
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol., 31 March 2015
Sec. Pharmacology of Anti-Cancer Drugs
Volume 6 - 2015 | https://doi.org/10.3389/fphar.2015.00070
The casein kinase 2 (CK2) protein kinase is a pro-survival kinase and therapeutic target in treatment of various human cancers. CK2 overexpression has been demonstrated in hematological malignancies, including chronic lymphocytic leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, and multiple myeloma. CX-4945, also known as Silmitasertib, is an orally administered, highly specific, ATP-competitive inhibitor of CK2. CX-4945 induces cytotoxicity and apoptosis and is currently being evaluated in clinical trials for treatment of many cancer types. In the past 2 years, the focus on the therapeutic potential of CX-4945 has shifted from solid tumors to hematological malignancies. CX-4945 exerts anti-proliferative effects in hematological tumors by downregulating CK2 expression and suppressing activation of CK2-mediated PI3K/Akt/mTOR signaling pathways. Furthermore, combination of CX-4945 with other inhibitors yielded synergistic effects in cell death induction. These new findings demonstrate that CK2 overexpression contributes to blood cancer cell survival and resistance to chemotherapy. Combinatorial use of CX-4945 is a promising therapeutic tool for treatment of hematological malignancies.
Among the diverse types of human cancers, hematologic or lymphoid malignancies present major therapeutic challenges due to their low survival rates and poor prognosis. New immuno-chemotherapeutic approaches have improved the survival rates overall; however, many patients with hematologic malignancies, such as chronic lymphocytic leukemia (CLL) and chronic myeloid leukemia (CML), still have poor outcomes due to resistance to chemotherapy, personal therapeutic limits, frequent metastasis, and relapse (Faderl et al., 2009; Gribben and O’Brien, 2011). Thus, there is a need for new and more sophisticated therapeutic strategies for treatment of hematologic malignancies.
The serine/threonine protein kinase, CK2 (casein kinase 2), modulates multiple signaling pathways involved in hematopoietic cell survival and function, and is therefore a promising drug target (Dominguez et al., 2009; Trembley et al., 2009; Piazza et al., 2012). CK2 is constitutively expressed in many cell types. In human cells it typically exists as a tetrameric complex comprising two catalytic alpha subunits (α and α′) and two regulatory (β) subunits (Litchfield, 2003). CK2 plays an important role in the regulation and phosphorylation of a broad range of cellular targets (Pinna, 1990; Litchfield and Lüscher, 1993; Allende and Allende, 1995; Pinna and Meggio, 1997; Guerra and Issinger, 1999; Faust and Montenarh, 2000). In mice, knockout of the CK2α′ subunit induces developmental defects and knockdown of the CK2α and β subunits results in embryonic lethality (Lou et al., 2008; Seldin et al., 2008). CK2 regulates hematopoiesis-associated signaling cascades as well as multiple biochemical processes involving tumor growth, proliferation, and resistance to cytotoxic agents (Piazza et al., 2012). In normal cells, CK2 shows ubiquitous localization throughout the nuclear and cytoplasmic compartments, whereas, in cancer cells, CK2 shows greater abundance in the nuclear compartment (Faust et al., 1999; Laramas et al., 2007). This difference in CK2 distribution may be significant with regard to its biochemical function in cancer. CK2 overexpression has been observed in many hematologic cancers, including CLL (Martins et al., 2010), multiple myeloma (MM; Piazza et al., 2006), T-cell acute lymphocytic leukemia (T-ALL; Silva et al., 2008), and acute myeloid leukemia (AML; Kim et al., 2007; Quotti Tubi et al., 2013). These studies found that CK2α/β mRNA or protein level was increased in cells from several AML patients (approximately 2- to 14-fold more, compared to controls; Quotti Tubi et al., 2013) or from CLL patients (approximately twofold more, compared controls; Martins et al., 2010), as well as plasma cells from MM patients (CK2α, 88% and CK2β, 64% of MM patients analyzed; Manni et al., 2013). Moreover, CK2 upregulation was correlated with poor prognosis (Trembley et al., 2009). These studies identify CK2 as a promising therapeutic target for the development of anti-cancer agents for treatment of many hematological cancers.
