
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 18 March 2025
Sec. Pediatric Surgery
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1558362
This article is part of the Research TopicAdvances and Challenges in Neonatal Surgery: Congenital and Acquired ConditionsView all 5 articles
 Jiahui Liu
Jiahui Liu Takuya Maeda
Takuya Maeda Chiyoe Shirota
Chiyoe Shirota Takahisa Tainaka
Takahisa Tainaka Wataru Sumida
Wataru Sumida Satoshi Makita
Satoshi Makita Yousuke Gohda
Yousuke Gohda Yoichi Nakagawa
Yoichi Nakagawa Aitaro Takimoto
Aitaro Takimoto Yaohui Guo
Yaohui Guo Daiki Kato
Daiki Kato Akihiro Yasui
Akihiro Yasui Akinari Hinoki
Akinari Hinoki Hiroo Uchida*
Hiroo Uchida*
Background: Robot-assisted surgery (RS) has gained popularity due to its potential advantages over conventional laparoscopic surgery (LS). However, the specific suturing steps that benefit most from RS in terms of efficiency remain unclear. This study aimed to compare the suturing performance and learning curves of RS and LS during hepaticojejunostomy.
Methods: We retrospectively analyzed surgical videos of patients who underwent hepaticojejunostomy performed by the same surgeon between 2016 and 2023. Cases with incomplete data or conversion to open surgery were excluded. Suturing efficiency, anastomotic precision, and learning curves were evaluated using standardized metrics.
Results: A total of 33 patients were included in the final analysis (17 RS, 16 LS). The median suture time per stitch was significantly shorter in the RS group (P = 0.017). The greatest efficiency gains were observed at the second (P = 0.041) and final stitches (P = 0.041). Complication rates were comparable between the two groups (P = 0.986).
Conclusion: RS significantly improves efficiency at challenging suturing steps and provides a more consistent learning curve, highlighting its potential advantage for complex pediatric procedures such as hepaticojejunostomy. Future multicenter studies with larger sample sizes and longer follow-up are needed to validate these results and explore long-term outcomes.
Extrahepatic biliary resection and hepaticojejunostomy are the standard surgical treatments for congenital biliary dilatation (CBD) (1, 2). However, suture failure in hepaticojejunostomy remains a major concern, as it can lead to serious complications such as bile leakage, cholangitis, and anastomotic stenosis, which can prolong hospitalization and contribute to long-term issues like hepatolithiasis (3, 4).
Over the years, advances in surgical methods have led to the development of two primary techniques: conventional laparoscopic surgery (LS) and robot-assisted surgery (RS). RS has been increasingly utilized in complex biliary procedures due to its potential advantages in precision and dexterity (5, 6). Villegas et al. compared RS and LS in a porcine model and found that RS resulted in shorter anastomosis times and fewer suture failures. Moreover, they found that once surgeons were trained in laparoscopic vivo suturing, the learning curve for RS became significantly shorter (7).
Several retrospective studies comparing RS and LS in hepaticojejunostomy have also shown that RS significantly reduces operative time and anastomotic complications (8, 9). Furthermore, Leijte et al. demonstrated that the learning curve for RS in minimally invasive suturing is steeper than that of LS, indicating a faster adaptation to robotic techniques (10).
To objectively evaluate surgical proficiency and technical consistency, cumulative sum (CUSUM) analysis is a commonly used method to visualize learning curves. By tracking deviations from the mean performance time, CUSUM analysis allows for the identification of key proficiency milestones, making it particularly useful for assessing complex surgical steps in minimally invasive procedures (11). Previous studies have applied CUSUM analysis to robotic-assisted urethral and gastrointestinal anastomoses, demonstrating that robotic systems can significantly shorten the learning phase and stabilize performance (12, 13). However, existing research on hepaticojejunostomy has primarily focused on overall anastomosis time rather than specific suturing steps, leaving a gap in understanding how RS enhances precision at different stages of the procedure.
