
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 11 November 2014
Sec. Experimental Pharmacology and Drug Discovery
Volume 5 - 2014 | https://doi.org/10.3389/fphar.2014.00248
This article is part of the Research Topic Transporters, physiology, efflux inhibition and the challenge of clinical implementation View all 9 articles
A commentary has been posted on this article:
Corrigendum: The role of drug transporters in the kidney: lessons from tenofovir
Tenofovir disoproxil fumarate, the prodrug of nucleotide reverse transcriptase inhibitor tenofovir, shows high efficacy and relatively low toxicity in HIV patients. However, long-term kidney toxicity is now acknowledged as a modest but significant risk for tenofovir-containing regimens, and continuous use of tenofovir in HIV therapy is currently under question by practitioners and researchers. Co-morbidities (hepatitis C, diabetes), low body weight, older age, concomitant administration of potentially nephrotoxic drugs, low CD4 count, and duration of therapy are all risk factors associated with tenofovir-associated tubular dysfunction. Tenofovir is predominantly eliminated via the proximal tubules of the kidney, therefore drug transporters expressed in renal proximal tubule cells are believed to influence tenofovir plasma concentration and toxicity in the kidney. We review here the current evidence that the actions, pharmacogenetics, and drug interactions of drug transporters are relevant factors for tenofovir-associated tubular dysfunction. The use of creatinine and novel biomarkers for kidney damage, and the role that drug transporters play in biomarker disposition, are discussed. The lessons learnt from investigating the role of transporters in tenofovir kidney elimination and toxicity can be utilized for future drug development and clinical management programs.
Tenofovir, administered as the prodrug tenofovir disoproxil fumarate, is a nucleotide reverse transcriptase inhibitor which is recommended for use in first-line treatment of HIV infection. The drug has many beneficial characteristics, including once-daily dosing, high efficacy, and lack of interaction with cytochrome P450 enzymes (Boffito et al., 2005). Tenofovir shows a favorable safety profile compared to other nucleoside reverse transcriptase inhibitors. However, long-term kidney toxicity is acknowledged as a modest but significant risk for tenofovir-containing regimens (Cooper et al., 2010). It has been observed in a particular clinic that tenofovir-associated nephrotoxicity is the most common single reason for HIV-related referral to specialist renal services, accounting for more than 20% of consultations (Hall et al., 2011). The mechanisms involved in the observed kidney tubular dysfunction are not fully understood, but direct mitochondrial toxicity by tenofovir, interference with normal tubular cell function, or a combination of both have been suggested (Hall et al., 2011). Co-morbidities (hepatitis C, diabetes), low body weight, older age, concomitant administration of potentially nephrotoxic drugs, low CD4 count, and duration of therapy are all risk factors associated with tubular dysfunction (Rodriguez-Novoa et al., 2010). Risk factors may also involve drug transporters expressed in renal proximal tubule cells. Indeed, evidence is emerging that high concentrations of tenofovir in plasma are associated with development of kidney damage, and it is likely that drug transporters play a role in this association (Barditch-Crovo et al., 2001; Rodriguez-Novoa et al., 2009a) as well as in perturbations of the commonly used biomarker, creatinine (Fernandez-Fernandez et al., 2011).
Drug transporters can be divided into two superfamilies; the solute carrier (SLC) superfamily and the ATP binding cassette (ABC) superfamily. It is acknowledged that drug transporters play a significant role in the absorption, distribution, metabolism, elimination (ADME), efficacy, and toxicity of numerous drugs. They are detectable in virtually all tissues, although the precise orientation and function of many transporters are not fully understood (Bleasby et al., 2006). Drug transporters play a key role in controlling the movement of drugs between the blood and the liver (Faber et al., 2003), intestine (Estudante et al., 2013), and kidney (Morrissey et al., 2013). Furthermore, drug transporters are involved in the penetration of drugs into target tissues such as the lymphatic system in antiretroviral treatment (Ford et al., 2004), and also act to protect tissues such as the central nervous system from potentially toxic drugs and xenobiotics (Ballabh et al., 2004). Prior to the licensing of a new drug, the Food and Drug Administration (FDA) and European Medicines Agency (EMA) require that certain tests are performed which determine if a drug is a substrate or inhibitor of a selection of clinically relevant transporters (Table 1).
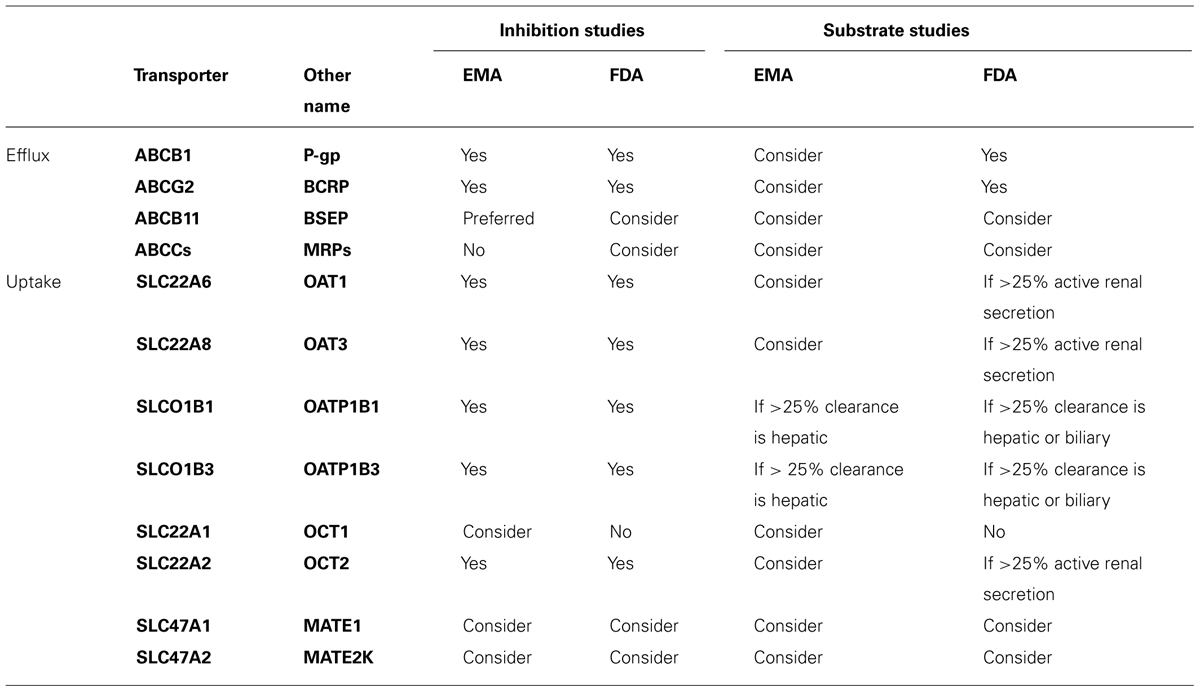
TABLE 1. Recommendations for drug transporter testing as outlined in the EMA Guideline on Investigation of Drug Interactions, July 2012, and the FDA Draft Guidance on Drug Interaction Studies, February 2012.
Tenofovir is predominantly eliminated via the proximal tubules of the kidney, and this review summarizes our current understanding of how kidney transporter polymorphisms and drug interactions may influence tenofovir-associated nephrotoxicity. The implications and knowledge gaps are also described, along with suggestions for future transporter studies. The lessons learnt from investigating the role of transporters in tenofovir kidney elimination and toxicity can be utilized for future drug development and clinical management, which is discussed in this review.
The kidney, along with the liver, is a key organ involved in systemic clearance of drugs, with around 32% of currently used drugs in the USA exhibiting significant (>25%) renal elimination (Morrissey et al., 2013). Elimination can occur via glomerular filtration, tubular secretion, or a combination of both pathways. The process of tubular secretion is twofold: (1) the drug requires access to the proximal tubule cells from the blood via the basolateral membrane, and (2) the drug is removed into the luminal fluid via the apical membrane. This process can occur passively, but in many cases drug transporter proteins are involved in facilitating drug movement across membranes and actively transporting drugs against concentration gradients.
Transporters in the kidney are involved in drug–drug interactions, particularly in cases where transport is the main or rate-limiting transmembrane route for a drug. The kidney transporters which are the focus of this review are those where a functional role in drug disposition has been demonstrated or is suspected (Table 2) and have been separated into cationic transporters, anionic transporters, transporters with less or unknown specificity in substrate charge, and ABC efflux transporters. It is important to recognize that transporter expression is often not exclusive to a single site in the body, and many have well-defined involvement in tissues other than the kidney (Kis et al., 2010; DeGorter et al., 2012). Several kidney transporters are capable of influencing the elimination of antiretroviral drugs, including tenofovir (Kis et al., 2010). The interactions between tenofovir and kidney transporters are discussed in more detail in a later section.
SLC22A1, SLC22A2, and SLC22A3 are organic cation transporters expressed on the basolateral membrane of proximal tubule cells. They control the entry of cationic small molecules, including creatinine and numerous drug substrates, into the epithelial cells (Gorboulev et al., 1997; Grundemann et al., 1999; Dresser et al., 2001; Kimura et al., 2002; Urakami et al., 2004; Zhu et al., 2010; Ciarimboli et al., 2012; Tzvetkov et al., 2013). Transporters relevant to this review along with representative drug and endogenous substrates are shown in Table 2. Transport is driven by electrochemical potential but is not altered by sodium or proton gradients (Nies et al., 2011). SLC47A1 and SLC47A2, also known as multidrug and toxin extrusion (MATE) transporters, are efflux transporters of cationic substrates (Masuda et al., 2006; Ohta et al., 2006; Chen et al., 2007; Tanihara et al., 2007; Martinez-Guerrero and Wright, 2013). SLC47A1 is highly expressed in the kidney and liver and SLC47A2 is almost exclusively expressed in the kidney, with both showing localization to the apical membrane of proximal tubule cells (Tanihara et al., 2007). Many of the substrates and inhibitors of SLC47 transporters overlap with those of SLC22A1, SLC22A2, and SLC22A3 (Nies et al., 2011). For example, SLC47A1 and SLC47A2 work in cooperation with SLC22A2 to control the concentration of several substrates within proximal tubule cells, such as creatinine (Motohashi and Inui, 2013).
