
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 04 April 2025
Sec. Pediatric Nephrology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1554929
Objective: To assess hospitalization costs in pediatric chronic kidney disease (CKD) patients, compare the economic burden between those with and without infections, and identify key factors influencing these costs, emphasizing the significant financial impact on families and healthcare systems.
Methods: This retrospective analysis included pediatric patients with CKD hospitalized between May 2011 and April 2020. Clinical characteristics, including demographics, etiology, urinary protein level, estimated glomerular filtration rate, and CKD stage, were analyzed. Hospitalization costs were compared between groups with and without infection using appropriate statistical methods.
Results: Among 721 pediatric CKD patients included in this study, 388 had primary kidney disease and 333 had secondary kidney disease. Patients in the infection group had significantly higher urine protein levels, longer hospital stays, and higher total hospital fees than those without infection (all P < 0.05). In the primary kidney disease cohort, patients aged 14–18 years incurred the highest costs (16,706 CNY, P = 0.009), while those with 1 + urine protein levels had expenses averaging 29,813 CNY (P = 0.035). In the secondary kidney disease cohort, the 3 + urine protein group had the highest costs (62,841 CNY, P < 0.001). Multiple linear regression identified age, urine protein level, and length of hospital stay as significant cost determinants. Patients with infection in the secondary kidney disease cohort had an average additional expenditure of 13,572.55 CNY compared to those without infection (P = 0.001).
Conclusion: This study highlights the economic burden of infection during pediatric CKD hospitalization, emphasizing the need for effective infection management strategies to reduce financial strain and improve outcomes.
Chronic kidney disease (CKD) in children has emerged as a significant global health issue, affecting an estimated 15–74.7 cases per million children globally and posing considerable risks to pediatric populations. CKD is defined as an abnormality in kidney structure or function that lasts more than 3 months (1, 2). Additionally, this condition can persist into adulthood, impacting up to 10%–15% of the adult population globally (2, 3). Recent epidemiological studies have reported a rising prevalence of CKD among children, positioning it as one of the most serious threats to pediatric health today (4). The etiology of pediatric CKD is multifaceted, encompassing primary kidney diseases ·like congenital anomalies and secondary conditions arising from systemic illnesses, such as systemic lupus erythematosus and Henoch-Schönlein purpura. This complexity leads to varied rates of disease progression and distinct hospitalization patterns, placing considerable strain on healthcare resources and underscoring the urgent need for effective management strategies (5, 6).
The increasing incidence of pediatric CKD has profound implications for healthcare systems, particularly concerning the economic burdens borne by families and healthcare providers. The costs associated with managing CKD in children can be substantial, often resulting in significant financial distress for families navigating the complexities of long-term treatment and care (7, 8). Unlike adult CKD patients, who commonly experience complications such as hypertension and diabetes, children with CKD are at risk for a greater variety of complications, including hypertension, anemia, electrolyte imbalance, and increased susceptibility to infection, which have become more prevalent, especially during the coronavirus disease 2019 (COVID-19) pandemic (9). Infections not only complicate the clinical management of CKD but also substantially increase hospitalization costs, exacerbating the economic burden on both families and the healthcare system. Despite the recognized significance of these complications, comprehensive studies addressing their impact on hospitalization costs for pediatric CKD patients are lacking.
As the understanding of pediatric CKD evolves, the results of economic analyses must be integrated into clinical care and healthcare policies to effectively address the needs of these patients. While the existing literature has primarily focused on the relationship between specific comorbidities and CKD outcomes, the broader context of hospitalization expenses has been largely overlooked (10, 11). The present study aimed to analyze hospitalization costs and their components in a cohort of 721 pediatric CKD patients with a systematic investigation of the interplay between common infections and hospitalization outcomes. By providing insights into the economic burden associated with pediatric CKD, the findings may inform targeted interventions aimed at reducing hospitalization costs and improving patient outcomes, ultimately guiding healthcare providers to make informed decisions regarding resource allocation and management strategies for this vulnerable population.
This study was conducted using the renal disease database of the Third Affiliated Hospital of Sun Yat-sen University. A retrospective analysis was conducted on hospitalization costs for pediatric patients under 18 years of age diagnosed with CKD. Additionally, the study compared the hospitalization costs between CKD patients who did or did not experience infection.
The study received approval from the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University [approval number: (2019)02-427-01] and complied with the principles outlined in the Declaration of Helsinki. Informed written consent was obtained from the patients' parents or guardians.
This study included pediatric patients aged 18 years or younger who were hospitalized at the Third Affiliated Hospital of Sun Yat-sen University between May 2011 and April 2020. A total of 721 patients were included based on the definitions and criteria for CKD.