Overexpression of CK2 has been observed in many cancers, including hematologic cancers such as AML, CLL, T-ALL, and MM (Piazza et al., 2006; Kim et al., 2007; Silva et al., 2008; Martins et al., 2010). Downregulation of CK2, either by transfection of specific siRNA or plasmid-based expression of kinase-inactive CK2, resulted in reduction of cancer cell viability and induction of apoptosis (Faust et al., 2000; Wang et al., 2001; Slaton et al., 2004). Similar to studies addressing non-hematological cancers, RNA interference that targets CK2 was found to induce apoptosis in MM, AML, CLL, and CML (Borgo et al., 2013; Manni et al., 2013; Quotti Tubi et al., 2013; Martins et al., 2014). These reports suggest that downregulation of CK2 by RNA interference or CX-4945 treatment enhances cytotoxicity of hematological cancer cells. Consistent with the overexpression-related observations, these downregulation experiments indicate that CK2 may be a valid druggable anti-cancer target for use in treatment of hematological malignancies, not only human solid tumors (Sarno et al., 2002; Martins et al., 2010; Kim and Kim, 2012).
CX-4945 (Silmitasertib) is an orally administered, ATP-competitive inhibitor of both CK2α and CK2α′ catalytic subunits that was first developed by Cylene Pharmaceuticals Inc. (Siddiqui-Jain et al., 2010; Pierre et al., 2011b). CX-4945 has been investigated in human cancer studies worldwide and is currently in Phase I and II clinical trials (ClinicalTrials.gov Identifier: NCT02128282). The Phase I trial addresses the safety and tolerability of increasing doses of CX-4945 in combination with gemcitabine plus cisplatin, to determine the maximum tolerated dose (MTD). The subsequent Phase II trial is a randomized study of antitumor activity in cholangiocarcinoma patients, comparing the standard-of-care protocol of gemcitabine plus cisplatin against treatment with CX-4945 in combination with gemcitabine plus cisplatin at the combination MTD determined in the preceding trial.
In the molecular model of inhibition, hydrophobic residues in the small and flat ATP binding site of the CK2α subunit can bind ATP or CK2 inhibitors (Sarno et al., 2005). Downregulation of CK2 kinase activity is expected to be due to the ability of inhibitors to establish polar interactions with the active conformation of CK2α. CX-4945 showed a strong interaction with the ATP binding pocket of CK2, with a Ki = 0.38 [0.02 nM with the recombinant human holoenzyme (ααββ; Ferguson et al., 2011)]. This strong binding interaction between CX-4945 and the ATP binding site of CK2 reduces the enzymatic activity and attenuates the downstream, CK2-regulated PI3K/Akt signaling pathway (Pierre et al., 2011a). The mechanistic relationship between CK2 inhibition by CX-4945, the downstream signaling pathways, and cancer cell survival remains to be fully elucidated.
The efficacy of CX-4945 has been evaluated with a broad range of human hematologic tumors, including CLL, ALL, AML, and lymphomas (Prins et al., 2013). These studies demonstrated that CX-4945 exerts strong anti-proliferative activity in CLL biopsy samples. As well as decreasing CLL cell viability (IC50 < 1 μM) when used alone, CX-4945 exerted synergistic effects in combination with several other inhibitors, including GS-1101, ibrutinib, and fludarabine, which regulate B-cell receptor (BCR)-mediated signaling cascades or downstream mediators. CK2 inhibition downregulates signaling mediators that act downstream of BCR, including PI3K and Akt (Martins et al., 2010, 2011; Ruzzene and Pinna, 2010; Piazza et al., 2012).