SLC22A6, SLC22A7, and SLC222A8 are influx transporters expressed on the basolateral membrane of proximal tubule cells, where they transport small anionic molecules into the cell. SLC22A11 is a related transporter located on the apical membrane and contributes to renal excretion and reabsorption of anionic substrates, as movement of substrates can occur in both directions (Kusuhara et al., 1999; Cha et al., 2000; Kobayashi et al., 2005; Hagos et al., 2007; Moss et al., 2011). Transporters relevant to this review along with representative drug and endogenous substrates are shown in Table 2. SLC22A12 is expressed on the apical surface of proximal tubule cells and, in conjunction with SLC22A11, mediates the reabsorption of uric acid from the urine, thereby regulating blood uric acid levels (Enomoto et al., 2002; Vitart et al., 2008). Disruption of SLC22A12 activity through genetic predisposition or drug interactions can cause toxicity, therefore the transporter is considered pharmacologically relevant (Shafiu et al., 2012). The bidirectional transporter SLCO4C1 is highly expressed in the kidney and is located on the apical surface of proximal tubule cells (Bleasby et al., 2006). Substrates of SLCO4C1 include steroid conjugates, thyroid hormones, anti-cancer drugs, and antibiotics (Yamaguchi et al., 2010).
SLC15A1 and SLC15A2 are proton-coupled co-transporters of many diverse peptide and peptidomimetic substrates, but not amino acids (Ganapathy et al., 1995, 1998; Liang et al., 1995; Shu et al., 2001; Daniel and Kottra, 2004; Tramonti et al., 2006). SLC15A1 is expressed on the apical surface of intestinal enterocytes and, to a lesser degree, the apical surface of renal proximal tubule cells, whereas SLC15A2 is expressed predominantly on the apical surface of renal proximal tubule cells. SLC15A2 undertakes the reabsorption of peptide-bound amino nitrogen from the glomerular filtrate, which is important in nitrogen homeostasis (Kamal et al., 2008). Nucleoside transporter proteins are divided into two families; the sodium-dependent, solute carrier family 28 (SLC28), and the equilibrative, solute carrier family 29 (SLC29), where the endogenous substrates are nucleosides or nucleoside-like drugs (Nagai et al., 2006; Endres et al., 2009; Sato et al., 2009; Bhutia et al., 2011; Choi et al., 2014). Again, representative drug and endogenous substrates for these transporters are shown in Table 2.
Multidrug resistance related proteins (ABCCs) and multidrug resistance protein ABCB1 are members of the ABC superfamily, which can be identified by the presence of a highly conserved ATP binding motif (DeGorter et al., 2012). ABCCs are found in multiple tissues throughout the body, including in relevant ADME tissues such as the small intestine, lymphatic system, liver and kidney, and function in an ATP-dependent process. In the kidney, ABCC2 and ABCC4 are expressed on the apical membrane of proximal tubule cells and efflux anionic substrates such as weakly acidic drugs, glutathione, sulfates, and xenobiotics (DeGorter et al., 2012). ABCC1, ABCC3, and ABCC6 are expressed on the basolateral membrane of proximal tubule cells. ABCC1 does not appear to play a significant role in the absorption or elimination of drugs, but is involved in resistance development of anticancer drugs and in the inflammatory response (Deeley et al., 2006; Bakos and Homolya, 2007). ABCC3 is predominantly expressed in the liver, where it is involved in the regulation of bile salt enterohepatic recirculation, but mRNA is also detectable in numerous other tissues including the kidney (Kool et al., 1999b; Scheffer et al., 2002; Zhou et al., 2008). High ABCC6 mRNA has been detected in both the liver and kidney (Kool et al., 1999a). However, the exact range of substrates for ABCC6 has not yet been determined, but preliminary investigations suggest that ABCC6 may be involved in the transport of anticancer drugs. ABCC10 is a recent addition to the potentially clinically relevant ABC multidrug resistance proteins, with high mRNA expression found in numerous tissues including the kidney, liver, and intestine (Bleasby et al., 2006). Specificity of expression (i.e., apical or basolateral) is unknown in the proximal tubules, and substrate specificity is limited. However, increasing numbers of drugs, including anticancer and antiretroviral drugs, have been shown to be substrates (Chen et al., 2003; Pushpakom et al., 2011; Liptrott et al., 2012; Sun et al., 2013). ABCB1 is widely distributed in the kidney, liver, small intestine, and brain and is integral for limiting the absorption of potentially toxic xenobiotics into tissues. In the kidney, ABCB1 is expressed on the apical membrane and has broad substrate specificity, although substrates are usually hydrophobic and either neutral or cationic (DeGorter et al., 2012). ABCG2 plays a similar role to ABCB1 in drug disposition, is generally expressed in the same tissues, and contributes to renal excretion of some drugs (Kage et al., 2002; Jani et al., 2009; Beery et al., 2011). Unlike, ABCB1, the substrate preference for ABCG2 includes hydrophilic conjugated organic anions, particularly the sulfate forms. Despite the recent progress made, several drug transporters in the kidney have not been well characterized, and expression levels, locations and substrate affinity remain undetermined.
Tenofovir is predominantly eliminated via the kidney by a combination of glomerular filtration and active tubular secretion. Both influx and efflux transporters are known to influence tenofovir elimination rate, although a complete understanding of the process has not yet been achieved. The efflux transporters ABCC2 (MRP2) and ABCC4 (MRP4) are expressed at the apical surface of proximal tubule cells and actively remove substrates into the renal lumen (Smeets et al., 2004). The level of transport of tenofovir by ABCC2 was found not to be significant (Imaoka et al., 2007; Neumanova et al., 2014). Conversely, ABCC4 has been shown to transport tenofovir and is believed to be the main tenofovir transporter on the apical surface of proximal tubule cells (Kohler et al., 2011). The efflux transporters ABCB1 and ABCG2 are expressed at many membrane barriers in the body, including at the apical surface of proximal tubule cells (Tanigawara, 2000; Woodward et al., 2009). The extent of tenofovir transport by ABCB1 and ABCG2 was assessed in vitro and in rodents and found to be not significant (Ray et al., 2006; Neumanova et al., 2014). The Neumanova study also found that the tenofovir prodrug, tenofovir disoproxil fumarate, was a substrate for both transporters. However, it is unlikely that orally administered tenofovir disoproxil fumarate is present at the blood-kidney barrier, as esterase activity rapidly degrades the prodrug in intestinal tissue and plasma following absorption (van Gelder et al., 2002). Nonetheless, ABCB1 and ABCG2 are heavily expressed at the apical surface of the intestinal wall, which is therefore likely to be the major cite where orally administered tenofovir disoproxil fumarate could encounter these transporters. Therefore, it may well be that tenofovir plasma concentrations, and therefore the extent of tenofovir-exposure-associated nephrotoxicity, are influenced by the actions of these transporters on tenofovir disoproxil fumarate absorption. The efflux transporter ABCC10 is known to confer resistance to several anti-cancer drugs (Hopper-Borge et al., 2009; Sun et al., 2013, 2014), and there is growing evidence that it plays a role in tenofovir-associated kidney toxicity. ABCC10 RNA is detectable at high levels in several pharmacologically relevant tissues, including the intestine, liver, brain, and kidney (Bleasby et al., 2006), although protein expression levels, orientation at blood-tissue membrane barriers and substrate specificity are not fully understood. The transport of tenofovir by ABCC1 has been demonstrated in vitro (ABCB10-transfected HEK293 cells) and ex vivo (ABCC10 siRNA knockdown in CD4+ T cells; Pushpakom et al., 2011). However, the potential impact of kidney expression of this transporter in vivo has not otherwise been well characterized.
Tenofovir contains a phosphate group with a negative charge at physiological pH, and this gives the drug an affinity for anion-specific influx transporters. Tenofovir is transported by SLC22A6 and, to a lesser extent, SLC22A8 (Uwai et al., 2007). Although affinity of tenofovir for SLC22A6 transporter is greater, SLC22A8 shows higher expression levels in the kidney. As such, this low-affinity high-capacity SLC22A8 transport route may also be important in tenofovir elimination. There remain several kidney-expressed transporters which may be involved in tenofovir-associated nephrotoxicity but which have not been comprehensively assessed for tenofovir transport. The influx transporter SLC22A7 is expressed on the basolateral surface of proximal tubule cells and may work in conjunction with the similar transporters SLC22A6 and SLC22A8 in tenofovir excretion. SLC22A11 is expressed on the apical surface of proximal tubule cells and is able to transport substrates in both directions. The concentrative nucleoside transporters SLC28A1 and SLC28A2 are expressed on the apical surface of proximal tubule cells. Concentrative nucleoside transporters are known to transport the anti-HIV nucleoside analog zidovudine (Hagos and Wolff, 2010) but transport of tenofovir has not been investigated. It is unknown if SLC28A1, SLC28A2, SLC22A7, or SLC22A11 transport tenofovir, and this is certainly worthy of clarification (Hagos and Wolff, 2010).
It has been proposed that genetic polymorphisms in renal transporters may predispose individuals to have high intracellular tenofovir concentrations, thus increasing the chance of developing tubular toxicity. ABCC2 polymorphisms have been evaluated, and the haplotype “CATC” [a combination of the polymorphisms at positions –24 (rs717620), 1249 (rs2273697), 3563 (rs8187694), and 3972 (rs3740066) within the ABCC1 gene] and the allele -24C > T (rs717620) have both been associated with an increased incidence of tenofovir-associated tubular toxicity (Izzedine et al., 2006; Rodriguez-Novoa et al., 2009b). In a study in Japanese HIV+ patients, the ABCC2 –24C > T and 1249G > A polymorphisms were found to be protective for tenofovir-induced kidney toxicity (Nishijima et al., 2012). These observations are difficult to rationalize because tenofovir is not a substrate for ABCC2, which conversely would suggest that ABCC2 activity and expression would not be relevant to tenofovir-associated kidney toxicity in vivo (Imaoka et al., 2007; Neumanova et al., 2014). It may be the case that an endogenous substrate for ABCC2 exacerbates the toxicity of tenofovir or competes with tenofovir for transport by ABCC4. Also, the ABCC2 genotypes may be in linkage disequilibrium with other polymorphisms in genes coding for unidentified factors which exacerbate tenofovir toxicity.
Currently, it is a matter of controversy whether ABCC4 polymorphisms alter the risk of tenofovir-induced kidney toxicity. A study in HIV+ patients found that a 669C > T (rs899494) polymorphism in the ABCC4 gene was associated with tenofovir-induced kidney toxicity, but this was not found in a subsequent study (Izzedine et al., 2006; Rodriguez-Novoa et al., 2009b). Several additional single nucleotide polymorphisms in ABCC4 were investigated [559G > T (rs11568658), 912G > T (rs2274407), 951G > T (rs2274406), 969G > A (rs2274405), 1497C > T (rs1557070), 3310T > C (rs11568655), and 3348A > G (rs1751034)] but no associations with tenofovir-induced kidney toxicity were found. The ABCC4 polymorphism 4131T > C (rs3742106) has been associated with increased concentrations of tenofovir diphosphate (35% higher than homozygotes for the common allele) in human peripheral blood mononuclear cells (PBMCs) 24 h post-dose (Kiser et al., 2008a). The ABCC10 efflux transporter is capable of transporting tenofovir in vitro and subsequently polymorphisms of ABCC10 may influence tenofovir disposition. In patients taking tenofovir therapy, two ABCC10 polymorphisms [526G > A (rs9349256) and 2843T > C (rs2125739)] were associated with kidney toxicity (Pushpakom et al., 2011) but no replication studies have been conducted.