Inclusion criteria: According to the 2012 clinical practice guidelines from Kidney Disease: Improving Global Outcomes (KDIGO), CKD was defined as abnormalities in kidney structure or function lasting for more than 3 months, with health implications, and meeting at least one of the following criteria (12): (1) proteinuria (urinary protein excretion rate ≥30 mg/24 h; urine protein-to-creatinine ratio ≥30 mg/g); (2) urinary sediment abnormalities; (3) electrolyte and other abnormalities due to tubular disorders; (4) histological abnormalities; (5) structural abnormalities detected by imaging; (6) history of kidney transplantation; (7) decreased glomerular filtration rate (GFR) for at least 3 months; and (8) estimated glomerular filtration rate (eGFR) < 60 ml/min·1.73 m².
Exclusion criteria: (1) acute kidney injury at time of hospitalization; (2) significant comorbidities affecting kidney function; (3) recent exposure to nephrotoxic agents; and (4) incomplete medical records or insufficient lab data.
To assess the factors influencing hospitalization costs for pediatric patients with CKD, data for a variety of variables were collected from the renal disease database. Key demographic variables, such as gender and age, were recorded for analysis of their impact on treatment and costs. Additionally, the etiology of CKD was documented for use in investigating the underlying causes of the disease.
Clinical metrics included disease history, urinary protein level, eGFR, CKD stage, and complications such as the site and severity of infections, all aimed at evaluating disease severity and progression. The length of hospitalization also was recorded for analysis of its impact on overall costs. On qualitative urine protein testing (indicator protein error method), the results were categorized as negative, trace, 1+, 2+, and 3+, corresponding to no proteinuria, trace, mild, moderate, and heavy proteinuria, respectively. CKD was staged based on the eGFR as follows: eGFR ≥90 ml/min·1.73 m² as stage 1, corresponding to normal kidney function with kidney damage; eGFR 60–89 ml/min·1.73 m² as stage 2, reflecting a mild decrease in kidney function; eGFR 30–59 ml/min·1.73 m² as stage 3, indicating moderate impairment; eGFR 15–29 ml/min·1.73 m² as stage 4, indicating severe kidney dysfunction; and eGFR <15 ml/min·1.73 m² as stage 5, representing end-stage renal disease.
The economic burden data included all in-hospital fees, with detailed records of medical insurance expenses and out-of-pocket costs incurred by families. Specific fees related to diagnosis, treatment, nursing care, laboratory tests, imaging, and clinical assessments were documented. Additionally, costs associated with surgical treatments, expenses for Western and traditional Chinese medicine, and material costs were recorded. This comprehensive data collection was assembled for use in identifying key cost drivers, with the goal of informing strategies to improve care while alleviating the financial burden on families managing pediatric CKD.
All statistical analyses were conducted using Stata/SE version 17.0 (StataCorp LP, College Station, TX, USA). The normality of all continuous variables was assessed using the Kolmogorov–Smirnov test. As none of the continuous variables met the normality assumption, they were reported using median and interquartile range (IQR, PR25, PR75) values. Comparisons between patients with and without infection were conducted using the Mann–Whitney U test. Categorical variables were presented as counts and percentages, and comparisons were made using the chi-square test or Fisher's exact test (for expected values ≤5). The analysis of total hospitalization costs was also stratified by age group and urine protein level, with intergroup comparisons performed using one-way analysis of variance (ANOVA) followed by Fisher's least significant difference (LSD) post-hoc test. A multiple linear regression model was utilized to investigate the linear relationship between overall infection and total hospitalization cost, with adjustments for clinical characteristics as covariates. Statistical significance was defined as P < 0.05 for all tests, with a two-tailed approach.
The study population included a total of 721 pediatric patients with CKD, categorized into two distinct cohorts: those with primary kidney disease (n = 388) and those with secondary kidney disease (n = 333). In the primary kidney disease cohort, 276 patients (71.13%) were male and 112 (28.87%) were female, with a median age of 12 years (IQR: 5.25–16 years). The secondary kidney disease cohort comprised 66 males (19.82%) and 267 females (80.18%), with a median age of 15 years (IQR: 13–17 years).
Table 1 presents comparisons of the clinical characteristics and medical costs between CKD patients with and without infection from the primary kidney disease cohort. As indicated, compared to patients without infection, those who experienced infection were relatively younger and had a lower prevalence of congenital anomalies of the kidney and urinary tract (CAKUT). However, the patients with infection had higher urine protein levels, and longer hospital stays. In terms of medical costs, patients in the infection group had significantly higher in-hospital total fees, out-of-pocket fees, medical services fees, therapeutic procedure fees, nursing fees, laboratory diagnosis fees, non-surgical treatment fees, Western drug fees, antibacterial drug fees, and treatment materials fees (all P < 0.05). Despite these elevated costs, the infected patients had a relatively lower average daily cost, likely attributable to their extended hospital stays (P = 0.025).
