
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 11 April 2025
Sec. Pediatric Infectious Diseases
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1561600
Background: Visceral leishmaniasis (VL) can lead to complications such as hemophagocytic lymphohistiocytosis (HLH) in children. The clinical features of VL overlap with that of HLH, and thus the diagnosis of VL-induced HLH can be challenging for clinicians.
Methods: We describe two pediatric cases and systematically review all reported cases of pediatric VL-related HLH in literatures until May 2024.
Results: The demographic characteristics, clinical manifestations, treatment and prognosis of our reported cases are presented. The systematic review included 29 articles with a total of 135 cases. More than half of the children (89/125, 71.2%) were under 3 years old, and 8.9% (n = 12/135) had specific epidemiological histories. The main clinical presentations were hypertriglyceridemia (34/45, 75.6%), hypofibrinogenemia (24/36, 66.7%), and hyperferritinemia (95/132, 72.0%). Bone marrow aspiration (BMA) analysis indicated positive evidence of leishmania infection in 84.7% (83/98) of cases, while 37.8% (14/37) of patients tested negative for leishmania on the first BMA smear. All patients were treated against leishmania with amphotericin B (76/135, 56.3%) or antimony (77/135, 57.0%), and 13.3% (n = 18/135) of patients received both medications, in which amphotericin B was used as rescue treatment. The prognosis was favorable, with the exception of two deaths.
Conclusions: Vigilance towards screening for leishmania infection induced HLH is imperative, particularly when there is a suspicious epidemiological history, ineffective chemotherapy, or prior to bone marrow transplantation. Early recognition, accurate diagnosis, and prompt treatment initiation can significantly alter the course of the disease and favor the prognosis in childhood with HLH secondary to VL.
Hemophagocytic lymphohistiocytosis (HLH) is a lethal condition characterized by immunological overactivation of cytotoxic T cell natural killer cells (NK) and macrophages leading to overproduction of pro-inflammatory cytokines and injury of multiple organ systems (1). The etiology of HLH can be broadly categorized into primary (familial) HLH and secondary (sporadic) HLH. Primary HLH is caused by gene mutations and primary immunodeficiency that regulate the granulose-dependent cytotoxicity of natural killer cells and cytotoxic T lymphocytes (CTLs), including familial HLHs (FHLHs) and related immunodeficiency diseases, which mainly occur in children. Secondary HLH can be triggered in the context of various infections, definite rheumatic immune disease (macrophage activation syndrome, MAS), malignancy, and iatrogenic immune activation, affecting all age groups (2). Infection is the most common cause of secondary HLH, while leishmania is the most common protozoan infection-induced HLH (3).
Visceral leishmaniasis (VL), also known as Kala-azar, is caused by infection with leishmania and transmitted by the bite of female phlebotomine sandflies (4). It is estimated that 700,000–1 million new cases occur annually in more than 90 countries, especially in East Africa, the Mediterranean basin, Southeast Asia, and Latin America (5). According to a systematic review, a total of 150,072 VL patients have been reported in China, and 7,847 (5.2%) of them have died (6). It is primarily distributed in the northwest, with Xinjiang Uygur Autonomous Region, Gansu Province, and Sichuan Province being the top three affected areas (7). VL exhibits a broad spectrum of clinical manifestations, ranging from asymptomatic infection to persistent fever, hepatosplenomegaly, and pancytopenia, which has overlapping clinical features with HLH. It has been reported that VL-related HLH is rare in childhood, and the mortality could reach 100% without early diagnosis and treatment (8). Early recognition and treatment of VL-related HLH is critical to improving outcomes.
Recent studies have highlighted the importance of various diagnostic methods in diagnosing VL-HLH. Bone marrow aspiration (BMA) is a traditional method for diagnosing VL, but it may require multiple tests to detect the parasite in some cases (9). The rK39 rapid diagnostic test (RDT) is quick and sensitive, but it can yield false negatives in certain situations (10, 11). Serological tests, which involve detecting Leishmania antibodies or antigens, aid in auxiliary diagnosis (9). Polymerase chain reaction (PCR) and reverse transcription polymerase chain reaction (RT-PCR) molecular detection offer higher sensitivity and specificity for detecting VL-HLH, particularly when bone marrow aspiration results are uncertain (12, 13). Furthermore, when traditional methods fail, next-generation sequencing (NGS) provides a new diagnostic approach (14, 15).
VL-associated HLH is a relatively rare disease with significant diagnostic and management implications. Information on the clinical and laboratory findings and the outcome of children diagnosed with VL-associated HLH is scarce. Herein, we describe two children who were initially diagnosed with HLH but were ultimately diagnosed with VL and systematically review all reported cases of pediatric HLH secondary to VL focusing on the clinical manifestations, diagnostic methods, treatment used and outcomes, to provide evidence and reference for clinician with their early identification and treatment.
A 15-month-old girl residing in Wen County, Gansu Province, China, was admitted to our hospital with recurrent high fever for 13 days, accompanied by cough and nausea. Her family histories were unremarkable. The patient received empirical treatment for presumed bacterial infection and platelet transfusions in the local hospital, but her symptoms were not relieved. Upon admission, physical examination revealed pallor, rales in both lungs and hepatosplenomegaly (hepatomegaly of 8 cm below the right costal margin and splenomegaly of 5 cm below the left costal margin). Hematological examinations confirmed pancytopenia, with neutropenia (0.59 × 109 L), anemia (80 g/L) and thrombocytopenia (53 × 109 L). The laboratory testing revealed hypertriglyceridemia (2.72 mmol/L), hypofibrinogenemia (104 mg/dl) and hyperferriinemia (>16,500 ng/ml). Serological tests for rK39, Epstein–Barrvirus (EBV), cytomegalovirus, hepatitis B, syphilis, and human immunodeficiency virus (HIV) were performed and proved to be negative (Table 1). BMA revealed signs of hemophagocytosis and Leishman-Donovan bodies (Figure 1A). Given of five out of eight diagnostic criteria for HLH, including fever, pancytopenia, hepatosplenomegaly, hypofibrinogenemia and hyperferriinemia were fulfilled (16), the leishmania amastigotes were also observed in the BMA, leading to a diagnosis of VL with secondary HLH. The patient was treated with a total of 227 mg/kg of antimony gluconate over 9 intravenous doses. Her clinical conditions dramatically improved as early as the third day of treatment. By the 14th day, fever and hepatosplenomegaly were relieved, and blood counts almost normalized. Follow-up examinations conducted over one year showed complete remission of VL without recurrence.