Unlike previous research, this study provides a detailed analysis of specific suturing positions, particularly the second and final stitches, which are among the most technically challenging steps in hepaticojejunostomy. By focusing on these key suturing points, we aim to provide new insights into how RS stabilizes surgical performance and enhances precision in complex suturing tasks.
Thus, the purpose of this study is to comprehensively compare RS and LS in hepaticojejunostomy by analyzing performance at specific suture sites. A better understanding of these differences may guide clinical decision-making and improve surgical training in minimally invasive pediatric surgery.
This retrospective study included pediatric and adult patients with CBD who underwent RS or LS hepaticojejunostomy at Nagoya University Hospital between November 2016 and July 2023. All procedures were performed by a single board-certified surgeon. Patients with incomplete data or those requiring conversion to open surgery were excluded from the final analysis.
All procedures were performed by the same surgeon following a standardized surgical protocol. RS and LS were performed using similar steps, differing only in port placements. In both groups, hepaticojejunostomy was performed using interrupted 5–0 absorbable sutures. In the LS group, electrocautery was used for dissection around the bile duct using monopolar scissors. In the RS group, monopolar scissors and bipolar Maryland forceps were used for dissection (14).
The hepaticojejunostomy was performed in a standardized manner:
1. Posterior wall suturing was completed first, starting from the right edge and proceeding leftward.
2. The final stitch of the posterior wall was placed at the leftmost edge.
3. The anterior wall suturing was then performed in the same manner, ensuring completion of the anastomosis.
Measurement of operative parameters:
1. Suture time per stitch: Defined as the time from the surgeon holding the needle to the completion of knotting.
2. Anastomotic diameter: Measured intraoperatively or from surgical videos, using the diameter of the forceps as a reference. The anastomotic diameter was defined as the distance between the right and left ends of the hilar bile duct.
3. Suture pitch: Measured intraoperatively using video analysis, with the forceps diameter as a reference
The surgical videos were reviewed by the primary author, who independently assessed the suturing process. To ensure consistency and minimize bias, all videos were analyzed under identical viewing conditions, without patient identifiers or surgical outcome information. Postoperative bile leakage and cholangitis was used as an indicator of suture precision.
Cumulative sum (CUSUM) analysis was performed to assess the learning curve of robot-assisted surgery (RS) and laparoscopic surgery (LS) in hepaticojejunostomy. CUSUM analysis detects sequential changes in procedural performance by tracking deviations in anastomosis time from the group mean, allowing identification of the proficiency threshold.
The CUSUM score for each case was calculated as follows:
Where Xn represents the anastomosis time for case n, and Xmean is the overall mean anastomosis time across all cases.
The learning curve was divided into two phases:
Phase I (learning phase): Characterized by a continuous increase in the CUSUM curve, indicating the surgeon's skill acquisition and adaptation to the procedure.
Phase II (proficiency phase): Defined as the point where the CUSUM curve reaches its first major peak, followed by a sustained decline, indicating stabilization of performance.
Instead of applying a predefined statistical threshold, we determined the proficiency threshold based on the first major peak in the CUSUM curve, followed by a sustained decline, marking the transition from Phase I to Phase II.
Categorical variables were described as frequencies and percentages, while continuous variables were reported as medians with interquartile ranges. Mann–Whitney U-tests were used to compare continuous variables, with statistical significance defined as P < 0.05.
Statistical analyses were performed using SPSS version 29.0.2.0 (IBM Corp., Armonk, NY, USA).
All procedures in this study complied with the ethical standards of the institutional and national research committees and the 1964 Declaration of Helsinki. This study was approved by the Ethics Review Board of Nagoya University Graduate School of Medicine (approval number: 2022-0474).
A total of 34 patients were initially enrolled, but one RS case was converted to open surgery due to duodenal diverticulum perforation and was excluded, leaving 33 cases for analysis (17 RS, 16 LS). All LS cases were completed successfully.
Baseline characteristics, including age, sex, weight, and Todani classification, were comparable between the RS and LS groups, with no statistically significant differences (Table 1).