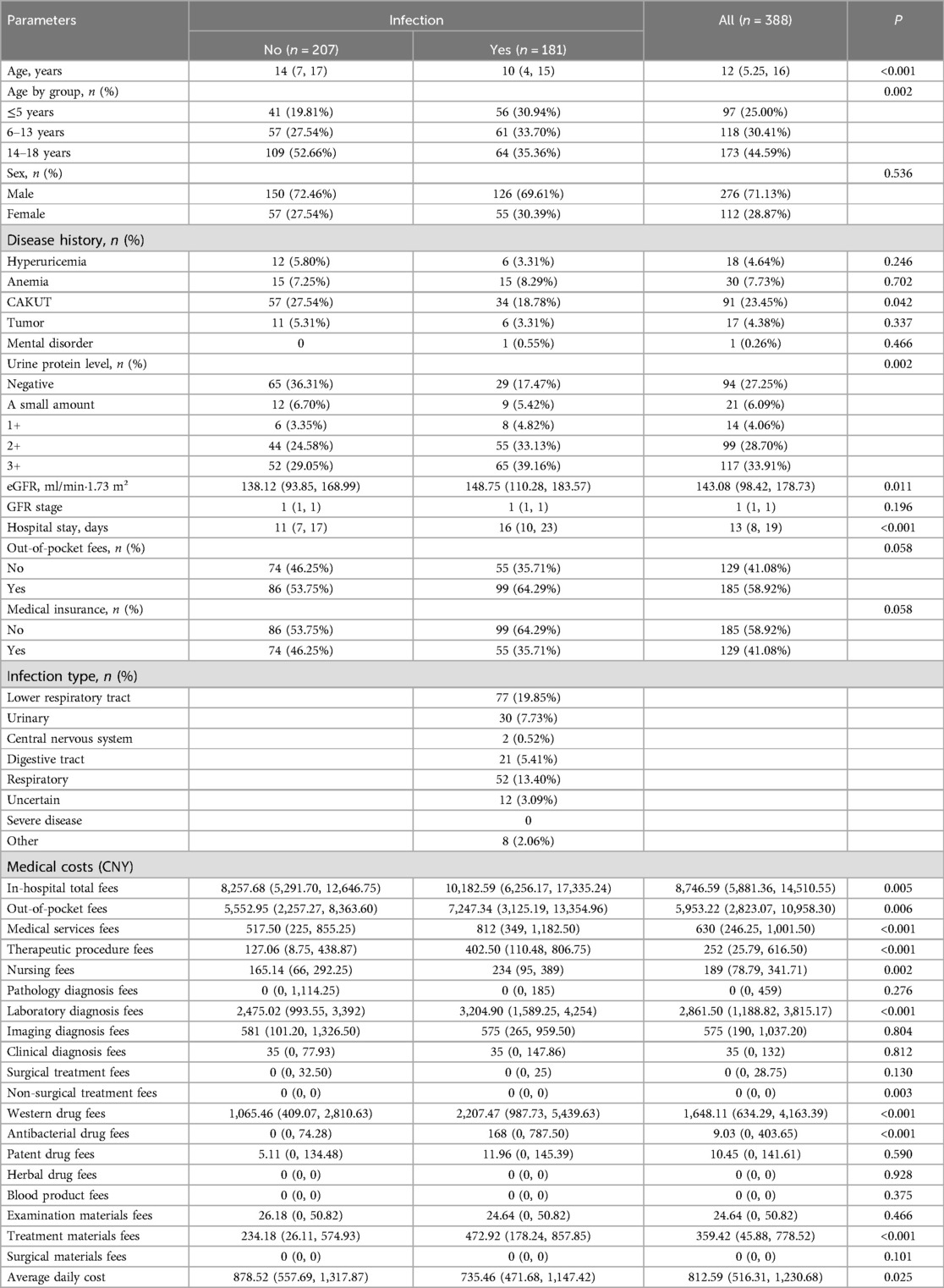
Table 1. Comparison of clinical characteristics and medical costs between patients with or without infection within the primary kidney disease cohort.
Table 2 presents comparisons of the clinical characteristics and medical costs between CKD patients with and without infection from the secondary kidney disease cohort. As indicated, compared to patients without infection, those who experienced infection had a higher rate of anemia, higher urine protein levels, lower eGFR and GFR stages, and longer hospital stays. With respect to medical costs, while the pathology diagnosis fees, patent drug fees, and herbal drug fees did not differ significantly between the groups, all other cost items were significantly higher in the infection group than in the non-infection group (all P < 0.05).
