- 1Department of Pharmacology and Toxicology, Faculty of Pharmacy, Madonna University, Port Harcourt, Nigeria
- 2Department of Pharmacology, Faculty of Basic Medical Sciences, College of Health Sciences, University of Port Harcourt, Port Harcourt, Nigeria
- 3Department of Experimental Pharmacology and Toxicology, Faculty of Pharmacy, University of Port Harcourt, Port Harcourt, Nigeria
The high rate of male infertility and the meager resources to manage same in sub Saharan Africa have necessitated the search for cost effective and available alternatives. Mushrooms have been used traditionally in folk medicine and as nutraceuticals. This study has investigated the effect of the wild mushroom Pleurotus tuber-regium on carbon tetrachloride (CCl4) deleterious effects on the reproductive system of male rats. Thirty six rats were divided into six groups of six animals each. Group I (negative control) received 10 ml/kg olive oil intraperitoneal weekly in addition to feed and water ad libitum. Group II (positive control) received CCl4 10 ml/kg (30% in Olive oil) weekly. Group III, IV, and V received 100 mg, 20 0mg, and 500 mg P. tuber-regium (33.3% in feed) daily in addition to 10 ml/kg CCl4 weekly. Group VI received 500 mg P. tuber-regium (33.3% in feed) daily. After 4 weeks, sperm motility, epididymal count and vitality were determined. Serum malondialdehyde (MDA), testosterone, Luteinizing hormone (LH), Follicle stimulating hormone (FSH), prolactin and oestradiol were estimated using enzyme-linked immunosorbent assay (ELISA) kits. Histopathologic examinations of the testis were carried out. Carbon tetrachloride significantly reduced the sperm motility (54.33 ± 3.79%), epididymal count (28.73 ± 2.86 × 106/ml, vitality (4.96 ± 0.62), LH (0.88 ± 0.14), FSH (2.04 ± 0.33), and Testosterone (2.02 ± 0.24) when compared with control (89.33 ± 9.01), 91.91 ± 1.92 × 106/ml, 13.12 ± 0.19, 2.74 ± 0.32, 3.64 ± 0.62, and 4.16 ± 0.23, respectively, which were reversed by P. tuber-regium administration. Co-administration of P. tuber-regium plus CCl4 significantly reduced MDA level. P. tuber-regium showed dose dependent ameliorative activity against CCl4 deleterious action on the testis and may be beneficial in the management of male infertility.
Introduction
Infertility affects at least 20% of married couples and growing evidence suggest an increasing incidence of male reproductive problems (Kolettis, 2003; Nayernia et al., 2004). In Africa, infertility prevalence rates are feared to be higher and range from 20 to 35% (Eze and Okonofua, 2015). The “infertility belt,” geographical regions with high infertility prevalence, is well-known to Africa, stretching from West Africa, through Central to East Africa (Irvine, 1998; Larsen, 2000; Etuk, 2009). Sperm count declined to a mean of 71.2 million/ml in Ibadan, Nigeria, 54.6 million/ml in Lagos, Nigeria, 65.0 million/ml in Salem, Libya, 66.9 million/ml in Dar Es salaam and Tanzania (Elkin and Fenster, 1997). Nigeria has about twelve million infertile persons (Giwa-Osagie, 2003). Although there is a general documented belief that the most common cause of infertility in Nigeria is infection (Cates et al., 1985; Orisakwe et al., 2004), cases abound where infection have been treated without correction of infertility (Giwa-Osagie, 2003). Environmental pollution and indiscriminate use of herbal medications have tended to add burden on male infertility in sub Sahara Africa (Amadi et al., 2011; Orisakwe et al., 2014). In Nigeria there are higher rates of irreversible oligospermia or azoospermia than most other causes of infertility and less resources for the management of infertility (Osegbe and Amaku, 1985).
Mushrooms are regarded as functional foods and have been in use for decades in folk medicine. Most of the edible mushrooms belong to two major superfamily; Ascomcota and Basidiomycota (Dandapat and Sinha, 2015) with over 600 species possessing therapeutic activity (Wasser and Weis, 1999). Diabetic rats fed some mshroom fruiting bodies exhibit significant anti-glycemic and anti-hypercholesterolemic effects (Jeong et al., 2010; Volman et al., 2010). Similarly, mushrooms show positive influence on lipid metabolism, liver function and decreased severity of streptozotocin-induced diabetes in rats with considerable protective effects on the pancreas and apparent repopulation. Many edible mushrooms contain pharmacological active agents which are mainly secondary metabolites like alkaloids, tannins, polysaccharides, phenolics, flavonoids, etc. (Dandapat and Sinha, 2015). Pleurotus tuber-regium is an edible mushroom found in Nigeria (Okjuoya, 1991) with several medicinal and nutritional properties (Alobo, 2003; Hu et al., 2006; Ngai and Ng, 2006).
