- 1Department of Pediatrics, Kansai Medical University, Osaka, Japan
- 2Department of Industrial Systems Engineering, Osaka Metropolitan University College of Technology, Osaka, Japan
1 Introduction
Orthostatic intolerance (OI) is common in pediatric practice, and children with OI have difficulty enduring an upright posture (1). Being a syndrome, the main pathologies of OI include orthostatic hypotension (OH), postural orthostatic tachycardia syndrome (POTS), and postural syncope (2). Healthy children can rise from the supine position and perform their daily activities, whereas children with OI exhibit various symptoms when moving from the supine position to an upright position, such as dizziness, lightheadedness, headache, palpitations, and fatigue, which are caused by cerebral hypoperfusion (1–4). The upright posture stresses regulatory capabilities and causes venous pooling (2). Muscle pumps usually push blood back to the heart when upright and during exercise, enabling the skeletal muscle pump to form an important class of physical countermeasures against OI (5). Conversely, if muscle pump function is impaired, OI symptoms will worsen.
Cardiovascular deconditioning due to physical inactivity has been reported to cause OI in healthy young individuals (6, 7). Gravitational deconditioning encompasses a markedly reduced blood volume, blood volume redistribution, trophic cardiac atrophy, baroreflex changes, diminished vasoconstriction to pressor drugs, skeletal muscle atrophy resulting in the loss of the skeletal muscle pump, and osteoporosis (1, 8). In patients confined to bed, OI may take the form of POTS, OH, or vasovagal syncope, and bed rest worsens these states if they are already present (1, 2). Deconditioning was observed in healthy children during the COVID-19 pandemic lockdown (9). Physical inactivity can either worsen existing OI symptoms or result in OI through deconditioning. From this perspective, physical exercise is one way to improve the chronic symptoms of OI.
The expert consensus on POTS states that it is essentially managed by nonpharmacological therapy, such as fluid intake and aerobic exercise, which are more effective than pharmacological treatment in adult patients (10). Exercise is considered an essential treatment for both OH and POTS (2). However, because children suffering from severe OI have deteriorated exercise tolerance, exercise must be started at a mild intensity level and then increased gradually.
Through this narrative review, we found the four key points for effective exercise training for children with OI: exercise at approximately 70% of maximum oxygen intake using heart rate (HR) as an indicator; exercise should be started in a supine or semi-supine position; it should consist of a warm-up, target exercise, and cool down; and as exercise tolerance improves, the exercise intensity and duration should also increase (11). Non-experts in exercise therapy may find it challenging to provide appropriate exercise for children with OI; therefore, training methods and equipment for those with a high evidence level are strongly needed.
To promote appropriate exercise training for children with OI, we are developing an ergometer with a monitor that will display the appropriate exercise training that children with severe OI can perform in the recumbent position.
2 Case presentation of a boy with severe OI who underwent recumbent exercise training
2.1 Case presentation
The patient was a 13-year-old boy, first-year junior high school student. He entered junior high school in April. Three months later, in July, he began to experience stomachache, vomiting, and headaches. After the summer vacation, he began to wake up feeling uncomfortable. He was a member of the basketball club activity; however, after the summer vacation, he was unable to go to school and attend the basketball club. He stayed lying down all day in his home. Subsequently, he was diagnosed with OI at a hospital and was started on pharmacotherapy, which was ineffective. His condition worsened, even short walks caused fatigue, and he became housebound. Six months later, he was referred to our hospital for specialized training for OI. On the first visit to our hospital, he complained of severe general fatigue and dizziness with slight movements. He was instructed to drink fluids and exercise; however, he complained that even short walks were tiring. He became aware of his declining physical strength. He therefore requested to undergo bed ergometer exercise training.
2.2 Exercise training regimen
The exercise regimens were based on several reviews and guidelines (1, 11–13) and should be performed once a day for 30 min for 5 days a week.
