- Department of Pathology and Biological Responses, Nagoya University Graduate School of Medicine, Nagoya, Japan
Iron is the most abundant metal in the human body and mainly works as a cofactor for proteins such as hemoglobin and various enzymes. No independent life forms on earth can survive without iron. However, excess iron is intimately associated with carcinogenesis by increasing oxidative stress via its catalytic activity to generate hydroxyl radicals. Biomolecules with redox-active sulfhydryl function(s) (thiol compounds) are necessary for the maintenance of mildly reductive cellular environments to counteract oxidative stress, and for the execution of redox reactions for metabolism and detoxification. Involvement of glutathione S-transferase and thioredoxin has long attracted the attention of cancer researchers. Here, I update recent findings on the involvement of iron and thiol compounds during carcinogenesis and in cancer cells. It is now recognized that the cystine/glutamate transporter (antiporter) is intimately associated with ferroptosis, an iron-dependent, non-apoptotic form of cell death, observed in cancer cells, and also with cancer stem cells; the former with transporter blockage but the latter with its stabilization. Excess iron in the presence of oxygen appears the most common known mutagen. Ironically, the persistent activation of antioxidant systems via genetic alterations in Nrf2 and Keap1 also contributes to carcinogenesis. Therefore, it is difficult to conclude the role of iron and thiol compounds as friends or foes, which depends on the quantity/distribution and induction/flexibility, respectively. Avoiding further mutation would be the most helpful strategy for cancer prevention, and myriad of efforts are being made to sort out the weaknesses of cancer cells.
Introduction
During the past 6–7 decades following World War II, the average human lifespan has been enormously extended from less than 50 years to nearly or more than 80 years in most developed countries (https://www.cia.gov/library/publications/the-world-factbook/rankorder/2102rank.html). This has been achieved at least in part by the discovery of antibiotics against bacterial infections such as tuberculosis, which had been continuously present deadly diseases until that period (Zhang, 2005). After the major human conquest over bacterial diseases, two pathologic conditions, atherosclerosis and cancer, have become the most common causes of human mortality. Atherosclerosis via the thickening of the arterial intima and rupture of atheromatous plaques causes myocardial and cerebral infarctions, which either kill the patients or dramatically decrease their quality of lives (Beckman et al., 2002). Atherosclerosis is, in a sense, an “analog” disease in that apparently nobody can escape from it (Kumar et al., 2013); rather, the speed of disease progression is much different among individuals, which is revealed with mathematical models (Hao and Friedman, 2014) (Figure 1). In contrast, cancer is a “digital” disease, which means that it consists of stepwise processes and is all or nothing for the generation of a malignant tumor (Weinberg, 2013) (Figure 1). Some patients develop secondary or even tertiary cancers after successful cancer treatments (Travis et al., 2013). In this review, I will focus on the recent advancements in the understanding of cancer regarding iron and thiol compounds that appears independent but are interdependent in many aspects.
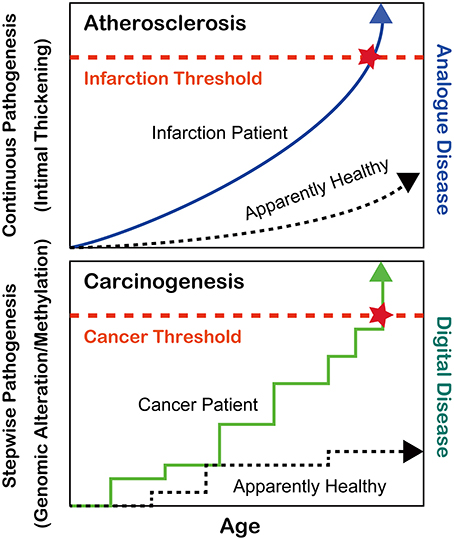
Figure 1. Differences between atherosclerosis and carcinogenesis as oxidative stress-induced diseases.
Cancer as a Genomic Disease
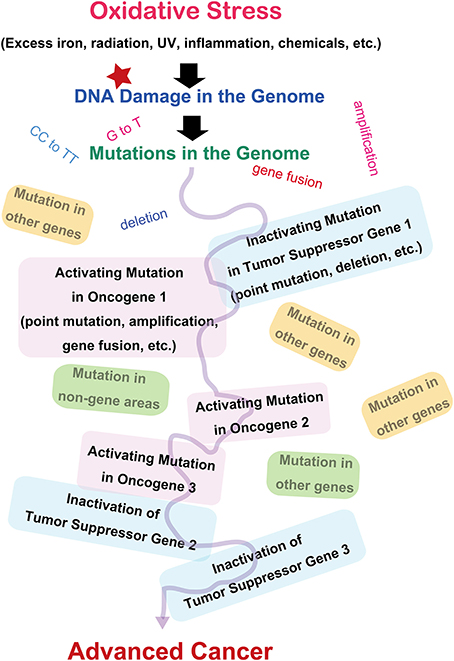
Since the discovery of oncogenes, decades of studies have revealed that cancer is basically a disease of the genomic alteration (Weinberg, 2013). Alteration of genome information after genomic damage and its inadequate repair is responsible for cancer development, and the alterations should occur in specific genes designated as oncogenes or tumor suppressor genes (Figure 2). There are more than 100 oncogenes identified thus far, and all of these genes are associated with cellular proliferation (Weinberg, 2013). Oncogene activation is the result of specific mutations in genes, leading to persistent activation of cellular signals toward proliferation. The genetic alterations include point mutations, gene amplifications, or gene fusions (Stratton et al., 2009; Pleasance et al., 2010). Tumor suppressor genes work as guardians of the genome by arresting the cell cycle, repairing the genome, and even inducing apoptosis after unrepairable excessive genomic injury occurs. These genes are inactivated during the carcinogenic process. It is now recognized that several (e.g., 5–8) independent or interdependent events of mixed activation of oncogenes and inactivation of tumor suppressor genes are necessary to generate a malignant tumor (Hanahan and Weinberg, 2000). Certain genetic alterations work as an instigator, facilitating the occurrence of sequential independent alterations at a significantly higher incidence (mutator phenotype) (Loeb, 2001). This is especially true of repair genes for the genome. Both alleles have to be disrupted to inactivate tumor suppressor genes. In addition, not only genetic alterations but also epigenetic changes (methylation of the promoter region) are important in halting the expression of specific genes (Feinberg et al., 2006).
As an exception, epigenetic alterations may lead to a malignant tumor in childhood cancer (Esteller and Herman, 2002). Recently, it was shown that doxycycline-controlled reprogrammable transgenic mice overexpressing the four Yamanaka factors (Oct3/4, Sox2, Klf4, and c-Myc) for induced pluripotent stem cell generation develop several cancers similar to childhood blastoma-type cancer that do not revert to normal and continue dysplastic growth, even after switching off those genes. Surprisingly, these cancers reportedly do not have major alterations of the genes, suggesting the importance of epigenetic mechanisms as well (Ohnishi et al., 2014). However, it was recently determined by next generation sequencing that genes regulating epigenetic mechanisms are one of the major targets of carcinogenesis in certain cancers such as leukemia and breast carcinoma (Smith et al., 2010; Dawson and Kouzarides, 2012). Thus, in most cases, genetic alterations regulate epigenetic mechanisms.
Iron as a Risk of Cancer
Iron is the most abundant metal in the human body. Approximately 4 g is present in normal adult humans. Thus, far, no life on earth can live without iron. Simultaneously, most of higher organisms, including humans, cannot survive without oxygen for 5 min. Oxygen is transported throughout the body by the heme moiety of hemoglobin, which contains as much as ~60% of the total iron in the body (Wriggleworth and Baum, 1980; Toyokuni, 2011a). Thus, there is a natural affinity between iron and oxygen. The most important characteristic of molecular oxygen is that it is easy to be reduced via one or more of four one-electron transfer processes, ending with the formation of water (Figure 3). During this process, superoxide, hydrogen peroxide, and hydroxyl radicals may be generated as intermediates either via enzymatic or chemical reactions. Hydroxyl radicals are most reactive among chemical species of the biological system. Fortunately, unfavorable reactions of this kind is usually prevented via antioxidative mechanisms (Halliwell and Gutteridge, 2007; Toyokuni, 2011b).
