
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 23 September 2015
Sec. Ethnopharmacology
Volume 6 - 2015 | https://doi.org/10.3389/fphar.2015.00211
 Yeng Chen1,2*
Yeng Chen1,2* Wai-Mei Phang1
Wai-Mei Phang1 Alan K.-W. Mu3
Alan K.-W. Mu3 Choon-Keat Chan4
Choon-Keat Chan4 Bin-Seng Low5,6
Bin-Seng Low5,6 Sreenivasan Sasidharan7
Sreenivasan Sasidharan7 Kit-Lam Chan5
Kit-Lam Chan5Eurycoma longifolia is a Malaysian native herb that has been widely used as an aphrodisiac and a remedy for andropause. Although the physiological effects of the plant extract were predicted as a result of the alterations in protein expression, the key protein(s) involved in these alterations are still unclear. In the present study, we have investigated the effect of standardized E. longifolia extract on serum protein expression up to 28 days following oral administration in rats. Serum protein profiles were analyzed by 2-dimensional electrophoresis, and altered proteins were identified via mass spectrometry. We observed that alpha-2-HS glycoprotein (AHS) was significantly decreased in the serum of experimentally treated rats compared to pre-treated animals. Moreover, reduction in AHS was confirmed using competitive enzyme-linked immunosorbent assay. AHS expression is known to be associated with insulin resistance and diabetes. Our data indicated that serum AHS was reduced in rats treated with standardized E. longifolia extract, and therefore form a prelude for further investigation into the effects of this natural extract in animal models involving infertility and diabetes.
Eurycoma longifolia Jack is a tall, slender shrub-tree that is commonly found in the lowland forests up to 500 m above sea level in Southeast Asia (Kuo et al., 2004). The root of E. longifolia is widely used in folk medicine as a recuperative tonic after childbirth and for treating fever, boils, wound ulcers, syphilis, and bleeding gums (Burkill, 1966). It has also been employed as a traditional remedy for malaria, high blood pressure, fatigue, loss of sexual desire, and impotence (Chan et al., 1998). Notably, it was demonstrated that eurycomanone, a quassinoid isolated from E. longifolia roots, displayed cytotoxicity against cancer cells as well as antimalarial and antiulcer activities (Bhat and Karim, 2010). Furthermore, several studies showed that E. longifolia extracts could improve sexual performance (Ang and Sim, 1998; Zanoli et al., 2009) and sperm quality in rats (Noor et al., 2004; Chan et al., 2009; Wahab et al., 2010). Clinical studies confirmed that sperm quality and testosterone levels in infertile males improved upon treatment with E. longifolia (Tambi and Imran, 2010; Tambi et al., 2012).
We recently provided evidence that eurycomanone obtained from the standardized extract F2 could improved spermatogenesis and fertility in rats (Low et al., 2013). The validated HPLC method for the phytochemical analysis of the active constituents in the standardized extract F2 was found to contain 34.14% w/w of the major bioactive quassinoids, comprising 13α(21)-epoxyeurycomanone (1) (7.39 ± 0.17%, w/w), eurycomanone (2) (14.49 ± 0.26%, w/w), 13,21-dihydroeurycomanone (3) (0.72 ± 0.06%, w/w) and eurycomanol (4) (9.54 ± 0.22%, w/w) (Figure 1) (Low et al., 2013). In fact, we observed elevation of testosterone, luteinizing hormone, and follicle stimulating hormone in plasma, along with suppression of estrogen, after oral administration of E. longifolia extract, revealing its effect on the hypothalamic-pituitary-gonadal axis (Low et al., 2013). This increase in male fertility and hormones following treatment with the quassinoid-rich E. longifolia extract suggests that it may alter animal physiology. However, no study has identified the protein(s) linking eurycomanone to its in vivo physiological effects. Although it is predicted that these physiological changes result from alterations in protein expression following E. longifolia treatment, it remains unclear which proteins might be affected (Tsai and Wiltbank, 1998). Thus, the aim of this study was to investigate serum proteomic profile changes following administration of standardized bioactive E. longifolia in an animal model. E. longifolia-treated male Sprague-Dawley rats were compared to pre-treated animals over 28 days, and serum proteins were analyzed using two-dimensional gel electrophoresis (2-DE) and subsequently identified via mass spectrometry (MS).
