
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 20 March 2025
Sec. Neonatology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1562736
This article is part of the Research Topic Evaluating Efficacy and Outcomes in Neonatal HIE Treatment: A Global Perspective View all articles
Objective: To investigate the safety and efficacy of servo-controlled cooling during the transport of neonates with perinatal asphyxia.
Methods: We conducted a retrospective non-randomized case-control study at a single-center,which included 65 neonates diagnosed with Hypoxic-Ischemic Encephalopathy (HIE). These neonates were referred by the Shenzhen Children's Hospital medical transport team between January 2020 and June 2024. All subjects received 72 h of mild hypothermia treatment upon admission. Participants were categorized into an active group and a control group based on the use of servo-controlled cooling during transport. To evaluate differences in clinical characteristics, transport variables, and hospitalization outcomes between the two groups, we employed independent samples t-tests, Mann–Whitney U tests, and χ2 tests for inter-group comparison.
Results: Among the 65 subjects, there were 42 males and 23 females. The active group comprised 17 patients, while the control group included 48. No statistically significant differences were observed in sex, gestational age, birth weight, or HIE grade between the two groups (P > 0.05). In comparison to the control group, the active group experienced a shorter duration from leaving the referral center to reaching the target temperature (1 h vs. 2.67 h, Z = −4.513, P < 0.05), arrived at the treatment center at a lower temperature (34.03°C vs. 35.6°C, t = −4.991, P < 0.05), and demonstrated a higher proportion of patients within the target temperature range upon arrival [88.2% (15/17) vs. 16.7% (8/48), χ2 = −0.774, P < 0.05]. Additionally, the length of hospitalization was shorter for the active group (15 days vs. 19 days, Z = −2.835, P < 0.05). The proportion of patients in the severe range on the aEEG recorded on the third day of cooling was higher in the control group [45.8% (22/48) vs. 11.8% (2/17), Z = −2.042, P < 0.05].
Conclusion: Active therapeutic hypothermia during transport is both safe and feasible.It enables a more rapid and stable achievement of the target temperature, enhances short-term EEG outcomes, and may serve as the preferred method for transporting neonates with hypoxic-ischemic encephalopathy(HIE).
Hypoxic-Ischemic Encephalopathy (HIE) is a significant contributor to neonatal mortality and disability. Statistics indicate that the incidence of HIE is 0.15% in developed countries, while it ranges from 0.23% to 2.65% in developing countries. Approximately 20% of infants with HIE may die during the neonatalperiod, and 40% of survivors may experience neurological and other disabilities (1). Without treatment, 62% of neonates with HIE infants will develop moderate to severe disabilities or die by 18–22 months; however, this risk can be reduced to 41% with appropriate intervention (2, 3). Evidence suggests that controlled hypothermia therapy effectively reduces the likelihood of death or severe neurodevelopmental impairment in neonates with moderate to severe HIE, establishing it as the standard treatment for neuroprotection in this condition, with early initiation being critical (1, 4–7). However, managing moderate to severe HIE and implementing hypothermia requires multidisciplinary collaboration and advanced care (5). Some eligible HIE infants are born in centers lacking hypothermia treatment, which poses the risk of delays due to long-distance transfers that may exceed the recommended initiation timeline. Currently, there is limited research in China regarding the implementation and outcomes of hypothermia during transport for cases of asphyxia. This study analyzes the clinical data of asphyxia cases referred to our center, exploring the safety and efficacy of active hypothermia during transport, thereby providing a reference for clinical implementation.