In primary CLL cells and in the stable CLL cell line MO1043, CX-4945 treatment led to decreased phosphorylation of Akt and PKC, which are downstream targets of PTEN and PI3K (Martins et al., 2014). Consistent with the in vitro effects observed in CLL cells, CX-4945 also showed anti-tumor activity in a mouse xenograft model. CX-4945 treatment caused delayed tumor growth, and treatment with CX-4945 plus fludarabine showed synergistic effects. This pre-clinical evidence suggests that CX-4945 is likely to show therapeutic activity, and that it represents a good candidate for CLL treatment in combination with other anti-tumor agents.
CK2 overexpression is a hallmark of ALL, and two recent studies investigated the relationship between increased CK2 expression and the cytotoxic activity of CX-4945 in T-cell ALL and B-cell ALL (Buontempo et al., 2014; Gomes et al., 2014). CK2 was found to induce phosphorylation of the PTEN tumor suppressor and thereby to activate PI3K/Akt/mTOR, which is a signaling axis that is important for cell survival in ALL (Torres and Pulido, 2001; Vázquez-Franco et al., 2012; Huang et al., 2013; Carnero and Paramio, 2014). CX-4945 treatment resulted in apoptosis of T-cell ALL and B-cell ALL cells (Buontempo et al., 2014; Gomes et al., 2014).
The therapeutic activity of CX-4945 was also evaluated in CML and AML, respectively. CML is characterized by a translocation known as the “Philadelphia chromosome,” which results in the fusion protein Bcr-Abl, a protein tyrosine kinase that plays a crucial role in cell proliferation and in maintenance of the CML phenotype (Goldman and Melo, 2003). A relationship between Bcr-Abl and CK2 has been previously suggested (Hériché and Chambaz, 1998; Mishra et al., 2003, 2007). Borgo et al. (2013) demonstrated that CX-4945 showed anti-tumor activity in imatinib-resistant CML cells. Downregulation of CK2 by CX-4945 or siRNA contributed to the induction of apoptotic cell death. Furthermore, CK2 inhibition affected the sensitivity of AML cells to chemotherapy. Downregulation of CK2 by CX-4945, K27, or siRNA showed synergistic effects on cytotoxicity and apoptosis in acute, primary blasts as well as in AML cell lines (Quotti Tubi et al., 2013). Moreover, CX-4945 increased the chemotherapeutic activity of daunorubicin in AML.
Inhibition of CK2 expression could also be useful in combination therapies for treatment of MM and mantle cell lymphoma (MCL). A recent report demonstrated CK2 overexpression in MM and MCL cells and that downregulation of CK2 with CK2 inhibitors, such as CX-4945 and K27, induced apoptosis (Manni et al., 2013). Bortezomib, a proteasome inhibitor, exerted anti-tumor activity in MM and MCL cells by stabilization of IκBα in the NF-κB signaling pathway; however, bortezomib alone proved to be insufficient for effective treatment. When used in conjunction with bortezomib, CX-4945 inhibition of CK2 enhanced the cytotoxic activity and mitochondrial-dependent cell death in MM and MCL cells (Manni et al., 2013).
Numerous studies have demonstrated the anti-tumor effects of CX-4945 in leukemias or lymphomas, resulting from inhibition of CK2 expression (Figure 1). Based on these results, we propose that CX-4945 has a potential role in novel therapeutic strategies in the future. Additionally, the combination of CX-4945 with various other anti-cancer drugs may be a useful therapeutic strategy for treatment of hematological cancers (Table 1).
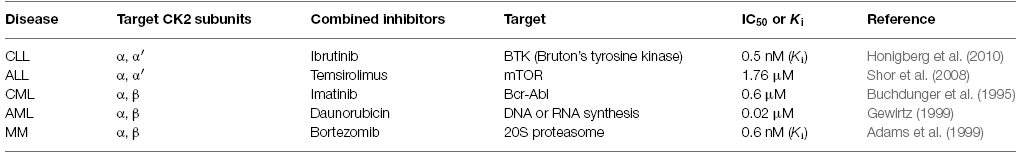
Table 1. Anti-cancer drugs for potential combination therapy with CX-4945 in treatment of human hematological cancers.
