (BiTE) direct to CD19 and CD3, is used as monotherapy in relapsed and refractory ALL, prolonging relapse free survival (Benjamin and Stein, 2016; Le Jeune and Thomas, 2016). Inotuzumab ozogamicin, an anti-CD22 antibody conjugated with a toxin, alone and in combination with chemotherapy, has been promising in relapsed and refractory B ALL (Yilmaz et al., 2015). Several newer monoclonal antibodies (ofatumumab, obinutuzumab, epratuzumab, denintuzumab mafodotin and, moxetumomab pasudotox) are currently under investigation as single agents or in combination with a chemotherapeutic back bone (Farhadfar and Litzow, 2016).
Other novel clinical approaches are related to immunotherapy by engineering of T-cells, derived from patients or allogeneic donors, with synthetic chimeric antigen receptors (CAR T-cells) that activate T cells enhancing their function (Maude et al., 2015; Sadelain et al., 2015).
To improve the outcome of B-ALL patients, novel therapeutic strategies have been developed, like the reactivation of apoptotic pathway by inhibiting MDM2 protein.
Zhang et al. (2014) demonstrated that Nilotinib, a second generation TKI inhibitor, inhibited MDM2 in both Ph+ and Ph- ALL cell lines with high MDM2 expression. This inhibition activated a p53-independent apoptosis by down-regulation of the anti-apoptotic protein XIAP. Gu et al. (2008) instead showed a cytotoxic activity of Nutlin-3a, a cis-imidazoline small molecules antagonizing Mdm2-p53 binding, in pediatric ALL with p53 wild-type and over-expressing MDM2. Moreover, they also found the positive correlation between MDM2 expression and Nutlin-3A cytotoxicity in ALL. In fact, a major effect of Nutlin was observed in cells over-expressing MDM2 respect to MDM2-negative ALL cells, probably due to the higher induction of p53, p21, Bax, and PUMA (Gu et al., 2008).
Moreover, Zhu et al. (2008) performed in vitro experiments with Nutlin and the inhibitor of antiapoptotic PI3K/AKT pathway that is frequently activated in different cancer cell types. They demonstrated the synergic effect of these drugs in inducing apoptosis in ALL cells.
Recently, we observed the effects of Nutlin-3a in adult B-ALL confirming the activation of p53-mediated pathway in wild-type p53 ALL cells (Trino et al., 2016). Given the clinical significance of BCR-ABL1 mutations in inducing resistance to conventional therapy (Soverini et al., 2016), we analyzed the efficacy of Nutlin-3a in Ph+ ALL resistant patients carrying the T315I BCR-ABL1 mutation. Interestingly, we observed that this drug is able to reduce in vitro cell viability in this subtype of resistant ALL suggesting its potential therapeutic application in resistant clinical setting of patients (Trino et al., 2016).
Moreover, due to the evidences that ETV6/RUNX1 (E/R), the most common fusion gene in childhood ALL, impaired p53 signaling, Kaindl et al. (2014) investigated the effect of Nutlin in E/R ALL cells. They demonstrated that MDM2 was over-expressed in E/R-positive respect to E/R-negative primary B-cell precursor-ALL samples, showing also that E/R transcription factor binds to the MDM2 P2 promoter and consequently up-regulates MDM2 in a direct and p53-independent manner. Nutlin-3 treatment reactivated p53 function in E/R-expressing leukemic cell lines, leading to cell cycle arrest, enhanced apoptosis, and increased expression of p53 direct targets p21, MDM2, and the pro-apoptotic BAX and PUMA (Kaindl et al., 2014).
Furthermore, Richmond et al. (2015) carried out a preclinical study in a specific subset of infant ALL patients carrying the translocation in the mixed-lineage leukemia (MLL) oncogene, associated with a lower survival rate. They demonstrated that RG7112, the analog of Nutlin-3a, induced regression and prolonged progression delay in a panel of patient-derived infant MLL-ALL xenografts, and p53 upregulation, cell cycle arrest and induction of apoptosis.
Kang et al. (2016) instead tested the efficacy of another inhibitor of MDM2, MK-8242, in in vitro and in vivo tumor panels and compared this study with their previous evaluation of RG7112 in the same cell line models (Carol et al., 2013). For both agents, they demonstrated that the in vitro ALL cell line sensitivity correlated with TP53 mutation status. Moreover, for in vivo experiments, the response of the leukemia xenografts was similar between MK-8242 and RG7112; in particular, xenografts from two MLL-rearranged cell lines achieved or maintained complete responses. Other non-MLL ALL xenografts had partial responses to MK-8242.
Interestingly, emerging literature data reported that MDM2 inhibition played a role not only in apoptosis induction but also in autophagy activation in different hematological malignancies, like multiple myeloma (Gu et al., 2014) and acute myeloid leukemia (AML; Borthakur et al., 2015).
