- Drug and Herbal Research Centre, Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
Tinospora crispa (L.) Hook. f. & Thomson (Menispermaceae), found in the rainforests or mixed deciduous forests in Asia and Africa, is used in traditional medicines to treat numerous health conditions. This review summarizes the up-to-date reports about the ethnobotany, phytochemistry, pharmacological activities, toxicology, and clinical trials of the plant. It also provides critical assessment about the present knowledge of the plant which could contribute toward improving its prospect as a source of lead molecules for drug discovery. The plant has been used traditionally in the treatment of jaundice, rheumatism, urinary disorders, fever, malaria, diabetes, internal inflammation, fracture, scabies, hypertension, reducing thirst, increasing appetite, cooling down the body temperature, and maintaining good health. Phytochemical analyses of T. crispa revealed the presence of alkaloids, flavonoids, and flavone glycosides, triterpenes, diterpenes and diterpene glycosides, cis clerodane-type furanoditerpenoids, lactones, sterols, lignans, and nucleosides. Studies showed that the crude extracts and isolated compounds of T. crispa possessed a broad range of pharmacological activities such as anti-inflammatory, antioxidant, immunomodulatory, cytotoxic, antimalarial, cardioprotective, and anti-diabetic activities. Most pharmacological studies were based on crude extracts of the plant and the bioactive compounds responsible for the bioactivities have not been well identified. Further investigations are required to transform the experience-based claims on the use of T. crispa in traditional medicine practices into evidence-based information. The plant extract used in pharmacological and biological studies should be qualitatively and quantitatively analyzed based on its biomarkers. There should be detail in vitro and in vivo studies on the mechanisms of action of the pure bioactive compounds and more elaborate toxicity study to ensure safety of the plant for human use. More clinical trials are encouraged to be carried out if there are sufficient preclinical and safety data.
Introduction
Herbs are the sources of crude drugs that are used to treat pathologic conditions, often chronic in nature, or to achieve or retain a state of improved health. Several cultures have distinct uses of plants for the treatment of various diseases (Wyk and Wink, 2004). This traditional knowledge has been vocally passed on through a number of generations; therefore these traditional remedies are still in practice. This knowledge on traditional medical practice, collected over the centuries by trial and error using the patient as the experimental animal throughout, must contain some material worthy of additional research. This, consequently, calls to carry out scientific studies on such plants to confirm the claims of community folks on their medicinal effects.
Tinospora crispa (L.) Hook. f. & Thomson is a medicinal plant belongs to the genus Tinospora of Menispermaceae family. It is prevalent in primary rainforests or mixed deciduous forests of South East Asia and Africa including Thailand, Malaysia, and Indonesia (Pathak et al., 1995). It has been used in conventional medicine to treat numerous pathologies in Malaysia (Najib Nik a Rahman et al., 1999), Indonesia (Dweck and Cavin, 2006), Thailand (Kongsaktrakoon et al., 1984), and the Philippines (Quisumbing, 1951). There was a previous review of the secondary metabolites and biological activities of T. crispa (Koay and Amir, 2013), however, critical assessment of the present knowledge is needed to provide the perspectives and directions for future research and potential applications. The purpose of this review is to provide an updated and complete overview of the botany, phytochemistry, traditional uses, and pharmacological activities of T. crispa. Moreover, the present knowledge obtained mainly from experimental studies was critically assessed to provide evidences and justifications for local and traditional uses of T. crispa and to propose future research prospects and potential therapeutic uses for this plant.
Vernacular Names
T. crispa, is known as “Patawali,” “Akar Patawali,” “Seruntum,” or “Akar Seruntum” in Malaysia (Noor et al., 1989), “Brotawali,” “Antawali,” and “Andawali” in Indonesia (Roosita et al., 2008; Koay and Amir, 2013), “Makabuhay” (meaning “You may live”) in Philippines, (Quisumbing, 1951), “Boraphet” in Thailand, “Da ye ruanjinteng” in China (Li et al., 2006), “Banndol Pech” in Cambodia (Hout et al., 2006) “Guloncho-ban” or “Golonchi” in Bangladesh (Rahmatullah et al., 2011), and “Lyann span Zeb kayenn” in Martinique island (Longuefosse and Nossin, 1996).
Plant Description
T. crispa is an herbaceous vine which extensively grows in tropical and subtropical regions of Southeast Asia (Pathak et al., 1995). The old stems of T. crispa are fleshy, with prominent blunt tubercles whereas younger stems are slightly fleshy, thin epidermis, membranous, brownish, and glabrous. The leaves are large, heart shaped 6–12 cm long and 7–12 cm wide. Petioles are glabrous and 5–15 cm long. Leaf blade is slightly fleshy, both surfaces glabrous and very delicate when dried (Figure 1). The herb contains two or three small and yellow or greenish yellow color flowers which are fascicled. Male inflorescences is very slender, 5–10 cm or longer. Male flower has six green and glabrous sepals in two whorls. Outer three are ovate (1 mm) while inner three are obovate. There are 3–6 yellow color petals and six stamens equivalent in length to petals. Female inflorescences are 2–6 cm long, mostly one flower per node. Female flower has sepals and petals as in male. The fruit is 7–8 mm in length.
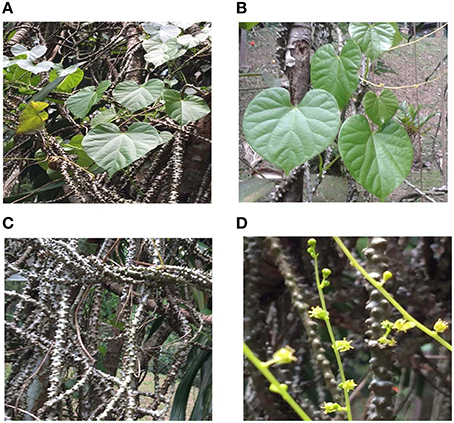
Figure 1. (A) whole plant of Tinospora crispa (B) leaves of Tinospora crispa (C) dried stem of Tinospora crispa (D) flowers of Tinospora crispa.
The whole plant of T. crispa was obtained from Marang, Kuala Terengganu, Malaysia and a voucher specimen (No. UKMB 40178) was identified and deposited at the Herbarium of Universiti Kebangsaan Malaysia (UKM), Bangi, Malaysia. The collection of the plant sample did not involve endangered or protected species.
Traditional Uses
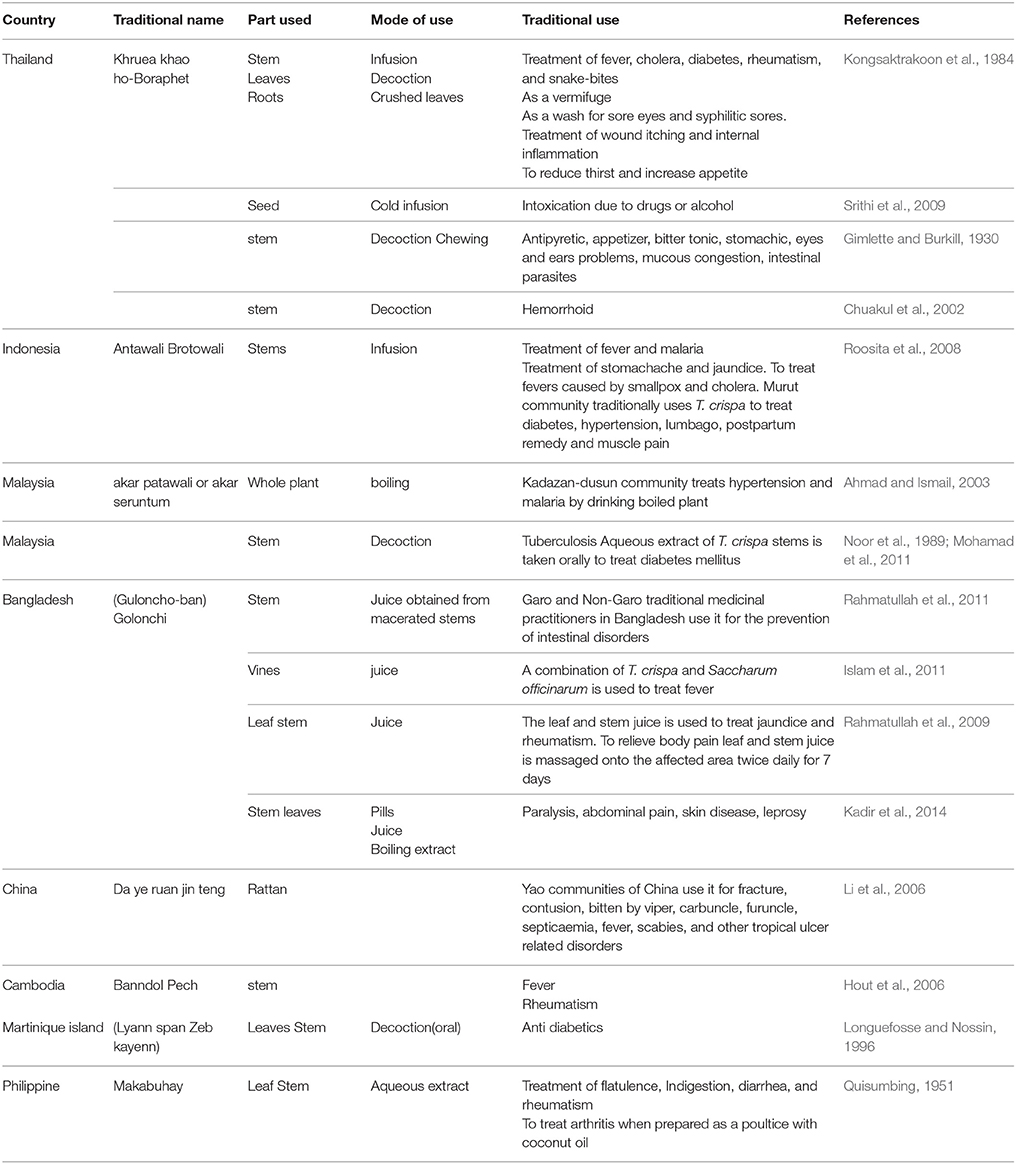
T. crispa is an ingredient in Thai folk remedies. Decoction from the stem of T. crispa has been used as an antipyretic, in the treatment of internal inflammations, decreasing thirst, enhancing hunger, cooling down body temperature, and for the maintenance of good health (Kongsaktrakoon et al., 1984; Dweck and Cavin, 2006). The cold infusion of the seed has been used to treat intoxication caused by drugs or alcohol. An infusion of its stem is drunk as vermifuge, a decoction of the stem is used to wash aching eyes and syphilitic sores, the crushed leaves are applied on wounds and made into dressing for itch. In Indonesia (Borneo) it has been used for the treatment of diabetes, hypertension, and backache (Dweck and Cavin, 2006). T. crispa has been used conventionally against a wide variety of health ailments by Yao communities of China. They used it to treat bruises, septicemia, fever, fracture, scabies, and other tropical ulcer-related disorders (Li et al., 2006). In Malaysia, T. crispa is used traditionally for numerous therapeutic purposes like diabetes, hypertension, stimulation of appetite, and protection from mosquito bites (Gimlette and Burkill, 1930). The infusion from the stems is used as a vermifuge. Personal communications with local traditional medicine practitioners highlighted its popular use as a general tonic. Moreover, it is used as an anti-parasitic agent in both humans and domestic animals (Noor et al., 1989). In Bangladesh, the juice of stem is used in the treatment of intestinal disorders, jaundice, rheumatism, body pain, paralysis, skin disease, and leprosy. The aqueous leaf extract is used to treat flatulence, dyspepsia, diarrhea, and rheumatism by traditional therapists in the Philippines. It is also used to prepare a poultice with coconut oil to treat arthritis. The traditional uses of T. crispa are summarized in Table 1 along with the parts used and methods of administration.