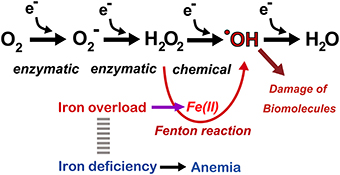
Figure 3. Oxygen as a medium for electron flow and the associated role of catalytic ferrous iron (Fe[II]) toward Fenton reaction. Note that only a small fraction generates hydroxyl radicals (·OH). Refer to text and Figure 7.
Whereas iron is an essential component, stable Fe[III] is hardly soluble at neutral pH (10−17 M) (Lippard and Berg, 1994). Therefore, precise and overlapping regulatory mechanisms exist in iron metabolism, and thus, only subtle amounts of catalytic iron are present in normal physiological conditions. Pathologic conditions, such as failure of iron regulatory sensors, repeated hemorrhage and chronic inflammation concomitant with continued parenchymal cell death, result in iron overload in the corresponding locations (Ganz, 2003), leading to oxidative damage through the Fenton reaction (Minotti and Aust, 1989; Miller et al., 1990; Halliwell and Gutteridge, 2007). Hydroxyl radicals are the most reactive species in the biological system, and the Fenton reaction in vivo appears to occur in the presence of catalytic ferrous iron (Samuni et al., 1983; Toyokuni, 2009), leading to the extensive scission, modification (Dizdaroglu, 1991) and cross-linking of biomolecules (Dizdaroglu, 1991). Such oxidative molecules eventually induce genomic alterations, increasing the risk of carcinogenesis. Recently, prompt formation of mono- or poly-iron FeIV = O (ferryl) species was suggested at the aqueous interface (Enami et al., 2014).
Indeed, iron overload has been associated with carcinogenesis both in human and animal experiments. It is well known that there is no active excretion pathway for iron except for hemorrhage, presumably due to the supreme biological importance of iron. Intriguingly, a US epidemiological study published in 2008 reported that for peripheral arterial disease patients, phlebotomy twice a year reduced the incidence of visceral cancer by 35% and the cancer mortality by 61% in a randomized trial involving 1277 patients. In this study, iron reduction did not stop the progression of atherosclerosis, as originally intended, but did unexpectedly prevent carcinogenesis, including that of most common cancers (lung, colon, prostate, etc.) (Zacharski et al., 2007).
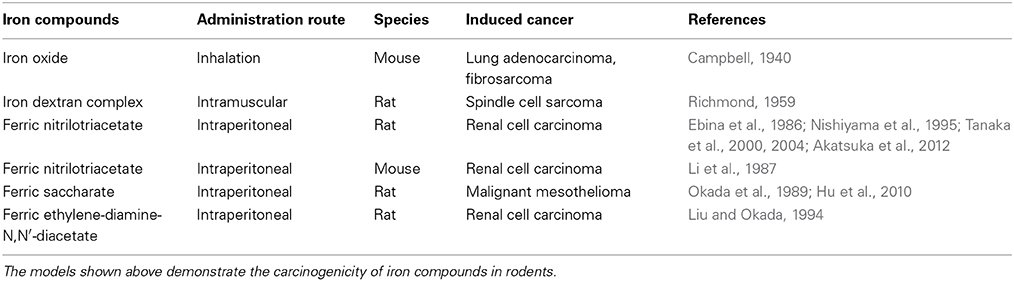
There are several review articles published on the direct demonstration of iron overload and carcinogenesis in animal experiments (Toyokuni, 1996, 2002, 2009; Beguin et al., 2014), which I summarize as Table 1. Now, the role of iron in human carcinogenesis remains under intensive discussion (Cho et al., 2013; Fonseca-Nunes et al., 2014). Here, I discuss the recent results from a genuine Fenton reaction-induced carcinogenesis model generated by intraperitoneal injections of an iron chelate, ferric nitrilotriacetate (Fe-NTA), to rodents (Ebina et al., 1986; Li et al., 1987). Nitrilotriacetate (NTA) has been used as a component of detergents in Canada because of its potent chelating activity with a variety of metals, including iron (Anderson et al., 1982). First, Fe-NTA was used to load Fe[III] to transferrin, a serum iron transporting protein, in biochemistry laboratories (Pootrakul et al., 1977). Then, it was used by Awai et al. to generate an animal model of hemochromatosis (Awai et al., 1979). Of note, Okada and Midorikawa found that Fe-NTA induces renal cell carcinoma (RCC) after repeated intraperitoneal administration in wild-type rats (Figure 4) that were accidentally under observation for more than 1 year after the confirmation of iron accumulation in the liver (Okada and Midorikawa, 1982). Fe-NTA is soluble at neutral pH and is the most potent catalyst thus far of Fenton reaction with 3–4 free iron ligands (Toyokuni and Sagripanti, 1992, 1993).
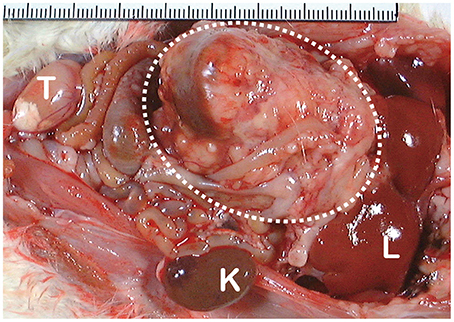
Figure 4. Macroscopic appearance of ferric nitrilotriacetate (Fe-NTA)-induced renal cell carcinoma (interrupted circle; tumor diameter is more than 40 mm). K, normal kidney of the opposite side; L, liver; T, testis.
After intraperitoneal injection, Fe-NTA is absorbed through the peritoneum into the portal vein and then enters the systemic blood flow. Thereafter, Fe-NTA is filtered through the glomeruli into the lumina of the renal proximal tubules, where Fe(III)-NTA is reduced to Fe(II)-NTA, presumably by the presence of L-cysteine (Okada et al., 1993; Okada, 1996) (Figure 5). The Fenton reaction indeed occurs in vivo in rats and mice because a variety of modified products are demonstrated in this model, including 4-hydroxy-2-nonenal (HNE) (Toyokuni et al., 1997a), HNE-modified proteins (Toyokuni et al., 1994b; Fukuda et al., 1996b), other lipid peroxidation products (Toyokuni et al., 1990; Uchida et al., 1995), 8-oxoguanine (Toyokuni et al., 1994a, 1997b), thymine-tyrosine cross-links (Toyokuni et al., 1995a) and other oxidative DNA base modifications (Toyokuni et al., 1994a). DNA single- and double-stranded breaks have also been shown in vitro (Toyokuni and Sagripanti, 1993, 1996).
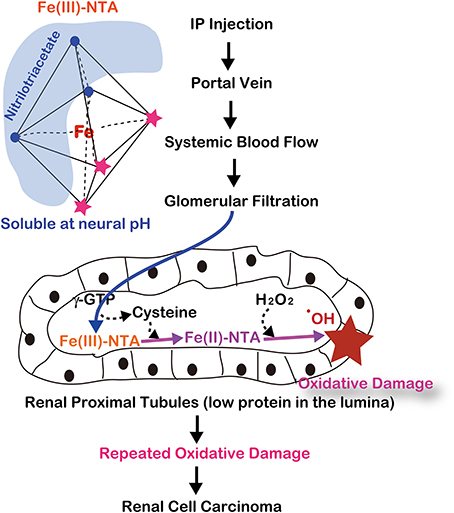
Figure 5. Molecular mechanism of ferric nitrilotriacetate (Fe-NTA)-induced oxidative damage of renal proximal tubules after a single intraperitoneal injection. This depends on two distinct characteristics of renal proximal tubular lumina: paucity of antioxidattive proteins such as albumin and reductive environment through the presence of L-cysteine with GSH cycles.
Recently, we demonstrated an abundance of Fe(II) in the lumina of renal proximal tubules after Fe-NTA injection (Mukaide et al., 2014) with a novel fluorescent probe (RhoNox-1) specific for catalytic ferrous iron (Hirayama et al., 2013). Furthermore, we showed that the induced RCCs have massive chromosomal alterations similar to those of human cancers, of which the amplification of c-Met (receptor for hepatocyte growth factor) and deletion of Cdkn2a/2b (p16Ink4a/p15Ink4b tumor suppressor genes) are most common (Tanaka et al., 1999; Akatsuka et al., 2012). The latter occurs early in carcinogenesis (Hiroyasu et al., 2002). Considering that it is rare thus far to find massive chromosomal alterations in any other carcinogenesis model using wild-type animals, we believe that iron overload is one of the most important risk factors in human carcinogenesis as well. Of note, asbestos- (Jiang et al., 2012) and multi-walled carbon nanotube-induced (Nagai et al., 2011) mesothelial carcinogenesis in wild-type rats are the models that confer massive chromosomal alterations including homozygous deletion of Cdkn2a/2b. We believe that these are through local iron overload (Toyokuni, 2013b).