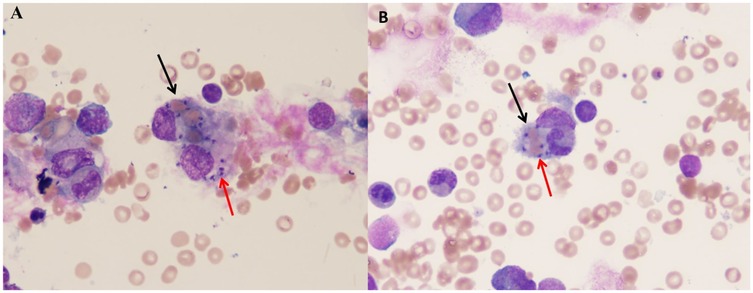
Figure 1. The picture showing extracellular Leishman-Donovan bodies and hemophagocytic cells in BMA [(A) for case 1, (B) for case 2]. The black arrow shows the hemophagocytic cell. The red arrow shows the Leishman-Donovan bodies.
A previously 29-month-old boy was referred to us with a persistent fever and pancytopenia that had persisted for 14 days. Family history was non-contributory. Physical examination on admission revealed pallor, hepatomegaly, significant splenomegaly, and mild enlargement of cervical lymph nodes. Laboratory investigations showed low levels of hemoglobin 81 g/dl, leukocyte 1.68 × 109 L, absolute neutrophil 0.657 × 109 L, and platelet 65 × 109 L. High levels of ferritin (1,437.34 ng/ml), alanine aminotransferase (ALT) 102 U/L, AST 110 U/L and fibrinogen 1.8 g/L. Travelling to Heishui County, Sichuan Province half a month ago leads to the suspicion of VL (Table 1). Multiple hematological examinations confirmed leukopenia, anemia and thrombocytopenia. A BMA examination was performed and revealed the presence of both Leishman-Donovan bodies (Figure 1B) and hemophagocytosis. rK39 enzyme-linked immunosorbent assay (ELISA) was negative. The diagnosis of HLH secondary to VL was considered. Prompt treatment with antimony gluconate was initiated, with dosages of 0.2 g on the first day, 0.6 g on the second day, 0.3 g for the next four days, and 0.4 g for the following three days. The patient's fever, pancytopenia, and organomegaly were relieved rapidly. On the 13th day of postadmission, blood routine examination before discharge indicated improvement particularly with a decrease C-reactive protein (CRP, 1.58 mg/L) and increase in platelet 212.0 × 109/L, neutrophil 2.03 × 109/L, red blood cell 3.52 × 1012/L, hemoglobin 91.0 g/L. Abdominal examination showed the liver was around 2.5 cm subcostal, and the spleen measured about 6 cm along the I line. The patient was discharged on the 15th day and followed up for one year without any recurrence.
A systematic search was performed in MEDLINE, Web of Science, EMBASE, CNKI, VIP, CBM independently using the following terms “visceral leishmaniasis”, “black fever”, “kala-azar”, and “child”, “children”, “pediatric”, and “hemophagocytic lymphohistiocytosis”. The search was limited to articles published from January 2013 to May 2024.
Two researchers independently screened the literature and extracted data. In cases where there were controversies, a third researcher was consulted until a consensus was reached. Duplicate articles were excluded during the screening process. Both abstracts and full articles were reviewed, and only articles that fulfilled the criteria of both VL and HLH were included (16, 17). Articles or cases in which primary HLH or secondary HLH was caused by other factors, including other pathogenic infections, autoimmune diseases, tumors, etc., were excluded. The extracted information from included studies included authors' names, publication year, demographic characteristics, clinical characteristics, laboratory test results, treatment, and outcomes of patients. Case reports were evaluated using the JBI criteria (18), with a total score of 8 points. The case series were evaluated using the quality evaluation tool developed by the Institute of Health Economics (IHE) in 2012, with literature meeting the requirement of 14 or more being considered high-quality (19).
Descriptive analysis was performed on the small number of cases of HLH secondary to VL using frequency percentages (%), as part of the systematic evaluation. Data analysis was conducted using SPSS 23 software and Excel.
Initial database searches identified 215 articles. After removing 94 duplicates and excluding 65 through title/abstract screening, 56 underwent full-text review. Ultimately, 29 studies (24 case reports, 5 case series) encompassing 135 cases were included (Figure 2).
The quality of included studies was assessed using JBI criteria for 24 case reports (scores: 6–8) and the IHE tool for 5 case series (scores: 10–15). Results are detailed in Table 2.
The study included 135 children with ages ranging from 3.5 months to 168 months. The male-to-female ratio was 1.3:1. Of these, 71.2% (89/125) were under 3 years old, followed by 3–6 years old (19/125, 15.2%) and over 6 years old (17/125, 13.6%). 8.9% (12/135) of the patients had positive epidemiological history, while the remaining cases were from regions where previous VL cases have been reported or VL endemic areas (Table 3). The most commonly seen countries of infection were Brazil (78/135, 57.8%) and China (36/135, 26.7%), followed by Spain (6/135, 4.4%), and Italy (3/135, 2.2%) (Tables 3, 4).
Children included in the study presented with fever (89/89, 100%), splenomegaly (130/134, 97.0%), and hepatomegaly (94/116, 81.0%). Other symptoms were also observed in the children (33/135, 24.4%), including lymphadenopathy, edema, jaundice, and erythema (Tables 3, 4). The majority of cases had a fever duration within 30 days (n = 76/89, 85.4%), followed by over 60 days (9/89, 10.1%) and between 30 and 60 days (4/89, 4.5%).
