
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr. , 08 April 2025
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1574462
 Jennifer Thompson1
Jennifer Thompson1 Wenly Ruan1
Wenly Ruan1 Douglas S. Fishman1
Douglas S. Fishman1 Matthew Giefer2
Matthew Giefer2 Kyung Mo Kim3
Kyung Mo Kim3 Mercedes Martinez4
Mercedes Martinez4 Luigi Dall'Oglio5
Luigi Dall'Oglio5 Valerio Balassone5
Valerio Balassone5 Filippo Torroni5
Filippo Torroni5 Paola De Angelis5
Paola De Angelis5 Simona Faraci5
Simona Faraci5 Cynthia Tsai1
Cynthia Tsai1 Michael Wilsey6
Michael Wilsey6 Racha Khalaf7
Racha Khalaf7 Petar Mamula8
Petar Mamula8 Quin Liu9
Quin Liu9 Yuhua Zheng10
Yuhua Zheng10 Bradley A. Barth11,12
Bradley A. Barth11,12 David Michael Troendle11,12*
David Michael Troendle11,12*
Patients with hemolytic diseases are at increased risk for gallstone-related complications. Modified scoring systems have been developed to assess which pediatric patients would benefit from endoscopic retrograde cholangiopancreatography (ERCP) to treat choledocholithiasis. This study aimed to evaluate the ability of the available criteria to determine which pediatric patients with hemolytic diseases are likely to benefit from ERCP. A secondary analysis was performed using the Pediatric ERCP Database Initiative database, which contains prospectively collected data from 1,124 ERCPs at tertiary-care institutions. We compared patients with a hemolytic disease to those without. Data was analyzed by two-tailed Fisher’s exact test and paired student t-test. Of the 47 (17.0%) patients who had a hemolytic disease, 34 (72.3%) had one or more common bile duct (CBD) stones at the time of ERCP. Among patients with hemolytic diseases, there were no differences in pre-ERCP imaging or laboratory findings between those with a CBD stone removed at ERCP and those without. Patients with hemolytic diseases did not fit the current choledocholithiasis selection criteria well: 80% in the no-stone at ERCP group met the American Society of Gastrointestinal Endoscopy high-risk criteria, and 90% met the 2016 modified Baylor pediatric criteria. Although not statistically significant, there was an increased number of adverse events in patients with hemolytic diseases. Existing ERCP criteria perform poorly in patients with hemolytic diseases, overestimating their risk of choledocholithiasis. Peri-procedure evaluations such as endoscopic ultrasound, magnetic resonance cholangiopancreatography, and intraoperative cholangiography appear underutilized and may be essential modalities in this population.
Pediatric patients with hemolytic diseases, including sickle cell disease, thalassemia, and red cell membrane disorders like hereditary spherocytosis, are at increased risk for gallstone-related complications such as choledocholithiasis (1, 2). However, risk stratification for patients who would benefit from endoscopic retrograde cholangiopancreatography (ERCP) in this population is less straightforward than the general pediatric population given baseline elevations in bilirubin and transaminases in the setting of ongoing hemolysis. Additionally, the role of intrahepatic sickling, with or without infarction, in sickle cell disease is often difficult to assess when there is co-existing gallbladder disease. Chronic liver injury secondary to iron overload can also contribute to baseline laboratory abnormalities in patients who receive frequent blood transfusions (2).
The ability to accurately predict choledocholithiasis using non-invasive measures such as laboratory markers and imaging is of great interest to avoid non-therapeutic ERCP and its associated risks and costs (3). Non-therapeutic ERCP refers to ERCP in which a gallstone is not identified in the common bile duct during the procedure. Recent guidelines have moved to increase specificity in the parameters to minimize these “negative” ERCPs (3). Modified pediatric scoring systems have been developed to assess which patients would benefit from ERCP. These scoring systems are primarily based on bilirubin and common bile duct (CBD) diameter (4–6). Specifically, conjugated bilirubin has been found to have a higher sensitivity than total bilirubin in predicting choledocholithiasis in pediatric patients (5). With this in mind, the primary aim of this study was to evaluate the ability of the currently recommended criteria to determine which pediatric patients with hemolytic diseases would benefit from ERCP to treat choledocholithiasis.