This study has evaluated the ameliorative effects of P. tuber-regium in carbon tetrachloride induced testicular damage in Sprague Dawley rats.
Materials and Methods
Harvesting of the Mushroom
Fresh fruiting bodies of wild P. tuber-regium were collected from a forest at the back of University of Nigeria Nsukka by a taxonomist working with the university. These fresh fruiting bodies were cleaned and air dried away from direct sunlight. The mushroom were ground and stored in a clean dry plastic container until use (El-kholy et al., 2013).
Carbon Tetrachloride
Thirty percent carbon tetrachloride (Sigma–Aldrich) in Olive oil (Khan and Ahmed, 2009) was used to induce renal and hepatic damage at a dose of 10 ml/kg (i.p) (Karadeniz et al., 2009).
Animal Husbandry
Thirty six male Sprague-Dawley rats with body weights 180–200 g acclimatized for 2 weeks were maintained under controlled conditions of temperature (23 ± 2°C) and humidity (50 ± 5%) and a 12-h light–dark cycle, were used for the experiment. The animals were housed in sanitized polypropylene cages containing sterile paddy husk as bedding. The bedding of the cages was changed daily and the cages were cleaned as well. They had free access to standard rat pellet diet and water ad libitum. The procedures were performed according to the guidelines on the use of animals and approved by the Institutional Animal Ethical Committee.
Acute Toxicity Studies
Different concentrations of P. tuber-regium (50 – 5000 mg/kg b.w.) were administered orally to male rats. These animals were observed daily for toxicological manifestations like behavioral changes, neural and autonomic toxicities, feeding pattern changes, etc. There was no mortality recorded during this period even up to the dose of 5000 mg/kg (OECD, 1994).
Experimental Design
The animals were divided into six groups with each group consisting of six animals each. The animals were treated as follows;
Group I – normal control received olive oil 10 ml/kg i.p. weekly in addition to standard food and water.
Group II – Positive control received CCl4 (30% CCl4 in olive oil) at a dose of 10 ml/kg weekly in addition to standard feed and water.
Group III – rats were treated orally with 100 mg/kg b.w. of P. tuber-regium in feed (33.3% w/w) along with 10 ml/kg CCl4 (30% in olive oil) weekly.
Group IV – rats were treated orally with 200 mg/kg b.w. of P. tuber-regium in feed (33.3% w/w) along with 10 ml/kg CCl4 (30% in olive oil) weekly.
Group V – rats were treated orally with 500 mg/kg b.w. of P. tuber-regium in feed (33.3% w/w) along with 10 ml/kg CCl4 (30% in olive oil) weekly.
Group VI – rats were treated orally with 500 mg/kg b.w. of P. tuber-regium in feed (33.3% w/w) only with standard feed and water.
Necropsy
Treatments continued for 4 weeks. Blood was collected by retero orbital sinus puncture and serum was separated by centrifugation at 3000 r.p.m. Rats were sacrificed under ether anesthesia; the testis was excised, weighed, rinsed clean in saline, weighed and preserved in 10% formalin for histopathological study. Sperm was harvested from the caudal epididymis and mounted on a slide to determine sperm motility at 40× magnification. The motility assessment was expressed as percentage motile forms. The epididymal filtrate was then mixed in equal volume with eosin-nigrosin stain and a smear made of it was used for epididymal sperm vitality (Lasley et al., 1944). The caudal epididymal sperm reserve was determined using standard hemocytometric method (Amann and Almquist, 1961).
Lipid Peroxidation Assay
Serum lipid peroxidation was quantified as malondialdehyde (MDA) according to the method described by Ohkawa et al. (1979). The MDA level was calculated according to the method of Todorova et al. (2005) and expressed as μmol MDA/mg protein.
Sex Hormonal Assay
Serum levels of testosterone, Luteinizing hormone (LH), Follicle stimulating hormone (FSH), prolactin and oestradiol were estimated using enzyme-linked immunosorbent assay (ELISA) kits (Diagnostic System Laboratories Inc., USA), according to the manufacturer’s instruction.