Laboratory findings at admission showed that most patients had low levels of hemoglobin (≤90 g/L, 128/132, 96.9%), platelet (≤100/L, 95/97, 97.9%), and leukocyte (≤1,000/ml, 37/48, 77.1%). Hypertriglyceridemia (34/45, 75.6%), hypofibrinogenemia (24/36, 66.7%), and hyperferritinemia (95/132, 72.0%) were found in more than half of patients. Almost all children (133 cases) were subjected to at least one bone marrow puncture, among the cases in which the number of bone marrow punctures was specified, 37.8% (n = 14/37) were negative for the first BMA smear. Positive evidence of VL infection found in BMA accounted for 84.7% (83/98). Patients who underwent BMA or blood PCR testing were positive in 13/13 (100%) patients, and BMA cultures were positive in 3/4 (75%) patients. More than half of bone marrow smear samples observed hemophagocytosis (66/100, 66.0%). Serological and rK39 tests were positive in 90.9% (n = 70/77) and 92.7% (n = 51/55), respectively (Table 4). Evidence of leishmania infection was detected in one child using NGS of blood and sputum due to negative results from two BMAs and serological tests (Table 3) (15).
Prior to confirmation of leishmaniasis infection, the included children who had received HLH-04 chemotherapy and corticosteroid therapy were 9.6% (n = 13/135) and 24.4% (n = 33/135) of patients, respectively. Additionally, one child has completed Hematopoietic Stem Cell Transplantation (HSCT), and another one is scheduled to undergo the procedure. Following diagnosis, amphotericin B (76/135, 56.3%) and antimony (77/135, 57.0%) administration were the most commonly used treatment; 13.3% (n = 18/135) of patients received both treatments, with amphotericin B used as rescue therapy. Regarding the duration of remission of fever, half of the patients achieved remission within 3 days of medication (9/18, 50%), one third (7/18, 38.8%) of patients within 4–6 days, one-ninth (2/18, 11.1%) of patients at 7 and 10 days after medication (23, 45). Among the patients who received treatments, more than half (10/17, 58.8%) of the patients have restored to normal within 1 month, and the remaining patients (7/17, 41.2%) have recovered for more than one month or even three months based on their clinical laboratory indexes. Regarding mortality, two patients died, one of whom likely suffered from acute kidney injury (AKI) (26), the other case involved a 9-month-old infant who was treated with antimony on the 27th day but succumbed to disseminated intravascular coagulation (DIC) three days later (47). No adverse reactions were observed in the two patients reported in our study (Table 4).
HLH is a life-threatening immunological syndrome characterized by fever, hepatosplenomegaly, pancytopenia, hypertriglyceridemia, hypofibrinemia, hyperferritinemia, and hemophagocytosis in bone marrow aspirate (16). Primary HLH, MAS, and partial EBV-related HLH require immediate immunochemotherapy and timely HSCT for patient survival (48). Other viruses, Mycobacterium tuberculosis, varicella and leishmania have been reported to trigger infection associated HLH (49). VL infection which is essentially caused by Leishmania donovani and Leishmania infantum, is also an important cause of HLH because it is often not suspected (50). VL has been reported in various regions with varying prevalence, with rates of 2.1% in Germany (25), 27.5% in Brazil (26), and 41.7% in Spain (51). This variability may be attributed to regional specificity. According to our literature reports, the countries where the included children may acquire VL infection were mainly Brazil and China, which is consistent with the geographical distribution of VL reported before. HLH secondary to previously reported pediatric VL patients were mostly under 5 years old (52). For the first time, we counted 71% of the children with HLH secondary to VL were younger than three years old. This may be related to the high incidence of HLH at this age (53), or the species of leishmania infected. Previous literature suggests that children are at a higher risk of developing clinical disease as a result of Leishmania infantum (54). Unfortunately, most of the articles we included failed to further identify the species of leishmania.
Mortality rates of HLH in children are estimated to range from 8% to 22%, while in adults, the rate is estimated to be 40% (1). Most cases of secondary HLH can be effectively managed by controlling the underlying trigger. However, due to the extensive clinical spectrum of VL and the overlap of clinical features between VL and HLH may lead to a diagnostic delay in forms of HLH secondary to VL (55). Therefore, identifying the underlying causes of HLH post-diagnosis is crucial. Epidemiological history investigation is useful in clinical practice for recognizing the underlying causes of secondary HLH. However, a positive epidemiological history is sometimes difficult to obtain. There were only 12 (8.9%) cases with a specific VL endemic travel history in our review. The long incubation period (which can last from 2 to 6 months), endemic specificity and nonspecific clinical symptoms of VL infection may contribute to underestimated infectious agents of secondary HLH. With the growth of the economy, international trade, tourism etc., transmission of kala-azar to non-endemic areas has been reported (56), reflecting the geographic spread of this disease and the importance of considering VL in the differential diagnosis for HLH. Furthermore, investigating the travel history of the mother during pregnancy may help to identify possible fetal transmission of leishmania in young infants (27, 32). Clinicians should gather detailed epidemiological history to identify possible pediatric kala-azar infections when common causes of HLH have been ruled out.