ABCB1 is unlikely to transport tenofovir at the kidney, but the prodrug tenofovir disoproxil fumarate may be influenced by ABCB1 activity at the intestine (as discussed above). Several ABCB1 polymorphisms [1236C > T (rs1128503), 2677G > T/A (rs2032582), and 3435C > T (rs1045642)] have been analyzed and were found not to be associated with tenofovir-induced kidney toxicity or alteration in tenofovir renal clearance (Izzedine et al., 2006; Rodriguez-Novoa et al., 2009b). Regarding influx transporters, SLC22A6 polymorphisms 453G > A (rs4149170) and 728G > A (rs11568626) have been analyzed and were found not to be associated with kidney toxicity or alteration in tenofovir renal clearance (Kiser et al., 2008b; Rodriguez-Novoa et al., 2009b).
Pharmacogenetics of relevant drug transporters provides a tool for identifying patients at risk when taking tenofovir. However, pharmacogenetics studies in this context have met with mixed success. Only ABCC2 has shown strong evidence of association with kidney damage phenotypes in patients taking tenofovir. Other associations have been contradicted in further studies, been performed in too few patients to make reliable conclusions or else no replication studies have been attempted. Since non-genetic factors, such as old age, low body weight, co-administered medicines, and co-morbidities are important; it seems likely that transporter genetics will not be fully predictive of the toxicity. Further investigations into the actions of drug transporters may improve our understanding of factors controlling tenofovir disposition and elimination. The pharmacogenetics of the nuclear receptors which control expression of certain transporters, such as the pregnane X receptor and the constitutive androstane receptor, may also be relevant factors, as has been shown for other pharmacological phenotypes involving transporters (Owen et al., 2004; Johnson et al., 2008; Martin et al., 2008; Siccardi et al., 2008; Schipani et al., 2010; Wyen et al., 2011).
When co-administered with tenofovir in highly active antiretroviral therapy (HAART), ritonavir-boosted protease inhibitors have been shown to increase tenofovir plasma exposure. An increase in tenofovir AUC of 37 and 32% was observed following co-administration of atazanavir and lopinavir, respectively (Tong et al., 2007). Less substantial increases have been observed for co-administered darunavir (22%), and saquinavir (14%). Ritonavir, and lopinavir inhibit relevant transporters SLC22A8 and ABCC4 in vitro, and a transporter-mediated drug interaction at the kidney may explain the elevated tenofovir concentrations when using these drugs (Cihlar et al., 2007). Proteinuria, the presence of an excess of serum protein in the urine, is indicative of kidney functional impairment. The co-administration of protease inhibitors with tenofovir increased the frequency of proteinuria development by sevenfold, compared to tenofovir treatment not containing protease inhibitors (Kelly et al., 2013). This is supported by a further publication that showed use of protease inhibitors to be a predictor of tubular toxicity in tenofovir-containing regiments (Calza et al., 2011). The authors hypothesized that the causes of this association include ritonavir-driven inhibition of enzymes involved in tenofovir elimination from the kidney. However, ritonavir is not known to be involved in affecting metabolism of tenofovir at the kidney, and it seems more likely that ritonavir and other protease inhibitors may inhibit the removal of tenofovir from the kidney proximal tubule cells by inhibiting kidney-expressed transporters, or by preventing tenofovir disoproxil fumarate degradation at the intestine (Tong et al., 2007). Interestingly, a further study by Calza et al. (2013) found that both the development of proteinuria associated with tenofovir use was more pronounced when co-administered with atazanavir, compared to tenofovir co-administered with lopinavir. This data is supported by a further study showing lopinavir to have less severe toxicity-associations compared to other atazanavir, when co-administered with tenofovir (Young et al., 2012). These data suggest that, to reduce the occurance of proteinuria in patients, certain protease inhibitors may be a more suitable addition in a tenofovir-containing regiment.
Other classes of antiretroviral have led to drug interactions with tenofovir. The co-administration of the integrase inhibitor raltegravir with tenofovir disoproxil fumarate resulted in a moderate increase (49%) in tenofovir AUC (Wenning et al., 2008). This interaction may in part be explained by an interaction involving SLC22A6, as raltegravir is capable of inhibiting SLC22A6 in vitro (Moss et al., 2011). However, the clinical significance of this interaction is unknown. The use of tenofovir disoproxil fumarate with the nucleoside analog didanosine has been associated with severe side effects, including a reduction in CD4+ cell count, pancreatitis, and hyperglycemia. Tenofovir and didanosine are both nephrotoxic and therefore the interaction may result from the additive toxic effects of both drugs. Additionally, tenofovir is capable of increasing didanosine AUC by 44%, which may involve inhibition of SLC22A6-mediated excretion of didanosine via the kidney (Ray et al., 2004). Due to the severity of the drug interaction, co-administration of tenofovir disoproxil fumarate and didanosine is not recommended.
In addition to co-administered antiretrovirals, any other drug which has the potential to compete with tenofovir for kidney excretion via drug transporters may alter tenofovir exposure. In a study using HIV patients, co-administration of the non-steroidal anti-inflammatory drug diclofenac with tenofovir led to a high (14.6%) occurrence of acute kidney injury, compared to tenofovir treatment without diclofenac (0%; Bickel et al., 2013). Diclofenac is an inhibitor of SLC22A6 and ABCC4 and the increased frequency of acute kidney injury in the diclofenac-administered group may be due to inhibition of transporter-associated tenofovir renal excretion (El-Sheikh et al., 2007; Juhasz et al., 2013). However, tenofovir plasma concentrations were not measured in the study and other mechanisms may also be responsible. Further information about drug interactions with tenofovir can be found at the Liverpool drug interactions website (www.HIV-druginteractions.org).
A new prodrug of tenofovir, tenofovir alafenamide fumarate, has been developed which is able to target HIV-susceptible CD4+ cells by selective intracellular hydrolysis by enzymes expressed within these cells. This has led to a greatly reduced dose of tenofovir being required for effective treatment, as the prodrug is relatively stable in plasma (Markowitz et al., 2014; Sax et al., 2014). Tenofovir alafenamide fumarate is not transported by SLC22A6, meaning that concentrations of drug in the kidney are unlikely to be high (Bam et al., 2014). A lower dose and less propensity for concentrating in the kidney suggest that tenofovir alafenamide fumarate is a potential solution to the issues associated with tenofovir disoproxil fumarate. However, it should be noted that the toxicities associated with tenofovir alafenamide fumarate have not been fully investigates in long-term studies. Furthermore, tenofovir disoproxil fumarate is about to enter the generic drugs market, making it potentially more easily available for widespread distribution in developing countries, and the use of the drug in pre-exposure prophylaxis trials has shown continued success (Bender, 2013). For this to occur successfully, it will still be beneficial for any related renal toxicities to be predictable and preferably avoidable.
Clinically relevant renal drug interactions are rare, but drug transporters are believed to be involved in the majority of reported cases. A well-established inhibitor of anionic transporters is probenecid, which has been used to enhance the activity of penicillin by inhibiting anionic transporters (SLC22A6 and SLC22A8) in the kidney (Robbins et al., 2012). Subsequently, clinical interactions have been observed between probenecid and other drugs, where reduced renal clearance has been observed for acyclovir (↓32%), cefmetazole (↓40%), cidofovir (↓38%), fexofenadine (↓68%), and oseltamivir (↓52%), following probenecid co-administration (Laskin et al., 1982; Ko et al., 1989; Cundy et al., 1995; Hill et al., 2002; Yasui-Furukori et al., 2005). Metformin is a substrate for SLC22A2 and SLC47A1, and these transporters are believed to be involved in the observed reduction in metformin renal clearance when co-administered with cimetidine (↓27%; Somogyi et al., 1987; Tsuda et al., 2009). Digoxin is a substrate for ABCB1, and renal clearance of the drug is reduced when co-administered with ABCB1 inhibitors ritonavir (↓35%) and quinidine (↓34%; Fenster et al., 1980; De Lannoy et al., 1992; Ding et al., 2004).
There are several nephrotoxic drugs, such as didanosine (Cote et al., 2006), cidofovir (Ortiz et al., 2005), cisplatin (Goren et al., 1986) and adefovir (Izzedine et al., 2009), which cause renal failure by accumulating in proximal tubule cells. In these and other cases, targeted inhibition of cellular uptake may reduce nephrotoxicity risks. An example of this strategy is represented by probenecid (an inhibitor of SLC22A6) being used to minimize concentrations of cidofovir in proximal tubule cells (Ho et al., 2000). Prophylaxis with probenecid can be considered in patients receiving cidofovir who have a baseline creatinine serum level of more than 1.5 mg/dL (Choudhury and Ahmed, 2006).
Creatinine is an endogenous waste product of skeletal muscle metabolism and is widely used as a biomarker for renal health. Excretion of creatinine occurs predominantly through glomerular filtration, with proximal tubular secretion accounting for around 15% of total renal clearance. Creatinine is transported into proximal tubule cells by SLC22A7 with a threefold higher affinity than that seen for transport via SLC22A2 and SLC22A3, and efflux into the proximal lumen occurs via SLC47A1 and SLC47A2 by low-affinity high-capacity transport (Urakami et al., 2004; Lepist et al., 2014). Baseline serum creatinine concentration in the blood varies depending on multiple factors, as previously described by Goicoechea et al. (2008). Increase in the serum concentration of creatinine is commonly regarded as an indicator of declining renal health, although serum creatinine concentration has been suggested to poorly represent actual filtration rate (Urakami et al., 2004).