Phytochemistry
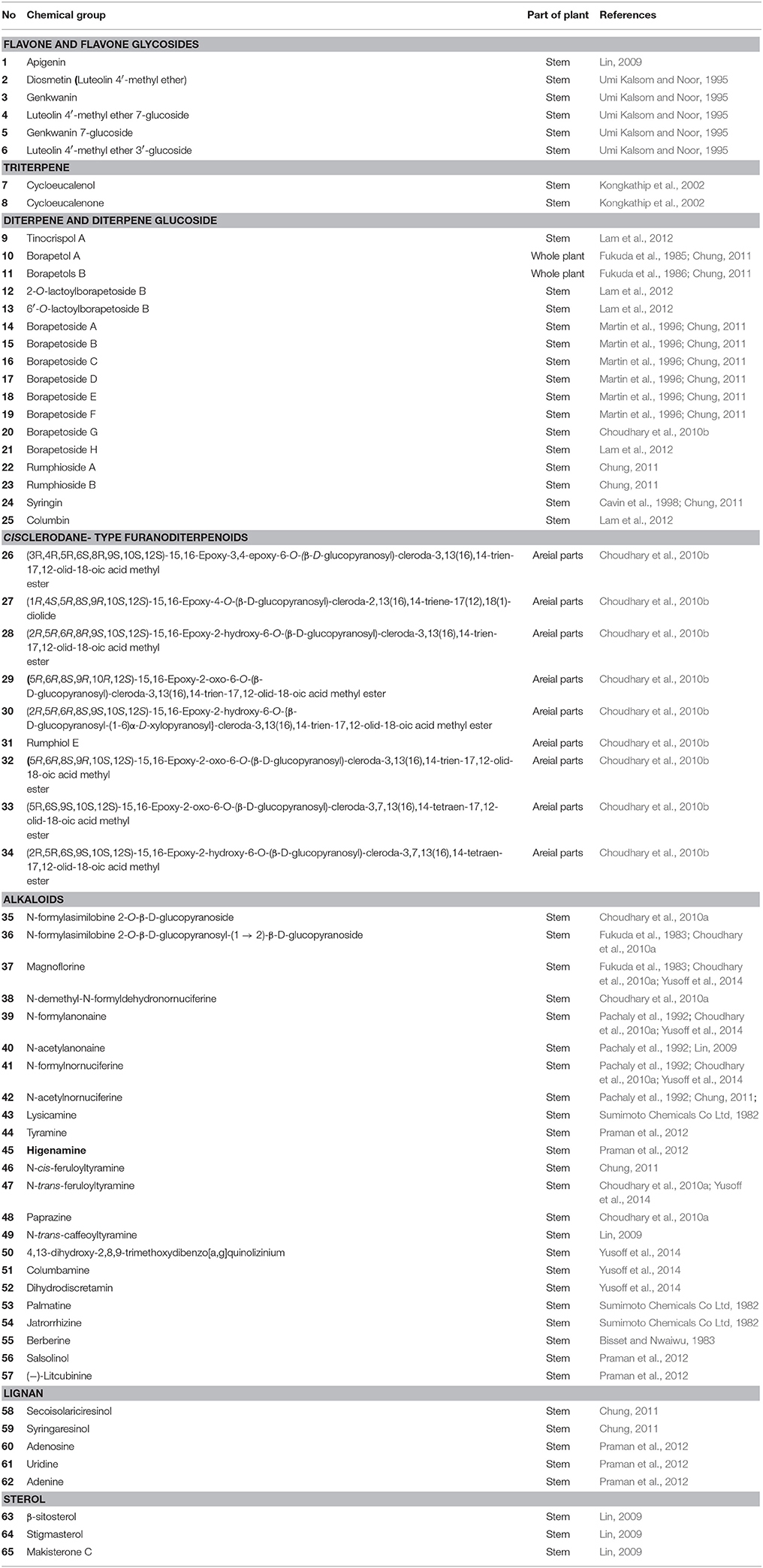
T. crispa comprises of a diversity of secondary metabolites. A number of studies have been carried out on the constituents of T. crispa, and more than 65 compounds have been isolated and identified such as furanoditerpenes, lactones, steroids, flavonoids, lignans, and alkaloids (Table 2). Among these isolated compounds, clerodane-type furanoditerpenes are the characteristic compounds of T. crispa.
Flavonoids
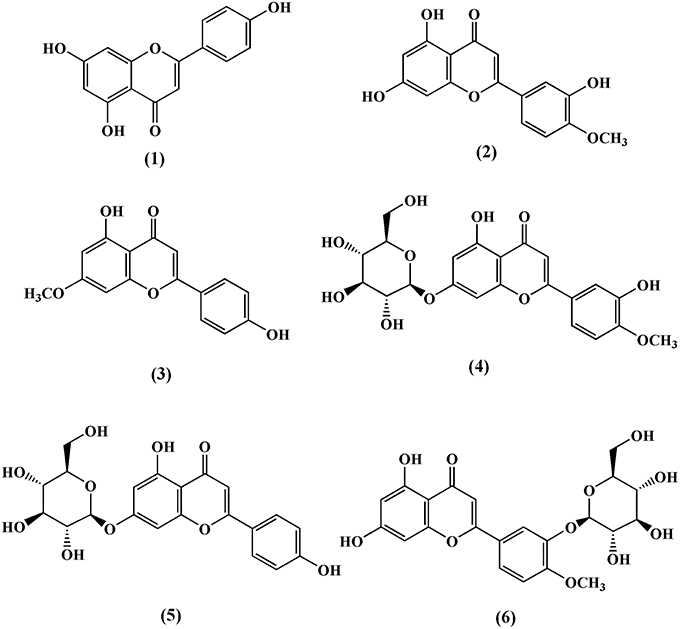
Till date, three flavones and three flavone glycosides have been identified from the stem of T. crispa, namely, apeginin (1) (Lin, 2009), diosmetin (2), genkwanin (3), luteolin 4′-methyl ether 7-glucoside (4), genkwanin 7-glucoside (5), and luteolin 4′-methyl ether 3′-glucoside (6) (Umi Kalsom and Noor, 1995; Figure 2).
Terpenoids
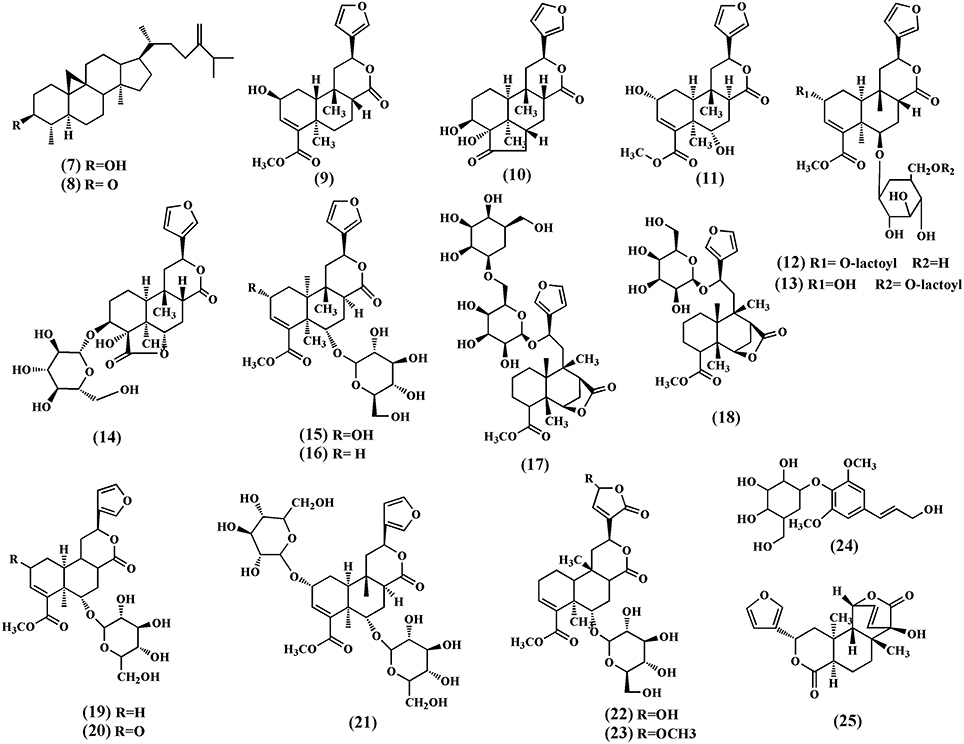
A number of terpenoids (7–33), classified as triterpenoids (7–8), and diterpenoids (9–34), have been isolated from different parts of T. crispa. The triterpenoids, cycloeucalenol (7) and cycloeucalenone (8) were also isolated from the stem (Kongkathip et al., 2002). Diterpenoids and their glycosides are the main terpenoids in T. crispa and the most common are the clerodane-type furanoditerpenoids. Diterpenoids, tinocrispol A (9) (Lam et al., 2012), borapetol A (10), borapetols B (11), were isolated from the ethanol extract of T. crispa vines (Fukuda et al., 1985, 1986; Chung, 2011; Figure 3).
Diterpenoid glycosides, 2-O-lactoylborapetoside B (12), 6′-O-lactoylborapetoside B (13), borapetoside A (14), borapetoside B (15), borapetoside C (16), borapetoside D (17), borapetoside E (18), borapetoside F (19) (Martin et al., 1996), borapetoside G (20), borapetoside H (21), rumphioside A (22), rumphioside B (23), rumphioside C, rumphioside F, rumphioside I, syringin (24), columbin (25), tinocrisposide A, tinocrisposide B, tinocrisposide C, and tinocrisposide D were isolated from the methanol extract of T. crispa (Chung, 2011; Lam et al., 2012; Figure 3).