Statistical Analysis
Data were expressed as mean ± SD of the number of animals used in each group of the experiment. One way analysis of variance (ANOVA) was used to analyze the difference among the groups followed by Bonferroni’s posttest. Values of p < 0.05 were considered significant. Graph pad prism 5.0 software was used for statistical analysis.
Results
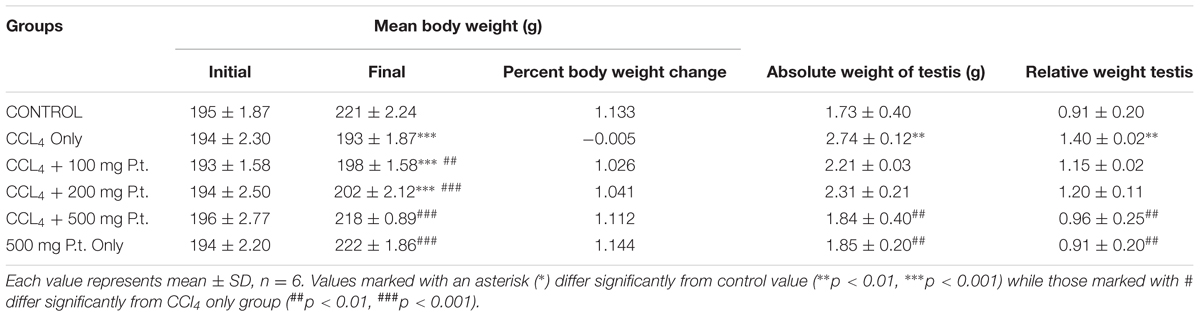
Table 1 shows the effect of P. tuber-regium on the testis and body weight CCl4 treated rats administration and treatment on the body weight of rats. Carbon tetrachloride significantly decreased the body weight [from 221 ± 2.24 (control) to 193 ± 1.87 g (CCl4 treated groups)] and increased the absolute (from 1.73 ± 0.40 (control) to 2.74 ± 0.12 g CCl4 group and relative weight of the testis [0.91 ± 0.20 (control) to 1.40 ± 0.02 CCl4 treated group p < 0.01]. Treatment with 100, 200, and 500 mg P. tuber-regium gave significant and dose-dependent reversal in the body weight and testis weight in CCl4 only treated group.
The effect of P. tuber-regium on sperm motility, epididymal count, vitality and (MDA of CCl4 treated rats is shown on Table 2. Carbon tetrachloride significantly reduced the sperm motility (54.33 ± 3.79%), epididymal count (28.73 ± 2.86 × 106/ml and vitality (4.96 ± 0.62) when compared with control (89.33 ± 9.01), 91.91 ± 1.92 × 106/ml and 13.12 ± 0.19, respectively. There was significant increase in the sperm motility, epididymal count and vitality following P. tuber-regium administration. The reversal effect of P. tuber-regium on these seminal parameters were dose-dependent. There was significant difference between the MDA level (14.00 ± 3.50) in the CCl4 treated rats and the untreated control (1.40 ± 0.32 μg/mg protein). Following the co-administration of 100, 200, and 500 mg P. tuber-regium plus CCl4 there was significant reduction in the level of the MDA (3.50 ± 0.61, 1.60 ± 0.36, and 1.70 ± 0.15 μmol/mg protein, respectively) in dose-dependent manner.
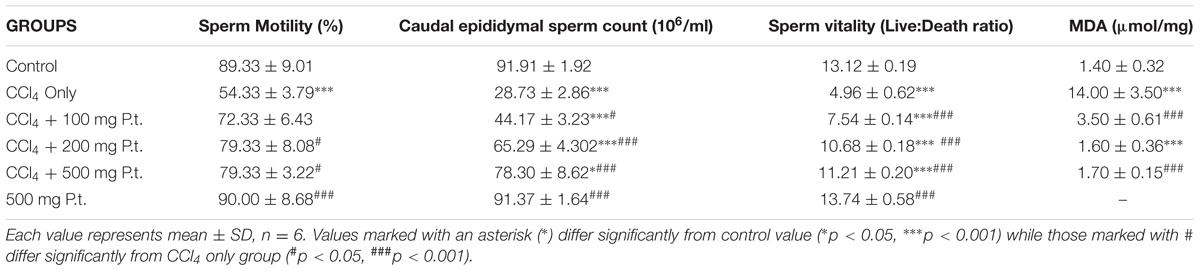
TABLE 2. Effect of P. tuber-regium on sperm motility, epididymal count, vitality and malondialdehyde (MDA) of CCl4 treated rats.