When glomerular filtration rate is low, the serum creatinine concentration and creatinine clearance rate are higher than the actual glomerular filtration rate (Urakami et al., 2004) and this is due to proximal tubule cells secreting creatinine into the tubular lumen. In this circumstance it may be necessary to measure serum creatinine concentrations alongside creatinine clearance to estimate filtration rate in the glomerulus more accurately. Estimated glomerular filtration rate can be calculated through several predictive equations, the most clinically useful being the Cockcroft–Gault and the Modification of Diet in Renal Disease (MDRD) equation (Robertshaw et al., 1989; Estrella and Fine, 2010). Both of these equations are known to have diminished precision at higher glomerular filtration rates (Estrella and Fine, 2010). The site of tenofovir toxicity is believed to be the mitochondria of proximal tubule cells and is achieved by inhibition of mitochondrial DNA polymerase γ (Pushpakom et al., 2011). This toxicity can produce both acute and chronic kidney injury and, less commonly, Fanconi syndrome defined as tubular proteinuria, aminoaciduria, phosphaturia, glycosuria, and bicarbonate wasting (Fernandez-Fernandez et al., 2011; Hall et al., 2011). The effect of tenofovir on creatinine concentration is generally reversible once the tenofovir regimen has ended, but for actual tenofovir-induced kidney tubule dysfunction this is not necessarily the case and therefore the distinction between these scenarios is essential in patients taking tenofovir disoproxil fumarate as part of HAART (Gupta et al., 2014; Solomon et al., 2014). Appropriate screening for abnormal proximal tubule function is necessary throughout a tenofovir regimen and this is achieved through calculating the retinol binding protein to creatinine ratio, a widely used reliable marker for proximal tubule damage (Bernard et al., 1987; Hall et al., 2011; Del Palacio et al., 2012).
Studies investigating the relationship between tenofovir exposure and kidney function have produced mixed results (Hall et al., 2011). Overall, tenofovir is not believed to produce glomerular toxicity (Hall et al., 2011). As creatinine is only excreted by proximal tubule cells to a small degree, a modest decline in estimated glomerular filtration rate may be observed in tubule toxicity. In the case of tenofovir, creatinine is unlikely to be an adequate indicator of renal toxicity and may provide a false positive for reduced glomerular filtration. Further investigation is required in order to elucidate the mechanism of this tenofovir/creatinine interaction.
Multiple drugs have been reported to alter estimated glomerular filtration rate with minimal evidence of actual kidney damage (Berglund et al., 1975; Van Acker et al., 1992; Lepist et al., 2014). The second generation integrase inhibitor dolutegravir and the pharmacological booster cobicistat are two examples with well-characterized mechanisms of creatinine transporter inhibition in the proximal tubule. Cobicistat inhibits SLC47A1 and dolutegravir inhibits SLC22A2, which both transport creatinine through to the proximal lumen (German et al., 2012; Koteff et al., 2013; Lepist et al., 2014).
The contribution of transporter-interaction to the apparent unreliability of creatinine as a biomarker for kidney damage necessitates further research for more appropriate biomarkers. Greater precedence has been given to the development of novel biomarkers with the aim of identifying those that can detect acute kidney injury and progression to chronic kidney damage. To avoid similar issues to those previously discussed with creatinine it is imperative that these biomarkers do not interact with kidney transporters, and this will aid successful intervention before permanent damage to the kidneys occurs. Although no consensus has yet been reached, promising novel biomarkers include cystatin C, asymmetric dimethylarginine (ADMA), neutrophil gelatinase-associated lipocalin, and KIM-1 amongst others (Table 3; Han et al., 2002; Herget-Rosenthal et al., 2004; Devarajan, 2008; Estrella and Fine, 2010; Fassett et al., 2011; Schwedhelm and Böger, 2011; de Geus et al., 2012). ADMA has a relatively low molecular weight compared to the other biomarker in Table 3, and similarly to creatinine is showing affinity for transporters involved in drug interactions. The biomarkers in Table 3 with large molecular weights are unlikely to be a substrate for drug transporters. However, transport of albumin via the megalin/cubilin system is the topic of current research, as albumin elevation in plasma has been associated with damage to proximal tubule cells (Dickson et al., 2014).
As our understanding of drug transporters improves, it is becoming clear that transporters can play an important role in disease development. Experiments with transgenic mice have shown that genetic knockdown of transporters can cause numerous kidney-related morbidities, developmental abnormalities, and even death (Table 4). Genetic associations with disease traits (in the absence of drugs) can also be useful for defining mechanisms. The genetics of hyperuricemia and gout is known to involve transporters expressed in the proximal tubule cells. In 2002, genetic variants in SLC22A12 were found to predict occurrence of gout, and this association was joined by further transporters in 2007 (SLC2A9), 2008 (ABCG2, SLC17A3, SLC17A1, SLC16A9, SLC22A11), and 2011 (SLC2A12; Reginato et al., 2012). Understanding that multiple transporters are usually involved in the movement of a drug through the proximal tubule, it can be misleading or even counterproductive to focus on individual transporters in order to discover the “major” players in the elimination of the drug for future pharmacogenetic and interaction studies. There is limited understanding of how kidney transporter expression and activity differ between men and women (Morris et al., 2003), and in special populations, such as in specific disease groups (Lalande et al., 2014), pediatrics (Shen et al., 2001) and geriatrics, and this area requires further investigation.
Despite showing a favorable toxicity profile in initial treatment, the long-term use of tenofovir disoproxil fumarate in HIV therapy is currently under question by practitioners and researchers (Fernandez-Fernandez et al., 2011). Large-scale and long-term studies are continuing to appear which suggest an association between tenofovir use and kidney damage. Despite this, tenofovir is included in first-line therapy for both treatment naive and experienced patients as it is very effective at reducing and controlling HIV replication in patients. Because of this, and due to the life-long nature of antiretroviral therapy, it is essential that a reliable strategy be developed to detect and preferably avoid tenofovir-associated kidney toxicity. It is clear from the summarized evidence that tenofovir plasma concentrations are linked to renal toxicity, and it is also clear that drug transporters, particularly those expressed in the kidney, are able to influence the clearance rate of tenofovir (Figure 1) and also interfere with the utility of creatinine clearance as a biomarker.
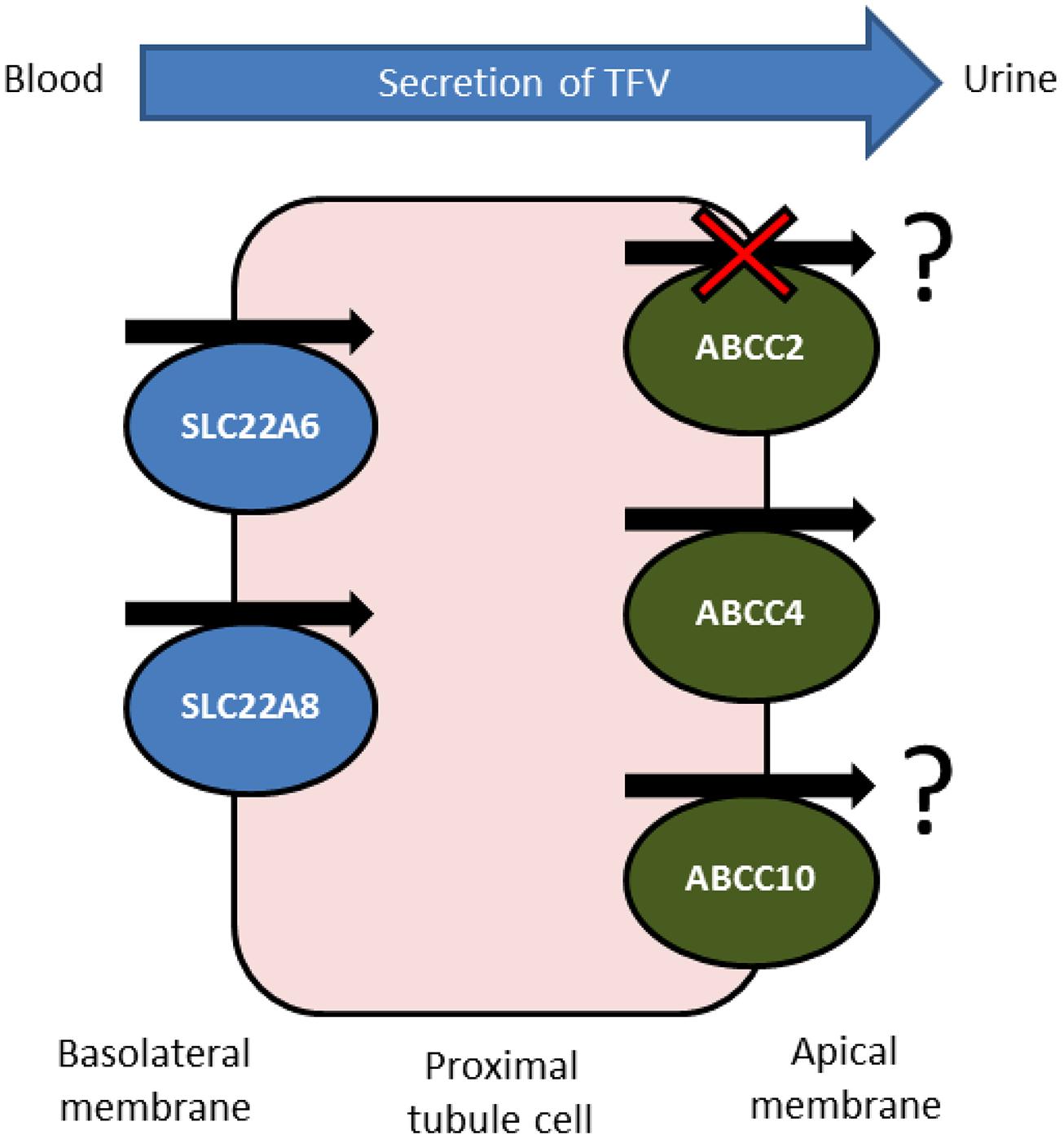
FIGURE 1. Confirmed and potential transporters involved in active tubular secretion of tenofovir into urine. Tenofovir is removed from the circulating blood and enters the proximal tubule cells by the actions of basolaterally expressed SLC22A6 and, to a lesser extent, SLC22A8. Tenofovir is then removed into the tubular lumen by apically expressed ABCC4. ABCC2 does not transport tenofovir in vitro but pharacogenetics suggests ABCC2 has a role in tenofovir-induced renal toxicity. The orientation of ABCC10 in proximal tubule cells is unknown, but in vitro and pharmacogenetic data suggest that expression may be localized to the apical membrane, facilitating tenofovir secretion.
When looked at more broadly, for the majority of drugs the potential for clinically relevant renal transporter-mediated drug interactions is low, and reported cases are limited. Renal excretion of drugs may be achieved by glomerular filtration as well as tubular secretion, and transporters are only likely to be influential in drug elimination when tubular secretion is the major pathway. Additionally, transporters in the kidney often show overlapping substrate affinity (see Table 2) and therefore the inhibition of a single transporter may not produce significant alterations in drug elimination in vivo. However, in certain cases the actions of transporters in the kidney can have clinical implications, as emphasized with tenofovir.