Etiological tests for HLH secondary to VL currently available include parasitological, immunological, and molecular methods. Parasitological diagnosis is the golden standard. Directly finding LD in tissues like the spleen, bone marrow, and lymph nodes could confirm VL infection. The sensitivity of tests depends on the tissue type. Splenic aspirate has a high sensitivity of up to 95% for the diagnosis of VL, but its use in clinical practice is limited due to the high risk of hemorrhage by unskilled persons (57). In our review, only one patient underwent a spleen puncture, but the result was negative (25). While lymph node sample collection is easier, its sensitivity is relatively low, ranging from 53% to 65%( 58). Moreover, our statistics indicate that lymph node enlargement is a rare manifestation among children with VL-HLH, which limits the clinical applicability of this approach. Conversely, bone marrow specimens are relatively easy to obtain and have demonstrated higher sensitivity in detecting Leishmania infection in patients with VL-HLH. The sensitivity of bone marrow smears ranges from 52% to 85% (59). The two cases we reported relied on positive BMA results to facilitate rapid diagnosis. Our study found that bone marrow smears had a positive detection rate of 82.5% for Leishmania infection in patients with VL-HLH. However, it has been reported that Leishmania amastigotes were not initially detected in BMA smears but were identified retrospectively after diagnosis (28). According to literature reports, 64% of specimens from the initial BMA test are negative (50). In our review, we analyzed the outcomes of BMAs and discovered that 37.8% of patients had negative results on their initial BMA smear. This highlights the challenges associated with detecting the Leishmania parasite in clinical samples, possibly due to the parasite's scarcity post-infection and the laboratory's testing expertise. To enhance diagnostic accuracy, repeated BMAs might be required to detect leishmania amastigotes. Parasitological culture of VL is a tedious and time-consuming procedure, which restricts its clinical application despite its high sensitivity of 97%–100%. In our review, there are only four patients underwent BMA culture, with a positivity rate of 75%. The serological tests, such as ELISA, direct agglutination test (DAT), immunofluorescence assay (IFA), and immuno-chromatographic test (ICT) is a non-invasive, rapid screening methods for VL, which are used in both endemic and non-endemic areas due to its low cost and quick results. However, its sensitivity and specificity can vary by region (60). In the cases we reported, both children's tests for rK39 antigen were negative. Statistics suggest that around 50% of patients with VL might have negative serological test results, possibly because these tests are less specific and sensitive in the early stages of the disease (27). Molecular diagnosis, including PCR techniques such as standard, nested, multiplex PCR and RT-PCR, as well as NGS plays a crucial role in detecting the parasite. In a report, the PCR-positive rate was up to 83% in serum and 100% in bone marrow aspirate samples of pediatric VL related HLH (25). It's especially useful in cases with multiple negative bone marrow smears but a strong suspicion of VL, which could reduce underdiagnosis due to limited diagnostic experience. In a systematic review of VL-related HLH cases collected before 2013, there were two cases using PCR to diagnose VL (31), while 13 cases were reported and all were positive for VL in our literature review. Although PCR for detecting VL is with high sensitivity, its use in clinical settings is limited by high costs and inadequate infrastructure in some regions. With the development of technology, the application of NGS has shown promise in detecting VL in previously intractable cases (15, 61). In summary, combining various diagnostic methods, such as multiple bone marrow smears and PCR when needed, may help in diagnosing VL at an early stage. Following a comprehensive literature evaluation, we developed a subsequent diagnostic algorithm (Figure 3) for patients fulfilling HLH diagnostic criteria with relevant travel history when initial bone marrow screening yields negative results for leishmania infection.
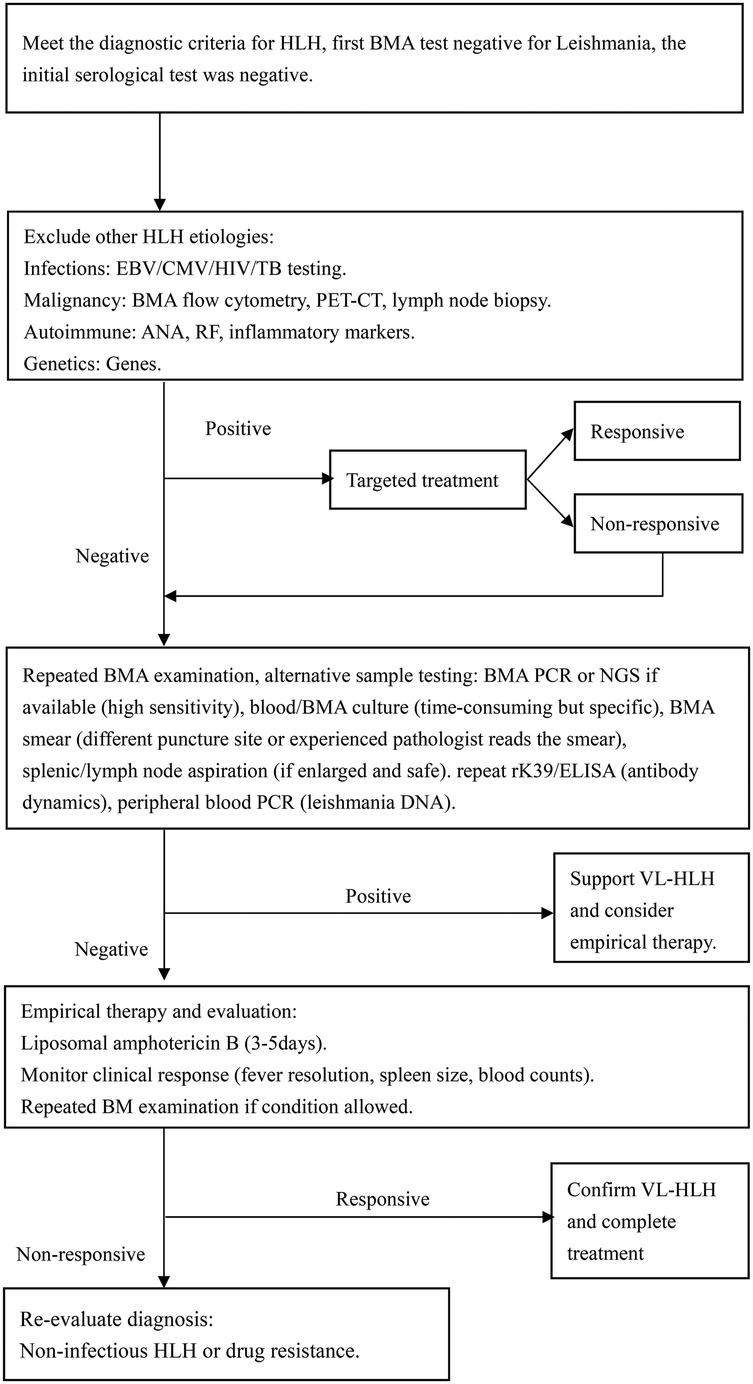
Figure 3. Diagnostic algorithm following an initial negative BMA examination for leishmania. CMV, cytomegalovirus; TB, tuberculosis; PET-CT, positron emission tomography—computed tomography; ANA, antinuclear antibody; RF, rheumatoid factor; DNA, deoxyribonucleic acid.