The standardized E. longifolia extract was prepared and quassinoid content was determined through a high performance liquid chromatography (HPLC)-based method as described previously (Teh et al., 2011; Chan et al., 2012). E. longifolia roots were purchased from Biotropics Malaysia Berhad, Kuala Lumpur, Malaysia. A voucher specimen of the plant has been deposited at Penang Botanical Garden, Malaysia, with a reference no. of JTB1189 (Low et al., 2013). Briefly, the E. longifolia roots were ground into a powder, and ∼100 kg were extracted with 10 L of methanol at 45°C (8 h per day, replacing fresh solvent for five consecutive days). Filtrates of the crude extract were combined and evaporated to dryness in vacuo. The dried methanolic extract was subjected to a resin-packed column and gradually eluted with a MeOH–H2O mixture (1:0–0:1 ratio; Chan et al., 2012). The quassinoid-containing fraction was identified as the standardized E. longifolia extract and was dried before oral administration.
Six Sprague-Dawley male rats (12 weeks old, weighing 290–310 g) were obtained from the Animal Research and Service Centre, University of Science Malaysia, Penang, Malaysia. The animals were fed with a commercial pellet diet (Gold Coin, Penang, Malaysia) and the water was provided ad libitum. The rats were individually caged and housed in a 12 h light-dark cycle at ambient room temperature. All animal experimentation was humanely conducted and maintained in accordance with Guide for the Care and Use of Laboratory Animals 2010 Animal Research and Service Centre, Universiti Sains Malaysia and European legislation (EEC n. 86/609). The experimental designs and procedures were approved by the institutional Animal Ethics Committee with Approval No: USM/Animal Ethics Approval/2012/(79) (386).
Prior to experimentation, the rats were kept for 1 week upon husbandry in accordance with guidelines set by the European Agency for the Evaluation of Medicinal Products. Animals were kept in cages with free access to food and water and were orally given standardized E. longifolia extract at a dose of 50 mg/kg for 28 days, consecutively. Blood samples (∼0.15 mL at pre-treatment and day 1; 0.2 mL at day 7, 14, 21, and 28) were collected from the tail veins of treated rats in accordance to the guidelines set by the John Hopkins Medicine Guidelines for multiple blood draws (John Hopkins Medicine, 2012). Sera were separated through centrifugation at 3,000 rpm for 15 min and stored at -20°C prior to analysis.
The protein concentrations of each samples group (n = 6) were quantified using 660 nm Protein Assay kit (Thermoscientific, Rockford, IL, USA). The serum samples were diluted in 1:1000 factor and then added into microplate wells (10 μl each). One hundred fifty micro liter of the protein assay reagent was added into each well and then incubated for 5 min. The absorbance was measured using μQuantTM microplate spectrometer.
Six biologically replicates for each treatment (pre-treatment, days 1, 7, 14, 21, and 28) were analyzed by 2-DE (Chen et al., 2008; Chen et al., 2009; Mu et al., 2012). Briefly, the serum samples (∼3 μl each) were rehydrated in 11 cm precast Immobilline Drystrips at pH 4–7 (GE Healthcare Biosciences, Uppsala, Sweden) for 16 h. The rehydrated strips were subjected to isoelectric focusing with IPGphor 3 (GE Healthcare Biosciences, Uppsala, Sweden) for a total duration of 14 kV/h (Phase 1: 500 V, 50 mA, 500 V/h; Phase 2: 1,000 V, 50 mA, 1,000 V/h; Phase 3: 8,000 V, 50 mA, 12.5 V/h). Focused strips were equilibrated with equilibration buffer (1.5 M Tris-HCl, pH8.8; 6 M urea; 2% w/v SDS; 30% v/v glycerol) containing 0.06 M DTT for 15 min. They were further incubated in equilibration buffer containing 4.5% v/v iodoacetamide for 15 min. For the second dimension, the focused strips were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) using 8–18% gradient gels. According to the optimized protocol (Phase 1: 50 V, 17 mA, 15 W for 30 min; Phase 2: 600 V, 25 mA, 15 W for 2 h), electrophoresis was performed using the SE 600 Ruby Electrophoresis System and Power supply EPS601 (GE Healthcare, Uppsala, Sweden).
