- 1Department for Children and Adolescents, Goethe-University Frankfurt, Frankfurt, Germany
- 2Interdisciplinary Center for Clinical Trials (IZKS), University Medical Center Mainz, Mainz, Germany
- 3Children’s Hospital, Evangelisches Krankenhaus Düsseldorf, Düsseldorf, Germany
- 4Pediatric Allergology, Department of Pediatrics, Dr. von Hauner Children’s University Hospital, LMU Munich-Member of the German Center for Lung Research-DZL-LMU Munich, Munich, Germany
- 5Department of Pediatrics, Medical Center, University of Leipzig, Leipzig, Germany
- 6Klinik für Kinder- und Jugendmedizin, Universität Jena, Jena, Germany
- 7Department for Pediatric Cardiology, Intensive Care and Neonatology, University Medicine, Göttingen, Germany
- 8Kinderlungen-Facharzt, Gemeinschaftspraxis in Mannheim, Mannheim, Germany
- 9Department of Respiratory Medicine, Immunology and Critical Care Medicine, Charité Universitätsmedizin Berlin, Berlin, Germany
- 10Department of Pediatrics, Children’s Center Bethel, University Medicine, Bielefeld, Germany
- 11Klinik und Poliklinik für Kinder- und Jugendmedizin, Universitätsklinikum Carl Gustav Carus an der Technischen Universität, Dresden, Germany
- 12Kinderklinik, Universitätsklinikum Augsburg, Augsburg, Germany
- 13Pediatric Pneumology, Allergology, Neonatology, Hannover Medical School, Hannover, Germany
Objective: Children with preschool asthma suffer disproportionally more often from severe asthma exacerbations with emergency visits and hospital admissions than school children. However, there are only a few reports on characteristics, hospitalization, phenotypes and symptoms in this age cohort.
Patients and methods: This analysis of an ongoing prospective trial of Tiotropium bromide in preventing severe asthma exacerbations (the TIPP study) assessed baseline characteristics, hospitalizations and symptoms in 100 children with severe preschool asthma. Children aged 1–5 years were analyzed at study enrollment and daily symptoms were recorded by an electronic diary [Pediatric Asthma Caregiver Diary (PACD)] for the following four weeks until randomization.
Results: At enrollment, the total number of severe asthma exacerbations, defined as three days systemic steroid use or hospitalization in the last 24 months, was mean (±SD) 5.8 ± 5.7 and the test for respiratory and asthma control in kids (TRACK) was mean 46.9 ± 19.0. Daily recording of symptoms by the PACD revealed that only 7 patients were controlled at randomization, whereas 35 were partially and 58 were uncontrolled according to GINA.
Conclusion: Despite protective therapy with inhaled corticosteroids (ICS), most children of this severe asthma cohort were only partially or uncontrolled according to GINA guidelines.
Research in context
• Evidence before this studyWe reviewed literature on PubMed before writing this manuscript for recent publications on severe asthma in preschool children. Despite this high disease burden, limited detailed reports on characteristics, hospitalization, phenotypes and symptoms in this particularly severe asthma cohort are available. The preschool asthma cohort is often described as exacerbation prone with relatively limited impairment.
• Added value of this studyThis analysis provides additional evidence that hospitalizations are frequent, and the electronic diary record showed a high symptom burden despite regular ICS treatment.
• Implications of all the available evidenceDespite protective therapy with ICS, most children of this severe preschool asthma cohort are only partial or uncontrolled according to GINA guidelines. Current care and treatment are not sufficient to adequately control severe asthma in preschool children.
Introduction
Asthma is the most common chronic disease in children, imposing a high lifetime burden on individuals, their caregivers, and healthcare systems (1, 2). The prevalence of childhood asthma has increased over the last 20 years, most likely due to a greater awareness of this condition and changes in diagnostic practice (1, 3). The global prevalence of diagnosed current asthma in children 5 years and younger cannot be well estimated due to the lack of international consensus on diagnostic criteria (4). A recent ERS task force suggested that the criteria used to define wheezing disorders in preschool children should include age of diagnosis (0 to <6 years) and confirmation of wheezing on more than one occasion (5).
Of note is the diagnostic uncertainty among young children in whom wheezing is more likely to be associated with lower respiratory tract infection and often is transient (5–9). Although asthma prevalence is higher in school children than in toddlers, severe asthma exacerbations with emergency admissions and hospitalizations occur disproportionally more often in preschool asthma (10, 11). In addition, young children are more susceptible to adverse outcomes than older children, due to their small airways and possibly increased bronchial airway reactivity (12, 13). Respiratory distress in the setting of infection can rapidly become life-threatening. This explains the relatively high use of emergency visits and hospitalizations among preschool children.
Many children with preschool asthma are not well controlled by inhaled corticosteroids (ICS), which is a significant healthcare concern (14). Failure to control asthma has a negative impact on patients' quality of life, and increases the risk of future exacerbations, which in turn leads to an increased need for medical care and higher costs (15, 16). The management of children with preschool asthma is complicated by a paucity of high-quality clinical trials in this age group. The preschool asthma population is often described as being susceptible to exacerbations with relatively limited impairment (17). However, young children with frequent severe exacerbations are common and caregivers are complaining about frequent coughing and wheezing and missed days in daycare center, especially in winter (18, 19).
Although studies are limited, data suggest that an asthma-like inflammation (presence of eosinophils and allergic sensitization) may be present at a very early age in some children with recurrent wheeze (20, 21). However, approximately half of preschool asthma patients do not present with TH2- inflammation and may show a TH1-like neutrophilic airway inflammation (11). These children are often younger and suffer from severe RSV and Rhinovirus infections and may outgrow this condition at school-age (11, 22–24). Number of exacerbations in the last 24 months, sensitization and analysis of eosinophils are useful for predicting future exacerbations and can identify children who are most likely to respond to daily ICS treatment (11, 25, 26). Nevertheless, current treatment guidelines are not based on the underlying asthma phenotype (27). This might explain why ICS is effective in reducing severe exacerbations by only 36% in preschool asthma patients (11, 27). At present, there are insufficient data to recommend additional controller therapies in this age group, such as combinations of ICS with LABAs or LAMAs (28, 29). In the Respimat, Tiotropium bromide has been approved by the European Medicines Agency for use in adults, and adolescents (12 years and older), and in children (6–11 years), as add-on maintenance treatment for severe asthma, who experienced at least one asthma exacerbation in the preceding year. Adding Tiotropium to ICS might be a new promising treatment option for severe uncontrolled preschool asthma (30, 31). However, the number of patients included in both studies was small, and further research is required to confirm the beneficial effect on asthma phenotypes, symptom control and exacerbations in this age group.