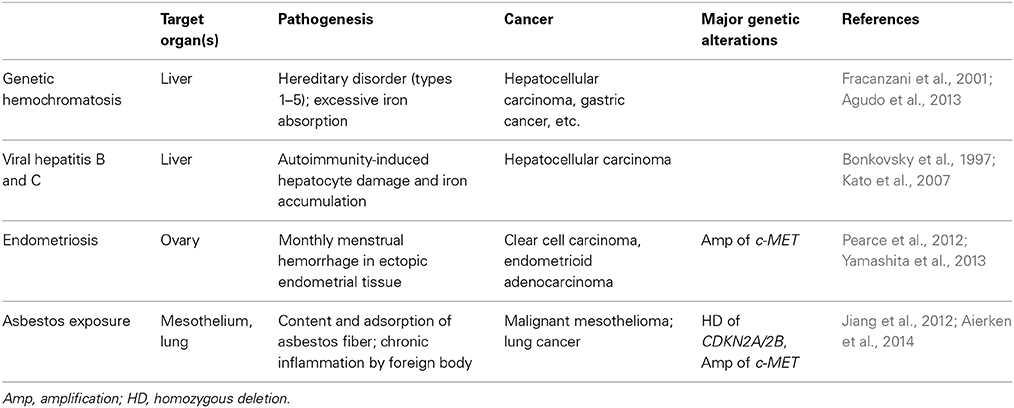
There are several distinct human diseases that preclude cancer via iron overload (Table 2). Endometriosis is defined by the presence of endometrial tissue outside of the uterine cavity and occurs in as many as 10% of women in their reproductive years. An epidemiological study revealed that ovarian endometriosis is associated with a high risk for clear cell carcinoma (Pearce et al., 2012). Because monthly menstrual hemorrhage occurs in these ectopic tissues, local iron overload is generated in situ (Yamaguchi et al., 2008). We recently studied endometriosis-associated ovarian clear cell carcinoma with an array-based comparative genome hybridization and found that c-Met (the same target gene as those of Fe-NTA-induced RCCs) is the most frequently amplified gene (Yamashita et al., 2013). Recently, it was shown with the use of knockout mice for Cdkn2a/2b and/or Pten that DNA double-stranded breaks cooperate with the loss of Ink4 and Arf (protein products from Cdkn2a/2b after alternative splicing) tumor suppressors to generate glioblastomas with frequent c-Met amplification (Camacho et al., 2014). It is remarkable that all of the necessary genomic alterations occurred in the Fe-NTA-induced RCC model of wild-type animals.
Together, these results indicate that the Fenton reaction can induce deletion/amplification mutations in target genes during carcinogenesis, presumably through DNA double-stranded breaks (Figure 6). Currently, next-generation sequencing studies are in progress for the above-mentioned tumors and may help determine whether iron overload can induce driver point mutations and create fusion genes through chromosomal translocations.
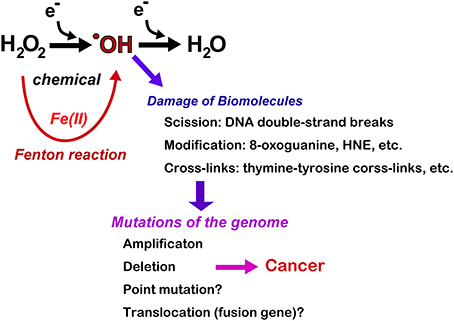
Figure 6. Consequences of Fenton reaction in the genome DNA during carcinogenesis. HNE, 4-hydroxy-2-nonenal.
Regulation of Hydrogen Peroxide via Thiol Compounds
Here I discuss the other partner of Fenton reaction, hydrogen peroxide. Hydrogen peroxide, a non-radical species, is a normal metabolite occurring at an approximately 10 nM intracellular concentration. Its increase in concentration may initiate Fenton reaction in the presence of catalytic Fe[II]. In the liver, which has one of the highest metabolic activities in the body, hydrogen peroxide is produced at 50 nmol/min/g tissue (Sies, 2014). In the cells and in tissues with iron overload, the regulation of the concentration and localization of hydrogen peroxide is a critical issue. It is now widely accepted that hydrogen peroxide is utilized in metabolic regulation in ways similar to diffusible gasses such as CO, NO, and H2S (Yang et al., 2008).
A major source of hydrogen peroxide comes from the dismutation of the superoxide anion radical, which is mainly generated through NAD(P)H oxidases operated under the control of growth factors and cytokines, such as interleukin-1 and tumor necrosis factor-α (Jiang et al., 2011). This mechanism is actively used for antibacterial defense in neutrophils and macrophages during inflammation (Bedard and Krause, 2007). Another major source of hydrogen peroxide resides in the physiological mitochondrial processes through Complex I, II, and III (Cadenas and Davies, 2000).
The metabolic elimination of hydrogen peroxide includes the catalytic reaction, which is performed by catalase in peroxisomes as well as by numerous peroxidases (Figure 7). In addition, in tissues, hydrogen peroxide diffuses away from its source across the plasma membrane to the extracellular space, or even to adjoining cells, occurs (Giorgio et al., 2007). Various peroxidases are under the control of metabolic signals, and the most potent peroxidase is peroxiredoxins (Rhee et al., 2005). The 106-fold higher rate constant of the reaction of hydrogen peroxide with the cysteine thiolate in peroxiredoxins using thioredoxin as a substrate in comparison to most other deprotonated thiol compounds gives them a major role in the biological chemistry of hydrogen peroxide removal (Winterbourn, 2013). However, cysteine residues of peroxiredoxins are easily hyperoxidized to cysteine sulfinic acid, resulting in the inactivation of peroxidase activity. As a result, if this occurs, there is an accumulation of hydrogen peroxide, allowing the oxidation of specific target proteins, a phenomenon that is comparable to the opening of a gate for signaling (Wood et al., 2003). This is the molecular basis for hydrogen peroxide compartmentation in signaling (Antunes and Cadenas, 2000) (Figure 7). This activation is finally shut down by sulfiredoxin, which recovers hyperoxidized peroxiredoxins (Jeong et al., 2012). Glutathione peroxidases in various distinct subcellular compartments and cells play a major function in the regulation of hydrogen peroxide and lipid peroxides (Brigelius-Flohe and Maiorino, 2013). Glutathione reductase and the efflux of oxidized glutathione (GSSG) allow the maintenance of the fraction of reduced glutathione (Sies, 1999). Thioredoxin peroxidase, a selenium-dependent enzyme, also has an important role in the elimination of hydrogen peroxide (Lu and Holmgren, 2014).
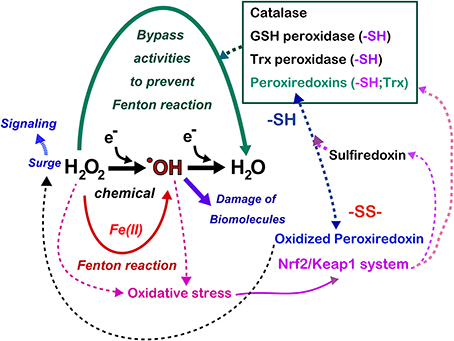
Figure 7. Antagonizing role of iron and thiol compounds. Numerous overlapping mechanisms using thiol compounds exist to decompose hydrogen peroxide to water in order to bypass the generation of hydroxyl radicals. Hydrogen peroxide is now recognized as a signaling molecule whose main regulator is the peroxiredoxin/sulfiredoxin systems, which are at least partially under the control of the Nrf2/Keap1 system. Some cancers hijack Nrf2/Keap1 system with mutation in these genes, which persistently activate antioxidant systems in the cancer cells. Refer to text and Figure 8 for details.