The anastomotic diameter was significantly larger in the RS group [10 (7.85-12.9) mm] than in the LS group [7.3 (5.78–9.78) mm, P = 0.037]. Similarly, the RS group used more suture needles (median 14 vs. 11, P < 0.001), whereas suture pitch was comparable between the groups (P = 0.846).
Suture time per stitch was significantly shorter in the RS group (179.5 s vs. 201.75 s, P = 0.017), indicating improved efficiency in robotic suturing.
The complication rates were comparable between groups (RS: 11.8% vs. LS: 12.4%, P = 0.986), with bile leakage and cholangitis observed in both groups at similar rates (Table 2).
Suturing times at key positions were compared (Table 3):
Right edge stitch: No significant difference between RS and LS groups (278 s vs. 255.5 s, P = 1).
Second stitch: The RS group was significantly faster (181 s vs. 240.5 s, P = 0.041).
Left edge stitch: No significant difference was noted (182 s vs. 165 s, P = 0.763).
Last stitch: The RS group was significantly faster than the LS group (148 s vs. 197 s, P = 0.041).
These findings suggest that robotic assistance improves efficiency, particularly in complex suturing steps (second and last stitches), while maintaining consistency in simpler steps.
The CUSUM analysis (Figure 1, Table 4) demonstrated notable differences in learning efficiency at different suturing positions:
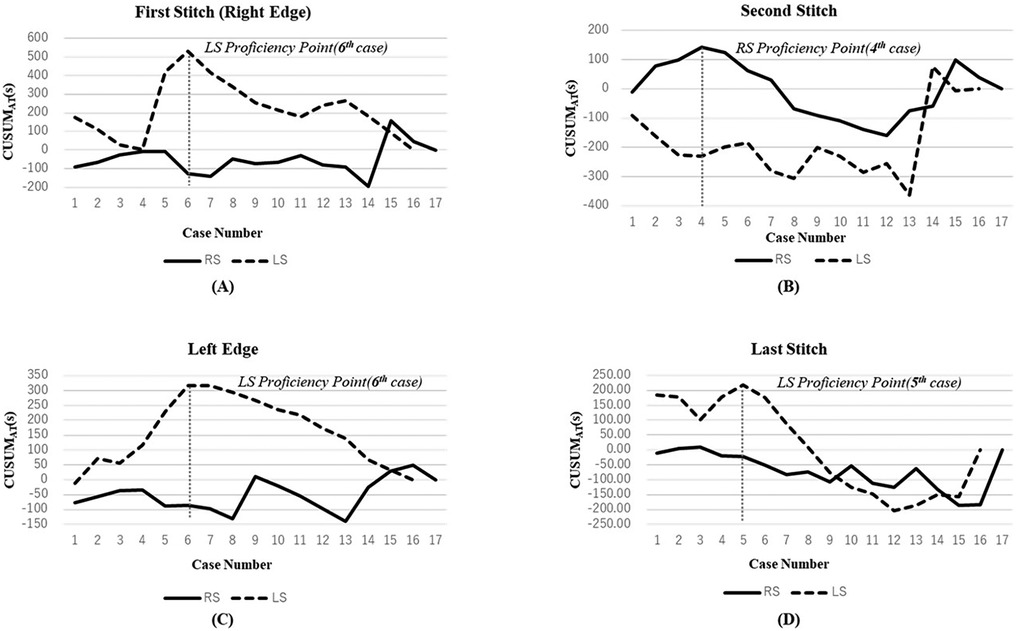
Figure 1. CUSUM analysis of suturing steps in hepaticojejunostomy. (A) First stitch (right edge); (B) Second stitch; (C) Left edge; (D) Last stitch. Solid lines and dashed lines represent RS and LS respectively. Vertical dashed lines indicate the transition point between Phase I (learning phase) and Phase II (proficiency phase).