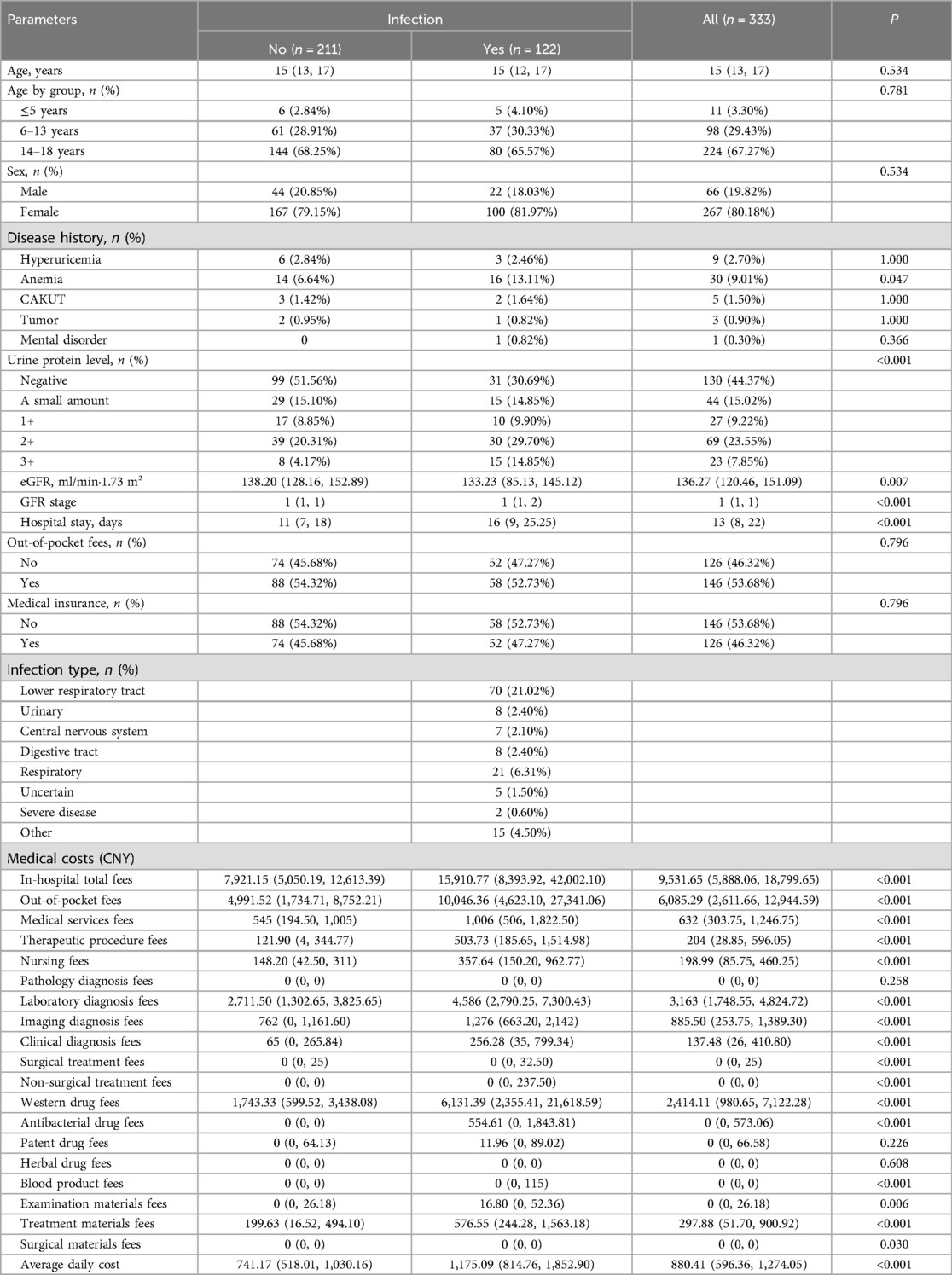
Table 2. Comparison of clinical characteristics and medical costs between patients with or without infection within the secondary kidney disease cohort.
Figure 1 illustrates the in-hospital total fees for patients stratified by age groups or by urine protein levels within the primary and secondary kidney disease cohorts.

Figure 1. In-hospital total fees of patients stratified by age and urine protein level within the primary and secondary kidney disease cohorts. (A,B) Cost comparisons among patients with primary kidney disease stratified by age and urine protein level. (C,D) Cost comparisons among patients with secondary kidney disease stratified by age and urine protein level.
In the primary kidney disease cohort, both age group and urine protein level were key factors in categories with particularly high costs. As shown in Figure 1, the oldest age group (14–18 years) incurred the highest costs, reaching 16,706 CNY (F = 4.80, P = 0.009). This amount was significantly greater than the costs for the groups aged 3–5 years (P = 0.040) and 6–13 years (P = 0.005) on post-hoc comparisons. Additionally, the 1 + urine protein level group had significantly higher medical costs, averaging 29,813 CNY (F = 2.62, P = 0.035), and in post-hoc comparisons, the costs for this group were significantly higher than those for the other four urine protein level groups (P = 0.003, 0.005, 0.004, and 0.011, respectively).