After 2-DE protein separation, the gels were fixed and silver-stained according to Mortz et al. (2001). For protein identification by tryptic digestion and MS, a modified silver-staining method was performed according to Shevchenko et al. (1996).
The silver-stained gels were scanned using the ImageScanner III (GE Healthcare Biosciences, Uppsala, Sweden). The Image Master Platinum 7.0 software (GE Healthcare Biosciences, Uppsala, Sweden) was used to evaluate the 2-DE profiles of serum samples obtained from all the six rats pre-treated and treated with E. longifolia. The percentage volume contribution of protein spots was calculated (i.e., the spot volume of a protein expressed as a percentage of the total spot volume of all detected proteins).
All values were expressed as mean ± SD. The Student’s t-test was used to compare the experimental groups with regard to mean percentage volume contribution for the detected spots. Student’s t-test was also used to compare the mean percentage inhibition of AHS following the treatment at various intervals compared to that of pre-treatment. A p-value of <0.05 was considered significant.
For MALDI-TOF-MS, the gels plugs containing proteins of interest from the 2-DE gels were subjected to in-gel digestion based on the manufacturer’s instruction (Pierce, Rockford, IL, USA). The MS analysis was performed using Applied Biosystem 4800 Proteomic analyzer. Mass spectra data were submitted to the Mascot search engine (Matrix Science Ltd, London, UK) for protein identification against Rattus entries in the NCBI database (accessed on 15 Nov 2012). Proteins were considered positively identified as described by Delahunty and Yates (2005).
Decreased expression of alpha-2-HS glycoprotein (AHS) was validated using a modified version of the competitive ELISA as previously described (Mu et al., 2013). Serum samples (n = 6 for each group; pre-treatment, days 1, 7, 14, 21, and 28) were diluted with 0.05 M sodium bicarbonate buffer (pH 9.6) and loaded into microtiter plates (Nunc, Denmark) for overnight coating of wells at 4°C. Plates were washed three times with 100 μl of PBST (phosphate-buffered saline [PBS] with 0.05% [v/v] Tween-20), and the wells were subsequently blocked with 100 μl of 0.5% gelatin in PBST for 30 min at room temperature. After blocking, the plates were washed with PBST and then incubated with goat anti-rat AHS (1:1,000 in PBST) for 1 h at room temperature (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Positive controls were prepared without serum, and blanks contained washing buffer. After incubation, the wells were washed with PBST, and 200 μl of horseradish peroxidase (HRP)-labeled secondary anti-goat IgG (1:5,000) was added to each well. The plate was then incubated for 1 h at room temperature, followed by washing with PBST. 100 μl of 3,3′,5,5′-tetramethylbenzidine substrate (Sigma–Aldrich Company, St. Louis, MO, USA) was added to each well and the plate was incubated for 15 min at room temperature in the dark, followed by reaction termination (100 μl 2 M sulphuric acid per well). Absorbance was read at 450 nm using an ELISA plate reader. The amount of AHS was determined by the percentage inhibition of substrate hydrolysis as described previously (Reddington et al., 1991).