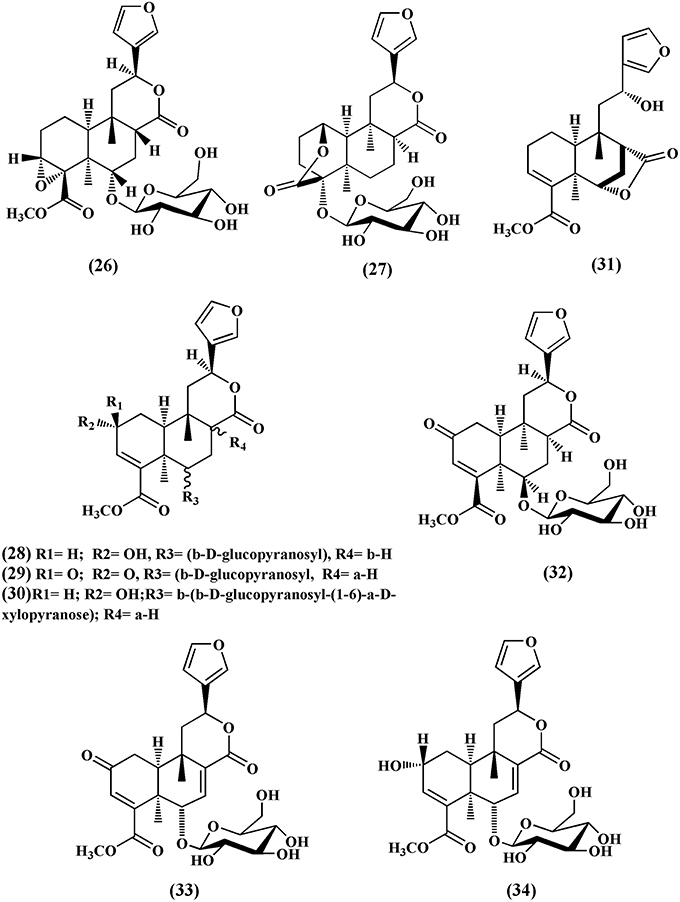
Choudhary et al. (2010b) also isolated nine new cis clerodane-type furanoditerpenoids, from aerial parts of T. crispa, viz. (3R,4R,5R,6S,8R,9S,10S,12S)-15,16-epoxy-3,4-epoxy-6-O-(β-D- glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (26), (1R,4S,5R,8S,9R,10S,12S)-15,16-epoxy-4-O -(β-D-glucopyranosyl) -cleroda-2,13(16),14-triene-17(12),18(1)-diolide (27), (2R,5R,6R,8R,9S,10S,12S)-15,16-epoxy-2 -hydroxy-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (28), (5)R,6R, 8S,9R,10R,12S)-15,16-epoxy-2-oxo-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (29), (2R,5R,6R,8S,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-{β-D-glucopyranosyl-(1-6)α-D-xylopyranosyl}-cleroda-3,13(16),14- trien-17,12-olid-18-oic acid methyl ester (30), rumphiol E (31), (5R,6R,8S,9R,10S,12S)-15,16-epoxy-2-oxo-6-O-(β-D-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester (32), (5R,6S,9S,10S,12S)-15,16-epoxy-2-oxo-6-O-(β-D-glucopyranosyl)-cleroda-3,7,13(16),14-tetraen-17,12-olid-18-oic acid methyl ester (33), and (2R,5R,6S,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-(β-D-glucopyranosyl)-cleroda-3,7,13(16),14-tetraen-17,12-olid-18-oic acid methyl ester (34) (Figure 4).
Alkaloids
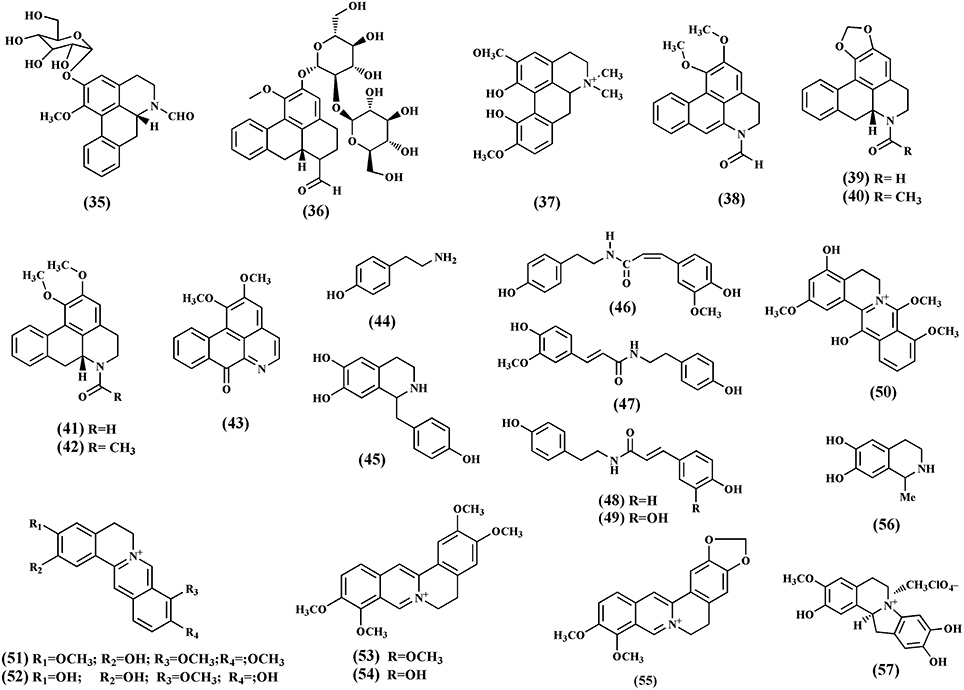
Alkaloids are important secondary metabolites from the plant. To date, 21 quaternary alkaloids have been isolated (35-57) and classified into protoberberine, furonoquinolone, and aporphine alkaloids (Figure 5). The most common alkaloids found in T. crispa are aporphines. These include N-formylasimilobine 2-O-β-D-glucopyranoside (35), N-formylasimilobine 2-O-β- D-glucopyranosyl-(1 → 2)-β-D-glucopyranoside (tinoscorside A) (36), magnoflorine (37), N-demethyl-N-formyldehydronornuciferine (38) (Fukuda et al., 1983; Choudhary et al., 2010a), N-formylanonaine (39), N-acetylanonaine (40), N-formylnornuciferine (41), N-acetylnornuciferine (42) (Pachaly et al., 1992; Na et al., 2005), and lysicamine (43) (Sumimoto Chemicals Co Ltd, 1982). The furquinolone alkaloids isolated from T. crispa comprise tyramine (44), higenamine (45) (Praman et al., 2012), N-cis-feruloyltyramine (46), N-trans-feruloyltyramine (47), paprazine (48), and N-trans-caffeoyltyramine (49) (Naomichi et al., 1983; Chung, 2011). The protoberberine alkaloids include 4,13-dihydroxy-2,8,9-trimethoxydibenzo[a,g]quinolizinium (50), columbamine (51), dihydrodiscretamin (52) (Yusoff et al., 2014), palmatine (53), jatrorrhizine (54) (Sumimoto Chemicals Co Ltd, 1982), and berberine (55) (Bisset and Nwaiwu, 1983). Salsolinol (56) (a tetrahydroisoquinoline) and (−)-Litcubinine (57) (a dibenzopyrrocoline type alkaloid) were identified from n-butanol fraction of T. crispa stem (Praman et al., 2012).
Lignans, Nucleosides, and Sterols
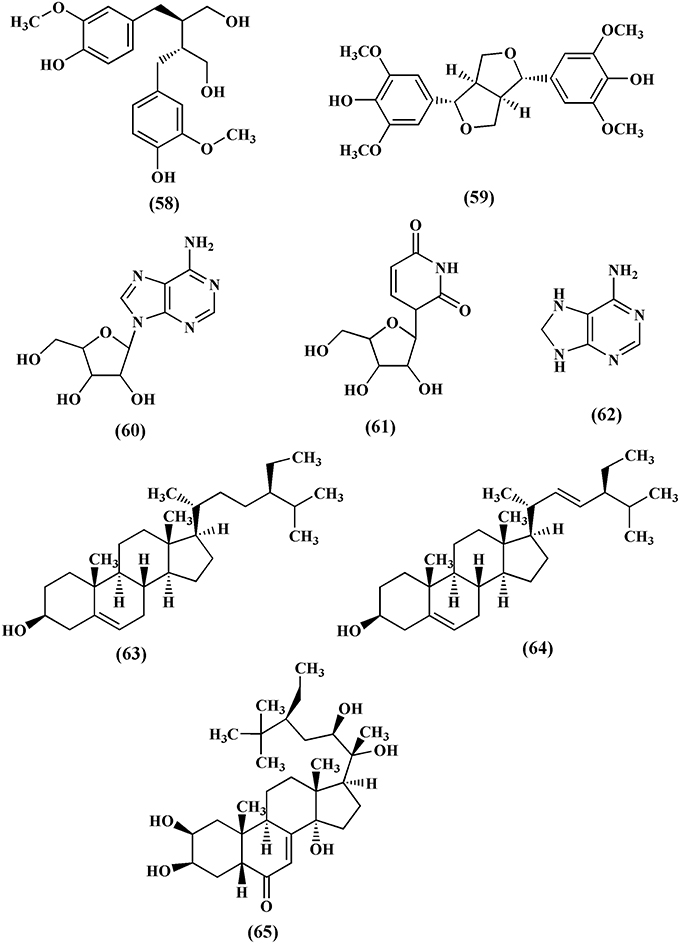
Lignans are group of compounds that arise from the shikimic acid pathway. Secoisolariciresinol (58) and syringaresinol (59) are lignans isolated from the methanol extract of T. crispa (Chung, 2011). Adenosine (60), uridine (61), and adenine (62) are the nucleosides isolated from the n-butanol fraction of T. crispa stem (Praman et al., 2012; Figure 6). Sterols like β-sitosterol (63), stigmasterol (64) and makisterone C (65) have also been isolated from T. crispa (Lin, 2009; Figure 6).
