
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Pharmacol. , 11 February 2015
Sec. Pharmacology of Ion Channels and Channelopathies
Volume 6 - 2015 | https://doi.org/10.3389/fphar.2015.00022
This article is part of the Research Topic Phosphoinositides and their phosphatases: Linking electrical and chemical signals in biological processes View all 6 articles
 Masayuki X. Mori1*
Masayuki X. Mori1* Kyohei Itsuki2
Kyohei Itsuki2 Hideharu Hase1
Hideharu Hase1 Seishiro Sawamura1
Seishiro Sawamura1 Tatsuki Kurokawa1
Tatsuki Kurokawa1 Yasuo Mori1
Yasuo Mori1 Ryuji Inoue3
Ryuji Inoue3Transient receptor potential canonical (TRPC) channels are Ca2+-permeable, nonselective cation channels that carry receptor-operated Ca2+ currents (ROCs) triggered by receptor-induced, phospholipase C (PLC)-catalyzed hydrolysis of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. Within the vasculature, TRPC channel ROCs contribute to smooth muscle cell depolarization, vasoconstriction, and vascular remodeling. However, TRPC channel ROCs exhibit a variable response to receptor-stimulation, and the regulatory mechanisms governing TRPC channel activity remain obscure. The variability of ROCs may be explained by their complex regulation by PI(4,5)P2 and its metabolites, which differentially affect TRPC channel activity. To resolve the complex regulation of ROCs, the use of voltage-sensing phosphoinositide phosphatases and model simulation have helped to reveal the time-dependent contribution of PI(4,5)P2 and the possible role of PI(4,5)P2 in the regulation of ROCs. These approaches may provide unprecedented insight into the dynamics of PI(4,5)P2 regulation of TRPC channels and the fundamental mechanisms underlying transmembrane ion flow. Within that context, we summarize the regulation of TRPC channels and their coupling to receptor-mediated signaling, as well as the application of voltage-sensing phosphoinositide phosphatases to this research. We also discuss the controversial bidirectional effects of PI(4,5)P2 using a model simulation that could explain the complicated effects of PI(4,5)P2 on different ROCs.
Calcium is a ubiquitous and fundamental messenger that triggers numerous downstream cellular events, including hormone secretion, vasoconstriction, and activity-dependent gene expression, to name a few (Berridge, 2012). There are several mechanisms by which Ca2+ signals are generated (Parekh and Putney, 2005). These include voltage-dependent Ca2+ influx, Ca2+ release from intracellular stores, and store-operated Ca2+ influx in response to depletion of the Ca2+ stores. In this paper, we will focus on phospholipase C (PLC)-driven Ca2+ influx, often described as receptor-operated Ca2+ currents (ROCs). The Ca2+ signal mediated by ROCs differs from that provided by voltage-dependent Ca2+ influx (Somlyo and Somlyo, 1994). However, comparatively little is known about the properties of ROCs and their underlying mechanisms. It is known that in various vertebrate cell types ROCs are generated through the breakdown of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] by PLC upon receptor stimulation and are marked by Ca2+/Na+ influx (Bolton, 1979, Putney and Tomita, 2012). The role of ROCs has also been studied in the Drosophila phototransduction pathway, wherein TRP channels mediate cation currents in response to photoreceptor activation (Hardie, 2011; Montell, 2011).
Several TRP channel homologs, known as transient receptor potential canonical (TRPC; canonical), have been cloned from mammalian (Ramsey et al., 2006). Among of these, TRPC2, 3, 6, 7 channels can be activated by diacylglycerol (DAG), a potent lipid messenger produced from PI(4,5)P2 by PLC activation (Hofmann et al., 1999). It has been suggested that activation of TRPC4, 5 is PLC-dependent, but with no detectable contribution of DAG (Schaefer et al., 2000). Nevertheless, the linkage between PLC-coupled receptors and TRPC channels is almost universally accepted, and the resulting Ca2+ influx is considered to be a ROC (Figure 1A).