The concentrations of serum protein samples were determined using a protein estimation kit. Estimation of the protein concentration was based on the preparation of several dilutions of BSA as the standard. All samples have protein concentration with variation not more than 25% (Table 1), which indicated that their protein amounts were almost equal.
Serum proteins from the pre-treatment and treated (days 1, 7, 14, 21, and 28) rat groups were separated by 2-DE on the basis of their isoelectric points (pIs) and molecular weights, yielding complex protein profiles consisting of hundreds of highly-resolved protein spots. Figure 2 illustrates representative serum protein profiles of rats before and after treatment with E. longifolia extract.
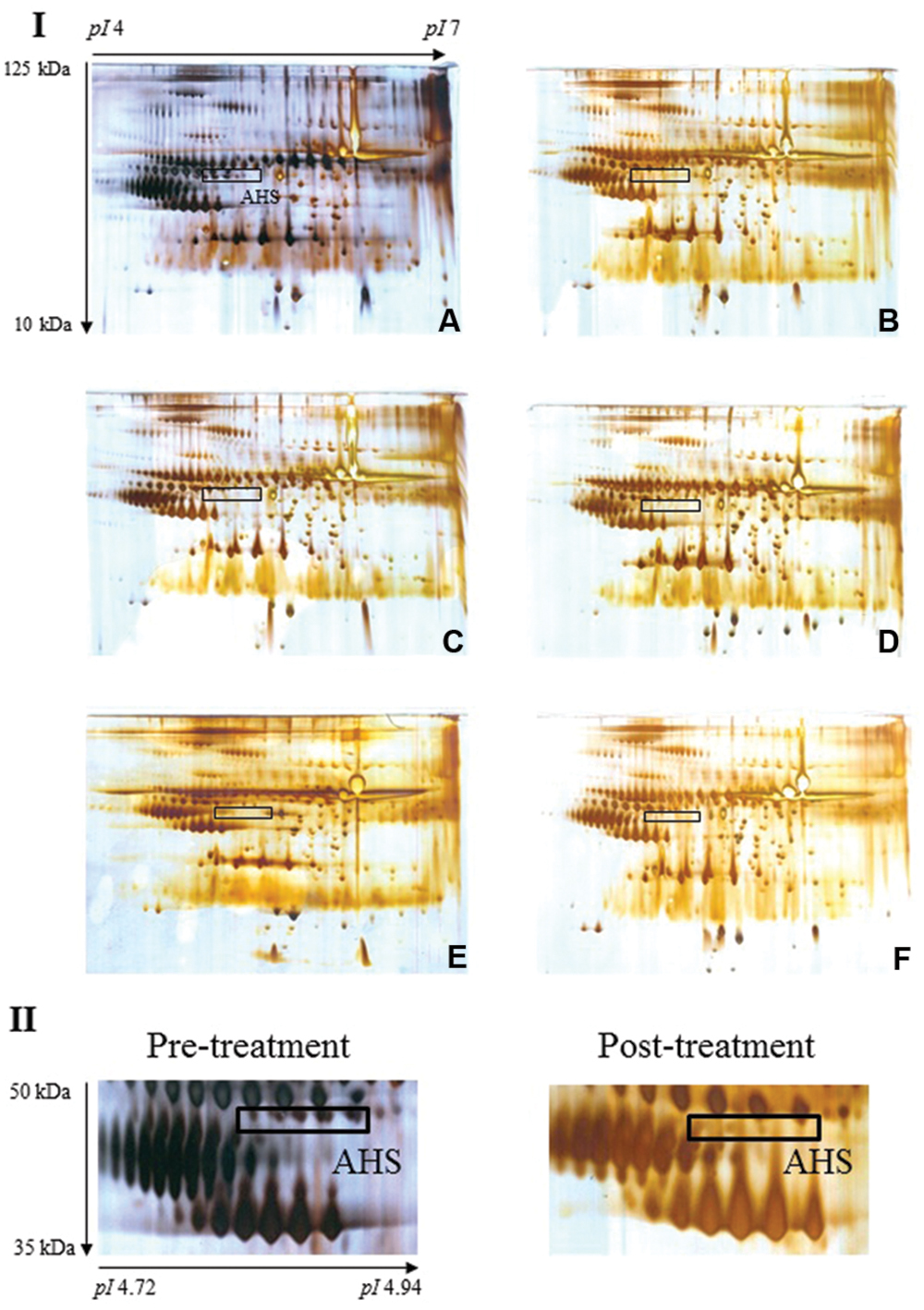
FIGURE 2. Typical 2-DE profiles of rat serum samples. (A–F) of section I illustrate representative 2-DE profiles of rat serum from pre-treatment and days 1, 7, 14, 21, and 28, respectively. The boxed protein cluster corresponds to AHS, which was lost in the serum samples from rats treated with E. longifolia extract (section II displays cropped images). Gel acidity and relative molecular weights are indicated.