A secondary analysis was performed using the Pediatric ERCP Database Initiative (PEDI) database. This international database contains prospectively collected data from 1,124 ERCPs performed at 13 large, tertiary-care institutions. This study was part of a distinct substudy involving 8 of the 13 centers. Inclusion criteria were age less than 19 years and ERCP performed for suspected choledocholithiasis. Patients were excluded if they had a prior ERCP or intervention for choledocholithiasis, such as laparoscopic common bile duct exploration (LCBDE). Detailed information on this cohort has been published (7). The study was approved by each individual center's institutional review board, and informed consent was obtained as per each institution's institutional review board requirements.
Information was collected on patient demographics [e.g., age, sex, race, and body mass index (BMI)] and clinical presentation, including right upper quadrant pain at rest, right upper quadrant pain with palpation, jaundice, and nausea. It was also noted whether the patient had a diagnosis of gallstone pancreatitis or cholangitis. For patients over two years of age, obesity was defined as BMI greater than or equal to the 95th percentile for age and sex.
Patients' pre-ERCP imaging findings were assessed for the presence of a common bile duct stone. Imaging techniques used at the various institutions included ultrasound, magnetic resonance cholangiopancreatography (MRCP), intraoperative cholangiography (IOC), and endoscopic ultrasound (EUS). The presence of a common bile duct stone on intraoperative cholangiography was defined as no duodenal filling of contrast with filling of the CBD or identification of a fixed or mobile filling defect in the CBD. In the other imaging modalities, the presence of a common bile duct stone was identified visually, by shadowing on the sonograms or a filling defect on MRCP. Laboratory indices were also collected, including total bilirubin, conjugated or direct bilirubin, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, and alkaline phosphatase, at the time of admission and within 24 h of ERCP. ERCP outcomes were defined as whether a stone was identified and whether it was removed from the common bile duct during ERCP. Adverse events were categorized using the American Society for Gastrointestinal Endoscopy (ASGE) lexicon as pancreatitis, bleeding, failed cannulation, or other adverse events (8).
Patient data was evaluated in relation to the 2019 ASGE high-risk criteria, which include common bile duct diameter greater than 6 mm, total bilirubin greater than 4 mg/dl, clinical ascending cholangitis, or stone seen on ultrasound or cross-sectional imaging (3), and the 2016 modified Baylor pediatric criteria, which include common bile duct diameter greater than 6 mm and conjugated bilirubin greater than or equal to 0.5 mg/dl (5).
Study data were collected and managed using Research Electronic Data Capture (REDCap Software, Vanderbilt University) tool hosted at University of Texas Southwestern Medical Center. REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) methods for importing data from external sources. This study was supported by the UT Southwestern Academic Information System (CTSA NIH Grant UL1-RR024982) and the REDCap project. Data was analyzed by two-tailed Fisher's exact test and paired student t-test, with statistical significance drawn at p < 0.05.
296 patients who required ERCP were enrolled from 8 of the 13 sites within the PEDI registry. Of those, 11 patients without hemolytic diseases were excluded for prior ERCP, and 8 patients with hemolytic diseases were excluded for prior ERCP or LCBDE. Of the 277 patients meeting inclusion criteria, 47 (17.0%) had a hemolytic condition.
Demographics differed between the patients with hemolytic diseases and those without. Patients with hemolytic diseases were more likely to be of African American race (42.6% vs. 10.0%, p = 0.0001). Patients without hemolytic diseases were more likely to be of Hispanic ethnicity (60.0% vs. 17.0%, p = 0.0001), female sex (80.0% vs. 44.7%, p = 0.0001), and to have obesity (40.2% vs. 10.9%, p = 0.0001) (Table 1a).
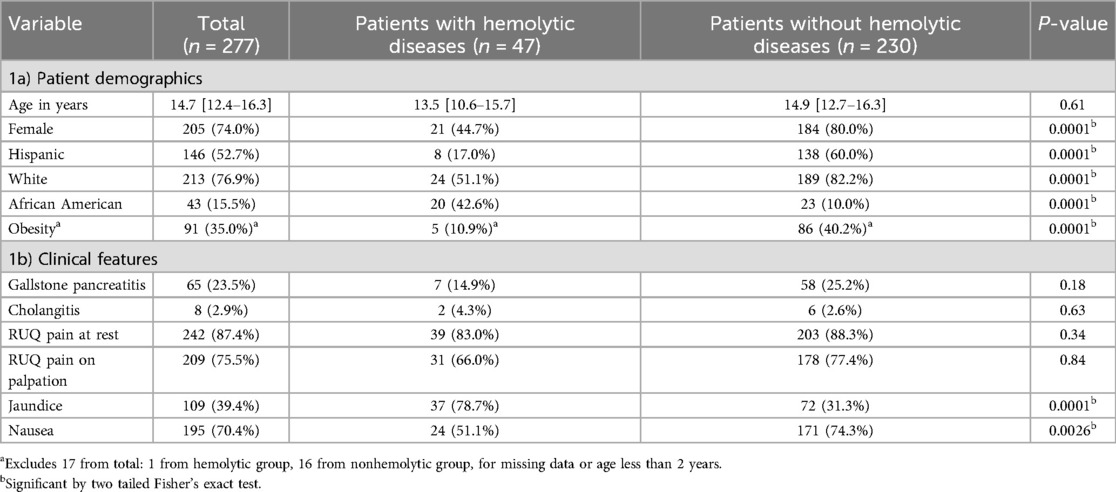
Table 1. Demographics and clinical features of patients with and without hemolytic diseases who received ERCP.