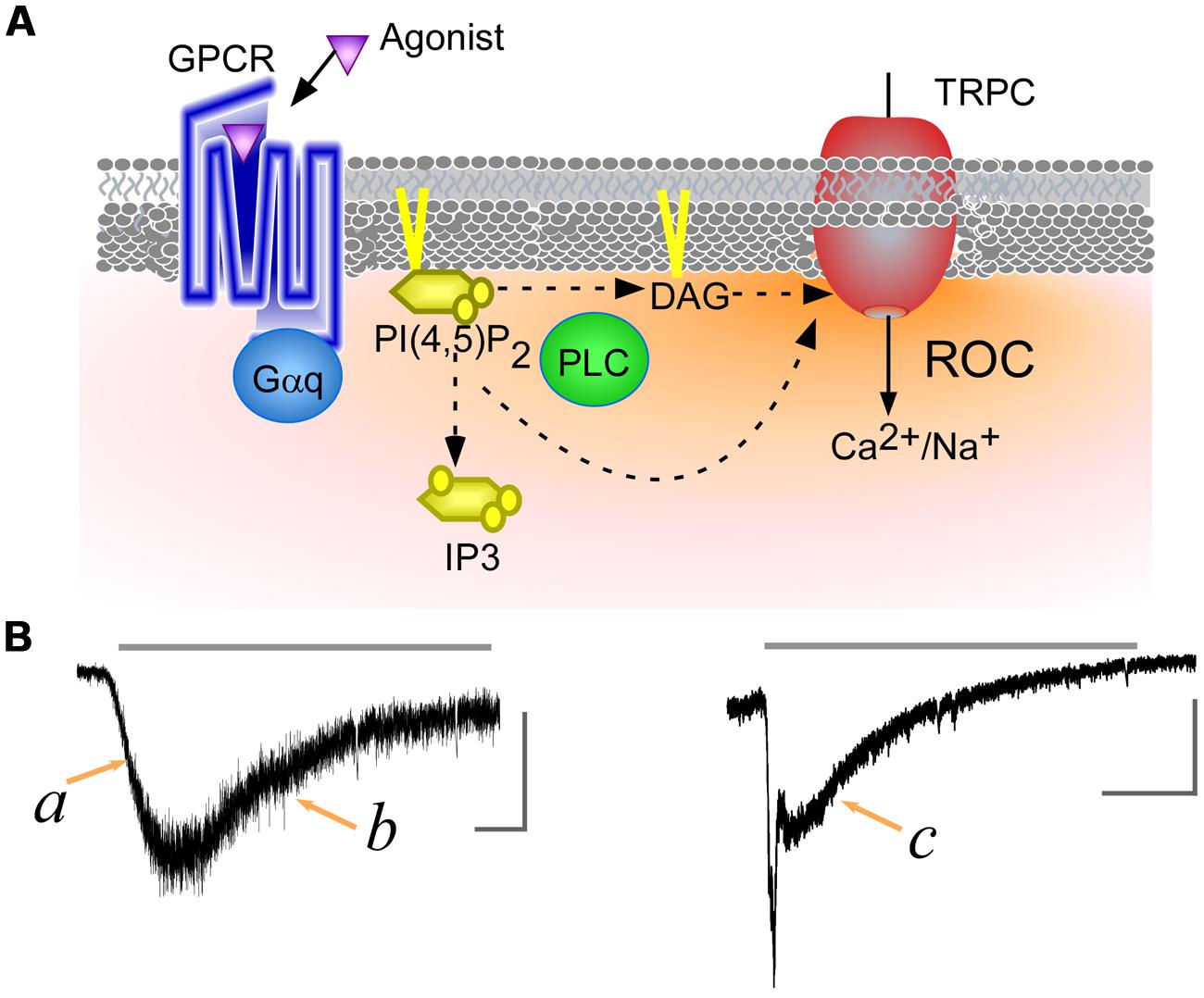
FIGURE 1. Transient receptor potential canonical (TRPC) channels receptor-operated Ca2+ currents (ROCs). (A) Schematic representation of ROCs. Binding of an agonist to a Gq-protein-coupled receptor leads to phospholipase C (PLC) activation. The activated PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to produce of diacylglycerol (DAG) and IP3. DAG and the reduction of PI(4,5)P2 levels directly contribute to TRPC channels activation, while IP3 triggers Ca2+ release from intracellular stores. (B) ROCs through TRPC7 channels. TRPC7 currents were induced using carbachol, a muscarinic receptor agonist (gray line, 100 μM). Left and right panels respectively display currents observed with low and high levels of muscarinic receptor expression (data from Itsuki et al., 2014). The vertical and horizontal gray scale bars indicate 200 pA and 15 s, respectively.