This retrospective non-randomized case-control study collected data from neonates with asphyxia who received controlled hypothermia treatment in the Neonatal Intensive Care Unit (NICU) of Shenzhen Children's Hospital from January 2020 to June 2024. The inclusion criteria were as follows (8, 9): (1) gestational age ≥35 weeks or birth weight ≥2,000 g; (2) evidence of hypoxia-ischemia meeting at least one of the following conditions: clear evidence of fetal distress (e.g., uterine rupture, placental abruption, abnormal fetal heart rate); 5 min Apgar score ≤5; resuscitation requiring positive pressure ventilation for more than 10 min; umbilical blood gas analysis indicating pH ≤7.10 or base excess ≤−12 mmol/L within one hour after birth; and (3) neurological assessment indicating moderate to severe HIE according to the modified Sarnat criteria (10). If mild HIE is diagnosed, the decision to initiate cooling should be made after consultation with a neonatal specialist. Exclusion criteria included: (1) severe congenital malformations; (2) traumatic brain injury or moderate to severe intracranial hemorrhage; (3) congenital metabolic diseases; (4) age at cooling initiation greater than 24 h or cessation of cooling within 72 h. Patients were categorized into two groups based on the use of servo-controlled cooling during transport: the Active Group, in which cooling was administered using a servo-controlled cooling device (Tecotherm Neo, Inspiration Healthcare, LTD; implemented at our center since September 2022), and the Control Group, in which infants were transported in incubators with temperatures maintained at 32–34°C without active cooling. The study received approval from the ethics committee of Shenzhen Children's Hospital (202403202), and informed consent was obtained from the guardians.
Clinical Data: Information was gathered through a review of the hospital's electronic medical record system, focusing on three main categoies: (1) Baseline Data: gestational age, gender, birth weight, mode of delivery, Apgar score, and the first blood gas analysis (umbilical/arterial/venous) as well as the severity classification of HIE (10); (2) Transport Data: the age at which the neonate commenced hypothermia, th age at which the target temperature was achieved, the age upon arrival at the treatment center, the duration from departure at the referral center to achieving the target temperature (33°C-34°C), the temperature upon arrival at the treatment center, the proportion of neonates whose temperature fell within the target range upon arrival, heart rate, mean blood pressure, and blood gas analysis (pH, base excess) before and after transport; (3) Hospitalization Data: administration of vasopressors, duration of mechanical ventilation, length of hospital stay, seizures confirmed by electroencephalogram, amplitude-integrated electroencephalogram (aEEG) on days 1 and 3 of cooling, classification of HIE-related brain injury and affected areas on cranial MRI, and mortality rate.
All neonates underwent continuous video electroencephalogram (EEG) monitoring for four hours on the first and third days of cooling. This monitoring was performed at the bedside using the Nicolet Monitor (Natus, Middleton, WI, USA) and was conducted by trained neonatal specialist nurses. In accordance with the “Chinese Expert Consensus on the Clinical Application of aEEG in Neonates” (11), the aEEG was classified based on the upper and lower voltage limits of the background as follows: (1) Normal: upper boundary of the EEG activity amplitude spectrum ≥10 μV, lower boundary ≥5 μV; (2) Mildly abnormal: upper boundary >10 μV, lower boundary <5 μV; or normal amplitude with convulsive episodes; 3. Severely abnormal: upper boundary <10 μV, lower boundary <5 μV; or abnormal amplitude with convulsive episodes.
All neonates underwent cranial MRI examinations within 7–14 days after birth. These examinations included T1 and T2-weighted imaging, as well as diffusion-weighted series. In term neonates, two primary injury patterns associated with HIE are observable on MRI (12): (1) basal ganglia-thalamic pattern, which primarily affects the bilateral central gray nuclei (ventrolateral thalamus and posterior putamen) and the periventricular cortex; and (2) the watershed pattern, which involves the watershed areas (anterior-middle cerebral artery and posterior-middle cerebral artery), affecting the white matter. Based on the regions of brain injury, classifications include (12, 13): (1) deep gray matter (thalamus, basal ganglia, posterior limb of the internal capsule, brainstem, periventricular cortex, and hippocampus); (2) cerebral white matter/cortex (cortex, white matter, optic radiation, corpus callosum, punctate white matter lesions, and intraparenchymal hemorrhage); and (3) cerebellum (cerebellum and cerebellar hemorrhage).