Recently, hydrogen peroxide has been shown to use water channels, the aquaporins, to cross the lipid membrane more rapidly than diffusion allows (Bienert et al., 2006). Specific aquaporins promote the diffusion of hydrogen peroxide and are thus referred to as peroxiporins (Bienert et al., 2007). Aquaporin-8 can modulate the transport of hydrogen peroxide produced by NAD(P)H oxidase in leukemia cells (Bienert et al., 2007), suggesting novel targets for cancer therapy in that, in general, cancer cells are under persistent oxidative stress (Toyokuni et al., 1995b).
Thiol-Dependent Antioxidant Systems and Cancer
The GSH and thioredoxin systems are generally activated in cancer (Tanaka et al., 1997; Dutta et al., 2005; Nogueira and Hay, 2013; Penney and Roy, 2013; Traverso et al., 2013). Among them, there has been much interest in the overexpression of GSH S-transferase in rodent hepatocarcinogenesis (Hatayama et al., 1993) and in human cancers (Huang et al., 2013; Tang et al., 2013). GSH S-transferase pi has now been connected with peroxiredoxin-6 for its recovery of peroxidase activity (Zhou et al., 2013). Acute temporary as well as persistent overexpression of GSH S-transferase pi was observed during renal carcinogenesis induced by Fe-NTA (Fukuda et al., 1996a; Tanaka et al., 1998).
Nrf2 and Keap1 are now recognized as a master regulatory transcription system for antioxidant enzymes (GSH synthesis, hydrogen peroxide removal, detoxification, drug excretion, and NADPH synthesis) (Suzuki et al., 2013). Under normal conditions, Nrf2 is constitutively produced but is inactivated in the cytoplasm following its interaction with Keap1 by ubiquitination and proteasomal degradation. Keap1 is indeed a sensor molecule for oxidative stress. The multiple cysteine residues on Keap1, which are ultrasensitive to electrophiles, are critically important for the binding with Nrf2 (Itoh et al., 1997, 1999; Mitsuishi et al., 2012) (Figure 8).
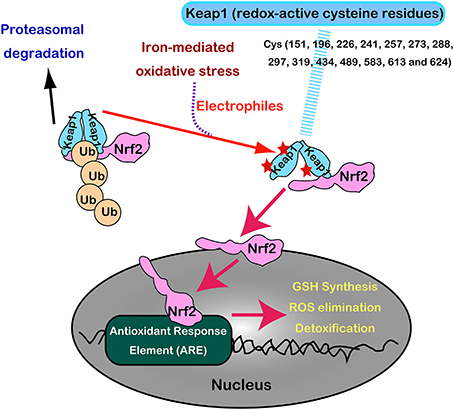
Figure 8. Regulation of Nrf2 transcription machinery through oxidative stress sensor, Keap1, with numerous redox-reactive cysteine residues.
With the aid of next generation sequencing, it has become clear that Nrf2 is consistently activated in certain cancers with various mutations. Mutually exclusive Nrf2 mutations or Keap1 mutations are observed in cancers for Nrf2 to be localized in the nucleus (Mitsuishi et al., 2012). Sulfiredoxin is also under the transcriptional regulation of Nrf2 (Jeong et al., 2012). Thus, cancer cells have hijacked this system, making them consistently more resistant to oxidative stress (Figure 7). Recently, peroxiredoxin 1, 4, and 6 were shown either to enhance tumor progression or to promote metastasis (Ishii et al., 2012).
Cancer Stem Cells and Thiol Metabolism
Stem cells are defined as undifferentiated immature cells that, upon certain stimuli or signaling, differentiate into a planned type of mature cell(s). Cancer stem cells represent a distinct subset, namely, cells that have acquired all the necessary genetic and epigenetic alterations but are usually quiescent and divide only if necessary (Holland et al., 2013). This feature is in contrast to the cancer tissue as a whole, which is exposed to persistent oxidative stress (Toyokuni et al., 1995b). In this sense, cancer stem cells constitute small heavenly territories in cancer tissue. In transplant experiments, theoretically, even a single cancer stem cell can generate a large tumor. Thus, the existence of cancer stem cells has been used to explain chemotherapy-resistance in such a dormant state. These cancer stem cells are more resistant than non-stem cancer cells to cytotoxic chemicals.
Recently, Hideyuki Saya's group reported that cancer stem cells in certain cancers present CD44 variant 8-10 (CD44v) isoform on the plasma membrane, which stabilizes the cystine/glutamate transporter (antiporter; xCT), leading to increased GSH (Ishimoto et al., 2011). This may at least partially explain the robustness of cancer cell defenses against oxidative stress, including those involved in chemotherapy-resistance. There is already an xCT antagonist, sulfasalazine, and clinical trials are underway in advanced gastric cancer in Japan (Figure 9A).
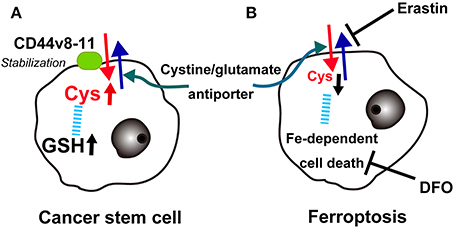
Figure 9. Key role of the cystine/glutamate antiporter in cancer cells. (A) Overexpression of CD44v(8-11) stabilizes the cystine/glutamate antiporter to increase cysteine and glutathione (GSH) in cancer stem cells. (B) Conversely, erastin blocks the cystine/glutamate antiporter, lowers cysteine and finally induces iron-dependent cancer cell death (ferroptosis), which can be blocked with deferoxamine (DFO).
Ferroptosis
Recently, a different type of cell death other than necrosis, apoptosis, or autophagy was reported in cancer cells. The oncogenic RAS-selective lethal small molecule erastin triggers a unique iron-dependent form of non-apoptotic cell death called ferroptosis (Dixon et al., 2012). Similar to glutamate, erastin inhibits cystine uptake by the cystine/glutamate antiporter, inducing a void of antioxidant defenses, resulting in iron-dependent oxidative stress. Interestingly, this type of cell death is inhibited by an iron chelator, deferoxamine, which removes cellular iron (Figure 9B). Deferoxamine functions in clear contrast to NTA in that it blocks all 6 ligands of iron (Toyokuni and Sagripanti, 1992). The authors suggest that one of more yet unidentified iron-dependent enzymes are functioning as a core lethal mechanism for ferroptosis (Dixon et al., 2012).
Future Cancer Prevention and Cancer Therapeutics
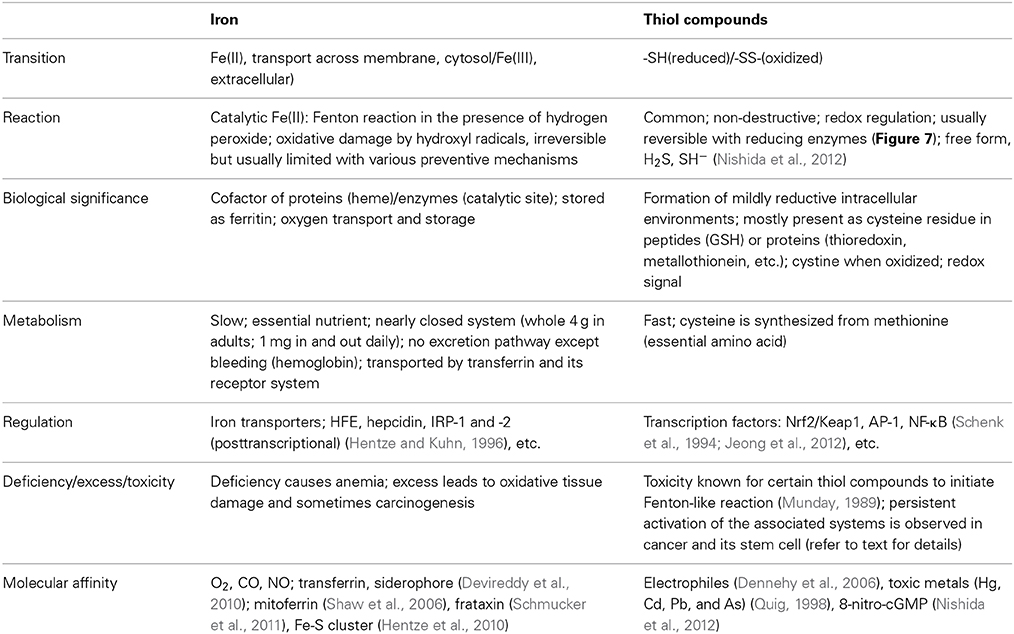
It is generally accepted that chronic oxidative stress via excess iron leads to carcinogenesis, presumably through hydroxyl radicals, and that most of the defense system is associated with thiol compounds whereas iron and thiol compounds are apparently essential elements. These two are antagonistic in abundance, and their characteristics are distinct (Table 3).
