
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 01 April 2025
Sec. Pediatric Critical Care
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1570859
Objective: The present study aimed to analyze the incidence of hyperchloremia and compare the time to reach DKA resolution with different parameters.
Methods: A chart review of patients diagnosed with DKA and aged <18 years was conducted. DKA was defined as serum glucose ≧200 mg/dl, venous pH (vpH) <7.30, serum bicarbonate <15 mmol/L, and presence of ketonemia, or ketonuria. Electrolytes and blood gases were recorded at 6-h intervals after treatment initiation.
Results: Overall, 84 patients were admitted because of DKA. The initial biomedical parameters in the emergency department were as follows: median glucose, 497 mg/dl; vpH, 7.1; serum HCO3, 6.6 mmol/L; anion gap (AG), 24.7 mmol/L; and ketone, 5.7 mmol/L. After treatment, the incidence of hyperchloremia increased progressively from 15.4% at treatment initiation to 80% at 18 h. The median time to resolution defined by AG ≦12 mmol/L was 12 h, which was significantly faster than the recovery of vpH ≧7.3 (median time, 17 h) and HCO3 >15 mmol/L (median time, 18 h). Approximately 63 (75%) patients reached the endpoints of AG ≦12 mmol/L prior, 14 (16.6%) patients reached the endpoints of vpH ≧7.3 prior, 7 (8.4%) patients reached the endpoints of HCO3 >15 mmol/L prior.
Conclusions: Hyperchloremic metabolic acidosis (HMA) was a common entity in pediatric DKA following treatment. The median time of AG ≦ 12 mmol/L was approximately 12 h and was the parameter that can identify DKA resolution at a faster rate, i.e., approximately 5, and 6 h faster than the normalization of vpH and HCO3, respectively. Future studies were warranted to use AG ≦12 mmol/L as the endpoint of DKA treatment and check if the treatment course and incidence of HMA could be reduced.
Diabetic ketoacidosis (DKA) is a potentially critical complication of type 1 diabetes mellitus (T1D) that mainly causes diabetes-related comorbidity and mortality in pediatric T1D. DKA is present at the diagnosis of T1D in 30%–40% of children in the USA (1–3). Furthermore, approximately one-half of these patients with DKA were diagnosed with moderate-to-severe DKA (pH <7.2) (2, 4). A more severe DKA was associated with higher rates of complications, indicating the need for management by an experienced diabetes team best in the pediatric intensive care unit (PICU).
The common therapeutic protocol for DKA included checking blood sugar hourly and serum electrolytes and blood gases every 4–6 h. The laboratory testing for defining DKA resolution included venous pH (vpH), plasma bicarbonate (HCO3), anion gap (AG), and ketone bodies [blood beta-hydroxybutyrate (BOHB) and urine acetoacetate]. The common cutoff value of these biomedical parameters for identifying DKA resolution included blood sugar <200 mg/dl, vpH ≧7.3, HCO3 >15 mmol/L, AG ≦12 mmol/L, and BOHB ≦1 mmol/L (5, 6).
Volume expansion with isotonic saline (0.9% sodium chloride) to restore the effective circulating volume is the first step of resuscitation for DKA. Hyperchloremic metabolic acidosis (HMA) usually develops after replacement with large amounts of isotonic saline solution. HMA would result in HCO3 loss to maintain electroneutrality. A previous study demonstrated that HMA was noted in nearly 94% of the patients with DKA after 20 h of fluid resuscitation (7). Recently, von Oettingen et al. reported that the single parameter serum HCO3 >15 mmol/L can be used to identify DKA resolution (8). However, previous studies have reported that HCO3 >15 mmol/L is probably slower than the recovery of vpH ≧7.3 and AG ≦12 mmol/L in patients with DKA N (7–10). Many clinicians may focus on HCO3 >15 mmol/L to indicate DKA resolution. However, HCO3 recovery may be masked by HMA, which would result in additional fluid replacement and intravenous (IV) insulin therapy. This additional therapy may result in complications such as cerebral edema and increasing the length of PICU stay (11, 12).