In the Active Group, the transport team initiated active cooling immediately upon arrival at the referral center. Cooling was maintained continuously during transport, alongside intensive care management that included respiratory support, hemodynamic stabilization, and seizure control. A servo-controlled cooling device, which could be securely mounted on the transport platform, was utilized. The neonate was positioned on the mattress of the transport incubator with minimal clothing. The target temperature was achieved by circulating water through the device and the mattress. A core temperature monitoring probe was connected to one end of the device and inserted 4–5 cm into the infant's rectum at the other end. The device effectively maintained the set temperature range of 33℃–34℃ by continuously monitoring the infant's rectal temperature (14). Throughout the process, the nursing staff ensured that the temperature probe remained securely in place.
In cases where the asphyxiated neonate initially did not meet the established criteria for cooling, the neonate's clinical condition was closely monitored throughout transport. The evolution of the condition was dynamically assessed to determine the necessity for cooling intervention. Due to the early manifestation of symptoms and the limited diagnostic capabilities at the referral center, the severity of HIE could not be promptly evaluated. If there was significant suspicion of potential progression to moderate or severe HIE, cooling intervention was initiated following consultation with a neonatal specialist. Upon arrival at the NICU, aEEG was employed to assist in assessing the severity of HIE (15).
In the control group, neonates were transported in an incubator set to 32℃–34°C, receiving only intensive care management without any controlled cooling until their arrival at the NICU, where hypothermia treatment was subsequently initiated.
During transport, respiratory support was customized according to the neonate's respiratory condition, utilizing invasive synchronized intermittent mandatory ventilation (SIMV) mode, non-invasive nasal intermittent positive pressure ventilation (NIPPV) mode, or withholding support when deemed unnecessary. Sedation was administered as required, primarily using phenobarbital (10–20 mg/kg) or midazolam (1–3 µg/kg/min), and was withheld if not indicated.
Data analysis was conducted using SPSS 27.0 statistical software. Normally distributed measurement data were presented as mean ± standard deviation (SD), and inter-group comparisons were performed using the independent sample t-test. Non-normally distributed measurement data were represented as median (Q1, Q3), with inter-group comparisons conducted using the Mann–Whitney U test. Categorical data were expressed as cases (%), and analyzed using the χ2 test for inter-group comparisons. Similarly, ordinal data were reported as cases (%) and compared between groups using the Mann–Whitney U test. A two-sided P-value of <0.05 was deemed statistically significant.
A total of 72 neonates with perinatal asphyxia were included for transport. After excluding 2 cases in which families abandoned treatment within 24 h of admission, 3 cases where hypothermia treatment was not completed within 72 h, and 2 cases where the age at hypothermia initiation exceeded 24 h, 65 cases were ultimately included (42 males and 23 females), with 17 in the active group and 48 in the control group. Except for the lower 1 min Apgar score in the active group (P < 0.05), no statistically significant differences were observed in gestational age, sex, birth weight, mode of delivery, Apgar scores at 5 and 10 min, initial blood gas pH and BE, or HIE severity between the two groups (all P > 0.05) (Table 1).
In comparison to the control group, the active group exhibited a lower average temperature upon arrival at the treatment center (34.03 ± 0.64°C vs. 35.60 ± 1.23°C, Z = −4.9921, P < 0.05). A greater proportion of the active group arrived within the target temperature range [88.2% (15/17) vs. 16.7% (8/48), χ2 = −0.774, P < 0.001] (Figure 1). The median time required to reach the target temperature from the moment of departure from the referral center was reduced by 1.67 h in the active group (P < 0.05). Additionally,the temperature difference observed before and after transport was greater in the active group (P < 0.05), with no cases of hypothermia (<33°C) occurring in this group; however, one case (2.1%) of hypothermia was reported in the control group. The median age at which both groups initiated hypothermia treatment was within 6 h post-birth, with the active group starting earlier, although these differences were not statistically significant (P > 0.05).