In the secondary kidney disease cohort, no significance differences in costs were observed among the age groups (F = 0.54, P = 0.586). According to the urine protein level though, the 3 + urine protein level group incurred significantly higher medical costs than the other groups, averaging 62,841 CNY (F = 7.19, P < 0.001), and on post-hoc comparisons, the costs for this group were significantly higher than the costs for each of the other four groups (all P < 0.001).
Table 3 presents the results of the multiple linear regression analysis conducted within the primary kidney disease cohort. With adjustments for age, sex, urine protein level, and length of hospital stay, total medical costs (in-hospital total fees) did not differ significantly between the groups with and without infection (P = 0.250). However, the model still revealed significant associations between patient age, urine protein level, length of hospital stay, and total medical costs (all P < 0.05). Overall, for each additional year of age, the average medical costs increased by 771.40 CNY. Moreover, each additional day of hospitalization added 684.20 CNY to the total cost. Patients with the 1 + urine protein level in this cohort incurred an average additional total medical cost of 13,442.22 CNY compared with those with negative urine protein, while for patients with the 3 + urine protein level, the total medical cost was on average 5,753.90 CNY less than that for patients with negative urine protein.

Table 3. Multiple linear regression analysis of factors influencing in-hospital total fees within the primary kidney disease cohort.
The multiple linear regression analysis results for the secondary kidney disease cohort are presented in Table 4. As indicated, patients in the infection group, on average, incurred an additional expenditure of 13,572.55 CNY compared with those in the non-infection group (P = 0.001). Of the covariates, each additional day of hospitalization resulted in an extra cost of 1953.23 CNY (P < 0.001). Patients with the 3 + urine protein level in this cohort incurred an average additional total medical cost of 20,360.05 CNY relative to patients with negative urine protein (P = 0.008).
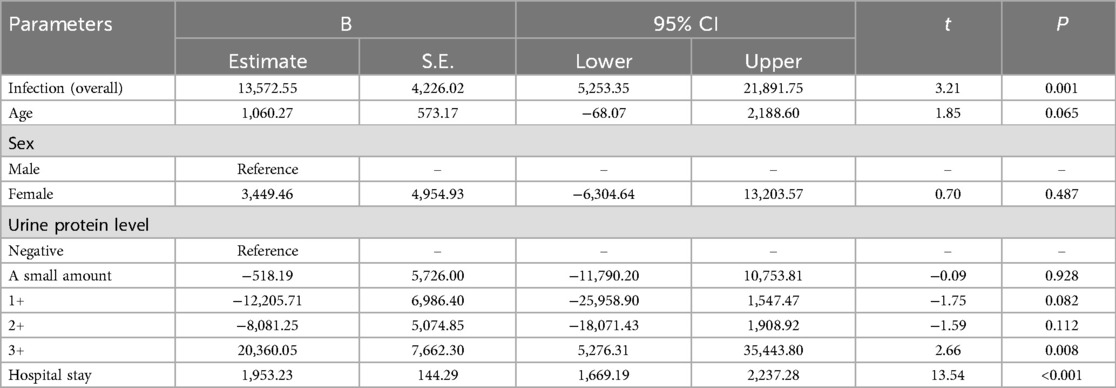
Table 4. Multiple linear regression analysis of factors influencing in-hospital total fees within the secondary kidney disease cohort.
Research to date regarding the hospitalization costs and specific components for pediatric patients with CKD has been limited. Unlike adult patients, children with CKD rarely present with comorbidities such as hypertension, diabetes, or coronary heart disease; instead, infection has become a prevalent complication, particularly since the onset of the COVID-19 pandemic (9, 13). The present study included 721 pediatric CKD patients and revealed that hospitalization costs vary significantly based on infection status, age, and CKD classification (primary vs. secondary kidney disease). Patients with infections incurred substantially higher hospitalization costs than those without, primarily due to longer lengths of stay and increased medical service expenses. Additionally, age and urinary protein level emerged as critical determinants of hospitalization costs, with older patients and those with higher urinary protein levels facing greater financial burdens. These findings underscore the urgent need for effective infection management to alleviate economic pressures on families and the healthcare system, while also supporting targeted interventions based on identified risk factors.
The economic burden associated with infection in pediatric CKD patients is significant, and our findings align with the existing literature, which has consistently identified infection as a major complication in this population (14). Infections can worsen the progression of CKD, leading to further kidney damage and increasing the risk of severe complications such as sepsis. The associated decline in health often results in longer hospital stays, which directly increases hospitalization costs. Multiple studies (15, 16) have shown that the occurrence of infection substantially contributes to elevated medical expenses, placing additional financial strain on families and healthcare systems. Moreover, recurrent infections can negatively impact a child's overall health and development, resulting in missed educational opportunities and increased psychological stress. The results of our present study underscore the stark cost differences between cases with and without infection, emphasizing the urgent need for the development and implementation of effective infection prevention strategies. Targeted interventions aimed at reducing infection rates are crucial for alleviating these economic burdens and improving patient outcomes (17).