Table 3 shows the effect of P. tuber-regium on LH, FSH, Testosterone, Prolactin, and Estradiol of CCl4 treated rats. Administration of carbon tetrachloride significantly decreased the levels of LH (0.88 ± 0.14), FSH (2.04 ± 0.33), and Testosterone (2.02 ± 0.24) compared to control 2.74 ± 0.32, 3.64 ± 0.62, and 4.16 ± 0.23, respectively. The CCl4, however, increased significantly the levels of prolactin from 1.06 ± 0.21 to 3.58 ± 0.48 and oestradiol from 0.81 ± 0.10 (control) to 2.89 ± 0.18 in the CCl4 treated group. There was dose-dependent reversal of the effects of CCl4 on these hormonal parameters in the P. tuber-regium treated groups.
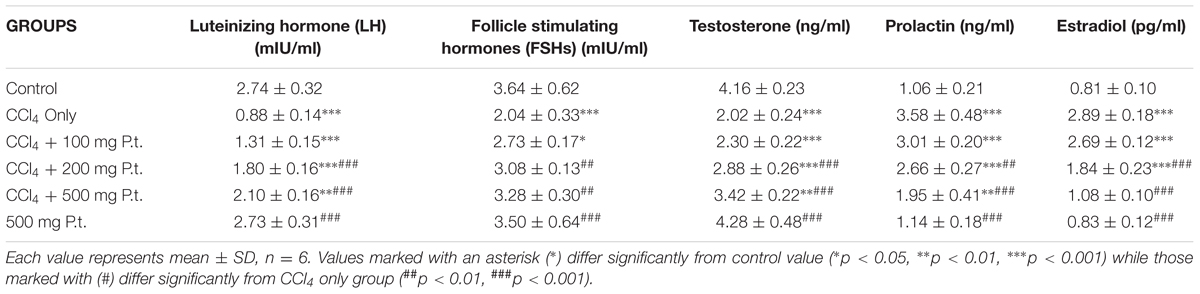
TABLE 3. Effect of P. tuber-regium on FSH, LH, Testosterone, Prolactin, and Estradiol of CCl4 treated rats.
Effect of P. tuber-regium on Histology of the Testis
Figure 1 present the histological changes in the CCl4 exposed animals. Administration of CCl4 to the animals lead to degeneration of the seminiferous tubules with different degrees of sperm cell arrest when compared to control. However, treatments with P. tuber-regium lead to improvement in sperm cell population while the 500 mg P. tuber-regium only group had there seminiferous tubules and sperm cell population similar to control.
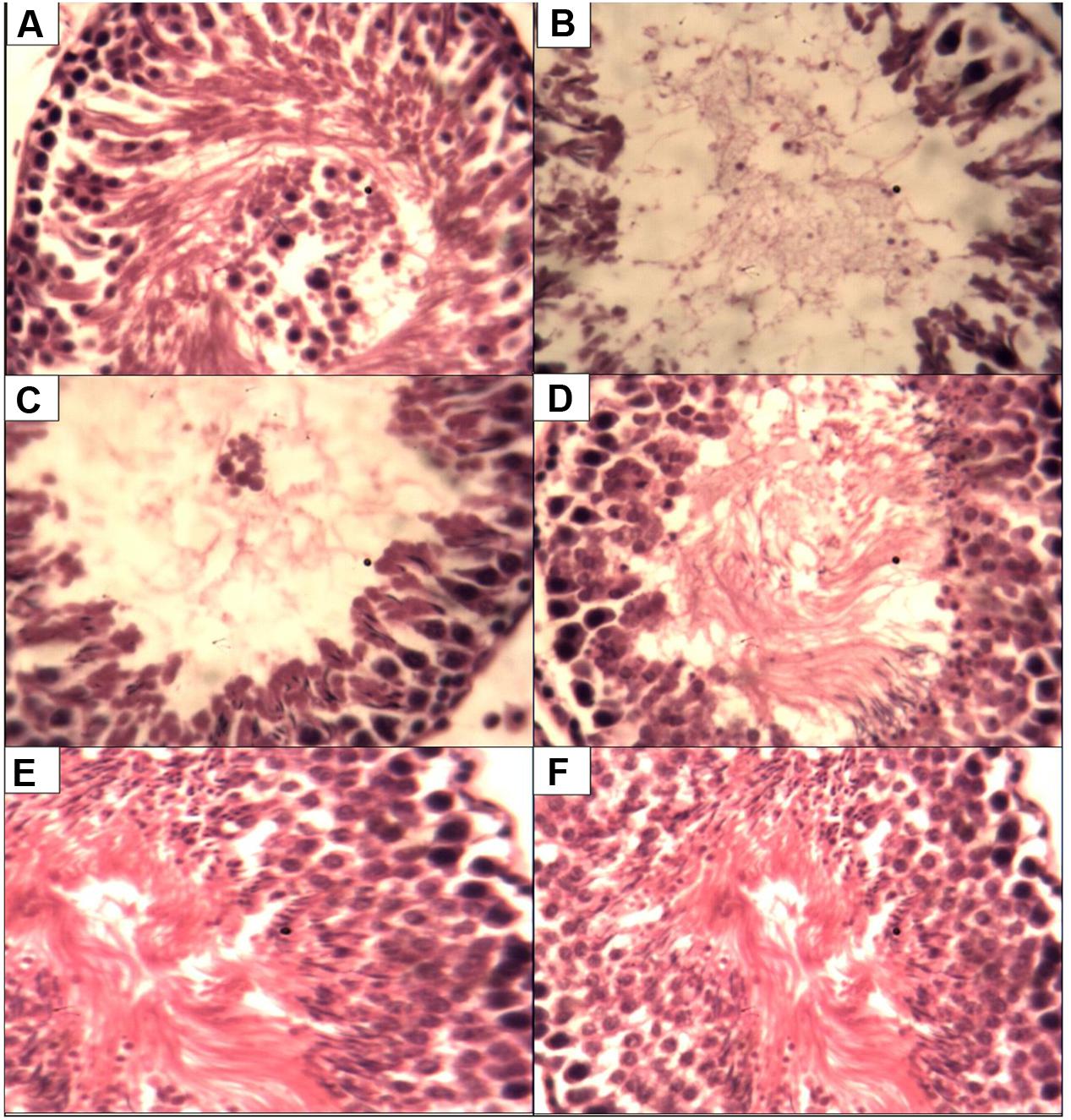
FIGURE 1. Histopathological changes in the testes of rats (magnification H & E ×400). (A) Control testis showing normal histology. (B) CCU only group showing with scanty spermatogenic cells. (C) CCU + l00 mg P.t. group showing seminiferous tubules without full population of spermatozoa. (D) CCU + 200 mg P.t. group showing seminiferous tubules without full population. (E) Received CCl4 + Pleurotus tuber-regium at a dose of 500 mg b.w. daily. (F) Received 500 mg Pleurotus tuber-regium only, i.e., treated negative control.
Discussion
Infertility which is usually defined as a couple’s inability to achieve pregnancy following 1 year of unprotected intercourse and considered one of the main public health issues (Jungwirth et al., 2012). Male factor infertility is present in approximately half of all infertile couples (Thonneau et al., 1991). Spermatogenesis takes place in the seminiferous tubular structures of the testis which are lined with germ and sertoli