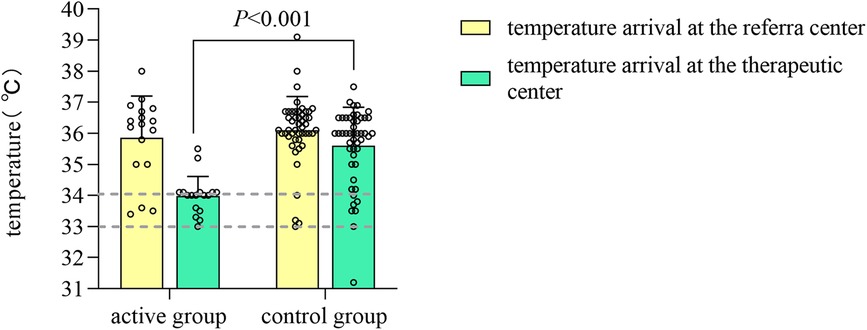
Figure 1. Bar chart of temperature measurements in the active and control groups before and after transport.
The pH and BE values before and after transport were comparable between the two groups, however, changes in BE values were significant (P < 0.05), with negative values decreasing, indicating that cooling during transport does not exacerbate acidosis (Table 2).
The median length of stay in the control group was increased by 4 days compared to the active group (P < 0.05), and the median duration of mechanical ventilation was extended by 63.5 h in the control group (P < 0.05) (Table 3).
Statistical differences in aEEG grading on day 3 of cooling were observed between the two groups (P < 0.05), with a higher proportion of patients in the severe range in the control group [45.8% (22/48) vs. 11.8% (2/17)]. Furthermore,the proportion of neonates in the active group classified in the severe range on day 3 was significantly reduced compared to day 1 (P < 0.05), while no statistically significant difference was found in the control group between day 1 and day 3 (P > 0.05) (Table 4).
Other assessments of neonatal outcomes, including the use of vasoactive drugs, seizures, distribution of HIE-related cranial MRI injury areas, and mortality rates, revealed no statistically significant differences between the two groups (P > 0.05) (Table 3).
Evidence indicates that the duration of the latent period between primary and secondary energy failure is inversely proportional to the severity of primary asphyxia injury. Clinical trials demonstrate that initiating cooling within six hours after birth can significantly reduce the risk of death or disability, establishing HIE as a time-dependent emergency (15–18). International guidelines emphasize that initiating hypothermia within 6 h of birth can maximize its neuroprotective effects (19). Clinical trials have demonstrated that commencing cooling within this time frame can significantly reduce the risk of death or disability (20–23). Furthermore, the earlier cooling is initiated, the more favorable the outcomes tend to be (16, 18, 24). Numerous studies have been conducted internationally that compare cooling protocols for HIE during transport (14, 21, 25–29). Servo-controlled cooling devices are increasingly recommended for neonatal transport owing to their convenience, precision, and stability (7, 21, 23, 26, 28, 30). Our center is the first NICU in the country to utilize active servo-controlled devices for transport, having successfully completed nearly 20 cases of active hypothermic transport for neonates with HIE. However, delays in cooling may occur due to factors such as families’ hesitancy to transfer patients, remote referral centers, and limited medical resources, which result in the inability to promptly assess brain injury and effectively implement therapeutic hypothermia. Many countries, including China, are currently facing the challenge of delayed hypothermia initiation, underscoring the critical need to commence therapeutic hypothermia as early as possible. In this study, however, the active group achieved the target temperature more rapidly. According to Laptook et al., initiating cooling treatment for HIE infants between 6 and 24 h after birth decreases the probability of death or disability by 76% compared to no cooling, with a 64% likelihood of achieving at least a 2% reduction in death or disability at 18–22 months (31). In this study, all neonates initiated therapeutic hypothermia within 24 h. We conducted a detailed analysis comparing cooling methods with and without servo-controlled devices during transport, focusing on the parameters of neonatal transport and the outcomes for the neonates. The results indicate that therapeutic hypothermia during transport is both safe and feasible. Additionally, servo-controlled active cooling facilitates a faster and more stable attainment of the target temperature, resulting in improved EEG outcomes.