Despite decades of research into drug transporters, the recommendations for drug interaction studies provided by the FDA and EMA include testing strategies for only a small fraction of the total expressed transporters in the human body (Table 1) and it is unknown whether transporter-associated drug interactions in the kidney will obtain the same relevance as seen with drug metabolizing enzymes and transporters in the intestine and liver. As the investigations into tenofovir elimination have emphasized, determination of the actions of individual transporters in drug elimination from the kidney, even when found to be relevant in vitro, often may not be clinically implementable, as drugs are often substrates for several transporters. Indeed, multiple transporters and metabolism enzymes, as well as other biological and drug-specific factors, work in concert to determine the overall disposition of a drug. This should be taken into consideration in future drug development strategies with the use of improved in vitro methodologies and the introduction of predictive physiologically based in silico modeling.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by internal funding.
Bakos, E., and Homolya, L. (2007). Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1). Pflugers Arch. 453, 621–641. doi: 10.1007/s00424-006-0160-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ballabh, P., Braun, A., and Nedergaard, M. (2004). The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13. doi: 10.1016/j.nbd.2003.12.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bam, R. A., Yant, S. R., and Cihlar, T. (2014). Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir. Ther. doi: 10.3851/IMP2770 [Epub ahead of print].
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barditch-Crovo, P., Deeks, S. G., Collier, A., Safrin, S., Coakley, D. F., Miller, M.,et al. (2001). Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45, 2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barone, S., Amlal, H., Xu, J., and Soleimani, M. (2012). Deletion of the Cl-/HCO3- exchanger pendrin downregulates calcium-absorbing proteins in the kidney and causes calcium wasting. Nephrol. Dial. Transplant. 27, 1368–1379. doi: 10.1093/ndt/gfr505
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Beery, E., Rajnai, Z., Abonyi, T., Makai, I., Bansaghi, S., Erdo, F.,et al. (2011). ABCG2 modulates chlorothiazide permeability in vitro – characterization of the interaction. Drug Metab. Pharmacokinet. 27, 349–353. doi: 10.2133/dmpk.DMPK-11-NT-068
Bender, B. S. (2013). Prophylactic tenofovir reduced HIV infection in injectable drug users. Ann. Intern. Med. 159, JC8. doi: 10.7326/0003-4819-159-6-201309170-02008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Berglund, F., Killander, J., and Pompeius, R. (1975). Effect of trimethoprim-sulfamethoxazole on the renal excretion of creatinine in man. J. Urol. 114, 802–808.
Bernard, A. M., Vyskocil, A., Mahieu, P., and Lauwerys, R. (1987). Assessment of urinary retinol-binding protein as an index of proximal tubular injury. Clin. Chem. 33, 775–779.
Bhutia, Y. D., Hung, S. W., Patel, B., Lovin, D., and Govindarajan, R. (2011). CNT1 expression influences proliferation and chemosensitivity in drug-resistant pancreatic cancer cells. Cancer Res. 71, 1825–1835. doi: 10.1158/0008-5472.CAN-10-2736
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bickel, M., Khaykin, P., Stephan, C., Schmidt, K., Buettner, M., Amann, K.,et al. (2013). Acute kidney injury caused by tenofovir disoproxil fumarate and diclofenac co-administration. HIV Med. 14, 633–638. doi: 10.1111/hiv.12072
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bleasby, K., Castle, J. C., Roberts, C. J., Cheng, C., Bailey, W. J., Sina, J. F.,et al. (2006). Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica 36, 963–988. doi: 10.1080/00498250600861751
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Boffito, M., Pozniak, A., Kearney, B. P., Higgs, C., Mathias, A., Zhong, L.,et al. (2005). Lack of pharmacokinetic drug interaction between tenofovir disoproxil fumarate and nelfinavir mesylate. Antimicrob. Agents Chemother. 49, 4386–4389. doi: 10.1128/AAC.49.10.4386-4389.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Braun, D., Wirth, E. K., Wohlgemuth, F., Reix, N., Klein, M. O., Gruters, A.,et al. (2011). Aminoaciduria, but normal thyroid hormone levels and signalling, in mice lacking the amino acid and thyroid hormone transporter Slc7a8. Biochem. J. 439, 249–255. doi: 10.1042/BJ20110759
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Calza, L., Trapani, F., Salvadori, C., Magistrelli, E., Manfredi, R., Colangeli, V.,et al. (2013). Incidence of renal toxicity in HIV-infected, antiretroviral-naive patients starting tenofovir/emtricitabine associated with efavirenz, atazanavir/ritonavir, or lopinavir/ritonavir. Scand. J. Infect. Dis. 45, 147–154. doi: 10.3109/00365548.2012.712213
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Calza, L., Trapani, F., Tedeschi, S., Piergentili, B., Manfredi, R., Colangeli, V.,et al. (2011). Tenofovir-induced renal toxicity in 324 HIV-infected, antiretroviral-naive patients. Scand. J. Infect. Dis. 43, 656–660. doi: 10.3109/00365548.2011.572906
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cha, S. H., Sekine, T., Kusuhara, H., Yu, E., Kim, J. Y., Kim, D. K.,et al. (2000). Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J. Biol. Chem. 275, 4507–4512. doi: 10.1074/jbc.275.6.4507
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, Y., Zhang, S., Sorani, M., and Giacomini, K. M. (2007). Transport of paraquat by human organic cation transporters and multidrug and toxic compound extrusion family. J. Pharmacol. Exp. Ther. 322, 695–700. doi: 10.1124/jpet.107.123554
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, Z. S., Hopper-Borge, E., Belinsky, M. G., Shchaveleva, I., Kotova, E., and Kruh, G. D. (2003). Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10). Mol. Pharmacol. 63, 351–358. doi: 10.1124/mol.63.2.351
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Choi, M. K., Kim, M. H., Maeng, H. J., and Song, I. S. (2014). Contribution of CNT1 and ENT1 to ribavirin uptake in human hepatocytes. Arch. Pharm. Res. doi: 10.1007/s12272-014-0437-y [Epub ahead of print].
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Choudhury, D., and Ahmed, Z. (2006). Drug-associated renal dysfunction and injury. Nat. Clin. Pract. Nephrol. 2, 80–91. doi: 10.1038/ncpneph0076
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Christiansen-Weber, T. A., Voland, J. R., Wu, Y., Ngo, K., Roland, B. L., Nguyen, S.,et al. (2000). Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am. J. Pathol. 157, 1017–1029. doi: 10.1016/S0002-9440(10)64614-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ciarimboli, G., Lancaster, C. S., Schlatter, E., Franke, R. M., Sprowl, J. A., Pavenstadt, H.,et al. (2012). Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin. Cancer Res. 18, 1101–1108. doi: 10.1158/1078-0432.CCR-11-2503
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cihlar, T., Ray, A. S., Laflamme, G., Vela, J. E., Tong, L., Fuller, M. D.,et al. (2007). Molecular assessment of the potential for renal drug interactions between tenofovir and HIV protease inhibitors. Antivir. Ther. 12, 267–272.
Cooper, R. D., Wiebe, N., Smith, N., Keiser, P., Naicker, S., and Tonelli, M. (2010). Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin. Infect. Dis. 51, 496–505. doi: 10.1086/655681
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cote, H. C., Magil, A. B., Harris, M., Scarth, B. J., Gadawski, I., Wang, N.,et al. (2006). Exploring mitochondrial nephrotoxicity as a potential mechanism of kidney dysfunction among HIV-infected patients on highly active antiretroviral therapy. Antivir. Ther. 11, 79–86.
Cundy, K. C., Petty, B. G., Flaherty, J., Fisher, P. E., Polis, M. A., Wachsman, M.,et al. (1995). Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 39, 1247–1252. doi: 10.1128/AAC.39.6.1247
Daniel, H., and Kottra, G. (2004). The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 447, 610–618. doi: 10.1007/s00424-003-1101-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dawson, P. A., Beck, L., and Markovich, D. (2003). Hyposulfatemia, growth retardation, reduced fertility, and seizures in mice lacking a functional NaSi-1 gene. Proc. Natl. Acad. Sci. U.S.A. 100, 13704–13709. doi: 10.1073/pnas.2231298100
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dawson, P. A., Russell, C. S., Lee, S., Mcleay, S. C., Van Dongen, J. M., Cowley, D. M.,et al. (2010). Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J. Clin. Invest. 120, 706–712. doi: 10.1172/JCI31474
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
de Geus, H. R., Betjes, M. G., and Bakker, J. (2012). Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin. Kidney J. 5, 102–108. doi: 10.1093/ckj/sfs008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De Lannoy, I. A., Koren, G., Klein, J., Charuk, J., and Silverman, M. (1992). Cyclosporin and quinidine inhibition of renal digoxin excretion: evidence for luminal secretion of digoxin. Am. J. Physiol. 263, F613–F622.
Deeley, R. G., Westlake, C., and Cole, S. P. (2006). Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev. 86, 849–899. doi: 10.1152/physrev.00035.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
DeGorter, M. K., Xia, C. Q., Yang, J. J., and Kim, R. B. (2012). Drug transporters in drug efficacy and toxicity. Annu. Rev. Pharmacol. Toxicol. 52, 249–273. doi: 10.1146/annurev-pharmtox-010611-134529
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Del Palacio, M., Romero, S., and Casado, J. L. (2012). Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. 14, 179–187.