Collectively, these different studies indicated that MDM2 inhibition could be a new promising target therapy in hematological malignancies.
Since drug resistance to MDM2 inhibitors or current therapeutic agents can be acquired by tumor cells, pharmacological combination could be a successful strategy to improve the treatment outcome and to reduce the side-effects of the drugs. In this regard, different groups evaluated in vitro the combinatory effects between Nutlin-3a and conventional drugs used in ALL therapy. Kaindl et al. (2014) reported that co-exposure of Nutlin-3a and chemotherapeutic drugs (daunorubicin, asparaginase, vincristine) reduced cell viability and potentiated apoptosis in a childhood ALL cell line, with E/R fusion gene.
In our previous study, we evaluated in vitro the co-treatment of Nutlin-3a with TKIs in Ph+ cell lines. In particular, the combination between Nutlin-3a and Imatinib, Dasatinib or Nilotinib showed significant effect in reducing cell viability of a Ph+ cell line in comparison with the effect of the single TKI treatment (Trino et al., 2016).
Another study by Richmond et al. (2015), showed that combining RG7112 with an induction type regimen (vincristine, dexamethasone, and L-asparaginase) significantly enhanced objective responses and prolonged leukemia regression in vivo MLL-ALL xenografts.
On the light of these pre-clinical evidences, literature data underline that targeting the p53-MDM2 axis in combination with established drugs for the management of ALL warrants further investigations.
As previously described, different preclinical studies demonstrated the in vitro and in vivo effects of MDM2 inhibitors to kill wild-type p53 tumor cells. Therefore, due to their promising anticancer abilities, these drugs are now translated into clinical trials to better assess their biological effects and toxicities in patients. RG7112 was the first MDM2 inhibitor entered clinical evaluation. Recently, a multicenter phase I trial of RG7112 was conducted in patients with hematological malignancies, including ALL (Andreeff et al., 2016). This study confirmed p53 stabilization and transcriptional activation of p53 target genes after MDM2 antagonist treatment, also demonstrating clinical activity in patients with poor prognosis, relapsed, or refractory. To identify the effective biomarkers of response, in this study were evaluated the p53 status by detection of single nucleotide substitution or deletion in exons 2-11 as well as their splice sites. Moreover, mRNA expression, by quantitative real-time PCR, of 24 direct and indirect p53 target genes and MDM2 transcript was also examined. By analyzing patient data the authors did not find any molecular marker predicting response to RG7112. Since this inhibitor was effective in patients with at least 1 wild-type TP53 allele, TP53 mutation status alone did not define pharmacological response. Furthermore, baseline MDM2 expression levels were found positively correlated with clinical response, but also this was not sufficient to define MDM2 as a single predictive marker of sensitivity to treatment. The analysis of p53 target genes showed 10, among 24, p53 target genes significantly modulated but only in p53 wild-type samples. Among those, the most induced genes were CDKN1A/p21, a crucial p53-mediator of cell-cycle arrest, and BBC3/PUMA, an important mediator of p53 dependent apoptosis (Andreeff et al., 2016).
However, from a clinical point of view, RG7112 showed several disadvantages as the gastrointestinal intolerance due to a high dose required for drug efficacy and variability of exposure at the maximum tolerate dose. To overcome these limitations, recently a new potent MDM2 inhibitor RG7388, also known as Idasanutlin, has been discovered (Ding et al., 2013) and actually entered in a phase 1/1b study in relapsed/refractory AML. Recent data about this trial revealed that MDM2 protein expression levels in leukemic blasts and stem cells were associated with Idasanutlin-induced complete remission in AML patients (Reis et al., 2016). Moreover, the same trial evaluated Idasanutlin as monotherapy or in combination with cytarabine in relapsed/refractory AML patients (Reis et al., 2016). No current data are available on ALL.
P53 pathway is often altered in ALL, in particular due to the overexpression of MDM2 and deletion of CDKN2A, the two main regulator of p53. Thus, targeting of MDM2-p53 axis could represent an attractive cancer therapeutic strategy in ALL. Nodaway, potent and selective MDM2 inhibitor drugs are available, such as Nutlins (Figure 1). These small molecules not only showed a preclinical evidence to restore p53 pathway, but also had a pharmaceutical properties and entered into clinical trials.