Hagan et al. (29) conducted a meta-analysis of eight published studies, demonstrating that the use of servo-controlled cooling devices during transport enables a greater number of infants to reach the target core temperature upon arrival at the treatment center, while also reducing temperature fluctuations during transport (25). Chaudhary reported a success rate of 100% (22), while Akula reported 80% (21), in this study, the rate was 88.2%, which may be related to shorter transport distances in individual cases. Although the median age of the active group upon arrival at the treatment center exceeded 6 h, this may be attributed to longer transport distances. These findings strongly support the feasibility of servo-controlled cooling devices and highlight the necessity for early implementation of hypothermia during transport. This study found that neonates in the active group reached the target temperature more quickly than those in the control group. Despite longer transport distances, a higher proportion of neonates in the active group were within the target temperature range upon arrival, with no incidents of hypothermia, consistent with the results from Nitin Goel et al. (14) The servo-controlled device continuously monitors core temperature via a rectal probe to gradually achieve the set temperature, allowing for precise control. Furthermore, stable core temperatures minimize excessive fluctuations in physiological status, thereby reducing the risk of hypothermia.
If neonates are exposed to a cold environment without being dried, they can lose heat at a rate of 0.2–1°C/min within minutes after birth. Asphyxiated neonates experience a more rapid decline in body temperature compared to non-asphyxiated ones due to reduced heat production. Robertson et al. reported that despite standard rewarming methods, infants in the standard care group could remain in a state of “natural” hypothermia for up to 15 h (32). This study found that the hypothermia rate in the control group was 2.1%, while no incidents occurred in the active group, which was attributed to continuous core temperature monitoring and timely temperature feedback from the servo-controlled device. Hypothermia can lead to complications such as arrhythmias and pulmonary hypertension, which are associated with morbidity and mortality (21, 33). Gloria et al. reported that hypothermia during transport (temperature <33°C) increases the risk of neurological damage before discharge (34). Existing studies have shown that hypothermia increases the demand for inhaled nitric oxide and ECMO treatment, prolongs the duration of oxygen therapy, and is associated with a higher incidence of bradycardia and mortality (35, 36).
In this study, the duration of hospital stay and mechanical ventilation for newborns in the active group were significantly shorter than those in the control group. One possible explanation for this difference is that the initiation of cooling and the achievement of the target temperature occurred earlier in the active group compared to the control group. Although the differences in these two parameters were not statistically significant, they may be influenced by the longer transport distance in the active group and the relatively small sample size. Furthermore, this study is retrospective, and the management of respiratory care and the initiation of enteral nutrition for patients with HIE at our center have gradually evolved, which may account for the observed differences. Additional randomized controlled studies are necessary to confirm the effects of active hypothermia on these outcomes. For instance, a study by Eniko et al. indicated that the median age at which cooling was initiated in the active group was 2.58 h earlier (P < 0.0001), and the median age at which the target temperature was reached was 1.83 h earlier (P < 0.0001) (27).
Research conducted by Nash (37) and Bourel-Ponchel (38) underscores the critical role of continuous EEG monitoring in patients with HIE. This monitoring technique is effective in accurately detecting electrographic seizures and possesses a high predictive value regarding mortality and neurodevelopmental outcomes in HIE patients. Additionally, Saliba et al. (39) further emphasized the significance of EEG in both the diagnosis and prognostic evaluation of HIE. Thoresen et al. indicated that normalizing aEEG background patterns within 48 h of hypothermia treatment is predictive of improved outcomes for affected infants (40). However, bedside aEEG is not available in most of the district referral hospitals, making the dianosis of HIE incomplete. In fact, we are building integrative aEEG equipments in our transfer platform now, hopefully could move forward the moniter time. The study demonstrates a significant reduction in the proportion of severe aEEG classifications on the third day of hypothermia in the active group compared to the control group, indicating that earlier cooling yields more favorable results. Nicolet Monitor offers convenience for bedside use, unrestricted by time or space, allowing for real-time monitoring of brain activity to assess brain damage and identify seizures. Furthermore, aEEG is particularly user-friendly for clinicians with limited experience. Consequently, it is strongly recommended to conduct continuous EEG monitoring for neonates with HIE during therapeutic hypothermia.