Accordingly, we present the characteristics of a severe preschool asthma cohort, such as number of severe exacerbations, level of asthma control, daily asthma symptoms monitored with an electronic diary for four weeks, and TH2-phenotype at enrollment in the ongoing TIPP study (TIPP study: Tiotropium as add-on to ICS; EudraCT 2021-000190-81).
Methods
The TIPP study is an ongoing, prospective multicenter placebo-controlled trial to evaluate the efficacy and safety of Tiotropium inhalation solution 2.5 µg daily in preventing severe asthma exacerbations in partial and uncontrolled preschool asthma. It is planned to enroll approximately 150 patients at 13 study sites in Germany. The present manuscript describes the baseline characteristics of the first 100 patients enrolled in the study. The study was registered at the “EU Clinical Trials Registry” (EudraCT 2021-000190-81, https://www.clinicaltrialsregister.eu/) and transitioned according to EU Clinical Trial Regulation 536/2014 (EU CT 2024-513916-84-00). The complete protocol of the study is provided in the Supplementary Material. Ethics approval was obtained from the leading Ethics Committee of the Goethe-University in Frankfurt (application number 2021-443-AMG) and all participating Ethics Committees. Written informed consent of both parents, or the custodial parent, was obtained before participation of the child.
All children were analyzed at study enrollment (visit 1) and daily symptoms were recorded with an electronic diary [Pediatric Asthma Caregiver Diary (PACD)] for the following four weeks until randomization (visit 2).
Main diagnosis for study entry
Patients aged 1–5 years with a history of at least one severe asthma exacerbation requiring hospitalization in the last 24 months and/or treated with 2 courses of systemic steroids before visit 1. In addition, all patients must have been on maintenance treatment with an ICS at a stable dose for at least 4 weeks before visit 1. At visit 1, and during the following four weeks of observation until visit 2, patients had to be symptomatic (partly or uncontrolled)—despite their current maintenance treatment—to be subsequently randomized to either the control group (placebo) or the intervention group (Tiotropium bromide).
Main inclusion criteria
Patients meeting the following criteria were recruited for the study:
1. All patients' parents (or legal guardians) must sign and date an informed consent, consistent with ICH-GCP guidelines and local legislation prior to participation in the trial.
2. Male or female patients with preschool asthma aged between 1 and 5 years (<6 at visit 1).
3. Physician diagnosed asthma with at least 6 months' history of asthma symptoms, including but not limited to wheezing, cough, and/or shortness of breath.
4. All patients must have been on maintenance treatment with an ICS at a stable dose, either as mono treatment or in combination with another controller medication, for at least 4 weeks before screening (visit 1) and randomization (visit 2).
5. Patient was hospitalized due to acute severe asthma and/or was treated with 2 courses of systemic steroids (three days of oral steroids or one day of rectal prednisolone 100 mg) in the last 24 months before visit 1.
6. Caregivers had to record daily symptoms by an electronic diary [Pediatric Asthma Caregiver Diary (PACD)] between visit 1 and visit 2 until randomization.
7. All patients must be symptomatic (partly or uncontrolled) as defined by the GINA guideline for children aged 5 years and in the week prior to randomization despite treatment with ICS between visit 1 and visit 2.
8. Ability of parents/legal guardians to understand nature, importance, and individual consequences of the trial.
9. Patients must be able to inhale from the Respimat® inhaler (with a spacer).
Main exclusion criteria
Patients presenting with any of the following criteria will not be included in the trial:
1. Patients with a significant disease other than asthma such as, but not limited to, the following diagnoses: cystic fibrosis, bronchopulmonary dysplasia, primary immune-deficiency, congenital heart disease, parasitic disease, and foreign body aspiration.
2. Patients with clinically relevant abnormal screening hematology or blood chemistry will be excluded if the abnormality defines a significant disease as defined in the exclusion criterion.
3. Patients with any acute asthma exacerbation or respiratory tract infection in the four weeks before visit 1 (screening). If an acute exacerbation or respiratory tract infection occurs 4 weeks before visit 1, this visit can be postponed for 4 weeks.
4. Patients with a history of congenital or acquired heart disease, or patients who have been hospitalized for cardiac syncope or failure during the past year.
5. Medical or psychological conditions that would jeopardize an adequate and orderly completion of the trial.
6. Patients with known hypersensitivity to anticholinergic drugs, or any other components of the Tiotropium inhalation solution.
Outcome parameters
Number of hospitalizations, number of systemic corticosteroids cycles (SCS) (defined as 3 days SCS use), number of exacerbations, night-time awakenings due to asthma symptoms as assessed by the patient`s electronical diary/PACD, percentage of days with asthma symptoms, percentage of days with use of salbutamol rescue medication, health utilization (missed days in daycare center, number of physician visits), were evaluated as outcome parameters.
To define the asthma phenotype, blood eosinophils, total Immunoglobulin E (IgE), and sIgE were measured. Asthma phenotypes were defined as recently described (26, 32):
1. Sensitization in patients (TH2-phenotype/possible allergy) was defined by measuring sIgE ≥ 0.75 K/UL to any of the tested allergens like birch, grass, mites, alternaria, cladosporium, cat, dog, milk, egg and peanut.
2. Non-allergic asthma was defined by absence of sIgE levels > 0.75 K/UL.
3. Patients without sIgE data (missing values) were classified as not defined.
Level of education of parents and/or caretakers
The information for parents and caregivers about the Tipp study, current asthma treatment in children 1–5 years and appropriate use of asthma medications like Salbutamol, Fluticasone and Fluticasone + LABA (Viani mite) were displayed during the recruitment period on two specialized websites. In addition, all parents and caregivers were educated about the need to inhale ICS on a regular basis. In addition, during every visit the inhalation technique was trained and the importance of adherence to treatment was taught by the study nurses and physicians. Children 1–<4 years inhaled ICS and tiotropium via an aerochamber with a face mask. In children >4 years, children and parents were trained to inhale via an areochamber using the mouthpiece.