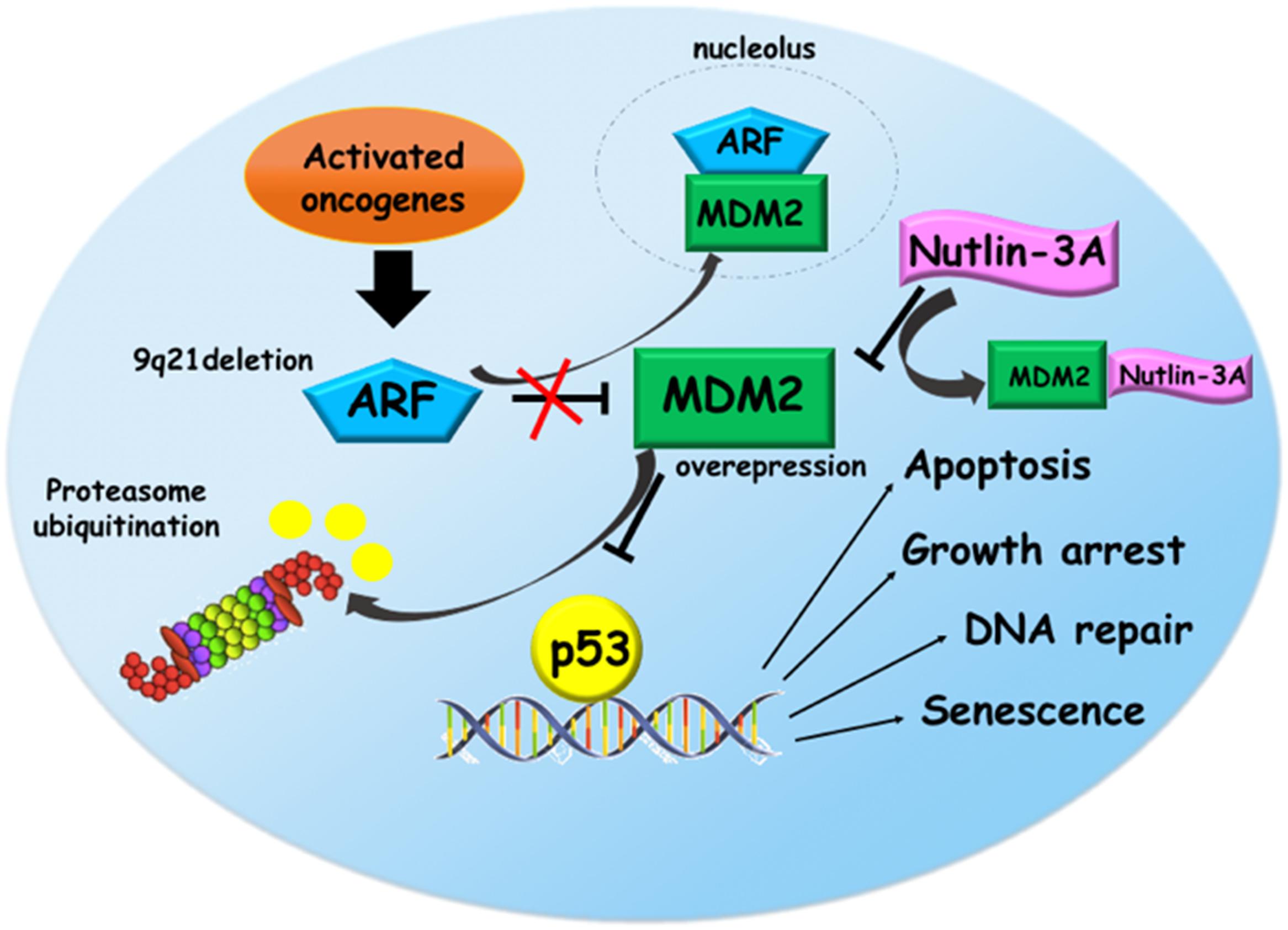
FIGURE 1. Reactivation of p53 pathway via Nutlin-3a in acute lymphoblastic leukemia (ALL). In response to oncogenic activation, ARF protein interacts with MDM2 sequestering it into the nucleolus. This binding prevents the proteasomal degradation of p53 that activates its target genes promoting several functions like apoptosis, growth arrest, DNA repair, and senescence. In ALL, 9p21 locus deletion and MDM2 overexpression eliminate the tumor surveillance mechanism based on ARF-MDM2 interaction leading to the p53 degradation. Nutlin-3a, a small molecule targeting MDM2, restores p53 pathway, suggesting a promising therapeutic option for ALL.
Clinical testing of Nutlin-3a and new agents activating p53 tumor suppressor functions may provide proof of concept for their therapeutic approaches in ALL.
ST and LDL revised the literature available on this topic and wrote the paper; IL and AC contributed in the scientific writing of the manuscript; LDV, GM, and PM revised the manuscript. All authors approved the paper for publication.