Devarajan, P. (2008). Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand. J. Clin. Lab. Invest. 68, 89–94. doi: 10.1080/00365510802150158
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dickson, L. E., Wagner, M. C., Sandoval, R. M., and Molitoris, B. A. (2014). The proximal tubule and albuminuria: really! J. Am. Soc. Nephrol. 25, 443–453. doi: 10.1681/ASN.2013090950
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ding, R., Tayrouz, Y., Riedel, K. D., Burhenne, J., Weiss, J., Mikus, G.,et al. (2004). Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin. Pharmacol. Ther. 76, 73–84. doi: 10.1016/j.clpt.2004.02.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dresser, M. J., Leabman, M. K., and Giacomini, K. M. (2001). Transporters involved in the elimination of drugs in the kidney: organic anion transporters and organic cation transporters. J. Pharm. Sci. 90, 397–421. doi: 10.1002/1520-6017(200104)90:4<397::AID-JPS1000>3.0.CO;2-D
El-Sheikh, A. A., Van Den Heuvel, J. J., Koenderink, J. B., and Russel, F. G. (2007). Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J. Pharmacol. Exp. Ther. 320, 229–235. doi: 10.1124/jpet.106.110379
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Endres, C. J., Moss, A. M., Ke, B., Govindarajan, R., Choi, D. S., Messing, R. O.,et al. (2009). The role of the equilibrative nucleoside transporter 1 (ENT1) in transport and metabolism of ribavirin by human and wild-type or Ent1–/– mouse erythrocytes. J. Pharmacol. Exp. Ther. 329, 387–398. doi: 10.1124/jpet.108.145854
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Enomoto, A., Kimura, H., Chairoungdua, A., Shigeta, Y., Jutabha, P., Cha, S. H.,et al. (2002). Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417, 447–452. doi: 10.1038/nature742
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eraly, S. A., Vallon, V., Rieg, T., Gangoiti, J. A., Wikoff, W. R., Siuzdak, G.,et al. (2008). Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol. Genomics 33, 180–192. doi: 10.1152/physiolgenomics.00207.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eraly, S. A., Vallon, V., Vaughn, D. A., Gangoiti, J. A., Richter, K., Nagle, M.,et al. (2006). Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J. Biol. Chem. 281, 5072–5083. doi: 10.1074/jbc.M508050200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Estrella, M. M., and Fine, D. M. (2010). Screening for chronic kidney disease in HIV-infected patients. Adv. Chronic Kidney Dis. 17, 26–35. doi: 10.1053/j.ackd.2009.07.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Estudante, M., Morais, J. G., Soveral, G., and Benet, L. Z. (2013). Intestinal drug transporters: an overview. Adv. Drug Deliv. Rev. 65, 1340–1356. doi: 10.1016/j.addr.2012.09.042
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Faber, K. N., Muller, M., and Jansen, P. L. (2003). Drug transport proteins in the liver. Adv. Drug Deliv. Rev. 55, 107–124. doi: 10.1016/S0169-409X(02)00173-4
Fassett, R. G., Venuthurupalli, S. K., Gobe, G. C., Coombes, J. S., Cooper, M. A., and Hoy, W. E. (2011). Biomarkers in chronic kidney disease: a review. Kidney Int. 80, 806–821. doi: 10.1038/ki.2011.198
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Feliubadalo, L., Arbones, M. L., Manas, S., Chillaron, J., Visa, J., Rodes, M.,et al. (2003). Slc7a9-deficient mice develop cystinuria non-I and cystine urolithiasis. Hum. Mol. Genet. 12, 2097–2108. doi: 10.1093/Hmg/Ddg228
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fenster, P. E., Powell, J. R., Graves, P. E., Conrad, K. A., Hager, W. D., Goldman, S.,et al. (1980). Digitoxin-quinidine interaction: pharmacokinetic evaluation. Ann. Intern. Med. 93, 698–701. doi: 10.7326/0003-4819-93-5-698
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fenton, R. A., Chou, C. L., Stewart, G. S., Smith, C. P., and Knepper, M. A. (2004). Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc. Natl. Acad. Sci. U.S.A. 101, 7469–7474. doi: 10.1073/pnas.0401704101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fernandez-Fernandez, B., Montoya-Ferrer, A., Sanz, A. B., Sanchez-Nino, M. D., Izquierdo, M. C., Poveda, J.,et al. (2011). Tenofovir nephrotoxicity: 2011 update. AIDS Res. Treat. 2011:354908. doi: 10.1155/2011/354908
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ford, J., Khoo, S. H., and Back, D. J. (2004). The intracellular pharmacology of antiretroviral protease inhibitors. J. Antimicrob. Chemother. 54, 982–990. doi: 10.1093/jac/dkh487
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ganapathy, M. E., Brandsch, M., Prasad, P. D., Ganapathy, V., and Leibach, F. H. (1995). Differential recognition of beta-lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J. Biol. Chem. 270, 25672–25677. doi: 10.1074/jbc.270.43.25672
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ganapathy, M. E., Huang, W., Wang, H., Ganapathy, V., and Leibach, F. H. (1998). Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem. Biophys. Res. Commun. 246, 470–475. doi: 10.1006/bbrc.1998.8628
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
German, P., Liu, H. C., Szwarcberg, J., Hepner, M., Andrews, J., Kearney, B. P.,et al. (2012). Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J. Acquir. Immune Defic. Syndr. 61, 32–40. doi: 10.1097/QAI.0b013e3182645648
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Goicoechea, M., Liu, S., Best, B., Sun, S., Jain, S., Kemper, C.,et al. (2008). Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J. Infect. Dis. 197, 102–108. doi: 10.1086/524061
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gorboulev, V., Ulzheimer, J. C., Akhoundova, A., Ulzheimer-Teuber, I., Karbach, U., Quester, S.,et al. (1997). Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 16, 871–881. doi: 10.1089/dna.1997.16.871
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Goren, M. P., Wright, R. K., and Horowitz, M. E. (1986). Cumulative renal tubular damage associated with cisplatin nephrotoxicity. Cancer Chemother. Pharmacol. 18, 69–73. doi: 10.1007/BF00253068
Grundemann, D., Liebich, G., Kiefer, N., Koster, S., and Schomig, E. (1999). Selective substrates for non-neuronal monoamine transporters. Mol. Pharmacol. 56, 1–10.
Gupta, S. K., Anderson, A. M., Ebrahimi, R., Fralich, T., Graham, H., Scharen-Guivel, V.,et al. (2014). Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case-control study of predictors and resolution in HIV-infected patients. PLoS ONE 9:e92717. doi: 10.1371/journal.pone.0092717
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hagos, Y., Stein, D., Ugele, B., Burckhardt, G., and Bahn, A. (2007). Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J. Am. Soc. Nephrol. 18, 430–439. doi: 10.1681/ASN.2006040415
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hagos, Y., and Wolff, N. A. (2010). Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins (Basel) 2, 2055–2082. doi: 10.3390/toxins2082055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hall, A. M., Hendry, B. M., Nitsch, D., and Connolly, J. O. (2011). Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am. J. Kidney Dis. 57, 773–780. doi: 10.1053/j.ajkd.2011.01.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Han, W. K., Bailly, V., Abichandani, R., Thadhani, R., and Bonventre, J. V. (2002). Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 62, 237–244. doi: 10.1046/j.1523-1755.2002.00433.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herget-Rosenthal, S., Marggraf, G., Hüsing, J., Göring, F., Pietruck, F., Janssen, O.,et al. (2004). Early detection of acute renal failure by serum cystatin C. Kidney Int. 66, 1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hill, G., Cihlar, T., Oo, C., Ho, E. S., Prior, K., Wiltshire, H.,et al. (2002). The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion-correlation of in vivo and in vitro studies. Drug Metab. Dispos. 30, 13–19. doi: 10.1124/dmd.30.1.13
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ho, E. S., Lin, D. C., Mendel, D. B., and Cihlar, T. (2000). Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J. Am. Soc. Nephrol. 11, 383–393.
Hopper-Borge, E., Xu, X., Shen, T., Shi, Z., Chen, Z. S., and Kruh, G. D. (2009). Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 69, 178–184. doi: 10.1158/0008-5472.CAN-08-1420
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Imaoka, T., Kusuhara, H., Adachi, M., Schuetz, J. D., Takeuchi, K., and Sugiyama, Y. (2007). Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol. Pharmacol. 71, 619–627. doi: 10.1124/mol.106.028233
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Izzedine, H., Hulot, J. S., Villard, E., Goyenvalle, C., Dominguez, S., Ghosn, J.,et al. (2006). Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J. Infect. Dis. 194, 1481–1491. doi: 10.1086/508546
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Izzedine, H., Kheder-Elfekih, R., Housset, P., Sarkozy, C., Brocheriou, I., and Deray, G. (2009). Adefovir dipivoxil-induced acute tubular necrosis and Fanconi syndrome in a renal transplant patient. AIDS 23, 544–545. doi: 10.1097/QAD.0b013e32832407f7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jani, M., Szabo, P., Kis, E., Molnar, E., Glavinas, H., and Krajcsi, P. (2009). Kinetic characterization of sulfasalazine transport by human ATP-binding cassette G2. Biol. Pharm. Bull. 32, 497–499. doi: 10.1248/bpb.32.497
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Johnson, D. J., Owen, A., Plant, N., Bray, P. G., and Ward, S. A. (2008). Drug-regulated expression of Plasmodium falciparum P-glycoprotein homologue 1: a putative role for nuclear receptors. Antimicrob. Agents Chemother. 52, 1438–1445. doi: 10.1128/AAC.01392-07
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jonker, J. W., Wagenaar, E., Van Eijl, S., and Schinkel, A. H. (2003). Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol. Cell. Biol. 23, 7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Juhasz, V., Beery, E., Nagy, Z., Bui, A., Molnar, E., Zolnerciks, J. K.,et al. (2013). Chlorothiazide is a substrate for the human uptake transporters OAT1 and OAT3. J. Pharm. Sci. 102, 1683–1687. doi: 10.1002/jps.23491
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kage, K., Tsukahara, S., Sugiyama, T., Asada, S., Ishikawa, E., Tsuruo, T.,et al. (2002). Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int. J. Cancer 97, 626–630. doi: 10.1002/ijc.10100
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kamal, M. A., Keep, R. F., and Smith, D. E. (2008). Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab. Pharmacokinet. 23, 236–242. doi: 10.2133/dmpk.23.236
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kelly, M. D., Gibson, A., Bartlett, H., Rowling, D., and Patten, J. (2013). Tenofovir-associated proteinuria. AIDS 27, 479–481. doi: 10.1097/QAD.0b013e32835883bf
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kimura, H., Takeda, M., Narikawa, S., Enomoto, A., Ichida, K., and Endou, H. (2002). Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J. Pharmacol. Exp. Ther. 301, 293–298. doi: 10.1124/jpet.301.1.293
Kis, O., Robillard, K., Chan, G. N., and Bendayan, R. (2010). The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol. Sci. 31, 22–35. doi: 10.1016/j.tips.2009.10.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kiser, J. J., Aquilante, C. L., Anderson, P. L., King, T. M., Carten, M. L., and Fletcher, C. V. (2008a). Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 47, 298–303. doi: 10.1097/QAI.0b013e31815e7478
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kiser, J. J., Carten, M. L., Aquilante, C. L., Anderson, P. L., Wolfe, P., King, T. M.,et al. (2008b). The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin. Pharmacol. Ther. 83, 265–272. doi: 10.1038/sj.clpt.6100269
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ko, H., Cathcart, K. S., Griffith, D. L., Peters, G. R., and Adams, W. J. (1989). Pharmacokinetics of intravenously administered cefmetazole and cefoxitin and effects of probenecid on cefmetazole elimination. Antimicrob. Agents Chemother. 33, 356–361. doi: 10.1128/AAC.33.3.356
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kobayashi, Y., Ohshiro, N., Sakai, R., Ohbayashi, M., Kohyama, N., and Yamamoto, T. (2005). Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J. Pharm. Pharmacol. 57, 573–578. doi: 10.1211/0022357055966
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kohler, J. J., Hosseini, S. H., Green, E., Abuin, A., Ludaway, T., Russ, R.,et al. (2011). Tenofovir renal proximal tubular toxicity is regulated by OAT1 and MRP4 transporters. Lab. Invest. 91, 852–858. doi: 10.1038/labinvest.2011.48
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kool, M., Van Der Linden, M., De Haas, M., Baas, F., and Borst, P. (1999a). Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 59, 175–182.