This study does have certain limitations. Firstly, it is a retrospective analysis, which may introduce potential information bias. Secondly, there is an absence of long-term neurodevelopmental follow-up data. Future multi-center, prospective randomized controlled trials are essential to further elucidate the safety and efficacy of active hypothermia during the transport of neonates with HIE.
In conclusion, servo-controlled cooling therapy during transport is both safe and effective. The servo-controlled device provides advantages such as precise temperature regulation and rapid cooling. From the perspective of the transport team, it ensures greater temperature stability with minimal personnel intervention, allowing the team to allocate more time and resources to patient monitoring and care. Medical institutions with suitable facilities and those involved in long-distance transport should consider implementing this method.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Medical Committee of Shenzhen Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
LH: Data curation, Methodology, Writing – original draft. QS: Formal Analysis, Investigation, Methodology, Writing – review & editing. WH: Methodology, Supervision, Writing – review & editing. XL: Investigation, Software, Writing – review & editing. YC: Formal Analysis, Software, Validation, Writing – original draft. XY: Writing – review & editing, Methodology, Validation. JJ: Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research and/or publication of this article. The payment for the article processing charges will be reimbursed by Shenzhen Children's Hospital, and the funding sources are supported by Sanming Project of Medicine in Shenzhen (Project No. SZSM202311027) and the Natural Science Foundation of Shenzhen City (JCYJ20220530155601004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang T, Li S. Efficacy of different treatment times of mild cerebral hypothermia on oxidative factors and neuroprotective effects in neonatal patients with moderate/severe hypoxic-ischemic encephalopathy. J Int Med Res. (2020) 48(9):300060520943770. doi: 10.1177/0300060520943770
2. Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. (2012) 366(22):2085–92. doi: 10.1056/NEJMoa1112066
3. Pierrat V, Haouari N, Liska A, Thomas D, Subtil D, Truffert P, et al. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch Dis Child Fetal Neonatal Ed. (2005) 90(3):F257–61. doi: 10.1136/adc.2003.047985
4. Wassink G, Davidson JO, Dhillon SK, Zhou K, Bennet L, Thoresen M, et al. Therapeutic hypothermia in neonatal hypoxic-ischemic encephalopathy. Curr Neurol Neurosci Rep. (2019) 19(1):2. doi: 10.1007/s11910-019-0916-0
5. Abate BB, Bimerew M, Gebremichael B, Mengesha Kassie A, Kassaw M, Gebremeskel T, et al. Effects of therapeutic hypothermia on death among asphyxiated neonates with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis of randomized control trials. PLoS One. (2021) 16(2):e0247229. doi: 10.1371/journal.pone.0247229
6. Lemyre B, Chau V. Hypothermia for newborns with hypoxic-ischemic encephalopathy. Paediatr Child Health. (2018) 23(4):285–91. doi: 10.1093/pch/pxy028
7. Mathew JL, Kaur N, Dsouza JM. Therapeutic hypothermia in neonatal hypoxic encephalopathy: a systematic review and meta-analysis. J Glob Health. (2022) 12:04030. doi: 10.7189/jogh.12.04030
8. Subspecialty Group of Neonatology tSoPCMA, Editorial Board CJoP. Editorial Board. Expert consensus on therapeutic hypothermia in neonates with hypoxic ischemic encephalopathy (2022). Zhonghua Er Ke Za Zhi. (2022) 60(10):983–9. doi: 10.3760/cma.j.cn112140-20220418-00344
9. The Subspecialty Group of Neonatology PS, Chinese Medical Association. Diagnostic criteria for neonatal hypoxic-ischemic encephalopathy. Chin J Pediatr. (2005) 7(2):97–8. doi: 10.3969/j.issn.1008-8830.2005.02.001
10. Queensland clinical guidelines: hypoxic ischaemic encephalopathy (HIE). (2021). Available at: http://www.health.qld.gov.au/qcg (Accessed October 01, 2024).