Pharmacological Properties
Anti-Inflammatory and Immunomodulatory Activities
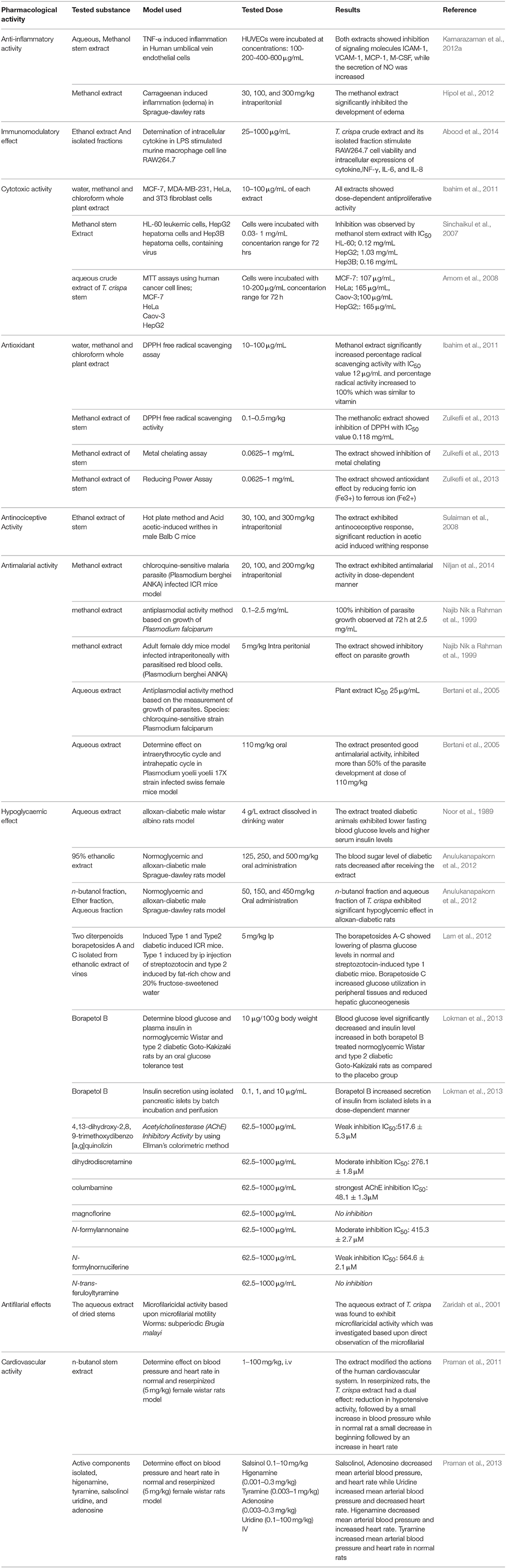
The crude ethanol extract of T. crispa together with other fractions were investigated for potential anti-inflammatory activity by evaluating their effect on expression of intracellular cytokine in LPS stimulated murine macrophage cell line RAW264.7 (murine macrophages from blood). T. crispa crude extract and its isolated fractions stimulated RAW264.7 proliferation in a dose dependent way. T. crispa crude extract at a dose of 25–800 μg/mL expressively increased RAW264.7 proliferation (Table 3). The ethanol extract of T. crispa and its fractions also improved intracellular expressions of cytokine, INF-γ, IL-6, and IL-8. Among all the fractions tested, ethyl acetate fraction was the most active which exhibited significant (P ≤ 0.05) increase in the intracellular expressions of cytokines in RAW264.7 macrophages (Abood et al., 2014). This suggested that the compounds which exhibited immunomodulatory activity were soluble in ethyl acetate. Four active compounds, i.e., cordioside, quercetin, paullinic acid, and boldine were identified by LC-MS analysis of the ethyl acetate fraction. However, chromatography of ethyl acetate fraction to further isolate and characterize the active constituents was not performed. Besides, the active constituents should also be studied at a molecular level to explore their mechanisms of action and role as immunomodulators.
The activity of the aqueous extract of T. crispa stem on nitric oxide (NO) production in LPS stimulated peritoneal macrophages was studied by Yokozawa et al. (2000) using Griess reagent method. The aqueous extract exhibited a dose-dependent (from 5 to 250 μg/mL) inhibition of NO production. Bioassay-guided isolation of the aqueous extract showed that N-trans-feruloyltyramine was the active constituent responsible for the inhibition of NO and this inhibition was linked with reduced levels of inducible NO synthase (iNOS) expression. (Yokozawa et al., 2000, 2001).
The secretion of macrophage colony stimulating factor (M-CSF), vascular cell adhesion molecule (VCAM-1), and intracellular cell adhesion molecule (ICAM-1) in TNF-α stimulated human umbilical vein endothelial cells (HUVECs) was reduced by the aqueous and methanol extracts of T. crispa stem (Kamarazaman et al., 2012a). These adhesion molecules (ICAM-1, VCAM-1) and an inflammatory signaling molecule (M-CSF) were reported to be up-regulated during an immune response. The recruitment of leukocytes was dependent on ICAM-1 and VCAM-1 (Kamarazaman et al., 2012a).
The effect of the aqueous and methanol extracts of T. crispa on cell-mediated immune response was evaluated by foot pad reaction. The development of edema was significantly inhibited by the aqueous extract at doses of 50, 100, and 150 mg/kg and the results were equivalent to ibuprofen (Hipol et al., 2012). The early and late phases of carrageenan-induced inflammation were affected by the extract. Furthermore, oral administration of 50% methanol extract of stem, at a dose of 10 mg/kg also inhibited the carrageenan-induced edema in rats as compared to control group (Higashino et al., 1992). However, these results differ from those reported by Aher and Kumar Wahi (2010) for the other plant of the same genus (T. cardifolia). According to Aher and Kumar Wahi (2010), the methanol extract of T. cardifolia increased the foot pad thickness in rats, at a dose of 100 mg/kg.
Apart from the above-mentioned reports, there is deficiency of data to offer proofs for anti-inflammatory activity. Actually, adequate studies have not been performed on the inflammatory cells, proinflammatory cytokines, and proinflammatory enzymes (PLA2, COX and LOX, PGE2). The assessment of the effect of pure compounds and fractions on the activity and gene expression of enzymes and cytokines involved in inflammation might be beneficial. Likewise, reactive oxygen species including superoxide anion, hydroxyl radical, hydrogen peroxide, and singlet oxygen play a vital part in pathogenesis of inflammation. It would be exciting to assess the effect on these reactive oxygen species. Taking together these results, it is quite premature to conclude regarding the anti-inflammatory activity of T. crispa.
Anticholinesterase Activity
The hydrolysis of acetylcholine to choline is catalyzed by an enzyme acetylcholinesterase (AChE). The hydrolysis of acetylcholine results in the end of nerve impulse transmission at the cholinergic synapses. The quarternary alkaloids, (4,13-dihydroxy-2,8,9-trimethoxydibenzo quinolizinium, magnoflorine, columbamine, N-formylannonaine, dihydrodiscretamine, N-formylnornuciferine, and N-trans-feruloyltyramine) isolated from T. crispa were investigated as inhibitors of AChE by using Ellman's colorimetric method. The isolated compounds showed different activity profiles. Among all the compounds, columbamine displayed the strongest AChE inhibitory activity with an IC50 value of 48.1 μM which was comparable to that of physostigmine (IC50 31.4 μM; Yusoff et al., 2014). A number of alkaloids isolated from medicinal plants have been reported for their AChE inhibitory activity. Alkaloids isolated from T. crispa should be evaluated for their AChE inhibitory activity. The AChE inhibition has therapeutic potential for treatment of parkinson's and alzheimer's diseases, senile dementia, ataxia, and myasthenia gravis. Nevertheless, the results presented in above-mentioned study are not sufficient to draw a meaningful conclusion. Hence, more cutting-edge and mechanistic studies are needed to better understand the anticholinesterase activity.
Antibacterial and Antifilarial Activities
Aqueous, ethanol and chloroform extracts of T. crispa were evaluated for their antimicrobial activity against some gram-positive (Bacillus cereus, Staphylococcus aureus, Listeria monoctogens, Streptococcus pneumonia, and Clostridium diphtheria) and gram-negative bacteria (Shigella flexneri, Salmonella typhi, Proteus vulgaris, Escherichia coli, and Klebsiella pneumonia). The activity of L. monoctogens and P. vulgaris was slightly inhibited by all the extracts. The ethanol extract was effective against S. pneumonia, S. aureus, S. flexneri, and C. diphtheria while chloroform extract inhibited the activities of S. flexneri, C. diphtheria, and S. pneumonia. However, E. coli, B. cereus, and S. typhi remained unaffected by all the extracts (Zakaria et al., 2006). The above-presented results do not coincide with the findings of Md and Mohammad (2011) and Chittur and Gunjan (2012). According to Md and Mohammad (2011), the chloroform extract of T. crispa inhibited E. coli, B. cereus, and S. typhi with zone of inhibitions of 7, 8, and 9 mm, respectively. On the other hand, Chittur and Gunjan (2012) reported that at a dose of 50 μg/mL, the aqueous and ethanol extracts of T. crispa inhibited E.coli with zone of inhibition of 2.6 and 3.6 mm, respectively. As no dose-dependent study was performed, it is difficult to evaluate the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC). The antibacterial studies carried out by Al-alusi et al. (2010) have shown worth mentioning antibacterial activity of T. crispa extracts against the methicillin-resistant S. aureus (MRSA) as compared to the control (vancomycin). The traditional use of T. crispa in the treatment of cholera and syphilitic sores could be validated by investigating its inhibitory effect on the activity of Vibrio cholera and Treponema pallidum. The antibacterial activity of T. crispa needs to be extensively studied and the mechanism involved in the antibacterial activity should also be further explored.
The aqueous extract of dried stems of T. crispa, investigated for in vitro antifilarial effects, showed moderate activity against the adult worms of sub periodic Brugia malayi whereby the value of relative movability values were used as a measure of the antifilarial activity (Zaridah et al., 2001). The aqueous extract of T. crispa exhibited microfilaricidal activity which was investigated based on direct observation of the microfilarial motility (Merawin et al., 2010). The bioactive compounds contributing to the antifilarial activity should be isolated and further studies need to be carried to study their mechanisms of action.
Antioxidant Activity
On the basis of DPPH, FRAP, and TBA tests, the aqueous crude extract of T. crispa stem was found to display high antioxidant activity and its antioxidative potency was equivalent to the previously established antioxidants like BHT and vitamin C (Amom et al., 2008; Zulkhairi et al., 2009). The antioxidant assay performed by Froemming (2011) exhibited that the methanol extract of T. crispa displayed the highest antioxidant activity which was determined by measuring total flavonoid content, total phenolic content, and DPPH free radical scavenging activity. The antioxidant activity could be attributed to the phenolic compounds present in T. crispa such as flavonoids that act as free radical scavengers. Cavin et al. (1998) isolated vanillin, syringin, N-formylannonain, N-formylnornuciferin, borapetosides B, C, and F, N cis- feruloyltyramine, N-trans-feruloyltyramine, and secoisolariciresinol from the dichloromethane extract of T. crispa. Antioxidant and free-radical scavenging potency of N-cis-feruloyltyramine, N-trans- feruloyltyramine, and secoisolariciresinol were higher than the synthetic antioxidant butylhydroxytoluene (BHT). The antioxidant activity could be of therapeutic importance in preventing oxidative stress involved in the development of several diseases including cardiovascular and neurological disorders.