For cancer prevention, we recognize that cancers of different tissues are completely different diseases. Specific risks are present for each type of cancer, and we have to decrease specific risks as early as possible in our lives. For example, asbestos is a definite carcinogen, causing malignant mesothelioma, and smoking increases the risk of more than 20 different cancers, including laryngeal and lung cancers (Toyokuni, 2013a). Today we demand practical methods for prevention of overall cancer. Antioxidant systems, if deficient, have to be supplemented. However, thus far, antioxidant supplementation has not officially been recommended based on the results of epidemiological studies (Bjelakovic et al., 2004, 2007). Excessive supplementation with β-carotene even increased the risk of lung cancer in smokers (Albanes et al., 1996). Here appropriate iron reduction via blood donation or phlebotomy may be a potential method of cancer prevention. Among the three components of iron, oxygen, and thiol compounds, we can modify only iron status after all. Humans live much longer than they did 70 years ago, following the conquest of major infectious diseases. After reaching middle age, iron is found in excess, especially in men of well-developed countries, because there is no other way to excrete iron (Toyokuni, 2011a). This theory requires further epidemiological studies and clinical trials for demonstration. Reportedly, cancer may hijack cytokine systems (e.g., SMAD4) via mutation to collect iron for proliferation (Wang et al., 2005). Notably, there is an opposing report that iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans (Noto et al., 2013). Iron deficiency appears to enhance the virulence of Helicobacter pylori, which definitely requires iron to live. This fact needs to be further discussed. Iron deficiency should be avoided because it causes anemia (hemoglobin) and muscle weakness (myoglobin).
Currently, antibody therapies (Scott et al., 2012) and small-molecular-weight kinase inhibitors (Fabbro et al., 2012) are popular and work well as individualized therapies for specific cancers. However, the drawbacks of these therapies include the acquisition of resistance and high medical costs. As an alternative approach, some scientists are already thinking of attacking the Achilles' heel of cancer. As foreseen from the presence of ferroptosis, cancers in general accumulate iron for proliferation, which may allow the abundance of catalytic iron in the cytoplasm. Indeed, hydrogen peroxide is not the only molecule to induce the Fenton reaction. Ascorbate (vitamin C) and L-cysteine as reducing agents can also initiate the Fenton reaction in the presence of catalytic Fe(III) (Toyokuni and Sagripanti, 1996). In light of this, high-dose ascorbate therapy is being tested in a clinical trial with a standard regimen of chemotherapy because ascorbate has long been proven to be a safe drug in humans (Welsh et al., 2013).
Epilog
Iron and sulfur are essential for life despite their presence in small amounts. Excessive iron cause oxidative damage in the genome, which can be a basis of somatic mutational evolution in search of resistance against oxidative stress and cellular proliferation. Apparently, iron and thiol compounds are antagonistic toward oxidative stress, but even thiol compounds can be our foes in cancer. Therefore, it is difficult to conclude the role of iron and thiol compounds as friends or foes, which depends on the quantity/distribution and induction/flexibility, respectively. Avoiding further mutation would be the most helpful strategy for cancer prevention, and myriad of efforts are being made to sort out the weaknesses of cancer cells.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by a grant-in-aid for research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (24390094; 221S0001-04; 24108001).
References
Agudo, A., Bonet, C., Sala, N., Munoz, X., Aranda, N., Fonseca-Nunes, A., et al. (2013). Hemochromatosis (HFE) gene mutations and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Carcinogenesis 34, 1244–1250. doi: 10.1093/carcin/bgt045
Aierken, D., Okazaki, Y., Chew, S. H., Sakai, A., Wang, Y., Nagai, H., et al. (2014). Rat model demostrates a high risk of tremolite but a low risk of anthophyllite for mesothelial carcinogenesis. Nagoya J. Med. Sci. 76, 149–160.
Akatsuka, S., Yamashita, Y., Ohara, H., Liu, Y. T., Izumiya, M., Abe, K., et al. (2012). Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLoS ONE 7:e43403. doi: 10.1371/journal.pone.0043403
Albanes, D., Heinonen, O., Taylor, P., Virtamo, J., Edwards, B., Rautalahti, M., et al. (1996). Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 88, 1560–1570. doi: 10.1093/jnci/88.21.1560
Anderson, R. L., Alden, C. L., and Merski, J. A. (1982). The effects of nitrilotriacetate on cation disposition and urinary tract toxicity. Food Chem. Toxicol. 20, 105–122. doi: 10.1016/S0278-6915(82)80017-3
Antunes, F., and Cadenas, E. (2000). Estimation of H2O2 gradients across biomembranes. FEBS Lett. 475, 121–126. doi: 10.1016/S0014-5793(00)01638-0
Awai, M., Narasaki, M., Yamanoi, Y., and Seno, S. (1979). Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate: a model of experimental hemochromatosis. Am. J. Pathol. 95, 663–674.
Beckman, J. A., Creager, M. A., and Libby, P. (2002). Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287, 2570–2581. doi: 10.1001/jama.287.19.2570
Bedard, K., and Krause, K. H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313. doi: 10.1152/physrev.00044.2005
Beguin, Y., Aapro, M., Ludwig, H., Mizzen, L., and Osterborg, A. (2014). Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis–a critical review. Crit. Rev. Oncol. Hematol. 89, 1–15. doi: 10.1016/j.critrevonc.2013.10.008
Bienert, G. P., Moller, A. L., Kristiansen, K. A., Schulz, A., Moller, I. M., Schjoerring, J. K., et al. (2007). Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192. doi: 10.1074/jbc.M603761200
Bienert, G. P., Schjoerring, J. K., and Jahn, T. P. (2006). Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 1758, 994–1003. doi: 10.1016/j.bbamem.2006.02.015
Bjelakovic, G., Nikolova, D., Gluud, L. L., Simonetti, R. G., and Gluud, C. (2007). Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297, 842–857. doi: 10.1001/jama.297.8.842
Bjelakovic, G., Nikolova, D., Simonetti, R. G., and Gluud, C. (2004). Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 364, 1219–1228. doi: 10.1016/S0140-6736(04)17138-9
Bonkovsky, H. L., Banner, B. F., and Rothman, A. L. (1997). Iron and chronic viral hepatitis. Hepatology 25, 759–768. doi: 10.1002/hep.510250345
Brigelius-Flohe, R., and Maiorino, M. (2013). Glutathione peroxidases. Biochim. Biophys. Acta 1830, 3289–3303. doi: 10.1016/j.bbagen.2012.11.020
Cadenas, E., and Davies, K. J. (2000). Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 29, 222–230. doi: 10.1016/S0891-5849(00)00317-8
Camacho, C. V., Todorova, P. K., Hardebeck, M. C., Tomimatsu, N., Gil Del Alcazar, C. R., Ilcheva, M., et al. (2014). DNA double-strand breaks cooperate with loss of Ink4 and Arf tumor suppressors to generate glioblastomas with frequent Met amplification. Oncogene. doi: 10.1038/onc.2014.29. [Epub ahead of print].
Campbell, J. A. (1940). Effects of precipitated silica and of iron oxide on the incidence of primary lung tumours in mice. Br. Med. J. August 31, 275–280. doi: 10.1136/bmj.2.4156.275
Cho, M., Eze, O. P., and Xu, R. (2013). A brief review of the controversial role of iron in colorectal carcinogenesis. J. Clin. Exp. Pathol. 3, 137. doi: 10.4172/2161-0681.1000137
Dawson, M. A., and Kouzarides, T. (2012). Cancer epigenetics: from mechanism to therapy. Cell 150, 12–27. doi: 10.1016/j.cell.2012.06.013
Dennehy, M. K., Richards, K. A., Wernke, G. R., Shyr, Y., and Liebler, D. C. (2006). Cytosolic and nuclear protein targets of thiol-reactive electrophiles. Chem. Res. Toxicol. 19, 20–29. doi: 10.1021/tx050312l
Devireddy, L. R., Hart, D. O., Goetz, D. H., and Green, M. R. (2010). A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell 141, 1006–1017. doi: 10.1016/j.cell.2010.04.040
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. doi: 10.1016/j.cell.2012.03.042
Dizdaroglu, M. (1991). Chemical determination of free radical-induced damage to DNA. Free Radic. Biol. Med. 10, 225–242. doi: 10.1016/0891-5849(91)90080-M
Dutta, K. K., Nishinaka, Y., Masutani, H., Akatsuka, S., Aung, T. T., Shirase, T., et al. (2005). Two distinct mechanisms for loss of thioredoxin-binding protein-2 in oxidative stress-induced renal carcinogenesis. Lab. Invest. 85, 798–807. doi: 10.1038/labinvest.3700280
Ebina, Y., Okada, S., Hamazaki, S., Ogino, F., Li, J. L., and Midorikawa, O. (1986). Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum- nitrilotriacetate complexes in rats. J. Natl. Cancer Inst. 76, 107–113.