Right-edge stitch (Figure 1A): The LS group exhibited a prolonged learning phase, stabilizing only after the sixth procedure (Phase Ⅰ: 350.5 s vs. Phase Ⅱ: 227.5 s, P = 0.056). This indicates that laparoscopic suturing at this position requires more practice to reach proficiency. In contrast, the RS group maintained stable performance from the first case.
Second stitch (Figure 1B): The RS group reached proficiency after four cases, showing a significant reduction in suturing time (Phase I: 233 s vs. Phase II: 178 s, P = 0.045).
This highlights the advantage of robotic assistance in handling complex suturing tasks.
Left-edge stitch (Figure 1C): The LS group's suturing time improved significantly after the sixth procedure (Phase Ⅰ: 256.5 s vs. Phase Ⅱ: 152.5 s, P ≤ 0.01). Conversely, the RS group maintained consistent precision from the first case, with minimal learning burden.
Last stitch (Figure 1D):
The LS group reached stability after five cases (Phase I: 251 s vs. Phase II: 170 s, P = 0.115).
The RS group demonstrated consistently low variance, with no clear “turning point”, suggesting immediate mastery of this simpler step.
These results indicate that robotic assistance accelerates proficiency in complex suturing steps while maintaining consistency across cases.
To further illustrate the differences in suturing techniques, Figure 2 presents representative intraoperative images of RS and LS hepaticojejunostomy.
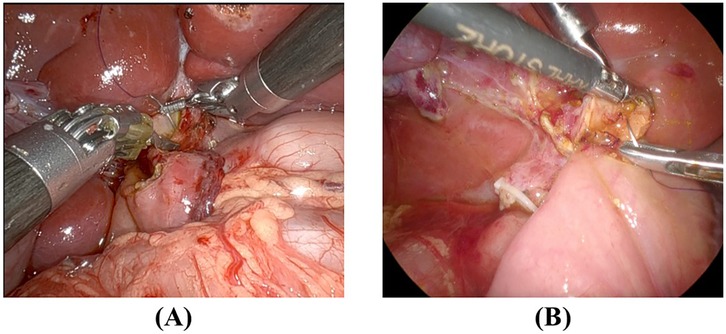
Figure 2. Intraoperative comparison of suturing techniques RS and LS (A) RS suturing at the hilar plate, demonstrating enhanced instrument articulation and stable needle control. The robotic arms provide tremor filtration and allow precise angulation. (B) LS suturing at the same location, requiring additional manual adjustments to maintain needle psitioning due to the limited range of motion of conventional laparoscopic instrument.
RS (Figure 2A) demonstrates enhanced instrument articulation and stable needle positioning, which facilitates precise suturing near the hilar plate.
LS (Figure 2B) requires additional manual adjustments due to limited instrument maneuverability, which may contribute to increased suturing time variability.
These intraoperative observations visually support the efficiency differences observed in our suturing time analysis.
Hepaticojejunostomy is a technically demanding procedure in congenital biliary dilatation (CBD) surgery, requiring high precision within a confined surgical field. This study is the first to utilize CUSUM analysis to assess suture efficiency at specific anastomotic steps, rather than relying solely on overall anastomosis time. By providing a more granular evaluation of suturing proficiency, our findings offer new insights into the technical advantages of robot-assisted surgery (RS) over laparoscopic surgery (LS) (15–19).
We found that RS significantly reduced suture time at challenging steps, such as the second and final stitches, whereas performance at simpler sites remained comparable between the groups. The second stitch is particularly complex due to its proximity to the hilar plate, requiring precise angulation within a narrow field. The CUSUM analysis showed that the RS group achieved proficiency after just four cases at this position (Phase Ⅰ: 233 s vs. Phase Ⅱ: 178 s, P = 0.045), whereas the LS group demonstrated significant variability and lacked a clear turning point. This underscores the benefits of robotic articulation and tremor filtration, which enhance precision in anatomically constrained regions.