HC, KB, and YL collected and analyzed the background research and created the figure and the table. JK wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was supported by Eulji University in 2014 and the Basic Science Research Program, through the National Research Foundation of Korea (NRF), funded by the Ministry of Science ICT and Future Planning (NRF-2014R1A1A1002349).
Adams, J., Palombella, V. J., Sausville, E. A., Johnson, J., Destree, A., Lazarus, D. D., et al. (1999). Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 59, 2615–2622.
Allende, J. E., and Allende, C. C. (1995). Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 9, 313–323.
Borgo, C., Cesaro, L., Salizzato, V., Ruzzene, M., Massimino, M. L., Pinna, L. A., et al. (2013). Aberrant signalling by protein kinase CK2 in imatinib-resistant chronic myeloid leukaemia cells: biochemical evidence and therapeutic perspectives. Mol. Oncol. 7, 1103–1115. doi: 10.1016/j.molonc.2013.08.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Buchdunger, E., Zimmermann, J., Mett, H., Meyer, T., Müller, M., Regenass, U., et al. (1995). Selective inhibition of the platelet-derived growth factor signal transduction pathway by a protein-tyrosine kinase inhibitor of the 2-phenylaminopyrimidine class. Proc. Natl. Acad. Sci. U.S.A. 92, 2558–2562. doi: 10.1073/pnas.92.7.2558
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Buontempo, F., Orsini, E., Martins, L. R., Antunes, I., Lonetti, A., Chiarini, F., et al. (2014). Cytotoxic activity of the casein kinase 2 inhibitor CX-4945 against T-cell acute lymphoblastic leukemia: targeting the unfolded protein response signaling. Leukemia 28, 543–553. doi: 10.1038/leu.2013.349
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carnero, A., and Paramio, J. M. (2014). The PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Front. Oncol. 4:252. doi: 10.3389/fonc.2014.00252
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dominguez, I., Sonenshein, G. E., and Seldin, D. C. (2009). Protein kinase CK2 in health and disease: CK2 and its role in Wnt and NF-kappaB signaling: linking development and cancer. Cell. Mol. Life Sci. 66, 1850–1857. doi: 10.1007/s00018-009-9153-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Faderl, S., Ferrajoli, A., Frankfurt, O., and Pettitt, A. (2009). Treatment of B-cell chronic lymphocytic leukemia with nonchemotherapeutic agents: experience with single-agent and combination therapy. Leukemia 23, 457–466. doi: 10.1038/leu.2008.322
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Faust, M., and Montenarh, M. (2000). Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res. 301, 329–340. doi: 10.1007/s004410000256
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Faust, R. A., Niehans, G., Gapany, M., Knapp, D., Cherwitz, D., Davis, A., et al. (1999). Subcellular immunolocalization of protein kinase CK2 in squamous cell carcinomas of the head and neck. Int. J. Biochem. Cell Biol. 31, 941–949. doi: 10.1016/S1357-2725(99)00050-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Faust, R. A., Tawfic, S., Davis, A. T., Bubash, L. A., and Ahmed, K. (2000). Antisense oligonucleotides against protein kinase CK2-alpha inhibit growth of squamous cell carcinoma of the head and neck in vitro. Head Neck 22, 341–346. doi: 10.1002/1097-0347(200007)22:4<341::AID-HED5>3.0.CO;2-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ferguson, A. D., Sheth, P. R., Basso, A. D., Paliwal, S., Gray, K., Fischmann, T. O., et al. (2011). Structural basis of CX-4945 binding to human protein kinase CK2. FEBS Lett. 585, 104–110. doi: 10.1016/j.febslet.2010.11.019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gewirtz, D. A. (1999). A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 57, 727–741. doi: 10.1016/S0006-2952(98)00307-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Goldman, J. M., and Melo, J. V. (2003). Chronic myeloid leukemia—advances in biology and new approaches to treatment. N. Engl. J. Med. 349, 1451–1464. doi: 10.1056/NEJMra020777