This paper was supported by Italian Ministry of Health, Current Research Funds for IRCCS, CUP E66J12000230001.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AR and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Agirre, X., Vizmanos, J. L., Calasanz, M. J., Garcia-Delgado, M., Larrayoz, M. J., and Novo, F. J. (2003). Methylation of CpG dinucleotides and/or CCWGG motifs at the promoter of TP53 correlates with decreased gene expression in a subset of acute lymphoblastic leukemia patients. Oncogene 22, 1070–1072. doi: 10.1038/sj.onc.1206236
Al Ustwani, O., Gupta, N., Bakhribah, H., Griffiths, E., Wang, E., and Wetzler, M. (2016). Clinical updates in adult acute lymphoblastic leukemia. Crit. Rev. Oncol. Hematol 99, 189–199. doi: 10.1016/j.critrevonc.2015.12.007
Andreeff, M., Kelly, K. R., Yee, K., Assouline, S., Strair, R., Popplewell, L., et al. (2016). Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in Leukemia. Clin. Cancer Res 22, 868–876. doi: 10.1158/1078-0432
Benjamin, J. E., and Stein, A. S. (2016). The role of blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Ther. Adv. Hematol. 7, 142–156. doi: 10.1177/2040620716640422
Bieging, K. T., Mello, S. S., and Attardi, L. D. (2014). Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 14, 359–370. doi: 10.1038/nrc3711
Biondi, A., Schrappe, M., De Lorenzo, P., Castor, A., Lucchini, G., Gandemer, V., et al. (2012). Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 13, 936–945. doi: 10.1016/S1470-2045(12)70377-7
Bleckmann, K., and Schrappe, M. (2016). Advances in therapy for Philadelphia-positive acute lymphoblastic leukaemia of childhood and adolescence. Br. J. Haematol. 172, 855–869. doi: 10.1111/bjh.13896
Borthakur, G., Duvvuri, S., Ruvolo, V., Tripathi, D. N., Piya, S., Burks, J., et al. (2015). MDM2 inhibitor, nutlin 3a, induces p53 dependent autophagy in acute leukemia by AMP kinase activation. PLoS ONE 10:e0139254. doi: 10.1371/journal.pone.0139254
Brady, C. A., and Attardi, L. D. (2010). p53 at a glance. J. Cell Sci. 123, 2527–2532. doi: 10.1242/jcs.064501
Burgess, A., Chia, K. M., Haupt, S., Thomas, D., Haupt, Y., and Lim, E. (2016). Clinical Overview of MDM2/X-targeted therapies. Front. Oncol. 6:7. doi: 10.3389/fonc.2016.00007
Carol, H., Reynolds, C. P., Kang, M. H., Keir, S. T., Maris, J. M., Gorlick, R., et al. (2013). Initial testing of the MDM2 inhibitor RG7112 by the pediatric preclinical testing program. Pediatr. Blood Cancer 60, 633–641. doi: 10.1002/pbc.24235
Carter, B. Z., Mak, P. Y., Mak, D. H., Ruvolo, V. R., Schober, W., Mcqueen, T., et al. (2015). Synergistic effects of p53 activation via MDM2 inhibition in combination with inhibition of Bcl-2 or Bcr-Abl in CD34+ proliferating and quiescent chronic myeloid leukemia blast crisis cells. Oncotarget 6, 30487–30499. doi: 10.18632/oncotarget.5890
Chiaretti, S., Brugnoletti, F., Tavolaro, S., Bonina, S., Paoloni, F., Marinelli, M., et al. (2013). TP53 mutations are frequent in adult acute lymphoblastic leukemia cases negative for recurrent fusion genes and correlate with poor response to induction therapy. Haematologica 98, e59–e61. doi: 10.3324/haematol.2012.076786
Curran, E., and Stock, W. (2015). How I treat acute lymphoblastic leukemia in older adolescents and young adults. Blood 125, 3702–3710. doi: 10.1182/blood-2014-11-551481
Davies, A. J., Lee, A. M., Taylor, C., Clear, A. J., Goff, L. K., Iqbal, S., et al. (2005). A limited role for TP53 mutation in the transformation of follicular lymphoma to diffuse large B-cell lymphoma. Leukemia 19, 1459–1465. doi: 10.1038/sj.leu.2403802
Demidenko, Z. N., Korotchkina, L. G., Gudkov, A. V., and Blagosklonny, M. V. (2010). Paradoxical suppression of cellular senescence by p53. Proc. Natl. Acad. Sci. U.S.A. 107, 9660–9664. doi: 10.1073/pnas.1002298107