Image analysis was performed on the silver-stained 2-DE gels obtained from each serum sample of pre-treatment and extract-treated rats. Notably, expression of most of the detected proteins was comparable with the exception of a protein cluster designated AHS. Protein spots corresponding to AHS were present in pre-treatment sera but were barely visible in treated samples. Table 2 displays the average percentage of volume contribution of AHS detected in pre-treatment sera.
Analysis of the differential protein cluster with MALDI-TOF MS/MS revealed more than twenty peaks with different m/z, and submission of the MS data to the Mascot search engine allowed confident identification of AHS. Table 3 shows the data for mass spectrometric identification of AHS after searching the NCBI database.
To validate AHS reduction upon extract treatment, competitive ELISA was performed on the serum samples (n = 6 for each group; pre-treatment, days 1, 7, 14, 21, and 28) using polyclonal antisera against AHS. We observed that AHS expression was significantly higher in pre-treatment sera compared to post-treatment sera (Figure 3).
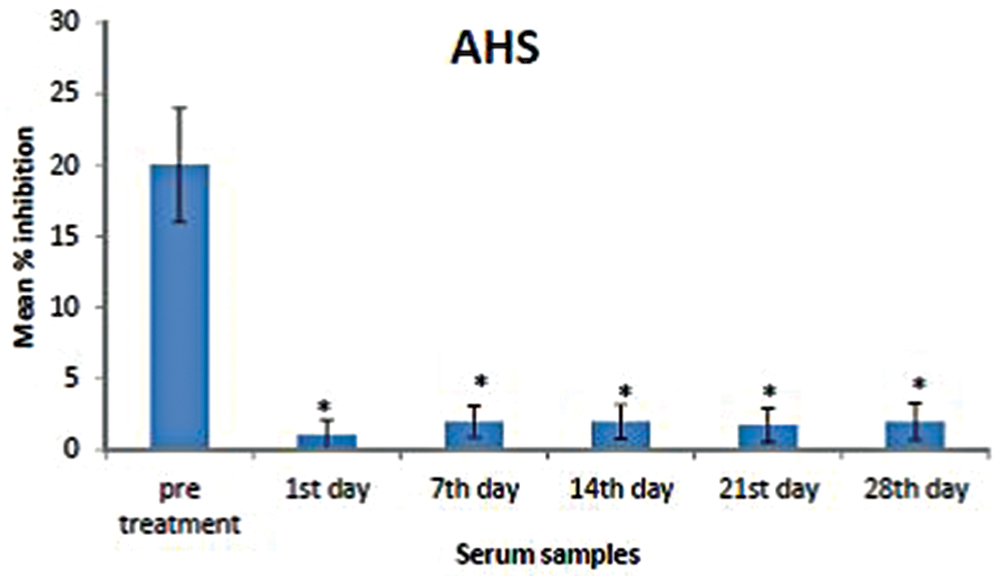
FIGURE 3. Mean percentage of inhibition of AHS in the pre-treatment and post-treatment serum samples. Analysis was performed by competitive ELISA. AHS concentrations in the pre-treatment sera were significantly higher than those in the post-treatment samples (days 1, 7, 14, 21, and 28). The amount of AHS present was proportional to the percentage inhibition of substrate hydrolysis. Asterisk denotes statistical significance (p < 0.05) of post-treatment samples compared to that of pre-treatment sample (n = 6).