cells. Spermatogenesis consists of three major phases; Proliferation, reduction-division and differentiation with distinct and specific germ cell types being associated with each phase (Hess, 1990). The present study has investigated the effect of P. tuber-regium on carbon tetrachloride induced testicular injury in animal model. After hepatic handling CCl4 generates chloride and trichloromethyl radicals CCl3, which reacts with oxygen to produce CCl3 O2. CCl3 bind to fatty acids to generate alkoxy and peroxy radicals (Bruckner et al., 2002). The increased lipid peroxidation provoke destruction of sperm (Aitken et al., 2014). P. tuber-regium co-administered with carbon tetrachloride produced a significant dose dependent decrease in the level of MDA. Reduced lipid peroxidation will promote spermatogenesis.
There are estrogen receptors in the hypothalamic nuclei and in pituitary gonadotropes, which act on the hypothalamus to affect gonadotrophin releasing hormone GnRH pulses and at the pituitary level to regulate FSH and LH secretion (Cabler et al., 2010). The excessive conversion of testosterone to estrogen in peripheral adipose tissue has been known to cause secondary hypogonadism through hypothalamic-pituitary-gonadal axis inhibition (Cabler et al., 2010). Estrogens affect spermatogenesis directly within the testis by alterations in gonadotropin secretion by the pituitary gland. High levels of circulating oestradiol and/or elevated oestradiol/testosterone ratios which is a feature of male infertility (Cabler et al., 2010), was observed in rats treated with CCl4 in this study. P. tuber-regium significantly reversed the oestradiol level in this study. Although there is a school of thought that normal levels of gonadotropins in the context of low free testosterone may signify the effective suppression of the hypothalamic-pituitary axis, resulting in subclinical hypogonadotropic hypogonadism (Agarwal et al., 2006). In this study, however, P. tuber-regium significantly reversed the CCl4, LH, FSH, and testosterone lowering and oestradiol increase. P. tuber-regium may be competitively bind to estrogen receptors on the hypothalamus and pituitary gland to decrease estrogenic firing to increase the release of LH, which increases testosterone production by the testes.