11. Subspecialty Group of Neonatal Electroencephalogram SoEaN. Chinese Expert consensus on clinical application of amplitude-integrated electroencephalography in neonates (2023). Chin J Neonatol. (2023) 38(3):129–35. doi: 10.3760/cma.j.issn.2096-2932.2023.03.001
12. de Vries LS, Groenendaal F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology. (2010) 52(6):555–66. doi: 10.1007/s00234-010-0674-9
13. Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr. (2018) 192:33–40.e2. doi: 10.1016/j.jpeds.2017.09.043
14. Sinha A, Kempley S, Ratnavel N, Mohinuddin S, Goel N. Comparison of passive and servo-controlled active cooling for infants with hypoxic-ischemic encephalopathy during neonatal transfers. Am J Perinatol. (2016) 34(01):19–25. doi: 10.1055/s-0036-1584151
15. Saliba E, Debillon T. Neuroprotection par hypothermie contrôlée dans l’encéphalopathie hypoxique-ischémique du nouveau-né à terme. Arch Pédiatr. (2010) 17:S67–77. doi: 10.1016/S0929-693X(10)70904-0
16. Iwata O, Iwata S, Thornton JS, De Vita E, Bainbridge A, Herbert L, et al. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. (2007) 1154:173–80. doi: 10.1016/j.brainres.2007.03.083
17. Thoresen M, Tooley J, Liu X, Jary S, Fleming P, Luyt K, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology. (2013) 104(3):228–33. doi: 10.1159/000353948
18. Sabir H, Scull-Brown E, Liu X, Thoresen M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 h in neonatal rats. Stroke. (2012) 43(12):3364–70. doi: 10.1161/STROKEAHA.112.674481
19. Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. (1997) 99(2):248–56. doi: 10.1172/JCI119153
20. European standards of care for newborn health: postnatal management of newborn infants with hypoxic ischaemic encephalopathy (HIE) European Foundation for the Care of Newborn Infants (EFCNI). (2018). Available at: https://newborn-health-standards.org (Accessed February 18, 2025).
21. Akula VP, Joe P, Thusu K, Davis AS, Tamaresis JS, Kim S, et al. A randomized clinical trial of therapeutic hypothermia mode during transport for neonatal encephalopathy. J Pediatr. (2015) 166(4):856–61.e1-2. doi: 10.1016/j.jpeds.2014.12.061
22. Chaudhary R, Farrer K, Broster S, McRitchie L, Austin T. Active versus passive cooling during neonatal transport. Pediatrics. (2013) 132(5):841–6. doi: 10.1542/peds.2013-1686
23. Karlsson M, Tooley JR, Satas S, Hobbs CE, Chakkarapani E, Stone J, et al. Delayed hypothermia as selective head cooling or whole body cooling does not protect brain or body in newborn pig subjected to hypoxia-ischemia. Pediatr Res. (2008) 64(1):74–80. doi: 10.1203/PDR.0b013e318174efdd
24. Leben M, Nolimal M, Vidmar I, Grosek S. Passive therapeutic hypothermia during ambulance and helicopter secondary neonatal transport in neonates with hypoxic brain injury: a 10-year retrospective survey. Childs Nerv Syst. (2018) 34(12):2463–9. doi: 10.1007/s00381-018-3914-7
25. Kendall GS, Kapetanakis A, Ratnavel N, Azzopardi D, Robertson NJ. Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. (2010) 95(6):F408–12. doi: 10.1136/adc.2010.187211
26. Momin S, Thomas S, Zein H, Scott JN, Leijser LM, Vayalthrikovil S, et al. Comparing three methods of therapeutic hypothermia among transported neonates with hypoxic-ischemic encephalopathy. Ther Hypothermia Temp Manag. (2023) 13(3):141–8. doi: 10.1089/ther.2022.0048
27. Sharma A. Provision of therapeutic hypothermia in neonatal transport: a longitudinal study and review of literature. Cureus. (2015) 7(5):e270. doi: 10.7759/cureus.270