In the present study, age was identified as a critical factor influencing hospitalization costs for pediatric CKD. Age is known to significantly impact the progression of CKD due to physiological changes in kidney function and the accumulation of comorbidities over time. Younger patients may experience different disease manifestations and treatment responses than older patients, whose kidneys may already be compromised by age-related factors and other health issues. Our findings indicate that adolescents aged 14–18 years incur significantly higher expenses than younger children, likely due to the complexities of managing CKD in this age group, which often faces unique psychosocial challenges and issues related to treatment adherence (18, 19). Effective management of pediatric CKD must address these age-specific challenges through tailored strategies, such as counseling on treatment adherence and providing psychosocial support to enhance overall care.
The present study also identified urinary protein level as a significant influencing factor for hospitalization costs in pediatric CKD patients. Urinary protein is well established as a critical biomarker for assessing kidney damage and disease progression in CKD. Elevated proteinuria is associated with deteriorating kidney function and an increased risk of cardiovascular complications, negatively affecting overall prognosis (20–22). Effective monitoring and management of urinary protein can provide valuable insights into disease severity and inform treatment strategies. The identification of urinary protein level as a significant determinant of hospitalization costs aligns with the understanding that higher proteinuria often indicates more severe disease, leading to greater healthcare utilization. However, it is important to note that severe proteinuria may be caused by systemic infections, complicating the relationship between proteinuria and hospitalization costs. While controlling proteinuria is crucial for long-term disease outcomes, it is debatable whether it alone can significantly reduce hospitalization costs. Factors such as infection management and complications also play critical roles in influencing hospitalization expenses. Therefore, comprehensive strategies addressing multiple aspects of CKD management may be more effective for reducing costs (20).
The findings of the present study can significantly inform clinical practice for the management of pediatric CKD. A multidisciplinary approach including nephrologists, pediatricians, and infectious disease specialists is essential for enhancing patient outcomes while mitigating the economic burdens of treatment. This collaborative care model can address the multifaceted health needs of the children by focusing not only on disease management but also on psychosocial support. Consistently, previous studies have found that integrating different specialties improves treatment adherence and overall health outcomes in pediatric populations facing complex health challenges (3, 23). Additionally, the identification of high-risk groups, particularly infants who may transition from acute kidney injury to chronic disease, allows for timely intervention (24). Early identification through routine screening for urinary tract anomalies in newborns can significantly prevent CKD progression and reduce long-term healthcare costs (25). Furthermore, the implementation of family education programs that emphasize infection prevention and management can empower families to not only avoid some infections but also to recognize symptoms and seek timely medical attention when infections do occur, thereby reducing hospitalization risks and associated expenses. This proactive approach will not only enhance clinical outcomes but also foster a supportive environment for families navigating the complexities of pediatric CKD.
From a healthcare policy perspective, the findings of the present study underscore the necessity for targeted resource allocation to enhance infection prevention programs specifically for pediatric CKD patients. Policymakers should prioritize funding initiatives focused on vaccinations, early screening, and educational efforts for families and caregivers, as effective programs will raise awareness about infection prevention and potentially reduce infection rates and related hospitalization costs (26, 27). Additionally, the development of cost-effective care pathways that incorporate preventive measures would help to optimize healthcare expenditures while improving patient outcomes. In recent years, Diagnosis-Related Groups (DRGs) have emerged as a significant payment method in China's healthcare system, referring to a model in which providers are reimbursed based on specific procedures or diagnoses rather than the length of hospital stay (28, 29). A data-driven approach to refining DRG classifications, based on the cost structures identified in this study, can improve reimbursement models for healthcare providers. By pinpointing high-cost factors, healthcare systems can implement preventive strategies, enabling more accurate assessment of pediatric CKD hospitalization costs. This approach will reduce the financial strain on families while strengthening healthcare infrastructure, and ensuring better long-term care for pediatric CKD patients.
This study provides valuable insights but has several limitations. First, the retrospective design may introduce selection bias, as only hospitalized patients were included, potentially omitting cases managed successfully on an outpatient basis. Future studies should incorporate outpatient data for a more comprehensive analysis of the economic burden of pediatric CKD. Additionally, most CKD cases in the study cohort were stages 1–3, which is typical in pediatric populations, and this early-stage predominance may have skewed the economic burden analysis. The use of a single-center database also limits the generalizability of the findings, as regional differences in medical practices, resources, and patient populations could influence the results. Future research should include multi-center data and longitudinal studies to better understand the long-term economic impact and the effectiveness of intervention strategies in diverse healthcare settings.