Interestingly, RS also demonstrated superior efficiency at the final stitch, despite its lower complexity. The RS group exhibited consistently low variance across all cases, with no clear “turning point,” indicating immediate mastery of this step. In contrast, the LS group required five cases to stabilize (Phase Ⅰ: 251 s vs. Phase Ⅱ: 170 s, P = 0.115). This finding aligns with previous studies on robot-assisted vesicourethral anastomosis, where RS outperforms LS in repetitive fine motor tasks due to its ergonomic instrument design (20).
The left-edge stitch analysis further highlighted RS's advantage in maintaining performance consistency. While the LS group exhibited a steep learning curve, stabilizing only after six cases (Phase Ⅰ: 256.5 s vs. Phase Ⅱ: 152.5 s, P ≤ 0.01), the RS group demonstrated stable performance from the first case onward, minimizing the learning burden. These results reinforce the hypothesis that robotic systems enhance precision while reducing operator fatigue, particularly in prolonged procedures requiring sustained dexterity.
Unlike previous studies that primarily focused on total anastomosis time, our analysis of suture time per stitch provides a more precise assessment of technical performance at each step of the procedure. Total anastomosis time can be influenced by multiple factors, including anastomotic diameter and the number of stitches. In this study, the RS group had a larger anastomotic diameter and required more stitches (median 14 vs. 11, P < 0.001), yet maintained a consistent suture pitch (P = 0.846), demonstrating greater precision without compromising spacing. The reason for the larger anastomotic diameter in the RS group is not entirely clear, but it is possible that a better view of the hepatic hilum allows for a more precise lateral incision to enlarge the bile duct diameter.
Furthermore, our complication analysis showed no significant difference between RS and LS (11.8% vs. 12.4%, P = 0.986), suggesting that the improved efficiency with RS does not compromise patient safety. However, long-term outcomes such as anastomotic stenosis or hepatolithiasis were not systematically assessed, warranting further follow-up studies.
Consistent with prior studies (21, 22), RS demonstrated a shorter learning phase in complex suturing steps, such as the second stitch, achieving proficiency after four cases, compared to six cases in LS. Additionally, at the left-edge and final stitches, RS maintained stable performance throughout, while LS showed greater variability during the early phase. These findings underscore the ergonomic and technical benefits of robotic systems in delicate, high-precision tasks. Our intraoperative analysis further supports these findings (Figure 2). The superior articulation of RS instruments reduced the need for repeated adjustments, allowing for greater consistency in suturing time, particularly at the second and final stitches. These observations align with our CUSUM analysis, which demonstrated a shorter learning phase and greater stability in RS compared to LS. In contrast, the increased manual adjustments required in LS may contribute to the greater variability in suturing efficiency observed in our study.
Despite these promising findings, our study has limitations. First, it was a retrospective, single-surgeon study with a relatively small sample size, which may limit its generalizability. Second, this analysis was based on the suturing of a highly experienced surgeon who had already performed many other LS procedures before transitioning to RS. Therefore, it may differ from an analysis conducted on beginners. Third, blinding was not feasible during video evaluation due to the nature of the recorded surgical procedures, which could introduce potential bias in the assessment.
Future research should focus on multicenter studies with diverse surgical teams, incorporating long-term follow-up to evaluate anastomotic patency, stenosis rates, and overall clinical outcomes in pediatric hepaticojejunostomy.
Our study demonstrated that RS significantly reduced suturing time compared to conventional LS, particularly at challenging steps such as the second and final stitches, while maintaining comparable complication rates. The CUSUM analysis further highlighted the shorter learning phase and greater procedural consistency of RS, suggesting an advantage in mastering complex suturing tasks.
These findings suggest that RS may be preferable for technically demanding pediatric procedures, such as hepaticojejunostomy, as it not only enhances surgical precision but also reduces the learning burden for trainees. Integrating robotic platforms into pediatric surgical training programs could accelerate skill acquisition and improve long-term proficiency.