Atherosclerosis Inhibitory Activity
Amom et al. (2011) discovered that the aqueous extract from T. crispa stem administered to hypercholesterolemic rabbits delayed the development of atherosclerosis by suppressing the levels of total cholesterol, triglycerides and low density lipoproteins. In contrast, the level of high density lipoproteins was found to be significantly increased. Furthermore, they also demonstrated that the aqueous and methanol extracts of T. crispa decreased the malondialdehyde level in a dose-dependent manner by increasing the activity of antioxidant enzymes (catalase, superoxide dismutase, and glutathione peroxidase) in H2O2 induced HUVECs (Kamarazaman et al., 2012b). These antioxidant enzymes have been reported to inhibit the reactive oxygen species that actively oxidize the LDL in blood and result in the development of atherosclerosis. The above-mentioned studies do not provide comprehensive and sufficient information. The range of the tested doses was very narrow and no information about the positive control, EC50 and IC50 have been provided. Hence, it is too early to conclude about the antiatherosclerosis activity of T. crispa. However, these findings showed that T. crispa possesses the potential activity and could be explored further as an atherosclerosis inhibitory drug.
Antiparasitic Activity
The methanol extract from the whole plant of T. crispa exhibited 100% inhibition of Plasmodium falciparum growth after 72 h at a dose of 2.5 mg/mL (Najib Nik a Rahman et al., 1999). The chloroquine-sensitive strain of P. falciparum w2 was also inhibited by the aqueous extract of the plant (IC50 25 μg/mL). Bertani et al. (2005) studied the effect of aqueous extract on intra erythrocytic and intra hepatic cycle. In this experiment, Swiss female mice infected with P. yoelii 17X were used. At a dose of 110 mg/kg, the extract inhibited more than 50% of the parasite development (Bertani et al., 2005). Recently, Niljan et al. (2014) determined the antimalarial activity of the methanol extact of T. crispa in ICR mice infected with chloroquine-sensitive malaria parasite P. berghei ANKA. It was discovered that the crude extract of T. crispa exhibited inhibitory effect on the growth of plasmodium in a dose-dependent way. Rungruang and Boonmars (2009) investigated the in vivo antimalarial effect of the crude extract of T. crispa. The mice administrated with a daily dose of 80 mg/kg of the extract exhibited promising inhibitory activity against the parasite, P. yoelii. Though, only crude extracts of T. crispa have been evaluated for its antimalarial activity and no mechanism of action has been described, still these results are in favor of the traditional use of T. crispa as an antimalarial agent. The above- mentioned findings should motivate the researchers to investigate the antiplasmodial activity of pure compounds from T. crispa for further characterization of their antimalarial activity.
Cytotoxic Activity
The cytotoxic activities of different extracts of T. crispa had been studied. The cytotoxic activity of the aqueous crude extract of T. crispa stem was assessed against various human cancer cell lines like MCF-7, HeLa (Henrietta Lacks), Caov-3 (Homo sapiens ovary adenocarcinoma cell line), and HepG2. The cytotoxic effect exerted by the aqueous extract of T. crispa stem was comparable to cisplatin and tamoxifen, with IC50 values as follows: MCF-7 (IC50: 107 μg/mL), HeLa (IC50: 165 μg/mL), Caov-3 (IC50: 100 μg/mL), and HepG2 (IC50: 165 μg/mL; Amom et al., 2008). In another study, Froemming (2011) investigated the cytotoxic effect of the methanol extract of T. crispa on MDA-MB-231 (human breast adenocarcinoma cell line) and MCF-7 cancer cell lines. The methanol extract of T. crispa exhibited a dose-dependent cytotoxic effect on MDA-MB-231 and MCF-7 cancer cell lines with IC50 values of 44.8 and 33.8 μg/mL, respectively. Water, methanol and chloroform extracts of the whole plant exhibited dose-dependent antiproliferative activity against MCF-7, MDA-MB-231, HeLa, and 3T3 (swiss albino mouse embryo fibroblast) cells lines (Ibahim et al., 2011). The growth of human cancer cell lines including HL-60 (human promyelocytic leukemia cells), HepG2 and virus infected Hep3B was inhibited by the methanol extract of T. crispa stem. The methanol extract of T. crispa exerted its effect in a dose- and time-dependent manner (Sinchaikul et al., 2007).
Most of the Tinospora species studied have similar chemical classes of isolates or same chemical constituents but their reported pharmacological properties were different and some showed opposite responses. Amom et al. (2008) investigated the anti-proliferative activity of the aqueous crude extract of T. crispa and found that the extract exhibited moderate anti-proliferative activity on selected human cancer cell lines (IC50 MCF-7: 107 μg/ml, HeLa: 165 μg/ml, Caov-3: 100 μg/ml, and HepG2: 165 μg/ml). While Jagetia et al. (1998) evaluated the antineoplastic activity of Tinospora cordifolia in cultured HeLa cells and found that exposure of HeLa cells to 0, 5, 10, 25, 50, and 100 mg/mL of the extracts (methanol, aqueous, and methylene chloride) resulted in a dose-dependent but significant increase in cell killing, when compared to non-drug-treated controls. This effect of Tinospora cordifolia extracts was comparable or better than doxorubicin treatment. These reported studies are quite preliminary in nature and were only carried out in vitro using different cancer cell lines. These results are currently premature to address the antitumor potential of T. crispa. The active constituents and underlying mechanisms responsible for antitumor properties are still unknown and are needed to be discovered. Moreover, future studies validating therapeutic effect in in vivo model are required.
Cardio-Protective Activity
T. crispa extracts and isolated active compounds showed effects on cardiovascular system both in vitro and in vivo. A study revealed that the crude alcohol extract from the stems of T. crispa caused an increase in blood pressure with a reduction in heart rate in anesthetized dogs (Mokkhasmit et al., 1971). A study conducted on the n-butanol extract of T. crispa revealed the presence of at least three different cardiovascular-active components which exerted their effect through β2-adrenergic receptors to cause a decrease in blood pressure, β1 and β2-adrenergic receptors to cause an increase in heart rate, α-adrenergic receptors to bring about an increase in blood pressure and heart rate, and a nonadrenergic and noncholinergic pathway to cause a decrease in MAP and heart rate (Praman et al., 2011, 2013). Bioassay guided fractionation of the n-butanol extract of the stems of T. crispa led to isolation of five active compounds namely adenosine, uridine, salsolinol, higenamine, and tyramine. The compounds exhibited effect on the mechanisms of blood pressure and heart rate in anesthetized, normal, and reserpinized rats. Salsolinol and adenosine decreased mean arterial blood pressure and heart rate, whereas uridine increased mean arterial blood pressure and decreased heart rate. Higenamine decreased mean arterial blood pressure and increased heart rate, moreover, tyramine increased mean arterial blood pressure and heart rate in normal rats. Salsolinol, tyramine, and higenamine acted via the adrenoreceptors, while uridine and adenosine acted via the purinergic adenosine A2 and P2 receptors to decrease blood pressure with a transitory decrease of heart rate followed by an increase. The crude extract of T. crispa along with the isolated compounds exerted a positive ionotropic effect on the rat isolated left atria stimulated with electrical field. Higenamine, salsolinol (at low concentrations) and tyramine acted through the adrenergic receptors to increase the force of the atrial contraction, however a high concentration of salsolinol acted secondarily by stimulating the release of acetylcholine. Adenosine and uridine acted through the purinergic pathways to cause negative ionotropic effects on the isolated left atria (Praman et al., 2013). The two isolated triterpenes, namely, cycloeucalenol and cycloeucalenone from the chloroform extract of the dried stems of T. crispa. Both of the isolated triterpenes further indicated mild cardiotonic effects, where cycloeucalenol showed slight increase in the right atrial contraction and initial reduction followed by 10% of sustained reduction on the left atria of the rat in vitro meanwhile cycloeucalenone, showed slight change on the right and left atrial contraction (Kongkathip et al., 2002). Imphanban et al. (2009) isolated an aporphine alkaloid, namely (−)-N-formylnornuciferine from the stems of T. crispa, which exhibited in vitro cardiotonic activity. Synthesis of the mixture, (±)-N-formylnornuciferine, by palladium-catalyzed coupling reaction, showed significant reduction in the force of contraction and the heart rate.
Antinociceptive Activity
The dried extract of the stem of T. crispa at a dose of 666 mL exhibited promising central analgesic activity (Almeida et al., 2001). However, the number of tested doses is not sufficient to highlight a dose-dependent effect. Owing to the lack of tested doses and a negative control, it is difficult to draw a conclusion from this study. Sulaiman et al. (2008) reported that the ethanol extract of T. crispa reduced acetic acid-induced writhes in mice in a dose-dependent manner. It was shown that the ethanol extract at a dose of 300 mg/kg exhibited higher analgesic response (92%) than 100 mg/kg of acetyl salicylic acid (81%). Further investigations are needed to provide an evidence for its traditional use against pain.
Cytochromes Inhibitory Activities
Cytochromes P450 (CYPs) are the principal enzymes that catalyze the oxidative metabolism of drugs and other xenobiotics. Isoforms of CYP such as CYP3A4, CYP2D6, CYP2C9, and CYP2E1 have been reported to be involved in the metabolism. The inhibition of CYP results in unexpected adverse drug interactions due to changes in metabolic clearance of co-administered drug. A radiometric assay carried out by Usia et al. (2006a,b) against CYP3A4 and CYP2D6 revealed that T. crispa exhibited an inhibitory activity over 70% on the metabolism mediated by CYP3A4. To better understand the inhibitory mechanism, N-methyl-14C]erythromycin and [O-methyl- 14C]dextromethorphan were used as substrates in human liver microsomes and the activity of CYP was determined by measuring the production of 14C-formaldehyde. At a dose of 0.5 mg/mL, T. crispa methanol extract exhibited more than 30% increase in CYP3A4 inhibition (Subehan et al., 2006). These results suggest an inhibitory effect of T. crispa on CYP3A4 and CYP2D6. The effect of T. crispa on other isomers of CYP viz CYP2C9 and CYP2E1 also needs to be investigated to determine the potential drug-drug interactions. Moreover, further work is needed to purify and identify active constituents responsible for the inhibitory effect.