Here, we have analyzed serum from rats treated with standardized E. longifolia extract by 2-DE and observed the obvious loss of a particular protein cluster upon treatment. Subsequently, we identified this protein cluster to be AHS, which could be seen in sera from untreated rats but was hardly detectable in sera from rats treated with standardized E. longifolia extract for 1–28 days. Finally, we confirmed the extract-induced reduction in AHS using competitive ELISA.
Alpha-2-HS glycoprotein, also known as alpha2-Heremans-Schmid glycoprotein or fetuin A, is a serum protein that participates in bone metabolism since it is one of the major components of non-collagenous bone matrix (Dickson et al., 1975). It accumulates in the skeleton due to its high affinity for hydroxyapatite. Also, several reports have suggested that AHS is involved in host defense, particularly in phagocytosis and innate immunity (Arnaud et al., 1988). Indeed, AHS is a negative acute phase protein and its concentration decreases during infection.
In addition, AHS has been linked to insulin resistance and diabetes (Stefan et al., 2006). The insulin receptor is a disulfide-linked heterotetrameric protein complex consisting of two hormone-binding α subunits and two signaling β subunits containing tyrosine kinase activity (Srinivas et al., 1993). Insulin binds to this receptor located on the surface of insulin responsive cells, leading to the activation of the tyrosine kinase and subsequently the autophosphorylation of β subunit in order to activate the function of insulin toward the physiological function (Myers and White, 1996). The phosphorylated form of AHS acts as a natural competitve inhibitor of insulin, which blocks both activities, potentially contributing to the development of type-2 diabetes (Srinivas et al., 1993). It was also reported that testosterone levels correlated positively with insulin sensitivity (Pitteloud et al., 2005). Thus, testosterone, which can be produced in response to insulin, is a modulator of insulin sensitivity. Therefore, we propose that the quassinoid-containing E. longifolia extract affects male infertility by suppressing AHS expression, which indirectly increases insulin sensitivity and testosterone levels in accordance with our previous findings (Low et al., 2013). Our earlier study showed that the testosterone enhancement activity of E. longifolia has been prolonged in the treated rats after the 28 days treatment of E. longifolia extract when they had displayed significantly higher sperm count (P < 0.001) than that of the untreated control (Low et al., 2013). In addition, the present finding also proposed that the E. longifolia extract could be used as a supplement to prevent insulin resistance by modulating phosphorylated AHS, which is present in healthy individuals (Haglund et al., 2001).
Taken together, our findings indicate that serum AHS was reduced in rats treated with standardized E. longifolia extract, and provide rational for further investigation into the effects of this natural extract in animal models involving infertility and diabetes.
YC, B-SL, and K-LC contributed to the design of the study and drafting the manuscript. W-MP and SS participated in the data analysis and critically revised the manuscript. AK-W and C-KC carried out the experiments and analyzed the data. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by University of Malaya Research Grants (UMRG) RG454-12HTM and High Impact Research MoE Grants UM.C/625/1/HIR/MOE/DENT/09 and UM.C/625/1/HIR/MOHE/MED/16/5 from the Ministry of Education Malaysia.
Ang, H. H., and Sim, M. K. (1998). Eurycoma longifolia JACK and orientation activities in sexually experienced male rats. Biol. Pharm. Bull. 21, 153–155. doi: 10.1248/bpb.21.153
Bhat, R., and Karim, A. (2010). Tongkat Ali (Eurycoma longifolia Jack): a review on its ethnobotany and pharmacological importance. Fitoterapia 81, 669–679. doi: 10.1016/j.fitote.2010.04.006
Burkill, I. H. (1966). A Dictionary of the Economic Products of the Malay Peninsula. Kuala Lumpur: Governments of Malaysia and Singapore by the Ministry of Agriculture and Cooperatives.