Cardiopulmonary exercise testing (CPX) is performed to assess the exercise tolerance of patients before and after the exercise training. CPX includes the measurement of respiratory gas and is the gold standard for evaluating aerobic fitness and examining integrated physiological responses to exercise in pediatric medicine (14). CPX is usually used for the evaluation of adult patients with cardiac or respiratory diseases (15), and an exercise prescription is one of the potential indications (15). Children with chronic conditions often limit their participation in physical activities, which leads to deconditioning, reduced functional ability, and downward spiral of further hypoactivity (14). It is also a possible indication of exercise training assessed by CPX. The measurement of the maximal oxygen uptake (VO2) or peak VO2 during a progressive CPX up to maximal exertion is widely considered the gold standard for assessing aerobic fitness (14). For exercise prescription, the knowledge of anaerobic threshold (AT) can also be useful (15), so we used HR, peak VO2, and AT VO2.
One approach aims for 60%–70% of a patient's HR achieved during maximal exercise testing beginning with semirecumbent exercise. This involves 5 min of warm-up to achieve the target, 15 min at the target, and then 5 min of cooling down (1). As exercise tolerance improves, 5 min is added per session at the target until one can complete 30 min at the target HR (1). Our exercise regimen was modified to their one with the patient in a recumbent position, i.e., the spine position, rather than a semirecumbent position at the beginning of the exercise training, and then after 2 weeks, when the patient's exercise tolerance had improved, the bed was raised to a semirecumbent position, and the exercise load was increased.1
2.3 Monitoring of exercise with sensors on the pedals
The combination of frequency, intensity, and duration of chronic exercises was found to be effective in triggering a training effect (14). It should be helpful to perform exercises to strengthen endurance at a constant speed, and this helps visualize the appropriateness of the exercise. Then, a monitor on the pedals of the recumbent ergometer used in the exercise training was set for this case. This monitor measured the pressure with which the patient pressed the pedals and the speed at which they pedaled. This monitor used Bluetooth and could be monitored from a PC located away from the bed (16).
During monitoring on the first day of exercise, the pedaling speed was unstable, even though the exercise load was kept at 20 watts (W). After the first day of exercise therapy, he complained of muscle pain and severe fatigue. By week 4, he could perform ergometer exercises with his upper body almost entirely raised in bed, and the load had been increased to 50 W. The pedaling speed remained constant even when the load was increased, and he was able to perform a stable exercise (Figure 1).
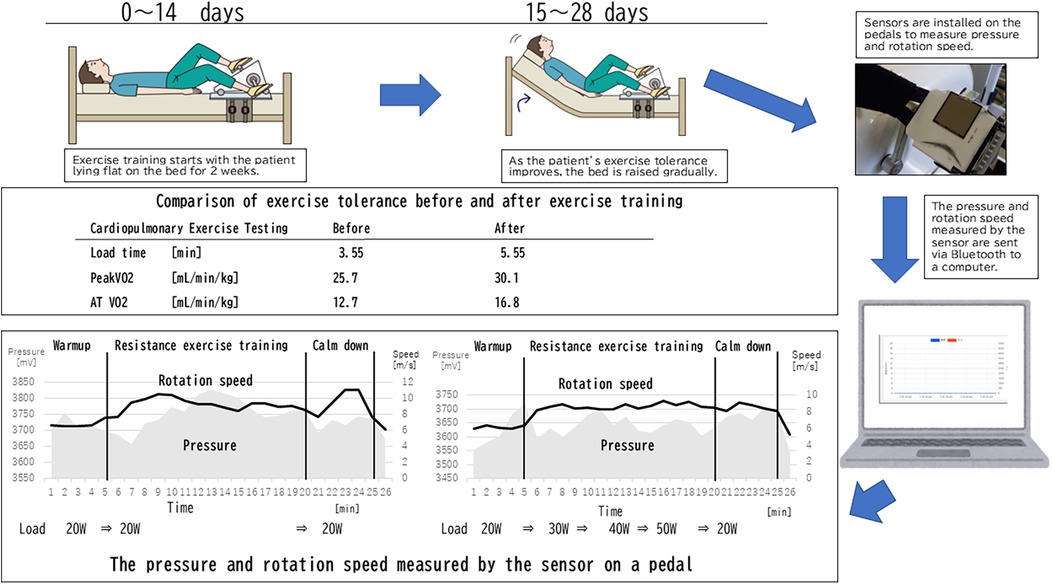
Figure 1. Exercise training, cardiopulmonary exercise testing, and monitoring diagram. The pressure value is the voltage converted into a number, and the actual pressure P [N] can be expressed by the following formula, assuming the voltage on the graph is V [mV]: .