Antidiabetic Effect
The research undertaken by Noor and Ashcroft (1998) indicated that the orally administrated extract of T. crispa displayed significant antihyperglycaemic effect. The extract might comprise of compounds which initiated the insulin secretion by the modulation of β-cell Ca2+ concentration. Therefore, it can be additionally used as an antidiabetic agent for the treatment of type II diabetes. A potent in vitro insulinotropic activity in the human and rat islets and HIT-T15 (syrian hamster islet cells) B cells was observed after an oral administration of T. crispa extract (Noor et al., 1989). Sriyapai et al. (2009) studied the dry powder of T. crispa for hypoglycemic effect on patients with metabolic syndrome. Twice daily administration of 250 mg T. crispa dry powder significantly decreased fasting blood glucose from the baseline. Noipha and Ninla-Aesong, 2011) indicated that the extract of T. crispa enhanced glucose uptake by in L6 myotubes which was linked to the increased levels of GLUT1 transporter, AMPKα, and PPARγ transcript. Likewise, among twelve furanoditerpenoids isolated from the ethanol extract of T. crispa, borapetosides A, and borapetosides C showed hypoglycemic effect in ICR diabetic mice. These compounds reduced plasma glucose levels in normal and streptozotocin-induced type-1 diabetic mice (Lam et al., 2012). In addition bropetoside C increased glucose utilization in peripheral tissues and decreased hepatic gluconeogenesis, thus accounting for the hypoglycemic effect. Ruan et al (2012) studied the molecular mechanism of borapetoside C for hypoglycemic effects in normal and diabetic mice. The findings of the study revealed that hypoglycemic effect of borapetoside C was mediated via insulin receptor, protein kinase and glucose transporter-2 pathway. They witnessed that borapetoside C increased the glycogen level in skeletal muscle. Borapetoside C increased the expression of glucose transporter-2 as well as phosphorylation of insulin receptor and protein kinase B. Borapetoside A increased the glycogen level in skeletal muscle C2C12 and human hepatocellular carcinoma Hep3B cell lines (Ruan et al, 2013). The report suggested that borapetoside A exerted its hypoglycemic effect, primarily via augmentation of glucose utilization of skeletal muscle and liver. Borapetoside A exerted its hypoglycemic action by the stimulation of insulin receptor, protein kinase and glucose transporter-2 pathway, and the suppression of phosphoenolpyruvate carboxykinase enzymes which regulate hepatic gluconeogenesis and glucolysis (Pilkis and Granner, 1992). The borapetol B, isolated from the methanol and water extracts of T. crispa stem showed anti-diabetic activity in normoglycemic wistar and spontaneously type 2 diabetic Goto-Kakizaki rats. The blood glucose levels were significantly decreased by borapetol B at a dose of 10 μg/100 g in normoglycemic and in type 2 diabetic rats, while the insulin level was significantly increased. Borapetol B dose-dependently stimulated the secretion of insulin from pancreatic islets isolated from rats without damaging islet beta cells (Lokman et al., 2013). Taken together, these results support the traditional use of T. crispa as an antidiabetic agent. Although T. crispa extract and isolated pure compounds have exhibited antidiabetic activity both in vitro and in vivo, the function in humans is still unconvincing as humans were not involved in those studies. Hence, T. crispa is worthwhile to be considered in human diabetes treatment and, therefore, should be extensively studied.
Clinical Trials
A randomized double blind placebo controlled trial was carried out to investigate the efficacy of T. crispa as an additional treatment in patients with type 2 diabetes mellitus who refused insulin injection and did not respond to oral hypoglycemic drugs (Sangsuwan et al., 2004). Twenty patients were apportioned to receive T. crispa powder in capsule form at a dose of 1 g thrice daily for 6 months. Twenty patients received a placebo. The main results were alterations in glycosylated hemoglobin, insulin, and fasting plasma glucose levels. The baseline features of the patients in both groups were not considerably different. There were no significant alterations in glycosylated hemoglobin, insulin and fasting plasma glucose levels between the patients within the group as well as between groups. Two patients who received T. crispa exhibited noticeable rise of liver enzymes that reverted to normal after withdrawing T. crispa. Furthermore, patients in the T. crispa group had noteworthy weight decrease and cholesterol elevation while taking T. crispa. It is hence concluded that there is no proof to support the use of T. crispa 3 g a day for additional therapy in patients with type 2 diabetes mellitus that refused insulin injection and did not respond to oral hypoglycemic drugs. The patients receiving T. crispa might have a greater risk of hepatic dysfunction. Currently, only one study related to antipyretic effect of T. crispa could be found on http://www.clinicaltrials.gov/. In this double blind, interventional, randomized, and placebo controlled phase II trial, safety, acceptability and effectiveness of T. crispa extract will be determined in patients with body temperature 37.8–38.5°C. The patients will receive 500 mg of T. crispa extract after every 4–6 h. Besides, the efficacy and safety results of T. crispa extract will also be compared with those of acetaminophen. Therefore, this interesting study may lead to insightful development of knowledge regarding its clinical efficacy. Nonetheless, more operationally thorough randomized controlled trials are required.
Toxicology
Although several studies have assessed the pharmacological properties of T. crispa, few data are available concerning its toxicity. Chavalittumrong et al. (1997) carried out studies to determine the acute and chronic toxicity of T. crispa. The acute toxicity study revealed that the ethanol extract of T. crispa stem did not cause any signs of toxicity or animal death at a dose of 4.0 g/kg of body weight (g/kg BW). However, the chronic toxicity test for 6 months exhibited that administration of the ethanol extract at a dose of 9.26 g/kg BW/day to rats caused hepatic and renal toxicities. Histopathological examination revealed higher frequency of bile duct proliferation and focal liver cell hyperplasia. Significant rise in alkaline phosphatase (ALP), alanine aminotransferase (ALT) creatinine levels and relative liver weights was also witnessed.
Kadir et al. (2011) reported that oral administration of the ethanol extract of T. crispa at doses of 100 and 200 mg/kg for 8 weeks potentiated the thioacetamide induced hepatotoxicity in rats. Moreover, they reported that the ethanol extract of T. crispa contained certain hepatotoxins which may be responsible for this effect (Kadir et al., 2011). A human hepatotoxicity case was reported due to chronic over use of herbal preparation of T. crispa stem as a prophylactic agent against malaria (Denis et al., 2007). Recently, Langrand et al. (2014) reported an incidence of toxic hepatitis linked with chronic use of high doses of T. crispa. They observed that a patient who received pellets of T. crispa had problem of dark urine and pale stools, linked with asthenia and right hypochondrial pain which lead to jaundice. The histopathological results also confirmed a toxic reaction. The herbal medicine was withdrawn on admission and the patient completely recovered without treatment, with normal liver function 2 months after the acute episode. The data reported about toxicity of T. crispa are very limited, so toxicological aspects of T. crispa need to be investigated comprehensively.
Conclusions and Future Directions
Herein, we documented the existing phytochemistry, pharmacological properties, and application researches on T. crispa. The amount of experimental data evidenced rich nutrients and vast biological active substances in T. crispa. A peruse of available scientific references show that the traditional medical uses of T. crispa have been evaluated by modern pharmacological studies. T. crispa has the potential multiple pharmacological and therapeutic activities in the management of hypertension, lumbago, postpartum remedy, tuberculosis, hemorrhoids, wound healing, itching, muscle pain, etc., which can be explained by the presence of various terpenoids, alkaloids, lignans and nucleosides in the herb. The biological activities and chemical nature of the bioactive compounds must be of great attention for the researchers. Diterpenoid glycosides from T. crispa have shown promising antidiabetic activity. Further investigations on the terpenoids offer great potential upon which they can be predicted to be successful clinical trial candidates in antidiabetic therapy. Similarly, authentication of all the secondary metabolites should be performed carefully by advanced analytical techniques to approve the quality and conforming biological activity. Most of the mentioned pharmacological studies have provided some suggestive scientific evidence for its various traditional uses in fever, internal inflammation (Yokozawa et al., 2000, 2001; Kamarazaman et al., 2012a), pain (Almeida et al., 2001; Sulaiman et al., 2008), antibacterial (Zakaria et al., 2006; Chittur and Gunjan, 2012), malaria (Niljan et al., 2014), diabetes (Lokman et al., 2013; Ruan et al, 2013), and hypertension (Praman et al., 2011, 2013) as in Asian countries, especially in Malaysia, Indonesia, Philippines, China, Cambodia, and Bangladesh.
In a word, T. crispa has received much interest. However, future studies are necessary to address issues regarding composition of the extract, explicability of preclinical experiments, and lack of transformation of the preclinical results to clinical efficacy. As a result, T. crispa was still employed as folk prescription and the related health products are unpersuasive. Thereby, it is extremely important to conduct detailed investigations on the composition and pharmacological significance of medicinal plants and standardize the formulations based on ingredients. Further systematic studies are necessary to evaluate the efficacy using standardized extracts of T. crispa, and to identify the bioactive molecules responsible for the biological activities so that cost-effective, potential medicinal drug and health products can be developed at a large scale. Also, attempts should be made to conduct serious randomized human trials and determine modes or mechanisms of action, bioavailability, pharmacokinetics, and physiological pathways for specific bioactives of T. crispa which might be responsible behind the protective effects offered by extracts rich in flavonoids and terpenoids in many pharmacological studies. As more scientific evidences on therapeutic effects of T. crispa will be found, products (e.g., health care products) based on it might boom in the future.
Author Contributions
Concept, Editing, Final Approval IJ participated in the concept, editing and gave the final approval of the final version of the manuscript to be submitted for publication. WA drafted the manuscript and SB was involved in the editing process.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Arus Perdana grant from Universiti Kebangsaan Malaysia (AP2014-023).
Abbreviations
Tinospora crispa (L.) Hook. f. & Thomson, T. crispa; LPS, lipopolysaccharide; ICAM, intracellular cell adhesion molecule; M-CSF, macrophage colony stimulating factor; VCAM, vascular cell adhesion molecule; MCP, Monocyte chemoattractant protein; AChE, acetylcholinesterase; MRSA, methicillin-resistantstaphylococcus aureus; NO, nitric oxide; DPPH, 2,2-diphenyl-1-picrylhydrazyl; FRAP, fluorescence recovery after photobleaching, BHT, butylhydroxytoluene; ICR, institute for cancer research;; IC50, half maximal inhibitory concentration; CYP3A4, cytochrome P450 3A4; GLUT 1, glucose transporter 1; AMPK, adenosine monophosphate-activated protein kinase; PPAR, peroxisome proliferator-activated receptor; BW, body weight; ALP, alkaline phosphatase; ALT, alanine aminotransferase.
References
Abood, W. N., Fahmi, I., Abdulla, M. A., and Ismail, S. (2014). Immunomodulatory effect of an isolated fraction from Tinospora crispa on intracellular expression of INF-gamma, IL-6 and IL-8. BMC Complement. Altern. Med. 14:205. doi: 10.1186/1472-6882-14-205
Aher, V. Kumar Wahi, A. (2010). Pharmacological study of Tinospora cordifolia as an immunomodulator. Int. J. Curr. Pharm. Res. 2, 52–54.
Ahmad, F. B., and Ismail, G. (2003). Medicinal plants used by Kadazandusun communities around crocker range, ASEAN Review of Biodiversity and Environmental Conservation (ARBEC). Available online at: http://kdca.org.my/wp-content/files/medicinal_crange.pdf
Al-alusi, N., Kadir, F., Ismail, S., and Abdullah, M. (2010). In vitro interaction of combined plants: Tinospora crispa and Swietenia mahagoni against methicillinresistant Staphylococcus aureus (MRSA). Afr. J. Microbiol. Res. 4, 2309–2312.
Almeida, R. N., Navarro, D. S., and Barbosa-Filho, J. M. (2001). Plants with central analgesic activity. Phytomedicine 8, 310–322. doi: 10.1078/0944-7113-00050
Amom, Z., Azman, K. F., Ismail, N. A., Shah, Z. M., and Arshad, M. S. M. (2011). An aqueous extract of Tinospora crispa possesses antioxidative properties and reduces atherosclerosis in hypercholesterolemic-induced rabbits. J. Food Biochem. 35, 1083–1098. doi: 10.1111/j.1745-4514.2010.00436.x
Amom, Z., Md Akim, A., Nik Hassan, M. K., Ibrahim, N., Moklas, M., Aris, M., et al. (2008). Biological properties of Tinospora crispa (akar patawali) and its antiproliferative activities on selected human cancer cell lines. Malays. J. Nutr. 14, 173–187.