28. Szakmar E, Kovacs K, Meder U, Nagy A, Szell A, Bundzsity B, et al. Feasibility and safety of controlled active hypothermia treatment during transport in neonates with hypoxic-ischemic encephalopathy. Pediatr Crit Care Med. (2017) 18(12):1159–65. doi: 10.1097/PCC.0000000000001339
29. Hagan JL. Meta-analysis comparing temperature on arrival at the referral hospital of newborns with hypoxic ischemic encephalopathy cooled with a servo-controlled device versus no device during transport. J Neonatal Perinatal Med. (2021) 14(1):29–41. doi: 10.3233/NPM-200464
30. Torre Monmany N, Behrsin J, Leslie A. Servo-controlled cooling during neonatal transport for babies with hypoxic-ischaemic encephalopathy is practical and beneficial: experience from a large UK neonatal transport service. J Paediatr Child Health. (2019) 55(5):518–22. doi: 10.1111/jpc.14232
31. Sibrecht G, Borys F, Campone C, Bellini C, Davis P, Bruschettini M. Cooling strategies during neonatal transport for hypoxic-ischaemic encephalopathy. Acta Paediatr. (2023) 112(4):587–602. doi: 10.1111/apa.16632
32. Laptook AR, Shankaran S, Tyson JE, Munoz B, Bell EF, Goldberg RN, et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA. (2017) 318(16):1550–60. doi: 10.1001/jama.2017.14972
33. Robertson NJ, Kendall GS, Thayyil S. Techniques for therapeutic hypothermia during transport and in hospital for perinatal asphyxial encephalopathy. Semin Fetal Neonatal Med. (2010) 15(5):276–86. doi: 10.1016/j.siny.2010.03.006
34. Hallberg B, Olson L, Bartocci M, Edqvist I, Blennow M. Passive induction of hypothermia during transport of asphyxiated infants: a risk of excessive cooling. Acta Paediatr. (2009) 98(6):942–6. doi: 10.1111/j.1651-2227.2009.01303.x
35. Troncoso G, Agudelo-Perez S, Thorin N, Diaz C, Vargas A. Short-term neurological injury in newborns infants with overcooling in passive hypothermia and transferred to reference hospital in Colombia. Acta Paediatr. (2023) 112(11):2346–51. doi: 10.1111/apa.16921
36. Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. (2014) 312(24):2629–39. doi: 10.1001/jama.2014.16058
37. Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA. (2017) 318(1):57–67. doi: 10.1001/jama.2017.7218
38. Nash KB, Bonifacio SL, Glass HC, Sullivan JE, Barkovich AJ, Ferriero DM, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. (2011) 76(6):556–62. doi: 10.1212/WNL.0b013e31820af91a
39. Bourel-Ponchel E, Querne L, Flamein F, Ghostine-Ramadan G, Wallois F, Lamblin MD. The prognostic value of neonatal conventional-EEG monitoring in hypoxic-ischemic encephalopathy during therapeutic hypothermia. Dev Med Child Neurol. (2023) 65(1):58–66. doi: 10.1111/dmcn.15302
Keywords: asphyxia, hypoxic-ischemic encephalopathy, therapeutic hypothermia, transport, servo-controlled cooling
Citation: Huang L, Su Q, Huang W, Lu X, Chen YL, Yang X and Jiang J (2025) Single-center analysis of servo-controlled cooling during the transport of neonates with perinatal asphyxia. Front. Pediatr. 13:1562736. doi: 10.3389/fped.2025.1562736
Received: 18 January 2025; Accepted: 27 February 2025;
Published: 20 March 2025.
Edited by:
Hemmen Sabir, University Hospital Bonn, GermanyReviewed by:
Herica Dutra, Juiz de Fora Federal University, BrazilCopyright: © 2025 Huang, Su, Huang, Lu, Chen, Yang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Jingbo, YmFsbC4xMzYxQGFsaXl1bi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.