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gomes, A. M., Soares, M. V., Ribeiro, P., Caldas, J., Póvoa, V., Martins, L. R., et al. (2014). Adult B-cell acute lymphoblastic leukemia cells display decreased PTEN activity and constitutive hyperactivation of PI3K/Akt pathway despite high PTEN protein levels. Haematologica 99, 1062–1068. doi: 10.3324/haematol.2013.096438
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gribben, J. G., and O’Brien, S. (2011). Update on therapy of chronic lymphocytic leukemia. J. Clin. Oncol. 29, 544–550. doi: 10.1200/JCO.2010.32.3865
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Guerra, B., and Issinger, O.-G. (1999). Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 20, 391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hériché, J. K., and Chambaz, E. M. (1998). Protein kinase CK2alpha is a target for the Abl and Bcr-Abl tyrosine kinases. Oncogene 9, 13–18. doi: 10.1038/sj.onc.1201900
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Honigberg, L. A., Smith, A. M., Sirisawad, M., Verner, E., Loury, D., Chang, B., et al. (2010). The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U.S.A. 107, 13075–13080. doi: 10.1073/pnas.1004594107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huang, F. F., Wu, D. S., Zhang, L., Yu, Y. H., Yuan, X. Y., Li, W. J., et al. (2013). Inactivation of PTEN increases ABCG2 expression and the side population through the PI3K/Akt pathway in adult acute leukemia. Cancer Lett. 336, 96–105. doi: 10.1016/j.canlet.2013.04.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, J., and Kim, S. H. (2012). Druggability of the CK2 inhibitor CX-4945 as an anticancer drug and beyond. Arch. Pharm. Res. 35, 1293–1296. doi: 10.1007/s12272-012-0800-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, J. S., Eom, J. I., Cheong, J. W., Choi, A. J., Lee, J. K., Yang, W. I., et al. (2007). Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin. Cancer Res. 13, 1019–1028. doi: 10.1158/1078-0432.CCR-06-1602
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Laramas, M., Pasquier, D., Filhol, O., Ringeisen, F., Desxotes, J.-L., and Cochet, C. (2007). Nuclear localization of protein kinase CK2 catalytic subunit (CK2a) is associated with poor prognostic factors in human prostate cancer. Eur. J. Cancer 43, 928–934. doi: 10.1016/j.ejca.2006.11.021
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Litchfield, D. W. (2003). Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 369, 1–15. doi: 10.1042/BJ20021469
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Litchfield, D. W., and Lüscher, B. (1993). Casein kinase II in signal transduction and cell cycle regulation. Mol. Cell. Biochem. 127/128, 187–200. doi: 10.1007/BF01076770
Lou, D. Y., Dominguez, I., Toselli, P., Landesman-Bollag, E., O’Brien, C., and Seldin, D. C. (2008). The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol. Cell. Biol. 28, 131–139. doi: 10.1128/MCB.01119-07
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Manni, S., Brancalion, A., Mandato, E., Tubi, L. Q., Colpo, A., Pizzi, M., et al. (2013). Protein kinase CK2 inhibition down modulates the NF-κB and STAT3 survival pathways, enhances the cellular proteotoxic stress and synergistically boosts the cytotoxic effect of bortezomib on multiple myeloma and mantle cell lymphoma cells. PLoS ONE 8:e75280. doi: 10.1371/journal.pone.0075280
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martins, L. R., Lúcio, P., Melão, A., Antunes, I., Cardoso, B. A., Stansfield, R., et al. (2014). Activity of the clinical-stage CK2-specific inhibitor CX-4945 against chronic lymphocytic leukemia. Leukemia 28, 179–182. doi: 10.1038/leu.2013.232