2.4 Evaluation of the effectiveness of exercise training
The exercise program calculated from the CPX results was a target HR of 100/m and an exercise load intensity of 46 W. The actual exercise loads were a maximum load of 30, 40, and 50 W in weeks 1, 2, and 3 and 4. After 4 weeks, the exercise training was completed without any accidents.
The CPX results before and after the exercise training are shown in Figure 1. At the beginning, the patient had low exercise tolerance compared with his peers and had improved after 4 weeks of training. Various indices have improved as follows: load time, which is possible exercise duration (min), at 3.55–5.55, peak VO2 (ml/min/kg), of 25.7–30.1, and AT VO2 (ml/min/kg) of 12.7–16.8, respectively. Before the exercise training, the patient experienced fatigue and had been confined to his home. After the training, he became confident in his physical strength and could engage in the sports activity without complaining OI symptoms.
The summary of the standing test before and after exercise training is as follows: (1) At the beginning of the exercise training, the patient complained of fatigue and stopped halfway through the 10-min standing test. However, after 4 weeks of training, the patient was able to complete the entire test; (2) The maximum heart rate at the beginning was 123 (beats/min), at which point the test was stopped. After exercise training, the maximum heart rate increased to 142 (beats/min); (3) The change in pulse pressure was 56 (beats/min) to 47 (beats/min); (4) No decrease in blood pressure was observed in any of the tests.
The results of the standing test suggest an improvement in orthostatic tolerance, as the patient was able to complete the 10-min standing test after training. Additionally, the small change in pulse pressure during the standing test likely indicates improved circulation while standing.
3 Perspective
To manage the exercise training of children with severe OI, sensors were installed on the pedals to show the pressure and speed that demonstrate appropriate training. Exercise must be continued at a constant pressure and at a speed to improve endurance. When performing the ergometer exercise on a bed, children tend to pedal faster and gain momentum or stop exercising without supervision. However, it is not easy for experts to be with the children every day and supervise their exercise. Thus, we developed this sensing system. We separately reported on the effectiveness of exercise training for children with OI (see text footnote 1), and in conducting the study, a specialist for exercise training accompanied the children during their exercise for 30 min every day. However, this method cannot be widely disseminated. Therefore, convenient tools that will not only prove the effectiveness of exercise therapy for children with OI but also enable exercise therapy in medical institutions without specialists must be developed. If exercise training can be implemented in ordinary homes, the deterioration of OI due to deconditioning can be prevented, which improves the quality of life of children with OI.
Author contributions
YI: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. YY: Data curation, Investigation, Project administration, Writing – review & editing. AY: Methodology, Project administration, Software, Writing – review & editing. KH: Data curation, Software, Supervision, Validation, Visualization, Writing – review & editing. KK: Conceptualization, Supervision, Visualization, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was funded by the Japan Society for the Promotion of Science (JSPS KAKENHI 21K07874) and the KMUOD Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnote
1. ^Yanagimoto Y, Ishizaki Y, Yoshida R, Terashima T, Ishitani K, Haraguchi K, et al. Evaluation of effectiveness of exercise training therapy for circulatory dynamics of children with orthostatic dysregulation. Front Pediatr.