Transient receptor potential canonical channels are also widely distributed in various other tissues (Beech, 2013). Thus, the upstream of TRPC channels can be diverse, reflecting the physiological context. Within the autonomic nervous system, receptor ligands (e.g., noradrenaline and acetylcholine) are released as transmitters from efferent and afferent sympathetic and parasympathetic nerve fibers at target organs. The effects of noradrenaline are mediated via activation of adrenergic receptors, including the α1A receptor, which is abundantly expressed on venous smooth muscle cells, and has been shown to induce ROCs through TRPC6 channels (Inoue et al., 2001). In cerebellar granule cells, brain-derived neurotrophic factor (BDNF)-induced Ca2+ elevation by TRPC channels has been shown to play an essential role in nerve growth cones guidance (Li et al., 2005). Furthermore, pathological contributions of ROCs of TRPC channels have been shown in development of hypertrophy (Onohara et al., 2006; Wu et al., 2010; Seo et al., 2014) and genetic kidney disease ‘focal segmental glomerulosclerosis (FSGS)’ (Mukerji et al., 2007). To emphasize physiological contribution of ROCs, a list of the agonists, receptors, PLC subtypes, and TRPC channels, and their confirmed linkage is presented in the supplementary material (Table S1).
Transient receptor potential canonical channel ROCs can exhibit slow u-shaped time dependence or an initial rapid spike followed by a sustained response, depending on the strength of the receptor stimulation (Figure 1B). The activation phase of ROCs shows a facilitative or growing curve, irrespective of the agonist concentration applied (Figure 1B,a). It has been demonstrated that cytosolic Ca2+ potentiates activity of TRPC5 channels (Blair et al., 2009), however, the facilitative responses induced by receptor agonists are less clear to PI(4,5)P2. For example, PI(4,5)P2 exerts an inhibitory effect on the Drosophila TRPC channel, which prompted an intriguing proposal that reductions in the PI(4,5)P2 concentration due to PLC hydrolysis may be sufficient to evoke ROCs. In a recent study, however, reducing PI(4,5)P2 levels in the absence of PLC activity through rapamycin-induced yeast PI(4,5)P2 phosphatase had no effect on TRPL channel activation (Lev et al., 2012).
The decay phase following the peak exhibits an even more curious. Under a low-dose of an agonist application, ROCs gradually disappear without a plateau phase (Figure 1B,b). On the other hand, at higher agonist doses ROCs often demonstrate fast inactivation followed by a plateau phase (Figure 1B,c). The plateau phase of ROCs also appears in Drosophila photoreceptors, where it is known to be dependent on the intensity of the light stimulation. When the light stimulus is very dim, the photoreceptor-operated currents decay to baseline without a clear plateau phase. Brighter stimuli elicit a plateau phase and shortened the decay time from the plateau to baseline (Minke, 1982). In mammalian TRPC channels, such low vs. high doses of receptor agonist application, which would turn out to impact PI(4,5)P2 hydrolysis, may evoke this opposing effect, but the pattern is not clearly understood yet. In addition, little is known that receptor’s down-regulation, heteromerization of channel subunits, and their regulatory molecules exert an inhibitory effect on ROCs, and an important goal of future experiments will be to identify and characterize the factors critically involved to generating the ROCs.
Phosphatidylinositol 4,5-bisphosphate is located in the inner leaflet of the plasma membrane. In addition to being a substrate for hydrolysis by PLC, PI(4,5)P2 plays key roles in the regulation of cytoskeletal organization, cell motility, and a number ion conducting proteins (Balla, 2013). In mammals, 20 of the 28 known TRP channel subtypes are regulated by PI(4,5)P2. However, studies of PI(4,5)P2 regulation have often reached differing conclusions. For example, in TRPV1, one of the best characterized members of the TRP superfamily, PI(4,5)P2 may positively or negatively regulate channel activity (for review, see Rohacs, 2013). In addition, TRPV4 channels, recently further added to the controversy surrounding PI(4,5)P2 function. PI(4,5)P2 appears to suppress TRPV4 channel activity by binding to an ankyrin domain, while on the other hand PI(4,5)P2 can also facilitate TRPV4 channel activity through binding to a N-terminal region separate from the ankyrin domain (Garcia-Elias et al., 2013; Takahashi et al., 2014), which implies domain-specific regulation of TRPV4 activation. Furthermore, the effect of PI(4,5)P2 on TRPV4 channel activation can depend on the stimulus in the heat or osmo vs chemical (4-α-PPD) stimulations. Under physiological conditions, the breakdown of PI(4,5)P2 by PLC is required for activation of TRPC and TRPL channels. Nonetheless, as with the aforementioned TRPV channels, the effect of PI(4,5)P2 is controversial. It is noteworthy that the controversial reports, which are well-reviewed (Rohacs, 2013), utilized different cell settings and different approaches to manipulating PI(4,5)P2 levels, which raises questions as to whether the PI(4,5)P2 manipulations in these studies are comparable (Mori and Inoue, 2014). To overcome that potential limitation, the clear-cut effects of the voltage-sensing phosphatase (VSP) are discussed in the next section.