Since only a few studies have analyzed the incidence of HMA in pediatric DKA after treatment and the time it takes for different biomedical parameters to reach DKA resolution. This study aimed to analyze the effect of HMA and compare the time to DKA resolution with different parameters.
This retrospective study analyzed all pediatric patients diagnosed with DKA and admitted to the PICU of Chang Gung Children's Hospital between January 2016 and December 2020. This study was approved by the institutional review board of Chang Gung Memorial Hospital (No. 202300264B0).
DKA was defined as serum glucose ≧200 mg/dl, vpH <7.30, serum bicarbonate <15 mmol/L, and presence of ketonemia, or ketonuria (1).
This study enrolled all patients diagnosed with DKA and aged <18 years. Patients who had other notable metabolic disorders were excluded. Electrolytes and blood gases were recorded at 6-h intervals after treatment initiation.
The severity of DKA was categorized into three groups according to the acid–base status (8): mild (vpH 7.2 ≤7.3), moderate (vpH 7.1 ≤7.2), and severe (vpH <7.1).
AG was defined as sodium—(chloride + bicarbonate) (reference range 12 ± 2 mmol/L), and AG closure was defined as AG ≦12 mmol/L.
Hyperchloremia was defined as the ratio of chloride to sodium (Cl: Na) >0.79 (9).
HMA was defined as hyperchloremia accompanied by non-AG metabolic acidosis (normal AG).
DKA resolution was defined as vpH ≧7.3, AG ≦12 mmol/L, or bicarbonate >15 mmol/L (5, 8). The treatment protocol for DKA in our hospital recommended normal saline administration during the initial resuscitation in the emergency department. Then, a two-bag system, which had been utilized since the 1990s, was administered in our PICU (10). The two-bag system contains two identical amounts of electrolytes (potassium chloride and potassium phosphate) but different concentrations of glucose. The initial insulin infusion rate was 0.1 u/kg/h. By adjusting the rate of the two bags, different rates of dextrose administration can be accomplished (11).
The primary outcome was to analyze the incidence of HMA after treatment. The secondary outcome was to analyze the time required for the three important biomedical parameters (vpH, HCO3, and AG) to reach the cutoff values of DKA resolution, and identify which biomedical parameters reach the cutoff values first (e.g., vpH ≧7.3, HCO3 >15 mmol/L, or AG ≦12 mmol/L).
Data were reported as mean ± standard deviation (SD) or median (interquartile range).
The Kruskal–Wallis test was used to examine differences in continuous variables.
Univariate analyses were performed using the chi-square test, Fisher's exact test, or Mann–Whitney U test, as appropriate. A P-value of <0.05 was considered statistically significant. The IBM SPSS Statistics for Windows version 20.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
In total, 84 patients diagnosed with DKA were enrolled in this study (Table 1). The mean age was 11.1 years, with a male-to-female ratio of 0.8. Approximately half of the patients (n = 43, 51.2%) had severe DKA. With increasing DKA severity, fluid administration also increased significantly. The median glycated hemoglobin (HbA1c) was 13.9. The initial biomedical parameters in the emergency department were as follows: median glucose, 497 mg/dl; vpH, 7.1; serum HCO3, 6.6 mmol/L; AG, 24.7 mmol/L; and ketone, 5.7 mmol/L.
Table 2 shows the temporal profile of the biomedical parameters during the first 24 h of in-hospital treatment. Metabolic acidosis persisted at 18 h (median pH, 7.32; base deficit, 10.7 mmol/L; Figure 1A), although the normalization of bicarbonate (from 6.7 to 15.5 mmol/L, Figure 1B) suggested DKA resolution. High AG persisted for 12 h (from 24.7 to 12 mmol/L, Figure 1C). The incidence of hyperchloremia increased progressively from 15.4% at treatment initiation to 80% at 18 h (Figure 1D).