References
1. Stewart JM, Boris JR, Chelimsky G, Fischer PR, Fortunato JE, Grubb BP, et al. Pediatric disorders of orthostatic intolerance. Pediatrics. (2018) 141:e20171673. doi: 10.1542/peds.2017-1673
2. Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics. (2013) 131:968–80. doi: 10.1542/peds.2012-2610
3. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. (2019) 285:352–66. doi: 10.1111/joim.12852
4. Boris JR. Postural orthostatic tachycardia syndrome in children and adolescents. Auton Neurosci. (2018) 215:97–101. doi: 10.1016/j.autneu.2018.05.004
5. Miller JD, Pegelow DF, Jacques AJ, Dempsey JA. Skeletal muscle pump ver sus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J Physiol. (2005) 563:925–43. doi: 10.1113/jphysiol.2004.076422
6. Ishizaki Y, Fukuoka H, Ishizaki Y, Kino M, Higashino H, Ueda N, et al. Measurement of inferior vena cava diameter for evaluation of venous return in subjects on day 10 of a bed-rest experiment. J Appl Physiol. (2004) 96:2179–86. doi: 10.1152/japplphysiol.01097.2003
7. Takenaka K, Suzuki Y, Uno K, Sato M, Komuro T, Haruna R, et al. Effects of rapid saline infusion on orthostatic intolerance and autonomic tone after 20 days bed rest. Am J Cardiol. (2002) 89:557–61. doi: 10.1016/S0002-9149(01)02296-2
8. Sekiguchi C. Issues of health care under weightlessness. Acta Physiol Scand Suppl. (1994) 616:89–97.8042531
9. Dayton JR, Ford K, Carroll SJ. The deconditioning effect of the COVID-19 pandemic on unaffected healthy children. Pediatr Cardiol. (2021) 42:554–9. doi: 10.1007/s00246-020-02513-w
10. Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 Heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. (2015) 12:e41–63. doi: 10.1016/j.hrthm.2015.03.029
11. Ishizaki Y, Yanagimoto Y, Yoshida S, Kaneko K. Exercise training as a non-pharmacological treatment for children with orthostatic dysregulation: narrative review. J Jap Soc Pediatr Psychosom. (2022) 30:546–9. (In Japanese).
12. Fu Q, Levine BD. Exercise and non-pharmacological treatment of POTS. Auton Neurosci. (2018) 215:20–7. doi: 10.1016/j.autneu.2018.07.001
13. Pollock ML, Gaesser GA, Butcher JD, Després JP, Dishman RK, Franklin BA, et al. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. (1998) 30(6):975–91. doi: 10.1097/00005768-199806000-00032
14. Takken T, Bongers BC, van Brussel M, Haapala EA, Hulzebos EHJ. Cardiopulmonary exercise testing in pediatrics. Ann Am Thorac Soc. (2017) 14:S123–8. doi: 10.1513/AnnalsATS.201611-912FR
15. Milani RV, Lavie CJ, Mehra MR, Ventura HO. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. (2006) 81:1603–11. doi: 10.4065/81.12.1603
Keywords: orthostatic intolerance, child, exercise training, deconditioning, monitoring
Citation: Ishizaki Y, Yanagimoto Y, Yoshida A, Hayakawa K and Kaneko K (2025) Development of equipment that promotes exercise training for children with orthostatic intolerance. Front. Pediatr. 13:1577253. doi: 10.3389/fped.2025.1577253
Received: 15 February 2025; Accepted: 25 March 2025;
Published: 9 April 2025.
Edited by:
Leandro Campos de Brito, Oregon Health & Science University, United StatesReviewed by:
Yoshie Shigeyasu, Okayama University, JapanCopyright: © 2025 Ishizaki, Yanagimoto, Yoshida, Hayakawa and Kaneko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuko Ishizaki, aXNoaXpha3lAdGFraWkua211LmFjLmpw
 Yuko Ishizaki
Yuko Ishizaki Yoshitoki Yanagimoto
Yoshitoki Yanagimoto Asahi Yoshida2
Asahi Yoshida2 Kazunari Kaneko
Kazunari Kaneko