Chan, K.-L., Choo, C.-Y., Morita, H., and Itokawa, H. (1998). High performance liquid chromatography in phytochemical analysis of Eurycoma longifolia. Planta Med. 64, 741–745. doi: 10.1055/s-2006-957570
Chan, K. L., Low, B. S., and Ho, D. S. S. (2012). Polar Organic Extract of Eurycoma longifolia. Malaysian Patent Grant No. MY-146000-A.
Chan, K.-L., Low, B.-S., Teh, C.-H., and Das, P. K. (2009). The effect of Eurycoma longifolia on sperm quality of male rats. Nat. Prod. Commun. 4, 1331–1336.
Chen, Y., Lim, B.-K., and Hashim, O. H. (2009). Different altered stage correlative expression of high abundance acute-phase proteins in sera of patients with epithelial ovarian carcinoma. J. Hematol. Oncol. 2, 37. doi: 10.1186/1756-8722-2-37
Chen, Y., Lim, B.-K., Peh, S.-C., Abdul-Rahman, P. S., and Hashim, O. H. (2008). Profiling of serum and tissue high abundance acute-phase proteins of patients with epithelial and germ line ovarian carcinoma. Proteome Sci. 6, 20. doi: 10.1186/1477-5956-6-20
Delahunty, C., and Yates, J. R. III. (2005). Protein identification using 2D-LC-MS/MS. Methods 35, 248–255. doi: 10.1016/j.ymeth.2004.08.016
Dickson, I. R., Poole, A. R., and Veis, A. (1975). Localisation of plasma α2HS glycoprotein in mineralising human bone. Nature 256, 430–432. doi: 10.1038/256430a0
Haglund, A. C., Ek, B., and Ek, P. (2001). Phosphorylation of human plasma α2-Heremans–Schmid glycoprotein (human fetuin) in vivo. Biochem. J. 357, 437–445. doi: 10.1042/bj3570437
John Hopkins Medicine (2012). Guidelines for multiple blood draws [Online]. Available at: http://www.hopkinsmedicine.org/animalresources/Clinical_Services/MultipleblooddrawsDSR.html [Accessed April 10, 2012].
Kuo, P.-C., Damu, A. G., Lee, K.-H., and Wu, T.-S. (2004). Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg. Med. Chem. 12, 537–544. doi: 10.1016/j.bmc.2003.11.017
Low, B.-S., Das, P. K., and Chan, K.-L. (2013). Standardized quassinoid-rich Eurycoma longifolia extract improved spermatogenesis and fertility in male rats via the hypothalamic–pituitary–gonadal axis. J. Ethnopharmacol. 145, 706–714. doi: 10.1016/j.jep.2012.11.013
Mortz, E., Krogh, T. N., Vorum, H., and Görg, A. (2001). Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics 1, 1359–1363. doi: 10.1002/1615-9861(200111)1:11<1359::AID-PROT1359>3.0.CO;2-Q
Mu, A. K.-W., Lim, B.-K., Hashim, O. H., and Shuib, A. S. (2012). Detection of differential levels of proteins in the urine of patients with endometrial cancer: analysis using two-dimensional gel electrophoresis and O-glycan binding lectin. Int. J. Mol. Sci. 13, 9489–9501. doi: 10.3390/ijms13089489
Mu, A. K.-W., Lim, B.-K., Hashim, O. H., and Shuib, A. S. (2013). Identification of O-glycosylated proteins that are aberrantly excreted in the urine of patients with early stage ovarian cancer. Int. J. Mol. Sci. 14, 7923–7931. doi: 10.3390/ijms14047923
Myers, M. G. Jr., and White, M. F. (1996). Insulin signal transduction and the IRS proteins. Annu. Rev. Pharmacol. Toxicol. 36, 615–658. doi: 10.1146/annurev.pa.36.040196.003151
Noor, M. M., Nor, M. M., and Hassan, L. C. (2004). The effect of Eurycoma longifolia Jack (Tongkat Ali) on sexual behaviour and sperm quality in rats. Malays. J. Pharm. Sci. 2, 53–60.