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martins, L. R., Lúcio, P., Silva, M. C., Anderes, K. L., Gameiro, P., Silva, M. G., et al. (2010). Targeting CK2 overexpression and hyperactivation as a novel therapeutic tool in chronic lymphocytic leukemia. Blood 15, 2724–2731. doi: 10.1182/blood-2010-04-277947
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martins, L. R., Lúcio, P., Silva, M. C., Gameiro, P., Silva, M. G., and Barata, J. T. (2011). On CK2 regulation of chronic lymphocytic leukemia cell viability. Mol. Cell. Biochem. 356, 51–55. doi: 10.1007/s11010-011-0947-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mishra, S., Pertz, V., Zhang, B., Kaur, P., Shimada, H., Groffen, J., et al. (2007). Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia 21, 178–180. doi: 10.1038/sj.leu.2404460
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mishra, S., Reichert, A., Cunnick, J., Senadheera, D., Hemmeryckx, B., Heisterkamp, N., et al. (2003). Protein kinase CKIIalpha interacts with the Bcr moiety of Bcr/Abl and mediates proliferation of Bcr/Abl-expressing cells. Oncogene 22, 8255–8262. doi: 10.1038/sj.onc.1207156
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Piazza, F., Manni, S., Ruzzenem, M., Pinna, L. A., Gurrieri, C., and Semenzato, G. (2012). Protein kinase CK2 in hematologic malignancies: reliance on a pivotal cell survival regulator by oncogenic signaling pathways. Leukemia 26, 1174–1179. doi: 10.1038/leu.2011.385
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Piazza, F. A., Ruzzene, M., Gurrieri, C., Montini, B., Bonanni, L., Chioetto, G., et al. (2006). Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood 108, 1698–1707. doi: 10.1182/blood-2005-11-013672
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pierre, F., Chua, P. C., O’Brien, S. E., Siddiqui-Jain, A., Bourbon, P., Haddach, M., et al. (2011a). Pre-clinical characterization of CX-4945, a potent and selective small molecule inhibitor of CK2 for the treatment of cancer. Mol. Cell. Biochem. 356, 37–43. doi: 10.1007/s11010-011-0956-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pierre, F., Chua, P. C., O’Brien, S. E., Siddiqui-Jain, A., Bourbon, P., Haddach, M., et al. (2011b). Discovery and SAR of 5-(3-chlorophenylamino)benzo [c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer. J. Med. Chem. 54, 635–654. doi: 10.1021/jm101251q
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pinna, L., and Meggio, F. (1997). Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog. Cell Cycle Res. 3, 77–97. doi: 10.1007/978-1-4615-5371-7_7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pinna, L. A. (1990). Casein kinase 2: an ‘eminence grise’ in cellular regulation? Biochim. Biophys. Acta 1054, 267–284. doi: 10.1016/0167-4889(90)90098-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Prins, R. C., Burke, R. T., Tyner, J. W., Druker, B. J., Loriaux, M. M., and Spurgeon, S. E. (2013). CX-4945, a selective inhibitor of casein kinase-2 (CK2), exhibits anti-tumor activity in hematologic malignancies including enhanced activity in chronic lymphocytic leukemia when combined with fludarabine and inhibitors of the B-cell receptor pathway. Leukemia 27, 2094–2096. doi: 10.1038/leu.2013.228
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Quotti Tubi, L., Gurrieri, C., Brancalion, A., Bonaldi, L., Bertorelle, R., Manni, S., et al. (2013). Inhibition of protein kinase CK2 with the clinical-grade small ATP-competitive compound CX-4945 or by RNA interference unveils its role in acute myeloid leukemia cell survival, p53-dependent apoptosis and daunorubicin-induced cytotoxicity. J. Hematol. Oncol. 6:78. doi: 10.1186/1756-8722-6-78