PACD
The PACD was used to evaluate daily asthma symptoms in children aged 1–5 years (33). The diary consists of three questions to be answered each morning when the child wakes up, and seven questions to be answered each evening, right after the child goes to bed. The combined daytime score is the average of scores from questions 4–7 in the diary which are questions regarding severity of cough, wheezing, trouble breathing, and interference with activities, scores for each question range from 0 (best) to 5 (worst). The PACD questions were collected electronically by a self-developed diary App (https://apps.apple.com/de/app/tipp-diary/id1598597989).
TRACK test
The test for respiratory and asthma control in kids (TRACK) was used to evaluate the respiratory and asthma control at visit 1 (34).
Laboratory parameters
The following parameters were analyzed at visit 1: Blood count with eosinophils, a safety lab (CRP, liver enzymes, creatinine), and total IgE and sIgE to 10 allergens [birch, grass, house dust mites (Dermatophagoides pteronyssinus), alternaria, cladosporium, cat, dog, milk, egg and peanut].
Statistics
Basic descriptive statistics including absolute and relative frequency distributions are reported. Clinical characteristics were compared by exploratory F-tests, chi-squared tests or Fisher's exact tests, if appropriate. Statistical analysis was performed with SAS Version 9.4.
Results
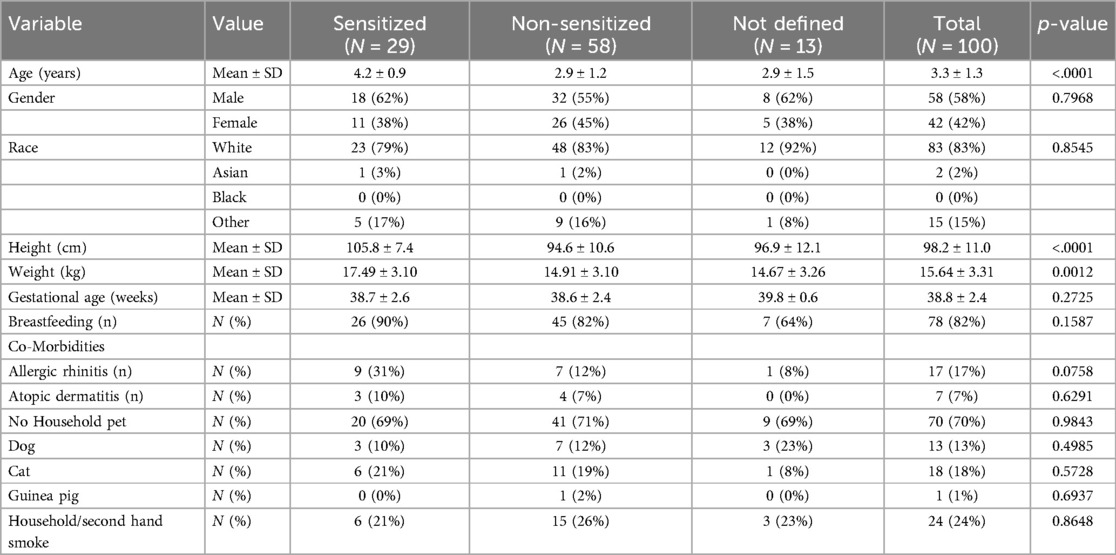
Between February 2022 and March 2024, 100 patients with severe asthma defined by hospitalization due to acute severe asthma and/or treatment with 2 courses of systemic steroids in the last 24 months were enrolled at 13 study centers. Of these patients, 58 (58%) were males. The male predominance was more pronounced in the sensitized patients, 18 (62%) vs. female, 11 (38%). In addition, the group of non-sensitized patients was significantly younger (p < 0.01). Accordingly, their size and weight were smaller (Table 1). All other parameters were equally distributed.
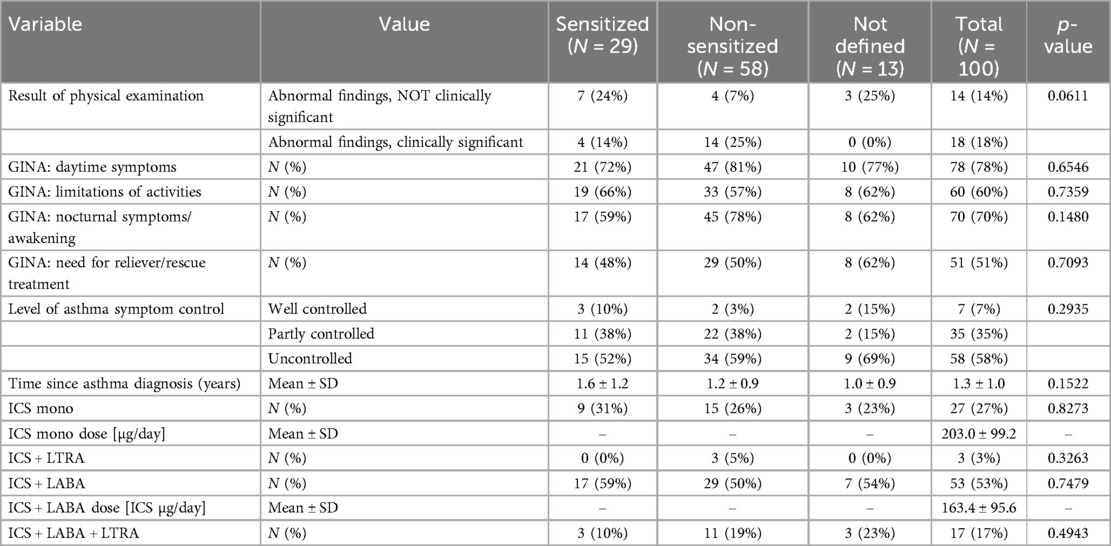
The maintenance therapy showed that ICS mono treatment was given in 27 (27%) patients, an ICS + LABA combination in 53 (53%) patients and an additional controller treatment with LTRA was given in 20 (20%) patients only (Table 2).