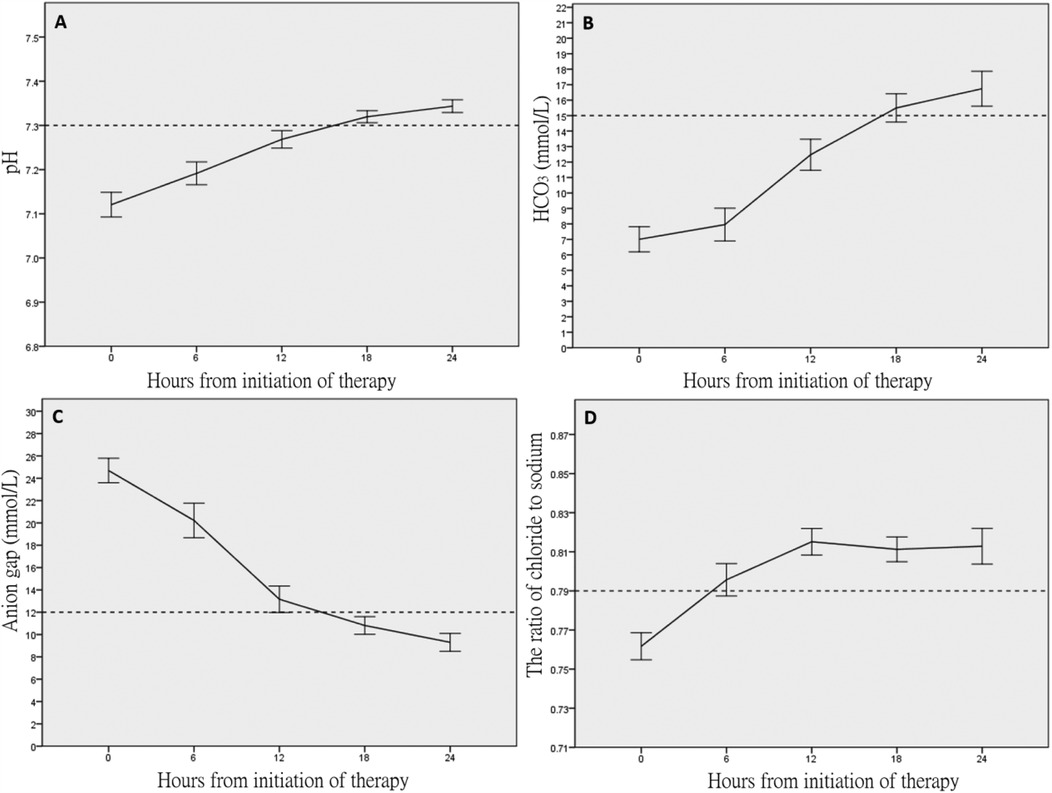
Figure 1. Temporal profiles of the acid–base and electrolyte parameters within 24 h after treatment. (A) vpH, (B) HCO3 (mmol/L), (C) anion gap, and (D) ratio of chloride to sodium.
Table 3 reports the time to DKA resolution. The median time to DKA resolution (defined as vpH ≧7.3 and AG ≦12 mmol/L) was 18 h [interquartile range (IQR) 12–24]. Not unexpectedly, mild DKA (median time to resolution, 12 h) resolved more rapidly than moderate DKA (median time to resolution, 18 h) and severe DKA (median time to resolution, 24 h). The time to resolution defined by AG ≦12 mmol/L was significantly shorter than the resolution time defined by vpH and AG, although shorter than the two discrete endpoints (vpH ≧7.3 or HCO3 >15 mmol/L). Approximately 63 (75%) patients reached the endpoints of AG ≦12 mmol/L prior, 14 (16.6%) reached the endpoints of vpH ≧7.3 prior, 7 (8.4%) reached the endpoints of HCO3 >15 mmol/L prior.