Luteinizing hormone binds to its receptors to activate G-proteins and, in turn, adenylate cyclase, which can increase cyclic AMP formation. cAMP will then stimulate protein kinase A (PKA), which will phosphorylate proteins. The phosphorylated proteins will further phosphorylate other proteins or induce new protein synthesis, i.e., steroidogenic acute regulatory protein (Manna et al., 2013). The function of steroidogenic acute regulatory protein is to transfer free cholesterol from the cytoplasm into the inner membrane of mitochondria, where cytochrome P450 side-chain cleavage enzyme converts cholesterol to pregnenolone (Stocco, 2002; Manna and Stocco, 2005). Pregnenolone will then be transported to smooth endoplasmic reticulum for testosterone synthesis, which is an essential steroid hormone for reproduction in males (Liu et al., 2007). It has also been shown that activation of the protein kinase C (PKC) signal pathway can strongly modulate Leydig cell steroidogenesis (Hirakawa et al., 2002). Chen et al. (2005) explained that Clonorchis sinensis activated both PKA and PKC signal transduction pathways to stimulate cell steroidogenesis. Although there seem to be no significant association between semen parameters and prolactin levels (Lotti et al., 2013), prolactin can affect steroidogenesis by modulating the expression of LH receptors (Dombrowicz et al., 1992), or by regulating the activity of steroidogenetic enzymes (Chandrashekar and Bartke, 1988) and has a trophic effect on male seminal accessory glands.
Histological examination suggests the consequences of oxidative stress in spermatogenesis with sperm cell maturation arrest. The result conform with the sperm parameter result of decreased sperm count since maturation arrest will ultimately decrease the number of sperm reaching the epididymis (El-kholy et al., 2013).
Conclusion
In this study has shown that P. tuber-regium may protect the testis from CCl4 induced damage as evidenced by the by the ameliorative effects on the epididymal sperm count, motility, viability, sex hormones, MDA, and testicular histomorphology. Since biochemical hypogonadism is considered a main feature of male infertility, dietary supplementation with P. tuber-regium with its significant reversal of all the deleterious testicular effects in CCl4 treated rats may hold some promise in the management of involuntary childlessness amongst married couples.
Author Contributions
KO: Designed study, carried out the bench work and analysed data, IS: Design, and OO: Designed the study, analysed data and write up.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agarwal, A., Gupta, S., and Sikka, S. (2006). The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol. 18, 325–332. doi: 10.1097/01.gco.0000193003.58158.4e
Aitken, R. J., Smith, T. B., Jobling, M. S., Baker, M. A., and De Iuliis, G. N. (2014). Oxidative stress and male reproductive health. Asian J. Androl. 16, 31–38. doi: 10.4103/1008-682X.122203
Alobo, A. P. (2003). Proximate composition and functional properties of Pleurotus tuberregium sclerotia flour and protein concentrate. Plant Foods Hum. Nutr. 58, 1–9. doi: 10.1023/B:QUAL.0000040319.61845.c2
Amadi, C. N., Siminialayi, I. M., and Orisakwe, O. E. (2011). Male infertility and herbal supplements: an update. Pharmacologia 2, 323–348. doi: 10.5567/pharmacologia.2011.323.348
Amann, R. P., and Almquist, J. O. (1961). Reproductive capacity of dairy bulls. I. Technique for direct measurement of gonadal and extra-gonadal sperm reserves. J. Dairy Sci. 44, 1537–1543. doi: 10.3168/jds.S0022-0302(61)89916-5
Bruckner, J. V., Ramanathan, R., Lee, K. M., and Muralidhara, S. (2002). Mechanisms of circadian rhythmicity of carbon tetrachloride hepatotoxicity. J. Pharmacol. Exp. Ther. 300, 273–281. doi: 10.1124/jpet.300.1.273
Cabler, S., Agarwal, A., Flint, M., and Du Plessis, S. S. (2010). Obesity: modern man’s fertility nemesis. Asian J. Androl. 12, 480–489. doi: 10.1038/aja.2010.38
Cates, W., Farley, T. M., and Rowe, P. J. (1985). Worldwide patterns of infertility: is Africa different? Lancet 326, 596–598. doi: 10.1016/S0140-6736(85)90594-X
Chandrashekar, V., and Bartke, A. (1988). Influence of endogenous prolactin on the luteinizing hormone stimulation of testicular steroidogenesis and the role of prolactin in adult male rats. Steroids 51, 559–576. doi: 10.1016/0039-128X(88)90052-9
Chen, Y. C., Huang, Y. L., and Huang, B. M. (2005). Cordyceps sinensis mycelium activates PKA and PKC signal pathways to stimulate steroidogenesis in MA-10 mouse Leydig tumor cells. Int. J. Biochem. Cell Biol. 37, 214–223. doi: 10.1016/j.biocel.2004.05.019
Dandapat, S., and Sinha, M. P. (2015). Antioxidant and anti-inflammatory activity of Pleurotus tuber- regium (Rumph. ex Fr.) Singer. Adv. Biol. Res. 9, 140–145.