Voltage-sensing phosphatase is a newly standardized tool for studying ion channel regulation by phosphoinositides that provides high temporal resolution and is controllable through the membrane potential. So far, two VSPs from aquatic species have been being applied to ion channel studies. Ciona intestinalis (Ci) VSP was the first to be identified as a voltage-sensing phosphoinositide phosphatase (Murata et al., 2005). Identified later was the VSP from Danio rerio (Dr), which exhibits only small differences in the substrate specificity from Ci-VSP, mainly in its voltage-sensitivity (Hossain et al., 2008).
Membrane depolarization under voltage-clamp activates VSPs within a few milliseconds, enabling detection of transient effects on ion channels (Figure 2A, onset). Hille’s group utilized Dr-VSP, and by measuring onset time course of the current reduction, they estimated the residence time of PI(4,5)P2 on the KCNQ2/3 channels to be less than 10 ms, which is more than 400 times faster than the previous estimation (Falkenburger et al., 2010). Because VSPs are only activated during membrane depolarization, upon repolarization of the membrane, PI(4,5)P2 levels, and thus channel currents, are able to recover due the presence of endogenous phosphatidylinositol 4-phosphate 5-kinase (PIP5K), which catalyzes position 5 of the inositol ring (Di Paolo and De Camilli, 2006). The time course of the channel current recovery (Figure 2A, recovery) is illuminating and reflects the time constant for the functional re-association of PI(4,5)P2 with the ion channel. Intriguingly, the recovery constant for the KCNQ2/3 channel and N-type Ca2+ channel inhibition is more than 10 s, which is much slower than in TRPC3/6/7 channels (1–4 s) or TRPM8 channels (4 s; Falkenburger et al., 2010; Suh et al., 2010; Yudin et al., 2011; Imai et al., 2012). According to the time constants of onset and recovery, higher affinity of PI(4,5)P2 for TRPC3 and TRPC6 channels has suggested than that for the KCNQ2/3 or N-type Ca2+ channels.
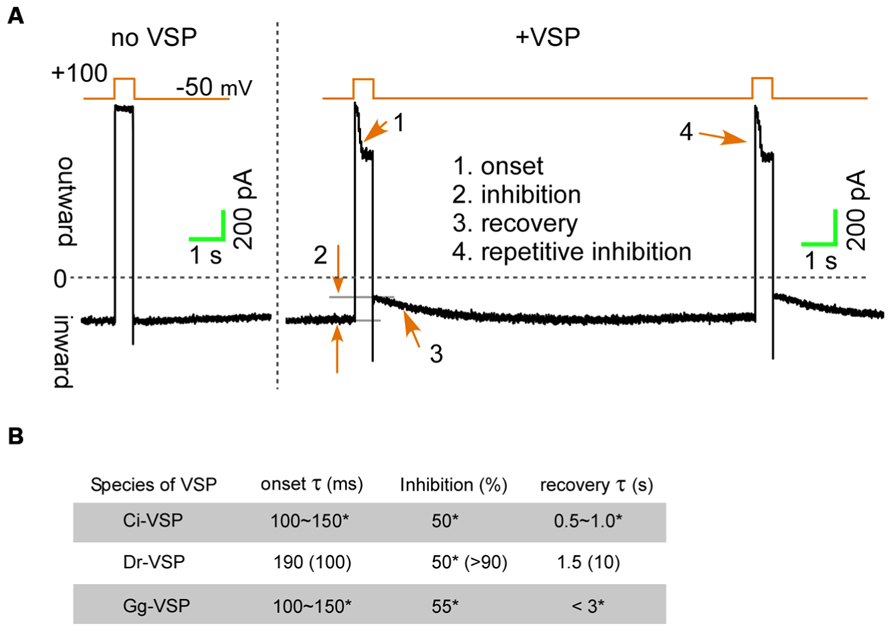
FIGURE 2. The inhibition upon the voltage-sensing phosphatases (VSPs) activation. (A) TRPC6 currents induced by a DAG analog (OAG) are transiently inhibited by Dr-VSP activation (Imai et al., 2012). (B) Inhibitory effects of VSPs on TRPC6 and KCNQ2/3 channels. Data for TRPC6 channels are from Imai et al. (2012). Parenthesizes indicate values for KCNQ2/3 channels (Falkenburger et al., 2010). *Unpublished data from experiments in which equal amounts of plasmid harboring cDNA encoding Ci-, Dr-, or Gg-VSP, and TRPC6 were co-transfected into HEK293 cells. Currents were evoked using 50 μM OAG.