Dias, A., Kenderian, S. J., Westin, G. F., and Litzow, M. R. (2016). Novel therapeutic strategies in acute lymphoblastic leukemia. Curr. Hematol. Malig. Rep. 11, 253–264. doi: 10.1007/s11899-016-0326-1
Ding, Q., Zhang, Z., Liu, J. J., Jiang, N., Zhang, J., Ross, T. M., et al. (2013). Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J. Med. Chem. 56, 5979–5983. doi: 10.1021/jm400487c
Eischen, C. M., and Lozano, G. (2009). p53 and MDM2: antagonists or partners in crime? Cancer Cell 15, 161–162. doi: 10.1016/j.ccr.2009.02.004
Faderl, S., Kantarjian, H. M., Estey, E., Manshouri, T., Chan, C. Y., Rahman Elsaied, A., et al. (2000). The prognostic significance of p16(INK4a)/p14(ARF) locus deletion and MDM-2 protein expression in adult acute myelogenous leukemia. Cancer 89, 1976–1982. doi: 10.1002/1097-0142(20001101)89:9<1976::AID-CNCR14>3.3.CO;2-E
Farhadfar, N., and Litzow, M. R. (2016). New monoclonal antibodies for the treatment of acute lymphoblastic leukemia. Leuk. Res. 49, 13–21. doi: 10.1016/j.leukres.2016.07.009
Fedorov, V. D., Upadhyay, V. A., and Fathi, A. T. (2016). The approach to acute lymphoblastic leukemia in older patients: conventional treatments and emerging therapies. Curr. Hematol. Malig. Rep. 11, 165–174. doi: 10.1007/s11899-016-0316-3
Fielding, A. K. (2015). Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: a broader range of options, improved outcomes, and more therapeutic dilemmas. Am. Soc. Clin. Oncol. Educ. Book 35, e352–e359. doi: 10.14694/EdBook_AM.2015.35.e352
Fielding, A. K., Rowe, J. M., Buck, G., Foroni, L., Gerrard, G., Litzow, M. R., et al. (2014). UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood 123, 843–850. doi: 10.1182/blood-2013-09-529008
Foa, R., Vitale, A., Vignetti, M., Meloni, G., Guarini, A., De Propris, M. S., et al. (2011). Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 118, 6521–6528. doi: 10.1182/blood-2011-05-351403
Gibbons, D. L., Byers, L. A., and Kurie, J. M. (2014). Smoking, p53 mutation, and lung cancer. Mol. Cancer Res. 12, 3–13. doi: 10.1158/1541-7786
Gu, D., Wang, S., Kuiatse, I., Wang, H., He, J., Dai, Y., et al. (2014). Inhibition of the MDM2 E3 Ligase induces apoptosis and autophagy in wild-type and mutant p53 models of multiple myeloma, and acts synergistically with ABT-737. PLoS ONE 9:e103015. doi: 10.1371/journal.pone.0103015
Gu, L., Zhu, N., Findley, H. W., and Zhou, M. (2008). MDM2 antagonist nutlin-3 is a potent inducer of apoptosis in pediatric acute lymphoblastic leukemia cells with wild-type p53 and overexpression of MDM2. Leukemia 22, 730–739. doi: 10.1038/leu.2008.11
Haidar, M. A., El-Hajj, H., Bueso-Ramos, C. E., Manshouri, T., Glassman, A., Keating, M. J., et al. (1997). Expression profile of MDM-2 proteins in chronic lymphocytic leukemia and their clinical relevance. Am. J. Hematol. 54, 189–195. doi: 10.1002/(SICI)1096-8652(199703)54:3<189::AID-AJH3>3.3.CO;2-X
Hernandez, L., Bea, S., Pinyol, M., Ott, G., Katzenberger, T., Rosenwald, A., et al. (2005). CDK4 and MDM2 gene alterations mainly occur in highly proliferative and aggressive mantle cell lymphomas with wild-type INK4a/ARF locus. Cancer Res. 65, 2199–2206. doi: 10.1158/0008-5472.CAN-04-1526
Hof, J., Krentz, S., Van Schewick, C., Korner, G., Shalapour, S., Rhein, P., et al. (2011). Mutations and deletions of the TP53 gene predict nonresponse to treatment and poor outcome in first relapse of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 29, 3185–3193. doi: 10.1200/JCO.2011.34.8144
Holmfeldt, L., Wei, L., Diaz-Flores, E., Walsh, M., Zhang, J., Ding, L., et al. (2013). The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat. Genet. 45, 242–252. doi: 10.1038/ng.2532