Anulukanapakorn, K., Pancharoen, O., and Bansiddhi, J. (2012). Hypoglycemic effect of Tinospora crispa (Linn.) Mier ex Hook F. & Thorns (Menispermaceae) in Rats. Bull. Depart. Med. Sci. 41, 231–243.
Bertani, S., Bourdy, G., Landau, I., Robinson, J. C., Esterre, P., and Deharo, E. (2005). Evaluation of French Guiana traditional antimalarial remedies. J. Ethnopharmacol. 98, 45–54. doi: 10.1016/j.jep.2004.12.020
Bisset, N. G., and Nwaiwu, J. (1983). Quaternary alkaloids of Tinospora species. Planta Med. 48, 275–279. doi: 10.1055/s-2007-969933
Cavin, A., Hostettmann, K., Dyatmyko, W., and Potterat, O. (1998). Antioxidant and lipophilic constituents of Tinospora crispa. Planta Med. 64, 393–396. doi: 10.1055/s-2006-957466
Chavalittumrong, P., Attawish, A., Chuthaputti, A., and Chuntapet, P. (1997). Toxicological study of crude extract of Tinospora crispa Mier ex Hook F. & Thoms. Thai. J. Pharm. Sci. 21, 199–210.
Chittur, M. A. I., and Gunjan, M. (2012). Antimicrobial activity of Tinospora crispa root extracts. Int. J. Res. Ayurveda Pharm. 3, 417–419.
Choudhary, M. I., Ismail, M., Ali, Z., Shaari, K., and Lajis, N. H. (2010a). Alkaloidal constituents of Tinospora crispa. Nat. Prod. Commun. 5, 1747–1750.
Choudhary, M. I., Ismail, M., Shaari, K., Abbaskhan, A., Sattar, S. A., and Lajis, N. H. (2010b). cis-Clerodane-type furanoditerpenoids from Tinospora crispa. J. Nat. Prod. 73, 541–547. doi: 10.1021/np900551u
Chuakul, W., Saralamp, P., and Boonpleng, A. (2002). Medicinal plants used in the Kutchum district, Yasothon Province, Thailand. Thai. J. Phytopharm. 9, 22–49.
Chung, S. Y. (2011). Studies on the Constituents of the Dry Stem of Tinospora crispa (Lour.) Merr. Masters dissertation, China Medical University 2011.
Denis, G., Gerard, Y., Sahpaz, S., Laporte, R., Viget, N., Ajana, F., et al. (2007). Malarial prophylaxis with medicinal plants: toxic hepatitis due to Tinospora crispa. Therapie 62, 271–272. doi: 10.2515/therapie:2007036
Dweck, A. C., and Cavin, J. P. (2006). Andawali (Tinospora crispa): a review. Pers. Care Mag. 7, 33–39.
Froemming, G. (2011). Anti-proliperative and antioxidant effects of Tinospora crispa (Batawali). Biomed. Res. 22, 57–62.
Fukuda, N., Yonemitsu, M., and Kimura, T. (1983). Studies on the constituents of the stems of Tinospora tuberculata Beumee. I. N-trans-and N-cis-feruloyl tyramine, and a new phenolic glucoside, tinotuberide. Chem. Pharm. Bull. 31, 156–161. doi: 10.1248/cpb.31.156
Fukuda, N., Yonemitsu, M., and Kimura, T. (1986). Studies on the constituents of the stems of Tinospora tuberculata Beumee, III: new diterpenoids, borapetoside B and borapetol B. Chem. Pharm. Bull. 34, 2868–2872. doi: 10.1248/cpb.34.2868
Fukuda, N., Yonemitsu, M., Kimura, T., Hachiyama, S., Miyahara, K., and Kawasaki, T. (1985). Studies on the constituents of the stems of Tinospora tuberculata Beumee. II. New diterpenoids, borapetoside A and borapetol A. Chem. Pharm. Bull. 33, 4438–4444. doi: 10.1248/cpb.33.4438
Gimlette, J. D., and Burkill, I. H. (1930). The Medical Book of Malayan Medicine. Singapore: Botanic Gardens.
Higashino, H., Suzuki, A., Tanaka, Y., and Pootakham, K. (1992). Inhibitory effects of Siamese Tinospora crispa extracts on the carrageenin-induced foot pad edema in rats (the 1st report). Nippon. Yakurigaku Zasshi 100, 339–344. doi: 10.1254/fpj.100.339
Hipol, R. L. B., Cariaga, M. F. N. M., and Hipol, R. M. (2012). Anti- inflammatory activities of the aqueous extract of the stem of Tinospora crispa (Family Menispermaceae). J. Nat. Stud. 11, 88–95.
Hout, S., Chea, A., Bun, S. S., Elias, R., Gasquet, M., Timon-David, P., et al. (2006). Screening of selected indigenous plants of Cambodia for antiplasmodial activity. J. Ethnopharmacol. 107, 12–18. doi: 10.1016/j.jep.2006.01.028
Ibahim, M., I'zzah, W. N. W., Narimah, A., Asyikin, N. Z., Shafinas, S.-N. S., and Froemming, G. (2011). Anti-proliperative and antioxidant effects of Tinospora crispa (Batawali). Biomed. Res. India 22, 57–62.
Imphanban, K., Kongkathip, N., Dhumma-Upakorn, P., Mesripong, R., and Kongkathip, B. (2009). Synthesis of N-formylnornuciferine with cardiotonic activity. Nat. Sci. 43, 738–744.
Islam, F., Jahan, F. I., Seraj, S., Malek, I., Sadat, A., Bhuiyan, M. S. A., et al. (2011). Variations in diseases and medicinal plant selection among folk medicinal practitioners: a case study in Jessore district, Bangladesh. Am. Eurasian J. Sustain. Agric. 5, 282–291.
Jagetia, G. C., Nayak, V., and Vidyasagar, M. S. (1998). Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in cultured HeLa cells. Cancer Lett. 127, 71–82. doi: 10.1016/S0304-3835(98)00047-0
Kadir, F. A., Othman, F., Abdulla, M. A., Hussan, F., and Hassandarvish, P. (2011). Effect of Tinospora crispa on thioacetamide-induced liver cirrhosis in rats. Indian J. Pharmacol. 43, 64–68. doi: 10.4103/0253-7613.75673
Kadir, M. F., Bin Sayeed, M. S., Setu, N. I., Mostafa, A., and Mia, M. M. (2014). Ethnopharmacological survey of medicinal plants used by traditional health practitioners in Thanchi, Bandarban Hill Tracts, Bangladesh. J. Ethnopharmacol. 155, 495–508. doi: 10.1016/j.jep.2014.05.043
Kamarazaman, I. S., Amorn, Z., and Ali, R. M. (2012a). Inhibitory properties of Tinospora crispa extracts on TNF-α induced inflammation on human umbilical vein endothelial cells (HUVECS). Int. J. Trop. Med. 7, 24–29. doi: 10.3923/ijtmed.2012.24.29
Kamarazaman, I. S., Amorn, Z., and Ali, R. M. (2012b). Protective effects of Tinospora crispa extracts on H2O2 induced oxidative stress and TNF-α-induced inflammation on human umbilical vein endothelial cells (HUVECs). J. Med. Plants Res. 6, 3013–3021. doi: 10.5897/JMPR11.1510
Koay, Y. C., and Amir, F. (2013). A review of the secondary metabolites and biological activities of Tinospora crispa (Menispermaceae). Trop. J. Pharm. Res. 12, 641–649. doi: 10.4314/tjpr.v12i4.30
Kongkathip, N., Dhumma-Upakorn, P., Kongkathip, B., Chawananoraset, K., Sangchomkaeo, P., and Hatthakitpanichakul, S. (2002). Study on cardiac contractility of cycloeucalenol and cycloeucalenone isolated from Tinospora crispa. J. Ethnopharmacol. 83, 95–99. doi: 10.1016/S0378-8741(02)00210-6
Kongsaktrakoon, B., Temsiririrkkul, R., Suvitayavat, W., Nakornchai, S., and Wongkrajang, Y. (1984). The antipyretic effect of Tinospora crispa Mier ex Hook. f. & Thoms. Mahidol Univ. J. Pharm. Sci. 21, 1–6.
Lam, S. H., Ruan, C. T., Hsieh, P. H., Su, M. J., and Lee, S. S. (2012). Hypoglycemic diterpenoids from Tinospora crispa. J. Nat. Prod. 75, 153–159. doi: 10.1021/np200692v
Langrand, J., Regnault, H., Cachet, X., Bouzidi, C., Villa, A. F., Serfaty, L., et al. (2014). Toxic hepatitis induced by a herbal medicine: Tinospora crispa. Phytomedicine 21, 1120–1123. doi: 10.1016/j.phymed.2014.04.031
Li, S., Long, C., Liu, F., Lee, S., Guo, Q., Li, R., et al. (2006). Herbs for medicinal baths among the traditional Yao communities of China. J. Ethnopharmacol. 108, 59–67. doi: 10.1016/j.jep.2006.04.014
Lin, Y. H. (2009). Studies on the Chemical Constituents of Tinospora crispa and Synthesis of the Analogous of Penta-o-Galloyl-d-Glucopyranose. Masters dissertation, China Medical University 2009.
Lokman, F. E., Gu, H. F., Wan Mohamud, W. N., Yusoff, M. M., Chia, K. L., and Ostenson, C. G. (2013). Antidiabetic effect of oral borapetol B compound, isolated from the plant Tinospora crispa, by stimulating insulin release. Evid. Based Complement. Alternat. Med. 2013:727602. doi: 10.1155/2013/727602
Longuefosse, J. L., and Nossin, E. (1996). Medical ethnobotany survey in Martinique. J. Ethnopharmacol. 53, 117–142. doi: 10.1016/0378-8741(96)01425-0
Martin, T. S., Ohtani, K., Kasai, R., and Yamasaki, K. (1996). Furanoid diterpene glucosides from Tinospora rumphii. Phytochemistry 42, 153–158. doi: 10.1016/0031-9422(95)00902-7
Md, H. A., and Mohammad, S. (2011). Antimicrobial, cytotoxicity and antioxidant activity of Tinospora crispa. J. Pharm. Biomed. Sci. 13, 1–4.