Enami, S., Sakamoto, Y., and Colussi, A. J. (2014). Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. U.S.A. 111, 623–628. doi: 10.1073/pnas.1314885111
Esteller, M., and Herman, J. G. (2002). Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J. Pathol. 196, 1–7. doi: 10.1002/path.1024
Fabbro, D., Cowan-Jacob, S. W., Mobitz, H., and Martiny-Baron, G. (2012). Targeting cancer with small-molecular-weight kinase inhibitors. Methods Mol. Biol. 795, 1–34. doi: 10.1007/978-1-61779-337-0_1
Feinberg, A. P., Ohlsson, R., and Henikoff, S. (2006). The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 7, 21–33. doi: 10.1038/nrg1748
Fonseca-Nunes, A., Jakszyn, P., and Agudo, A. (2014). Iron and cancer risk–a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol. Biomarkers Prev. 23, 12–31. doi: 10.1158/1055-9965.EPI-13-0733
Fracanzani, A., Conte, D., Fraquelli, M., Taioli, E., Mattioli, M., Losco, A., et al. (2001). Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology 33, p647–p651. doi: 10.1053/jhep.2001.22506
Fukuda, A., Oda, H., Toyokuni, S., Hiai, H., Satoh, K., and Uchida, K. (1996a). Oxidative stress response in iron-induced renal carcinogenesis: acute nephrotoxicity mediates the enhanced expression of glutathione S-transferase Yp isozyme. Arch. Biochem. Biophys. 329, 39–46.
Fukuda, A., Osawa, T., Hitomi, K., and Uchida, K. (1996b). 4-Hydroxy-2-nonenal cytotoxicity in renal proximal tubular cells: protein modification and redox alteration. Arch. Biochem. Biophys. 333, 419–426.
Ganz, T. (2003). Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102, 783–788. doi: 10.1182/blood-2003-03-0672
Giorgio, M., Trinei, M., Migliaccio, E., and Pelicci, P. G. (2007). Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 8, 722–728. doi: 10.1038/nrm2240
Halliwell, B., and Gutteridge, J. M. C. (2007). Free Radicals in Biology and Medicine. New York, NY: Oxford University Press.
Hanahan, D., and Weinberg, R. A. (2000). The hallmarks of cancer. Cell 100, 57–70. doi: 10.1016/S0092-8674(00)81683-9
Hao, W., and Friedman, A. (2014). The LDL-HDL profile determines the risk of atherosclerosis: a mathematical model. PLoS ONE 9:e90497. doi: 10.1371/journal.pone.0090497
Hatayama, I., Nishimura, S., Narita, T., and Sato, K. (1993). Sex-dependent expression of class pi glutathione S-transferase during chemical hepatocarcinogenesis in B6C3F1 mice. Carcinogenesis 14, 537–538. doi: 10.1093/carcin/14.3.537
Hentze, M., and Kuhn, L. (1996). Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 93, p8175–p8182. doi: 10.1073/pnas.93.16.8175
Hentze, M. W., Muckenthaler, M. U., Galy, B., and Camaschella, C. (2010). Two to tango: regulation of Mammalian iron metabolism. Cell 142, 24–38. doi: 10.1016/j.cell.2010.06.028
Hirayama, T., Okuda, K., and Nagasawa, H. (2013). A highly selective turn-on fluorescent probe fro iron(II) to visualize labile iron in living cells. Chem. Sci. 4, 1250–1256. doi: 10.1039/c2sc21649c
Hiroyasu, M., Ozeki, M., Kohda, H., Echizenya, M., Tanaka, T., Hiai, H., et al. (2002). Specific allelic loss of p16 (INK4A) tumor suppressor gene after weeks of iron-mediated oxidative damage during rat renal carcinogenesis. Am. J. Pathol. 160, 419–424. doi: 10.1016/S0002-9440(10)64860-2
Holland, J. D., Klaus, A., Garratt, A. N., and Birchmeier, W. (2013). Wnt signaling in stem and cancer stem cells. Curr. Opin. Cell Biol. 25, 254–264. doi: 10.1016/j.ceb.2013.01.004
Hu, Q., Akatsuka, S., Yamashita, Y., Ohara, H., Nagai, H., Okazaki, Y., et al. (2010). Homozygous deletion of CDKN2A/2B is a hallmark of iron-induced high-grade rat mesothelioma. Lab. Invest. 90, 360–373. doi: 10.1038/labinvest.2009.140
Huang, S. X., Wu, F. X., Luo, M., Ma, L., Gao, K. F., Li, J., et al. (2013). The glutathione S-transferase P1 341C>T polymorphism and cancer risk: a meta-analysis of 28 case-control studies. PLoS ONE 8:e56722. doi: 10.1371/journal.pone.0056722
Ishii, T., Warabi, E., and Yanagawa, T. (2012). Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J. Clin. Biochem. Nutr. 50, 91–105. doi: 10.3164/jcbn.11-109
Ishimoto, T., Nagano, O., Yae, T., Tamada, M., Motohara, T., Oshima, H., et al. (2011). CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19, 387–400. doi: 10.1016/j.ccr.2011.01.038
Itoh, K., Chiba, T., Takahashi, S., Ishii, T., Igarashi, K., Katoh, Y., et al. (1997). An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322. doi: 10.1006/bbrc.1997.6943
Itoh, K., Wakabayashi, N., Katoh, Y., Ishii, T., Igarashi, K., Engel, J. D., et al. (1999). Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13, 76–86. doi: 10.1101/gad.13.1.76
Jeong, W., Bae, S. H., Toledano, M. B., and Rhee, S. G. (2012). Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression. Free Radic. Biol. Med. 53, 447–456. doi: 10.1016/j.freeradbiomed.2012.05.020
Jiang, F., Zhang, Y., and Dusting, G. J. (2011). NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 63, 218–242. doi: 10.1124/pr.110.002980
Jiang, L., Akatsuka, S., Nagai, H., Chew, S. H., Ohara, H., Okazaki, Y., et al. (2012). Iron overload signature in chrysotile-induced malignant mesothelioma. J. Pathol. 228, 366–377. doi: 10.1002/path.4075
Kato, J., Miyanishi, K., Kobune, M., Nakamura, T., Takada, K., Takimoto, R., et al. (2007). Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J. Gastroenterol. 42, 830–836. doi: 10.1007/s00535-007-2095-z
Kumar, V., Abbas, A. K., and Aster, J. C. (2013). Robbins Basic Pathology. Philadelphia, PA: Elsevier Saunders.
Li, J. L., Okada, S., Hamazaki, S., Ebina, Y., and Midorikawa, O. (1987). Subacute nephrotoxicity and induction of renal cell carcinoma in mice treated with ferric nitrilotriacetate. Cancer Res. 47, 1867–1869.
Lippard, S. J., and Berg, J. M. (1994). Principles of Bioorganic Chemistry. Mill Valley, CA: University Science Books.