Clinically, patients with hemolytic diseases were more likely to have jaundice (78.7% vs. 31.3%, p = 0.0001) and less likely to have nausea (51.5% vs. 74.3%, p = 0.0026) than their non-hemolytic counterparts. There was no difference in right upper quadrant pain at rest (83.0% vs. 88.3%, p = 0.34) or with palpation (66.0% vs. 77.4%, p = 0.84) between the two groups. The incidence of gallstone pancreatitis (14.9% vs. 25.2%, p = 0.18) and cholangitis (4.3% vs. 2.6%, p = 0.63) did not differ between the two groups (Table 1b).
Among the 47 patients with hemolytic diseases, 34 (72.3%) had a CBD stone identified at the time of ERCP. The remaining 13 patients underwent ERCP but did not have a stone in the common bile duct at the time of the procedure, resulting in non-therapeutic ERCP. This is similar to previously published data on patients without hemolytic diseases from the PEDI Database, where 69 out of 95 (72.6%) had a CBD stone removed at ERCP (4). Among patients with hemolytic diseases, those with a stone removed at ERCP were more likely to present with jaundice (82.4% vs. 46.2%, p = 0.03). There was no difference in age, race, ethnicity, sex, obesity, gallstone pancreatitis, or cholangitis between the patients with hemolytic diseases who had a stone removed at ERCP and those who did not (Tables 2a,b). There was also no significant difference in pre-ERCP laboratory or imaging findings, including common bile duct stone on ultrasound, MRCP, intraoperative cholangiography, or endoscopic ultrasound (Tables 3a,b). However, the number of patients who received endoscopic ultrasound or intraoperative cholangiography was low (n = 6 and 1, respectively).
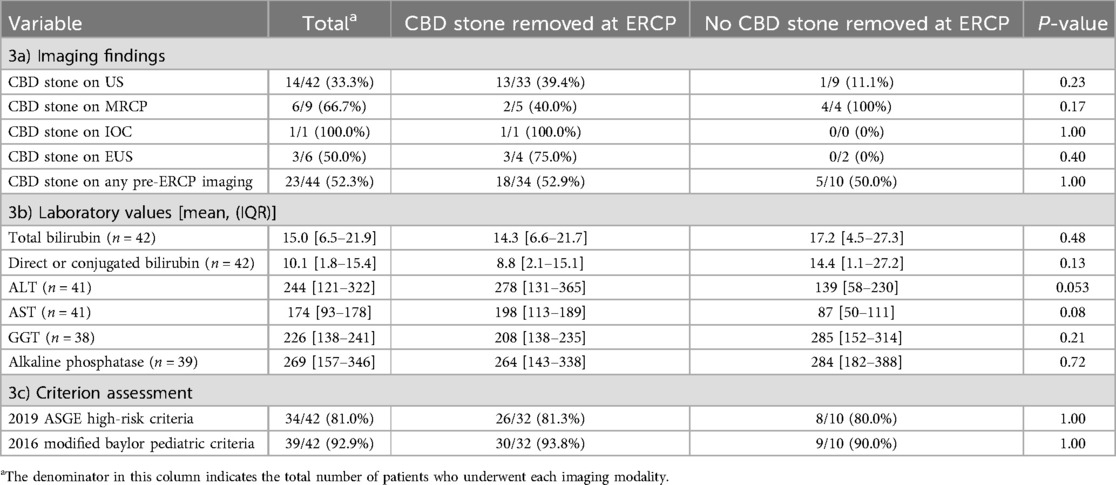
Table 3. Imaging findings and laboratory values of patients with hemolytic diseases who received ERCP.