Correct identification of leishmania is crucial for timely treatment of children with VL-HLH. In our systematic review, 33 children received corticosteroid therapy to control excessive inflammation. However, Misuse of immunosuppressive agents may lead to the need for higher doses and a longer duration of anti-leishmanial therapy upon revised diagnosis (24, 30). Currently, VL is treated with a limited number of drugs, such as pentavalent antimony, meglumine antimoniate, injectable paromomycin, oral miltefosine, and amphotericin B (62). Amphotericin B and pentavalent antimony are the most commonly used treatments in clinical practice. In our cases, all the children included were treated with antimony or amphotericin B, and almost all children in China receive antimony due to the difficulty in obtaining amphotericin B. However, the intravenous administration of pentavalent antimony over 20–30 days is associated with significant side effects, such as cardiotoxicity, pancreatitis, and nephrotoxicity (63). The case report limitations in our study prevented us from assessing the incidence of adverse reactions to antimony. Furthermore, the rising number of reports on pentavalent antimonial resistance highlights the urgent need for more effective and safer treatments for leishmaniasis (64). In our review, 77 children were treated with antimony, and 18 experienced treatment failure or complications that resolved with remedial treatment using amphotericin B. The remaining 58 children, who were treated with amphotericin B as the first choice, were cured, except one fatality. Modified amphotericin B, which has shown efficacy and safety in regions where VL is endemic, is now recommended as a first-line anti-leishmanial drug. It offers a shorter treatment course and improved safety compared to pentavalent antimony, making it a promising alternative for treating leishmaniasis (63). Previous reports have indicated that severe HLH can lead to mortality due to multiple organ failure (65), secondary septic shock (55), hemorrhagic shock, and antimony-related myocarditis (66). In our systematic review, two deaths were reported, attributed to DIC and possible AKI. Generally, early recognition, accurate diagnosis, and prompt treatment initiation can significantly alter the course of the disease and favor the prognosis in children with HLH secondary to VL.
As an important drug for the treatment of HLH secondary to VL, the research of amphotericin B liposomal (L-AmB) has mainly focused on the optimization of dosing regimens, the efficacy of special populations and the combination therapy strategy. In a case study conducted at the First Affiliated Hospital of Xi'an Jiaotong University in China, a low-dose (0.15 mg/kg) escalating L-AmB regimen was used to achieve negative bone marrow PCR conversion with a cumulative dose of 10 mg/kg without serious adverse reactions (67). This regimen is particularly appropriate for patients with complications such as renal dysfunction or HLH. A 4.5-month-old HLH patient with H1N1 infection in Turkey had complete resolution of symptoms after treatment with L-AmB (3 mg/kg/day × 10 days), and there was no recurrence after 1 year of follow-up (68). In patients with HIV and HLH, L-AmB results in clinical improvement in 83% of patients with initial treatment, but it is important to note that immune reconstitution syndrome may exacerbate HLH manifestations (69). A study in Guyana, France, showed that early empiric use of L-AmB resulted in a 71% survival rate for HIV-associated HLH (69). Although L-AmB is more than 80% effective in treatment VL-HLH, the recurrence rate in immunocompromised patients is still as high as 30% (70). In this regard, some studies have recommended an extended course of therapy or a combination of immunomodulatory therapies (e.g., dexamethasone, immunoglobulin) (71).
Secondary HLH associated with VL is relatively rare and has non-specific early symptoms. Therefore, thorough medical history, and multiple and precise diagnostic tests are essential for making a timely and effective clinical diagnosis and initiating treatment. We suggest screening all children with HLH for leishmaniasis in endemic areas, particularly before starting HLH chemotherapy or HSCT. Quick remission with anti-leishmanial therapy suggests a good prognosis for children with HLH caused by VL.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the ethics committee of the Public Health Clinical Center of Chendu. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
ZC: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. YG: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. CZ: Project administration, Supervision, Validation, Writing – review & editing. JM: Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. (2020) 34(4):101515. doi: 10.1016/j.berh.2020.101515
2. Esteban YM, de Jong JLO, Tesher MS. An overview of hemophagocytic lymphohistiocytosis. Pediatr Ann. (2017) 46(8):e309–e13. doi: 10.3928/19382359-20170717-01
3. Cascio A, Pernice LM, Barberi G, Delfino D, Biondo C, Beninati C, et al. Secondary hemophagocytic lymphohistiocytosis in zoonoses. A systematic review. Eur Rev Med Pharmacol Sci. (2012) 16:1324–37. Available at: https://www.europeanreview.org/article/149123104648
4. Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. (2018) 392(10151):951–70. doi: 10.1016/S0140-6736(18)31204-2
5. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. (2012) 7(5):e35671. doi: 10.1371/journal.pone.0035671
6. Abongomera C, van Henten S, Vogt F, Buyze J, Verdonck K, van Griensven J. Prognostic factors for mortality among patients with visceral leishmaniasis in East Africa: systematic review and meta-analysis. PLoS Negl Trop Dis. (2020) 14(5):e0008319. doi: 10.1371/journal.pntd.0008319
7. Lun ZR, Wu MS, Chen YF, Wang JY, Zhou XN, Liao LF, et al. Visceral leishmaniasis in China: an endemic disease under control. Clin Microbiol Rev. (2015) 28(4):987–1004. doi: 10.1128/CMR.00080-14
8. Gradoni L, López-Vélez R, Mokni M. Manual on case management and surveillance of leishmaniases in the WHO European Region (2017). Available at: https://www.who.int/publications/i/item/9789289052511 (Accessed July 05, 2017).