Liu, M., and Okada, S. (1994). Induction of free radicals and tumors in the kidneys of Wistar rats by ferric ethylenediamine-N,N′-diacetate. Carcinogenesis 15, 2817–2821. doi: 10.1093/carcin/15.12.2817
Lu, J., and Holmgren, A. (2014). The thioredoxin antioxidant system. Free Radic. Biol. Med. 66, 75–87. doi: 10.1016/j.freeradbiomed.2013.07.036
Miller, D. M., Buettner, G. R., and Aust, S. D. (1990). Transition metals as catalysts of autoxidation reactions. Free Radic. Biol. Med. 8, 95–108. doi: 10.1016/0891-5849(90)90148-C
Minotti, G., and Aust, S. D. (1989). The role of iron in oxygen radical mediated lipid peroxidation. Chem. Biol. Interact. 71, 1–19. doi: 10.1016/0009-2797(89)90087-2
Mitsuishi, Y., Motohashi, H., and Yamamoto, M. (2012). The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front. Oncol. 2:200. doi: 10.3389/fonc.2012.00200
Mukaide, T., Hattori, Y., Misawa, N., Funahashi, S., Jiang, L., Hirayama, T., et al. (2014). Histological detection of catalytic ferrous iron with the selective turn-on fluorescent probe RhoNox-1 in a Fenton reaction-based rat renal carcinogenesis model. Free Radic. Res. 48, 990–995. doi: 10.3109/10715762.2014.898844
Munday, R. (1989). Toxicity of thiols and disulphides: involvement of free-radical species. Free Radic. Biol. Med. 7, 659–673. doi: 10.1016/0891-5849(89)90147-0
Nagai, H., Okazaki, Y., Chew, S., Misawa, N., Yamashita, Y., Akatsuka, S., et al. (2011). Diameter of multi-walled carbon nanotubes is a critical factor in mesothelial injury and subsequent carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 108, E1330–E1338. doi: 10.1073/pnas.1110013108
Nishida, M., Sawa, T., Kitajima, N., Ono, K., Inoue, H., Ihara, H., et al. (2012). Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 8, 714–724. doi: 10.1038/nchembio.1018
Nishiyama, Y., Suwa, H., Okamoto, K., Fukumoto, M., Hiai, H., and Toyokuni, S. (1995). Low incidence of point mutations in H-, K- and N-ras oncogenes and p53 tumor suppressor gene in renal cell carcinoma and peritoneal mesothelioma of Wistar rats induced by ferric nitrilotriacetate. Jpn. J. Cancer Res. 86, 1150–1158. doi: 10.1111/j.1349-7006.1995.tb03308.x
Nogueira, V., and Hay, N. (2013). Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 19, 4309–4314. doi: 10.1158/1078-0432.CCR-12-1424
Noto, J. M., Gaddy, J. A., Lee, J. Y., Piazuelo, M. B., Friedman, D. B., Colvin, D. C., et al. (2013). Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J. Clin. Invest. 123, 479–492. doi: 10.1172/JCI64373
Ohnishi, K., Semi, K., Yamamoto, T., Shimizu, M., Tanaka, A., Mitsunaga, K., et al. (2014). Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell 156, 663–677. doi: 10.1016/j.cell.2014.01.005
Okada, S. (1996). Iron-induced tissue damage and cancer: the role of reactive oxygen free radicals. Pathol. Int. 46, 311–332. doi: 10.1111/j.1440-1827.1996.tb03617.x
Okada, S., Hamazaki, S., Toyokuni, S., and Midorikawa, O. (1989). Induction of mesothelioma by intraperitoneal injections of ferric saccharate in male Wistar rats. Br. J. Cancer 60, 708–711. doi: 10.1038/bjc.1989.344
Okada, S., and Midorikawa, O. (1982). Induction of rat renal adenocarcinoma by Fe-nitrilotriacetate (Fe-NTA). Jpn. Arch. Intern. Med. 29, 485–491.
Okada, S., Minamiyama, Y., Hamazaki, S., Toyokuni, S., and Sotomatsu, A. (1993). Glutathione cycle dependency of ferric nitrilotriacetate-induced lipid peroxidation in mouse proximal renal tubules. Arch. Biochem. Biophys. 301, 138–142. doi: 10.1006/abbi.1993.1125
Pearce, C. L., Templeman, C., Rossing, M. A., Lee, A., Near, A. M., Webb, P. M., et al. (2012). Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 13, 385–394. doi: 10.1016/S1470-2045(11)70404-1
Penney, R. B., and Roy, D. (2013). Thioredoxin-mediated redox regulation of resistance to endocrine therapy in breast cancer. Biochim. Biophys. Acta 1836, 60–79. doi: 10.1016/j.bbcan.2013.02.005
Pleasance, E. D., Cheetham, R. K., Stephens, P. J., McBride, D. J., Humphray, S. J., Greenman, C. D., et al. (2010). A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463, 191–196. doi: 10.1038/nature08658
Pootrakul, P., Christensen, A., Josephson, B., and Finch, C. A. (1977). Role of transferrin in determining internal iron distribution. Blood 49, 957–966.
Rhee, S. G., Chae, H. Z., and Kim, K. (2005). Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 38, 1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026
Richmond, H. G. (1959). Induction of sarcoma in the rat by iron-dextran complex. Br. Med. J. 1, 947–949. doi: 10.1136/bmj.1.5127.947
Samuni, A., Aronovich, J., Godinger, D., Chevion, M., and Czapski, G. (1983). On the toxicity of vitamin C and metal ions: a site-specific Fenton mechanism. Eur. J. Biochem. 137, 119–124. doi: 10.1111/j.1432-1033.1983.tb07804.x
Schenk, H., Klein, M., Erdbruegger, W., Droege, W., and Schulze-Osthoff, K. (1994). Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kB and AP-1. Proc. Natl. Acad. Sci. U.S.A. 91, 1672–1676. doi: 10.1073/pnas.91.5.1672
Schmucker, S., Martelli, A., Colin, F., Page, A., Wattenhofer-Donze, M., Reutenauer, L., et al. (2011). Mammalian frataxin: an essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS ONE 6:e16199. doi: 10.1371/journal.pone.0016199
Scott, A. M., Wolchok, J. D., and Old, L. J. (2012). Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287. doi: 10.1038/nrc3236
Shaw, G. C., Cope, J. J., Li, L., Corson, K., Hersey, C., Ackermann, G. E., et al. (2006). Mitoferrin is essential for erythroid iron assimilation. Nature 440, 96–100. doi: 10.1038/nature04512
Sies, H. (1999). Glutathione and its role in cellular functions. Free Radic. Biol. Med. 27, 916–921. doi: 10.1016/S0891-5849(99)00177-X
Sies, H. (2014). Role of metabolic H2O2 generation: redox signaling and oxidative stress. J. Biol. Chem. 289, 8735–8741. doi: 10.1074/jbc.R113.544635
Smith, A. E., Mohamedali, A. M., Kulasekararaj, A., Lim, Z., Gaken, J., Lea, N. C., et al. (2010). Next-generation sequencing of the TET2 gene in 355 MDS and CMML patients reveals low-abundance mutant clones with early origins, but indicates no definite prognostic value. Blood 116, 3923–3932. doi: 10.1182/blood-2010-03-274704
Stratton, M. R., Campbell, P. J., and Futreal, P. A. (2009). The cancer genome. Nature 458, 719–724. doi: 10.1038/nature07943
Suzuki, T., Motohashi, H., and Yamamoto, M. (2013). Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 34, 340–346. doi: 10.1016/j.tips.2013.04.005
Tanaka, T., Akatsuka, S., Ozeki, M., Shirase, T., Hiai, H., and Toyokuni, S. (2004). Redox regulation of annexin 2 and its implications for oxidative stess-induced renal carcinogenesis and metastasis. Oncogene 23, 3980–3989. doi: 10.1038/sj.onc.1207555
Tanaka, T., Iwasa, Y., Kondo, S., Hiai, H., and Toyokuni, S. (1999). High incidence of allelic loss on chromosome 5 and inactivation of p15 INK4B and p16 INK4A tumor suppressor genes in oxystress-induced renal cell carcinoma of rats. Oncogene 18, 3793–3797. doi: 10.1038/sj.onc.1202707
Tanaka, T., Kondo, S., Iwasa, Y., Hiai, H., and Toyokuni, S. (2000). Expression of stress-response and cell proliferation genes in renal cell carcinoma induced by oxidative stress. Am. J. Pathol. 156, p2149–p2157. doi: 10.1016/S0002-9440(10)65085-7
Tanaka, T., Nishiyama, Y., Okada, K., Hirota, K., Matsui, M., Yodoi, J., et al. (1997). Induction and nuclear translocation of thioredoxin by oxidative damage in the mouse kidney: independence of tubular necrosis and sulfhydryl depletion. Lab. Invest. 77, 145–155.