Previously published selection criteria, including the ASGE high-risk criteria and the 2016 modified Baylor pediatric criteria, performed poorly in the patients with hemolytic diseases. Of the patients with hemolytic diseases who did not have a stone at the time of ERCP, 80.0% met the ASGE high-risk criteria, and 90.0% met the modified Baylor pediatric criteria. By comparison, 81.3% of patients with hemolytic diseases who had one or more stones removed at the time of ERCP met the ASGE high-risk criteria (p = 1) and 93.8% met the modified Baylor pediatric criteria (p = 1) (Table 3c).
While procedural technical success between patients with and without hemolytic diseases was similar (95.7% vs. 97.8%, p = 0.34), adverse events were experienced twice as commonly in the hemolytic population. However, this was not statistically significant (12.8% vs. 5.2%, p = 0.09). Specifically, the difference in bleeding between the two groups approached significance, with 6.4% of the patients with hemolytic diseases and 1.7% of those without hemolytic diseases experiencing bleeding after ERCP (p = 0.10) (Table 4).
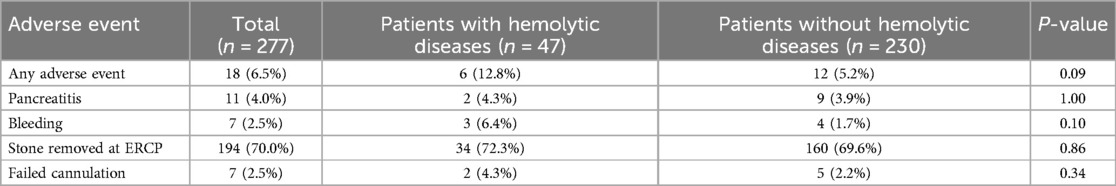
Table 4. Adverse events and technical outcomes among pediatric patients with and without hemolytic diseases who received ERCP.
This is the first pediatric study to specifically evaluate patients undergoing ERCPs with underlying hemolytic disorders. We found that existing ERCP criteria perform poorly in patients with hemolytic diseases. The existing criteria overestimate their risk of choledocholithiasis, possibly resulting in unnecessary ERCPs and their associated risks and costs. Current criteria rely on pre-procedure laboratory and imaging findings; in our cohort, there were no differences in these pre-ERCP evaluations in patients with hemolytic diseases with a common bile duct stone removed at ERCP and those without. These patients' clinical presentations and laboratory values, especially bilirubin, can be clouded by disease-specific factors such as ongoing hemolysis, intrahepatic sickling with or without infarction (in sickle cell disease), and iron overload related to blood transfusions. Total bilirubin includes components of conjugated or direct and unconjugated or indirect bilirubin. Unconjugated bilirubin in the setting of hemolysis is expected to be elevated. Therefore, risk stratification for choledocholithiasis is more complicated than in the general pediatric population and continues to be challenging. The most recent ASGE guideline made a conscious effort to increase specificity to greater than 90% by using more stringent criteria (3).
The poor performance of bile duct size and identification of a stone on ultrasound is consistent with non-hemolytic pediatric data that our group has previously published, in which there was no significant difference in ultrasound findings between patients who had a stone removed during ERCP and those who did not (4). Ultrasound overall seems to have poor sensitivity in detecting choledocholithiasis but is frequently used given its accessibility and lack of radiation.
Few patients in our study underwent other peri-procedure evaluations such as endoscopic ultrasound, MRCP, or intraoperative cholangiography, as the use and timing of these modalities is center-dependent. Nevertheless, these may be very important modalities given the poor ability to predict choledocholithiasis through labs and other imaging modalities in patients with hemolytic diseases. For example, a prior study demonstrated that MRCP better predicts choledocholithiasis in children than transabdominal ultrasound or CT, though it is important to consider the timing of MRCP in relation to ERCP as stones may pass if there is delay (4). The delay between MRCP and ERCP is of particular relevance in pediatrics, as many pediatric patients require anesthesia to undergo MRCP and therefore MRCP is oftentimes not performed on the same day as the ERCP, potentially allowing time for the stone to pass prior to ERCP. Additionally, some pediatric centers lack the ability to perform sedated MRCP on weekends.