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ruzzene, M., and Pinna, L. A. (2010). Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim. Biophys. Acta 1804, 499–504. doi: 10.1016/j.bbapap.2009.07.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sarno, S., Moro, S., Meggio, F., Zagotto, G., Dal Ben, D., Ghisellini, P., et al. (2002). Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol. Ther. 93, 159–168. doi: 10.1016/S0163-7258(02)00185-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sarno, S., Salvi, M., Battistutta, R., Zanotti, G., and Pinna, L. A. (2005). Features and potentials of ATP-site directed CK2 inhibitors. Biochim. Biophys. Acta 1754, 263–270. doi: 10.1016/j.bbapap.2005.07.043
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Seldin, D. C., Lou, D. Y., Toselli, P., Landesman-Bollag, E., and Dominguez, I. (2008). Gene targeting of CK2 catalytic subunits. Mol. Cell. Biochem. 316, 141–147. doi: 10.1007/s11010-008-9811-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shor, B., Zhang, W. G., Toral-Barza, L., Lucas, J., Abraham, R. T., Gibbons, J. J., et al. (2008). A new pharmacologic action of CCI-779 involves FKBP12-independent inhibition of mTOR kinase activity and profound repression of global protein synthesis. Cancer Res. 68, 2934–2943. doi: 10.1158/0008-5472.CAN-07-6487
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Siddiqui-Jain, A., Drygin, D., Streiner, N., Chua, P., Pierre, F., O’Brien, S. E., et al. (2010). CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 70, 10288–10298. doi: 10.1158/0008-5472.CAN-10-1893
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Silva, A., Yunes, J. A., Cardoso, B. A., Martins, L. R., Jotta, P. Y., Abecasis, M., et al. (2008). PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J. Clin. Invest. 118, 3762–3774. doi: 10.1172/JCI34616
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Slaton, J. W., Unger, G. M., Sloper, D. T., Davis, A. T., and Ahmed, K. (2004). Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model. Mol. Cancer Res. 2, 712–721.
Torres, J., and Pulido, R. (2001). The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 276, 993–998. doi: 10.1074/jbc.M009134200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Trembley, J. H., Wang, G., Unger, G., Slaton, J., and Ahmed, K. (2009). Protein kinase CK2 in health and disease: CK2: a key player in cancer biology. Cell. Mol. Life Sci. 66, 1858–1867. doi: 10.1007/s00018-009-9154-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vázquez-Franco, J. E., Reyes-Maldonado, E., Vela-Ojeda, J., Domínguez-López, M. L., and Lezama, R. A. (2012). Src, Akt, NF-κB, BCL-2 and c-IAP1 may be involved in an anti-apoptotic effect in patients with BCR-ABL positive and BCR-ABL negative acute lymphoblastic leukemia. Leuk. Res. 36, 862–867. doi: 10.1016/j.leukres.2012.03.020
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, H., Davis, A., Yu, S., and Ahmed, K. (2001). Response of cancer cells to molecular interruption of the CK2 signal. Mol. Cell. Biochem. 227, 167–174. doi: 10.1023/A:1013112908734
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: ALL, AML, CLL, CML, MM, CK2, CX-4945
Citation: Chon HJ, Bae KJ, Lee Y and Kim J (2015) The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front. Pharmacol. 6:70. doi: 10.3389/fphar.2015.00070
Received: 18 November 2014; Accepted: 16 March 2015;
Published: 31 March 2015.
Edited by:
Alexandre Arcaro, University of Bern, SwitzerlandReviewed by:
Revati Wani, Pfizer Inc., USACopyright © 2015 Chon, Bae, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiyeon Kim, Department of Biomedical Laboratory Science, School of Medicine, Eulji University, 143-5, Yongdu-dong, Jung-gu, Daejeon 301-746, South KoreaeWVvbkBldWxqaS5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.