Hunger, S. P., and Mullighan, C. G. (2015). Acute lymphoblastic leukemia in children. N. Engl. J. Med. 373, 1541–1552. doi: 10.1056/NEJMra1400972
Iacobucci, I., Ferrari, A., Lonetti, A., Papayannidis, C., Paoloni, F., Trino, S., et al. (2011). CDKN2A/B alterations impair prognosis in adult BCR-ABL1-positive acute lymphoblastic leukemia patients. Clin. Cancer Res. 17, 7413–7423. doi: 10.1158/1078-0432.CCR-11-1227
Isin, M., Yenerel, M., Aktan, M., Buyru, N., and Dalay, N. (2012). Analysis of p53 tumor suppressor pathway genes in chronic lymphocytic leukemia. DNA Cell Biol. 31, 777–782. doi: 10.1089/dna.2011.1314
Jabbour, E., O’brien, S., Ravandi, F., and Kantarjian, H. (2015). Monoclonal antibodies in acute lymphoblastic leukemia. Blood 125, 4010–4016. doi: 10.1182/blood-2014-08-596403
Kaindl, U., Morak, M., Portsmouth, C., Mecklenbrauker, A., Kauer, M., Zeginigg, M., et al. (2014). Blocking ETV6/RUNX1-induced MDM2 overexpression by Nutlin-3 reactivates p53 signaling in childhood leukemia. Leukemia 28, 600–608. doi: 10.1038/leu.2013.345
Kang, M. H., Reynolds, C. P., Kolb, E. A., Gorlick, R., Carol, H., Lock, R., et al. (2016). Initial testing (Stage 1) of MK-8242-A novel MDM2 inhibitor-by the pediatric preclinical testing Program. Pediatr. Blood Cancer 63, 1744–1752. doi: 10.1002/pbc.26064
Kojima, K., Ishizawa, J., and Andreeff, M. (2016). Pharmacological activation of wild-type p53 in the therapy of leukemia. Exp. Hematol. 44, 791–798. doi: 10.1016/j.exphem.2016.05.014
Kojima, K., Konopleva, M., Samudio, I. J., Shikami, M., Cabreira-Hansen, M., Mcqueen, T., et al. (2005). MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood 106, 3150–3159. doi: 10.1182/blood-2005-02-0553
Kryukov, F., Dementyeva, E., Kubiczkova, L., Jarkovsky, J., Brozova, L., Petrik, J., et al. (2013). Cell cycle genes co-expression in multiple myeloma and plasma cell leukemia. Genomics 102, 243–249. doi: 10.1016/j.ygeno.2013.06.007
Kupper, M., Joos, S., Von Bonin, F., Daus, H., Pfreundschuh, M., Lichter, P., et al. (2001). MDM2 gene amplification and lack of p53 point mutations in hodgkin and reed-sternberg cells: results from single-cell polymerase chain reaction and molecular cytogenetic studies. Br. J. Haematol. 112, 768–775. doi: 10.1046/j.1365-2141.2001.02566.x
Le Jeune, C., and Thomas, X. (2016). Potential for bispecific T-cell engagers: role of blinatumomab in acute lymphoblastic leukemia. Drug Des. Devel. Ther. 10, 757–765. doi: 10.2147/DDDT.S83848
Leenders, G. B., and Tuszynski, J. A. (2013). Stochastic and deterministic models of cellular p53 regulation. Front. Oncol. 3:64. doi: 10.3389/fonc.2013.00064
Maggi, L. B. Jr., Winkeler, C. L., Miceli, A. P., Apicelli, A. J., Brady, S. N., Kuchenreuther, M. J., et al. (2014). ARF tumor suppression in the nucleolus. Biochim. Biophys. Acta 1842, 831–839. doi: 10.1016/j.bbadis.2014.01.016
Malagola, M., Papayannidis, C., and Baccarani, M. (2016). Tyrosine kinase inhibitors in Ph+ acute lymphoblastic leukaemia: facts and perspectives. Ann. Hematol. 95, 681–693. doi: 10.1007/s00277-016-2617-y
Marks, D. I. (2015). The challenges of managing older patients with acute lymphoblastic leukemia. Am. Soc. Clin. Oncol. Educ. Book e343–e351. doi: 10.14694/EdBook_AM.2015.35.e343
Maude, S. L., Teachey, D. T., Porter, D. L., and Grupp, S. A. (2015). CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 125, 4017–4023. doi: 10.1182/blood-2014-12-580068
Maury, S., Chevret, S., Thomas, X., Heim, D., Leguay, T., Huguet, F., et al. (2016). Rituximab in B-lineage adult acute lymphoblastic leukemia. N. Engl. J. Med. 375, 1044–1053. doi: 10.1056/NEJMoa1605085
Muller, P. A., and Vousden, K. H. (2013). p53 mutations in cancer. Nat. Cell Biol. 15, 2–8. doi: 10.1038/ncb2641