While very few children in our study underwent intraoperative cholangiogram (IOC) or endoscopic ultrasound (EUS) (n = 1 and 6, respectively), these modalities appeared to perform well; the patient who had a positive IOC had a stone removed during ERCP, and all three of the patients who had a positive EUS had a stone removed during ERCP (Table 3). However, these are very small samples. EUS has been recommended in select patients to diagnose choledocholithiasis with good specificity (9, 10). However, in the adult literature, EUS is thought to have higher sensitivity for detecting small stones (9, 10). While EUS is a relatively recent development in pediatric gastroenterology, it has been shown to be safe and have good technical outcomes in children (11–13). EUS is radiation-sparing and, in at least two studies in the pediatric population, has been shown to successfully prevent a subset of patients from undergoing non-therapeutic ERCP (12, 13). Regarding IOC, there is some data that performing IOC prior to planned ERCP can prevent non-therapeutic ERCP in a subset of patients who have preoperative labs and imaging that are falsely concerning for choledocholithiasis (14).
Overall, increased use of peri-procedure evaluations may decrease the incidence of non-therapeutic ERCPs in the hemolytic disease population, but more data is needed to determine acceptable sensitivity and specificity of these modalities in pediatric patients. Additionally, the potential costs and risk of additional time spent under sedation to perform these evaluations must be weighed clinically. This dilemma is of particular relevance in the hemolytic disease population. For example, when considering patients with sickle cell disease who have a history of stroke or acute chest syndrome, one must balance the desire to avoid anesthesia exposure with potential ERCP complications such as pancreatitis, which could also prompt an episode of acute chest syndrome.
Finally, while not statistically significant, the increased number of adverse events in patients with hemolytic diseases highlights the importance of careful patient selection for ERCP. In particular, the suggestion of increased bleeding in patients with hemolytic diseases is of interest. Our incidence of bleeding in 2.5% of patients (7/277) is slightly higher than the previously published incidence of 1.2% from the PEDI database, though we did not sub-categorize by severity of bleeding in this substudy and the difference between patients with and without hemolytic diseases was not significant (7). Sickle cell disease, the most common hemolytic disease in our patient population, is typically conceptualized as a prothrombotic state that predisposes patients to cerebral infarction and venous thromboembolism (15); however, there are some reports of spontaneous bleeding events in adults with sickle cell disease, mainly in the gastrointestinal tract (16). A plausible contributing factor to bleeding is heavy NSAID use due to chronic sickle cell-related pain, but this is unlikely to fully explain the bleeding risk in this population. Overall, further investigation is needed to determine which factors would better predict the need for ERCP in patients with hemolytic diseases.
The datasets presented in this article are not readily available because data release is restricted by data use agreements between each of the participating sites and UT Southwestern Medical Center. Requests to access the datasets should be directed toZGF2aWQudHJvZW5kbGVAdXRzb3V0aHdlc3Rlcm4uZWR1.
The studies involving humans were approved by UT Southwestern Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
JT: Conceptualization, Writing – original draft, Writing – review & editing. WR: Writing – original draft, Writing – review & editing. DF: Conceptualization, Writing – original draft, Writing – review & editing. MG: Writing – original draft, Writing – review & editing. KK: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. LD: Writing – original draft, Writing – review & editing. VB: Writing – original draft, Writing – review & editing. FT: Writing – original draft, Writing – review & editing. PD: Writing – original draft, Writing – review & editing. SF: Writing – original draft, Writing – review & editing. CT: Writing – original draft, Writing – review & editing. MW: Writing – original draft, Writing – review & editing. RK: Writing – original draft, Writing – review & editing. PM: Writing – original draft, Writing – review & editing. QL: Writing – original draft, Writing – review & editing. YZ: Writing – original draft, Writing – review & editing. BB: Writing – original draft, Writing – review & editing. DT: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mohamed SOO, Ibrahim OAO, Mohammad DAA, Ali AHM. Correlates of gallbladder stones among patients with sickle cell disease: a meta-analysis. JGH Open. (2021) 5:997–1003. doi: 10.1002/jgh3.12622
2. Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol H. (2010) 8:483–9. doi: 10.1016/j.cgh.2010.02.016
3. Committee AS of P, Buxbaum JL, Fehmi SMA, Sultan S, Fishman DS, Qumseya BJ, et al. ASGE Guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc. (2019) 89:1075–105.e15. doi: 10.1016/j.gie.2018.10.001
4. Fishman DS, Barth B, Tsai CM-W, Giefer MJ, Martinez M, Wilsey M, et al. A prospective multicenter analysis from the pediatric ERCP database initiative: predictors of choledocholithiasis at ERCP in pediatric patients. Gastrointest Endosc. (2021) 94:311–17.e1. doi: 10.1016/j.gie.2021.01.030
5. Fishman DS, Chumpitazi BP, Raijman I, Tsai CM-W, Smith EO, Mazziotti MV, et al. Endoscopic retrograde cholangiography for pediatric choledocholithiasis: assessing the need for endoscopic intervention. World J Gastrointest Endosc. (2016) 8:425–32. doi: 10.4253/wjge.v8.i11.425
6. Capparelli MA, D'alessandro PD, Questa HA, Ayarzabal VH, Bailez MM, Barrenechea ME. Development of a risk score for choledocholithiasis in pediatric patients. Pediatr Surg Int. (2021) 37:1393–9. doi: 10.1007/s00383-021-04952-9
7. Troendle DM, Ruan W, Fishman DS, Barth BA, Liu QY, Giefer M, et al. Technical outcomes in pediatric endoscopic retrograde cholangiopancreatography. J Pediatr Gastroenterol Nutr. (2022) 75:755–60. doi: 10.1097/mpg.0000000000003612
8. Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. (2010) 71:446–54. doi: 10.1016/j.gie.2009.10.027
9. Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, et al. Endoscopic management of common bile duct stones: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. (2019) 51:472–91. doi: 10.1055/a-0862-0346
10. Meeralam Y, Al-Shammari K, Yaghoobi M. Diagnostic accuracy of EUS compared with MRCP in detecting choledocholithiasis: a meta-analysis of diagnostic test accuracy in head-to-head studies. Gastrointest Endosc. (2017) 86:986–93. doi: 10.1016/j.gie.2017.06.009
11. Mahajan R, Simon EG, Chacko A, Reddy DV, Kalyan PR, Joseph AJ, et al. Endoscopic ultrasonography in pediatric patients—experience from a tertiary care center in India. Indian J Gastroenterol. (2016) 35:14–9. doi: 10.1007/s12664-016-0619-2
12. Barakat MT, Cagil Y, Gugig R. Landscape of pediatric endoscopic ultrasound in a United States tertiary care medical center. J Pediatr Gastroenterol Nutr. (2022) 74:657–61. doi: 10.1097/mpg.0000000000003403
13. Scheers I, Ergun M, Aouattah T, Piessevaux H, Borbath I, Stephenne X, et al. Diagnostic and therapeutic roles of endoscopic ultrasound in pediatric pancreaticobiliary disorders. J Pediatr Gastroenterol Nutr. (2015) 61:238–47. doi: 10.1097/mpg.0000000000000692
14. Waldhausen JHT, Graham DD, Tapper D. Routine intraoperative cholangiography during laparoscopic cholecystectomy minimizes unnecessary endoscopic retrograde cholangiopancreatography in children. J Pediatr Surg. (2001) 36:881–4. doi: 10.1053/jpsu.2001.23960
15. Shet AS, Lizarralde-Iragorri MA, Naik RP. The molecular basis for the prothrombotic state in sickle cell disease. Haematologica. (2020) 105:2368–79. doi: 10.3324/haematol.2019.239350
Keywords: ERCP, choledocholithiasis, hemolytic disease, sickle cell, predictor
Citation: Thompson J, Ruan W, Fishman DS, Giefer M, Kim KM, Martinez M, Dall'Oglio L, Balassone V, Torroni F, De Angelis P, Faraci S, Tsai C, Wilsey M, Khalaf R, Mamula P, Liu Q, Zheng Y, Barth BA and Troendle DM (2025) Risk scores for choledocholithiasis perform poorly in patients with hemolytic diseases: a PEDI database report. Front. Pediatr. 13:1574462. doi: 10.3389/fped.2025.1574462
Received: 10 February 2025; Accepted: 17 March 2025;
Published: 8 April 2025.
Edited by:
Valeria Dipasquale, University of Messina, ItalyReviewed by:
Sravan Kumar Reddy Matta, Kaiser Permanente, United StatesCopyright: © 2025 Thompson, Ruan, Fishman, Giefer, Kim, Martinez, Dall'Oglio, Balassone, Torroni, De Angelis, Faraci, Tsai, Wilsey, Khalaf, Mamula, Liu, Zheng, Barth and Troendle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Troendle, ZGF2aWQudHJvZW5kbGVAdXRzb3V0aHdlc3Rlcm4uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.