Pitteloud, N., Mootha, V. K., Dwyer, A. A., Hardin, M., Lee, H., Eriksson, K.-F., et al. (2005). Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 28, 1636–1642. doi: 10.2337/diacare.28.7.1636
Reddington, J. J., Reddington, G. M., and Maclachlan, N. J. (1991). A competitive ELISA for detection of antibodies to the group antigen of bluetongue virus. J. Vet. Diagn. Invest. 3, 144–147. doi: 10.1177/104063879100300207
Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858. doi: 10.1021/ac950914h
Srinivas, P., Wagner, A. S., Reddy, L. V., Deutsch, D., Leon, M. A., Goustin, A. S., et al. (1993). Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol. Endocrinol. 7, 1445–1455. doi: 10.1210/mend.7.11.7906861
Stefan, N., Hennige, A. M., Staiger, H., Machann, J., Schick, F., Kröber, S. M., et al. (2006). α2-Heremans-schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29, 853–857. doi: 10.2337/diacare.29.04.06.dc05-1938
Tambi, M. I. B. M., and Imran, M. K. (2010). Eurycoma longifolia Jack in managing idiopathic male infertility. Asian J. Androl. 12, 376–380. doi: 10.1038/aja.2010.7
Tambi, M. I. B. M., Imran, M. K., and Henkel, R. R. (2012). Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late–onset hypogonadism? Andrologia 44, 226–230. doi: 10.1111/j.1439-0272.2011.01168.x
Teh, C.-H., Murugaiyah, V., and Chan, K.-L. (2011). Developing a validated liquid chromatography–mass spectrometric method for the simultaneous analysis of five bioactive quassinoid markers for the standardization of manufactured batches of Eurycoma longifolia Jack extract as antimalarial medicaments. J. Chromatogr. A 1218, 1861–1877. doi: 10.1016/j.chroma.2011.02.014
Tsai, S.-J., and Wiltbank, M. C. (1998). Prostaglandin F2alpha regulates distinct physiological changes in early and mid-cycle bovine corpora lutea. Biol. Reprod. 58, 346–352. doi: 10.1095/biolreprod58.2.346
Wahab, N. A., Mokhtar, N. M., Halim, W. N. H. A., and Das, S. (2010). The effect of Eurycoma longifolia Jack on spermatogenesis in estrogen-treated rats. Clinics 65, 93–98. doi: 10.1590/S1807-59322010000100014
Keywords: 2-dimensional electrophoresis, alpha-2-HS glycoprotein, Eurycoma longifolia, plant extract, mouse model
Citation: Chen Y, Phang W-M, Mu AK-W, Chan C-K, Low B-S, Sasidharan S and Chan K-L (2015) Decreased expression of alpha-2-HS glycoprotein in the sera of rats treated with Eurycoma longifolia extract. Front. Pharmacol. 6:211. doi: 10.3389/fphar.2015.00211
Received: 12 July 2015; Accepted: 10 September 2015;
Published: 23 September 2015.
Edited by:
Banasri Hazra, Jadavpur University, IndiaReviewed by:
Maria Teresa Riggio Lima-Landman, Universidade Federal de Sao Paulo, BrazilCopyright © 2015 Chen, Phang, Mu, Chan, Low, Sasidharan and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yeng Chen, Department of Oral Biology and Biomedical Sciences, Faculty of Dentistry, University of Malaya, Kuala Lumpur 50603, Malaysia,Y2hlbnllbmdAdW0uZWR1Lm15
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.