Naccarati, A., Polakova, V., Pardini, B., Vodickova, L., Hemminki, K., Kumar, R., et al. (2012). Mutations and polymorphisms in TP53 gene–an overview on the role in colorectal cancer. Mutagenesis 27, 211–218. doi: 10.1093/mutage/ger067
Nucera, S., Giustacchini, A., Boccalatte, F., Calabria, A., Fanciullo, C., Plati, T., et al. (2016). miRNA-126 orchestrates an oncogenic program in B cell precursor acute lymphoblastic leukemia. Cancer Cell 29, 905–921. doi: 10.1016/j.ccell.2016.05.007
Olivier, M., Hollstein, M., and Hainaut, P. (2010). TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2:a001008. doi: 10.1101/cshperspect.a001008
Pagnano, K. B., Vassallo, J., Lorand-Metze, I., Costa, F. F., and Saad, S. T. (2001). p53, Mdm2, and c-Myc overexpression is associated with a poor prognosis in aggressive non-Hodgkin’s lymphomas. Am. J. Hematol. 67, 84–92. doi: 10.1002/ajh.1084
Pui, C. H., Yang, J. J., Hunger, S. P., Pieters, R., Schrappe, M., Biondi, A., et al. (2015). Childhood acute lymphoblastic leukemia: progress through collaboration. J. Clin. Oncol. 33, 2938–2948. doi: 10.1200/JCO.2014.59.1636
Reis, B., Jukofsky, L., Chen, G., Martinelli, G., Zhong, H., So, W. V., et al. (2016). Acute myeloid leukemia patients’ clinical response to idasanutlin (RG7388) is associated with pre-treatment MDM2 protein expression in leukemic blasts. Haematologica 101, e185–e188. doi: 10.3324/haematol.2015.139717
Richmond, J., Carol, H., Evans, K., High, L., Mendomo, A., Robbins, A., et al. (2015). Effective targeting of the P53-MDM2 axis in preclinical models of infant MLL-rearranged acute lymphoblastic leukemia. Clin. Cancer Res. 21, 1395–1405. doi: 10.1158/1078-0432.CCR-14-2300
Riley, M. F., You, M. J., Multani, A. S., and Lozano, G. (2016). Mdm2 overexpression and p73 loss exacerbate genomic instability and dampen apoptosis, resulting in B-cell lymphoma. Oncogene 35, 358–365. doi: 10.1038/onc.2015.88
Ronson, A., Tvito, A., and Rowe, J. M. (2016). Treatment of relapsed/refractory acute lymphoblastic leukemia in adults. Curr. Oncol. Rep. 18:39. doi: 10.1007/s11912-016-0519-8
Sadelain, M., Brentjens, R., Riviere, I., and Park, J. (2015). CD19 CAR therapy for acute lymphoblastic leukemia. Am. Soc. Clin. Oncol. Educ. Book e360–e363. doi: 10.14694/EdBook_AM.2015.35.e360
Saha, M. N., Qiu, L., and Chang, H. (2013). Targeting p53 by small molecules in hematological malignancies. J. Hematol. Oncol. 6, 23. doi: 10.1186/1756-8722-6-23
Seiter, K. (2016). Therapy for relapsed acute lymphoblastic leukemia: still a role for standard chemotherapy regimens? Leuk. Res. 41, 1–2. doi: 10.1016/j.leukres.2015.12.004
Shi, D., and Gu, W. (2012). Dual roles of MDM2 in the regulation of p53: ubiquitination dependent and ubiquitination independent mechanisms of MDM2 repression of p53 activity. Genes Cancer 3, 240–248. doi: 10.1177/1947601912455199
Solenthaler, M., Matutes, E., Brito-Babapulle, V., Morilla, R., and Catovsky, D. (2002). p53 and mdm2 in mantle cell lymphoma in leukemic phase. Haematologica 87, 1141–1150.
Soverini, S., De Benedittis, C., Papayannidis, C., Polakova, K. M., Venturi, C., Russo, D., et al. (2016). Clinical impact of low-burden BCR-ABL1 mutations detectable by amplicon deep sequencing in Philadelphia-positive acute lymphoblastic leukemia patients. Leukemia 30, 1615–1619. doi: 10.1038/leu.2016.17
Stengel, A., Schnittger, S., Weissmann, S., Kuznia, S., Kern, W., Kohlmann, A., et al. (2014). TP53 mutations occur in 15.7% of ALL and are associated with MYC-rearrangement, low hypodiploidy, and a poor prognosis. Blood 124, 251–258. doi: 10.1182/blood-2014-02-558833
Teoh, G., Urashima, M., Ogata, A., Chauhan, D., Decaprio, J. A., Treon, S. P., et al. (1997). MDM2 protein overexpression promotes proliferation and survival of multiple myeloma cells. Blood 90, 1982–1992.