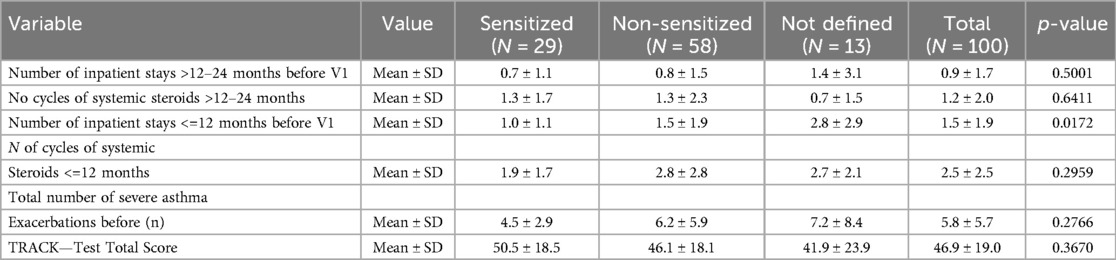
At enrollment, the number of asthma exacerbations and SCS cycles of the previous 12 and 24 months were recorded. As shown in Table 3, the total number of severe asthma exacerbations was mean (±SD) 5.8 ± 5.7. Accordingly, the TRACK test for respiratory and asthma control in kids was 46.9 ± 19.0, indicating severe uncontrolled preschool asthma. None of the children was fully controlled at enrollment.
Asthma phenotype
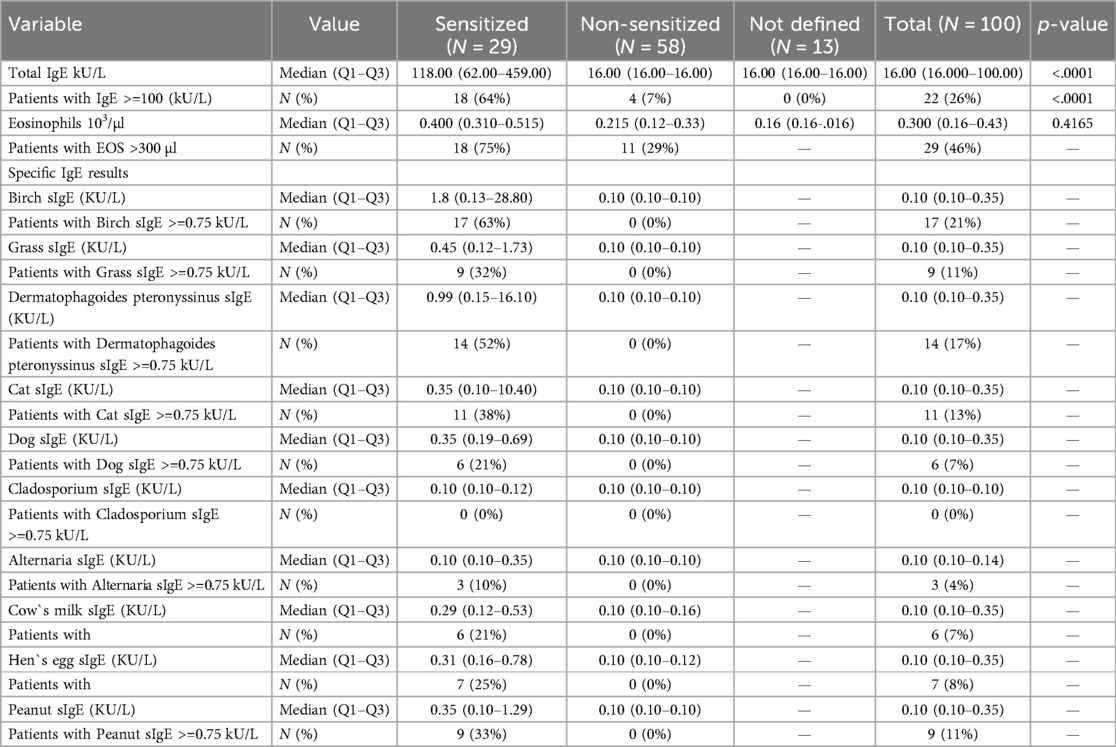
The determination of the asthma TH-2 phenotype (complete dataset) was possible in 87% of the 100 enrolled patients. The phenotype distribution of patients based on sIgE is shown in Table 4: Sensitized patients had significantly higher levels of total IgE and higher eosinophils than patients with non-allergic asthma (IgE 260.95 ± 335.46 vs. 27.90 ± 37.59; p < 0.01; eosinophils 0.723 ± 1.275 vs. 0.377 ± 0.826, n.s.).
Allergen sensitization
The distribution of sensitization to pollen, HDM, animal epithelial and food allergens is shown in Table 4. Birch pollen sensitization was found in 17 (63%) sensitized patients. HDM sensitization was present in 14 (52%) patients and cat sensitization in 11 (38%) patients. Peanut sensitization was found in 9 (33%) patients and egg sensitization in 7 (25%) patients.
Symptoms and treatment between V1 and V2
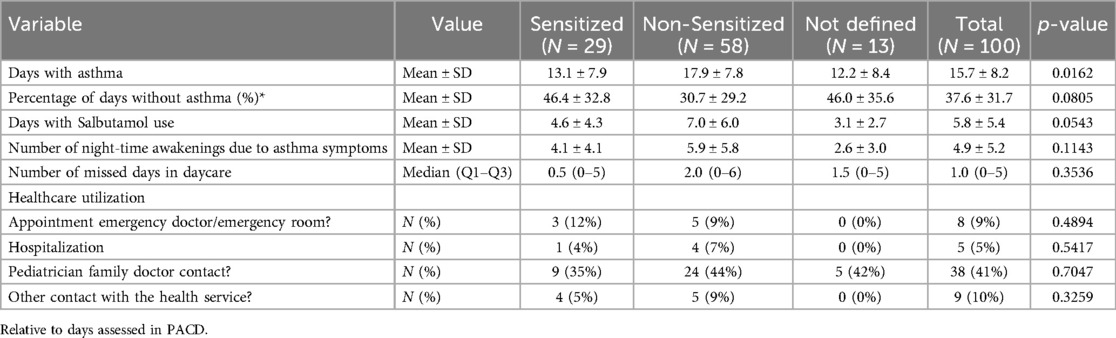
The compliance of the TIPP App diary use was high with a median (Q1–Q3) of 96% (86%–100%) documented days. As shown in Table 5, patients had symptoms on mean (± SD) 15.7 ± 8.2 days and night-time awakenings in mean 4.9 ± 5.2 of 28 days of observation. Daily salbutamol use was recorded at mean 5.8 ± 5.4 days and median (Q1–Q3) number of missed days in daycare was 1.0 (0–5 days (Table 5). Although the patients in TIPP are being cared for, healthcare utilization was high. The number of pediatric family contacts were 38 (41%), other health contacts 9 (10%) and emergency visits 8 (9%) during the four weeks observation (Table 5).