9. Qin Y, Lv X, Zheng Q, Wu Q, Zheng L, Kang M, et al. Case report: visceral leishmaniasis-associated hemophagocytic lymphohistiocytosis in adults: a case series and literature review. Am J Trop Med Hyg. (2022) 107(6):1203–9. doi: 10.4269/ajtmh.22-0361
10. Ranjan P, Kumar V, Ganguly S, Sukumar M, Sharma S, Singh N, et al. Hemophagocytic lymphohistiocytosis associated with visceral leishmaniasis: varied presentation. Indian J Hematol Blood Transfus. (2016) 32(Suppl 1):351–4. doi: 10.1007/s12288-015-0541-2
11. Wang L, Hu M, Wu X, Ma L, Yang H. Case report: diagnosis and treatment of two clinical cases of visceral leishmaniasis-related hemophagocytic lymphohistiocytosis. Am J Trop Med Hyg. (2023) 109(2):296–300. doi: 10.4269/ajtmh.22-0776
12. Badiola J, Munoz-Medina L, Callejas JL, Delgado-Garcia A, Jurado M, Hernandez-Quero J. Hemophagocytic lymphohistiocytosis associated with leishmania: a hidden passenger in endemic areas. Enferm Infecc Microbiol Clin. (2021) 39(4):188–91. doi: 10.1016/j.eimc.2020.04.012
13. Shi Q, Huang M, Li X, Zheng X, Wang F, Zou Y, et al. Clinical and laboratory characteristics of hemophagocytic lymphohistiocytosis induced by leishmania infantum infection. PLoS Negl Trop Dis. (2021) 15(11):e0009944. doi: 10.1371/journal.pntd.0009944
14. Chen C, Yu Y, Zhu Ma LZ, Nin J, Zhang D, Nong W, et al. Visceral leishmaniasis related secondary haemophagocytic syndrome: a case report. J Int Med Res. (2025) 53(2):3000605251318204. doi: 10.1177/03000605251318204
15. Guo F, Kang L, Xu M. A case of pediatric visceral leishmaniasis-related hemophagocytic lymphohistiocytosis diagnosed by mNGS. Int J Infect Dis. (2020) 97:27–9. doi: 10.1016/j.ijid.2020.05.056
16. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2007) 48(2):124–31. doi: 10.1002/pbc.21039
17. Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. (2016) 63(12):e202–e64. doi: 10.1093/cid/ciw670
18. Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, editors. JBI Manual for Evidence Synthesis. JBI (2024). Available at: https://synthesismanual.jbi.global (Accessed November 25, 2024).
19. Moga C, Guo B, Schopflocher D, Harstall C. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. Edmonton, AB: Institute of Health Economics (2012). Available at: https://www.ihe.ca/research-programs/rmd/cssqac/cssqac-info (Accessed March 07, 2012).
20. Bolia R, Singh A, Maji M, Misra M, Sharawat IK, Panda PK. Visceral leishmaniasis associated with hemophagocytic lymphohistiocytosis. Indian J Pediatr. (2021) 88(1):73. doi: 10.1007/s12098-020-03361-5
21. Brum NFF, Coelho JS, Carvalho LS, Vieira MNO, Bentes AA, Carellos EVM, et al. Hemophagocytic lymphohistiocytosis and visceral leishmaniasis in children: a series of cases and literature review. Rev Paul Pediatr. (2021) 40:e2020269. doi: 10.1590/1984-0462/2022/40/2020269
22. Carvalho FHG, Lula JF, Teles LF, Caldeira AP, Carvalho SFG. Hemophagocytic lymphohistiocytosis secondary to visceral leishmaniasis in an endemic area in the North of Minas Gerais, Brazil. Rev Soc Bras Med Trop. (2020) 53:e20190491. doi: 10.1590/0037-8682-0491-2019
23. Das S, Sarkar N, Chatterjee K, Aich B, Bhattacharya M. Portal hypertension and reactive hemophagocytosis in pediatric visceral leishmaniasis. Bangladesh J Med Sci. (2015) 14(4):409–12. doi: 10.3329/bjms.v14i4.25809
24. Yarali N, Hacisalihoglu S, Culha V, Altan O, Gurlek Gokcebay D. Hemophagocytic lymphohistiocytosis: a confusing problem of the diagnosis of visceral leishmaniasis. Indian J Hematol Blood Transfus. (2017) 34(1):161–2. doi: 10.1007/s12288-017-0838-4
25. Bode SF, Bogdan C, Beutel K, Behnisch W, Greiner J, Henning S, et al. Hemophagocytic lymphohistiocytosis in imported pediatric visceral leishmaniasis in a nonendemic area. J Pediatr. (2014) 165(1):147–53 e1. doi: 10.1016/j.jpeds.2014.03.047
26. Daher EF, Lima LL, Vieira AP, Nascimento LS, Soares DS, Abreu KL, et al. Hemophagocytic syndrome in children with visceral leishmaniasis. Pediatr Infect Dis J. (2015) 34(12):1311–4. doi: 10.1097/INF.0000000000000916
27. Mantadakis E, Alexiadou S, Totikidis G, Grapsa A, Chatzimichael A. A brief report and mini-review of visceral leishmaniasisassociated hemophagocytic lymphohistiocytosis in children. J Pediatr Hematol Oncol. (2021) 43(2):e223–6. doi: 10.1097/MPH.0000000000001747
28. Singh G, Shabani-Rad M-T, Vanderkooi OG, Vayalumkal JV, Kuhn SM, Guilche GMT, et al. Leishmania in HLH: a rare finding with significant treatment implications. J Pediatr Hematol Oncol. (2013) 35(3):e127–e9. doi: 10.1097/mph.0b013e318286d619
29. Higel L, Froehlich C, Pages MP, Dupont D, Collardeau-Frachon S, Dijoud F, et al. Macrophage activation syndrome and autoimmunity due to visceral leishmaniasis. Arch Pediatr. (2015) 22(4):397–400. doi: 10.1016/j.arcped.2014.11.025