To determine the affinity of PI(4,5)P2 for ion channels in another voltage clamp study, we controlled the magnitude of PI(4,5)P2 reductions by Dr-VSP activation using a step-pulse protocol, which enabled us to determine the affinities of PI(4,5)P2 for TRPC3/6/7 channels. The Kd values obtained ranged from 1 to 10 μM (Itsuki et al., 2014), indicating higher affinity than that of PI(4,5)P2 for KCNQ2/3 channels (EC50 ≈ 87 μM; Zhang et al., 2003). This raises the possibility that the affinity of PI(4,5)P2 for TRPC channels could be too strong to exert regulatory effects, given the physiological PI(4,5)P2 concentration. However, the PI(4,5)P2 concentration in cells has been estimated to range from 5 to 10 μM and may vary by around 50% upon activation of signaling (Xu et al., 2003; McLaughlin and Murray, 2005). This concentration range clearly overlaps the dissociation constants for TRPC channels and is consistent with the physiological relevance of PI(4,5)P2 regulation. Furthermore, evidence of physical coupling between PLC and TRPC channels has been provided (van Rossum et al., 2005), local PI(4,5)P2 depletion may possible to happen nearby TRPC channels.
Voltage-sensing phosphatase In addition to sea ascidian (Ci) and zebra fish (Dr), VSPs have also been identified in chicken (Gallus gallus, Gg; Yamaguchi et al., 2014). Among these three VSPs, Dr-VSP appears to be best for manipulating PI(4,5)P2, as it is more strongly expressed than Ci-VSP (Hossain et al., 2008) and has higher catalytic specificity toward PI(4,5)P2 than Gg-VSP (Yamaguchi et al., 2014). Nonetheless, we compared the effects of Ci-, Dr,- and Gg-VSP on TRPC6 currents. The extent to which the three homologs inhibited TRPC6 currents and the time-courses of their effects differed only with respect to recovery, and were otherwise nearly equal (Figure 2B). This finding supports the theory that Ci-, Dr-, and Gg-VSP are all valuable molecular tools for characterizing channel regulation by PI(4,5)P2.
As described above, reductions in PI(4,5)P2 can have opposite effects on TRPC channel activity. We therefore wondered whether this effect could explain the dynamics of both ROCs and the reductions in PI(4,5)P2 observed in our recent experiments (Itsuki et al., 2014). So far, no model has incorporated opposing effects of PI(4,5)P2 on the regulation of TRPC channel activity. We newly modified to add these effects into a promising model of TRPC6/7 currents, which we called the SPD model (Itsuki et al., 2014). When we then fit simulations generated with the new model to TRPC6 channel ROCs, which were recorded simultaneously with PI(4,5)P2 dynamics using a FRET sensor, the simulated ROC was well fitted to the experimentally observed ROC dynamics (Figure S1, upper right panel). Although the simulated PI(4,5)P2 dynamics diverged somewhat from the experimental data (Figure S1, lower right panel), we found that bi-directional PI(4,5)P2 regulation may be possible if the negative effect site of PI(4,5)P2 is a range from 20× less to greater affinity for PI(4,5)P2 than the positive effect site for PI(4,5)P2 (Table S2).
Phosphatidylinositol 4,5-bisphosphate association sites as well as its physiological importance remains to be addressed in future studies. TRPC channel ROCs show great variation, and the mechanisms, and physiological consequences of that variation are not yet fully understood. Precise detection of signals elicited by receptor stimulation and clear cut evidence of the regulatory factors, including PI(4,5)P2, Ca2+, will aid our understanding of ROCs within pathophysiological responses.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank to Dr. Y. Okamura (Osaka University) for providing Gg-VSP. This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Sciences (MXM).