Interestingly, the non-sensitized patients had significantly more days with asthma symptoms than the sensitized group (17.9 ± 7.8). However, in all other indices the symptom level of non-sensitized patients were similar or slightly higher (Table 5).
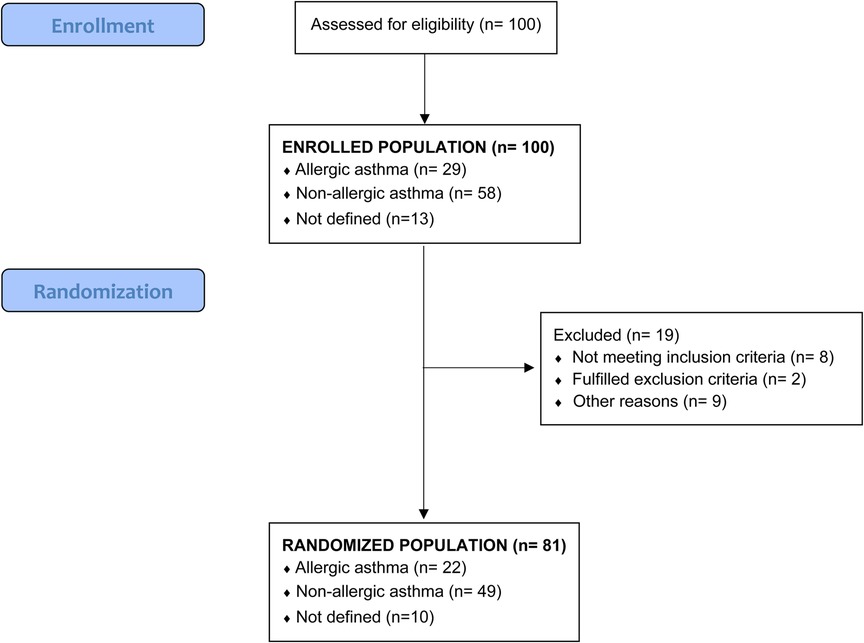
Daily symptoms recording by the PACD revealed that only 7 patients were controlled at randomization, whereas 35 were partially and 58 uncontrolled according to GINA. The GINA level in relation to the month of recruitment is shown in the supplement (Supplementary Figure 1). In addition, 10 patients were not randomized due to different reasons (Figure 1. Flow chart).
Discussion
Optimizing asthma control and management among preschool children is an unmet need since this age group experiences disproportional morbidity and health care utilization compared to school-age children with asthma (1, 11, 26, 28). Known risk factors for exacerbations include poor asthma control, previous hospitalization, viral infection, secondhand smoking and eosinophilic inflammation. So far, only a few studies have analyzed patients' characteristics and symptoms with acute severe asthma in preschoolers. This is the strength of our study, which analyzed such an age cohort at enrollment and monitored the symptom level for 4 weeks with a new App using the PACD questionnaire until randomization.
The age distribution of the present cohort showed that most children were around 3 years old, with a significant age difference between non-sensitized and sensitized children (2.9 vs. 4.2 years). Both phenotypes experienced a high number of severe asthma exacerbations defined by systemic steroid use in the last 24 months (with and without sensitization: 4.5 vs. 5.9)—although all patients were treated with ICS and often a second controller such as LTRA or LABA for more than 12 months. This is consistent with earlier reports showing that ICS and ICS + LABA combinations are less effective in this age group (27, 35). The reasons for the frequent hospitalizations and symptoms in preschool age, and especially in the non-sensitized younger patients, are manifold:
One of the main reasons are the “small airways”, or the narrowness of the still-growing bronchial system (12, 13, 36). Swelling of the mucous membranes by viral and bacterial pathogens can quickly lead to obstruction with increased airway resistance and oxygen requirements. In addition, uncontrolled asthma is associated with small airways dysfunction, bronchial hyperresponsiveness and inflammatory changes including increased airway smooth muscle mass, and eosinophilic inflammation (37–39). On the other hand, young children have to build up their immunity in infancy, when they come into contact with other children and multiple pathogens (40). Accordingly, immunizations to viral and bacterial antigens are important to prevent future exacerbations in children with asthma (41, 42).
The dogma that preschool asthma is often described as susceptible to exacerbations with relatively limited impairment is at least wrong for our cohort. The PACD dairy revealed that the percentage of days with asthma were 37,9%, and more than 38 patients (41%) had health care contacts with their local physicians in addition to the care they received in the TIPP study. The symptom burden and the healthcare utilization are much higher than in the famous study of Guilbert TW (43). In this study, the proportion of episode-free days at beginning of the ICS treatment was 72.6% only (43).
Interestingly, patients without sensitization had significantly more days with asthma, more days with salbutamol use, night-time awakenings and health care utilization than patients with allergic sensitization. At first glance this finding is surprising, since most long-term or birth cohort studies on asthma persistence in childhood showed that early sensitization—especially to HDM—is a strong indicator for asthma persistence later in life, whereas the non-sensitized patients have a greater chance to outgrow of this condition (32, 44). However, in real life, the non-sensitized preschoolers are often less well controlled by ICS and suffer from severe virus induced exacerbations, despite treatment. Several reasons could explain our findings. The non-sensitized patients were younger and smaller. Accordingly, they have increased bronchial vulnerability due to small airways and their immune system is less mature than in sensitized patients. Recently, we demonstrated that severe bronchial hyperresponsiveness (BHR) was present in both non-sensitized and sensitized preschool asthma patients (45). At follow-up, in patients without sensitization, BHR normalizes, whereas in sensitized patients HDM allergy indicates persistence of BHR and asthma beyond school-age (45).