Future multicenter studies with larger sample sizes and extended follow-up are warranted to validate these results and assess the long-term clinical outcomes, including anastomotic patency and postoperative complications.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Review Board of Nagoya University Graduate School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
JL: Formal Analysis, Validation, Visualization, Writing – original draft. TM: Data curation, Resources, Writing – review & editing. CS: Writing – review & editing. TT: Resources, Writing – review & editing. WS: Resources, Writing – review & editing. SM: Resources, Writing – review & editing. YoG: Data curation, Writing – review & editing. YN: Resources, Writing – review & editing. AT: Data curation, Writing – review & editing. YaG: Data curation, Writing – review & editing. DK: Data curation, Writing – review & editing. AY: Validation, Writing – review & editing. AH: Validation, Writing – review & editing. HU: Resources, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The author expresses gratitude to colleagues and mentors for their invaluable guidance and support throughout the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ishibashi H, Shimada M, Kamisawa T, Fujii H, Hamada Y, Kubota M, et al. Japanese Clinical practice guidelines for congenital biliary dilatation. J Hepato-Biliary-Pancreat Sci. (2017) 24(1):1–16. doi: 10.1002/jhbp.415
2. Ciccioli C, Mazza S, Sorge A, Torello Viera F, Mauro A, Vanoli A, et al. Diagnosis and treatment of choledochal cysts: a comprehensive review with a focus on choledochocele. Dig Dis Sci. (2025) 70(1):39–48. doi: 10.1007/s10620-024-08708-y
3. Haidar H, Manasa E, Yassin K, Suissa A, Kluger Y, Khamaysi I. Endoscopic treatment of post-cholecystectomy bile leaks: a tertiary center experience. Surg Endosc. (2021) 35(3):1088–92. doi: 10.1007/s00464-020-07472-0
4. Tang ST, Yang Y, Wang Y, Mao YZ, Li SW, Tong QS, et al. Laparoscopic choledochal cyst excision, hepaticojejunostomy, and extracorporeal Roux-en-Y anastomosis: a technical skill and intermediate-term report in 62 cases. Surg Endosc. (2011) 25(2):416–22. doi: 10.1007/s00464-010-1183-y
5. Marichez A, Adam JP, Laurent C, Chiche L. Hepaticojejunostomy for bile duct injury: state of the art. Langenbecks Arch Surg. (2023) 408(1):107. doi: 10.1007/s00423-023-02818-3
6. Cazares J, Koga H, Yamataka A. Choledochal cyst. Pediatr Surg Int. (2023) 39(1):209. doi: 10.1007/s00383-023-05483-1
7. Villegas L, Lagoo S, Schwartz T, Athar N, Greene R, Eubanks WS. Robotically assisted laparoscopic Roux-en-Y hepaticojejunostomy. JSLS. (2004) 8(3):239–44.15347111
8. Koga H, Murakami H, Ochi T, Miyano G, Lane GJ, Yamataka A. Comparison of robotic versus laparoscopic hepaticojejunostomy for choledochal cyst in children: a first report. Pediatr Surg Int. (2019) 35(12):1421–5. doi: 10.1007/s00383-019-04565-3
9. Chi S-Q, Cao G-Q, Li S, Guo J-L, Zhang X, Zhou Y, et al. Outcomes in robotic versus laparoscopic-assisted choledochal cyst excision and hepaticojejunostomy in children. Surg Endosc. (2021) 35(9):5009–14. doi: 10.1007/s00464-020-07981-y
10. Leijte E, De Blaauw I, Van Workum F, Rosman C, Botden S. Robot assisted versus laparoscopic suturing learning curve in a simulated setting. Surg Endosc. (2020) 34(8):3679–89. doi: 10.1007/s00464-019-07263-2
11. Nasseri Y, Stettler I, Shen W, Zhu R, Alizadeh A, Lee A, et al. Learning curve in robotic colorectal surgery. J Robot Surg. (2021) 15(3):489–95. doi: 10.1007/s11701-020-01131-1