In conclusion, the results of the present study underscore the significant economic burden of hospitalization among pediatric CKD patients, with infection, age, and urinary protein level serving as critical determinants of costs. Our findings indicate that patients who experience infection incur markedly greater hospitalization expenses, primarily due to longer stays and increased medical service fees. Our analysis revealed that among different age groups, adolescents face the highest costs, highlighting the need for age-specific management strategies that address unique challenges commonly faced by different age groups. Moreover, the association between a higher urinary protein level and increased healthcare utilization emphasizes the importance of effective interventions to manage this condition. A multidisciplinary approach involving nephrologists, pediatricians, and infectious disease specialists is essential for improving patient outcomes while reducing the financial strain on families. Policymakers should prioritize funding for infection prevention and educational initiatives for families to enhance care and reduce hospitalization rates. Overall, addressing these economic challenges is crucial for both supporting families and improving the quality of care for children with CKD. Future research should expand to include outpatient data to achieve a more comprehensive understanding of the economic impact of pediatric CKD and inform the development of effective intervention strategies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The study received approval from the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University [approval number: (2019)02-427-01] and complied with the principles outlined in the Declaration of Helsinki. Informed written consent was obtained from the patients' parents or guardians. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XS: Investigation, Project administration, Resources, Writing – original draft, Writing – review & editing. LL: Data curation, Project administration, Writing – review & editing. YZ: Data curation, Writing – original draft. XL: Project administration, Writing – original draft. YM: Project administration, Software, Visualization, Writing – original draft. LG: Conceptualization, Validation, Visualization, Writing – original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant Nos. 81370866 and 81070612), the China Postdoctoral Science Foundation (grant No. 201104335), Guangdong Science and Technology Plan (grant Nos. 2011B031800084 and 2013B021800190), the Fundamental Research Funds for the Central Universities (grant No. 11ykpy38), and the National Project of Scientific and Technical Supporting Programs Funded by Ministry of Science and Technology of China (grant No. 2018YFC1315400).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. (2024) 105(4S):S117–S314. doi: 10.1016/j.kint.2023.10.018
2. Becherucci F, Roperto RM, Materassi M, Romagnani P. Chronic kidney disease in children. Clin Kidney J. (2016) 9(4):583–91. doi: 10.1093/ckj/sfw047
3. Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. (2007) 22(12):1999–2009. doi: 10.1007/s00467-006-0410-1
4. Cirillo L, De Chiara L, Innocenti S, Errichiello C, Romagnani P, Becherucci F. Chronic kidney disease in children: an update. Clin Kidney J. (2023) 16(10):1600–11. doi: 10.1093/ckj/sfad097
5. Panzarino V, Lesser J, Cassani FA. Pediatric chronic kidney disease. Adv Pediatr. (2022) 69(1):123–32. doi: 10.1016/j.yapd.2022.03.008
6. Shimonov D, Tummalapalli SL, Donahue S, Narayana V, Wu S, Walters LS, et al. Clinical outcomes of a novel multidisciplinary care program in advanced kidney disease (PEAK). Kidney Int Rep. (2024) 9(10):2904–14. doi: 10.1016/j.ekir.2024.07.018
7. Darwish MM, Hassan SH, Taha SF, Abd El-Megeed HS, Ismail TAM. Family impact and economic burden among caregivers of children with chronic kidney disease in Assiut, Egypt. J Egypt Public Health Assoc. (2020) 95(1):27. doi: 10.1186/s42506-020-00058-7
8. Harambat J, Madden I. What is the true burden of chronic kidney disease in children worldwide? Pediatr Nephrol. (2023) 38(5):1389–93. doi: 10.1007/s00467-022-05816-7
9. Natale P, Zhang J, Scholes-Robertson N, Cazzolli R, White D, Wong G, et al. The impact of the COVID-19 pandemic on patients with CKD: systematic review of qualitative studies. Am J Kidney Dis. (2023) 82(4):395–409.e1. doi: 10.1053/j.ajkd.2023.04.001
10. Adamczuk D, Roszkowska-Blaim M. Long-term outcomes in children with chronic kidney disease stage 5 over the last 40 years. Arch Med Sci. (2017) 13(3):635–44. doi: 10.5114/aoms.2017.67283