The presence of sensitization, measured by specific IgE to common allergens, especially HDM, is one of the strongest associations for persistent childhood asthma (46, 47). In our cohort, 33% of tested patients had at least one sIgE ≥ 0.75 KU/L to the allergen tested. The sensitization pattern showed that pollen allergen (birch, grass) and HDM are the main allergens for preschool children. Surprisingly, peanut sensitization was the main allergen for food allergens and the prevalence was much higher than in the Unites states. The high prevalence of peanut sensitization may be in part due to cross-reactivity to pollen (48) and it is well known that the incidence of food allergy in asthma is higher than in the normal population (49). Among pollen allergens, birch sensitization was found in 63% and grass sensitization in 32% of sensitized patients. HDM sensitization was present in 14 patients (52%) and cat sensitization in 11 (38%) patients of sensitized children. It is well-known that early sensitization to pollen, HDM, and cat, is an indicator of a TH-2 high phenotype (high blood eosinophilia and atopy); and that these patients were more likely to develop persistent asthma at school-age (44, 46–51). Moreover, asthma patients showing a TH-2 phenotype with high total, specific IgE and elevated eosinophils are more likely to respond to ICS than the non-TH-2 phenotype (11, 28).
Our study has several limitations: First, a complete data set with eosinophils and IgE results was only available in 87% of patients. This was mostly due to problems in shipping the material and sometimes to difficulties in successfully taking blood samples from young children.
Second, it is very difficult to estimate how often such severe preschool asthma occurs in Germany. According to the recent German “Weißbuch Lunge 2023” using insurance data, at least 193,186 patients were classified as having asthma in the age group 1–4 years (52). We estimate that at least 5% of these patients suffer from partial and uncontrolled preschool asthma. Our experience with recruiting patients for the TIPP study has taught us that only 10–20 percent of parents caring for children with severe asthma have given their consent to participate in this study. These parents were mostly employed in the medical field and were less hesitant to take part in study.
Conclusion: Our data showed that a significant number of preschool children with severe asthma are not fully controlled by ICS. This is consistent with earlier reports reporting that ICS and ICS + LABA combinations are less effective in this age group. The symptom burden of this cohort was high, especially in the non-sensitized younger patients. Thus, new treatment options are urgently needed. Inhaled anticholinergic agents are a new option which, in addition to inhaled beta2-agonists, reduce the number of hospital admissions in this age group (53). At least two studies showed that Tiotropium was well-tolerated and efficacious as add-on therapy to ICS plus one or more controller medications (30, 31). However, further prospective studies such as the ongoing TIPP study (study protocol in the Supplementary Material) are required before recommending Tiotropium as additional controller therapy in severe preschool asthma.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethics approval was obtained from the leading Ethics Committee of the Goethe-University in Frankfurt (application number 2021-443-AMG) and all participating Ethics Committees. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
SZ: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization. JW: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft. NW: Data curation, Project administration, Validation, Supervision, Methodology, Writing – review & editing. TN: Data curation, Investigation, Methodology, Supervision, Validation, Writing – review & editing. CG: Investigation, Writing – review & editing. DS: Investigation, Writing – review & editing. FS: Investigation, Writing – review & editing. FP: Investigation, Writing – review & editing. ML: Investigation, Writing – review & editing. BS: Investigation, Writing – review & editing. CL: Investigation, Writing – review & editing. MD: Investigation, Writing – review & editing. JT: Investigation, Software, Supervision, Writing – review & editing. HD: Investigation, Supervision, Writing – review & editing. SL: Investigation, Writing – review & editing. EH: Investigation, Writing – review & editing. CV: Investigation, Writing – review & editing. MG: Investigation, Writing – review & editing. MW: Investigation, Writing – review & editing. RS: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing. LS: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. MG: Formal Analysis, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from the German Federal Ministry of Education and Research (01KG2030). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. This was an independent investigator-initiated study supported by Boehringer Ingelheim. Boehringer Ingelheim provided the placebo and tiotropium respimat inhaler. However, Boehringer Ingelheim had no role in the design, analysis or interpretation of the results in this study.
Acknowledgments
We thank all site investigators and study nurses involved in this study, the members of the Independent Data Monitoring Committee, the team of AppCologne GmbH for developing the TIPP diary app, and BI for their support. The corresponding author confirms that he had full access to all the data in the study and had final responsibility for the decision to submit for publication. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to Boehringer Ingelheim substances, as well as intellectual property considerations.
Conflict of interest
SZ reports grants and personal fees from Engelhard-Arzneimittel GmbH, personal fees from Stallergen, AstraZeneca, Sanofi/Pasteur, Erydel, and Allergy therapeutics outside the submitted work.
The remaining authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1558256/full#supplementary-material
Supplementary File 1 | Protocol of the TIPP study.
Supplementary Figure S1 | GINA level in relation to months of enrollment.
Abbreviations
BHR, bronchial hyperresponsiveness; ICS, inhaled corticosteroids; EU, European Union; GINA, global initiative for asthma; HDM, house dust mite; IgE, immunoglobulin E; LTRA, leukotriene receptor antagonist; LABA, long-acting beta agonist; LAMA, long-acting Muscarin receptor agonist; PACD, pediatric asthma caregiver diary; sIgE, specific immunoglubulin E; ICMJE, Committee of Medical Journal Editors; TH-1, T helper-cells-1; TH-2, T helper-cells-2; TRACK, test for respiratory and asthma control in kids.
References
1. Ferrante G, La Grutta S. The burden of pediatric asthma. Front Pediatr. (2018) 6:186. doi: 10.3389/fped.2018.00186
2. European Respiratory Society. European lung white book—Chapter 11 Childhood asthma. Available online at: https://www.erswhitebook.org/chapters/childhood-asthma/ (accessed November 15, 2019).
3. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatrics. (2019) 7:246. doi: 10.3389/fped.2019.00246
4. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. (2007) 42(8):723–8. doi: 10.1002/ppul.20644
5. Makrinioti H, Fainardi V, Bonnelykke K, Custovic A, Cicutto L, Coleman C, Eiwegger T, Kuehni C, Moeller A, Pedersen E, Pijnenburg M. European Respiratory society statement on preschool wheezing disorders: updated definitions, knowledge gaps and proposed future research directions. Eur Respir J. 2024;64(3):2400624. doi: 10.1183/13993003.00624-2024
6. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med. (1995) 332:113–82. doi: 10.1056/NEJM199501193320301
7. Carlsen KH. What distinguishes the asthmatic amongst the infant wheezers? Pediatr Allergy Immunol. (1997) 8:S40–45. doi: 10.1111/j.1399-3038.1997.tb00163.x
8. Kurukulaaratchy RJ, Matthews S, Holgate ST, Arshad SH. Predicting persistent disease among children who wheeze during early life. Eur Respir J. (2003) 22(5):767–71. doi: 10.1183/09031936.03.00005903
9. Inoue Y, Shimojo N. Epidemiology of virus-induced wheezing/asthma in children. Front Microbiol. (2013) 4:391. doi: 10.3389/fmicb.2013.00391
10. Akinbami L, Moorman J, Garbe P, Sondik E. Status of childhood asthma in the United States, 1980–2007. Pediatrics. (2009) 123:S131–45. doi: 10.1542/peds.2008-2233C
11. Beigelman A, Bacharier LB. Management of preschool recurrent wheezing and asthma: a phenotype-based approach. Curr Opin Allergy Clin Immunol. (2017) 17(2):131–8. doi: 10.1097/ACI.0000000000000344
12. Hogg JC, Paré PD, Hackett T-L. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev. (2017) 97(2):529–52. doi: 10.1152/physrev.00025.2015
13. Carr TF, Altisheh R, Zitt M. Small airways disease and severe asthma. World Allergy Organ J. (2017) 10(1):20. doi: 10.1186/s40413-017-0153-4
14. Liu AH, Gilsenan AW, Stanford RH, Lincourt W, Ziemiecki R, Ortega H. Status of asthma control in pediatric primary care: results from the pediatric asthma control characteristics and prevalence survey study (ACCESS). J Pediatr. (2010) 157:276–81. doi: 10.1016/j.jpeds.2010.02.017
15. Guilbert TW, Garris C, Jhingran P, Bonafede M, Tomaszewski KJ, Bonus T, et al. Asthma that is not well-controlled is associated with increased healthcare utilization and decreased quality of life. J Asthma. (2011) 48:126–32. doi: 10.3109/02770903.2010.535879
16. Haselkorn T, Fish JE, Zeiger RS, Szefler SJ, Miller DP, Chipps BE, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the expert panel report 3 guidelines, increases risk for future severe asthma exacerbations in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) study. J Allergy Clin Immunol. (2009) 124:895–902.e4. doi: 10.1016/j.jaci.2009.07.035
17. Bacharier LB, Guilbert TW. Diagnosis and management of early asthma in preschool-aged children. J Allergy Clin Immunol. (2012) 130(2):287–96; quiz 297–8. doi: 10.1016/j.jaci.2012.04.025
18. Marbury MC, Maldonado G, Waller L. Lower respiratory illness, recurrent wheezing, and day care attendance. Am J Respir Crit Care Med. (1997) 155(1):156–61. doi: 10.1164/ajrccm.155.1.9001305
19. Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten J, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. (2005) 171(4):315–22. doi: 10.1164/rccm.200407-894OC
20. Delmas MC, Marguet C, Raherison C, Nicolau J, Fuhrman C. Readmissions for asthma in France in 2002–2005. Rev Mal Respir. (2011) 28:e115–22. doi: 10.1016/j.rmr.2011.09.023
21. Laitinen LA, Altraja A, Karjalainen EM, Laitinen A. Early interventions in asthma with inhaled corticosteroids. J Allergy Clin Immunol. (2000) 105(2 Pt 2):S582–5. doi: 10.1016/s0091-6749(00)90062-9
22. Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. (2003) 22(2 Suppl):S76–82. doi: 10.1097/01.inf.0000053889.39392.a7
23. Jartti T, Liimatainen U, Xepapadaki P, Vahlberg T, Bachert C, Finotto S, et al. Clinical correlates of rhinovirus infection in preschool asthma. Allergy. (2021) 76(1):247–54. doi: 10.1111/all.14479
24. Makrinioti H, Hasegawa K, Lakoumentas J, Xepapadaki P, Tsolia M, Castro-Rodriguez JA, et al. The role of respiratory syncytial virus- and rhinovirus-induced bronchiolitis in recurrent wheeze and asthma-A systematic review and meta-analysis. Pediatr Allergy Immunol. (2022) 33(3):e13741. doi: 10.1111/pai.13741
25. Fitzpatrick AM, Bacharier LB, Guilbert TW, Jackson DJ, Szefler SJ, Beigelman A, et al. Phenotypes of recurrent wheezing in preschool children: identification by latent class analysis and utility in prediction of future exacerbation. J Allergy Clin Immunol Pract. (2019) 7(3):915–24. doi: 10.1016/j.jaip.2018.09.016
26. Donath H, Kluge S, Sideri G, Trischler J, Jerkic SP, Schulze J, et al. Hospitalization, asthma phenotypes, and readmission rates in pre-school asthma. Front Pediatr. (2020) 8:562843. doi: 10.3389/fped.2020.562843
27. Kaiser SV, Huynh T, Bacharier LB, Rosenthal JL, Bakel LA, Parkin PC, et al. Preventing exacerbations in preschoolers with recurrent wheeze: a meta-analysis. Pediatrics. (2016) 137(6):e20154496. doi: 10.1542/peds.2015-4496
28. Fainardi V, Caffarelli C, Deolmi M, Skenderaj K, Meoli A, Morini R, et al. Management of preschool wheezing: guideline from the Emilia-romagna asthma (ERA) study group. J Clin Med. (2022) 11(16):4763. doi: 10.3390/jcm11164763
29. Global Initiative for Asthma. Global Strategy for asthma management and prevention (2019 update). Available online at: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf (Last accessed June 2019).