12. Gil PJ, Ruiz-Manzanera JJ, De Angulo D R, Munitiz V, Ferreras D, López V, et al. Learning curve for laparoscopic sleeve gastrectomy: a cumulative summation (CUSUM) analysis. Obes Surg. (2022) 32(8):2598–604. doi: 10.1007/s11695-022-06145-2
13. Guo Y, Hinoki A, Deie K, Tainaka T, Sumida W, Makita S, et al. Anastomotic time was associated with postoperative complications: a cumulative sum analysis of thoracoscopic repair of tracheoesophageal fistula in a single surgeon’s experience. Surg Today. (2023) 53(12):1363–71. doi: 10.1007/s00595-023-02687-9
14. Maeda T, Liu J, Uchida H, Amano H, Shirota C, Tainaka T, et al. Robotic versus laparoscopic radical surgery for pediatric congenital biliary dilatation: a comparison of surgical outcomes of a single surgeon’s initial experience. Pediatr Surg Int. (2023) 39(1):261. doi: 10.1007/s00383-023-05548-1
15. Rha SY, Stovroff MC, Glick PL, Allen JE, Ricketts RR. Choledochal cysts: a ten year experience. Am Surg. (1996) 62(1):30–4.8540642
16. Pasticier G, Rietbergen JBW, Guillonneau B, Fromont G, Menon M, Vallancien G. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol. (2001) 40(1):70–4. doi: 10.1159/000049751
17. Ou YC, Yang CR, Wang J, Cheng CL, Patel VR. Learning curve of robotic-assisted radical prostatectomy with 60 initial cases by a single surgeon. Asian J Surg. (2011) 34(2):74–80. doi: 10.1016/S1015-9584(11)60023-7
18. Grivas N, Zachos I, Georgiadis G, Karavitakis M, Tzortzis V, Mamoulakis C. Learning curves in laparoscopic and robot-assisted prostate surgery: a systematic search and review. World J Urol. (2022) 40(4):929–49. doi: 10.1007/s00345-021-03815-1
19. Jaffe J, Castellucci S, Cathelineau X, Harmon J, Rozet F, Barret E, et al. Robot-Assisted laparoscopic prostatectomy: a single-institutions learning curve. Urology. (2009) 73(1):127–33. doi: 10.1016/j.urology.2008.08.482
20. Heemskerk J, Van Gemert WG, De Vries J, Greve J, Bouvy ND. Learning curves of robot-assisted laparoscopic surgery compared with conventional laparoscopic surgery: an experimental study evaluating skill acquisition of robot-assisted laparoscopic tasks compared with conventional laparoscopic tasks in inexperienced users. Surg Laparosc Endosc Percutan Tech. (2007) 17(3):171–4. doi: 10.1097/SLE.0b013e31805b8346
21. Menon M, Shrivastava A, Tewari A, Sarle R, Hemal A, Peabody JO, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. (2002) 168(3):945–9. doi: 10.1016/S0022-5347(05)64548-X
Keywords: hepaticojejunostomy, robot-assisted surgery, laparoscopic surgery, CUSUM analysis, congenital biliary dilatation, surgical approach
Citation: Liu J, Maeda T, Shirota C, Tainaka T, Sumida W, Makita S, Gohda Y, Nakagawa Y, Takimoto A, Guo Y, Kato D, Yasui A, Hinoki A and Uchida H (2025) Learning curve comparison of robot-assisted and laparoscopic hepaticojejunostomy: a focus on critical suturing. Front. Pediatr. 13:1558362. doi: 10.3389/fped.2025.1558362
Received: 10 January 2025; Accepted: 5 March 2025;
Published: 18 March 2025.
Edited by:
Simonetta Costa, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Alessandro Boscarelli, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyCopyright: © 2025 Liu, Maeda, Shirota, Tainaka, Sumida, Makita, Gohda, Nakagawa, Takimoto, Guo, Kato, Yasui, Hinoki and Uchida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroo Uchida, dWNoaWRhLmhpcm9vLmY1QGYubWFpbC5uYWdveWEtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.