Tanaka, T., Nishiyama, Y., Okada, K., Satoh, K., Fukuda, A., Uchida, K., et al. (1998). Over-expression of glutathione S-transferase Yp isozyme and concomitant down-regulation of Ya isozyme in renal cell carcinoma of rats induced by ferric nitrilotriacetate. Carcinogenesis 19, 897–903. doi: 10.1093/carcin/19.5.897
Tang, S. C., Wu, C. H., Lai, C. H., Sung, W. W., Yang, W. J., Tang, L. C., et al. (2013). Glutathione S-transferase mu2 suppresses cancer cell metastasis in non-small cell lung cancer. Mol. Cancer Res. 11, 518–529. doi: 10.1158/1541-7786.MCR-12-0488
Toyokuni, S. (1996). Iron-induced carcinogenesis: the role of redox regulation. Free Radic. Biol. Med. 20, 553–566. doi: 10.1016/0891-5849(95)02111-6
Toyokuni, S. (2002). Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep. 7, 189–197. doi: 10.1179/135100002125000596
Toyokuni, S. (2009). Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 100, 9–16. doi: 10.1111/j.1349-7006.2008.01001.x
Toyokuni, S. (2011a). Iron as a target of chemoprevention for longevity in humans. Free Radic. Res. 45, 906–917. doi: 10.3109/10715762.2011.564170
Toyokuni, S. (2011b). Mysterious link between iron overload and CDKN2A/2B. J. Clin. Biochem. Nutr. 48, 46–49. doi: 10.3164/jcbn.11-001FR
Toyokuni, S. (2013a). Genotoxicity and carcinogenicity risk of carbon nanotubes [epub ahead of print] [Record Supplied By Publisher]. Adv. Drug Deliv. Rev. 65, 2098–2110. doi: 10.1016/j.addr.2013.05.011
Toyokuni, S. (2013b). Iron overload as a major targetable pathogenesis of asbestos-induced mesothelial carcinogenesis. Redox Rep. 19, 1–7. doi: 10.1179/1351000213Y.0000000075
Toyokuni, S., Luo, X. P., Tanaka, T., Uchida, K., Hiai, H., and Lehotay, D. C. (1997a). Induction of a wide range of C2-12 aldehydes and C7-12 acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic. Biol. Med. 22, 1019–1027.
Toyokuni, S., Tanaka, T., Hattori, Y., Nishiyama, Y., Ochi, H., Hiai, H., et al. (1997b). Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab. Invest. 76, 365–374.
Toyokuni, S., Mori, T., and Dizdaroglu, M. (1994a). DNA base modifications in renal chromatin of Wistar rats treated with a renal carcinogen, ferric nitrilotriacetate. Int. J. Cancer 57, 123–128.
Toyokuni, S., Uchida, K., Okamoto, K., Hattori-Nakakuki, Y., Hiai, H., and Stadtman, E. R. (1994b). Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc. Natl. Acad. Sci. U.S.A. 91, 2616–2620.
Toyokuni, S., Mori, T., Hiai, H., and Dizdaroglu, M. (1995a). Treatment of Wistar rats with a renal carcinogen, ferric nitrilotriacetate, causes DNA-protein cross-linking between thymine and tyrosine in their renal chromatin. Int. J. Cancer 62, 309–313.
Toyokuni, S., Okamoto, K., Yodoi, J., and Hiai, H. (1995b). Persistent oxidative stress in cancer. FEBS Lett. 358, 1–3.
Toyokuni, S., Okada, S., Hamazaki, S., Minamiyama, Y., Yamada, Y., Liang, P., et al. (1990). Combined histochemical and biochemical analysis of sex hormone dependence of ferric nitrilotriacetate-induced renal lipid peroxidation in ddY mice. Cancer Res. 50, 5574–5580.
Toyokuni, S., and Sagripanti, J. L. (1992). Iron-mediated DNA damage: sensitive detection of DNA strand breakage catalyzed by iron. J. Inorg. Biochem. 47, 241–248. doi: 10.1016/0162-0134(92)84069-Y
Toyokuni, S., and Sagripanti, J.-L. (1993). DNA single- and double-strand breaks produced by ferric nitrilotriacetate in relation to renal tubular carcinogenesis. Carcinogenesis 14, 223–227. doi: 10.1093/carcin/14.2.223
Toyokuni, S., and Sagripanti, J.-L. (1996). Association between 8-hydroxy-2′-deoxyguanosine formation and DNA strand breaks mediated by copper and iron. Free Radic. Biol. Med. 20, 859–864. doi: 10.1016/0891-5849(95)02184-1
Traverso, N., Ricciarelli, R., Nitti, M., Marengo, B., Furfaro, A. L., Pronzato, M. A., et al. (2013). Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 972913. doi: 10.1155/2013/972913
Travis, L. B., Demark Wahnefried, W., Allan, J. M., Wood, M. E., and Ng, A. K. (2013). Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat. Rev. Clin. Oncol. 10, 289–301. doi: 10.1038/nrclinonc.2013.41
Uchida, K., Fukuda, A., Kawakishi, S., Hiai, H., and Toyokuni, S. (1995). A renal carcinogen ferric nitrilotriacetate mediates a temporary accumulation of aldehyde-modified proteins within cytosolic compartment of rat kidney. Arch. Biochem. Biophys. 317, 405–411. doi: 10.1006/abbi.1995.1181
Wang, R. H., Li, C., Xu, X., Zheng, Y., Xiao, C., Zerfas, P., et al. (2005). A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2, 399–409. doi: 10.1016/j.cmet.2005.10.010
Weinberg, R. A. (2013). The Biology of Cancer, 2nd Edn. New York, NY: Garland Science; Tailor & Francis Group; LLC.
Welsh, J. L., Wagner, B. A., Van't Erve, T. J., Zehr, P. S., Berg, D. J., Halfdanarson, T. R., et al. (2013). Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 71, 765–775. doi: 10.1007/s00280-013-2070-8
Winterbourn, C. C. (2013). The biological chemistry of hydrogen peroxide. Meth. Enzymol. 528, 3–25. doi: 10.1016/B978-0-12-405881-1.00001-X
Wood, Z. A., Poole, L. B., and Karplus, P. A. (2003). Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300, 650–653. doi: 10.1126/science.1080405
Wriggleworth, J. M., and Baum, H. (1980). “The biochemical function of iron,” in Iron in Biochemistry and Medicine,Vol. 2, eds A. Jacobs, and M. Worwood (London: Academic Press), 29–86.
Yamaguchi, K., Mandai, M., Toyokuni, S., Hamanishi, J., Higuchi, T., Takakura, K., et al. (2008). Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 14, p32–p40. doi: 10.1158/1078-0432.CCR-07-1614
Yamashita, Y., Akatsuka, S., Shinjo, K., Yatabe, Y., Kobayashi, H., Seko, H., et al. (2013). Met is the most frequently amplified gene in endometriosis-associated ovarian clear cell adenocarcinoma and correlates with worsened prognosis. PLoS ONE 8:e57724. doi: 10.1371/journal.pone.0057724
Yang, G., Wu, L., Jiang, B., Yang, W., Qi, J., Cao, K., et al. (2008). H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322, 587–590. doi: 10.1126/science.1162667
Zacharski, L., Chow, B., Howes, P., Shamayeva, G., Baron, J., Dalman, R., et al. (2007). Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA 297, 603–610. doi: 10.1001/jama.297.6.603
Zhang, Y. (2005). The magic bullets and tuberculosis drug targets. Annu. Rev. Pharmacol. Toxicol. 45, 529–564. doi: 10.1146/annurev.pharmtox.45.120403.100120
Keywords: iron, carcinogenesis, glutathione, oxidative stress, cancer stem cell, ferroptosis, peroxiredoxins, Nrf2
Citation: Toyokuni S (2014) Iron and thiols as two major players in carcinogenesis: friends or foes? Front. Pharmacol. 5:200. doi: 10.3389/fphar.2014.00200
Received: 17 May 2014; Accepted: 12 August 2014;
Published online: 28 August 2014.
Edited by:
Alfonso Pompella, Università di Pisa, ItalyReviewed by:
Joao Batista Teixeira Rocha, Universidade Federal de Santa Maria, BrazilPaolo Arosio, University of Brescia, Italy
Copyright © 2014 Toyokuni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinya Toyokuni, Department of Pathology and Biological Responses, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan e-mail:dG95b2t1bmlAbWVkLm5hZ295YS11LmFjLmpw
 Shinya Toyokuni
Shinya Toyokuni