Teoh, P. J., Chung, T. H., Sebastian, S., Choo, S. N., Yan, J., Ng, S. B., et al. (2014). p53 haploinsufficiency and functional abnormalities in multiple myeloma. Leukemia 28, 2066–2074. doi: 10.1038/leu.2014.102
Timofeev, O., Schlereth, K., Wanzel, M., Braun, A., Nieswandt, B., Pagenstecher, A., et al. (2013). p53 DNA binding cooperativity is essential for apoptosis and tumor suppression in vivo. Cell Rep 3, 1512–1525. doi: 10.1016/j.celrep.2013.04.008
Trino, S., Iacobucci, I., Erriquez, D., Laurenzana, I., De Luca, L., Ferrari, A., et al. (2016). Targeting the p53-MDM2 interaction by the small-molecule MDM2 antagonist Nutlin-3a: a new challenged target therapy in adult Philadelphia positive acute lymphoblastic leukemia patients. Oncotarget 7, 12951–12961. doi: 10.18632/oncotarget.7339
Trotta, R., Vignudelli, T., Candini, O., Intine, R. V., Pecorari, L., Guerzoni, C., et al. (2003). BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell 3, 145–160. doi: 10.1016/S1535-6108(03)00020-5
Usvasalo, A., Savola, S., Raty, R., Vettenranta, K., Harila-Saari, A., Koistinen, P., et al. (2008). CDKN2A deletions in acute lymphoblastic leukemia of adolescents and young adults: an array CGH study. Leuk. Res. 32, 1228–1235. doi: 10.1016/j.leukres.2008.01.014
Van Maerken, T., Rihani, A., Van Goethem, A., De Paepe, A., Speleman, F., and Vandesompele, J. (2014). Pharmacologic activation of wild-type p53 by nutlin therapy in childhood cancer. Cancer Lett 344, 157–165. doi: 10.1016/j.canlet.2013.11.002
Verduci, L., Azzalin, G., Gioiosa, S., Carissimi, C., Laudadio, I., Fulci, V., et al. (2015). microRNA-181a enhances cell proliferation in acute lymphoblastic leukemia by targeting EGR1. Leuk. Res. 39, 479–485. doi: 10.1016/j.leukres.2015.01.010
Vignetti, M., Fazi, P., Cimino, G., Martinelli, G., Di Raimondo, F., Ferrara, F., et al. (2007). Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood 109, 3676–3678. doi: 10.1182/blood-2006-10-052746
Vilas-Zornoza, A., Agirre, X., Martin-Palanco, V., Martin-Subero, J. I., San Jose-Eneriz, E., Garate, L., et al. (2011). Frequent and simultaneous epigenetic inactivation of TP53 pathway genes in acute lymphoblastic leukemia. PLoS ONE 6:e17012. doi: 10.1371/journal.pone.0017012
Vivo, M., Matarese, M., Sepe, M., Di Martino, R., Festa, L., Calabro, V., et al. (2015). MDM2-mediated degradation of p14ARF: a novel mechanism to control ARF levels in cancer cells. PLoS ONE 10:e0117252. doi: 10.1371/journal.pone.0117252
Wilda, M., Bruch, J., Harder, L., Rawer, D., Reiter, A., Borkhardt, A., et al. (2004). Inactivation of the ARF-MDM-2-p53 pathway in sporadic Burkitt’s lymphoma in children. Leukemia 18, 584–588. doi: 10.1038/sj.leu.2403254
Yilmaz, M., Richard, S., and Jabbour, E. (2015). The clinical potential of inotuzumab ozogamicin in relapsed and refractory acute lymphocytic leukemia. Ther. Adv. Hematol. 6, 253–261. doi: 10.1177/2040620715596715
Zhang, H., Gu, L., Liu, T., Chiang, K. Y., and Zhou, M. (2014). Inhibition of MDM2 by nilotinib contributes to cytotoxicity in both Philadelphia-positive and negative acute lymphoblastic leukemia. PLoS ONE 9:e100960. doi: 10.1371/journal.pone.0100960
Zhou, M., Gu, L., Abshire, T. C., Homans, A., Billett, A. L., Yeager, A. M., et al. (2000). Incidence and prognostic significance of MDM2 oncoprotein overexpression in relapsed childhood acute lymphoblastic leukemia. Leukemia 14, 61–67. doi: 10.1038/sj.leu.2401619
Zhou, M., Yeager, A. M., Smith, S. D., and Findley, H. W. (1995). Overexpression of the MDM2 gene by childhood acute lymphoblastic leukemia cells expressing the wild-type p53 gene. Blood 85, 1608–1614.
Keywords: acute lymphoblastic leukemia, p53, MDM2, Nutlin-3a, target therapy
Citation: Trino S, De Luca L, Laurenzana I, Caivano A, Del Vecchio L, Martinelli G and Musto P (2016) P53-MDM2 Pathway: Evidences for A New Targeted Therapeutic Approach in B-Acute Lymphoblastic Leukemia. Front. Pharmacol. 7:491. doi: 10.3389/fphar.2016.00491
Received: 30 September 2016; Accepted: 30 November 2016;
Published: 16 December 2016.
Edited by:
Daniele Tibullo, University of Catania, ItalyReviewed by:
Sara Galimberti, University of Pisa, ItalyCopyright © 2016 Trino, De Luca, Laurenzana, Caivano, Del Vecchio, Martinelli and Musto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Trino, c3RlZmFuaWEudHJpbm9AY3JvYi5pdA==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.