In this study, approximately four-fifths of the patients were diagnosed with moderate-to-severe DKA on the initial presentation. Although the complications were rare in children with DKA, such a high proportion of moderate-to-severe DKA still reminds clinicians that the majority of pediatric patients with DKA need intensive care because these patients need frequent follow-up of biomedical data, more fluid resuscitation, and insulin dosing. Hourly monitoring of vital signs and neurologic symptoms is important in those patients. In addition, the high rate of hyperchloremia increased progressively with time, which indicated that the majority of patients experienced HMA during treatment, which would prolong recovery from DKA. In most of the patients (75%), the AG decreased to normal before the normalization of vpH and serum HCO3 levels.
A previous study reported that the development of hyperchloremia during treatment for adult DKA was associated with worsening clinical outcomes such as longer time to DKA resolution, higher rates of acute kidney injury, and longer hospital stay (12). The present study identified a high proportion of hyperchloremia during DKA resuscitation that increased with time, which were comparable with the results of previous pediatric studies (7, 13, 14). Initially, all patients presented with pure keto-acidosis (high AG acidosis), and then 75% experienced secondary HMA (normal AG) during the first 24 h of treatment. The phenomenon was caused by chloride-rich fluids used during treatment, following the renal excretion of anionic ketones (as HCO3 precursors), and then chloride was reabsorbed for maintaining electroneutrality (15, 16). The HMA may sometimes mask DKA resolution if the intensivists were not cautious during treatment.
In our cohort, the normalization of AG reflected resolution of DKA faster than vpH and HCO3. A previous study reported that HCO3 >15 mmol/L maybe a reliable parameter in DKA prediction (8). HCO3 recovery was significantly slower than AG closure, and DKA resolution based on the recovery of HCO3 and vpH may result in unnecessary treatment. The study identified that AG closure was a reliable parameter for detecting pediatric DKA resolution and occurred 5 and 6 h faster than the normalization of vpH and HCO3, respectively.
In clinical practice in the PICU, patients were not transferred to the general ward from the ICU until oral intake and subcutaneous insulin were started; however, this usually did not happen until acidosis had been resolved. HMA developed in the majority of patients with DKA and would prolong the therapeutic course. Previous studies recommended that AG closure can be an early parameter to determine DKA resolution (7, 8), and this study also demonstrated this phenomenon. By using AG ≦12 mmol/L to determine DKA resolution, overtreatment could be avoided (fluid therapy and insulin infusion), oral intake started early, subcutaneous insulin, and ultimately reduction of e the length of PICU stay and medical costs. Future prospective studies are warranted to use AG closure as the endpoint of DKA treatment to determine if it can reduce the incidence of HMA and therapeutic course.
This study is limited by the retrospective design, small sample size, and single-center setting, which could have resulted in information bias. Future studies with a larger number of patients at different centers are needed.
HMA was a common entity in pediatric DKA after treatment. The median time of AG closure was approximately 12 h and was the parameter that can detect DKA resolution at faster rates, i.e., approximately 5, and 6 h faster than the normalization of vpH and HCO3, respectively. Future studies should use AG ≦12 mmol/L as the endpoint of DKA treatment and determine whether the treatment course and incidence of HMA could be reduced.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Institutional Review Board of Chang Gung Memorial Hospital, (No. 202300264B0). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this is a routine medical treatment and examination, no consent is required. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because this study is part of routine medical care and testing and no consent is required.
E-PL: Conceptualization, Writing – review & editing. Y-HL: Writing – original draft. Y-TS: Formal analysis, Investigation, Writing – original draft. J-JL: Data curation, Methodology, Writing – original draft. O-WC: Resources, Software, Validation, Writing – original draft. C-WY: Methodology, Writing – original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Chang Gung Memorial Hospital (CORPG3M0341 and CORPG3M0351).