30. Johnson SM, Gilmour K, Samarasinghe S, Bamford A. Haemophagocytic lymphohistiocytosis complicating visceral leishmaniasis in the UK: a case for detailed travel history, a high index of suspicion and timely diagnostics. BMJ Case Rep. (2019) 12(7):e228307. doi: 10.1136/bcr-2018-228307
31. Scalzone M, Ruggiero A, Mastrangelo S, Trombatore G, Ridola V, Maurizi P, et al. Hemophagocytic lymphohistiocytosis and visceral leishmaniasis in children: case report and systematic review of literature. J Infect Dev Ctries. (2016) 10(1):103–8. doi: 10.3855/jidc.6385
32. Russo A, Alt F, Neu MA, Eder S, Wingerter A, El Malki K, et al. Hemophagocytic lymphohistiocytosis in early infancy- pitfall of differentiation between hereditary and infectious reasons. Blood. (2018) 132(Supplement 1):4961. doi: 10.1182/blood-2018-99-117519
33. Visentin S, Baudesson de Chanville A, Loosveld M, Chambost H, Barlogis V. Infantile visceral leishmaniasis, an etiology of easily curable hemophagocytic lymphohistiocytosis syndrome. Arch Pediatr. (2013) 20(11):1225–9. doi: 10.1016/j.arcped.2013.08.003
34. Zhang Z. A case of leishmania associated hemophagocytic syndrome. J Lanzhou Univ. (2018) 44:82–4. (In Chinese). doi: 10.13885/j.issn.1000-2812.2018.06.015
35. Yi D. A case report of leishmaniasis complicated with hemophagocytic syndrome. Chin J Pract Pediatr. (2017) 32:639–40. (In Chinese). doi: 10.19538/j.ek20170806020
36. Shi H, Hao G, Wang X. Clinical analysis of eleven children with visceral leishmaniasis. Proc Clin Med. (2017) 26:245–9. (In Chinese). doi: 10.16047/j.cnki.cn14-1300/r.2017.04.002
37. Chao R, Wang L, Zhu S. Two cases of hemophagocytic syndrome secondary to viseral leishmaniasis in children and literature review. Mod Med J. (2021) 49:453–6. (In Chinese). doi: 10.3969/j.issn.1671-7562.2021.04.018
38. Suo T, Guoping H, Xiaohuan W, Yanli C, Haiyan L, Jing W, et al. Single-center clinical analysis of hemophagocytic syndrome secondary to leishmaniasis in children. Shanxi Med J. (2020) 49:1721–3. (In Chinese). doi: 10.3969/j.issn.0253-9926.2020.13.035
39. Dai W, Lei C, Fuqin Z. A case of hemophagocytic syndrome secondary to visceral leishmaniasis in infant. Chin J Parasitol Parasit Dis. (2019) 37:518–9. (In Chinese). doi: 10.12140/j.issn.1000-7423.2019.05.024
40. Arora P, Sangwan P, Jaedi H, Puthiyachirakka M, Hassan F, Hassanein M, et al. Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis: case report. Pediatr Blood Cancer. (2015) 62(S2):S21–119. doi: 10.1002/pbc.25540
41. Leblanc C, Nouar D, Izri A, Brun S, Marty P, Gaudelus J, et al. Visceral leishmaniasis without splenomegaly. A pediatric case report. Arch Pediatr. (2016) 23(4):378–81. doi: 10.1016/j.arcped.2015.12.007
42. Oudaina W, Assini K, El Ouardi M, Tligui H. Severe macrophage activation syndrome following visceral leishmaniasis in a child. Med Sante Trop. (2014) 24(2):221–3. doi: 10.1684/mst.2014.0335
43. Melchionda F, Varani S, Carfagnini F, Belotti T, Di Muccio T, Tigani R, et al. Spleen nodules: a potential hallmark of visceral leishmaniasis in young children. BMC Infect Dis. (2014) 14:620. doi: 10.1186/s12879-014-0620-2
44. Shi SL, Zhao H, Zhou BJ, Ma MB, Li XJ, Xu J, et al. Diagnostic value of bone marrow cell morphology in visceral leishmaniasis-associated hemophagocytic syndrome: two case reports. World J Clin Cases. (2022) 10(16):5463–9. doi: 10.12998/wjcc.v10.i16.5463
45. Li W, Yongjun W, Donghai L, Xuemei D, Wenyuan W, Qian H. Hemophagocytic syndrome caused by visceral leishmaniasis in children: a report of four cases and literature review. J Clin Pediatr. (2022) 41:54–9. doi: 10.12372/jcp.2023.22e0096
46. Zhang P, Wen H-L, Yang Y-L, Wang C-H. Analysis of three cases of infantile leishmaniasis misdiagnosed as hemophagocytic syndrome. Clin Misdiagn Misther. (2015) 28:57–9. (In Chinese). doi: 10.3969/j.issn.1002-3492.2015.01.018
47. Li M, Jiawu Y, Tingyun Y, Hongmin F. A case of visceral leishmaniasis complicated with hemophagocytic lymphohistiocytosis in an infant in Yunnan province. Chin Pediatr Emerg Med. (2022) 29:754–6. (In Chinese). doi: 10.3760/cma.j.issn.1673-4912.2022.05.021
48. Morimoto A, Nakazawa Y, Ishii E. Hemophagocytic lymphohistiocytosis: pathogenesis, diagnosis, and management. Pediatr Int. (2016) 58(9):817–25. doi: 10.1111/ped.13064
49. Mottaghipisheh H, Kalantar K, Amanati A, Shokripour M, Shahriari M, Zekavat OR, et al. Comparison of the clinical features and outcome of children with hemophagocytic lymphohistiocytosis (HLH) secondary to visceral leishmaniasis and primary HLH: a single-center study. BMC Infect Dis. (2021) 21(1):732. doi: 10.1186/s12879-021-06408-w
50. Rajagopala S, Dutta U, Chandra KS, Bhatia P, Varma N, Kochhar R. Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis–case report and systematic review. J Infect. (2008) 56(5):381–8. doi: 10.1016/j.jinf.2008.02.013