Dombrowicz, D., Sente, B., Closset, J., and Hennen, G. (1992). Dose-dependent effects of human prolactin on the immature hypophysectomized rat testis. Endocrinology 130, 695–700. doi: 10.1210/en.130.2.695
El-kholy, T. A., Hassanen, N. H. M., and Abbas, H. Y. (2013). Protection of the Mushroom (shiitake ”Lentinus-edodes) against Carbon-Tetrachloride-Induced renal injury in rats. Life Sci. J. 10, 1701–1708.
Elkin, E. P., and Fenster, L. (1997). Have sperm densities declined? A re-analysis of global trend data. Environ. Health Perspect. 105, 1228–1232.
Etuk, S. J. (2009). Reproductive health: global infertility trend. Niger. J. Physiol. Sci. 24, 85–90.
Eze, U. A., and Okonofua, F. E. (2015). Editorial: high prevalence of male infertility in africa: are mycotoxins to blame? Afr. J. Reprod. Health 19, 9–17.
Hess, R. A. (1990). Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion- fixed and plastic-embedded testes. Biol. Reprod. 43, 525–542. doi: 10.1095/biolreprod43.3.525
Hirakawa, T., Galet, C., and Ascoli, M. (2002). MA-10 cells transfected with the human lutropin/choriogonadotropin receptor (hLHR): a novel experimental paradigm to study the functional properties of the hLHR. Endocrinology 143, 1026–1035. doi: 10.1210/endo.143.3.8702
Hu, S. H., Liang, Z. C., Chia, Y. C., Lien, J. L., Chen, K. S., Lee, M. Y., et al. (2006). Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. J. Agric. Food Chem. 54, 2103–2110. doi: 10.1021/jf052890d
Irvine, D. S. (1998). Epidemiology and aetiology of male infertility. Hum. Reprod. 13(Suppl. 1), 33–44.
Jeong, S. C., Jeong, Y. T., Yang, B. K., Islam, R., Koyyalamudi, S. R., Pang, G., et al. (2010). White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr. Res. 30, 49–56. doi: 10.1016/j.nutres.2009.12.003
Jungwirth, A., Diemer, T., Dohle, G. R., Giwercman, A., Kopa, Z., Krausz, C., et al. (2012). EAU guidelines on male infertility. Eur. Urol. 62, 324–332. doi: 10.1016/j.eururo.2012.04.048
Karadeniz, A., Yıldırım, A., Karakoç, A., Kalkan, Y., and Celebi, F. (2009). Protective effect of Panax ginseng on carbon tetrachloride induced liver, heart and kidney injury in rats. Revue Med. Vet. 160, 237–243.
Khan, M. R., and Ahmed, D. (2009). Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem. Toxicol. 47, 1393–1399. doi: 10.1016/j.fct.2009.03.020
Larsen, U. (2000). Primary and secondary infertility in sub-Saharan Africa. Int. J. Epidemiol. 29, 285–291. doi: 10.1093/ije/29.2.285
Lasley, J. F., Easely, G. T., and McKenzie, F. F. (1944). staining method for the differentiation of live and dead spermatozoa. Anat. Rec. 82, 167–174.