The authors thank the statistician at Chang Gung Memorial Hospital for the statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wolfsdorf J, Glaser N, Sperling MA, Association AD. Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. (2006) 29:1150–9. doi: 10.2337/diacare.2951150
2. Klingensmith GJ, Tamborlane WV, Wood J, Haller MJ, Silverstein J, Cengiz E, et al. Diabetic ketoacidosis at diabetes onset: still an all too common threat in youth. J Pediatr. (2013) 162:330–4.e1. doi: 10.1016/j.jpeds.2012.06.058
3. Dabelea D, Rewers A, Stafford JM, Standiford DA, Lawrence JM, Saydah S, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. (2014) 133:e938–45. doi: 10.1542/peds.2013-2795
4. Jensen ET, Stafford JM, Saydah S, D'Agostino RB, Dolan LM, Lawrence JM, et al. Increase in prevalence of diabetic ketoacidosis at diagnosis among youth with type 1 diabetes: the SEARCH for diabetes in youth study. Diabetes Care. (2021) 44:1573–8. doi: 10.2337/dc20-0389
5. Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser N, Jain V, et al. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. (2014) 15:154–79. doi: 10.1111/pedi.12165
6. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. (2009) 32:1335–43. doi: 10.2337/dc09-9032
7. Taylor D, Durward A, Tibby SM, Thorburn K, Holton F, Johnstone I, et al. The influence of hyperchloraemia on acid base interpretation in diabetic ketoacidosis. Intensive Care Med. (2006) 134:295–301. doi: 10.1007/s00134-005-0009-1
8. von Oettingen JE, Rhodes ET, Wolfsdorf JI. Resolution of ketoacidosis in children with new onset diabetes: evaluation of various definitions. Diabetes Res Clin Pract. (2018) 135:76–84. doi: 10.1016/j.diabres.2017.09.011
9. Durward A, Skellett S, Mayer A, Taylor D, Tibby SM, Murdoch IA. The value of the chloride: sodium ratio in differentiating the aetiology of metabolic acidosis. Intensive Care Med. (2001) 27:828–35. doi: 10.1007/s001340100915
10. Grimberg A, Cerri RW, Satin-Smith M, Cohen P. The “two bag system” for variable intravenous dextrose and fluid administration: benefits in diabetic ketoacidosis management. J Pediatr. (1999) 134:376–8. doi: 10.1016/S0022-3476(99)70469-5
11. Poirier MP, Greer D, Satin-Smith M. A prospective study of the “two-bag system” in diabetic ketoacidosis management. Clin Pediatr. (2004) 43:809–13. doi: 10.1177/000992280404300904
12. Goad NT, Bakhru RN, Pirkle JL, Kenes MT. Association of hyperchloremia with unfavorable clinical outcomes in adults with diabetic ketoacidosis. J Intensive Care Med. (2020) 35:1307–13. doi: 10.1177/0885066619865469
13. Mrozik LT, Yung M. Hyperchloraemic metabolic acidosis slows recovery in children with diabetic ketoacidosis: a retrospective audit. Aust Crit Care. (2009) 22:172–7. doi: 10.1016/j.aucc.2009.05.001
14. Basnet S, Venepalli PK, Andoh J, Verhulst S, Koirala J. Effect of normal saline and half normal saline on serum electrolytes during recovery phase of diabetic ketoacidosis. J Intensive Care Med. (2014) 29:38–42. doi: 10.1177/0885066612467149
15. Adrogue HJ, Wilson H, Boyd AE III, Suki WN, Eknoyan G. Plasma acid-base patterns in diabetic ketoacidosis. N Engl J Med. (1982) 307:1603–10. doi: 10.1056/NEJM198212233072603
Keywords: diabetic ketoacidosis, children, hyperchloremia, resolution, anion gap
Citation: Liu Y-H, Su Y-T, Lin J-J, Chan O-W, Yen C-W and Lee E-P (2025) Early parameter to detect the resolution of pediatric diabetic ketoacidosis. Front. Pediatr. 13:1570859. doi: 10.3389/fped.2025.1570859
Received: 4 February 2025; Accepted: 19 March 2025;
Published: 1 April 2025.
Edited by:
Adnan Bhutta, Riley Hospital for Children, United StatesReviewed by:
Jason Custer, University of Maryland, United StatesCopyright: © 2025 Liu, Su, Lin, Chan, Yen and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: En-Pei Lee, cGlsaWNocmlzbG5wQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.