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fphar.2015.00022/abstract
Balla, T. (2013). Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137. doi: 10.1152/physrev.00028.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Beech, D. J. (2013). Characteristics of transient receptor potential canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circ. J. 77, 570–579. doi: 10.1253/circj.CJ-13-0154
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Berridge, M. J. (2012). Calcium signalling remodelling and disease. Biochem. Soc. Trans. 40, 297–309. doi: 10.1042/BST20110766
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Blair, N. T., Kaczmarek, J. S., and Clapham, D. E. (2009). Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J. Gen. Physiol. 133, 525–546. doi: 10.1085/jgp.200810153
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bolton, T. B. (1979). Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 59, 606–718.
Di Paolo, G., and De Camilli, P. (2006). Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657. doi: 10.1038/nature05185
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Falkenburger, B. H., Jensen, J. B., and Hille, B. (2010). Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 135, 99–114. doi: 10.1085/jgp.200910345
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Garcia-Elias, A., Mrkonjic, S., Pardo-Pastor, C., Inada, H., Hellmich, U. A., Rubio-Moscardo, F.,et al. (2013). Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc. Natl. Acad. Sci. U.S.A. 110, 9553–9558. doi: 10.1073/pnas.1220231110
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hardie, R. C. (2011). A brief history of trp: commentary and personal perspective. Pflugers. Arch. 461, 493–498. doi: 10.1007/s00424-011-0922-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T., and Schultz, G. (1999). Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263. doi: 10.1038/16711
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hossain, M. I., Iwasaki, H., Okochi, Y., Chahine, M., Higashijima, S., Nagayama, K.,et al. (2008). Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J. Biol. Chem. 283, 18248–18259. doi: 10.1074/jbc.M706184200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Imai, Y., Itsuki, K., Okamura, Y., Inoue, R., and Mori, M. X. (2012). A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P(2)-diacylglycerol signalling. J. Physiol. 590, 1101–1119. doi: 10.1113/jphysiol.2011.221358
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Inoue, R., Okada, T., Onoue, H., Hara, Y., Shimizu, S., Naitoh, S.,et al. (2001). The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ. Res. 88, 325–332. doi: 10.1161/01.RES.88.3.325
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Itsuki, K., Imai, Y., Hase, H., Okamura, Y., Inoue, R., and Mori, M. X. (2014). PLC-mediated PI(4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels. J. Gen. Physiol. 143, 183–201. doi: 10.1085/jgp.201311033
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lev, S., Katz, B., Tzarfaty, V., and Minke, B. (2012). Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: implications on gating of Drosophila TRPL (transient receptor potential-like) channel. J. Biol. Chem. 287, 1436–1447. doi: 10.1074/jbc.M111.266585
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Li, Y., Jia, Y. C., Cui, K., Li, N., Zheng, Z. Y., Wang, Y. Z.,et al. (2005). Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434, 894–898. doi: 10.1038/nature03477
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McLaughlin, S., and Murray, D. (2005). Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611. doi: 10.1038/nature04398
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Minke, B. (1982). Light-induced reduction in excitation efficiency in the trp mutant of Drosophila. J. Gen. Physiol. 79, 361–385. doi: 10.1085/jgp.79.3.361
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Montell, C. (2011). The history of TRP channels, a commentary and reflection. Pflugers. Arch. 461, 499–506. doi: 10.1007/s00424-010-0920-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mori, M. X., and Inoue, R. (2014). New experimental trends for phosphoinositides research on ion transporter/channel regulation. J. Pharmacol. Sci. 126, 186–197. doi: 10.1254/jphs.14R14CP
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mukerji, N., Damodaran, T. V., and Winn, M. P. (2007). TRPC6 and FSGS: the latest TRP channelopathy. Biochim. Biophys. Acta 1772, 859–868. doi: 10.1016/j.bbadis.2007.03.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Murata, Y., Iwasaki, H., Sasaki, M., Inaba, K., and Okamura, Y. (2005). Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435, 1239–1243. doi: 10.1038/nature03650