Merawin, L. T., Arifah, A. K., Sani, R. A., Somchit, M. N., Zuraini, A., Ganabadi, S., et al. (2010). Screening of microfilaricidal effects of plant extracts against Dirofilaria immitis. Res. Vet. Sci. 88, 142–147. doi: 10.1016/j.rvsc.2009.05.017
Mohamad, S., Zin, N. M., Wahab, H. A., Ibrahim, P., Sulaiman, S. F., Zahariluddin, A. S., et al. (2011). Antituberculosis potential of some ethnobotanically selected Malaysian plants. J. Ethnopharmacol. 133, 1021–1026. doi: 10.1016/j.jep.2010.11.037
Mokkhasmit, M., Ngarmwathana, W., Sawasdimongkol, K., and Permphiphat, U. (1971). Pharmacological evaluation of Thai medicinal plants. J. Med. Assoc. Thai. 54, 490–503.
Na, B., Sadikun, A., Choon, T., Ying, T., and Asmawi, M. (2005). Aporphine alkaloids isolated from the cardiovascular active fraction of Tinospora crispa. Malays. J. Sci. 24. 161–165.
Najib Nik a Rahman, N., Furuta, T., Takane, K., and Ali Mohd, M. (1999). Antimalarial activity of extracts of Malaysian medicinal plants. J. Ethnopharmacol. 64, 249–254. doi: 10.1016/S0378-8741(98)00135-4
Naomichi, F., Michiko, Y., and Takeatsu, K. (1983). Studies on the constituents of the stems of Tinospora tuberculata Beumee. IN-trans and Ncis-Feruloyl tyramine and a new phenolic glucoside, Tinotuberide. Chem. Pharm. Bull. 31, 156–161. doi: 10.1248/cpb.31.156
Niljan, J., Jaihan, U., Srichairatanakool, S., Uthaipibull, C., and Somsak, V. (2014). Antimalarial activity of stem extract of Tinospora crispa against plasmodium berghei infection in mice. J. Health Res. 28, 199–204.
Noipha, K., and Ninla-Aesong, P. (2011). The Activation of GLUT1, AMPK alpha and PPAR gamma by Tinospora crispa in L6 Myotubes. Spatula DD. 1, 245–249. doi: 10.5455/spatula.20111206115628
Noor, H., and Ashcroft, S. J. (1998). Pharmacological characterisation of the antihyperglycaemic properties of Tinospora crispa extract. J. Ethnopharmacol. 62, 7–13. doi: 10.1016/S0378-8741(98)00008-7
Noor, H., Hammonds, P., Sutton, R., and Ashcroft, S. J. (1989). The hypoglycaemic and insulinotropic activity of Tinospora crispa: studies with human and rat islets and HIT-T15 B cells. Diabetologia 32, 354–359. doi: 10.1007/BF00277258
Pachaly, P., Adnan, A. Z., and Will, G. (1992). NMR-assignments of N-acylaporphine alkaloids from Tinospora crispa. Planta Med. 58, 184–187. doi: 10.1055/s-2006-961425
Pathak, A. K., Jain, D. C., and Sharma, R. P. (1995). Chemistry and biological activities of the genera Tinospora. Pharm. Biol. 33, 277–287. doi: 10.3109/13880209509065379
Pilkis, S. J., and Granner, D. K. (1992). Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 54, 885–909. doi: 10.1146/annurev.ph.54.030192.004321
Praman, S., Mulvany, M. J., Allenbach, Y., Marston, A., Hostettmann, K., Sirirugsa, P., et al. (2011). Effects of an n-butanol extract from the stem of Tinospora crispa on blood pressure and heart rate in anesthetized rats. J. Ethnopharmacol. 133, 675–686. doi: 10.1016/j.jep.2010.10.052
Praman, S., Mulvany, M. J., Williams, D. E., Andersen, R. J., and Jansakul, C. (2012). Hypotensive and cardio-chronotropic constituents of Tinospora crispa and mechanisms of action on the cardiovascular system in anesthetized rats. J. Ethnopharmacol. 140, 166–178. doi: 10.1016/j.jep.2012.01.006
Praman, S., Mulvany, M. J., Williams, D. E., Andersen, R. J., and Jansakul, C. (2013). Crude extract and purified components isolated from the stems of Tinospora crispa exhibit positive inotropic effects on the isolated left atrium of rats. J. Ethnopharmacol. 149, 123–132. doi: 10.1016/j.jep.2013.06.010
Rahmatullah, M., Azam, M. N. K., Rahman, M. M., Seraj, S., Mahal, M. J., Mou, S. M., et al. (2011). A survey of medicinal plants used by Garo and non-Garo traditional medicinal practitioners in two villages of Tangail district, Bangladesh. Am. Eurasian J. Sustain. Agric. 5, 350–357.
Rahmatullah, M., Noman, A., Hossan, M. S., Rashid, M., Rahman, T., Chowdhury, M. H., et al. (2009). A survey of medicinal plants in two areas of Dinajpur district, Bangladesh including plants which can be used as functional foods. Am. Eurasian J. Sustain. Agric. 3, 862–876.
Roosita, K., Kusharto, C. M., Sekiyama, M., Fachrurozi, Y., and Ohtsuka, R. (2008). Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J. Ethnopharmacol. 115, 72–81. doi: 10.1016/j.jep.2007.09.010
Ruan, C.-T., Lam, S.-H., Chi, T.-C., Lee, S.-S., and Su, M.-J. (2012). Borapetoside C from Tinospora crispa improves insulin sensitivity in diabetic mice. Phytomedicine 19, 719–724. doi: 10.1016/j.phymed.2012.03.009
Ruan, C.-T., Lam, S.-H., Lee, S.-S., and Su, M.-J. (2013). Hypoglycemic action of borapetoside A from the plant Tinospora crispa in mice. Phytomedicine 20, 667–675. doi: 10.1016/j.phymed.2013.02.009
Rungruang, T., and Boonmars, T. (2009). In vivo antiparasitic activity of the Thai traditional medicine plant-Tinospora crispa-against plasmodium Yoelii. Southeast Asian J. Trop. Med. Pub. Health 40, 898.
Sangsuwan, C., Udompanthurak, S., Vannasaeng, S., and Thamlikitkul, V. (2004). Randomized controlled trial of Tinospora crispa for additional therapy in patients with type 2 diabetes mellitus. J. Med. Assoc. Thai. 87, 543–546.
Sinchaikul, S., Chen, S. T., and Sookkheo, B. (2007). Tumor cell selective antiproliferative effect of the extract from Tinospora crispa (borapet), Bull. Health Sci. Tech. 7, 75–84.
Srithi, K., Balslev, H., Wangpakapattanawong, P., Srisanga, P., and Trisonthi, C. (2009). Medicinal plant knowledge and its erosion among the Mien (Yao) in northern Thailand. J. Ethnopharmacol. 123, 335–342. doi: 10.1016/j.jep.2009.02.035
Sriyapai, C., Dhumma-Upakorn, R., Sangwatanaroj, S., Kongkathip, N., and Krittiyanunt, S. (2009). Hypoglycemic effect of Tinospora crispa dry powder in outpatients with metabolic syndrome at King Chulalongkorn Memorial Hospital. J. Health Res. 23, 125–133.
Subehan, U. T., Iwata, H., Kadota, S., and Tezuka, Y. (2006). Mechanism-based inhibition of CYP3A4 and CYP2D6 by Indonesian medicinal plants. J. Ethnopharmacol. 105, 449–455. doi: 10.1016/j.jep.2005.12.001
Sulaiman, M., Zakaria, Z., and Lihan, R. (2008). Antinociceptive and anti-inflamatory activities of Tinospora crispa in various animal models. Int. J. Top. Med. 3, 66–69.
Sumimoto Chemicals Co Ltd (1982). Berberine alkaloid production by tissue culture. Jpn Kokai Tokkyo Koho 57, 144, 992 (CI C 12pl7/18).
Umi Kalsom, Y., and Noor, H. (1995). Flavone O-glycosides from Tinospora crispa. Fitoterapia 66, 280.
Usia, T., Iwata, H., Hiratsuka, A., Watabe, T., Kadota, S., and Tezuka, Y. (2006a). CYP3A4 and CYP2D6 inhibitory activities of Indonesian medicinal plants. Phytomedicine 13, 67–73. doi: 10.1016/j.phymed.2004.06.022
Usia, T., Iwata, H., Kadota, S., and Tezuka, Y. (2006b). Mechanism-based inhibition of CYP3A4 and CYP2D6 by Indonesian medicinal plants. J. Ethnopharmacol. 105, 449–455. doi: 10.1016/j.jep.2005.12.001
Wyk, B. E. V., and Wink, M. (2004). Medicinal Plants of the World: An Illustrated Scientific Guide to Important Medicinal Plants and their Uses. Portland, OR: Timber Press.
Yokozawa, T., Tanaka, T., and Kimura, T. (2001). Examination of the nitric oxide production-suppressing component in Tinospora tuberculata. Biol. Pharm. Bull. 24, 1153–1156. doi: 10.1248/bpb.24.1153
Yokozawa, T., Wang, T. S., Chen, C. P., and Hattori, M. (2000). Inhibition of nitric oxide release by an aqueous extract of Tinospora tuberculata. Phytother. Res. 14, 51–53. doi: 10.1002/(sici)1099-1573(200002)14:1<51::aid-ptr545>3.0.co;2-k
Yusoff, M., Hamid, H., and Houghton, P. (2014). Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa. Molecules 19, 1201–1211. doi: 10.3390/molecules19011201
Zakaria, Z. A., Mat Jais, A. M., Henie, E. F. P., Zaiton, H., Somchit, M. N., Sulaiman, M. R., et al. (2006). The in vitro antibacterial activity of Tinospora crispa extracts. J. Biol. Sci. 6, 398–401. doi: 10.3923/jbs.2006.398.401
Zaridah, M. Z., Idid, S. Z., Omar, A. W., and Khozirah, S. (2001). In vitro antifilarial effects of three plant species against adult worms of subperiodic Brugia malayi. J. Ethnopharmacol. 78, 79–84. doi: 10.1016/S0378-8741(01)00286-0
Zulkefli, H. N., Mohama, J., and Abidin, N. Z. (2013). Antioxidant activity of methanol extract of Tinospora crispa and Tabernaemontana corymbosa. Sains Malays. 42, 697–706.
Keywords: Tinospora crispa, traditional uses, phytochemistry, pharmacological activities, toxicity studies, clinical trials
Citation: Ahmad W, Jantan I and Bukhari SNA (2016) Tinospora crispa (L.) Hook. f. & Thomson: A Review of Its Ethnobotanical, Phytochemical, and Pharmacological Aspects. Front. Pharmacol. 7:59. doi: 10.3389/fphar.2016.00059
Received: 23 December 2015; Accepted: 29 February 2016;
Published: 21 March 2016.
Edited by:
Aiping Lu, Hong Kong Baptist University, ChinaReviewed by:
Subhalakshmi Ghosh, Jadavpur University, IndiaRajasekaran Subbiah, BIT-Campus, Anna University, India
Copyright © 2016 Ahmad, Jantan and Bukhari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibrahim Jantan, profibj@gmail.com
 Waqas Ahmad
Waqas Ahmad Ibrahim Jantan
Ibrahim Jantan Syed N. A. Bukhari
Syed N. A. Bukhari