11. Chou HH, Lin CY, Chiou YH, Tain YL, Wang YF, Wang HH, et al. Clinical characteristics and prevalence of complications of chronic kidney disease in children: the Taiwan pediatric renal collaborative study. Pediatr Nephrol. (2016) 31(7):1113–20. doi: 10.1007/s00467-016-3325-5
12. Eknoyan G, Lameire N, Eckardt K, Kasiske B, Wheeler D, Levin A, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney int. (2013) 3(1):5–14. doi: 10.1038/kisup.2012.77
13. Piotrowski CC, Strong J, Giesbrecht A, Goldberg A, Kudar K, Pappas K, et al. Coping with COVID-19: perspectives of caregivers of children and young people with chronic kidney disease. Pediatr Transplant. (2024) 28(5):e14823. doi: 10.1111/petr.14823
14. Ishigami J, Matsushita K. Clinical epidemiology of infectious disease among patients with chronic kidney disease. Clin Exp Nephrol. (2019) 23(4):437–47. doi: 10.1007/s10157-018-1641-8
15. Huang W, Li B, Jiang N, Zhang F, Shi W, Zuo L, et al. Impact of the COVID-19 pandemic on patients with chronic kidney disease: a narrative review. Medicine (Baltimore). (2022) 101(24):e29362. doi: 10.1097/md.0000000000029362
16. Prasad N, Bhatt M, Agarwal SK, Kohli HS, Gopalakrishnan N, Fernando E, et al. The adverse effect of COVID pandemic on the care of patients with kidney diseases in India. Kidney Int Rep. (2020) 5(9):1545–50. doi: 10.1016/j.ekir.2020.06.034
17. Doshi S, Wish JB. Strategies to reduce rehospitalization in patients with CKD and kidney failure. Clin J Am Soc Nephrol. (2021) 16(2):328–34. doi: 10.2215/cjn.02300220
18. Kang NR, Ahn YH, Park E, Choi HJ, Kim SH, Cho H, et al. Mental health and psychosocial adjustment in pediatric chronic kidney disease derived from the KNOW-Ped CKD study. Pediatr Nephrol. (2019) 34(10):1753–64. doi: 10.1007/s00467-019-04292-w
19. Zhang Y, Gutman T, Tong A, Craig JC, Sinha A, Dart A, et al. Child and caregiver perspectives on access to psychosocial and educational support in pediatric chronic kidney disease: a focus group study. Pediatr Nephrol. (2023) 38(1):249–60. doi: 10.1007/s00467-022-05551-z
20. Zeng D, Wang B, Xiao Z, Wang X, Tang X, Yao X, et al. Early diagnosis and treatment of kidney injury: a focus on urine protein. Int J Mol Sci. (2024) 25(20):11171. doi: 10.3390/ijms252011171
21. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. (2015) 4(1):57–73. doi: 10.5527/wjn.v4.i1.57
22. Carbayo J, Verdalles Ú, Díaz-Crespo F, Lázaro A, González-Nicolás M, Arroyo D, et al. Tubular biomarkers in proteinuric kidney disease: histology correlation and kidney prognosis of tubular biomarkers. Clin Kidney J. (2024) 17(5):sfae146. doi: 10.1093/ckj/sfae146
23. Plevinsky JM, Gutierrez-Colina AM, Carmody JK, Hommel KA, Crosby LE, McGrady ME, et al. Patient-reported outcomes for pediatric adherence and self-management: a systematic review. J Pediatr Psychol. (2020) 45(3):340–57. doi: 10.1093/jpepsy/jsz096
24. Robinson CH, Iyengar A, Zappitelli M. Early recognition and prevention of acute kidney injury in hospitalised children. Lancet Child Adolesc Health. (2023) 7(9):657–70. doi: 10.1016/s2352-4642(23)00105-0
25. Walawender L, Becknell B, Matsell DG. Congenital anomalies of the kidney and urinary tract: defining risk factors of disease progression and determinants of outcomes. Pediatr Nephrol. (2023) 38(12):3963–73. doi: 10.1007/s00467-023-05899-w
26. Donskey CJ. Empowering patients to prevent healthcare-associated infections. Am J Infect Control. (2023) 51(11, Supplement):A107–A13. doi: 10.1016/j.ajic.2023.03.008
27. Chen W, Lynd LD, FitzGerald JM, Marra CA, Balshaw R, To T, et al. Excess medical costs in patients with asthma and the role of comorbidity. Eur Respir J. (2016) 48(6):1584–92. doi: 10.1183/13993003.01141-2016
28. Chen YJ, Zhang XY, Yan JQ, Xue T, Qian MC, Ying XH. Impact of diagnosis-related groups on inpatient quality of health care: a systematic review and meta-analysis. Inquiry. (2023) 60:469580231167011. doi: 10.1177/00469580231167011
Keywords: chronic kidney disease, children, hospitalization costs, infection, urine protein
Citation: Shi X, Li L, Zhu Y, Liu X, Mou Y and Guo L (2025) Economic burden of hospitalization for Chinese children with chronic kidney disease: a comparison between patients with and without infection. Front. Pediatr. 13:1554929. doi: 10.3389/fped.2025.1554929
Received: 14 January 2025; Accepted: 24 March 2025;
Published: 4 April 2025.
Edited by:
Orkun Tolunay, Adana Faculty of Medicine/University of Health Sciences, TürkiyeReviewed by:
Sevgin Taner, Ege University, TürkiyeCopyright: © 2025 Shi, Li, Zhu, Liu, Mou and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yikun Mou, bXlrNjYzMjFAMTYzLmNvbQ==; Lei Guo, Z3VvbGVpNUBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.