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Onohara, N., Nishida, M., Inoue, R., Kobayashi, H., Sumimoto, H., Sato, Y.,et al. (2006). TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 25, 5305–5316. doi: 10.1038/sj.emboj.7601417
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Parekh, A. B., and Putney, J. W. Jr. (2005). Store-operated calcium channels. Physiol. Rev. 85, 757–810. doi: 10.1152/physrev.00057.2003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Putney, J. W., and Tomita, T. (2012). Phospholipase C signaling and calcium influx. Adv. Biol. Regul. 52, 152–164. doi: 10.1016/j.advenzreg.2011.09.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ramsey, I. S., Delling, M., and Clapham, D. E. (2006). An introduction to TRP channels. Annu. Rev. Physiol. 68, 619–647. doi: 10.1146/annurev.physiol.68.040204.100431
Rohacs, T. (2013). Regulation of transient receptor potential channels by the phospholipase C pathway. Adv. Biol. Regul. 53, 341–355. doi: 10.1016/j.jbior.2013.07.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schaefer, M., Plant, T. D., Obukhov, A. G., Hofmann, T., Gudermann, T., and Schultz, G. (2000). Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 275, 17517–17526. doi: 10.1074/jbc.275.23.17517
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Seo, K., Rainer, P. P., Shalkey Hahn, V., Lee, D. I., Jo, S. H., Andersen, A.,et al. (2014). Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 111, 1551–1556. doi: 10.1073/pnas.1308963111
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Somlyo, A. P., and Somlyo, A. V. (1994). Signal transduction and regulation in smooth muscle. Nature 372, 231–236. doi: 10.1038/372231a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Suh, B. C., Leal, K., and Hille, B. (2010). Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron 67, 224–238. doi: 10.1016/j.neuron.2010.07.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Takahashi, N., Hamada-Nakahara, S., Itoh, Y., Takemura, K., Shimada, A., Ueda, Y.,et al. (2014). TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P2. Nat. Commun. 5, 4994. doi: 10.1038/ncomms5994
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
van Rossum, D. B., Patterson, R. L., Sharma, S., Barrow, R. K., Kornberg, M., Gill, D. L.,et al. (2005). Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature 434, 99–104. doi: 10.1038/nature03340
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wu, X., Eder, P., Chang, B., and Molkentin, J. D. (2010). TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 107, 7000–7005. doi: 10.1073/pnas.1001825107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xu, C., Watras, J., and Loew, L. M. (2003). Kinetic analysis of receptor-activated phosphoinositide turnover. J. Cell Biol. 161, 779–791. doi: 10.1083/jcb.200301070
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yamaguchi, S., Kurokawa, T., Taira, I., Aoki, N., Sakata, S., Okamura, Y.,et al. (2014). Potential role of voltage-sensing phosphatases in regulation of cell structure through the production of PI(3,4)P2. J. Cell. Physiol. 229, 422–433. doi: 10.1002/jcp.24463
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yudin, Y., Lukacs, V., Cao, C., and Rohacs, T. (2011). Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J. Physiol. 589, 6007–6027. doi: 10.1113/jphysiol.2011.220228
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, H., Craciun, L. C., Mirshahi, T., Rohacs, T., Lopes, C. M., Jin, T.,et al. (2003). PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 37, 963–975. doi: 10.1016/S0896-6273(03)00125-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: receptor-operated calcium current, TRPC channels, PIP2, voltage-sensing phosphatase, Ca2+ signaling, smooth muscle
Citation: Mori MX, Itsuki K, Hase H, Sawamura S, Kurokawa T, Mori Y and Inoue R (2015) Dynamics of receptor-operated Ca2+ currents through TRPC channels controlled via the PI(4,5)P2-PLC signaling pathway. Front. Pharmacol. 6:22. doi: 10.3389/fphar.2015.00022
Received: 01 November 2014; Accepted: 24 January 2015;
Published online: 11 February 2015.
Edited by:
Carlos Alberto Villalba-Galea, Virginia Commonwealth University School of Medicine, USAReviewed by:
Baruch Minke, Hebrew University of Jerusalem, IsraelCopyright © 2015 Mori, Itsuki, Hase, Sawamura, Kurokawa, Mori and Inoue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masayuki X. Mori, Department of Synthetic and Biological Chemistry, School of Engineering, Kyoto University, Katsura campus, Nishikyo-ku, Kyoto-city, Kyoto 615-8510, Japan e-mail:bXhtb3JpQHNiY2hlbS5reW90by11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.