51. Blazquez-Gamero D, Dominguez-Pinilla N, Chicharro C, Negreira S, Galan P, Perez-Gorricho B, et al. Hemophagocytic lymphohistiocytosis in children with visceral leishmaniasis. Pediatr Infect Dis J. (2015) 34(6):667–9. doi: 10.1097/INF.0000000000000685
52. Abdinia B, Oliaei-Motlagh M, Teimouri-Dereshki A. Pediatric visceral leishmaniasis in northwest of Iran. Medicine. (2016) 95(44):e5261. doi: 10.1097/MD.0000000000005261
53. Zhou YH, Han XR, Xia FQ, Poonit ND, Liu L. Clinical features and prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: a retrospective analysis of 227 cases. J Pediatr Hematol Oncol. (2022) 44(1):e217–e22. doi: 10.1097/MPH.0000000000002283
54. Scarpini S, Dondi A, Totaro C, Biagi C, Melchionda F, Zama D, et al. Visceral leishmaniasis: epidemiology, diagnosis, and treatment regimens in different geographical areas with a focus on pediatrics. Microorganisms. (2022) 10(10):1887. doi: 10.3390/microorganisms10101887
55. Celik U, Alabaz D, Alhan E, Bayram I, Celik T. Diagnostic dilemma in an adolescent boy: hemophagocytic syndrome in association with kala azar. Am J Med Sci. (2007) 334(2):139–41. doi: 10.1097/MAJ.0b013e31812e97f4
56. Qing-xiang N, Wei R, Ling-ling Z, Hua-liang C, Li-nong Y. Epidemiological investigation of a child visceral leishmaniasis case in Wenzhou, Zhejiang Province. Chin J Parasitol Parasit Dis. (2019) 37:108–10. doi: 10.12140/j.issn.1000-7423.2019.01.021
57. Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. (2002) 9(5):951–8. doi: 10.1128/cdli.9.5.951-958.2002
58. Singh OP, Sundar S. Developments in diagnosis of visceral leishmaniasis in the elimination era. J Parasitol Res. (2015) 2015:239469. doi: 10.1155/2015/239469
59. Srivastava P, Dayama A, Mehrotra S, Sundar S. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. (2011) 105(1):1–6. doi: 10.1016/j.trstmh.2010.09.006
60. Kumari D, Perveen S, Sharma R, Singh K. Advancement in leishmaniasis diagnosis and therapeutics: an update. Eur J Pharmacol. (2021) 910:174436. doi: 10.1016/j.ejphar.2021.174436
61. Zhang X, Liu Y, Zhang M, Wang Z, Feng X, Yang L, et al. Case report: diagnosis of visceral leishmaniasis using metagenomic next-generation sequencing and bone marrow smear. Front Cell Infect Microbiol. (2022) 12(1095072):1–10. doi: 10.3389/fcimb.2022.1095072
62. Alves F, Bilbe G, Blesson S, Goyal V, Zijlstra EE, Monnerat S, et al. Recent development of visceral leishmaniasis treatments: successes, pitfalls, and perspectives. Clin Microbiol Rev. (2018) 31:e00048-18. doi: 10.1128/CMR.00048-18
63. van Griensven J, Diro E. Visceral leishmaniasis: recent advances in diagnostics and treatment regimens. Infect Dis Clin North Am. (2019) 33(1):79–99. doi: 10.1016/j.idc.2018.10.005
64. Maltezou HC. Drug resistance in visceral leishmaniasis. J Biomed Biotechnol. (2010) 2010:617521. doi: 10.1155/2010/617521
65. Bouguila J, Chabchoub I, Moncef Y, Mlika A, Saghrouni F, Boughamoura L, et al. Treatment of severe hemophagocytic syndrome associated with visceral leishmaniasis. Arch Pediatr. (2010) 17(11):1566–70. doi: 10.1016/j.arcped.2010.08.018
66. Pahwa R, Singh T, Khurana N. Hemophagocytic syndrome in malaria and kala-azar. Indian J Pathol Microbiol. (2004) 47(3):348–50.16295421
67. Ren D, Cao W, Liu X, Han Q, Fan W, Li G, et al. Case report: use of liposomal amphotericin B in low doses in patients with visceral leishmaniasis. Front Med. (2021) 8:766400. doi: 10.3389/fmed.2021.766400
68. Ay Y, Yildiz B, Unver H, Karapinar DY, Vardar F. Hemophagocytic lymphohistiocytosis associated with H1N1 virus infection and visceral leishmaniasis in a 4.5-month-old infant. Rev Soc Bras Med Trop. (2012) 45(3):407–9. doi: 10.1590/S0037-86822012000300026
69. Nguyen D, Nacher M, Epelboin L, Melzani A, Demar M, Blanchet D, et al. Hemophagocytic lymphohistiocytosis during HIV infection in cayenne hospital 2012–2015: first think histoplasmosis. Front Cell Infect Microbiol. (2020) 10:574584. doi: 10.3389/fcimb.2020.574584
70. Coukell AJ, Brogden RN. Liposomal amphotericin B. Therapeutic use in the management of fungal infections and visceral leishmaniasisDrugs. Drugs. (1998) 55(4):585–612. doi: 10.2165/00003495-199855040-00008
Keywords: visceral leishmaniasis, hemophagocytic lymphohistiocytosis, children, amphotericin B, systematic review
Citation: Chen Z, Gao Y, Zhang C and Mao J (2025) Hemophagocytic lymphohistiocytosis secondary to visceral leishmaniasis in children: case report and systematic review. Front. Pediatr. 13:1561600. doi: 10.3389/fped.2025.1561600
Received: 16 January 2025; Accepted: 24 March 2025;
Published: 11 April 2025.
Edited by:
Maurizio Aricò, ASL Pescara, ItalyReviewed by:
Desiree Caselli, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, ItalyCopyright: © 2025 Chen, Gao, Zhang and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Gao, c2VuaW9yeWkxMjNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.