Liu, T., Wimalasena, J., Bowen, R. L., and Atwood, C. S. (2007). Luteinizing hormone receptor mediates neuronal pregnenolone production via up-regulation of steroidogenic acute regulatory protein expression. J. Neurochem. 100, 1329–1339. doi: 10.1111/j.1471-4159.2006.04307.x
Lotti, F., Corona, G., Maseroli, E., Rossi, M., Silverii, A., Degl’Innocenti, S., et al. (2013). Clinical implications of measuring prolactin levels in males of infertile couples. Andrology 1, 764–771. doi: 10.1111/j.2047-2927.2013.00114.x
Manna, P. R., Slominski, A. T., King, S. R., Stetson, C. L., and Stocco, D. M. (2013). Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: involvement of cAMP/PKA signaling. Endocrinology 155, 576–591. doi: 10.1210/en.2013-1694
Manna, P. R., and Stocco, D. M. (2005). Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr. Drug Targets Immune Endocr. Metabol. Disord. 5, 93–108. doi: 10.2174/1568008053174714
Nayernia, K., Li, M., Jaroszynski, L., Khusainov, R., Wulf, G., Schwandt, I., et al. (2004). Stem cell based therapeutical approach of male infertility by teratocarcinoma derived germ cells. Hum. Mol. Genet. 13, 1451–1460. doi: 10.1093/hmg/ddh166
Ngai, P. H., and Ng, T. B. (2006). A hemolysin from the mushroom Pleurotus eryngii. Appl. Microbiol. Biotechnol. 72, 1185–1191. doi: 10.1007/s00253-006-0406-6
Ohkawa, H., Ohishi, N., and Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. doi: 10.1016/0000203-2697(79)90738-3
Okjuoya, J. A. (1991). “Cultivation of Pleurotus tuber-regium (Fr) Sing on various farm wastes,” in Proceedings of the Oklahoma Academy of Science, Vol. 71, Benin City, 1–3.
Orisakwe, O. E., Akumka, D. D., Njan, A. A., and Afonne, O. J. (2004). Testicular toxicity of Nigerian bonny light crude oil in male albino rats. Reprod. Toxicol. 18, 439–442. doi: 10.1016/j.reprotox.2004.02.002
Orisakwe, O. E., Blum, J. L., Sujak, S., and Zelikoff, J. T. (2014). Metal pollution in Nigeria: a biomonitoring update. J. Health Pollut. 4, 40–52. doi: 10.5696/2156-9614-4-6.40
Osegbe, D. N., and Amaku, E. O. (1985). The causes of male infertility in 504 consecutive Nigerian patients. Int. Urol. Nephrol. 17, 349–358. doi: 10.1007/BF02083505
Stocco, D. M. (2002). Clinical disorders associated with abnormal cholesterol transport: mutations in the steroidogenic acute regulatory protein. Mol. Cell. Endocrinol. 191, 19–25. doi: 10.1016/S0303-7207(02)00048-5
Thonneau, P., Marchand, S., Tallec, A., Ferial, M. L., Ducot, B., Lansac, J., et al. (1991). Incidence and main causes of infertility in a resident population (1 850 000) of three French regions (1988–1989). Hum. Reprod. 6, 811–816.
Todorova, I., Simeonova, G., Kyuchukova, D., Dinev, D., and Gadjeva, V. (2005). Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp. Clin. Pathol. 13, 190–194. doi: 10.1007/s00580-005-0547-5
Volman, J. J., Mensink, R. P., van Griensven, L. J., and Plat, J. (2010). Effects of alpha-glucans from Agaricus bisporus on ex vivo cytokine production by LPS and PHA-stimulated PBMCs; a placebo controlled study in slightly hypercholesterolemic subjects. Eur. J. Clin. Nutr. 64, 720–726. doi: 10.1038/ejcn.2010.32
Keywords: male infertility, testicular toxicity, sex hormone, mushroom, carbon tetrachloride treatment
Citation: Okolo KO, Siminialayi IM and Orisakwe OE (2016) Protective Effects of Pleurotus tuber-regium on Carbon- Tetrachloride Induced Testicular Injury in Sprague Dawley Rats. Front. Pharmacol. 7:480. doi: 10.3389/fphar.2016.00480
Received: 11 September 2016; Accepted: 24 November 2016;
Published: 15 December 2016.
Edited by:
Judith Maria Rollinger, University of Vienna, AustriaReviewed by:
Keliang Xie, Tianjin Medical University, ChinaBenedict Green, Agricultural Research Service (USDA), USA
Copyright © 2016 Okolo, Siminialayi and Orisakwe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orish E. Orisakwe, b3Jpc2hlYmVyZUBnbWFpbC5jb20=
 Kenneth O. Okolo1
Kenneth O. Okolo1 Orish E. Orisakwe
Orish E. Orisakwe