Kool, M., van Der Linden, M., de Haas, M., Scheffer, G. L., de Vree, J. M., Smith, A. J.,et al. (1999b). MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc. Natl. Acad. Sci. U.S.A. 96, 6914–6919. doi: 10.1073/pnas.96.12.6914
Koteff, J., Borland, J., Chen, S., Song, I., Peppercorn, A., Koshiba, T.,et al. (2013). A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br. J. Clin. Pharmacol. 75, 990–996. doi: 10.1111/j.1365-2125.2012.04440.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kusuhara, H., Sekine, T., Utsunomiya-Tate, N., Tsuda, M., Kojima, R., Cha, S. H.,et al. (1999). Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J. Biol. Chem. 274, 13675–13680. doi: 10.1074/jbc.274.19.13675
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lalande, L., Charpiat, B., Leboucher, G., and Tod, M. (2014). Consequences of renal failure on non-renal clearance of drugs. Clin. Pharmacokinet. 53, 521–532. doi: 10.1007/s40262-014-0146-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Laskin, O. L., De Miranda, P., King, D. H., Page, D. A., Longstreth, J. A., Rocco, L.,et al. (1982). Effects of probenecid on the pharmacokinetics and elimination of acyclovir in humans. Antimicrob. Agents Chemother. 21, 804–807. doi: 10.1128/AAC.21.5.804
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, H. W., Verlander, J. W., Bishop, J. M., Igarashi, P., Handlogten, M. E., and Weiner, I. D. (2009). Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am. J. Physiol. Renal Physiol. 296, F1364–F1375. doi: 10.1152/ajprenal.90667.2008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lepist, E.-I., Zhang, X., Hao, J., Huang, J., Kosaka, A., Birkus, G.,et al. (2014). Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int. 86, 350–357. doi: 10.1038/ki.2014.66
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leviel, F., Hubner, C. A., Houillier, P., Morla, L., El Moghrabi, S., Brideau, G.,et al. (2010). The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J. Clin. Invest. 120, 1627–1635. doi: 10.1172/JCI40145
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liang, R., Fei, Y. J., Prasad, P. D., Ramamoorthy, S., Han, H., Yang-Feng, T. L.,et al. (1995). Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J. Biol. Chem. 270, 6456–6463.
Liptrott, N. J., Pushpakom, S., Wyen, C., Fatkenheuer, G., Hoffmann, C., Mauss, S.,et al. (2012). Association of ABCC10 polymorphisms with nevirapine plasma concentrations in the German Competence Network for HIV/AIDS. Pharmacogenet. Genomics 22, 10–19. doi: 10.1097/FPC.0b013e32834dd82e
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Markowitz, M., Zolopa, A., Squires, K., Ruane, P., Coakley, D., Kearney, B.,et al. (2014). Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J. Antimicrob. Chemother. 69, 1362–1369. doi: 10.1093/jac/dkt532
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martin, P., Riley, R., Back, D. J., and Owen, A. (2008). Comparison of the induction profile for drug disposition proteins by typical nuclear receptor activators in human hepatic and intestinal cells. Br. J. Pharmacol. 153, 805–819. doi: 10.1038/sj.bjp.0707601
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martinez-Guerrero, L. J., and Wright, S. H. (2013). Substrate-dependent inhibition of human MATE1 by cationic ionic liquids. J. Pharmacol. Exp. Ther. 346, 495–503. doi: 10.1124/jpet.113.204206
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Masuda, S., Terada, T., Yonezawa, A., Tanihara, Y., Kishimoto, K., Katsura, T.,et al. (2006). Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol. 17, 2127–2135. doi: 10.1681/ASN.2006030205
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Morris, M. E., Lee, H. J., and Predko, L. M. (2003). Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol. Rev. 55, 229–240. doi: 10.1124/pr.55.2.1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Morrissey, K. M., Stocker, S. L., Wittwer, M. B., Xu, L., and Giacomini, K. M. (2013). Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 53, 503–529. doi: 10.1146/annurev-pharmtox-011112-140317
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moss, D. M., Kwan, W. S., Liptrott, N. J., Smith, D. L., Siccardi, M., Khoo, S. H.,et al. (2011). Raltegravir is a substrate for SLC22A6: a putative mechanism for the interaction between raltegravir and tenofovir. Antimicrob. Agents Chemother. 55, 879–887. doi: 10.1128/AAC.00623-10
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Motohashi, H., and Inui, K.-I. (2013). Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J. 15, 581–588. doi: 10.1208/s12248-013-9465-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nagai, K., Nagasawa, K., Koma, M., Hotta, A., and Fujimoto, S. (2006). Cytidine is a novel substrate for wild-type concentrative nucleoside transporter 2. Biochem. Biophys. Res. Commun. 347, 439–443. doi: 10.1016/j.bbrc.2006.06.103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Neumanova, Z., Cerveny, L., Ceckova, M., and Staud, F. (2014). Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta. AIDS 28, 9–17. doi: 10.1097/QAD.0000000000000112
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nies, A. T., Koepsell, H., Damme, K., and Schwab, M. (2011). Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb. Exp. Pharmacol. 201, 105–167. doi: 10.1007/978-3-642-14541-4_3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nishijima, T., Komatsu, H., Higasa, K., Takano, M., Tsuchiya, K., Hayashida, T.,et al. (2012). Single nucleotide polymorphisms in ABCC2 associate with tenofovir-induced kidney tubular dysfunction in Japanese patients with HIV-1 infection: a pharmacogenetic study. Clin. Infect. Dis. 55, 1558–1567. doi: 10.1093/cid/cis772
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ocheltree, S. M., Shen, H., Hu, Y., Keep, R. F., and Smith, D. E. (2005). Role and relevance of peptide transporter 2 (PEPT2) in the kidney and choroid plexus: in vivo studies with glycylsarcosine in wild-type and PEPT2 knockout mice. J. Pharmacol. Exp. Ther. 315, 240–247. doi: 10.1124/jpet.105.089359
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ohana, E., Shcheynikov, N., Moe, O. W., and Muallem, S. (2013). SLC26A6 and NaDC-1 transporters interact to regulate oxalate and citrate homeostasis. J. Am. Soc. Nephrol. 24, 1617–1626. doi: 10.1681/ASN.2013010080
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ohi, A., Hanabusa, E., Ueda, O., Segawa, H., Horiba, N., Kaneko, I.,et al. (2011). Inorganic phosphate homeostasis in sodium-dependent phosphate cotransporter Npt2b(+)/(–) mice. Am. J. Physiol. Renal Physiol. 301, F1105–F1113. doi: 10.1152/ajprenal.00663.2010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ohta, K. Y., Inoue, K., Hayashi, Y., and Yuasa, H. (2006). Molecular identification and functional characterization of rat multidrug and toxin extrusion type transporter 1 as an organic cation/H+ antiporter in the kidney. Drug Metab. Dispos. 34, 1868–1874. doi: 10.1124/dmd.106.010876
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ortiz, A., Justo, P., Sanz, A., Melero, R., Caramelo, C., Guerrero, M. F.,et al. (2005). Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir. Ther. 10, 185–190.
Owen, A., Chandler, B., Back, D. J., and Khoo, S. H. (2004). Expression of pregnane-X-receptor transcript in peripheral blood mononuclear cells and correlation with MDR1 mRNA. Antivir. Ther. 9, 819–821.
Preitner, F., Bonny, O., Laverriere, A., Rotman, S., Firsov, D., Da Costa, A.,et al. (2009). Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc. Natl. Acad. Sci. U.S.A. 106, 15501–15506. doi: 10.1073/pnas.0904411106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pushpakom, S. P., Liptrott, N. J., Rodriguez-Novoa, S., Labarga, P., Soriano, V., Albalater, M.,et al. (2011). Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J. Infect. Dis. 204, 145–153. doi: 10.1093/infdis/jir215
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Quan, H., Athirakul, K., Wetsel, W. C., Torres, G. E., Stevens, R., Chen, Y. T.,et al. (2004). Hypertension and impaired glycine handling in mice lacking the orphan transporter XT2. Mol. Cell. Biol. 24, 4166–4173. doi: 10.1128/MCB.24.10.4166-4173.2004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ray, A. S., Cihlar, T., Robinson, K. L., Tong, L., Vela, J. E., Fuller, M. D.,et al. (2006). Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 50, 3297–3304. doi: 10.1128/AAC.00251-06
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ray, A. S., Olson, L., and Fridland, A. (2004). Role of purine nucleoside phosphorylase in interactions between 2′,3′-dideoxyinosine and allopurinol, ganciclovir, or tenofovir. Antimicrob. Agents Chemother. 48, 1089–1095. doi: 10.1128/AAC.48.4.1089-1095.2004
Reginato, A. M., Mount, D. B., Yang, I., and Choi, H. K. (2012). The genetics of hyperuricaemia and gout. Nat. Rev. Rheumatol. 8, 610–621. doi: 10.1038/nrrheum.2012.144
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Robbins, N., Koch, S. E., Tranter, M., and Rubinstein, J. (2012). The history and future of probenecid. Cardiovasc. Toxicol. 12, 1–9. doi: 10.1007/s12012-011-9145-8
Robertshaw, M., Lai, K., and Swaminathan, R. (1989). Prediction of creatinine clearance from plasma creatinine: comparison of five formulae. Br. J. Clin. Pharmacol. 28, 275–280. doi: 10.1111/j.1365-2125.1989.tb05427.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rodriguez-Novoa, S., Alvarez, E., Labarga, P., and Soriano, V. (2010). Renal toxicity associated with tenofovir use. Expert Opin. Drug Saf. 9, 545–559. doi: 10.1517/14740331003627458
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rodriguez-Novoa, S., Labarga, P., and Soriano, V. (2009a). Pharmacogenetics of tenofovir treatment. Pharmacogenomics 10, 1675–1685. doi: 10.2217/pgs.09.115
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rodriguez-Novoa, S., Labarga, P., Soriano, V., Egan, D., Albalater, M., Morello, J.,et al. (2009b). Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin. Infect. Dis. 48, e108–e116. doi: 10.1086/598507
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sato, K., Sai, Y., Nishimura, T., Chishu, T., Shimpo, S., Kose, N.,et al. (2009). Influx mechanism of 2′,3′-dideoxyinosine and uridine at the blood-placenta barrier. Placenta 30, 263–269. doi: 10.1016/j.placenta.2008.11.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sax, P. E., Zolopa, A., Brar, I., Elion, R., Ortiz, R., Post, F.,et al. (2014). Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J. Acquir. Immune Defic. Syndr. 67, 52–58. doi: 10.1097/QAI.0000000000000225
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Scheffer, G. L., Kool, M., De Haas, M., De Vree, J. M., Pijnenborg, A. C., Bosman, D. K.,et al. (2002). Tissue distribution and induction of human multidrug resistant protein 3. Lab. Invest. 82, 193–201. doi: 10.1038/labinvest.3780411
Schipani, A., Siccardi, M., D’Avolio, A., Baietto, L., Simiele, M., Bonora, S.,et al. (2010). Population pharmacokinetic modeling of the association between 63396C- > T pregnane X receptor polymorphism and unboosted atazanavir clearance. Antimicrob. Agents Chemother. 54, 5242–5250. doi: 10.1128/AAC.00781-10
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schultheis, P. J., Clarke, L. L., Meneton, P., Miller, M. L., Soleimani, M., Gawenis, L. R.,et al. (1998). Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat. Genet. 19, 282–285. doi: 10.1038/969
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schwedhelm, E., and Böger, R. H. (2011). The role of asymmetric and symmetric dimethylarginines in renal disease. Nat. Rev. Nephrol. 7, 275–285. doi: 10.1038/nrneph.2011.31
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shafiu, M., Johnson, R. J., Turner, S. T., Langaee, T., Gong, Y., Chapman, A. B.,et al. (2012). Urate transporter gene SLC22A12 polymorphisms associated with obesity and metabolic syndrome in Caucasians with hypertension. Kidney Blood Press. Res. 35, 477–482. doi: 10.1159/000337370
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shen, H., Smith, D. E., and Brosius, F. C. III. (2001). Developmental expression of PEPT1 and PEPT2 in rat small intestine, colon, and kidney. Pediatr. Res. 49, 789–795. doi: 10.1203/00006450-200106000-00013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shu, C., Shen, H., Hopfer, U., and Smith, D. E. (2001). Mechanism of intestinal absorption and renal reabsorption of an orally active ace inhibitor: uptake and transport of fosinopril in cell cultures. Drug Metab. Dispos. 29, 1307–1315.