30. Vrijlandt E, El Azzi G, Vandewalker M, Rupp N, Harper T, Graham L, et al. Safety and efficacy of tiotropium in children aged 1–5 years with persistent asthmatic symptoms: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. (2018) 6(2):127–37. doi: 10.1016/S2213-2600(18)30012-2
31. Zielen S, Reichert G, Donath H, Trischler J, Schulze J, Eickmeier O, et al. Tiotropium as an add-on treatment option for severe uncontrolled asthma in preschool patients. J Asthma Allergy. (2021) 14:23–30. doi: 10.2147/JAA.S274544
32. Oksel C, Granell R, Haider S, Fontanella S, Simpson A, Turner S, et al. Distinguishing wheezing phenotypes from infancy to adolescence: a pooled analysis of five birth cohorts. Ann Am Thorac Soc. (2019) 16:868–76. doi: 10.1183/13993003
33. Santanello NC, DeMuro-Mercon C, Davies G, Ostrom N, Noonan M, Rooklin A, et al. Validation of a pediatric asthma caregiver diary. J Allergy Clin Immunol. (2000) 106(5):861–6. doi: 10.1067/mai.2000.110478
34. Chipps B, Zeiger RS, Murphy K, Mellon M, Schatz M, Kosinski M, et al. Longitudinal validation of the test for respiratory and asthma control in kids in pediatric practices. Pediatrics. (2011) 127(3):e737–47. doi: 10.1542/peds.2010-1465
35. Yoshihara S, Tsubaki T, Ikeda M, Lenney W, Tomiak R, Hattori T, et al. The efficacy and safety of fluticasone/salmeterol compared to fluticasone in children younger than four years of age. Pediatr Allergy Immunol. (2019) 30(2):195–203. doi: 10.1111/pai.13010
36. Cottini M, Lombardi C, Berti A, Comberiati P. Small-airway dysfunction in paediatric asthma. Curr Opin Allergy Clin Immunol. (2021) 21(2):128–34. doi: 10.1097/ACI.0000000000000728
37. Martin G, Duguet A, Eidelman DH. The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease. Eur Respir J. (2000) 16(2):349–54. doi: 10.1034/j.1399-3003.2000.16b25.x
38. Shi Y, Aledia AS, Tatavoosian AV, Vijayalakshmi S, Galant SP, George SC. Relating small airways to asthma control by using impulse oscillometry in children. Allergy Clin Immunol. (2012) 129(3):671–8. doi: 10.1016/j.jaci.2011.11.002
39. Denlinger LC, Heymann P, Lutter R, Gern JE. Exacerbation-prone asthma. J Allergy Clin Immunol Pract. (2020) 8(2):474–82. doi: 10.1016/j.jaip.2019.11.009
40. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci. (2015) 282(1821):20143085. doi: 10.1098/rspb.2014.3085
41. Martínez-Baz I, Navascués A, Casado I, Portillo ME, Guevara M, Gómez-Ibáñez C, et al. Effect of influenza vaccination in patients with asthma. CMAJ. (2021) 193(29):E1120–8. doi: 10.1503/cmaj.201757
42. Gao YD, Xepapadaki P, Cui YW, Stanic B, Maurer DJ, Bachert C, et al. Effect of Haemophilus influenzae, Streptococcus pneumoniae and influenza vaccinations on infections, immune response and asthmacontrol in preschool children with asthma. Allergy. (2023) 78(6):1473–88. doi: 10.1111/all.15551
43. Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. (2006) 354(19):1985–97. doi: 10.1056/NEJMoa051378
44. Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. (2006) 368(9537):763–70. doi: 10.1016/S0140-6736(06)69286-6
45. Donath H, Klenner H, Hutter M, Meoli A, Trischler J, Schulze J, et al. Severe bronchial hyperresponsiveness along with house dust mite allergy indicates persistence of asthma in young children. Pediatr Allergy Immunol. (2023) 34(12):e14047. doi: 10.1111/pai.14047
46. Oksel C, Custovic A. Development of allergic sensitization and its relevance to paediatric asthma. Curr Opin Allergy Clin Immunol. (2018) 18:109–16. doi: 10.1097/ACI.0000000000000430
47. Bacharier LB, Guilbert TW, Jartti T, Saglani S. Which wheezing preschoolers should be treated for asthma? J Allergy Clin Immunol Pract. (2021) 9(7):2611–8. doi: 10.1016/j.jaip.2021.02.045
48. Niggemann B, Schmitz R, Schlaud M. The high prevalence of peanut sensitization in childhood is due to cross-reactivity to pollen. Allergy. (2011) 66(7):980–1. doi: 10.1111/j.1398-9995.2011.02561.x
49. Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the national health and nutrition examination survey 2005-2006. J Allergy Clin Immunol. (2010) 126(4):798–806.e13. doi: 10.1016/j.jaci.2010.07.026
50. Sonntag HJ, Filippi S, Pipis S, Custovic A. Blood biomarkers of sensitization and asthma. Front Pediatr. (2019) 7:251. doi: 10.3389/fped.2019.00251
51. Laubhahn K, Schaub B. From preschool wheezing to asthma: immunological determinants. Review Pediatr Allergy Immunol. (2023) 34(10):e14038. doi: 10.1111/pai.14038
52. Gillissen A, Jany B, Randerath W. Weißbuch Lunge 2023. Pneumologische Erkrankungen in Deutschland-Zahlen und Fakten. 5 edn. Langenhagen, Germany: Deutsche Lungenstiftung (2023). Available online at: https://www.lungenstiftung.de/blog/weissbuch-lunge-neuauflage-2023
Keywords: preschool asthma, uncontrolled asthma, PACD, hospitalization, asthma phenotypes, tiotropium bromide
Citation: Zielen S, Wosniok J, Wollscheid N, Nickolay T, Grimmel C, Scheele D, Sattler F, Prenzel F, Lorenz M, Schaub B, Lex C, Dahlheim M, Trischler J, Donath H, Lau S, Hamelmann E, Vogelberg C, Gerstlauer M, Wetzke M, Schubert R, Schollenberger L and Gappa M (2025) Characteristics of children with severe preschool asthma prior to starting the TIPP study. Front. Pediatr. 13:1558256. doi: 10.3389/fped.2025.1558256
Received: 9 January 2025; Accepted: 6 February 2025;
Published: 5 March 2025.
Edited by:
Ting Fan Leung, The Chinese University of Hong Kong, ChinaReviewed by:
Arturo Solis-Moya, Dr. Carlos Sáenz Herrera National Children's Hospital, Costa RicaKenan Haver, Boston Children's Hospital and Harvard Medical School, United States
Copyright: © 2025 Zielen, Wosniok, Wollscheid, Nickolay, Grimmel, Scheele, Sattler, Prenzel, Lorenz, Schaub, Lex, Dahlheim, Trischler, Donath, Lau, Hamelmann, Vogelberg, Gerstlauer, Wetzke, Schubert, Schollenberger and Gappa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Zielen, cy56aWVsZW5AbWVkYWltdW4uZGU=
 S. Zielen
S. Zielen J. Wosniok2
J. Wosniok2 C. Grimmel
C. Grimmel M. Lorenz
M. Lorenz B. Schaub
B. Schaub J. Trischler
J. Trischler S. Lau
S. Lau M. Gerstlauer
M. Gerstlauer