Siccardi, M., D’Avolio, A., Baietto, L., Gibbons, S., Sciandra, M., Colucci, D.,et al. (2008). Association of a single-nucleotide polymorphism in the pregnane X receptor (PXR 63396C– > T) with reduced concentrations of unboosted atazanavir. Clin. Infect. Dis. 47, 1222–1225. doi: 10.1086/592304
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smeets, P. H., Van Aubel, R. A., Wouterse, A. C., Van Den Heuvel, J. J., and Russel, F. G. (2004). Contribution of multidrug resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J. Am. Soc. Nephrol. 15, 2828–2835. doi: 10.1097/01.ASN.0000143473.64430.AC
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Solomon, M. M., Lama, J. R., Glidden, D. V., Mulligan, K., Mcmahan, V., Liu, A. Y.,et al. (2014). Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS 28, 851–859. doi: 10.1097/QAD.0000000000000156
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Somogyi, A., Stockley, C., Keal, J., Rolan, P., and Bochner, F. (1987). Reduction of metformin renal tubular secretion by cimetidine in man. Br. J. Clin. Pharmacol. 23, 545–551. doi: 10.1111/j.1365-2125.1987.tb03090.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sun, Y. L., Chen, J. J., Kumar, P., Chen, K., Sodani, K., Patel, A.,et al. (2013). Reversal of MRP7 (ABCC10)-mediated multidrug resistance by tariquidar. PLoS ONE 8:e55576. doi: 10.1371/journal.pone.0055576
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sun, Y. L., Kumar, P., Sodani, K., Patel, A., Pan, Y., Baer, M. R.,et al. (2014). Ponatinib enhances anticancer drug sensitivity in MRP7-overexpressing cells. Oncol. Rep. 31, 1605–1612. doi: 10.3892/or.2014.3002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tanigawara, Y. (2000). Role of P-glycoprotein in drug disposition. Ther. Drug Monit. 22, 137–140. doi: 10.1097/00007691-200002000-00029
Tanihara, Y., Masuda, S., Sato, T., Katsura, T., Ogawa, O., and Inui, K. (2007). Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem. Pharmacol. 74, 359–371. doi: 10.1016/j.bcp.2007.04.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thangaraju, M., Ananth, S., Martin, P. M., Roon, P., Smith, S. B., Sterneck, E.,et al. (2006). c/ebp delta null mouse as a model for the double knock-out of slc5a8 and slc5a12 in kidney. J. Biol. Chem. 281, 26769–26773. doi: 10.1074/jbc.C600189200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tong, L., Phan, T. K., Robinson, K. L., Babusis, D., Strab, R., Bhoopathy, S.,et al. (2007). Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob. Agents Chemother. 51, 3498–3504. doi: 10.1128/AAC.00671-07
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Trajkovic-Arsic, M., Visser, T. J., Darras, V. M., Friesema, E. C., Schlott, B., Mittag, J.,et al. (2010). Consequences of monocarboxylate transporter 8 deficiency for renal transport and metabolism of thyroid hormones in mice. Endocrinology 151, 802–809. doi: 10.1210/en.2009-1053
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tramonti, G., Xie, P., Wallner, E. I., Danesh, F. R., and Kanwar, Y. S. (2006). Expression and functional characteristics of tubular transporters: P-glycoprotein, PEPT1, and PEPT2 in renal mass reduction and diabetes. Am. J. Physiol. Renal Physiol. 291, F972–F980. doi: 10.1152/ajprenal.00110.2006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tsuda, M., Terada, T., Ueba, M., Sato, T., Masuda, S., Katsura, T.,et al. (2009). Involvement of human multidrug and toxin extrusion 1 in the drug interaction between cimetidine and metformin in renal epithelial cells. J. Pharmacol. Exp. Ther. 329, 185–191. doi: 10.1124/jpet.108.147918
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tzvetkov, M. V., Dos Santos Pereira, J. N., Meineke, I., Saadatmand, A. R., Stingl, J. C., and Brockmoller, J. (2013). Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochem. Pharmacol. 86, 666–678. doi: 10.1016/j.bcp.2013.06.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Urakami, Y., Kimura, N., Okuda, M., and Inui, K.-I. (2004). Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm. Res. 21, 976–981. doi: 10.1023/B:PHAM.0000029286.45788.ad
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Uwai, Y., Ida, H., Tsuji, Y., Katsura, T., and Inui, K. (2007). Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm. Res. 24, 811–815. doi: 10.1007/s11095-006-9196-x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vallon, V., Platt, K. A., Cunard, R., Schroth, J., Whaley, J., Thomson, S. C.,et al. (2011). SGLT2 mediates glucose reabsorption in the early proximal tubule. J. Am. Soc. Nephrol. 22, 104–112. doi: 10.1681/ASN.2010030246
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Acker, B., Koopman, M., Arisz, L., Koomen, G., and De Waart, D. (1992). Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 340, 1326–1329. doi: 10.1016/0140-6736(92)92502-7
van Gelder, J., Deferme, S., Naesens, L., De Clercq, E., Van Den Mooter, G., Kinget, R.,et al. (2002). Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug Metab. Dispos. 30, 924–930. doi: 10.1124/dmd.30.8.924
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vitart, V., Rudan, I., Hayward, C., Gray, N. K., Floyd, J., Palmer, C. N.,et al. (2008). SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 40, 437–442. doi: 10.1038/ng.106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wenning, L. A., Friedman, E. J., Kost, J. T., Breidinger, S. A., Stek, J. E., Lasseter, K. C.,et al. (2008). Lack of a significant drug interaction between raltegravir and tenofovir. Antimicrob. Agents Chemother. 52, 3253–3258. doi: 10.1128/AAC.00005-08
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Woodward, O. M., Kottgen, A., Coresh, J., Boerwinkle, E., Guggino, W. B., and Kottgen, M. (2009). Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. U.S.A. 106, 10338–10342. doi: 10.1073/pnas.0901249106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wyen, C., Hendra, H., Siccardi, M., Platten, M., Jaeger, H., Harrer, T.,et al. (2011). Cytochrome P450 2B6 (CYP2B6) and constitutive androstane receptor (CAR) polymorphisms are associated with early discontinuation of efavirenz-containing regimens. J. Antimicrob. Chemother. 66, 2092–2098. doi: 10.1093/jac/dkr272
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xu, J., Song, P., Nakamura, S., Miller, M., Barone, S., Alper, S. L.,et al. (2009). Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J. Biol. Chem. 284, 29470–29479. doi: 10.1074/jbc.M109.044396
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yamaguchi, H., Sugie, M., Okada, M., Mikkaichi, T., Toyohara, T., Abe, T.,et al. (2010). Transport of estrone 3-sulfate mediated by organic anion transporter OATP4C1: estrone 3-sulfate binds to the different recognition site for digoxin in OATP4C1. Drug Metab. Pharmacokinet. 25, 314–317. doi: 10.2133/dmpk.25.314
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yasui-Furukori, N., Uno, T., Sugawara, K., and Tateishi, T. (2005). Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. Clin. Pharmacol. Ther. 77, 17–23. doi: 10.1016/j.clpt.2004.08.026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Young, J., Schafer, J., Fux, C. A., Furrer, H., Bernasconi, E., Vernazza, P.,et al. (2012). Renal function in patients with HIV starting therapy with tenofovir and either efavirenz, lopinavir or atazanavir. AIDS 26, 567–575. doi: 10.1097/QAD.0b013e32834f337c
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhou, S. F., Wang, L. L., Di, Y. M., Xue, C. C., Duan, W., Li, C. G.,et al. (2008). Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Med. Chem. 15, 1981–2039. doi: 10.2174/092986708785132870
Zhu, H. J., Appel, D. I., Grundemann, D., and Markowitz, J. S. (2010). Interaction of organic cation transporter 3 (SLC22A3) and amphetamine. J. Neurochem. 114, 142–149. doi: 10.1111/j.1471-4159.2010.06738.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: tenofovir, drug transporters, pharmacokinetics, kidney, toxicity
Citation: Moss DM, Neary M and Owen A (2014) The role of drug transporters in the kidney: lessons from tenofovir. Front. Pharmacol. 5:248. doi: 10.3389/fphar.2014.00248
Received: 08 September 2014; Accepted: 24 October 2014;
Published online: 11 November 2014.
Edited by:
George Tegos, University of New Mexico, USAReviewed by:
Olaf Grisk, University of Greifswald, GermanyCopyright © 2014 Moss, Neary and Owen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew Owen, Department of Molecular and Clinical Pharmacology, University of Liverpool, 70 Pembroke Place, Liverpool L69 3GF, UK e-mail:YW93ZW5AbGl2ZXJwb29sLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.